Extending the Hierarchy of Heterogeneous Catalysis to Substituted Derivatives of Benzimidazole Synthesis: Transition Metals Decorated CNTs
Abstract
1. Introduction
2. Results and Discussion
2.1. Physical Characterization of the Catalysts
2.2. Catalysts Screening
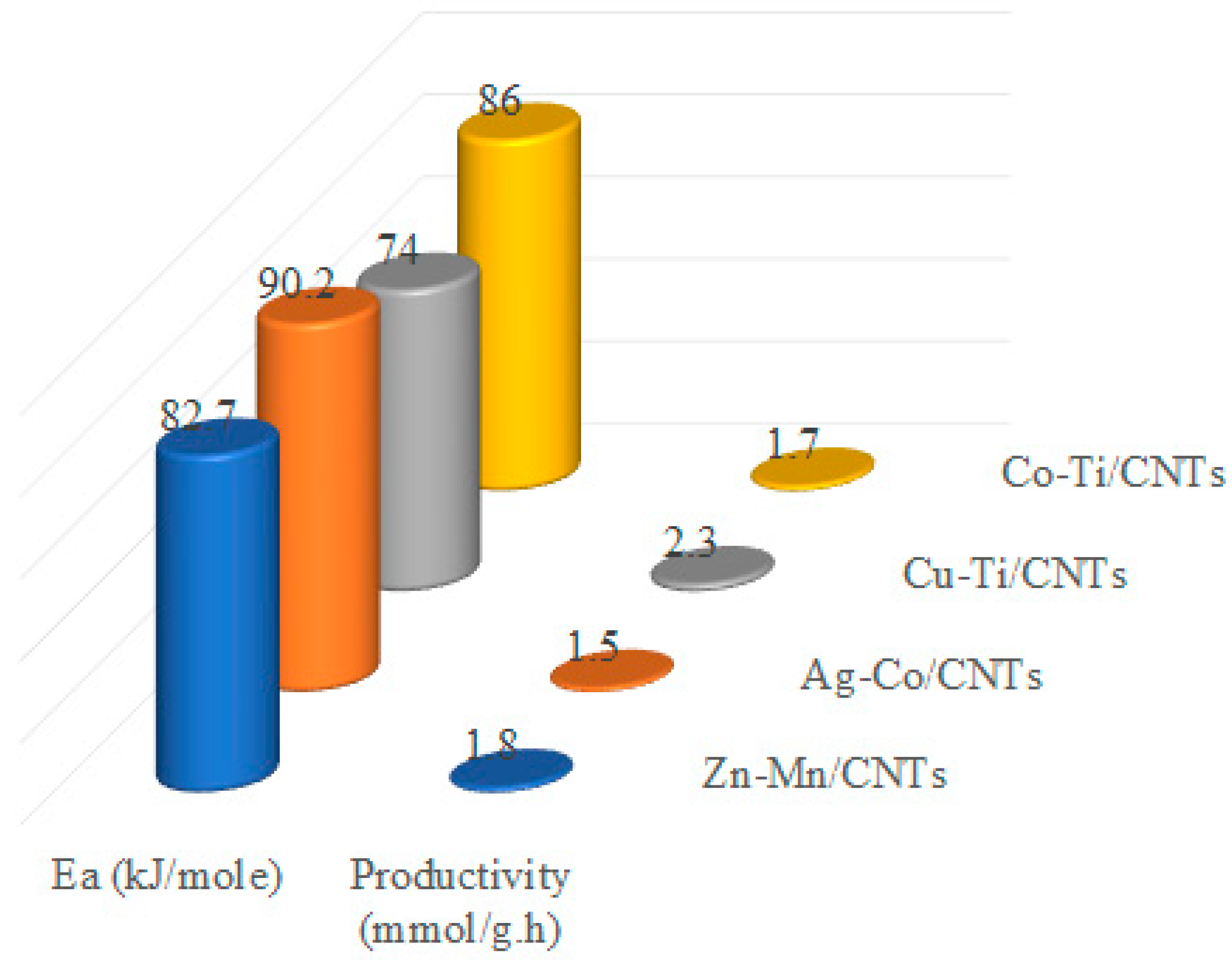
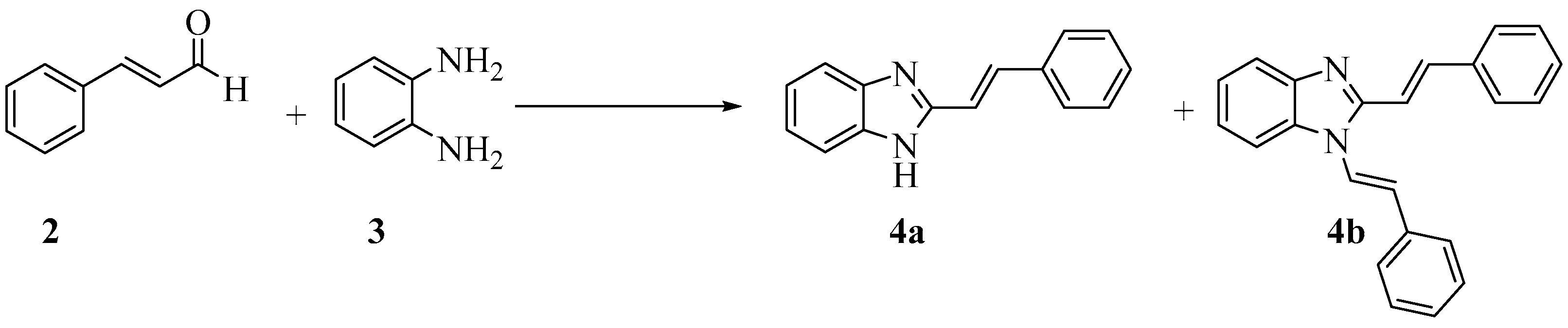
2.3. Time Profile Study
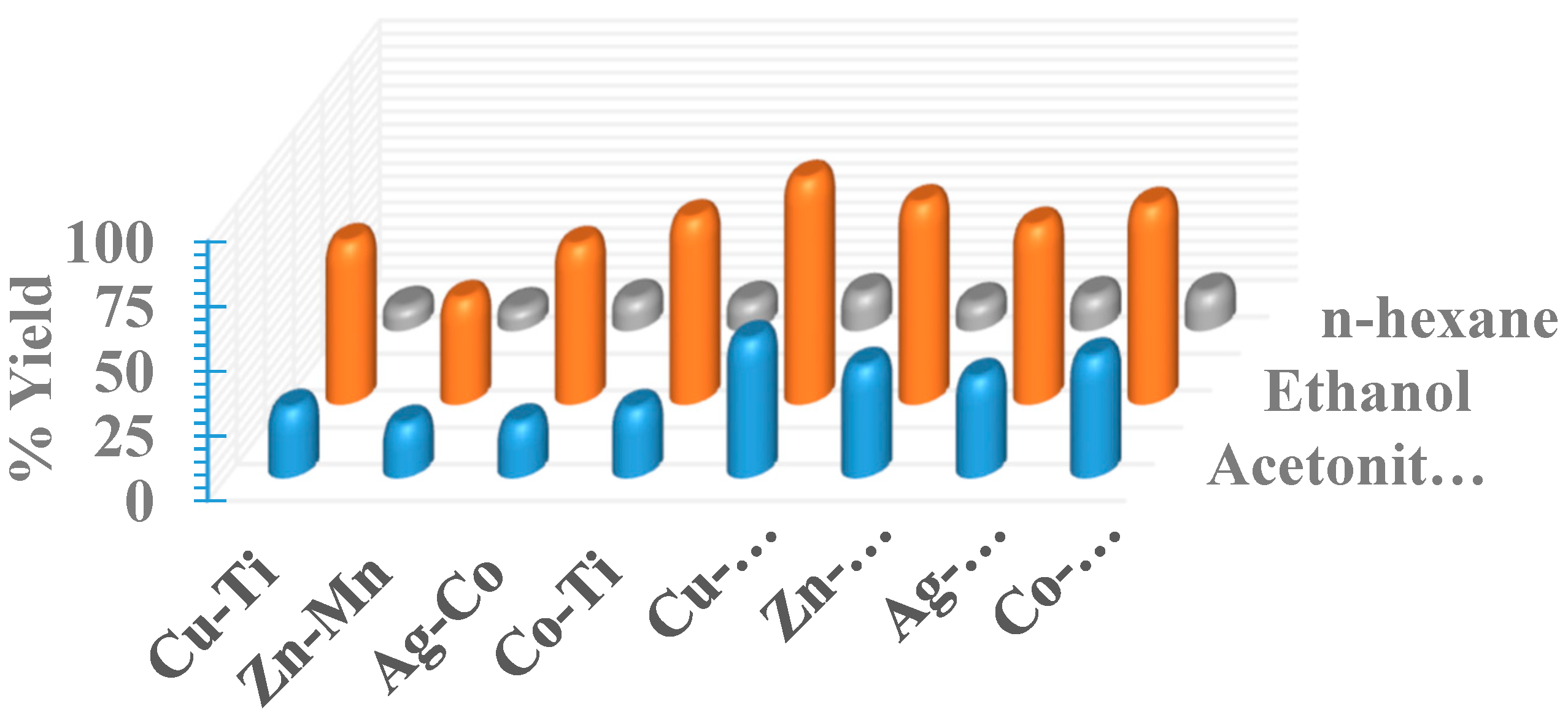
2.4. Solvent Effect
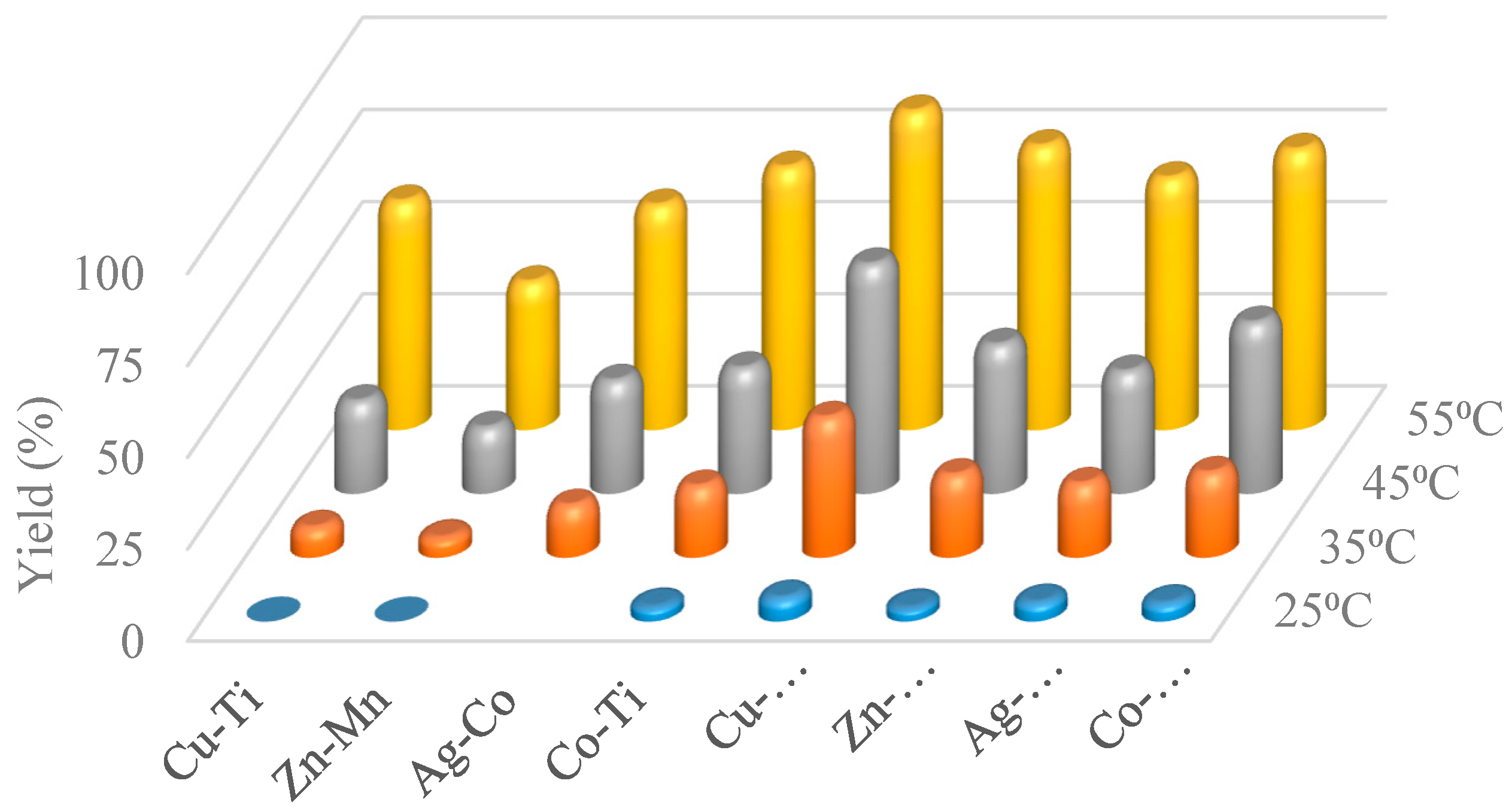
2.5. Thermal Effect
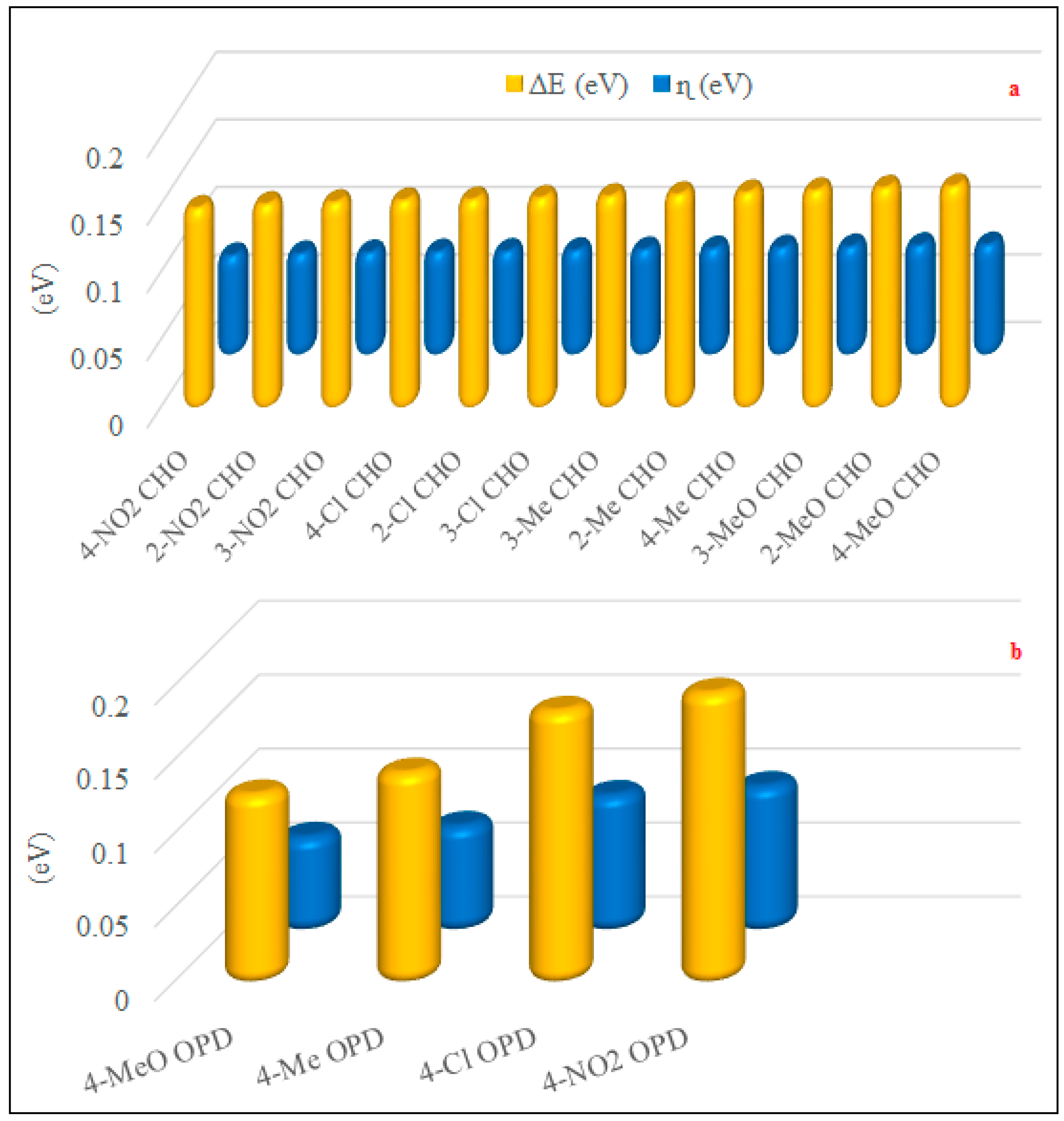
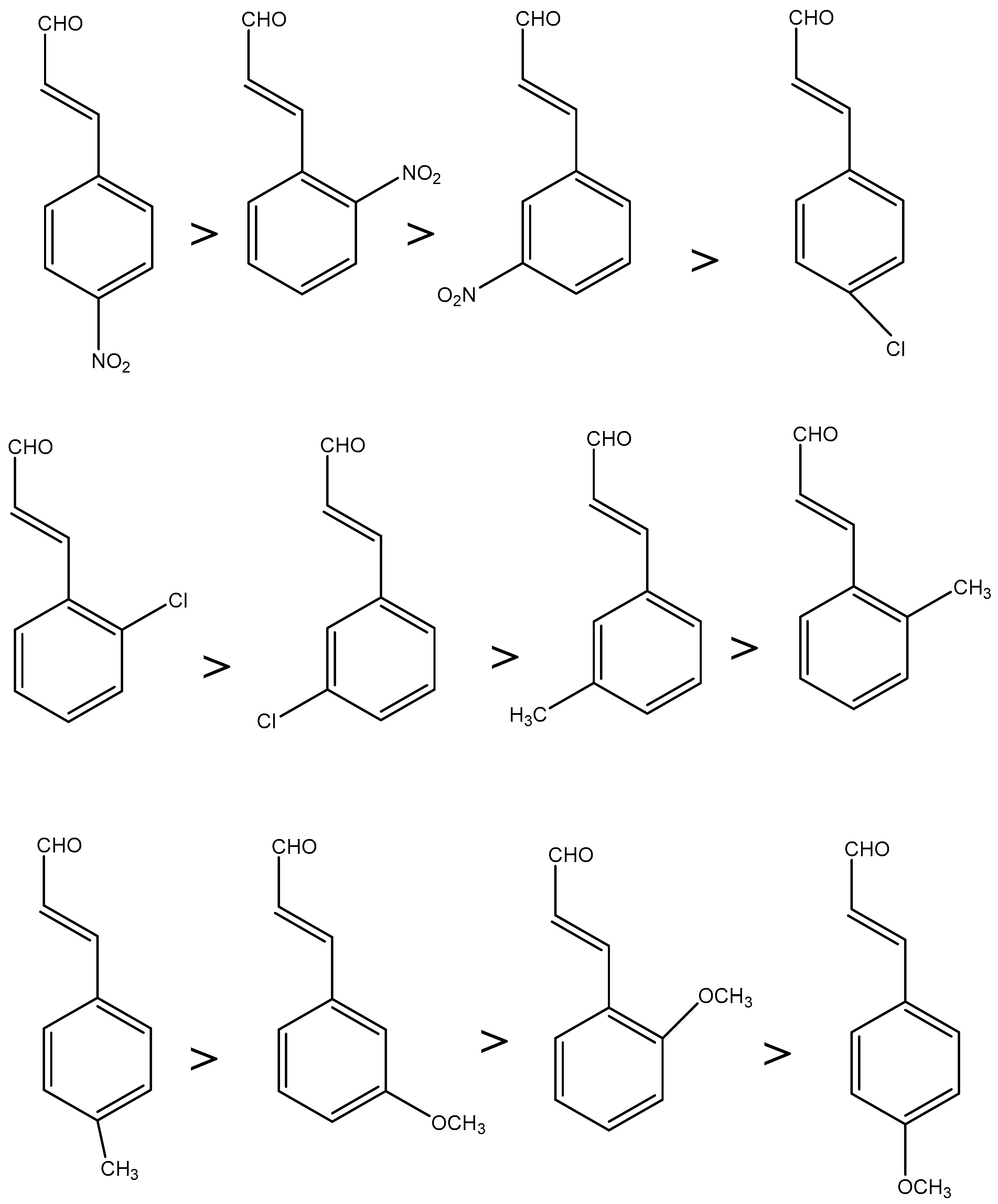
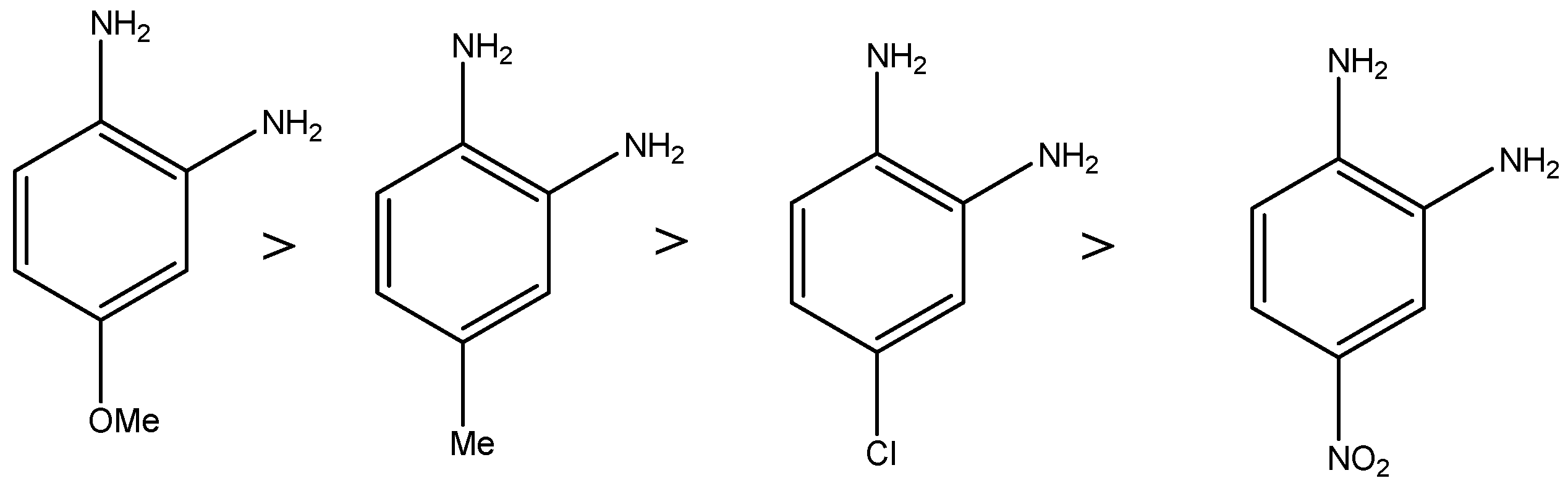
2.6. Structural and Electronic Properties
3. Experimental
3.1. Functionalization of MWCNTs
3.2. Synthesis of the Nanoparticles
3.3. Synthesis of Supported Nanoparticles
3.4. Characterization of Catalysts
3.5. Catalytic Test
3.6. Computational Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arulmurugan, S.; Kavitha, H.P.; Venkatraman, B. Biological activities of Schiff base and its complexes: A review. Rasayan J. Chem. 2010, 3, 385–410. [Google Scholar]
- Venkateswarlu, Y.; Kumar, S.R.; Leelavathi, P. Facile and efficient one-pot synthesis of benzimidazoles using lanthanum chloride. Org. Med. Chem. Lett. 2013, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Hisano, T.; Ichikawa, M.; Tsumoto, K.; Tasaki, M. Synthesis of benzoxazoles, benzothiazoles and benzimidazoles and evaluation of their antifungal, insecticidal and herbicidal activities. Chem. Pharm. Bull. 1982, 30, 2996–3004. [Google Scholar] [CrossRef]
- Kumar, B.V.S.; Vaidya, S.D.; Kumar, R.V.; Bhirud, S.B.; Mane, R.B. Synthesis and anti-bacterial activity of some novel 2-(6-fluorochroman-2-yl)-1-alkyl/acyl/aroyl-1H-benzimidazoles. Eur. J. Med. Chem. 2006, 41, 599–604. [Google Scholar] [CrossRef]
