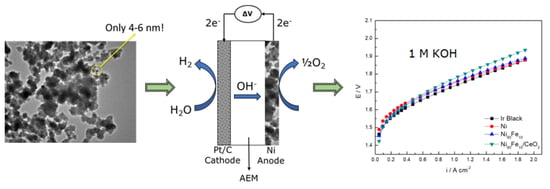
The Performance of Nickel and Nickel-Iron Catalysts Evaluated As Anodes in Anion Exchange Membrane Water Electrolysis
Abstract
1. Introduction
2. Results and Discussion
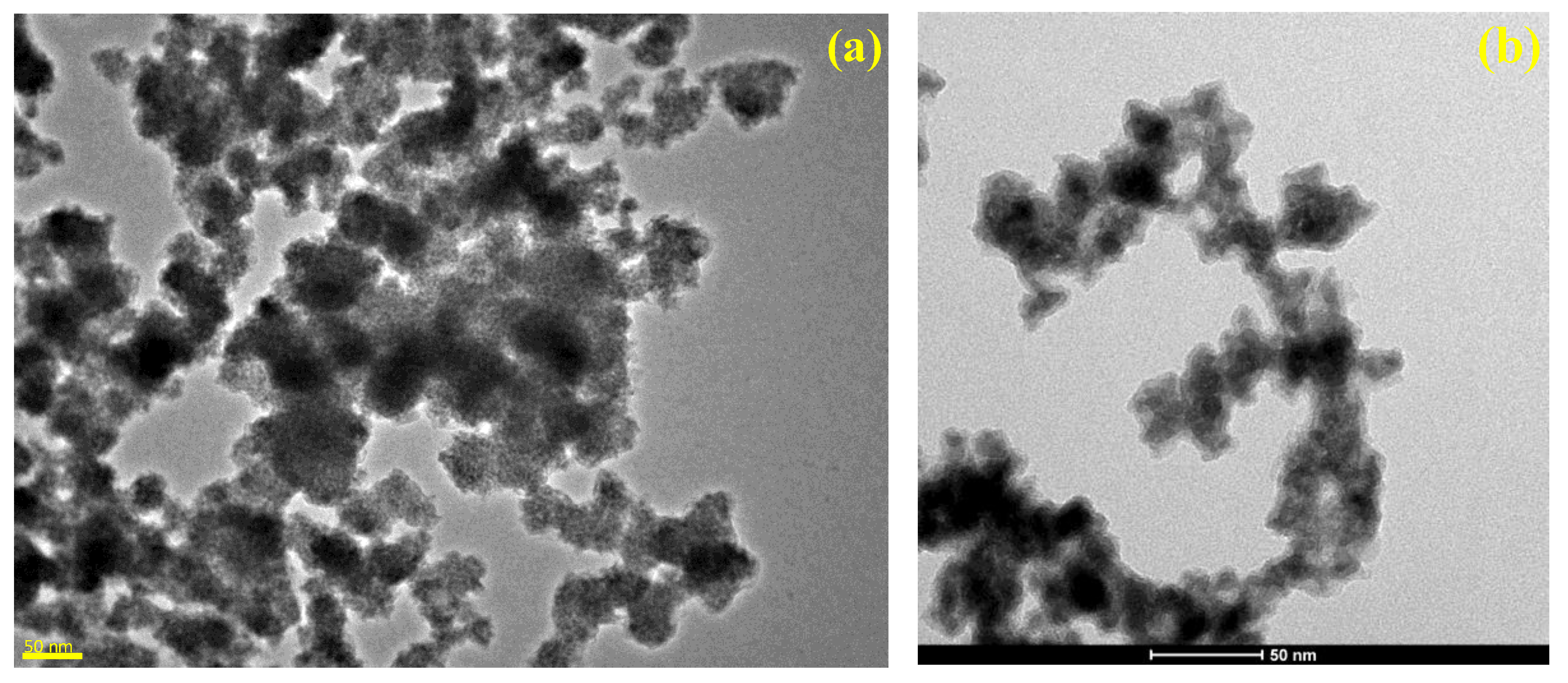
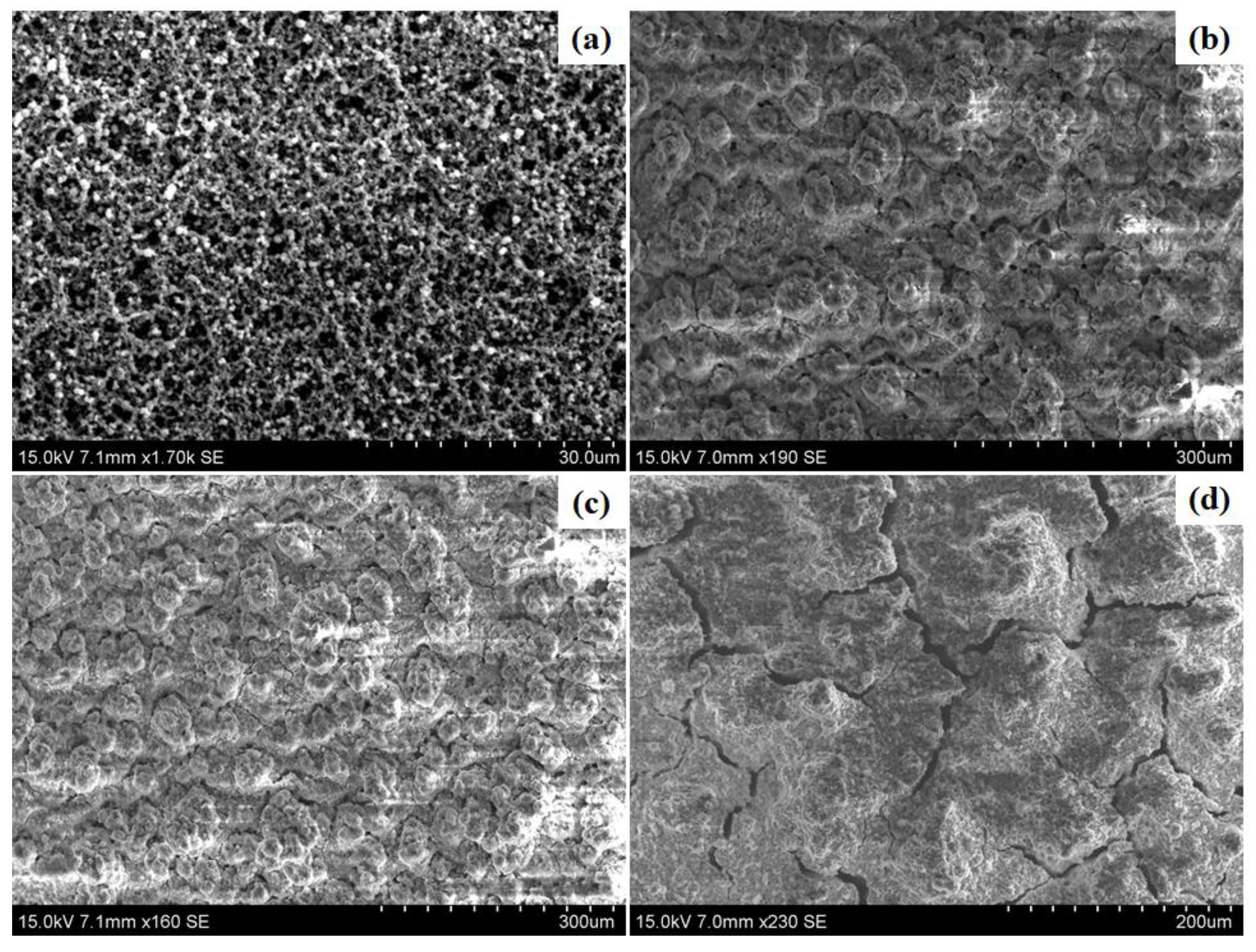
2.1. Scanning and Transmission Electron Microscopy
2.2. X-ray Diffraction
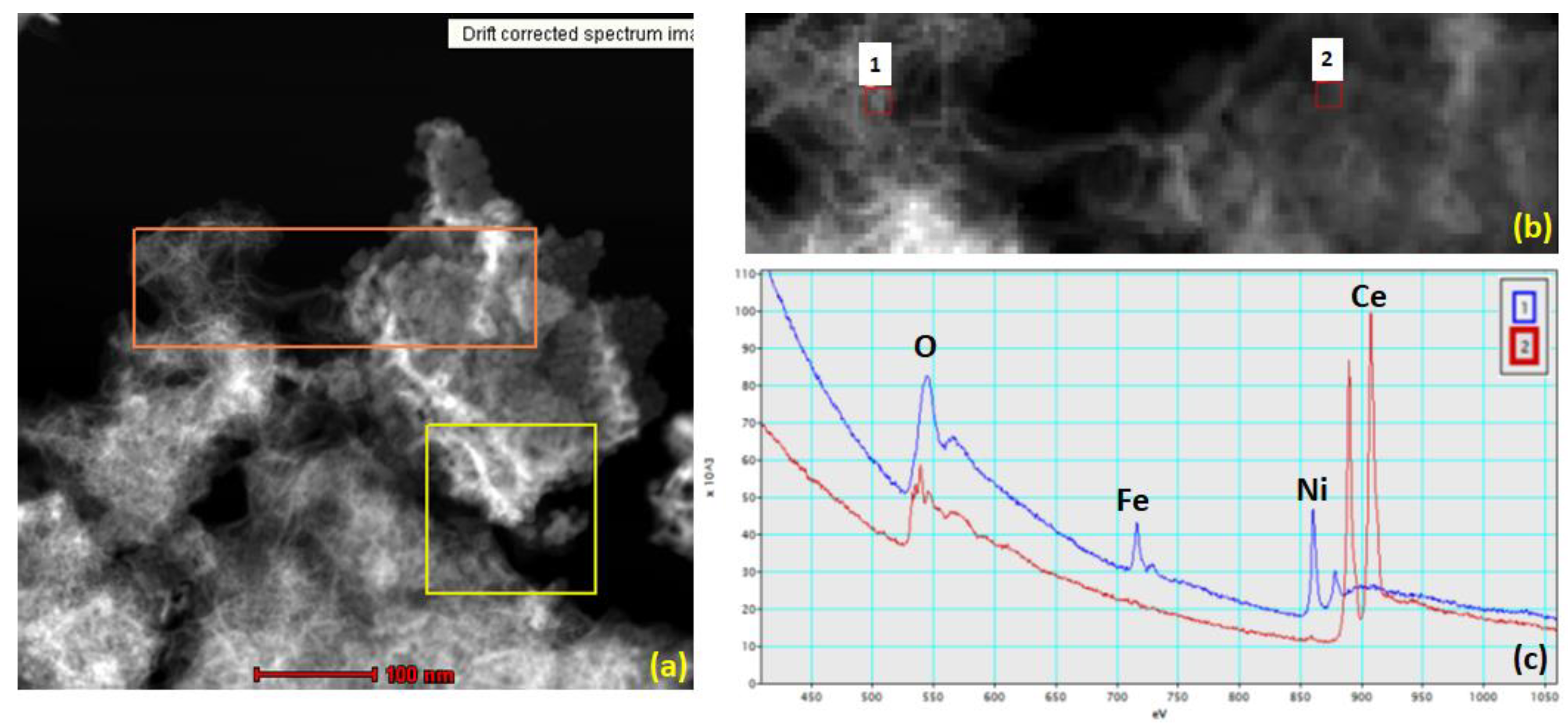
2.3. Electron Energy-Loss Spectroscopy
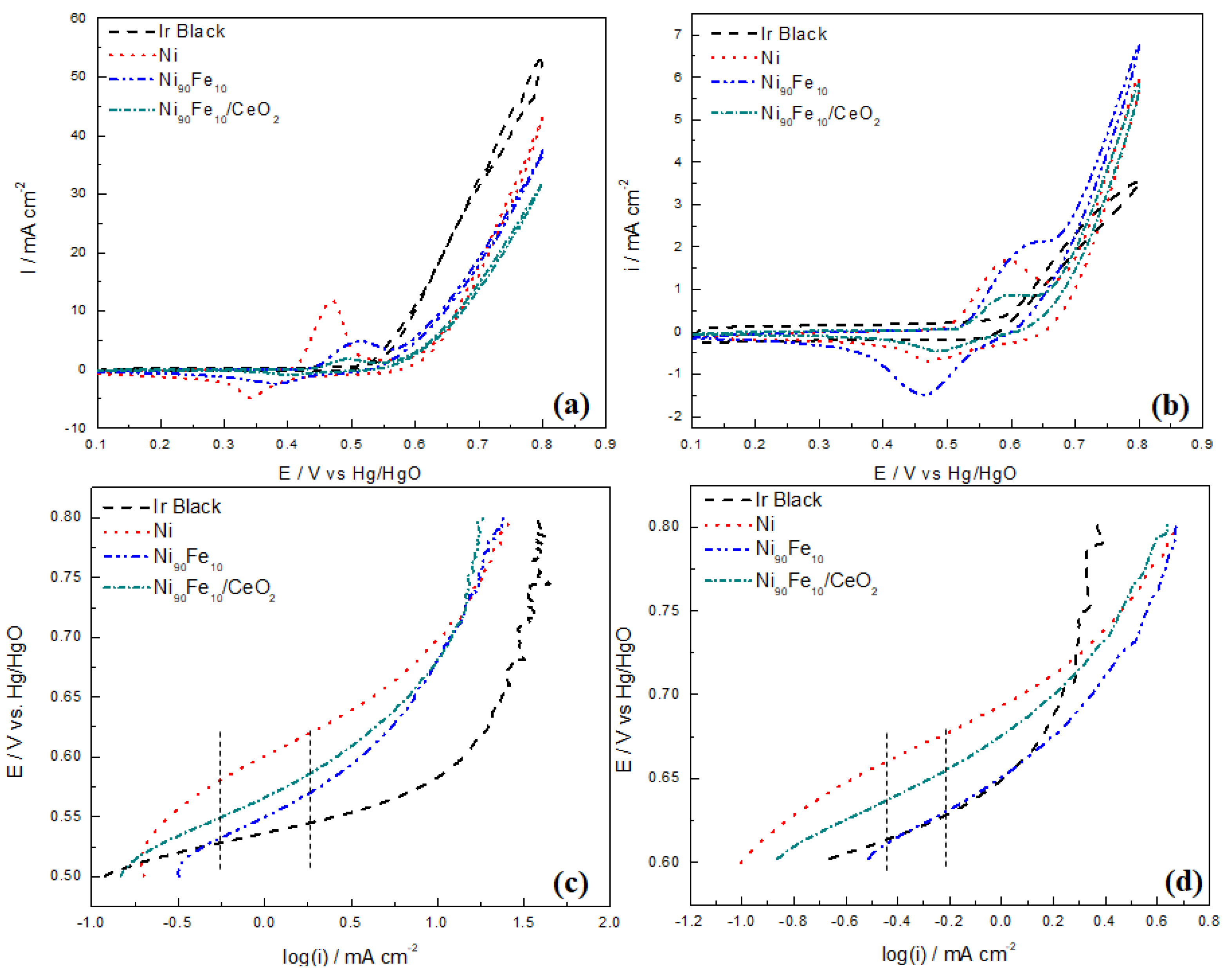
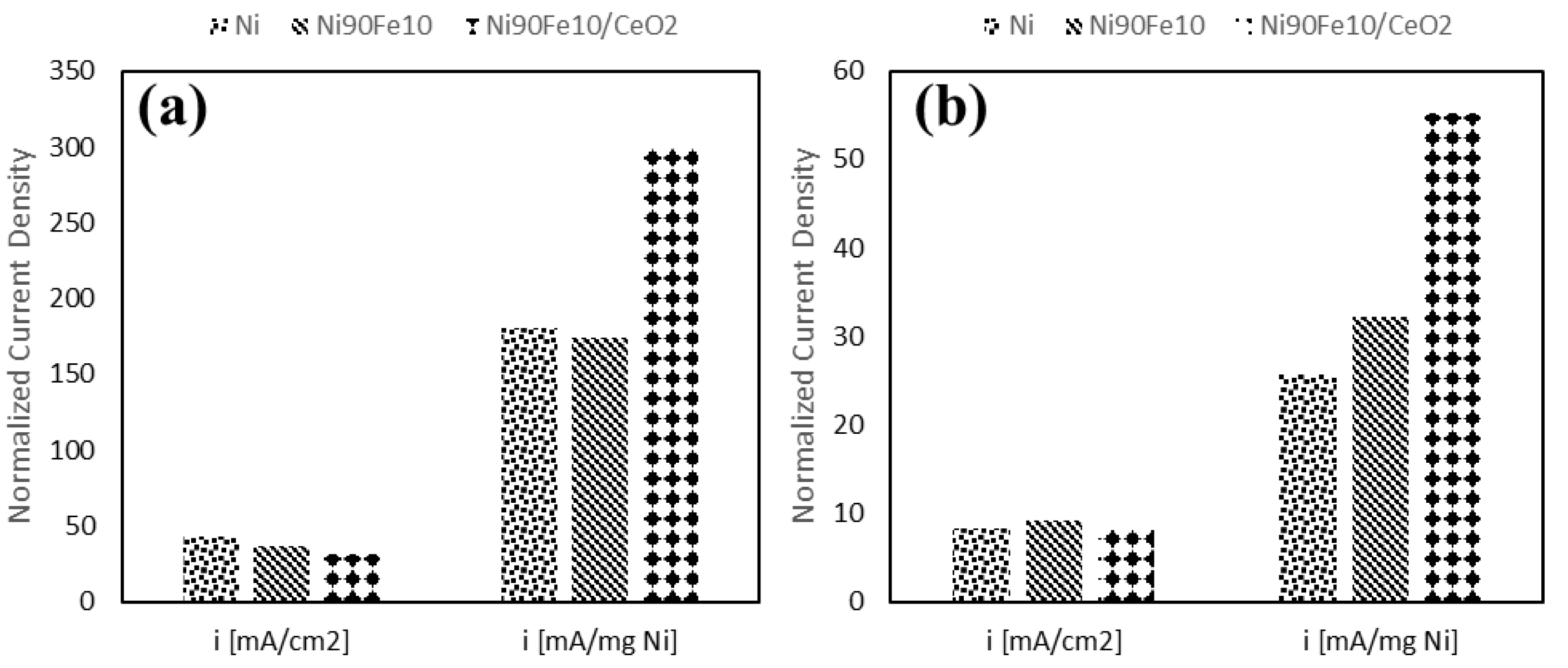
2.4. OER Experiments
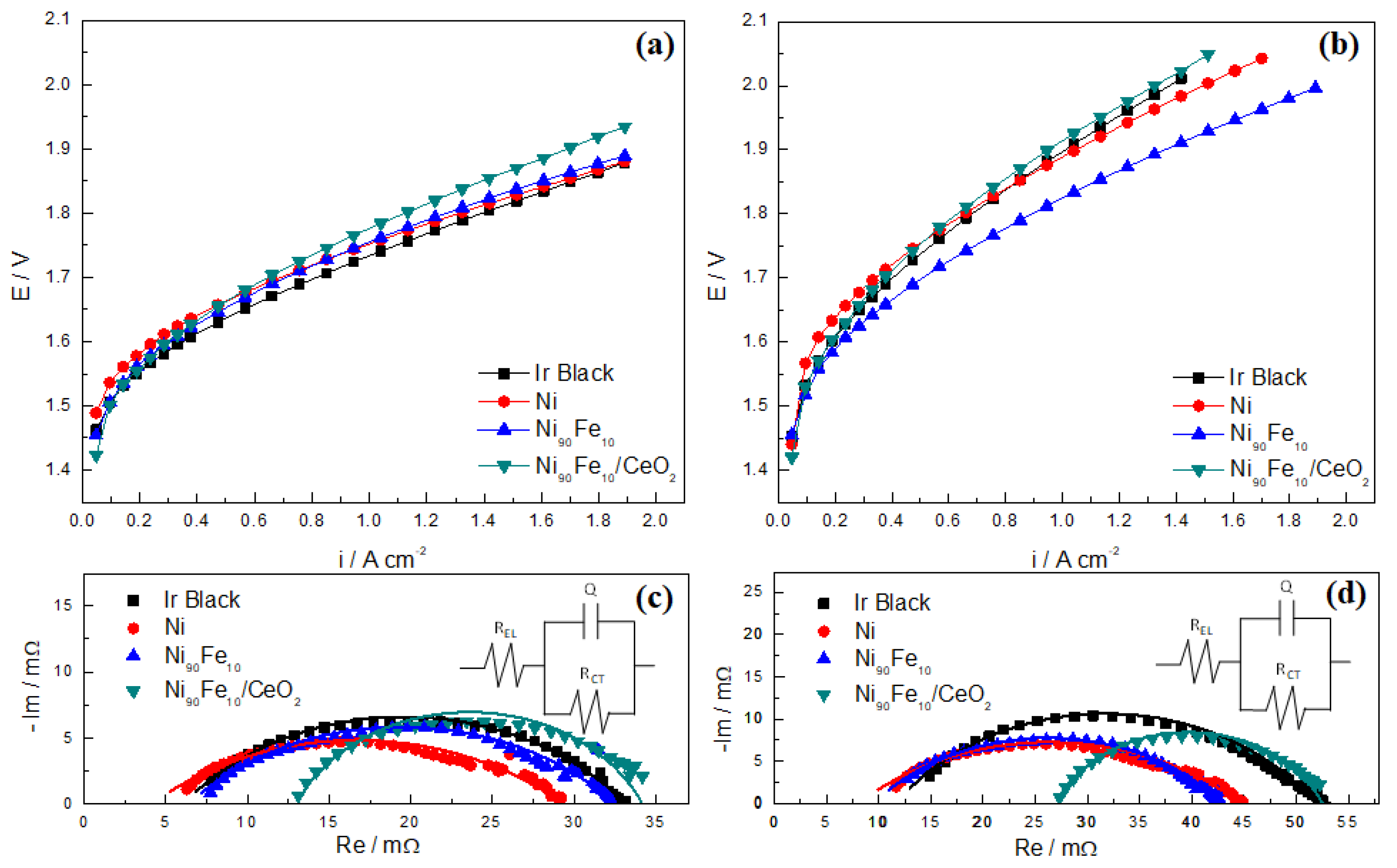
2.5. AEMWE Experiments
3. Materials and Methods
3.1. Synthesis of Ni-Based Materials by Chemical Reduction
3.2. Material Characterization
3.3. Ex-Situ Experiments
3.3.1. Cell Set-Up
3.3.2. OER Experiments
3.4. In Situ Experiments
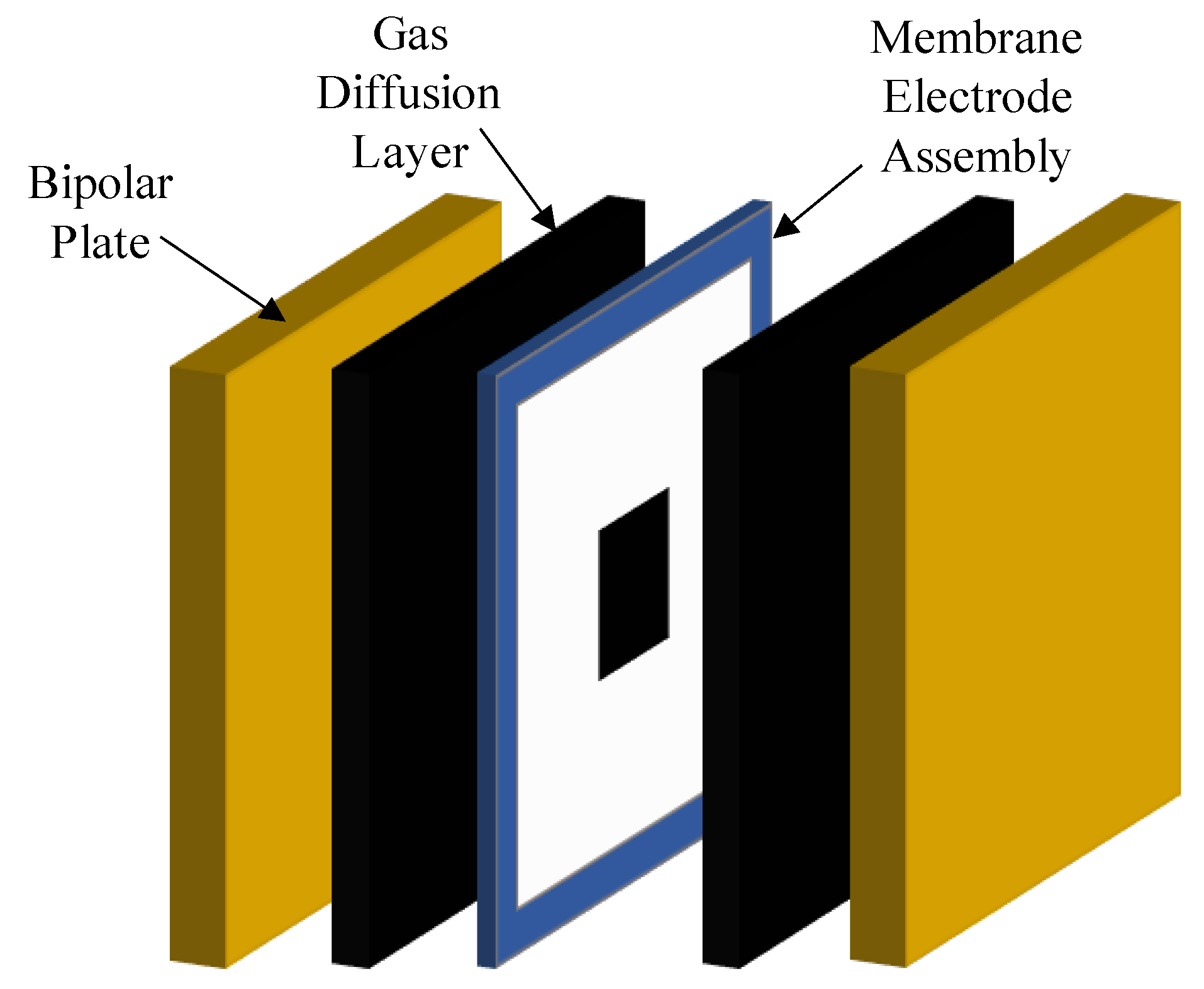
3.4.1. Cell Set-Up
3.4.2. AEMWE Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clark, D.; Brandon, N. What’s the “Hydrogen Economy”? The Guardian: London, UK, 2012. [Google Scholar]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Leng, Y.; Chen, G.; Mendoza, A.J.; Tighe, T.B.; Hickner, M.A.; Wang, C.Y. Solid-state water electrolysis with an alkaline membrane. J. Am. Chem. Soc. 2012, 134, 9054–9057. [Google Scholar] [CrossRef] [PubMed]
- Varcoe, J.R.; Atanassov, P.; Dekel, D.R.; Herring, A.M.; Hickner, M.A.; Kohl, P.A.; Kucernak, A.R.; Mustain, W.E.; Nijmeijer, K.; Scott, K.; et al. Anion-exchange membranes in electrochemical energy systems. Energy Environ. Sci. 2014, 7, 3135–3191. [Google Scholar] [CrossRef]
- Cho, M.K.; Park, H.Y.; Lee, H.J.; Kim, H.J.; Lim, A.; Henkensmeier, D.; Yoo, S.J.; Kim, J.Y.; Lee, S.Y.; Park, H.S.; et al. Alkaline anion exchange membrane water electrolysis: Effects of electrolyte feed method and electrode binder content. J. Power Sources 2018, 382, 22–29. [Google Scholar] [CrossRef]
