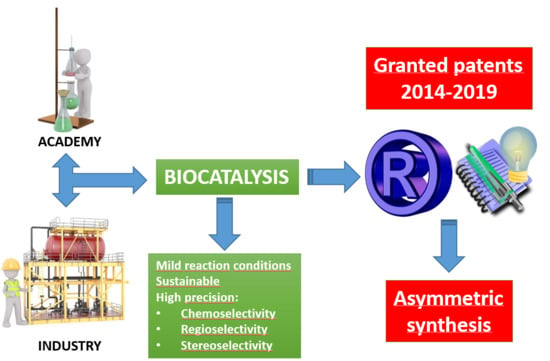
Biocatalysis as Useful Tool in Asymmetric Synthesis: An Assessment of Recently Granted Patents (2014–2019)
Abstract
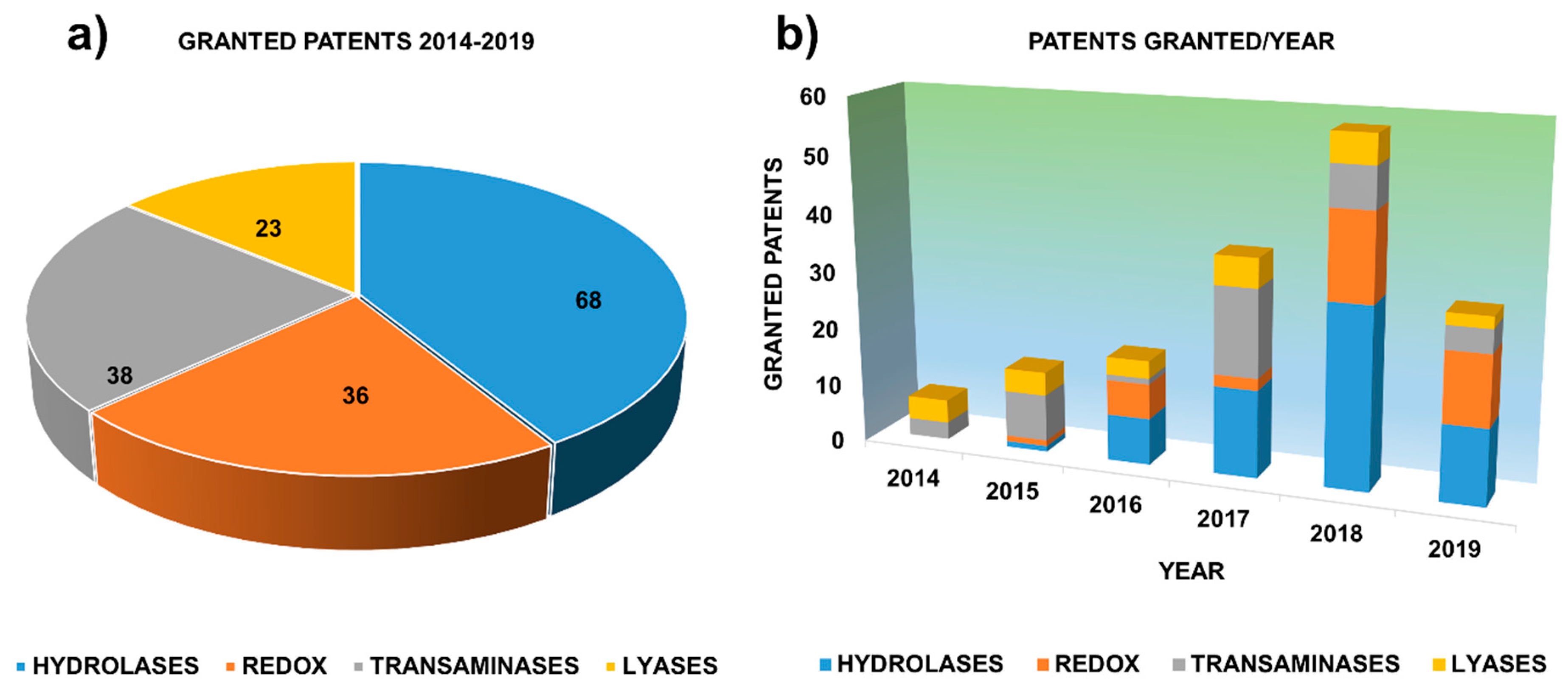
1. Motivation. The Role of Granted Patents as Key Actors in Innovation
2. Granted Patents Related to Hydrolases in Asymmetric Synthesis
2.1. Lipases
2.1.1. Stereoselective Hydrolysis Catalyzed by Lipases
2.1.2. Regio- and Chemoselective Hydrolysis Catalyzed by Lipases
2.1.3. Stereoselective Acyl-Transfer Catalyzed by Lipases
2.1.4. Site-selective Acyl-Transfer Catalyzed by Lipases
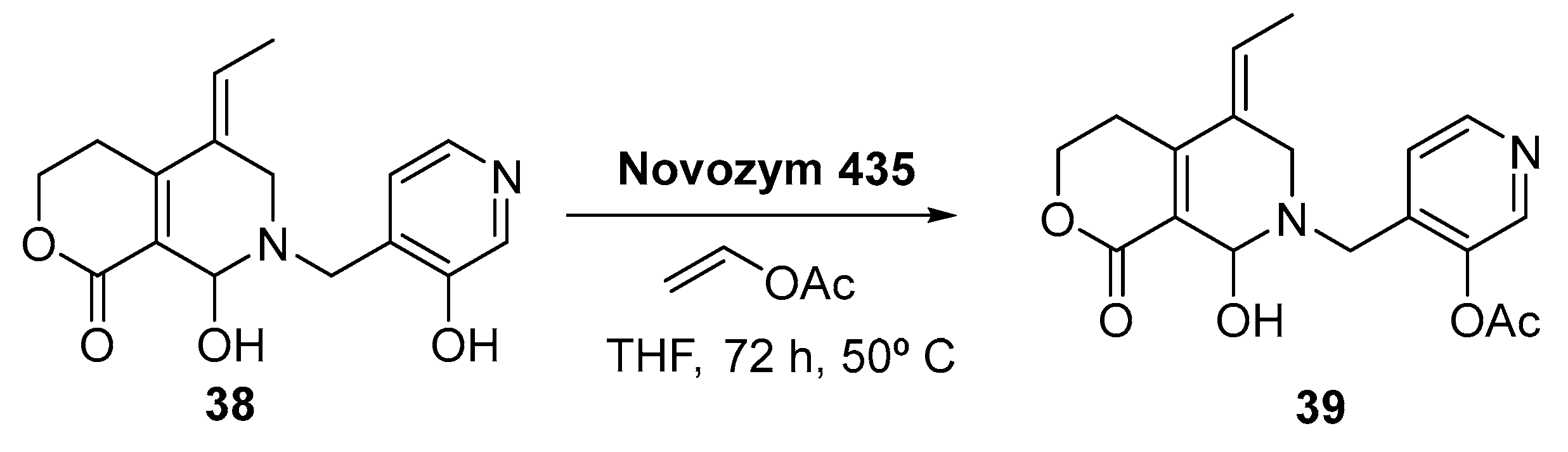
2.1.5. Other Recently-Granted Patents Using Lipases
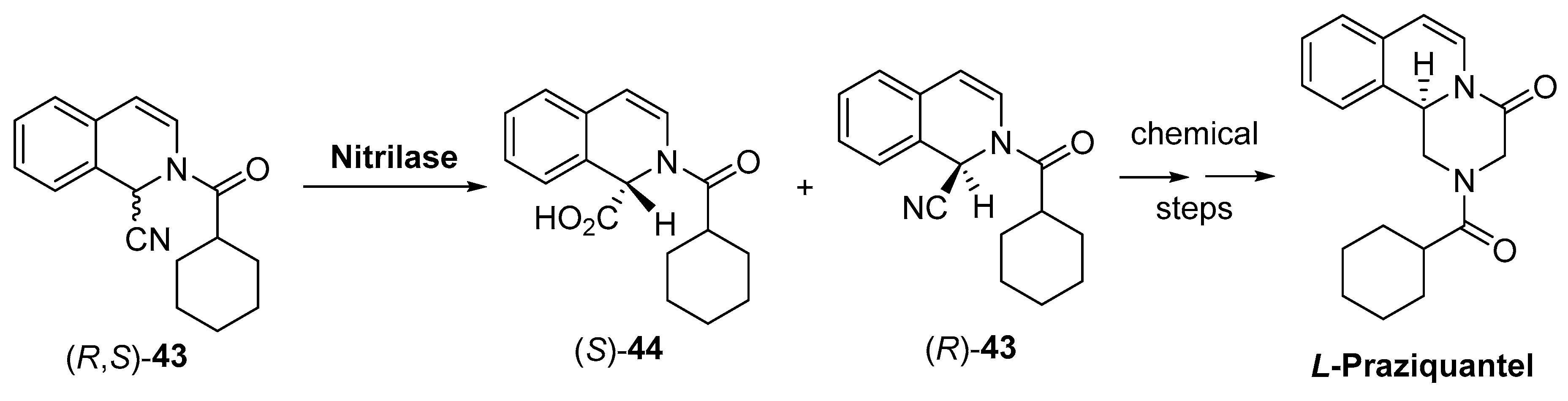
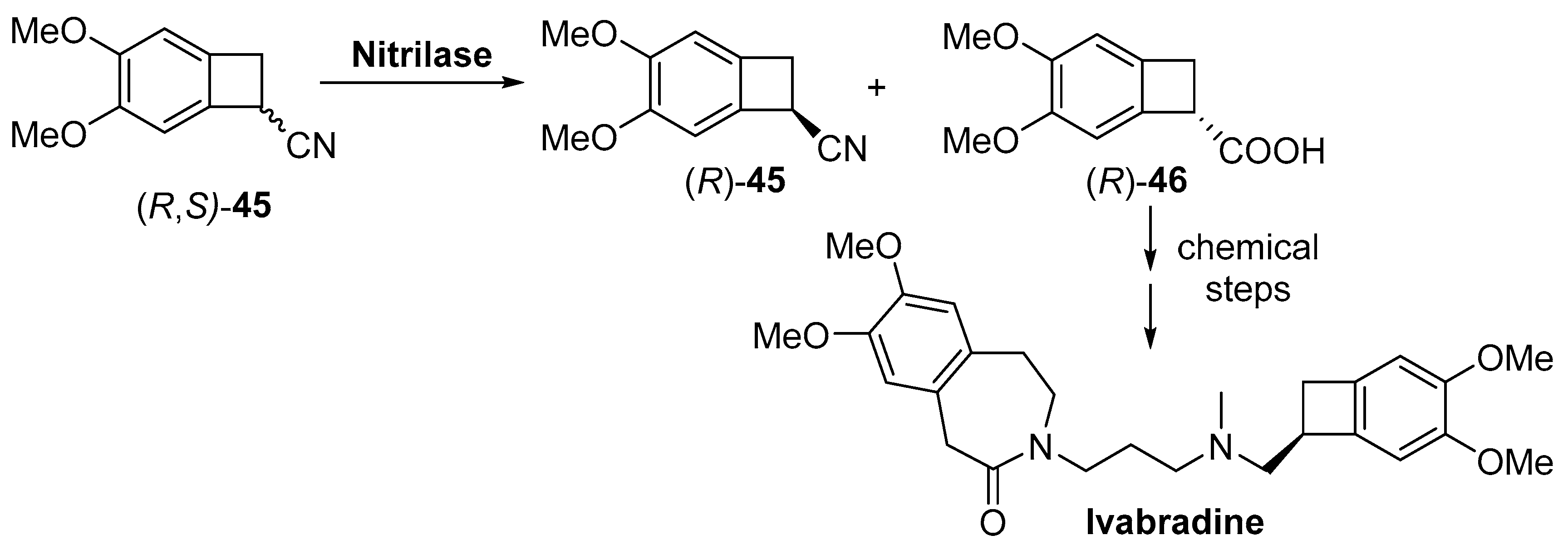
2.2. Other Hydrolases
3. Granted Patents Related to Oxidoreductases in Asymmetric Synthesis
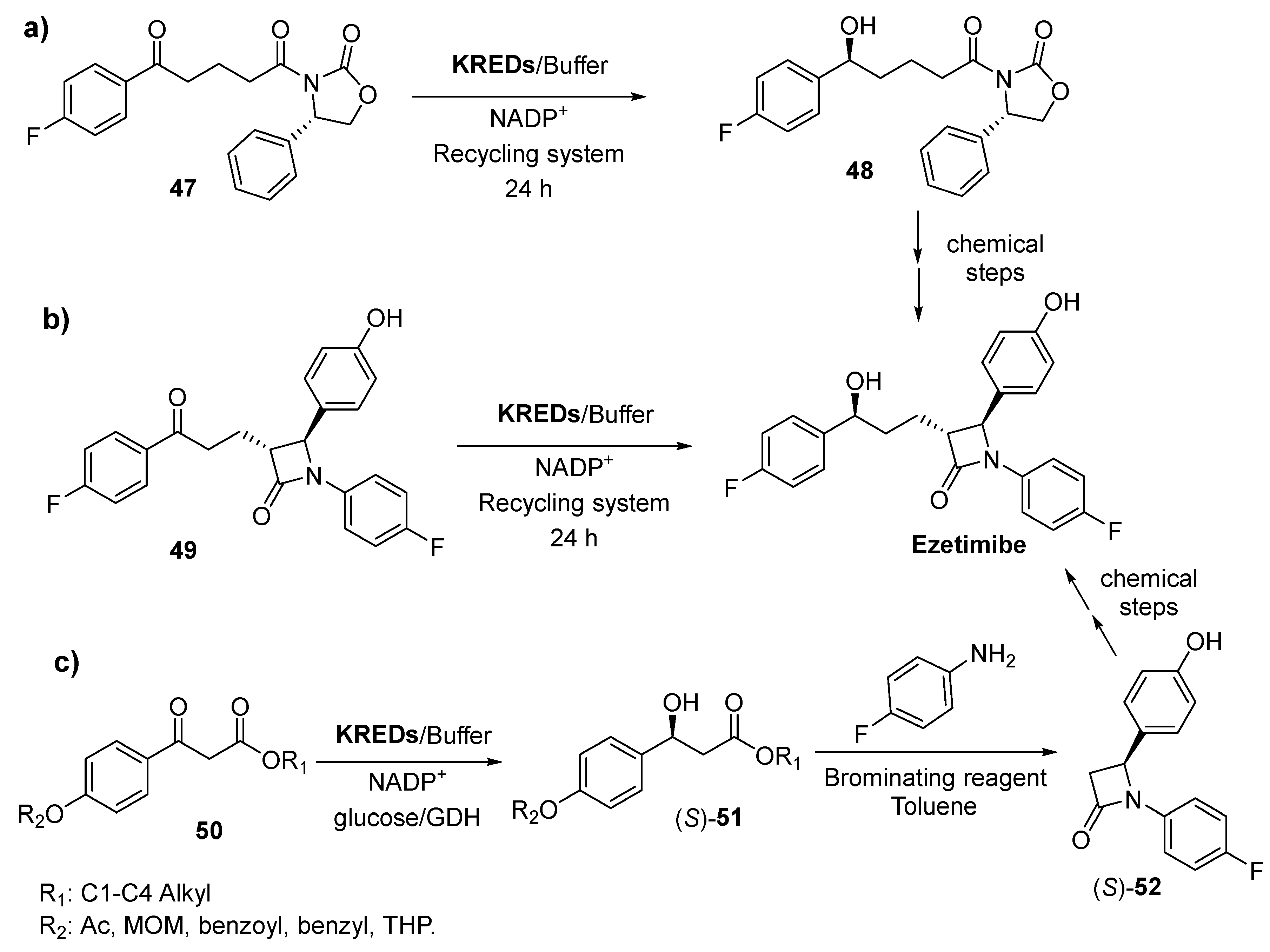
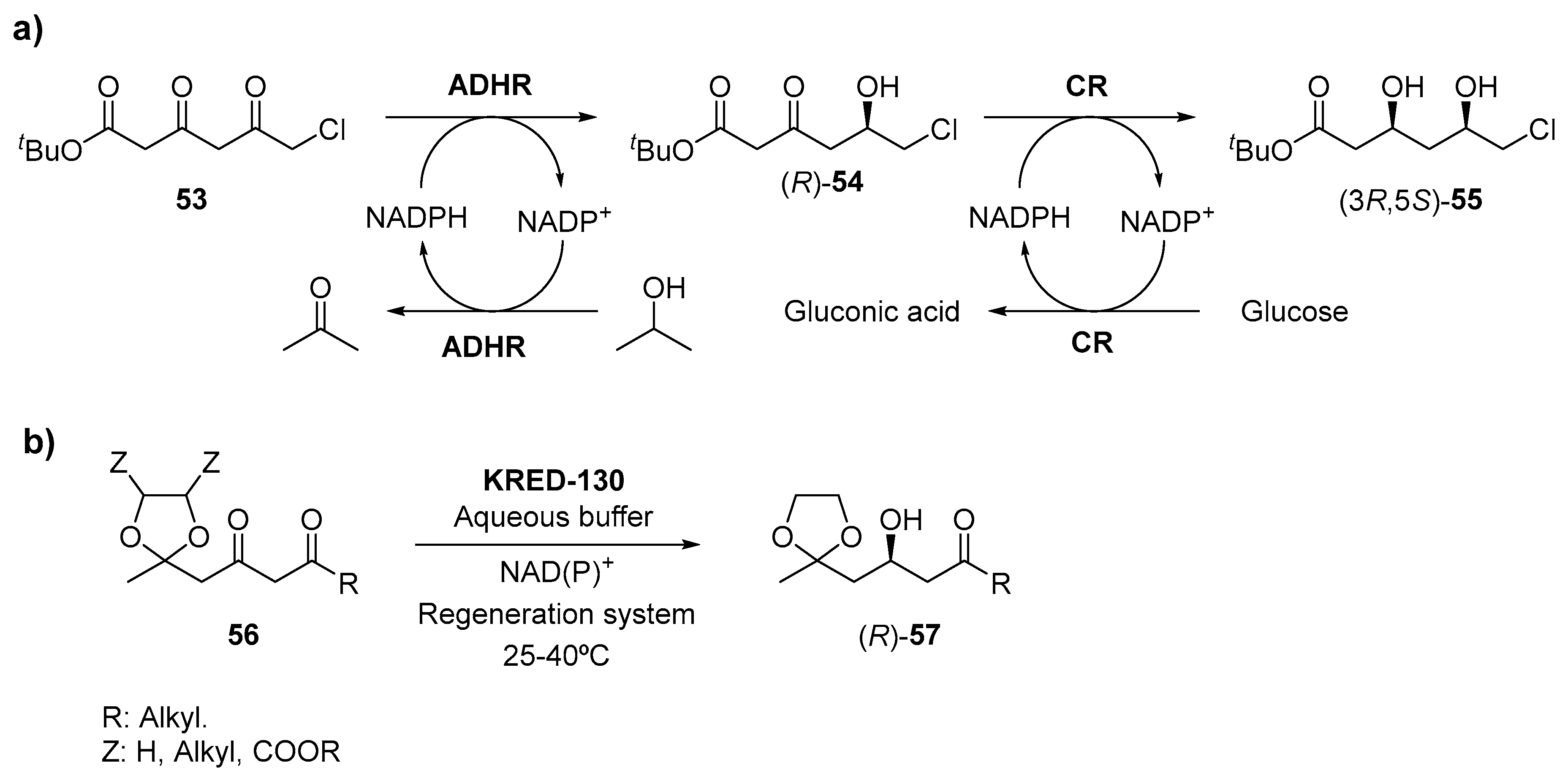
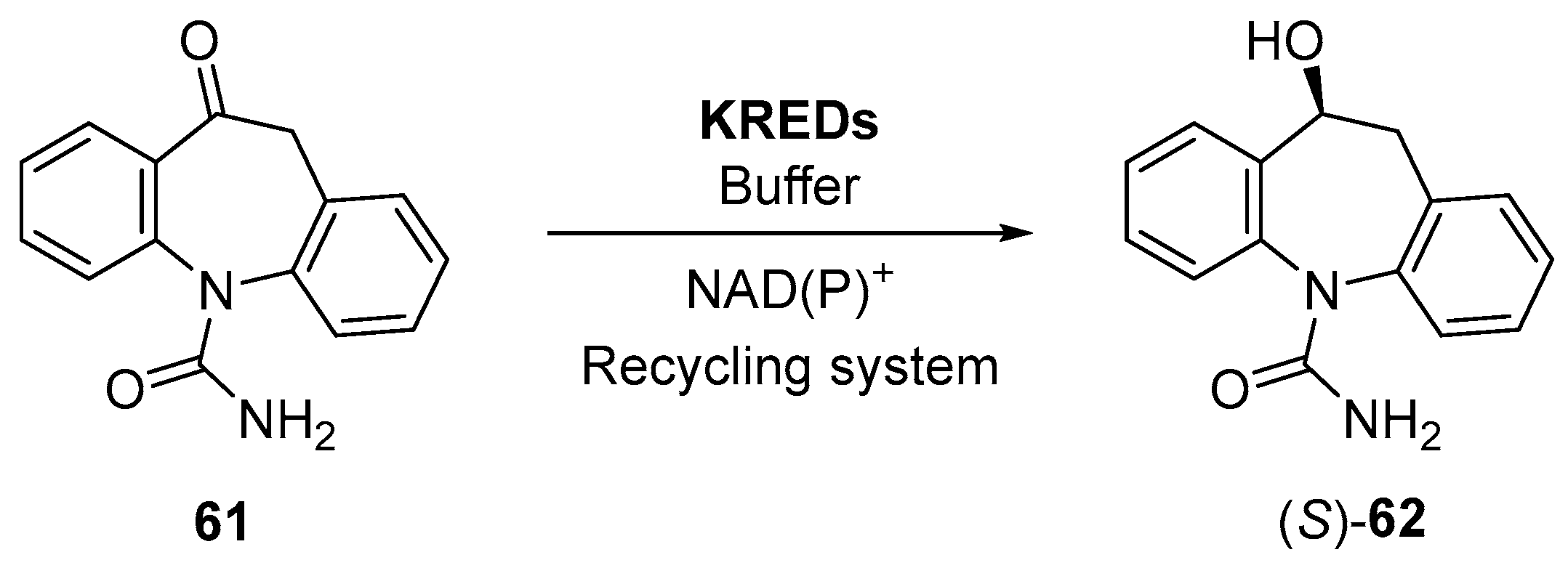
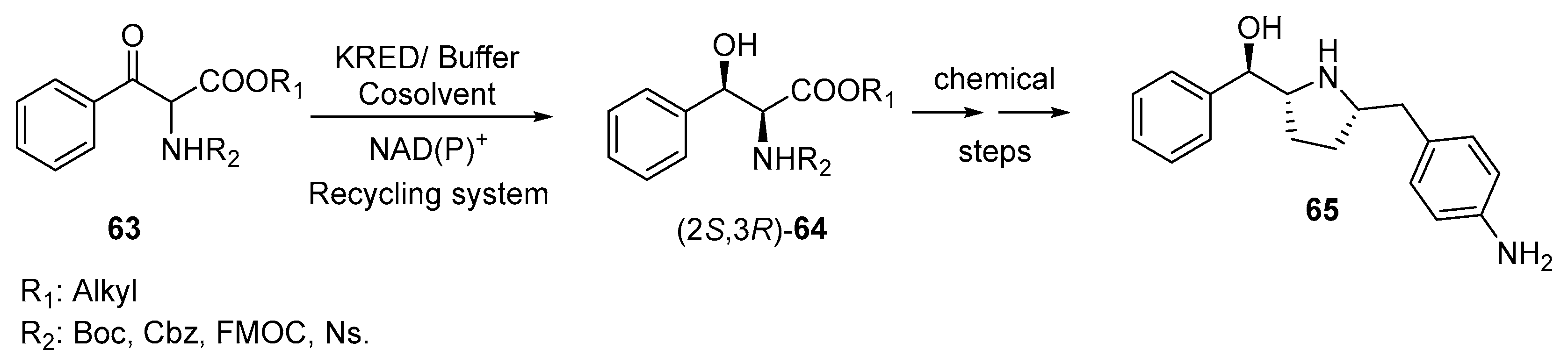
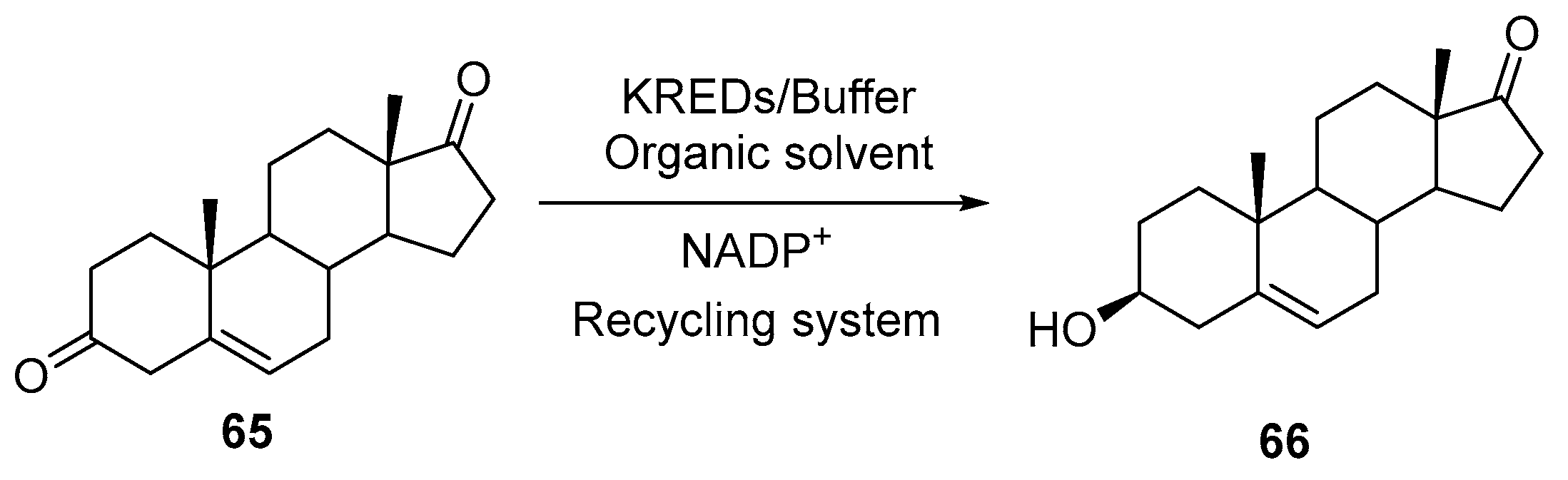
3.1. Ketone Reductions
3.2. Imine Reductions
3.3. Oxidations
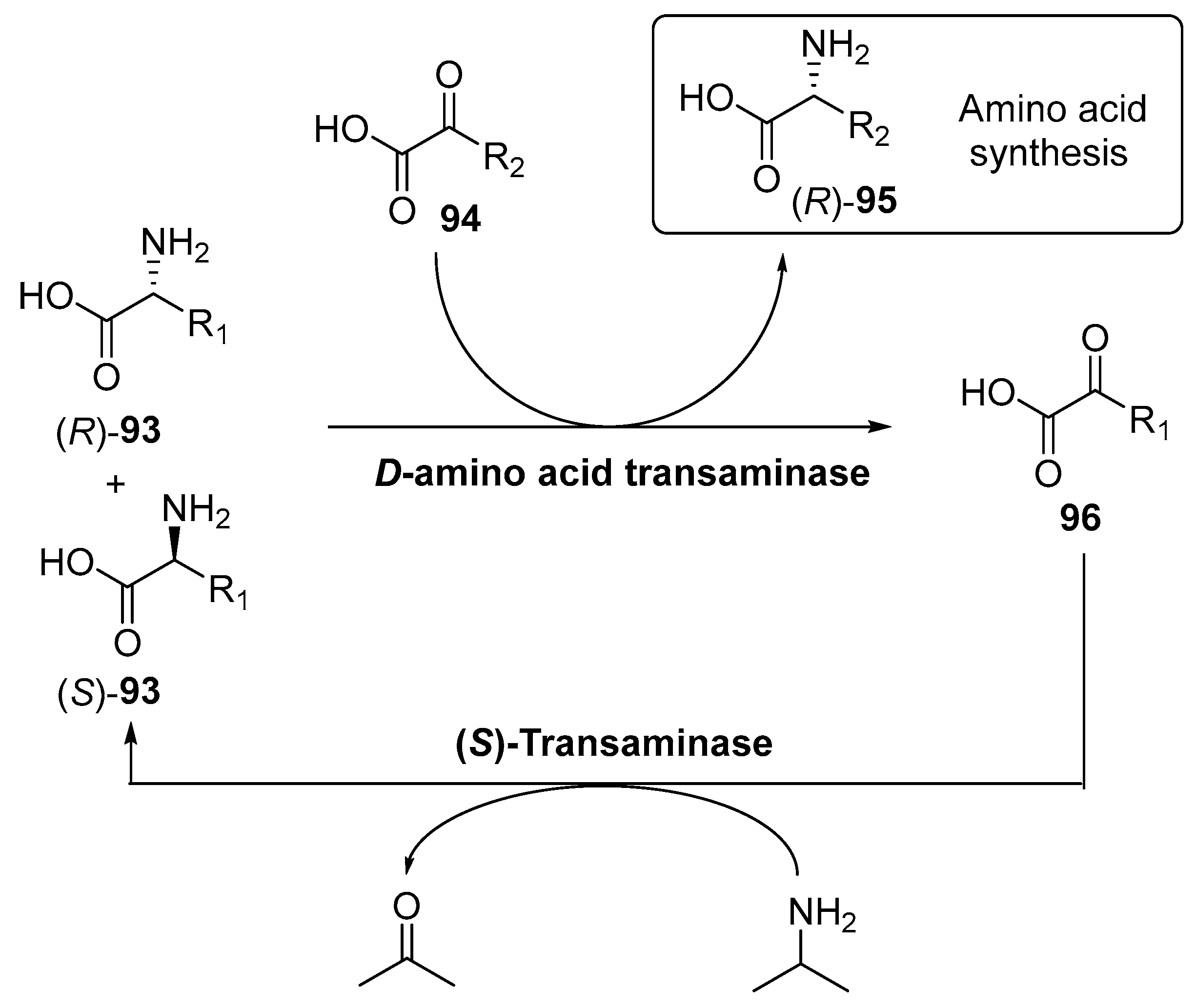
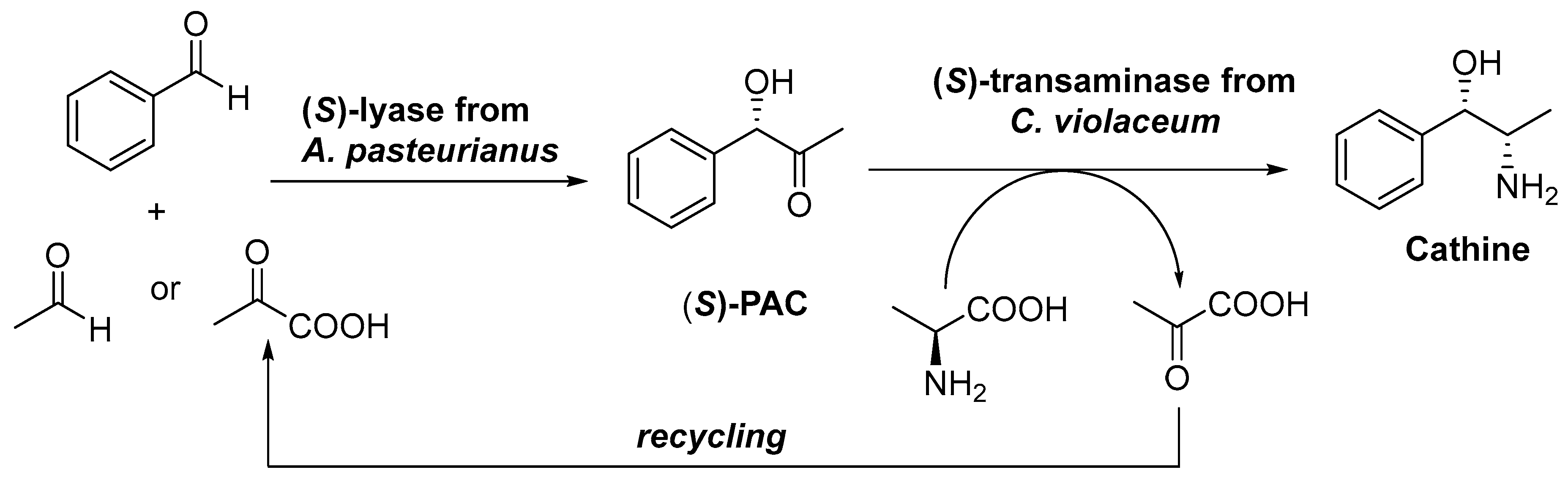
4. Granted Patents Related to Transaminases in Asymmetric Synthesis
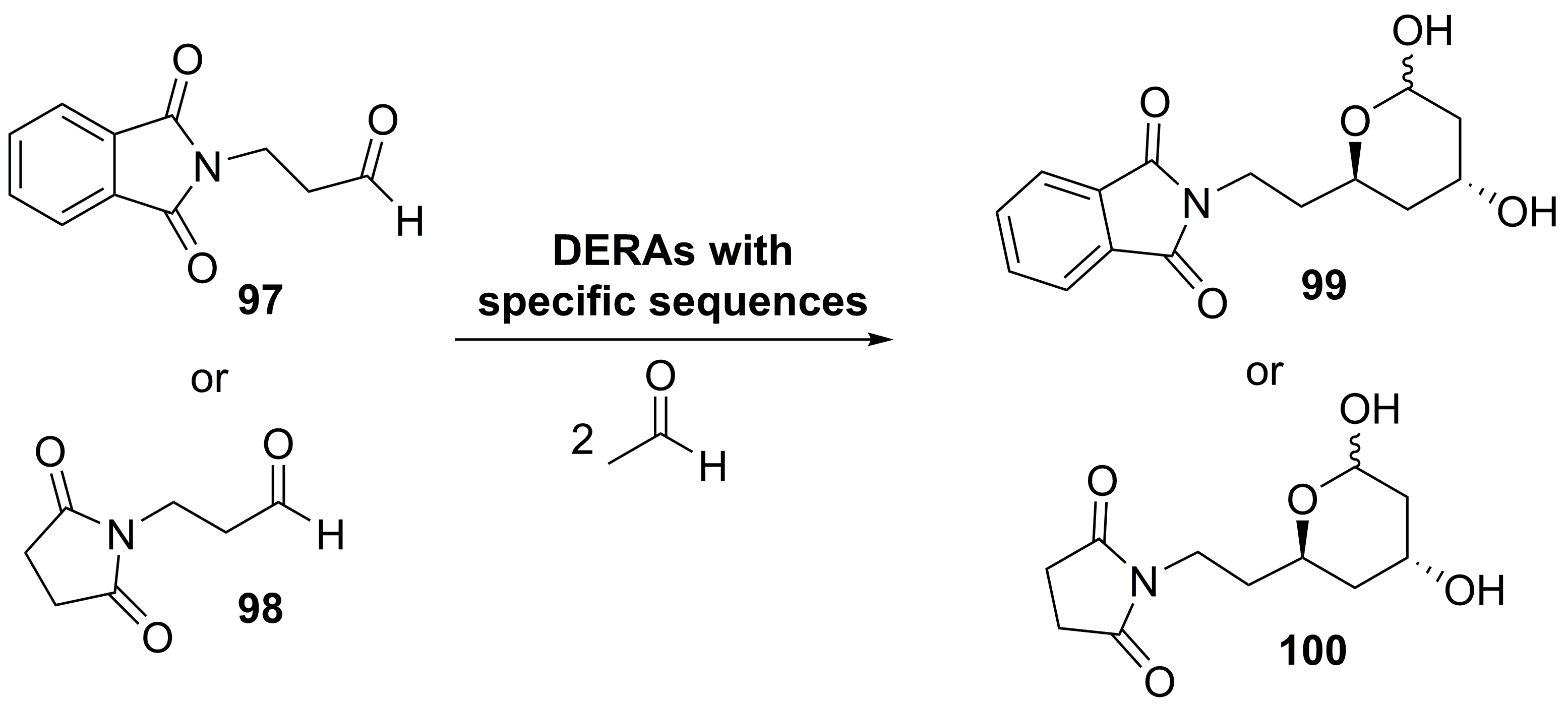
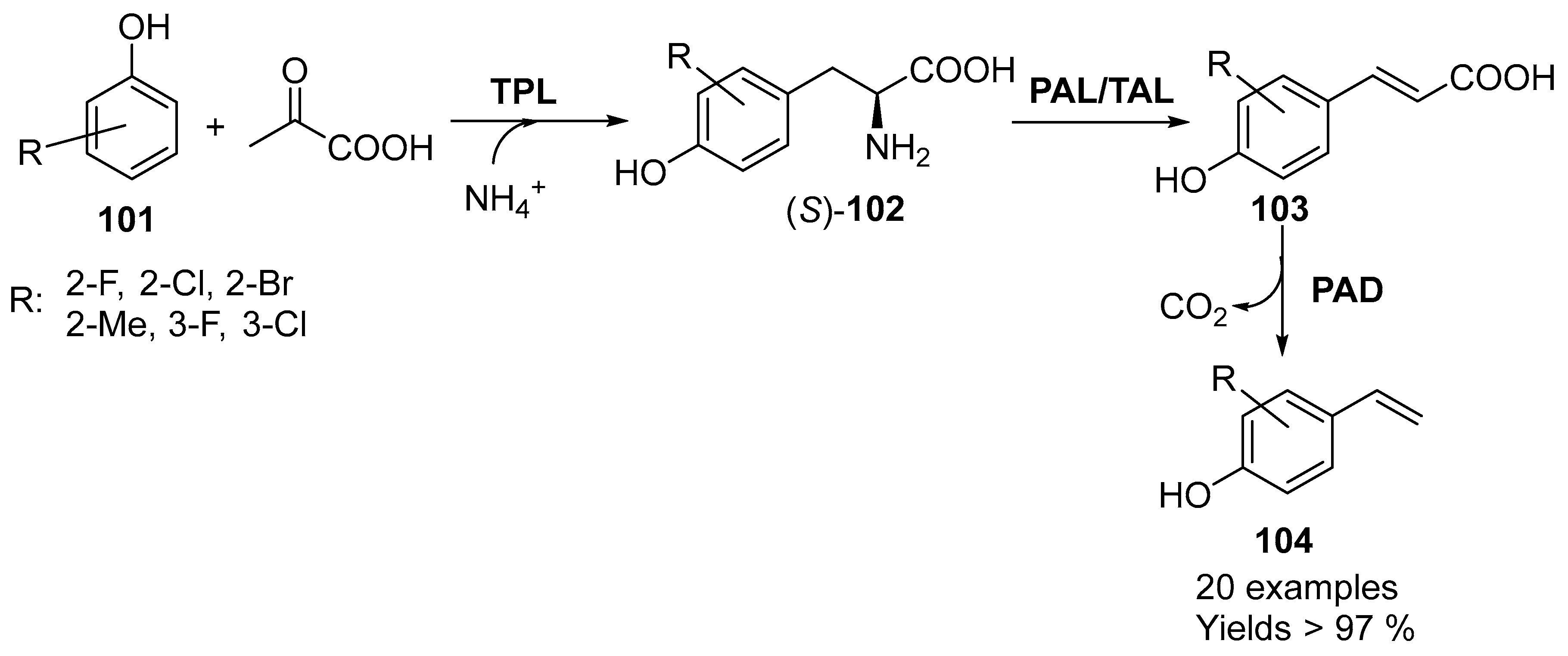
5. Granted Patents Related to Lyases in Asymmetric Synthesis
6. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Turner, N.J.; Kumar, R. Editorial overview: Biocatalysis and biotransformation: The golden age of biocatalysis. Curr. Opin. Chem. Biol. 2018, 43, A1–A3. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Brady, D. The limits to biocatalysis: Pushing the envelope. Chem. Commun. 2018, 54, 6088–6104. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, K.; Lutz, S. Recent developments and challenges of biocatalytic processes in the pharmaceutical industry. Curr. Opin. Green Sustain. Chem. 2018, 11, 58–64. [Google Scholar] [CrossRef]
