Abstract
Several organic templates were introduced during acid or alkaline treatment to optimize pore structures of hierarchical HY zeolites. The influences of category and concentration of templates on the pore structures and acidity of hierarchical HY zeolites were systemically studied. The N2 adsorption-desorption showed that the micropore amount of the optimized HY zeolites obviously increased, while both the large mesopore size and amount remained almost unchanged. The XRD and NH3-TPD revealed that the optimized HY zeolites exhibited higher relative crystallinity and medium-strong acid sites amount than those of hierarchical HY zeolites produced without the addition of templates. The optimized HY zeolites were used for the synthesis of methyl methoxyacetate (MMAc) from dimethoxymethane (DMM) carbonylation. In comparison with parent HY, the conversion and the selectivity clearly increased from 36.43% to 96.32% and from 11.06% to 92.35%, respectively. The stability of the optimized zeolite was also conducted under the same conditions. The conversion and the selectivity remained nearly unchanged even through 24 h reaction, showing that the performance was extremely stable. The TG-DTA and GC-MS also indicated that the generation of coke was effectively inhibited. This catalyst treatment method, which is facile and highly efficient, provided a route for producing mesoporous zeolites.
1. Introduction
Hierarchical zeolites, which have microporous and mesoporous structure, have received wide attention in the past decade, since they display superior catalytic activity compared to traditional microporous zeolites in several reactions [1,2,3]. For instance, the performance and catalyst lifetime for catalytic cracking of large molecules are significantly improved with hierarchical zeolites in place of micropore catalysts due to increased diffusion [4]. Hierarchical ZSM-5 zeolite employed in the benzylation of benzyl alcohol with benzene reaction can clearly enhance the mass transfer and improve the product selectivity [5]. Furthermore, the activity of hierarchical SAPO-34 for the MTO process is obviously enhanced [6].
Methyl methoxyacetate (MMAc) is one of the significant fine chemicals and can be applied to produce protectants, pharmaceuticals, and vitamin B6 [7,8]. MMAc is also an important intermediate for the preparation of ethylene glycol [9], a bulk chemical widely employed in polyester fiber, antifreeze, surface active agents, and textiles [10].
In our previous study [11], a sequential acid and alkaline solution treatment was applied for the preparation of hierarchical HY zeolites with greater mesopore volume and surface area. The pretreated samples displayed much higher catalytic activity and stability than those of parent HY zeolites for the dimethoxymethane (DMM) carbonylation to MMAc because of higher mass transfer efficiency and less carbon deposition, and the conversion of DMM was closely relevant to the amount of medium-strong acid sites. However, the selective extraction of aluminum and silicon from the framework caused the formation of vacancies and amorphous species [12], leading to a decrease of the relative crystallinity. Besides, the alkaline treatment could influence the state of framework aluminum [13], resulting in the decrease of the acidity. Therefore, it was important to protect crystallinity and acidity during acid or alkaline treatment.
The introduction of organic templates was an effective method to protect crystallinity and acidity during acid or alkaline treatment [14,15,16,17,18,19,20,21,22]. These templates, which are named as external pore directing agents (PDAs) [15], could be absorbed on the external surface of zeolites. Tetrapropyl ammonium (TPA+) or tetramethyl ammonium (TMA+) have been proved to be helpful in the formation of directing mesopores during the desilication process [19]. Another branch of excellent PDAs involved cationic surfactants such as cetyltrimethylammonium (CTA+) [18,19]. This surfactant could promote a reassembly of the dissolved aluminum and silicon species [19]. In 1995, Ankica Čižmek et al. [14] first investigated the desilication of high-silica ZSM-5 zeolites in the presence of TPA+. The results indicated that the introduction of TPA+ could control the dissolution of ZSM-5 and thus protect the framework silica. Petra E. de Jongh et al. [20] reported that templated ZSM-5, ZSM-12, and Beta zeolites could introduce mesopores via sodium hydroxide treatment, without changing crystallinity and acidity. Javier Pérez-Ramírez et al. [15,16] revealed that the addition of TPA+ during alkaline treatment could obtain mesoporous Beta and USY zeolites with high crystallinity. Their studies also indicated that the tetraalkylammonium cations with long alkyl chains were more conducive to the formation of zeolite pores [15,17]. Javier Garcia-Martinez et al. [22] demonstrated that the introduction of surfactants during acid treatment could lead to the generation of the ordered mesoporous zeolites. Furthermore, many articles and reviews have reported some synthetic strategies and characterization methods of hierarchical materials. Monoj Ghosh et al. [23] prepared mesoporous TiO2 nanofibers via a facile gas jet fiber spinning process. The porous structure was controlled by using a polymer template (polyvinylpyrrolidone) and sol-gel chemistry. Liu et al. [24] reviewed the synthesis strategies for hierarchical zeolites through the “direct synthesis” route, using a series of templates. Bai et al. [25] reviewed various synthesis methods in the production of hierarchical zeolites by means of mesoporogen, mesoporogen-free and demetallization strategies. K.S.W. Sing et al. [26] summarized characterization methods and physisorption data for the determination of porosity and surface area.
In this work, commercial HY zeolite (Si/Al = 2.7) was employed as the primary precursor. Several organic templates (tetrapropy lammonium bromide (TPABr), tetrapropy lammonium hydroxide (TPAOH), tetraethyl ammonium hydroxide (TEAOH), and tetramethylammonium hydroxide (TMAOH)) were introduced during acid or alkaline treatment to optimize pore structures of hierarchical HY zeolites. The effects of category and concentration of templates on the relative crystallinity, pore structures, and acidity of hierarchical HY zeolites were systemically investigated and discussed, combined with XRD, N2 adsorption-desorption and NH3-TPD results. The carbonylation of DMM to MMAc was employed as a model reaction to assess the parent HY and the optimized HY zeolites, and the differences in the catalytic activity were related to physiochemical properties of the optimized HY zeolites. The catalytic stability of parent HY, HY produced without the addition of templates, as well as HY produced with 0.10 mol/L TPAOH were also carried out under the same conditions. The coke generation and coke species of the used HY zeolites after 24 h reaction was identified by TG-DTA and GC-MS analysis. This optimized HY zeolite with high activity, selectivity, and excellent stability exhibited enormous potential for industrial production of MMAc from DMM carbonylation.
