Endogenous Roles of Mammalian Flavin-Containing Monooxygenases
Abstract
1. Introduction
2. Developmental Stage- and Tissue-Specific Regulation of Expression of FMOs in Human and Mouse
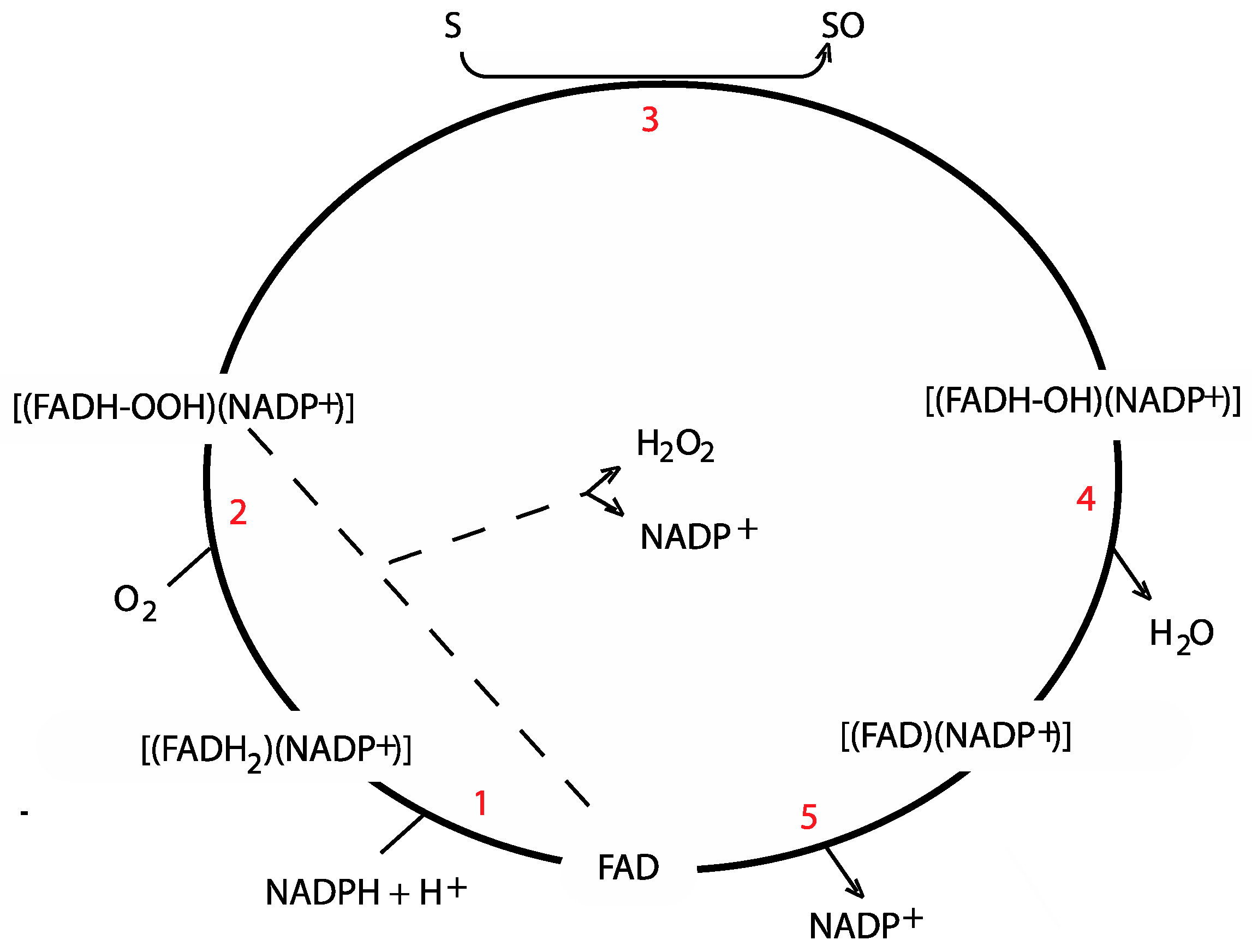
3. Catalytic Mechanism
4. Endogenous Substrates of FMOs
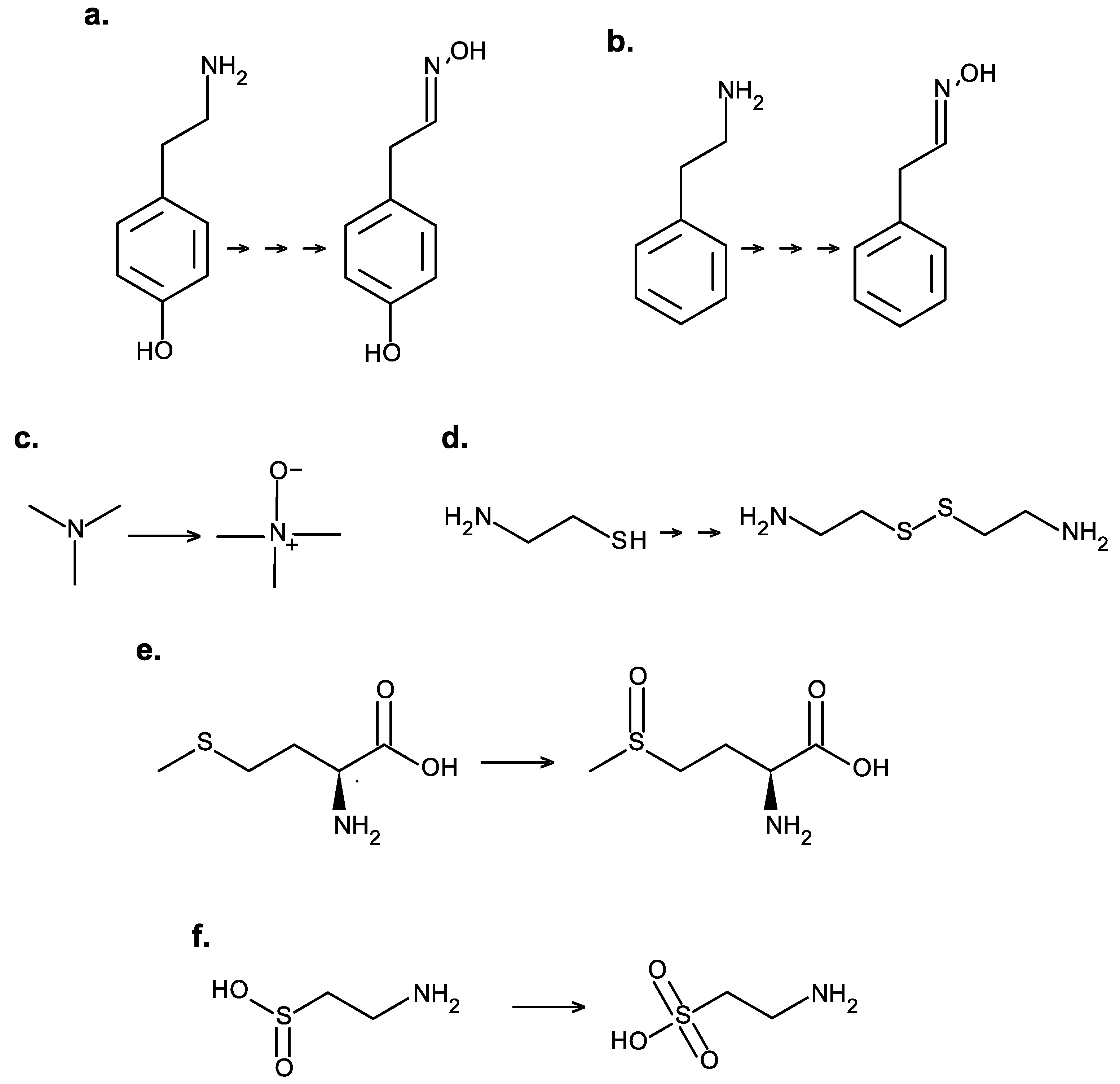
4.1. Tyramine and Phenethylamine
4.2. Trimethylamine
4.3. Cysteamine
4.4. Methionine
4.5. Lipoic Acid and Lipoamide
5. Evidence for Involvement of FMOs in Endogenous Metabolic Processes
5.1. FMO1
5.2. FMO3
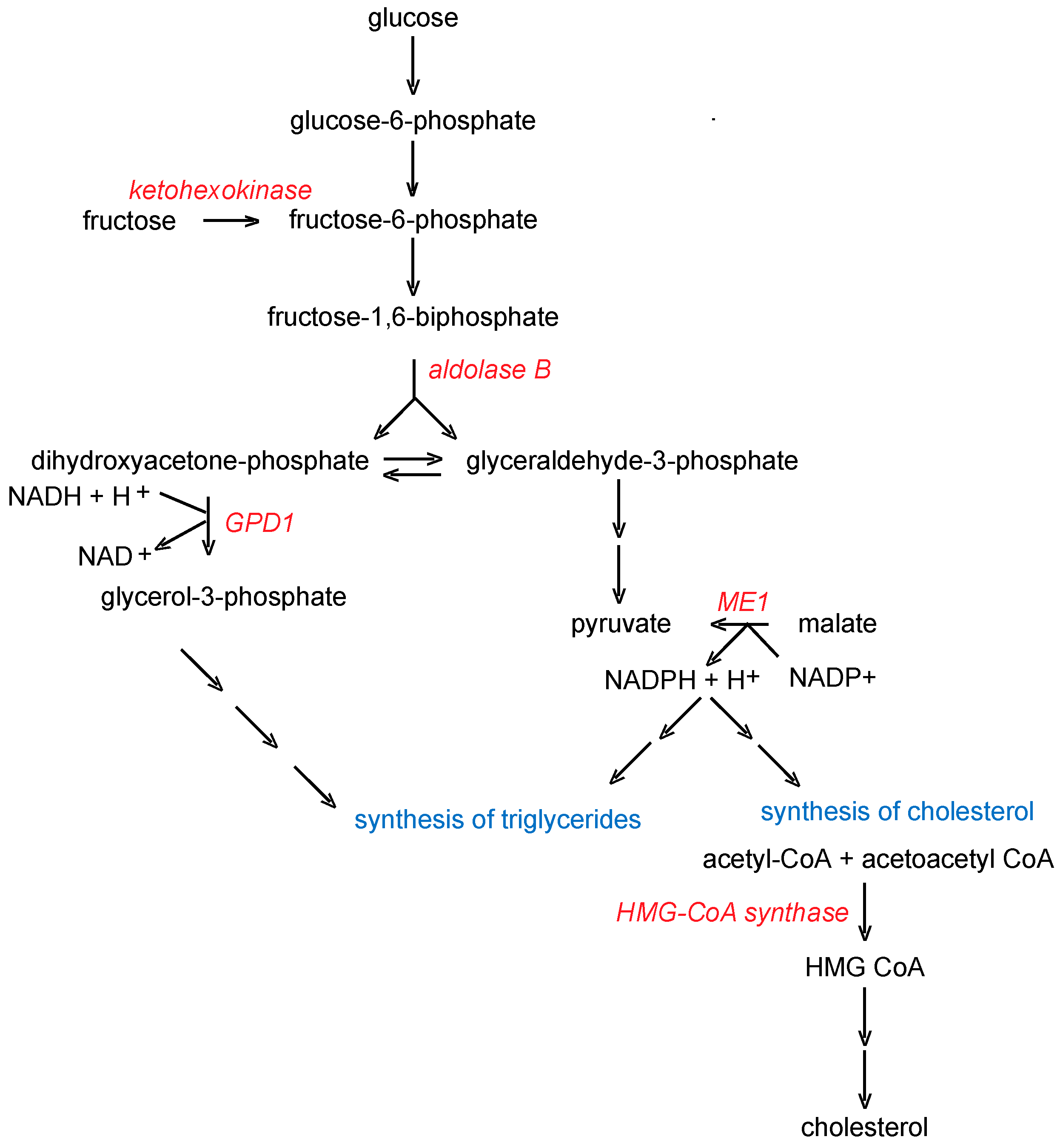
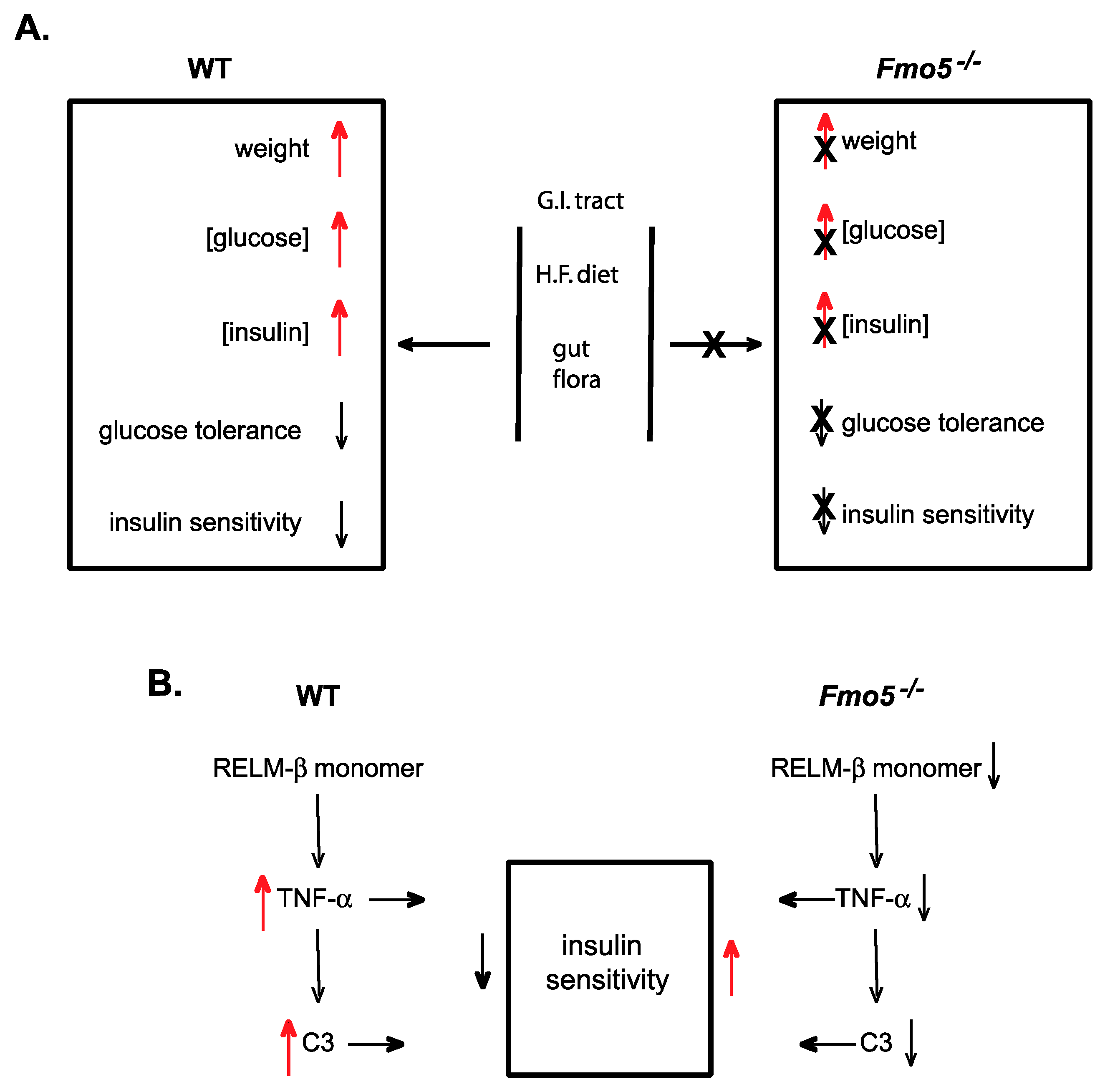
5.3. FMO5
6. The Use of Knockout-Mouse Lines to Identify Endogenous Roles of FMOs
6.1. Identification of FMO1 as a Novel Regulator of Energy Balance
6.2. FMO1 Catalyzes the Formation of Taurine from Hypotaurine
6.3. Identification of Endogenous Roles for FMO5
7. Conclusions
Funding
Conflicts of Interest
References
- Krueger, S.K.; Williams, D.E. Mammalian flavin-containing monooxygenases: Structure/function, genetic polymorphisms and role in drug metabolism. Pharmacol. Ther. 2005, 106, 357–387. [Google Scholar] [CrossRef] [PubMed]
- Cashman, J.R.; Zhang, J. Human flavin-containing monooxygenases. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 65–100. [Google Scholar] [CrossRef] [PubMed]
- Phillips, I.R.; Francois, A.A.; Shephard, E.A. The flavin-containing monoooxygenases (FMOs): Genetic variation and its consequences for the metabolism of therapeutic drugs. Curr. Pharmacogenomics 2007, 5, 292–313. [Google Scholar] [CrossRef]
- Phillips, I.R.; Shephard, E.A. Drug metabolism by flavin-containing monooxygenases of human and mouse. Expert Opin. Drug Metab. Toxicol. 2017, 13, 167–181. [Google Scholar] [CrossRef]
- Lawton, M.P.; Cashman, J.R.; Cresteil, T.; Dolphin, C.T.; Elfarra, A.A.; Hines, R.N.; Hodgson, E.; Kimura, T.; Ozols, J.; Phillips, I.R.; et al. A nomenclature for the mammalian flavin-containing monooxygenase gene family based on amino acid sequence identities. Arch. Biochem. Biophys. 1994, 308, 254–257. [Google Scholar] [CrossRef]
- Phillips, I.R.; Dolphin, C.T.; Clair, P.; Hadley, M.R.; Hutt, A.J.; McCombie, R.R.; Smith, R.L.; Shephard, E.A. The molecular biology of the flavin-containing monooxygenases of man. Chem. Biol. Interact. 1995, 96, 17–32. [Google Scholar] [CrossRef]
- Hernandez, D.; Janmohamed, A.; Chandan, P.; Phillips, I.R.; Shephard, E.A. Organization and evolution of the flavin-containing monooxygenase genes of human and mouse: Identification of novel gene and pseudogene clusters. Pharmacogenetics 2004, 14, 117–130. [Google Scholar] [CrossRef]
- Hines, R.N.; Hopp, K.A.; Franco, J.; Saeian, K.; Begun, F.P. Alternative processing of the human FMO6 gene renders transcripts incapable of encoding a functional flavin-containing monooxygenase. Mol. Pharmacol. 2002, 62, 320–325. [Google Scholar] [CrossRef]
- Dolphin, C.; Shephard, E.A.; Povey, S.; Palmer, C.N.A.; Ziegler, D.M.; Ayesh, R.; Smith, R.L.; Phillips, I.R. Cloning, primary sequence, and chromosomal mapping of a human flavin-containing monooxygenase (FMO1). J. Biol. Chem. 1991, 266, 12379–12385. [Google Scholar]