- Vincent, I.; Bessarabov, D. Low cost hydrogen production by anion exchange membrane electrolysis: A review. Renew. Sustain. Energy Rev. 2018, 81, 1690–1704. [Google Scholar] [CrossRef]
- Phillips, R.; Edwards, A.; Rome, B.; Jones, D.R.; Dunnill, C.W. Minimising the ohmic resistance of an alkaline electrolysis cell through effective cell design. Int. J. Hydrog. Energy 2017, 42, 23986–23994. [Google Scholar] [CrossRef]
- Park, J.E.; Kang, S.Y.; Oh, S.H.; Kim, J.K.; Lim, M.S.; Ahn, C.Y.; Cho, Y.H.; Sung, Y.E. High-performance anion-exchange membrane water electrolysis. Electrochim. Acta 2019, 295, 99–106. [Google Scholar] [CrossRef]
- Hnát, J.; Paidar, M.; Schauer, J.; Žitka, J.; Bouzek, K. Polymer anion-selective membranes for electrolytic splitting of water. Part II: Enhancement of ionic conductivity and performance under conditions of alkaline water electrolysis. J. Appl. Electrochem. 2012, 42, 545–554. [Google Scholar] [CrossRef]
- Chanda, D.; Hnát, J.; Bystron, T.; Paidar, M.; Bouzek, K. Optimization of synthesis of the nickel-cobalt oxide based anode electrocatalyst and of the related membrane-electrode assembly for alkaline water electrolysis. J. Power Sources 2017, 347, 247–258. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.N.; Yoo, C.Y.; Lee, K.B.; Lee, W.M. Low-cost and energy-efficient asymmetric nickel electrode for alkaline water electrolysis. Int. J. Hydrog. Energy 2015, 40, 10720–10725. [Google Scholar] [CrossRef]
- Schalenbach, M.; Kasian, O.; Mayrhofer, K.J.J. An alkaline water electrolyzer with nickel electrodes enables efficient high current density operation. Int. J. Hydrog. Energy 2018, 43, 11932–11938. [Google Scholar] [CrossRef]
- Liu, Z.; Sajjad, S.D.; Gao, Y.; Yang, H.; Kaczur, J.J.; Masel, R.I. The effect of membrane on an alkaline water electrolyzer. Int. J. Hydrog. Energy 2017, 42, 29661–29665. [Google Scholar] [CrossRef]
