Carbozincation of Substituted 2-Alkynylamines, 1-Alkynylphosphines, 1-Alkynylphosphine Sulfides with Et2Zn in the Presence of Catalytic System of Ti(O-iPr)4 and EtMgBr
Abstract
1. Introduction
2. Results and Discussions
2.1. Ti-Mg-Catalyzed Carbozincation of Substituted 1-Alkynylphosphine Sulfides with Et2Zn
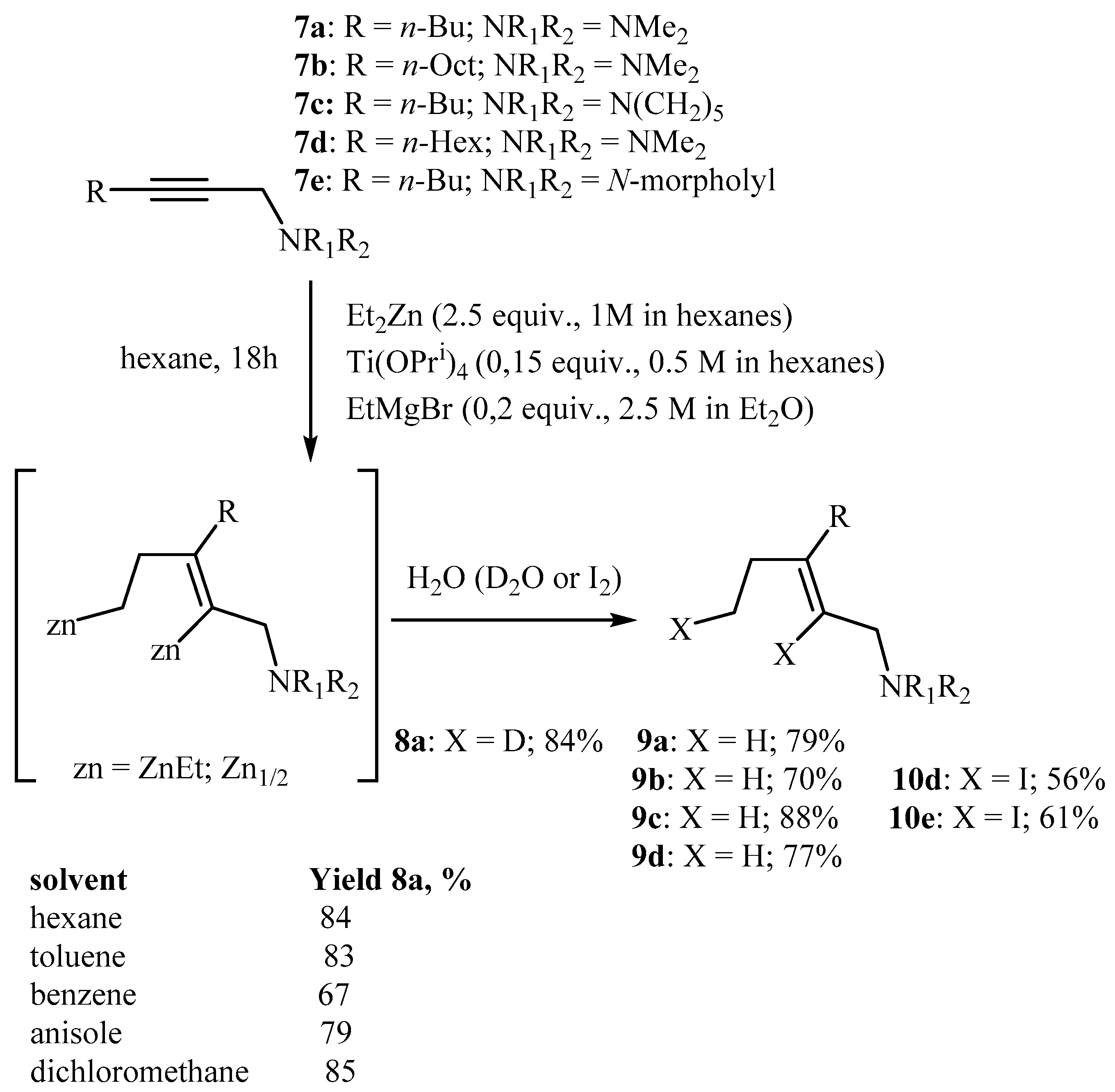
2.2. The EtMgBr and Ti(O-iPr)4-Catalyzed 2-Zincoethylzincation of Substituted Substituted Propargylamines with Et2Zn
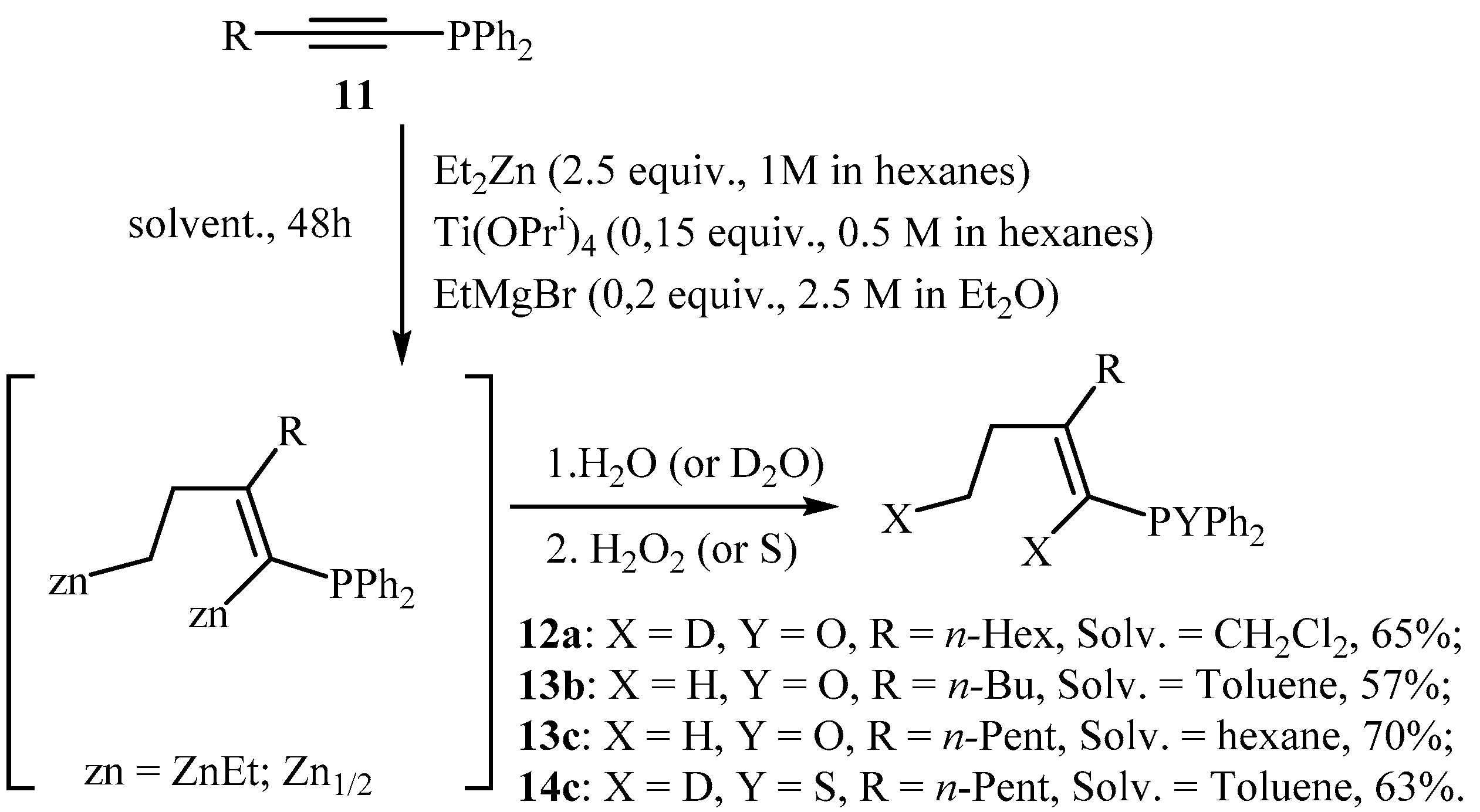
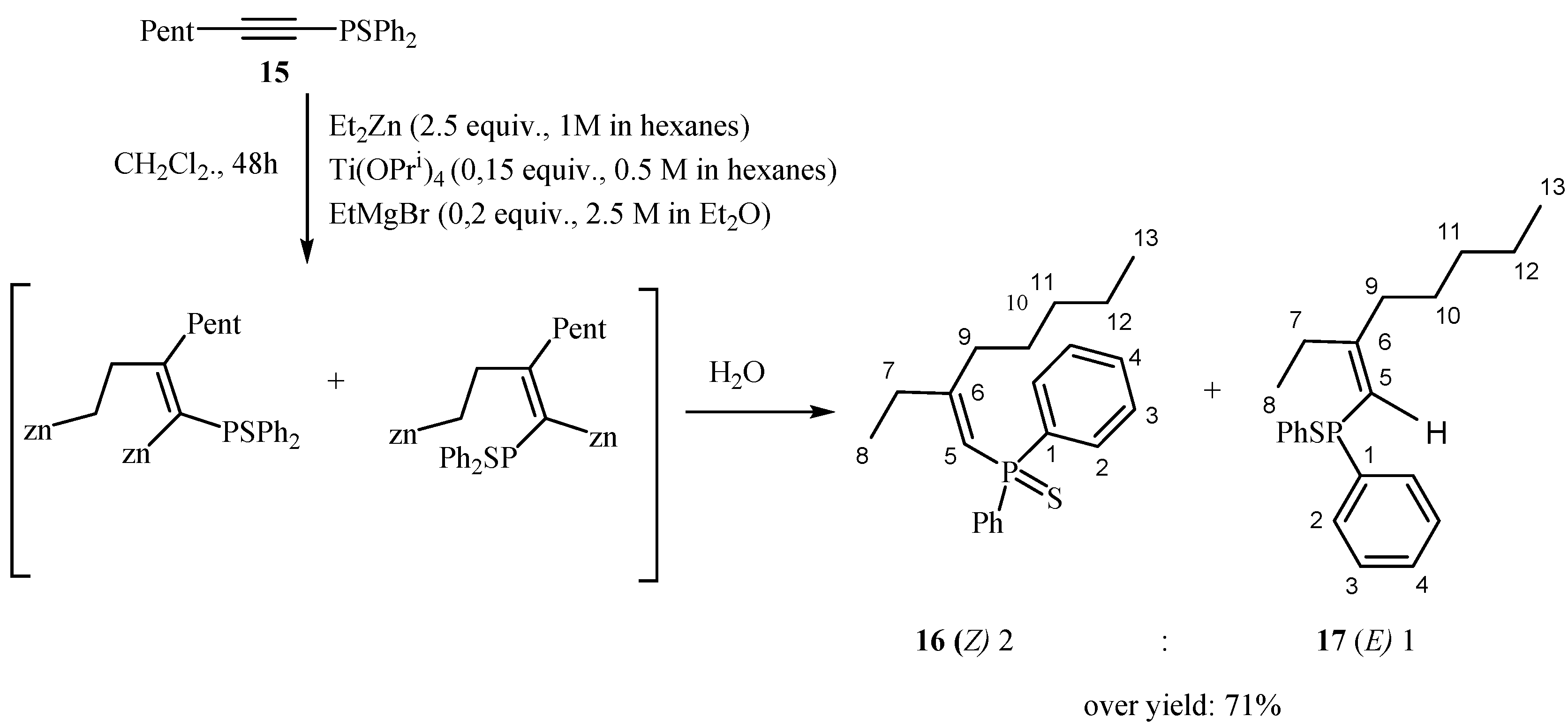
2.3. EtMgBr and Ti(O-iPr)4-Catalyzed 2-Zincoethylzincation of Substituted 1-Alkynylphosphines with Diethylzinc
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of 1-Alkenyl Phosphine Sulfides 3a–c, 4a and 5, 6 via Ethylmagnesium Bromide and Titanium(IV) Isopropoxide-Catalyzed Reaction of 1-Alkynyl Phosphine Sulfides with Et2Zn
3.2.2. Preparation of 1-Alkenyl Phosphine Oxides 12a, 13b, 13c, 14c, 16, and 17 via Titanium(IV) Isopropoxide and Ethylmagnesium Bromide-Catalyzed Reaction of 1-Alkynyl Phosphines with Et2Zn
3.2.3. Preparation of Allylic Amines 8a, 9a–d via Titanium(IV) Isopropoxide and Ethylmagnesium Bromide-Catalyzed Reaction of 2-Alkynylamines with Et2Zn
3.2.4. The Iodination of Intermediate Organozinc Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kadikova, R.N.; Ramazanov, I.R.; Mozgovoi, O.S.; Gabdullin, A.M.; Dzhemilev, U.M. 2-Zincoethylzincation of 2-Alkynylamines and 1-Alkynylphosphines Catalyzed by Titanium(IV) Isopropoxide and Ethylmagnesium Bromide. Synlett 2019, 30, 311–314. [Google Scholar] [CrossRef]
