Abstract
Enantiopure β-amino alcohols constitute one of the most significant building blocks for the synthesis of active pharmaceutical ingredients. Despite the availability of a range of chiral β-amino alcohols from a chiral pool, there is a growing demand for new enantioselective synthetic routes to vicinal amino alcohols and their derivatives. In the present study, an asymmetric 2-step catalytic route that converts 4-anisaldehyde into a β-amino alcohol derivative, (S)-tembamide, with excellent enantiopurity (98% enantiomeric excess) has been developed. The recently published initial step consists in a concurrent biocatalytic cascade for the synthesis of (S)-4-methoxymandelonitrile benzoate. The O-benzoyl cyanohydrin is then converted to (S)-tembamide in a hydrogenation reaction catalyzed by Raney Ni. To achieve hydrogenation of the nitrile moiety with highest chemoselectivity and enantioretention, various parameters such as nature of the catalyst, reaction temperature and hydrogen pressure were studied. The reported strategy might be transferrable to the synthesis of other N-acyl-β-amino alcohols.
1. Introduction
1,2-amino alcohols are a common moiety present in numerous biologically active compounds and, therefore, play an increasingly important role as precursors in the synthesis of active pharmaceutical ingredients (APIs). Enantiomerically pure β-amino alcohols have traditionally been synthesized from the limited chiral pool of amino acids [1]. However, in view of their high potential for the synthesis of APIs, considerable efforts have been directed towards the development of new asymmetric synthetic routes to vicinal amino alcohols and their derivatives [2,3,4,5,6,7,8,9].
In the present study, a 2-step fully catalytic enantioselective route that converts 4-anisaldehyde into a β-amino alcohol derivative, (S)-tembamide, with excellent enantiopurity has been developed. Its N-acyl-β-amino alcohol moiety is found in various biologically active natural and synthetic compounds, as shown in Figure 1.

Figure 1.
Biologically active natural and synthetic molecules carrying the N-acyl-β-amino alcohol motif.
Tembamide is a natural compound that can be isolated from various members of the Rutaceae family [10,11]. Extracts from Aegle marmelos Correa containing tembamide were used in traditional Indian medicine because of its hypoglycemic activity [12]. This amino alcohol derivative also shows adrenaline-like activity and mild insecticidal properties [13], furthermore (S)-tembamide has been reported to exhibit anti-HIV activity [14]. Although tembamide possesses a chiral center, it has only been isolated from natural sources either as a racemate or with low enantiomeric excess (e.e.) [15], prompting scientists to develop enantioselective synthetic routes towards both enantiomers [16,17,18,19,20,21,22,23,24,25,26,27,28,29].
All reported pathways achieve the formation of the amide bond by acylating the amino alcohol precursor with benzoyl chloride. Furthermore, most of the routes starting from inexpensive substrates involve at least four steps, with the exception of the 3-step synthesis reported by Brown et al., in which asymmetric hydrocyanation of 4-anisaldehyde, catalyzed by a tripeptide, is followed by the reduction of the isolated chiral cyanohydrin with LiAlH4, and completed with acylation using benzoyl chloride [27].
The current trend towards more efficient chemical transformations promotes not only the use of catalysts, rather than using activated compounds, but also the reduction of work-up steps by combining two or more reactions into sequential or concurrent cascades [30,31,32,33,34,35]. Considering this trend, a two-step route to (S)-tembamide ((S)-4) starting from 4-anisaldehyde (1) was envisioned (see Scheme 1).

Scheme 1.
2-step catalytic asymmetric route towards (S)-tembamide.
The first step consists in a concurrent bi-enzymatic synthesis of (S)-4-methoxymandelonitrile benzoate ((S)-3), using immobilized Manihot esculenta hydroxynitrile lyase (MeHNL) and Candida antarctica lipase A (CALA) as catalysts [36]. In a second step, the nitrile group is catalytically reduced to give (S)-tembamide. The catalytic hydrogenation of acylated cyanohydrins is an approach reported by Veum et al. [37] which initially forms an amino ester intermediate that spontaneously undergoes acyl transfer to yield the corresponding N-(β-hydroxy)amide. Our designed pathway constitutes a fully catalytic route that reduces the number of work-up steps with respect to the reported routes and avoids the isolation of the unstable cyanohydrin (S)-2.
The combination of MeHNL and CALA to convert aldehyde 1 into (S)-3 in organic solvent could be accomplished at low water activity by carefully selecting the acylating agent and further optimizing the reaction conditions [36]. The present work focuses on the identification of a proper catalytic system to reduce the chiral benzoylated cyanohydrin (S)-3 to form (S)-tembamide.
The reduction of nitriles is usually achieved either by stoichiometric amounts of metal hydrides [38,39,40] or via catalytic hydrogenation using heterogeneous transition metal catalysts [37,41,42,43,44,45]. While metal hydrides are generally effective, their use results in the co-production of stoichiometric amounts of metal salts, which may cause elaborate work-up procedures. The alternative catalytic hydrogenation typically yields secondary and tertiary amines as side products, due to the condensation reactions that take place between the reduction intermediate imines (II) with the formed amines (III and VI) as shown in Scheme 2 [46,47,48].
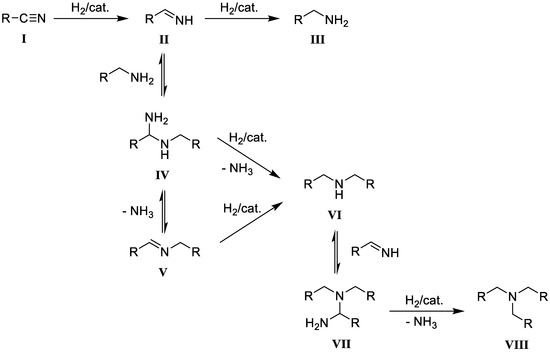
Scheme 2.
Mechanism for the formation of primary (III), secondary (VI) and tertiary (VIII) amines in the catalytic hydrogenation of nitriles as proposed by Braun [49] and modified by Greenfield [50].
Besides the intrinsic side reactions associated with the catalytic reduction shown above, selectivity is very important, since the reactions at other functional groups in the molecule would result in undesired side products. For example, the C–O bond of the benzyloxy group is especially susceptible to hydrogenolysis, due to the phenyl ring of the cyanohydrin fragment (see Scheme 3) [37,51,52].
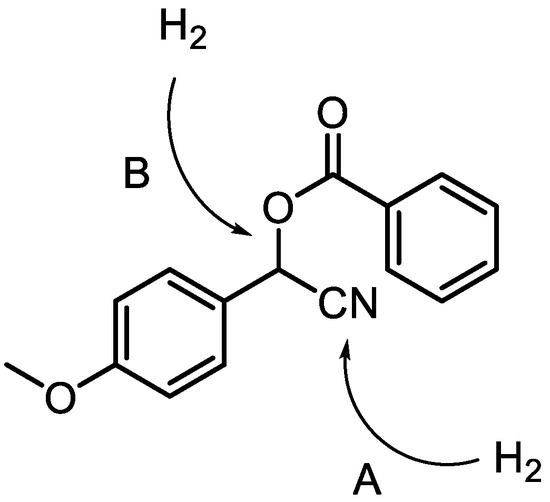
Scheme 3.
In addition to the desired hydrogenation of the nitrile group (A), 4-methoxymandelonitrile benzoate may undergo hydrogenolysis of the C–O bond in the benzyloxy group (B) under catalytic hydrogenation conditions.
The chemoselectivity of the catalytic hydrogenation of nitriles depends on the substrate structure, the nature and loading of catalyst, the reaction temperature, the hydrogen pressure, the selected solvent and the presence of basic additives such as ammonia [46,47,48]. Furthermore, the hydrogenation conditions may affect the chiral center of the reactant resulting in racemization [53]. For example, even under optimized conditions, Veum et al. observed in their study on the catalytic hydrogenation of (S)-mandelonitrile acetate a decrease in enantiopurity of the resulting (S)-N-(2-hydroxy-2-phenylethyl)acetamide (i.e., a decrease in e.e. from 95% to 75%) [37]. In contrast, Hertzberg et al. accomplished the hydrogenation of benzylic O-acyl cyanohydrins with little or no racemization using milder temperature [54,55].
