Performance Improvement in Direct Methanol Fuel Cells by Using CaTiO3-δ Additive at the Cathode
Abstract
:1. Introduction
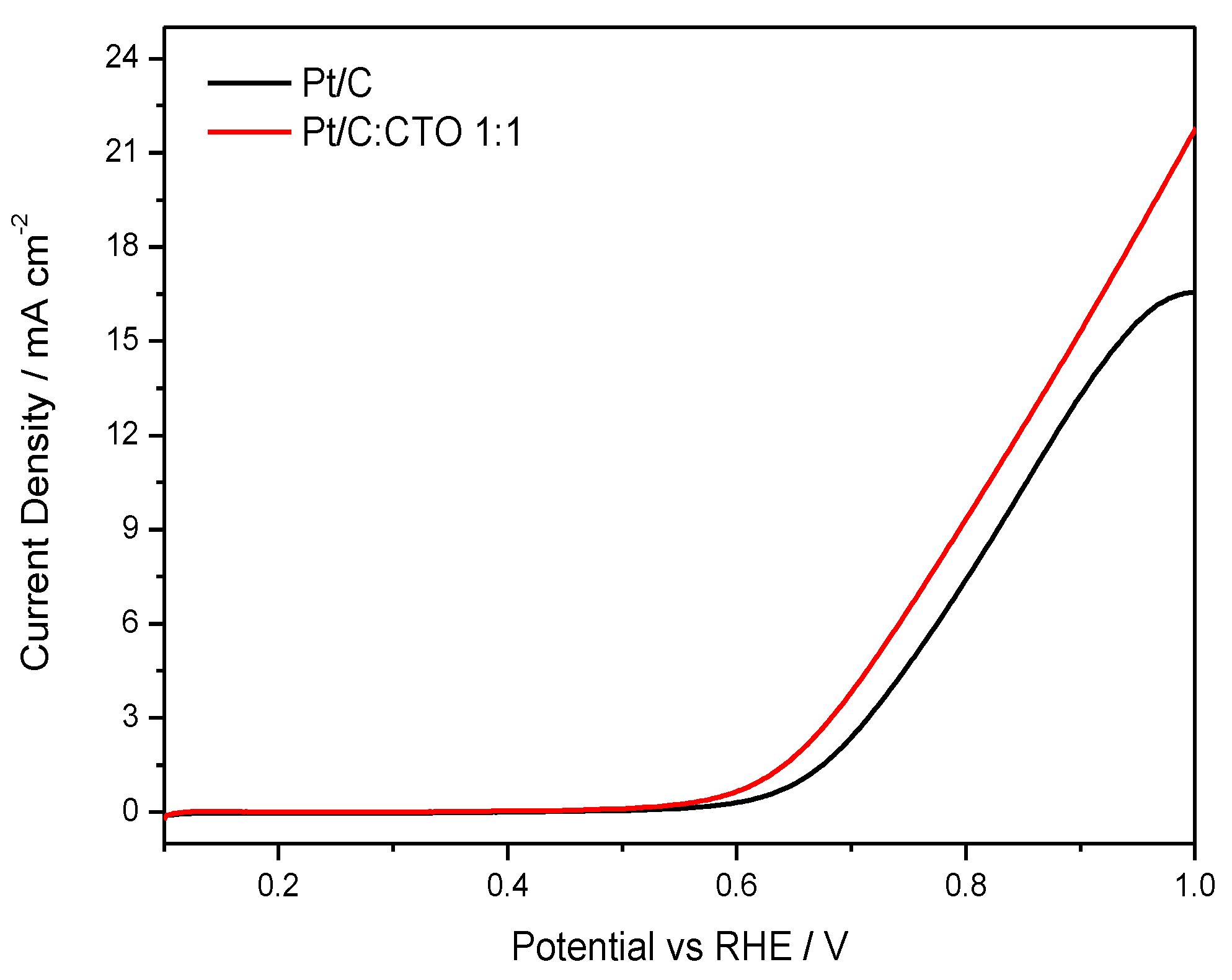
2. Results
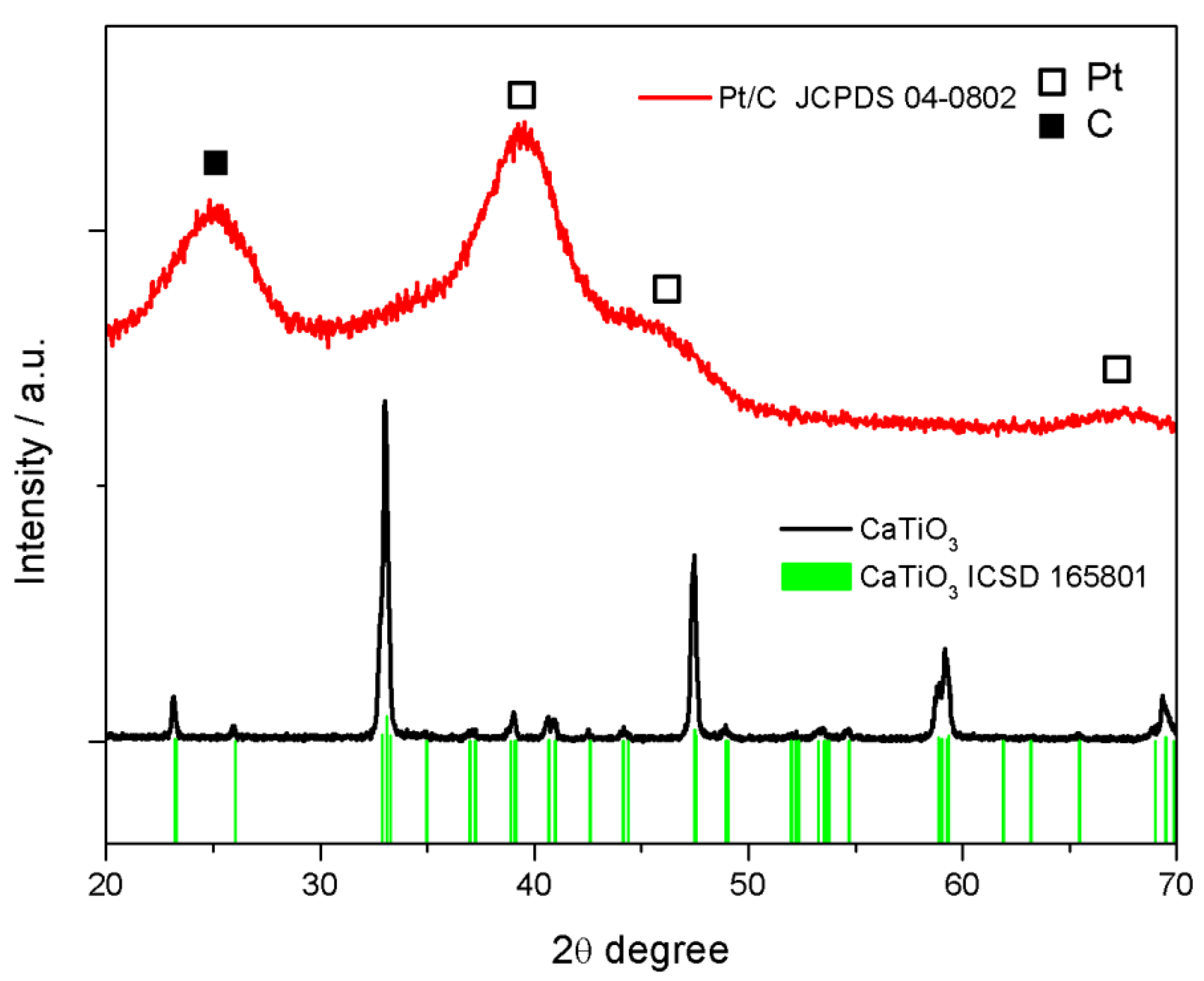
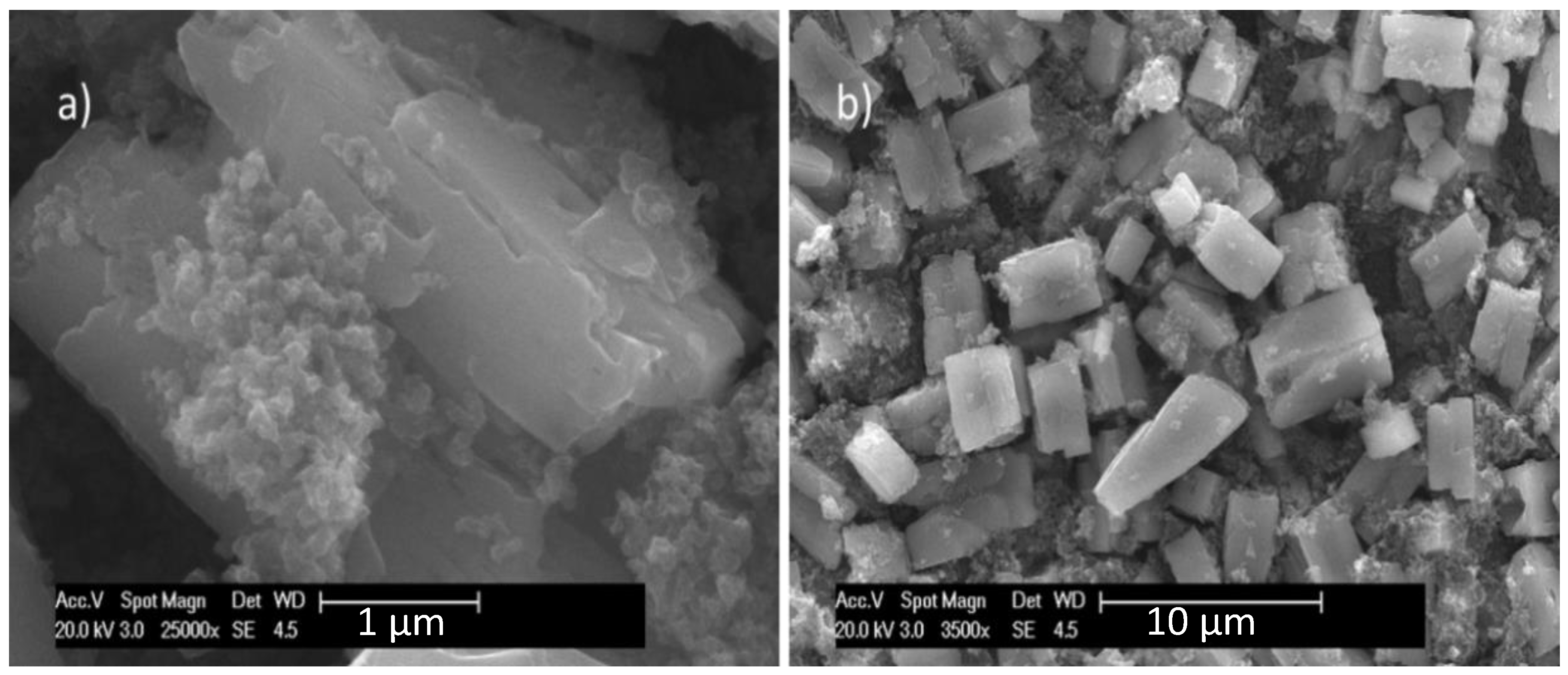
2.1. Physicochemical Characterization
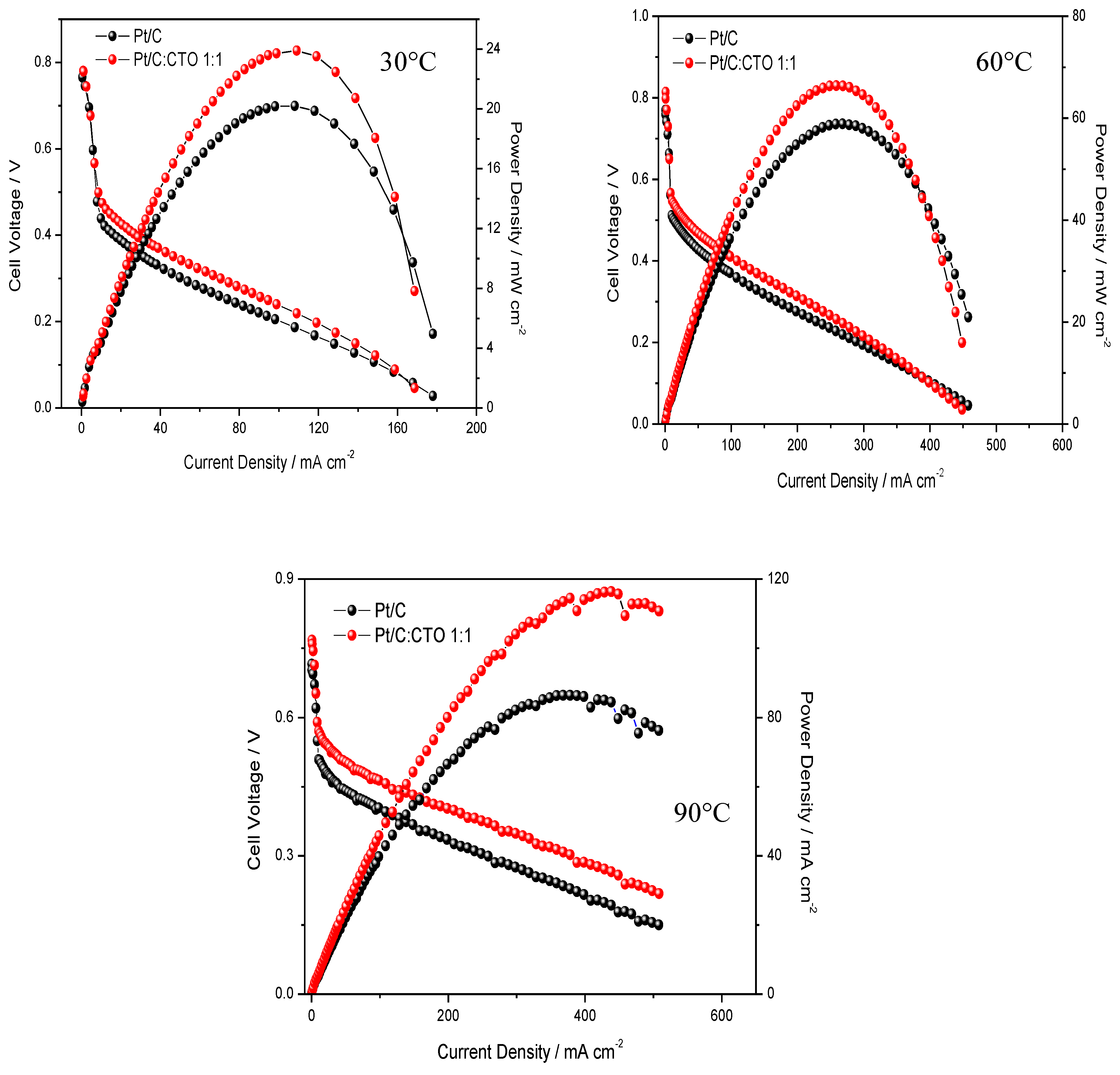
2.2. DMFC Results
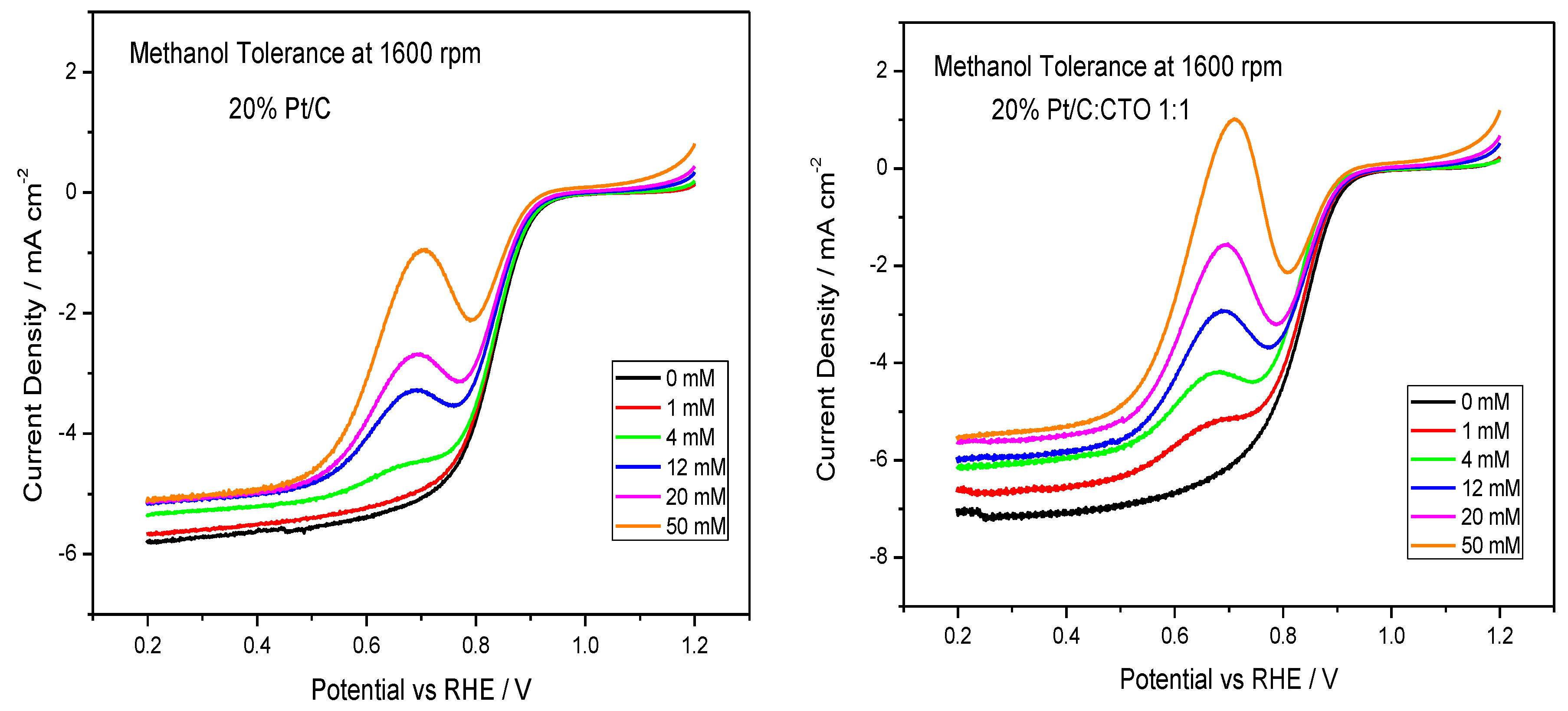
2.3. Methanol Tolerance Characteristics of the Composite Electrode: Ex-Situ Experiments
3. Materials and Methods
3.1. Synthesis of CaTiO3-δ
3.2. Physical-Chemical Characterizations
3.3. Electrochemical Investigations
3.3.1. Rotating Disk Electrode (RDE) Screening
3.3.2. Direct Methanol Fuel Cell (DMFC) Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vielstich, W.; Lamm, A.; Gasteiger, H. Handbook of Fuel Cells—Fundamentals, Technology and Applications; Wiley: West Sussex, UK, 2003. [Google Scholar]
- Aricò, A.S.; Baglio, V.; Antonucci, V. Direct methanol fuel cells: Hystory, status and perspectives, Chapter 1. In Electrocatalysis of Direct Methanol Fuel Cells: From Fundamentals to Applications; Liu, H., Zhang, J., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; pp. 1–78. [Google Scholar]
- Zeng, J.; Francia, C.; Gerbaldi, C.; Baglio, V.; Specchia, S.; Aricò, A.S.; Spinelli, P. Hybrid ordered mesoporous carbons doped with tungsten trioxide as supports for Pt electrocatalysts for methanol oxidation reaction. Electrochim. Acta 2013, 94, 80–91. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Kocha, S.S.; Sompalli, B.; Wagner, F.T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B 2005, 56, 9–35. [Google Scholar] [CrossRef]
