Abstract
The main objective of this study focused on the sustainable production of cellobiose and other cellulose-derived oligosaccharides from non-edible sources, more specifically, from forest residues. For this purpose, a fine-tuning of the performance of the commercially available enzyme mixture Celluclast® was conducted towards the optimization of cellobiose production. By enzyme reaction engineering (pH, multi-stage hydrolysis with buffer exchange, addition of β-glucosidase inhibitor), a cellobiose-rich product with a high cellobiose to glucose ratio (37.4) was achieved by utilizing organosolv-pretreated birch biomass. In this way, controlled enzymatic hydrolysis combined with efficient downstream processing, including product recovery and purification through ultrafiltration and nanofiltration, can potentially support the sustainable production of food-grade oligosaccharides from forest biomass. The potential of the hydrolysis product to support the growth of two Lactobacilli probiotic strains as a sole carbon source was also demonstrated.
1. Introduction
Nowadays, food and pharmaceutical industries show a growing interest in the development of the so-called functional foods. This term is used to describe products that demonstrate various beneficial effects for the consumer, such as improving the bioavailability of a particular component and, eventually, the reduction of the risk of certain diseases and the general amelioration of the person’s well-being [1]. The main target for these high added-value food ingredients are the non- digestible oligosaccharides (NDOs), including carbohydrates with a low degree of polymerization (DP), between 3–10 sugar moieties. Their importance arises from the fact that these oligosaccharides are not digested by the enzymes of the gastrointestinal system, therefore they are available to be fermented by the human intestinal flora and promote the growth of beneficial gut bacteria in the colon, such as Bifidobacteria and Lactobacilli [2]. Over 20 different types of NDOs are currently available on the market and they are either extracted from natural sources, obtained by enzyme processing, or produced chemically [3]. The preferable process targeted for NDOs production involves the controlled enzymatic degradation, since it offers mild reaction conditions and less chemically harsh byproducts.
A novel source of NDOs are plant cell wall polysaccharides. The inability of humans to digest NDOs derived from cellulose and hemicellulose structures is due to the fact that NDOs sugar units are linked by glucosidic bonds that have a β-configuration and thus cannot be degraded by human gastrointestinal enzymes since they are specific for compounds with an α-configuration [3]. Such plant polysaccharides are often present in large amounts in fiber-rich byproducts and wastes, such as lignocellulosic residues. These residues are currently being underutilized and burned for energy production with a low sale price; this biomass can serve instead as a starting material for many valuable products. There is a rapidly growing interest in new technologies that can convert renewable, low-cost biomass from the forest into high-value bulk chemicals and materials, including NDOs and other food ingredients [4]. The availability of well-defined enzymes or enzyme combinations for the production of NDOs from these substrates is a prerequisite [5]. The plant cell wall polysaccharides as a novel source of prebiotic oligosaccharides have gained increasing attention, since they offer a sustainable and attractive utilization of the agricultural and industrial residues leading to the development of a bio-based economy.
Among the lignocellulosic-derived NDOs, those produced by cellulose hydrolysis, namely cello-oligosaccharides (COS), represent a group of novel oligosaccharides with exceptional interest and numerous potential applications as potential prebiotic ingredients. These oligosaccharides, especially cellobiose, have been shown to promote the growth of Bifidobacterium species and exhibit a higher prebiotic potential than other widely used oligosaccharides, such those from fructose [6]. The bioavailability of cellobiose in humans has already been evaluated with cellobiose tolerance tests [7]. It has consequently been observed that after ingestion, cellobiose can be fermented by the gut microflora and that it cannot be hydrolyzed by the digestive enzymes, therefore it reaches the colon undigested [8]. Additional studies have been carried out with humans and rodents and suggested the beneficial effects of cellobiose on carbohydrate metabolism, diabetes and obesity [9,10].
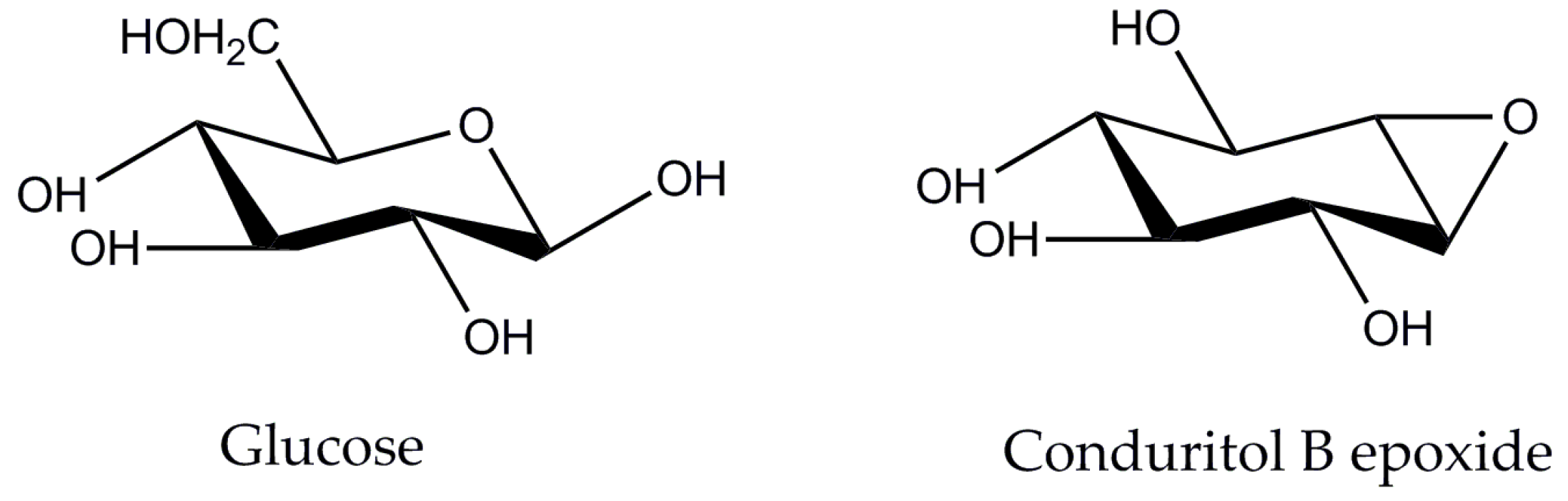
Several strategies for enzyme-mediated production of oligosaccharides have been proposed. These include, among others, the design of tailor-made enzymatic cocktails that offer a controlled polysaccharide cleavage breaking and produce less monomers [11], the modification of reaction conditions (e.g., buffer exchange to abolish the end-product inhibition of enzymatic activity) [12] or the fine-tuning of the performance of commercially available enzyme cocktails (e.g., addition of β-glucosidase inhibitor [13]). Out of these strategies, the construction of customized enzyme mixtures may offer great advantages, such as the ability to adapt the mixture composition to different substrates according to their structural properties, but there are many bottlenecks that hamper the scaling-up of such processes. These bottlenecks arise either from the production costs of different monoenzymes or the laborious, time-consuming techniques related to molecular cloning and heterologous production methods and protocols. On the contrary, tuning the performance of commercially available cellulase mixtures could be a promising solution for the valorization of available biomass wastes. These enzyme cocktails comprise of a unique set of various activities that act synergistically to release the desired product. By modifying the reaction conditions, such as pH or temperature, it is possible to boost the activity of specific enzymatic activities of interest, such as cellobiohydrolases and endoglucanases in the case of cellobiose production, selectively. Combining this selectivity together with blocking the activity of β-glucosidase with inhibitory compounds can theoretically lead to the accumulation of cellobiose. Conduritol-B-epoxide mimics the structure of β-glucose, as shown in Figure 1, and it is a potential irreversible inhibitor of β-glucosidases [14]. Opening of the epoxide by a nucleophilic residue of the enzyme active site enables the interaction with the OH groups of the inhibitor, the formation of a stable ester bond and, subsequently, the specific binding to the enzyme [15].
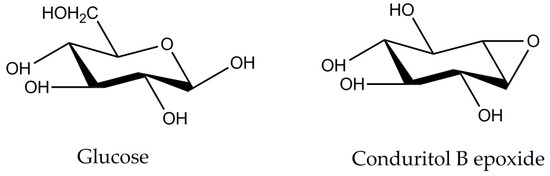
Figure 1.
Structures of glucose and conduritol-B-epoxide, two potent inhibitors of enzymes with cellobiohydrolase activity.
In the present study, different strategies were followed including modification of the reaction conditions, such as pH, temperature, addition of conduritol-B-epoxide at various concentrations, as well as change of hydrolysis strategies, such as buffer exchange, supplementation of enzyme and conduritol. Celluclast®, an enzyme preparation for breakdown of cellulose into glucose, cellobiose and longer glucose polymers, produced by fungus Trichoderma reesei, was selected for the study due to the limited activity of β-glucosidase that it is a native characteristic property of the cocktail [16]. As this enzyme preparation is lacking lytic polysaccharide monooxygenase (LPMO) activities, the synergistic effect of cellulases with external addition of LPMO towards the production of cellobiose was also evaluated. This class of enzymes has been reported to cleave cellulose chains in an oxidative way and to create nicking points [17], thus providing new chain ends for the processive enzymes to act. Moreover, a comparative study of the performance of the optimized cocktail on different substrates under optimal reaction conditions is presented. Organosolv-pretreated birch and spruce woodchips [18,19] were used as substrates to determine the production of cellobiose. Finally, the substrate that was degraded to the greatest extent was assessed on a scale-up reaction and the whole process, including product recovery and purification as well as evaluation of prebiotic potential on different Lactobacilli species, is described.
