Energy-Saving UHMW Polymeric Flow Aids: Catalyst and Polymerization Process Development
Abstract
:1. Introduction
- ▪
- Low catalyst activity;
- ▪
- Requirements of 0 °C and below, and very long polymerization time (≥12 h) to synthesize the DRP;
- ▪
- Low drag reduction (~13.4%); and
- ▪
- Use of hazardous catalyst adjuvant(s), and costly gel-prone aluminoxane cocatalysts.
2. Results and Discussion
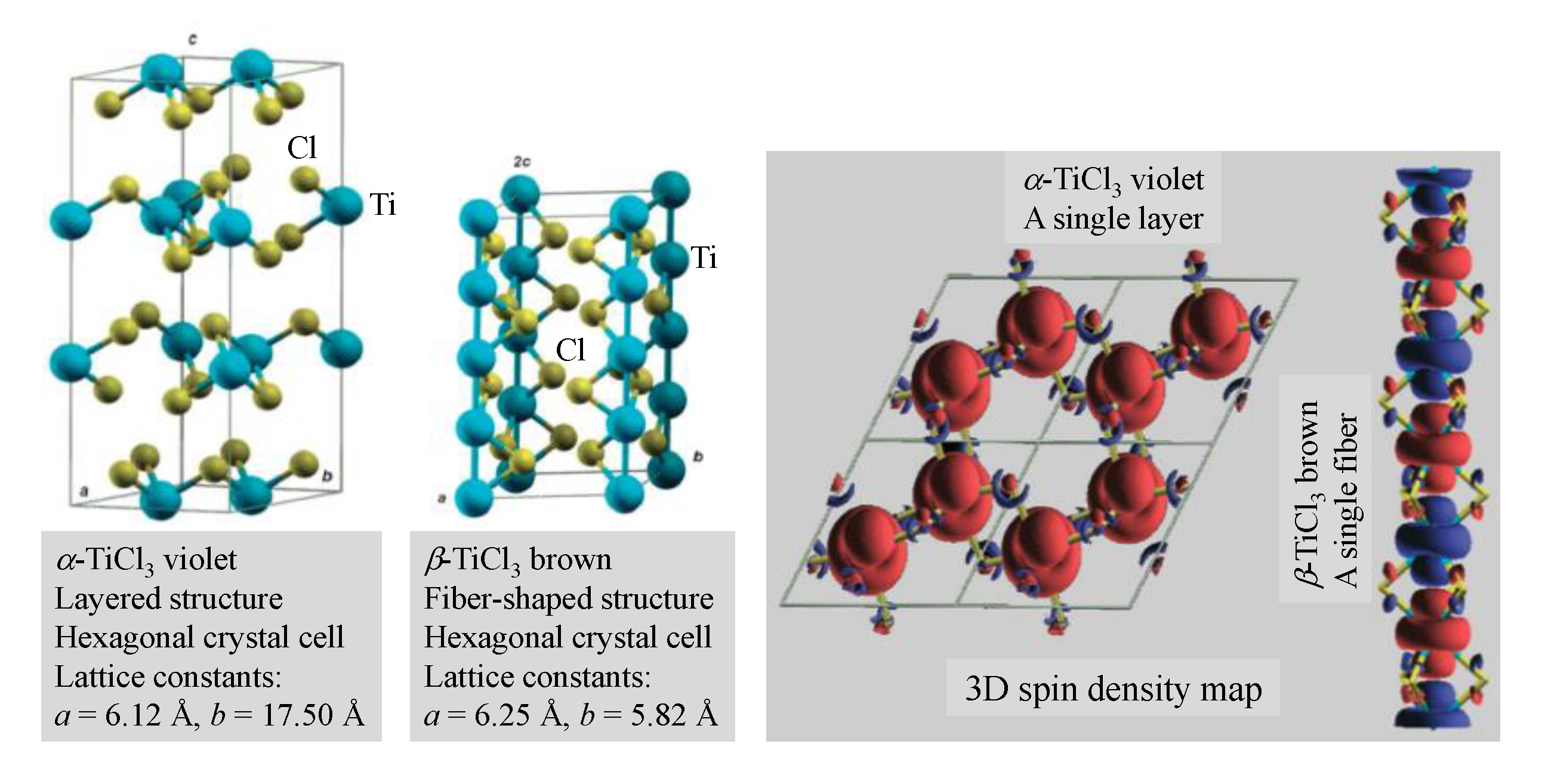