- Sgroi, M.F.; Zedde, F.; Barbera, O.; Stassi, A.; Sebastián, D.; Lufrano, F.; Baglio, V.; Aricò, A.S.; Bonde, J.L.; Schuster, M. Cost analysis of direct methanol fuel cell stacks for mass production. Energies 2016, 9, 1008. [Google Scholar] [CrossRef]
- Morozan, A.; Jousselme, B.; Palacin, S. Low-platinum and platinum-free catalysts for the oxygen reduction reaction at fuel cell cathodes. Energy Environ. Sci. 2011, 4, 1238–1254. [Google Scholar] [CrossRef]
- Nie, Y.; Li, L.; We, Z. Recent advancements in Pt and Pt-free, catalysts for oxygen reduction reaction. Chem. Soc. Rev. 2015, 44, 2168–2201. [Google Scholar] [CrossRef]
- Lo Vecchio, C.; Aricò, A.S.; Monforte, G.; Baglio, V. EDTA-derived Co-N-C and Fe-N-C electro-catalysts for the oxygen reduction reaction in acid environment. Renew. Energy 2018, 120, 342–349. [Google Scholar] [CrossRef]
- Li, Z.; Gao, Q.; Zhang, H.; Tian, W.; Tan, Y.; Qian, W.; Liu, Z. Low content Pt nanoparticles anchored on N-doped reduced graphene oxide with high and stable electrocatalytic activity for oxygen reduction reaction. Sci. Rep. 2017, 7, 43352. [Google Scholar] [CrossRef]
- Lo Vecchio, C.; Sebastiàn, D.; Làzaro, M.J.; Aricò, A.S.; Baglio, V. Methanol-tolerant M–N–C catalysts for oxygen reduction reactions in acidic media and their application in direct methanol fuel cells. Catalysts 2018, 8, 650. [Google Scholar] [CrossRef]
- Sebastián, D.; Serov, A.; Artyushkova, K.; Atanassov, P.; Aricò, A.S.; Baglio, V. Performance, methanol tolerance and stability of Fe-aminobenzimidazole derived catalyst for direct methanol fuel cells. J. Power Sources 2016, 319, 235–246. [Google Scholar] [CrossRef]