- Dolphin, C.T.; Cullingford, T.E.; Shephard, E.A.; Smith, R.L.; Phillips, I.R. Differential developmental and tissue-specific regulation of expression of the genes encoding three members of the flavin-containing monooxygenase family of man, FMO1, FMO3 and FM04. Eur. J. Biochem. 1996, 235, 683–689. [Google Scholar] [CrossRef]
- Koukouritaki, S.B.; Simpson, P.; Yeung, C.K.; Rettie, A.E.; Hines, R.N. Human Hepatic Flavin-Containing Monooxygenases 1 (FMO1) and 3 (FMO3) Developmental Expression. Pediatr. Res. 2002, 51, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Shephard, E.A.; Chandan, P.; Stevanovic-Walker, M.; Edwards, M.; Phillips, I.R. Alternative promoters and repetitive DNA elements define the species-dependent tissue-specific expression of the FMO1 genes of human and mouse. Biochem. J. 2007, 406, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.K.; Lang, D.H.; Thummel, K.E.; Rettie, A.E. Immunoquantitation of FMO1 in human liver, kidney, and intestine. Drug Metab. Dispos. 2000, 28, 1107–1111. [Google Scholar] [PubMed]
- Zhang, J.; Cashman, J.R. Quantitave analysis of FMO gene mRNA levels in human tissues. Drug Metab. Dispos. 2006, 34, 19–26. [Google Scholar] [CrossRef]
- Cherrington, N.J.; Falls, J.G.; Rose, R.L.; Clements, K.M.; Philpot, R.M.; Levi, P.E.; Hodgson, E. Molecular cloning, sequence, and expression of mouse flavin-containing monooxygenases 1 and 5 (FMO1 and FMO5). J. Biochem. Mol. Toxicol. 1998, 12, 205–212. [Google Scholar] [CrossRef]
- Janmohamed, A.; Hernandez, D.; Phillips, I.R.; Shephard, E.A. Cell-, tissue-, sex- and developmental stage-specific expression of mouse flavin-containing monooxygenases (Fmos). Biochem. Pharmacol. 2004, 68, 73–83. [Google Scholar] [CrossRef]
- Veeravalli, S.; Omar, B.A.; Houseman, L.; Hancock, M.; Gonzalez Malagon, S.G.; Scott, F.; Janmohamed, A.; Phillips, I.R.; Shephard, E.A. The phenotype of a flavin-containing monooyxgenase knockout mouse implicates the drug-metabolizing enzyme FMO1 as a novel regulator of energy balance. Biochem. Pharmacol. 2014, 90, 88–95. [Google Scholar] [CrossRef]
- Siddens, L.K.; Henderson, M.C.; VanDyke, J.E.; Williams, D.E.; Krueger, S.K. Characterization of mouse flavin-containing monooxygenase transcript levels in lung and liver, and activity of expressed isoforms. Biochem. Pharmacol. 2008, 75, 570–579. [Google Scholar] [CrossRef]
- Dolphin, C.T.; Beckett, D.J.; Janmohamed, A.; Cullingford, T.E.; Smith, R.L.; Shephard, E.A.; Phillips, I.R. The flavin-containing monooxygenase 2 gene (FMO2) of humans, but not of other primates, encodes a truncated, nonfunctional protein. J. Biol. Chem. 1998, 273, 30599–30607. [Google Scholar] [CrossRef]
- Veeramah, K.R.; Thomas, M.G.; Weale, M.E.; Zeitlyn, D.; Tarekegn, A.; Bekele, E.; Mendell, N.R.; Shephard, E.A.; Bradman, N.; Phillips, I.R. The potentially deleterious functional variant flavin-containing monooxygenase 2*1 is at high frequency throughout sub-Saharan Africa. Pharmacogenet. Genomics 2008, 18, 877–886. [Google Scholar] [CrossRef]