- Vinodkumar, R.; Vaidya, S.D.; Kumar, B.V.S.; Bhise, U.N.; Bhirud, S.B.; Mashelkar, U.C. Synthesis, anti-bacterial, anti-asthmatic and anti-diabetic activities of novel N-substituted-2-(4-phenylethynyl-phenyl)-1H-benzimidazoles and N-substituted 2 [4-(4, 4-dimethyl-thiochroman-6-yl-ethynyl)-phenyl)-1H-benzimidazoles. Eur. J. Med. Chem. 2008, 43, 986–995. [Google Scholar] [CrossRef]
- Mann, J.; Baron, A.; Opoku-Boahen, Y.; Johansson, E.; Parkinson, G.; Kelland, L.R.; Neidle, S. A new class of symmetric bisbenzimidazole-based DNA minor groove-binding agents showing antitumor activity. J. Med. Chem. 2001, 44, 138–144. [Google Scholar] [CrossRef]
- Patil, A.; Ganguly, S.; Surana, S. A systematic review of benzimidazole derivatives as an antiulcer agent. Rasayan J. Chem. 2008, 1, 447–460. [Google Scholar]
- Shah, K.; Chhabra, S.; Shrivastava, S.K.; Mishra, P. Benzimidazole: A promising pharmacophore. Med. Chem. Res. 2013, 22, 5077–5104. [Google Scholar] [CrossRef]
- UŞ, C.K.; Altanlar, N. Synthesis of some new benzimidazole carbamate derivatives for evaluation of antifungal activity. Turk. J. Chem. 2003, 27, 35–40. [Google Scholar]
- Mohamed, B.G.; Abdel-Alim, A.-A.M.; Hussein, M.A. Synthesis of 1-acyl-2-alkylthio-1, 2, 4-triazolobenzimidazoles with antifungal, anti-inflammatory and analgesic effects. Acta Pharm. 2006, 56, 31–48. [Google Scholar]