2. Results and Discussion
2.1. XRD Analysis
The power XRD patterns of parent HY, HY-H4EDTA (only treated by 0.11 mol/L H4EDTA), HY-H4EDTA-TPABr (by 0.11 mol/L H4EDTA and 0.10 mol/L TPABr), HY-H4EDTA-NH4OH (further treated by 0.10 mol/L NH4OH founded on HY-H4EDTA), and HY-H4EDTA-NaOH (by 0.10 mol/L NaOH) are shown in Figure 1A. The relative crystallinity of HY-H4EDTA, HY-H4EDTA-TPABr, HY-H4EDTA-NH4OH, and HY-H4EDTA-NaOH, which were calculated by comparing the relative intensity divided by the reference at 2 θ of 23.58°, were 58.39%, 61.80%, 53.50%, and 40.98%, respectively, supposing that the reference HY crystallinity was 100% (Table 1). As compared with the reference HY zeolite, the crystallinity of HY-H4EDTA significantly decreased due to the formation of vacancies and amorphous aluminum species during the dealumination process [12]. After dealumination via H4EDTA, further desilication through NH4OH or NaOH solution caused more severe crumbling of zeolite crystals, owing to the breakage of Si-O-Si and Si-O-Al by OH- [27,28]. In comparison with HY-H4EDTA, the crystallinity of HY-H4EDTA-TPABr slightly improved to 61.80%, indicating that the introduction of TPABr during H4EDTA treatment could effectively protect the crystallinity of zeolites. The introduced TPA+ cations could be adsorbed on the external surface of zeolites through a monolayer adsorption mode [15,29]. On the one hand, the adsorbed TPA+ cations blockaded most of the usable outer surface and thus controlled the dissolution of the zeolite crystals [15,19]. On the other hand, the adsorbed TPA+ cations also largely restrained the surface realumination, leading to the permanent departure of aluminum species from zeolite crystals [15]. Accordingly, HY-H4EDTA-TPABr zeolite treated by H4EDTA and TPA+ exhibited greater crystallinity than that of HY-H4EDTA zeolite handled by pure H4EDTA.
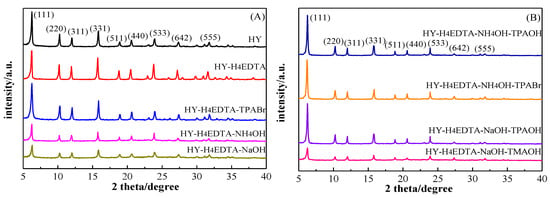
Figure 1.
XRD patterns of the parent HY and the optimized hierarchical HY zeolites (A) and (B).

Table 1.
The crystallinity and acidity distribution of pretreated zeolites.
As displayed in Figure 1B and Table 1, the relative crystallinity of HY-H4EDTA-NH4OH-TPAOH (further treated by 0.10 mol/L NH4OH and 0.10 mol/L TPAOH founded on HY-0.11), HY-H4EDTA-NH4OH-TPABr (by 0.10 mol/L NH4OH and 0.10 mol/L TPABr), HY-H4EDTA-NaOH-TPAOH (by 0.10 mol/L NaOH and 0.10 mol/L TPAOH), and HY-H4EDTA-NaOH-TMAOH (by 0.10 mol/L NaOH and 0.10 mol/L TMAOH) were 79.82%, 79.39%, 60.08%, and 48.47%, respectively. When HY-0.11 was only handled by 0.10 mol/L NH4OH for desilication, the crystallinity of HY-H4EDTA-NH4OH was only 53.50%. With the addition of 0.10 mol/L TPAOH or TPABr during NH4OH treatment, the crystallinity of HY-H4EDTA-NH4OH-TPAOH and HY-H4EDTA-NH4OH-TPABr obviously increased to 79.82% and 79.39%, respectively. Similarly, with only 0.10 mol/L NaOH for desilication, the crystallinity of HY-H4EDTA-NaOH was as low as 40.98%. When 0.10 mol/L TPAOH or TMAOH was added in NaOH solution, the crystallinity of HY-H4EDTA-NaOH-TPAOH and HY-H4EDTA-NaOH-TMAOH clearly increased to 60.08% and 48.47%, respectively. The above results demonstrate that the addition of TPA+ or TMA+ in either NH4OH or NaOH treatment evidently protected the crystallinity of zeolites. According to previous reports [15,29], the TPA+ or TMA+ molecules were absorbed by zeolites via a monolayer adsorption mode, sealing off a majority of the available outer surface and thereby protecting the zeolite structure [18]. Furthermore, the absorbed TPA+ or TMA+ species could largely suppress zeolite recrystallization during alkaline treatment, resulting in the generation of mono-phase, highly crystalline in the zeolite crystals [16]. Therefore, hierarchical HY zeolites optimized by TPA+ or TMA+ displayed higher crystallinity.
As shown in Table 1, the categories of organic templates also influenced the crystallinity of zeolites during alkaline treatment. The HY-H4EDTA-NH4OH-TPAOH (79.82%) exhibited similar crystallinity to that of the HY-H4EDTA-NH4OH-TPABr (79.39%), showing that the anion of templates had almost no effect on the crystallinity. However, it was obvious that the crystallinity of HY-H4EDTA-NaOH-TPAOH (60.08%) was much higher than that of HY-H4EDTA-NaOH-TMAOH (48.47%), demonstrating that the cation with short alkyl chain (TMA+) was not beneficial to protecting crystallinity because a short alkyl chain could not generate a tightly packed adsorption layer [8], resulting in a weak protection role of the zeolitic structure. Hence, HY-H4EDTA-NaOH-TPAOH zeolite treated by NaOH and TPA+ exhibited higher crystallinity than that of HY-H4EDTA-NaOH-TMAOH zeolite handled by NaOH and TMA+.
2.2. NH3-TPD Analysis
The acidity and acid amounts of optimized HY zeolites was gauged by NH3-TPD analysis and shown in Figure 2A,B. All the zeolites exhibited two desorption peaks of NH3. The low temperature desorption peak was ascribed to weak acid centers, while the high temperature peak was assigned to medium-strong acid centers [30,31,32,33,34,35]. The acid amount was computed in the light of the desorbed NH3 amount and is listed in Table 1. The weak acid amounts of HY, HY-H4EDTA, HY-H4EDTA-TPABr, HY-H4EDTA-NH4OH, HY-H4EDTA-NaOH, HY-H4EDTA-NH4OH-0.05TPAOH, HY-H4EDTA-NH4OH-TPAOH, HY-H4EDTA-NH4OH-0.20TPAOH, HY-H4EDTA-0.20NH4OH-TPAOH, and HY-H4EDTA-NaOH-TPAOH were 1.68, 0.72, 0.59, 0.63, 0.69, 0.61, 0.60, 0.59, 0.62, and 0.63 mmol·g−1, while their medium-strong acid amounts were 0.53, 0.21, 0.32, 0.20, 0.07, 0.25, 0.31, 0.32, 0.26, and 0.23 mmol·g−1. As compared with the parent HY zeolite, the acid amount obviously reduced with H4EDTA treatment. After dealumination and further desilication by NH4OH or NaOH, the amount of acid also clearly dropped. In comparison with HY-H4EDTA, the HY-H4EDTA-TPABr zeolite treated by H4EDTA and TPABr resulted in a decrease of the weak acid amount from 0.72 to 0.59 mmol·g−1 and an increase of the medium-strong acid amount from 0.21 to 0.32 mmol·g−1. When the HY-H4EDTA zeolite was only treated by 0.10 mol/L NH4OH, the weak and medium-strong acid amounts were 0.63 and 0.20 mmol·g−1, respectively. With improving the concentration of TPAOH from 0 to 0.10 mol/L, the weak acid amount decreased and showed 0.60 mmol·g−1, while the medium-strong acid amount increased and exhibited 0.32 mmol·g−1 at 0.10 mol/L TPAOH. Further enhancing the TPAOH concentration to 0.20 mol/L, the amount of weak acid centers slightly dropped from 0.60 to 0.59 mmol·g−1, yet the amount of medium-strong acid sites briefly improved from 0.31 to 0.32 mmol·g−1. The change trend of the total acid amount was consistent with that of the medium-strong acid amount and constantly increased and exhibited the maximum value (0.92 mmol·g−1) at 0.20 mol/L TPAOH. When the TPAOH concentration was fixed at 0.10 mol/L, the amount of weak acid centers slowly increased from 0.60 to 0.62 mmol·g−1, and the amount of medium-strong acid sites gradually decreased from 0.32 to 0.26 mmol·g−1, with improvements in the NH4OH concentration from 0.10 to 0.20 mol/L. The above results clearly indicate that the introduction of TPAOH during the dealumination or desilication process could increase the medium-strong acid amount of as-treated HY zeolites. Furthermore, the acid amount was closely related to the concentration of TPAOH, and higher TPAOH concentration led to a greater medium-strong acid amount. It has been reported [36] that the medium-strong acid amount is associated with the amount of framework aluminum with tetrahedral coordination. As discussed above, the introduction of TPA+ could control the dissolution of the zeolite crystal and protect the framework aluminum, leading to an increase of the medium-strong acid amount.
