Insight into the Microstructure and Deactivation Effects on Commercial NiMo/γ-Al2O3 Catalyst through Aberration-Corrected Scanning Transmission Electron Microscopy
Abstract
:1. Introduction
2. Results and Discussion
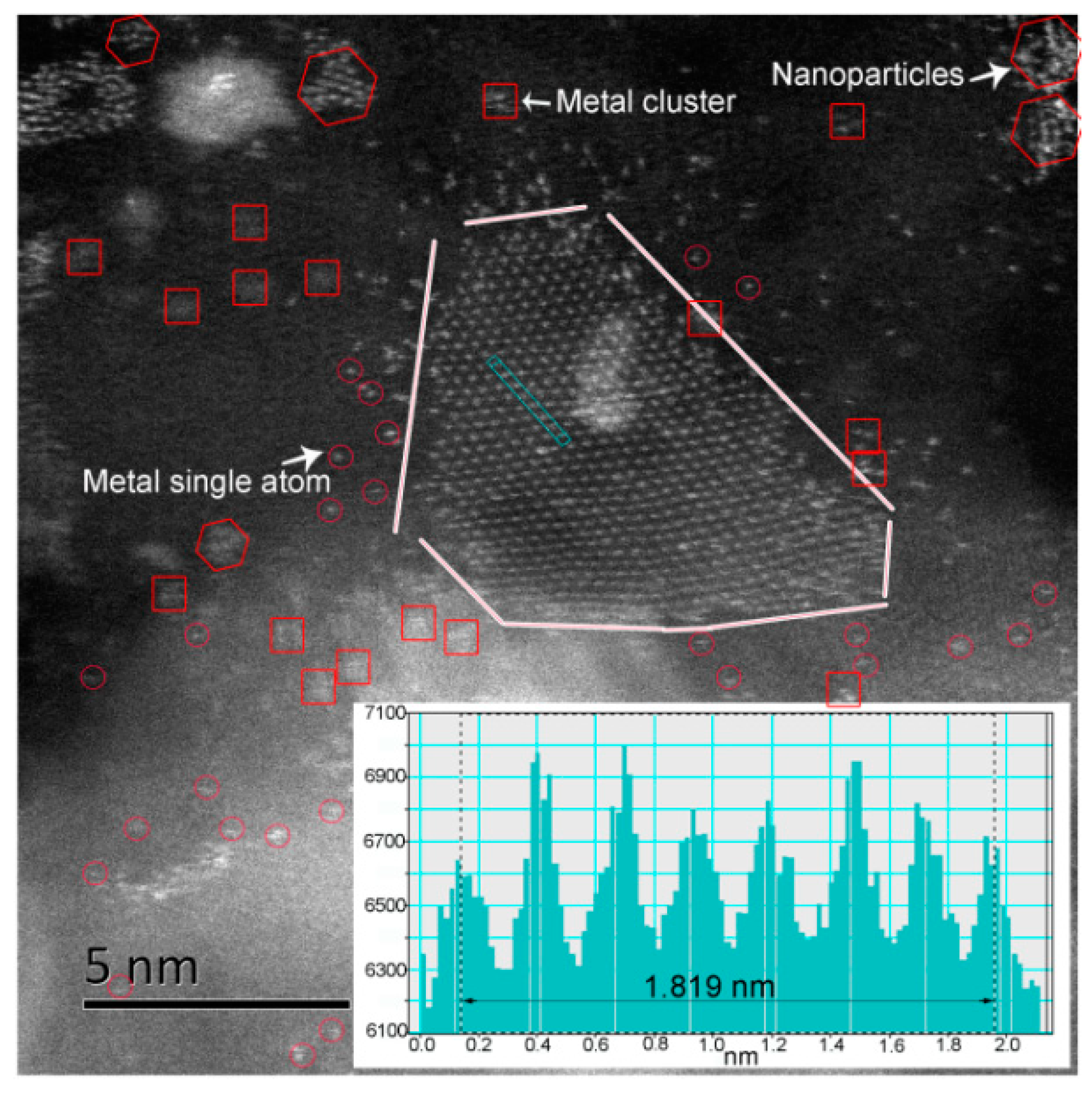
2.1. Atomic-Resolution Microstructure
2.2. Structural Variations with the Decreasing of Catalytic Activity
2.3. Deactivation Effects
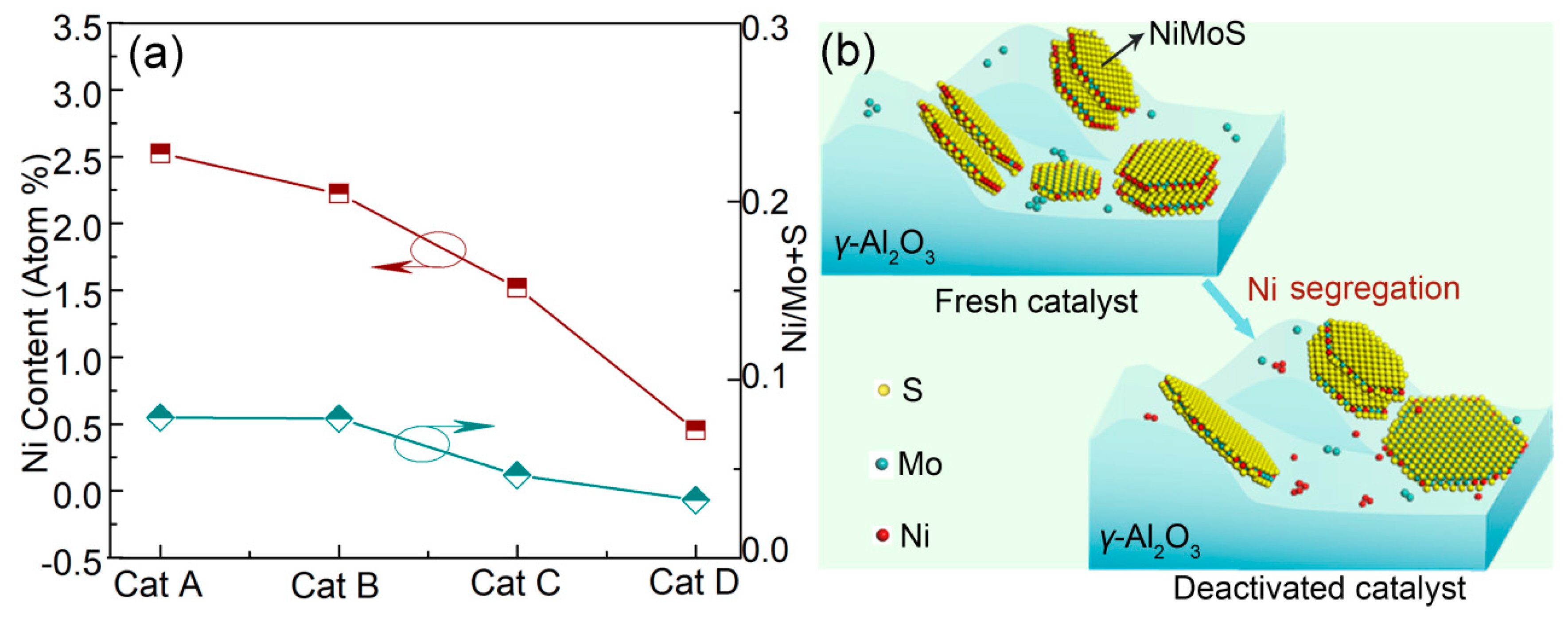
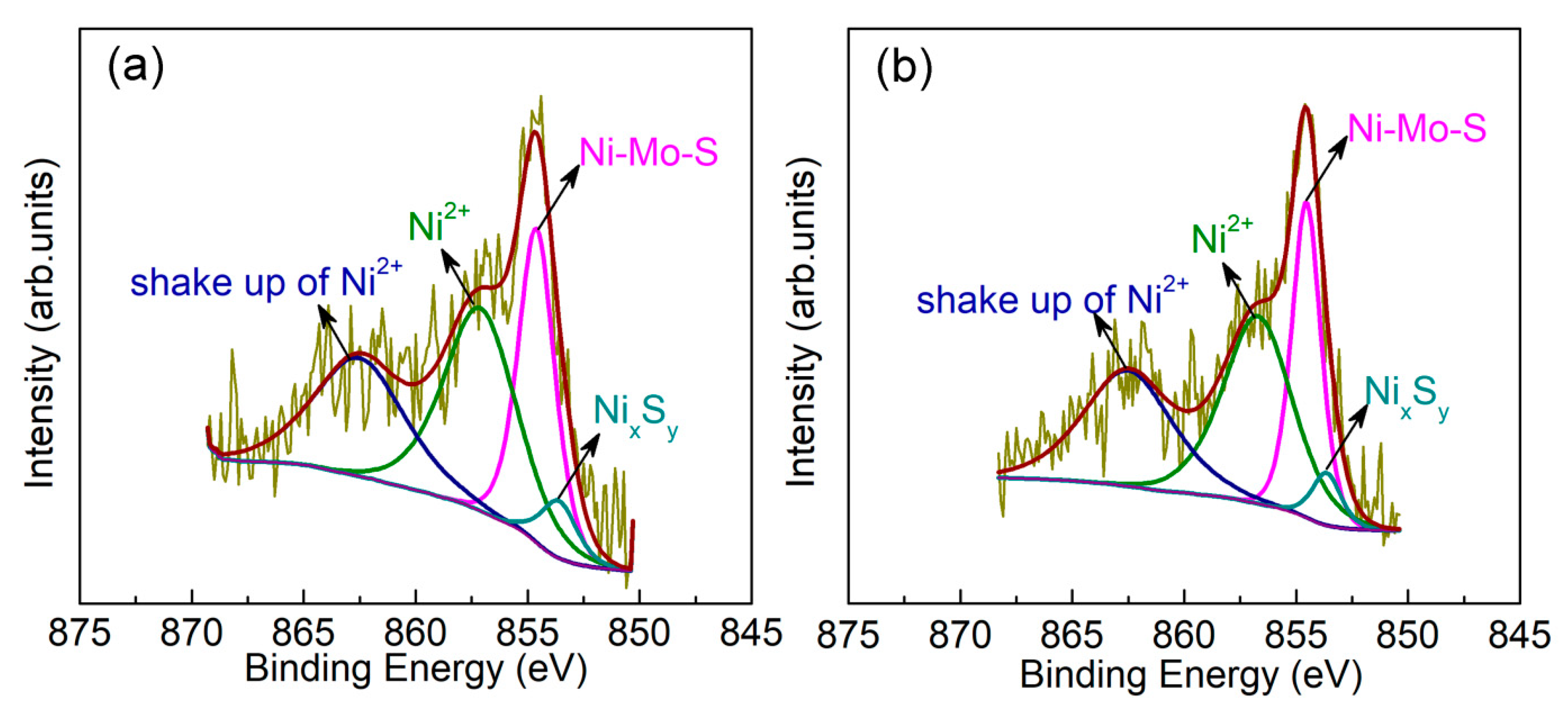
2.3.1. Ni Segregation from the Active Phase
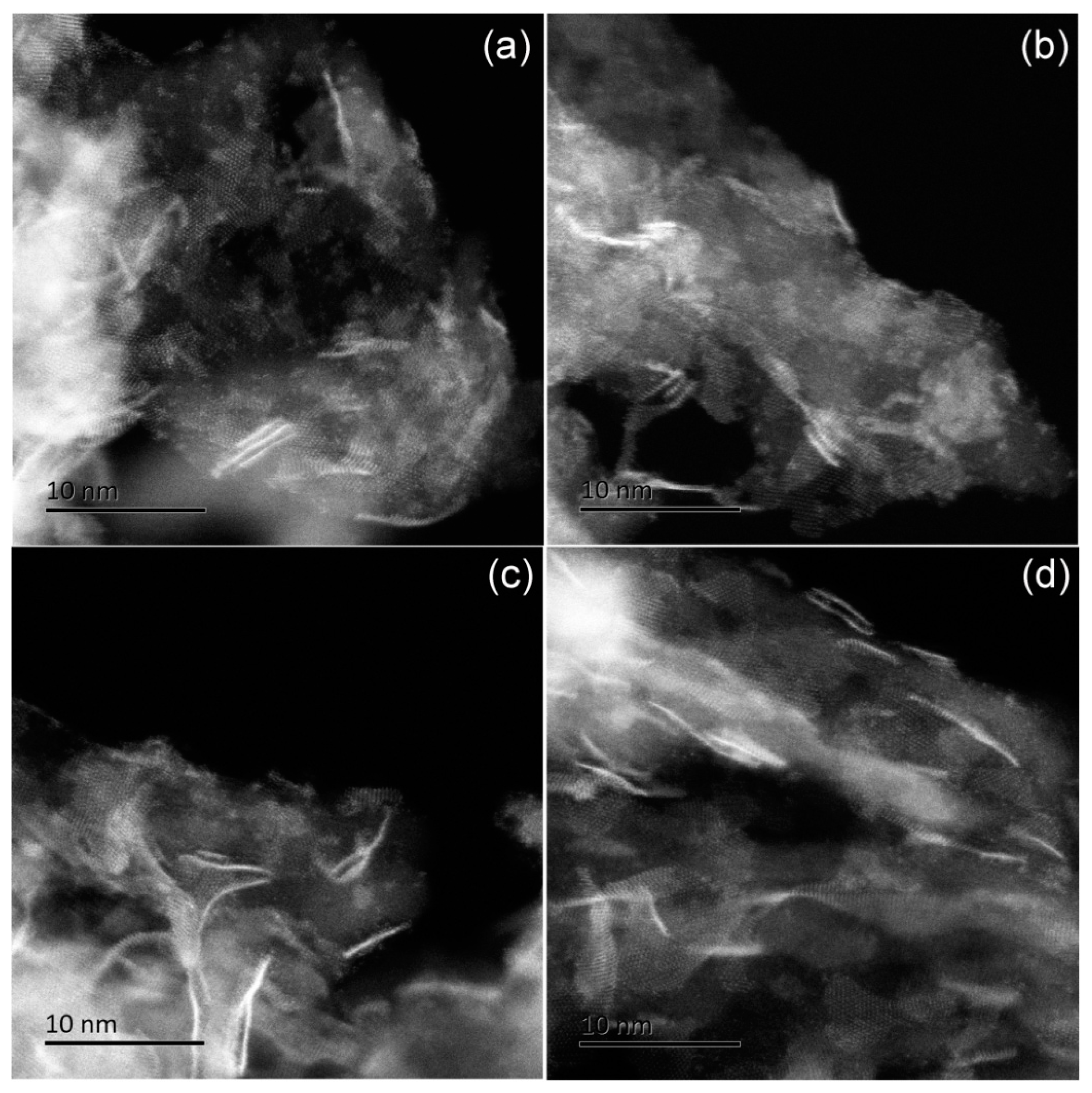
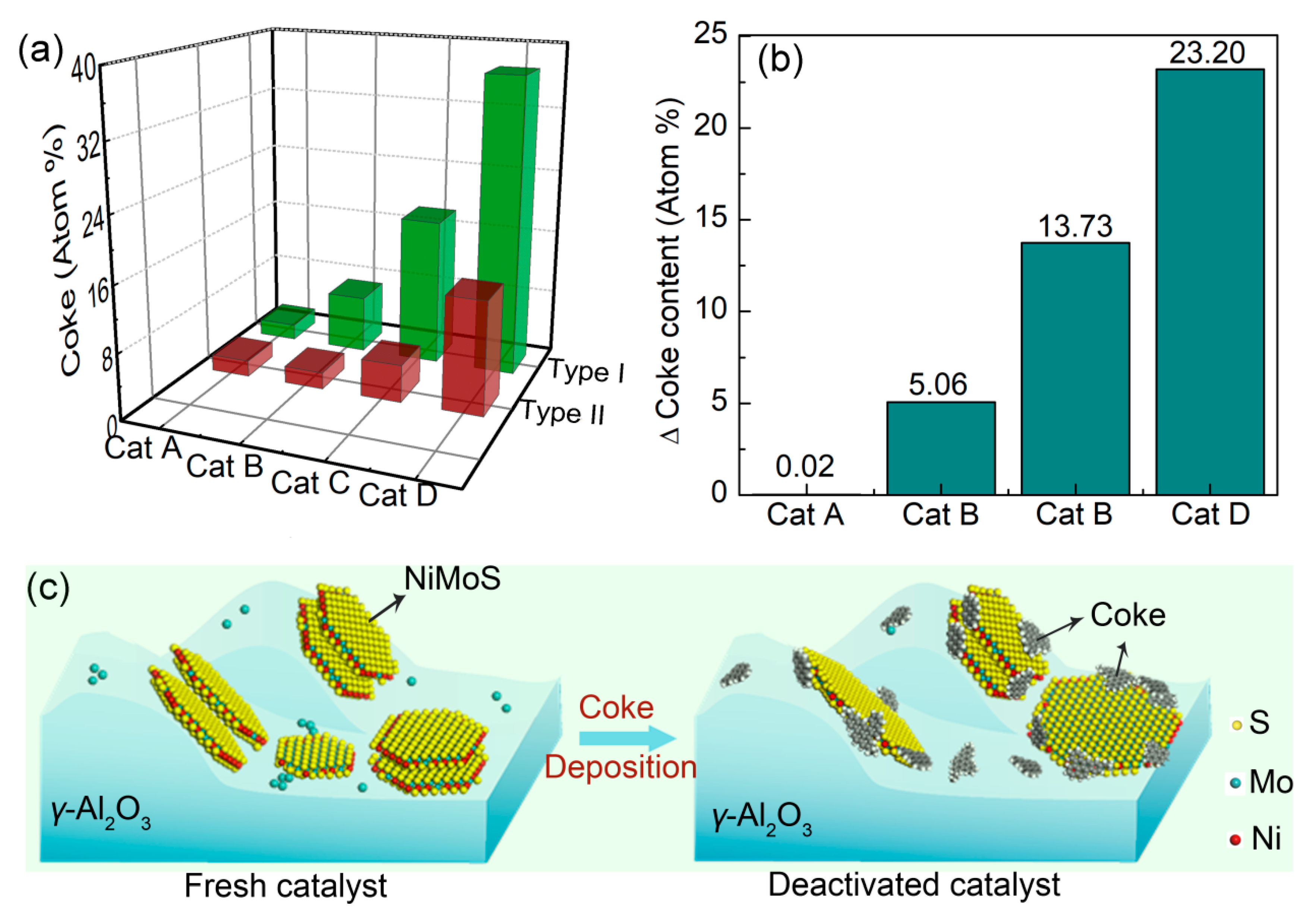
2.3.2. Coke Deposition
3. Materials and Methods
3.1. Catalyst Preparations
3.2. Catalytic Activity Evaluation
3.3. Catalyst Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Girleanu, M.; Alphazan, T.; Boudene, Z.; Bonduelle-Skrzypczak, A.; Legens, C.; Gay, A.S.; Copret, C.; Ersen, O.; Raybaud, P. Magnifying the morphology change induced by a nickel promoter in tungsten (IV) sulfide industrial hydrocracking catalyst: A HAADF-STEM and DFT Study. ChemCatChem 2014, 6, 1594–1598. [Google Scholar] [CrossRef]
- Allard, L.F.; Borisevich, A.; Deng, W.; Si, R.; Flytzani-Stephanopoulos, M.; Overbury, S.H. Evolution of gold structure during thermal treatment of Au/FeOx catalysts revealed by aberration-corrected electron microscopy. J. Electron Microsc. 