- Kulkarni, M.V.; Patil, V.D. Synthesis Spectral Studies and Anti-Inflammatory Activity of Some New 2-Aryloxybenzimidazoles. Arch. Pharm. 1981, 314, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Mariappan, G.; Hazarika, R.; Alam, F.; Karki, R.; Patangia, U.; Nath, S. Synthesis and biological evaluation of 2-substituted benzimidazole derivatives. Arab. J. Chem. 2015, 8, 715–719. [Google Scholar] [CrossRef]
- Stevenson, C.; Davies, R.J.H. Photosensitization of guanine-specific DNA damage by 2-phenylbenzimidazole and the sunscreen agent 2-phenylbenzimidazole-5-sulfonic acid. Chem. Res. Toxicol. 1999, 12, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, E.; Yoneoka, A.; Suzuki, K.; Kato, T.; Kitazume, T.; Yanagi, K. Reductive transformation of α, β-epoxy ketones and other compounds promoted through photoinduced electron transfer processes with 1, 3-dimethyl-2-phenylbenzimidazoline (DMPBI). Tetrahedron 1999, 55, 12957–12968. [Google Scholar] [CrossRef]
- Figge, A.; Altenbach, H.J.; Brauer, D.J.; Tielmann, P. Synthesis and resolution of 2-(2-diphenylphosphinyl-naphthalen-1-yl)-1-isopropyl-1H-benzoimidazole; a new atropisomeric P, N-chelating ligand for asymmetric catalysis. Tetrahedron Asymmetry 2002, 13, 137–144. [Google Scholar] [CrossRef]
- Savini, L.; Chiasserini, L.; Pellerano, C.; Filippelli, W.; Falcone, G. Synthesis and pharmacological activity of 1, 2, 4-triazolo [4, 3-a] quinolones. II Farm. 2001, 56, 939–945. [Google Scholar]
- Chari, M.A.; Shobha, D.; Sasaki, T. Room temperature synthesis of benzimidazole derivatives using reusable cobalt hydroxide (II) and cobalt oxide (II) as efficient solid catalysts. Tetrahedron Lett. 2011, 52, 5575–5580. [Google Scholar] [CrossRef]
- Rithe, S.; Jagtap, R.; Ubarhande, S. One pot synthesis of substituted benzimidazole derivatives and their characterization. Rasayan J. Chem. 2015, 8, 213–217. [Google Scholar]