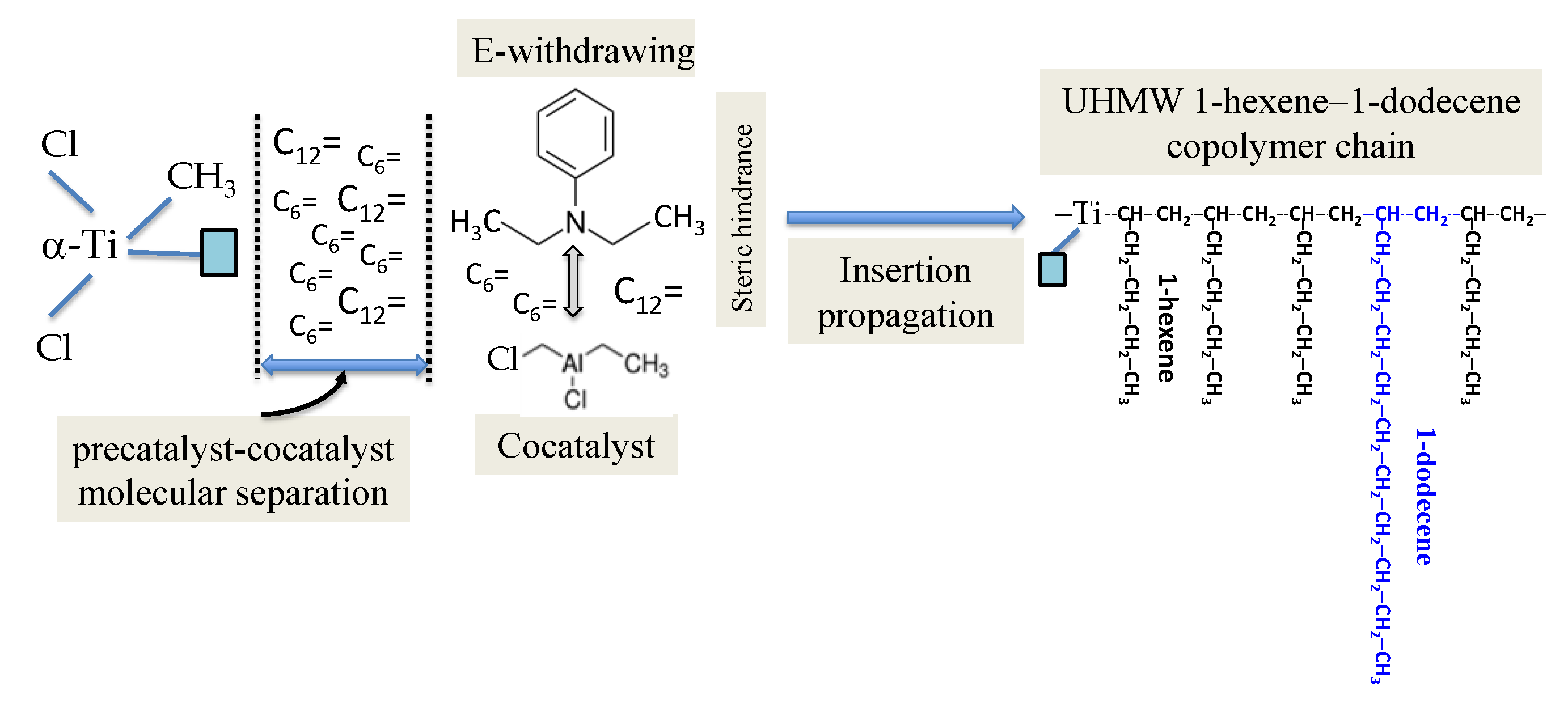
2.1. Present Patented Catalyst Design Concept and Composition
- ▪
- They simultaneously show steric hindrance and electronic effect. Steric hindrance is achieved by the bulky substituents around the heteroatom, which reduces the access of the related base functionality to the Lewis acid alkyl aluminum cocatalyst.
- ▪
