Novel Nickel- and Magnesium-Modified Cenospheres as Catalysts for Dry Reforming of Methane at Moderate Temperatures
Abstract
1. Introduction
2. Results and Discussion
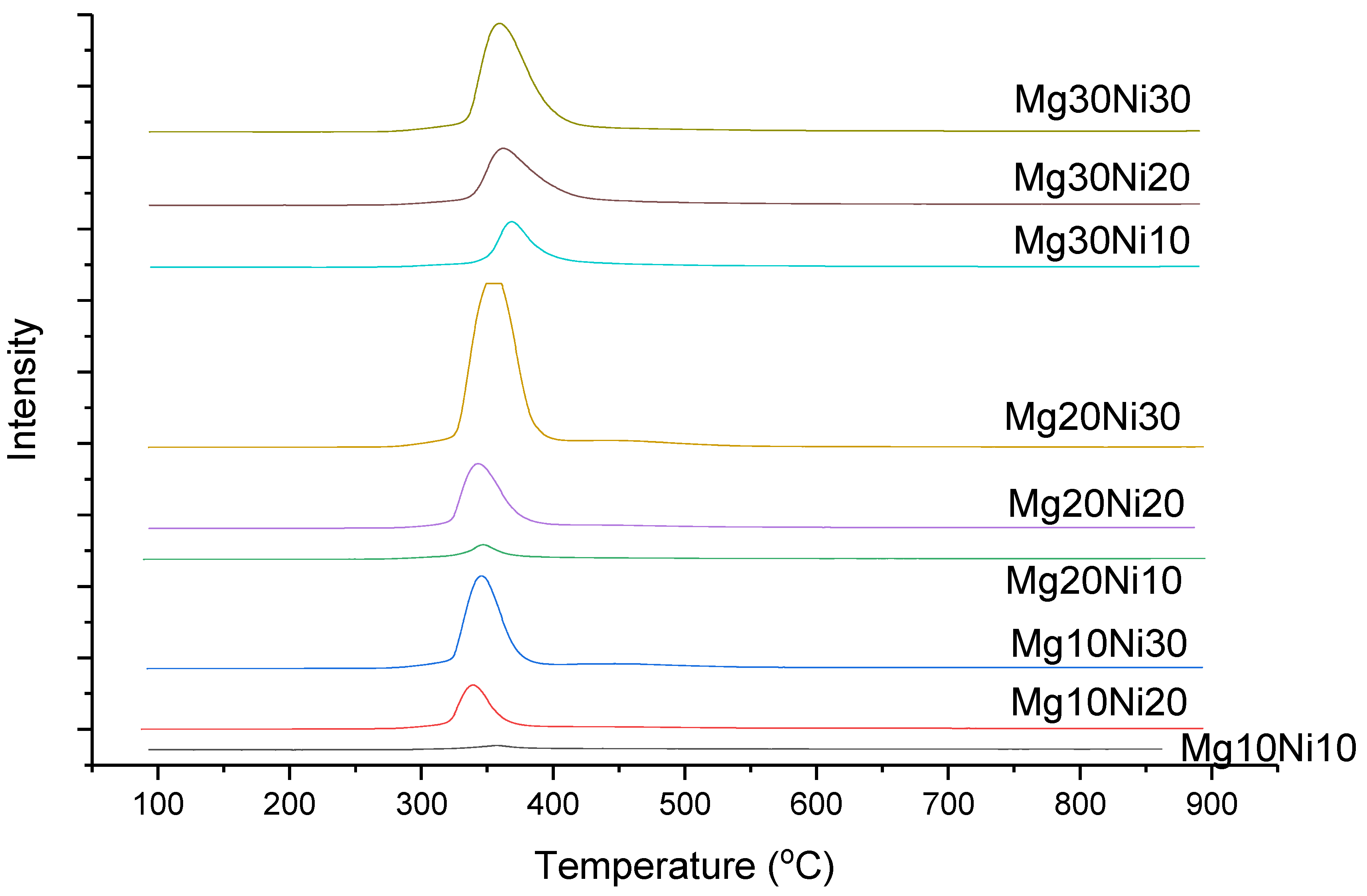
2.1. Catalyst Characterization
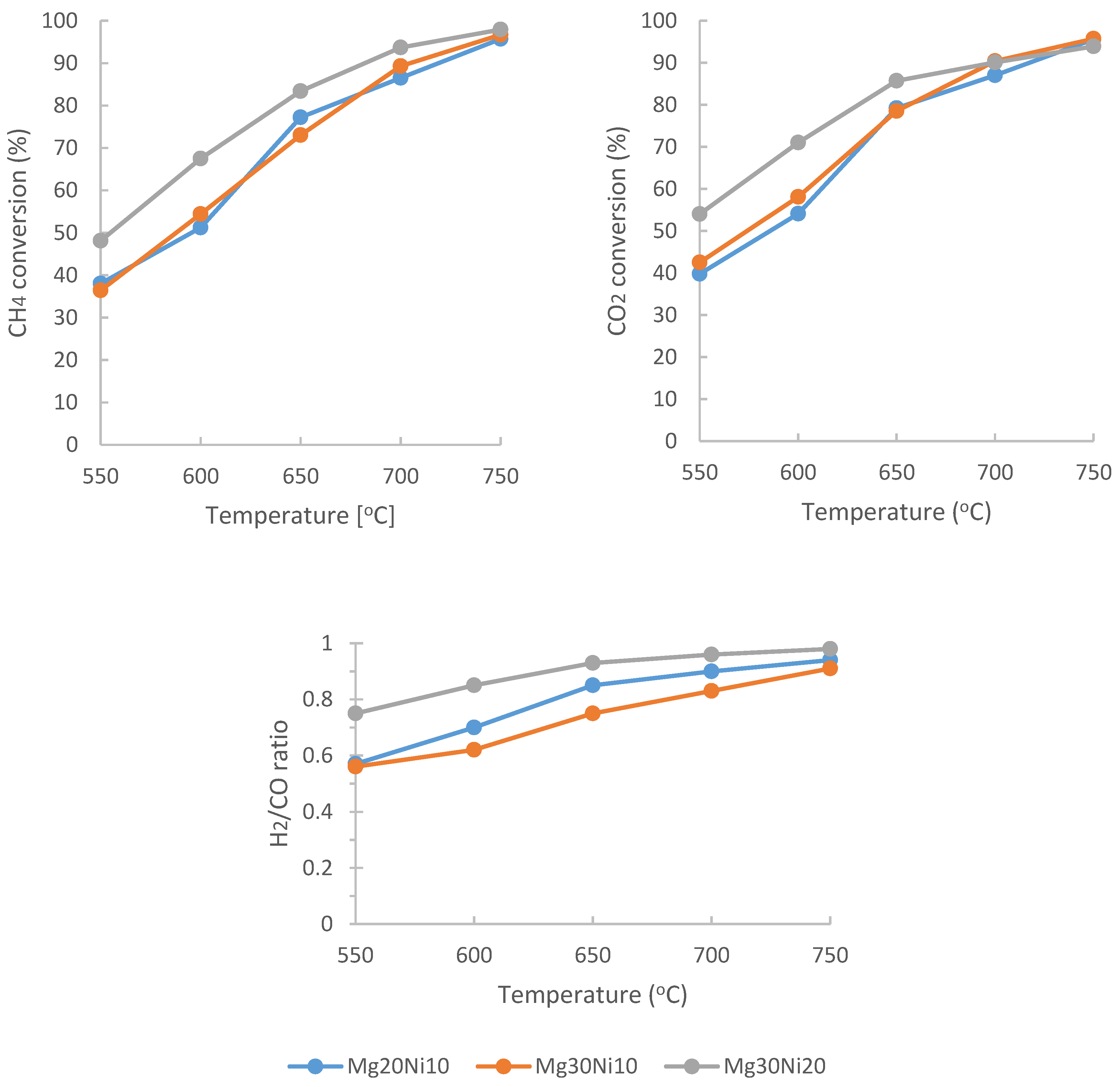
2.2. DRM Catalytic Tests
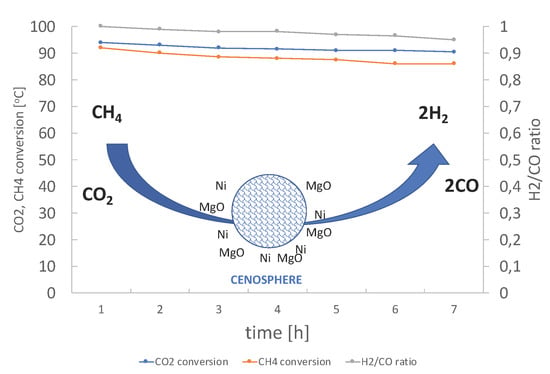
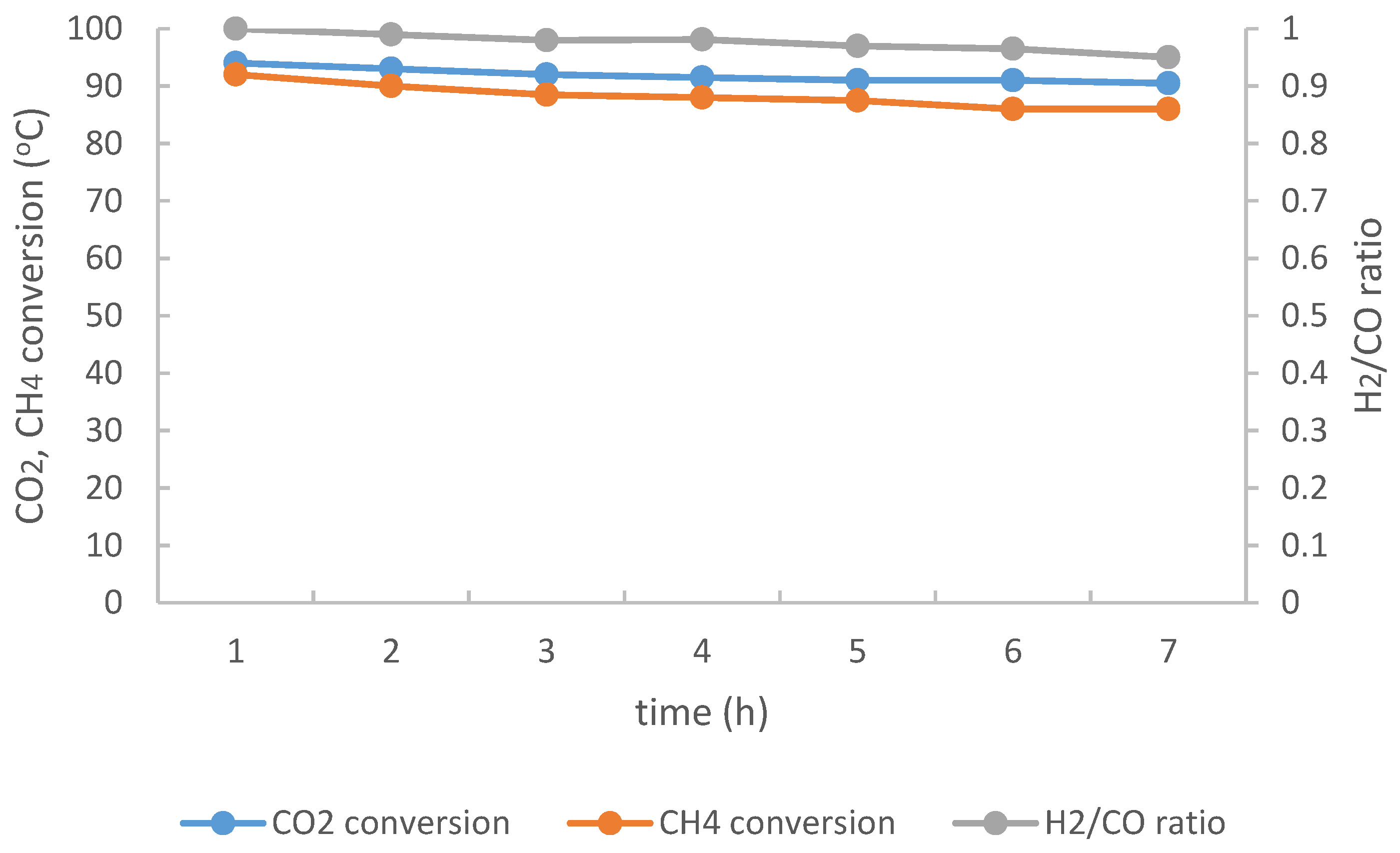
2.3. Stability Test
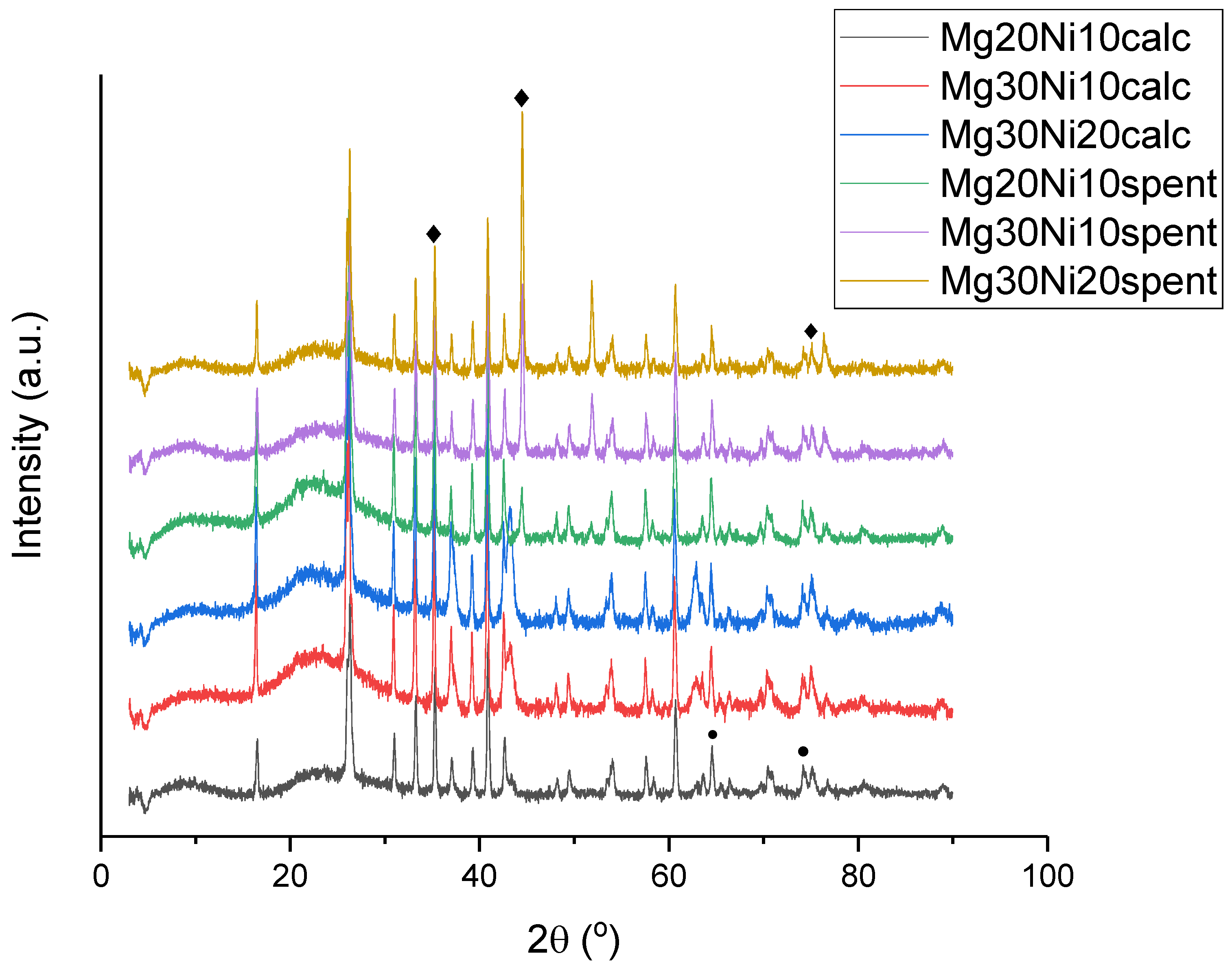
2.4. Post-Test Characterization of the Catalysts
3. Materials and Methods
3.1. Ni/Mg Cenosphere Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lecomte, T.; Ferrería De La Fuente, F.J.; Neuwahl, F.; Canova, M.; Pinasseau, A.; Jankov, I.; Brinkmann, T.; Roudier, S.; Delgado Sancho, L. Best Available Techniques (BAT) Reference Document for Large Combustion Plants-Industrial Emissions Directive 2010/75/EU (Integrated Pollution Prevention and Control); Publications Office of the European Union: Luxembourg, 2017; ISBN 978-92-79-74303-0. [Google Scholar]
- Cuéllar-Franca, R.M.; Azapagic, A. Carbon capture, storage and utilisation technologies: A critical analysis and comparison of their life cycle environmental impacts. J. CO2 Util. 2015, 9, 82–102. [Google Scholar] [CrossRef]
- Wee, J.-H. A review on carbon dioxide capture and storage technology using coal fly ash. Appl. Energy 2013, 106, 143–151. [Google Scholar] [CrossRef]
- Acar, I.; Atalay, M.U. Recovery potentials of cenospheres from bituminous coal fly ashes. Fuel 2016, 180, 97–105. [Google Scholar] [CrossRef]
- Żyrkowski, M.; Costa, R.; Santos, L.F.; Witkowski, K. Characterization of fly-ash cenospheres from coal-fired power plant unit. Fuel 2016, 174, 49–53. [Google Scholar] [CrossRef]
