Shapes Control of Bi2WO6 Nano-Structures as Photo-Fenton Catalysts for Pulping Wastewater Treatment
Abstract
1. Introduction
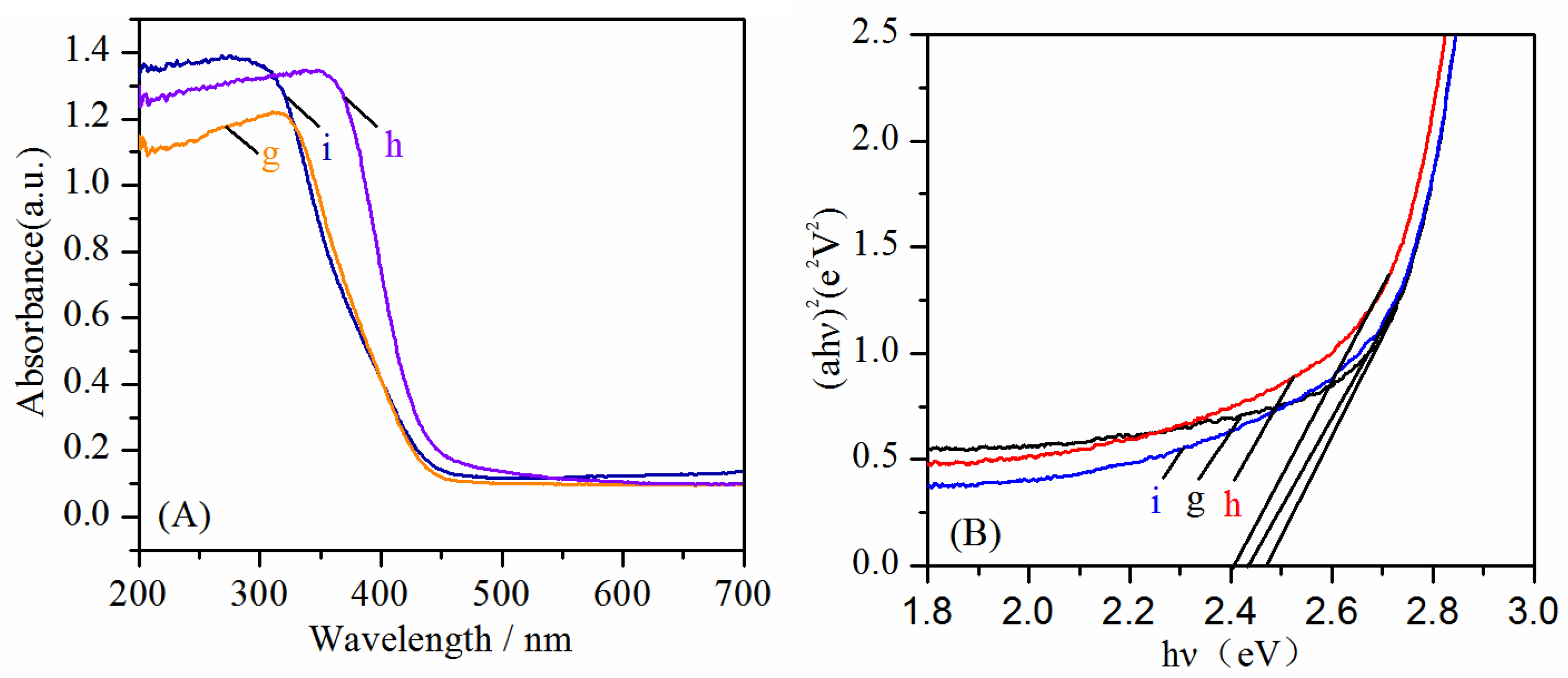
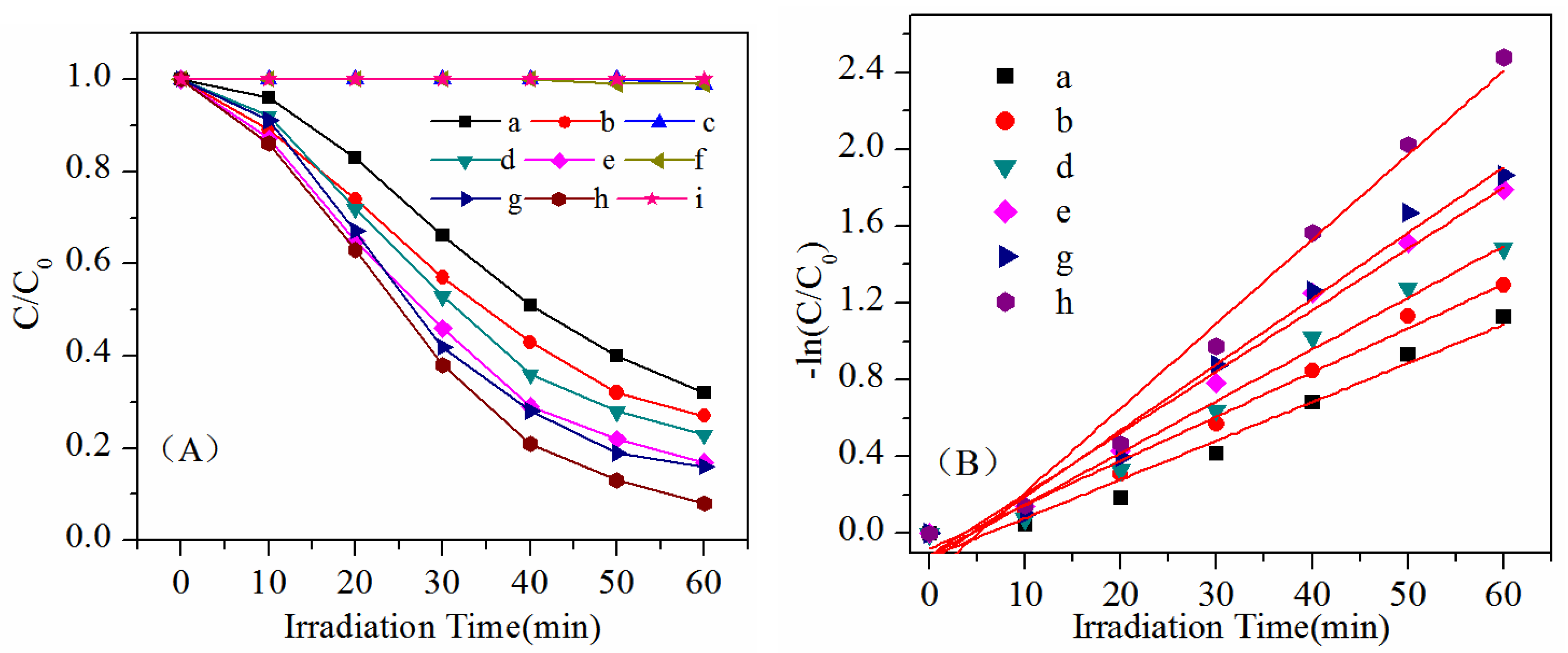
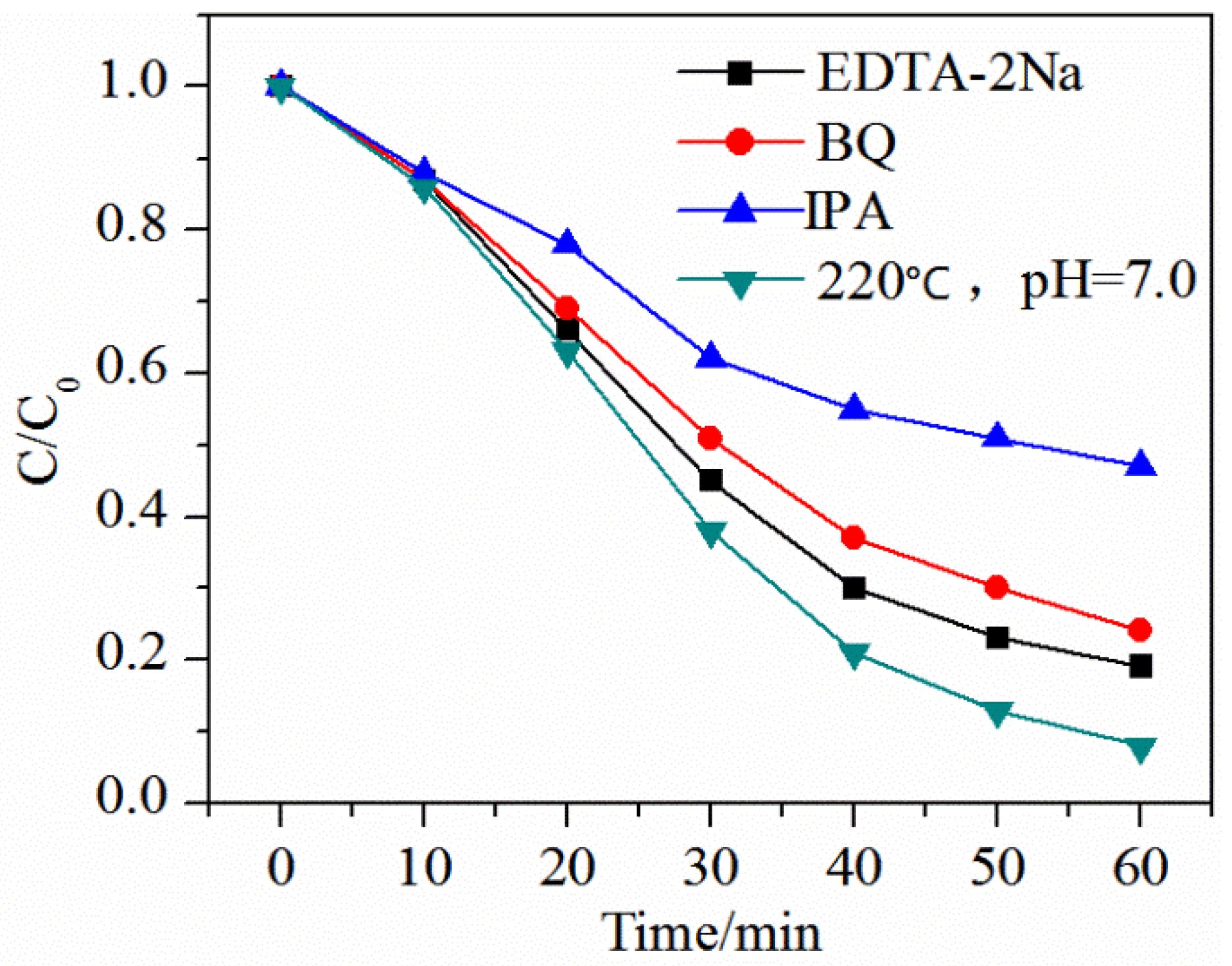
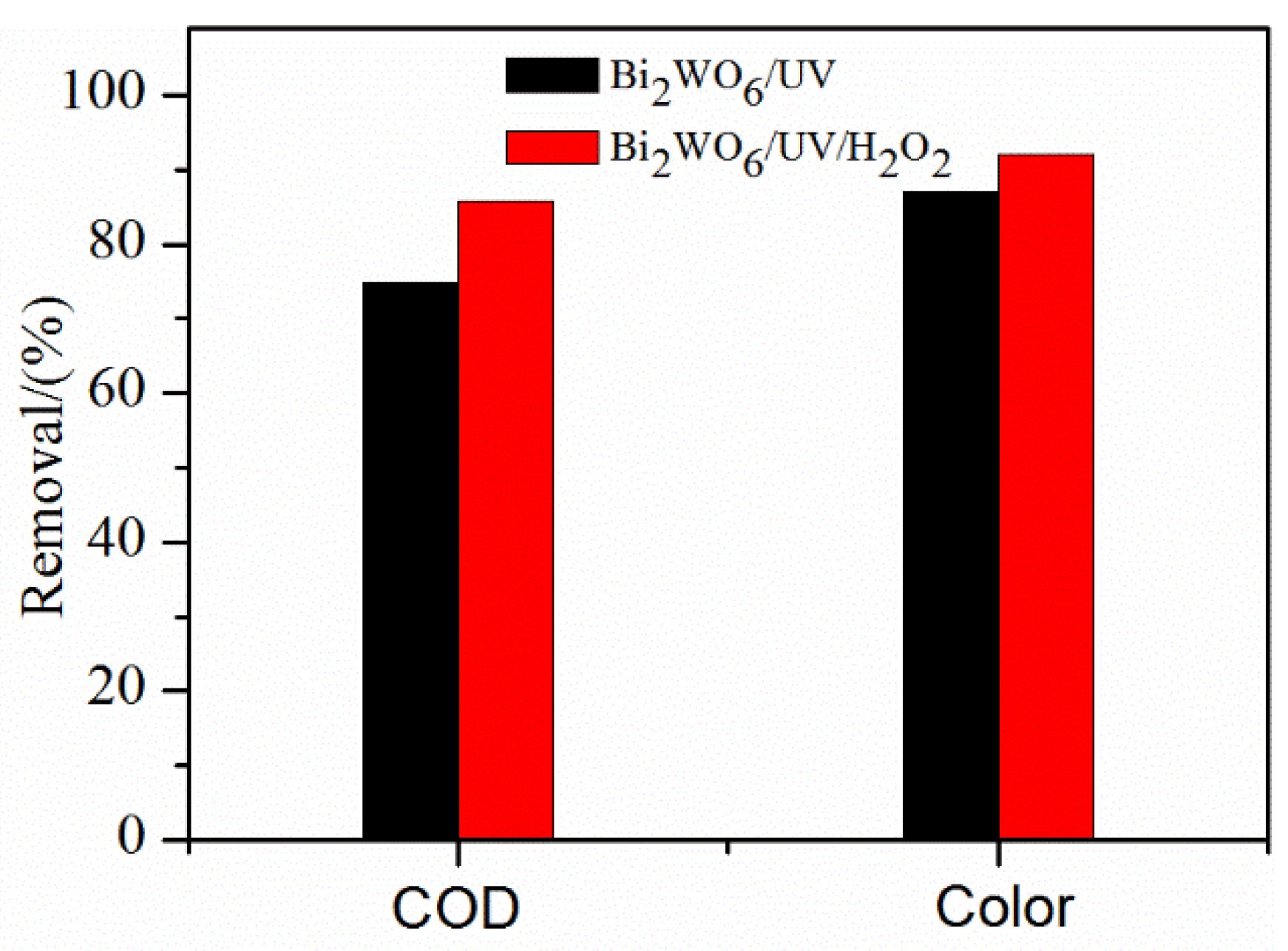
2. Results and Discussion
3. Experimental Section
3.1. Synthesis of the Samples
3.2. Characterization
3.3. Photocatalytic Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kamali, M.; Khodaparast, Z. Review on recent developments on pulp and paper mill wastewater treatment. Ecotoxicol. Environ. Saf. 2015, 114, 326–342. [Google Scholar] [CrossRef] [PubMed]
- Machani, M.; Nourelfath, M.; D’Amours, S. A mathematically-based framework for evaluating the technical and economic potential of integrating bioenergy production within pulp and paper mills. Biomass Bioenergy 2014, 63, 126–139. [Google Scholar] [CrossRef]
- Ashrafi, O.; Yerushalmi, L.; Haghighat, F. Application of dynamic models to estimate greenhouse gas emission by wastewater treatment plants of the pulp and paper industry. Environ. Sci. Pollut. Res. Int. 2013, 20, 1858–1869. [Google Scholar] [CrossRef] [PubMed]
