Synthesis, Characterization, Solution Behavior and Theoretical Studies of Pd(II) Allyl Complexes with 2-Phenyl-3H-indoles as Ligands
Abstract
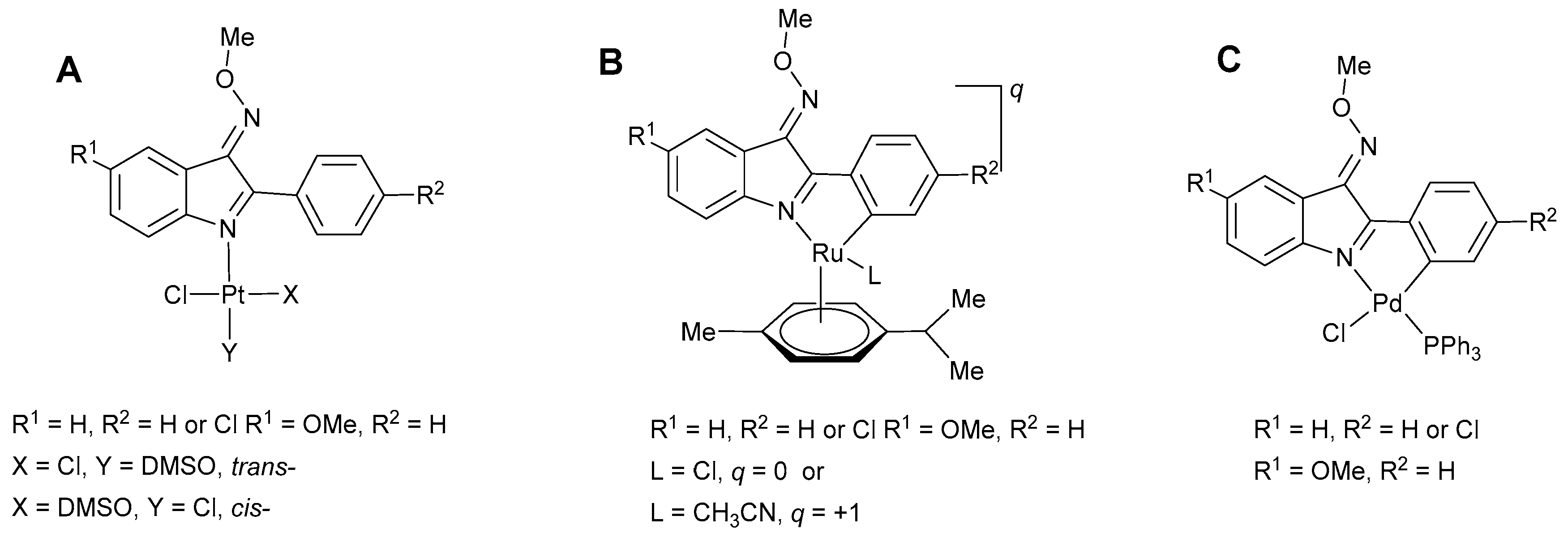
1. Introduction
2. Results and Discussion
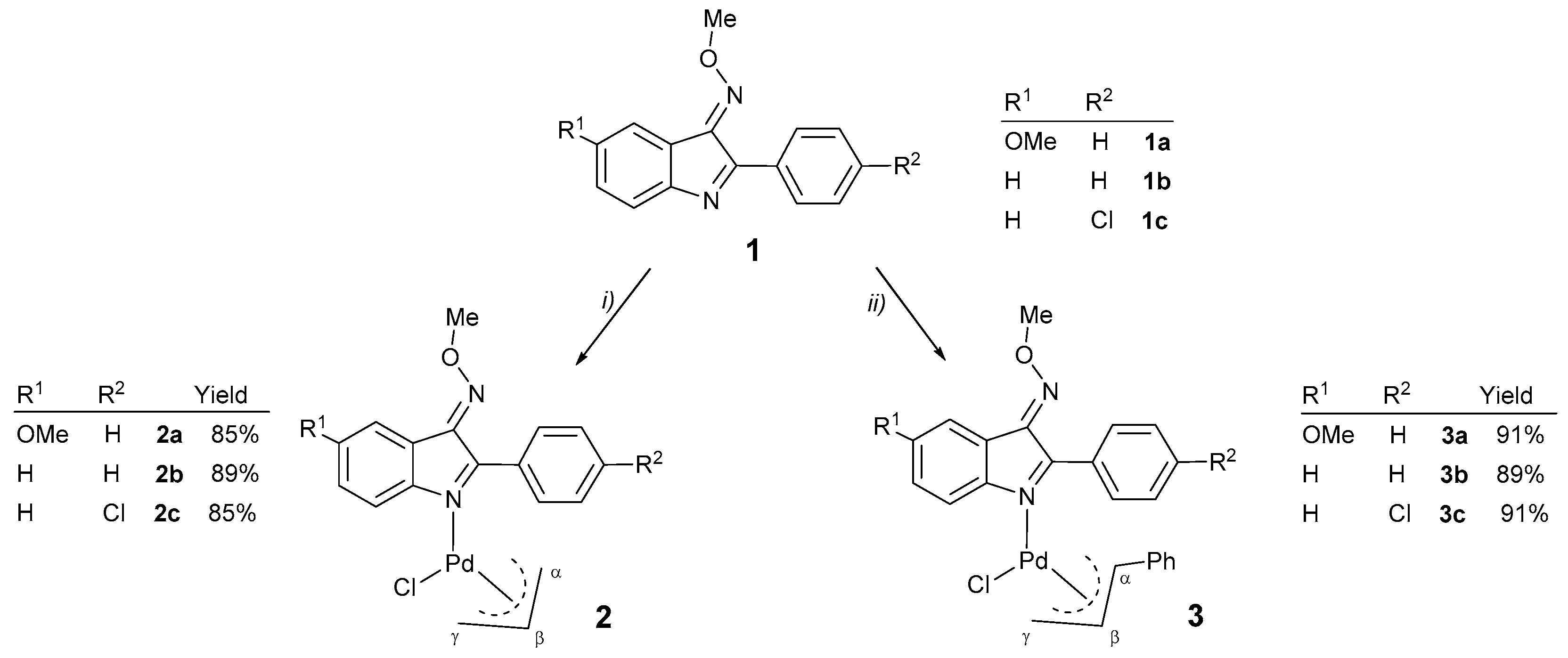
2.1. Synthesis of the Pd(II) Compounds
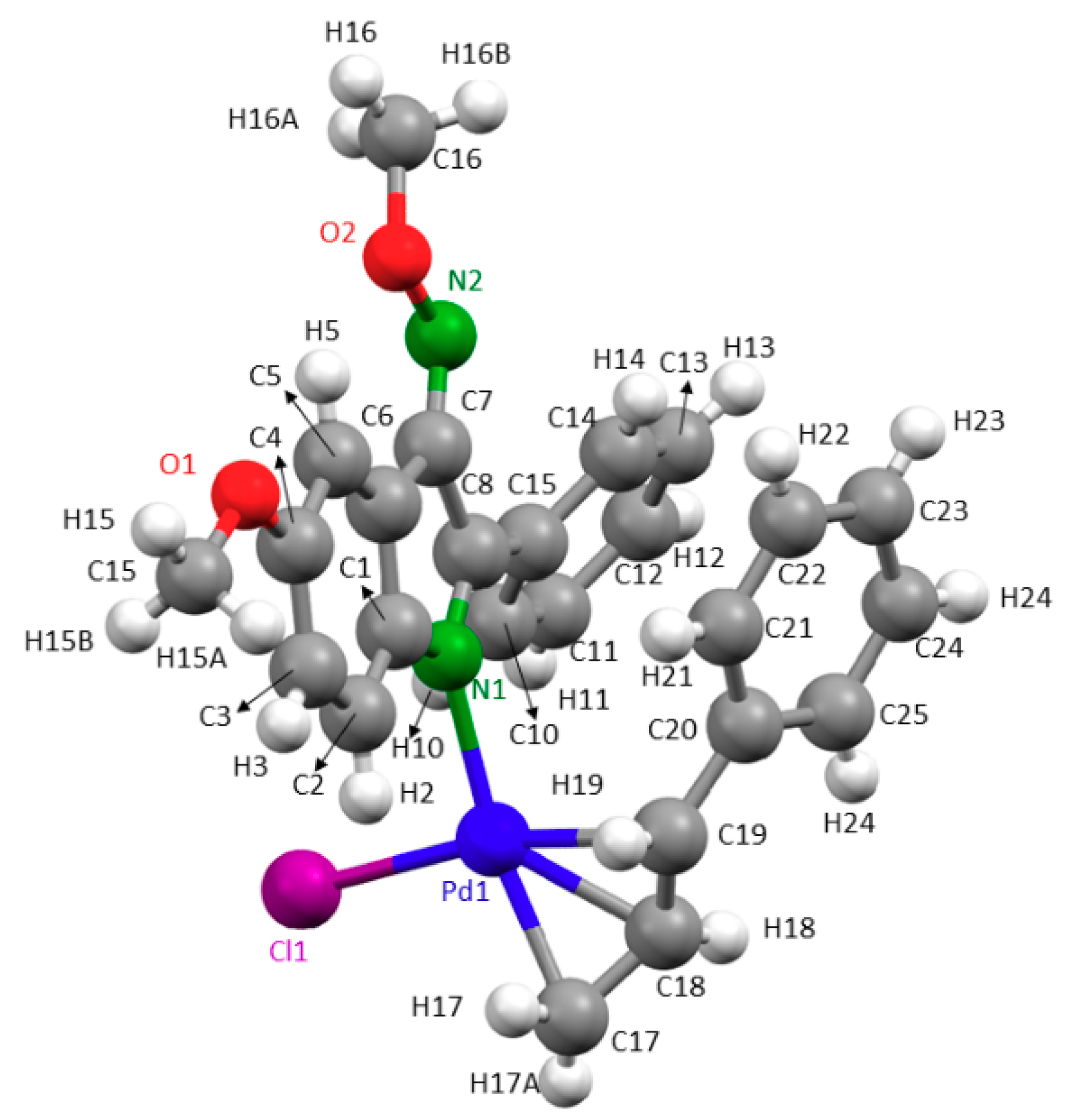
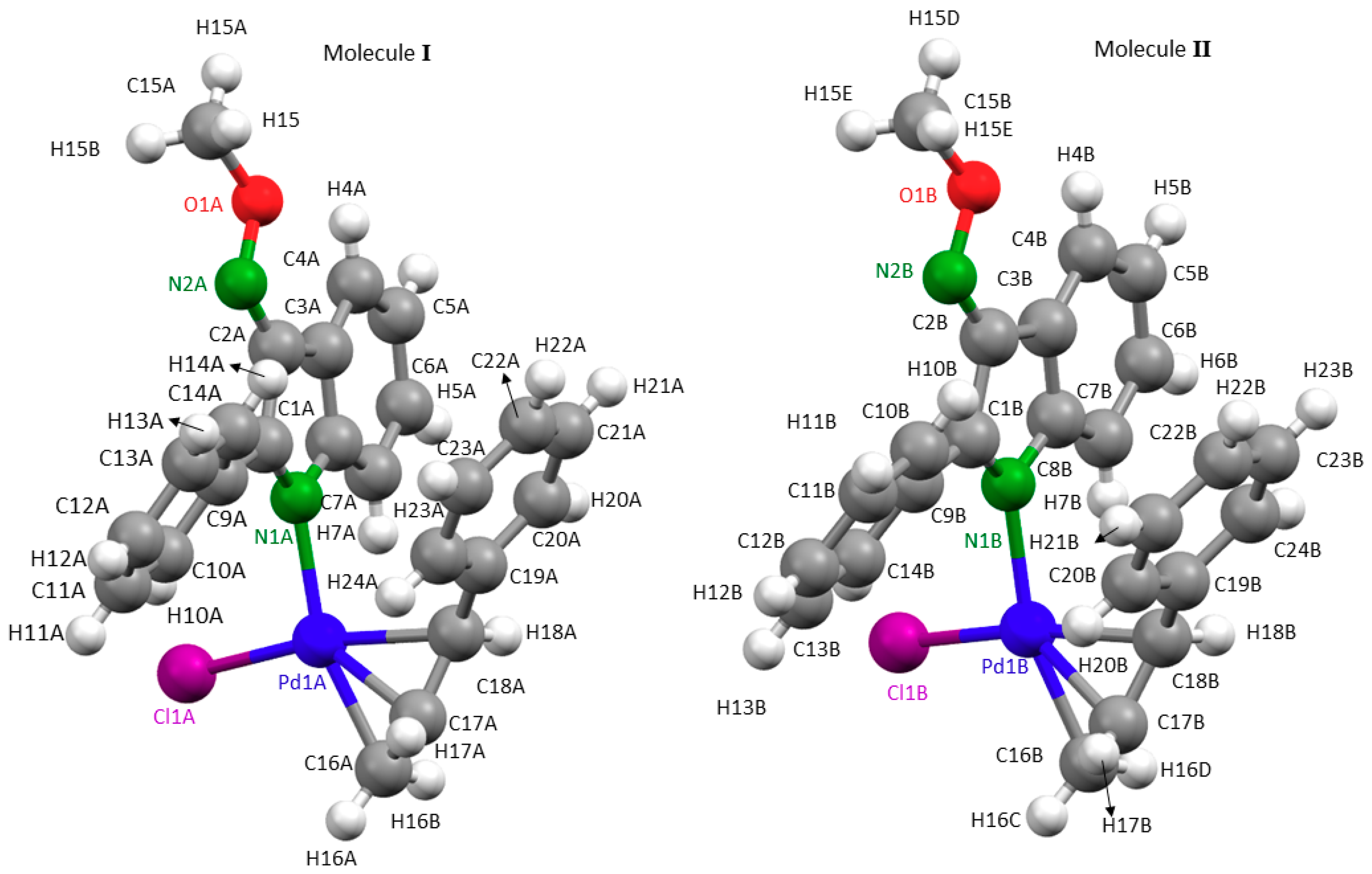
2.2. Description of the Crystal Structures of Compounds 2a, 3a and 3b
2.3. Solution Studies
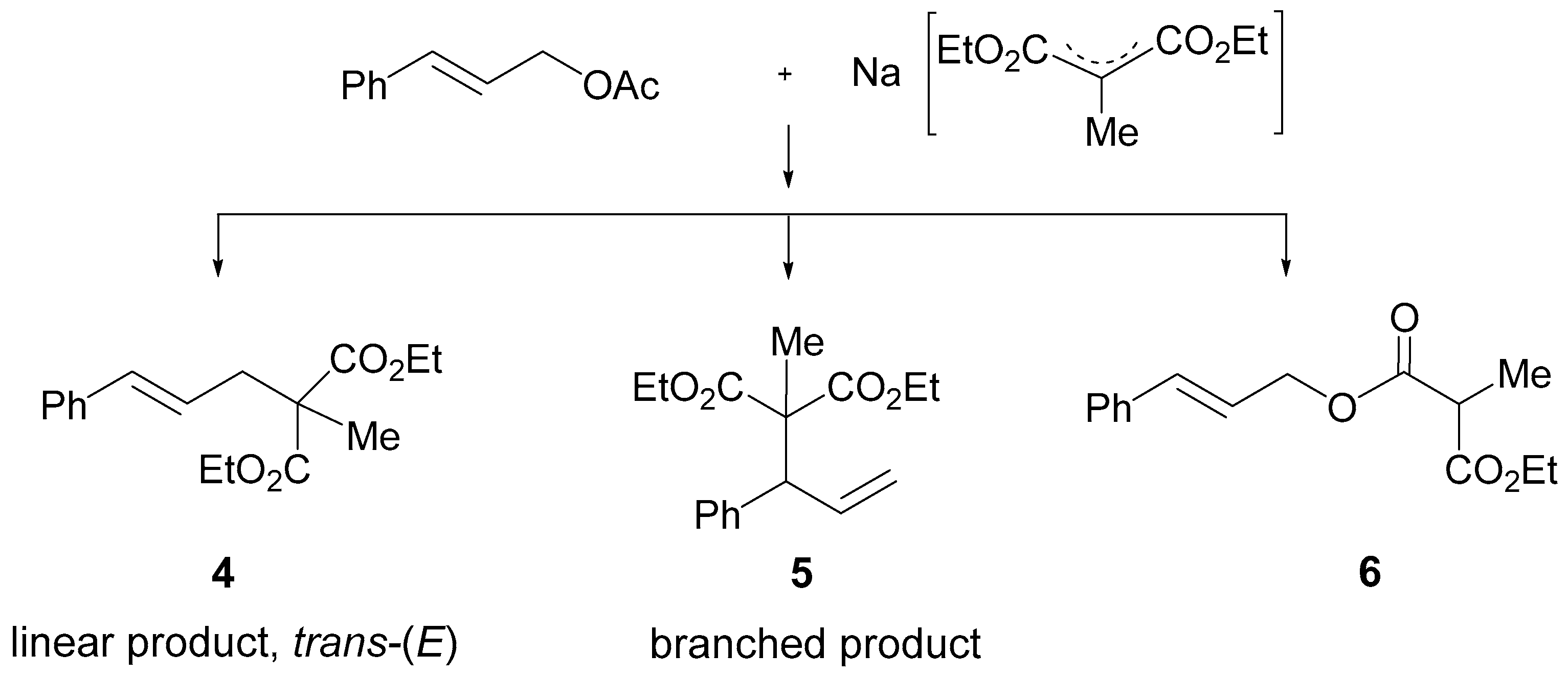
2.3.1. Allylic Alkylation Reactions
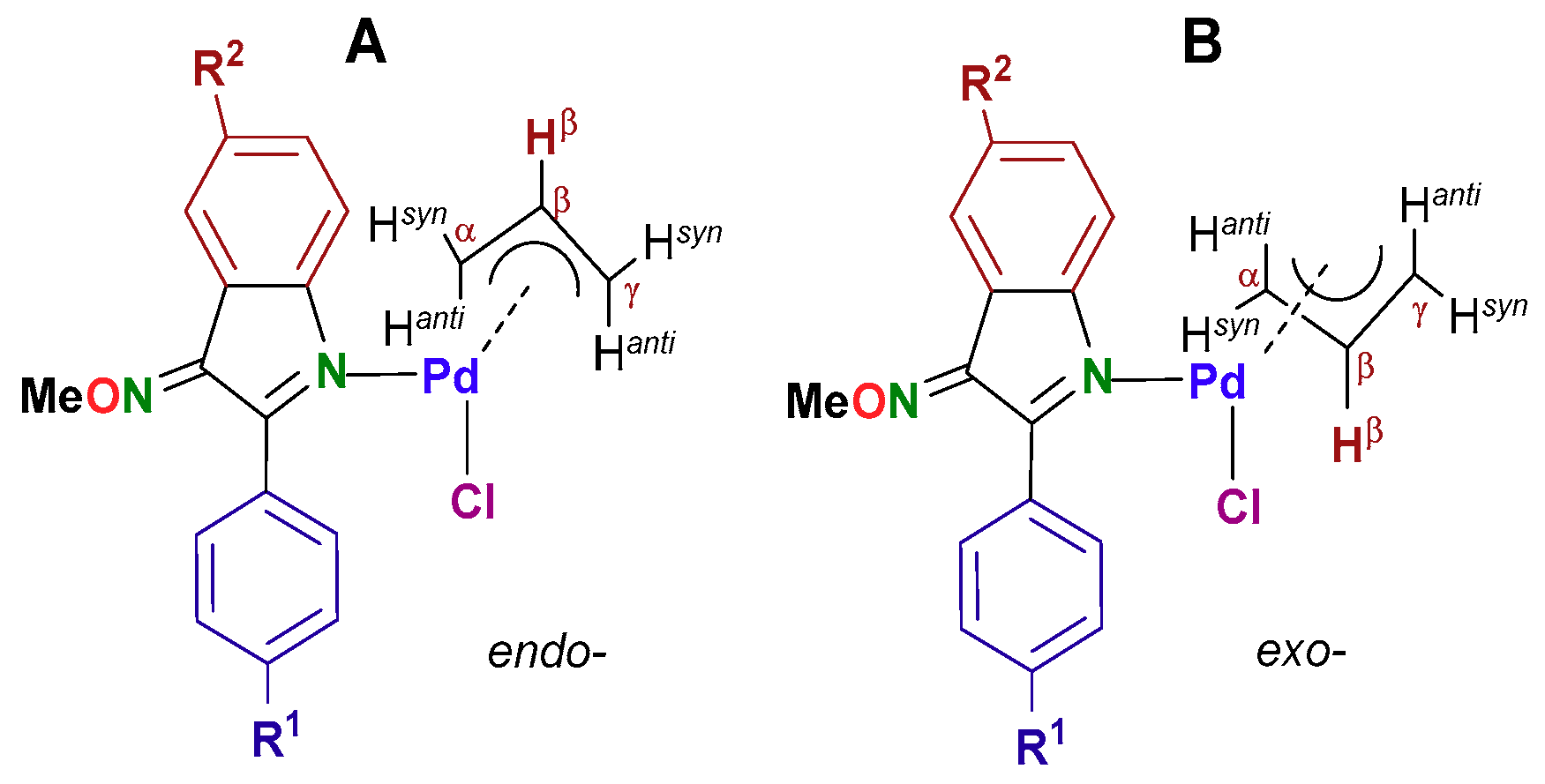
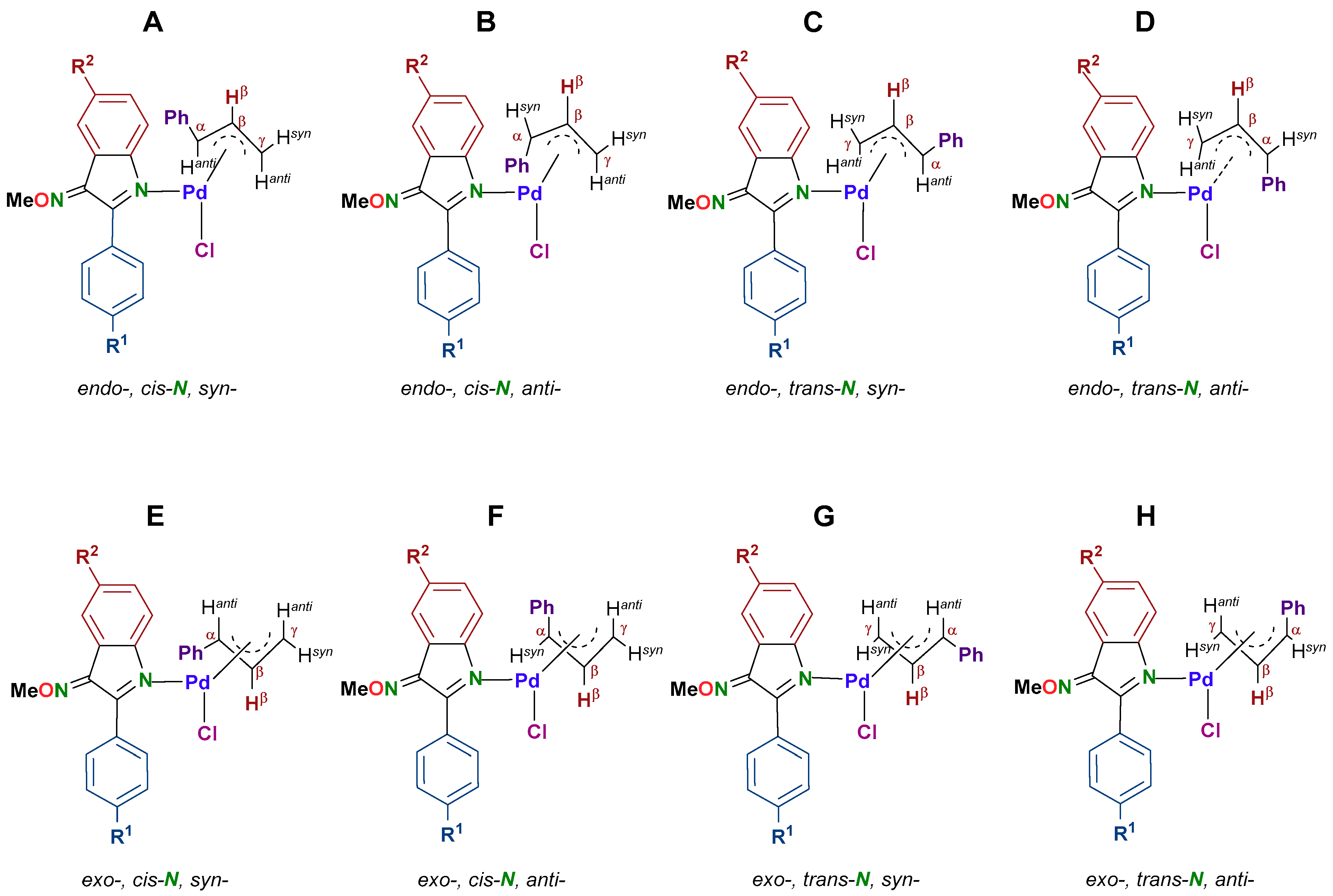
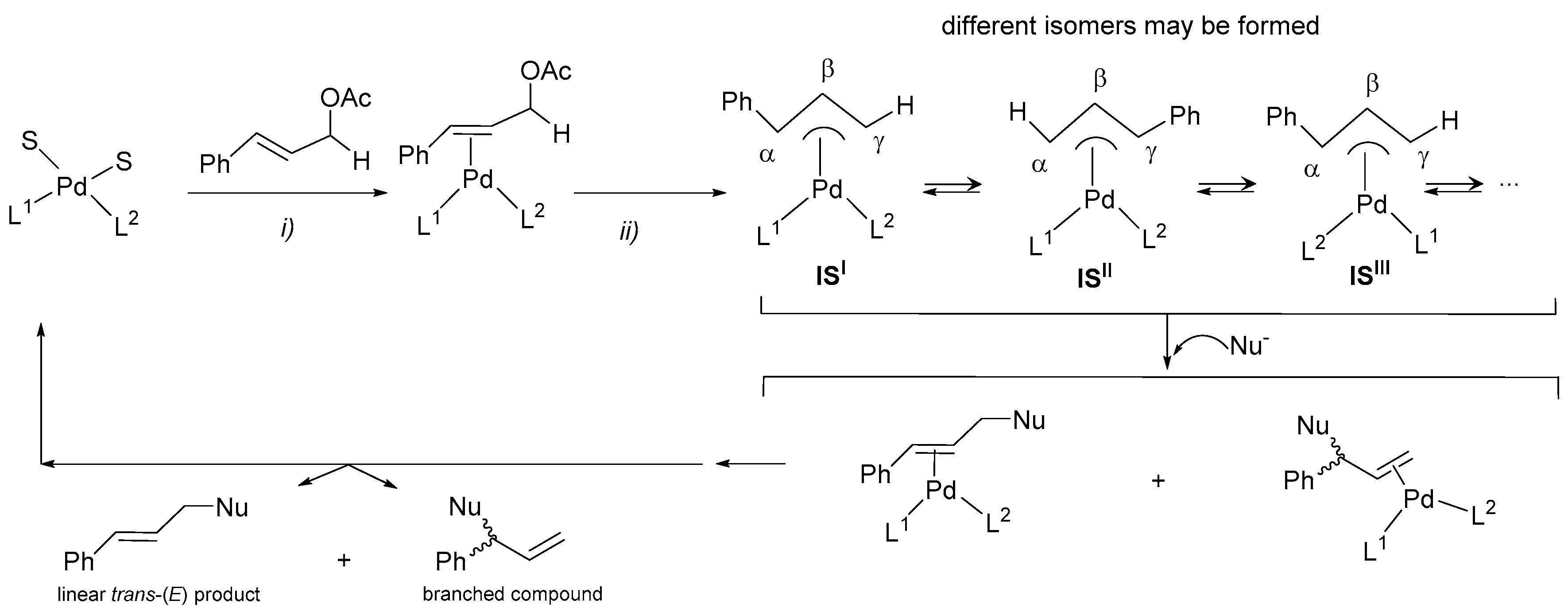
2.3.2. Study of the Solution Behaviour of the New Pd(II) Allyl Compounds
2.4. Computational Studies
3. Experimental Section
3.1. Materials and Methods
3.2. Preparation of the Compounds
3.2.1. General Procedure for the Synthesis of Compounds [Pd(η3-C3H5){C8H3N-2-(C6H4-4-R1)-3-NOMe-5-R2}Cl] [with R1 = H, R2 = OMe (2a); R1 = R2 = H (2b) or R1 = Cl, R2 = H (2c)]
3.2.2. General Procedure for the Synthesis of Compounds [Pd(η3-1-PhC3H4){C8H3N-2-(C6H4-4-R1)-3-NOMe-5-R2}Cl] [with R1 = H, R2 = OMe (3a); R1 = R2 = H (3b) or R1 = Cl, R2 = H (3c)]
3.3. Crystallography
3.4. Typical Procedure for Allylic Alkylation
