Rh(III)-Catalyzed C–H Bond Activation for the Construction of Heterocycles with sp3-Carbon Centers
Abstract
1. Introduction
2. Construction of Five-Member Heterocycles
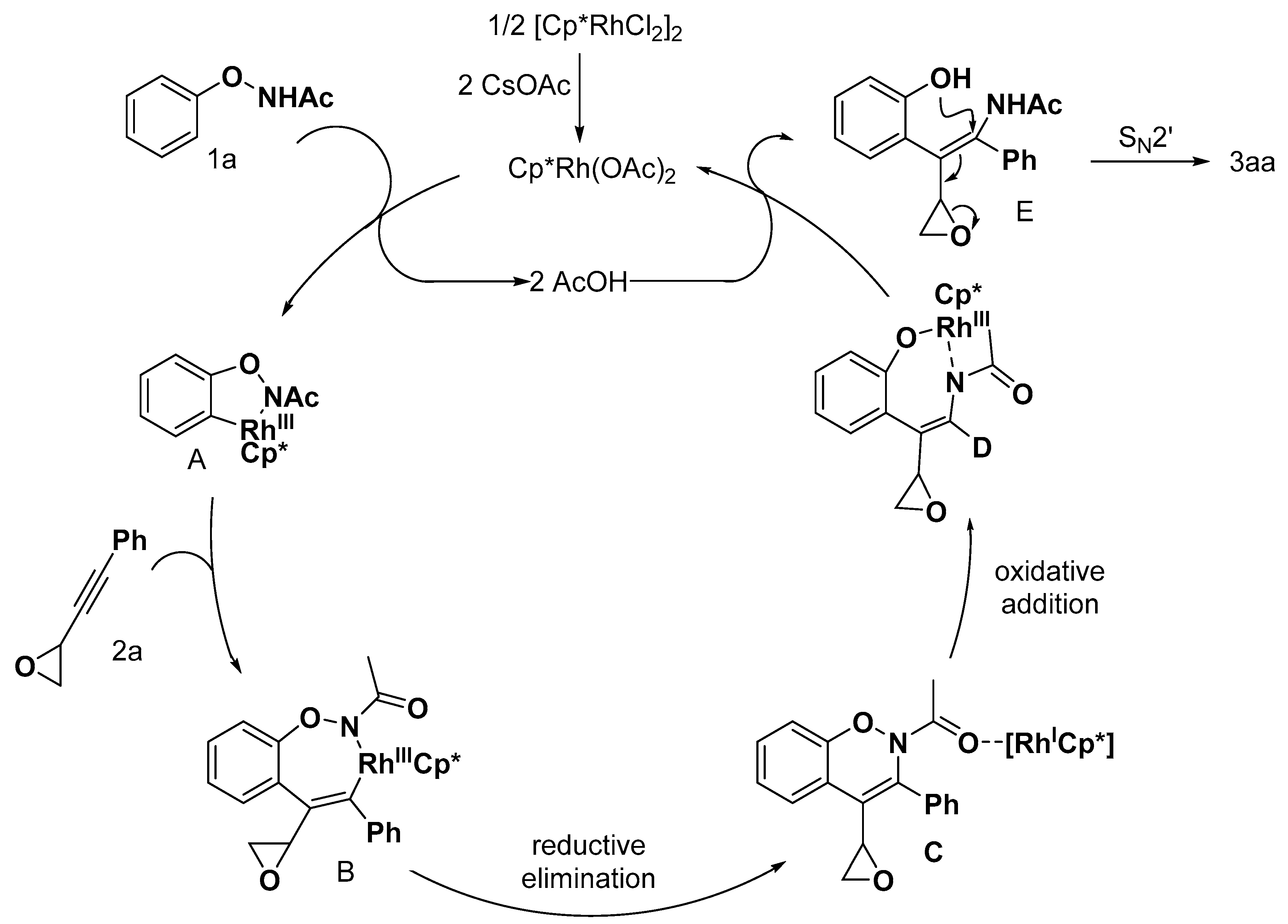
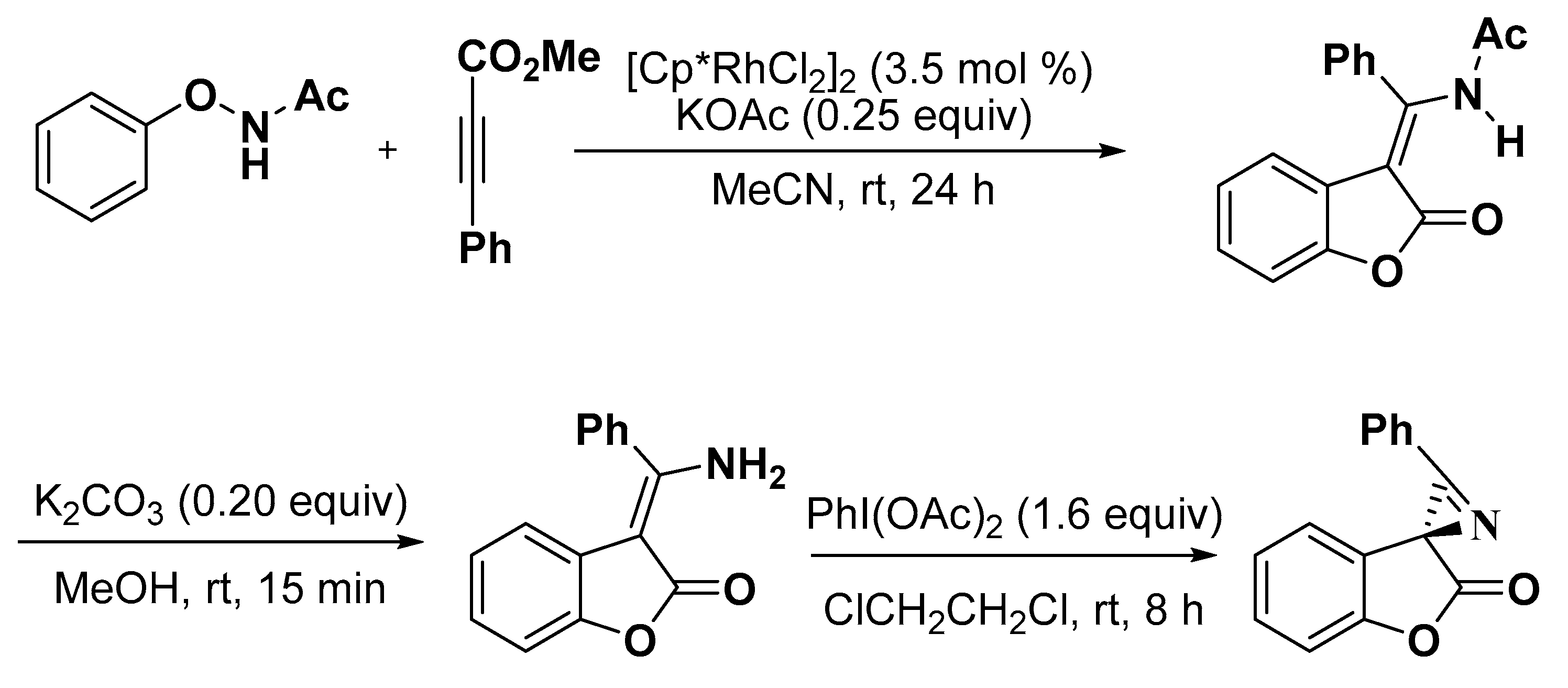
2.1. Benzofuran Derivatives
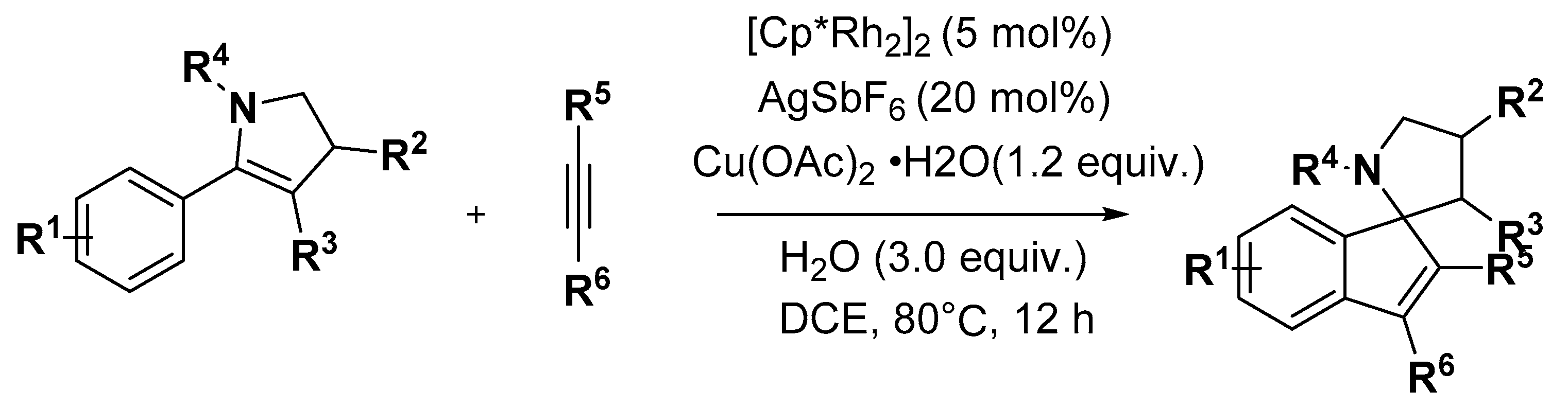
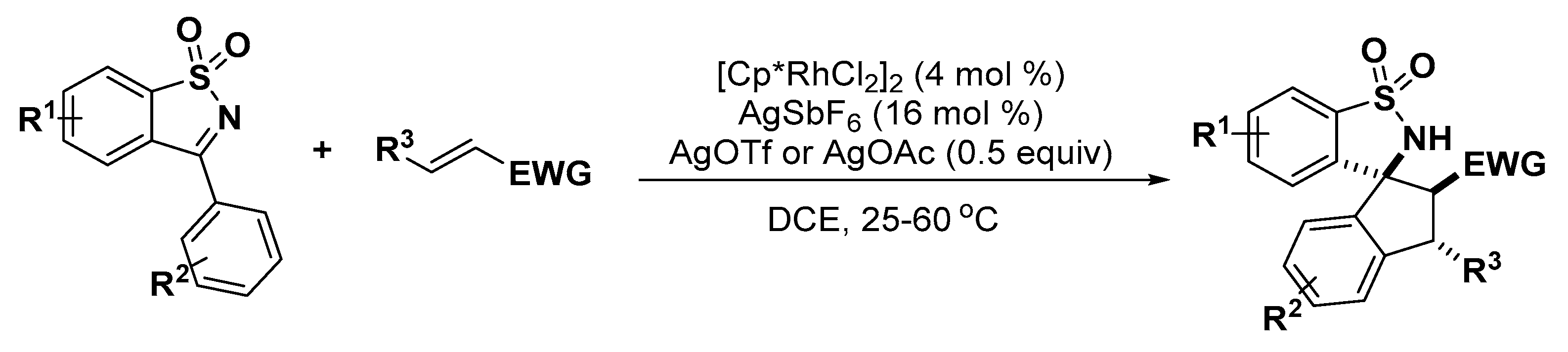
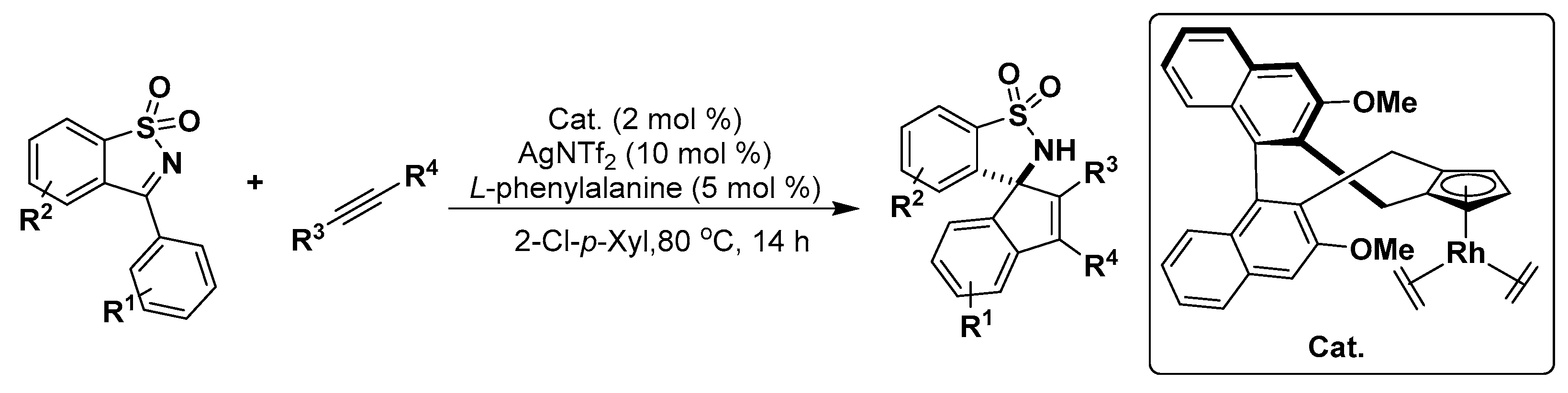
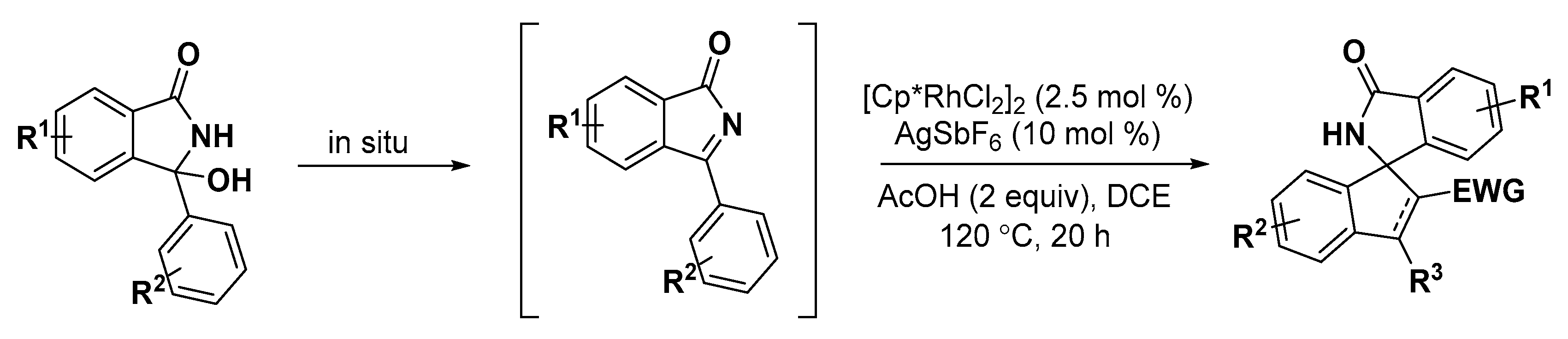
2.2. Indole Derivatives
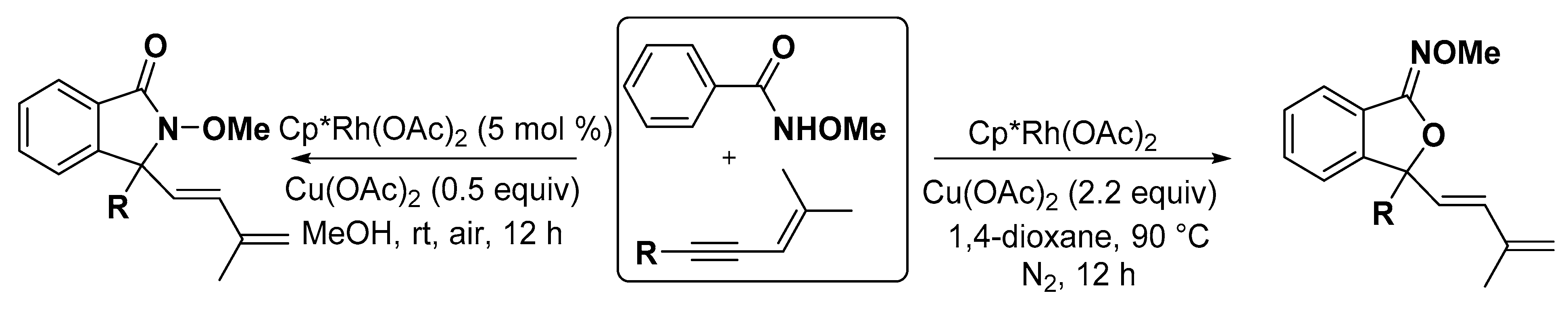
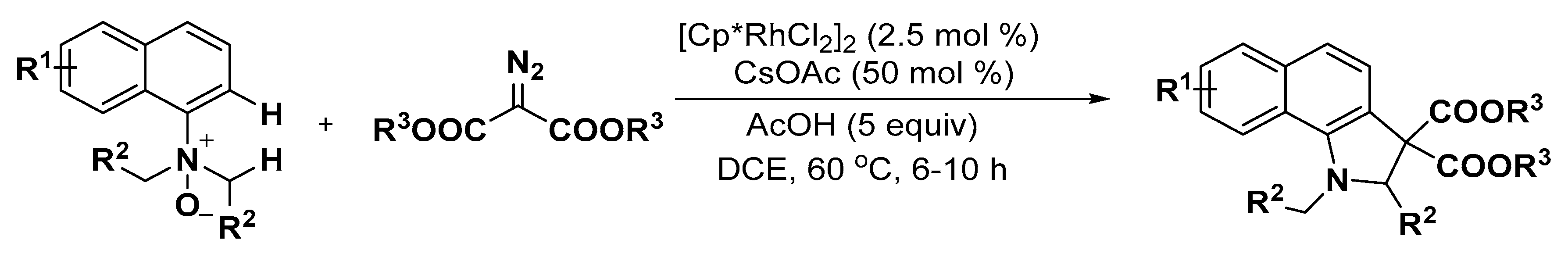
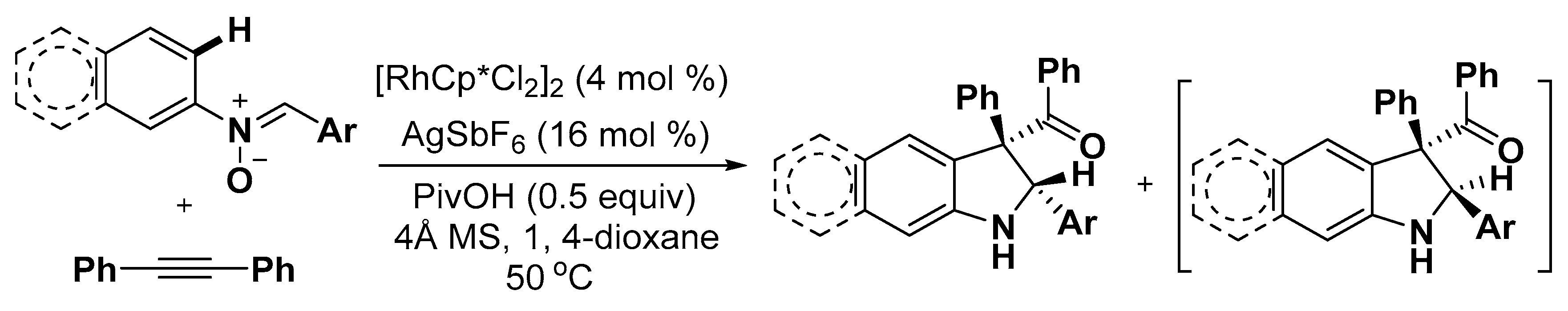
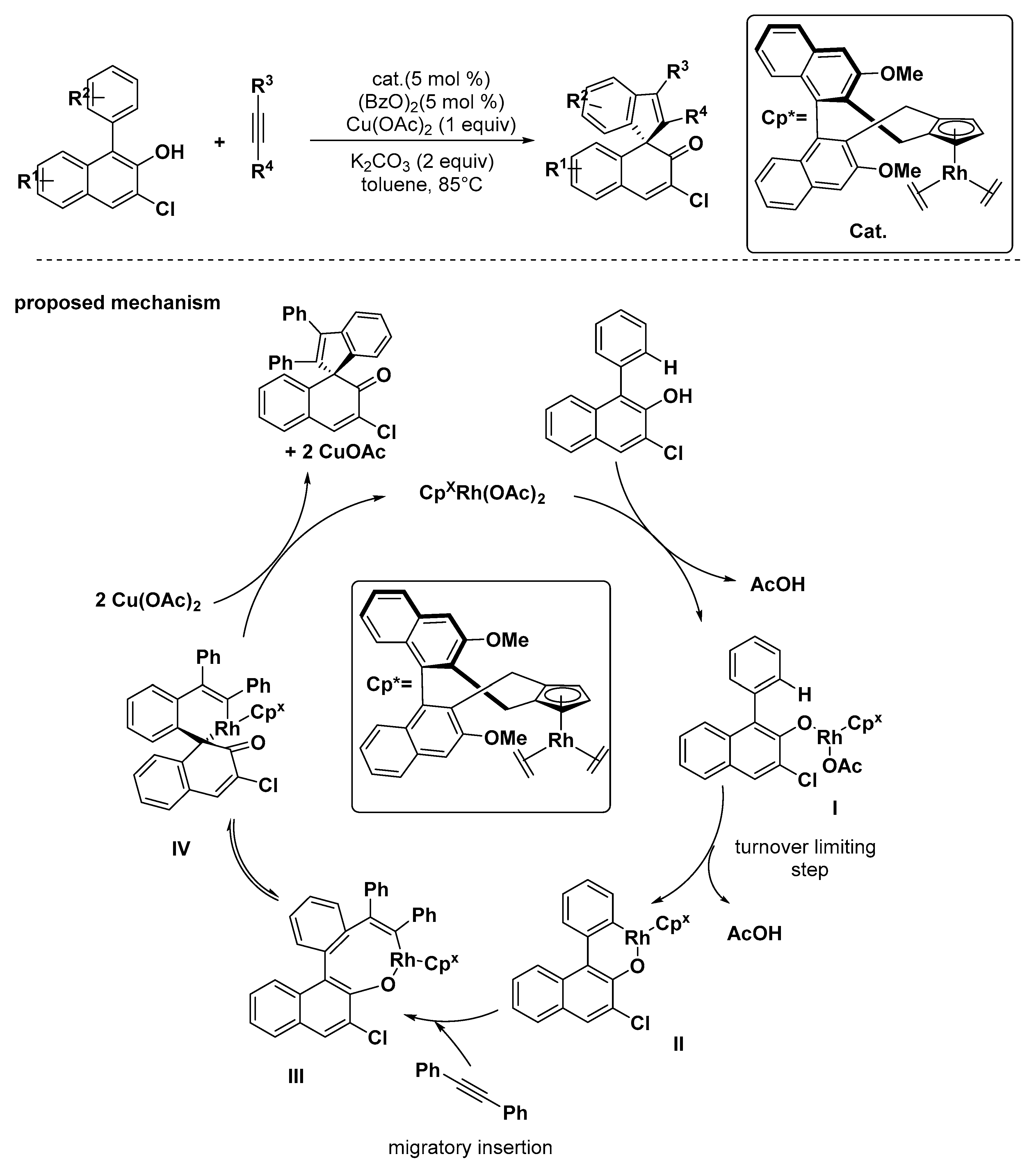
2.3. Others
3. Construction of Six-Member Heterocycles
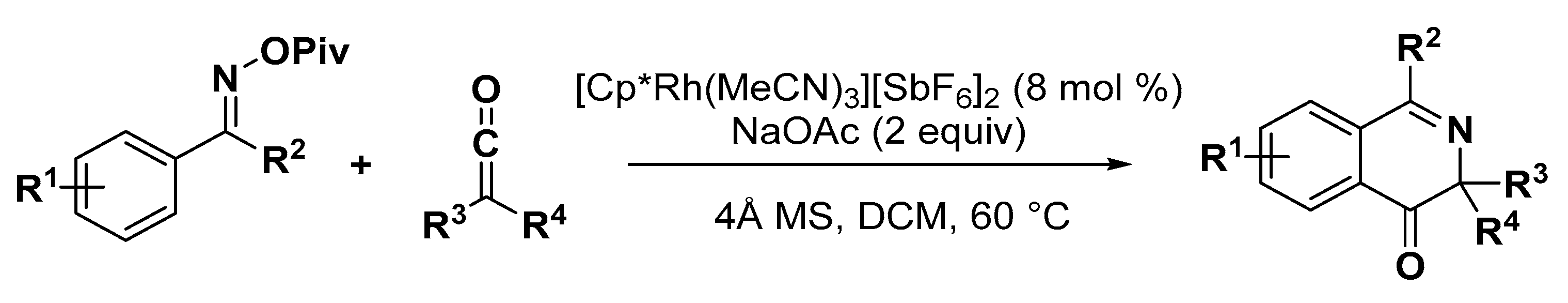
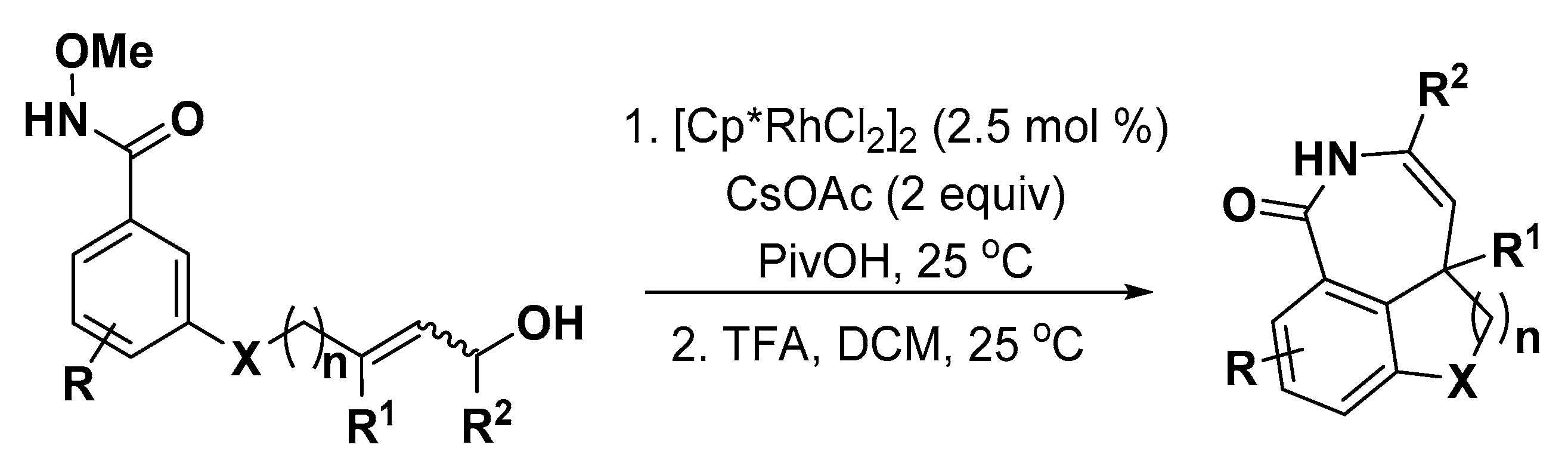
3.1. Nitrogen Heterocycles
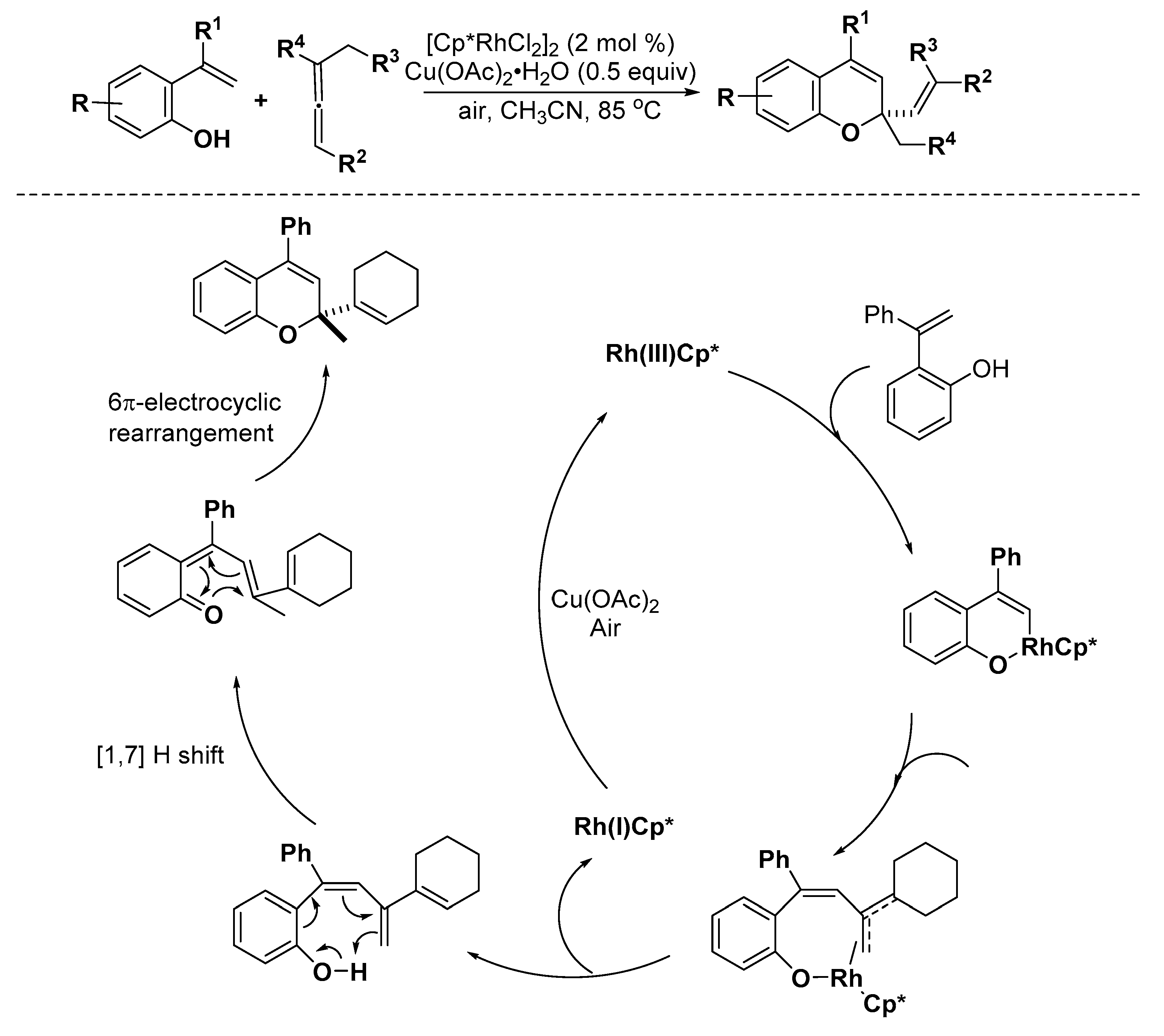
3.2. Oxygen Heterocycles
4. Construction of Seven-Member Heterocycles
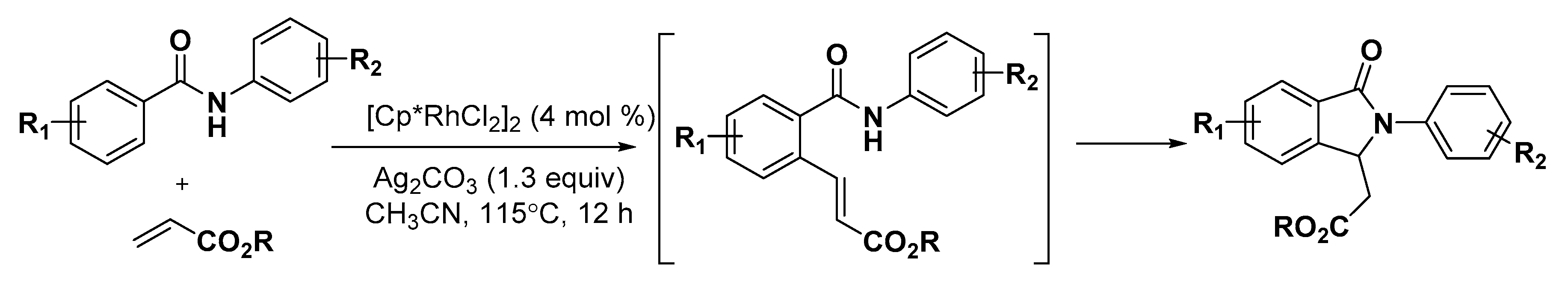
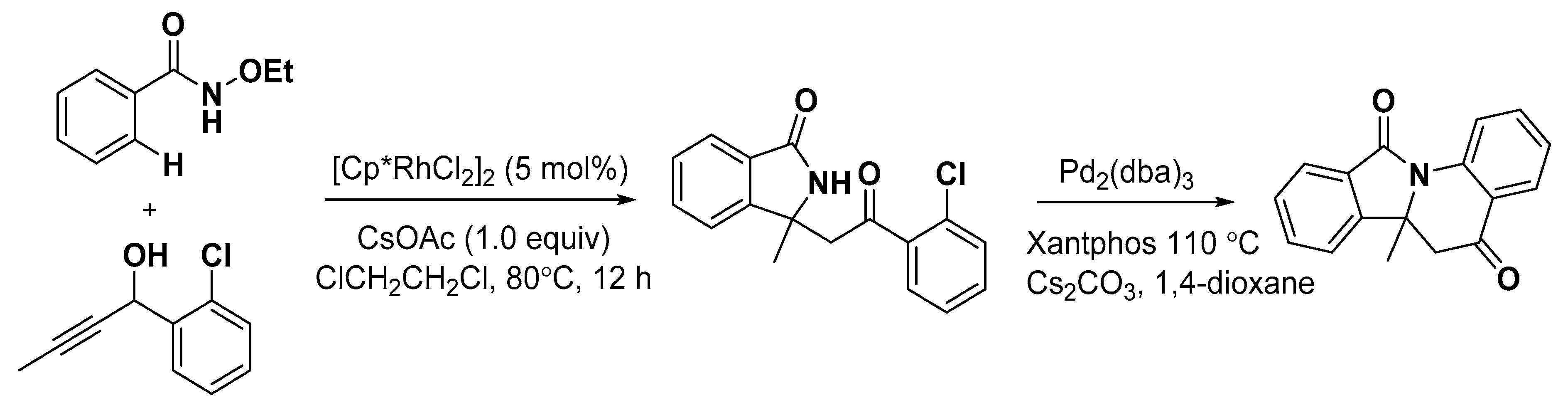
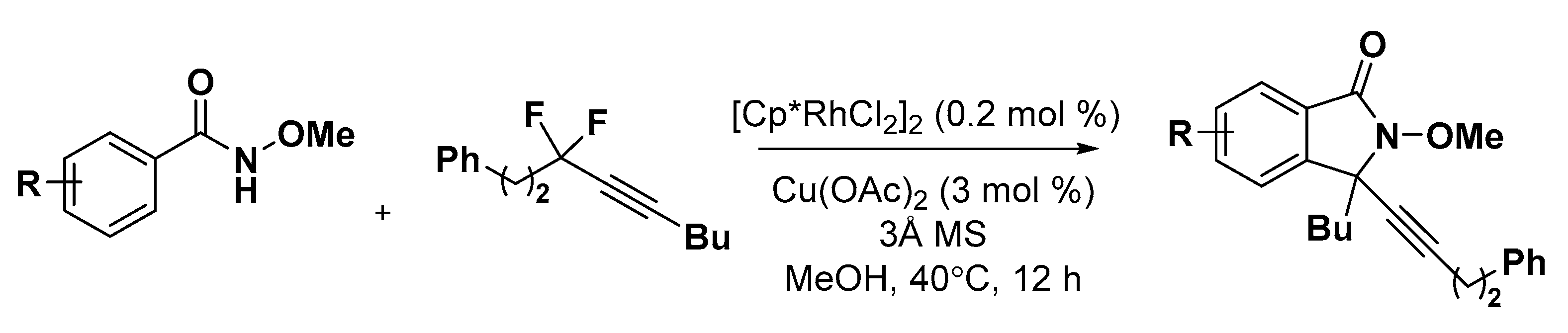
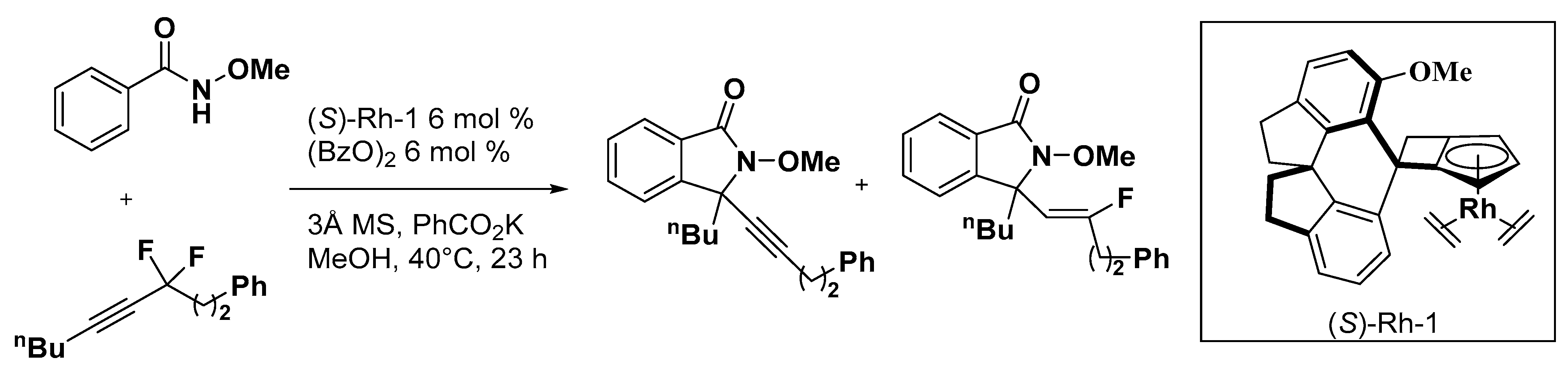
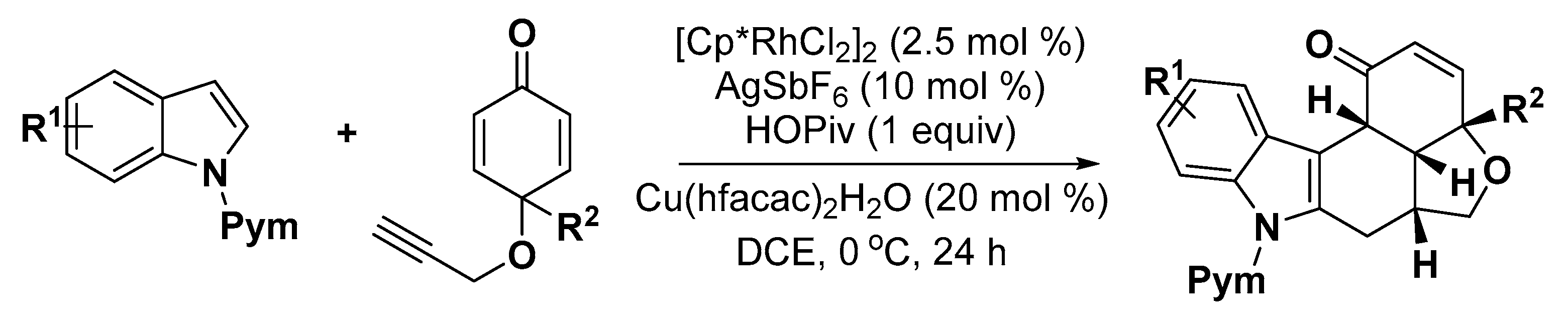
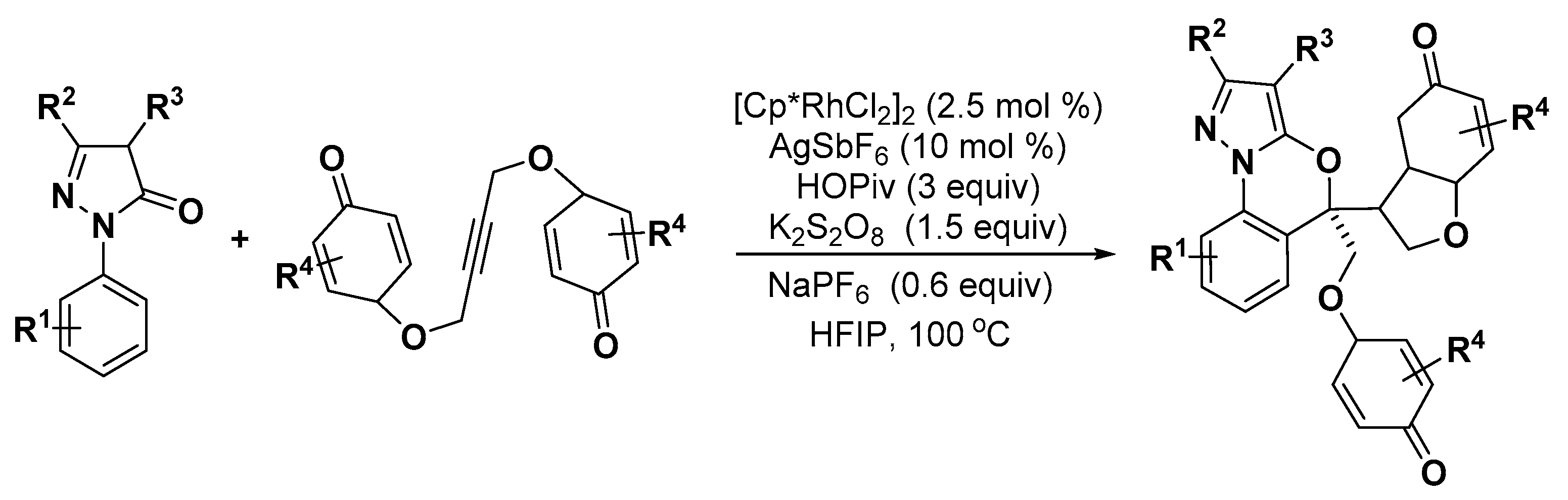
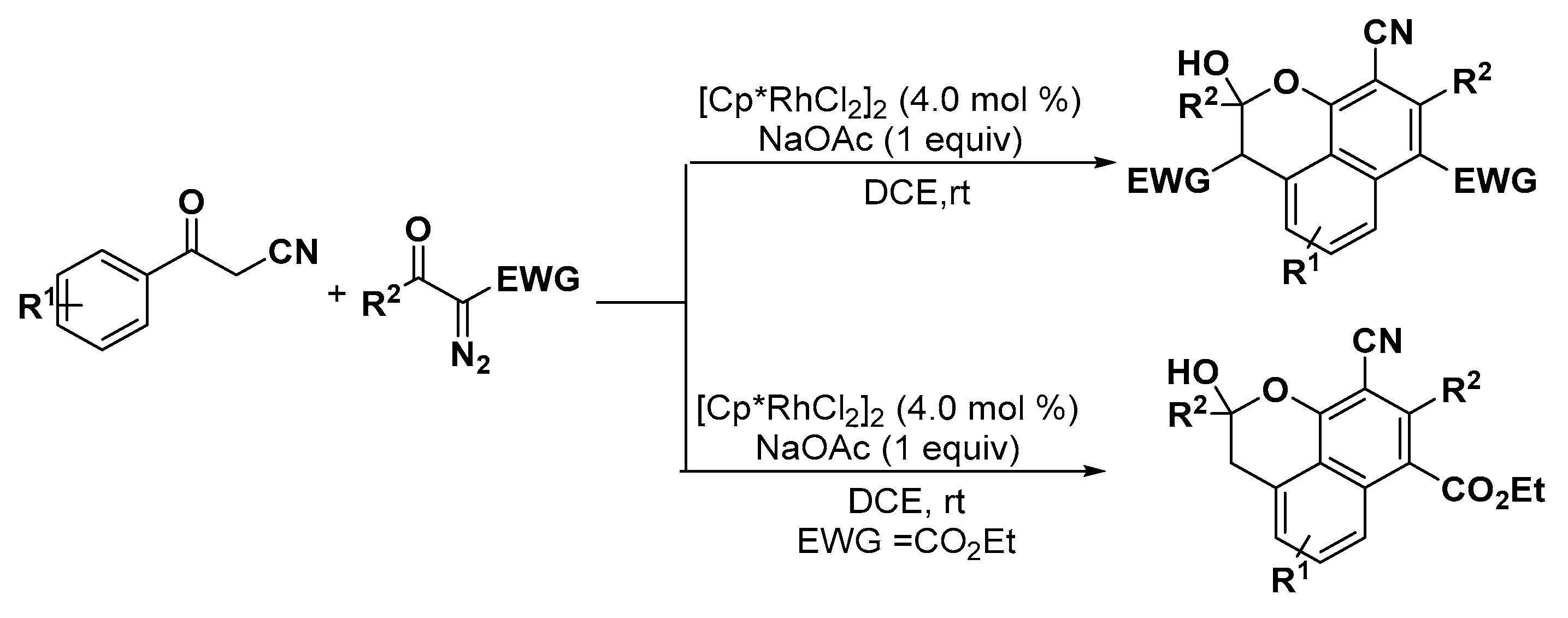
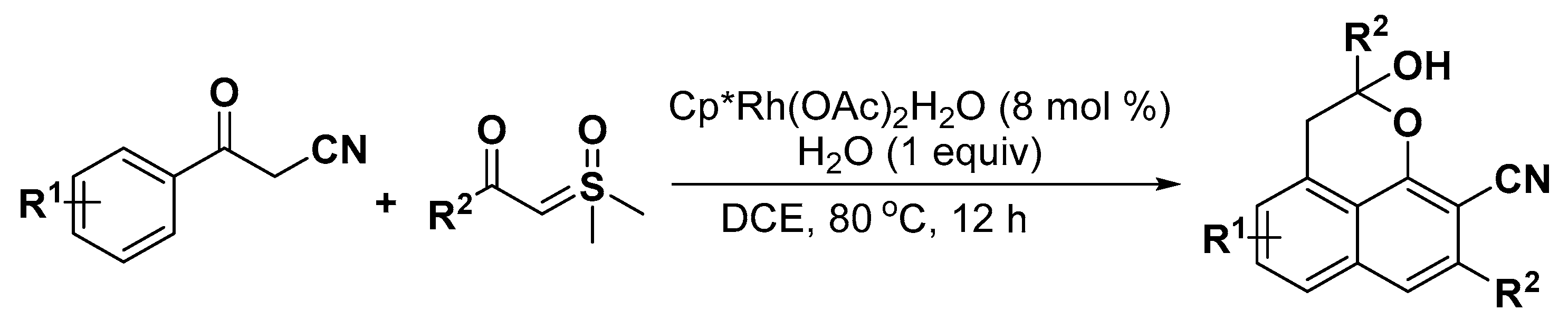
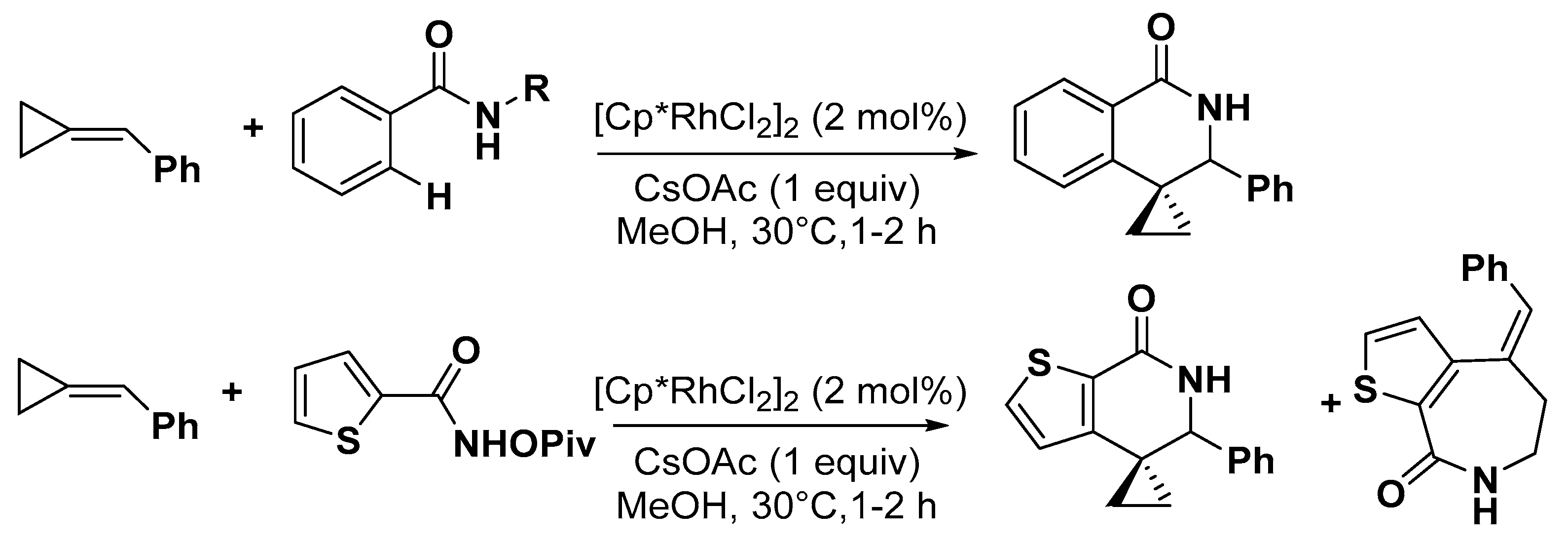
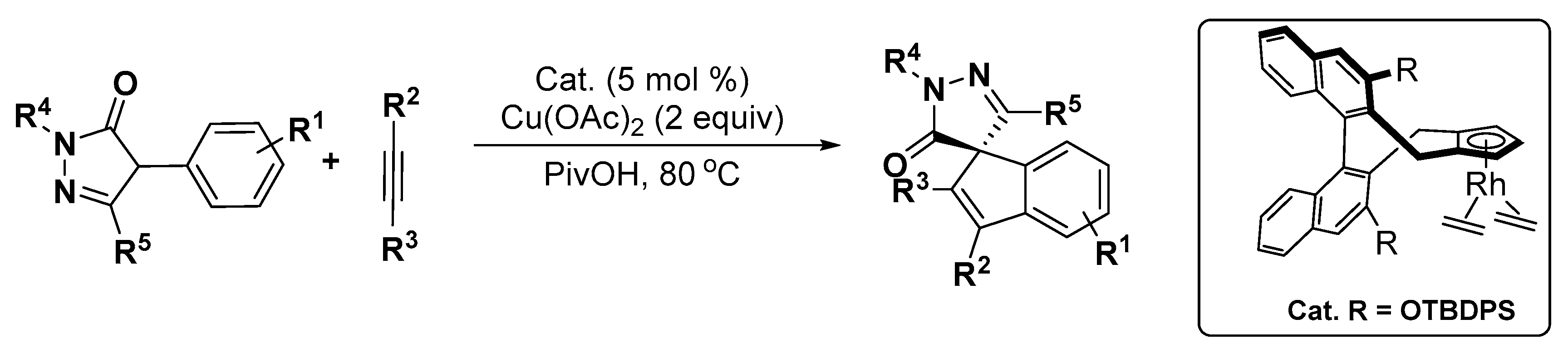
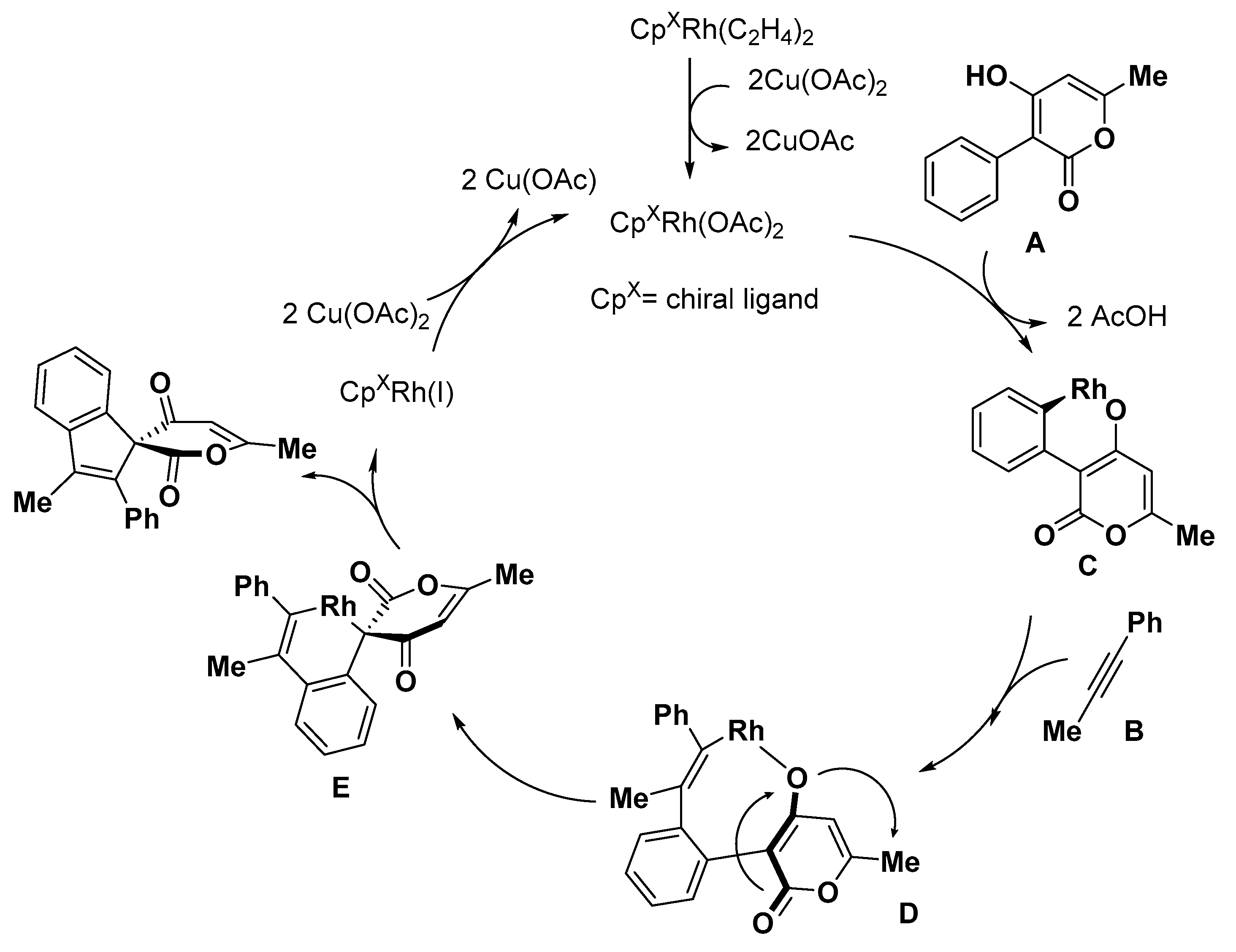
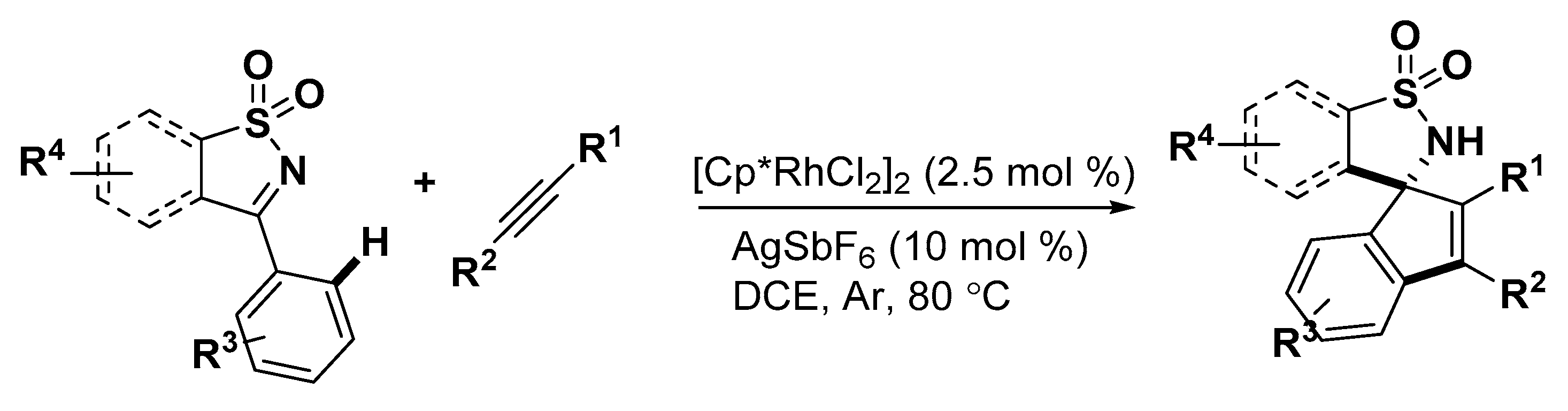
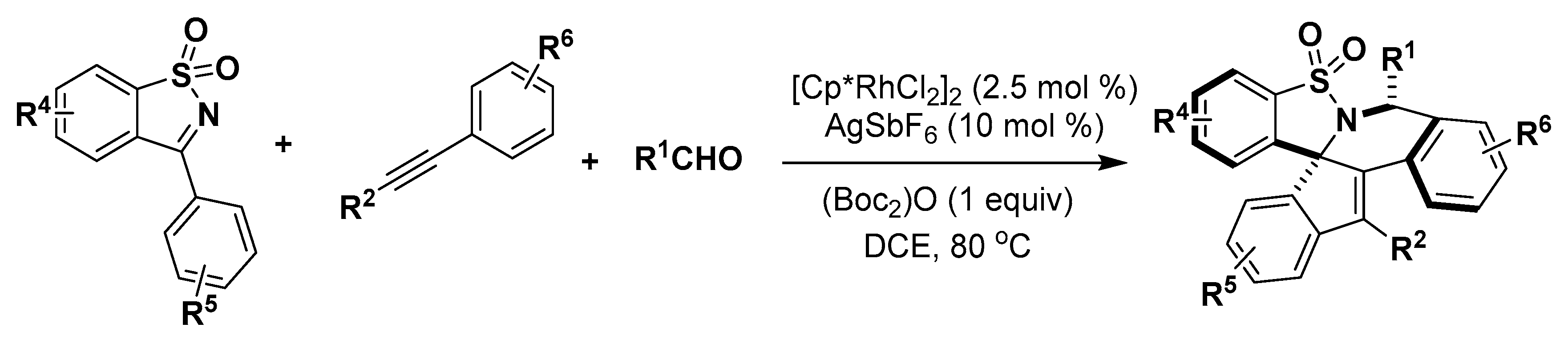
5. Construction of Spiroheterocycles
6. Conclusions
Funding
Conflicts of Interest
References
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Delost, M.D.; Qureshi, M.H.; Smith, D.T.; Njardarson, J.T. A survey of the structures of US FDA approved combination drugs. J. Med. Chem. 2019, 62, 4265–4311. [Google Scholar] [CrossRef] [PubMed]
- Delost, M.D.; Smith, D.T.; Anderson, B.J.; Njardarson, J.T. From oxiranes to oligomers: Architectures of U.S. FDA approved pharmaceuticals containing oxygen heterocycles. J. Med. Chem. 2018, 61, 10996–11020. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.A.; Willis, M.C. Tandem inverse-electron-demand hetero-/retro-Diels-Alder reactions for aromatic nitrogen heterocycle synthesis. Chem. Soc. Rev. 2013, 42, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Rotstein, B.H.; Zaretsky, S.; Rai, V.; Yudin, A.K. Small heterocycles in multicomponent reactions. Chem. Rev. 2014, 114, 8323–8359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Studer, A. Recent advances in the synthesis of nitrogen heterocycles via radical cascade reactions using isonitriles as radical acceptors. Chem. Soc. Rev. 2015, 44, 3505–3521. [Google Scholar] [CrossRef] [PubMed]
- Jacques Royer, M.B.; Micouin, L. Chiral heterocycles by iminium ion cyclization. Chem. Rev. 2004, 104, 2311–2352. [Google Scholar] [CrossRef]
- Villar, H.; Frings, M.; Bolm, C. Ring closing enyne metathesis: A powerful tool for the synthesis of heterocycles. Chem. Soc. Rev. 2007, 36, 55–66. [Google Scholar] [CrossRef]
- Chen, J.R.; Hu, X.Q.; Lu, L.Q.; Xiao, W.J. Exploration of visible-light photocatalysis in heterocycle synthesis and functionalization: Reaction design and beyond. Acc. Chem. Res. 2016, 49, 1911–1923. [Google Scholar] [CrossRef]
- Xuan, J.; Lu, L.Q.; Chen, J.R.; Xiao, W.J. Visible-light-driven photoredox catalysis in the construction of carbocyclic and heterocyclic ring systems. Eur. J. Org. Chem. 2013, 2013, 6755–6770. [Google Scholar] [CrossRef]
- Itaru Nakamura, Y.Y. Transition-metal-catalyzed reactions in heterocyclic synthesis. Chem. Rev. 2004, 104, 2127–2198. [Google Scholar] [CrossRef] [PubMed]
- Barluenga, J.; Santamaria, J.; Tomás, M. Synthesis of heterocycles via group VI fischer carbene complexes. Chem. Rev. 2004, 1014, 2259–2283. [Google Scholar] [CrossRef] [PubMed]
- Jagodzin´ski, T.S. Thioamides as useful synthons in the synthesis of heterocycles. Chem. Rev. 2003, 103, 197–227. [Google Scholar] [CrossRef] [PubMed]
- Han, F.S. Transition-metal-catalyzed Suzuki-Miyaura cross-coupling reactions: A remarkable advance from palladium to nickel catalysts. Chem. Soc. Rev. 2013, 42, 5270–5298. [Google Scholar] [CrossRef] [PubMed]
- Phapale, V.B.; Cardenas, D.J. Nickel-catalysed Negishi cross-coupling reactions: Scope and mechanisms. Chem. Soc. Rev. 2009, 38, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.M.; Hajiabbasi, P. Recent advances in Kumada-Tamao-Corriu cross-coupling reaction catalyzed by different ligands. Monatshefte Chem. Chem. Mon. 2012, 143, 1575–1592. [Google Scholar] [CrossRef]