- Katsounaros, I.; Cherevko, S.; Zeradjanin, A.R.; Mayrhofer, K.J.J. Oxygen electrochemistry as a cornerstone for sustainable energy conversion. Angew. Chem. Int. Ed. 2014, 53, 102–121. [Google Scholar] [CrossRef] [PubMed]
- Marini, S.; Salvi, P.; Nelli, P.; Pesenti, R.; Villa, M.; Berrettoni, M.; Zangari, G.; Kiros, Y. Advanced alkaline water electrolysis. Electrochim. Acta 2012, 82, 384–391. [Google Scholar] [CrossRef]
- Faid, A.; Oyarce Barnett, A.; Seland, F.; Sunde, S. Highly active nickel-based catalyst for hydrogen evolution in anion exchange membrane electrolysis. Catalysts 2018, 8, 614. [Google Scholar] [CrossRef]
- Fabbri, E.; Habereder, A.; Waltar, K.; Kötz, R.; Schmidt, T.J. Developments and perspectives of oxide-based catalysts for the oxygen evolution reaction. Catal. Sci. Technol. 2014, 4, 3800–3821. [Google Scholar] [CrossRef]
- Li, X.; Walsh, F.C.; Pletcher, D. Nickel based electrocatalysts for oxygen evolution in high current density, alkaline water electrolysers. Phys. Chem. Chem. Phys. 2011, 13, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.K.; Lim, A.; Lee, S.Y.; Kim, H.; Yoo, S.J.; Sung, Y.; Park, H.S.; Jang, J.H. A review on membranes and catalysts for anion exchange membrane water electrolysis single cells. J. Electrochem. Sci. Technol. 2017, 8, 183–196. [Google Scholar] [CrossRef]
- Trotochaud, L.; Ranney, J.K.; Williams, K.N.; Boettcher, S.W. Solution-cast metal oxide thin film electrocatalysts for oxygen evolution. J. Am. Chem. Soc. 2012, 134, 17253–17261. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Dai, H. A mini review of NiFe-based materials as highly active oxygen evolution reaction electrocatalysts. Nano Res. 2015, 8, 23–39. [Google Scholar] [CrossRef]
- Long, X.; Ma, Z.; Yu, H.; Gao, X.; Pan, X.; Chen, X.; Yang, S.; Yi, Z. Porous FeNi oxide nanosheets as advanced electrochemical catalysts for sustained water oxidation. J. Mater. Chem. A 2016, 4, 14939–14943. [Google Scholar] [CrossRef]
