Surfactant Imprinting Hyperactivated Immobilized Lipase as Efficient Biocatalyst for Biodiesel Production from Waste Cooking Oil
Abstract
1. Introduction
2. Results and Discussion
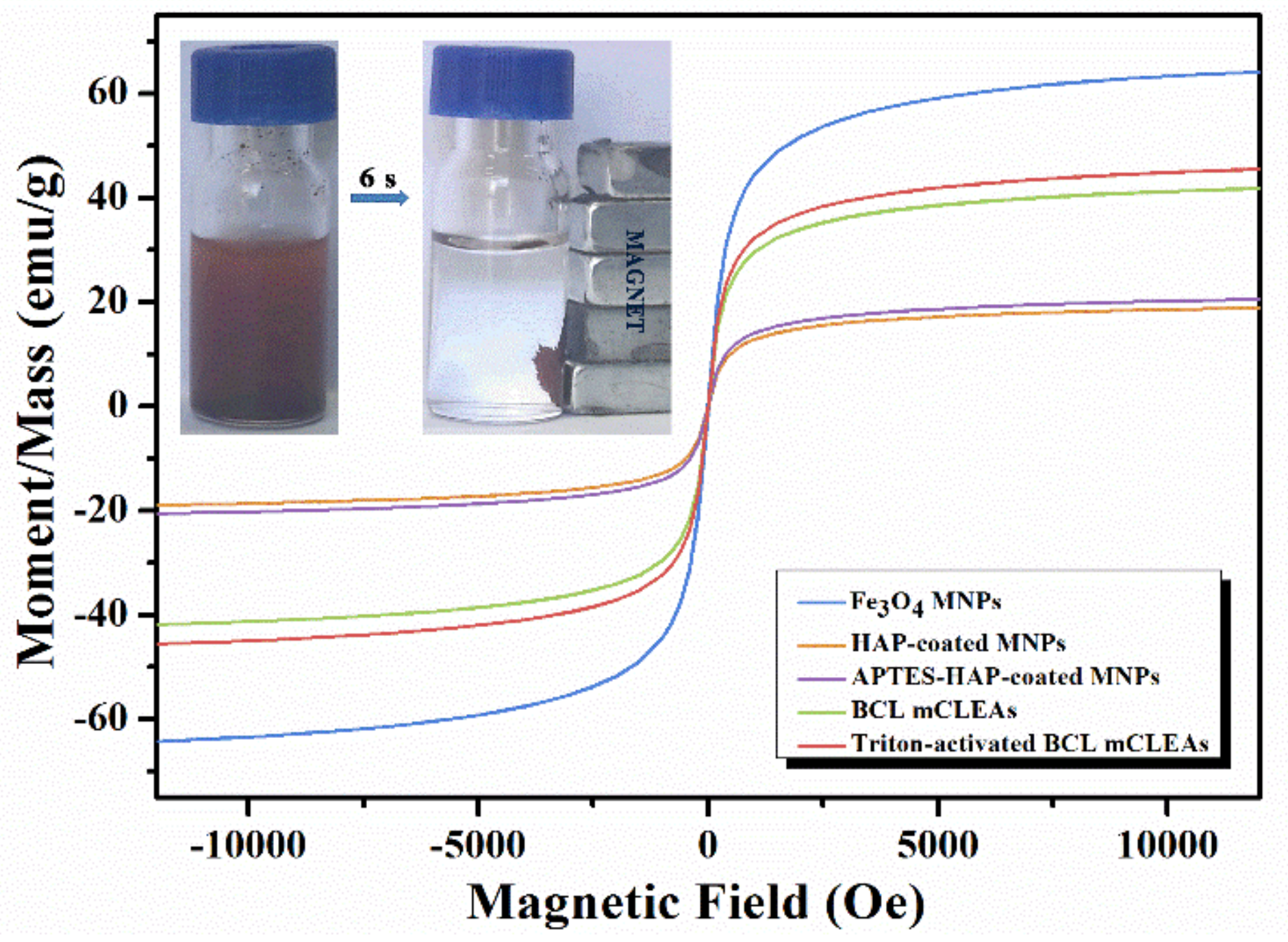
2.1. Preparation and Characterization of Immobilized Lipase
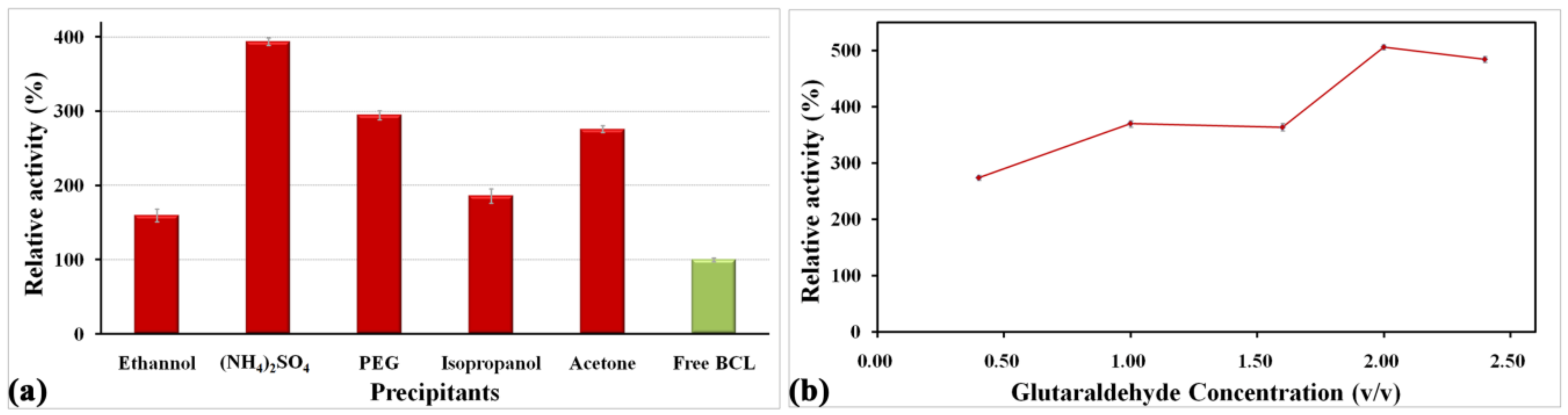
2.2. Optimization of the Immobilization Conditions
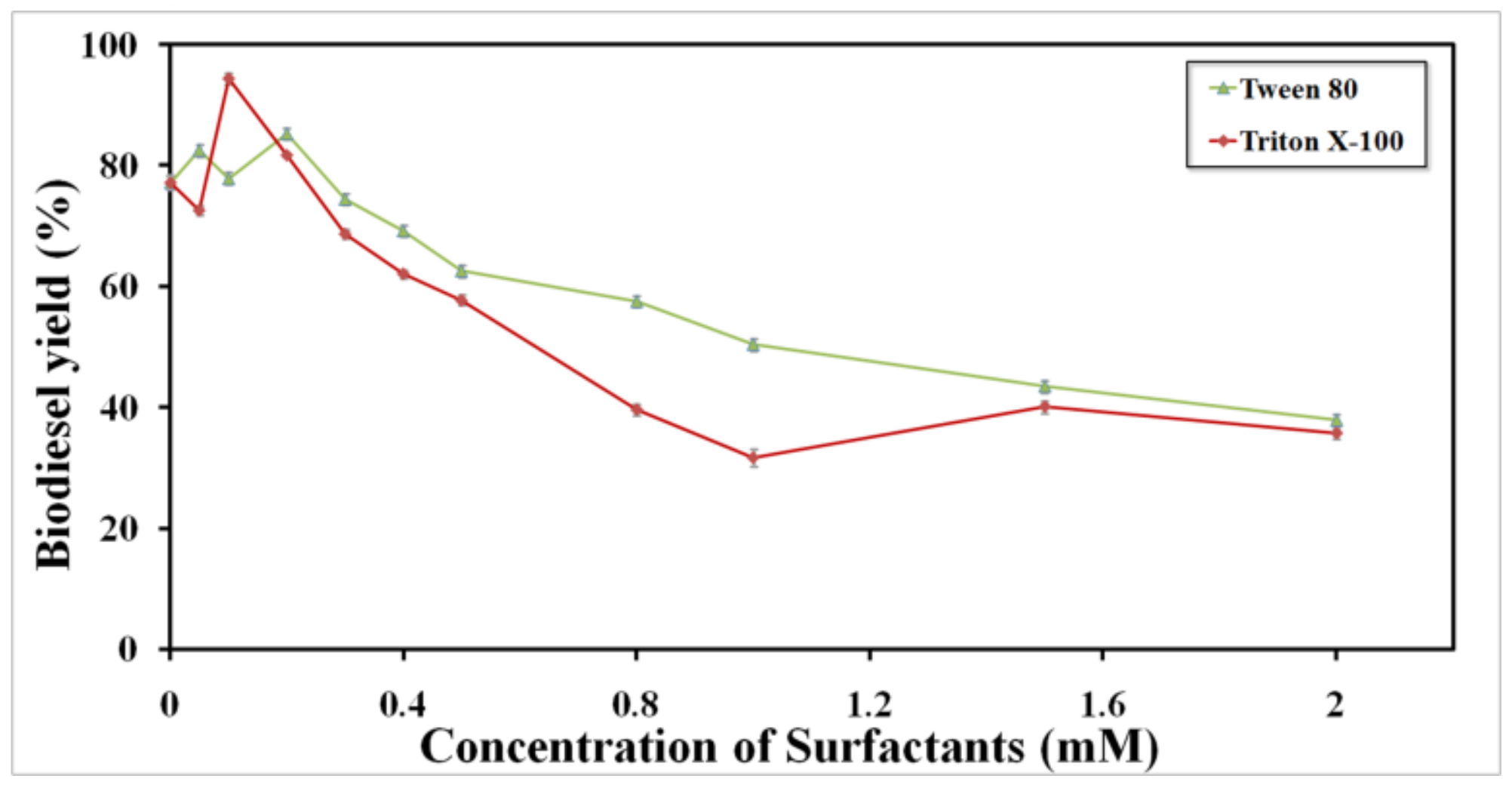
2.3. Hyperactivation of BCL mCLEAs with Surfactants
2.4. Biodiesel Production
2.5. Reusability
3. Materials and Methods
3.1. Materials
3.2. Preparation of Magnetic Support
3.3. Lipase Immobilization
3.4. Characterization
3.5. Activity Assay
3.6. Enzymatic Transesterification for Biodiesel Production
3.7. Analytical Methods
3.8. Reusability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mahmudul, H.M.; Hagos, F.Y.; Mamat, R.; AbdulAdam, A.; Ishak, W.F.W.; Alenezi, R. Production, characterization and performance of biodiesel as an alternative fuel in diesel engines-a review. Renew. Sustain. Energy Rev. 2017, 72, 497–509. [Google Scholar] [CrossRef]
- Laesecke, J.; Ellis, N.; Kirchen, P. Production, analysis and combustion characterizationof biomass fast pyrolysis oil-biodiesel blends for use in diesel engines. Fuel 2017, 199, 346–357. [Google Scholar] [CrossRef]
- Jamil, F.; Alhaj, L.; Almuhtaseb, A.H.; Baawain, M.; Rashid, U.; Ahmad, M.N.M. Current scenario of catalysts for biodieselproduction: A critical review. Rev. Chem. Eng. 2018, 34, 267–297. [Google Scholar] [CrossRef]
- Banković-Ilić, I.B.; Stamenković, O.S.; Veljković, V.B. Biodiesel production fromnon-edible plant oils. Renew. Sustain. Energy Rev. 2012, 16, 3621–3647. [Google Scholar] [CrossRef]
- Hama, S.; Noda, H.; Kondo, A. How lipase technology contributes to evolution ofbiodiesel production using multiple feedstocks. Curr. Opin. Biotechnol. 2018, 50, 57–64. [Google Scholar] [CrossRef]