- Pokhrel, D.; Viraraghavan, T. Treatment of pulp and paper mill wastewater--a review. Sci. Total Environ. 2004, 333, 37–58. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Sreekrishnan, T.R. Aquatic toxicity from pulp and paper mill effluents a review. Adv. Environ. Res. 2001, 5, 175–196. [Google Scholar] [CrossRef]
- Karrasch, B.; Parra, O.; Cid, H.; Mehrens, M.; Pacheco, P.; Urrutia, R.; Valdovinos, C.; Zaror, C. Effects of pulp and paper mill effluents on the microplankton and microbial self-purification capabilities of the Biobio River, Chile. Sci. Total Environ. 2006, 359, 194–208. [Google Scholar] [CrossRef]
- Chandra, R.; Sharma, P.; Yadav, S.; Tripathi, S. Biodegradation of Endocrine-Disrupting Chemicals and Residual Organic Pollutants of Pulp and Paper Mill Effluent by Biostimulation. Front. Microbiol. 2018, 9, 960. [Google Scholar] [CrossRef]
- Kamali, M.; Alavi-Borazjani, S.A.; Khodaparast, Z.; Khalaj, M.; Jahanshahi, A.; Costa, E.; Capela, I. Additive and additive-free treatment technologies for pulp and paper mill effluents: Advances, challenges and opportunities. Water Resour. Ind. 2019, 21. [Google Scholar] [CrossRef]
- Ashrafi, O.; Yerushalmi, L.; Haghighat, F. Wastewater treatment in the pulp-and-paper industry: A review of treatment processes and the associated greenhouse gas emission. J. Environ. Manag. 2015, 158, 146–157. [Google Scholar] [CrossRef]