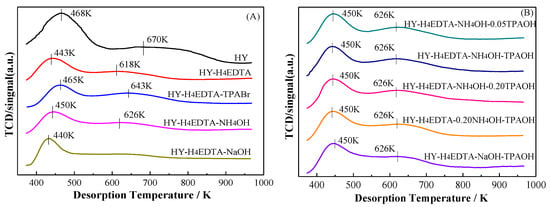
Figure 2.
NH3-TPD analysis of the parent HY and the optimized hierarchical HY zeolites (A) and (B).
After dealumination by H4EDTA, the peak of weak acid sites transformed from 468 to 443 K, demonstrating that the intensity of weak acid centers dropped. After further desilication by 0.10 mol/L NH4OH or NaOH, the peak changed to 450 or 440 K. The desorption temperature of the medium-strong acid peak dropped from 670 to 618 K when the parent HY zeolite was only treated by H4EDTA. After further desilication by 0.10 mol/L NH4OH, the second peak shifted to 626 K. With desilication by the same concentration of NaOH, the medium-strong acid peak practically vanished. It was obvious that the intensity of acid sites decreased after dealumination or desilication. As compared with HY-H4EDTA, the two desorption peaks of HY-H4EDTA-TPABr zeolite shifted to a higher temperature range, suggesting that the strength of both weak acid and medium-strong acid sites clearly increased. This result indicates that the introduction of TPAOH could protect acidity during acid treatment. In comparison with HY-H4EDTA-NH4OH and HY-H4EDTA-NaOH, the desorption temperature remained nearly unchanged (450 and 626 K, respectively) with the variation of TPAOH concentration, indicating that the addition of TPAOH could not change the strength of acid sites during alkaline treatment. The above results are in good agreement with the literature reports [20].
2.3. N2 Adsorption-Desorption Analysis
The isotherms of HY, HY-H4EDTA, HY-H4EDTA-TPABr, HY-H4EDTA-NH4OH, and HY-H4EDTA-NH4OH-TPAOH are exhibited in Figure 3. The pretreated samples displayed Type IV isotherm, including the hysteresis loop at P/P0 greater than 0.4, which demonstrated the presence of mesoporous structure [21].
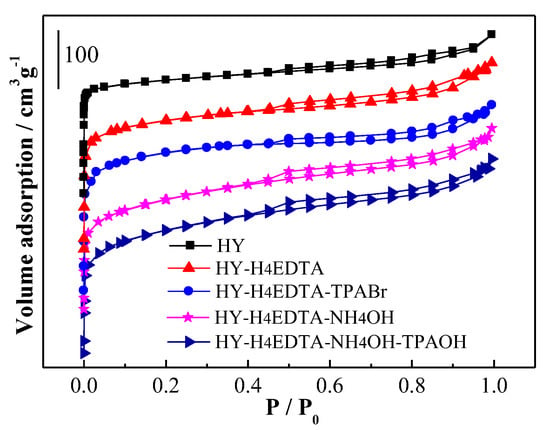
Figure 3.
N2 adsorption-desorption isotherms of the parent HY and the optimized hierarchical HY zeolites.
The micropore sizes distribution determined by the Horváth–Kawazoe (HK) method as well as the mesopore sizes distribution calculated by the Barrett–Joyner–Halenda (BJH) method [23,24,25,26] are displayed in Figure 4A,B. As exhibited in Figure 4A, all the zeolites exhibited a unimodal in the microporous area. The parent HY displayed a micropore size of 0.56 nm with 1.56 cm3 g−1 nm−1 intensity. When HY zeolite was treated by 0.11 mol/L H4EDTA, the micropore size of HY-H4EDTA dropped to 0.50 nm, while the intensity obviously enhanced to 2.53 cm3 g−1 nm−1, which was extremely greater than that of HY, indicating that the amount of micropore clearly improved. After dealumination and further desilication by 0.10 mol/L NH4OH, the micropore size of HY-H4EDTA-NH4OH slightly enhanced to 0.59 nm, and the peak intensity markedly dropped to 1.21 cm3 g−1 nm−1, showing that the micropore amount evidently decreased with the alkaline treatment. As compared with HY-H4EDTA, the peak intensity of HY-H4EDTA-TPABr increased to 2.62 cm3 g−1 nm−1, although the micropore size slightly dropped to 0.47 nm, suggesting that the micropore amount increased with the introduction of TPA+ during acid treatment. In comparison with HY-H4EDTA-NH4OH, the micropore size of HY-H4EDTA-NH4OH-TPAOH remained nearly unchanged (0.58 nm), while the intensity rapidly increased to 1.52 cm3 g−1 nm−1, indicating that the addition of TPA+ could also enhance the micropore amount during alkaline treatment. As shown in Figure 4B, the reference HY exhibited two peaks in mesopore area. The first peak was ascribed to small mesoporous (3.41 nm), while the second peak associated well with large mesoporous (17.51 nm) [11]. The intensity corresponding to these two peaks were 0.10 and 0.09 cm3 g−1 nm−1, respectively. When HY zeolite was treated by 0.11 mol/L H4EDTA, the small mesopore size of HY-H4EDTA remained almost the same, yet the intensity dropped to 0.09 cm3 g−1 nm−1; the large mesoporous rose to 18.32 nm with 0.10 cm3 g−1 nm−1 intensity. After dealumination and further desilication by 0.10 mol/L NH4OH, the small mesoporous of HY-H4EDTA-NH4OH disappeared, while the large mesopore size decreased to 17.44 nm with 0.11 cm3 g−1 nm−1 intensity. As compared with HY-H4EDTA, the small mesoporous of HY-H4EDTA-TPABr vanished, while both the large mesopore size and intensity stayed at constant values (18.34 nm and 0.10 cm3 g−1 nm−1). Similarly, in comparison with HY-H4EDTA-NH4OH, the small mesoporous of HY-H4EDTA-NH4OH-TPAOH also disappeared, while the large mesopore size and intensity also remained nearly unchanged (17.43 nm and 0.10 cm3 g−1 nm−1).