- Espinet, P.; Echavarren, A.M. The mechanisms of the Stille reaction. Angew. Chem. Int. Ed. 2004, 43, 4704–4734. [Google Scholar]
- Monfared, A.; Mohammadi, R.; Ahmadi, S.; Nikpassand, M.; Hosseinian, A. Recent advances in the application of nano-catalysts for Hiyama cross-coupling reactions. RSC Adv. 2019, 9, 3185–3202. [Google Scholar] [CrossRef]
- He, J.; Wasa, M.; Chan, K.S.L.; Shao, Q.; Yu, J.Q. Palladium-catalyzed transformations of alkyl C–H bonds. Chem. Rev. 2017, 117, 8754–8786. [Google Scholar] [CrossRef] [PubMed]
- Arockiam, P.B.; Bruneau, C.; Dixneuf, P.H. Ruthenium(II)-catalyzed C–H bond activation and functionalization. Chem. Rev. 2012, 112, 5879–5918. [Google Scholar] [CrossRef]
- Colby, D.A.; Bergman, R.G.; Ellman, J.A. Rhodium-catalyzed C–C bond formation via heteroatom-directed C–H bond activation. Chem. Rev. 2010, 110, 624–655. [Google Scholar] [CrossRef] [PubMed]
- Boorman, T.C.; Larrosa, I. Gold-mediated C–H bond functionalisation. Chem. Soc. Rev. 2011, 40, 1910–1925. [Google Scholar] [CrossRef]
- Davies, D.L.; Al-Duaij, O.; Fawcett, J.; Giardiello, M.; Hilton, S.T.; Russell, D.R. Room-temperature cyclometallation of amines, imines and oxazolines with [MCl2Cp*]2(M = Rh, Ir) and [RuCl2(p-cymene)]2. Dalton Trans. 2003, 4132–4138. [Google Scholar] [CrossRef]
- Ueura, K.; Satoh, T.; Miura, M. An efficient waste-free oxidative coupling via regioselective C−H bond cleavage: Rh/Cu-catalyzed reaction of benzoic acids with alkynes and acrylates under air. Org. Lett. 2007, 9, 1407–1409. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Miura, M. Oxidative coupling of aromatic substrates with alkynes and alkenes under rhodium catalysis. Chem. Eur. J. 2010, 16, 11212–11222. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Li, X. Substrate activation strategies in Rhodium(III)-catalyzed selective functionalization of arenes. Acc. Chem. Res. 2015, 48, 1007–1020. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, N.; Schröder, N.; Glorius, F. Formal SN-type reactions in Rhodium(III)-catalyzed C–H bond activation. Adv. Synth. Catal. 2014, 356, 1443–1460. [Google Scholar] [CrossRef]
- Colby, D.A.; Tsai, A.S.; Bergman, R.G.; Ellman, J.A. Rhodium catalyzed chelation-assisted C–H bond functionalization reactions. Acc. Chem. Res. 2012, 45, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.F.; Neumann, H.; Beller, M. Synthesis of heterocycles via Palladium-catalyzed carbonylations. Chem. Rev. 2013, 113, 1–35. [Google Scholar] [CrossRef]
- Chemler, S.R.; Fuller, P.H. Heterocycle synthesis by copper facilitated addition of heteroatoms to alkenes, alkynes and arenes. Chem. Soc. Rev. 2007, 36, 1153–1160. [Google Scholar] [CrossRef]
- Duarah, G.; Kaishap, P.P.; Begum, T.; Gogoi, S. Recent advances in Ruthenium(II)-catalyzed C−H bond activation and alkyne annulation reactions. Adv. Synth. Catal. 2019, 361, 654–672. [Google Scholar] [CrossRef]
- Neuhaus, J.D.; Willis, M.C. Homogeneous Rhodium(I)-catalysis in de novo heterocycle syntheses. Org. Biomol. Chem. 2016, 14, 4986–5000. [Google Scholar] [CrossRef]
- Alvarez-Corral, M.; Munoz-Dorado, M.; Rodriguez-Garcia, I. Silver-mediated synthesis of heterocycles. Chem. Rev. 2008, 108, 3174–3198. [Google Scholar] [CrossRef]
- Sreedevi, R.; Saranya, S.; Rohit, K.R.; Anilkumar, G. Recent trends in Iron-catalyzed reactions towards the synthesis of nitrogen-containing heterocycles. Adv. Synth. Catal. 2019, 361, 2236–2249. [Google Scholar] [CrossRef]
- Kaur, N. Nickel catalysis: Six membered heterocycle syntheses. Synth. Commun. 2019, 49, 1103–1133. [Google Scholar] [CrossRef]
- Keerthi Krishnan, K.; Ujwaldev, S.M.; Saranya, S.; Anilkumar, G.; Beller, M. Recent advances and perspectives in the synthesis of heterocycles via Zinc catalysis. Adv. Synth. Catal. 2018, 361, 382–404. [Google Scholar] [CrossRef]
- Nitin, T.; Patil, Y.Y. Coinage metal-assisted synthesis of heterocycles. Chem. Rev. 2008, 108, 3395–3442. [Google Scholar]
- Marco, B. Au-Catalyzed Synthesis and Functionalization of Heterocycles, 1st ed.; Springer: Berlin, Germany, 2016; pp. 1–294. [Google Scholar]
- Zhou, Z.B.; Luo, J.G.; Pan, K.; Shan, S.M.; Zhang, W.; Kong, L.Y. Bioactive benzofuran neolignans from Aristolochia fordiana. Planta Med. 2013, 79, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Naima, B.; Francois, F. Flavonoids from dalbergia louvelii and their antiplasmodial activity. J. Nat. Prod. 2003, 66, 1447–1450. [Google Scholar]
