Metal Complexes Containing Redox-Active Ligands in Oxidation of Hydrocarbons and Alcohols: A Review
Abstract
1. Introduction
2. Metal Complexes Containing Redox-Active Ligands
3. New Methods of Study of Alkane Oxidation Reactions
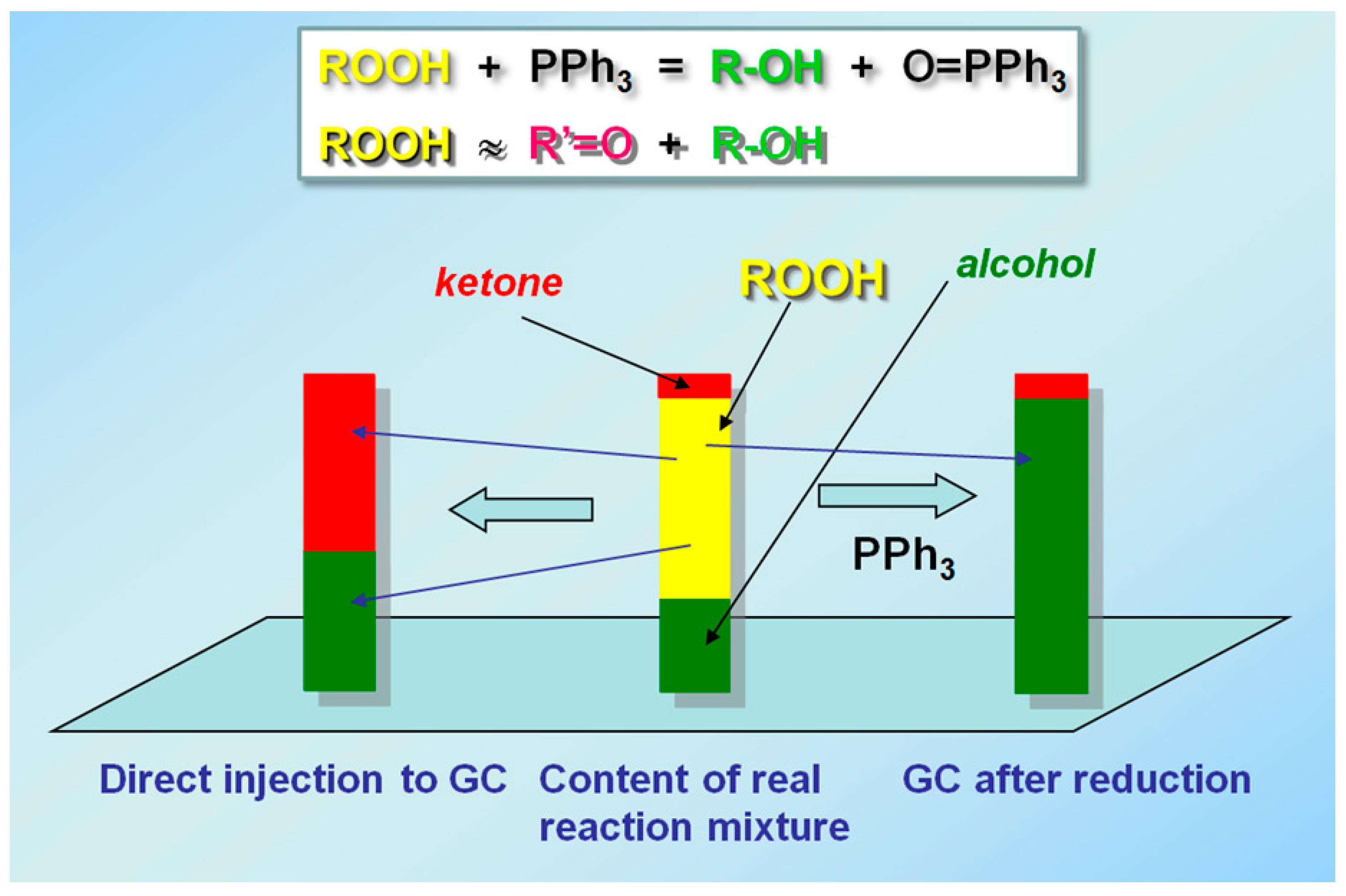
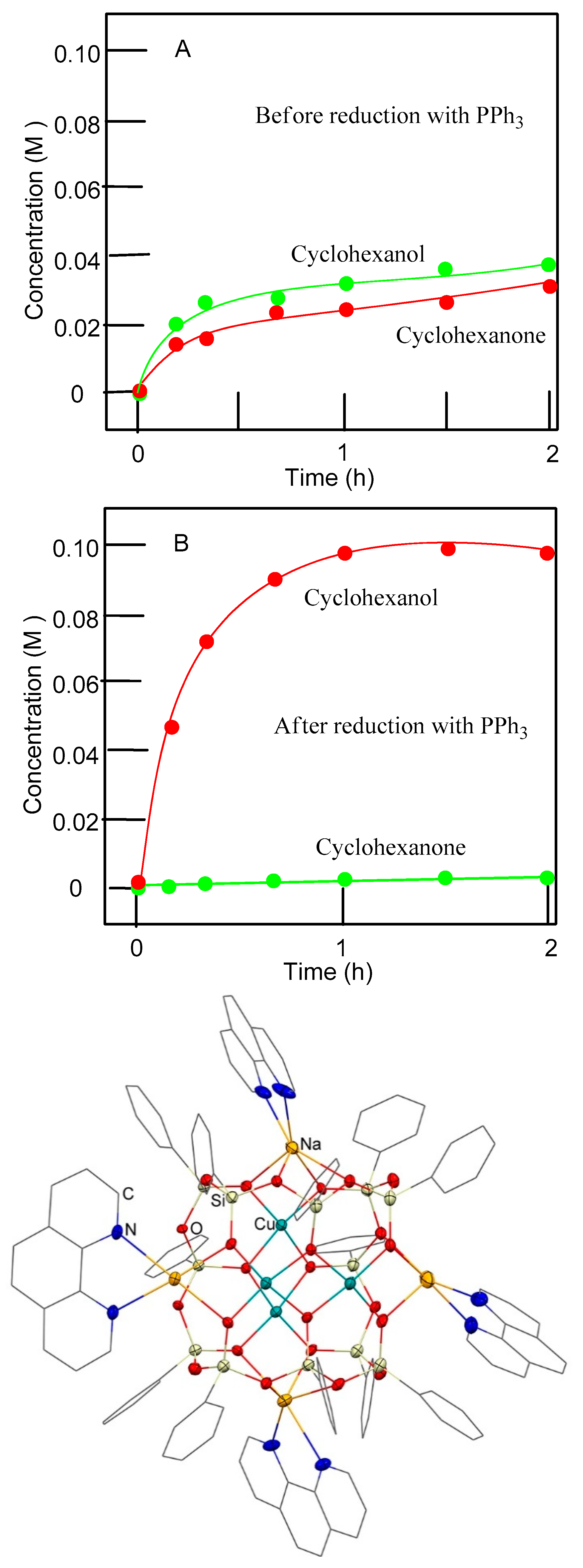
3.1. Detection of Alkyl Hydroperoxide by the Chromatography (GC) Before and After Treatment with PPh3 (the Shul’pin Method)
3.2. A Competitive Oxidation of Cyclohexane and Acetonitrile as a Method for Detection of Hydroxyl Radicals
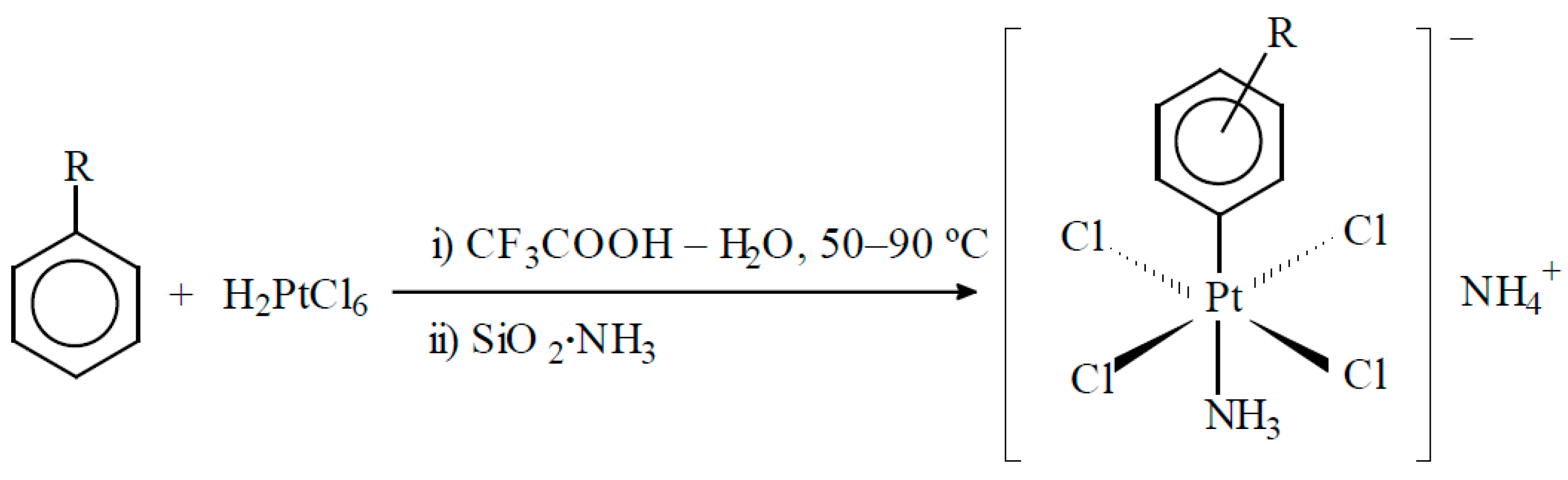
4. Activation of Hydrocarbons in the Presence of Chloride Platinum Complexes
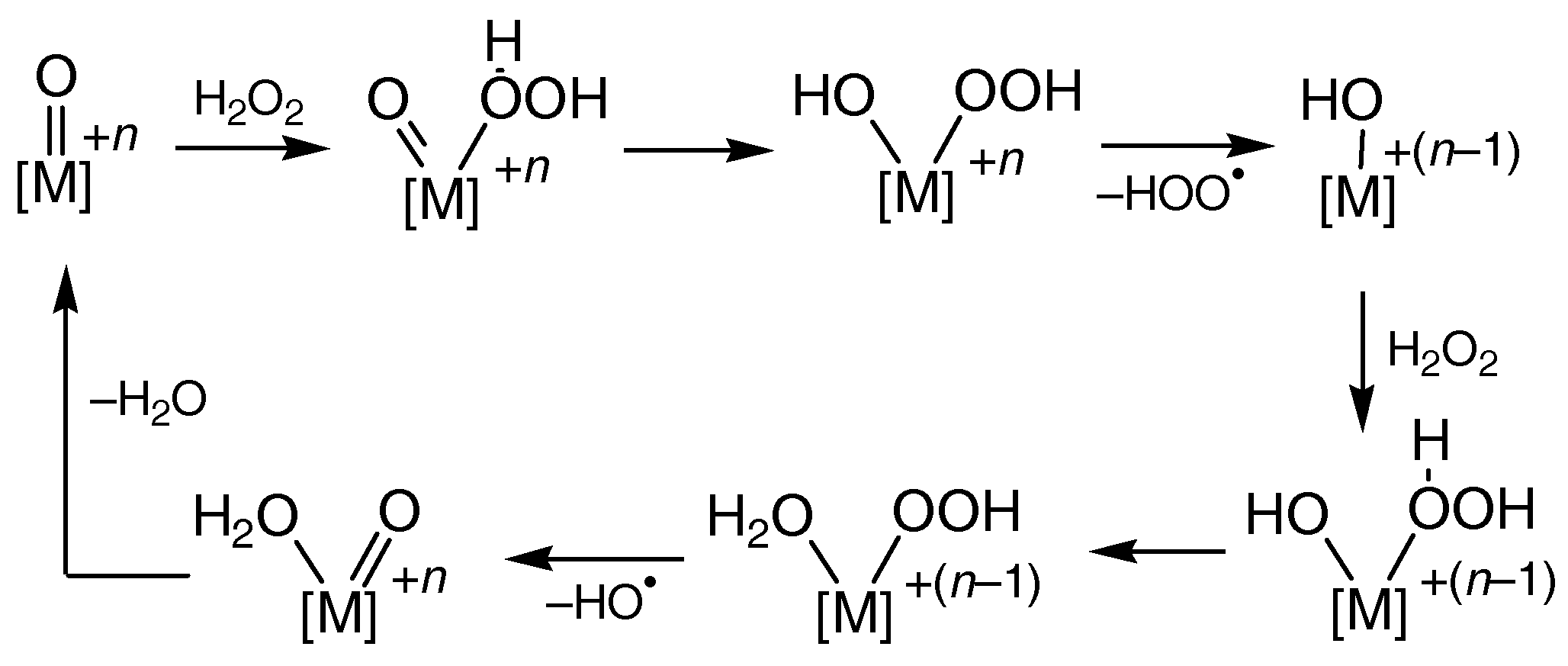
5. A Hydrogen Peroxide Molecule Can Act Both as Oxidizing and as Reducing Reagent
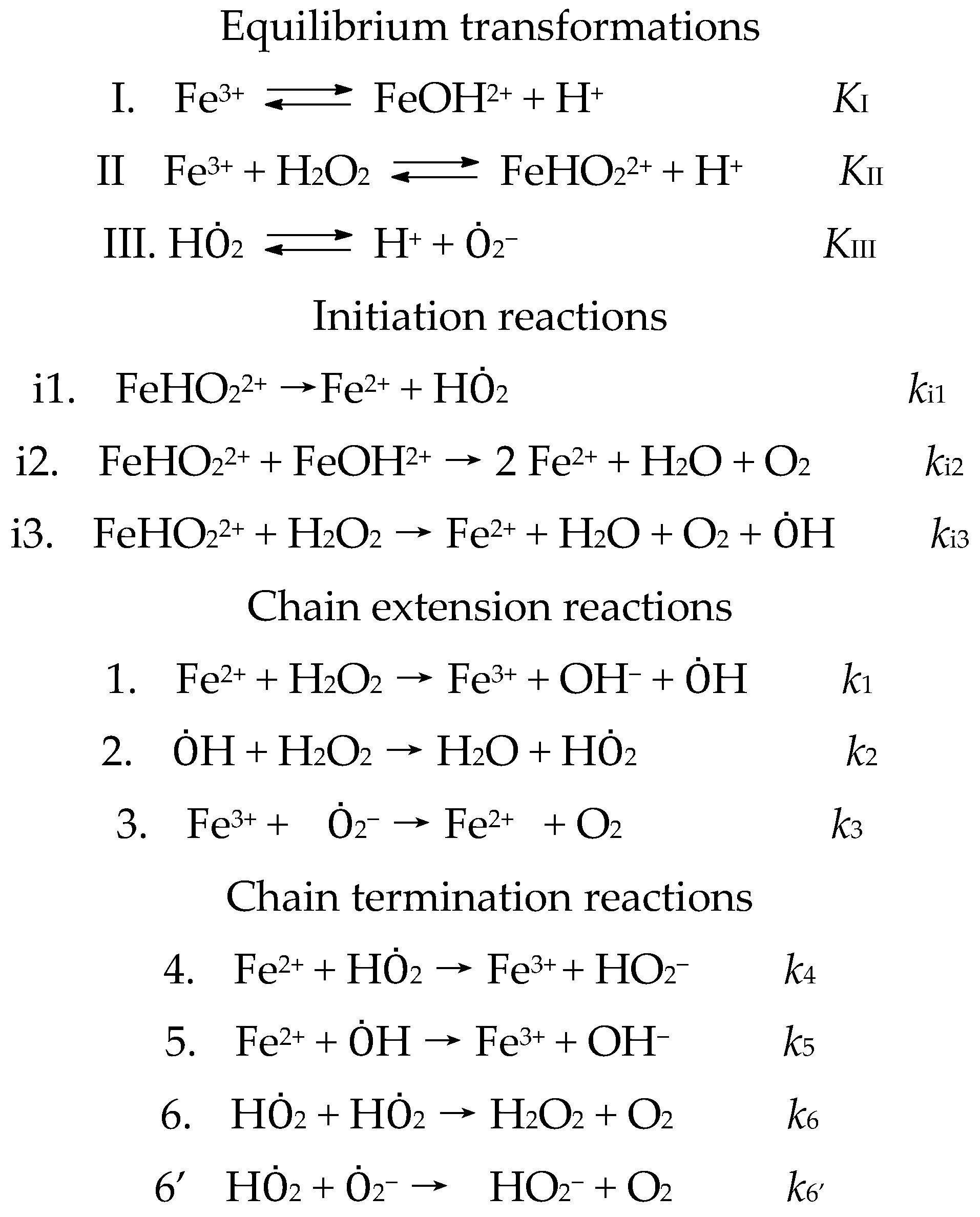
5.1. Oxidation and Reduction with H2O2
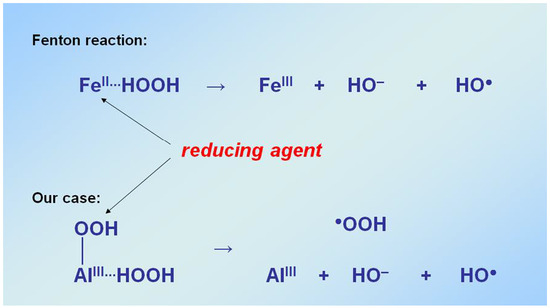
5.2. Decomposition of H2O2 to Afford Hydroxyl Radicals Occurs with the Simultaneous Participation of Two Molecules H2O2
6. Oxidation of Hydrocarbons and Alcohols with Peroxides in the Presence of Metal Complexes Bearing Nitrogen-Containing Ligands
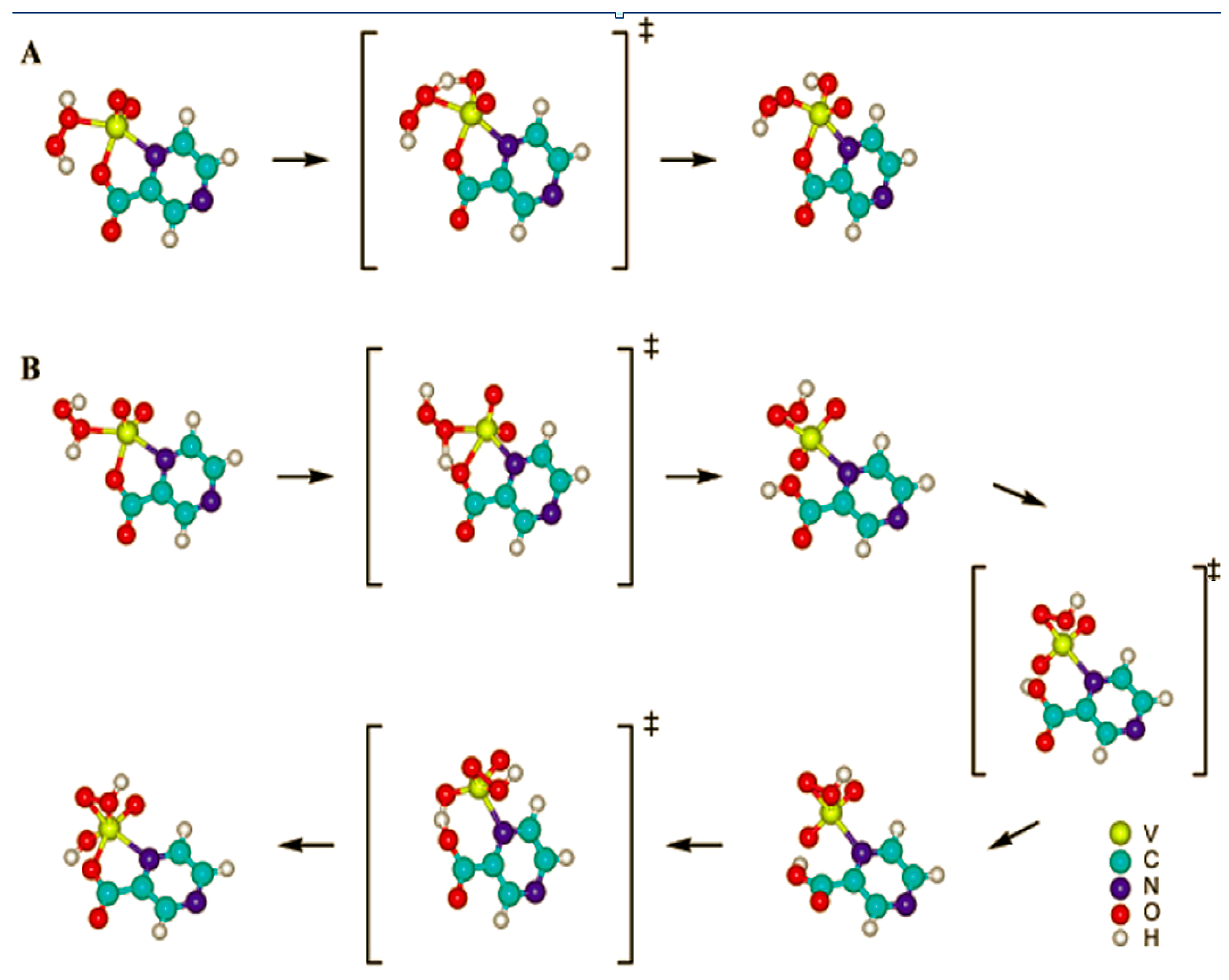
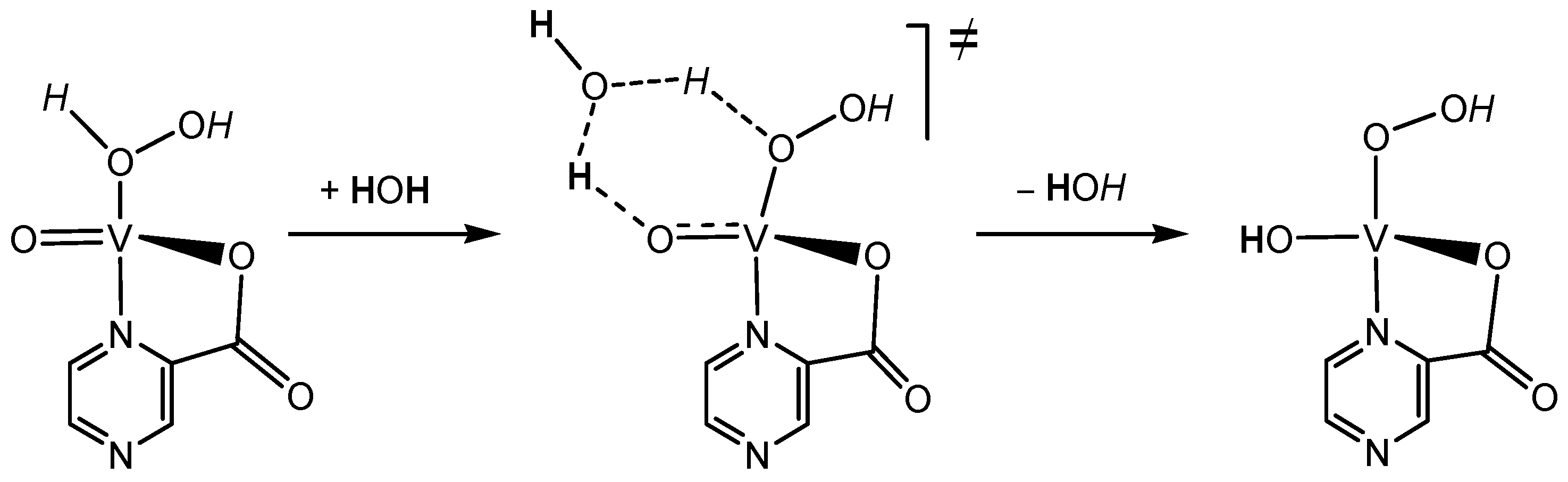
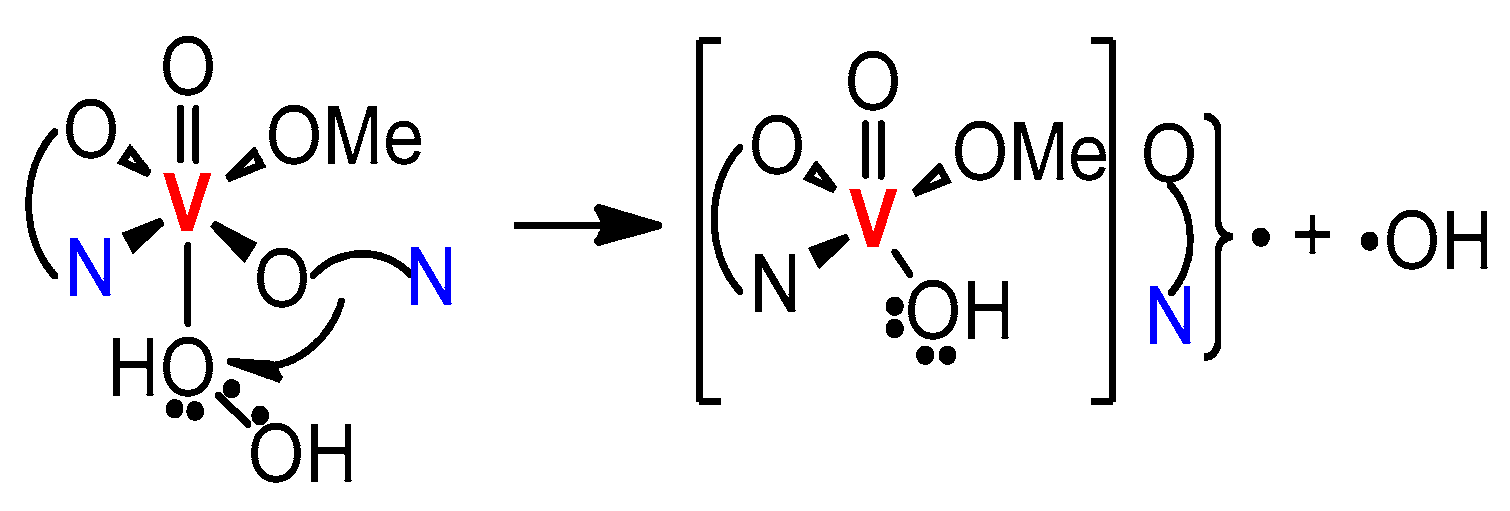
6.1. Pyrazinecarboxylic Acid (PCA) is a Unique Powerful Cocatalyst in the Oxidation of Organic Compounds with Hydrogen Peroxide. Reagent “H2O2—Derivative of Vanadium—Pyrazinecarboxylic Acid”
6.2. Oxidation of Hydrocarbons and Alcohols with Peroxides Catalyzed by Various Amine-Containing Complexes
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wójtowicz-Młochowska, H. Synthetic utility of metal catalyzed hydrogen peroxide oxidation of C-H, C-C and C = C bonds in alkanes, arenes and alkenes: Recent advances. Arch. Org. Chem. 2017, 2017, 12–58. [Google Scholar]
- Shilov, A.E.; Shul’pin, G.B. Activation and Catalytic Reactions of Saturated Hydrocarbons in the Presence of Metal Complexes; Kluwer Academic Publishers: New York, NY, USA; Dordrecht, The Netherlands; Boston, MA, USA; London, UK; Moscow, Russia, 2002. [Google Scholar]
- Shul’pin, G.B. Organometallic Complexes as Catalysts in Oxidation of C–H Compounds. In Advances in Organometallic Chemistry and Catalysis; Pombeiro, A.J.L., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014; Chapter 1; pp. 3–14. [Google Scholar]
- Shul’pin, G.B. Alkane-Oxidizing Systems Based on Metal Complexes. Radical Versus Non-Radical Mechanisms. In Alkane Functionalization; Pombeiro, A.J.L., Guedes da Silva, F.C., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2018; Chapter 3; pp. 47–72. [Google Scholar]
- Shul’pin, G.B. Metal-Catalyzed Oxidation of C-H Compaunds with Peroxides in Unconventional Solvents. In Frontiers of Green Catalytic Selective Oxidations; Bryliakov, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2020; Chapter 1; pp. 1–34. [Google Scholar]
- Shul’pin, G.B. Organic Reactions Catalyzed by Metal Complexes; URSS: Moscow, Russia, 2019. [Google Scholar]
- Shul’pin, G.B. New Trends in Oxidative Functionalization of Carbon–Hydrogen Bonds: A Review. Catalysts 2016, 6, 50. [Google Scholar] [CrossRef]
- Levitsky, M.M.; Bilyachenko, A.N.; Shul’pin, G.B. Oxidation of C-H compounds with peroxides catalyzed by polynuclear transition metal complexes in Si- or Ge-sesquioxane frameworks: A review. J. Organomet. Chem. 2017, 849–850, 201–218. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Vinogradov, M.M.; Shul’pina, L.S. Oxidative functionalization of C–H compounds induced by extremely efficient osmium catalysts (A review). Catal. Sci. Technol. 2018, 8, 4287–4313. [Google Scholar] [CrossRef]
- Shilov, A.E.; Shul’pin, G.B. Activation of C–H Bonds by Metal Complexes. Chem. Rev. 1997, 97, 2879–2932. [Google Scholar] [CrossRef]
- Chirik, P.J.; Wieghardt, K.W. Radical ligands confer nobility of base-metal catalysts. Science 2010, 327, 794–795. [Google Scholar] [CrossRef]
- Blanchard, S.; Derat, E.; Desage-El Murr, M.; Fensterbank, L.; Malacria, M.; Mouriès-Mansuy, V. Non-Innocent Ligands: New Opportunities in Iron Catalysis. Eur. J. Inorg. Chem. 2012, 3, 376–389. [Google Scholar] [CrossRef]