- Janmohamed, A.; Dolphin, C.T.; Phillips, I.R.; Shephard, E.A. Quantification and cellular localization of expression in human skin of genes encoding flavin-containing monooxygenases and cytochromes P450. Biochem. Pharmacol. 2001, 62, 777–786. [Google Scholar] [CrossRef]
- Zhang, A.Q.; Mitchell, S.C.; Smith, R.L. Exacerbation of symptoms of fish-odour syndrome during menstruation. Lancet 1996, 348, 1740–1741. [Google Scholar] [CrossRef]
- Shimizu, M.; Cashman, J.R.; Yamazaki, H. Transient trimethylaminuria related to menstruation. BMC Med. Genet. 2007, 8, 2. [Google Scholar] [CrossRef]
- Falls, J.G.; Blake, B.L.; Cao, Y.; Levi, P.E.; Hodgson, E. Gender differences in hepatic expression of flavin-containing monooxygenase isoforms (FMO1, FMO3, and FMO5) in Mice. J. Biochem. Toxicol. 1995, 10, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.D.; Selwyn, F.P.; Cui, J.Y.; Klaassen, C.D. RNA sequencing quantification of xenobiotic-processing genes in various sections of the intestine in comparison to the liver of male mice. Drug Metab. Dispos. 2016, 44, 842–856. [Google Scholar] [CrossRef] [PubMed]
- Scott, F.; Gonzalez Malagon, S.G.; O’Brien, B.A.; Fennema, D.; Veeravalli, S.; Coveney, C.R.; Phillips, I.R.; Shephard, E.A. Identification of flavin-containing monooxygenase 5 (FMO5) as a regulator of glucose homeostasis and a potential sensor of gut bacteria. Drug Metab. Dispos. 2017, 45, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D. Recent studies on the structure and function of multisubstrate flavin-containing monooxygenases. Annu. Rev. Pharmacol. Toxicol. 1993, 33, 179–199. [Google Scholar] [CrossRef]
- Poulsen, L.L.; Ziegler, D.M. Multisubstrate flavin-containing monooxygenases: Applications of mechanism to specificity. Chem. Biol. Interact. 1995, 96, 57–73. [Google Scholar] [CrossRef]
- Ziegler, D.M. An overview of the mechanism, substrate specificities, and structure of FMOs. Drug Metab. Rev. 2002, 34, 503–511. [Google Scholar] [CrossRef]
- Guengerich, F.P. Mechanisms of cytochrome P450 substrate oxidation: MiniReview. J. Biochem. Mol. Toxicol. 2007, 21, 163–168. [Google Scholar] [CrossRef]
- Siddens, L.K.; Krueger, S.K.; Henderson, M.C.; Williams, D.E. Mammalian flavin-containing monooxygenase (FMO) as a source of hydrogen peroxide. Biochem. Pharmacol. 2014, 89, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Ziegler, D.M. Size limits of thiocarbamides accepted as substrates by human flavin-containing monooxygenase 1. Drug Metab. Dispos. 2000, 28, 1003–1006. [Google Scholar] [PubMed]
- Lai, W.G.; Farah, N.; Moniz, G.A.; Wong, Y.N. A Baeyer-Villiger oxidation specifically catalyzed by human flavin-containing monooxygenase 5. Drug Metab. Dispos. 2011, 39, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, F.; Geier, M.; Binda, C.; Winkler, M.; Faber, K.; Hall, M.; Mattevi, A. Biocatalytic characterization of human FMO5: Unearthing Baeyer-Villiger reactions in humans. ACS Chem. Biol. 2016, 11, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.R.; Yang, S. Hydrogen peroxide: A signaling messenger. Antioxid. Redox Signal. 2006, 8, 243–270. [Google Scholar] [CrossRef] [PubMed]