- Izadi, A.; Hosseini, M.; Najafpour Darzi, G.; Nabi Bidhendi, G.; Pajoum Shariati, F. Treatment of paper-recycling wastewater by electrocoagulation using aluminum and iron electrodes. J. Environ. Health Sci. Eng. 2018, 16, 257–264. [Google Scholar] [CrossRef]
- Sandberg, M.; Venkatesh, G.; Granström, K. Experimental study and analysis of the functional and life-cycle global warming effect of low-dose chemical pre-treatment of effluent from pulp and paper mills. J. Clean. Prod. 2018, 174, 701–709. [Google Scholar] [CrossRef]
- Mansour, L.B.; Ksentini, I.; Elleuch, B. Treatment of wastewaters of paper industry by coagulation-electroflotation. Desalination 2007, 208, 34–41. [Google Scholar] [CrossRef]
- Toczyłowska-Mamińska, R. Limits and perspectives of pulp and paper industry wastewater treatment—A review. Renew. Sustain. Energy Rev. 2017, 78, 764–772. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2018, 17, 145–155. [Google Scholar] [CrossRef]
- Kamali, M.; Gameiro, T.; Costa, M.E.V.; Capela, I. Anaerobic digestion of pulp and paper mill wastes—An overview of the developments and improvement opportunities. Chem. Eng. J. 2016, 298, 162–182. [Google Scholar] [CrossRef]
- Savant, D.V.; Abdul-Rahman, R.; Ranade, D.R. Anaerobic degradation of adsorbable organic halides (AOX) from pulp and paper industry wastewater. Bioresour. Technol. 2006, 97, 1092–1104. [Google Scholar] [CrossRef]