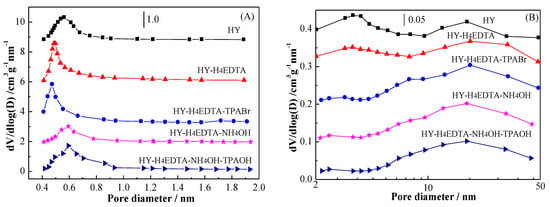
Figure 4.
The micropore pore size (A) and mesopore pore size (B) distribution of the parent HY and the optimized hierarchical HY zeolites.
The above results indicate that 0.11 mol/L H4EDTA treatment could increase the amount of micropore and large mesopore but reduce micropore sizes due to the formation of amorphous aluminum species [12]. Dealumination and further desilication by 0.10 mol/L NH4OH led to the increase of micropore size but the decrease of large mesopore size as well as micropore amount. With the introduction of TPA+ during H4EDTA and NH4OH treatment, the micropore amount clearly increased, while both the large mesopore size and amount remained almost unchanged, showing that the added TPA+ largely protected microporosity. This result is consistent with that of the literature reports [16] and is further proved by the following pore volumes analysis.
The characterization results of pore volumes and BET surface areas are exhibited in Table 2. The external surface area, computed by t-plot method [37] and associated with mesoporous surface area [38], of HY, HY-H4EDTA, HY-H4EDTA-TPABr, HY-H4EDTA-NH4OH, and HY-H4EDTA-NH4OH-TPAOH were 126, 264, 138, 237, and 158 m2/g, respectively. The corresponding mesopore volumes were 0.13, 0.24, 0.20, 0.26, and 0.22 cm3/g, respectively. The micropore volumes of these samples were 0.27, 0.22, 0.26, 0.19, 0.23 cm3/g, respectively. The above results demonstrate that the mesoporous surface area and volume of HY-H4EDTA were approximately twofold larger than those of the parent HY zeolite, while the micropore volume slightly decreased, indicating that H4EDTA treatment led to the collapse of microporous and caused the formation of mesoporous [38,39]. Dealumination and further desilication by 0.10 mol/L NH4OH resulted in the decrease of mesoporous surface area and micropore volume but the increase of mesopore volume, showing that NH4OH treatment further resulted in the generation of mesoporous [27]. In comparison with HY-H4EDTA, the mesoporous surface area and volume of HY-H4EDTA-TPABr clearly decreased, while the micropore volume slightly increased. A similar result was also observed during alkaline treatment in the presence of TPA+. The HY-H4EDTA-NH4OH-TPAOH exhibited smaller mesoporous surface area and volume as well as higher micropore volume than those of HY-H4EDTA-NH4OH. These results were in good agreement with the previous discussion, which indicates that the introduction of TPA+ could effectively protect microporous structure during acid or alkaline treatment.

Table 2.
Characterization results of pore volumes and BET surface areas of pretreated zeolites.
2.4. TG-DTA and GC-MS Analysis
The TG-DTA plots of the used HY and HY-H4EDTA-0.10NH4OH-0.10TPAOH after 24 h reaction is exhibited in Figure 5A,B. The DTA curves of HY and HY-H4EDTA-NH4OH-TPAOH displayed four exothermic peaks in the temperature range of 300 to 1080 K. The first exothermic peak of HY as well as the first and second exothermic peaks of HY-H4EDTA-NH4OH-TPAOH were attributed to the desorption of some light components remaining in zeolite pores [11]. The second exothermic peak of HY and the third exothermic peak of HY-H4EDTA-NH4OH-TPAOH were attributed to the combustion of soft coke [40,41] that was hemiacetal or methoxyacetyl specie generated during the carbonylation process. The third and fourth exothermic peaks of HY as well as the fourth exothermic peak of HY-H4EDTA-NH4OH-TPAOH were attributed to the burning of the heavy coke [42,43,44]. For the as-used HY, the burnt temperature of soft coke was 682 K and the amount was 5.29%. The heavy coke combustion temperatures were 859 and 1034 K and the amount was 4.43% (3.63% + 0.80%). For HY-H4EDTA-NH4OH-TPAOH after 24 h reaction, the soft coke combustion temperature (647 K) and amount (2.42%) as well as the heavy coke burnt temperature (985 K) and amount (0.55%) were lower than those of the parent HY, demonstrating that the coke generation was effectively inhibited in the HY-H4EDTA-NH4OH-TPAOH during the carbonylation procedure. The deposition of carbon might be closely relevant to the mesoporous structure and acid strength of the pretreated catalysts [11]. The higher mesoporous surface area and volume, as well as the weaker acid strength, could contribute to the coke reduction. As compared with HY, the HY-H4EDTA-NH4OH-TPAOH displayed lower acid strength and greater mesoporous surface area and volume, leading to less generation of carbon deposition.
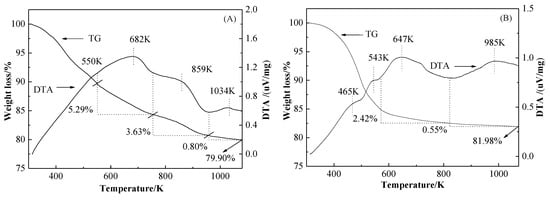
Figure 5.
The TG-DTA plots of the used HY (A) and HY-H4EDTA-NH4OH-TPAOH (B) zeolites after 24 h reaction.
In order to confirm the coke species of the used HY and HY-H4EDTA-NH4OH-TPAOH, about 0.20 g of the catalysts were dissolved with 20% HF solution, and then the coke components were extracted by CH2Cl2 solvent. The coke species were identified by GC-MS and shown in Figure 6. For HY after reaction, the coke species mainly included methoxy species, aromatic compounds (anisole, phenol, 2,4-dimethylbenzaldehyde, 2,4-di-tert-butylphenol), nonanal, aliphatic compounds (2,4-dimethylheptane, 5-ethyl-2-methyloctane, C14-C20 alkane), and ester compounds (isopropyl myristate, diisobutyl phthalate, methyl palmitate, isopropyl palmitate, and methyl stearate). For the used HY-H4EDTA-NH4OH-TPAOH, the coke species consisted of methoxy species, aromatic compound (anisole, 2,4-dimethylbenzaldehyde, 2,4-di-tert-butylphenol), aliphatic compound (C20 alkane), methyl palmitate, and methyl stearate. It was obvious that the coke species deposited on the HY-H4EDTA-NH4OH-TPAOH were extremely less than those of HY, especially the heavy coke species. This result was in good agreement with the former TG-DTA analysis.
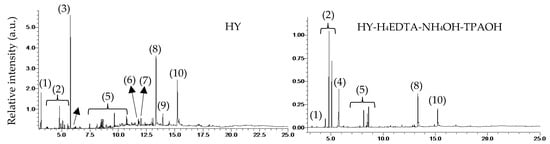
Figure 6.