2009, 58, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Besenbacher, F.; Brorson, M.; Clausen, B.S.; Helveg, S.; Hinnemann, B.; Kibsgaard, J.; Lauritsen, J.V.; Moses, P.G.; Nørskov, J.K.; Topsøe, H. Recent STM, DFT and HAADF-STEM studies of sulfide-based hydrotreating catalysts: Insight into mechanistic, structural and particle size effects. Catal. Today 2008, 130, 86–96. [Google Scholar] [CrossRef]
- Castro-Guerrero, C.F.; Deepak, F.L.; Ponce, A.; Cruz-Reyes, J.; Valle Granados, M.D.; Fuentes-Moyado, S.; Galva’n, D.H.; Jose’-Yacama’n, M. Structure and catalytic properties of hexagonal molybdenum disulfide nanoplates. Catal. Sci. Technol. 2011, 1, 1024–1031. [Google Scholar] [CrossRef]
- Baubet, B.; Girleanu, M.; Gay, A.S.; Taleb, A.L.; Moreaud, M.; Wahl, F.; Delattre, V.; Devers, E.; Hugon, A.; Ersen, O.; et al. Quantitative Two-Dimensional (2D) Morphology—Selectivity relationship of CoMoS nanolayers: A combined high-resolution high-angle annular dark field scanning transmission electron microscopy (HR HAADF- STEM) and Density Functional Theory (DFT) Study. ACS Catal. 2016, 6, 1081–1092. [Google Scholar] [CrossRef]
- Nellist, P.D.; Pennycook, S.J. Direct imaging of the atomic configuration of ultradispersed catalysts. Science 1996, 274, 413–415. [Google Scholar] [CrossRef]