- Raveendran, S.; Parameswaran, B.; Ummalyma, S.B.; Abraham, A.; Mathew, A.K.; Madhavan, A.; Rebello, S.; Pandey, A. Applications of microbial enzymes in food industry. Food Technol. Biotechnol. 2018, 56, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Y.; Wang, J.B.; Reetz, M.T. Biocatalysts for the pharmaceutical industry created by structure-guided directed evolution of stereoselective enzymes. Bioorg. Med. Chem. 2018, 26, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.; Lewis, J.C. Introduction: Biocatalysis in Industry. Chem. Rev. 2018, 118, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.A.; Pohle, S.; Wharry, S.; Mix, S.; Allen, C.C.R.; Moody, T.S.; Gilmore, B.F. Application of Omega-Transaminases in the Pharmaceutical Industry. Chem. Rev. 2018, 118, 349–367. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.L. Biocatalysis in drug development-highlights of the recent patent literature. Org. Process Res. Dev. 2018, 22, 1063–1080. [Google Scholar] [CrossRef]
- Dorr, B.M.; Fuerst, D.E. Enzymatic amidation for industrial applications. Curr. Opin. Chem. Biol. 2018, 43, 127–133. [Google Scholar] [CrossRef]
- Bornscheuer, U.T. The fourth wave of biocatalysis is approaching. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2018, 376, 20170063. [Google Scholar] [CrossRef]
- Chapman, J.; Ismail, A.E.; Dinu, C.Z. Industrial Applications of Enzymes: Recent Advances, Techniques, and Outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef]
- Guajardo, N.; Domínguez de María, P. Continuous biocatalysis in environmentally-friendly media: A triple synergy for future sustainable processes. ChemCatChem 2019, 11, 3128–3137. [Google Scholar] [CrossRef]
- Woodley, J.M. Accelerating the implementation of biocatalysis in industry. Appl. Microbiol. Biotechnol. 2019, 103, 4733–4739. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Brady, D. Broadening the Scope of Biocatalysis in Sustainable Organic Synthesis. ChemSusChem 2019, 12, 2859–2881. [Google Scholar] [CrossRef] [PubMed]