- Overby, L.H.; Carver, G.C.; Philpot, R.M. Quantitation and kinetic properties of hepatic microsomal and recombinant flavin-containing monooxygenases 3 and 5 from humans. Chem. Biol. Interact. 1997, 106, 29–45. [Google Scholar] [CrossRef]
- Broadley, K.J. The vascular effects of trace amines and amphetamines. Pharmacol. Ther. 2010, 125, 363–375. [Google Scholar] [CrossRef]
- Khan, M.Z.; Nawaz, W. The emerging roles of human trace amines and human trace amine-associated receptors (hTAARs) in central nervous system. Biomed. Pharmacother. 2016, 83, 439–449. [Google Scholar] [CrossRef]
- Miller, G.M. The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity. J. Neurochem. 2011, 116, 164–176. [Google Scholar] [CrossRef]
- Pei, Y.; Asif-Malik, A.; Canales, J.J. Trace amines and the trace amine-associated receptor 1: Pharmacology, neurochemistry, and clinical implications. Front. Neurosci. 2016, 10, 148. [Google Scholar] [CrossRef]
- Paterson, I.A.; Juorio, A.V.; Boulton, A.A. 2-Phenylethylamine: A modulator of catecholamine transmission in the mammalian central nervous system? J. Neurochem. 1990, 55, 1827–1837. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.D. Mammalian central nervous system trace amines. Pharmacologic amphetamines, physiologic neuromodulators. J. Neurochem. 2004, 90, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Cashman, J.R. Detoxication of tyramine by the flavin-containing monooxygenase: Stereoselective formation of the trans oxime. Chem. Res. Toxicol. 1997, 10, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Cashman, J.R. N-oxygenation of phenethylamine to the trans-oxime by adult human liver flavin-containing monooxygenase and retroreduction of phenethylamine hydroxylamine by human liver microsomes. J. Pharmacol. Exp. Ther. 1997, 282, 1269–1279. [Google Scholar]
- Mitchell, S. Trimethylaminuria (fish-odour syndrome) and oral malodour. Oral Dis. 2005, 11, 10–13. [Google Scholar] [CrossRef]
- Mackay, R.J.; McEntyre, C.J.; Henderson, C.; Lever, M.; George, P.M. Trimethylaminuria: Causes and diagnosis of a socially distressing condition. Clin. Biochem. Rev. 2011, 32, 33–43. [Google Scholar]
- Al-Waiz, M.; Mikov, M.; Mitchell, S.C.; Smith, R.L. The exogenous origin of trimethylamine in the mouse. Metabolism. 1992, 41, 135–136. [Google Scholar] [CrossRef]
- Fennema, D.; Phillips, I.R.; Shephard, E.A. Trimethylamine and trimethylamine N-oxide, a flavin-containing monooxygenase 3 (FMO3)-mediated host-microbiome metabolic axis implicated in health and disease. Drug Metab. Dispos. 2016, 44, 1839–1850. [Google Scholar] [CrossRef]
- Higgins, T.; Chaykin, S.; Hammond, K.B.; Humbert, J.R. Trimethylamine N-oxide synthesis: A human variant. Biochem. Med. 1972, 6, 392–396. [Google Scholar] [CrossRef]