- Saberi, A. Efficient synthesis of Benzimidazoles using zeolite, alumina and silica gel under microwave irradiation. Iran. J. Sci. Technol. 2015, 39, 7–10. [Google Scholar]
- Reddy, P.L.; Arundhathi, R.; Tripathi, M.; Rawat, D.S. CuI nanoparticles mediated expeditious synthesis of 2-substituted benzimidazoles using molecular oxygen as the oxidant. RSC Adv. 2016, 6, 53596–53601. [Google Scholar] [CrossRef]
- Aman, R.; Sadiq, S.; Ali, M.; Sadiq, M.; Gul, J.; Saeed, K.; Khan, A.A.; Shah, S.H. Facile route for green synthesis of N-benzylideneaniline over bimetallic reduced graphene oxide: Chemical reactivity of 2, 3, 4-substituted derivatives of aniline. Res. Chem. Intermed. 2019, 45, 2947–2961. [Google Scholar] [CrossRef]
- Trivedi, R.; De, S.K.; Gibbs, R.A. A convenient one-pot synthesis of 2-substituted benzimidazoles. J. Mol. Catal. A Chem. 2006, 245, 8–11. [Google Scholar] [CrossRef]
- Srinivasulu, R.; Kumar, K.R.; Satyanarayana, P.V.V. Facile and efficient method for synthesis of benzimidazole derivatives catalyzed by zinc triflate. Green Sustain. Chem. 2014, 4, 33. [Google Scholar] [CrossRef]
- Lu, J.; Yang, B.; Bai, Y. Microwave irradiation synthesis of 2-substituted benzimidazoles using PPA as a catalyst under solvent-free conditions. Synth. Commun. 2002, 32, 3703–3709. [Google Scholar] [CrossRef]
- Riadi, Y.; Mamouni, R.; Azzalou, R.; El Haddad, M.; Routier, S.; Guillaumet, G.; Lazar, S. An efficient and reusable heterogeneous catalyst animal bone meal for facile synthesis of benzimidazoles, benzoxazoles, and benzothiazoles. Tetrahedron Lett. 2011, 52, 3492–3495. [Google Scholar] [CrossRef]