- Electronic effect is obtained by placing electron-withdrawing substituents on the heteroatom to reduce its electron density. Consequently, the (electrophilic) Lewis acid alkyl aluminum cocatalyst●Lewis base complex presumably dissociates (in a nonpolar medium) under the DRP synthesis condition, and consequently the catalyst productivity increases. The introduction of this dissociation phenomenon and the control of precatalyst–cocatalyst molecular separation are the key to the subject novel catalyst design. Aromatic substituents are especially useful because they are relatively inert toward other catalyst components.
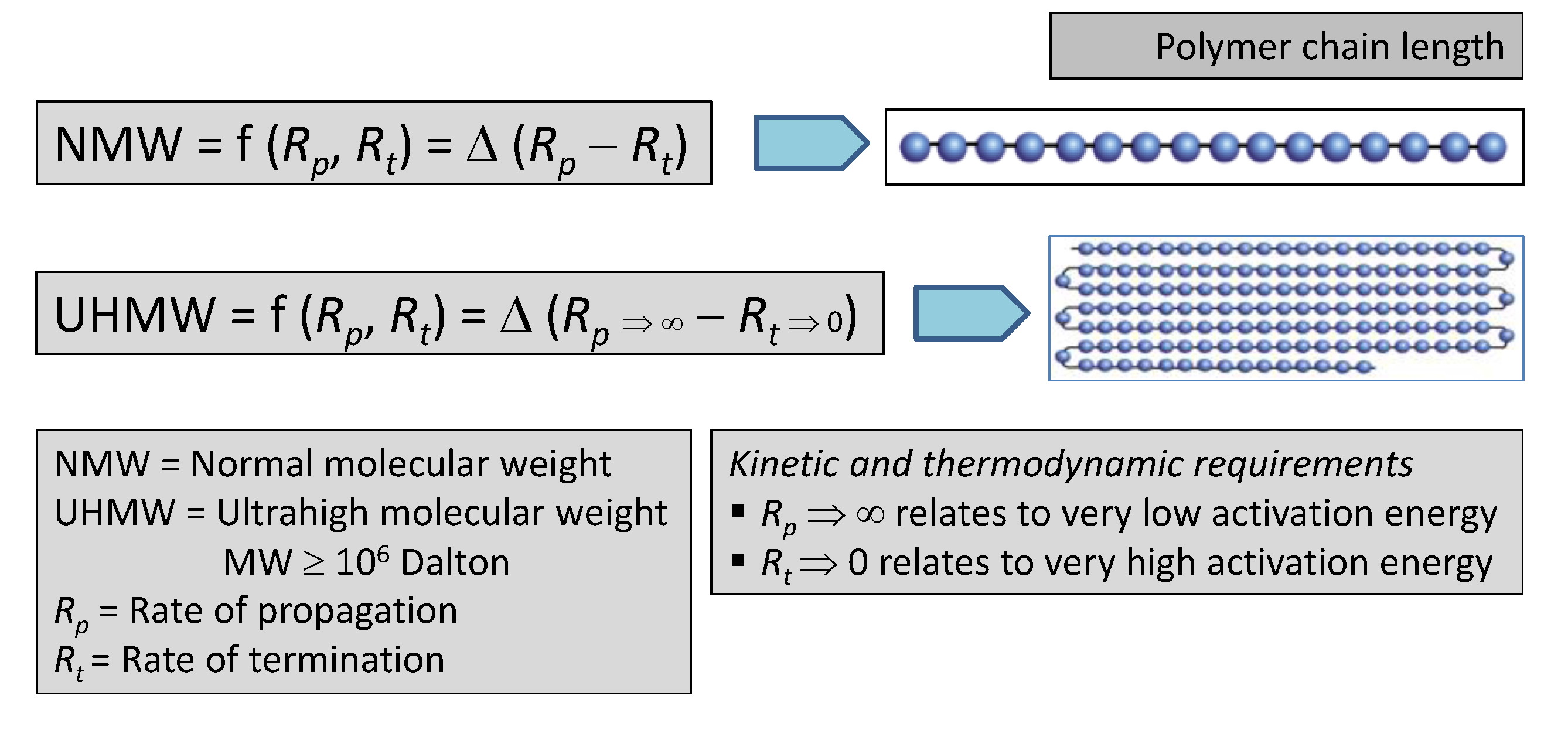
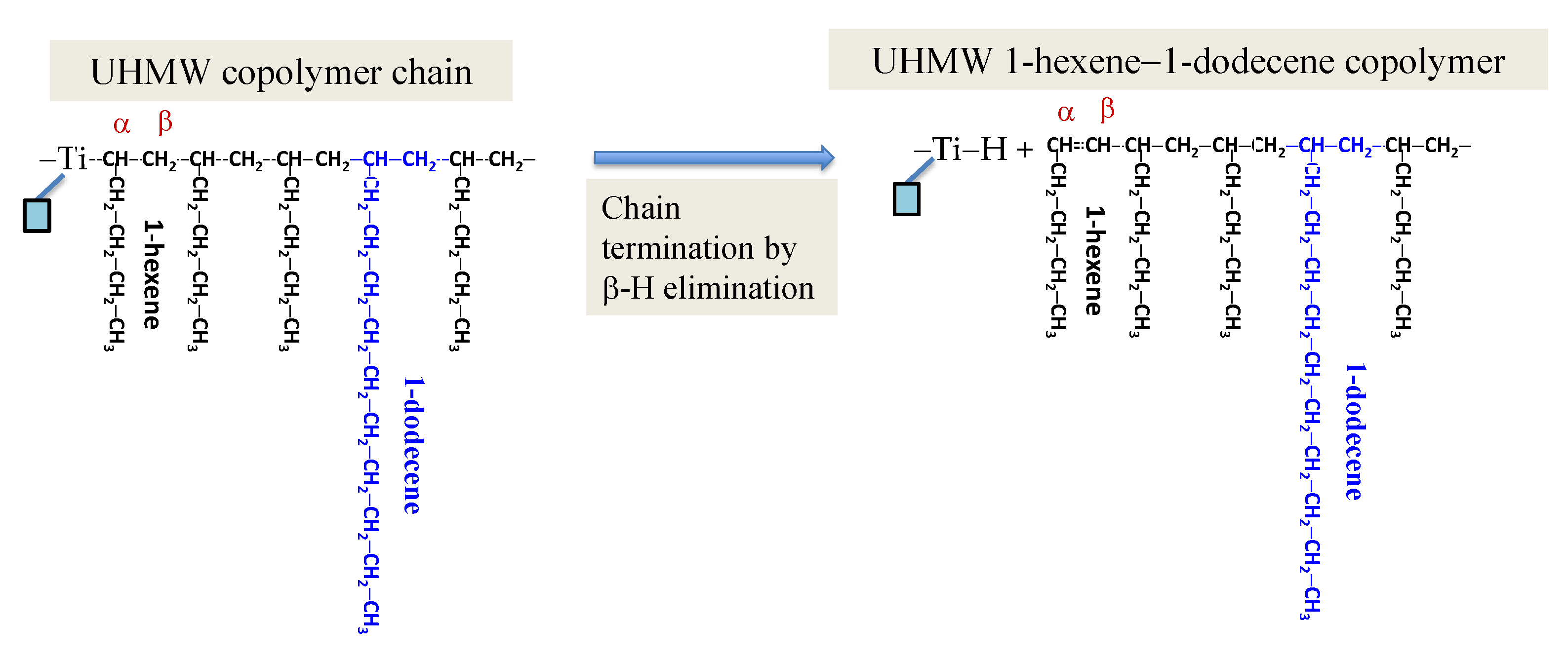
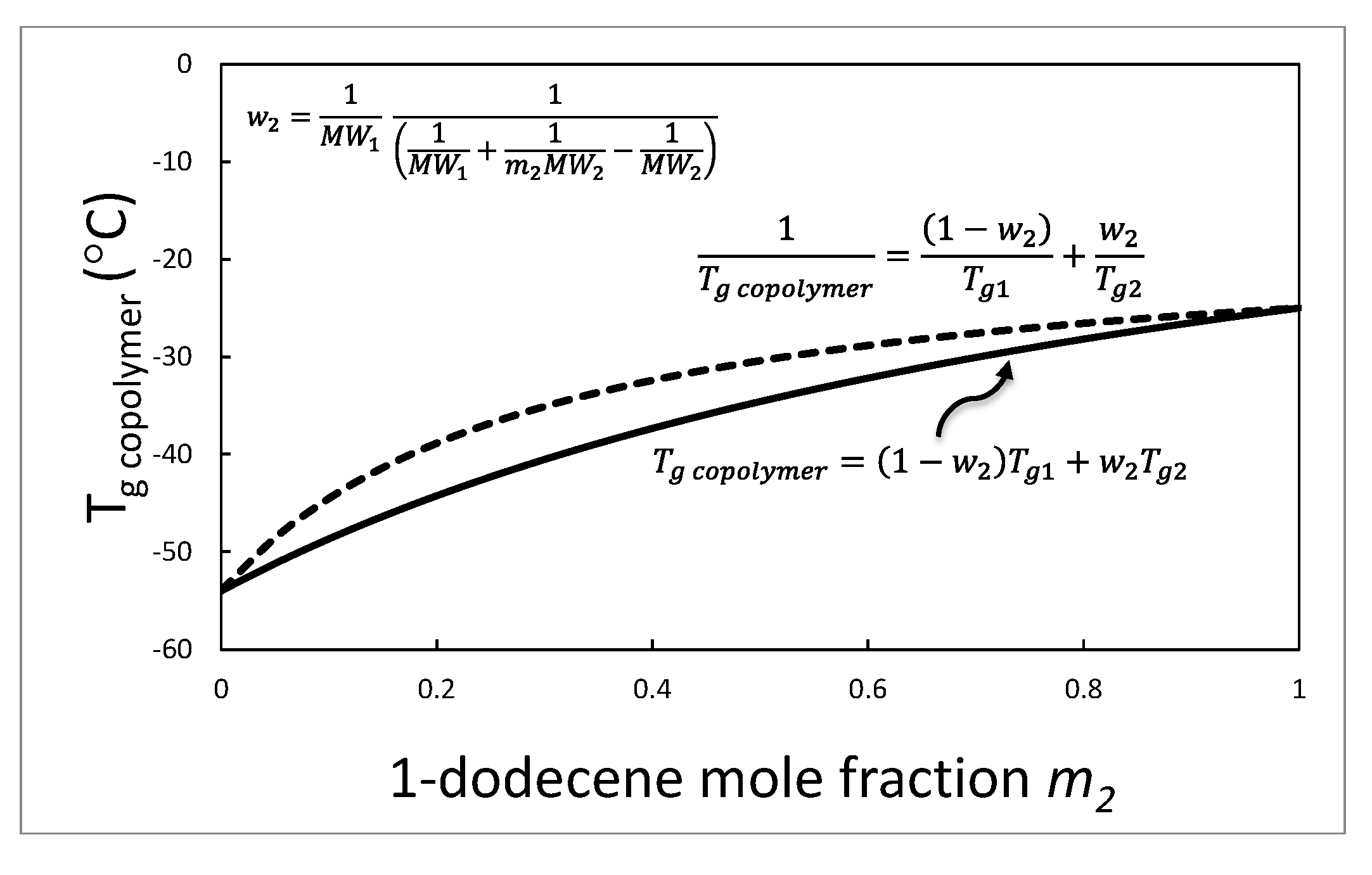
2.2. Catalyst and Polymer Science Underlying the Present Patented Catalyst System
- ▪
- Stretching the copolymer chain with respect to equilibrium conformations;
- ▪