- Tezgerevska, T.; Alley, K.G.; Boskovic, C. Valence Tautomerism in Metal Complexes: Stimulated and Reversible Intramolecular Electron Transfer between Metal Centers and Organic Ligands. Coord. Chem. Rev. 2014, 268, 23–40. [Google Scholar] [CrossRef]
- Römelt, C.; Weyhermüller, T.; Wieghardt, K. Structural characteristics of redox-active pyridine-1,6-diimine complexes: Electronic structures and ligand oxidation levels. Coord. Chem. Rev. 2019, 380, 287–317. [Google Scholar] [CrossRef]
- Sinha, S.; Das, S.; Sikari, R.; Parua, S.; Brandao, P.; Demeshko, S.; Meyer, F.; Paul, N.D. Redox Noninnocent Azo-Aromatic Pincers and Their Iron Complexes. Isolation, Characterization, and Catalytic Alcohol Oxidation. Inorg. Chem. 2017, 56, 14084–14100. [Google Scholar] [CrossRef]
- Kaim, W.; Schwederski, B. Non-innocent ligands in bioinorganic chemistry: An overview. Coord. Chem. Rev. 2010, 254, 1580–1588. [Google Scholar] [CrossRef]
- Que, L.; Tolman, W.B. Biologically inspired oxidation catalysis. Nature 2008, 455, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Lyaskovskyy, V.; de Bruin, B. Redox Non-Innocent Ligands: Versatile New Tools to Control Catalytic Reactions. ACS Catal. 2012, 2, 270–279. [Google Scholar] [CrossRef]
- Sherbow, T.J.; Fettinger, J.C.; Berben, L.A. Control of Ligand pKa Values Tunes the Electrocatalytic Dihydrogen Evolution Mechanism in a Redox-Active Aluminum (III) Complex. Inorg. Chem. 2017, 56, 8651–8660. [Google Scholar] [CrossRef] [PubMed]
- Dub, P.A.; Gordon, J.C. Metal–Ligand Bifunctional Catalysis: The “Accepted” Mechanism, the Issue of Concertedness, and the Function of the Ligand in Catalytic Cycles Involving Hydrogen Atoms. ACS Catal. 2017, 7, 6635–6655. [Google Scholar] [CrossRef]
- Kaim, W. The Shrinking World of Innocent Ligands: Conventional and Non-Conventional Redox-Active Ligands. Eur. J. Inorg. Chem. 2012, 2012, 343–348. [Google Scholar] [CrossRef]
- Mitra, M.; Shteinman, A.A. Synthesis and characterization of a new ortho palladed complex via C-H activation of redox non-innocent 2-(arylazo)-N-phenyl aniline. J. Appl. Chem. 2018, 7, 417–425. [Google Scholar]
- Razborov, D.A. Metal Coplexes Based on Monoiminoacenaphtenone: Synthesis, Structure and Reactivity. Ph.D. Thesis, Lobachevsky State University of Nizhni Novgorod, Novgorod, Russia, 2015. [Google Scholar]
- Olivo, G.; Lanzalunga, O.; Di Stefano, S. Non-Heme Imine-Based Iron Complexes as Catalysts for Oxidative Processes. Adv. Synth. Catal. 2016, 358, 843–863. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Metal-catalyzed hydrocarbon oxygenations in solutions: The dramatic role of additives: A review. J. Mol. Catal. A Chem. 2002, 189, 39–66. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Metal-catalysed hydrocarbon oxidations. C. R. Chim. 2003, 6, 163–178. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kozlov, Y.N.; Shul’pina, L.S.; Kudinov, A.R.; Mandelli, D. Extremely Efficient Alkane Oxidation by a New Catalytic Reagent H2O2/Os3(CO)12/Pyridine. Inorg. Chem. 2009, 48, 10480–10482. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B.; Kozlov, Y.N.; Shul’pina, L.S.; Petrovskiy, P.V. Oxidation of alkanes and alcohols with hydrogen peroxide catalyzed by complex Os3(CO)10(mu-H)2. Appl. Organometal. Chem. 2010, 24, 464–472. [Google Scholar]
- Shul’pin, G.B.; Gradinaru, J.; Kozlov, Y.N. Alkane hydroperoxidation with hydroperoxides catalysed by copper complexes. Org. Biomol. Chem. 2003, 1, 3611–3617. [Google Scholar] [CrossRef] [PubMed]
- Astakhov, G.S.; Levitsky, M.M.; Korlyukov, A.A.; Shul’pina, L.S.; Shubina, E.S.; Ikonnikov, N.S.; Vologzhanina, A.V.; Bilyachenko, A.N.; Dorovatovskii, P.V.; Kozlov, Y.N.; et al. New Cu4Na4- and Cu5-Based Phenylsilsesquioxanes. Synthesis via Complexation with 1,10-Phenanthroline, Structures and High Catalytic Activity in Alkane Oxidations with Peroxides in Acetonitrile. Catalysts 2019, 9, 701. [Google Scholar] [CrossRef]