- Gong, M.; Li, Y.; Wang, H.; Liang, Y.; Wu, J.Z.; Zhou, J.; Wang, J.; Regier, T.; Wei, F.; Dai, H. An advanced Ni-Fe layered double hydroxide electrocatalyst for water oxidation. J. Am. Chem. Soc. 2013, 135, 8452–8455. [Google Scholar] [CrossRef] [PubMed]
- Bode, H.; Dehmelt, K.; White, J. Zur kenntnis der nickelhydroxidelektrode—I.Über das nickel (II)-hydroxidhydrat. Electrochim. Acta 1966, 11, 1079–1087. [Google Scholar] [CrossRef]
- Diaz-Morales, O.; Ferrus-Suspedra, D.; Koper, M.T.M. The importance of nickel oxyhydroxide deprotonation on its activity towards electrochemical water oxidation. Chem. Sci. 2016, 7, 2639–2645. [Google Scholar] [CrossRef] [PubMed]
- Surendranath, Y.; Kanan, M.W.; Nocera, D.G. Mechanistic studies of the oxygen evolution reaction by a cobalt-phosphate catalyst at neutral pH. J. Am. Chem. Soc. 2010, 132, 16501–16509. [Google Scholar] [CrossRef] [PubMed]
- Takashima, T.; Hashimoto, K.; Nakamura, R. Mechanisms of pH-dependent activity for water oxidation to molecular oxygen by MnO2 electrocatalysts. J. Am. Chem. Soc. 2012, 134, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Lyons, M.E.G.; Cakara, A.; O’Brien, P.; Godwin, I.; Doyle, R.L. Redox, pH sensing and electrolytic water splitting properties of electrochemically generated nickel hydroxide thin films in aqueous alkaline solution. Int. J. Electrochem. Sci. 2012, 7, 11768–11795. [Google Scholar]
- Lyons, M.E.G.; Doyle, R.L.; Brandon, M.P. Redox switching and oxygen evolution at oxidized metal and metal oxide electrodes: Iron in base. Phys. Chem. Chem. Phys. 2011, 13, 21530–21551. [Google Scholar] [CrossRef]
- Panaritis, C.; Edake, M.; Couillard, M.; Einakchi, R.; Baranova, E.A. Insight towards the role of ceria-based supports for reverse water gas shift reaction over RuFe nanoparticles. J. CO2 Util. 2018, 26, 350–358. [Google Scholar] [CrossRef]
- Dole, H.A.E.; Baranova, E.A. Ethylene oxidation in an oxygen-deficient environment: Why ceria is an active support? ChemCatChem 2016, 8, 1977–1986. [Google Scholar] [CrossRef]