2. Results
2.1. Effect of β-Glucosidase Inhibitor
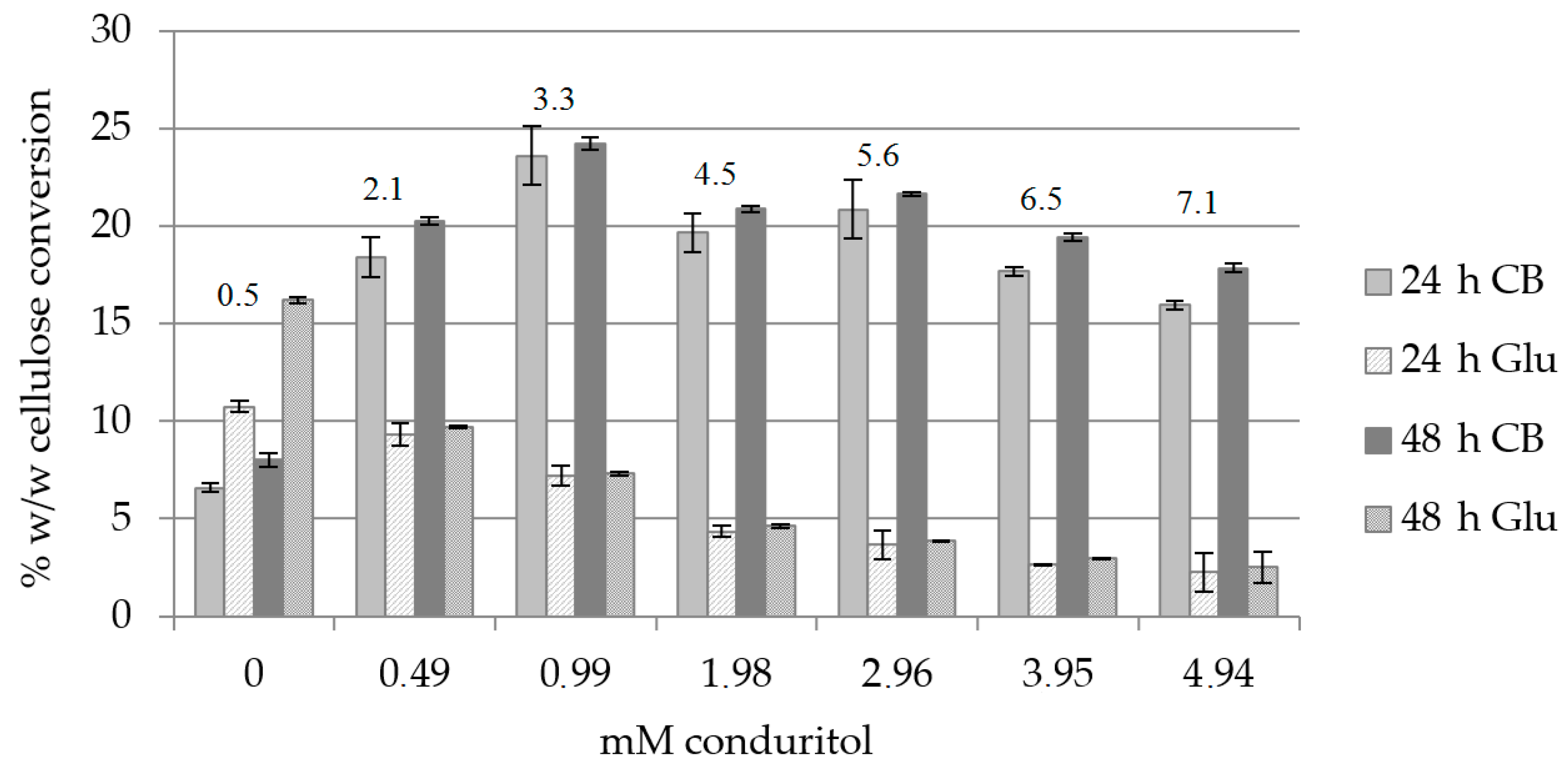
The effect of conduritol-B-epoxide, which binds covalently to the catalytic site of β-glucosidase, towards the production of cellobiose was evaluated. Organosolv-pretreated birch B1 (the composition is described in Section 4.1), was used as a substrate. Since the inhibition effect is usually dose- and time-dependent, different concentrations of conduritol were tested in order to find the minimum amount that caused inhibition of the activity of β-glucosidase. Buffer exchange was applied at 24 h, as from preliminary results it was found that it increased the hydrolysis yield by 23% (data not shown). Total hydrolysis time was 48 h. As depicted in Figure 2 and Supplementary Table S1, the highest cellulose conversion to cellobiose was observed with a concentration of 0.99 mM conduritol and corresponded to 35.2% (w/w) for 48 h of hydrolysis, while the cellobiose (CB): glucose (Glu) ratio was equal to 3.3. When conduritol was added to a concentration of 3.95 mM, the ratio of cellobiose to glucose was 6.5, with a total production of 168.5 mg cellobiose/g biomass and 25.8 mg Glu/g biomass. In presence of 4.94 mM conduritol, the ratio of CB to Glu was 7.1 and was the highest among all conditions tested, leading to 154 mg CB/g of substrate. In order to combine the maximum ratio of cellobiose to glucose to get a cellobiose-rich hydrolysis product with the minimum amount of conduritol, addition of 3.95 mM conduritol was selected as the optimum condition for further experiments.
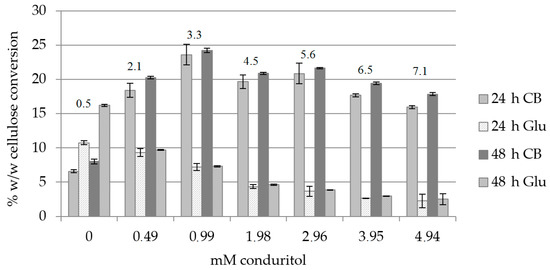
Figure 2.
Cellulose conversion (% w/w) to cellobiose (CB) and glucose (Glu) under addition of various concentrations of conduritol-B-epoxide, upon buffer exchange at 24 h. The CB:Glu ratio is also described for 48 h of hydrolysis.
2.2. Effect of Enzyme and Conduritol Mixture Preincubation
Preincubation of enzyme with conduritol was investigated in order to check whether the inhibitor could bind onto the enzyme under a time-dependent mode. The preincubation took place at room temperature for 60 min. Hydrolysis took place under the same conditions as described above and the results were compared to those from a hydrolysis reaction without preincubation. The results, described in Table 1, showed that preincubation did not result in further improvement of the activity of Celluclast® towards the production of CB showing that the covalent bonding between the enzyme and inhibitor is instant. Moreover, the CB:Glu ratio was slightly lower after preincubation.

Table 1.
Cellulose conversion (% w/w) to CB and Glu with and without preincubation of the enzyme with the inhibitor compound. Birch B1 was used as a substrate.
2.3. Effect of pH and Enzyme Loading
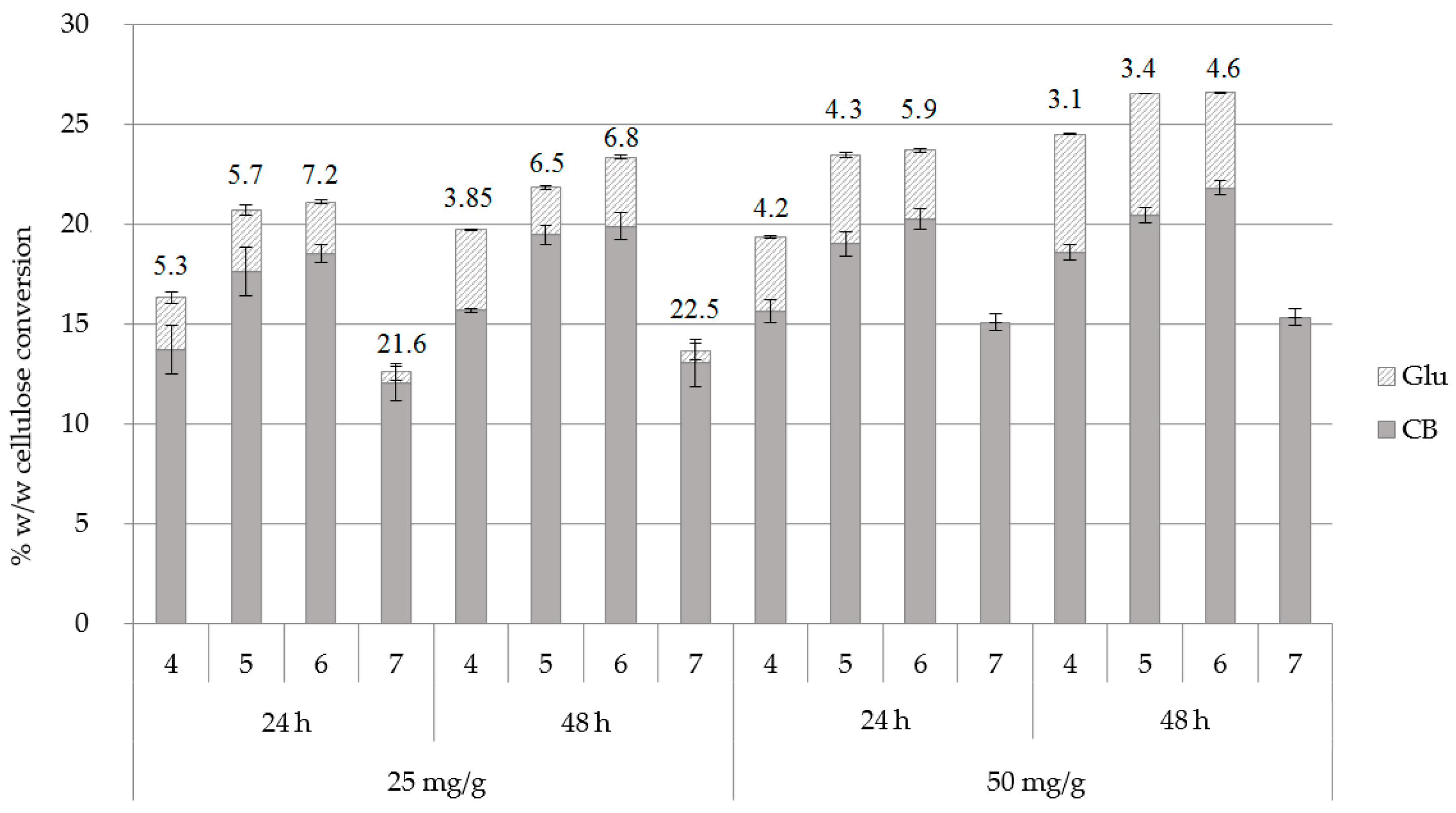
The effect of different pH conditions was investigated with an enzyme loading of 25 and 50 mg/g substrate, for 24 and 48 h of hydrolysis, with buffer exchange at 24 h. From the results, as shown in Figure 3 and Supplementary Table S2, it can be concluded that an increase of enzyme loading leads to a concurrent rise of the overall cellulose conversion but it does not favor the production of CB over Glu. It is only at pH 7.0 that the addition of 50 mg enzyme/g of substrate results in production of CB as the sole product in the absence of Glu, with a CB yield of 133 mg/g of substrate after 48 h of hydrolysis confirming previous observations that the inhibition from conduritol-B-epoxide is pH-dependent [20].
