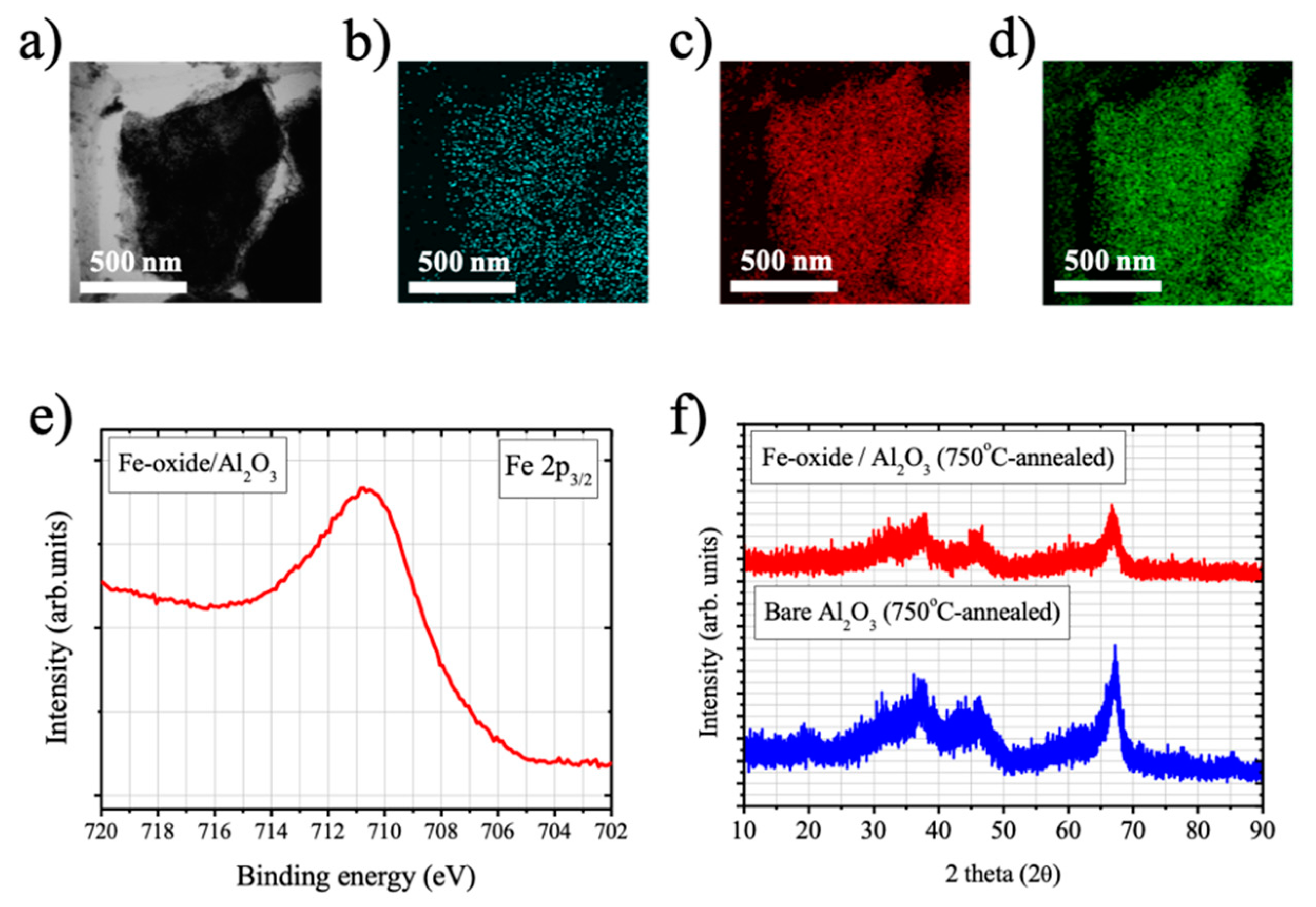
2.1. Characterization Results
A STEM image and STEM-EDS mapping images of Fe, Al, and O elements were measured at the sample position of Fe-oxide/Al
2O
3 particles and they indicate that the Fe atoms were evenly distributed on Al
2O
3 particles (
Figure 1a–d).
Figure 1e shows a Fe 2p
3/2 core-level X-ray photoelectron spectrum (XPS) of Fe-oxide/Al
2O
3 and a broad peak extended from 715 to 706 eV was found in the Fe 2p
3/2 binding energy region, indicating that distributed Fe atoms in oxidized states. Binding energy position of Fe 2p
3/2 XPS peak can differ upon the oxidation state of Fe-oxides, e.g., Fe
3O
4 (~710.4 eV) and α-Fe
3O
4 (~710.4 eV). However, the inherent complexity and broadness of Fe 2p core-level XPS spectrum results from the existence of multiple elements due to the final state relaxation effects prevent us from determining the exact oxidation state of Fe-oxide. It is likely that Fe-oxide consisted of a complex mixture of various oxidized Fe-oxide, such as Fe
3O
4 and α-Fe
3O
4, which was indicated by our previous analysis on the Fe-oxide/Al
2O
3 sample that was prepared via a similar TR-CVD and post-annealing process by means of Mössbauer spectroscopy [
24].
The crystallinities of bare Al
2O
3 and Fe-oxide/Al
2O
3 samples were also studied by measuring their XRD patterns (
Figure 1f). A pronounce XRD peak at 67° was observed with broad feature consist of multiple peaks in the 2θ range of 20~50° for the case of bare Al
2O
3, which can be attributed to χ-phase of Al
2O
3 [
24]. No additional XRD peaks appeared upon the Fe-oxide deposition and subsequent 750 °C-annealing, and the Fe-oxide/Al
2O
3 sample also exhibited very similar XRD patterns with bare Al
2O
3, which can be attributed to the small size of Fe-oxide moiety existed in Fe-oxide/Al
2O
3. However, it is noticeable that the intensity of the XRD peak at 67° decreased upon Fe-oxide deposition, followed by 750 °C-annealing, which implies that Fe-oxide deposited on Al
2O
3 can disturb the lattice structure of Al
2O
3 during the annealing process (750 °C, 8 h).