- Rao, G.R.; Mishra, B.G. Structural redox and catalytic chemistry of ceria based materials. Bull. Catal. Soc. India 2003, 2, 122–134. [Google Scholar]
- Feng, J.-X.; Ye, S.-H.; Xu, H.; Tong, Y.-X.; Li, G.-R. Design and synthesis of FeOOH/CeO2 heterolayered nanotube electrocatalysts for the oxygen evolution reaction. Adv. Mater. 2016, 28, 4698–4703. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Kronawitter, C.X.; Yang, X.; Yeh, Y.W.; Yao, N.; Koel, B.E. The promoting effect of tetravalent cerium on the oxygen evolution activity of copper oxide catalysts. Phys. Chem. Chem. Phys. 2017, 19, 31545–31552. [Google Scholar] [CrossRef] [PubMed]
- Favaro, M.; Drisdell, W.S.; Marcus, M.A.; Gregoire, J.M.; Crumlin, E.J.; Haber, J.A.; Yano, J. An operando investigation of (Ni-Fe-Co-Ce)Ox system as highly efficient electrocatalyst for oxygen evolution reaction. ACS Catal. 2017, 7, 1248–1258. [Google Scholar] [CrossRef]
- Haber, J.A.; Cai, Y.; Jung, S.; Xiang, C.; Mitrovic, S.; Jin, J.; Bell, A.T.; Gregoire, J.M. Discovering Ce-rich oxygen evolution catalysts, from high throughput screening to water electrolysis. Energy Environ. Sci. 2014, 7, 682–688. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef] [PubMed]
- Seetharaman, S.; Balaji, R.; Ramya, K.; Dhathathreyan, K.S.; Velan, M. Graphene oxide modified non-noble metal electrode for alkaline anion exchange membrane water electrolyzers. Int. J. Hydrog. Energy 2013, 38, 14934–14942. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, S.; Pan, J.; Yang, C.; He, M.; Zhuang, L.; Lu, J. First implementation of alkaline polymer electrolyte water electrolysis working only with pure water. Energy Environ. Sci. 2012, 5, 7869–7871. [Google Scholar] [CrossRef]
- Ayers, K.E.; Anderson, E.B.; Capuano, C.B.; Niedzwiecki, M.; Hickner, M.A.; Wang, C.-Y.; Leng, Y.; Zhao, W. Characterization of anion exchange membrane technology for low cost electrolysis. ECS Trans. 2013, 45, 121–130. [Google Scholar] [CrossRef]