GC-MS analysis of the used HY and HY-H4EDTA-NH4OH-TPAOH zeolites after 24 h reaction. The coke species were: (1) methoxy species; (2) aromatic compounds; (3) 2,4-dimethylbenzaldehyde; (4) nonanal; (5) C14-C20 alkane; (6) isopropyl myristate; (7) diisobutyl phthalate; (8) methyl palmitate; (9) isopropyl palmitate; (10) methyl stearate.
2.5. Catalytic Performances of Different Samples for the Carbonylation of DMM
The parent HY and the optimized HY zeolites were used for the DMM carbonylation to MMAc at 100 °C and 5.0 MPa. As displayed in Figure 7A, the DMM conversion was as low as 36.43% for the HY zeolite. After dealumination by H4EDTA, the DMM conversion of HY-H4EDTA clearly increased to 51.17%. With the addition of TPABr during H4EDTA treatment, the DMM conversion of HY-H4EDTA-TPABr further increased and was as high as 96.29%. After dealumination and further desilication by NaOH, the DMM conversion of HY-H4EDTA-NaOH was 49.72%. With the introduction of different organic templates (TPAOH, TEAOH, or TMAOH) during NaOH treatment, the DMM conversion of HY-H4EDTA-NaOH-TPAOH, HY-H4EDTA-NaOH-TEAOH, and HY-H4EDTA-NaOH-TMAOH evidently increased to 63.15%, 59.25%, and 55.11%, respectively. As displayed in Figure 7B, when HY-H4EDTA was treated by NH4OH for desilication, the DMM conversion of HY-H4EDTA-NH4OH was 57.01%. With the addition of different concentrations (0.05–0.20 mol/L) of TPAOH during NH4OH treatment, the DMM conversion of HY-H4EDTA-NH4OH-0.05TPAOH, HY-H4EDTA-NH4OH-TPAOH, HY-H4EDTA-NH4OH-0.20TPAOH, and HY-H4EDTA-0.20NH4OH-TPAOH clearly increased to 88.20%, 95.94%, 96.32%, and 90.09%, respectively. In comparison with the NH3-TPD analysis in Table 1, the amount of medium-strong acid sites (except for HY-H4EDTA-NaOH-TEAOH and HY-H4EDTA-NaOH-TMAOH) were 0.21, 0.32, 0.20, 0.07, 0.25, 0.31, 0.32, 0.26, and 0.23 mmol·g−1. It was clear that the conversion of DMM was consistent with the amount of medium-strong acid sites. This result was in good agreement with our previous report [11], which indicated that higher medium-strong acid sites amount led to higher conversion. Although HY had the largest amount (0.53 mmol·g−1) of medium-strong acid sites, the parent HY exhibited the lowest DMM conversion. This could be attributed to the lower mass-transfer efficiency because of the microporous structure.
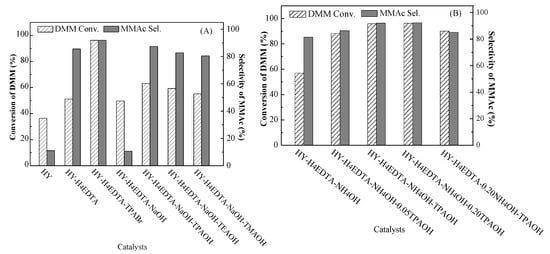
Figure 7.
The effect of different catalysts (A) and (B) on the carbonylation of dimethoxymethane (DMM) to methyl methoxyacetate (MMAc). Reaction conditions: catalyst weight, 1.0 g; reaction temperature, 100 °C; reaction pressure, 5.0 MPa; total CO flow rate, 100 mL/min; time on stream, 2 h.
As shown in Figure 7A, the parent HY exhibited only 11.06% selectivity of MMAc. When HY was treated by H4EDTA for dealumination, the MMAc selectivity of HY-H4EDTA sharply increased to 85.55%. After dealumination by H4EDTA and TPABr, the MMAc selectivity of HY-H4EDTA-TPABr further improved and reached 91.92%. When the HY-H4EDTA was treated by NaOH for desilication, the MMAc selectivity of HY-H4EDTA-NaOH was as low as 10.65%, much lower than that of HY because a high concentration of NaOH resulted in the fractional collapsion of the zeolitic structure [28]. With the addition of TPAOH, TEAOH, or TMAOH during NaOH treatment, the MMAc selectivity of HY-H4EDTA-NaOH-TPAOH, HY-H4EDTA-NaOH-TEAOH, and HY-H4EDTA-NaOH-TMAOH obviously increased to 87.37%, 82.72%, and 80.52%, respectively. As exhibited in Figure 7B, after dealumination and further desilication by NH4OH, the MMAc selectivity of HY-H4EDTA-NH4OH was 81.44%. When HY-H4EDTA was treated by NH4OH and different concentrations of TPAOH for desilication, the MMAc selectivity of HY-H4EDTA-NH4OH-0.05TPAOH, HY-H4EDTA-NH4OH-TPAOH, HY-H4EDTA-NH4OH-0.20TPAOH, and HY-H4EDTA-0.20NH4OH-TPAOH evidently improved to 86.25%, 92.04%, 92.35%, and 85.17%, respectively. We believe that the efficiency of mass transfer and the diffusion of MMAc were highly limited in microporous HY zeolite due to its high molecular weight. Accordingly, the lower mass transfer efficiency could cause residue of the heavy products on the acid sites, leading to the generation of the carbon deposition, which easily resulted in the deactivation of the catalyst. Therefore, the optimized HY zeolites with greater mesopore volume and surface area obviously improved the efficiency of mass transfer, resulting in the improvement of MMAc selectivity.
2.6. Catalytic Stability of HY, HY-H4EDTA-NH4OH, and HY-H4EDTA-NH4OH-TPAOH
The stability of HY, HY-H4EDTA-0.10NH4OH, and HY-H4EDTA-NH4OH-TPAOH was conducted under the same conditions. The DMM conversion and the MMAc selectivity with 24 h reaction is exhibited in Figure 8. At the initial 10 h, the conversion of HY rapidly decreased from 37.15% to 17.96%, and the MMAc selectivity remained nearly unchanged at about 11.06%. After 10 h reaction, the parent HY gradually lost its performance from 17.96% to 9.19% until 24 h when the selectivity of MMAc slowly decreased from 11.06% to 10.71%. For HY-H4EDTA-NH4OH, the variation trend of DMM conversion was almost consistent with that of HY, and rapidly declined from 57.16% to 43.04% at the initial 10 h and further dropped to 39.60% after 24 h reaction. The selectivity of HY-H4EDTA-NH4OH remained at a constant value of about 81.44% during 24 h reaction, much higher than that of HY, since the efficiency of mass transfer clearly improved due to the increased mesoporous surface area and volume. For HY-H4EDTA-NH4OH-TPAOH, it was obvious that the DMM conversion (95.94%) and the MMAc selectivity (92.04%) stayed almost unchanged even through 24 h reaction, showing that the behavior of the optimized HY-H4EDTA-NH4OH-TPAOH zeolite was extremely stable. We considered that the superior performance and stability of HY-H4EDTA-NH4OH-TPAOH were attributed to the increased medium-strong acid sites amount as well as the improved efficiency of mass transfer in mesoporous structure. The reason for the deactivation of the catalysts was attributed to the carbon deposition because all the components in this study system were neutral and would not result in the removal of aluminum and silicon from the framework. The TG-DTA and GC-MS analysis also revealed that HY-H4EDTA-NH4OH-TPAOH was counteractive to the generation of coke. Hence, the optimized HY-H4EDTA-NH4OH-TPAOH zeolite displayed higher mass transfer efficiency, leading to outstanding stability during 24 h reaction.