- Kokare, N.D.; Sangshetti, J.N.; Shinde, D.B. One-pot efficient synthesis of 2-aryl-1-arylmethyl-1H-benzimidazoles and 2, 4, 5-triaryl-1H-imidazoles using oxalic acid catalyst. Synthesis 2007, 2007, 2829–2834. [Google Scholar] [CrossRef]
- Chen, Y.X.; Qian, L.F.; Zhang, W.; Han, B. Efficient Aerobic Oxidative Synthesis of 2-Substituted Benzoxazoles, Benzothiazoles, and Benzimidazoles Catalyzed by 4-Methoxy-TEMPO. Angew. Chem. Int. Ed. 2008, 47, 9330–9333. [Google Scholar] [CrossRef]
- Zada, N.; Khan, I.; Saeed, K. Synthesis of multiwalled carbon nanotubes supported manganese and cobalt zinc oxides nanoparticles for the photodegradation of malachite green. Sep. Sci. Technol. 2017, 52, 1477–1485. [Google Scholar] [CrossRef]
- Shakirullah, M.; Ahamd, I.; Zada, N.; Ishaq, M.; Ahmad, W.; Saeed, K.; Mohammadzai, I.U. Thermocatalytic Conversion of Coal Soot to Carbon Nanorods, Fullerenes. Fuller. Nanotub. Carbon Nanostruct. 2013, 21, 171–182. [Google Scholar] [CrossRef]
- Saeed, K.; Zada, N.; Khan, I. Photocatalytic degradation of alizarin red dye in aqueous medium using carbon nanotubes/Cu–Ti oxide composites. Sep. Sci. Technol. 2018, 1–9. [Google Scholar] [CrossRef]
- Monazzam, P.; Kisomi, B.F. Co/TiO2 nanoparticles: Preparation, characterization and its application for photocatalytic degradation of methylene blue. Desalin. Water Treat. 2017, 63, 283–292. [Google Scholar]
- Rekha, M.; Hamza, A.; Venugopal, B.; Nagaraju, N. Synthesis of 2-substituted benzimidazoles and 1, 5-disubstituted benzodiazepines on alumina and zirconia catalysts. Chin. J. Catal. 2012, 33, 439–446. [Google Scholar] [CrossRef]