- Kulakova, A.N.; Korlyukov, A.A.; Zubavichus, Y.V.; Khrustalev, V.N.; Bantreil, X.; Shul’pina, L.S.; Levitsky, M.M.; Ikonnikov, N.S.; Shubina, E.S.; Lamaty, F.; et al. Hexacoppergermsesquioxanes as complexes with N-ligands: Synthesis, structure and catalytic properties. J. Organometal. Chem. 2019, 884, 17–28. [Google Scholar] [CrossRef]
- Garcia-Bosch, I.; Siegel, M.A. Copper-Catalyzed Oxidation of Alkanes with H2O2 under a Fenton-like Regime. Angew. Chem. 2016, 55, 12873–12876. [Google Scholar] [CrossRef]
- Maksimov, A.L.; Kardasheva, Y.S.; Predeina, V.V.; Kluev, M.V.; Ramazanov, D.N.; Talanova, M.Y.; Karakhanov, E.A. Iron and copper complexes with nitrogen-containing ligands as catalysts for cyclohexane oxidation with hydrogen peroxide under mild reaction conditions. Pet. Chem. 2012, 52, 318–326. [Google Scholar] [CrossRef]
- Kim, A.R.; Ahn, S.; Yoon, T.U.; Notestein, J.M.; Farha, O.K.; Bae, Y.S. Fast Cyclohexane Oxidation under Mild Reaction Conditions through a Controlled Creation of Redox-active Fe (II/III). Sites in a Metal-organic Framework. ChemCatChem 2019, 11, 5650–5656. [Google Scholar] [CrossRef]
- Leckie, L.; Mapolie, S.F. Triazole complexes of ruthenium immobilized on mesoporous silicaas recyclable catalysts for octane oxidation. Catal. Commun. 2019, 131, 105803. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Nizova, G.V.; Kozlov, Y.N.; Gonzalez Cuervo, L.; Süss-Fink, G. Hydrogen peroxide oxygenation of alkanes including methane and ethane catalyzed by iron complexes in acetonitrile. Adv. Synth. Catal. 2004, 346, 317–332. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Rozenberg, L.P.; Shibaeva, R.P.; Shilov, A.E. Synthesis and structure of the sigma-naphthyl derivative of platinum (IV) formed by the reaction of naphthalene with H2PtCl6. Kinet. Catal. 1979, 20, 1296–1298. [Google Scholar]
- Shulpin, G.B.; Nizova, G.V.; Nikitaev, A.T. The reaction of PtCl62− with aromatic compounds to afford anionic sigma-aryl complexes of Pt (IV). VIII. Kinetics and mechanisms of thermal, photochemical and gamma-induced reactions with arenes and arylmercury compounds (electrophilic substitution involving electron transfer. J. Organometal. Chem. 1984, 276, 115–153. [Google Scholar]
- Rachmilovich-Calis, S.; Masarwa, A.; Meyerstein, N.; Meyerstein, D.; van Eldik, R. New Mechanistic Aspects of the Fenton Reaction. Chem. Eur. J. 2009, 15, 8303–8309. [Google Scholar] [CrossRef] [PubMed]
- Ramua, R.; Wannaa, W.H.; Janmanchia, D.; Tsaia, Y.F.; Liua, C.C.; Mou, C.Y.; Yu, S.S.F. Mechanistic study for the selective oxidation of benzene and toluenecatalyzed by Fe(ClO4)2 in an H2O2-H2O-CH3CN system. Mol. Catal. 2017, 441, 114–121. [Google Scholar] [CrossRef]
- Oszajca, M.; Brindell, M.; Orzeł, Ł.; Dąbrowski, J.M.; Klaudyan, Ś.; Łabuz, P.; Pacia, M.; Stochel-Gaudyn, A.; Macyk, W.; van Eldik, R.; et al. Mechanistic studies on versatile metal-assisted hydrogen peroxide activation processes for biomedical and environmental incentives. Coord. Chem. Rev. 2016, 327–328, 143–165. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Navalon, S.; Alvaro, M.; Garcia, H. Metal Nanoparticles as Heterogeneous Fenton Catalysts. ChemSusChem 2012, 5, 46–64. [Google Scholar] [CrossRef]
- Das, B.; Al-Hunaiti, A.; Haukka, M.; Demeshko, S.; Meyer, S.; Shteinman, A.A.; Meyer, F.; Repo, T.; Nordlander, E. Catalytic Oxidation of Alkanes and Alkenes by H2O2 with a μ-Oxido Diiron (III) Complex as Catalyst/Catalyst Precursor. Eur. J. Inorg. Chem. 2015, 2015, 3590–3601. [Google Scholar] [CrossRef]
- Petit, A.S.; Pennifold, R.C.R.; Harvey, J.N. Electronic Structure and Formation of Simple Ferryloxo Complexes: Mechanism of the Fenton Reaction. Inorg. Chem. 2014, 53, 6473–6481. [Google Scholar] [CrossRef]
- Shtamm, E.V.; Purmal, A.P.; Skurlatov, Y.I. Catalysis of the oxidation of ascorbic acid by copper ions. Viii. The Cu2+-DH2-H2O2 system as a source of ȮH radicals. J. Phys. Chem. 1977, LI, 3136–3139. (In Rusian) [Google Scholar]
- Duca, G.G.; Scurlatov, Y.I.; Sychev, A.Y. Redox Catalysis and Ecological Chemistry; State University of Moldova: Chisinau, Moldova, 2002. [Google Scholar]