- Montchamp, J.-L.; Negishi, E.-I. Carbozincation of Enynes Catalyzed by Titanium(IV) Alkoxides and Alkylmagnesium Derivatives. J. Am. Chem. Soc. 1998, 120, 5345–5346. [Google Scholar] [CrossRef]
- Sklute, G.; Bolm, C.; Marek, I. Regio- and stereoselective copper-catalyzed carbozincation reactions of alkynyl sulfoximines and sulfones. Org. Lett. 2007, 9, 1259–1261. [Google Scholar] [CrossRef]
- Maezaki, N.; Sawamoto, H.; Yoshigami, R.; Suzuki, T.; Tanaka, T. Geometrically Selective Synthesis of Functionalized β,β-Disubstituted Vinylic Sulfoxides by Cu-Catalyzed Conjugate Addition of Organozinc Reagents to 1-Alkynyl Sulfoxides. Org. Lett. 2003, 5, 1345–1347. [Google Scholar] [CrossRef]
- Stüdemann, T.; Ibrahim-Ouali, M.; Knochel, P. A nickel-catalyzed carbozincation of aryl-substituted alkynes. Tetrahedron 1998, 54, 1299–1316. [Google Scholar] [CrossRef]
- Yasui, H.; Nishikawa, T.; Yorimitsu, H.; Oshima, K. Bull. Cobalt-Catalyzed Allylzincations of Internal Alkynes. Chem. Soc. Jpn. 2006, 79, 1271–1274. [Google Scholar] [CrossRef]
- Gourdet, B.; Lam, H.W. Stereoselective Synthesis of Multisubstituted Enamides via Rhodium-Catalyzed Carbozincation of Ynamides. J. Am. Chem. Soc. 2009, 131, 3802–3803. [Google Scholar] [CrossRef]
- Sallio, R.; Corpet, M.; Habert, L.; Durandetti, M.; Gosmini, C.; Gillaizeau, I. Cobalt-Catalyzed Carbozincation of Ynamides. J. Org. Chem. 2017, 82, 1254–1259. [Google Scholar] [CrossRef]
- Stüdemann, T.; Knochel, P. New Nickel-Catalyzed Carbozincation of Alkynes: A Short Synthesis of (2)-Tamoxifen. Angew. Chem. Int. Ed. 1997, 36, 93–95. [Google Scholar] [CrossRef]