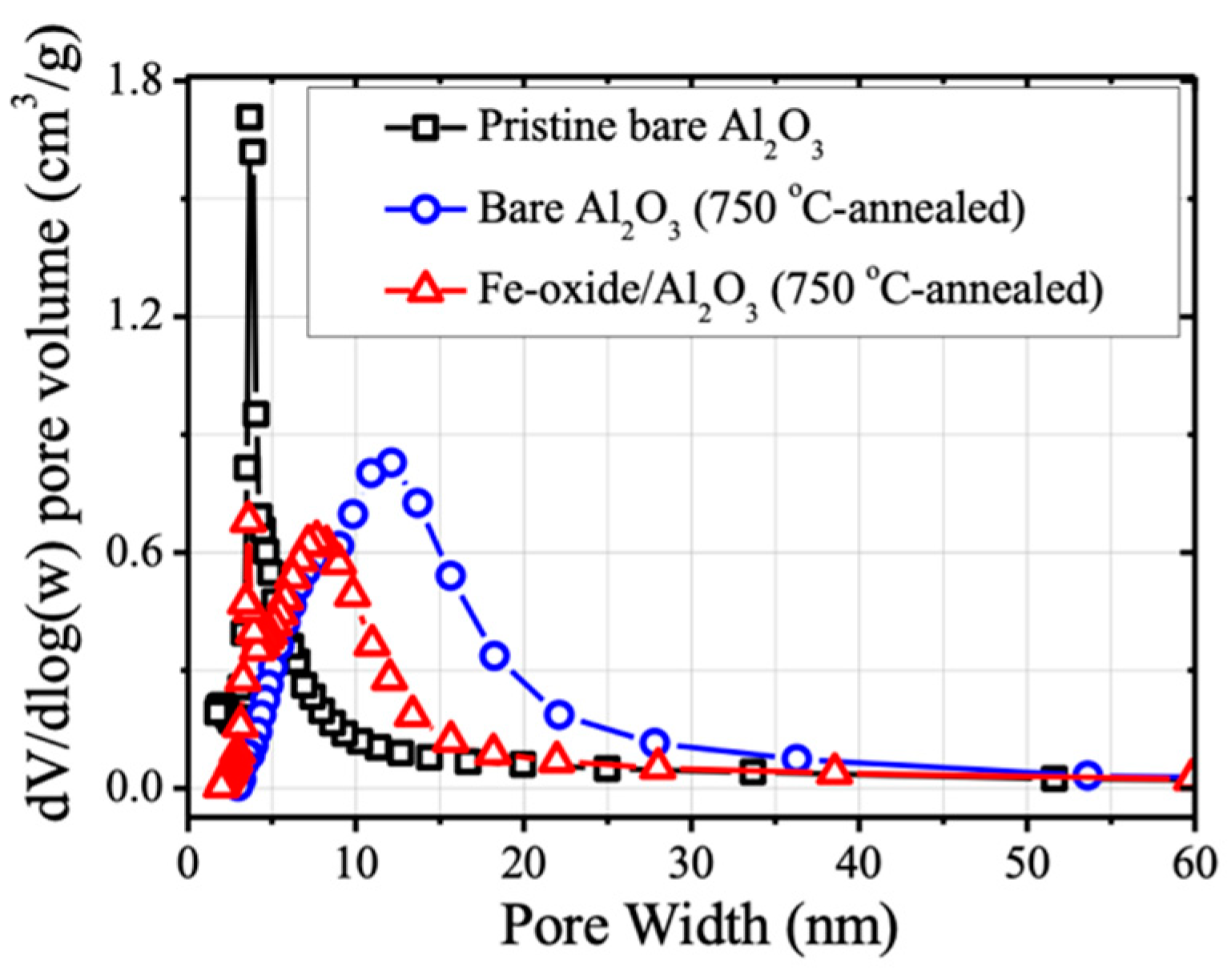
Figure 2 shows the pore size distributions of pristine Al
2O
3 (none-annealed), bare Al
2O
3 (750 °C annealed), and Fe-oxide/Al
2O
3 (750 °C annealed), and
Table 1 summarizes their BET specific surface areas and average pore diameters. The pore size distribution curve of pristine Al
2O
3 underwent significant changes upon 750 °C-annealing. The average pore diameter Al
2O
3 increased from 4.38 nm to 9.56 nm, accompanied with a decrease of surface area of Al
2O
3 (from 334.2 m
2/g to 138.6 m
2/g). These results indicated that the porous structure of pristine Al
2O
3 was significantly ruptured upon 750 °C-annealing. Similar phenomenon was observed when the Fe-oxide were deposited on Al
2O
3; an increase of average pore diameter from 4.38 to 6.46 nm and decrease of surface area from 334.2 to 171.8 m
2/g upon 750 °C-annealing were observed. However, the structural changes due to collapsing of the original porous structure of Al
2O
3 upon 750 °C annealing were less significant when Fe-oxide was deposited on Al
2O
3, resulting in higher BET surface area and smaller average pore diameter of Fe-oxide/Al
2O
3 (750 °C-annealed) than bare Al
2O
3 (750 °C-annealed) (
Figure 2 and
Table 1). Those results implied that the thermal stability of the porous structure of Al
2O
3 particle can be improved in the presence of TR-CVD deposited Fe-oxide on Al
2O
3, which can be attributed to the interaction between Fe-oxide and Al
2O
3.