- Gebremariam, S.N.; Marchetti, J.M. Economics of biodiesel production: Review. Energy Convers. Manag. 2018, 168, 74–84. [Google Scholar] [CrossRef]
- Moazeni, F.; Chen, Y.-C.; Zhang, G. Enzymatic transesterification for biodiesel production from usedcooking oil, a review. J. Clean. Prod. 2019, 216, 117–128. [Google Scholar] [CrossRef]
- Lopresto, C.; Naccarato, S.; Albo, L.; De Paola, M.; Chakraborty, S.; Curcio, S.; Calabro, V. Enzymatic transesterification of waste vegetable oil to producebiodiesel. Ecotoxicol. Environ. Saf. 2015, 121, 229–235. [Google Scholar] [CrossRef]
- Chhetri, A.; Watts, K.; Islam, M. Waste cooking oil as an alternate feedstock forbiodiesel production. Energies 2008, 1, 3–18. [Google Scholar] [CrossRef]
- Sabudak, T.; Yildiz, M. Biodiesel production from waste frying oils and itsquality control. Waste Manag. 2010, 30, 799–803. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, O.K.; Lee, E.Y. Nano-immobilized biocatalysts for biodiesel production from renewable and sustainable resources. Catalysts 2018, 8, 68. [Google Scholar] [CrossRef]
- Facin, B.R.; Melchiors, M.S.; Valério, A.; Oliveira, J.V.; Oliveira, D. Driving immobilized lipases as biocatalysts: 10 years state of the art and future prospects. Ind. Eng. Chem. Res. 2019, 58, 5358–5378. [Google Scholar] [CrossRef]
- Filho, D.G.; Silva, A.G.; Guidini, C.Z. Lipases: Sources, immobilization methods, and industrial applications. Appl. Microbiol. Biotechnol. 2019, 103, 7399–7423. [Google Scholar] [CrossRef]
- Xu, M.-Q.; Wang, S.-S.; Li, L.-N.; Gao, J.; Zhang, Y.-W. Combined cross-linked enzyme aggregatesas biocatalysts. Catalysts 2018, 8, 460. [Google Scholar] [CrossRef]
- Sheldon, R.A. CLEAs, combi-CLEAs and ‘smart’ magnetic CLEAs: Biocatalysis in a bio-based economy. Catalysts 2019, 9, 261. [Google Scholar] [CrossRef]
- Liu, D.-M.; Chen, J.; Shi, Y.-P. Advances on methods and easy separated support materials forenzymes immobilization. TrAC-Trends Anal. Chem. 2018, 102, 332–342. [Google Scholar] [CrossRef]