- Negishi, E.; Van Horn, D.E.; Yoshida, T.; Rand, C.L. Selective carbon-carbon bond formation via transition metal catalysis. 31. Controlled Carbometallation. 15. Zirconium-Promoted Carbozincation of Alkynes. Organometallics 1983, 2, 563–565. [Google Scholar] [CrossRef]
- Negishi, E.; Miller, J.A. Selective carbon-carbon bond formation via transition metal catalysis. 37. Controlled carbometalation. 16. Novel syntheses of .alpha.,.beta.-unsaturated cyclopentenones via allylzincation of alkynes. J. Am. Chem. Soc. 1983, 105, 6761–6763. [Google Scholar] [CrossRef]
- Rezaei, H.; Marek, I.; Normant, J.F. Diastereoselective carbozincation of propargylic amines. Tetrahedron 2001, 57, 2477–2483. [Google Scholar] [CrossRef]
- Bertrand, G. Phosphorus chemistry: Introduction. Chem. Rev. 1994, 94, 1161–1162. [Google Scholar] [CrossRef]
- Kollár, L.; Keglevich, G.P. Heterocycles as Ligands in Homogeneous Catalytic Reactions. Chem. Rev. 2010, 110, 4257–4302. [Google Scholar] [CrossRef]
- Kulinkovich, O.G.; Sviridov, S.V.; Vasilevskii, D.A.; Pritytskaya, T.S. Reaction of ethylmagnesium bromide with carboxylic esters in the presence of tetraisopropoxytitanium. Zh. Org. Khim. 1989, 25, 2244–2245. [Google Scholar]
- Lewis, D.P.; Whitby, R.J. The mechanism of the zirconium catalysed ethyl- and 2-magnesioethyl-magnesiation of unactivated alkenes. Tetrahedron 1995, 51, 4541–4550. [Google Scholar] [CrossRef]
- Tipper, C.F.H. Some Reactions of cycloPropane, and a Comparison with the Lower Olefins. Part II.* Some Platinous-cycloPropane Complexes. J. Chem. Soc. 1955, 2045–2046, Reprint Order No. 6013. [Google Scholar]