- Prier, C.K.; Kosjek, B. Recent preparative applications of redox enzymes. Curr. Opin. Chem. Biol. 2019, 49, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Bornscheuer, U.T.; Huisman, G.W.; Kazlauskas, R.J.; Lutz, S.; Moore, J.C.; Robins, K. Engineering the third wave of biocatalysis. Nature 2012, 485, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Foley, A.M.; Maguire, A.R. The Impact of Recent Developments in Technologies which Enable the Increased Use of Biocatalysts. Eur. J. Org. Chem. 2019, 2019, 3713–3734. [Google Scholar] [CrossRef]
- Adams, J.P.; Brown, M.J.B.; Diaz-Rodriguez, A.; Lioyd, R.C.; Roiban, G.D. Biocatalysis: A Pharma Perspective. Adv. Synth. Catal. 2019, 361, 2421–2432. [Google Scholar] [CrossRef]
- Domínguez de María, P.; de Gonzalo, G. Biocatalysis: An Industrial Perspective; Royal Society of Chemistry: London, UK, 2018. [Google Scholar]
- Woodley, J.M. Bioprocess intensification for the effective production of chemical products. Comput. Chem. Eng. 2017, 105, 297–307. [Google Scholar] [CrossRef]
- Ramesh, H.; Nordblad, M.; Whittall, J.; Woodley, J.M. Considerations for the Application of Process Technologies in Laboratory- and Pilot-Scale Biocatalysis for Chemical Synthesis. In Practical Methods for Biocatalysis and Biotransformations 3; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 1–30. [Google Scholar]
- Woodley, J.M. Scale-Up and Development of Enzyme-Based Processes for Large-Scale Synthesis Applications. In Biocatalysis in Organic Synthesis, 2015 ed.; Faber, K., Fessner, W.D., Turner, N.J., Eds.; Georg Thieme Verlag: Stuttgart, Germany, 2015; pp. 515–546. [Google Scholar]
- Lindeque, R.M.; Woodley, J.M. Reactor Selection for Effective Continuous Biocatalytic Production of Pharmaceuticals. Catalysts 2019, 9, 262. [Google Scholar] [CrossRef]
- Reetz, M.T. Directed Evolution of Selective Enzymes: Catalysts for Organic Chemistry and Biotechnology; Wiley-VCH: Weinheim, Germany, 2017. [Google Scholar]