- Liu, J. Aberration-corrected scanning transmission electron microscopy in single-atom catalysis: Probing the catalytically active centers. Chin. J. Catal. 2017, 38, 1460–1472. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Ramasse, Q.M.; Brorson, M.; Moses, P.G.; Hansen, L.P.; Kisielowski, C.F.; Helveg, S. Visualizing the stoichiometry of industrial-style Co-Mo-S catalysts with single-atom sensitivity. Angew. Chem. Int. Ed. 2014, 53, 10723–10727. [Google Scholar] [CrossRef] [PubMed]
- Lauritsen, J.V.; Kibsgaard, J.; Olesen, G.H.; Moses, P.G.; Hinnemann, B.; Helveg, S.; Nørskov, J.K.; Clausen, B.S. Topsøe, H.; Lægsgaard, E.; et al. Location and coordination of promoter atoms in Co- and Ni-promoted MoS2-based hydrotreating catalysts. J. Catal. 2007, 249, 220–233. [Google Scholar] [CrossRef]
- Topsøe, H. The role of Co-Mo-S type structures in hydrotreating catalysts. Appl. Catal. A Gen. 2007, 322, 3–8. [Google Scholar] [CrossRef]
- Helveg, S.; Lauritsen, J.V.; Lægsgaard, E.; Stensgaard, I.; Nørskov, J.K.; Clausen, B.S.; Topsøe, H.; Besenbacher, F. Atomic-scale structure of single-layer MoS2 nanoclusters. Phys. Rev. Lett. 2000, 84, 951–954. [Google Scholar] [CrossRef] [PubMed]
- Lauritsen, J.V.; Helveg, S.; Lægsgaard, E.; Stensgaard, I.; Clausen, B.S.; Topsøe, H.; Besenbacher, F. Atomic-scale structure of Co-Mo-S nanoclusters in hydrotreating catalysts. J. Catal. 2001, 197, 1–5. [Google Scholar] [CrossRef]