Those are somewhat different situations from our previous studies, where the formation of crystalline Fe-oxide nanoparticles was observed on mesoporous materials via a TR-CVD of Fe-oxide and subsequent thermal annealing [
25]. In the previous case, the original pore structure of mesoporous SiO
2 was mostly maintained, so that TR-CVD deposited Fe-oxide can be accumulated inside a specific pore generating crystalline Fe-oxide nanoparticles during the post-annealing process [
25]. Additionally, it resulted in a decrease of pore volume with a specific size (~10 nm) of which size was correlated with the diameter (~10 nm) of crystalline Fe-oxide nanoparticles directly measured by the size of Fe-oxide lattice fringes on TEM images [
25].
In contrast, in this case of study, the mesoporous structure Al
2O
3 underwent significant changes during the annealing process. Thus, it was likely that the accumulation of Fe-oxide inside a specific size of pore originally existed on pristine Al
2O
3 took place. Instead, Fe-oxide might accumulate inside a newly formed pores (>10 nm) of the 750 °C-annealed sample. However, no indication of the formation of Fe-oxide nanoparticles (>10 nm) was found by HRTEM and XRD analyses, although the even distribution of Fe atoms on Al
2O
3 was confirmed by STEM-EDS analysis (
Figure 1). It is worth mentioning that the formation of Fe-oxide nanoparticles (~10 nm) was confirmed by TEM and XRD analyses in our previous studies [
25]. Therefore, we conclude that Fe-oxide impregnation into mesoporous Al
2O
3 took place, rather than specific sized Fe-oxide nanoparticles forming by accumulation of temperature regulated chemical vapor deposition (TR-CVD) deposited Fe-oxide inside the pores of Al
2O
3.
2.2. DMMP Exposure at Room Temperature
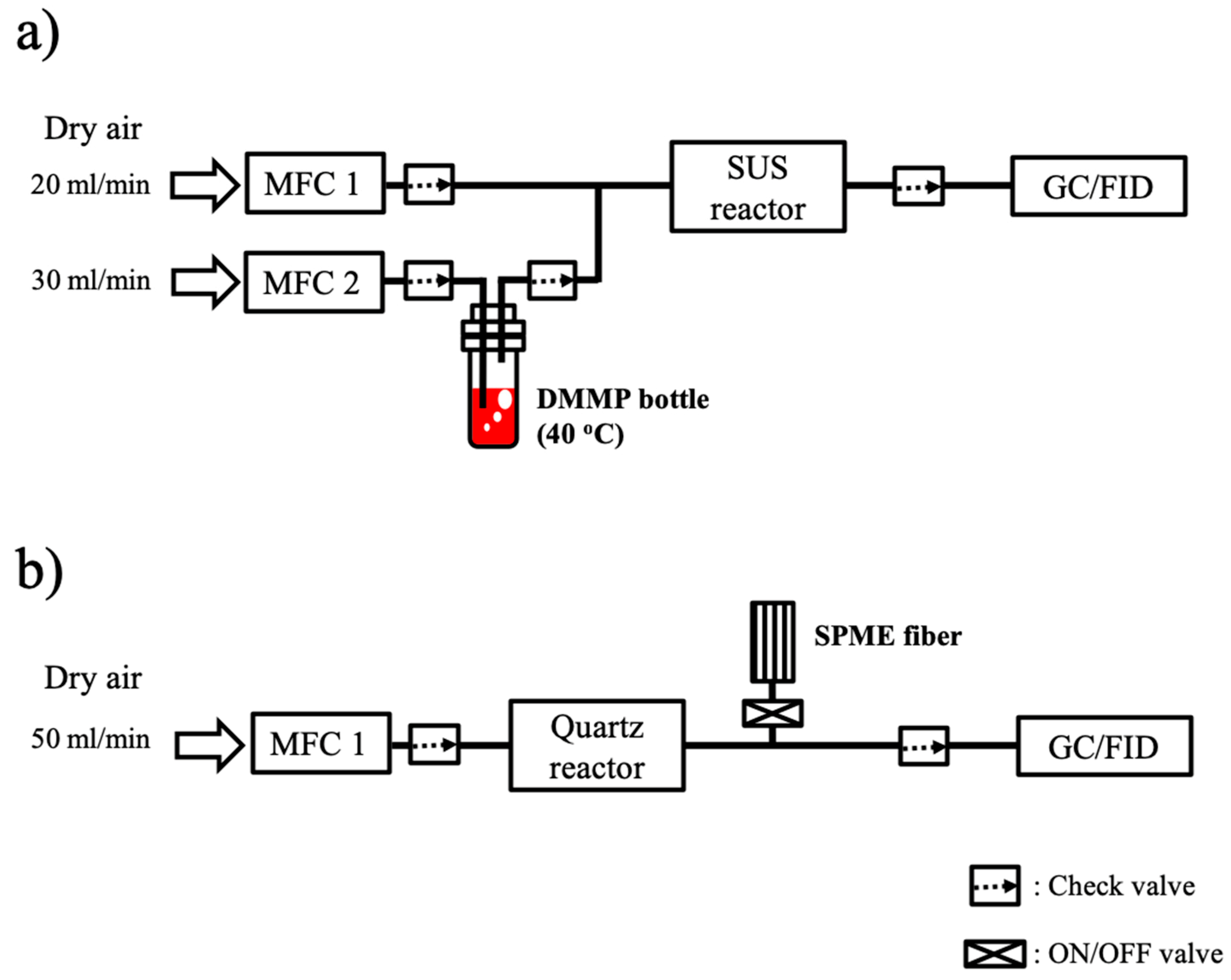
DMMP containing dry air (total flow of 50 mL/min) flowed into the SUS reactor that was filled with 2.0 g of each sample (bare Al
2O
3 and Fe-oxide/Al
2O
3 particles) at room temperature and the gas that passed through the reactor was analyzed by online GC. Two peaks were detected by online GC at different retention times, one centered at ~4.46 min and the other one at ~2.27 min, and they corresponded to the DMMP and CO
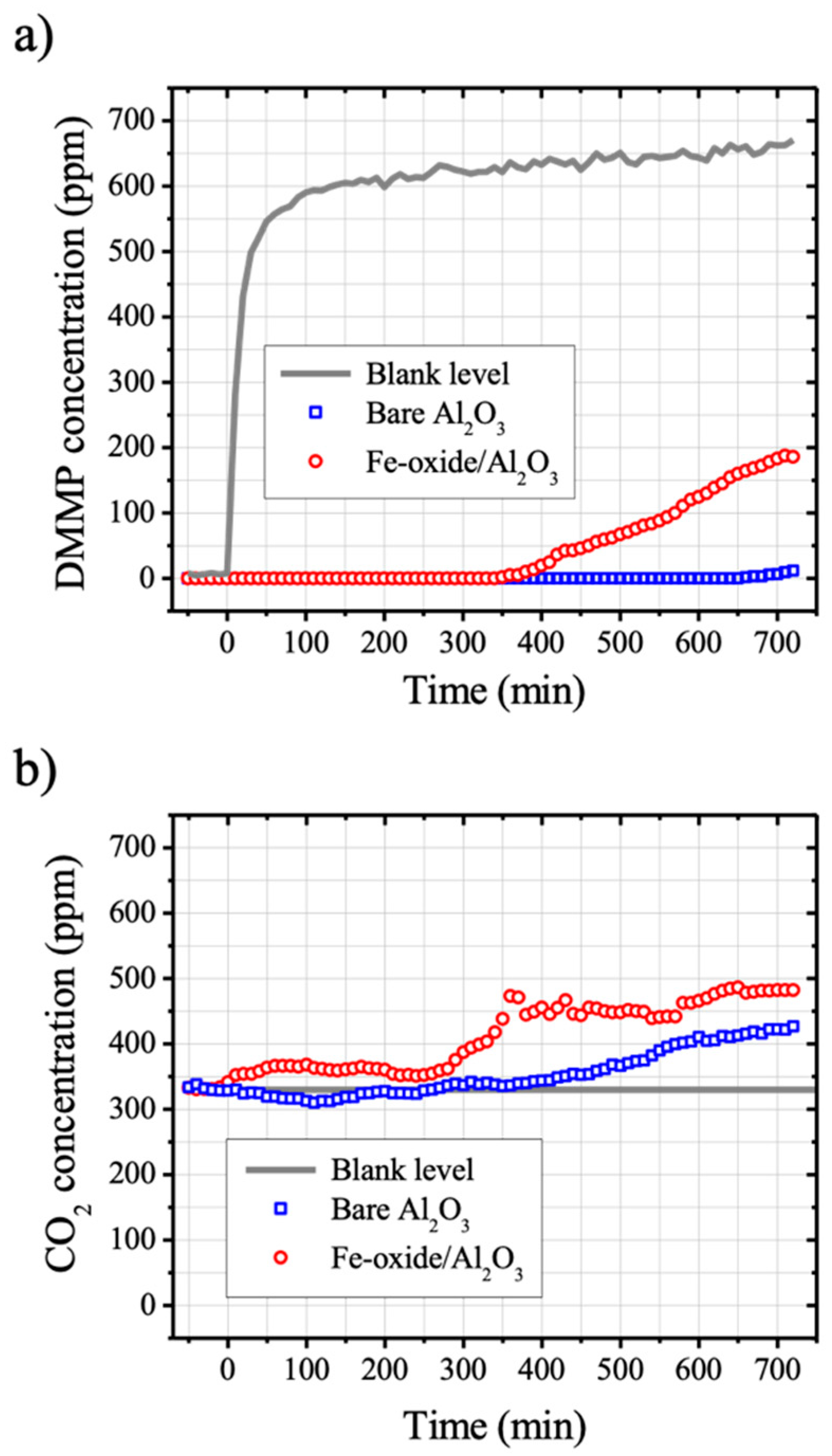