- Madhavan, A.; Sindhu, R.; Binod, P.; Sukumaran, R.K.; Pandey, A. Strategies for design of improved biocatalysts for industrial applications. Bioresour. Technol. 2017, 245, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Rigoldi, F.; Donini, S.; Redaelli, A.; Parisini, E.; Gautieri, A. Review: Engineering of thermostable enzymes for industrial applications. APL Bioeng. 2018, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Zeymer, C.; Hilvert, D. Directed evolution of protein catalysts. In Annual Review of Biochemistry; Kornberg, R.D., Ed.; Annual Reviews: Palo Alto, CA, USA, 2018; Volume 87, pp. 131–157. [Google Scholar]
- Arnold, F.H. Directed Evolution: Bringing New Chemistry to Life. Angew. Chem. Int. Ed. 2018, 57, 4143–4148. [Google Scholar] [CrossRef] [PubMed]
- Bornscheuer, U.T.; Hauer, B.; Jaeger, K.E.; Schwaneberg, U. Directed evolution empowered redesign of natural proteins for the sustainable production of chemicals and pharmaceuticals. Angew. Chem. Int. Ed. 2019, 58, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Iqbal, H.M.N. Tailoring Multipurpose Biocatalysts via Protein Engineering Approaches: A Review. Catal. Lett. 2019, 149, 2204–2217. [Google Scholar] [CrossRef]
- De Gonzalo, G.; Alcántara, A.R.; Domínguez de María, P. Cyclopentyl Methyl Ether (CPME): A versatile eco-friendly solvent for applications in biotechnology and biorefineries. ChemSusChem 2019, 12, 2083–2097. [Google Scholar] [CrossRef]
- Shanmuganathan, S.; Natalia, D.; van den Wittenboer, A.; Kohlmann, C.; Greiner, L.; Domínguez de María, P. Enzyme-catalyzed C–C bond formation using 2-methyltetrahydrofuran (2-MTHF) as (co)solvent: efficient and bio-based alternative to DMSO and MTBE. Green Chem. 2010, 12, 2240–2245. [Google Scholar] [CrossRef]
- Pace, V.; Hoyos, P.; Castoldi, L.; Domínguez de María, P.; Alcántara, A.R. 2-Methyltetrahydrofuran (2-MeTHF): A Biomass-Derived Solvent with Broad Application in Organic Chemistry. ChemSusChem 2012, 5, 1369–1379. [Google Scholar] [CrossRef]
- Hernáiz, M.; Alcántara, A.R.; García, J.I.; Sinisterra, J.V. Applied Biotransformations in Green Solvents. Chem. Eur. J. 2010, 16, 9422–9437. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. Green solvents for green technologies. J. Chem. Technol. Biotechnol. 2015, 90, 1631–1639. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.-C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef] [PubMed]