- Tambosi, J.L.; Di Domenico, M.; Schirmer, W.N.; José, H.J.; Moreira, F.P.M. Treatment of paper and pulp wastewater and removal of odorous compounds by a Fenton-like process at the pilot scale. J. Chem. Technol. Biotechnol. 2006, 81, 1426–1432. [Google Scholar] [CrossRef]
- Catalkaya, E.C.; Kargi, F. Color, TOC and AOX removals from pulp mill effluent by advanced oxidation processes: A comparative study. J. Hazard. Mater. 2007, 139, 244–253. [Google Scholar] [CrossRef]
- Ginni, G.; Adishkumar, S.; Rajesh Banu, J.; Yogalakshmi, N. Treatment of pulp and paper mill wastewater by solar photo-Fenton process. Desalin. Water Treat. 2013, 52, 2457–2464. [Google Scholar] [CrossRef]
- Neoh, C.H.; Noor, Z.Z.; Mutamim, N.S.A.; Lim, C.K. Green technology in wastewater treatment technologies: Integration of membrane bioreactor with various wastewater treatment systems. Chem. Eng. J. 2016, 283, 582–594. [Google Scholar] [CrossRef]
- Pirilä, M.; Saouabe, M.; Ojala, S.; Rathnayake, B.; Drault, F.; Valtanen, A.; Huuhtanen, M.; Brahmi, R.; Keiski, R.L. Photocatalytic Degradation of Organic Pollutants in Wastewater. Top. Catal. 2015, 58, 1085–1099. [Google Scholar] [CrossRef]