2, respectively. The area of each GC peak was converted to mol-ppm and its change with increasing DMMP exposure time is shown in
Figure 3. The grey solid lines in
Figure 3 correspond to the blank level of each species measured with empty SUS reactor under the same conditions of gas flow and temperature.
Figure 3a shows the DMMP breakthrough curves of bare Al
2O
3 and Fe-oxide/Al
2O
3. The breakthrough times of bare Al
2O
3 and Fe-oxide/Al
2O
3 where the DMMP concentration at effluent gas was higher than 1 percent of the blank level were determined to be 710 min and 380 min, respectively (
Table 2). For both cases of bare Al
2O
3 and Fe-oxide/Al
2O
3, increases of CO
2 level as a function of exposure time were observed before the respective breakthrough time, which implies that the degradation of DMMP molecules occurred on both samples surface at room temperature (
Figure 3b). There were certain induction periods of gaseous CO
2 evolution from the surfaces of both samples, and Fe-oxide/Al
2O
3 exhibited shorter induction time when compared to the bare Al
2O
3 (
Figure 3b). The time when more than 20% of increase from the blank level of CO
2 concentration (330 ppm) was found was taken as an induction time since there was a fluctuation of CO
2 concentration level (±9%) under our experimental conditions. The induction time of Fe-oxide/Al
2O
3 for gaseous CO
2 was determined to be 310 min, whereas that of bare Al
2O
3 was determined to be 560 min (
Table 2).
Gaseous CO
2 evolution from the metal-oxide surface at room temperature has been previously reported [
11,
16,
17,
26], and the relationship between the induction period of CO
2 evolution and surface area has been suggested; the higher the surface area, the longer the CO
2 induction time [
16,
17]. However, in our case of study, the different induction time for CO
2 evolution between two samples (bare Al
2O
3 and Fe-oxide/Al
2O
3) was not likely attributed to the difference in surface area. Fe-oxide/Al
2O
3 with higher surface area (171.8 m
2/g) exhibited shorter CO
2 induction time (310 min) than bare Al
2O
3 (560 min) with lower surface area (138.6 m
2/g) in this study. Alternatively, the shorter induction CO
2 time of Fe-oxide/Al
2O
3 samples than that of bare Al
2O
3 can be attributed to the co-existence of Fe-oxides and Al-oxides, which can provide additional sites being able to facilitate DMMP degradation.