- Lomba, L.; Zuriaga, E.; Giner, B. Solvents derived from biomass and their potential as green solvents. Curr. Opin. Green Sustain. Chem. 2019, 18, 51–56. [Google Scholar] [CrossRef]
- Sheldon, R.A. The greening of solvents: Towards sustainable organic synthesis. Curr. Opin. Green Sustain. Chem. 2019, 18, 13–19. [Google Scholar] [CrossRef]
- Florindo, C.; Lima, F.; Ribeiro, B.D.; Marrucho, I.M. Deep eutectic solvents: Overcoming 21st century challenges. Curr. Opin. Green Sustain. Chem. 2019, 18, 31–36. [Google Scholar] [CrossRef]
- Tomé, L.I.N.; Baião, V.; da Silva, W.; Brett, C.M.A. Deep eutectic solvents for the production and application of new materials. Appl. Mater. Today 2018, 10, 30–50. [Google Scholar] [CrossRef]
- Guajardo, N.; Muller, C.R.; Schrebler, R.; Carlesi, C.; de Maria, P.D. Deep Eutectic Solvents for Organocatalysis, Biotransformations, and Multistep Organocatalyst/Enzyme Combinations. ChemCatChem 2016, 8, 1020–1027. [Google Scholar] [CrossRef]
- Gotor-Fernandez, V.; Paul, C.E. Deep eutectic solvents for redox biocatalysis. J. Biotechnol. 2019, 293, 24–35. [Google Scholar] [CrossRef]
- Paiva, A.; Matias, A.A.; Duarte, A.R.C. How do we drive deep eutectic systems towards an industrial reality? Curr. Opin. Green Sustain. Chem. 2018, 11, 81–85. [Google Scholar] [CrossRef]
- Juneidi, I.; Hayyan, M.; Hashim, M.A. Intensification of biotransformations using deep eutectic solvents: Overview and outlook. Process Biochem. 2018, 66, 33–60. [Google Scholar] [CrossRef]
- Maugeri, Z.; de Maria, P.D. Whole-Cell Biocatalysis in Deep-Eutectic-Solvents/Aqueous Mixtures. ChemCatChem 2014, 6, 1535–1537. [Google Scholar] [CrossRef]
- Lin, B.; Tao, Y. Whole-cell biocatalysts by design. Microb. Cell Factories 2017, 16, 106. [Google Scholar] [CrossRef] [PubMed]