- Chandane, V.S.; Rathod, A.P.; Wasewar, K.L.; Sonawane, S.S. Efficient cenosphere supported catalyst for the esterification of n-octanol with acetic acid. C. R. Chim. 2017, 20, 818–826. [Google Scholar] [CrossRef]
- Ranjbar, N.; Kuenzel, C. Cenospheres: A review. Fuel 2017, 207, 1–12. [Google Scholar] [CrossRef]
- Fomenko, E.V.; Anshits, N.N.; Solovyov, L.A.; Mikhaylova, O.A.; Anshits, A.G. Composition and Morphology of Fly Ash Cenospheres Produced from the Combustion of Kuznetsk Coal. Energy Fuels 2013, 27, 5440–5448. [Google Scholar] [CrossRef]
- Samojeden, B.; Drużkowska, J.; Duraczyńska, D.; Poddębniak, M.; Motak, M. Use of iron and copper-promoted cenospheres as catalysts in the selective catalytic reduction of nitrogen(II) oxide with ammonia. Przem. Chem. 2019, 1, 55–59. [Google Scholar]
- Zhang, J.; Wang, B.; Cui, H.; Li, C.; Zhai, J.; Li, Q. Synthesis of CeO2/fly ash cenospheres composites as novel photocatalysts by modified pyrolysis process. J. Rare Earths 2014, 32, 1120–1125. [Google Scholar] [CrossRef]
- Hosseini Asl, S.M.; Ghadi, A.; Sharifzadeh Baei, M.; Javadian, H.; Maghsudi, M.; Kazemian, H. Porous catalysts fabricated from coal fly ash as cost-effective alternatives for industrial applications: A review. Fuel 2018, 217, 320–342. [Google Scholar] [CrossRef]
- Samojeden, B. The current and future trends in chemical CO2 utilization. In Contemporary Problems of Power Engineering and Environmental Protection; Pikoń, K., Czarnowska, L., Eds.; Silesian University of Technology: Gliwice, Poland, 2018; pp. 215–226. ISBN 978-83-950087-1-9. [Google Scholar]