- Dhopte, K.B.; Zambare, R.S.; Patwardhan, A.V.; Nemade, P.R. Role of graphene oxide as heterogeneous acid catalyst and benign oxidant for synthesis of benzimidazoles and benzothiazoles. RSC Adv. 2016, 6, 8164–8172. [Google Scholar] [CrossRef]
- Rajabi, F.; De, S.; Luque, R. An Efficient and Green Synthesis of Benzimidazole Derivatives Using SBA-15 Supported Cobalt Nanocatalysts. Catal. Lett. 2015, 145, 1566–1570. [Google Scholar] [CrossRef]
- Baltork, I.M.; Moghadam, M.; Tangestaninejad, S.; Mirkhani, V.; Zolfigolb, M.A.; Hojatia, S.F. Silica Sulfuric Acid Catalyzed Synthesis of Benzoxazoles, Benzimidazoles and Oxazolo[4,5-b] pyridines Under Heterogeneous and Solvent-Free Conditions. J. Iran. Chem. Soc. 2008, 5, S6S–S70. [Google Scholar]
- Senapak, W.; Saeeng, R.; Jaratjaroonphong, J.; Promarak, V.; Sirion, U. Metal free selective synthesis of 2-substituted benzimidazoles catalysed by Bronsted acidic ionic liquid: Convenient access to one pot synthesis of N-alkylated 1,2-disubstituted benzimidazoles. Tetrahedron 2019, 75, 3543–3552. [Google Scholar] [CrossRef]
- Kalhor, M.; Mobinikhaledi, A.; Jamshidi, J. Synthesis of 2-arylbenzimidazoles catalyzed by transition metal nitrates. Res. Chem. Intermed. 2013, 39, 3127–3133. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Špirtović-Halilović, S.; Salihović, M.; Veljović, E.; Osmanović, A.; Trifunović, S.; Završnik, D. Chemical reactivity and stability predictions of some coumarins by means of DFT calculations. Glas. Hem. Tehnol. Bosne Herceg. 2014, 43, 57–60. [Google Scholar]

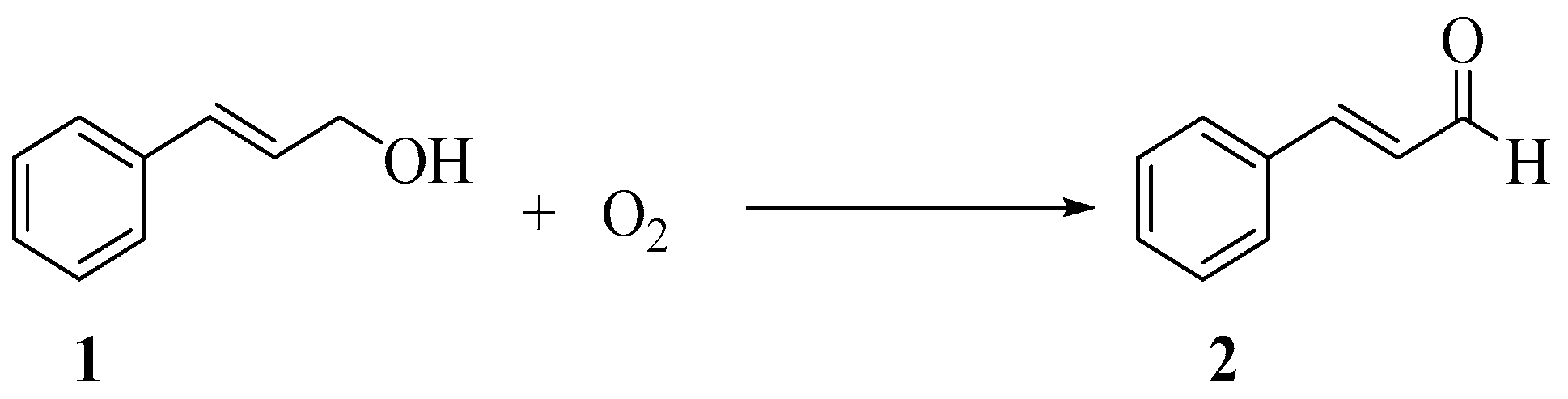
| S.No | Catalyst | SEM | nm * | Morphology | XRD | 2θ Values | nm ** | BET (m2/g) |
|---|---|---|---|---|---|---|---|---|
