Facile Fabrication of Metal Oxide Based Catalytic Electrodes by AC Plasma Deposition and Electrochemical Detection of Hydrogen Peroxide
Abstract
:1. Introduction
2. Results
2.1. Characterization of the Catalytic Electrode Prepared by AC Plasma Deposition
2.1.1. Contact angle and Wettability
2.1.2. Scanning Electron Microscopy (SEM)
2.1.3. Microstructure Analysis
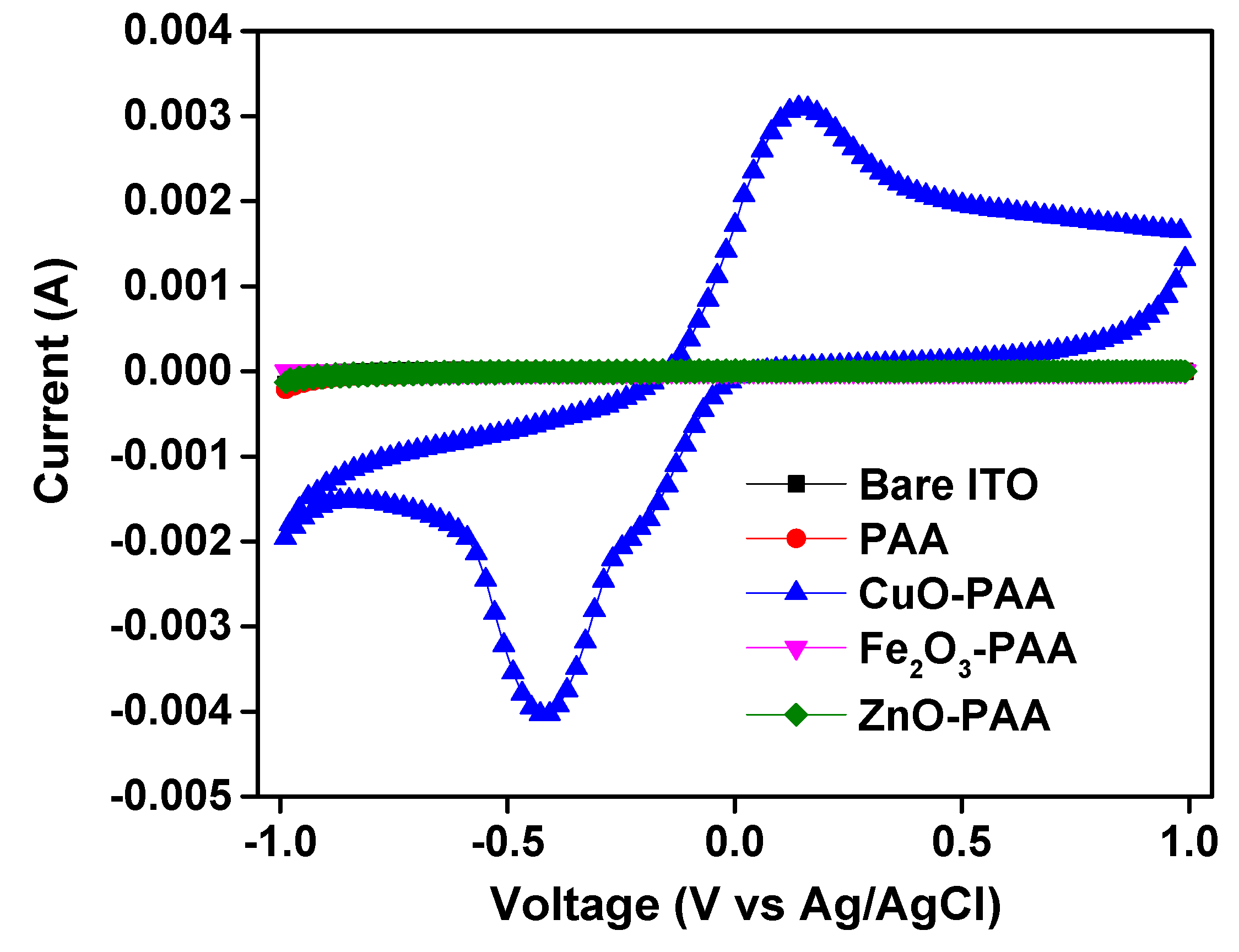
2.1.4. Cyclic Voltammetry
2.2. Electrocatalytic Determination of H2O2
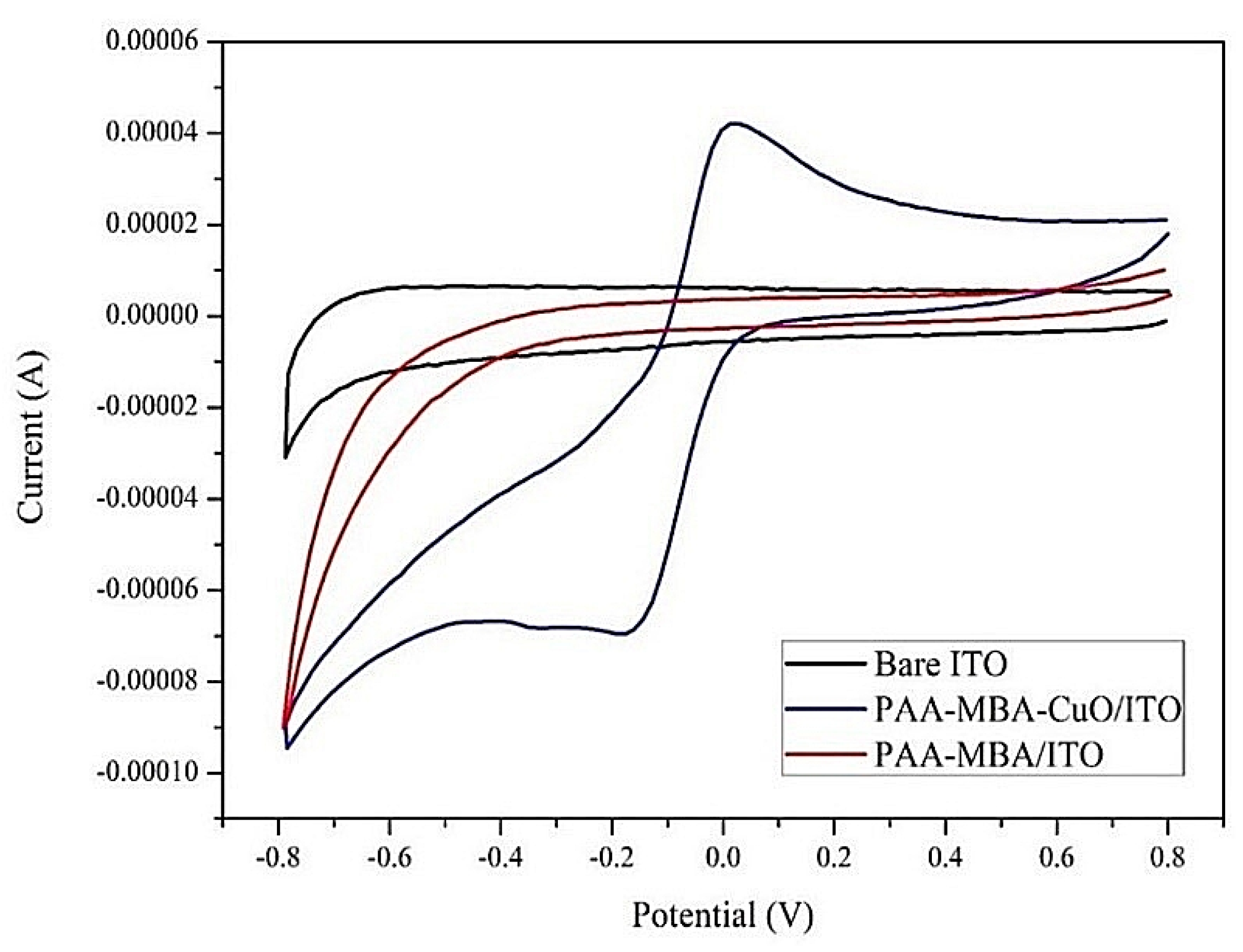
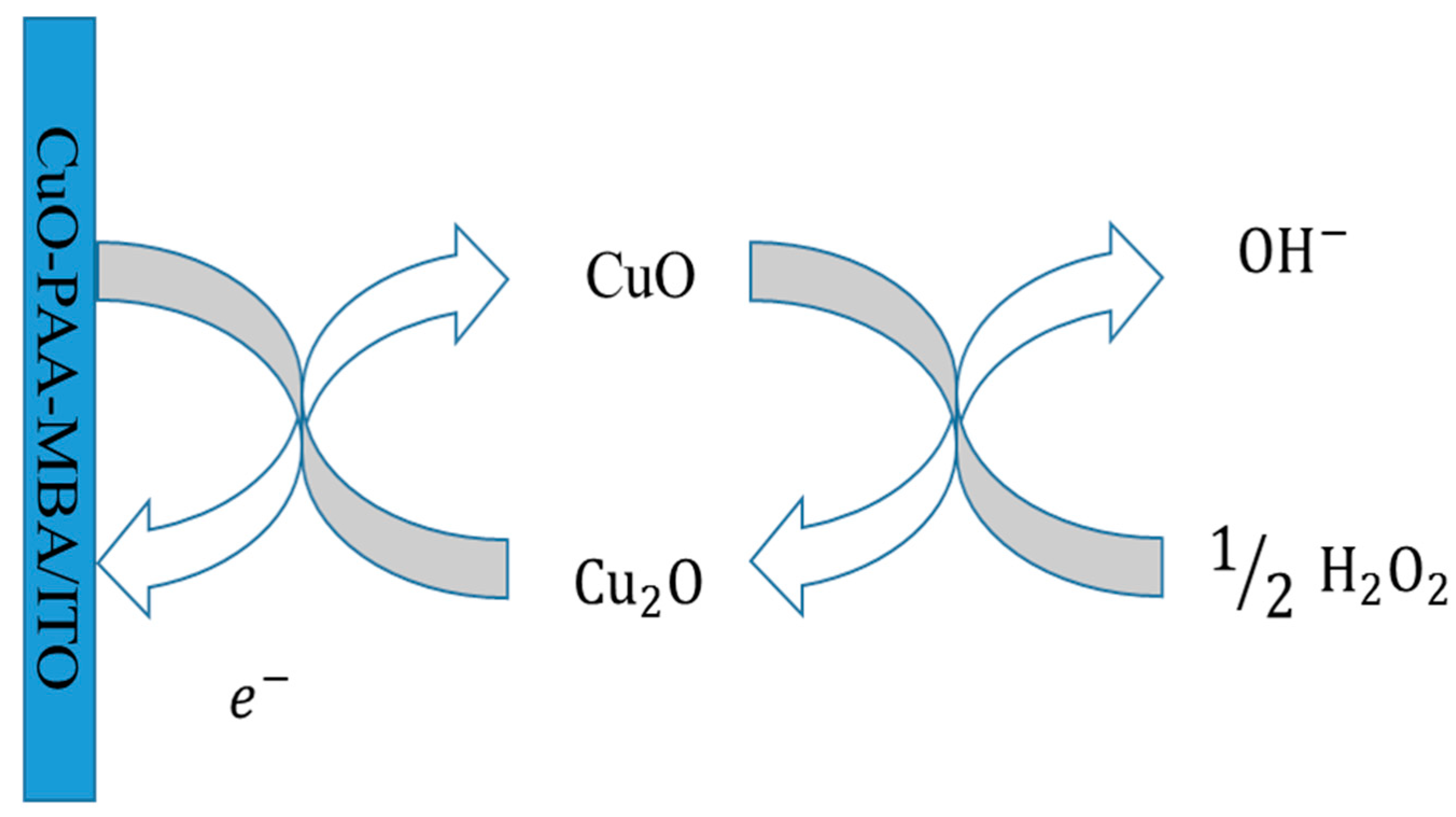
2.2.1. Electrode Catalytic Activity
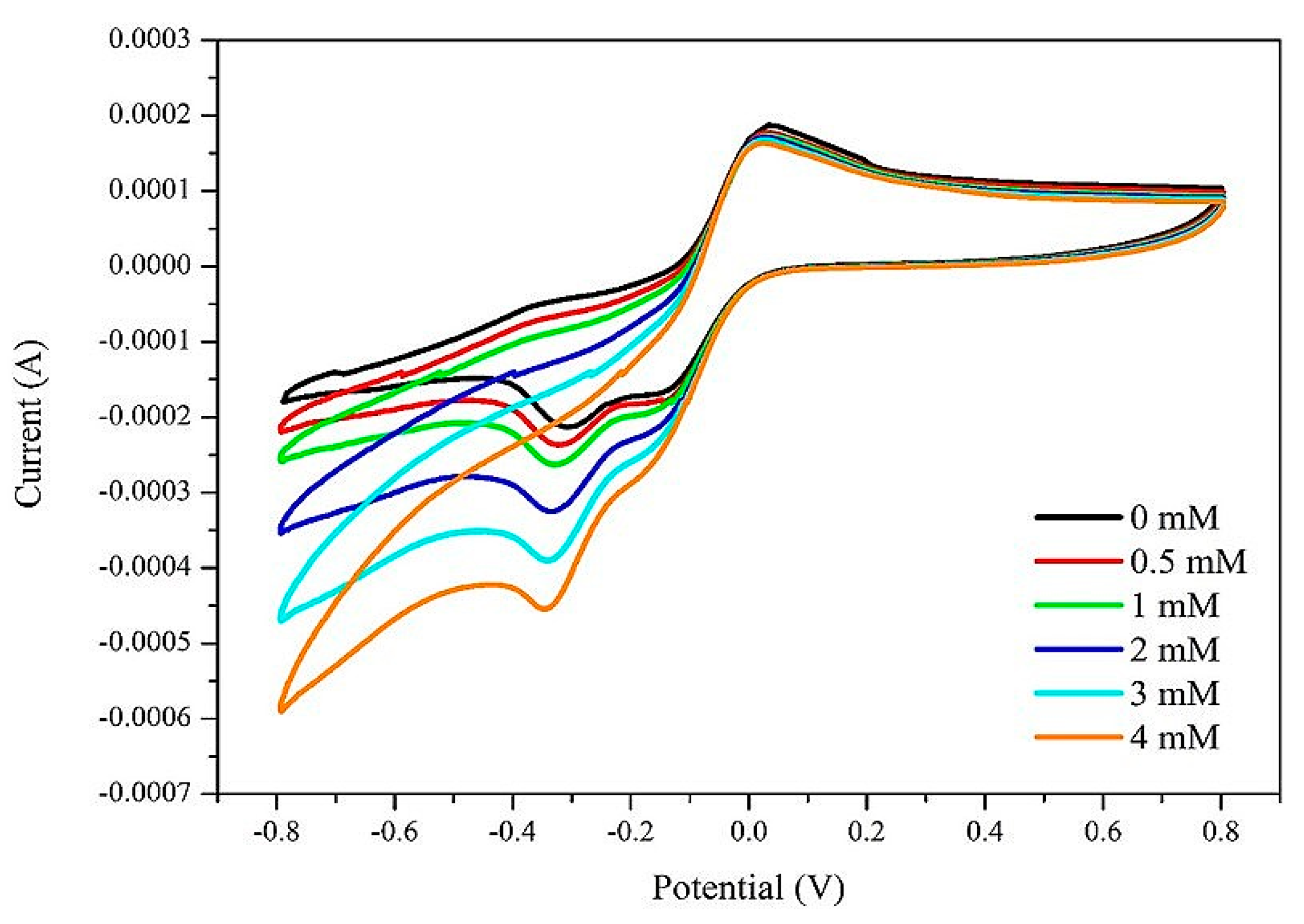
2.2.2. Feasibility Analysis of Electrochemical H2O2 Detection
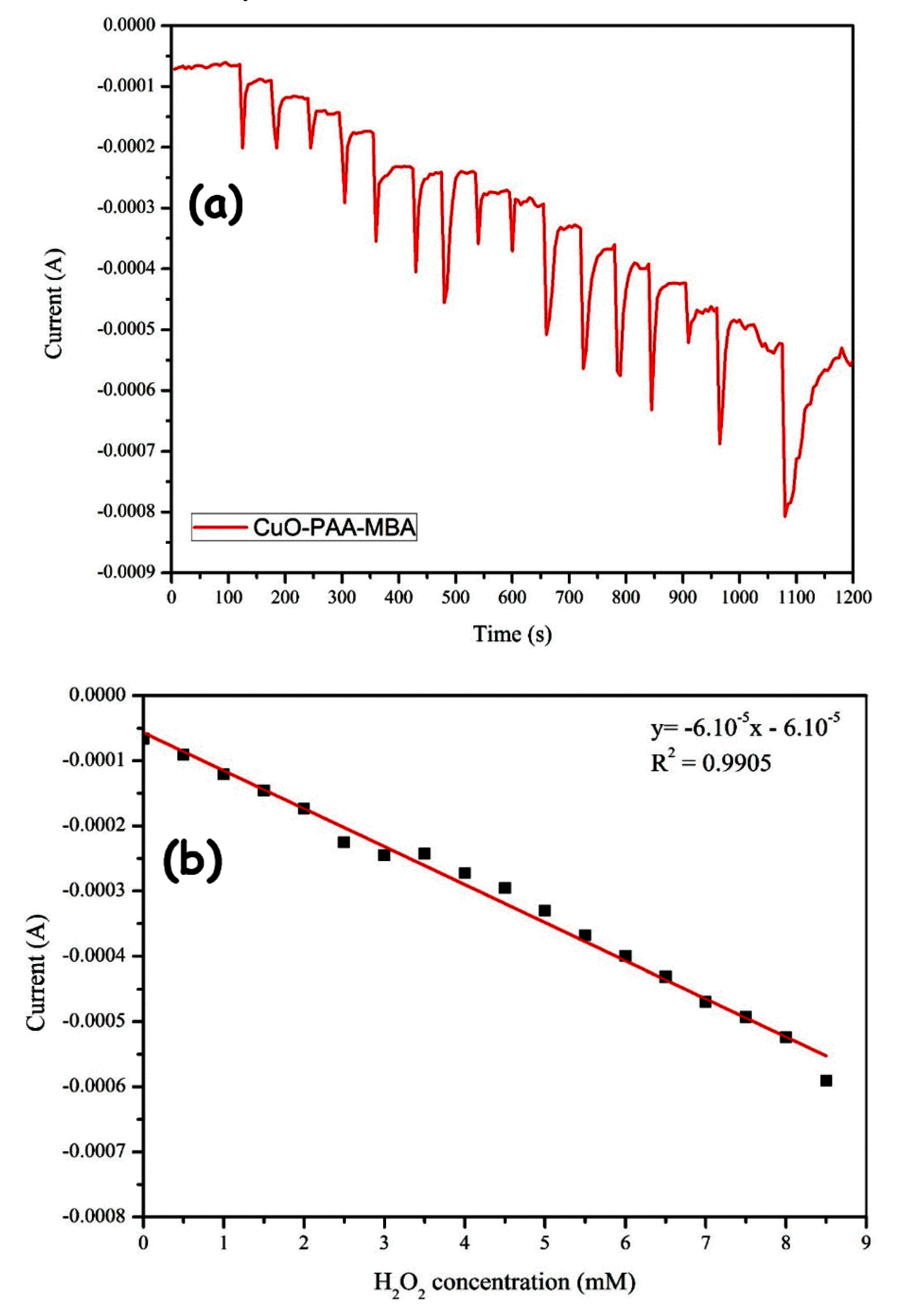
2.2.3. Amperometric H2O2 Detection and Sensor Performances
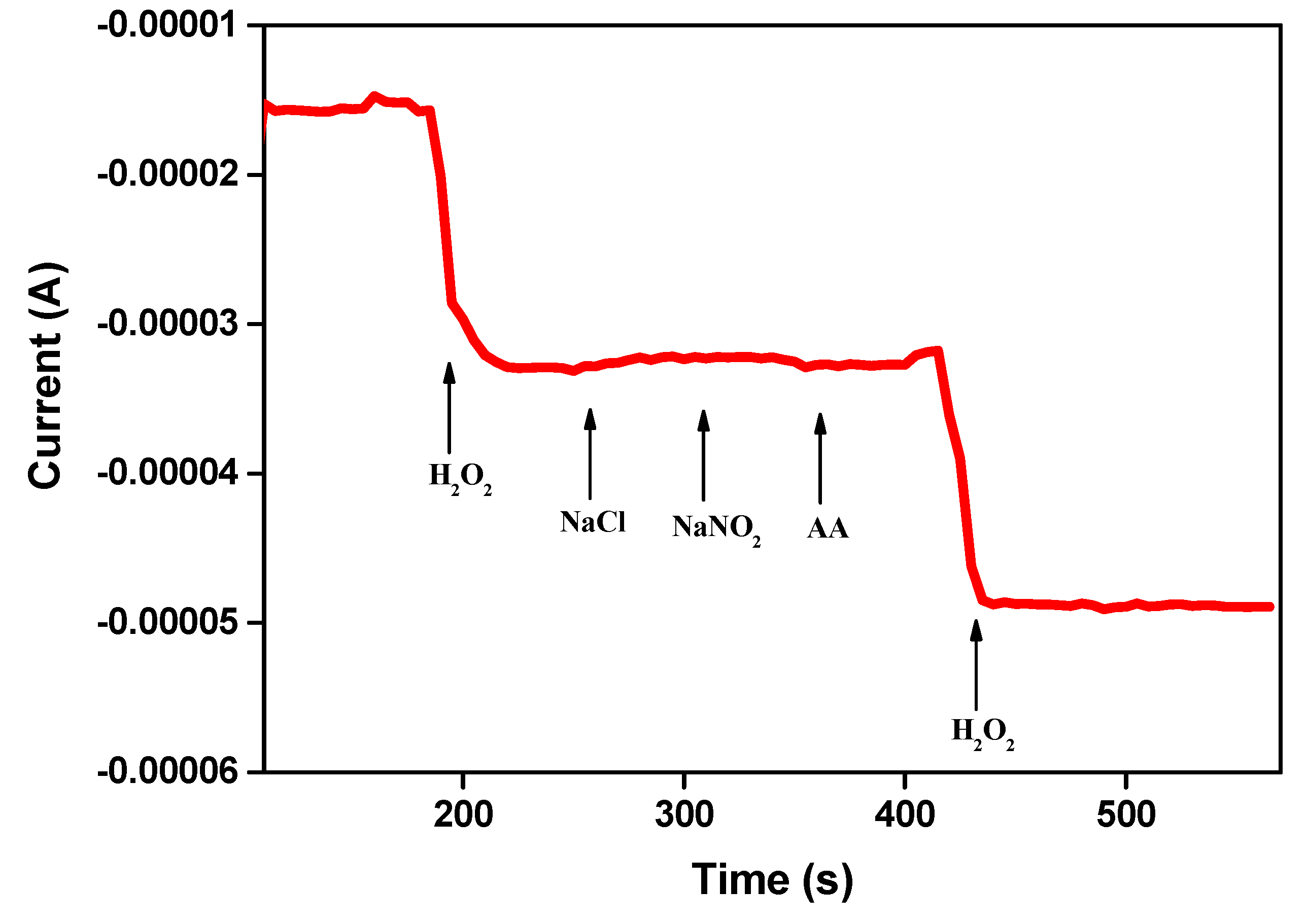
2.2.4. Selectivity Measurement of the Fabricated Catalytic Electrode
2.2.5. Stability Measurement of the Fabricated Catalytic Electrode
3. Discussion
Electrode Fabrication by Plasma Treatment
4. Materials and Methods
4.1. Reagents
4.2. Instruments
4.3. Fabrication of Catalytic Electrode by AC Plasma Deposition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Freakley, S.J.; He, Q.; Harrhy, J.H.; Lu, L.; Crole, D.A.; Morgan, D.J.; Ntainjua, E.N.; Edwards, J.K.; Carley, A.F.; Borisevich, A.Y.; et al. Palladium-tin catalysts for the direct synthesis of H2O2 with high selectivity. Science 2016, 351, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Wang, L.; Li, G.; Guo, H. A review on research progress in the direct synthesis of hydrogen peroxide from hydrogen and oxygen: Noble-metal catalytic method, fuel-cell method and plasma method. Catal. Sci. Technol. 2016, 6, 1593–1610. [Google Scholar] [CrossRef]
- Benzigar, M.R.; Joseph, S.; Saianand, G.; Gopalan, A.-I.; Sarkar, S.; Srinivasan, S.; Park, D.-H.; Kim, S.; Talapaneni, S.N.; Ramadass, K.; et al. Highly ordered iron oxide-mesoporous fullerene nanocomposites for oxygen reduction reaction and supercapacitor applications. Microporous Mesoporous Mater. 2019, 285, 21–31. [Google Scholar] [CrossRef]