- Increasing solution extensional viscosity;
- ▪
- Dampening turbulent eddies; and
- ▪
- Thickening of the viscous laminar sublayer.
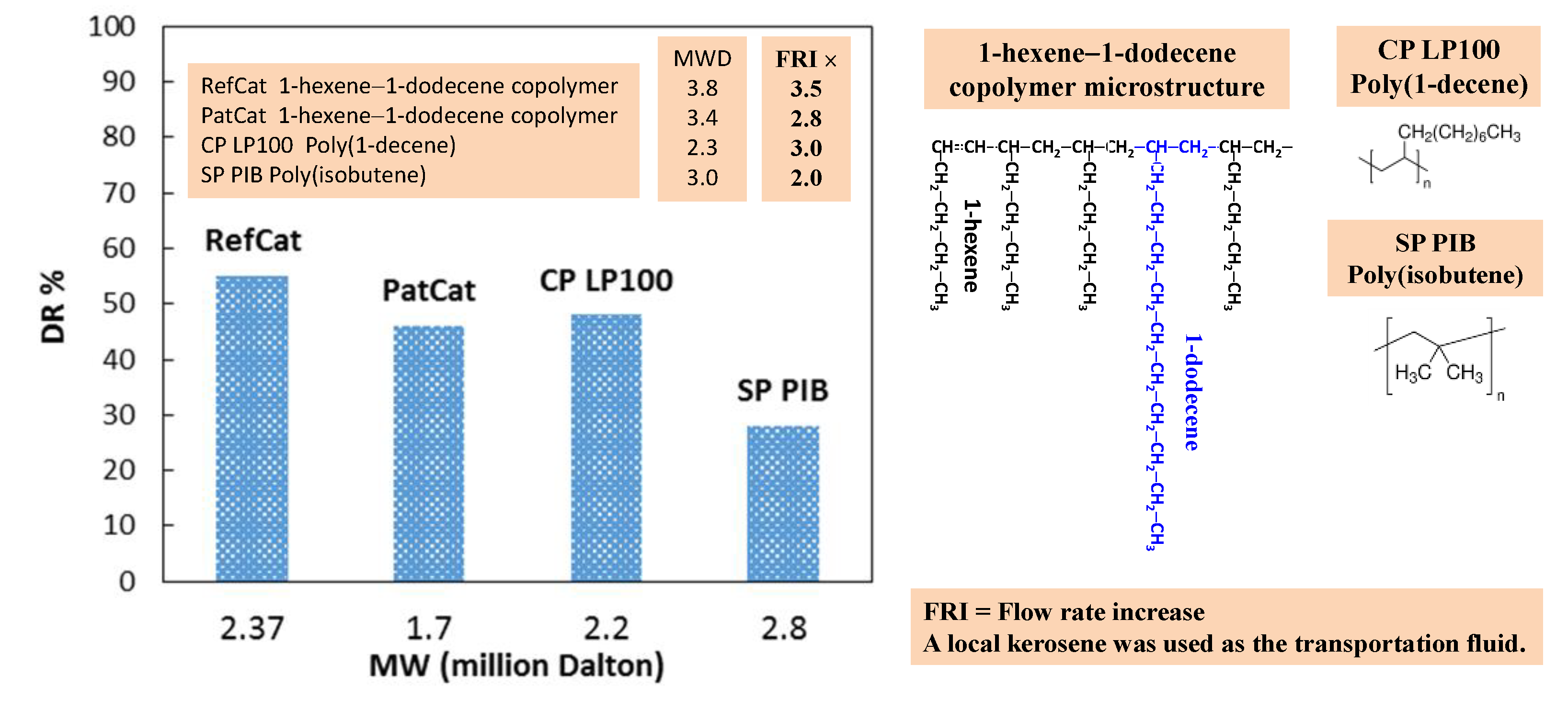
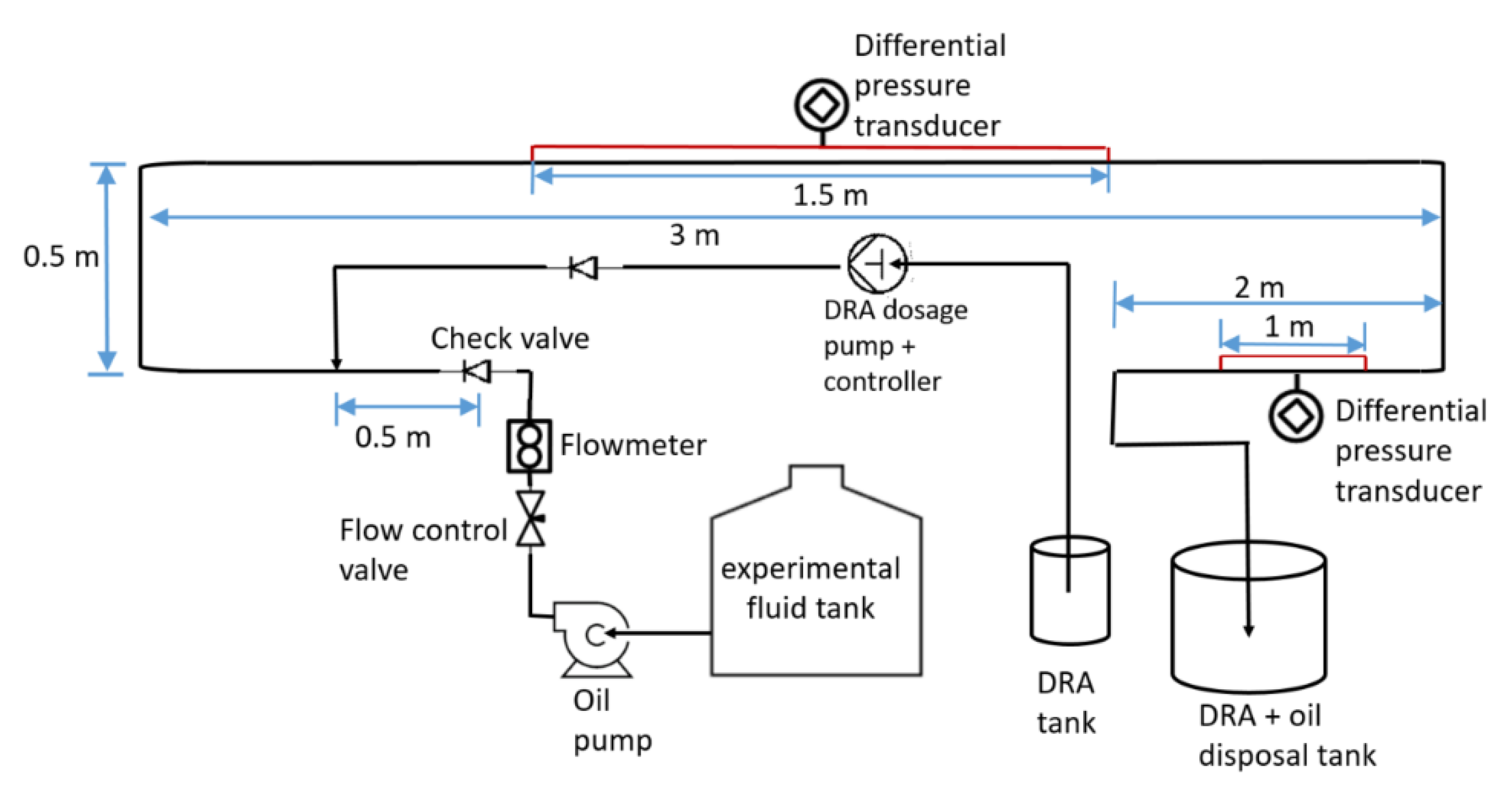
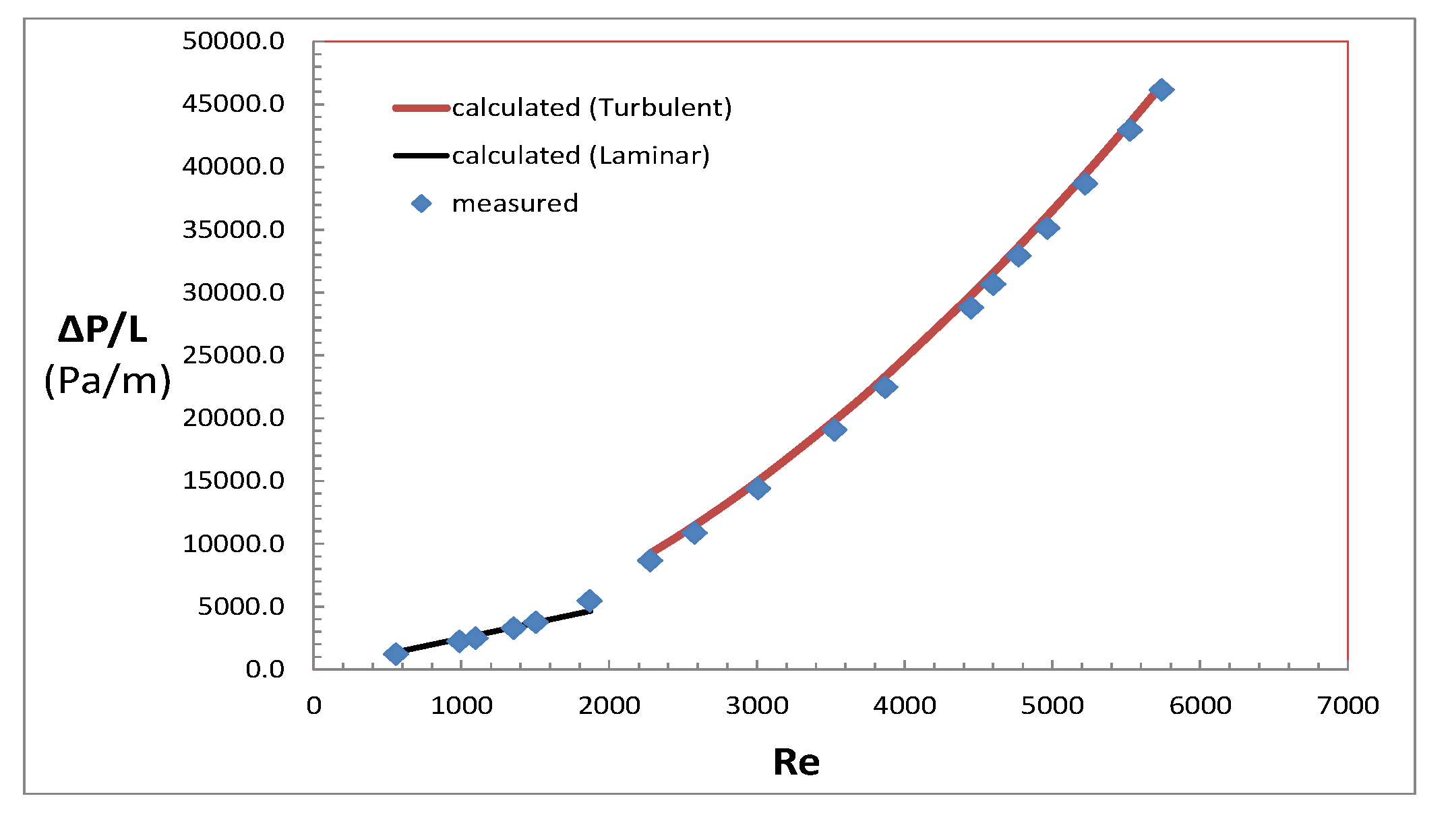
3. Materials and Methods
- ▪
- Dosage pump flow rate;
- ▪
- Flow rate of the experimental transportation fluid in the pipe line; and
- ▪
- The concentration of the DRP master solution.