- Sychev, A.Y.; Travin, S.O.; Duka, G.G.; Skurlatov, Y.I. Catalytic Reactions and Environmental Protection; Stiinza: Chisinau, Moldova, 1983. [Google Scholar]
- Kozlov, Y.N.; Nadezhdin, A.D.; Purmal, A.P. The Mechanism of Initiation in the System Fe3+ + H2O2. Kinet. Katal. 1973, 14, 452–457. [Google Scholar]
- Popivker, I.; Zilbermann, I.; Maimon, E.; Cohenc, H.; Meyerstein, D. The “Fenton like reaction of MoO43− involves two H2O2 molecules. Dalton Trans. 2013, 42, 16666–166668. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, M.L.; Kozlov, Y.N.; Mandelli, D.; Pombeiro, A.J.L.; Shul’pin, G.B. Mechanism of Al3+-Catalyzed Oxidations of Hydrocarbons: Dramatic Activation of H2O2 toward O–O Homolysis in Complex [Al(H2O)4(OOH)(H2O2)]2+ Explains the Formation of HO Radicals. Inorg. Chem. 2011, 50, 3996–4005. [Google Scholar] [CrossRef] [PubMed]
- Novikov, A.S.; Kuznetsov, M.L.; Pombeiro, A.J.L.; Bokach, N.A.; Shul’pin, G.B. Generation of HO Radical from Hydrogen Peroxide Catalyzed by Aqua Complexes of the Group III Metals [M(H2O)n]3+ (M = Ga, In, Sc, Y, or La): A theoretical Study. ACS Catal. 2013, 3, 1195–1208. [Google Scholar] [CrossRef]
- Kuznetsov, M.L.; Teixeira, F.A.; Bokach, N.A.; Pombeiro, A.J.L.; Shul’pin, G.B. Radical decomposition of hydrogen peroxide catalyzed by aqua complexes [M(H2O)n]2+ (M = Be, Zn, Cd). J. Catal. 2014, 313, 135–148. [Google Scholar] [CrossRef]
- Rocha, B.G.M.; Kuznetsov, M.L.; Kozlov, Y.N.; Pombeiro, A.J.L.; Shul’pin, G.B. Simple soluble Bi (III) salts as efficient catalysts for the oxidation of alkanes with H2O2. Catal. Sci. Technol. 2015, 5, 2174–2187. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Attanasio, D.; Suber, L. Efficient H2O2 oxidation of alkanes and arenes to alkyl peroxides and phenols catalyzed by the system vanadate-pyrazine-2-carboxylic acid. J. Catal. 1993, 142, 147–152. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Attanasio, D.; Suber, L. Oxidations by a H2O2-VO3−-pyrazine-2-carboxylic acid reagent. 1. Oxidations of alkanes in CH3CN to produce alkyl peroxides. Russ. Chem. Bull. 1993, 42, 55–59. [Google Scholar] [CrossRef]
- Süss-Fink, G.; Stanislas, S.; Shul’pin, G.B.; Nizova, G.V.; Stoeckli-Evans, H.; Neels, A.; Bobillier, C.; Claude, S. Oxidative functionalisation of alkanes: Synthesis, molecular structure and catalytic implications of anionic vanadium (V) oxo and peroxo complexes containing bidentate N, O ligands. J. Chem. Soc. Dalton Trans. 1999, 18, 3169–3175. [Google Scholar] [CrossRef]
- Kirillova, M.V.; Kuznetsov, M.L.; Romakh, V.B.; Shul’pina, L.S.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L.; Shul’pin, G.B. Mechanism of oxidations with H2O2 catalyzed by vanadate anion or oxovanadium(V) triethanolaminate (vanadatrane) in combination with pyrazine-2-carboxylic acid (PCA): Kinetic and DFT studies. J. Catal. 2009, 267, 140–157. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Druzhinina, A.N.; Nizova, G.V. Oxidation with the H2O2-VO3−-pyrazine-2-carboxylic acid reagent. 2. Oxidation of alcohols and aromatic hydrocarbons. Russ. Chem. Bull. 1993, 42, 1327–1329. [Google Scholar]
- Nizova, G.V.; Shul’pin, G.B. Oxidation by a H2O2-vanadium complex-2-pyrazinecarboxylic acid reagent. 3. Evidence for hydroxyl radical formation. Russ. Chem. Bull. 1994, 43, 1146–1148. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Süss-Fink, G. Oxidations by the reagent “H2O2-vanadium complex-pyrazine-2-carboxylic acid”. Part 4. Oxidation of alkanes, benzene and alcohols by an adduct of H2O2 with urea. J. Chem. Soc. Perkin. Trans. 1995, 1459–1463. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Drago, R.S.; Gonzalez, M. Oxidations by a “H2O2-vanadium complex-pyrazine-2-carboxylic acid” reagent. 5. Oxidation of lower alkanes with the formation of carbonyl compounds. Russ. Chem. Bull. 1996, 45, 2386–2388. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Guerreiro, M.C.; Schuchardt, U. Oxidations by the reagent O2-H2O2-vanadium complex-pyrazine-2-carboxylic acid. Part 7. Hydroperoxidation of higher alkanes. Tetrahedron 1996, 52, 13051–13062. [Google Scholar] [CrossRef]