- Xu, L.; Song, H.; Chou, L. Ordered mesoporous MgO–Al2O3 composite oxides supported Ni based catalysts for CO2 reforming of CH4: Effects of basic modifier and mesopore structure. Int. J. Hydrog. Energy 2013, 38, 7307–7325. [Google Scholar] [CrossRef]
- Jeong, M.; Nunotani, N.; Imanaka, N. Relationship between the conductivities of CeO2-ZrO2-MOx (M = Bi, Ca, Sn, Ni, Fe) solid solutions and catalytic activities during methane oxidation. Bull. Chem. Soc. Jpn. 2018, 91, 158–164. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.F.; Chen, X.L.; Wang, A.J.; Han, D.M.; Wang, Z.G.; Feng, J.J. Facile solvothermal synthesis of Pt71Co29 lamellar nanoflowers as an efficient catalyst for oxygen reduction and methanol oxidation reactions. J. Colloid Interface Sci. 2019, 536, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Torimoto, M.; Murakami, K.; Sekine, Y. Low-temperature heterogeneous catalytic reaction by surface protonics. Bull. Chem. Soc. Jpn. 2019, 92, 1785–1792. [Google Scholar] [CrossRef]
- Mota, F.M.; Kim, D.H. From CO2 methanation to ambitious long-chain hydrocarbons: Alternative fuels paving the path to sustainability. Chem. Soc. Rev. 2019, 48, 205–259. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst design for dry reforming of methane: Analysis review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Albarazi, A.; Beaunier, P.; Da Costa, P. Hydrogen and syngas production by methane dry reforming on SBA-15 supported nickel catalysts: On the effect of promotion by Ce0.75Zr0.25O2 mixed oxide. Int. J. Hydrog. Energy 2013, 38, 127–139. [Google Scholar] [CrossRef]
- Duan, Y.; Shang, R.; Zhong, X.; Xie, W.; Wang, X.; Huang, L. In-situ synthesis of NiMo2C/Al2O3 catalysts for dry reforming of methane. Int. J. Hydrog. Energy 2016, 41, 21955–21964. [Google Scholar] [CrossRef]