In this study, a careful screening of catalysts and reaction conditions has been performed in order to achieve the hydrogenation of 3 with the highest chemoselectivity and highest enantioretention to yield (S)-tembamide. As a proof of concept, the optimized hydrogenation reaction was combined with the enzyme-catalyzed synthesis of (S)-3 in a preparative 2-step chemoenzymatic cascade synthesis of (S)-tembamide.
2. Results and Discussion
2.1. Catalyst Screening
A crucial factor influencing selectivity in the catalytic hydrogenation of nitriles is the nature of the catalyst [48,49,50,51,52,53,54,55,56]. In order to hydrogenate the nitrile in compound (S)-3 with the highest yield towards (S)-tembamide, minimizing the hydrogenolysis of the C–O bond from the benzyloxy group and the formation of secondary and tertiary amines, a transition metal catalyst screening was performed.
With the idea of achieving the total synthesis of tembamide in a one-pot-two-step fashion, the initial catalyst screening was performed under mild conditions that could facilitate the overall process, including potential recycling of the enzymes. Additionally, by working under milder conditions, we hoped to improve the enantioretention of the chiral center with respect to the procedure reported by Veum et al. [37]. The hydrogenation reactions were carried out at room temperature under 1 bar of H2 in dioxane, this being the solvent of choice in the optimized hydrogenation reactions previously reported by Veum et al.
Table 1 shows the results of the metal-catalyzed conversion of (±)-3 under the conditions mentioned above. Although conversion of the substrate was observed in all cases, not all the catalysts afforded tembamide as a product, and only low chemoselectivity towards the desired product was observed in the cases where tembamide was formed.

Table 1.
Catalyst screening for the catalytic hydrogenation of 19 mM (±)-3 at room temperature (25 °C) under 1 bar of H2. Reactions stopped after 24 h.
While Pd on carbon afforded full conversion of (±)-3, tembamide was not formed. 1H NMR spectroscopy identified benzoic acid as one of the reaction products and a control reaction under N2 atmosphere afforded no conversion of the acyl cyanohydrin, indicating that benzoic acid was formed via hydrogenolysis (see Table 1, entry 1).
Raney Co and Ni on Al2O3/SiO2 (Ni@Al2O3/SiO2 65%) catalyzed the hydrogenation of (±)-3 with low conversion (see Table 1, entries 2 and 3). Raney Co is frequently used at high H2 pressure and elevated temperature [57], which might explain its poor performance under the initial screening conditions. It is, nevertheless, an attractive catalyst because of its typically high chemoselectivity, especially when seeking to reduce nitrile groups in the presence of other potentially reactive moieties [57]. Regarding Ni@Al2O3/SiO2 65%, it has been shown that the catalytic activity of Ni on alumina can be enhanced after a pre-activation treatment under high H2 pressure and high temperature [37]. This might also be the case for Rh on Al2O3 (Table 1, entry 7), which afforded tembamide in negligible yields.
Raney Ni, Rh on silica (Rh@SiO2 1%) and Rh on carbon exhibited high conversion of the ester (>90%) along with similar chemoselectivity towards tembamide, with yields ranging between 4 and 5% (see Table 1, entries 4, 5 and 6).
RhCl3·3H2O and RuO2·H2O, which catalyzed the hydrogenation of (±)-3 with lower conversion, but without tembamide formation, were not further considered (see Table 1, entries 8 and 9).
The hydrogenation using Rh@SiO2 1% was also performed in diisopropyl ether (iPr2O), resulting in higher selectivity compared to the reaction in dioxane (Table 1, entry 10). Since the first two enzymatic reactions of the final cascade are performed in diisopropyl ether, this solvent was chosen for further optimization.
In contrast to the results obtained by Veum et al. using 120 °C and 20 bar of H2 as optimized conditions [37], our initial catalyst screening results, performed at room temperature and 1 bar of H2, afforded only very low yields towards tembamide. Hence, we speculated that the catalysts’ chemoselectivity to hydrogenate the nitrile moiety instead of the C–O bond could be enhanced by increasing the temperature and/or H2 pressure. Additionally, the intramolecular acyl migration might be facilitated by increased temperatures. Therefore, a second catalyst screening was performed at 100 °C under 5 bar of H2 (see Table 2). For this purpose, the catalysts that had afforded tembamide under the initial conditions, as well as Ni@Al2O3/SiO2 65% (which was expected to perform better after pre-activation under high temperature and H2 pressure) were selected.

Table 2.
Catalyst screening for the catalytic hydrogenation of (±)-3 19 mM at 100 °C under 5 bar of H2 in diisopropyl ether. Reactions stopped after 2.5 h.
As expected, the pre-activation of Ni@Al2O3/SiO2 65% and higher temperature and hydrogen pressure allowed for the formation of tembamide (see Table 2, entry 1). In the case of the Raney catalysts, the selectivity towards tembamide was greatly increased with respect to the initial screening (Table 2, entries 2 and 3). Surprisingly, none of the rhodium catalysts afforded the desired product under the new reaction conditions, although full conversion was reached in these cases (see Table 2, entries 4, 5 and 6). Although the catalytic hydrogenation of nitriles has been widely studied, there is no general method that affords the desired products with highest selectivity and yields, and therefore, optimization of the reaction parameters is always required for optimal results. In this context, there appears to be no clear trend in the reported effect of temperature and H2 pressure on the reaction selectivity [43,46,56,58]. The effect of H2 pressure has been reported to directly influence the relative hydrogenation/condensation rates involved in the synthesis of primary, secondary and tertiary amines [43]. The higher yields observed when increasing the temperature in the case of Ni and Co catalysts may be attributed to a positive change in the relative ratios of the hydrogenation/hydrogenolysis and the hydrogenation/condensation rates.
In order to better understand the reaction and the processes leading to the formation of undesired products, the stability of (±)-tembamide was evaluated in parallel under hydrogenating conditions in the presence of Ni@Al2O3/SiO2 65%. After 2 h of reaction, 30% of tembamide had been depleted and several degradation products were observed in HPLC. A control in the absence of catalyst showed no reaction, indicating that the degradation of tembamide was due to the hydrogenation process.
2.2. Selection of Catalyst and Optimization of Reaction Temperature
Given the superiority of the Raney catalysts in terms of selectivity towards tembamide, a reaction temperature optimization was performed under 5 bar of H2 in order to select the best catalyst (see Figure 2). Raney Ni showed a temperature optimum around 100 °C, while lower yields of tembamide were obtained at higher and lower temperatures. Raney Co performed best also at 100 °C, while increasing the temperature up to 140 °C did not afford higher conversion or yield towards tembamide. Both catalysts were then tested at 100 °C and 10 bar of H2. In the case of Raney Ni, lower selectivity towards tembamide was observed (100% conversion, 21% tembamide yield), whereas Raney Co afforded slightly higher conversion (76%) and tembamide yield (17%) than at lower pressure. The results indicate that Raney Co generally requires higher temperature and H2 pressure to afford tembamide with similar selectivity as Raney Ni, but with lower reaction rate. Since variations in the H2 pressure did not have a strong effect on the reaction performance, this parameter was set at 5 bar and Raney Ni was selected for further optimization of the reaction conditions.
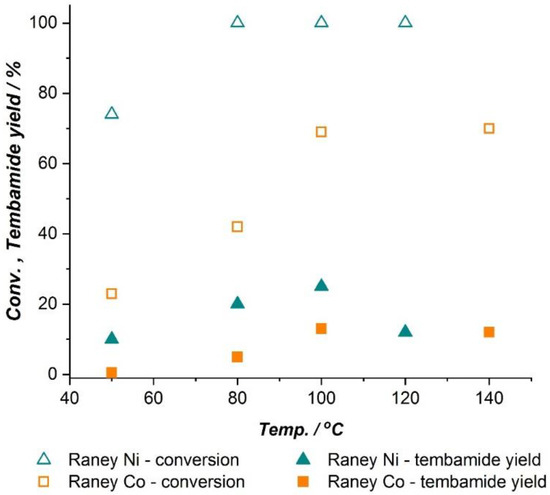
Figure 2.