- Guerreiro, M.C.; Schuchardt, U.; Shul’pin, G.B. Oxidation with the “O2-VO3−-pyrazine-2-carboxylic acid” reagent. Part 6. Oxidation of n-heptane and cyclohexane. Direct determination of alkyl hydroperoxides by gas-liquid chromatography. Russ. Chem. Bull. 1997, 46, 749–754. [Google Scholar] [CrossRef]
- Nizova, G.V.; Süss-Fink, G.; Shul’pin, G.B. Oxidations by the reagent O2-H2O2-vanadium complex-pyrazine-2-carboxylic acid—8. Efficient oxygenation of methane and other lower alkanes in acetonitrile. Tetrahedron 1997, 53, 3603–3614. [Google Scholar] [CrossRef]
- Schuchardt, U.; Guerreiro, M.C.; Shul’pin, G.B. Oxidation with the ‘O2-H2O2-vanadium complex-pyrazine-2-carboxylic acid’ reagent. 9. Oxidation of cyclohexene and decalin. Russ. Chem. Bull. 1998, 47, 247–252. [Google Scholar] [CrossRef]
- Süss-Fink, G.; Nizova, G.V.; Stanislas, S.; Shul’pin, G.B. Oxidations by the reagent ‘O2-H2O2-vanadate anion-pyrazine-2-carboxylic acid’. Part 10. Oxygenation of methane in acetonitrile and water. J. Mol. Catal. A Chem. 1998, 130, 163–170. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Ishii, Y.; Sakaguchi, S.; Iwahama, T. Oxidations with the “O2-H2O2-vanadium complex-pyrazine-2-carboxylic acid” reagent. 11. Oxidation of styrene, phenylacetylene, and their derivatives with the formation of benzaldehyde and benzoic acid. Russ. Chem. Bull. 1999, 48, 887–890. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kozlov, Y.N.; Nizova, G.V.; Süss-Fink, G.; Stanislas, S.; Kitaygorodskiy, A.; Kulikova, V.S. Oxidations by the reagent “O2-H2O2-vanadium derivative-pyrazine-2-carboxylic acid” Part 12. Main features, kinetics and mechanism of alkane hydroperoxidation. J. Chem. Soc. Perkin. Trans. 2001, 1351–1371. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Lachter, E.R. Aerobic hydroxylation of hydrocarbons catalysed by vanadate ion. J. Mol. Catal. A Chem. 2003, 197, 65–71. [Google Scholar] [CrossRef]
- De la Cruz, M.H.C.; Kozlov, Y.N.; Lachter, E.R.; Shul’pin, G.B. Oxidations by the reagent “O2-H2O2-vanadium derivative-pyrazine-2-carboxylic acid”. Part 13. Kinetics and mechanism of the benzene hydroxylation. New J. Chem. 2003, 27, 634–638. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Shul’pin, G.B. Pyrazinecarboxylic acid and analogs: Highly efficient co-catalysts in the metal-complex-catalyzed oxidation of organic compounds. Coord. Chem. Rev. 2013, 257, 732–754. [Google Scholar] [CrossRef]
- Süss-Fink, G.; Gonzalez Cuervo, L.; Therrien, B.; Stoeckli-Evans, H.; Shul’pin, G.B. Mono and oligonuclear vanadium complexes as catalysts for alkane oxidation: Synthesis, molecular structure and catalytic potential. Inorg. Chim. Acta 2004, 357, 475–484. [Google Scholar] [CrossRef]
- Kozlov, Y.N.; Romakh, V.B.; Kitaygorodskiy, A.; Buglyó, P.; Süss-Fink, G.; Shul’pin, G.B. Oxidation of 2-Propanol and Cyclohexane by the Reagent “Hydrogen Peroxide-Vanadate Anion-Pyrazine-2-carboxylic Acid”: Kinetics and Mechanism. J. Phys. Chem. A 2007, 111, 7736–7752. [Google Scholar] [CrossRef] [PubMed]
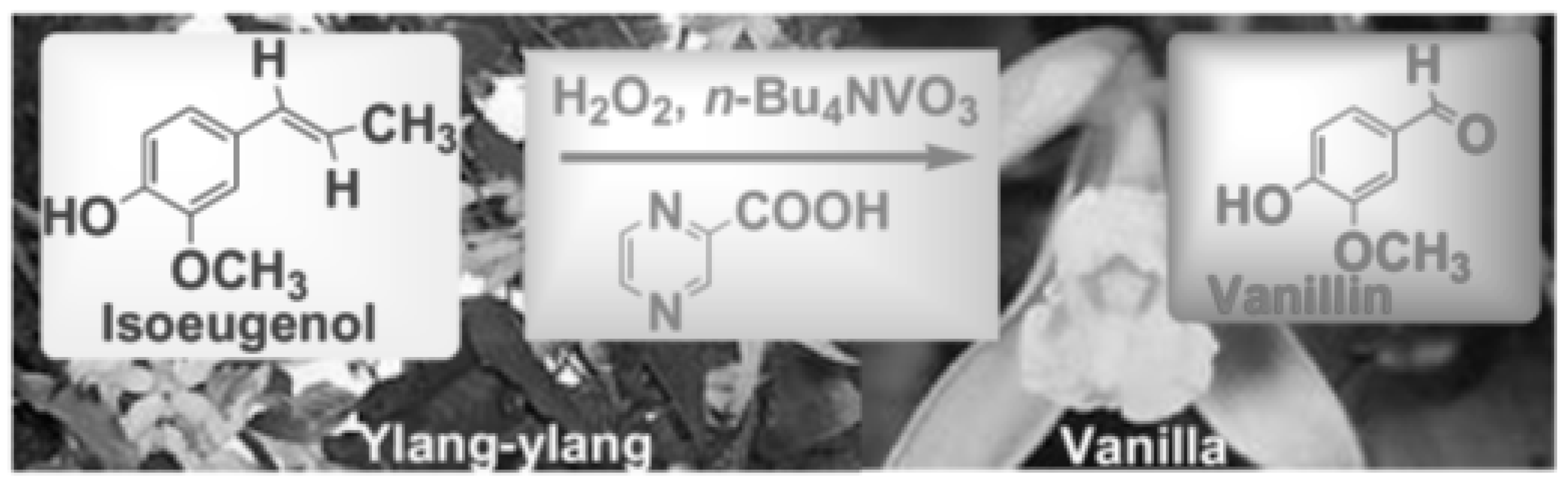
- Gusevskaya, E.V.; Menini, L.; Parreira, L.A.; Mesquita, R.A.; Kozlov, Y.N.; Shul’pin, G.B. Oxidation of isoeugenol to vanillin by the “H2O2–vanadate–pyrazine-2-carboxylic acid” reagent” <Part 17 of the series “Oxidations by the reagent ‘H2O2–vanadium derivative–pyrazine-2-carboxylic acid. J. Mol. Catal. A Chem. 2012, 363–364, 140–147. [Google Scholar] [CrossRef]
- Kirillova, M.V.; Kuznetsov, M.L.; Kozlov, Y.N.; Shul’pina, L.S.; Kitaygorodskiy, A.; Pombeiro, A.J.L.; Shul’pin, G.B. Participation of Oligovanadates in Alkane Oxidation with H2O2 Catalyzed by Vanadate Anion in Acidified Acetonitrile: Kinetic and DFT Studies. ACS Catal. 2011, 1, 1511–1520. [Google Scholar] [CrossRef]
- Khaliullin, R.Z.; Bell, A.T.; Head-Gordon, M. A Density Functional Theory Study of the Mechanism of Free Radical Generation in the System Vanadate/PCA/H2O2. J. Phys. Chem. B 2005, 109, 17984–17992. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E. More Hydra than Janus- non-classical coordination modes in complexes of oligopyridine ligands. Coord. Chem. Rev. 2017, 350, 84–104. [Google Scholar] [CrossRef]