- He, D.; Wang, L.; Xu, D.; Zhai, J.; Wang, D.; Xie, T. Investigation of photocatalytic activities over Bi(2)WO(6)/ZnWO(4) composite under UV light and its photoinduced charge transfer properties. ACS Appl. Mater. Interfaces 2011, 3, 3167–3171. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wang, W.; Zhang, L.; Gao, E.; Jiang, D.; Sun, Y.; Xie, Y. Ultrathin {001}-oriented bismuth tungsten oxide nanosheets as highly efficient photocatalysts. ChemSusChem 2013, 6, 1873–1877. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Sang, Y.; Yu, G.; Jiang, H.; Mu, X.; Liu, H. A Bi2WO6-based hybrid photocatalyst with broad spectrum photocatalytic properties under UV, visible, and near-infrared irradiation. Adv. Mater. 2013, 25, 5075–5080. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Shan, X.; Yang, W.; Chen, Y. Structure regulation and photocatalytic activity enhancement of Bi2WO6 by “double-effect modification” mode. Int. J. Energy Res. 2019. [Google Scholar] [CrossRef]
- Long, M.; Cai, W.; Kisch, H. Photoelectrochemical properties of nanocrystalline Aurivillius phase Bi2MoO6 film under visible light irradiation. Chem. Phys. Lett. 2008, 461, 102–105. [Google Scholar] [CrossRef]
- Liang, Y.; Shi, J.; Fang, B. Synthesis and electrochemical performance of bismuth tungsten oxides with different composition and morphology. Chem. Phys. Lett. 2019, 716, 112–118. [Google Scholar] [CrossRef]