- Izadia, A.; Meshkinia, A.; Entezari, M.H. Mesoporous superparamagnetic hydroxyapatite nanocomposite: Amultifunctional platform for synergistic targeted chemo-magnetotherapy. Mater. Sci. Eng. C Mater. 2019, 101, 27–41. [Google Scholar] [CrossRef]
- Sánchez, D.A.; Tonetto, G.M.; Ferreira, M.L. Burkholderia cepacia lipase: A versatile catalyst in synthesisreactions. Biotechnol. Bioeng. 2018, 115, 6–24. [Google Scholar] [CrossRef]
- Barbe, S.; Lafaquiere, V.; Guieysse, D.; Monsan, P.; Remaud-Siméon, M.; Andre, I. Insights into lid movements of Burkholderia cepacialipase inferred from molecular dynamics simulations. Proteins 2009, 77, 509–523. [Google Scholar] [CrossRef]
- Gabriele, F.; Spreti, N.; Giacco, T.D.; Germani, R.; Tiecco, M. Effect of surfactant structure on the superactivity of Candida rugosalipase. Langmuir 2018, 34, 11510–11517. [Google Scholar] [CrossRef]
- Zhang, N.; Gao, T.; Wang, Y.; Wang, Z.; Zhang, P.; Liu, J. Environmental pH-controlledloading and release of protein on mesoporous hydroxyapatite nanoparticles forbone tissue engineering. Mater. Sci. Eng. C Mater. 2015, 46, 158–165. [Google Scholar] [CrossRef]
- Kannan, S.; Marudhamuthu, M. Development of chitin cross-linked enzyme aggregates of L-methioninase for upgraded activity, permanence andapplication as efficient therapeutic formulations. Int. J. Biol. Macromol. 2019, 141, 218–231. [Google Scholar] [CrossRef]
- Shaarani, S.M.; Jahim, J.M.; Rahman, R.A.; Idris, A.; Murad, A.M.A.; Illias, R.M. Silanized maghemite for cross-linked enzyme aggregates of recombinantxylanase from Trichoderma reesei. J. Mol. Catal. B Enzym. 2016, 133, 65–76. [Google Scholar] [CrossRef]
- Rehman, S.; Bhatti, H.N.; Bilal, M.; Asgher, M. Cross-linked enzyme aggregates (CLEAs) of Pencilluim notatum lipaseenzymewith improved activity, stability and reusabilitycharacteristics. Int. J. Biol. Macromol. 2016, 91, 1161–1169. [Google Scholar] [CrossRef]