2.3. IR Analysis
The surface of each sample (bare Al
2O
3 and Fe-oxide/Al
2O
3) before and after DMMP exposure at room temperature was analyzed by means of IR absorption spectroscopy in order to understand different behaviors of bare Al
2O
3 and Fe-oxide/Al
2O
3 observed during the DMMP exposure experiments at room temperature (
Figure 4). The conditions of DMMP exposure to prepare the samples for IR analysis were same as those of the aforementioned DMMP exposure experiments. However, the DMMP exposure time was reduced to 360 min from 720 min, i.e., DMMP exposure was ended before the DMMP breakthrough times of both samples (
Table 1) to keep the amount of adsorbed DMMP on both samples surface at same level.
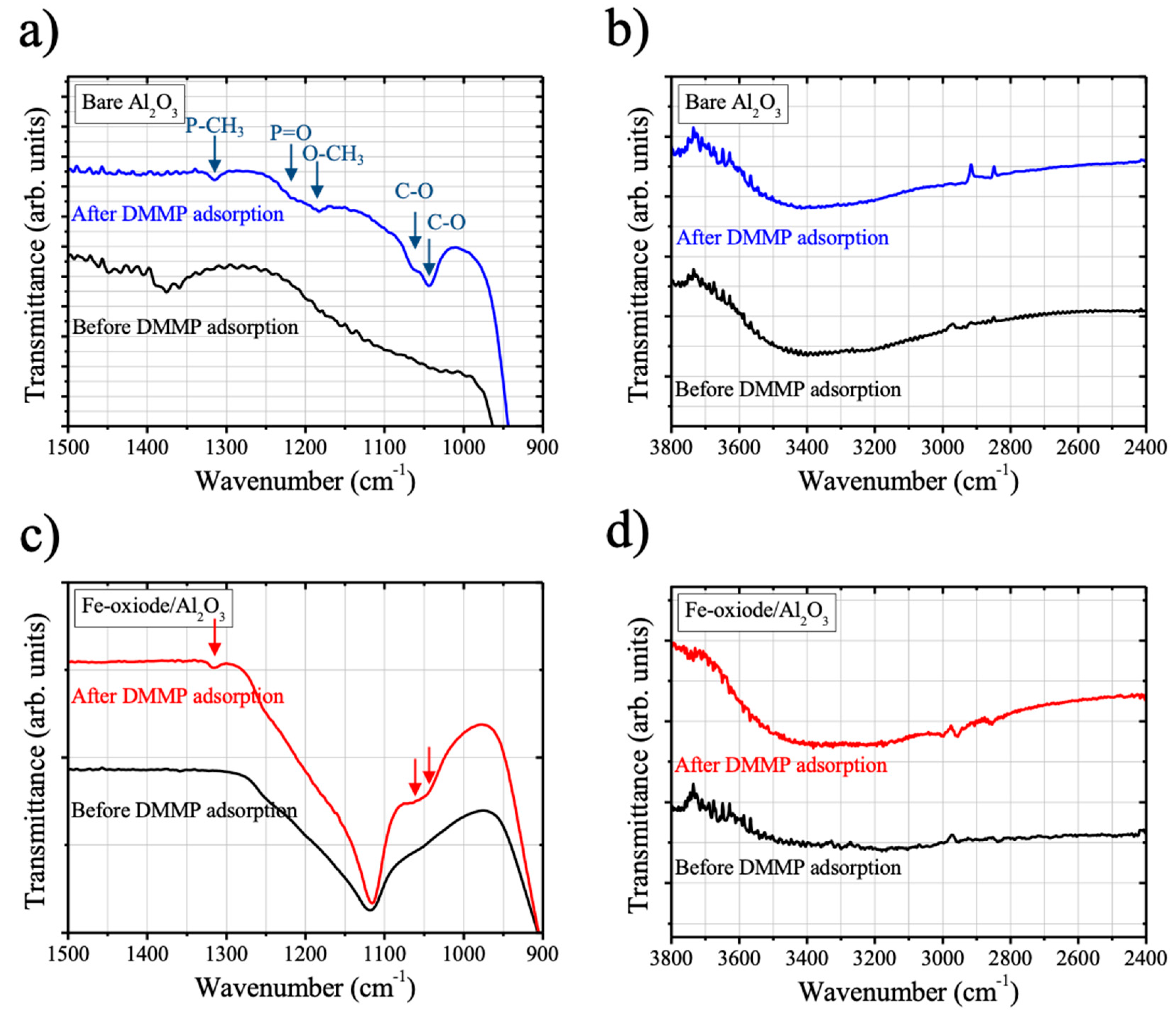
Figure 4 shows the IR absorption spectra of bare Al
2O
3 and Fe-oxide/Al
2O
3 measured before and after the DMMP exposure. The spectra are displayed in two wavenumber ranges (lower wavenumber range of 900–1500 cm
−1 and higher wavenumber range of 2400–3800 cm
−1), respectively, and they were only vertically shifted without background subtraction. Background subtracted IR spectra in higher wavenumber range are additionally provided in the
supplementary material Figure S1.