4. Future Challenges and Research Niches
4.1. DRP Catalyst and Process Development
4.2. Surfactant-Based DRP Formulation Development
- ▪
- Viscofication-free pumpable and stable slurry;
- ▪
- Tendency to aggregate, gel, and layer with the passage of time;
- ▪
- Phase separation instability;
- ▪
- Transportation inconvenience;
- ▪
- Inadequate DRP dissolution within the turbulent mixing length; and
- ▪
- Difficulty in achieving very high extensional viscosity.
- ▪
- Surfactant properties;
- ▪
- DRP swelling;
- ▪
- Surface interaction;
- ▪
- Self-assembly of surfactants;
- ▪
- Surfactant micelle scission free energy;
- ▪
- Interaction and repulsive parameters; and
- ▪
- Surfactant critical micelle concentration.
4.3. Turbulent Mixing and Dispersion Models in Transportation Pipeline and DRP Injection
- ▪
- Inconsistent pumping and feeding;
- ▪
- Inadequate dispersion and mixing under turbulent fluid flow; and
- ▪
- Turbulence- and shear-induced mechanical degradation.
5. Conclusions and Perspectives
5.1. Conclusions
5.2. Perspectives
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crude Oil Production Statistics | Crude Oil | Enerdata. Available online: https://yearbook.enerdata.net/crude-oil/production-statistics.html.
- Oil Consumption by Region | Oil Energy Consumption Statistics | Enerdata. Available online: https://yearbook.enerdata.net/oil-products/world-oil-domestic-consumption-statistics.html.
- Mowla, D.; Naderi, A. Experimental study of drag reduction by a polymeric additive in slug two-phase flow of crude oil and air in horizontal pipes. Chem. Eng. Sci. 2006, 61, 1549–1554. [Google Scholar] [CrossRef]
- Hasan, S.W.; Ghannam, M.T.; Esmail, N. Heavy crude oil viscosity reduction and rheology for pipeline transportation. Fuel 2010, 89, 1095–1100. [Google Scholar] [CrossRef]
- Martínez-Palou, R.; Mosqueira, M.d.L.; Zapata-Rendón, B.; Mar-Juárez, E.; Bernal-Huicochea, C.; de la Cruz Clavel-López, J.; Aburto, J. Transportation of heavy and extra-heavy crude oil by pipeline: A review. J. Pet. Sci. Eng. 2011, 75, 274–282. [Google Scholar] [CrossRef]
- Alsurakji, I.; Al-Sarkhi, A.; Atiqullah, M.; Alhems, L.; El Nakla, M. Study of oil-soluble and water-soluble drag reducing polymers in multiphase flows. Can. J. Chem. Eng. 2018, 96, 1012–1028. [Google Scholar] [CrossRef]
- Al-Roomi, Y.; George, R.; Elgibaly, A.; Elkamel, A. Use of a novel surfactant for improving the transportability/transportation of heavy/viscous crude oils. J. Pet. Sci. Eng. 2004, 42, 235–243. [Google Scholar] [CrossRef]
- Toms, B.A. Some observations on the flow of linear polymer solutions through straight tubes at large Reynolds numbers. In Proceedings of the First International Congress on Rheology, Scheveningen, The Netherlands, 21–24 September 1948; Volume 2, pp. 135–141. [Google Scholar]
- Motier, J.F.; Carrier, A.M. Recent studies on polymer drag reduction in commercial pipelines. In Drag Reduction in Fluid Flows: Techniques for Friction Control; Sellin, R., Moses, R., Eds.; Ellis Horwood: West Sussex, UK, 1989; pp. 197–204. [Google Scholar]
- Nijs, L. New generation drag reducer. In 2nd Pipeline Technology; Ostend, B., Denys, R., Eds.; Elsevier: Amsterdam, The Netherlands, 1995; pp. 143–149. [Google Scholar]
- Dujmovich, T.; Gallegos, A. Drag reducers improve throughput, cut costs. Offshore 2005, 65, 1–4. [Google Scholar]
- Motier, J.F.; Chou, L.C.; Kommareddi, N.S. Commercial drag reduction past, present and future. In Proceedings of the ASME Fluids Engineering Division Summer Meeting; ASME: San Diego, CA, USA, 1996; pp. 229–234. [Google Scholar]
- Burger, E.D.; Munk, W.R.; Wahl, H.A. Flow increase in the trans alaska pipeline through use of a polymeric drag-reducing additive. J. Pet. Technol. 1982, 34, 377–386. [Google Scholar] [CrossRef]
- Wang, Y. Reynolds stress model for viscoelastic drag-reducing flow induced by polymer solution. Polymers 2019, 11, 1659. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, B.; Zakin, J.L.; Shi, H. Review on Drag Reduction and Its Heat Transfer by Additives. Adv. Mech. Eng. 2011, 478749. [Google Scholar] [CrossRef]
- Research Institute. Development of Novel Poly(Alpha-Olefin) Drag Reducing Agents for Saudi Aramco Oil and Refinery Products; Final Report CRP02253; King Fahd University of Petroleum & Minerals: Dhahran, Saudi Arabia, 2015. [Google Scholar]