Effect of reaction temperature on the hydrogenation of 19 mM (±)-3 catalyzed by Raney Ni and Raney Co under 5 bar of H2, using 3.5 gram of catalyst per gram of substrate. Reactions performed at 80 °C and below were run for 5 h, whereas reactions run at 100 °C and above where stopped after 2.5 h.
Having selected the catalyst, a test was performed to evaluate the enantioretention of the chiral center during the hydrogenation reaction. The hydrogenation of 19 mM (R)-4-methoxymandelonitrile benzoate (99% e.e.) catalyzed by Raney Ni at 100 °C under 5 bar of H2, afforded 25% (R)-tembamide with 98% e.e..
2.3. Identification of Side Products and Further Optimization of Reaction Parameters
Using high resolution GC-MS analysis of a crude reaction mixture obtained after hydrogenation, three major side products were identified (Figure 3, compounds 5–7, see Supporting Information, Figures S1–S8, for GC-MS spectra). Formation of benzoic acid (5) and 4-methoxy benzyl cyanide (7) can be explained by hydrogenation of the benzylic C–O bond of compound 3. The presence of the secondary amine 6 is supported by the mechanism proposed in Scheme 2, involving reduction products of compound 7 and possibly other reaction intermediates. We infer that hydrogenolysis of the benzylic C–O bond is one of the major side reactions, which leads to products, such as 6 and 7, that can trigger the formation of other side products such as secondary and tertiary amines, further depleting the tembamide yield. Previous reports have indicated that the addition of NH3 minimizes the formation of secondary and tertiary amines [47,48]. However, this additive can also result in the formation of other undesired products, as observed by Veum et al. for the catalytic hydrogenation of acylated cyanohydrins [37]. Furthermore, we surmised that if the nitrile hydrogenation takes place selectively, the β-amino ester quickly undergoes transacylation to tembamide and, thus, will not participate in secondary amine formation pathways. Based on these considerations, no efforts were made towards the addition of additives such as NH3 to avoid secondary and tertiary amine formation.
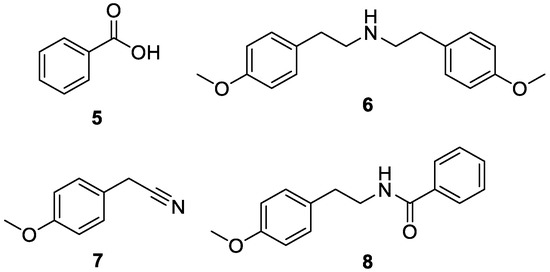
Figure 3.
Major side products of the hydrogenation of 4-methoxymandelonitrile benzoate.
Additionally, NMR analysis of a column chromatography fraction established the presence of the side product 8 (see Supporting Information, Figures S9 and S10 for NMR spectra). The amine formed via hydrogenation of compound 7 may react with another species bearing a benzoyl group in a nucleophilic acyl substitution to yield compound 8. Alternatively, this side product may result from the hydrogenolysis of the C–OH bond after tembamide formation, considering that tembamide was shown to react under hydrogenating conditions. Therefore, the identification of an optimal reaction time to avoid tembamide degradation appears to be crucial.
The presence of water in the reaction medium also influences the catalytic hydrogenation of nitriles, but, so far, the studies on the specific effect of water on the product distribution and reaction rate are inconclusive. Volf et al. reported increased reaction rate and selectivity towards primary amines upon addition of water when using unsupported Ni and Co catalysts, while the contrary effect was observed when using supported catalysts [59]. In the case of Pt(C)-catalyzed hydrogenation of benzonitrile, increased selectivity towards primary amines was reported when small quantities of water were added [50]. However, it has also been claimed that water increases the reaction rate, while having a negligible effect on the chemoselectivity when supported Pt and Ru catalysts were used [60]. Veum et al. observed a slightly positive effect on the reaction yield when adding water in the hydrogenation of aliphatic cyanohydrins but reported no effect on benzylic acylated cyanohydrins [37]. Additionally, studies on acyl migration of monoacylglycerols have shown that water addition influences the migration rate, which could also affect the yield of tembamide in our reaction [61]. Furthermore, the addition of water could increase the risk of hydrolysis of benzoylated species. To evaluate the effect of water on the hydrogenation of (±)-3, two experiments were carried out (Table 3, entries 1 and 2) where water was either removed or added to the system. The addition of 4 Å molecular sieves to remove water had a slightly negative effect on the yield towards tembamide compared to reactions without molecular sieves (Table 2, entry 2). This might imply that small quantities of water have a beneficial effect on the catalyst performance, making complete removal counterproductive. The addition of 2.5% v/v water, however, resulted in a dramatically lower yield of tembamide and a higher concentration of benzoic acid (5.7 mM when using molecular sieves vs. 8 mM with water addition). Unfortunately, we cannot differentiate to what extent this is caused by increased hydrogenolysis, hydrolysis or other processes. Therefore, no further experiments were directed towards optimizing the amount of water in the reaction medium and it was decided to proceed without further removal or addition of water.

Table 3.
Optimization of parameters influencing the yield towards tembamide in the hydrogenation of (±)-3 catalyzed by Raney Ni at 100 °C under 5 bar of H2 in diisopropyl ether.
The catalyst loading and substrate concentration were also expected to impact the reaction performance. First, the catalyst loading was adjusted (see Table 3, entries 3–5). A reduction of the catalyst/substrate ratio was desirable in terms of process economy and could potentially alter the selectivity of the reaction. An experiment was performed decreasing the Raney Ni loading to 0.5 g per g of substrate, whereupon a slight increase in selectivity was observed. Further decreasing the catalyst loading to 0.25 g/g resulted in a lower tembamide yield. Thus, for future experiments the catalyst loading was set to 0.5 g/g.
In order to evaluate the effect exerted by the substrate concentration, a hydrogenation experiment was performed starting from a saturated solution of substrate (Table 3, entry 6). Also, based on the concentration of the ester obtained via the biocatalytic cascade, a hydrogenation reaction using 84 mM substrate was performed (Table 3, entry 7). Increasing the concentration of 4-methoxymandelonitrile benzoate had a negative influence on the selectivity of the hydrogenation reaction, leading to a decreased tembamide yield. We hypothesize that this detrimental effect associated with an increased substrate concentration results from a competition between the O-benzoyl cyanohydrin and the partially reduced imino intermediate (see Scheme 2, compound II) on the catalyst surface, in which compound 3 outcompetes the imino intermediate at higher concentrations. This situation, combined with the higher concentration of free amines would increase the rate of the condensation reaction between the imino intermediate and the amines, and therefore limit the hydrogenation of intermediate II that would eventually lead to tembamide.
2.4. Preparative Synthesis of (S)-Tembamide
Once all hydrogenation conditions had been optimized, we aimed for a one-pot, two-step catalytic synthesis of (S)-tembamide. Based on our previous studies [36], the enzymatic cascade leading to (S)-3 afforded a crude solution containing approximately 80–85 mM (S)-3, 10–15 mM 2, 5–10 mM 1, 180 mM phenyl benzoate, 40 mM benzoic acid and a maximum of 550 mM HCN. Given the high concentration of HCN and its susceptibility to hydrogenation [62,63], its effect on the hydrogenation of 3 was evaluated. For optimal selectivity, a concentration of 3 in the range of 20 mM was selected, for which the biocatalytic crude reaction mixture would undergo a 4-fold dilution prior to the reduction step. Therefore, the final HCN concentration in the autoclave was estimated to be around 120 mM. Thus, a hydrogenation reaction of 19 mM (±)-3 with addition of 120 mM HCN and a control reaction without HCN were performed. For safety reasons, a setup was used that limited the reaction temperature to 70 °C. After 1 h, the control reaction had afforded 60% conversion of (±)-3, whereas the reactor containing HCN showed no conversion of the benzoylated cyanohydrin. This result established the need to evaporate the crude reaction mixture obtained from the enzymatic cascade in order to eliminate the HCN, followed by subsequent re-dissolution in iPr2O prior to the hydrogenation step.