Upon the DMMP exposure at room temperature, appearances of characteristic IR absorption peaks of DMMP molecules were observed on the bare Al
2O
3 samples, which were P-CH
3 deformation (~1315 cm
−1), P=O stretching (~1215 cm
−1), O–CH
3 deformation (~1181 cm
−1), and asymmetric and symmetric stretching of C–O (~1065 and 1045 cm
−1, respectively) (
Figure 4a) [
22]. Apart from the appearances of characteristic DMMP peaks, other changes were not noticeable for the case of bare Al
2O
3 upon the DMMP exposure. It has been reported that the DMMP adsorption on aluminum oxide took place via an electrophilic attack of the surface on DMMP [
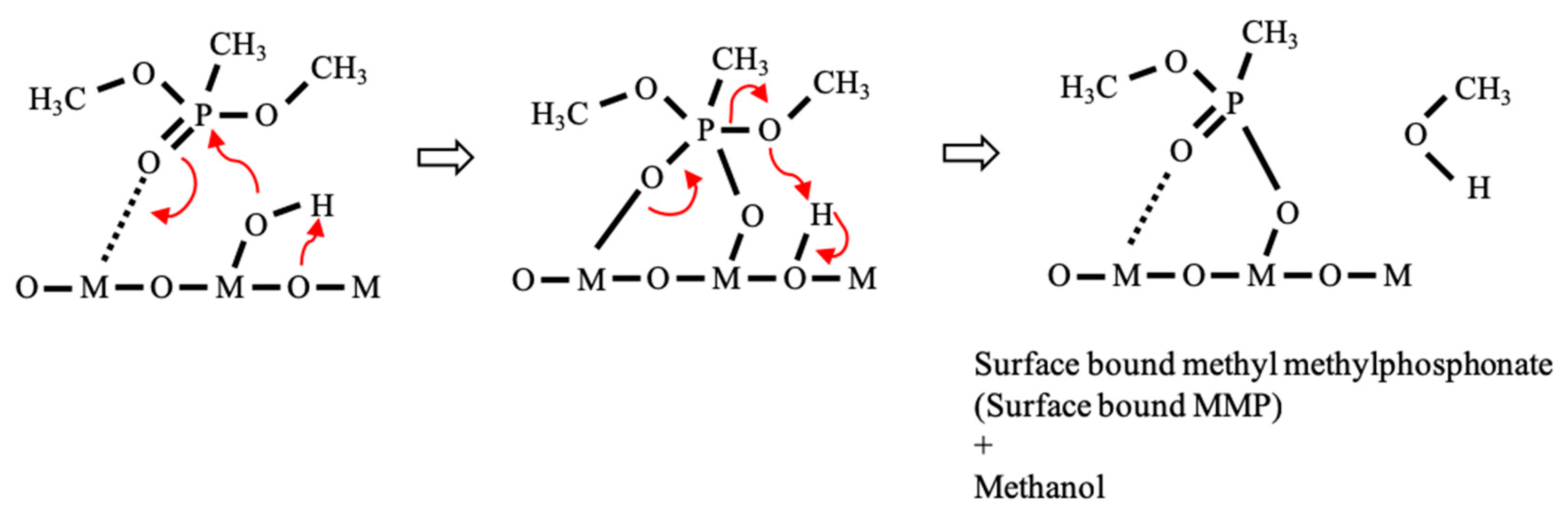
10]; the P=O bond of DMMP molecule interacts with coordinatively unsaturated aluminum (Lewis acid) sites and phosphoryl group of DMMP molecule interacts with a surface hydroxyl group (
Figure 5). Subsequently, it was followed by the loss of one methoxy group from surface-bound DMMP molecule, leaving a surface-bridging methyl methylphosphonate (MMP) on the surface [
10]. As temperature increases, the other methoxy group remained at MMP can be lost, forming a surface-bound methylphosphonate (MP). The formation of surface-bound MMP and MP was evidenced by the shift of P=O stretching vibration towards lower wavenumber from 1216 cm
−1 to ~1190 cm
−1 [
10]. However, in our case of study, it is not clear whether the surface-bound MMP or MP was formed on bare Al
2O
3 surface under our experimental conditions, since the reported IR absorption peak position of surface-bound MMP and MP was very close to the peak of O–CH
3 deformation (~1181 cm
−1).
Characteristic IR absorption peaks of DMMP molecules were also detected on the surface of Fe-oxide/Al
2O
3 after the DMMP exposure at room temperature, which were P–CH
3 deformation at ~1315 cm
−1 and asymmetric and symmetric stretching of C–O at ~1065 and 1045 cm
−1, respectively (
Figure 4c). However, in contrast to the case of bare Al
2O
3 sample, the IR peak at ~1114 cm
−1 increased in its intensity and P=O stretching (~1215 cm
−1), O–CH
3 deformation (~1181 cm
−1) peaks of DMMP molecules were no longer observable after the DMMP exposure. The peak at 1114 cm
−1 existed on the surface of Fe-oxide/Al
2O
3 before the DMMP adsorption was attributed to oxidized carbon impurities (C–O stretching mode), which were likely to be formed on the surface of Fe-oxide/Al
2O
3 during the sample preparation, e.g., oxidation of adsorbed ferrocene molecules. The increase of IR peak intensity at ~1114 cm
−1 can be attributed to the P=O stretching mode of the surface-bound MMP or MP molecules formed on the surface of Fe-oxide/Al
2O
3 (
Figure 4c) during DMMP exposure. The difference in IR absorption peak position of surface-bound MMP or MP species between on the surface of Fe-oxide/Al
2O
3 and on previously studied alumina surface can be ascribed to the different nature of the surfaces, e.g., the degree of Lewis acidity of the surfaces or coordination of surface-bound species on the surfaces [
10].
Those results indicate that the interaction between DMMP molecules and Al
2O
3 surface was enhanced by Fe-oxide impregnation, which facilitated the dissociation of DMMP molecules on the surface formation of surface-bound MMP or MP and methanol species (
Figure 5). It is also worth mentioning that surface-bound methanol species can be also formed, which can contribute to the intensity increase of IR absorption peak at 1114 cm
−1 (C–O stretching mode of methanol) and increase of –OH stretching IR band (3600~3200 cm
−1) (
Figure 4d) [
27]. The surface-bound formate species might also be formed during the DMMP conversion into CO
2; however, in our case of study, there was no IR absorption peak that appeared at 1600 cm
−1 (antisymmetric COO stretching mode) upon DMMP exposure for both samples (bare Al
2O
3, and Fe-oxide/Al
2O
3).