- Wachtmeister, J.; Rother, D. Recent advances in whole cell biocatalysis techniques bridging from investigative to industrial scale. Curr. Opin. Biotech. 2016, 42, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Garzón-Posse, F.; Becerra-Figueroa, L.; Hernández-Arias, J.; Gamba-Sánchez, D. Whole cells as biocatalysts in organic transformations. Molecules 2018, 23, 1265. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, P. Immobilized Biocatalysts. Catalysts 2018, 8, 386. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Virgen-Ortiz, J.J.; Dos Santos, J.C.S.; Berenguer-Murcia, A.; Alcantara, A.R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports: Immobilization mechanism, advantages, problems, and solutions. Biotechnol. Adv. 2019, 37, 746–770. [Google Scholar] [CrossRef]
- Bernal, C.; Rodriguez, K.; Martinez, R. Integrating enzyme immobilization and protein engineering: An alternative path for the development of novel and improved industrial biocatalysts. Biotechnol. Adv. 2018, 36, 1470–1480. [Google Scholar] [CrossRef]
- Facin, B.R.; Melchiors, M.S.; Valerio, A.; Oliveira, J.V.; de Oliveira, D. Driving immobilized lipases as biocatalysts: 10 years’ state of the art and future prospects. Ind. Eng. Chem. Res. 2019, 58, 5358–5378. [Google Scholar] [CrossRef]
- Rueda, N.; dos Santos, J.C.S.; Ortiz, C.; Torres, R.; Barbosa, O.; Rodrigues, R.C.; Berenguer-Murcia, A.; Fernandez-Lafuente, R. Chemical modification in the design of immobilized enzyme biocatalysts: Drawbacks and opportunities. Chem. Rec. 2016, 16, 1436–1455. [Google Scholar] [CrossRef]
- DiCosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Bilal, M.; Cui, J.D.; Iqbal, H.M.N. Tailoring enzyme microenvironment: State-of-the-art strategy to fulfill the quest for efficient bio-catalysis. Int. J. Biol. Macromol. 2019, 130, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Britton, J.; Majumdar, S.; Weiss, G.A. Continuous flow biocatalysis. Chem. Soc. Rev. 2018, 47, 5891–5918. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. The E factor 25 years on: The rise of green chemistry and sustainability. Green Chem. 2017, 19, 18–43. [Google Scholar] [CrossRef]
- Tickner, J.A.; Becker, M. Mainstreaming green chemistry: The need for metrics. Curr. Opin. Green Sustain. Chem. 2016, 1, 1–4. [Google Scholar] [CrossRef]