3.5. Computational Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gribble, W. Heterocyclic Scaffolds II: Reactions and Applications of Indoles, Topics in Heterocyclic Chemistry; Book Series; Springer: Berlin, Germany, 2010; Volume 26. [Google Scholar] [CrossRef]
- Nylund, K.; Johansson, P. Heterocyclic Compounds: Synthesis, Properties and Applications; Chemistry Research and Applied Series; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2010; ISBN 10: 1608763684. [Google Scholar]
- Ghinea, I.O.; Dinica, R.D. Breakthoughts in Indole and Indolizine Chemistry, New Synthetic Pathways, New Applications, in Scope of Selective Heterocycles from Organic and Pharmaceutical Perspective; Varala, V., Ed.; IntechOpen: Rijeka, Croatia, 2016; Chapter 5; pp. 1043–1561. [Google Scholar] [CrossRef]
- Sonsona, I.G. Indole, a Privileged Structural Core Motif. Synlett 2015, 26, 2325–2326. [Google Scholar] [CrossRef][Green Version]
- Ziarani, G.M.; Moradi, R.; Ahmadi, T.; Lashgar, N. Recent advances in the application of indoles in multicomponent reactions. RSC Adv. 2018, 8, 12069–12103. [Google Scholar] [CrossRef]
- Ila, H.; Markiewicz, J.T.; Malakhov, V.; Knochel, P. Metalated Indoles, Indazoles, Benzimidazoles, and Azaindoles and Their Synthetic Applications. Synthesis 2013, 45, 2343–2371. [Google Scholar] [CrossRef]
- Bandini, M. Electrophilicity: The “dark-side” of indole chemistry. Org. Biomol. Chem. 2013, 11, 5206–5212. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.; Kaushik, N.; Attri, P.; Kumar, N. Biomedical Importance of Indole. Molecules 2013, 18, 6620–6666. [Google Scholar] [CrossRef] [PubMed]