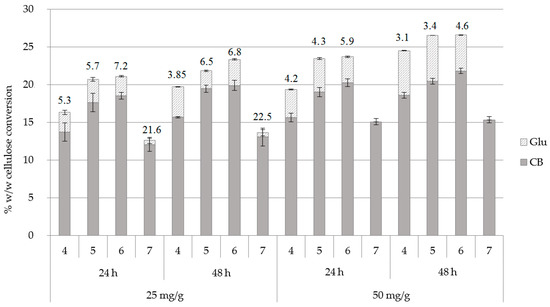
Figure 3.
Cellulose conversion (% w/w) to cellobiose (CB) and glucose (Glu) in different pH conditions and at an enzyme loading of 25 and 50 mg/g of substrate, upon buffer exchange at 24 h. The CB:Glu ratio is also described for 24 and 48 h of hydrolysis.
The trials at different pH values showed that there is a gradual increase both in total hydrolysis yields and the CB:Glu ratio when the pH of the reaction increases from 4.00 to 6.00, while the optimum CB:Glu ratio is achieved at pH 7.0 (21.6 and 22.5 after 24 and 48 h of hydrolysis, respectively, for 25 mg enzyme/g of substrate). Interestingly, the overall cellulose conversion is lower at this condition, and this is apparently due to the lower activity of a fraction of enzymes that are included in Celluclast® mixture. With the aim to minimize the enzyme usage and, since the amount of CB produced from both enzyme loading conditions was approximately the same, while the amount of glucose was negligible (0.56% w/w), the enzyme loading of 25 mg enzyme/g substrate and pH 7.0 was selected as the optimal condition to proceed further. Trials at pH 7.5 and 8.0 showed a reduced enzyme activity that reached 9.27% and 1.81% w/w cellulose conversion to CB, respectively (data not shown).
2.4. Effect of Hydrolysis Time, Inhibitor Concentration and Reaction Temperature at pH 7.0
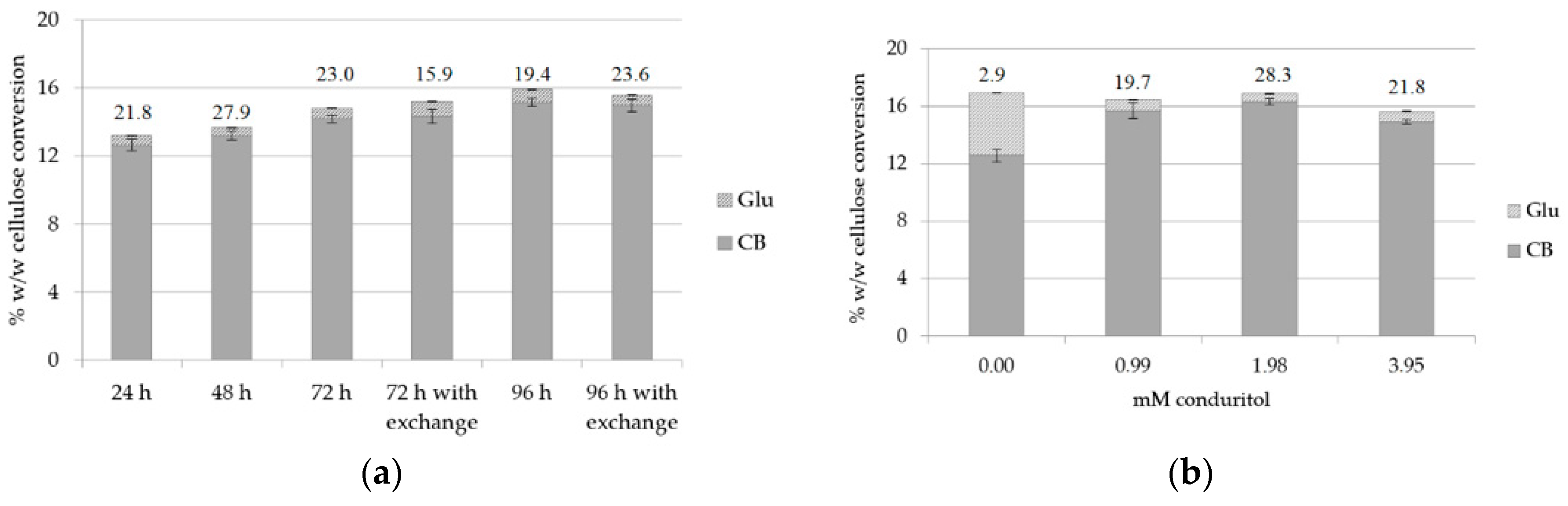
The hydrolysis rates after 24, 48, 72 and 96 h incubation were evaluated at pH 7.00, both with and without buffer exchange at 48 and 72 h, in order to evaluate whether it was possible for the reaction to continue leading to an increase of cellobiose production. Buffer exchange at 24 h was applied in both cases. The results, as depicted in Figure 4a and Supplementary Table S3, showed that the rate is higher during the first 24 h of reaction, with a CB yield of 109.5 mg/g substrate and a CB:Glu ratio of 21.8, which in accordance with the previous results, and it remains high after 48 h. Comparison of 72 and 96 h of hydrolysis with and without buffer exchange did not show significant difference, therefore this strategy was not used further. This can be attributed to the fact that enzymes, though bound onto the substrate, have lost a part of their activity after 96 h of hydrolysis, therefore they are not able to continue the hydrolysis of the substrate and contribute further to the increase of the CB yield. As the greater amount of cellobiose was produced within the first 24 h, this condition was selected in order to minimize the hydrolysis time and, consequently, the possible overall costs of the process.
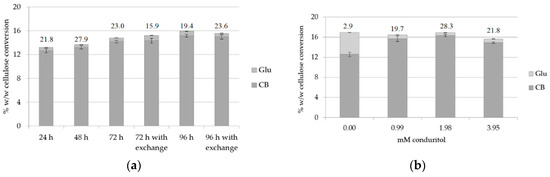
Figure 4.
Cellulose conversion (% w/w) to CB and Glu: (a) for different hydrolysis time and evaluation of buffer exchange after 72 (buffer exchange at 48 h) and 96 h (buffer exchange at 72 h) of hydrolysis; (b) for various concentrations of conduritol-B-epoxide at pH 7.0. The CB:Glu ratio is also described for: (a) different hydrolysis time; (b) 24 h of reaction.
As pH 7.0 was proved to be a condition that does not favor the activity of β-glucosidase, since the enzyme was apparently less active, and with the aim to reduce the addition of conduritol as much as possible, another set of experiments was set up in order to test different concentrations of the inhibitory compound. Conduritol-B-epoxide was added in concentrations varying within the range of 0–3.95 mM and the reaction was incubated for 24 h. In Figure 4b and Supplementary Table S4, it can be observed that a concentration of 1.98 mM conduritol is sufficient to suppress the activity of β-glucosidase and lead to a total production of 141.7 mg CB/g of substrate, with a CB:Glu ratio of 28.3. Trials with different temperature conditions were also conducted in order to evaluate whether a temperature change within the range of 40–50 °C (optimal temperature conditions for the performance of Celluclast® according to [10]), could have a beneficial effect towards the increase of cellobiose production. The hydrolysis yield and the CB:Glu ratio were evaluated after 12 and 24 h of hydrolysis, after applying buffer exchange at 12 h. The results, summarized in Table 2, showed that 45 °C is the optimal temperature that maximizes the cellobiose production, but the CB:Glu ratio is much lower. Therefore, the condition of 1.98 mM conduritol, 50 °C and 24 h of reaction was chosen as the optimal.

Table 2.
Trials with different temperature and incubation time. All experiments have been conducted upon the addition of 1.98 mM conduritol-B-epoxide and a pH 7.0, while buffer exchange was applied at 12 h.