- Al-waiz, M.; Mitchell, S.C.; Idle, J.R.; Smith, R.L. The metabolism of 14 C-labelled trimethylamine and its N-oxide in man. Xenobiotica 1987, 17, 551–558. [Google Scholar] [CrossRef]
- Lang, D.; Yeung, C.; Peter, R.; Ibarra, C.; Gasser, R.; Itagaki, K.; Philpot, R.; Rettie, A. Isoform specificity of trimethylamine N-oxygenation by human flavin-containing monooxygenase (FMO) and P450 enzymes. Biochem. Pharmacol. 1998, 56, 1005–1012. [Google Scholar] [CrossRef]
- Dolphin, C.T.; Janmohamed, A.; Smith, R.L.; Shephard, E.A.; Phillips, I.R. Missense mutation in flavin-containing mono-oxygenase 3 gene, FMO3, underlies fish-odour syndrome. Nat. Genet. 1997, 17, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Treacy, E.P.; Akerman, B.R.; Chow, L.M.L.; Youil, R.; Bibeau, C.; Lin, J.; Bruce, A.G.; Knight, M.; Danks, D.M.; Cashman, J.R.; et al. Mutations of the flavin-containing monooxygenase gene (FMO3) cause trimethylaminuria, a defect in detoxication. Hum. Mol. Genet. 1998, 7, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Dolphin, C.T.; Janmohamed, A.; Smith, R.L.; Shephard, E.A.; Phillips, I.R. Compound heterozygosity for missense mutations in the flavin-containing monooxygenase 3 (FM03) gene in patients with fish-odour syndrome. Pharmacogenetics 2000, 10, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Ayesh, R.; Mitchell, S.C.; Zhang, A.; Smith, R.L. The fish odour syndrome: Biochemical, familial, and clinical aspects. BMJ 1993, 307, 655–657. [Google Scholar] [CrossRef]
- Mitchell, S.C.; Smith, R.L. Trimethylaminuria: The fish malodor syndrome. Drug Metab. Dispos. 2001, 29, 517–521. [Google Scholar]
- Phillips, I.R.; Shephard, E.A. Flavin-containing monooxygenases: Mutations, disease and drug response. Trends Pharmacol. Sci. 2008, 29, 294–301. [Google Scholar] [CrossRef]
- Shephard, E.A.; Treacy, E.P.; Phillips, I.R. Clinical utility gene card for: Trimethylaminuria—Update 2014. Eur. J. Hum. Genet. 2015, 23, 1269. [Google Scholar] [CrossRef]
- Phillips, I.R.; Shephard, E.A. Primary Trimethylaminuria. In GeneReviews®; Adam, M.P., Ardinger, H.H., Pagon, R.A., Eds.; University of Washington, Seattle: Seattle, WA, USA, 2015. Available online: https://www.ncbi.nlm.nih.gov/books (accessed on 18 October 2019).
- Yamazaki, H.; Shimizu, M. Survey of variants of human flavin-containing monooxygenase 3 (FMO3) and their drug oxidation activities. Biochem. Pharmacol. 2013, 85, 1588–1593. [Google Scholar] [CrossRef]
- Gao, C.; Catucci, G.; Di Nardo, G.; Gilardi, G.; Sadeghi, S.J. Human flavin-containing monooxygenase 3: Structural mapping of gene polymorphisms and insights into molecular basis of drug binding. Gene 2016, 593, 91–99. [Google Scholar] [CrossRef][Green Version]
- Hernandez, D.; Addou, S.; Lee, D.; Orengo, C.; Shephard, E.A.; Phillips, I.R. Trimethylaminuria and a human FMO3 mutation database. Hum. Mutat. 2003, 22, 209–213. [Google Scholar] [CrossRef] [PubMed]
- LOVD3. Available online: https://databases.lovd.nl/shared/genes/FMO3 (accessed on 18 October 2019).