- Sun, X.X.; Zhang, H.H.; Li, G.H.; Meng, L.; Shi, F. Diastereo-and enantioselective construction of an indole-based 2,3-dihydrobenzofuran scaffold via catalytic asymmetric [3 + 2] cyclizations of quinone monoimides with 3-vinylindoles. Chem. Commun. 2016, 52, 2968–2971. [Google Scholar] [CrossRef]
- Chen, J.; Shao, Y.; Zheng, H.; Cheng, J.; Wan, X. Metal-free coupling of 2-vinylphenols and carboxylic acids: An access to 3-acyloxy-2,3-dihydrobenzofurans. J. Org. Chem. 2015, 80, 10734–10741. [Google Scholar] [CrossRef]
- Wang, D.H.; Yu, J.Q. Highly convergent total synthesis of (+)-lithospermic acid via a late-stage intermolecular C–H olefination. J. Am. Chem. Soc. 2011, 133, 5767–5769. [Google Scholar] [CrossRef]
- Kuppusamy, R.; Gandeepan, P.; Cheng, C.H. Rh(III)-Catalyzed [4 + 1] Annulations of 2-hydroxy-and 2-aminobenzaldehydes with allenes: A simple method toward 3-coumaranones and 3-indolinones. Org. Lett. 2015, 17, 3846–3849. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Wang, F.; Li, X. C–C, C–O and C–N bond formation via Rhodium(III)-catalyzed oxidative C–H activation. Chem. Soc. Rev. 2012, 41, 3651–3678. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, Z.; Yang, Y.; Zhu, W.; Zhou, B. Rh(III)-catalyzed redox-neutral unsymmetrical C-Halkylation and amidation reactions of N-phenoxyacetamides. J. Am. Chem. Soc. 2018, 140, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, D.; Tang, Y.; He, X.; Xu, S. Rhodium(III)-catalyzed redox-neutral c-h activation/annulation of n-aryloxyacetamides with alkynyloxiranes: Synthesis of highly functionalized 2,3-dihydrobenzofurans. J. Org. Chem. 2018, 83, 9464–9470. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.L.; Xie, P.; Chen, C.; Hao, Y.; Liu, C.; Bai, H.Y.; Ding, J.; Wang, L.R.; Xia, Y.; Zhang, S.Y. Rhodium(III)-catalyzed redox-neutral cascade [3 + 2] annulation of N-phenoxyacetamides with propiolates via C–H functionalization/isomerization/lactonization. Org. Lett. 2018, 20, 7131–7136. [Google Scholar] [CrossRef]
- Sun, J.; Bai, D.; Wang, P.; Wang, K.; Zheng, G.; Li, X. Chemodivergent oxidative annulation of benzamides and enynes via 1,4-Rhodium migration. Org. Lett. 2019, 21, 1789–1793. [Google Scholar] [CrossRef] [PubMed]
- Bronner, S.M.; Garg, N.K. Heterocycles in Natural Product Synthesis, 1st ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; pp. 221–265. [Google Scholar]
- Cacchi, S.; Fabrizi, G. Synthesis and functionalization of indoles through Palladium-catalyzed reactions. Chem. Rev. 2005, 105, 2873–2920. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, G.; Dalpozzo, R.; Nardi, M. Applications of Bartoli indole synthesis. Chem. Soc. Rev. 2014, 43, 4728–4750. [Google Scholar] [CrossRef]
- Robinson, B. Studies on the Fischer indole synthesis. Chem. Rev. 1969, 69, 227–250. [Google Scholar] [CrossRef]
- Stephen, W.; Wright, L.D.M.; Hageman, D.L. A Convenient modification of the Gassman oxindole synthesis. Tetrahedron Lett. 1996, 37, 4631–4634. [Google Scholar]
- Menéndez, J.C.; Sridharan, V.; Perumal, S.; Avendaño, C. Microwave-assisted, solvent-free Bischler indole synthesis. Synlett 2006. [Google Scholar] [CrossRef]
- Satoshi Sakami, M.M.; Koji, K.; Takumi, A.; Kuniaki, K.; Hideaki, F.; Ko, H.; Mayumi, N.; Takashi, E.; Shinya, U.; Tsuyoshi, I.; et al. Structure-antitussive activity relationships of naltrindole derivatives. identification of novel and potent antitussive agents. J. Med. Chem. 2008, 51, 4404–4411. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Vasquez-Cespedes, S.; Glorius, F. Rhodium(III)-catalyzed cyclative capture approach to diverse 1-aminoindoline derivatives at room temperature. Angew. Chem. Int. Ed. 2015, 54, 1657–1661. [Google Scholar] [CrossRef]
- Li, B.J.; Wang, H.Y.; Zhu, Q.L.; Shi, Z.J. Rhodium/copper-catalyzed annulation of benzimides with internal alkynes: Indenone synthesis through sequential C–H and C–N cleavage. Angew. Chem. Int. Ed. 2012, 51, 3948–3952. [Google Scholar] [CrossRef] [PubMed]
- Muralirajan, K.; Parthasarathy, K.; Cheng, C.H. Regioselective synthesis of indenols by rhodium-catalyzed C–H activation and carbocyclization of aryl ketones and alkynes. Angew. Chem. Int. Ed. 2011, 50, 4169–4172. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Chen, Z.; Yang, Y.; Ai, W.; Tang, H.; Wu, Y.; Zhu, W.; Li, Y. Redox-neutral Rhodium-catalyzed C–H functionalization of arylamine N-oxides with diazo compounds: Primary C(sp3)-H/C(sp2)-H activation and oxygen-atom transfer. Angew. Chem. Int. Ed. 2015, 54, 12121–12126. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, D.; Yan, H.; Wang, H.; Pan, B.; Xin, X.; Li, X.; Wu, F.; Wan, B. Rhodium-catalyzed cyclization of diynes with nitrones: A formal [2 + 2 + 5] approach to bridged eight-membered heterocycles. Angew. Chem. Int. Ed. 2014, 53, 11940–11943. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, Z.; Li, X. Rhodium(III)-catalyzed C–C and C–O coupling of quinoline N-oxides with alkynes: Combination of C–H activation with O-atom transfer. Angew. Chem. Int. Ed. 2014, 53, 10794–10798. [Google Scholar] [CrossRef]