- Lukoyanov, A.N.; Ulivanova, E.A.; Razborov, D.A.; Khrizanforova, V.; Budnikova, Y.H.; Makarov, S.G.; Rumyantcev, R.V.; Ketkov, S.Y.; Fedushkin, I.L. One-electron reduction of mono-iminoacenaphthenone dpp-mian (dpp-mian = 2-mono(2,6-diisopropylphenylimino)acenaphthene-1-one). Chem. Eur. J. 2019, 25, 3858–3866. [Google Scholar] [CrossRef]
- Gryca, I.; Czerwińska, K.; Machura, B.; Chrobok, A.; Shul’pina, L.S.; Kuznetsov, M.L.; Nesterov, D.S.; Kozlov, Y.N.; Pombeiro, A.J.L.; Varyan, I.A.; et al. High Catalytic Activity of Vanadium Complexes in Alkane Oxidations with Hydrogen Peroxide: An Effect of 8-Hydroxyquinoline Derivatives as Noninnocent Ligands. Inorg. Chem. 2018, 57, 1824–1839. [Google Scholar]
- Gryca, I.; Machura, B.; Małecki, J.G.; Kusz, J.; Shul’pina, L.S.; Ikonnikov, N.S.; Shul’pin, G.B. p-Tolylimido rhenium(V) complexes with phenolate-based ligands: Synthesis, X-ray studies and catalytic activity in oxidationQwith tert-butylhydroperoxide. Dalton Trans. 2016, 45, 334–351. [Google Scholar] [CrossRef] [PubMed]
- Gryca, I.; Machura, B.; Shul’pina, L.S.; Shul’pin, G.B. Synthesis, structures and catalytic activity of p-tolylimido rhenium(V) complexes incorporating quinoline-derived ligands. Inorg. Chim. Acta 2017, 455, 683–695. [Google Scholar] [CrossRef]
- Czerwińska, K.; Machura, B.; Kula, S.; Krompiec, S.; Erfurt, K.; Roma-Rodrigues, C.; Fernandes, A.R.; Shul’pina, L.S.; Ikonnikov, N.S.; Shul’pin, G.B. Copper (II) complexes of functionalized 2,2′:6′,2″-terpyridines and 2,6-di(thiazol-2-yl)pyridine: Structure, spectroscopy, cytotoxicity and catalytic activity. Dalton Trans. 2017, 46, 9591–9604. [Google Scholar] [CrossRef] [PubMed]
- Choroba, K.; Machura, B.; Kula, S.; Raposo, L.R.; Fernandes, A.R.; Kruszynski, R.; Erfurt, K.; Shul’pina, L.S.; Kozlov, Y.N.; Shul’pin, G.B. Copper (II) complexes with 2,2′:6′,2″-terpyridine, 2,6-di(thiazol-2-yl)pyridine and 2,6-di(pyrazin-2-yl)pyridine substituted with quinolines. Synthesis, structure, antiproliferative activity, and catalytic activity in oxidation of alkanes and alcohols with peroxides. Dalton Trans. 2019, 48, 12656–12673. [Google Scholar] [PubMed]
- Fomenko, I.S.; Gushchin, A.L.; Shul’pina, L.S.; Ikonnikov, N.S.; Abramov, P.A.; Romashev, N.F.; Poryvaev, A.S.; Sheveleva, A.M.; Bogomyakov, A.S.; Shmelev, N.Y.; et al. New oxidovanadium(IV) complex with redox-active acenaphthene-1,2-diimine ligand: Synthesis, structure, redox properties and catalytic activity in alkane oxidations with hydrogen peroxide. New J. Chem. 2018, 42, 16200–16210. [Google Scholar] [CrossRef]
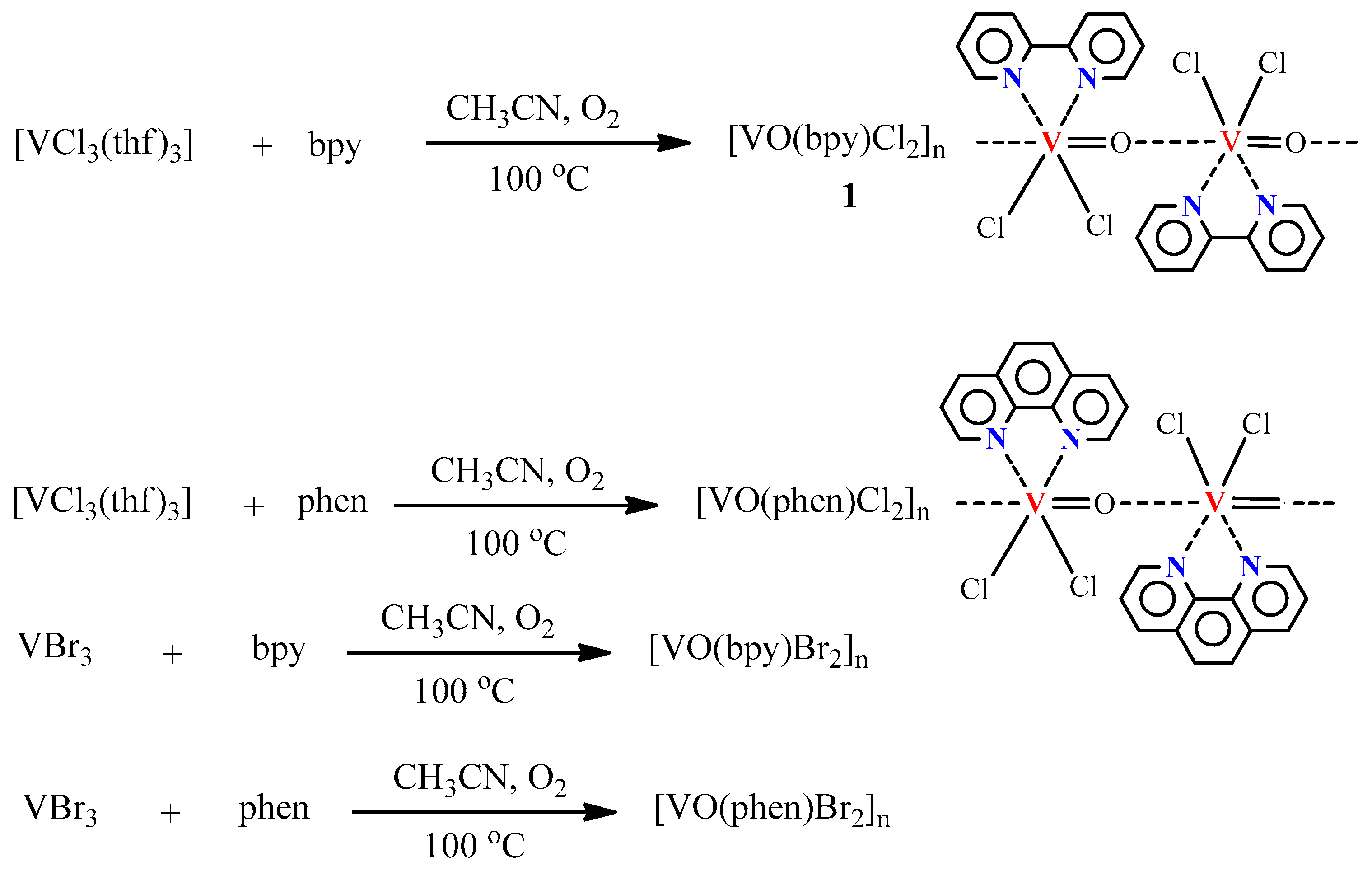
- Fomenko, I.S.; Gushchin, A.I.; Abramov, P.A.; Sokolov, M.N.; Shul’pina, L.S.; Ikonnikov, N.S.; Kuznetsov, M.L.; Pombeiro, A.J.L.; Kozlov, Y.N.; Shul’pin, G.B. New oxidovanadium(IV) complexes with 2,2′-bipyridine and 1,10-phenathroline Ligands. Synthesis, structure and high catalytic activity in oxidations of alkanes and alcohols with peroxides. Catalysts 2019, 9, 217. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Dronova, M.S.; Yalymov, A.I.; Korlyukov, A.A.; Shul’pina, L.S.; Arkhipov, D.E.; Shubina, E.S.; Levitsky, M.M.; Kirilin, A.D.; Shul’pin, G.B. New binuclear cage-like copper(II) silsesquioxane (“Cooling Tower”); its high catalytic activity in oxidation of benzene and alcohols. Eur. J. Inorg. Chem. 2013, 2013, 5240–5246. [Google Scholar] [CrossRef]
- Dronova, M.S.; Bilyachenko, A.N.; Yalymov, A.I.; Kozlov, Y.N.; Shul’pina, L.S.; Korlyukov, A.A.; Arkhipov, D.E.; Levitsky, M.M.; Shubina, E.S.; Shul’pin, G.B. Solvent-controlled synthesis of tetranuclear cage-like copper (II) silsesquioxanes. Remarkable features of the cage structures and their high catalytic activity in oxidation with peroxides. Dalton Trans. 2014, 43, 872–882. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Dronova, M.S.; Yalymov, A.I.; Lamaty, F.; Bantreil, X.; Martinez, J.; Bizet, C.; Shul’pina, L.S.; Korlyukov, A.A.; Arkhipov, D.E.; et al. Cage-like Copper(II) Silsesquioxanes: Transmetalation Reactions, Structural, Quantum Chemical and Catalytic Studies. Chem. Eur. J. 2015, 21, 8758–8770. [Google Scholar] [CrossRef]