- Goldfuss, B.; Schleyer, P.V.R.; Hampel, F. A “Lithium-Bonded” Cyclopropyl Edge: The X-ray Crystal Structure of [Li−O−C(Me)−(c-CHCH2CH2)2]6 and Computational Studies. J. Am. Chem. Soc. 1996, 118, 12183–12189. [Google Scholar] [CrossRef]
- Harvey, B.G.; Ernst, R.D. Transition-Metal Complexes with (C–C)→M Agostic Interactions. Eur. J. Inorg. Chem. 2017, 1205–1226. [Google Scholar] [CrossRef]
- Sato, F.; Okamoto, S. The Divalent Titanium Complex Ti(O-i-Pr)4/2 i-PrMgX as an Efficient and Practical Reagent for Fine Chemical Synthesis. Adv. Synth. Catal. 2001, 343, 759–784. [Google Scholar] [CrossRef][Green Version]
- Davis, M.; Deady, L.W.; Finch, A.J.; Smith, J.F. Some reactions of grignard reagents with chloroform and carbon tetrachloride in the presence of cyclohexene. Tetrahedron 1973, 29, 349–352. [Google Scholar] [CrossRef]
- Gartiaa, Y.; Biswasb, A.; Stadlerc, M.; Nasinia, U.B.; Ghosha, A. Cross coupling reactions of multiple CCl bonds of polychlorinated solvents with Grignard reagent using a pincer nickel complex. J. Mol. Catal. A Chem. 2012, 363, 322–327. [Google Scholar] [CrossRef]
- Berding, J.; Lutz, M.; Spek, A.L.; Bouwman, E. Synthesis of Novel Chelating Benzimidazole-Based Carbenes and Their Nickel(II) Complexes: Activity in the Kumada Coupling Reaction. Organometallics 2009, 28, 1845–1854. [Google Scholar] [CrossRef]
- Hayashi, T.; Tajika, M.; Tamao, K.; Kumada, M. High stereoselectivity in asymmetric Grignard cross-coupling catalyzed by nickel complexes of chiral (aminoalkylferrocenyl)phosphines. J. Am. Chem. Soc. 1976, 98, 3718–3719. [Google Scholar] [CrossRef]