In our first attempt to synthesize (S)-tembamide from the re-dissolved biocatalytic reaction crude mixture containing 19 mM (S)-3 (97% e.e.), the hydrogenation reaction was stopped after 40 minutes. With such short reaction time, we aimed at a conversion close to 100% and at a maximum yield of tembamide by avoiding excessive product degradation due to a long reaction time. However, the reaction proved to be much slower than when starting from pure 3, resulting in a conversion of 45% and affording tembamide with 9% yield (95% e.e.). The decrease in reaction rate was attributed to the presence of other species that can also undergo hydrogenation, especially phenyl benzoate, which is present in much higher concentration than (S)-3. Therefore, a longer reaction time was required to achieve full conversion. Regarding the enantioretention, the enantiomeric excess of tembamide experienced only a minor loss, as had been confirmed previously during the hydrogenation of (R)-3.
Once all hydrogenation reaction parameters were defined, the synthesis of (S)-tembamide starting from 300 mg 4-anisaldehyde was performed. The scaled-up biocatalytic cascade afforded (S)-3 with 80% yield and 99% e.e. After separation of the immobilized enzymes and evaporation of the volatiles, the crude product was re-dissolved in diisopropyl ether to a concentration of 21 mM and the hydrogenation reaction was performed under the optimal conditions for 90 minutes. Chiral HPLC analysis showed that (S)-tembamide had been successfully synthetized with 32% yield and 98% e.e., while compound 3 was fully converted. Column chromatography purification, followed by recrystallization in heptane/diethyl ether, afforded 91 mg of pure colorless crystals (isolated overall yield of 15%, see Supporting Information, Figures S11 and S12 for NMR spectra). We cannot determine to what extent the factors that differentiate this experiment from the optimized hydrogenation of pure 3, such as the presence of other species combined with the longer reaction time or the larger reaction volume, may have influenced the reaction chemoselectivity in order to afford a higher yield.
3. Materials and Methods
3.1. Enzymes
Hydroxynitrile lyase from Manihot esculenta was heterologously expressed in E. coli K12 Top 10F’ harboring the pSE420-MeHNL plasmid, which was kindly provided by the Austrian Centre of Industrial Biotechnology (ACIB GmbH), Graz, Austria. The procedure is described in detail in our previous publication [36]. Candida antarctica lipase A (Novozym® CALA L, LDN 00025) was purchased from Novozymes A/S, Bagsværd, Denmark.
3.1.1. Enzyme Immobilization
MeHNL and CALA were adsorbed on Celite R633 and Relizyme EXE309, respectively, following the previously described procedures [36].
3.1.2. Enzyme Activity Measurements
The enzymatic activity of immobilized MeHNL was determined following the hydrocyanation of 4-anisaldehyde [36]. The enzymatic activity of immobilized CALA was determined following the hydrolysis of 4-nitrophenyl butyrate [36].
3.2. Chemicals
Unless otherwise indicated, reagents and organic solvents were purchased from Fisher Scientific, Landsmeer, The Netherlands. TCI Europe N.V., Zwijndrecht, Belgium, Sigma Aldrich N.V., Zwijndrecht, The Netherlands and Acros Organics B.V.B.A., Geel, Belgium and were of the highest available purity.
Raney® Cobalt was kindly provided by Dr. Klaas van Gorp (Grace Catalysts Technologies, Grace GmbH & Co, Worms, Germany); Celite R633 was a gift from Imerys S.A., Paris, France; Relizyme EXE309 was kindly provided by Resindion S.r.l., Binasco, Italy. (S)-Tembamide used for construction of HPLC calibration curves had been previously synthetized by Joerg Schrittwieser at Frank Hollmann’s group [16].
The preparation of the hydrogen cyanide (HCN) solution, as well as the determination of the HCN concentration was performed according to the previously reported procedure [36].
3.2.1. Synthesis of (±)-4-Methoxymandelonitrile, (±)-2
(±)-4-Methoxymandelonitrile was synthetized via chemical hydrocyanation of 4-anisaldehyde following a modified literature procedure [64]. In a 100 mL round-bottom flask, NaCN (4.4 g, 90 mmol) was dissolved in water (30 mL) and NaHSO3 (9.4 g, 90 mmol) was slowly added. 4-anisaldehyde (2.4 g, 18 mmol) was dissolved in ethyl acetate (20 mL) and added to the reaction mixture. After 1 h of vigorous stirring, the flask was introduced in an ice-water bath and stirred for another 6 h. The reaction was followed by HPLC until it reached plateau after 90% conversion. Distilled water (30 mL) was added to dissolve the salts and the aqueous phase was extracted with ethyl acetate (2 × 10 mL). The combined organic phase was washed with brine, dried over anhydrous MgSO4 and concentrated on a rotary evaporator using a 10 °C bath, whereupon crystallization occurred. The crystals were washed with cold heptane/ethyl acetate (2:1) and stored at 5 °C. HPLC analysis showed that the (±)-4-methoxymandelonitrile crystals contained 3.0% 4-anisaldehyde impurity.
3.2.2. Synthesis of (±)-4-Methoxymandelonitrile Benzoate, (±)-3
The cyanohydrin ester was synthesized via chemical benzoylation of (±)-2 (97.0%). A sealed 50 mL round-bottom flask containing (±)-4-methoxymandelonitrile (2.02 g, 12 mmol) and benzoyl chloride (1.5 mL, 13.2 mmol) in dry dichloromethane (20 mL) under N2 atmosphere, was introduced in an ice-water bath and anhydrous pyridine (10.7 mL, 13.2 mmol) was added dropwise with a syringe. The reaction mixture was vigorously stirred for 2 h. The mixture was then transferred to a 100 mL separatory funnel and washed with demineralized water (2 × 20 mL), citrate 0.2 M pH 4 (2 × 20 mL) and phosphate 0.2 M pH 8 (2 × 20 mL). The organic phase was then dried over anhydrous MgSO4 and concentrated in a rotary evaporator. Recrystallization (dichloromethane/diethyl ether 1:4) afforded (±)-4-methoxymandelonitrile benzoate (1.90 g, 58% isolated yield).
3.2.3. Synthesis of (R)-4-methoxymandelonitrile benzoate, (R)-3
The same procedure as described for (±)-3 was followed using commercially available (R)-4-methoxymandelonitrile benzoate (0.50 g, 98.0%, 3 mmol), affording white crystals of (R)-4-methoxymandelonitrile benzoate in 60% yield.
3.3. Hydrogenation Reactions
3.3.1. Pretreatment of Catalysts
When mentioned, Ni@Al2O3/SiO2 65% and Rh@Al2O3 5% were preactivated overnight at 120 °C under 10 bar of H2 in diisopropyl ether. Unless otherwise noted, Raney catalysts were washed 2× with Milli-Q® water, then 2× with ethanol and finally 2× with the reaction solvent.
3.3.2. General Procedure for the Hydrogenation of (±)-3 at Room Temperature Under 1 Bar of H2 - Initial Catalyst Screening
In a 4 mL glass vial with septum cap containing a magnetic stir bar, the catalyst was weighed. A solution (2 mL) containing (±)-3 19 mM (38 µmol) and mesitylene 20 mM (40 µmol, internal standard) in dioxane or diisopropyl ether was added and, after flushing with N2, the gas was replaced with H2 using a balloon. Finally, the balloon was refilled with H2 and the reactions were stirred at 350 rpm at room temperature (25 °C) for 24 h. Samples (20 µL) were analyzed by HPLC.