- Yao, L.; Galvez, M.E.; Hu, C.; Da Costa, P. Mo-promoted Ni/Al2O3 catalyst for dry reforming of methane. Int. J. Hydrog. Energy 2017, 42, 23500–23507. [Google Scholar] [CrossRef]
- Dębek, R.; Motak, M.; Galvez, M.E.; Grzybek, T.; Da Costa, P. Promotion effect of zirconia on Mg(Ni,Al)O mixed oxides derived from hydrotalcites in CO2 methane reforming. Appl. Catal. B Environ. 2018, 223, 36–46. [Google Scholar] [CrossRef]
- Abdullah, B.; Abd Ghani, N.A.; Vo, D.V.N. Recent advances in dry reforming of methane over Ni-based catalysts. J. Clean. Prod. 2017, 162, 170–185. [Google Scholar] [CrossRef]
- BASF and Linde Successfully Complete Pilot Project at National Carbon Capture Center in Wilsonville, Alabama. Available online: https://www.linde-gaz.pl/pl/news_and_media/press_releases/news_20160719.html (accessed on 6 December 2019).
- Şener, A.N.; Günay, M.E.; Leba, A.; Yıldırım, R. Statistical review of dry reforming of methane literature using decision tree and artificial neural network analysis. Catalysis Today 2018, 299, 289–302. [Google Scholar]
- Dȩbek, R.; Wierzbicki, D.; Motak, M.; Galvez, M.E.; Da Costa, P.; Azzolina-Jury, F. Operando FT-IR study on basicity improvement of Ni(Mg,Al)O hydrotalcite-derived catalysts promoted by glow plasma discharge. Plasma Sci. Technol. 2019, 21, 045503. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Arishtirova, K.; Fierro, J.L.G. Ni-based catalysts for reforming of methane with CO2. Int. J. Hydrog. Energy 2012, 37, 15966–15975. [Google Scholar] [CrossRef]
- Mohamedali, M.; Henni, A.; Ibrahim, H. Recent Advances in Supported Metal Catalysts for Syngas Production from Methane. ChemEngineering 2018, 2, 9. [Google Scholar] [CrossRef]