- Saianand, G.; Gopalan, A.-I.; Lee, J.-C.; Sathish, C.I.; Gopalakrishnan, K.; Unni, G.E.; Shanbhag, D.; Dasireddy, V.D.B.C.; Yi, J.; Xi, S.; et al. Mixed copper/Copper-oxide anchored mesoporous fullerene nanohybrids as superior electrocatalysts toward Oxygen Reduction Reaction. Small 2019, 1903937. [Google Scholar] [CrossRef]
- Baglio, V.; Di Blasi, A.; D’Urso, C.; Antonucci, V.; Arico, A.S.; Ornelas, R.; Morales-Acosta, D.; Ledesma-Garcia, J.; Godinez, L.A.; Arriaga, L.G.; et al. Development of Pt and Pt-Fe catalysts supported on multiwalled carbon nanotubes for oxygen reduction in direct methanol fuel cells. J. Electrochem. Soc. 2008, 155, B829–B833. [Google Scholar] [CrossRef]
- Antolini, E.; Salgado, J.R.C.; Santos, L.G.R.A.; Garcia, G.; Ticianelli, E.A.; Pastor, E.; Gonzalez, E.R. Carbon supported Pt-Cr alloys as oxygen reduction catalysts for direct methanol fuel cells. J. Appl. Electrochem. 2006, 36, 355–362. [Google Scholar] [CrossRef]
- Toda, T.; Igarashi, H.; Uchida, M.; Watanabe, M. Enhancement of the electroreduction of oxygen on Pt alloys with Fe, Ni, and Co. J. Electrochem. Soc. 1999, 146, 3750–3756. [Google Scholar] [CrossRef]
- Mukerjee, S.; Srinivasan, S.; Soriaga, M.P.; McBreen, J. Role of structural and electronic properties of Pt and Pt alloys on electrocatalysis of oxygen reduction. J. Electrochem. Soc. 1995, 142, 1409–1422. [Google Scholar] [CrossRef]
- Shukla, A.K.; Raman, R.K.; Choudhury, N.A.; Priolkar, K.R.; Sarode, P.R.; Emura, S.; Kumashiro, R. Carbon-supported Pt–Fe alloy as a methanol-resistant oxygen-reduction catalyst for direct methanol fuel cells. J. Electroanal. Chem. 2004, 563, 181–190. [Google Scholar] [CrossRef]
- Scott, K.; Yuan, W.; Cheng, H. Feasibility of using PtFe alloys as cathodes in direct methanol fuel cells. J. Appl. Electrochem. 2007, 37, 21–26. [Google Scholar] [CrossRef]