- Anantha-Iyengar, G.; Shanmugasundaram, K.; Nallal, M.; Lee, K.-P.; Whitcombe, M.J.; Lakshmi, D.; Sai-Anand, G. Functionalized conjugated polymers for sensing and molecular imprinting applications. Prog. Polym. Sci. 2019, 88, 1–129. [Google Scholar] [CrossRef]
- Sai-Anand, G.; Sivanesan, A.; Benzigar, M.R.; Singh, G.; Gopalan, A.-I.; Baskar, A.V.; Ilbeygi, H.; Ramadass, K.; Kambala, V.; Vinu, A. Recent Progress on the sensing of pathogenic bacteria using advanced nanostructures. Bull. Chem. Soc. Jpn. 2019, 92, 216–244. [Google Scholar] [CrossRef]
- Komathi, S.; Gopalan, A.I.; Kim, S.-K.; Anand, G.S.; Lee, K.-P. Fabrication of horseradish peroxidase immobilized poly (N-[3-(trimethoxy silyl) propyl]aniline) gold nanorods film modified electrode and electrochemical hydrogen peroxide sensing. Electrochim. Acta 2013, 92, 71–78. [Google Scholar] [CrossRef]
- Gopalan, A.I.; Komathi, S.; Sai Anand, G.; Lee, K.-P. Nanodiamond based sponges with entrapped enzyme: A novel electrochemical probe for hydrogen peroxide. Biosens. Bioelectron. 2013, 46, 136–141. [Google Scholar] [CrossRef]
- Shanmugasundaram, K.; Sai-Anand, G.; Gopalan, A.-I.; Lee, H.-G.; Yeo, H.K.; Kang, S.-W.; Lee, K.-P. Direct electrochemistry of cytochrome c with three-dimensional nanoarchitectured multicomponent composite electrode and nitrite biosensing. Sens. Actuators B Chem. 2016, 228, 737–747. [Google Scholar] [CrossRef]
- Sai-Anand, G.; Gopalan, A.-I.; Kang, S.-W.; Komathi, S.; Lee, K.-P. One pot synthesis of new gold nanoparticles dispersed poly (2-aminophenyl boronic acid) composites for fabricating an affinity based electrochemical detection of glucose. Sci. Adv. Mater. 2014, 6, 1356–1364. [Google Scholar] [CrossRef]
- Thunkhamrak, C.; Chuntib, P.; Ounnunkad, K.; Banet, P.; Aubert, P.-H.; Saianand, G.; Gopalan, A.-I.; Jakmunee, J. Highly sensitive voltammetric immunosensor for the detection of prostate specific antigen based on silver nanoprobe assisted graphene oxide modified screen printed carbon electrode. Talanta 2019, 208, 120389. [Google Scholar] [CrossRef]