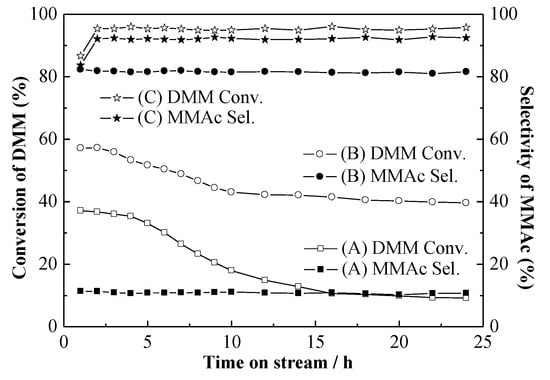
Figure 8.
The catalytic stability of HY (A), HY-H4EDTA-NH4OH (B), and HY-H4EDTA-NH4OH-TPAOH (C) for 24 h reaction. Reaction conditions: catalyst weight, 1.0 g; reaction temperature, 100 °C; reaction pressure, 5.0 MPa; total CO flow rate, 100 mL/min; time on stream, 24 h.
3. Materials and Methods
3.1. Materials
The parent HY zeolite (Si/Al = 2.7) was purchased form Nankai University Catalyst Co. Sodium hydroxide (NaOH, 98%), Ethylenediaminetetraacetic acid (H4EDTA, 99%), ammonium nitrate (NH4NO3, 99%), and ammonia aqueous solution (NH4OH) were obtained from Sinopharm Chemical Reagent Co., Ltd. Dimethoxymethane (DMM, 98%) was acquired from Sigma–Aldrich. Four organic templates, including tetrapropy lammonium bromide (TPABr, 99%), tetrapropy lammonium hydroxide (TPAOH, 99%), tetraethyl ammonium hydroxide (TEAOH, 99%), and tetramethylammonium hydroxide (TMAOH, 99%) were provided by Aladdin Co. All the reagents were directly used without further purification.
3.2. Catalyst Preparation
Dealumination: 13.40 g parent HY zeolite was first put into 200 mL 0.11 mol/L H4EDTA or the mixed solution of 0.11 mol/L H4EDTA and 0.10 mol/L TPABr, followed by stirring at 65 °C for 6 h. The suspension was filtered and washed until the pH value was 7.0. The desired sample was dried at 120 °C for 8 h and then calcined at 550 °C for 4 h to acquire the precursor, noted as “HY-H4EDTA”and “HY-H4EDTA-TPABr”.
Desilication: 1.70 g HY-H4EDTA was poured into a 50 mL mixed solution of 0.10 or 0.20 mol/Lalkali (NaOH or NH4OH) and 0.05–0.20 mol/L PDAs (TPABr, TPAOH, TEAOH, and TMAOH), followed by stirring at 65 °C for 0.5 h. The suspension was also filtered and washed until the pH value was 7.0. The obtained solid was dried at 120 °C and afterwards calcined in a muffle furnace at 550 °C for 4 h to gain the precursor. The NaOH and PDA treatments were noted as “HY-H4EDTA-NaOH-PDA”, while the NH4OH and PDA treatments were labeled as “HY-H4EDTA-NH4OH-PDA”.
After treatments of dealumination and desilication, 10 g of the precursor was exchanged with 1.0 mol/L NH4NO3 solution at 65 °C in order to convert it into the NH4+ form. The suspension was filtered and washed several times. The obtained precursor was dried at 120 °C, subsequently calcined at 550 °C for 6 h in a muffle furnace to receive the catalyst.
3.3. Catalyst Characterization
Powder X-ray diffraction (XRD) patterns were obtained with a Bruker D8 advance diffractometer using Cu K α (λ = 0.15406 nm) radiation. Data was recorded in the 2 θ range from 5 to 40° with a step size of 0.05° at 40 kV and 40 mA. The relative crystallinity of the as-treated HY zeolites was calculated by comparing the relative intensity divided by the reference at 2 θ of 23.58°, assuming that the crystallinity of reference HY was 100% [12].
N2 isotherms were measured in a Quantachrome (Autosorb iQ Statio) instrument at 77 K. The samples were first treated at 300 °C under vacuum for 3 h. The surface area was calculated by using a Brunauer–Emmett–Teller (BET) method. Barrett–Joyner–Halenda (BJH) and Horváth–Kawazoe (HK) methods were applied to determine the pore size distribution in the mesopore and micropore region, respectively.
The NH3-TPD experiments were carried out on a Builder PCA-1200. 200 mg sample was pretreated at 300 °C in a flow of 30 mL/min He for 1 h. After the pretreatment, the sample was cooled to 80 °C and exposed to NH3 for 10 min. Then, the physically adsorbed NH3 was removed by He at the same temperature until the baseline was stable. Thereafter, NH3-TPD was conducted in a constant flow of He (30 mL/min) from 80 to 700 °C at a heating rate of 10 °C/min. The amount of desorbed NH3 was detected by a thermal conductivity detector.
TG-DTA analysis proceeded in the thermal analysis equipment (STA 449C Jupiter, NETZSCH). 2 mg precursor was performed in a flow of 80 mL/min air with the temperature increasing from 40 to 800 °C at a heating rate of 10 °C /min.
The coke species were analyzed by GC-MS. First, about 0.2 g of the catalyst was dried at 200 °C for 1 h, then the sample was dissolved with 2 mL of 20% hydrofluoric acid (HF) solution according to the literature [45]. The coke species were extracted with dichloromethane (CH2Cl2) and were subsequently analyzed on a GCMS-TQ8040 instrument. The coke species were obtained by comparing chromatographic peaks with the NIST14 database.
3.4. Catalyst Test
The carbonylation reaction was conducted in a continuous fixed-bed reactor with an inner diameter of 8.5 mm by employing 1.0 g of the catalyst at 100 °C and 5.0 MPa CO pressure. 20 mL/min CO bubbled through a stainless-steel saturator filled with DMM kept at 25 °C, and 80 mL/min pure CO were mixed together and introduced into the reactor. All the reaction products were analyzed online by a gas chromatograph (GC-2014C) equipped with a HP-FFAP capillary column connected to a flame ionization detector. The conversion of DMM and the MMAc selectivity were calculated on the basis of weight, as follows [11,46]: DMM Conv. = (DMMin − DMMleft)/DMMin; MMAc selectivity was calculated on the basis of weight, MMAc Sel. = MMAcformation/the mass of all products.