| 1 | Zn–Mn | S1(a) | 33.8 | Agglomerated | S1(e) | 34.5°,37.1°,48°,61°,65.6°,75.1° | 30.5 | 34.5 |
| 2 | Zn–Mn/FMWCNTs | S1(b) | - | Dispersed and Agglomerated | S1 (f) | 34.5°,37.1°,48°,53.5°,61.3°,75.15°,79° | - | 229.3 |
| 3 | Ag–Co | S2(a) | 27.5 | Dispersed | S2 (e) | 32.8°,33.6°,38.1°,44.2°,55.2°,77.3° | 28.2 | 27.3 |
| 4 | Ag–Co/FMWCNTs | S2(b) | - | Dispersed and Agglomerated | S2(f) | 18.5°,32.8°,33.6°,33.7°,38.1°,38.2°,44°, 56.3°,77.3° | - | 231.5 |
| 5 | Cu–Ti | S3(a) | 22.7 | Irregular shaped | S3 (e) | 25.3°,35°,38.6°,48.1°,54° | 23.5 | 31.8 |
| 6 | Cu–Ti/FMWCNTs | S3(b) | - | Dispersed and agglomerated | S3 (f) | 25.3°,35°,38°,48.1°,54°,63° | - | 230.1 |
| 7 | Co–Ti | S4(a) | 22.3 | Dispersed | S4 (e) | 25.15°,38.45°,48°,54°,4°,69°,70°,76° | 22.9 | 26.4 |
| 8 | Co–Ti/FMWCNTs | S4(b) | - | Dispersed | S4 (f) | 25.3°,37.7°,48°,54°,64°,76° | - | 233.7 |

| ENTRY | R1 | R2 | % Yield |
|---|---|---|---|
| 1 | H | H | 73 |
| 2 | 4-OMe | 4-NO2 | 98 |
| 3 | 4-OMe | 4-Cl | 95 |
| 4 | 4-OMe | 3-Me | 82 |
| 5 | 4-OMe | 3-OMe | 77 |
| 6 | 4-Me | 4-NO2 | 94 |
| 7 | 4-Me | 4-Cl | 92 |
| 8 | 4-Me | 3-Me | 76 |
| 9 | 4-Me | 3-OMe | 69 |
| 10 | 4-Cl | 3-OMe | 66 |
| 11 | 4-Cl | 3-Me | 71 |
| 12 | 4-Cl | 4-NO2 | 88 |
| 13 | 4-NO2 | 3-Cl | 84 |
| 14 | 4-NO2 | 3-Me | 65 |
| 15 | 4-NO2 | 3-OMe | 61 |
| Catalyst | Reaction Conditions | % Yield | Reference |
|---|---|---|---|
| Co/SBA-15 nanocatalyst (0.014 g) | CHO (1 mmol), OPD (1 mmol), EtOH (2 mL), temp (60 °C), time (4 h) | 98 | [34] |
| Graphene Oxide (0.02 g) | CHO (1 mmol), OPD (1 mmol), MeOH (3 mL), temp (60 °C), time (4 h) | 81 | [33] |
| Silica sulfuric acid (0.05 g) | Orthoester (1.1 mmol), OPD (1 mmol), solvent free, temp (85 °C), time (5 h) | 90 | [35] |