- Baglio, V.; Aricò, A.S.; Stassi, A.; D’Urso, C.; Di Blasi, A.; Luna, A.M.C.; Antonucci, V. Investigation of Pt-Fe catalysts for oxygen reduction in low temperature direct methanol fuel cells. J. Power Sources 2006, 159, 900–904. [Google Scholar] [CrossRef]
- Yang, H.; Coutanceau, C.; Leger, J.-M.; Alonso-Vante, N.; Lamy, C. Methanol tolerant oxygen reduction on carbon-supported Pt–Ni alloy nanoparticles. J. Electroanal. Chem. 2005, 576, 305–313. [Google Scholar] [CrossRef]
- Antolini, E.; Salgado, J.R.C.; Gonzalez, E.R. The methanol oxidation reaction on platinum alloys with the first row transition metals: The case of Pt–Co and –Ni alloy electrocatalysts for DMFCs: A short review. Appl. Catal. B 2006, 63, 137–149. [Google Scholar] [CrossRef]
- Li, W.; Zhou, W.; Li, H.; Zhou, Z.; Zhou, B.; Sun, G.; Xin, Q. Nano-stuctured Pt–Fe/C as cathode catalyst in direct methanol fuel cell. Electrochim. Acta 2004, 49, 1045–1055. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Markovic, N.M.; Ross, P.N.; Cairns, E.J. Electro-oxidation of small organic molecules on well-characterized Pt-Ru alloys. Electrochim. Acta 1994, 39, 1825–1832. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Meng, X.; Zhong, Y.; Li, R.; Sun, X.; Ye, S.; Knights, S. Pt-SnO2/nitrogen-doped CNT hybrid catalysts for proton-exchange membrane fuel cells (PEMFC): Effects of crystalline and amorphous SnO2 by atomic layer deposition. J. Power Sources 2013, 238, 144–149. [Google Scholar] [CrossRef]
- Huang, S.Y.; Ganesan, P.; Popov, B.N. Titania supported platinum catalyst with high electrocatalytic activity and stability for polymer electrolyte membrane fuel cell. Appl. Catal. B 2011, 102, 71–77. [Google Scholar] [CrossRef]