- Dateer, R.B.; Chang, S. Selective cyclization of arylnitrones to indolines under external oxidant-free conditions: Dual role of Rh(III) catalyst in the C–H activation and oxygen atom transfer. J. Am. Chem. Soc. 2015, 137, 4908–4911. [Google Scholar] [CrossRef] [PubMed]
- Dateer, R.B.; Chang, S. Rh(III)-catalyzed C–H cyclization of arylnitrones with diazo compounds: Access to N-hydroxyindolines. Org. Lett. 2016, 18, 68–71. [Google Scholar] [CrossRef]
- Font, M.; Cendon, B.; Seoane, A.; Mascarenas, J.L.; Gulias, M. Rhodium(III)-catalyzed annulation of 2-alkenyl anilides with alkynes through C–H activation: Direct access to 2-substituted indolines. Angew. Chem. Int. Ed. 2018, 57, 8255–8259. [Google Scholar] [CrossRef] [PubMed]
- Lawson, E.C.; Luci, D.K.; Ghosh, S.; Kinney, W.A.; Reynolds, C.H.; Qi, J.; Smith, C.E.; Wang, Y.; Minor, L.K.; Haertlein, B.J.; et al. Nonpeptide urotensin-II receptor antagonists: A new ligand class based on piperazino-phthalimide and piperazino-isoindolinone subunits. J. Med. Chem. 2009, 52, 7432–7445. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Chen, D.; Pan, C.L.; Crabtree, R.H.; Li, X. Rh-catalyzed oxidative coupling between primary and secondary benzamides and alkynes: Synthesis of polycyclic amides. J. Org. Chem. 2010, 75, 7487–7490. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, F.; Yu, S.; Li, X. Rhodium(III)-catalyzed selective access to isoindolinones via formal [4 + 1] annulation of arylamides and propargyl alcohols. Chin. J. Catal. 2017, 38, 1390–1398. [Google Scholar] [CrossRef]
- Hyster, T.K.; Ruhl, K.E.; Rovis, T. A coupling of benzamides and donor/acceptor diazo compounds to form gamma-lactams via Rh(III)-catalyzed C–H activation. J. Am. Chem. Soc. 2013, 135, 5364–5367. [Google Scholar] [CrossRef] [PubMed]
- Guimond, N.; Gorelsky, S.I.; Fagnou, K. Rhodium(III)-catalyzed heterocycle synthesis using an internal oxidant: Improved reactivity and mechanistic studies. J. Am. Chem. Soc. 2011, 133, 6449–6457. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, Y.; Qi, Z.; Xie, F.; Lan, Y.; Li, X. Rhodium(III)-Catalyzed annulation between n-sulfinyl ketoimines and activated olefins: C–H activation assisted by an oxidizing N–S bond. ACS Catal. 2016, 6, 1971–1980. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Cui, S. Facile synthesis of isoindolinones via Rh(III)-catalyzed one-pot reaction of benzamides, ketones, and hydrazines. Org. Lett. 2015, 17, 2494–2497. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, G.; Lu, X. Regiocontrolled coupling of aromatic and vinylic amides with alpha-allenols to form gamma-lactams via Rhodium(III)-catalyzed C–H activation. Org. Lett. 2016, 18, 5668–5671. [Google Scholar] [CrossRef]
- Zhou, X.; Peng, Z.; Zhao, H.; Zhang, Z.; Lu, P.; Wang, Y. Rh-Catalyzed annulations of N-methoxybenzamides with ketenimines: Synthesis of 3-aminoisoindolinones and 3-diarylmethyleneisoindolinones with strong aggregation induced emission properties. Chem. Commun. 2016, 52, 10676–10679. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Wang, J.; Jiang, H.; Liu, H. Rhodium(III)-catalyzed site-selective C–H alkylation and arylation of pyridones using organoboron reagents. Org. Lett. 2016, 18, 5376–5379. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, B.; Zhou, Y.; Liu, H. Propargyl alcohols as one-carbon synthons: Redox-neutral Rhodium(III)-catalyzed C–H bond activation for the synthesis of isoindolinones bearing a quaternary carbon. Org. Lett. 2017, 19, 1294–1297. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Zhang, C.; Zhou, C.; Li, Y.; Zhou, Y.; Liu, H. Rh(III)-catalyzed C–H activation of benzoylacetonitriles and tandem cyclization with diazo compounds to substituted benzo[de]chromenes. Org. Lett. 2018, 20, 1720–1724. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Fang, F.; Cheng, Y.; Li, Y.; Liu, H.; Zhou, Y. Rhodium(III)-catalyzed C–H activation of benzoylacetonitriles and cyclization with sulfoxonium ylides to naphthols. Adv. Synth. Catal. 2018, 360, 2546–2551. [Google Scholar] [CrossRef]