2.5. Effect of Buffer Exchange, Enzyme and Inhibitor Supplementation
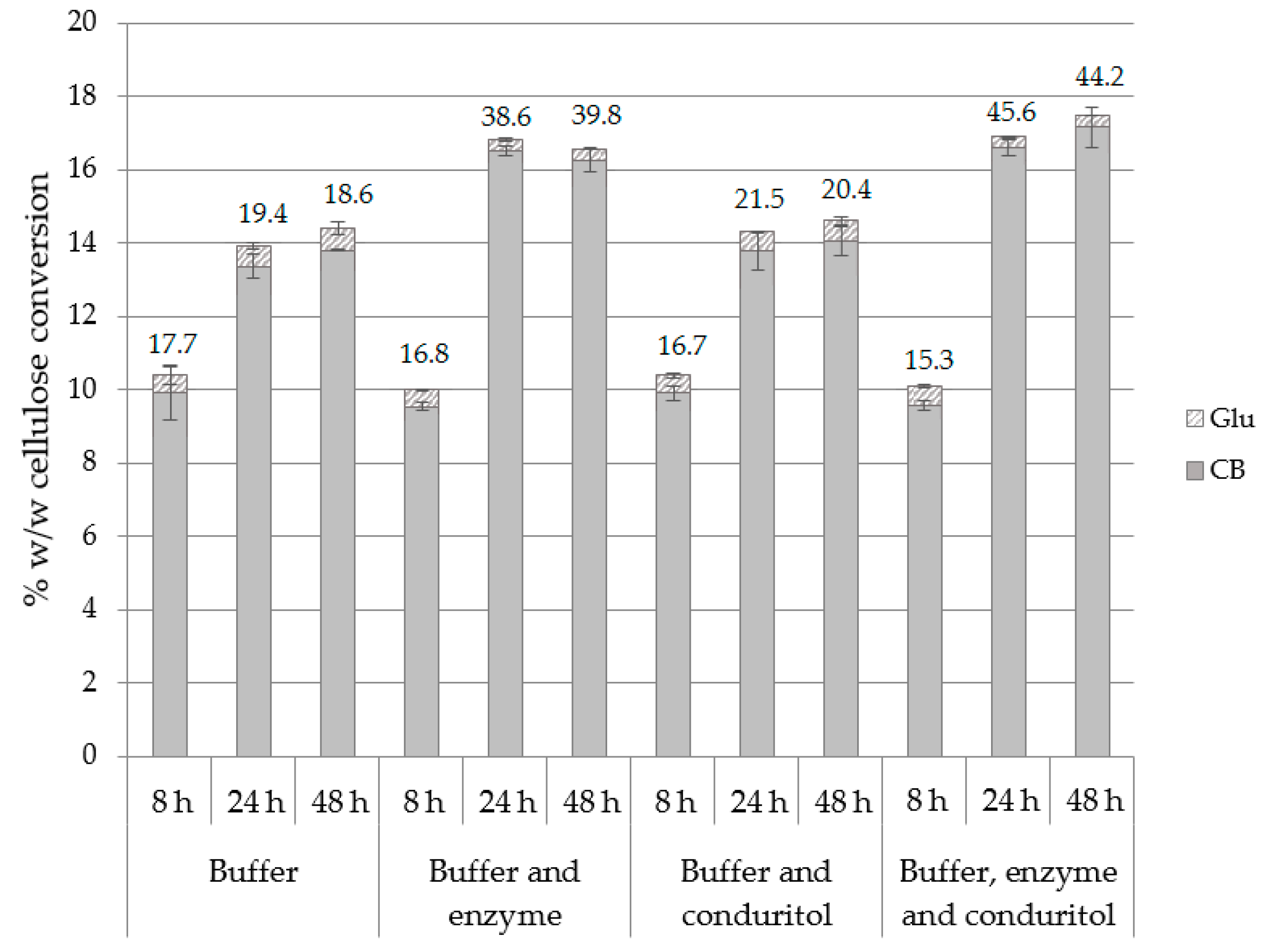
The effect of buffer exchange, as well as the supplementation with additional enzyme loading and/or conduritol-B-epoxide was assessed for 8, 24 and 48 h of hydrolysis. Buffer exchange and supplementation of enzyme and inhibitor occurred at 8, 24 and 48 h, while the amount of enzyme and inhibitor added each time was equal to the initial concentration. The results in Figure 5 show that buffer exchange not only improved the CB yield but also increased the CB:Glu ratio at 24 and 48 h. This can be attributed not only to the removal of produced cellobiose and, thus, elimination of end-product inhibition, but also due to the fact that β-glucosidase was removed in the supernatant while other cellulolytic enzymes remained bound onto the substrate. As a result, after the buffer exchange step, the majority of the enzymes that are present represent enzymes with activity of endo- and exo-cellulases, while the β-glucosidase fraction has been removed. Trials with supplementation of enzyme, conduritol or combination of both showed that the addition of enzyme loading boosts the hydrolysis towards the production of cellobiose and rapidly increases the CB:Glu ratio to 39.8, compared to 18.6 in case of buffer exchange. The highest performance is achieved when all three different constituents, namely buffer, enzyme and conduritol, are all supplemented, leading to 164.3 and 172.2 mg CB/g of substrate after 24 and 48 h of hydrolysis, respectively (Supplementary Table S5). Supplementation with conduritol alone does not further improve the product yield.
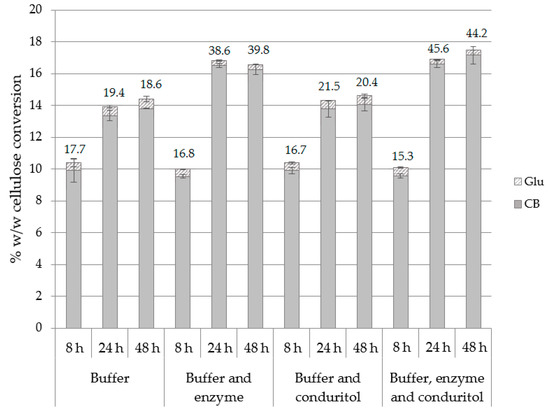
Figure 5.
Cellulose conversion (% w/w) to CB and Glu after applying buffer exchange and/or supplementation with additional enzyme loading or conduritol-B-epoxide. The CB:Glu ratio is also described for 8, 24 and 48 h of hydrolysis.
2.6. Evaluation of LPMO on the Production of Cellobiose
Synergistic effect of PcLPMO9D with cellulases was investigated in order to evaluate whether the addition of a C1 oxidizing enzyme can boost the production of cellobiose. The control reaction with 25 mg Celluclast®/g of substrate and addition of 1.98 mM of conduritol-B-epoxide resulted in the release of 145.7 mg CB/g of substrate, with a CB:Glu ratio equal to 21.8. The test reaction with an additional loading of 2.5 mg LPMO/g of substrate showed a substantial increase of cellobiose to 220.9 g CB/g of substrate. Interestingly, the data displayed in Table 3 show that, in parallel, there is a higher increase in the glucose production rate, which is translated into a decrease of the overall CB:Glu ratio. As a result, although the total sugar release is increased by around 58% in the LPMO supplemented reaction and there is a significant boost obtained in the case of cellobiose, the enormous enhancement in glucose levels causes the CB:Glu ratio to drop to 13.1. Interestingly, in the control reaction containing 27.5 mg Celluclast®/g of substrate, no significant changes in either cellobiose or glucose yields were observed. This is an indication that the increase of cellobiose yields can be attributed to the activity of LPMO and not to the effect of increased protein content. To the best in our knowledge this is the first report where the additional action of LPMO results not only in the increase of release of glucose but also in the increase of production of cellobiose.

Table 3.
Evaluation of synergistic effect of Celluclast® supplementation with PcLPMO9D towards the production of cellobiose.
2.7. Evaluation of Different Substrates
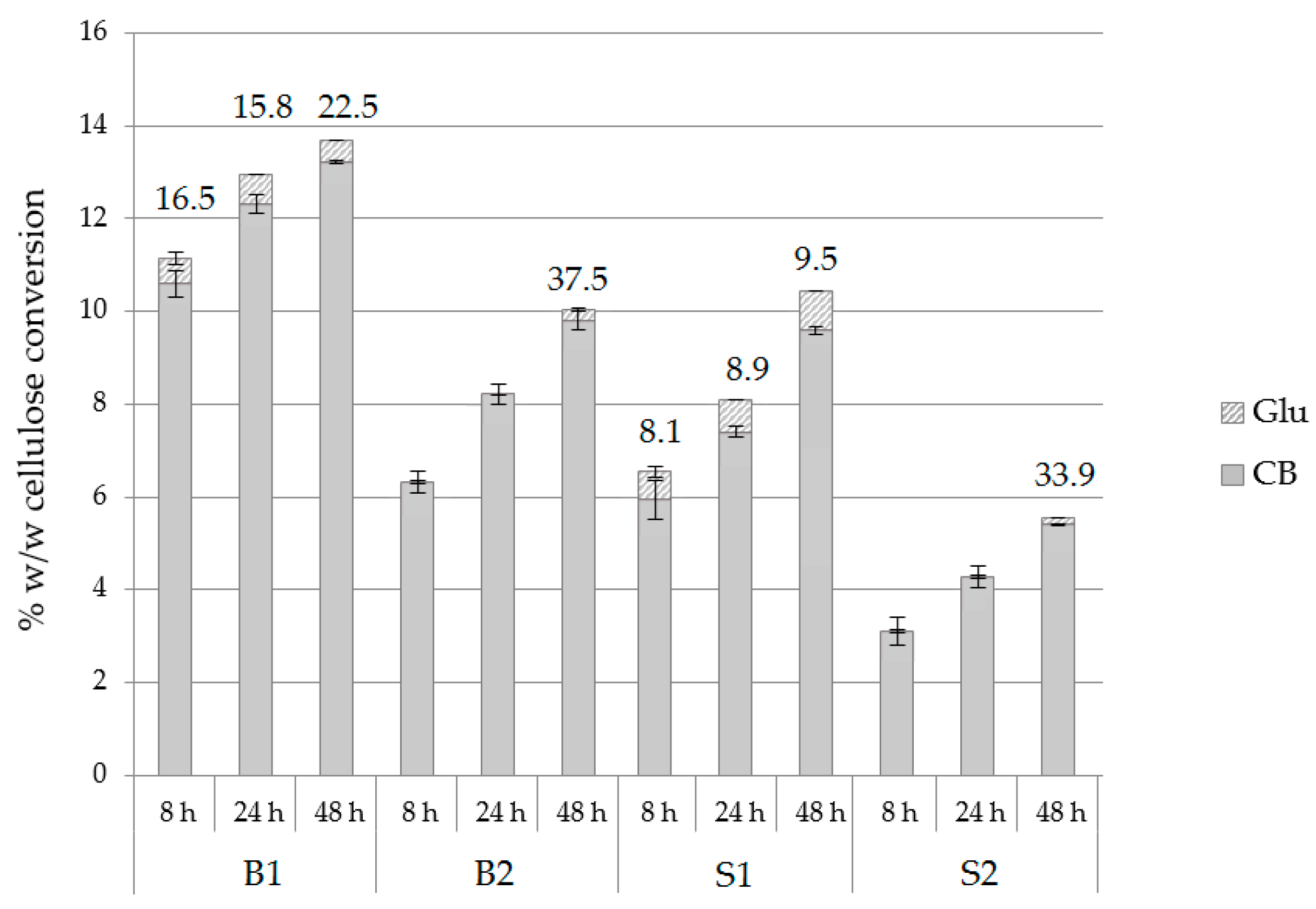
Different substrates were tested towards the production of cellobiose by using the optimal hydrolysis conditions, namely 1.98 mM of conduritol-B-epoxide at pH 7.0 with an enzyme loading of 25 mg/g substrate. Buffer exchange was applied at 8 and 24 h of hydrolysis, without any supplementation of conduritol or enzyme. The composition of each substrate is described in Section 4.1. According to the results depicted in Figure 6 and Supplementary Table S6, conversion of cellulose to cellobiose was higher in birch compared to spruce. Regarding the CB:Glu ratio, in the case of birch, this was lower for B1, and in case of spruce it was lower for S1; both were pretreated with acid catalyst. B1 was by far the best substrate among those tested for the production of cellobiose, therefore it was selected for the scale-up reactions.