- Sheldon, R.A. Reaction efficiencies and green chemistry metrics of biotransformations. In Biocatalysis for Green Chemistry and Chemical Process Development; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 67–88. [Google Scholar]
- De Maria, P.D.; Hollmann, F. On the (Un)greenness of Biocatalysis: Some Challenging Figures and Some Promising Options. Front. Microbiol. 2015, 6, 5. [Google Scholar]
- Daiha, K.D.; Angeli, R.; de Oliveira, S.D.; Almeida, R.V. Are lipases still important biocatalysts? A study of scientific publications and patents for technological forecasting. PLoS ONE 2015, 10, e0131624. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.L. Highlights of the Recent, U.S. Patent Literature: Focus on Biocatalysis. Org. Process Res. Dev. 2016, 20, 700–706. [Google Scholar] [CrossRef]
- Bornscheuer, U.; Kazlauskas, R.J. Hydrolases in Organic Synthesis. Regio- and Stereoselective Biotransformations, 2nd ed.; WILEY-VCH Verlag: Weinheim, Germany, 2006. [Google Scholar]
- Busto, E.; Gotor-Fernandez, V.; Gotor, V. Hydrolases: Catalytically promiscuous enzymes for non-conventional reactions in organic synthesis. Chem. Soc. Rev. 2010, 39, 4504–4523. [Google Scholar] [CrossRef]
- Siirola, E.; Frank, A.; Grogan, G.; Kroutil, W. C-C hydrolases for biocatalysis. Adv. Synth. Catal. 2013, 355, 1677–1691. [Google Scholar] [CrossRef]
- Lopez-Iglesias, M.; Gotor-Fernandez, V. Recent advances in biocatalytic promiscuity: Hydrolase-catalyzed reactions for nonconventional transformations. Chem. Rec. 2015, 15, 743–759. [Google Scholar] [CrossRef]
- Mendez-Sanchez, D.; Lopez-Iglesias, M.; Gotor-Fernandez, V. Hydrolases in organic chemistry. Recent achievements in the synthesis of pharmaceuticals. Curr. Org. Chem. 2016, 20, 1186–1203. [Google Scholar] [CrossRef]
- Kazlauskas, R. Chapter 5-Hydrolysis and formation of carboxylic acid and alcohol derivatives. In Organic Synthesis Using Biocatalysis; Goswami, A., Stewart, J.D., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 127–148. [Google Scholar]