Our IR analyses results cannot reveal the detailed mechanisms of the reactive DMMP adsorption on Fe-oxide/Al
2O
3 sample. Additionally, further investigations, e.g., operando FT-IR analyses during the DMMP exposure, are needed to follow the adsorption mechanisms that guarantee another subject of study. For instance, P–CH
3 bond breaking was not evidenced on the surface Fe-oxide/Al
2O
3, which is different from the previously reported study [
10], and this discrepancy likely arises from different nature of the sample. The previous study was performed with pure Fe-oxide surface; whereas, we studied Fe-oxide impregnated Al
2O
3, where the Fe-Al oxide interface sites as well as two oxidic surface (Fe- and Al-oxide surface) took part in the reactive DMMP adsorption process. However, this cannot be clearly addressed solely based on the presented IR analyses results and it requires further studies. Nevertheless, our experimental observations show that Fe-oxide impregnation in Al
2O
3 can promote the dissociation of adsorbed DMMP molecules on the surface, which can facilitate the degradation of DMMP into CO
2 on the surface of Fe-oxide/Al
2O
3, which reduces the CO
2 induction time (
Table 2).
2.4. Temperature Programmed Oxidation (TPO) Experiments and GC/MS Analysis
The TPO experiments were performed after 360 min of DMMP adsorption at room temperature with bare Al2O3 and Fe-oxide/Al2O3. Temperature was linearly increased (1 °C/min) from the room temperature to 750 °C at a constant dry air flow (50 mL/min) and the gaseous species evolved from sample surface was analyzed by online GC. During the TPO experiments, only one GC peak was detected around at ~2.27 min and no other GC peaks, including a DMMP peak (at ~4.46 min), were detected.
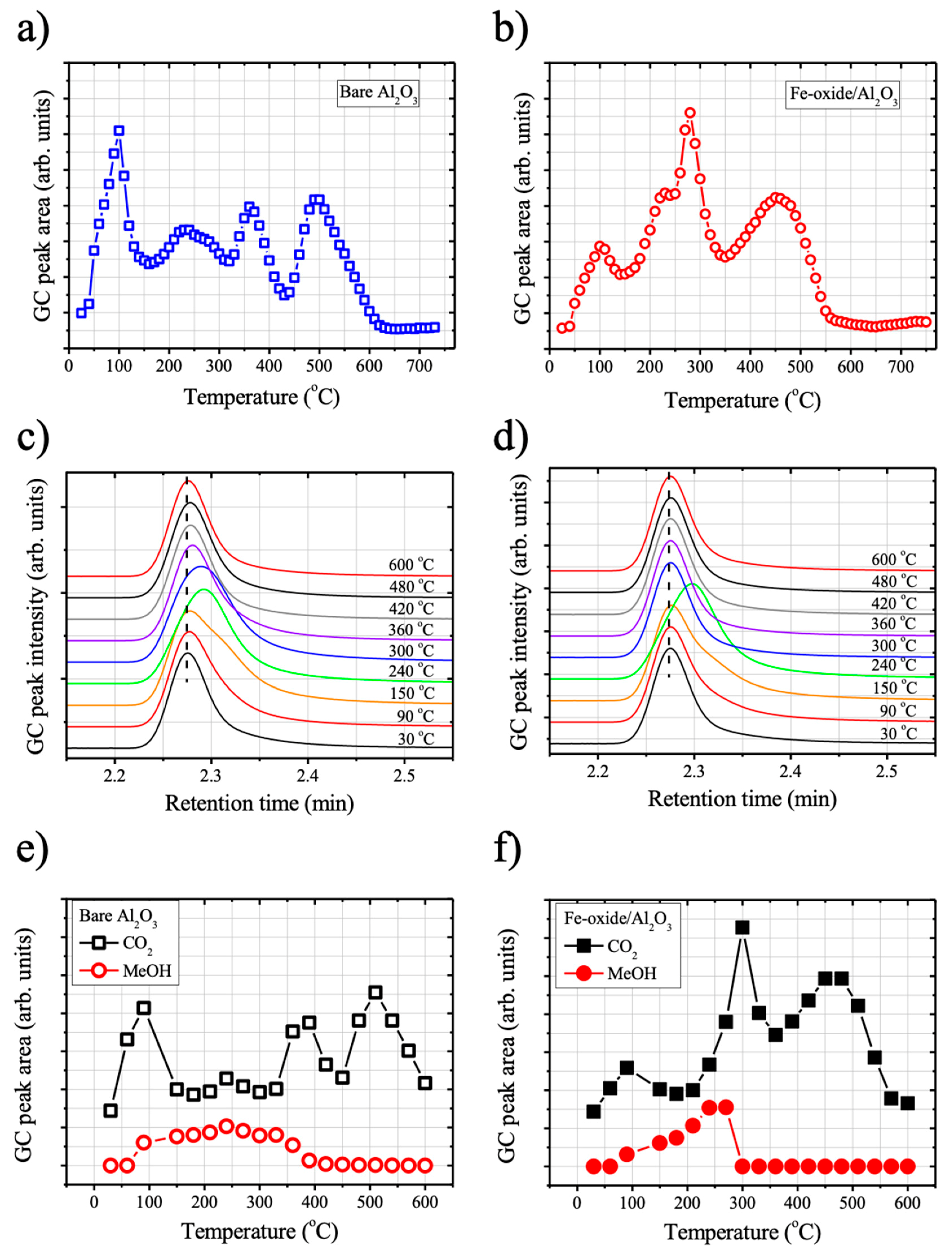
Figure 6a and b show the GC peak area as a function of temperature during TPO for either case of bare Al
2O
3 or Fe-oxide/Al
2O
3. The
y-axis values (GC peak area) of
Figure 6a,b are displayed in same
Y-axis scale, thus a direct comparison of the GC peak area value between bare Al
2O
3 and Fe-oxide/Al
2O
3 is possible, although they are displayed in arbitrary units.