- Zhou, J.; Li, J.; Li, Y.; Wu, C.; He, G.; Yang, Q.; Zhou, Y.; Liu, H. direct synthesis of 3-acylindoles through Rhodium(III)-catalyzed annulation ofN-phenylamidines with alpha-Cl ketones. Org. Lett. 2018, 20, 7645–7649. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Li, C.; Liu, H. Rh(III)-catalyzed annulation of Boc-protected benzamides with diazo compounds: Approach to isocoumarins. Molecules 2019, 24, 739. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.; Li, C.; Yu, X.; Liu, H. Rh(III)-catalyzed hydroarylation of alkyne MIDA boronates via C–H activation of indole derivatives. J. Org. Chem. 2019, 84, 6840–6850. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Han, X.; Zhang, R.; Liu, H.; Wang, J. Rhodium(III)-catalyzed [4+2] annulation via C–H activation: Synthesis of multi-substituted naphthalenone sulfoxonium ylides. Molecules 2019, 24, 1884. [Google Scholar] [CrossRef]
- Yang, L.; Li, C.; Wang, D.; Liu, H. Cp*Rh(III)-catalyzed C–H bond difluorovinylation of indoles with alpha,alpha-difluorovinyl tosylate. J. Org. Chem. 2019, 84, 7320–7330. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wu, C.; Zhou, J.; He, G.; Liu, H.; Zhou, Y. Highly selective C–H bond activation of N-arylbenzimidamide and divergent couplings with diazophosphonate compounds: A catalyst-controlled selective synthetic strategy for 3-phosphorylindoles and 4-phosphorylisoquinolines. Org. Chem. Front. 2019, 6, 393–398. [Google Scholar] [CrossRef]
- Wang, B.; Li, C.; Liu, H. Cp*Rh(III)-catalyzed directed C−H methylation and arylation of quinoline n-oxides at the C-8 position. Adv. Synth. Catal. 2017, 359, 3029–3034. [Google Scholar] [CrossRef]
- Wang, C.Q.; Ye, L.; Feng, C.; Loh, T.P. C–F bond cleavage enabled redox-neutral [4 + 1] annulation via C–H bond activation. J. Am. Chem. Soc. 2017, 139, 1762–1765. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhou, C.; Yan, X.; Wang, J. Solvent-dependent asymmetric synthesis of alkynyl and monofluoroalkenyl isoindolinones by Cp*Rh(III) -catalyzed C–H activation. Angew. Chem. Int. Ed. 2018, 57, 4048–4052. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Khullar, A.; Chou, S.; Sacramo, A.; Gerratana, B. Biosynthesis of sibiromycin, a potent antitumor antibiotic. Appl. Environ. Microbiol. 2009, 75, 2869–2878. [Google Scholar] [CrossRef] [PubMed]
- Magedov, I.V.; Luchetti, G.; Evdokimov, N.M.; Manpadi, M.; Steelant, W.F.; Van Slambrouck, S.; Tongwa, P.; Antipin, M.Y.; Kornienko, A. Novel three-component synthesis and antiproliferative properties of diversely functionalized pyrrolines. Bioorgan. Med. Chem. Lett. 2008, 18, 1392–1396. [Google Scholar] [CrossRef]
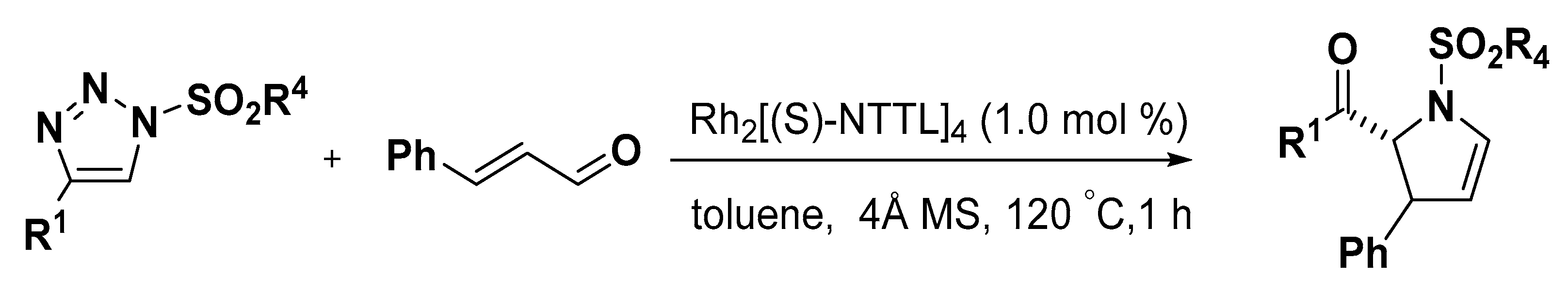
- Miura, T.; Tanaka, T.; Hiraga, K.; Stewart, S.G.; Murakami, M. Stereoselective synthesis of 2,3-dihydropyrroles from terminal alkynes, azides, and alpha,beta-unsaturated aldehydes via N-sulfonyl-1,2,3-triazoles. J. Am. Chem. Soc. 2013, 135, 13652–13655. [Google Scholar] [CrossRef] [PubMed]
- Nakamuro, T.; Hagiwara, K.; Miura, T.; Murakami, M. Enantioselective denitrogenative annulation of 1H-tetrazoles with styrenes catalyzed by Rhodium. Angew. Chem. Int. Ed. 2018, 57, 5497–5500. [Google Scholar] [CrossRef]
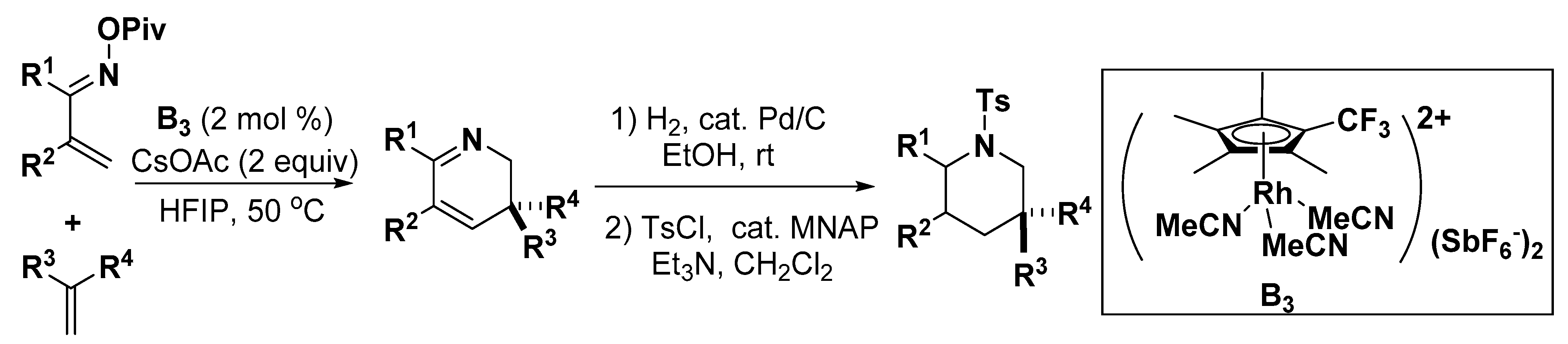
- Romanov-Michailidis, F.; Sedillo, K.F.; Neely, J.M.; Rovis, T. Expedient access to 2,3-dihydropyridines from unsaturated oximes by Rh(III)-catalyzed C–H activation. J. Am. Chem. Soc. 2015, 137, 8892–8895. [Google Scholar] [CrossRef]
- Shu Wen Li, M.G.N.; Donna, M.; Edwards, R.L.; Kisliuk, Y.; Gaumont, I.K.; Dev, D.S.; Duch, J.; Humphreys, G.K.; Smith, R.F. Folate analogues 35: Synthesis and biological evaluation of 1-deaza, 3-deaza, and bridge-elongated analogues of JV10-propargyl-5,8-dideazafolic acid. J. Med. Chem. 1990, 34, 2746–2754. [Google Scholar]
- Toshiaki Matsui, T.S.; Hisao, N.; Sadahiko, I.; Satoshi, S.; Hideo, T.; Yoshihiko, O.; Yuhki, N.; Yasuyuki, U.; Kazuyuki, O.; Hiroyuki, I.; et al. Novel 5-HT3 antagonists. isoquinolinones and 3-aryl-2-pyridones. J. Med. Chem. 1992, 35, 3319. [Google Scholar]
- Asano, Y.; Kitamura, S.; Ohra, T.; Itoh, F.; Kajino, M.; Tamura, T.; Kaneko, M.; Ikeda, S.; Igata, H.; Kawamoto, T.; et al. Discovery, synthesis and biological evaluation of isoquinolones as novel and highly selective JNK inhibitors (2). Bioorgan. Med. Chem. 2008, 16, 4699–4714. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.A.; Hyster, T.K.; Rovis, T. Rhodium(III)-catalyzed intramolecular hydroarylation, amidoarylation, and Heck-type reaction: Three distinct pathways determined by an amide directing group. Angew. Chem. Int. Ed. 2013, 52, 14181–14185. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, Z.; Zhao, H.; Lu, P.; Wang, Y. Rh-catalyzed annulations of n-methoxybenzamides and ketenimines: Sterically and electronically controlled synthesis of isoquinolinones and isoindolinones. J. Org. Chem. 2017, 82, 3787–3797. [Google Scholar] [CrossRef]