- Stassi, A.; Gatto, I.; Baglio, V.; Passalacqua, E.; Aricò, A.S. Oxide supported PtCo alloy catalyst for intermediate temperature polymer electrolyte fuel cells. Appl. Catal. B 2013, 142–143, 15–24. [Google Scholar] [CrossRef]
- Mittermeier, T.; Madkikar, P.; Wang, X.; Gasteiger, H.A.; Piana, M. ZrO2 based oxygen reduction catalysts for PEMFCs: Towards a better understanding. J. Electrochem. Soc. 2016, 163, F1543–F1552. [Google Scholar] [CrossRef]
- Baglio, V.; Amin, R.S.; El-Khatib, K.M.; Siracusano, S.; D’Urso, C.; Aricò, A.S. IrO2 as a promoter of Pt-Ru for methanol electro-oxidation. Phys. Chem. Chem. Phys. 2014, 16, 10414–10418. [Google Scholar] [CrossRef]
- Zuo, G.; Li, B.; Guo, Z.; Wang, L.; Yang, F.; Hou, W.; Zhang, S.; Zong, P.; Liu, S.; Meng, X.; et al. Efficient photocatalytic hydrogen peroxide production over TiO2 passivated by SnO2. Catalysts 2019, 9, 623. [Google Scholar] [CrossRef]
- Lee, J.-C.; Gopalan, A.-I.; Sainand, G.; Lee, K.-P.; Kim, W.-J. Preparation of visible light photocatalytic graphene embedded rutile titanium(IV) oxide composite nanowires and enhanced NOx removal. Catalysts 2019, 9, 170. [Google Scholar] [CrossRef]
- Abdullah, N.; Kamarudin, S.K. Titanium dioxide in fuel cell technology: An overview. J. Power Sources 2015, 278, 109–118. [Google Scholar] [CrossRef]
- Pena, M.A.; Fierro, J.L.G. Chemical structures and performance of perovskite oxides. Chem. Rev. 2001, 101, 1981–2018. [Google Scholar] [CrossRef]