- Eijsbouts, S.; Battiston, A.A.; Van Leerdam, G.C. Life cycle of hydro-processing catalysts and total catalyst management. Catal. Today 2008, 130, 361–373. [Google Scholar] [CrossRef]
- Kim, K.D.; Lee, Y.K. Active phase of dispersed MoS2 catalysts for slurry phase hydrocracking of vacuum residue. J. Catal. 2019, 369, 111–121. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Nishi, M.; Mochizuki, T.; Takagi, H.; Takatsuki, A.; Roschat, W.; Toba, M.; Yoshimura, Y. Co-processing of Jatropha-derived bio-oil with petroleum distillates over mesoporous CoMo and NiMo sulfide catalysts. Catalysts 2018, 8, 59. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, K.D.; Lee, Y.K. Effects of dispersed MoS2 catalysts and reaction conditions on slurry phase hydrocracking of vacuum residue. J. Catal. 2017, 347, 127–137. [Google Scholar] [CrossRef]
- Grønborg, S.S.; Salazar, N.; Bruix, A.; Rodríguez-Fernández, J.; Thomsen, S.D.; Hammer, B.; Lauritsen, J.V. Visualizing hydrogen-induced reshaping and edge activation in MoS2 and Co-promoted MoS2 catalyst clusters. Nat. Commun. 2018, 9, 2211. [Google Scholar] [CrossRef]
- Abdus Salam, M.; Creaser, D.; Arora, P.; Tamm, S.; Lind Grennfelt, E.; Olsson, L. Influence of bio-oil phospholipid on the hydrodeoxygenation activity of NiMoS/Al2O3 catalyst. Catalysts 2018, 8, 418. [Google Scholar] [CrossRef]