3.3.3. General Procedure for the Hydrogenation of (±)-3 in Autoclave Reactor
To a stainless-steel reactor (Büchi tinyclave) provided with a Teflon vessel with 10 mL maximum capacity and a magnetic stir bar, a solution (3.5 mL) containing (±)-3 21.7 mM (76 µmol) and mesitylene 22.9 mM (80 µmol) in diisopropyl ether was added. The pretreated catalyst was subsequently added, together with diisopropyl ether (0.5 mL). The autoclave was then tightly closed and flushed with four cycles of N2-vacuum. Finally, it was pressurized with 5 or 10 bar of H2, introduced in a preheated oil bath and stirring was set to 600 rpm. After the selected reaction time, the autoclave was removed from the oil bath and allowed to cool for 20 minutes. The reactor was then de-pressurized and dioxane was added to dissolve all species, since formation of crystals was observed. Samples (20 µL) were analyzed by HPLC.
3.3.4. Stability of Tembamide Under Hydrogenating Conditions in Autoclave Reactor
To a stainless-steel reactor (Büchi tinyclave) provided with a Teflon vessel with 10 mL maximum capacity and a magnetic stir bar, a solution (3.5 mL) containing (±)-tembamide 4.6 mM (16 µmol) and mesitylene 22.9 mM (80 µmol) in diisopropyl ether was added. (±)-Tembamide was prepared following the procedure described in 4.3.3. at 100 °C, under 5 bar H2 using pretreated Raney Ni as catalyst, and further purified using column chromatography (ethyl acetate/n-heptane). Pretreated Ni@Al2O3/SiO2 65% (5 mg) was subsequently added, together with diisopropyl ether (0.5 mL). For the control reaction, only diisopropyl ether (0.5 mL) was added. The autoclave was then tightly closed and flushed with four cycles of N2-vacuum. Finally, it was pressurized with 5 bar of H2, introduced in an oil bath preheated to 100 °C and stirring was set to 600 rpm. After 2 h, the autoclave was removed from the oil bath and allowed to cool for 20 minutes. The reactor was then de-pressurized and dioxane was added to dissolve all species. Samples (20 µL) were analyzed by HPLC.
3.3.5. Hydrogenation of (R)-3 in Autoclave Reactor
To a stainless-steel reactor (Büchi tinyclave) provided with a Teflon vessel with 10 mL maximum capacity and a magnetic stir bar, a solution (3.5 mL) containing (R)-3 21.7 (76 µmol) mM and mesitylene 22.9 mM (80 µmol) in dioxane was added. Pretreated Raney Ni was subsequently added, together with dioxane (0.5 mL). The autoclave was then tightly closed and flushed with four cycles of N2-vacuum. Finally, it was pressurized with 5 bar of H2, introduced in an oil bath preheated to 100 °C and stirring was set to 600 rpm. After 2.5 h, the autoclave was removed from the oil bath and allowed to cool for 20 minutes. The reactor was then de-pressurized and dioxane was added to dissolve all species. Samples (20 µL) were analyzed by HPLC.
3.3.6. Evaluation of the Effect of HCN on the Hydrogenation of (±)-3 in Autoclave Reactor
In a 25 mL glass reactor (Büchi tinyclave) provided with a magnetic stir bar, a solution (4 mL) containing (±)-3 23.8 mM (95 µmol) and mesitylene 25.0 mM (100 µmol) in diisopropyl ether was added. Pretreated Raney Ni was subsequently added, together with diisopropyl ether (0.5 mL) and, finally, HCN 1.2 M in diisopropyl ether (0.5 mL, 0.6 mmol) was added. For the control reaction, diisopropyl ether (0.5 mL) was added instead of the HCN solution. The autoclave was then tightly closed and flushed with four cycles of N2-vacuum. Finally, it was pressurized with 5 bar of H2, introduced in a steel cage and in an oil bath preheated to 70 °C and stirring was set to 600 rpm. After 1 h, the autoclave was removed from the oil bath and allowed to cool for 20 minutes. The reactor was then de-pressurized and dioxane was added to dissolve all species. Samples (20 µL) were analyzed by HPLC.
3.4. Preparative Synthesis of (S)-Tembamide
3.4.1. Biocatalytic Cascade Synthesis of (S)-3
In a 100 mL round-bottomed flask provided with a magnetic stir bar, immobilized MeHNL (190 mg, 0.03 U/mg) and immobilized CALA (137 mg, 0.11 U/mg) were weighed. Subsequently, a solution (22 mL) containing 4-anisaldehyde 100 mM (2.2 mmol), HCN 650 mM (14.3 mmol) and mesitylene 50 mM (1.1 mmol) (internal standard) was added. Finally, phenyl benzoate (440 mg, 2.2 mmol, 1 equivalent) was added at the beginning of the reaction, after 24 h and after 48 h (total of 3 equivalents). The mixture was stirred at 300 rpm and room temperature. The reaction was followed by HPLC and was stopped after 136 h, affording (S)-4-methoxymandelonitrile benzoate in 80% HPLC yield and 99% e.e. The mixture was then centrifuged (4000× g, 4 °C for 15 min) and the volatiles of the clear solution were evaporated using a rotary evaporator.
3.4.2. Catalytic Hydrogenation of (S)-3
The crude reaction mixture from the biocatalytic step was re-dissolved in diisopropyl ether (93 mL) and introduced in a stainless-steel reactor (Büchi miniclave) provided with a Teflon vessel with 300 mL maximum capacity, containing a magnetic stir bar. Raney Ni slurry (1 mL) was centrifuged and the aqueous phase was removed. After adjusting the moist weight, the catalyst (224 mg) was added together with diisopropyl ether (1 mL) to the reactor. The autoclave was then tightly closed and flushed with four cycles of N2-vacuum. Finally, it was pressurized with 5 bar of H2, introduced in an oil bath preheated to 100 °C and stirring was set to 600 rpm. After 1.5 h, the autoclave was removed from the oil bath and allowed to cool for 20 minutes. The reactor was then de-pressurized and dioxane was added to dissolve all species. A sample (20 µL) analyzed by HPLC showed that (S)-tembamide was synthetized with 32% yield and 98% e.e. The solvents were then evaporated using a rotary evaporator. Column chromatography purification using an eluent mixture of ethyl acetate and n-heptane followed by recrystallization of the enriched fractions (ethyl acetate/n-heptane 1:9) afforded 91 mg of pure colorless (S)-tembamide crystals (isolated overall yield of 15%). 1H-NMR (300 MHz, DMSO-d6): δ [ppm] = 3.25–3.34 (1H, m, CH2), 3.40–3.49 (1H, m, CH2), 3.73 (3H, s, OCH3), 4.72 (1H, dt, J = 7.5 Hz, 4.8 Hz, CH-OH), 5.41 (1H, d, J = 4.5 Hz, OH), 6.89 (2H, d, J = 8.7 Hz, Ar-m), 7.28 (2H, d, J = 8.7 Hz, Ar-o), 7.45 (2H, t, J = 6.9 Hz, Ar-m′), 7.51 (1H, tt, J = 6.9 Hz, 1.5 Hz, Ar-p′), 7.83 (2H, dd, J = 6.9 Hz, 1.5 Hz, Ar-o′), 8.47 (1H, t, J = 5.4 Hz, NH). 13C-NMR (300 MHz, DMSO-d6): δ [ppm] = 47.6, 54.9, 70.6, 113.3, 127.0, 127.1, 128.1, 130.9, 134.5, 135.7, 158.2, 166.2.