- Wallrabenstein, I.; Kuklan, J.; Weber, L.; Zborala, S.; Werner, M.; Altmüller, J.; Becker, C.; Schmidt, A.; Hatt, H.; Hummel, T.; et al. Human trace amine-associated receptor TAAR5 can be activated by trimethylamine. PLoS ONE 2013, 8, e54950. [Google Scholar] [CrossRef] [PubMed]
- Phillips, I.R.; Shephard, E.A. Flavin-containing monooxygenase 3 (FMO3): Genetic variants and their consequences for drug metabolism and disease. Xenobiotica 2019, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Bennett, B.J.; de Aguiar Vallim, T.Q.; Wang, Z.; Shih, D.M.; Meng, Y.; Gregory, J.; Allayee, H.; Lee, R.; Graham, M.; Crooke, R.; et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013, 17, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef]
- Obeid, R.; Awwad, H.M.; Rabagny, Y.; Graeber, S.; Herrmann, W.; Geisel, J. Plasma trimethylamine N-oxide concentration is associated with choline, phospholipids, and methyl metabolism. Am. J. Clin. Nutr. 2016, 103, 703–711. [Google Scholar] [CrossRef]
- Miller, C.A.; Corbin, K.D.; da Costa, K.-A.; Zhang, S.; Zhao, X.; Galanko, J.A.; Blevins, T.; Bennett, B.J.; O’Connor, A.; Zeisel, S.H. Effect of egg ingestion on trimethylamine-N-oxide production in humans: A randomized, controlled, dose-response study. Am. J. Clin. Nutr. 2014, 100, 778–786. [Google Scholar] [CrossRef]
- Fukami, K.; Yamagishi, S.; Sakai, K.; Kaida, Y.; Yokoro, M.; Ueda, S.; Wada, Y.; Takeuchi, M.; Shimizu, M.; Yamazaki, H.; et al. Oral L-carnitine supplementation increases trimethylamine-N-oxide but reduces markers of vascular injury in hemodialysis patients. J. Cardiovasc. Pharmacol. 2015, 65, 289–295. [Google Scholar] [CrossRef]
- Veeravalli, S.; Karu, K.; Scott, F.; Fennema, D.; Phillips, I.R.; Shephard, E.A. Effect of flavin-containing monooxygenase genotype, mouse strain, and gender on trimethylamine N-oxide production, plasma cholesterol concentration, and an index of atherosclerosis. Drug Metab. Dispos. 2018, 46, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Ussher, J.R.; Lopaschuk, G.D.; Arduini, A. Gut microbiota metabolism of l-carnitine and cardiovascular risk. Atherosclerosis 2013, 231, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Ufnal, M.; Zadlo, A.; Ostaszewski, R. TMAO: A small molecule of great expectations. Nutrition 2015, 31, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.E.; Caudill, M.A. Trimethylamine-N-oxide: Friend, foe, or simply caught in the cross-fire? Trends Endocrinol. Metab. 2017, 28, 121–130. [Google Scholar] [CrossRef]
- Nowiński, A.; Ufnal, M. Trimethylamine N-oxide: A harmful, protective or diagnostic marker in lifestyle diseases? Nutrition 2018, 46, 7–12. [Google Scholar] [CrossRef]
- Dominy, J.E.; Simmons, C.R.; Hirschberger, L.L.; Hwang, J.; Coloso, R.M.; Stipanuk, M.H. Discovery and characterization of a second mammalian thiol dioxygenase, cysteamine dioxygenase. J. Biol. Chem. 2007, 282, 25189–25198. [Google Scholar] [CrossRef]
- Gahl, W.A.; Thoene, J.G.; Schneider, J.A. Cystinosis. N. Engl. J. Med. 2002, 347, 111–121. [Google Scholar] [CrossRef]
- Nesterova, G.; Gahl, W.A. Cystinosis. In GeneReviews®; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Stephens, K., Amemiya, A., Eds.; University of Washington, Seattle: Seattle, WA, USA, 2017. Available online: https://www.ncbi.nlm.nih.gov/books (accessed on 18 October 2019).
- Besouw, M.; Masereeuw, R.; van den Heuvel, L.; Levtchenko, E. Cysteamine: An old drug with new potential. Drug Discov. Today 2013, 18, 785–792. [Google Scholar] [CrossRef]
- Shannon, K.M.; Fraint, A. Therapeutic advances in Huntington’s Disease. Mov. Disord. 2015, 30, 1539–1546. [Google Scholar] [CrossRef]
- Gibrat, C.; Cicchetti, F. Potential of cystamine and cysteamine in the treatment of neurodegenerative diseases. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 380–389. [Google Scholar] [CrossRef]
- Poulsen, L.L. Organic sulfur substrates for the microsomal flavin-containing monooxygenase. In Reviews in Biochemical Toxicology; Hodgson, E., Bend, J.R., Philpot, R.M., Eds.; Elsevier Press: New York, NY, USA, 1981; pp. 33–49. [Google Scholar]
- Jeitner, T.M. Mechanisms for the Cytotoxicity of Cysteamine. Toxicol. Sci. 2001, 63, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Mato, J.; Alvarez, L.; Ortiz, P.; Pajares, M.A. S-adenosylmethionine synthesis: Molecular mechanisms and clinical implications. Pharmacol. Ther. 1997, 73, 265–280. [Google Scholar] [CrossRef]
- Ripp, S.L.; Itagaki, K.; Philpot, R.M.; Elfarra, A.A. Methionine S-oxidation in human and rabbit liver microsomes: Evidence for a high-affinity methionine S-oxidase activity that is distinct from flavin-containing monooxygenase 3. Arch. Biochem. Biophys. 1999, 367, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Mayr, J.A.; Feichtinger, R.G.; Tort, F.; Ribes, A.; Sperl, W. Lipoic acid biosynthesis defects. J. Inherit. Metab. Dis. 2014, 37, 553–563. [Google Scholar] [CrossRef]
- Diesel, B.; Kulhanek-Heinze, S.; Höltje, M.; Brandt, B.; Höltje, H.-D.; Vollmar, A.M.; Kiemer, A.K. α-Lipoic acid as a directly binding activator of the insulin receptor: Protection from hepatocyte apoptosis. Biochemistry 2007, 46, 2146–2155. [Google Scholar] [CrossRef]
- Estrada, D.E.; Ewart, H.S.; Tsakiridis, T.; Volchuk, A.; Ramlal, T.; Tritschler, H.; Klip, A. Stimulation of glucose uptake by the natural coenzyme α-lipoic acid/thioctic acid: Participation of elements of the insulin signaling pathway. Diabetes 1996, 45, 1798–1804. [Google Scholar] [CrossRef]