- Kang, B.-H.; Kim, J.-S.; Lee, J.-S.; Lee, S.-W.; Sai-Anand, G.; Jeong, H.-M.; Lee, S.-H.; Kwon, D.-H.; Kang, S.-W. Solution processable CdSe/ZnS quantum dots light-emitting diodes using ZnO nanocrystal as electron transport layer. J. Nanosci. Nanotechnol. 2015, 15, 7416–7420. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-G.; Gopalan, A.-I.; Sai-Anand, G.; Lee, B.-C.; Kang, S.-W.; Lee, K.-P. Facile synthesis of functionalized graphene-palladium nanoparticle incorporated multicomponent TiO2 composite nanofibers. Mater. Chem. Phys. 2015, 154, 125–136. [Google Scholar] [CrossRef]
- Lee, S.-W.; Cha, S.-H.; Choi, K.-J.; Kang, B.-H.; Lee, J.-S.; Kim, S.-W.; Kim, J.-S.; Jeong, H.-M.; Gopalan, S.-A.; Kwon, D.-H.; et al. Low dark-current, high current-gain of PVK/ZnO nanoparticles composite-based UV photodetector by PN-heterojunction control. Sensors 2016, 16, 74. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-C.; Gopalan, A.-I.; Sai-Anand, G.; Lee, K.-P.; Kim, W.-J. Preparation of visible light photocatalytic graphene embedded rutile titanium (IV) oxide composite nanowires and enhanced nox removal. Catalysts 2019, 9, 170. [Google Scholar] [CrossRef]
- Lee, H.-G.; Sai-Anand, G.; Komathi, S.; Gopalan, A.-I.; Kang, S.-W.; Lee, K.-P. Efficient visible-light-driven photocatalytic degradation of nitrophenol by using graphene-encapsulated TiO2 nanowires. J. Hazard. Mater. 2015, 283, 400–409. [Google Scholar] [CrossRef]
- Sai-Anand, G.; Gopalan, A.-I.; Lee, K.-P.; Venkatesan, S.; Qiao, Q.; Kang, B.-H.; Lee, S.-W.; Lee, J.-S.; Kang, S.-W. Electrostatic nanoassembly of contact interfacial layer for enhanced photovoltaic performance in polymer solar cells. Sol. Energy Mater. Sol. Cells 2016, 153, 148–163. [Google Scholar] [CrossRef]