- Wu, J.Q.; Zhang, S.S.; Gao, H.; Qi, Z.; Zhou, C.J.; Ji, W.W.; Liu, Y.; Chen, Y.; Li, Q.; Li, X. Experimental and theoretical studies on rhodium-catalyzed coupling of benzamides with 2,2-difluorovinyl tosylate: Diverse synthesis of fluorinated heterocycles. J. Am. Chem. Soc. 2017, 139, 3537–3545. [Google Scholar] [CrossRef]
- Yang, X.; Liu, S.; Yu, S.; Kong, L.; Lan, Y.; Li, X. Redox-neutral access to isoquinolinones via Rhodium(III)-catalyzed annulations of O-pivaloyl oximes with ketenes. Org. Lett. 2018, 20, 2698–2701. [Google Scholar] [CrossRef]
- Fukui, Y.; Liu, P.; Liu, Q.; He, Z.T.; Wu, N.Y.; Tian, P.; Lin, G.Q. Tunable arylative cyclization of 1,6-enynes triggered by Rhodium(III)-catalyzed C–H activation. J. Am. Chem. Soc. 2014, 136, 15607–15614. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Pan, Y.; Li, X. Catalyst-controlled regiodivergent alkyne insertion in the context of C-H activation and Diels-Alder reactions: Synthesis of fused and bridged cycles. Angew. Chem. Int. Ed. 2017, 56, 8163–8167. [Google Scholar] [CrossRef]
- Cajaraville, A.; Suarez, J.; Lopez, S.; Varela, J.A.; Saa, C. Rh(III)-catalyzed [5+1] oxidative cycloaddition of arylguanidines with alkynes: A novel access to C4-disubstituted 1,4-dihydroquinazolin-2-amines. Chem. Commun. 2015, 51, 15157–15160. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, Z.; Lam, J.W.Y.; Zhao, Y.; Chen, Y.; Liu, Y.; Tang, B.Z. Cascade polyannulation of diyne and benzoylacetonitrile: A new strategy for synthesizing functional substituted poly(naphthopyran)s. Macromolecules 2015, 48, 4241–4249. [Google Scholar] [CrossRef]
- Basak, T.; Grudzień, K.; Barbasiewicz, M. Remarkable ability of the benzylidene ligand to control initiation of Hoveyda-Grubbs metathesis catalysts. Eur. J. Inorg. Chem. 2016, 2016, 3513–3523. [Google Scholar] [CrossRef]
- Casanova, N.; Seoane, A.; Mascarenas, J.L.; Gulias, M. Rhodium-catalyzed (5 + 1) annulations between 2-alkenylphenols and allenes: A practical entry to 2,2-disubstituted 2H-chromenes. Angew. Chem. Int. Ed. 2015, 54, 2374–2377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, K.; Wang, B.; Yi, H.; Hu, F.; Li, C.; Zhang, Y.; Wang, J. Rhodium(III)-catalyzed transannulation of cyclopropenes with N-phenoxyacetamides through C–H activation. Angew. Chem. Int. Ed. 2014, 53, 13234–13238. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Fan, Z.; Xiong, C.; Zhang, A. Highly stereoselective assembly of polycyclic molecules from 1,6-enynes triggered byRhodium(III)-catalyzed C–H activation. Org. Lett. 2018, 20, 3065–3069. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, Y.; Xu, Y.; Yang, S.; Liu, B.; Li, X. Construction of (dihydro)naphtha [1,8-bc] pyrans via Rh(III)-Catalyzed two-fold C–H activation of benzoylacetonitriles. Org. Lett. 2018, 20, 2160–2163. [Google Scholar] [CrossRef] [PubMed]
- Fliss, G.; Staab, A.; Tillmann, C.; Trommeshauser, D.; Schaefer, H.G.; Kloft, C. Population pharmacokinetic data analysis of cilobradine, an IF channel blocker. Pharm. Res. 2008, 25, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.W.; McClendon, K.S.; Riche, D.M. New obesity agents: Lorcaserin and phentermine/topiramate. Ann. Pharmacother. 2013, 47, 1007–1016. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, M.B.; Ouyang, X.H.; Pi, R.; Song, R.J.; Li, J.H. Rhodium(III)-catalyzed [3 + 2]/[5 + 2] annulation of 4-aryl 1,2,3-triazoles with internal alkynes through dual C(sp2)-H functionalization. Angew. Chem. Int. Ed. 2015, 54, 6595–6599. [Google Scholar] [CrossRef]
- Pandey, A.K.; Han, S.H.; Mishra, N.K.; Kang, D.; Lee, S.H.; Chun, R.; Hong, S.; Park, J.S.; Kim, I.S. Synthesis of 2-benzazepines from benzylamines and MBH adducts under Rhodium(III) catalysis via C(sp2)–H functionalization. ACS Catal. 2017, 8, 742–746. [Google Scholar] [CrossRef]
- Peneau, A.; Tricart, Q.; Guillou, C.; Chabaud, L. Mild Rhodium(III)-catalyzed intramolecular annulation of benzamides with allylic alcohols to access azepinone derivatives. Chem. Commun. 2018, 54, 5891–5894. [Google Scholar] [CrossRef]
- Yusuke Hirasawa, H.M.; Jun’ichi, K. Nankakurine A, a novel C16N2-type alkaloid from lycopodium hamiltonii. Org. Lett. 2004, 6, 3389–3391. [Google Scholar] [CrossRef]