- Alsurakji, I.H.; Al-Sarkhi, A.; Habib, M.; Badr, H.M. An experimental study on the performance of drag reducing polymers in single- and multiphase horizontal flow using particle image velocimetry. J. Energy Resour. Technol. 2018, 140, 052005. [Google Scholar] [CrossRef]
- Jing, X.; Liu, Y.; Li, W.; Xu, Y.; Huang, Z. Using water-miscible nonionic hydrophobic monomer associating HPAM as drag reducing agent. J. Appl. Polym. Sci. 2019, 136, 48362. [Google Scholar] [CrossRef]
- Jubran, B.A.; Zurigat, Y.H.; Goosen, M.F.A. Drag reducing agents in multiphase flow pipelines: Recent trends and future needs. Pet. Sci. Technol. 2005, 23, 1403–1424. [Google Scholar] [CrossRef]
- Manfield, P.D.; Lawrence, C.J.; Hewitt, G.F. Drag reduction with additives in multiphase flow: A literature survey. Multiph. Sci. Technol. 1999, 11, 197–221. [Google Scholar] [CrossRef]
- Al-Sarkhi, A. Drag reduction with polymers in gas-liquid/liquid-liquid flows in pipes: A literature review. J. Nat. Gas Sci. Eng. 2010, 2, 41–48. [Google Scholar] [CrossRef]
- Al-Yaari, M.; Soleimani, A.; Abu-Sharkh, B.; Al-Mubaiyedh, U.; Al-Sarkhi, A. Effect of drag reducing polymers on oil-water flow in a horizontal pipe. Int. J. Multiph. Flow 2009, 35, 516–524. [Google Scholar] [CrossRef]
- Warholic, M.D.; Massah, H.; Hanratty, T.J. Influence of drag reducing polymers on turbulence: Effects of Reynolds number, concentration, and mixing. Exp. Fluids 1999, 27, 461–472. [Google Scholar] [CrossRef]
- Dehm, D. Low Temperature Polymerization Process. U.S. Patent 4,384,089, 17 May 1983. [Google Scholar]
- Motier, J. Compositions for and Method of Reducing Hydrocarbon Fluid Friction Loss in Conduits. U.S. Patent 4,527,581, 9 July 1985. [Google Scholar]
- Smith, G.H.; Perry, D.C. Novel preparation of pure β-TiCl3 and its use in isoprene polymerization. J. Polym. Sci. Part A 1 Polym. Chem. 1969, 7, 707–713. [Google Scholar] [CrossRef]
- Sementa, L.; D’Amore, M.; Barone, V.; Busico, V.; Causa’, M. A quantum mechanical study of TiCl3 alpha, beta and gamma crystal phases: Geometry, electronic structure and magnetism. Phys. Chem. Chem. Phys. 2009, 11, 11264–11275. [Google Scholar] [CrossRef]
- Mack, P.; Decker, L.; Wallac, A. Method for the Preparation of Non-Crystalline Polymers of High Molecular Weight. U.S. Patent 4,358,572, 9 November 1982. [Google Scholar]
- Mack, P. Polymerization Process for Drag Reducing Substances. U.S. Patent 4493903 A, 15 January 1985. [Google Scholar]
- Mack, P. Catalyst and Method for Preparation of Drag Reducing Substances. U.S. Patent 4493904 A, 15 January 1985. [Google Scholar]
- Marullo, G.; Baroni, A.; Maffezzoni, U. Three Component Catalyst for Polymerization of 1-Olefins Containing Titanium Halide, Aluminum Alkyl, and Pyridine. U.S. Patent 3,139,418, 30 June 1964. [Google Scholar]
- Mack, P. Polymerization Process for Drag Reducing Substances. U.S. Patent 4,433,123, 21 February 1984. [Google Scholar]
- Eaton, G.; Monahan, M.; Tipton, R. Methods for Forming Amorphous Ultra-High Molecular Weight Polyalphaolefin Drag Reducing Agents. U.S. Patent 6,015,779, 18 Janurary 2000. [Google Scholar]
- Eaton, G.; Monahan, M.; Tipton, R. Methods for Forming Amorphous Ultra-High Molecular Weight Polyalphaolefin Drag Reducing Agents Using a Halocarbon. U.S. Patent 6,162,773, 23 August 2000. [Google Scholar]
- Eaton, G.; Monahan, M.; Tipton, R. Methods for forming Amorphous Ultra-High Molecular Weight Polyalphaolefin Drag Reducing Agents Using Non-Metallocene and Alkylaluminoxane. U.S. Patent 6,242,395, 5 June 2001. [Google Scholar]
- Eaton, G.; Monahan, M.; Ebert, A.; Tipton, R.; Baralt, E. Methods for Forming Amorphous Ultra-High Molecular Weight Polyolefins for Use as Drag Reducing Agents. U.S. Patent 6,730,750 B2, 4 May 2004. [Google Scholar]
- Eaton, G.; Monahan, M.; Tipton, R. Methods for Forming Amorphous Ultra-High Molecular Weight Polyolefins and Drag Reducing Compositions Comprising Amorphous Ultra-High Molecular Weight Polyolefins. U.S. Patent 6,730,752, 4 May 2004. [Google Scholar]
- Atiqullah, M.; Al-Sarkhi, A.; Al-Thenayan, F.; Al-Malki, A.; Xu, W.; Hossaen, A. Catalyst Composition for Making Ultrahigh Molecular Weight Poly(Alphaolefin) Drag Reducing Agents. U.S. Patent 10,301,410 B2, 28 May 2019. [Google Scholar]