- Lee, S.-W.; Choi, K.-J.; Kang, B.-H.; Lee, J.-S.; Kim, S.-W.; Kwon, J.-B.; Gopalan, S.-A.; Bae, J.-H.; Kim, E.-S.; Kwon, D.-H.; et al. Low dark current and improved detectivity of hybrid ultraviolet photodetector based on carbon-quantum-dots/zinc-oxide-nanorod composites. Org. Electron. 2016, 39, 250–257. [Google Scholar] [CrossRef]
- Liu, M.; Liu, R.; Chen, W. Graphene wrapped Cu2O nanocubes: Non-enzymatic electrochemical sensors for the detection of glucose and hydrogen peroxide with enhanced stability. Biosens. Bioelectron. 2013, 45, 206–212. [Google Scholar] [CrossRef]
- Xu, F.; Deng, M.; Li, G.; Chen, S.; Wang, L. Electrochemical behavior of cuprous oxide–reduced graphene oxide nanocomposites and their application in nonenzymatic hydrogen peroxide sensing. Electrochim. Acta 2013, 88, 59–65. [Google Scholar] [CrossRef]
- Jiang, L.-C.; Zhang, W.-D. A highly sensitive nonenzymatic glucose sensor based on CuO nanoparticles-modified carbon nanotube electrode. Biosens. Bioelectron. 2010, 25, 1402–1407. [Google Scholar] [CrossRef]
- Batchelor-McAuley, C.; Du, Y.; Wildgoose, G.G.; Compton, R.G. The use of copper (II) oxide nanorod bundles for the non-enzymatic voltammetric sensing of carbohydrates and hydrogen peroxide. Sens. Actuators B Chem. 2008, 135, 230–235. [Google Scholar] [CrossRef]
- Weng, S.; Zheng, Y.; Zhao, C.; Zhou, J.; Lin, L.; Zheng, Z.; Lin, X.J.M.A. CuO nanoleaf electrode: Facile preparation and nonenzymatic sensor applications. Microchim. Acta 2013, 180, 371–378. [Google Scholar] [CrossRef]
- Wang, X.; Hu, C.; Liu, H.; Du, G.; He, X.; Xi, Y. Synthesis of CuO nanostructures and their application for nonenzymatic glucose sensing. Sens. Actuators B Chem. 2010, 144, 220–225. [Google Scholar] [CrossRef]
- Wang, K.; Dong, X.; Zhao, C.; Qian, X.; Xu, Y. Facile synthesis of Cu2O/CuO/RGO nanocomposite and its superior cyclability in supercapacitor. Electrochim. Acta 2015, 152, 433–442. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Ni, Y.; Li, J.; Liao, K.; Zhao, G. Porous cuprous oxide microcubes for non-enzymatic amperometric hydrogen peroxide and glucose sensing. Electrochem. Commun. 2009, 11, 812–815. [Google Scholar] [CrossRef]