- Pavel, C.C.; Cecconi, F.; Emiliani, C.; Santiccioli, S.; Scaffidi, A.; Catanorchi, S.; Comotti, M. Highly efficient platinum group metal free based membrane-electrode assembly for anion exchange membrane water electrolysis. Angew. Chem. Int. Ed. 2014, 53, 1378–1381. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.P.; Li, X.Q.; Cao, J.; Zhang, W.X.; Wang, H.P. Characterization of zero-valent iron nanoparticles. Adv. Colloid Interface Sci. 2006, 120, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.S.; Lockwood, D.J.; Bock, C.; MacDougall, B.R. Nickel hydroxides and related materials: A review of their structures, synthesis and properties. Proc. R. Soc. A Math. Phys. Eng. Sci. 2015, 471, 20140792. [Google Scholar] [CrossRef] [PubMed]
- Baranova, E.A.; Le Page, Y.; Ilin, D.; Bock, C.; MacDougall, B.; Mercier, P.H.J. Size and composition for 1–5 nm Ø PtRu alloy nano-particles from Cu Kα X-ray patterns. J. Alloys Compd. 2009, 471, 387–394. [Google Scholar] [CrossRef][Green Version]
- Reksten, A.H.; Thuv, H.; Seland, F.; Sunde, S. The oxygen evolution reaction mechanism at IrxRu1−xO2 powders produced by hydrolysis synthesis. J. Electroanal. Chem. 2018, 819, 547–561. [Google Scholar] [CrossRef]
- Lyons, M.E.G.; Brandon, M.P. A comparative study of the oxygen evolution reaction on oxidised nickel, cobalt and iron electrodes in base. J. Electroanal. Chem. 2010, 641, 119–130. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, C. Electrodeposition of hierarchically structured three-dimensional nickel-iron electrodes for efficient oxygen evolution at high current densities. Nat. Commun. 2015, 6, 6616. [Google Scholar] [CrossRef]
- Tahir, M.; Pan, L.; Idrees, F.; Zhang, X.; Wang, L.; Zou, J.J.; Wang, Z.L. Electrocatalytic oxygen evolution reaction for energy conversion and storage: A comprehensive review. Nano Energy 2017, 37, 136–157. [Google Scholar] [CrossRef]