- Atiqullah, M.; Al-Sarkhi, A.; Al-Thenayan, F.; Al-Malki, A.; Xu, W.; Hossaen, A.A. Catalyst Composition and a Process for Making Ultrahigh Molecular Weight Poly(Alphaolefin) Drag Reducing Agents. U.S. Patent 9,969,826 B2, 15 May 2018. [Google Scholar]
- Atiqullah, M.; Al-Sarkhi, A.; Al-Thenayan, F.; Al-Malki, A.; Xu, W.; Hossaen, A. Method of Reducing Drag in a Conduit. U.S. Patent 10,287,374 B2, 14 May 2019. [Google Scholar]
- Bochmann, M. The chemistry of catalyst activation: The case of group 4 polymerization catalysts. Organometallics 2010, 29, 4711–4740. [Google Scholar] [CrossRef]
- Atiqullah, M.; Anantawaraskul, S.; Emwas, A.-H.M.; Al-Harthi, M.A.; Hussain, I.; Ul-Hamid, A.; Hossaen, A. Silica-supported (nBuCp)2ZrCl2: Effect of catalyst active center distribution on ethylene-1-hexene copolymerization. Polym. Int. 2014, 63, 955–972. [Google Scholar] [CrossRef]
- Ray Smith, B.E. Polymers: A Property Database, 2nd ed.; CRC Press, Taylor & Francis Group: New York, NY, USA, 2009; ISBN 978-0-8493-3940-0. [Google Scholar]
- Atiqullah, M.; Adamu, S.; Emwas, A.H.M. UHMW Ziegler–Natta polyethylene: Synthesis, crystallization, and melt behavior. J. Taiwan Inst. Chem. Eng. 2017, 76, 141–155. [Google Scholar] [CrossRef]
- Reding, F.P. Transitions in aliphatic olefin polymers. J. Polym. Sci. 1956, 21, 547–549. [Google Scholar] [CrossRef]
- Gordon, M.; Taylor, J.S. Ideal copolymers and the second-order transitions of synthetic rubbers. I. Non-crystalline copolymers. J. Appl. Chem. 2007, 2, 493–500. [Google Scholar] [CrossRef]
- Rodriguez, F.; Cohen, C.; Ober, C.K.; Archer, L.A. Principles of Polymer Systems, 6th ed.; Taylor & Francis Group: New York, NY, USA, 2014; ISBN 9781482223781. [Google Scholar]
- Elger, D.F.; LeBret, B.A.; Crowe, C.T.; Clayton, T.; Roberson, J.A. Engineering Fluid Mechanics, 11th ed.; Wiley: Hoboken, NJ, USA, 2016; ISBN 9781118880685. [Google Scholar]
- Swamee, P.; Jain, A. Explicit eqations for pipe-flow problems. ASCE J. Hydraul. Div. 1976, 102, 657–664. [Google Scholar]
- Subhash, N.S.; Ahmed, K.; Yunxu, Z. Drag reduction characteristics in straight and coiled tubing—An experimental study. J. Pet. Sci. Eng. 2006, 53, 179–188. [Google Scholar]
- Al-Wahaibi, T.; Smith, M.; Angeli, P. Effect of drag reducing polymers on horizontal oil-water flows. J. Pet. Sci. Eng. 2007, 57, 334–346. [Google Scholar] [CrossRef]
- Li, B.; Dong, G.; Zhang, C. Storage stability and solubility of poly(urea-formaldehyde) microcapsules containing α-olefin drag reducing polymer. J. Appl. Polym. Sci. 2011, 122, 1450–1456. [Google Scholar] [CrossRef]
- Wang, H.; Tang, X.; Eike, D.M.; Larson, R.G.; Koenig, P.H. Scission free energies for wormlike surfactant micelles: Development of a simulation protocol, application, and validation for personal care formulations. Langmuir 2018, 34, 1564–1573. [Google Scholar] [CrossRef]
- Hara, S.; Ii, R.; Tsukahara, T.; Kawaguchi, Y. Analysis of an organized turbulent structure using a pattern recognition technique in a drag-reducing surfactant solution flow. Int. J. Heat Fluid Flow 2017, 63, 56–66. [Google Scholar] [CrossRef]
- Al-Sarkhi, A. Effect of mixing on frictional loss reduction by drag reducing polymer in annular horizontal two-phase flows. Int. J. Multiph. Flow 2012, 39, 186–192. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atiqullah, M.; Al-Sarkhi, A.H.; Al-Thenayan, F.M.; Al-Malki, A.R.; Alasiri, H.S. Energy-Saving UHMW Polymeric Flow Aids: Catalyst and Polymerization Process Development. Catalysts 2019, 9, 1002. https://doi.org/10.3390/catal9121002
Atiqullah M, Al-Sarkhi AH, Al-Thenayan FM, Al-Malki AR, Alasiri HS. Energy-Saving UHMW Polymeric Flow Aids: Catalyst and Polymerization Process Development. Catalysts. 2019; 9(12):1002. https://doi.org/10.3390/catal9121002
Chicago/Turabian StyleAtiqullah, Muhammad, Abdelsalam H. Al-Sarkhi, Faisal M. Al-Thenayan, Abdullah R. Al-Malki, and Hassan S. Alasiri. 2019. "Energy-Saving UHMW Polymeric Flow Aids: Catalyst and Polymerization Process Development" Catalysts 9, no. 12: 1002. https://doi.org/10.3390/catal9121002
APA StyleAtiqullah, M., Al-Sarkhi, A. H., Al-Thenayan, F. M., Al-Malki, A. R., & Alasiri, H. S. (2019). Energy-Saving UHMW Polymeric Flow Aids: Catalyst and Polymerization Process Development. Catalysts, 9(12), 1002. https://doi.org/10.3390/catal9121002