4. Conclusions
A series of organic templates (TPABr, TPAOH, TEAOH, and TMAOH) were added during the dealumination or desilication process to optimize the pore structures of hierarchical HY zeolites. The XRD analysis revealed that the optimized HY zeolites displayed much higher relative crystallinity than that of hierarchical HY zeolites produced in the absence of templates. According to the N2 adsorption-desorption analysis, both the large mesopore size and amount of the optimized HY zeolites remained nearly unchanged, while the micropore amount clearly increased, showing that the introduced templates largely protected microporous structure. The NH3-TPD analysis showed that the introduction of templates during dealumination or desilication process could increase the medium-strong acid sites amount of as-treated HY zeolites. The conversion of DMM was strictly in accordance with the amount of medium-strong acid sites, and higher acid amount resulted in higher DMM conversion. The optimized HY zeolites displayed superior conversion of DMM and selectivity of MMAc since the larger mesoporous surface area and volume contributed to increasing the efficiency of mass transfer, resulting in significantly less carbon deposition. It was proved by TG-DTA and GC-MS analyses that the generation of coke was effectively inhibited. HY-H4EDTA-NH4OH-TPAOH exhibited outstanding performance (95.94% DMM conversion and 92.04% MMAc selectivity) and stability at 100 °C and 5.0 MPa during 24 h reaction procedure. This optimized HY zeolite, which was highly active and stable, exhibited tremendous potential for the industrial production of MMAc.
Author Contributions
Conceptualization and methodology, F.C., D.Z., L.S., and G.X.; investigation, F.C., D.Z., and Y.W.; writing—original draft preparation, F.C. and D.Z.; writing—review and editing, L.S. and G.X.
Funding
This research was funded by the Liaoning Provincial University Innovation Talent Project (LR 2016015).
Acknowledgments
The authors are grateful for the financial support from the Liaoning Provincial University Innovation Talent Project (LR 2016015).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ivanova, I.I.; Kuznetsov, A.S.; Yuschenko, V.V.; Knyazeva, E.E. Design of composite micro/mesoporous molecular sieve catalysts. Pure Appl. Chem. 2004, 76, 1647–1657. [Google Scholar] [CrossRef]
- Xing, C.; Sun, J.; Yang, G.H.; Shen, W.Z.; Tan, L.; Zhu, P.F.; Wei, Q.H.; Li, J.; Kyodo, M.; Yang, R.Q.; et al. Tunable isoparaffin and olefin synthesis in Fischer–Tropsch synthesis achieved by composite catalyst. Fuel Process. Technol. 2015, 136, 68–72. [Google Scholar] [CrossRef]
- Gueudré, L.; Milina, M.; Mitchell, S.; Pérez-Ramírez, J. Superior mass transfer properties of technical zeolite bodies with hierarchical porosity. Adv. Funct. Mater. 2014, 24, 209–219. [Google Scholar] [CrossRef]
- Lei, Q.; Zhao, T.B.; Li, F.Y.; Zhang, L.L.; Wang, Y. Catalytic cracking of large molecules over hierarchical zeolites. Chem. Commun. 2006, 16, 1769–1771. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.Y.; Prins, R. Friedel-Crafts alkylations over hierarchical zeolite catalysts. Appl. Catal. A Gen. 2008, 336, 11–16. [Google Scholar] [CrossRef]
- Li, Y.X.; Huang, Y.H.; Guo, J.H.; Zhang, M.Y.; Wang, D.Z.; Wei, F.; Wang, Y. Hierarchical SAPO-34/18 zeolite with low acid site density for converting methanol to olefins. Catal. Today 2014, 233, 2–7. [Google Scholar] [CrossRef]
- Kim, S.H.; Chun, D.B.; Yoon, S.D.; Byun, H.S. Phase Behavior for the CO2 + Methyl Methoxyacetate and CO2 + Methyl trans-3-Methoxyacrylate Systems at Pressures from (5 to 20) MPa and Various Temperatures. J. Chem. Eng. Data 2016, 61, 1101–1108. [Google Scholar] [CrossRef]
- Balkenhohl, F.; Ditrich, K.; Hauer, B.; Ladner, W. Optisch aktive amine durch lipase-katalysierte methoxyacetylierung. J. Prakt. Chem. 1997, 339, 381–384. [Google Scholar] [CrossRef]
- Celik, F.E.; Kim, T.J.; Bell, A.T. Effect of zeolite framework type and Si/Al ratio on dimethoxymethane carbonylation. J. Catal. 2010, 270, 185–195. [Google Scholar] [CrossRef]
- Yue, H.R.; Zhao, Y.J.; Ma, X.B.; Gong, J.L. Ethylene glycol: Properties, synthesis, and applications. Chem. Soc. Rev. 2012, 41, 4218–4244. [Google Scholar] [CrossRef]
- Zhang, D.X.; Shi, L.; Wang, Y.; Chen, F.; Yao, J.; Li, X.Y.; Ni, Y.M.; Zhu, W.L.; Liu, Z.M. Effect of mass-transfer control on HY zeolites for dimethoxymethane carbonylation to methyl methoxyacetate. Catal. Today 2018, 316, 114–121. [Google Scholar] [CrossRef]
- Verboekend, D.; Vilé, G.; Pérez-Ramírez, J. Hierarchical Y and USY zeolites designed by post-synthetic strategies. Adv. Funct. Mater. 2012, 22, 916–928. [Google Scholar] [CrossRef]
- Groen, J.C.; Abelló, S.; Villaescusa, L.A.; Pérez-Ramírez, J. Mesoporous beta zeolite obtained by desilication. Microporous Mesoporous Mater. 2008, 114, 93–102. [Google Scholar] [CrossRef]
- Čižmek, A.; Subotić, B.; Aiello, R.; Crea, F.; Nastro, A.; Tuoto, C. Dissolution of high-silica zeolites in alkaline solutions I. Dissolution of silicalite-1 and ZSM-5 with different aluminum content. Microporous Mater. 1995, 4, 159–168. [Google Scholar]
- Verboekend, D.; Pérez-Ramírez, J. Desilication mechanism revisited: Highly mesoporous all-silica zeolites enabled through pore-directing agents. Chem. Eur. J. 2011, 17, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Verboekend, D.; Milina, M.; Mitchell, S.; Pérez-Ramírez, J. Hierarchical zeolites by desilication: Occurrence and catalytic impact of recrystallization and restructuring. Cryst. Growth Des. 2013, 13, 5025–5035. [Google Scholar] [CrossRef]
- Verboekend, D.; Nuttens, N.; Locus, R.; Van Aelst, J.; Verolme, P.; Groen, J.C.; Pérez-Ramírez, J.; Sels, B.F. Synthesis, characterisation, and catalytic evaluation of hierarchical faujasite zeolites: Milestones, challenges, and future directions. Chem. Soc. Rev. 2016, 45, 3331–3352. [Google Scholar] [CrossRef]