- Vinogradov, M.M.; Kozlov, Y.N.; Bilyachenko, A.N.; Nesterov, D.S.; Shul’pina, L.S.; Zubavichus, Y.V.; Pombeiro, A.J.L.; Levitsky, M.M.; Yalymov, A.I.; Shul’pin, G.B. Alkane oxidation with peroxides catalyzed by cage-like copper (II) silsesquioxanes. New J. Chem. 2015, 39, 187–199. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Yalymov, A.I.; Shul’pina, L.S.; Mandelli, D.; Korlyukov, A.A.; Vologzhanina, A.V.; Es’kova, M.A.; Shubina, E.S.; Levitsky, M.M.; Shul’pin, G.B. Novel Cage-Like Hexanuclear Nickel(II) Silsesquioxane. Synthesis, Structure, and Catalytic Activity in Oxidations with Peroxides. Molecules 2016, 21, 665. [Google Scholar] [CrossRef] [PubMed]
- Bilyachenko, A.N.; Levitsky, M.M.; Yalymov, A.I.; Korlyukov, A.A.; Vologzhanina, A.V.; Kozlov, Y.N.; Shul’pina, L.S.; Nesterov, D.S.; Pombeiro, A.J.L.; Lamaty, F.; et al. A heterometallic (Fe6Na8) cage-like silsesquioxane: Synthesis, structure, spin glass behavior and high catalytic activity. RSC Adv. 2016, 6, 48165–48180. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Yalymov, A.I.; Korlyukov Alexander, A.; Long, J.; Larionova, J.; Guari, Y.; Vologzhanina, A.V.; Es’kova, M.A.; Shubina, E.S.; Levitsky, M.M. Unusual penta- and hexanuclear Ni(ii)-based silsesquioxane polynuclear complexes. Dalton Trans. 2016, 45, 7320–7327. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Yalymov, A.I.; Levitsky, M.M.; Korlyukov, A.A.; Es’kova, M.A.; Long, J.; Larionova, J.; Guari, Y.; Shul’pina, L.S.; Ikonnikov, N.S.; et al. First cage-like pentanuclear Co (II)-silsesquioxane. Dalton Trans. 2016, 45, 13663–13666. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Levitsky, M.M.; Yalymov, A.I.; Korlyukov, A.A.; Khrustalev, V.N.; Vologzhanina, A.V.; Shul’pina, L.S.; Ikonnikov, N.S.; Trigub, A.E.; Dorovatovsky, P.V.; et al. Cage-like Fe,Na-Germsesquioxanes: Structure, Magnetism, and Catalytic Activity. Angew. Chem. 2016, 55, 15360–15363. (In English) [Google Scholar] [CrossRef]
- Yalymov, A.I.; Bilyachenko, A.N.; Levitsky, M.M.; Korlyukov, A.A.; Khrustalev, V.N.; Shul’pina, L.S.; Dorovatovskii, P.V.; Es’kova, M.A.; Lamaty, F.; Bantreil, X.; et al. High Catalytic Activity of Heterometallic (Fe6Na7 and Fe6Na6) Cage Silsesquioxanes in Oxidations with Peroxides. Catalysts 2017, 7, 101. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Kulakova, A.N.; Levitsky, M.M.; Petrov, A.A.; Korlyukov, A.A.; Shul’pina, L.S.; Khrustalev, V.N.; Dorovatovskii, P.V.; Vologzhanina, A.V.; Tsareva, U.S.; et al. Unusual Tri-, Hexa- and Nonanuclear Organosilicon Copper Clusters: Synthesis, Structures and Catalytic Activity in Oxidations with Peroxides. Inorg. Chem. 2017, 56, 4093–4103. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Kulakova, A.N.; Levitsky, M.M.; Korlyukov, A.A.; Khrustalev, V.N.; Vologzhanina, A.V.; Titov, A.A.; Dorovatovskii, P.V.; Shul’pina, L.S.; Lamaty, F.; et al. Ionic Complexes of Tetra- and Nonanuclear Cage Copper (II) Phenylsilsesquioxanes: Synthesis and High Activity in Oxidative Catalysis. ChemCatChem 2017, 9, 4437–4447. [Google Scholar] [CrossRef]
- Kulakova, A.N.; Bilyachenko, A.N.; Levitsky, M.M.; Khrustalev, V.N.; Korlyukov, A.A.; Zubavichus, Y.V.; Dorovatovskii, P.V.; Lamaty, F.; Bantreil, X.; Villemejeanne, B.; et al. Si10Cu6N4 Cage Hexacoppersilsesquioxanes Containing N-Ligands: Synthesis, Structure, and High Catalytic Activity in Peroxide Oxidations. Inorg. Chem. 2017, 56, 15026–15040. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Khrustalev, V.N.; Zubavichus, Y.V.; Shul’pina, L.S.; Kulakova, A.N.; Bantreil, X.; Lamaty, F.; Levitsky, M.M.; Gutsul, E.I.; Shubina, E.S.; et al. Heptanuclear Fe5Cu2-Phenylgermsesquioxane containing 2,2′-Bipyridine: Synthesis, Structure, and Catalytic Activity in Oxidation of C-H Compounds. Inorg. Chem. 2018, 57, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Bilyachenko, A.N.; Levitsky, M.M.; Khrustalev, V.N.; Zubavichus, Y.V.; Shul’pina, L.S.; Shubina, E.S.; Shul’pin, G.B. Mild and Regioselective Hydroxylation of Methyl Group in Neocuproine: Approach to an N, O-Ligated Cu6 Cage Phenylsilsesquioxane. Organometallics 2018, 37, 168–171. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Kulakova, A.N.; Shul’pina, L.S.; Levitsky, M.M.; Korlyukov, A.A.; Khrustalev, V.N.; Zubavichus, Y.V.; Dorovatovskii, P.V.; Tsareva, U.S.; Shubina, E.S.; et al. Family of penta- and hexanuclear metallasilsesquioxanes: Synthesis, structure and catalytic properties in oxidations. J. Organomet. Chem. 2018, 867, 133–141. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Levitsky, M.M.; Korlyukov, A.A.; Khrustalev, V.N.; Zubavichus, Y.V.; Shul’pina, L.S.; Shubina, E.S.; Vologzhanina, A.V.; Shul’pin, G.B. Heptanuclear Cage Cu (II)—Silsesquioxanes. Features of Synthesis, Structure and Catalytic Activity. Eur. J. Inorg. Chem. 2018, 2018, 2505–2511. [Google Scholar] [CrossRef]
- Astakhov, G.S.; Bilyachenko, A.N.; Korlyukov, A.A.; Levitsky, M.M.; Shul’pina, L.S.; Bantreil, X.; Lamaty, F.; Vologzhanina, A.V.; Shubina, E.S.; Dorovatovskii, P.V.; et al. High cluster (Cu9) cage silsesquioxanes. Synthesis, structure and catalytic activity. Inorg. Chem. 2018, 57, 11524–11529. [Google Scholar] [CrossRef]
- Kulakova, A.N.; Bilyachenko, A.N.; Korlyukov, A.A.; Shul’pina, L.S.; Bantreil, X.; Lamaty, F.; Shubina, E.S.; Levitsky, M.M.; Ikonnikov, N.S.; Shul’pin, G.B. A new “bicycle helmet”—Like copper (II), sodiumphenylsilsesquioxane. Synthesis, structure and catalytic activity. Dalton Trans. 2018, 47, 15666–15669. [Google Scholar] [CrossRef]
- Kulakova, A.N.; Bilyachenko, A.N.; Khrustalev, V.N.; Zubavichus, Y.V.; Dorovatovskii, P.V.; Shul’pina, L.S.; Bantreil, X.; Lamaty, F.; Shubina, E.S.; Levitsky, M.M.; et al. Cu42Ge24Na4—A Giant Trimetallic Sesquioxane Cage: Synthesis, Structure, and Catalytic Activity. Catalysts 2018, 8, 484. [Google Scholar] [CrossRef]
- Kulakova, A.N.; Khrustalev, V.N.; Zubavichus, Y.V.; Shul’pina, L.S.; Shubina, E.S.; Levitsky, M.M.; Ikonnikov, N.S.; Bilyachenko, A.N.; Kozlov, Y.N.; Shul’pin, G.B. Palanquin-like Cu4Na4 Silsesquioxane. Synthesis (via oxidation of 1,1-bis (diphenyphosphino) methane), structure and catalytic activity in akane or alcohol oxidation with peroxides. Catalysts 2019, 9, 154. [Google Scholar] [CrossRef]
- Kulakova, A.N.; Sedykh, E.E.; Levitsky, M.M.; Dorovatovskii, P.V.; Khrustalev, V.N.; Shul’pina, L.S.; Shubina, E.S.; Kozlov, Y.N.; Ikonnikov, N.S.; Bilyachenko, A.N.; et al. The first tris-heteroleptic copper cage, ligated by germsesquioxanes, 2,2′-bipyridines and 3,5-dimethylpyrazolates. Synthesis, structure and unique catalytic activity in oxidation of alkanes and alcohols with peroxides. J. Organomet. Chem. 2019, 899, 120911. [Google Scholar] [CrossRef]
- Levitsky, M.M.; Bilyachenko, A.N. Modern concepts and methods in the chemistry of polyhedral metallasiloxanes. Coord. Chem. Rev. 2016, 306, 235–269. [Google Scholar] [CrossRef]
- Levitsky, M.M.; Bilyachenko, A.N.; Shubina, E.S. Cagelike metallagermanates and metallagermoxanes: Synthesis, structures and functional properties. Coord. Chem. Rev. 2019, 386, 209–239. [Google Scholar] [CrossRef]
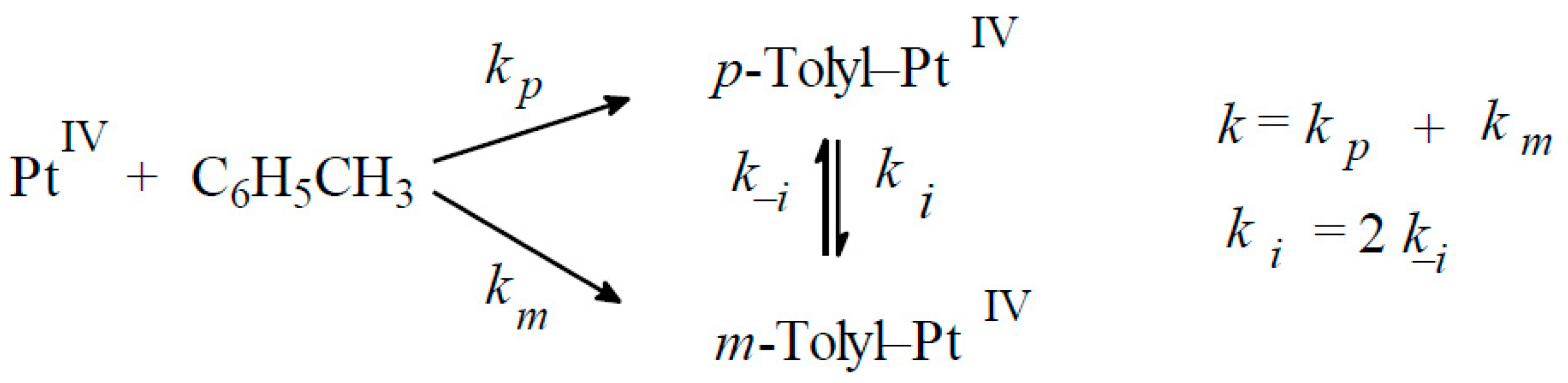




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shul’pin, G.B.; Kozlov, Y.N.; Shul’pina, L.S. Metal Complexes Containing Redox-Active Ligands in Oxidation of Hydrocarbons and Alcohols: A Review. Catalysts 2019, 9, 1046. https://doi.org/10.3390/catal9121046
Shul’pin GB, Kozlov YN, Shul’pina LS. Metal Complexes Containing Redox-Active Ligands in Oxidation of Hydrocarbons and Alcohols: A Review. Catalysts. 2019; 9(12):1046. https://doi.org/10.3390/catal9121046
Chicago/Turabian StyleShul’pin, Georgiy B., Yuriy N. Kozlov, and Lidia S. Shul’pina. 2019. "Metal Complexes Containing Redox-Active Ligands in Oxidation of Hydrocarbons and Alcohols: A Review" Catalysts 9, no. 12: 1046. https://doi.org/10.3390/catal9121046
APA StyleShul’pin, G. B., Kozlov, Y. N., & Shul’pina, L. S. (2019). Metal Complexes Containing Redox-Active Ligands in Oxidation of Hydrocarbons and Alcohols: A Review. Catalysts, 9(12), 1046. https://doi.org/10.3390/catal9121046