- Guichard, B.; Roy-Auberger, M.; Devers, E.; Legens, C.; Raybaud, P. Aging of Co(Ni)MoP/Al2O3 catalysts in working state. Catal. Today 2008, 130, 97–108. [Google Scholar] [CrossRef]
- Van Doorn, J.; Moulijn, J.A.; Djéga-Mariadassou, G. High-resolution electron microscopy of spent Ni-Mo/Al2O3 hydrotreating catalysts. Appl. Catal. 1990, 63, 77–90. [Google Scholar] [CrossRef]
- Marafi, M.; Stanislaus, A. Effect of initial coking on hydrotreating catalyst functionalities and properties. Appl. Catal. A Gen. 1997, 159, 259–267. [Google Scholar] [CrossRef]
- Shi, X.G.; Liu, Q.Y.; Liu, Z.Y.; Shi, L.; Han, W.; Li, M.F.; Zhang, L.; Nie, H. Coke removal from a deactivated industrial diesel hydrogenation catalyst by Tetralin at 300–400 °C. Energy Fuels 2019, 33, 2437–2444. [Google Scholar] [CrossRef]
- Marafi, M.; Furimsky, E. Hydroprocessing Catalysts containing noble metals: Deactivation, regeneration, metals reclamation, and environment and Safety. Energy Fuels 2017, 31, 5711–5750. [Google Scholar] [CrossRef]
- Tanaka, Y.; Shimada, H.; Matsubayashi, N.; Nishijima, A.; Nomura, M. Accelerated deactivation of hydrotreating catalysts: Comparison to long term deactivation in a commercial plant. Catal. Today 1998, 45, 319–325. [Google Scholar] [CrossRef]
- Song, S.-K.; Ihm, S.-K. Deactivation control through accelerated precoking for the CoMo/γ-Al2O3 catalysts in thiophene hydrodesulfurization. Korean J. Chem. Eng. 2003, 20, 284–287. [Google Scholar] [CrossRef]