3.5. HPLC Analysis
The quantification of 4-anisaldehyde, (S)- and (R)-4-methoxymandelonitrile, (S)- and (R)-4-methoxymandelonitrile benzoate, (S)- and (R)-tembamide and benzoic acid was achieved using chiral normal phase HPLC. Samples were diluted to a final mesitylene concentration of 1 mM with n-heptane/isopropanol 8:2 v/v and dried over anhydrous MgSO4. Analysis was performed using a Hitachi Elite LaChrom HPLC system, consisting of a VWR Hitachi L-2130 pump, L-2200 auto-sampler, L-2350 column oven, L-2400 UV detector using a D2 lamp, coupled to a Hitachi organizer module. Separation was achieved on a Chiralpak AD-H column (250 mm × 4.6 mm × 5 μm; Daicel, Japan), using n-heptane/isopropanol (82/18) at a flow rate of 0.65 mL/min. The column oven temperature was set at 35 °C and a detection wavelength of 225 nm was chosen. Under these conditions, the compounds were separated with the following retention times: mesitylene (internal standard) 5.0 min, benzoic acid 6.7 min, phenol 7.0 min, phenyl benzoate 7.3 min, 4-anisaldehyde 8.3 min, (S)-4-methoxymandelonitrile 9.6 min, (R)-4-methoxymandelonitrile 10.5 min, (S)-4-methoxymandelonitrile benzoate 13.1 min, (R)-4-methoxymandelonitrile benzoate 13.9 min, (S)-tembamide 16.4 min and (R)-tembamide 17.4 min.
Calibration curves were prepared using commercial 4-anisaldehyde (>99.0%), (R)-4-methoxymandelonitrile (98.0%) and benzoic acid (>99.5%) as well as chemically synthesized (±)-4-methoxymandelonitrile benzoate and (S)-tembamide (99%).
4. Conclusions
In the present work, the one-pot, two-step catalytic synthesis of (S)-tembamide was successfully accomplished on a preparative scale. Whereas the initial concurrent bienzymatic cascade step afforded the intermediate (S)-3 with a fair yield of 80% and excellent enantioselectivity (99% e.e.), the hydrogenation reaction proceeded with a maximum yield of 32% towards the desired product, significantly lowering the overall yield of the designed route. However, the enantiopurity of the synthesized (S)-tembamide (98% e.e.) was outstanding, with a minor loss of 1% during the hydrogenation step in contrast to the low enantioretention observed under the harsher conditions employed by Veum et al. on the hydrogenation of benzylic acyl cyanohydrins [37].
The identification of the major side products, together with the limited improvements on the yield of tembamide achieved during the reaction optimization, demonstrated the difficulty of controlling the reaction chemoselectivity. Nevertheless, we hypothesize that the yield might be further enhanced when working with a fed batch system, thereby lowering the observed detrimental effect of high substrate concentrations. Furthermore, on-line reaction monitoring would allow studying the effect of the reaction parameters on the rate of side product formation, which might further contribute to optimizing the overall reaction.
Our approach is potentially transferrable to the synthesis of other N-acyl-β-amino alcohols, for which higher selectivity is expected when using aliphatic aldehydes as starting material, since the C–O bond would be less susceptible to hydrogenolysis (see Scheme 3). However, based on our experience and literature examples, it seems likely that an optimization of the reaction parameters would be required in each case in order to maximize the yield, since there is still no general robust method for the selective hydrogenation of nitriles in compounds bearing other reactive groups [37,54,55].
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/9/10/822/s1, Figure S1: Gas chromatogram of the crude reaction mixture from the Raney Ni-catalyzed hydrogenation of (±)-4-methoxymandelonitrile benzoate at 100 °C under 5 bar of H2, Figure S2: mass spectrum of diacetone alcohol, Figure S3: mass spectrum of benzoic acid, Figure S4: mass spectrum of 4-methoxyphenylacetonitrile, Figure S5: mass spectrum of 1-iodonaphthalene, Figure S6: mass spectrum of 4-methoxymandelonitrile benzoate, Figure S7: mass spectrum of 4-methoxy-N-[2-(4-methoxyphenyl)ethyl] benzeneethanamine, Figure S8: mass spectrum of tembamide, Figure S9: 1H-NMR spectrum of a column chromatography fraction containing side products from the Raney Ni-catalyzed hydrogenation of (±)-4-methoxymandelonitrile benzoate at 100 °C under 5 bar of H2, Figure S10: 13C-NMR and DEPT-135 spectra of a column chromatography fraction containing side products from the Raney Ni-catalyzed hydrogenation of (±)-4-methoxymandelonitrile benzoate at 100 °C under 5 bar of H2, Figure S11: 1H-NMR spectrum of (S)-tembamide. Figure S12: 13C-NMR spectrum of (S)-tembamide.
Author Contributions
Conceptualization, L.M.v.L. and F.H.; methodology, L.L. and M.D.W.; validation, L.L., M.D.W., L.M.v.L. and A.S.; formal analysis, L.L.; investigation, L.L.; resources, F.H., L.M.v.L., A.S. and M.D.W.; data curation, L.L, L.M.v.L. and A.S.; writing—original draft preparation, L.L. and L.M.v.L.; writing—review and editing, M.D.W., A.S. and F.H.; visualization, L.L.; supervision, L.M.v.L., A.S. and M.D.W.; project administration, L.M.v.L., F.H., and A.S.; funding acquisition, L.M.v.L., F.H., and A.S.
Funding
This project was funded by the European Union’s Horizon 2020 MSCA ITN-EID program under grant agreement No 634200 (BIOCASCADES). This communication reflects only the beneficiary’s view and the European Commission is not responsible for any use that may be made of the information it contains.
Acknowledgments
We thank Antje Spieß (Institute of Biochemical Engineering, Technische Universität Braunschweig, Germany) for fruitful discussion and for providing an HPLC system for chiral analysis. L. Leemans also thanks Yvonne Goecke (Institute of Biochemical Engineering, Technische Universität Braunschweig, Germany) for technical support with the HPLC equipment. Further, Klaas van Gorp (Grace Catalysts Technologies, Grace GmbH & Co, Germany) is acknowledged for providing Raney® Cobalt.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Reetz, M.T. New Approaches to the Use of Amino Acids as Chiral Building Blocks in Organic Synthesis. Angew. Chem. Int. Ed. Engl. 1991, 30, 1531–1546. [Google Scholar] [CrossRef]
- Bergmeier, S.C. The Synthesis of Vicinal Amino Alcohols. Tetrahedron 2000, 56, 2561–2576. [Google Scholar] [CrossRef]
- Klingler, F.D. Asymmetric Hydrogenation of Prochiral Amino Ketones to Amino Alcohols for Pharmaceutical Use. Acc. Chem. Res. 2007, 40, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Métro, T.X.; Duthion, B.; Gomez Pardo, D.; Cossy, J. Rearrangement of β-amino alcohols via aziridiniums: A review. Chem. Soc. Rev. 2010, 39, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.; Zhang, H.; Xiong, X.; Lu, X.; Zhou, Y. Evolution of epoxides to synthesize β-amino alcohols. Asian J. Chem. 2014, 26, 3761–3768. [Google Scholar] [CrossRef]