- Abbey, E.R.; Liu, S.-Y. BN isosteres of indole. Org. Biomol. Chem. 2013, 11, 2060–2069. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.A.; Al-Omar, M.A.; Raish, M.; Ansari, M.A.; Abuelizz, H.A.; Bakheit, A.H.; Naglah, A.M. Indole Derivatives as Cyclooxygenase Inhibitors: Synthesis, Biological Evaluation and Docking Studies. Molecules 2018, 23, 1250. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, J.S.; Singla, R.; Mayank, J.V. Indole Derivatives as Anticancer Agents for Breast Cancer Therapy: A Review. Anti-Cancer Agents Med. Chem. 2016, 16, 160–173. [Google Scholar] [CrossRef]
- Mollica, A.; Locatelli, M.; Stefanucci, A.; Pinnen, F. Synthesis and Bioactivity of Secondary Metabolites from Marine Sponges Containing Dibrominated Indolic Systems. Molecules 2012, 17, 6083–6099. [Google Scholar] [CrossRef]
- Venkateswararao, A.; Tyagi, P.; Justin, T.K.R.; Chen, P.-W.; Ho, K.-C. Organic dyes containing indolo [2, 3-b]quinoxaline as a donor: Synthesis, optical and photovoltaic properties. Tetrahedron 2014, 70, 6318–6327. [Google Scholar] [CrossRef]
- Babu, D.D.; Gachumale, S.R.; Anandan, S.; Adhikari, A.V. New D-π-A type indole based chromogens for DSSC: Design, synthesis and performance studies. Dyes Pigments 2015, 112, 183–191. [Google Scholar] [CrossRef]