- Koh, J.H.; Lee, J.J.; Kim, H.; Cho, A.; Moon, S.H. Correlation of the deactivation of CoMo/Al2O3 in hydrodesulfurization with surface carbon species. Appl. Catal. B Environ. 2009, 86, 176–181. [Google Scholar] [CrossRef]
- Pacheco, M.E.; Martins Salim, V.M.; Pinto, J.C. Accelerated deactivation of hydrotreating catalysts by coke deposition. Ind. Eng. Chem. Res. 2011, 50, 5975–5981. [Google Scholar] [CrossRef]
- Faye, P.; Payen, E.; Bougeard, D. Density functional approach of a γ-Alumina supported MoS2 hydrotreating catalyst. J. Catal. 1998, 179, 560–564. [Google Scholar] [CrossRef]
- Deepak, F.L.; Esparza, R.; Borges, B.; Lopez-Lozano, X.; Jose-Yacaman, M. Direct imaging and identification of individual dopant atoms in MoS2 and WS2 catalysts by aberration corrected scanning transmission electron microscopy. ACS Catal. 2011, 1, 537–543. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Lauritsen, J.V.; Lægsgaard, E.; Clausen, B.S.; Topsøe, H.; Besenbacher, F. Cluster-support interactions and morphology of MoS2 nanoclusters in a graphite-supported hydrotreating model catalyst. J. Am. Chem. Soc. 2006, 128, 13950–13958. [Google Scholar] [CrossRef]