- Peng, H.; Fangli, Y.; Liuyang, B.; Jinlin, L.; Yunfa, C. Plasma synthesis of large quantities of zinc oxide nanorods. J. Phys. Chem. C 2007, 111, 194–200. [Google Scholar] [CrossRef]
- Langereis, E.; Creatore, M.; Heil, S.B.S.; Sanden, M.C.M.v.d.; Kessels, W.M.M. Plasma-assisted atomic layer deposition of Al2O3 moisture permeation barriers on polymers. Appl. Phys. Lett. 2006, 89, 081915. [Google Scholar] [CrossRef]
- Ben Salem, D.; Carton, O.; Fakhouri, H.; Pulpytel, J.; Arefi-Khonsari, F. Deposition of water stable plasma polymerized acrylic Acid/MBA organic coatings by atmospheric pressure Air Plasma Jet. Plasma Process. Polym. 2014, 11, 269–278. [Google Scholar] [CrossRef]
- Ishida, Y.; Motokane, Y.; Tokunaga, T.; Yonezawa, T. Plasma induced tungsten doping of TiO2 particles for enhancement of photocatalysis under visible light. Phys. Chem. Chem. Phys. 2015, 17, 24556–24559. [Google Scholar] [CrossRef]
- Hattori, Y.; Nomura, S.; Mukasa, S.; Toyota, H.; Inoue, T.; Kasahara, T. Synthesis of tungsten trioxide nanoparticles by microwave plasma in liquid and analysis of physical properties. J. Alloys Compd. 2013, 560, 105–110. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Zhang, H.; Duan, X.; Xu, J.; Wen, Y. Electrochemical sensing application of poly (acrylic acid modified EDOT-co-EDOT): PSS and its inorganic nanocomposite with high soaking stability, adhesion ability and flexibility. RSC Adv. 2015, 5, 12237–12247. [Google Scholar] [CrossRef]
- O’Hare, L.-A.; O’Neill, L.; Goodwin, A.J. Anti-microbial coatings by agent entrapment in coatings deposited via atmospheric pressure plasma liquid deposition. Surf. Interface Anal. 2006, 38, 1519–1524. [Google Scholar] [CrossRef]
- Nisol, B.; Batan, A.; Dabeux, F.; Kakaroglou, A.; De Graeve, I.; Van Assche, G.; Van Mele, B.; Terryn, H.; Reniers, F. Surface characterization of atmospheric pressure plasma-deposited allyl methacrylate and acrylic acid based coatings. Plasma Process. Polym. 2013, 10, 564–571. [Google Scholar] [CrossRef]