- Li, G.; Chang, H.T.; Barry Sharpless, K. Catalytic Asymmetric Aminohydroxylation (AA) of Olefins. Angew. Chem. Int. Ed. Engl. 1996, 35, 451–454. [Google Scholar] [CrossRef]
- Donohoe, T.J.; Callens, C.K.A.; Flores, A.; Lacy, A.R.; Rathi, A.H. Recent developments in methodology for the direct oxyamination of olefins. Chem. Eur. J. 2011, 17, 58–76. [Google Scholar] [CrossRef] [PubMed]
- Burchak, O.N.; Py, S. Reductive cross-coupling reactions (RCCR) between CN and CO for β-amino alcohol synthesis. Tetrahedron 2009, 65, 7333–7356. [Google Scholar] [CrossRef]
- Ye, C.X.; Melcamu, Y.Y.; Li, H.H.; Cheng, J.T.; Zhang, T.T.; Ruan, Y.P.; Zheng, X.; Lu, X.; Huang, P.Q. Dual catalysis for enantioselective convergent synthesis of enantiopure vicinal amino alcohols. Nat. Commun. 2018, 9, 410. [Google Scholar] [CrossRef]
- Cheng, M.-J.; Tsai, I.-L.; Chen, I.-S. Chemical Constituents from the Root Bark of Formosan Zanthoxylum Ailanthoides. J. Chin. Chem. Soc. 2003, 50, 1241–1246. [Google Scholar] [CrossRef]
- Johns, S.R.; Lamberton, J.A.; Tweeddale, H.J.; Willing, R.I. Alkaloids of Zanthoxylum Conspersipunctatum (Rutaceae): The Structure of a New Alkaloid Isomeric with Protopine. Aust. J. Chem. 1969, 22, 2233–2236. [Google Scholar] [CrossRef]
- Shoeb, A.; Kapil, R.S.; Popli, S.P. Coumarins and alkaloids of Aegle marmelos. Phytochemistry 1973, 12, 2071–2072. [Google Scholar] [CrossRef]
- Somanathan, R.; Aguilar, H.R.; Ventura, G.R.; Smith, K.M. Syntheses of natural hydroxyamides using trimethylsilyl cyanide. Synth. Commun. 1983, 13, 273–280. [Google Scholar] [CrossRef]
- Cheng, M.J.; Lee, K.H.; Tsai, I.L.; Chen, I.S. Two new sesquiterpenoids and anti-HIV principles from the root bark of Zanthoxylum ailanthoides. Bioorg. Med. Chem. 2005, 13, 5915–5920. [Google Scholar] [CrossRef] [PubMed]
- Albonico, S.M.; Kuck, A.M.; Deulofeu, V. Tembamide from Fagara hyemalis (St. Hill.) Engler. J. Chem. Soc. 1967, 22, 1327–1328. [Google Scholar] [CrossRef]
- Schrittwieser, J.H.; Coccia, F.; Kara, S.; Grischek, B.; Kroutil, W.; D’Alessandro, N.; Hollmann, F. One-pot combination of enzyme and Pd nanoparticle catalysis for the synthesis of enantiomerically pure 1,2-amino alcohols. Green Chem. 2013, 15, 3318–3331. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, P.T.; Nanda, S.; Bhaskar Rao, A. Stereoselective synthesis of (R)-(-)-denopamine, (R)-(-)-tembamide and (R)-(-)-aegeline via asymmetric reduction of azidoketones by Daucus carota in aqueous medium. Tetrahedron Asymmetry 2002, 12, 3381–3385. [Google Scholar] [CrossRef]
- Kamal, A.; Shaik, A.A.; Sandbhor, M.; Malik, M.S. Chemoenzymatic synthesis of (R)- and (S)-tembamide, aegeline and denopamine by a one-pot lipase resolution protocol. Tetrahedron Asymmetry 2004, 15, 3939–3944. [Google Scholar] [CrossRef]
- Buchanan, D.J.; Dixon, D.J.; Scott, M.S.; Lainé, D.I. Short asymmetric syntheses of bioactive β-aryl ethanolamine derivatives via the highly diastereoselective delta lactol oxy-Michael addition. Tetrahedron Asymmetry 2004, 15, 195–197. [Google Scholar] [CrossRef]
- Sadyandy, R. An asymmetric dihydroxylation route to (R)-(-)-octopamine, (R)-(-)-tembamide and (R)-(-)-aegeline. Arkivoc 2005, 2005, 36–43. [Google Scholar]
- Liardo, E.; González-Fernández, R.; Ríos-Lombardía, N.; Morís, F.; García-Álvarez, J.; Cadierno, V.; Crochet, P.; Rebolledo, F.; González-Sabín, J. Strengthening the Combination between Enzymes and Metals in Aqueous Medium: Concurrent Ruthenium-Catalyzed Nitrile Hydration-Asymmetric Ketone Bioreduction. ChemCatChem 2018, 10, 4676–4682. [Google Scholar] [CrossRef]
- Cortez, N.A.; Aguirre, G.; Parra-Hake, M.; Somanathan, R. Synthesis of (R)-tembamide and (R)-aegeline via asymmetric transfer hydrogenation in water. Tetrahedron Asymmetry 2013, 24, 1297–1302. [Google Scholar] [CrossRef]
- Tae Cho, B.; Kyu Kang, S.; Hye Shin, S. Application of optically active 1,2-diol monotosylates for synthesis of β-azido and β-amino alcohols with very high enantiomeric purity. Synthesis of enantiopure (R)-octopamine, (R)-tembamide and (R)-aegeline. Tetrahedron Asymmetry 2002, 13, 1209–1217. [Google Scholar] [CrossRef]
- Lee, D.M.; Lee, J.C.; Jeong, N.; Lee, K.I. Asymmetric transfer hydrogenation of 2-tosyloxy-1-(4-hydroxyphenyl)ethanone derivatives: Synthesis of (R)-tembamide, (R)-aegeline, (R)-octopamine, and (R)-denopamine. Tetrahedron Asymmetry 2007, 18, 2662–2667. [Google Scholar] [CrossRef]
- Zhu, C.; Xia, Q.; Chen, X.; Liu, Y.; Du, X.; Cui, Y. Chiral metal–Organic framework as a platform for cooperative catalysis in asymmetric cyanosilylation of aldehydes. ACS Catal. 2016, 6, 7590–7596. [Google Scholar] [CrossRef]
- Aguirre, G.; Salgado-Rodríguez, A.; Flores-López, L.Z.; Parra-Hake, M. Asymmetric Synthesis of Naturally Occurring β-Hydroxyamides (R)-Tembamide and (R)-Aegeline. J. Mex. Chem. Soc. 2001, 45, 21–24. [Google Scholar]
- Brown, R.F.C.; Donohue, A.C.; Jackson, W.R.; McCarthy, T.D. Synthetic applications of optically active cyanohydrins. Enantioselective syntheses of the hydroxyamides tembamide and aegeline, the cardiac drug denopamine, and some analogues of the bronchodilator salbutamol. Tetrahedron 1994, 50, 13739–13752. [Google Scholar] [CrossRef]
- Baeza, A.; Nájera, C.; Sansano, J.M.; Saá, J.M. Binolam-AlCl: A two-centre catalyst for the synthesis of enantioenriched cyanohydrin O-phosphates. Chem. Eur. J. 2005, 11, 3849–3862. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Choudhary, M.K.; Kureshy, R.I.; Roy, T.; Khan, N.U.H.; Abdi, S.H.R.; Bajaj, H.C. Enantioselective Henry and aza-Henry reaction in the synthesis of (R)-tembamide using efficient, recyclable polymeric CuII complexes as catalyst. ChemPlusChem 2014, 79, 1138–1146. [Google Scholar] [CrossRef]
- Gröger, H.; Hummel, W. Chemoenzymatic Multistep One-Pot Processes. In Cascade Biocatalysis: Integrating Stereoselective and Environmentally Friendly Reactions; Riva, S., Fessner, W.D., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; pp. 427–456. [Google Scholar]
- Gröger, H.; Hummel, W. Combining the “two worlds” of chemocatalysis and biocatalysis towards multi-step one-pot processes in aqueous media. Curr. Opin. Chem. Biol. 2014, 19, 171–179. [Google Scholar] [CrossRef]
- Schaaf, P.; Bayer, T.; Koley, M.; Schnürch, M.; Bornscheuer, U.T.; Rudroff, F.; Mihovilovic, M.D. Biocompatible metal-assisted C-C cross-coupling combined with biocatalytic chiral reductions in a concurrent tandem cascade. Chem. Commun. 2018, 54, 12978–12981. [Google Scholar] [CrossRef] [PubMed]