| MnZrO2 (0.2 g) | CHO (1mmol), OPD (1 mmol), EtOH (5 mL), temp (80 °C), time (2 h) | 92 | [32] |
| Cu–Ti/FMWCNTs (0.1 g) | CHO (0.89 mmol), OPD (1.07 mmol), EtOH (10 mL), temp (55 °C), time (4 h) | 98 | Present Study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, Z.; Sadiq, S.; Sadiq, M.; Ullah, I.; Fazal, Z.; Khan, D.; Bibi, N.; Saeed, K.; Khan, A.A.; Mabood, F.; et al. Extending the Hierarchy of Heterogeneous Catalysis to Substituted Derivatives of Benzimidazole Synthesis: Transition Metals Decorated CNTs. Catalysts 2019, 9, 1000. https://doi.org/10.3390/catal9121000
Iqbal Z, Sadiq S, Sadiq M, Ullah I, Fazal Z, Khan D, Bibi N, Saeed K, Khan AA, Mabood F, et al. Extending the Hierarchy of Heterogeneous Catalysis to Substituted Derivatives of Benzimidazole Synthesis: Transition Metals Decorated CNTs. Catalysts. 2019; 9(12):1000. https://doi.org/10.3390/catal9121000
Chicago/Turabian StyleIqbal, Zahoor, Saima Sadiq, Muhammad Sadiq, Inam Ullah, Zahid Fazal, Doctor Khan, Neelam Bibi, Khalid Saeed, Adnan Ali Khan, Fazal Mabood, and et al. 2019. "Extending the Hierarchy of Heterogeneous Catalysis to Substituted Derivatives of Benzimidazole Synthesis: Transition Metals Decorated CNTs" Catalysts 9, no. 12: 1000. https://doi.org/10.3390/catal9121000
APA StyleIqbal, Z., Sadiq, S., Sadiq, M., Ullah, I., Fazal, Z., Khan, D., Bibi, N., Saeed, K., Khan, A. A., Mabood, F., & Ali, M. (2019). Extending the Hierarchy of Heterogeneous Catalysis to Substituted Derivatives of Benzimidazole Synthesis: Transition Metals Decorated CNTs. Catalysts, 9(12), 1000. https://doi.org/10.3390/catal9121000