- Cherevko, S.; Geiger, S.; Kasian, O.; Kulyk, N.; Grote, J.-P.; Savan, A.; Shrestha, R.B.; Merzlikin, S.; Breitbach, B.; Ludwig, A.; et al. Oxygen and hydrogen evolution reactions on Ru, RuO2, Ir, and IrO2 thin film electrodes in acidic and alkaline electrolytes: A comparative study on activity and stability. Catal. Today 2015, 262, 170–180. [Google Scholar] [CrossRef]
- Lyons, M.E.G.; Floquet, S. Mechanism of oxygen reactions at porous oxide electrodes. Part 2—Oxygen evolution at RuO2, IrO2 and IrxRu1−xO2 electrodes in aqueous acid and alkaline solution. Phys. Chem. Chem. Phys. 2011, 13, 5314–5335. [Google Scholar] [CrossRef]
- Pi, Y.; Zhang, N.; Guo, S.; Guo, J.; Huang, X. Ultrathin laminar Ir superstructure as highly efficient oxygen evolution electrocatalyst in broad pH range. Nano Lett. 2016, 16, 4424–4430. [Google Scholar] [CrossRef]
- Tahir, M.; Mahmood, N.; Zhang, X.; Mahmood, T.; Butt, F.K.; Aslam, I.; Tanveer, M.; Idrees, F.; Khalid, S.; Shakir, I.; et al. Bifunctional catalysts of Co3O4@GCN tubular nanostructured (TNS) hybrids for oxygen and hydrogen evolution reactions. Nano Res. 2015, 8, 3725–3736. [Google Scholar] [CrossRef]
- Vincent, I.; Kruger, A.; Bessarabov, D. Development of efficient membrane electrode assembly for low cost hydrogen production by anion exchange membrane electrolysis. Int. J. Hydrogen Energy 2017, 42, 10752–10761. [Google Scholar] [CrossRef]
- Alsabet, M.; Grden, M.; Jerkiewicz, G. Electrochemical growth of surface oxides on nickel. Part 1: Formation of α-Ni(OH)2 in relation to the polarization potential, polarization time, and temperature. Electrocatalysis 2011, 2, 317–330. [Google Scholar] [CrossRef]
- Harrington, D. Complex Nonlinear Least Squares Fitting of Immittance Data. Available online: https://www.maplesoft.com/applications/view.aspx?SID=154540 (accessed on 26 August 2019).