- Fusao Kido, T.A.; Michiharu, K. Spiroannulation by the [2,3] sigmatropic rearrangement via the cyclic allylsulfonium ylide. a stereoselective synthesis of (+)-Acorenone, B. J. Chem. Soc. Perkin Trans. 1992, 33, 229–233. [Google Scholar] [CrossRef]
- Taylor, R.D.; MacCoss, M.; Lawson, A.D. Rings in drugs. J. Med. Chem. 2014, 57, 5845–5859. [Google Scholar] [CrossRef] [PubMed]
- Tobat, P.I.; Saragi, T.S.; Achim, S.; Thomas, F.L.; Josef, S. Spiro compounds for organic optoelectronics. Chem. Rev. 2007, 107, 1011–1065. [Google Scholar]
- Baihua Ye, N.C. Chiral Cyclopentadienyl Ligands as Stereocontrolling element in asymmetric C–H functionalization. Science 2012, 338, 504–508. [Google Scholar]
- Ding, K.; Han, Z.; Wang, Z. Spiro skeletons: A class of privileged structure for chiral ligand design. Chemistry 2009, 4, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Seoane, A.; Casanova, N.; Quinones, N.; Mascarenas, J.L.; Gulias, M. Rhodium(III)-catalyzed dearomatizing [3 + 2] annulation of 2-alkenylphenols and alkynes. J. Am. Chem. Soc. 2014, 136, 7607–7610. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Zhang, Y.; Wu, Q. Rh(III)-catalyzed C–H activation/cycloaddition of benzamides and methylenecyclopropanes: Divergence in ring formation. Chem. Sci. 2013, 4, 3421–3426. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Knecht, T.; Daniliuc, C.G.; Houk, K.N.; Glorius, F. Unprecedented dearomatized spirocyclopropane in a sequential Rhodium(III)-catalyzed C–H activation and rearrangement reaction. Angew. Chem. Int. Ed. 2018, 57, 5520–5524. [Google Scholar] [CrossRef]
- Rios, R. Enantioselective methodologies for the synthesis of spiro compounds. Chem. Soc. Rev. 2012, 41, 1060–1074. [Google Scholar] [CrossRef]
- Ding, A.; Meazza, M.; Guo, H.; Yang, J.W.; Rios, R. New development in the enantioselective synthesis of spiro compounds. Chem. Soc. Rev. 2018, 47, 5946–5996. [Google Scholar] [CrossRef]
- Moyano, A.; Rios, R. Asymmetric organocatalytic cyclization and cycloaddition reactions. Chem. Rev. 2011, 111, 4703–4832. [Google Scholar] [CrossRef]
- Pape, A.R.; Kaliappan, K.P.; Ku¨ndig, E.P. Transition-metal-mediated dearomatization reactions. Chem. Rev. 2000, 100, 2917–2940. [Google Scholar]
- Zhuo, C.X.; Zheng, C.; You, S.L. Transition-metal-catalyzed asymmetric allylic dearomatization reactions. Acc. Chem. Res. 2014, 47, 2558–2573. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, S.; Best, D.; Burns, D.J.; Lam, H.W. Synthesis of spirocyclic enones by Rhodium-catalyzed dearomatizing oxidative annulation of 2-alkenylphenols with alkynes and enynes. Chem. Eru. J. 2014, 20, 8599–8602. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wang, S.B.; Zheng, C.; You, S.L. Asymmetric dearomatization of naphthols via a Rh-catalyzed C(sp2)-H functionalization/annulation reaction. J. Am. Chem. Soc. 2015, 137, 4880–4883. [Google Scholar] [CrossRef]
- Zheng, C.; Zheng, J.; You, S.L. A DFT study on Rh-catalyzed asymmetric dearomatization of 2-naphthols initiated with C–H activation: A refined reaction mechanism and origins of multiple selectivity. ACS Catal. 2015, 6, 262–271. [Google Scholar] [CrossRef]
- Ye, B.; Donets, P.A.; Cramer, N. Chiral Cp-Rhodium(III)-catalyzed asymmetric hydroarylations of 1,1-disubstituted alkenes. Angew. Chem. Int. Ed. 2014, 53, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Cramer, N. A tunable class of chiral Cp ligands for enantioselective Rhodium(III)-catalyzed C–H allylations of benzamides. J. Am. Chem. Soc. 2013, 135, 636–639. [Google Scholar] [CrossRef]
- Madhukar, S.C.; Vijay, S. Synthesis and antimicrobial activity of novel spirocompounds with pyrazolone and pyrazolthione moiety. J. Heterocycl. Chem. 2007, 44, 49–53. [Google Scholar]
- Zheng, J.; Li, P.; Gu, M.; Lin, A.; Yao, H. Synthesis of spiropentadiene pyrazolones by Rh(III)-catalyzed formal sp3 C–H activation/annulation. Org. Lett. 2017, 19, 2829–2832. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, S.B.; Zheng, C.; You, S.L. Asymmetric synthesis of spiropyrazolones by Rhodium-catalyzed C(sp2)-H functionalization/annulation reactions. Angew. Chem. Int. Ed. 2017, 56, 4540–4544. [Google Scholar] [CrossRef] [PubMed]
- Burns, D.J.; Best, D.; Wieczysty, M.D.; Lam, H.W. All-carbon [3 + 3] oxidative annulations of 1,3-enynes by Rhodium(III)-catalyzed C–H functionalization and 1,4-migration. Angew. Chem. Int. Ed. 2015, 54, 9958–9962. [Google Scholar] [CrossRef] [PubMed]