- Dębek, R.; Motak, M.; Grzybek, T.; Galvez, M.; Da Costa, P. A Short Review on the Catalytic Activity of Hydrotalcite-Derived Materials for Dry Reforming of Methane. Catalysts 2017, 7, 32. [Google Scholar] [CrossRef]
- Dębek, R.; Motak, M.; Galvez, M.E.; Da Costa, P.; Grzybek, T. Catalytic activity of hydrotalcite-derived catalysts in the dry reforming of methane: On the effect of Ce promotion and feed gas composition. React. Kinet. Mech. Catal. 2017, 121, 185–208. [Google Scholar] [CrossRef]
- Liu, H.; Bel Hadjltaief, H.; Benzina, M.; Gálvez, M.E.; Da Costa, P. Natural clay based nickel catalysts for dry reforming of methane: On the effect of support promotion (La, Al, Mn). Int. J. Hydrog. Energy 2018, 1–10. [Google Scholar] [CrossRef]
- Christensen, K.O.; Chen, D.; Lødeng, R.; Holmen, A. Effect of supports and Ni crystal size on carbon formation and sintering during steam methane reforming. Appl. Catal. A Gen. 2006, 314, 9–22. [Google Scholar] [CrossRef]
- Molina, R.; Poncelet, G. α-Alumina-Supported Nickel Catalysts Prepared from Nickel Acetylacetonate: A TPR Study. J. Catal. 1998, 173, 257–267. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.-W. Temperature-programmed-reduction studies of nickel oxide/alumina catalysts: Effects of the preparation method. Thermochim. Acta 1995, 256, 457–465. [Google Scholar] [CrossRef]
- Dębek, R.; Motak, M.; Duraczyńska, D.; Launay, F.; Galvez, M.E.; Grzybek, T.; Da Costa, P. Methane dry reforming over hydrotalcite-derived Ni–Mg–Al mixed oxides: The influence of Ni content on catalytic activity, selectivity and stability. Catal. Sci. Technol. 2016, 6, 6705–6715. [Google Scholar] [CrossRef]