- Makosza, M.; Wojciechowski, K. Synthesis of Heterocycles via Nucleophilic Substitution of Hydrogen in Nitroarenes. Heterocycles 2014, 88, 75–101. [Google Scholar] [CrossRef]
- Zhuo, D.; Sun, J.; Yan, C.G. One-pot synthesis of 6, 11-dihydro-5H-indolizino[8,7-b]indoles via sequential formation of β-enamino ester, Michael addition and Pictet–Spengler reactions. RSC Adv. 2014, 4, 62817–62828. [Google Scholar] [CrossRef]
- Gosh, S.K.; Nagaraya, R. Total synthesis of cruciferane via epoxidation/tandem cyclization sequence. RSC Adv. 2014, 4, 63147–63149. [Google Scholar] [CrossRef]
- Shimazaki, Y.; Yajima, T.; Takani, M.; Yamauch, O. Metal complexes involving indole rings, structures and effects of metal-indole interactions. Coord. Chem Rev. 2009, 253, 479–492. [Google Scholar] [CrossRef]
- Khaledi, H.; Ali, H.M.; Olmstead, M.M. Versatile Coordination Modes of 2-(Diformylmethylene)-3,3-dimethylindole towards Late-Transition-Metal Ions: C–H Bond Activation and Formation of Cyclic Acyl–Palladium(II) Complexes. Eur. J. Inorg. Chem. 2011, 2011, 2394–2404. [Google Scholar] [CrossRef]
- Khaledi, H.; Olmstead, M.M.; Ali, H.M.; Thomas, N.F. Indolenine meso-Substituted Dibenzotetra-aza[14]annulene and Its Coordination Chemistry toward the Transition Metal Ions MnIII, FeIII, CoII, NiII, CuII, and PdII. Inorg. Chem. 2013, 52, 1926–1941. [Google Scholar] [CrossRef]