- Ge, X.; Sumboja, A.; Wuu, D.; An, T.; Li, B.; Goh, F.W.T.; Hor, T.S.A.; Zong, Y.; Liu, Z. Oxygen reduction in alkaline media: From mechanisms to recent advances of catalysts. ACS Catal. 2015, 5, 4643–4667. [Google Scholar] [CrossRef]
- Risch, M. Perovskite electrocatalysts for the oxygen reduction reaction in alkaline media. Catalysts 2017, 7, 154. [Google Scholar] [CrossRef] [Green Version]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Nakanishi, H.; Goodenough, J.B.; Shao-Horn, Y. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal-air batteries. Nat. Chem. 2011, 3, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, E.; Mohamed, R.; Levecque, P.; Conrad, O.; Kötza, R.; Schmidt, T.J. Unraveling the oxygen reduction reaction mechanism and activity of d-band perovskite electrocatalysts for low temperature alkaline fuel cells. ECS Trans. 2014, 64, 1081–1093. [Google Scholar] [CrossRef]
- Mazzapioda, L.; Lo Vecchio, C.; Paolone, A.; Aricò, A.S.; Baglio, V.; Navarra, M.A. Enhancing oxygen reduction reaction catalytic activity using a sub-stoichiometric CaTiO3-δ additive. ChemElectroChem 2019, in press. [Google Scholar] [CrossRef]
- Yu, L.; Xi, J. TiO2 nanoparticles promoted Pt/C catalyst for ethanol electro-oxidation. Electrochim. Acta 2012, 67, 166–171. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, G.; Xia, D.; Zhang, L. Studies on the electrocatalytic properties of PtRu/C-TiO2 toward the oxidation of methanol. J. Alloys Compd. 2008, 450, 148–151. [Google Scholar] [CrossRef]
- Tian, J.; Sun, G.; Jiang, L.; Yan, S.; Mao, Q.; Xin, Q. Highly stable PtRuTiOx/C anode electrocatalyst for direct methanol fuel cells. Electrochem. Commun. 2007, 9, 563–568. [Google Scholar] [CrossRef]