- Świrk, K.; Gálvez, M.E.; Motak, M.; Grzybek, T.; Rønning, M.; Da Costa, P. Yttrium promoted Ni-based double-layered hydroxides for dry methane reforming. J. CO2 Util. 2018, 27, 247–258. [Google Scholar] [CrossRef]
- Liu, H.; Wierzbicki, D.; Debek, R.; Motak, M.; Grzybek, T.; Da Costa, P.; Galvez, M.E. La-promoted Ni-hydrotalcite-derived catalysts for dry reforming of methane at low temperatures. Fuel 2016, 182, 8–16. [Google Scholar] [CrossRef]
- Titus, J.; Goepel, M.; Schunk, S.A.; Wilde, N.; Gläser, R. The role of acid/base properties in Ni/MgO-ZrO2–based catalysts for dry reforming of methane. Catal. Commun. 2017, 100, 76–80. [Google Scholar] [CrossRef]
- Świrk, K.; Gálvez, M.E.; Motak, M.; Grzybek, T.; Rønning, M.; Da Costa, P. Syngas production from dry methane reforming over yttrium-promoted nickel-KIT-6 catalysts. Int. J. Hydrog. Energy 2019, 4, 274–286. [Google Scholar] [CrossRef]
- Zhang, R.; Xia, G.; Li, M.; Wu, Y.; Nie, H.; Li, D. Effect of support on the performance of Ni-based catalyst in methane dry reforming. J. Fuel Chem. Technol. 2015, 43, 1359–1365. [Google Scholar] [CrossRef]
- Świrk, K.; Rønning, M.; Motak, M.; Beaunier, P.; Da Costa, P.; Grzybek, T. Ce-and Y-modified double-layered hydroxides as catalysts for dry reforming of methane: On the effect of yttrium promotion. Catalysts 2019, 9, 56. [Google Scholar] [CrossRef]
- Świrk, K.; Gálvez, M.E.; Motak, M.; Grzybek, T.; Rønning, M.; Da Costa, P. Dry reforming of methane over Zr- and Y-modified Ni/Mg/Al double-layered hydroxides. Catal. Commun. 2018, 117, 26–32. [Google Scholar] [CrossRef]