- Srinivasamurthy, V.S.T.; Böttcher, D.; Bornscheuer, U.T. A multi-enzyme cascade reaction for the production of 6-hydroxyhexanoic acid. Z. Nat.-Sect. C J. Biosci. 2019, 74, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Schrittwieser, J.H.; Velikogne, S.; Hall, M.; Kroutil, W. Artificial Biocatalytic Linear Cascades for Preparation of Organic Molecules. Chem. Rev. 2018, 118, 270–348. [Google Scholar] [CrossRef] [PubMed]
- Dawood, A.W.H.; Bassut, J.; de Souza, R.O.M.A.; Bornscheuer, U.T. Combination of the Suzuki–Miyaura Cross-Coupling Reaction with Engineered Transaminases. Chem. Eur. J. 2018, 24, 16009–16013. [Google Scholar] [CrossRef]
- Leemans, L.; van Langen, L.; Hollmann, F.; Schallmey, A. Bienzymatic Cascade for the Synthesis of an Optically Active O-benzoyl Cyanohydrin. Catalysts 2019, 9, 522. [Google Scholar] [CrossRef]
- Veum, L.; Pereira, S.R.M.; Van Der Waal, J.C.; Hanefeld, U. Catalytic hydrogenation of cyanohydrin esters as a novel approach to N-acylated β-amino alcohols—Reaction optimisation by a design of experiment approach. Eur. J. Org. Chem. 2006, 1664–1671. [Google Scholar] [CrossRef]
- Saavedra, J.Z.; Resendez, A.; Rovira, A.; Eagon, S.; Haddenham, D.; Singaram, B. Reaction of InCl 3 with various reducing agents: InCl 3-NaBH 4-mediated reduction of aromatic and aliphatic nitriles to primary amines. J. Org. Chem. 2012, 77, 221–228. [Google Scholar] [CrossRef]
- Haddenham, D.; Pasumansky, L.; DeSoto, J.; Eagon, S.; Singaram, B. Reductions of aliphatic and aromatic nitriles to primary amines with diisopropylaminoborane. J. Org. Chem. 2009, 74, 1964–1970. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.T.; Westcott, S.A. Hydroboration and diboration of imines. In Modern Reduction Methods; Anderson, P., Munslow, I.M., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 305–307. ISBN 9783527317240. [Google Scholar]
- Freifelder, M. A Low Pressure Process for the Reduction of Nitriles. Use of Rhodium Catalyst. J. Am. Chem. Soc. 1960, 82, 2386–2389. [Google Scholar] [CrossRef]
- Enthaler, S.; Junge, K.; Addis, D.; Erre, G.; Beller, M. A practical and benign synthesis of primary amines through ruthenium-catalyzed reduction of nitriles. ChemSusChem 2008, 1, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Segobia, D.J.; Trasarti, A.F.; Apesteguía, C.R. Hydrogenation of nitriles to primary amines on metal-supported catalysts: Highly selective conversion of butyronitrile to n-butylamine. Appl. Catal. A Gen. 2012, 445, 69–75. [Google Scholar] [CrossRef]
- Vilches-Herrera, M.; Werkmeister, S.; Junge, K.; Börner, A.; Beller, M. Selective catalytic transfer hydrogenation of nitriles to primary amines using Pd/C. Catal. Sci. Technol. 2014, 4, 629–632. [Google Scholar] [CrossRef]
- Schärringer, P.; Müller, T.E.; Lercher, J.A. Investigations into the mechanism of the liquid-phase hydrogenation of nitriles over Raney-Co catalysts. J. Catal. 2008, 253, 167–179. [Google Scholar] [CrossRef]
- Krupka, J.; Pasek, J. Nitrile Hydrogenation on Solid Catalysts—New Insights into the Reaction Mechanism. Curr. Org. Chem. 2012, 16, 988–1004. [Google Scholar] [CrossRef]
- Bagal, D.B.; Bhanage, B.M. Recent advances in transition metal-catalyzed hydrogenation of nitriles. Adv. Synth. Catal. 2015, 357, 883–900. [Google Scholar] [CrossRef]
- Lévay, K.; Hegedűs, L. Selective Heterogeneous Catalytic Hydrogenation of Nitriles to Primary Amines. Period. Polytech. Chem. Eng. 2018, 62, 476–488. [Google Scholar] [CrossRef]
- Braun, J.; Blessing, G.; Zobel, F. Katalytische Hydrierungen unter Druck bei Gegenwart von Nickelsalzen, VI.: Nitrile. Ber. Dtsch. Chem. Ges. 1923, 36, 1988–2001. [Google Scholar] [CrossRef]
- Greenfield, H. Hydrogenation of Benzonitrile to Dibenzylamine. Ind. Eng. Chem. Prod. Res. Dev. 1976, 15, 156–158. [Google Scholar] [CrossRef]
- Hartung, W.H. Catalytic reduction of nitriles and oximes. J. Am. Chem. Soc. 1928, 50, 3370–3374. [Google Scholar] [CrossRef]
- Rosenmund, K.W.; Schindler, H. Über die katalytische Reduktion von Mandelsäuren. Arch. Pharm. (Weinh.) 1928, 432, 281–283. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, G.; Li, Y.; Yan, X.; Chen, L. Racemization of R-2-amino-1-butanol catalyzed by a fixed-bed Raney cobalt catalyst. J. Mol. Catal. A Chem. 2006, 255, 269–274. [Google Scholar] [CrossRef]
- Hertzberg, R.; Dinér, P.; Moberg, C. Palladium-Catalyzed C(sp3)–C(sp2) Cross-Couplings of O-(α-Bromoacyl) Cyanohydrins with Boronic Acids: An Entry to Enantio-enriched N-Acylated β-Amino Alcohols. Synthesis (Stuttg.) 2016, 48, 3175–3182. [Google Scholar] [CrossRef]
- Hertzberg, R.; Monreal Santiago, G.; Moberg, C. Synthesis of the β3-adrenergic receptor agonist Solabegron and Analogous N-(2-Ethylamino)-β-amino alcohols from O-acylated cyanohydrins—Expanding the scope of minor enantiomer recycling. J. Org. Chem. 2015, 80, 2937–2941. [Google Scholar] [CrossRef]
- Gomez, S.; Peters, J.A.; Maschmeyer, T. The Reductive Animation of Aldehydes and Ketones and the Hydrogenation of Nitriles: Mechanistic Aspects and Selectivity Control. Adv. Synth. Catal. 2002, 344, 1037–1057. [Google Scholar] [CrossRef]
- Banwell, M.G.; Jones, M.T.; Reekie, T.A.; Schwartz, B.D.; Tan, S.H.; White, L.V. RANEY® cobalt-an underutilised reagent for the selective cleavage of C-X and N-O bonds. Org. Biomol. Chem. 2014, 12, 7433–7444. [Google Scholar] [CrossRef]
- Bhor, M.D.; Bhanushali, M.J.; Nandurkar, N.S.; Bhanage, B.M. Highly efficient chemoselective catalytic hydrogenation of diaryl substituted α,β-unsaturated nitriles/carbonyls using homogeneous Pd(OAc)2/PPh3 catalyst. Catal. Commun. 2007, 8, 2064–2068. [Google Scholar] [CrossRef]
- Volf, J.; Pasek, J. Hydrogenation of Nitriles. In Studies in Surface Science and Catalysis: Catalytic Hydrogenation; Cerveny, L., Ed.; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 1986; Volume 27, pp. 105–144. ISBN 0444426825. [Google Scholar]
- Huang, Y.; Adeeva, V.; Sachtler, W.M.H. Stability of supported transition metal catalysts in the hydrogenation of nitriles. Appl. Catal. A Gen. 2000, 196, 73–85. [Google Scholar] [CrossRef]
- Fureby, A.M.; Virto, C.; Adlercreutz, P.; Mattiasson, B. Acyl group migrations in 2-monoolein. Biocatal. Biotransform. 1996, 14, 89–111. [Google Scholar] [CrossRef]
- Hsiao, M.K.; Lo, W.T.; Wang, J.H.; Chen, H.L. Hydrogenation of Hydrogen Cyanide to Methane and Ammonia by a Metal Catalyst: Insight from First-Principles Calculations. J. Phys. Chem. C 2016, 120, 22946–22956. [Google Scholar] [CrossRef]
- Besson, P.; Thirion, P. Process for Manufacture of Methylamines. US3468953A, 23 September 1969. [Google Scholar]
- Malona, J.A.; Cariou, K.; Spencer, W.T.; Frontier, A.J. Total synthesis of (±)-rocaglamide via oxidation-initiated nazarov cyclization. J. Org. Chem. 2012, 77, 1891–1908. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).