- Beck, A.J.; Short, R.D.; Matthews, A. Deposition of functional coatings from acrylic acid and octamethylcyclotetrasiloxane onto steel using an atmospheric pressure dielectric barrier discharge. Surf. Coat. Technol. 2008, 203, 822–825. [Google Scholar] [CrossRef]
- Wei, C.; Sun, S.; Mandler, D.; Wang, X.; Qiao, S.Z.; Xu, Z.J. Approaches for measuring the surface areas of metal oxide electrocatalysts for determining their intrinsic electrocatalytic activity. Chem. Soc. Rev. 2019, 48, 2518–2534. [Google Scholar] [CrossRef]
- Cao, D.; Chao, J.; Sun, L.; Wang, G. Catalytic behavior of Co3O4 in electroreduction of H2O2. J. Power Sources 2008, 179, 87–91. [Google Scholar] [CrossRef]
- Polonskyi, O.; Ahadi, A.M.; Peter, T.; Fujioka, K.; Abraham, J.W.; Vasiliauskaite, E.; Hinz, A.; Strunskus, T.; Wolf, S.; Bonitz, M.; et al. Plasma based formation and deposition of metal and metal oxide nanoparticles using a gas aggregation source. Eur. Phys. J. D 2018, 72, 93. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Ghijsen, J.; Tjeng, L.H.; van Elp, J.; Eskes, H.; Westerink, J.; Sawatzky, G.A.; Czyzyk, M.T. Electronic structure of Cu2O and CuO. Phys. Rev. B 1988, 38, 11322–11330. [Google Scholar] [CrossRef]
- Heli, H.; Jafarian, M.; Mahjani, M.G.; Gobal, F. Electro-oxidation of methanol on copper in alkaline solution. Electrochim. Acta 2004, 49, 4999–5006. [Google Scholar] [CrossRef]
- Meng, F.; Yan, X.; Liu, J.; Gu, J.; Zou, Z. Nanoporous gold as non-enzymatic sensor for hydrogen peroxide. Electrochim. Acta 2011, 56, 4657–4662. [Google Scholar] [CrossRef]
- Tomida, T.; Hamaguchi, K.; Tunashima, S.; Katoh, M.; Masuda, S. Binding properties of a water-soluble chelating polymer with divalent metal ions measured by ultrafiltration. poly (acrylic acid). Ind. Eng. Chem. Res. 2001, 40, 3557–3562. [Google Scholar] [CrossRef]