- Tomé, M.; López, C.; González, A.; Ozay, B.; Quirante, J.; Font-Bardía, M.; Calvet, T.; Calvis, C.; Messeguer, R.; Baldomà, L.; et al. Trans-and cis-2-phenylindole platinum(II) complexes as cytotoxic agents against human breast adenocarcinoma cell lines. J. Mol. Struct. 2013, 1048, 88–97. [Google Scholar] [CrossRef]
- Belsa, L.; López, C.; González, A.; Font-Bardía, M.; Calvet, T.; Calvis, C.; Messeguer, R. Neutral and Ionic Cycloruthenated 2-Phenylindoles as Cytotoxic Agents. Organometallics 2013, 32, 7264–7267. [Google Scholar] [CrossRef]
- López, C.; González, A.; Moya, C.; Bosque, R.; Solans, X.; Font-Bardía, M. Cyclopalladation of 3-methoxyimino-2-phenyl-3H-indoles. J. Organomet. Chem. 2008, 693, 2877–2886. [Google Scholar] [CrossRef]
- Li, J.J.; Gribble, G.W. Palladium in Heterocyclic Chemistry; Pergamon: New York, NY, USA, 2000. [Google Scholar]
- Joule, J.A.; Mills, K. Heterocyclic Chemistry at a Glance; Chapters 4 Palladium in Heterocyclic Chemistry; Chapters 10 Indoles; Wiley: Hoboken, NJ, USA, 2012; pp. 21–32, 86–98. [Google Scholar] [CrossRef]
- Trost, B.M. Catalysis: Unlimited frontiers. Our early personal journey into the world of palladium, Topics in Organometallic Chemistry. In Inventing Reactions; Springer: Heildelberg, Germany, 2013; Volume 44, pp. 1–12. [Google Scholar] [CrossRef]
- Brase, S. Organopalladium Chemistry, in Organometallics in Synthesis: Third Manual; Schlosser, M., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 777–1000. [Google Scholar] [CrossRef]