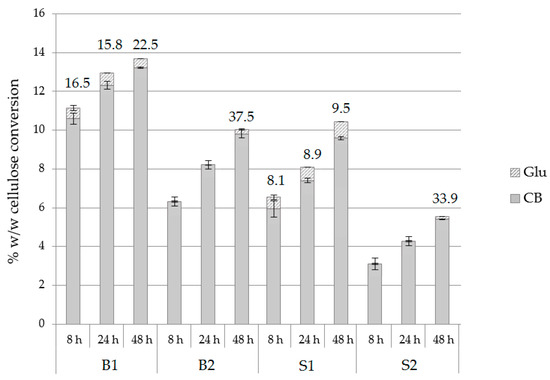
Figure 6.
Hydrolysis yields from birch and spruce substrates, described as % w/w cellulose conversion into cellobiose and glucose of at pH 7.0, upon the addition of 1.98 mM conduritol-B-epoxide, at an enzyme loading of 25 mg/g of substrate, with buffer exchange at 8 and 24 h. CB:Glu and total mg CB per gram of substrate are also described.
2.8. Scale-up Reaction and Downstream Processing for Product Recovery
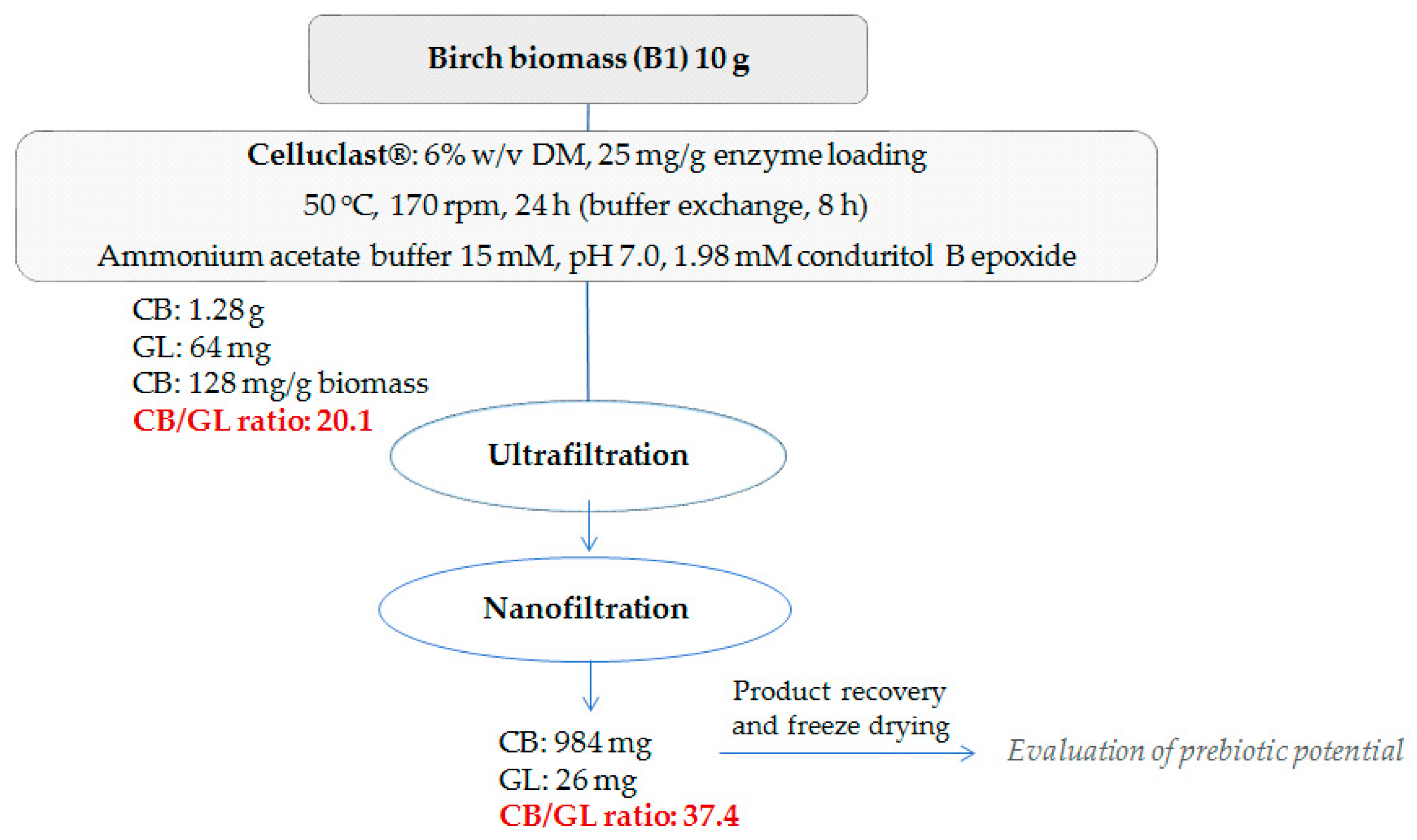
A scale-up reaction with a total volume of 60 mL was carried out using birch B1 as the substrate and the optimal reaction conditions to maximize the cellobiose production upon the minimum addition of conduritol-B-epoxide and enzyme loading, as determined in the previous experiments. The hydrolysis was carried out at pH 7.0, with addition of 1.98 mM conduritol-B-epoxide and an enzyme loading of 25 mg/g of substrate. Hydrolysis took place for 24 h and buffer exchange was applied after 8 h. After centrifuge and removal of the residual biomass, all fractions were collected and mixed (final reaction mixture together with that originating from the buffer exchange). Ultrafiltration and nanofiltration were applied in order to remove glucose and conduritol and to recover a cellobiose-rich liquor. Figure 7 represents the overall procedure for the enzymatic production of cellobiose from organosolv-pretreated birch B1, as well as the product yield and recovery in each stage. A total amount of 1.28 g of cellobiose was produced, corresponding to 128 mg of cellobiose/g of substrate, while after ultra- and nano-filtration, 984 mg of the final product remained. The nanofiltration step resulted in the removal of a great amount of glucose, leading to a final cellobiose to glucose ratio of 37.4. The protein content of the mixture was determined at 0.53% w/w. The product was freeze-dried (Figure 8) and used for evaluation of its prebiotic potential.
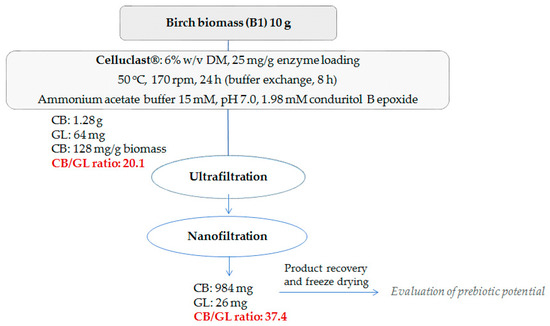
Figure 7.
Overall scheme of the cellobiose production from pretreated birch.

Figure 8.
Freeze-dried cellobiose-rich product from enzymatic hydrolysis of pretreated birch.
2.9. Evaluation of COS Prebiotic Activity
Growth Potential of Lactobacillus Strains on Pure Cellobiose and Birch-Derived Sugars
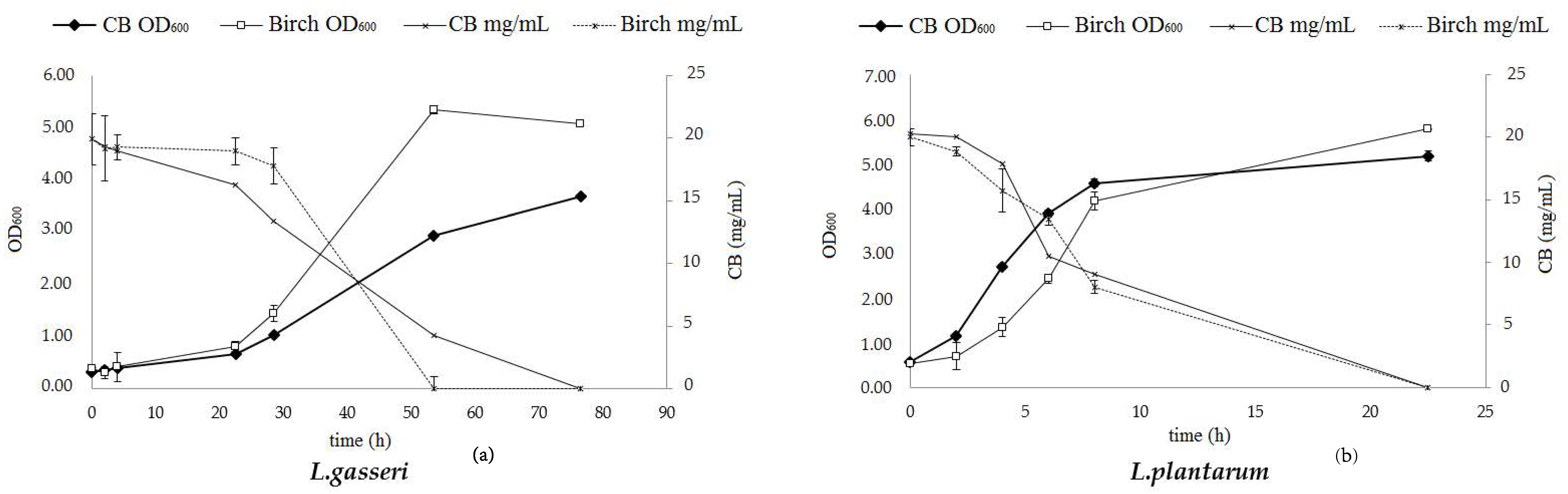
Two Lactobacilli strains were used for testing the prebiotic effect of the birch hydrolysates. The results, as evaluated by the increase of the optical density (OD600) and the carbohydrate accumulation, are depicted in Figure 9. Both strains (L. gasseri and L. plantarum) can efficiently utilize cellobiose which is demonstrated by the growth rate values that reach μ = 0.19 h−1 and μ = 0.78 h−1, respectively. L. plantarum exhibits a relatively higher growth in cellobiose (final OD600 = 5.18 ± 0.19) and consumes the total carbohydrate content within the first 23 h of hydrolysis. L. gasseri is slower (final OD600 = 5.08 ± 0.27) and incubation time over 50 h is required in order to consume the total amount of cellobiose. Lactic acid is the only metabolite that is produced by both L. gasseri and L. plantarum when grown on cellobiose, as depicted in Table 4, while no production of any short chain fatty acid (acetic, propionic or butyric acid) is detected.