- Topsøe, H.; Clausen, B.S.; Massoth, F.E. Hydrotreating Catalysis; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Tavizón-Pozos, J.A.; Santolalla-Vargas, C.E.; Valdés-Martínez, O.U.; de los Reyes Heredia, J.A. Effect of metal loading in unpromoted and promoted CoMo/Al2O3-TiO2 catalysts for the hydrodeoxygenation of phenol. Catalysts 2019, 9, 550. [Google Scholar] [CrossRef]
- Qiu, L.M.; Xu, G.T. Peak overlaps and corresponding solutions in the X-ray photoelectron spectroscopic study of hydrodesulfurization catalysts. Appl. Surf. Sci. 2010, 256, 3413–3417. [Google Scholar] [CrossRef]
- Guichard, B.; Roy-Auberger, M.; Devers, E.; Pichon, C.; Legens, C. Characterization of aged hydrotreating catalysts. Part II: The evolution of the mixed phase. Effects of deactivation, activation and/or regeneration. Appl. Catal. A Gen. 2009, 367, 9–22. [Google Scholar] [CrossRef]
- Yao, S.D.; Zheng, Y.; Ding, L.H.; Ng, S.; Yang, H. Co-promotion of fluorine and boron on NiMo/Al2O3 for hydrotreating light cycle oil. Catal. Sci. Technol. 2012, 2, 1925–1932. [Google Scholar] [CrossRef]

| Catalyst | HDS (%) | HDN (%) |
|---|---|---|
| Cat A | - | - |
| Cat B | 98.81 | 99.76 |
| Cat C | 88.50 | 85.43 |
| Cat D | 85.84 | 84.30 |
| Sample | Mean Atomic Ratio of Ni (%) | Ratio of Ni/Mo+S (%) | Number of Examined Regions |
|---|---|---|---|
| Cat A | 2.53 | 0.0788 | 50 |
| Cat B | 2.23 | 0.0782 | 50 |
| Cat C | 1.52 | 0.0466 | 50 |