- Crabtree, R.H.; Mingos, D.P. Comprehensive Organometallic Chemistry; Elsevier Ltd: Oxford, UK, 2007; Volume 8, Chapter 6; pp. 358–371. [Google Scholar]
- Canovese, L.; Visentin, F.; Levi, C.; Dolmella, A. Synthesis, characterization, dynamics and reactivity toward amination of η3-allyl palladium complexes bearing mixed ancillary ligands. Evaluation of the electronic characteristics of the ligands from kinetic data. Dalton Trans. 2011, 40, 966–981. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sha, S.-C.; Zhang, J.; Carroll, P.J.; Walsh, P.J. Raising the pKa Limit of “Soft” Nucleophiles in Palladium-Catalyzed Allylic Substitutions: Application of Diarylmethane Pronucleophiles. J. Am. Chem. Soc. 2013, 135, 17602–17609. [Google Scholar] [CrossRef] [PubMed]
- Canovese, L.; Visentin, F.; Santo, C.; Bertolasi, V. Insertion of Isocyanides across the Pd–C Bond of Phosphinoquinoline Allyl Palladium Complexes Bearing η1-and η3-Coordinated Allyl Groups. A Synthetic and Mechanistic Study. Organometallics 2014, 33, 1700–1709. [Google Scholar] [CrossRef]
- Pérez, S.; López, C.; Bosque, R.; Solans, X.; Font-Bardía, M.; Roig, A.; Molins, E.; van Leeuwen, P.W.N.M.; van Strijdonck, G.P.F.; Freixa, Z. Heterodimetallic Palladium(II) Complexes with Bidentate (N,S) or Terdentate (C,N,S)-Ferrocenyl Ligands. The Effect of the Ligand Donor Atoms on the Regioselectivity of the Allylic Alkylation of Cinnamyl Acetate. Organometallics 2008, 27, 4288–4299. [Google Scholar] [CrossRef]