- Baglio, V.; Zignani, S.C.; Siracusano, S.; Stassi, A.; D’Urso, C.; Aricò, A.S. Composite anode electrocatalyst for direct methanol fuel cells. Electrocatalysis 2013, 4, 235–240. [Google Scholar] [CrossRef]
- Sebastiàn, D.; Stassi, A.; Siracusano, S.; Lo Vecchio, C.; Aricò, A.S.; Baglio, V. Influence of metal oxide additives on the activity and stability of PtRu/C for methanol electro-oxidation. J. Electrochem. Soc. 2015, 162, F713–F717. [Google Scholar] [CrossRef]
- Thiagarajan, V.; Karthikeyan, P.; Thanarajan, K.; Neelakrishnan, S.; Manoharan, R.; Chen, R.; Fly, A.; Anand, R.; Karuppa Raj, T.R.; Kumar, N.S. Experimental investigation on DMFCs using reduced noble metal loading with NiTiO3 as supportive material to enhance cell performances. Int. J. Hydrogen Energy 2019, 44, 13415–13423. [Google Scholar] [CrossRef]
- Arico, A.S.; Baglio, V.; Di Blasi, A.; Modica, E.; Antonucci, P.L.; Antonucci, V. Analysis of the high-temperature methanol oxidation behaviour at carbon-supported Pt/Ru catalysts. J. Electroanal. Chem. 2003, 557, 167–176. [Google Scholar] [CrossRef]
- Tsiouvaras, N.; Martınez-Huerta, M.V.; Paschos, O.; Stimming, U.; Fierro, J.L.G.; Pena, M.A. PtRuMo/C catalysts for direct methanol fuel cells: Effect of the pretreatment on the structural characteristics and methanol electrooxidation. Int. J. Hydrogen Energy 2010, 35, 11478–11488. [Google Scholar] [CrossRef]
- Amin, R.S.; El-Khatib, K.M.; Siracusano, S.; Baglio, V.; Stassi, A.; Arico, A.S. Metal oxide promoters for methanol electro-oxidation. Int. J. Hydrogen Energy 2014, 39, 9782–9790. [Google Scholar] [CrossRef]
- Alegre, C.; Modica, E.; Rodlert-Bacilieri, M.; Mornaghini, F.C.; Aricò, A.S.; Baglio, V. Enhanced durability of a cost-effective perovskite-carbon catalyst for the oxygen evolution and reduction reactions in alkaline environment. Int. J. Hydrogen Energy 2017, 42, 28063–28069. [Google Scholar] [CrossRef]