| Cat D | 0.45 | 0.0326 | 50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.; Hu, A.; Qiu, L.; Wang, W.; Xiang, Y.; Han, W.; Xu, G.; Zhang, L.; Zheng, A. Insight into the Microstructure and Deactivation Effects on Commercial NiMo/γ-Al2O3 Catalyst through Aberration-Corrected Scanning Transmission Electron Microscopy. Catalysts 2019, 9, 810. https://doi.org/10.3390/catal9100810
He W, Hu A, Qiu L, Wang W, Xiang Y, Han W, Xu G, Zhang L, Zheng A. Insight into the Microstructure and Deactivation Effects on Commercial NiMo/γ-Al2O3 Catalyst through Aberration-Corrected Scanning Transmission Electron Microscopy. Catalysts. 2019; 9(10):810. https://doi.org/10.3390/catal9100810
Chicago/Turabian StyleHe, Wenhui, Anpeng Hu, Limei Qiu, Wei Wang, Yanjuan Xiang, Wei Han, Guangtong Xu, Le Zhang, and Aiguo Zheng. 2019. "Insight into the Microstructure and Deactivation Effects on Commercial NiMo/γ-Al2O3 Catalyst through Aberration-Corrected Scanning Transmission Electron Microscopy" Catalysts 9, no. 10: 810. https://doi.org/10.3390/catal9100810
APA StyleHe, W., Hu, A., Qiu, L., Wang, W., Xiang, Y., Han, W., Xu, G., Zhang, L., & Zheng, A. (2019). Insight into the Microstructure and Deactivation Effects on Commercial NiMo/γ-Al2O3 Catalyst through Aberration-Corrected Scanning Transmission Electron Microscopy. Catalysts, 9(10), 810. https://doi.org/10.3390/catal9100810