- Yao, S.; Wei, J.; Huang, B.; Feng, S.; Zhang, X.; Qin, X.; Wang, P.; Wang, Z.; Zhang, Q.; Jing, X.; et al. Morphology modulated growth of bismuth tungsten oxide nanocrystals. J. Solid State Chem. 2009, 182, 236–239. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Zhou, L.; Xu, H. Bi2WO6 nano- and microstructures: Shape control and associated visible-light-driven photocatalytic activities. Small 2007, 3, 1618–1625. [Google Scholar] [CrossRef]
- Shang, Y.; Cui, Y.; Shi, R.; Yang, P. Effect of acetic acid on morphology of Bi2WO6 with enhanced photocatalytic activity. Mater. Sci. Semicond. Process. 2019, 89, 240–249. [Google Scholar] [CrossRef]
- Hojamberdiev, M.; Katsumata, K.-I.; Morita, K.; Bilmes, S.A.; Matsushita, N.; Okada, K. One-step hydrothermal synthesis and photocatalytic performance of ZnWO4/Bi2WO6 composite photocatalysts for efficient degradation of acetaldehyde under UV light irradiation. Appl. Catal. A: Gen. 2013, 457, 12–20. [Google Scholar] [CrossRef]
- Wang, J.-J.; Tang, L.; Zeng, G.-M.; Zhou, Y.-Y.; Deng, Y.-C.; Fan, C.-Z.; Gong, J.-L.; Liu, Y.-N. Effect of bismuth tungstate with different hierarchical architectures on photocatalytic degradation of norfloxacin under visible light. Trans. Nonferrous Met. Soc. China 2017, 27, 1794–1803. [Google Scholar] [CrossRef]