- Dębek, R.; Zubek, K.; Motak, M.; Da Costa, P.; Grzybek, T. Effect of nickel incorporation into hydrotalcite-based catalyst systems for dry reforming of methane. Res. Chem. Intermed. 2015, 41, 9485–9495. [Google Scholar] [CrossRef]
- Izquierdo-Colorado, A.; Dębek, R.; Da Costa, P.; Gálvez, M.E. Excess-methane dry and oxidative reforming on Ni-containing hydrotalcite-derived catalysts for biogas upgrading into synthesis gas. Int. J. Hydrog. Energy 2018, 43, 11981–11989. [Google Scholar] [CrossRef]
- Kolebuk, I.; Samojeden, B. The Preparation and Proporties of Mg-and Ni-Modified Cenospheres; AGH University of Science and Technology: Kraków, Poland, 2018. [Google Scholar]
| Ni | SBET | H2 Consumption for the Calcined Samples | Basicity for the Calcined Samples after Reduction * | Basicity after DRM * | Nickel Crystallite Size for the Reduced Samples ** | Nickel Crystallite Size for the Spent Catalysts ** | Carbon Deposi-tion † | |
|---|---|---|---|---|---|---|---|---|
| wt % | m2/g | µmolH2/gcat | µmolCO2/gcat | µmolCO2/gcat | nm | nm | % | |
| Mg10 | ||||||||
| 10 | 1 | 53.4 | 35.0 | 29.0 | 20 | 23 | 0.5 | |
| 20 | 1 | 201.5 | 30.0 | 44.7 | 40 | 41 | 0.6 | |
| 30 | 2 | 455.5 | 27.1 | 28.1 | 36 | 39 | 0.9 | |
| Mg20 | ||||||||
| 10 | 1 | 82.86 | 29.7 | 19.6 | 23 | 24 | - | |
| 20 | 2 | 344.8 | 22.3 | 41.5 | 44 | 31 | - | |
| 30 | 4 | 947.5 | 18.3 | 21.5 | 43 | 41 | - | |
| Mg30 | ||||||||
| 10 | 2 | 233.7 | 19.9 | 36.0 | 32 | 34 | - | |
| 20 | 4 | 386.2 | 48.0 | 49.3 | 39 | 41 | 1.1 | |
| 30 | 4 | 612.3 | 26.0 | 35.9 | 36 | 37 | - |
| Catalyst | Ni Loading | Reaction Conditions | Conversion * | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| Temp. (°C) | CH4/CO2 | GHSV (h−1) | TOS (h) | CH4 (%) | CO2 (%) | H2/CO | |||