- Yaworsky, K.; Somwar, R.; Ramlal, T.; Tritschler, H.J.; Klip, A. Engagement of the insulin-sensitive pathway in the stimulation of glucose transport by α-lipoic acid in 3T3-L1 adipocytes. Diabetologia 2000, 43, 294–303. [Google Scholar] [CrossRef]
- Fratantonio, D.; Speciale, A.; Molonia, M.S.; Bashllari, R.; Palumbo, M.; Saija, A.; Cimino, F.; Monastra, G.; Virgili, F. Alpha-lipoic acid, but not di-hydrolipoic acid, activates Nrf2 response in primary human umbilical-vein endothelial cells and protects against TNF-α induced endothelium dysfunction. Arch. Biochem. Biophys. 2018, 655, 18–25. [Google Scholar] [CrossRef]
- Schupke, H.; Hempel, R.; Peter, G.; Hermann, R.; Wessel, K.; Engel, J.; Kronbach, T. New metabolic pathways of alpha-lipoic acid. Drug Metab. Dispos. 2001, 29, 855–862. [Google Scholar]
- Borbás, T.; Benkő, B.; Dalmadi, B.; Szabó, I.; Tihanyi, K. Insulin in flavin-containing monooxygenase regulation. Eur. J. Pharm. Sci. 2006, 28, 51–58. [Google Scholar] [CrossRef]
- Warrier, M.; Shih, D.M.; Burrows, A.C.; Ferguson, D.; Gromovsky, A.D.; Brown, A.L.; Marshall, S.; McDaniel, A.; Schugar, R.C.; Wang, Z.; et al. The TMAO-Generating Enzyme Flavin Monooxygenase 3 Is a Central Regulator of Cholesterol Balance. Cell Rep. 2015, 10, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Shih, D.M.; Wang, Z.; Lee, R.; Meng, Y.; Che, N.; Charugundla, S.; Qi, H.; Wu, J.; Pan, C.; Brown, J.M.; et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J. Lipid Res. 2015, 56, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Ling, A.V.; Manthena, P.V.; Gearing, M.E.; Graham, M.J.; Crooke, R.M.; Croce, K.J.; Esquejo, R.M.; Clish, C.B.; Vicent, D.; et al. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat. Commun. 2015, 6, 6498. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.M.; McManus, S.A.; Hughes, W.E.; Schmitz-Peiffer, C. Flavin-containing monooxygenase 3 reduces endoplasmic reticulum stress in lipid-treated hepatocytes. Mol. Endocrinol. 2016, 30, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Houseman, L.; Edwards, M.; Phillips, I.R.; Shephard, E.A. Isolation and culture of mouse hepatocytes: Gender-specific gene expression responses to chemical treatments. In Protocols in In Vitro Hepatocyte Research; Methods in Molecular Biology (Methods and Protocols); Vinken, M., Rogiers, V., Eds.; Humana Press: New York, NY, USA, 2015; Volume 1250, pp. 3–12. [Google Scholar] [CrossRef]
- Miller, M.M.; James, R.A.; Richer, J.K.; Gordon, D.F.; Wood, W.M.; Horwitz, K.B. Progesterone regulated expression of flavin-containing monooxygenase 5 by the B-isoform of progesterone receptors: Implications for tamoxifen carcinogenicity. J. Clin. Endocrinol. Metab. 1997, 82, 2956–2961. [Google Scholar] [CrossRef]
- Rae, J.M.; Johnson, M.D.; Lippman, M.E.; Flockhart, D.A. Rifampin is a selective, pleiotropic inducer of drug metabolism genes in human hepatocytes: Studies with cDNA and oligonucleotide expression arrays. J. Pharmacol. Exp. Ther. 2001, 299, 849–857. [Google Scholar]
- Krusekopf, S.; Roots, I. St. John’s wort and its constituent hyperforin concordantly regulate expression of genes encoding enzymes involved in basic cellular pathways. Pharmacogenet. Genomics 2005, 15, 817–829. [Google Scholar] [CrossRef]
- Hukkanen, J.; Hakkola, J.; Rysä, J. Pregnane X receptor (PXR)—A contributor to the diabetes epidemic? Drug Metab. Drug Interact. 2014, 29, 3–15. [Google Scholar] [CrossRef]
- Takamura, T.; Sakurai, M.; Ota, T.; Ando, H.; Kaneko, S.; Honda, M. Genes for systemic vascular complications are differentially expressed in the livers of Type 2 diabetic patients. Diabetologia 2004, 47, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Pravenec, M.; Saba, L.M.; Zídek, V.; Landa, V.; Mlejnek, P.; Šilhavý, J.; Šimáková, M.; Strnad, H.; Trnovská, J.; Škop, V.; et al. Systems genetic analysis of brown adipose tissue function. Physiol. Genomics 2018, 50, 52–66. [Google Scholar] [CrossRef]
- Chen, M.; Guan, B.; Xu, H.; Yu, F.; Zhang, T.; Wu, B. The molecular mechanism regulating diurnal rhythm of flavin-containing monooxygenase 5 in mouse liver. Drug Metab. Dispos. 2019, 47, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, D.; Chandan, P.; Janmohamed, A.; Phillips, I.R.; Shephard, E.A. Deletion of genes from the mouse genome using Cre/loxP technology. Methods Mol. Biol. 2006, 320, 307–319. [Google Scholar] [PubMed]
- Hernandez, D.; Melidoni, A.N.; Phillips, I.R.; Shephard, E.A. Microinjection of targeted embryonic stem cells and establishment of knockout mouse lines for Fmo genes. Methods Mol. Biol. 2006, 320, 329–341. [Google Scholar] [PubMed]
- Hernandez, D.; Janmohamed, A.; Chandan, P.; Omar, B.A.; Phillips, I.R.; Shephard, E.A. Deletion of the mouse Fmo1 gene results in enhanced pharmacological behavioural responses to imipramine. Pharmacogenet. Genomics 2009, 19, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Shephard, E.A.; Phillips, I.R. The potential of knockout mouse lines in defining the role of flavin-containing monooxygenases in drug metabolism. Expert Opin. Drug Metab. Toxicol. 2010, 6, 1083–1094. [Google Scholar] [CrossRef]
- Palmer, A.L.; Leykam, V.L.; Larkin, A.; Krueger, S.K.; Phillips, I.R.; Shephard, E.A.; Williams, D.E. Metabolism and pharmacokinetics of the anti-tuberculosis drug ethionamide in a flavin-containing monooxygenase null mouse. Pharmaceuticals 2012, 5, 1147–1159. [Google Scholar] [CrossRef]
- Whetstine, J.R.; Yueh, M.-F.; Hopp, K.A.; McCarver, D.G.; Williams, D.E.; Park, C.-S.; Kang, J.H.; Cha, Y.-N.; Dolphin, C.T.; Shephard, E.A.; et al. Ethnic differences in human flavin-containing monooxygenase 2 (FMO2) polymorphisms: Detection of expressed protein in African-Americans. Toxicol. Appl. Pharmacol. 2000, 168, 216–224. [Google Scholar] [CrossRef]