- Miao, X.-M.; Yuan, R.; Chai, Y.-Q.; Shi, Y.-T.; Yuan, Y.-Y. Direct electrocatalytic reduction of hydrogen peroxide based on Nafion and copper oxide nanoparticles modified Pt electrode. J. Electroanal. Chem. 2008, 612, 157–163. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, L.-C.; Zhang, W.-D.; Gunasekaran, S. A highly sensitive non-enzymatic glucose sensor based on a simple two-step electrodeposition of cupric oxide (CuO) nanoparticles onto multi-walled carbon nanotube arrays. Talanta 2010, 82, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Su, X.; Yuan, H.; Sun, Q.; Xiao, D.; Choi, M.M.F. An improved sensitivity non-enzymatic glucose sensor based on a CuO nanowire modified Cu electrode. Analyst 2008, 133, 126–132. [Google Scholar] [CrossRef]
- Reddy, S.; Kumara Swamy, B.E.; Jayadevappa, H. CuO nanoparticle sensor for the electrochemical determination of dopamine. Electrochim. Acta 2012, 61, 78–86. [Google Scholar] [CrossRef]
- Ahmad, R.; Tripathy, N.; Ahn, M.-S.; Bhat, K.S.; Mahmoudi, T.; Wang, Y.; Yoo, J.-Y.; Kwon, D.-W.; Yang, H.-Y.; Hahn, Y.-B. Highly efficient non-enzymatic glucose sensor based on CuO modified vertically-grown ZnO nanorods on electrode. Sci. Rep. 2017, 7, 5715. [Google Scholar] [CrossRef]
- Song, M.-J.; Hwang, S.W.; Whang, D. Non-enzymatic electrochemical CuO nanoflowers sensor for hydrogen peroxide detection. Talanta 2010, 80, 1648–1652. [Google Scholar] [CrossRef]





| Reagents | A-Type | B-Type | ||||
|---|---|---|---|---|---|---|
| PAA | CuO-PAA | Fe2O3-PAA | ZnO-PAA | MBA-PAA | CuO-MBA-PAA | |
| CuCl2.2H2O | - | 0.2 mM | - | - | - | 0.2 mM |
| FeCl3.6H2O | - | - | 0.2 mM | - | - | - |
| ZnCl2.2H2O | - | - | - | 0.2 mM | - | - |
| MBA | - | 0.5 g | 0.5 g | |||
| H2O | - | 0.5 mL | 0.5 mL | 0.5 mL | - | 0.5 mL |
| AA | 5.0 mL | 5.0 mL | 5.0 mL | 5.0 mL | 5.0 mL | 5.0 mL |
| Type of CuO Nanostructures | Preparation Route | Sensing Analyte | Sensitivity | Linear Response Range | Detection Limit | Reference |
|---|---|---|---|---|---|---|
| Nanoparticles (NPs) onto carbon nanotubes | Two-step electrodeposition of CuO nanoparticles onto vertically well-aligned array of multi-walled CNTs | Glucose | 2190 mA M−1 cm−2 | 800 nM–3 mM | 800 nN | [44] |