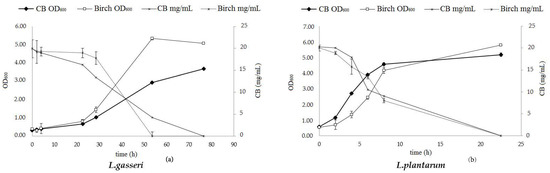
Figure 9.
Growth curve and carbohydrate consumption of (a) L. gasseri and (b) L. plantarum grown anaerobically, at 36 °C, on MRS culture media supplemented with 2% (w/v) cellobiose and with birch hydrolysate at an initial concentration corresponding to 2% (w/v) cellobiose.

Table 4.
Fermentation metabolites (mg/mL) of Lactobacilli strains upon growth on pure cellobiose, birch and spruce COS-rich hydrolysates. No significant amounts of formic or butyric acid were detected.
The results from the studies with birch hydrolysate showed that both strains are able to grow on birch-derived cellobiose as shown in Figure 9 by the increase of the optical density value and the consumption of the cellobiose content. The most effective strain was L. plantarum, with a growth rate of μ = 0.92 h−1, while L. gasseri could also utilize this carbon source, though it was much slower (μ = 0.22 h−1). Determination of the fermentation products (Table 4) reveals the presence of other sugars existing in the biomass hydrolysate that were not detected by HPLC and can be consumed by both strains, since the amount of the lactic acid produced is much higher than the cellobiose that is consumed. In fact, a low amount of glucose is present, but still the final concentration of lactic acid is much higher. Analysis of the product with HPAEC-PAD chromatography revealed traces of sugars with higher degree of polymerization, such as cellotriose and cellotetraose, leading to the conclusion that other oligosaccharides with higher degree of polymerization (DP) originating either for cellulose or hemicellulose exist in the hydrolysate and serve as a carbon source for the bacteria. In a similar way as for the cellobiose substrate, no production of acids was observed.
3. Discussion
Lignocellulosic biomass residues constitute an abundant, renewable source of energy-rich polysaccharides that, when subjected to enzymatic hydrolysis, are transformed into oligomeric and monomeric sugars. The latter can either be processed through chemical and/or biological treatment towards a repertoire of high added-value products or, depending on their structural properties, to be used as animal feed or human dietary supplements with prebiotic properties. Many tons of forest materials are annually made available by the forest industry and these residual streams are usually under-valorized. Biomass from birch trees consists of approximately 43.9% w/w cellulose, while this percentage reaches 42% w/w for spruce trees [21], underlining the great potential of these residues towards the production of COS. It has been already reported in the literature that COS produced by enzymatic hydrolysis of wheat straw, comprised of 84% of cellobiose, showed a substantial improvement of the microbial consortium of weaned pigs [22]. However, limited studies on production of COS from forest biomass have yet been reported. Production of non-digestible COS from forest residual biomass includes a complete process starting from physicochemical pretreatment and fractionation in order to obtain a cellulose-rich solid pulp, followed by controlled enzymatic hydrolysis in order to increase the ratio of oligomers over monomers, product recovery and purification and, eventually, evaluation of prebiotic activity. To the best of our knowledge, this is the first study to report the optimization of parameters for tuning the performance of the commercial cocktail Celluclast® towards the production of oligosaccharides and, moreover, this is one of the first reports that describes the whole procedure covering from the pretreatment/fractionation to the in vitro evaluation of COS prebiotic potential on Lactobacilli strains.
Lignocellulose degradation is a challenging process due a number of factors linked with the recalcitrance and complex nature of this material. The saccharification of the lignocellulosic biomass is hence limited due to these factors. Particularly, the hemicellulose and lignin removal, the decrease of the degree of polymerization of the cellulose chain and the decrease of the cellulose crystallinity index, which is also accompanied by the decrease of the particle size and thus the increase of the accessible surface area, are considered as major requirements for a hydrolysis process to be efficient [23,24]. Therefore, to boost the hydrolysis of lignocellulose towards the production of prebiotic oligosaccharides, there is a necessity to overcome the difficulty and complexity of the depolymerization of this substrate. Applying an efficient pretreatment/fractionation method, such as organosolv process, has proven a promising solution [18,19], particularly by employing food-grade aqueous ethanol solutions. Combining an efficient fractionation process with controlled enzymatic hydrolysis can boost the release of oligomers over monomers. Synthesis of cellobiose and other oligosaccharides with higher DP is also possible through enzyme-mediated condensation reaction of monosaccharides, catalyzed by β-glucosidases [25,26]. However, the reaction rate is quite slow, leading to very low oligosaccharide yields and thus, making the process not economically viable. Compared to synthesis, hydrolysis of cellulose-containing substrates, including lignocellulosic residues, is an attractive alternative. The main bottleneck of the process is the end-product inhibition, which is more severe in case of cellobiohydrolases [27] but can also affect many enzymatic activities that are present in a cellulase mixture. Additionally, the remaining hemicellulose-derived products on the substrate, like xylan, also impact the hydrolysis rates of cellulases [28]. Even though generally the inhibition is more apparent at high-solid concentrations it is also significant at low-solid concentrations [29] and therefore can occur under the conditions of the present study.
To eliminate the adverse effects of the end-product inhibition, we studied the effect of buffer exchange on the increase of cellobiose yields. A similar strategy described as a multistage degradation process of cellulose towards the production of cellobiose has been also reported in the literature [12]. This process is expected not only to alleviate the inhibitory reactions from present cellobiose and glucose, but also to enable the removal of unbound β-glucosidase in the washing fraction, while other cellulases containing a carbohydrate binding module (CBM) remain attached onto the substrate and continue the hydrolysis process. Moreover, we studied the addition of the β-glucosidase inhibitor conduritol-B-epoxide at different concentrations, which led to a substantial increase of the final cellobiose produced. Finally, modifying the reaction conditions and performing the hydrolysis at pH 7.0 resulted in a high ratio of CB:Glu. This is in accordance with data reported in the literature describing that activity of Celluclast® against pNP-glucopyranoside (indicative of β-glucosidase activity) is observed only in a narrow pH range between 4.0–5.5 [30]. By combining buffer exchange with addition of conduritol and changing the pH of the reaction, we achieved a CB:Glu ratio of 21.8, which is the highest among those reported. Ultrafiltration in order to remove the enzymes and possibly re-use them, combined with nanofiltration in order to remove glucose, conduritol and other low-molecular-weight compounds was also employed for the scale-up reactions [31].
LPMOs, a novel class of oxidative biocatalysts acting on carbohydrate-containing substrates, have been shown to boost the hydrolytic performance of cellulase cocktails by improving their accessibility to the cellulosic substrate [17]. LPMOs have been investigated for their implication in defibrillation and separation of cellulose fibrils, acting as “amorphogenesis”-inducing factors [32], thus providing to hydrolases novel cellulose sites for binding and cleavage. Since LPMOs have this mode of action, it was supposed that they can possibly boost the production of cellobiose. Indeed, as shown in our study, their incorporation in the cellulase mixture increased the yield of CB, but this was accompanied by a simultaneous increase of glucose and, as a result, the decrease of the ratio of CB:Glu. In our case, this condition was not selected further for the experimental purposes of the present project.
Testing different substrates showed that at least two-fold higher cellobiose yield was achieved in the case of birch compared to spruce, verifying that the performance of the cocktail differs among biomass samples and depends on the composition and structure of the lignocellulosic materials that differ according to the type of wood that is used. The main hemicellulolytic component of hardwoods (birch) is xylan, while for softwoods (spruce) is glucomannan. Consequently, the response of the different materials to the pretreatment method that is being used is also distinctive. It has been reported that after the organosolv pretreatment of both birch and spruce biomass the lignin removal is more significant for birch than for spruce and therefore, the enzymatic digestibility showed higher improvement in the case of birch than in the case of spruce [33,34], as it can also be observed by the composition of the OS pretreated substrates that were used for this study.
The results of this study clearly demonstrate that there are several possible strategies that allow a fine-tuning of the performance of the commercial enzyme mixture Celluclast® towards the production of cellobiose. This occurs in a way meaning that there is the option of choosing between high production yields and high purity of the obtained product. Consequently, the decision-making can be adjusted accordingly to the purpose of the experiment. In our case, the target was the production of a cellobiose-rich stream, as pure as possible, in order to be incorporated in products and foods with low glycemic index. This study was a part of ForceUp Value project, funded by Vinnova, Sweden, with the aim to provide functional products. The project’s overall approach is to utilize residual lignocellulosic biomass, namely forest feedstocks, for the production of prebiotics to be used as health-beneficial products for human consumption. To achieve this goal towards the production of food supplements with low glycemic index, the focus was on producing COS with a glucose content as low as possible, therefore all experimental design was based on that aspect. The results clearly demonstrate the successful production of COS from birch biomass, as well as their ability to be fermented by beneficial lactic acid bacterial species, which contributes to their prebiotic characteristic.
4. Materials and Methods
4.1. Enzymes and Substrates
For the production of cellobiose, organosolv-pretreated birch (B1: 200 °C for 30 min, 60% (v/v) EtOH, 1% (w/wbiomass) H2SO4; and B2: 200 °C for 15 min, 60% (v/v) EtOH) and spruce (S1: 200 °C for 30 min, 52% (v/v) EtOH, 1% (w/wbiomass) H2SO4; and S2: 200 °C for 30 min, 52% (v/v) EtOH) were used as substrates [18,19]. The compositional analysis of the materials was 77.9% (w/w) cellulose, 8.9% (w/w) hemicellulose, 7.0% (w/w) lignin for B1, 66.3% (w/w) cellulose, 22.0% (w/w) hemicellulose, 7.8% (w/w) lignin for B2 and 72.0% (w/w) cellulose, 4.0% (w/w) hemicellulose, 15.4% (w/w) lignin for S1 and 66.0% (w/w) cellulose, 6.0% (w/w) hemicellulose, 14.9% (w/w) lignin for S2 [18,19]. Glucose and cello-oligosaccharides (DP2-6), as well as weak acids (lactic acid, acetic acid, propionic acid) that were used as analytical standards, were obtained from Sigma-Aldrich (St. Louis, MO, USA). Cellulase mixture from Trichoderma reesei (Celluclast®) and conduritol-B-epoxide (1,2-anhydro-myo-inositol) was purchased from Millipore, Burlington, MA, USA.