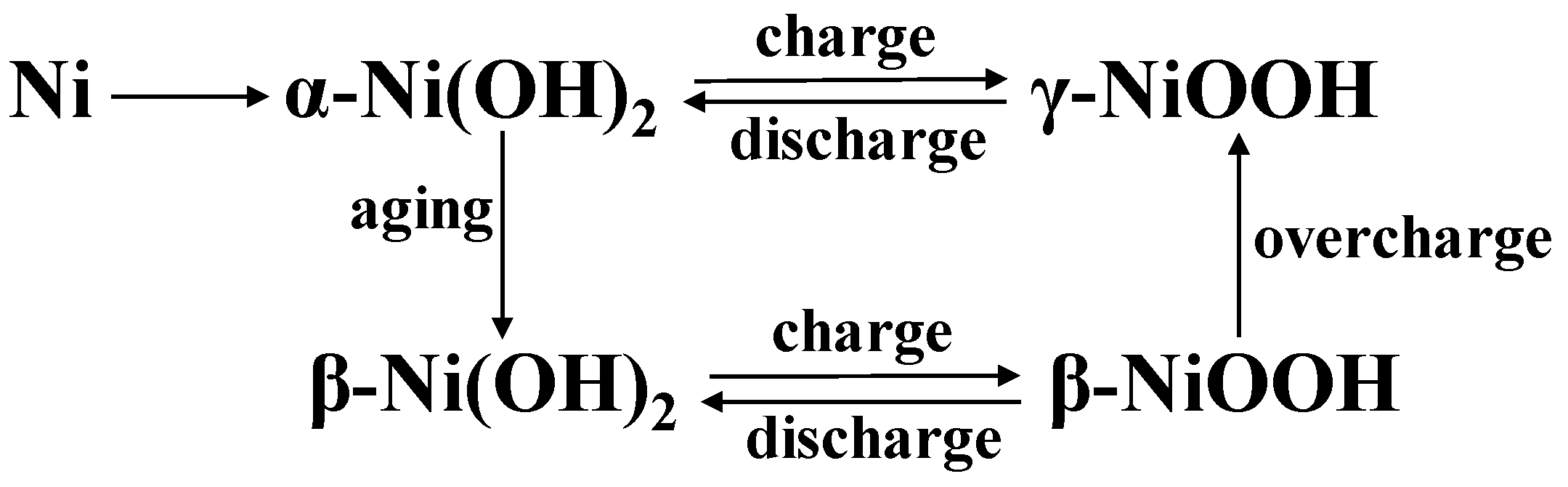

| Icon on Figure 4 | Experimental 2θ [deg] | Corresponding Compound; Reported 2θ [deg] |
|---|---|---|
Diamond  | 33.78 | β-Ni(OH)2 (100); 33.1 |
Pentagon  | 44.65 | Ni (111); 44.45 |
Circle  | 45.37 | Ni (111); 44.45 Fe (110); 44.9 |
Triangle  | 59.99 | β-Ni(OH)2 (110); 59.1 |
Square  | 70.68 | β-Ni(OH)2 (103); ~71 |
| Catalyst | KOH Conc. [M] | Tafel Slope [mV dec−1] | η @ 5 mA cm−2 [mV] | η @ 10 mA cm−2 [mV] |
|---|---|---|---|---|
| Ir Black | 1.0 | 32.0 | 268 | 295 |
| 0.1 | 70.6 | N/A | N/A | |
| Ni | 1.0 | 75.5 | 337 | 365 |
| 0.1 | 73.1 | 424 | N/A | |
| Ni90Fe10 | 1.0 | 71.9 | 298 | 341 |
| 0.1 | 83.3 | 404 | N/A | |
| Ni90Fe10/CeO2 | 1.0 | 70.7 | 323 | 369 |
| 0.1 | 82.1 | 424 | N/A |
| Catalyst. | KOH conc. [M] | E @ 0.4 A/cm2 [V] | E @ 1.4 A/cm2 [V] | REL [mΩ] | RCT [mΩ] | Y [10−4 F s(1−α)] | α |
|---|---|---|---|---|---|---|---|
| Ir Black | 1.0 | 1.608 | 1.804 | 6.0 ± 0.1 | 27 ± 0.1 | 3.6 ± 0.1 | 0.577 ± 0.005 |
| 0.1 | 1.691 | 2.011 | 11.6 ± 0.2 | 40.2 ± 0.3 | 11.5 ± 0.1 | 0.618 ± 0.007 | |
| Ni | 1.0 | 1.636 | 1.815 | 4.1 ± 0.2 | 25.2 ± 0.3 | 12.8 ± 0.8 | 0.465 ± 0.009 |
| 0.1 | 1.713 | 1.984 | 8.0 ± 0.3 | 36.7 ± 0.5 | 6.4 ± 0.4 | 0.478 ± 0.009 | |
| Ni90Fe10 | 1.0 | 1.622 | 1.823 | 6.3 ± 0.2 | 26.1 ± 0.3 | 6.6 ± 0.5 | 0.53 ± 0.01 |
| 0.1 | 1.659 | 1.911 | 9.4 ± 0.1 | 33.6 ± 0.2 | 2.8 ± 0.1 | 0.551 ± 0.005 | |
| Ni90Fe10/CeO2 | 1.0 | 1.627 | 1.854 | 13.0 ± 0.4 | 21.2 ± 0.2 | 10.1 ± 0.3 | 0.74 ± 0.04 |
| 0.1 | 1.703 | 2.023 | 27.0 ± 0.4 | 25.6 ± 0.6 | 0.5 ± 0.1 | 0.74 ± 0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cossar, E.; Oyarce Barnett, A.; Seland, F.; Baranova, E.A. The Performance of Nickel and Nickel-Iron Catalysts Evaluated As Anodes in Anion Exchange Membrane Water Electrolysis. Catalysts 2019, 9, 814. https://doi.org/10.3390/catal9100814
Cossar E, Oyarce Barnett A, Seland F, Baranova EA. The Performance of Nickel and Nickel-Iron Catalysts Evaluated As Anodes in Anion Exchange Membrane Water Electrolysis. Catalysts. 2019; 9(10):814. https://doi.org/10.3390/catal9100814
Chicago/Turabian StyleCossar, Emily, Alejandro Oyarce Barnett, Frode Seland, and Elena A. Baranova. 2019. "The Performance of Nickel and Nickel-Iron Catalysts Evaluated As Anodes in Anion Exchange Membrane Water Electrolysis" Catalysts 9, no. 10: 814. https://doi.org/10.3390/catal9100814
APA StyleCossar, E., Oyarce Barnett, A., Seland, F., & Baranova, E. A. (2019). The Performance of Nickel and Nickel-Iron Catalysts Evaluated As Anodes in Anion Exchange Membrane Water Electrolysis. Catalysts, 9(10), 814. https://doi.org/10.3390/catal9100814