- Liu, H.; Xie, S.J.; Xin, W.J.; Liu, S.L.; Xu, L.Y. Hierarchical ZSM-11 zeolite prepared by alkaline treatment with mixed solution of NaOH and CTAB: Characterization and application for alkylation of benzene with dimethyl Ether. Catal. Sci. Technol. 2016, 6, 1328–1342. [Google Scholar] [CrossRef]
- Verboekend, D.; Vilè, G.; Pérez-Ramírez, J. Mesopore formation in USY and beta zeolites by base leaching: Selection criteria and optimization of pore-directing agents. Cryst. Growth Des. 2012, 12, 3123–3132. [Google Scholar] [CrossRef]
- Van laak, A.N.C.; Zhang, L.; Parvulescu, A.N.; Bruijnincx, P.C.A.; Weckhuysen, B.M.; de Jong, K.P.; de Jongh, P.E. Alkaline treatment of template containing zeolites: Introducing mesoporosity while preserving acidity. Catal. Today 2011, 168, 48–56. [Google Scholar] [CrossRef]
- De Jong, K.P.; Zečević, J.; Friedrich, H.; de Jongh, P.E.; Bulut, M.; van Donk, S.; Kenmogne, R.; Finiels, A.; Hulea, V.; Fajula, F. Zeolite Y crystals with trimodal porosity as ideal hydrocracking catalysts. Angew. Chem. Int. Ed. 2010, 49, 10074–10078. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, J. Introduction of Mesoporosity into Inorganic Materials in The Presence of a Non-ionic Surfactant. US 20130183229A1, 18 July 2013. [Google Scholar]
- Ghosh, M.; Lohrasbi, M.; Chuang, S.S.C.; Jana, S.C. Mesoporous titanium dioxide nanofibers with a significantly enhanced photocatalytic activity. ChemCatChem 2016, 8, 2525–2535. [Google Scholar] [CrossRef]
- Liu, Z.H.; Hua, Y.J.; Wang, J.J.; Dong, X.L.; Tian, Q.W.; Han, Y. Recent progress in the direct synthesis of hierarchical zeolites: Synthetic strategies and characterization methods. Mater. Chem. Front. 2017, 1, 2195–2212. [Google Scholar] [CrossRef]
- Bai, R.S.; Song, Y.; Li, Y.; Yu, J.H. Creating Hierarchical Pores in Zeolite Catalysts. Trends Chem. 2019, 1, 601–611. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems-with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Perez-Ramirez, J.; Abello, S.; Villaescusa, L.A.; Bonilla, A. Toward Functional Clathrasils: Size-and Composition-Controlled Octadecasil Nanocrystals by Desilication. Angew. Chem. Int. Ed. 2008, 47, 7913–7917. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Okuhara, T. Change in pore structure of MFI zeolite by treatment with NaOH aqueous solution. Microporous Mesoporous Mater. 2001, 43, 83–89. [Google Scholar] [CrossRef]
- Li, X.; Shantz, D.F. PFG NMR Investigations of Tetraalkylammonium—Silica Mixtures. J. Phys. Chem. C 2010, 114, 8449–8458. [Google Scholar] [CrossRef]
- Zheng, J.J.; Yi, Y.M.; Wang, W.L.; Guo, K.; Ma, J.H.; Li, R.F. Synthesis of bi-phases composite zeolites MFZ and its hierarchical effects in isopropylbenzene catalytic cracking. Microporous Mesoporous Mater. 2013, 171, 44–52. [Google Scholar] [CrossRef]
- Yang, G.H.; Tsubaki, N.; Shamoto, J.; Yoneyama, Y.; Zhang, Y. Confinement Effect and Synergistic Function of H-ZSM-5/Cu-ZnO-Al2O3 Capsule Catalyst for One-Step Controlled Synthesis. J. Am. Chem. Soc. 2010, 132, 8129–8136. [Google Scholar] [CrossRef]
- Jin, D.F.; Zhu, B.; Hou, Z.Y.; Fei, J.H.; Lou, H.; Zheng, X.M. Dimethyl ether synthesis via methanol and syngas over rare earth metals modified zeolite Y and dual Cu–Mn–Zn catalysts. Fuel 2007, 86, 2707–2713. [Google Scholar] [CrossRef]
- Yaripour, F.; Baghaei, F.; Schmidt, I.; Perregaard, J. Catalytic dehydration of methanol to dimethyl ether (DME) over solid-acid catalysts. Catal. Commun. 2005, 6, 147–152. [Google Scholar] [CrossRef]
- Arena, F.; Dario, R.; Parmaliana, A. A characterization study of the surface acidity of solid catalysts by temperature programmed methods. Appl. Catal. A Gen. 1998, 170, 127–137. [Google Scholar] [CrossRef]
- Taufiqurrahmi, N.; Mohamed, A.R.; Bhatia, S. Nanocrystalline zeolite beta and zeolite Y as catalysts in used palm oil cracking for the production of biofuel. J. Nanoparticle Res. 2011, 13, 3177–3189. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Wang, A.; Li, X.; Zhou, F.; Hu, Y. Highly acidic mesoporous aluminosilicates assembled from zeolitic subunits generated by controllable desilication of ZSM-5 in Na2SiO3 solution. Microporous Mesoporous Mater. 2013, 180, 242–249. [Google Scholar] [CrossRef]
- Cranston, R.W.; Inkley, F.A. The determination of pore structures from nitrogen adsorption isotherms. Adv. Catal. 1957, 9, 143–154. [Google Scholar]
- Verboekend, D.; Pérez-Ramírez, J. Design of hierarchical zeolite catalysts by desilication. Catal. Sci. Technol. 2011, 1, 879–890. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Verboekend, D.; Bonilla, A.; Abelló, S. Zeolite catalysts with tunable hierarchy factor by pore-growth moderators. Adv. Funct. Mater. 2009, 19, 3972–3979. [Google Scholar] [CrossRef]
- Shi, L.; Yao, J.; Zhu, W.L.; Liu, Z.M. Efficient sulfonic acid resin catalysts for carbonylation ofdimethoxymethane to value-added methyl methoxyacetate. J. CIESC 2017, 68, 3739–3746. [Google Scholar]
- Shapovalov, V.; Bell, A.T. Theoretical study of zeolite-catalyzed dimethoxymethane carbonylation to methyl methoxyacetate. J. Phys. Chem. C 2010, 114, 17753–17760. [Google Scholar] [CrossRef]
- Camblor, M.A.; Corma, A.; Valencia, S. Characterization of nanocrystalline zeolite Beta. Microporous Mesoporous Mater. 1998, 25, 59–74. [Google Scholar] [CrossRef]
- Guisnet, M.; Magnoux, P. Organic chemistry of coke formation. Appl. Catal. A Gen. 2001, 212, 83–96. [Google Scholar] [CrossRef]
- Palumbo, L.; Bonino, F.; Beato, P.; Bjørgen, M.; Zecchina, A.; Bordiga, S. Conversion of methanol to hydrocarbons: Spectroscopic characterization of carbonaceous species formed over H-ZSM-5. J. Phys. Chem. C 2008, 112, 9710–9716. [Google Scholar] [CrossRef]
- Guisnet, M.; Costa, L.; Ribeiro, F.R. Prevention of zeolite deactivation by coking. J. Mol. Catal. A Chem. 2009, 305, 69–83. [Google Scholar] [CrossRef]
- Chen, F.; Shi, L.; Yao, J.; Wang, Y.; Zhang, D.X.; Zhu, W.L.; Liu, Z.M. A highly efficient sulfonic acid resin for liquid-phase carbonylation of dimethoxymethane. Catal. Sci. Technol. 2018, 8, 580–590. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).