- Amruta, J.-P.; Wang, C.-Y.; Biscoe, M.R. Nickel-Catalyzed Kumada Nickel-Catalyzed Kumada Cross-Coupling Reactions of Tertiary Alkylmagnesium Halides and Aryl Bromides/Triflates. J. Am. Chem. Soc. 2011, 133, 8478–8481. [Google Scholar] [CrossRef]
- Kumada, M.; Tamao, K.; Sumitani, K. Phosphine–nickel complex catalyzed cross-coupling of grignard reagents with aryl and alkenyl. Org. Synth. 1978, 58, 127–133. [Google Scholar] [CrossRef]
- Yang, X.; Matsuo, D.; Suzuma, Y.; Fang, J.-K.; Xu, F.; Orita, A.; Otera, J.; Kajiyama, S.; Koumura, N.; Hara, K. Ph2P(O) Group for Protection of Terminal Acetylenes. Synlett 2011, 16, 2402–2406. [Google Scholar] [CrossRef]
- Kondoh, A.; Yorimitsu, H.; Oshima, K. Synthesis of Bulky Phosphines by Rhodium-Catalyzed Formal [2 + 2 + 2] Cycloaddition Reactions of Tethered Diynes with 1-Alkynylphosphine Sulfides. J. Am. Chem. Soc. 2007, 129, 6996. [Google Scholar] [CrossRef]
- Shaibakova, M.G.; Titova, I.G.; Makhmudiyarov, G.A.; Ibragimov, A.G.; Dzhemilev, U.M. Synthesis of 2, 3-acetylenic amines by aminomethylation of acetylenes with geminal diamines. Russ. J. Org. Chem. 2010, 46, 44–48. [Google Scholar] [CrossRef]
- Bieber, L.W.; da Silva, M.F. Mild and efficient synthesis of propargylamines by copper-catalyzed Mannich reaction. Tetrahedron Lett. 2004, 45, 8281–8283. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian Inc.: Wallingford, UK, 2009. [Google Scholar]