- Edgar, S.E.; Hickman, M.A.; Marsden, M.M.; Morris, J.G.; Rogers, Q.R. Dietary cysteic acid serves as a precursor of taurine for cats. J. Nutr. 1994, 124, 103–109. [Google Scholar] [CrossRef]
- Cavallini, D.; De Marco, C.; Mondovi, B.; Stirpe, F. The biological oxidation of hypotaurine. Biochim. Biophys. Acta 1954, 15, 301–303. [Google Scholar] [CrossRef]
- Sumizu, K. Oxidation of hypotaurine in rat liver. Biochim. Biophys. Acta 1962, 63, 210–212. [Google Scholar] [CrossRef]
- Oja, S.S.; Kontro, P. Oxidation of hypotaurine in vitro by mouse liver and brain tissues. Biochim. Biophys. Acta-Gen. Subj. 1981, 677, 350–357. [Google Scholar] [CrossRef]
- Veeravalli, S.; Phillips, I.R.; Freire, R.T.; Varshavi, D.; Everett, J.R.; Shephard, E.A. FMO1 catalyses the production of taurine from hypotaurine. bioRxiv 2019, 750273. [Google Scholar] [CrossRef]
- Huxtable, R.J. Physiological actions of taurine. Physiol. Rev. 1992, 72, 101–163. [Google Scholar] [CrossRef] [PubMed]
- Lombardini, J.B. Effects of ATP and taurine on calcium uptake by membrane preparations of the rat retina. J. Neurochem. 1983, 40, 402–406. [Google Scholar] [CrossRef]
- Ripps, H.; Shen, W. Review: Taurine: A “very essential” amino acid. Mol. Vis. 2012, 18, 2673–2686. [Google Scholar]
- Yancey, P.H. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J. Exp. Biol. 2005, 208, 2819–2830. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Halliwell, B.; Hoey, B.M.; Butler, J. The antioxidant action of taurine, hypotaurine and their metabolic precursors. Biochem. J. 1988, 256, 251–255. [Google Scholar] [CrossRef]
- Furnes, B.; Feng, J.; Sommer, S.S.; Schlenk, D. Identification of novel variants of the flavin-containing monooxygenase gene family in African Americans. Drug Metab. Dispos. 2003, 31, 187–193. [Google Scholar] [CrossRef]
- Gonzalez Malagon, S.G.; Melidoni, A.N.; Hernandez, D.; Omar, B.A.; Houseman, L.; Veeravalli, S.; Scott, F.; Varshavi, D.; Everett, J.; Tsuchiya, Y.; et al. The phenotype of a knockout mouse identifies flavin-containing monooxygenase 5 (FMO5) as a regulator of metabolic ageing. Biochem. Pharmacol. 2015, 96, 267–277. [Google Scholar] [CrossRef]
- Mehrabian, M.; Callaway, K.A.; Clarke, C.F.; Tanaka, R.D.; Greenspan, M.; Lusis, A.J.; Sparkes, R.S.; Mohandas, T.; Edmond, J.; Fogelman, A.M. Regulation of rat liver 3-hydroxy-3-methylglutaryl coenzyme A synthase and the chromosomal localization of the human gene. J. Biol. Chem. 1986, 261, 16249–16255. [Google Scholar]
- Greenspan, M.D.; Yudkovitz, J.B.; Lo, C.Y.; Chen, J.S.; Alberts, A.W.; Hunt, V.M.; Chang, M.N.; Yang, S.S.; Thompson, K.L.; Chiang, Y.C. Inhibition of hydroxymethylglutaryl-coenzyme A synthase by L-659,699. Proc. Natl. Acad. Sci. USA 1987, 84, 7488–7492. [Google Scholar] [CrossRef] [PubMed]
- Krimi, R.B.; Kotelevets, L.; Dubuquoy, L.; Plaisancié, P.; Walker, F.; Lehy, T.; Desreumaux, P.; Van Seuningen, I.; Chastre, E.; Forgue-Lafitte, M.-E.; et al. Resistin-like molecule β regulates intestinal mucous secretion and curtails TNBS-induced colitis in mice. Inflamm. Bowel Dis. 2008, 14, 931–941. [Google Scholar] [CrossRef] [PubMed]
- McVay, L.D.; Keilbaugh, S.A.; Wong, T.M.H.; Kierstein, S.; Shin, M.E.; Lehrke, M.; Lefterova, M.I.; Shifflett, D.E.; Barnes, S.L.; Cominelli, F.; et al. Absence of bacterially induced RELMβ reduces injury in the dextran sodium sulfate model of colitis. J. Clin. Investig. 2006, 116, 2914–2923. [Google Scholar] [CrossRef] [PubMed]
- Cianflone, K.; Xia, Z.; Chen, L.Y. Critical review of acylation-stimulating protein physiology in humans and rodents. Biochim. Biophys. Acta-Biomembr. 2003, 1609, 127–143. [Google Scholar] [CrossRef]
- Kwon, H.; Pessin, J.E. Adipokines mediate inflammation and insulin resistance. Front. Endocrinol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Engstrom, G.; Hedblad, B.; Eriksson, K.-F.; Janzon, L.; Lindgarde, F. Complement C3 is a risk factor for the development of diabetes: A population-based cohort Study. Diabetes 2005, 54, 570–575. [Google Scholar] [CrossRef]
- Samaras, K.; Botelho, N.K.; Chisholm, D.J.; Lord, R.V. Subcutaneous and visceral adipose tissue gene expression of serum adipokines that predict Type 2 diabetes. Obesity 2010, 18, 884–889. [Google Scholar] [CrossRef]
- Hertle, E.; Stehouwer, C.D.A.; van Greevenbroek, M.M.J. The complement system in human cardiometabolic disease. Mol. Immunol. 2014, 61, 135–148. [Google Scholar] [CrossRef]
| Human | Mouse Male | Mouse Female | |
|---|---|---|---|
| FMO1 | Kidney | Liver, Lung, Kidney | Liver, Lung, Kidney |
| FMO2 | Lung | Lung | Lung |
| FMO3 | Liver | - | Liver |
| FMO5 | Liver, Gastrointestinal tract | Liver, Gastrointestinal tract | Liver, Gastrointestinal tract |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phillips, I.R.; Shephard, E.A. Endogenous Roles of Mammalian Flavin-Containing Monooxygenases. Catalysts 2019, 9, 1001. https://doi.org/10.3390/catal9121001
Phillips IR, Shephard EA. Endogenous Roles of Mammalian Flavin-Containing Monooxygenases. Catalysts. 2019; 9(12):1001. https://doi.org/10.3390/catal9121001
Chicago/Turabian StylePhillips, Ian R., and Elizabeth A. Shephard. 2019. "Endogenous Roles of Mammalian Flavin-Containing Monooxygenases" Catalysts 9, no. 12: 1001. https://doi.org/10.3390/catal9121001
APA StylePhillips, I. R., & Shephard, E. A. (2019). Endogenous Roles of Mammalian Flavin-Containing Monooxygenases. Catalysts, 9(12), 1001. https://doi.org/10.3390/catal9121001