4.2. Hydrolysis of Lignocellulosic Materials
Organosolv-pretreated birch B1 was used as a substrate for all experiments towards the optimization of the enzymatic hydrolysis conditions. Organosolv-pretreated birch (B1, B2) and spruce (S1, S2) were then tested as substrates to estimate the cellobiose production from different lignocellulosic feedstocks. All enzymatic treatments took place with a commercially available cellulase mixture from Trichoderma reesei (Celluclast®, Sigma-Aldrich). The initial dry matter (DM) in all experiments was 6% (w/v) and the enzyme loading was 25 or 50 mg/g substrate, as described below for each experimental run. Enzymatic reactions were performed in safe lock microtubes at 1.5 mL reaction volume, at 50 °C, under agitation of 1100 rpm. All reactions were performed in 100 mM phosphate-citrate buffer pH 5.0 and contained 0.02% (w/v) NaN3. Conduritol-B-epoxide was used as a β-glucosidase inhibitor. Buffer exchange was applied at 24 h, while the total hydrolysis time was 48 h. All trials were run in duplicates. At different time intervals (8, 12, 24, 48 h) according to the design and purpose of each experiment, samples were taken, boiled for 5 min for enzyme inactivation, centrifuged and the supernatant was filtered (0.22 μM pore size). As the main reaction products were cellobiose and glucose, sugar analysis was performed by isocratic ion-exchange chromatography, using an Aminex HPX-87H column (Bio-Rad Laboratories, Hercules, CA, USA) with a micro-guard column at 65 °C as previously described [35]. The % w/w cellulose conversion into cellobiose was calculated by following the equation below:
where the concentrations of cellobiose and initial substrate are calculated in mg/mL of reaction volume and 1.05 is the conversion rate of cellobiose to glucose. The % w/w cellulose for each substrate is described in Section 4.1. In case other oligosaccharides were present, the hydrolysates were analyzed with high performance anion exchange chromatography equipped with pulsed amperometric detection (HPAEC-PAD), as previously described [36].
4.3. Evaluation of Synergistic Effect of PcLPMO9D with Cellulases towards Cellobiose Production
Combined activity of cellulases with PcLPMO9D from Phanerochaete chrysosporium [37] towards the production of cellobiose was evaluated. The enzyme, acting on the C1 atom of the glucose molecules and producing lactones as oxidized products, was heterologously produced in Pichia pastoris and purified to its homogeneity according to previously described methods and protocols [37]. The reactions were performed with 6% (w/v) initial DM in 100 mM phosphate-citrate buffer pH 7.0, upon addition of 1.98 mM conduritol-B-epoxide, in the presence of 1 mM ascorbic acid as reducing agent, in safe lock microtubes at 1.5 mL reaction volume, at 50 °C, under agitation of 1100 rpm. Control reaction was performed with 25 mg/g of substrate Celluclast®. To test the effect of PcLPMO9D supplementation, the aforementioned enzyme load was supplemented with 2.5 mg/g of substrate of PcLPMO9D, with the rest of the conditions remaining the same. Another control reaction was also included, in which Celluclast® loading was 27.5 mg/g of substrate. Hydrolysis took place for 8 h. After centrifugation and boiling, the supernatants were filtered (0.22 μM pore size) and the released sugars were detected by HPLC chromatography using an Aminex HPX-87H column as previously described [35].
4.4. Scale-up Hydrolysis Reaction and Product Recovery
After identifying the optimal conditions to maximize the cellobiose yield from birch biomass, a scale-up reaction with B1 as a substrate was set up. The initial dry matter was 6% (w/v) and the enzyme loading was 25 mg/g substrate, all suspended in 15 mM ammonium acetate buffer pH 7.0. Ammonium acetate was selected as a sufficiently volatile salt and it was used as a buffer solution at a low concentration in order to minimize the amount of salts in the final product. The hydrolysis total volume was 60 mL in a 500 mL shake flask and the reaction took place at 50 °C, under continuous agitation of 160 rpm, for 24 h. The hydrolysate was collected after centrifugation, filtrated with 0.22 μm pore size filter and then samples were taken for HPLC analysis for identifying and quantifying the cellobiose and glucose content using an Aminex HPX-87H column, as previously described [35]. Then, the hydrolysate was further processed to ultrafiltration for the removal of the enzymes and nanofiltration for the glucose removal and concentration.
For the removal of the total protein, the hydrolysate was filtrated with a LabScale Tangential Flow Filtration system (TFF) (Millipore, Burlington, MA, USA) with exclusion membrane size 5 kDa (Pellicon XL Ultrafiltration Module Biomax 5 kDa, Millipore). The retentate, containing the concentrated solution of cellulases, was maintained in 4 °C for further use in other hydrolysis experiments. The removal of protein was quickly confirmed by determination of protein content with the Bradford method [38]. The permeate was then collected and applied to the nanofiltration system. Nanofiltration was employed for the removal of glucose, conduritol and other low-molecular-weight compounds, as well as for the concentration of birch hydrolysate. A system comprised of a HP4750 High Pressure Stirred Cell (Sterlitech, Kent, WA, USA) and NF270 (pore size ~200–400 Da, Polyamide-TFC, Flux (GFD/psi) 72-98/130, Dow Filmtec™) was employed. A constant pressure of 10 bar was provided by filling nitrogen gas into the cell, while temperature was set at 50 °C. During the nanofiltration process, samples of the permeate and the feed solutions were taken every 15 min on average depending on the flow rate of the permeate. At the end of the filtration, another sample was taken from the sugar mixture that was inside of the vessel (retentate). All samples were then filtrated with 0.22 μm pore size filters and were analyzed with HPLC chromatography using an Aminex HPX-87H column, as previously described in order to determine the sugar content and the presence of acids originating from biomass components (hemicellulose) or reaction conditions (buffer) [35]. The retentate was then collected, freeze-dried and stored in a dry place until further use.
4.5. Determination of Prebiotic Potential of Birch Hydrolysate
Birch hydrolysates produced after enzymatic hydrolysis were tested by prebiotic tests in order to identify whether they could be utilized as carbon sources and support the growth of probiotic strains. Lactobacillus gasseri DSM 20077 was purchased from DSMZ (Braunschweig, Germany) while Lactobacillus plantarum ATCC 8014 was from ATCC, Manassas, VA, USA. The growth medium for both Lactobacillus strains’ stock cultures was MRS medium with cysteine (Medium 232 DSMZ). Birch hydrolysate was tested at an initial cellobiose concentration of 2% (w/v) in MRS broth prepared at pH 6.0, in the absence of glucose or any other carbohydrate. The obtained media was then sterilized using 0.22 μm pore size filters. Bacteria cells grown in glucose precultures were centrifuged (4000 rpm, 10 min), collected and resuspended in 50 mL MRS medium containing birch-derived sugars. The cultures were incubated anaerobically, at 36 °C, without agitation, for a maximum of 80 h. Growth rate was monitored by identifying the cell density at 600 nm, while sugar consumption and release of fermentation products (lactic acid, acetic acid, etc.) were analyzed using HPLC chromatography with Aminex HPX-87H column as described above [35]. All trials were run in duplicates. Cultures with MRS media with 2% (w/v) cellobiose were used for comparison.
5. Conclusions
The abundance of the lignocellulosic biomass together with its ability to generate high added-value oligosaccharides, such as those derived from the cellulose fraction, through enzymatic treatment make it a sustainable source for the potential larger scale production of these novel food-grade ingredients. In this study, we modified the performance of the commercially available enzyme mixture, Celluclast®, towards the production of COS from birch biomass. The potential of the hydrolysis product to support the growth of two Lactobacilli probiotic strains as a sole carbon source was also demonstrated.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/9/11/897/s1, Table S1: Hydrolysis yields after 24 and 48 h of hydrolysis. Cellobiose (CB) and glucose (Glu) production is expressed in % w/w cellulose conversion. Table S2: Effect of pH and enzyme loading on the % w/w cellulose conversion into CB and Glu. Table S3: Cellulose conversion (% w/w) to CB and Glu for different hydrolysis time and evaluation of buffer exchange after 72 and 96 h of hydrolysis. Table S4: Effect of addition of various concentrations of conduritol-B-epoxide at pH 7.0 on the % w/w cellulose conversion into CB and Glu. Table S5: Effect of buffer exchange and/or supplementation with additional enzyme loading or conduritol-B-epoxide on the cellulose conversion (% w/w) to CB and Glu. Table S6: Hydrolysis yields from birch and spruce substrates, described as % w/w cellulose conversion into cellobiose and glucose of at pH 7.0, upon the addition of 1.98 mM conduritol-B-epoxide, at an enzyme loading of 25 mg/g of substrate, with buffer exchange at 8 and 24 h.
Author Contributions
Conceptualization, A.K., L.M., U.R. and P.C.; methodology, A.K. and L.M..; investigation, A.K., L.M., S.B., M.N.M.; data curation, A.K. and LM.; writing—original draft preparation, A.K.; writing—review and editing, L.M.; supervision, P.C. and U.R.; project administration, P.C. and U.R.; funding acquisition, U.R. All authors have read and approved the final manuscript.
Funding
This work was partially funded by Vinnova, BioInnovation Program Food-grade prebiotic oligosaccharide production, merging marine, and forest resources for moving up the cellulose value-chain (ForceUpValue).