- Tian, Q.-W.; Li, N.-X.; Liu, J.-H.; Wang, M.; Deng, J.-Q.; Zhou, J.-C.; Ma, Q.-H. Catalytic Hydrogenation of Alkali Lignin to Bio-oil Using Fullerene-like Vanadium Sulfide. Energy Fuels 2014, 29, 255–261. [Google Scholar] [CrossRef]
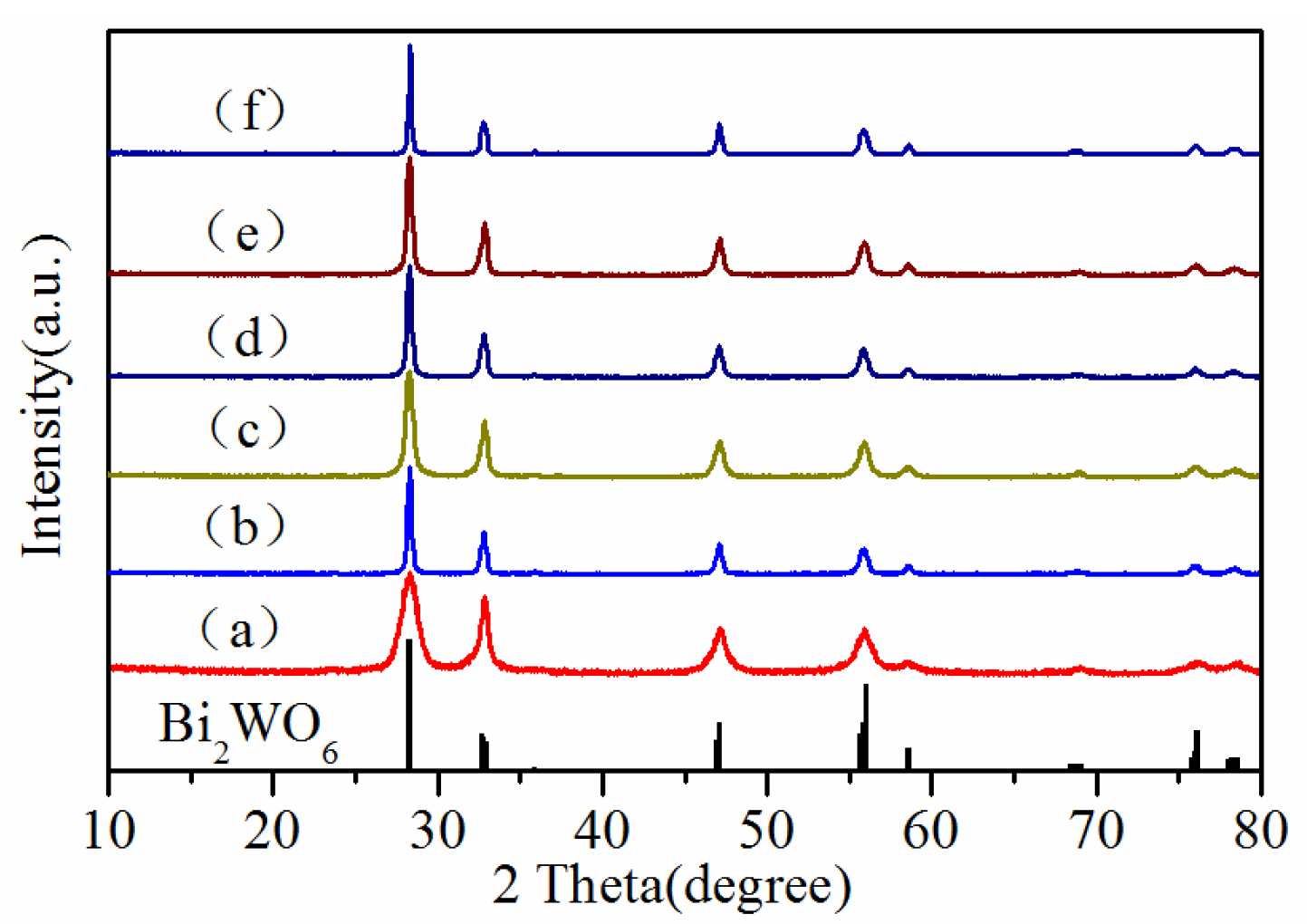
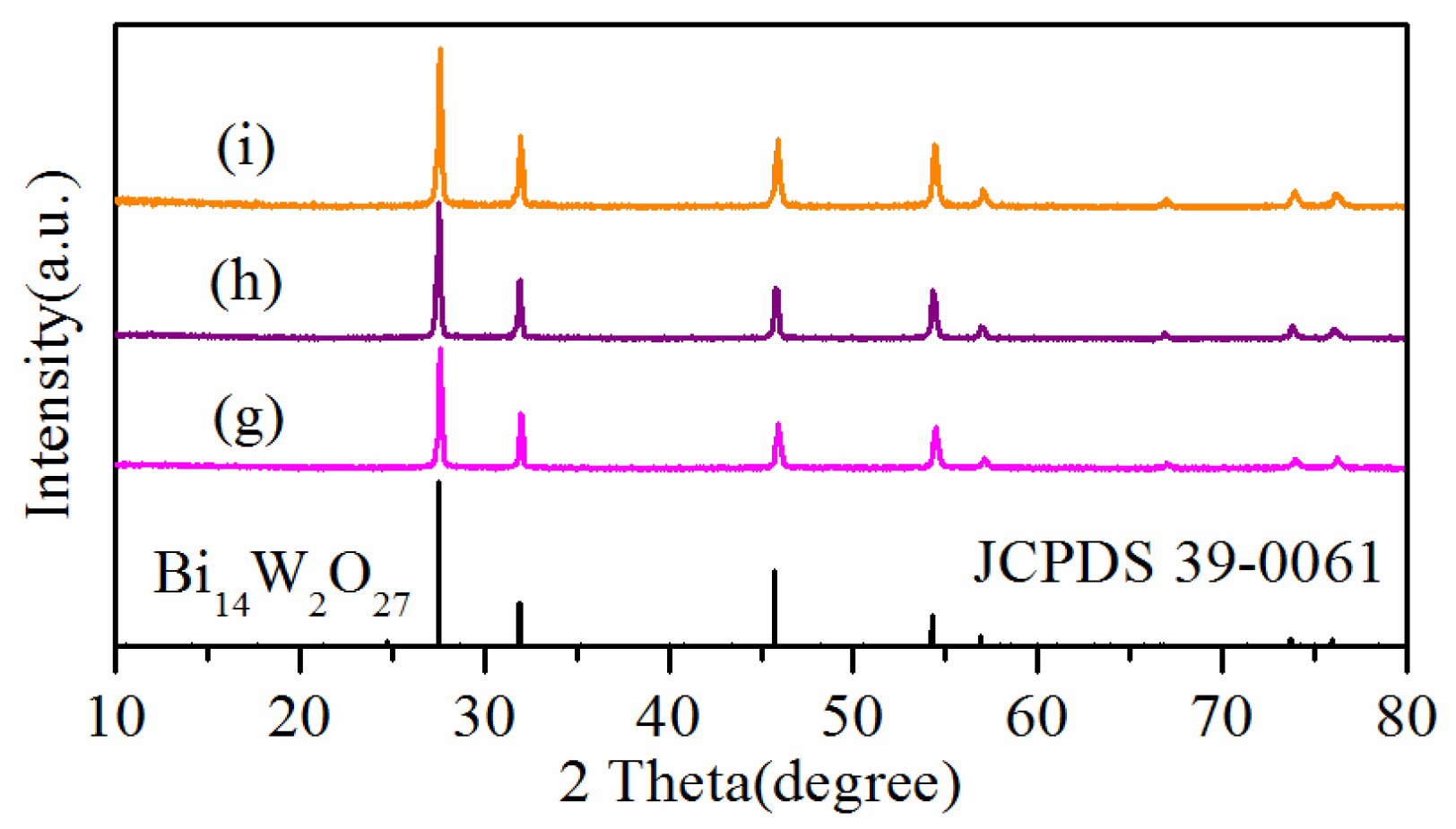
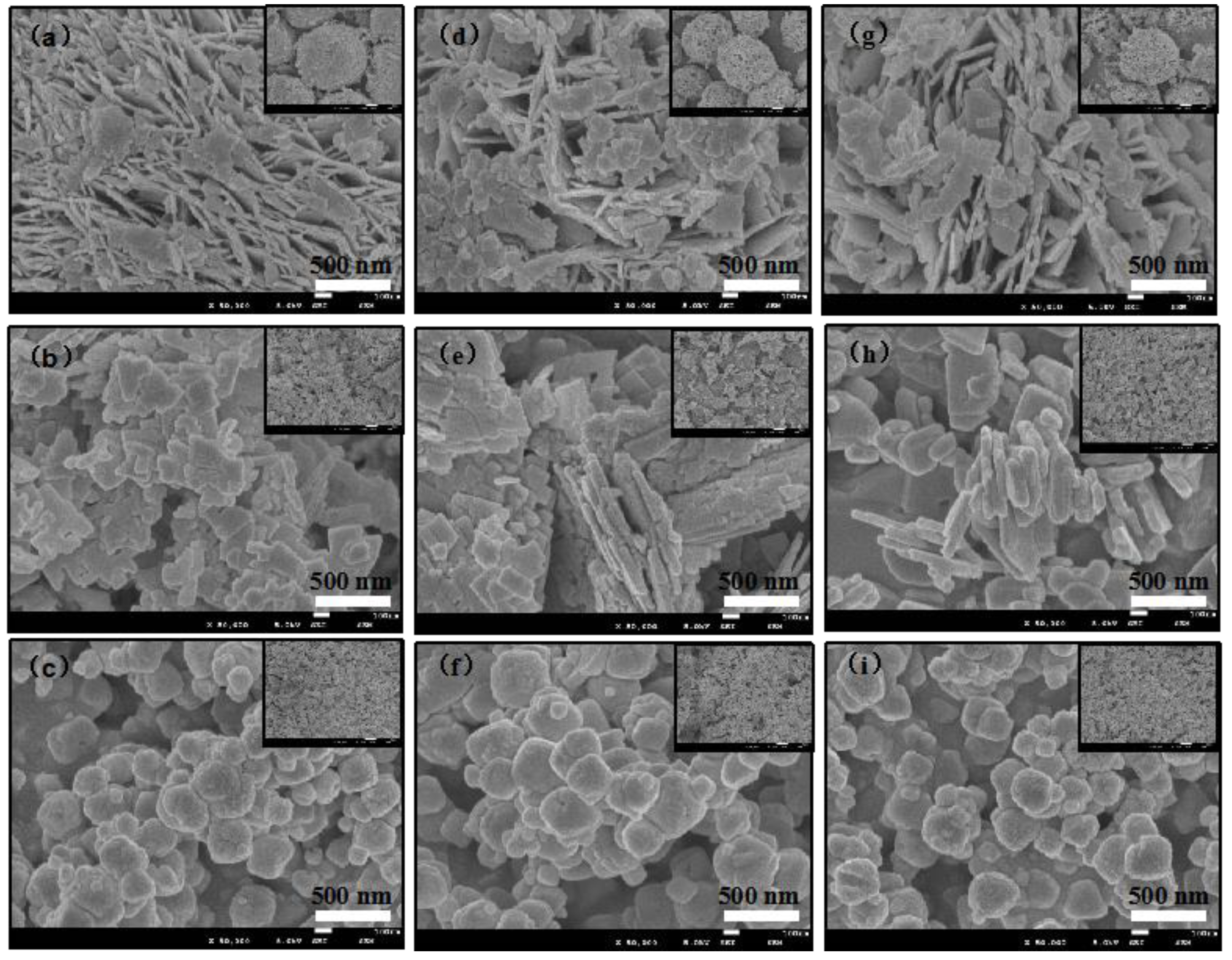
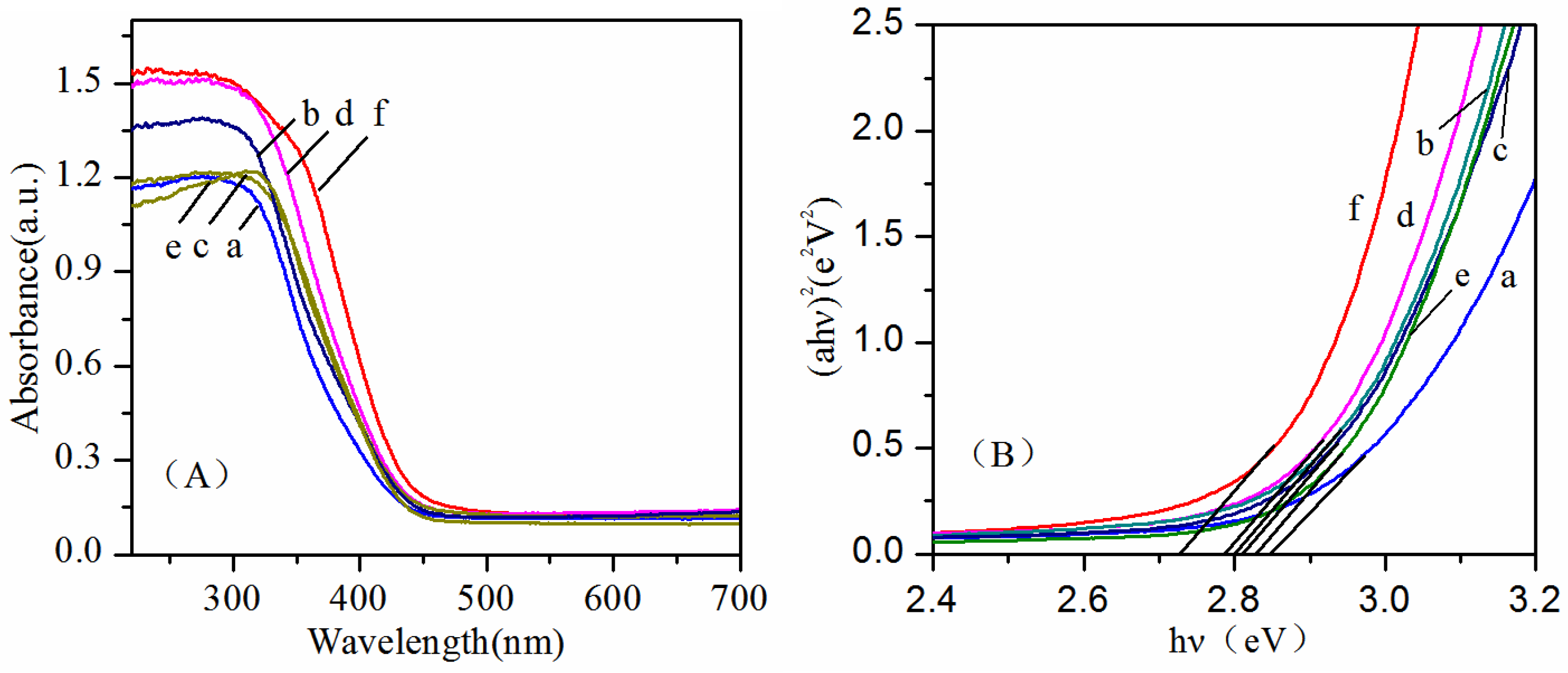
| Sample | BET Area (m2/g) | |
|---|---|---|
| a | Bi2WO6-140 °C, pH = 1 | 9.73 |
| b | Bi2WO6-140 °C, pH = 7 | 18.36 |
| c | Bi14W2O27-140 °C, pH = 13 | 5.12 |
| d | Bi2WO6-180 °C, pH = 1 | 11.88 |
| e | Bi2WO6-180 °C, pH = 7 | 22.51 |
| f | Bi14W2O27-180 °C, pH = 13 | 6.08 |
| g | Bi2WO6-220 °C, pH = 1 | 14.67 |
| h | Bi2WO6-220 °C, pH = 7 | 25.51 |
| i | Bi14W2O27-220 °C, pH = 13 | 5.89 |
| Sample | Hv (eV) | |
|---|---|---|
| a | Bi2WO6-140 °C, pH = 1 | 2.85 |
| b | Bi2WO6-140 °C, pH = 7 | 2.80 |
| c | Bi2WO6-180 °C, pH = 1 | 2.81 |
| d | Bi2WO6-180 °C, pH = 7 | 2.79 |
| e | Bi2WO6-220 °C, pH = 1 | 2.83 |
| f | Bi2WO6-220 °C, pH = 7 | 2.73 |
| g | Bi14W2O27-140 °C, pH = 13 | 2.47 |
| h | Bi14W2O27-180 °C, pH = 13 | 2.43 |
| i | Bi14W2O27-220 °C, pH = 13 | 2.41 |
| Sample | K (min−1) | R2 | |
|---|---|---|---|
| a | Bi2WO6-140 °C, pH = 1 | 0.020 | 0.966 |
| b | Bi2WO6-140 °C, pH = 7 | 0.023 | 0.986 |
| d | Bi2WO6-180 °C, pH = 1 | 0.032 | 0.984 |