- Cui, J.; Lin, T.; Feng, Y.; Tan, Z.; Jia, S. Preparation of spherical cross-linked lipaseaggregates with improved activity, stabilityand reusability characteristic in water-in-ionicliquid microemulsion. J. Chem. Technol. Biotechnol. 2017, 92, 1785–1793. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Kiyota, Y.; Miyazaki, M. Techniques for preparation of cross-linked enzyme aggregates and their applications in bioconversions. Catalysts 2018, 8, 174. [Google Scholar] [CrossRef]
- Bañó, M.C.; González-Navarro, H.; Abad, C. Long-chain fatty acyl-CoA esters induce lipase activation in the absence of a water-lipid interface. BBA Mol. Cell Biol. Lipids 2003, 1632, 55–61. [Google Scholar] [CrossRef]
- Delorme, V.; Dhouib, R.; Canaan, S.; Fotiadu, F.; Carriere, F.; Cavalier, J.F. Effects of surfactants on lipase structure, activity, and inhibition. Pharm. Res. 2011, 28, 1831–1842. [Google Scholar] [CrossRef]
- Mukherjee, J.; Gupta, M.N. Molecular bioimprinting of lipases with surfactantsand its functional consequences in low water media. Int. J. Biol. Macromol. 2015, 81, 544–551. [Google Scholar] [CrossRef]
- Zhang, W.-W.; Yang, X.-L.; Jia, J.Q.; Wang, N.; Hu, C.-L.; Yu, X.-Q. Surfactant-activated magnetic cross-linked enzyme aggregates (magnetic CLEAs) of Thermomyces lanugunosus lipase for biodiesel production. J. Mol. Catal. B Enzym. 2015, 115, 83–89. [Google Scholar] [CrossRef]
- Lee, H.V.; Yunus, R.; Juan, J.C.; Taufiq-Yap, Y.H. Process optimization design forjatropha-based biodiesel production using response surface methodology. Fuel Process. Technol. 2011, 92, 2420–2428. [Google Scholar] [CrossRef]
- Dong, T.; Zhao, L.; Huang, Y.; Tan, X. Preparation of cross-linked aggregates ofaminoacylase from Aspergillus melleus by using bovine serum albumin as aninert additive. Bioresour. Technol. 2010, 101, 6569–6571. [Google Scholar] [CrossRef]