- Alegre, C.; Modica, E.; Aricò, A.S.; Baglio, V. Bifunctional oxygen electrode based on a perovskite/carbon composite for electrochemical devices. J. Electroanal. Chem. 2018, 808, 412–419. [Google Scholar] [CrossRef]
- Mazzapioda, L.; Navarra, M.A.; Trequattrini, F.; Paolone, A.; Elamin, K.; Martinelli, A.; Palumbo, O. Composite Nafion membranes with CaTiO3-δ additive for possible applications in electrochemical devices. Membranes 2019, 9, 143. [Google Scholar] [CrossRef] [Green Version]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzapioda, L.; Lo Vecchio, C.; Aricò, A.S.; Navarra, M.A.; Baglio, V. Performance Improvement in Direct Methanol Fuel Cells by Using CaTiO3-δ Additive at the Cathode. Catalysts 2019, 9, 1017. https://doi.org/10.3390/catal9121017
Mazzapioda L, Lo Vecchio C, Aricò AS, Navarra MA, Baglio V. Performance Improvement in Direct Methanol Fuel Cells by Using CaTiO3-δ Additive at the Cathode. Catalysts. 2019; 9(12):1017. https://doi.org/10.3390/catal9121017
Chicago/Turabian StyleMazzapioda, Lucia, Carmelo Lo Vecchio, Antonino Salvatore Aricò, Maria Assunta Navarra, and Vincenzo Baglio. 2019. "Performance Improvement in Direct Methanol Fuel Cells by Using CaTiO3-δ Additive at the Cathode" Catalysts 9, no. 12: 1017. https://doi.org/10.3390/catal9121017
APA StyleMazzapioda, L., Lo Vecchio, C., Aricò, A. S., Navarra, M. A., & Baglio, V. (2019). Performance Improvement in Direct Methanol Fuel Cells by Using CaTiO3-δ Additive at the Cathode. Catalysts, 9(12), 1017. https://doi.org/10.3390/catal9121017