| Nanowires | Preparation of Cu(OH)2 nanowires and thermal decomposition at a higher temperature | Glucose | 0.49 μmdm−3 | 0.40–2.0 mM dm−3 | 49 nM dm−3 | [45] |
| Polyglycine/flake-like NPs | Co-precipitation and subsequent high temperature thermal treatment | Dopamine | - | - | 5.5*10−8 M | [46] |
| NPs over ZnO nanorod | formation of vertically-aligned ZnO nanorods on fluorine doped tin oxide electrodes and decoration of CuO over the nanorods | Glucose | 2961.7 μA mM−1cm−2 | 0.40 μM–8.45 mM | 0.40 μM | [47] |
| Nanoflowers | chemical treatment of copper foil and subsequent thermal treatment at 100 c for 12h | H2O2 | 88.4 μA mM−1cm−2 | 4.25*10−5–4*10−2 M | 0.167 μΜ | [48] |
| NPs dispersed in polymer matrix | One-step AC plasma treatment | H2O2 | 63.52 mA M−1cm−2 | 0.5–8.5 mM | 0.6 μΜ | Present work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bui, Q.-T.; Yu, I.-K.; Gopalan, A.I.; Saianand, G.; Kim, W.; Choi, S.-H. Facile Fabrication of Metal Oxide Based Catalytic Electrodes by AC Plasma Deposition and Electrochemical Detection of Hydrogen Peroxide. Catalysts 2019, 9, 888. https://doi.org/10.3390/catal9110888
Bui Q-T, Yu I-K, Gopalan AI, Saianand G, Kim W, Choi S-H. Facile Fabrication of Metal Oxide Based Catalytic Electrodes by AC Plasma Deposition and Electrochemical Detection of Hydrogen Peroxide. Catalysts. 2019; 9(11):888. https://doi.org/10.3390/catal9110888
Chicago/Turabian StyleBui, Quang-Tan, In-Keun Yu, Anantha Iyengar Gopalan, Gopalan Saianand, Woonjung Kim, and Seong-Ho Choi. 2019. "Facile Fabrication of Metal Oxide Based Catalytic Electrodes by AC Plasma Deposition and Electrochemical Detection of Hydrogen Peroxide" Catalysts 9, no. 11: 888. https://doi.org/10.3390/catal9110888
APA StyleBui, Q.-T., Yu, I.-K., Gopalan, A. I., Saianand, G., Kim, W., & Choi, S.-H. (2019). Facile Fabrication of Metal Oxide Based Catalytic Electrodes by AC Plasma Deposition and Electrochemical Detection of Hydrogen Peroxide. Catalysts, 9(11), 888. https://doi.org/10.3390/catal9110888