- Rehm, S.; Trodler, P.; Pleiss, J. Solvent-induced lid opening in lipases: A molecular dynamics study. Protein Sci. 2010, 19, 2122–2130. [Google Scholar] [CrossRef]
- Xie, W.; Huang, M. Immobilization of Candida rugosa lipase onto graphene oxide Fe3O4nanocomposite: Characterization and application for biodiesel production. Energy Convers. Manag. 2018, 159, 42–53. [Google Scholar] [CrossRef]
- Xie, W.; Zang, X. Covalent immobilization of lipase onto aminopropyl-functionalizedhydroxyapatite-encapsulated-γ-Fe2O3 nanoparticles: A magneticbiocatalyst for interesterification of soybean oil. Food Chem. 2017, 227, 397–403. [Google Scholar] [CrossRef]
- Zhang, W.-W.; Yang, H.-X.; Liu, W.-Y.; Wang, N.; Yu, X.-Q. Improved performance of magnetic cross-linked lipase aggregates by interfacial activation: A robustand magnetically recyclable biocatalyst for transesterification of Jatropha oil. Molecules 2017, 22, 2157. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Zhang, W. Surfactant Imprinting Hyperactivated Immobilized Lipase as Efficient Biocatalyst for Biodiesel Production from Waste Cooking Oil. Catalysts 2019, 9, 914. https://doi.org/10.3390/catal9110914
Yang H, Zhang W. Surfactant Imprinting Hyperactivated Immobilized Lipase as Efficient Biocatalyst for Biodiesel Production from Waste Cooking Oil. Catalysts. 2019; 9(11):914. https://doi.org/10.3390/catal9110914
Chicago/Turabian StyleYang, Huixia, and Weiwei Zhang. 2019. "Surfactant Imprinting Hyperactivated Immobilized Lipase as Efficient Biocatalyst for Biodiesel Production from Waste Cooking Oil" Catalysts 9, no. 11: 914. https://doi.org/10.3390/catal9110914
APA StyleYang, H., & Zhang, W. (2019). Surfactant Imprinting Hyperactivated Immobilized Lipase as Efficient Biocatalyst for Biodiesel Production from Waste Cooking Oil. Catalysts, 9(11), 914. https://doi.org/10.3390/catal9110914