- Platero-Prats, A.E.; Pérez, S.; López, C.; Solans, X.; Font-Bardía, M.; van Leeuwen, P.W.N.M.; van Strijdonck, G.P.F.; Freixa, Z. Palladium(II)–allyl complexes containing chiral N-donor ferrocenyl ligand. J. Organomet. Chem. 2007, 692, 4215–4226. [Google Scholar] [CrossRef]
- Li, M.-B.; Wang, Y.; Tian, S.-K. Regioselective and Stereospecific Cross-Coupling of Primary Allylic Amines with Boronic Acids and Boronates through PalladiumCatalyzed C−N Bond Cleavage. Angew. Chem. Int. Ed. 2012, 51, 2968–2971. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-B.; Li, H.; Wang, J.; Liu, C.-R.; Tian, S.-K. Catalytic stereospecific alkylation of malononitriles with enantioenriched primary allylic amines. Chem. Commun. 2013, 49, 8190–8192. [Google Scholar] [CrossRef] [PubMed]
- Szafran, Z.; Pike, P.E.; Singh, M.M. Microscale Inorganic Chemistry: A Comprehensive Laboratory Experience; John Wiley & Sons: New York, NY, USA, 1991. [Google Scholar]
- Auburn, P.R.; Mackenzie, P.B.; Bosnich, B. Asymmetric synthesis. Asymmetric catalytic allylation using palladium chiral phosphine complexes. J. Am. Chem. Soc. 1985, 107, 2033–2046. [Google Scholar] [CrossRef]
- Cambridge Crystallographic Data Centre (CCDC). Available online: http://www.ccdc.cam.uk/data (accessed on 20 June 2019).