| HTNi | 20 | 750 | 1/1 | 20,000 | 1 | 85 | 82 | 1.1 | [44] |
| HT-25Ni | 19.57 | 750 | 1/1 | 20,000 | 0.5 | 97 | 90 | 1.2 | [43] |
| HT | 20 | 750 | 1/1 | 20,000 | 0.5 | 82.5 | 86.5 | 0.93 | [41] |
| Mg20Ni20 | 20 | 750 | 1/1 | 20,000 | 0.5 | 97.9 | 93.9 | 0.98 | This work |
| Mg30Ni20 | 10 | 750 | 1/1 | 20,000 | 0.5 | 95.7 | 93.7 | 0.97 | This work |
| Mg30Ni20 | 20 | 750 | 1/1 | 20,000 | 0.5 | 96.7 | 93.8 | 0.91 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samojeden, B.; Kamienowska, M.; Izquierdo Colorado, A.; Galvez, M.E.; Kolebuk, I.; Motak, M.; Da Costa, P. Novel Nickel- and Magnesium-Modified Cenospheres as Catalysts for Dry Reforming of Methane at Moderate Temperatures. Catalysts 2019, 9, 1066. https://doi.org/10.3390/catal9121066
Samojeden B, Kamienowska M, Izquierdo Colorado A, Galvez ME, Kolebuk I, Motak M, Da Costa P. Novel Nickel- and Magnesium-Modified Cenospheres as Catalysts for Dry Reforming of Methane at Moderate Temperatures. Catalysts. 2019; 9(12):1066. https://doi.org/10.3390/catal9121066
Chicago/Turabian StyleSamojeden, Bogdan, Marta Kamienowska, Armando Izquierdo Colorado, Maria Elena Galvez, Ilona Kolebuk, Monika Motak, and Patrick Da Costa. 2019. "Novel Nickel- and Magnesium-Modified Cenospheres as Catalysts for Dry Reforming of Methane at Moderate Temperatures" Catalysts 9, no. 12: 1066. https://doi.org/10.3390/catal9121066
APA StyleSamojeden, B., Kamienowska, M., Izquierdo Colorado, A., Galvez, M. E., Kolebuk, I., Motak, M., & Da Costa, P. (2019). Novel Nickel- and Magnesium-Modified Cenospheres as Catalysts for Dry Reforming of Methane at Moderate Temperatures. Catalysts, 9(12), 1066. https://doi.org/10.3390/catal9121066