The peak around at 2.27 min can be tentatively assigned to gaseous CO
2 generated by DMMP decomposition during TPO, and, at a first glance, CO
2 evolution due to DMMP degradation at low temperature region (<200 °C) was pronounced on the surface of bare Al
2O
3 than Fe-oxide/Al
2O
3 (
Figure 6a,b). Thus, one might come to the conclusion that the bare Al
2O
3 surface was more active for DMMP degradation at a lower temperature region (<200 °C) when compared to the surface of Fe-oxide/Al
2O
3. Additionally, it is not correlated to the afore-discussed results (
Figure 3b,
Table 2, and
Figure 4), implying a facilitation of DMMP degradation into CO
2 or methanol in the presence of Fe-oxide on Al
2O
3 surface at room temperature.
However, it should be noted that, on the surface of Fe-oxide/Al
2O
3, gaseous CO
2 evolution and methanol generation resulted from DMMP degradation already took place during the 360 min of DMMP adsorption conducted before the TPO experiments, as pointed out by the aforementioned experimental results (
Figure 3,
Table 2, and
Figure 4). Whereas both the CO
2 evolution and methanol generation were not evidenced under the same conditions on the surface of bare Al
2O
3.
Further on, we carefully examined the GC spectra of TPO experiments in the range of 2.2~2.5 min for both cases of samples (bare Al
2O
3 and Fe-oxide/Al
2O
3), and the changes of shape and position of the GC peaks of TPO were examined (
Figure 6c,d). For the examination of shape and positions of the GC peaks, the peak height of each GC peak was normalized to that of the respective GC peak that was obtained with each sample (bare Al
2O
3 or Fe-oxide/Al
2O
3) at 30 °C and the GC spectra were vertically shifted for comparison (
Figure 6c,d).
The GC peaks centering at ~2.27 min can be attributed to gaseous CO2, which were observed in the beginning of TPO (at 30 °C) for both cases of samples. In the case of bare Al2O3, a falling edge of the GC peak was extended to a longer retention time as the temperature increased to 90 °C from 30 °C, attributed to an appearance of additional GC peak at a longer retention time. The extension was more pronounced by a further temperature increase to 150 °C due to an increase of the additional GC peak component in its intensity, and the contribution of the additional component to the GC spectrum reached its maximum at ~240 °C; the shift of the center peak position of GC peak to a longer retention time was most pronounced at 240 °C. Subsequently, the intensity of additional GC peak component with respect to the CO2 peak gradually decreased as the temperature increased from 240 °C. Finally, the GC spectrum became identical to the original GC spectrum that was measured at 30 °C when the temperature reached to 600 °C.
The GC spectra of Fe-oxide/Al2O3 samples behaved similarly upon the temperature increase during the TPO experiments that were performed under the same conditions. The initiation of GC peak extension to a longer retention time was observed at 90 °C due to an appearance of additional GC peak at longer time. The intensity of the additional GC peak component gradually increased as the temperature was ramped to 240 °C and then decreased by a further temperature increase (>240 °C). However, the contribution of the additional GC peak component was decreased much faster when compared to the bare Al2O3 case. The GC peak of Fe-oxide/Al2O3 recovered its original shapes and position at 30 °C when the temperature reached to 300 °C, whereas a significant shift of the center position of GC peak to a longer time was still found for the case of bare Al2O3 at 300 °C.
Gaseous molecules evolved from the surface of samples during the TPO experiments were collected by SPME fibers and analyzed by GC/MS in order to identify the chemical species responsible for the additional GC peak component observed during the TPO experiments with Fe-oxide/Al
2O
3 samples.
Figure 6 shows the results of GC/MS analysis with Fe-oxide/Al
2O
3 samples. The main GC/MS peak was in the retention time range of 1.0 to 2.0 min (
Figure 7a) and it mostly consisted of 18, 28, and 32 m/z species that were attributed to water vapor, N
2, and O
2 of ambient air, respectively (
Supplementary material Figure S2). Along with species that originated from ambient air adsorption on SPEM fiber, the existence of species corresponding to 44 m/z and 31 m/z signal in the retention time region of main GC/MS peak were evidenced (
Figure 7b,c). The 44 m/z signal that was attributed to CO
2, which was originated either from ambient air adsorption or adsorption of CO
2 evolved during the TPO experiment. On the other hand, the 31 m/z signal that can assigned to methanol did not originate from ambient air, since no 31 m/z signal was detected by SPME fiber injection exposed to ambient air (
Supplementary material Figure S2). The 31 m/z signal (methanol) was also found in the main GC/MS peak region measured with bare Al
2O
3 samples (
Supplementary material Figure S3). Those results allow for us to conclude that gaseous methanol molecules were generated together with CO
2 at a certain temperature range of the TPO, resulting in the broadening and shifting of GC peaks.
Further on, we deconvolute the GC peaks at various temperatures of TPO experiments while using two components (CO
2 and methanol components) (
Figure 6e,f). During the deconvolution process, only each peak intensity was varied, while the peak position and the peak width at half maximum (FWHM) of each peak were fixed. The deconvolution results show that the temperature window for the evolution of gaseous methanol of Fe-oxide/Al
2O
3 was smaller than that of bare Al
2O
3 (
Figure 5e,f). In the case of Fe-oxide/Al
2O
3, methanol formation was ended and the most of adsorbed DMMP molecules was converted to CO
2 at the temperature at 300 °C. Whereas, still, a significant amount of methanol molecules was evolved from the surface bare Al
2O
3 together with CO
2 by the degradation of adsorbed DMMP molecules until the temperature reached to 400 °C. It indicated that the minimum temperature required for total oxidation of DMMP molecules to CO
2 can be lowered in the presence of Fe-oxides on Al
2O
3. Additionally, one can suggest the utilization of Fe-oxide/Al
2O
3 at elevated temperature higher than 300 °C for a continuous total oxidation of DMMP molecules.