- Allen, F.H. The Cambridge Structural Database: a quarter of a million crystal structures and rising. Acta Crystallogr. Sect. B Struct. Sci. 2002, 58, 380–388. [Google Scholar] [CrossRef]
- Simpson, P.V.; Skelton, B.W.; Brown, D.H.; Baker, M.V. Synthesis and Characterisation of Mono-and Bidentate Alkoxybenzimidazolin-2-ylidene Palladium Complexes: Interesting Solution Behaviour and Application in Catalysis. Eur. J. Inorg. Chem. 2011, 2011, 1937–1952. [Google Scholar] [CrossRef]
- Bettucci, L.; Bianchini, C.; Filippi, J.; Lavacchi, A.; Oberhauser, W. Chemoselective Aerobic Diol Oxidation by Palladium(II)–Pyridine Catalysis. Eur. J. Inorg. Chem. 2011, 2011, 1797–1805. [Google Scholar] [CrossRef]
- Trost, B.M. Asymmetric Allylic Alkylation, an Enabling Methodology. J. Org. Chem. 2004, 69, 5813–5837. [Google Scholar] [CrossRef] [PubMed]
- Colacot, T.J. A concise update on the applications of chiral ferrocenyl phosphines in homogeneous catalysis leading to organic synthesis. Chem. Rev. 2003, 103, 3101–3118. [Google Scholar] [CrossRef] [PubMed]
- Helmchen, G.; Ernst, M.; Paradies, G. Application of allylic substitutions in natural products synthesis. Pure Appl. Chem. 2004, 76, 495–505. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 (Revission B.1); Gaussian Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Perrin, D.D. Purification of Laboratory Chemicals, 4th ed.; Butterworth-Heinemann: Oxford, UK, 1996. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. Influence of polarization functions on molecular-orbital hydrogenation energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Francl, M.M.; Petro, W.J.; Hehre, W.J.; Binkley, J.S.; Gordon, M.S.; DeFrees, D.J.; Pople, J.A. Self-Consistent Molecular Orbital Methods. 23. A polarization-type basis set for 2nd-row elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef]

| 2a | 3a | 3b | |
|---|---|---|---|
| Empirical formula | C19H19ClN2O2Pd | C25H23ClN2O2Pd | C24H21ClN2OPd |
| Formula weight | 449.21 | 525.30 | 495.28 |
| Crystal sizes/mm × mm × mm | 0.20 × 0.10 × 0.10 | 0.20 × 0.10 × 0.10 | 0.20 × 0.10 × 0.12 |
| Crystal system | Monoclinic | Monoclinic | Triclinic |
| Space group | C2/c | P21/c | P-1 |
| a/Å | 18.953(10) | 14.461(4) | 9.813(3) |
| b/Å | 7.137(3) | 15.481(4) | 14.180(4) |
| c/Å | 28.722(8) | 10.096(4) | 16.556(3) |
| α/deg. | 90.0 | 90.0 | 98.97(2) |
| β/deg. | 108.87(3) | 97.08(2) | 103.94(2) |
| γ/deg. | 90.0 | 90.0 | 98.88(2) |
| T/K | 293(2) | 293(2) | 293(2) |
| λ/Å | 0.71073 | 0.71073 | 0.71073 |
| V/Å3 | 3676(3) | 2243.0(12) | 2163.8(10) |
| Z | 8 | 4 | 4 |
| Dcalc/mg × m3 | 1.623 | 1.556 | 1.520 |
| F(000) | 1808 | 1064 | 1000 |
| μ/mm−1 | 1.169 | 0.971 | 0.998 |
| Θ range for data collection/deg. | from 2.271 to 32.345 | from 2.677 to 30.875 | from 1.761 to 30.361 |
| N. of collected reflections | 3861 | 19380 | 18112 |
| N. of unique reflections, [Rint] | 3866 [0.0543] | 5572 [0.0275] | 9882 [0.0207] |
| N. of parameters/N. of restrains | 214/6 | 283/0 | 524/0 |
| R indices, [I > 2 σ(I)] | R1 = 0.0534, wR2 = 0.1652 | R1 = 0.0428, wR2 = 0.1013 | R1 = 0.0424, wR2 = 0.1049 |
| R indices (all data) | R1 = 0.0608, wR2 = 0.1719 | R1 = 0.0446, wR2 = 0.1030 | R1 = 0.0462, wR2 = 0.1097 |
| Entry 1 | Ligand | T (h) | Conversion 2 (%) | Molar Ratio 3 4:5:6 |
|---|---|---|---|---|
| I | 1a | 24 | 89 | 76:6:18 |
| II | 1b | 24 | 87 | 74:7:19 |
| III | 1c | 24 | 91 | 83:7:10 |
| IV | 1a | 96 | >99 | 88:8:4 |
| V | 1b | 96 | >99 | 91:6:2 |
| VI | 1c | 96 | >99 | 92:9:0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomé, M.; Grabulosa, A.; Rocamora, M.; Aullón, G.; Font-Bardía, M.; Calvet, T.; López, C. Synthesis, Characterization, Solution Behavior and Theoretical Studies of Pd(II) Allyl Complexes with 2-Phenyl-3H-indoles as Ligands. Catalysts 2019, 9, 811. https://doi.org/10.3390/catal9100811
Tomé M, Grabulosa A, Rocamora M, Aullón G, Font-Bardía M, Calvet T, López C. Synthesis, Characterization, Solution Behavior and Theoretical Studies of Pd(II) Allyl Complexes with 2-Phenyl-3H-indoles as Ligands. Catalysts. 2019; 9(10):811. https://doi.org/10.3390/catal9100811
Chicago/Turabian StyleTomé, Maria, Arnald Grabulosa, Mercè Rocamora, Gabriel Aullón, Mercè Font-Bardía, Teresa Calvet, and Concepción López. 2019. "Synthesis, Characterization, Solution Behavior and Theoretical Studies of Pd(II) Allyl Complexes with 2-Phenyl-3H-indoles as Ligands" Catalysts 9, no. 10: 811. https://doi.org/10.3390/catal9100811
APA StyleTomé, M., Grabulosa, A., Rocamora, M., Aullón, G., Font-Bardía, M., Calvet, T., & López, C. (2019). Synthesis, Characterization, Solution Behavior and Theoretical Studies of Pd(II) Allyl Complexes with 2-Phenyl-3H-indoles as Ligands. Catalysts, 9(10), 811. https://doi.org/10.3390/catal9100811