Acknowledgments
Eva Grahn Håkansson from Essum Probiotics AB is greatly acknowledged for providing her expertise regarding the prebiotic activity tests. Sveaskog is greatly acknowledged for providing the forest materials. We would like to thank Bing Liu and Mats Sandgren from Swedish University of Agricultural Sciences, Uppsala, for providing the PcLPMO9D. Finally, Bio4Energy, a strategic research environment appointed by the Swedish government, is also acknowledged for supporting this work.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M. Prebiotics: The concept revisited. J. Nutr. 2007, 137, 830S–837S. [Google Scholar] [CrossRef] [PubMed]
- Swennen, K.; Courtin, C.M.; Delcour, J.A. Non-digestible oligosaccharides with prebiotic properties. Crit. Rev. Food Sci. Nutr. 2006, 46, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Belorkar, S.A.; Gupta, A.K. Oligosaccharides: A boon from nature’s desk. AMB Express 2016, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Mancilha, I.M. Non-digestible oligosaccharides: A review. Carbohydr. Polym. 2007, 68, 587–597. [Google Scholar] [CrossRef]
- Pokusaeva, K.; O’Connell-Motherway, M.; Zomer, A.; MacSharry, J.; Fitzgerald, G.F.; van Sinderen, D. Cellodextrin utilization by Bifidobacterium breve UCC2003. Appl. Environ. Microbiol. 2011, 77, 1681–1690. [Google Scholar] [CrossRef]
- Nakamura, S.; Oku, T.; Ichinose, M. Bioavailability of cellobiose by tolerance test and breath hydrogen excretion in humans. Nutrition 2004, 20, 979–983. [Google Scholar] [CrossRef]
- Basholli-Salihu, M.; Mueller, M.; Unger, F.M.; Viernstein, H. The use of cellobiose and fructooligosaccharide on growth and stability of Bifidobacterium infantis in fermented milk. Food Nutr. Sci. 2013, 4, 1301. [Google Scholar] [CrossRef]
- Satouchi, M.; Watanabe, T.; Wakabayashi, S.; Ohokuma, K.; Koshijima, T.; Kuwahara, M. Digestibility, absorptivity and physiological effects of cellooligosaccharides in human and rat. Nippon Eiyo Shokuryo Gakkaishi 1996, 49, 143–148. [Google Scholar] [CrossRef]
- Watanabe, T. Development of physiological functions of cellooligosaccharides. Cellul. Commun. 1998, 5, 91–97. [Google Scholar]
- Karnaouri, A.; Topakas, E.; Matsakas, L.; Rova, U.; Christakopoulos, P. Fine-tuned enzymatic hydrolysis of organosolv pretreated forest materials for the efficient production of cellobiose. Front. Chem 2018, 6, 128. [Google Scholar] [CrossRef] [PubMed]
- Vanderghem, C.; Boquel, P.; Blecker, C.; Paquot, M. A multistage process to enhance cellobiose production from cellulosic materials. Appl. Biochem. Biotechnol. 2010, 160, 2300–2307. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, A.; Tominaga, K.; Nishiyama, N.; Yuasa, K. Comprehensive enzymatic analysis of the cellulolytic system in digestive fluid of the Sea Hare Aplysia kurodai. Efficient glucose release from sea lettuce by synergistic action of 45 kDa endoglucanase and 210 kDa ß-glucosidase. PLoS ONE 2013, 8, e65418. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.L.; Kallemeijn, W.W.; Lelieveld, L.T.; Mirzaian, M.; Zoutendijk, I.; Vardi, A.; Futerman, A.H.; Meijer, A.H.; Spaink, H.P.; Overkleeft, H.S.; et al. In vivo inactivation of glycosidases by conduritol B epoxide and cyclophellitol as revealed by activity-based protein profiling. FEBS J. 2019, 286, 3. [Google Scholar] [CrossRef] [PubMed]
- Witte, M.D.; van der Marel, G.A.; Aerts, J.M.; Overkleeft, H.S. Irreversible inhibitors and activity-based probes as research tools in chemical glycobiology. Org. Biomol. Chem. 2011, 9, 5908–5926. [Google Scholar] [CrossRef]
- Rodrigues, A.C.; Haven, M.Ø.; Lindedam, J.; Felby, C.; Gama, M. Celluclast and Cellic® CTec2: Saccharification/fermentation of wheat straw, solid-liquid partition and potential of enzyme recycling by alkaline washing. Enzym. Microb. Technol. 2015, 79–80, 70–77. [Google Scholar] [CrossRef]
- Hu, J.; Arantes, V.; Pribowo, A.; Gourlay, K.; Saddler, J. Substrate factors that influence the synergistic interaction of AA9 and cellulases during the enzymatic hydrolysis of biomass. Energy Environ. Sci. 2014, 7, 2308–2315. [Google Scholar] [CrossRef]
- Matsakas, L.; Nitsos, C.; Raghavendran, V.; Yakimenko, O.; Persson, G.; Olsson, E.; Rova, U.; Olsson, L.; Christakopoulos, P. A novel hybrid organosolv: Steam explosion method for the efficient fractionation and pretreatment of birch biomass. Biotechnol. Biofuels 2018, 11, 1–14. [Google Scholar] [CrossRef]
- Matsakas, L.; Raghavendran, V.; Yakimenko, O.; Persson, G.; Olsson, E.; Rova, U.; Olsson, L.; Christakopoulos, P. Lignin-first biomass fractionation using a hybrid organosolv—Steam explosion pretreatment technology improves the saccharification and fermentability of spruce biomass. Bioresour. Technol. 2019, 273, 521–528. [Google Scholar] [CrossRef]
- Umezurike, G.M. The mechanism of action of beta-glucosidase from Botryodiplodia theobromae Pat. Biochem. J. 1987, 241, 455–462. [Google Scholar] [CrossRef]
- Willför, S.; Sundberg, A.; Hemming, J.; Holmbom, B. Polysaccharides in some industrially important softwood species. Wood Sci. Technol. 2005, 39, 245–257. [Google Scholar] [CrossRef]
- Jiao, L.F.; Ke, Y.L.; Xiao, K.; Song, Z.H.; Hu, C.H.; Shi, B. Effects of cello-oligosaccharide on intestinal microbiota and epithelial barrier function of weanling pigs. J. Anim. Sci. 2015, 93, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, S.D.; Mooney, C.; Saddler, J.N. Substrate and enzyme characteristics that limit cellulose hydrolysis. Biotechnol. Prog. 1999, 15, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, L.; Liu, D. Biomass recalcitrance. Part I: The chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels Bioprod. Biorefin. 2012, 6, 465–482. [Google Scholar] [CrossRef]
- Ajisaka, K.; Nishida, H.; Fujimoto, H. The synthesis of oligosaccharides by the reversed hydrolysis reaction of beta-glucosidase at high substrate concentration and at high temperature. Biotechnol. Lett 1987, 9, 243–248. [Google Scholar] [CrossRef]
- Bucke, C. Oligosaccharide synthesis using glycosidases. J. Chem. Technol. Biotechnol. 1996, 67, 217–220. [Google Scholar] [CrossRef]
- Murphy, L.; Bohlin, C.; Baumann, M.J.; Olsen, S.N.; Sørensen, T.H.; Anderson, L.; Borch, K.; Westh, P. Product inhibition of five Hypocrea jecorina cellulases. Enzym. Microb. Technol 2009, 52, 163–169. [Google Scholar] [CrossRef]
- Qing, Q.; Yang, B.; Wyman, C.E. Xylooligomers are strong inhibitors of cellulose hydrolysis by enzymes. Bioresour. Technol. 2010, 101, 9624. [Google Scholar] [CrossRef]
- Modenbach, A.A.; Nokes, S.E. Enzymatic hydrolysis of biomass at high-solids loadings—A review. Biosyst. Agric. Eng. Fac. Publ. 2013. [Google Scholar] [CrossRef]
- Herlet, J.; Kornberger, P.; Roessler, B.; Glanz, J.; Schwarz, W.H.; Liebl, W.; Zverlov, V.V. A new method to evaluate temperature vs. pH activity profiles for biotechnological relevant enzymes. Biotechnol. Biofuels 2017, 10, 234. [Google Scholar] [CrossRef]
- Qi, B.; Luo, J.; Chen, G.; Chen, X.; Wan, Y. Application of ultrafiltration and nanofiltration for recycling cellulase and concentrating glucose from enzymatic hydrolyzate of steam exploded wheat straw. Bioresour. Technol. 2012, 104, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Villares, A.; Moreau, C.; Bennati-Granier, C.; Garajova, S.; Foucat, L.; Falourd, X.; Saake, B.; Berrin, J.G.; Cathala, B. Lytic polysaccharide monooxygenases disrupt the cellulose fibers structure. Sci. Rep. 2017, 7, 40262. [Google Scholar] [CrossRef] [PubMed]
- Nitsos, C.; Stoklosa, R.; Karnaouri, A.; Vörös, D.; Lange, H.; Hodge, D.; Crestini, C.; Rova, U.; Christakopoulos, P. Isolation and characterization of organosolv and alkaline lignins from hardwood and softwood biomass. ACS Sustain. Chem. Eng. 2016, 4, 5181–5193. [Google Scholar] [CrossRef]
- Raghavendran, V.; Nitsos, C.; Matsakas, L.; Rova, U.; Christakopoulos, P.; Olsson, L. A comparative study of the enzymatic hydrolysis of batch organosolv-pretreated birch and spruce biomass. AMB Express 2018, 8, 114. [Google Scholar] [CrossRef]
- Karnaouri, A.; Rova, U.; Christakopoulos, P. Effect of different pretreatment methods on birch outer bark: New biorefinery routes. Molecules 2016, 21, 427. [Google Scholar] [CrossRef] [PubMed]
- Karnaouri, A.; Muraleedharan, M.N.; Dimarogona, M.; Topakas, E.; Rova, U.; Sandgren, M.; Christakopoulos, P. Recombinant expression of thermostable processive MtEG5 endoglucanase and its synergism with MtLPMO from Myceliophthora thermophila during the hydrolysis of lignocellulosic substrates. Biotechnol. Biofuels 2017, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Westereng, B.; Ishida, T.; Vaaje-Kolstad, G.; Wu, M.; Eijsink, V.G.; Igarashi, K.; Samejima, M.; Ståhlberg, J.; Horn, S.J.; Sandgren, M. The putative endoglucanase PcGH61D from Phanerochaete chrysosporium is a metal-dependent oxidative enzyme that cleaves cellulose. PLoS ONE 2011, 6, e27807. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).