- Ramazanov, I.R.; Kadikova, R.N.; Saitova, Z.R.; Dzhemilev, U.M. A Route to 1-alkenylphosphine derivatives via the Zr-catalyzed reaction of 1-alkynylphosphines with triethylaluminum. Asian J. Org. Chem. 2015, 4, 1301–1307. [Google Scholar] [CrossRef]
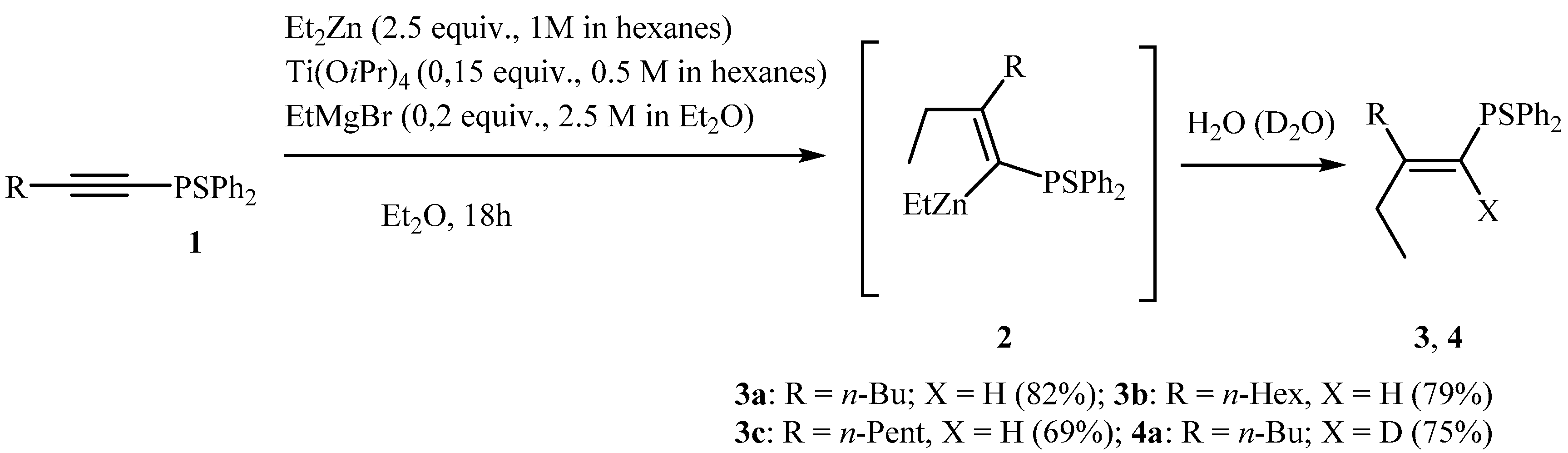



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadikova, R.N.; Ramazanov, I.R.; Gabdullin, A.M.; Mozgovoi, O.S.; Dzhemilev, U.M. Carbozincation of Substituted 2-Alkynylamines, 1-Alkynylphosphines, 1-Alkynylphosphine Sulfides with Et2Zn in the Presence of Catalytic System of Ti(O-iPr)4 and EtMgBr. Catalysts 2019, 9, 1022. https://doi.org/10.3390/catal9121022
Kadikova RN, Ramazanov IR, Gabdullin AM, Mozgovoi OS, Dzhemilev UM. Carbozincation of Substituted 2-Alkynylamines, 1-Alkynylphosphines, 1-Alkynylphosphine Sulfides with Et2Zn in the Presence of Catalytic System of Ti(O-iPr)4 and EtMgBr. Catalysts. 2019; 9(12):1022. https://doi.org/10.3390/catal9121022
Chicago/Turabian StyleKadikova, Rita N., Ilfir R. Ramazanov, Azat M. Gabdullin, Oleg S. Mozgovoi, and Usein M. Dzhemilev. 2019. "Carbozincation of Substituted 2-Alkynylamines, 1-Alkynylphosphines, 1-Alkynylphosphine Sulfides with Et2Zn in the Presence of Catalytic System of Ti(O-iPr)4 and EtMgBr" Catalysts 9, no. 12: 1022. https://doi.org/10.3390/catal9121022
APA StyleKadikova, R. N., Ramazanov, I. R., Gabdullin, A. M., Mozgovoi, O. S., & Dzhemilev, U. M. (2019). Carbozincation of Substituted 2-Alkynylamines, 1-Alkynylphosphines, 1-Alkynylphosphine Sulfides with Et2Zn in the Presence of Catalytic System of Ti(O-iPr)4 and EtMgBr. Catalysts, 9(12), 1022. https://doi.org/10.3390/catal9121022