| e | Bi2WO6-180 °C, pH = 7 | 0.034 | 0.977 |
| g | Bi2WO6-220 °C, pH = 1 | 0.035 | 0.980 |
| h | Bi2WO6-220 °C, pH = 7 | 0.044 | 0.975 |
| Sample | pH | CODCr/(mg/L) | Color/(Pt-Co) |
|---|---|---|---|
| PPW | 7.16 | 216 | 745 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ran, M.; Zou, X.; Tian, Q.; Liang, L.; Wu, T.; Pan, A.; Deng, Y.; Fang, G.; Ding, L. Shapes Control of Bi2WO6 Nano-Structures as Photo-Fenton Catalysts for Pulping Wastewater Treatment. Catalysts 2019, 9, 1065. https://doi.org/10.3390/catal9121065
Ran M, Zou X, Tian Q, Liang L, Wu T, Pan A, Deng Y, Fang G, Ding L. Shapes Control of Bi2WO6 Nano-Structures as Photo-Fenton Catalysts for Pulping Wastewater Treatment. Catalysts. 2019; 9(12):1065. https://doi.org/10.3390/catal9121065
Chicago/Turabian StyleRan, Miao, Xiuxiu Zou, Qingwen Tian, Long Liang, Ting Wu, Aixiang Pan, Yongjun Deng, Guigan Fang, and Laibao Ding. 2019. "Shapes Control of Bi2WO6 Nano-Structures as Photo-Fenton Catalysts for Pulping Wastewater Treatment" Catalysts 9, no. 12: 1065. https://doi.org/10.3390/catal9121065
APA StyleRan, M., Zou, X., Tian, Q., Liang, L., Wu, T., Pan, A., Deng, Y., Fang, G., & Ding, L. (2019). Shapes Control of Bi2WO6 Nano-Structures as Photo-Fenton Catalysts for Pulping Wastewater Treatment. Catalysts, 9(12), 1065. https://doi.org/10.3390/catal9121065