- Reddy Chidipudi, S.; Burns, D.J.; Khan, I.; Lam, H.W. Enantioselective synthesis of spiroindenes by enol-directed Rhodium(III)-catalyzed C–H functionalization and spiroannulation. Angew. Chem. Int. Ed. 2015, 54, 13975–13979. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.B.; Pi, R.; Hu, M.; Yang, Y.; Song, R.J.; Xia, Y.; Li, J.H. Rhodium(III)-catalyzed [3+2] annulation of 5-aryl-2,3-dihydro-1H-pyrroles with internal alkynes through C(sp2)-H/alkene functionalization. Angew. Chem. Int. Ed. 2014, 53, 11338–11341. [Google Scholar] [CrossRef]
- Singh, S.K.; Reddy, P.G.; Rao, K.S.; Lohray, B.B.; Misra, P.; Rajjak, S.A.; Rao, Y.K.; Venkateswarlu, A. Polar substitutions in the benzenesulfonamide ring of celecoxib afford a potent 1,5-diarylpyrazole class of COX-2 inhibitors. Bioorgan. Med. Chem. Lett. 2004, 14, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Qu, C.H.; Huang, J.R.; Zhang, W.; Zhang, Q.R.; Deng, J.G. Rhodium-catalyzed spirocyclic sultam synthesis by [3+2] annulation with cyclic N-sulfonyl ketimines and alkynes. Chem. Eur. J. 2013, 19, 16537–16540. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.R.; Song, Q.; Zhu, Y.Q.; Qin, L.; Qian, Z.Y.; Dong, L. Rhodium(III)-catalyzed three-component reaction of imines, alkynes, and aldehydes through C–H activation. Chem. Eur. J. 2014, 20, 16882–16886. [Google Scholar] [CrossRef]
- Liu, B.; Hu, P.; Zhang, Y.; Li, Y.; Bai, D.; Li, X. Rh(III)-catalyzed diastereodivergent spiroannulation of cyclic imines with activated alkenes. Org. Lett. 2017, 19, 5402–5405. [Google Scholar] [CrossRef]
- Mei, S.T.; Liang, H.W.; Teng, B.; Wang, N.J.; Shuai, L.; Yuan, Y.; Chen, Y.C.; Wei, Y. Spirocyclic sultam and heterobiaryl synthesis through Rh-catalyzed cross-dehydrogenative coupling of N-sulfonyl ketimines and thiophenes or furans. Org. Lett. 2016, 18, 1088–1091. [Google Scholar] [CrossRef]
- Franklin, A.; Davis, B.-C.C. Asymmetric hydroxylation of enolates with N-sulfonyloxaziridines. Chem. Rev. 1992, 92, 919–934. [Google Scholar]
- Kim, B.H.; Curran, D.P. Asymmetric thermal reactions with Oppolzer’s camphor sultam. Tetrahedron 1993, 49, 293–318. [Google Scholar]
- Pham, M.V.; Cramer, N. Enantioselective access to spirocyclic sultams by chiral Cp(x) -Rhodium(III)-catalyzed annulations. Chem. Eur. J. 2016, 22, 2270–2273. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Oh, Y.; Mishra, N.K.; De, U.; Jo, H.; Sachan, R.; Kim, H.S.; Jung, Y.H.; Kim, I.S. Rhodium-catalyzed [3 + 2] Annulation of cyclic n-acyl ketimines with activated olefins: Anticancer activity of spiroisoindolinones. J. Org. Chem. 2017, 82, 3359–3367. [Google Scholar] [CrossRef] [PubMed]
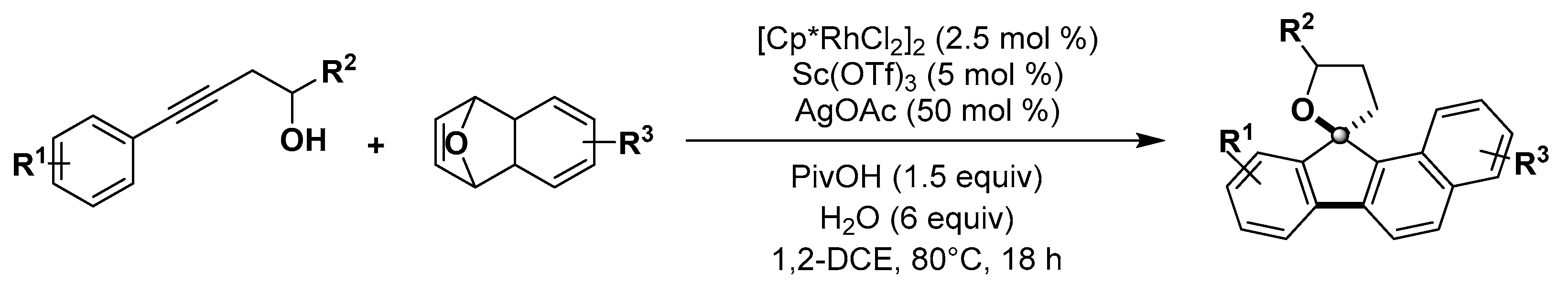
- Li, D.Y.; Jiang, L.L.; Chen, S.; Huang, Z.L.; Dang, L.; Wu, X.Y.; Liu, P.N. Cascade reaction of alkynols and 7-oxabenzonorbornadienes involving transient hemiketal group directed C-H activation and synergistic Rh(III)/Sc(III) catalysis. Org. Lett. 2016, 18, 5134–5137. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Luan, J.; Fang, J.; Zhao, X.; Wu, X.; Li, Y.; Luo, Y. A Rhodium-catalyzed [3 + 2] annulation of general aromatic aldimines/ketimines and N-Substituted maleimides. Org. Lett. 2018, 20, 5960–5963. [Google Scholar] [CrossRef] [PubMed]
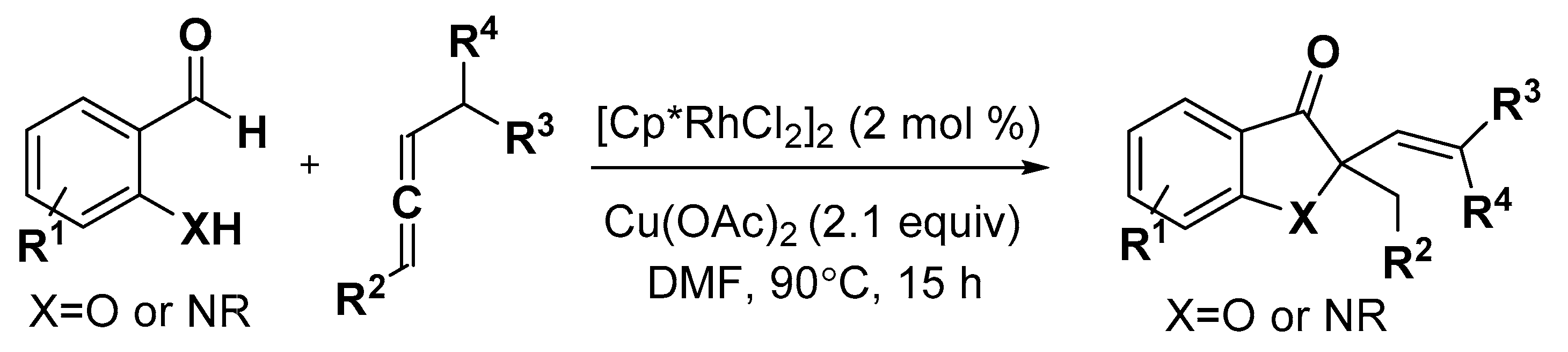

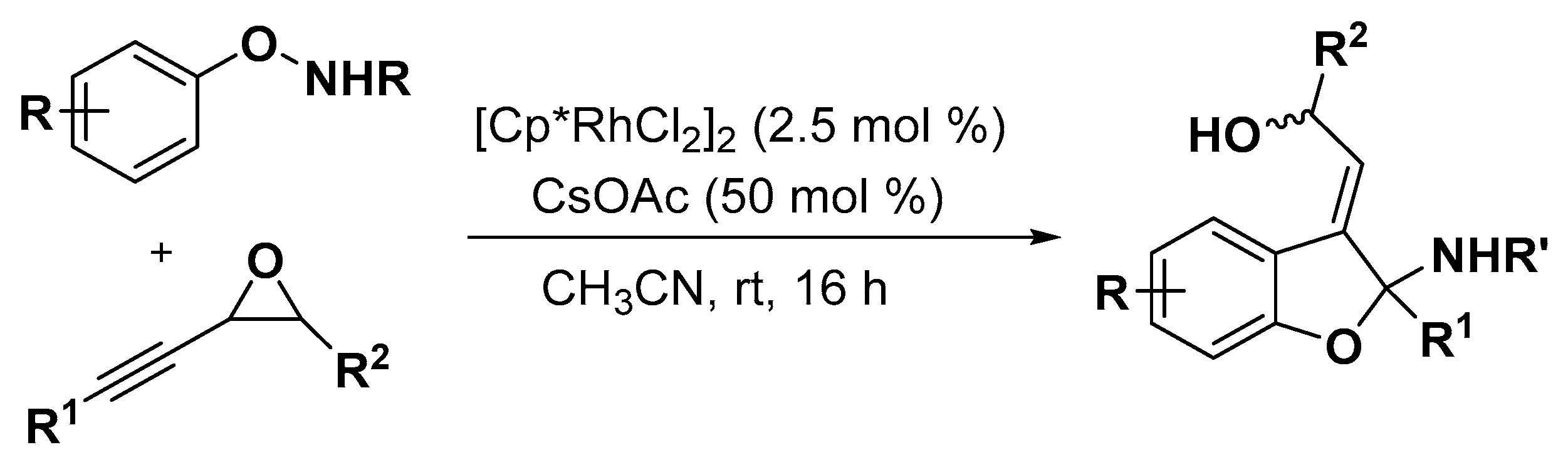

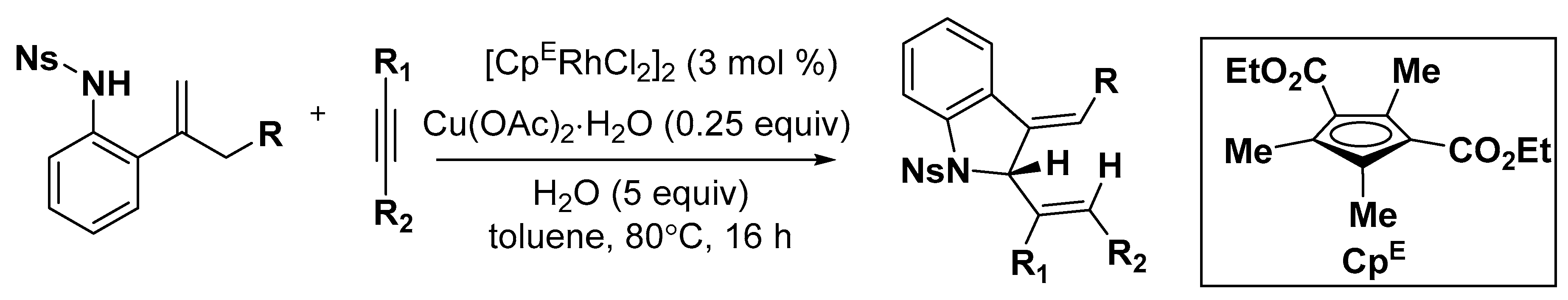

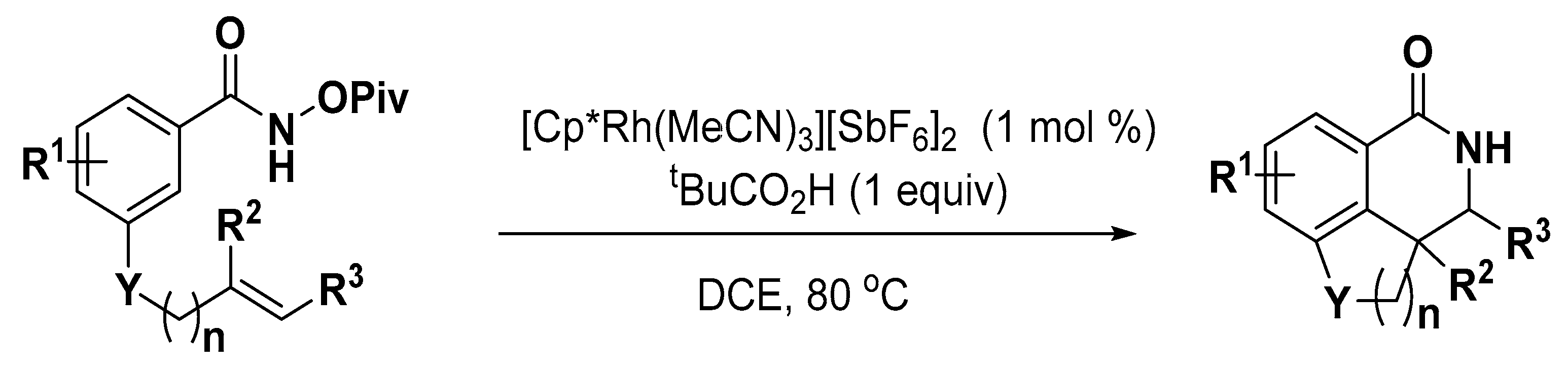
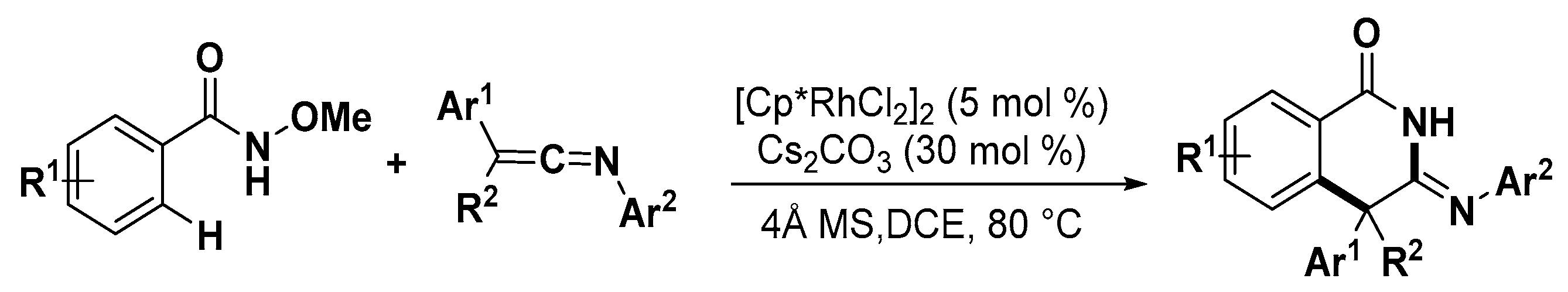


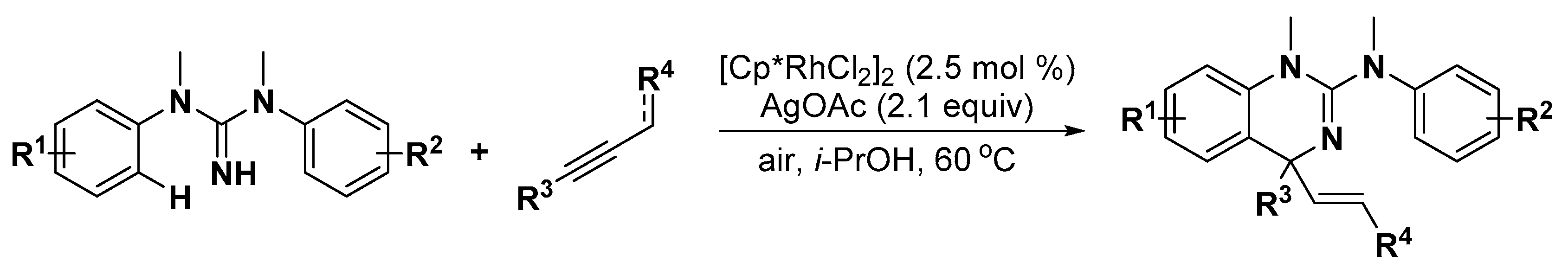


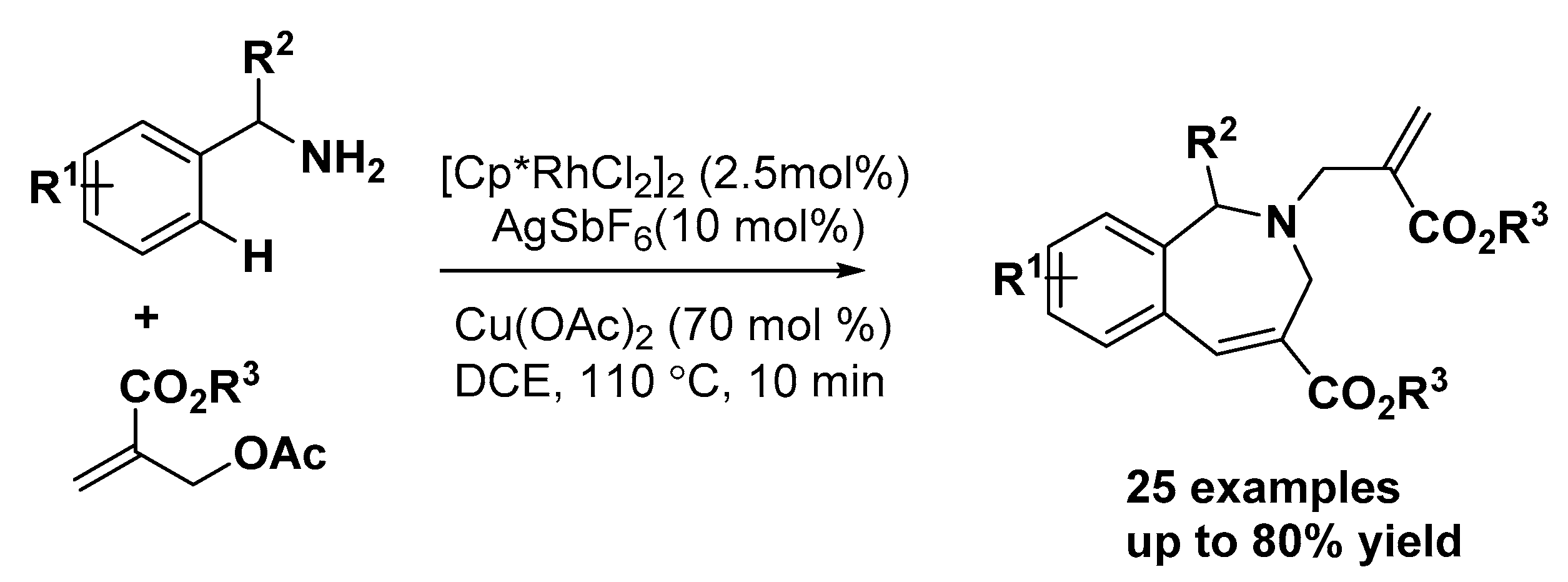
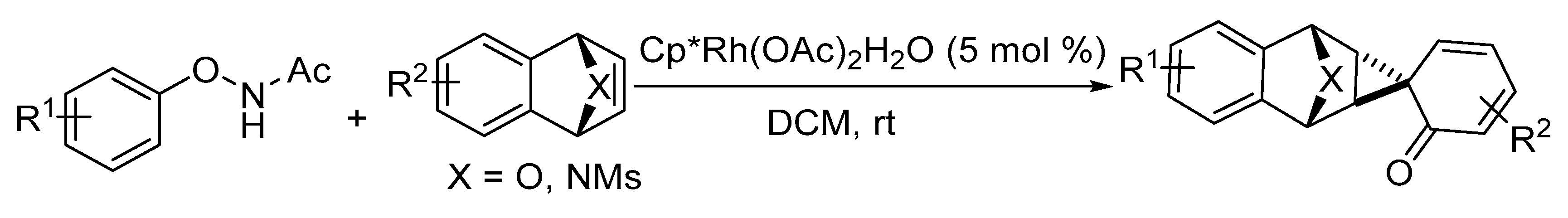

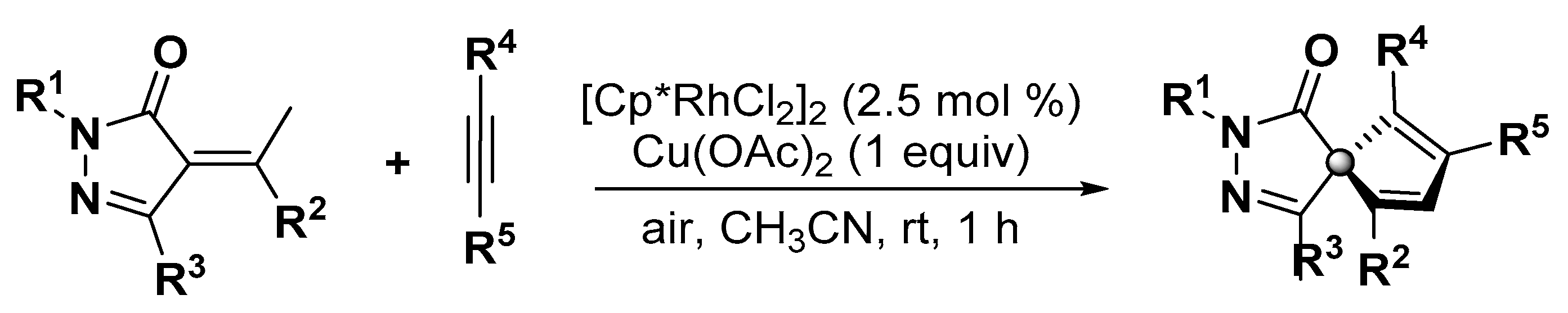
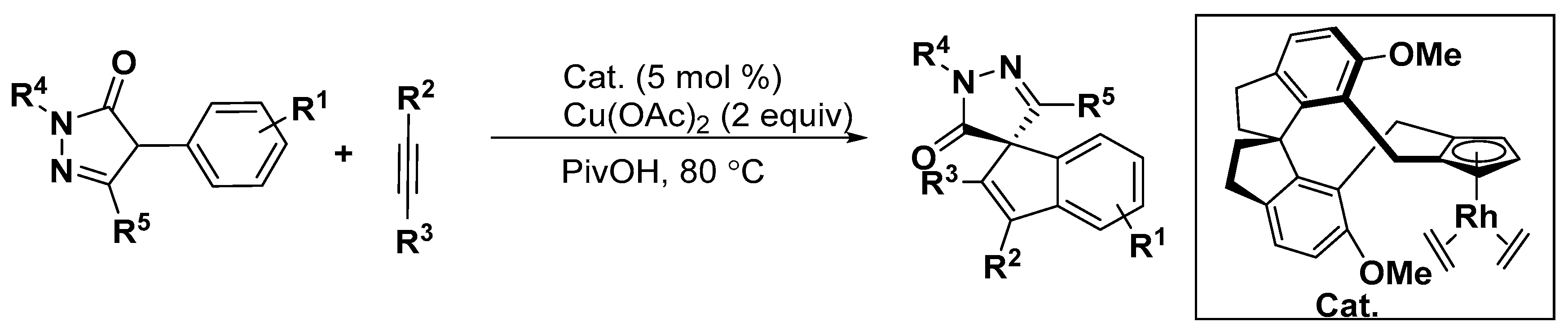
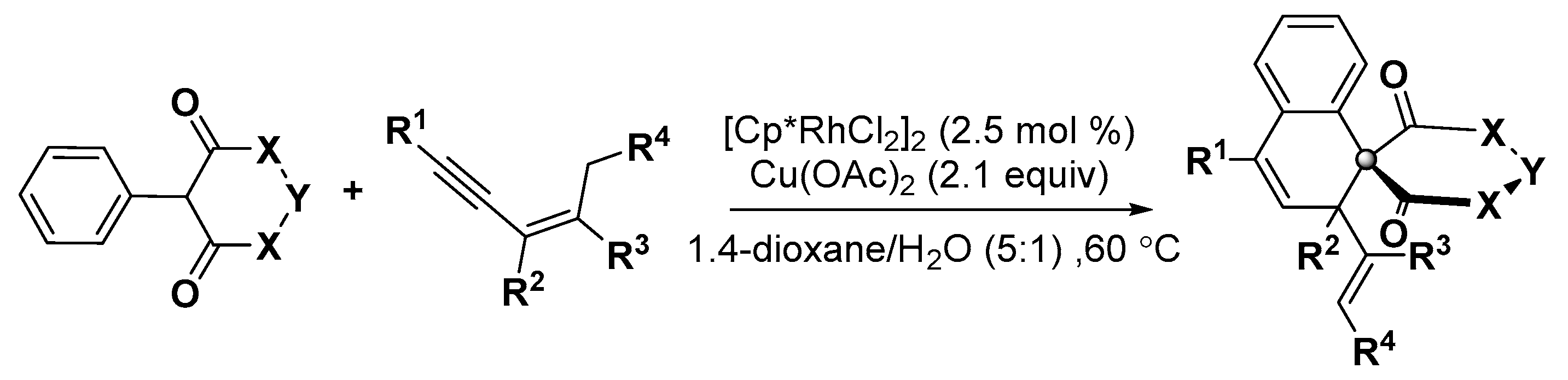
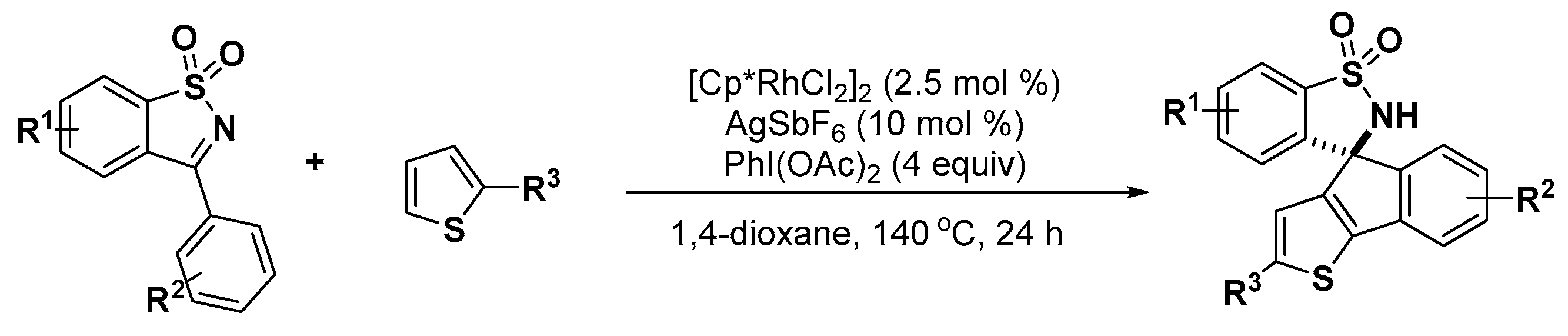
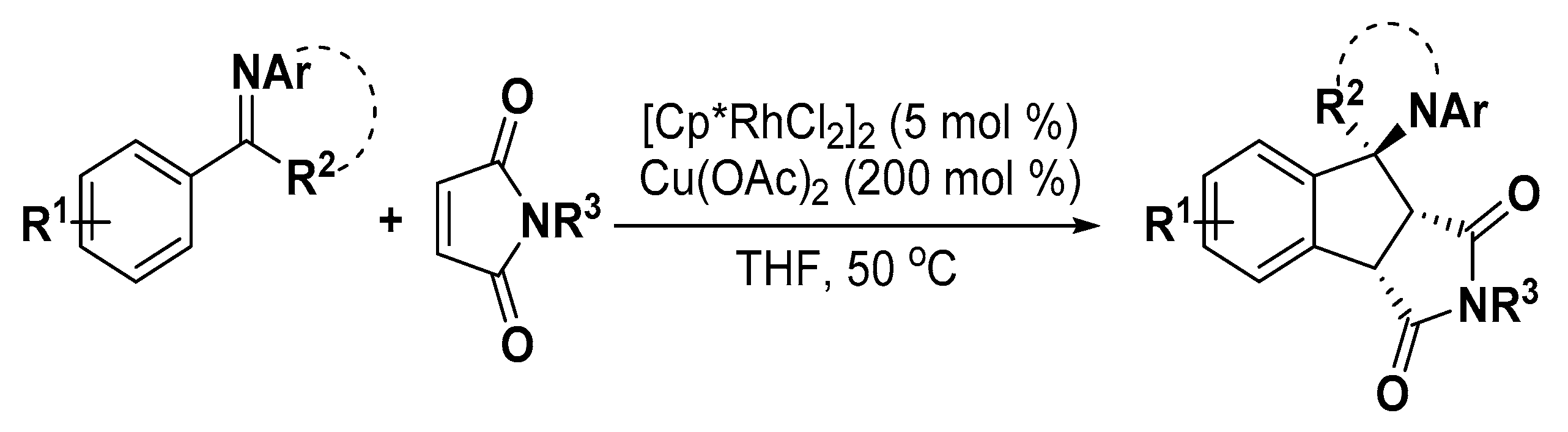
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Xie, X.; Liu, H.; Zhou, Y. Rh(III)-Catalyzed C–H Bond Activation for the Construction of Heterocycles with sp3-Carbon Centers. Catalysts 2019, 9, 823. https://doi.org/10.3390/catal9100823
Wang R, Xie X, Liu H, Zhou Y. Rh(III)-Catalyzed C–H Bond Activation for the Construction of Heterocycles with sp3-Carbon Centers. Catalysts. 2019; 9(10):823. https://doi.org/10.3390/catal9100823
Chicago/Turabian StyleWang, Run, Xiong Xie, Hong Liu, and Yu Zhou. 2019. "Rh(III)-Catalyzed C–H Bond Activation for the Construction of Heterocycles with sp3-Carbon Centers" Catalysts 9, no. 10: 823. https://doi.org/10.3390/catal9100823
APA StyleWang, R., Xie, X., Liu, H., & Zhou, Y. (2019). Rh(III)-Catalyzed C–H Bond Activation for the Construction of Heterocycles with sp3-Carbon Centers. Catalysts, 9(10), 823. https://doi.org/10.3390/catal9100823