- Bertau, M.; Jeromin, G.E. Hydrolysis and transacylation: Esterases, lipases, phosphatases, and phosphoryl transferases. In Biocatalysis in Organic Synthesis, 2015 ed.; Faber, K., Fessner, W.D., Turner, N.J., Eds.; Georg Thieme Verlag: Stuttgart, Germany, 2015; Volume 1, pp. 129–187. [Google Scholar]
- Liu, X.; Kokare, C. Microbial enzymes of use in Industry. In Biotechnology of Microbial Enzymes; Brahmachari, G., Ed.; Academic Press, Elsevier: Amsterdam, The Netherlands, 2017; pp. 267–298. [Google Scholar]
- Sanchez, S.; Demain, A.L. Useful Microbial Enzymes—An Introduction. In Biotechnology of Microbial Enzymes; Brahmachari, G., Ed.; Academic Press, Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–11. [Google Scholar]
- Reetz, M.T. Lipases as practical biocatalysts. Curr. Opin. Chem. Biol. 2002, 6, 145–150. [Google Scholar] [CrossRef]
- Roy, I.; Mukherjee, J.; Gupta, M.N. High Activity Preparations of Lipases and Proteases for Catalysis in Low Water Containing Organic Solvents and Ionic Liquids. In Immobilization of Enzymes and Cells, 3rd ed.; Guisan, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2013; Volume 1051, pp. 275–284. [Google Scholar]
- De Miranda, A.S.; Miranda, L.S.M.; de Souza, R. Lipases: Valuable catalysts for dynamic kinetic resolutions. Biotechnol. Adv. 2015, 33, 372–393. [Google Scholar] [CrossRef] [PubMed]
- Seddigi, Z.S.; Malik, M.S.; Ahmed, S.A.; Babalghith, A.O.; Kamal, A. Lipases in asymmetric transformations: Recent advances in classical kinetic resolution and lipase–metal combinations for dynamic processes. Co-ord. Chem. Rev. 2017, 348, 54–70. [Google Scholar] [CrossRef]
- Hills, G. Industrial use of lipases to produce fatty acid esters. Eur. J. Lipid Sci. Technol. 2003, 105, 601–607. [Google Scholar] [CrossRef]
- Bornscheuer, U.T.; Flickinger, M.C. Lipases, synthesis of chiral compounds, aqueous and organic solvents. In Encyclopedia of Industrial Biotechnology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; Volume 5, pp. 1–12. [Google Scholar]
- Tufvesson, P.; Tornvall, U.; Carvalho, J.; Karlsson, A.J.; Hatti-Kaul, R. Towards a cost-effective immobilized lipase for specialty chemicals. J. Mol. Catal. B Enzym. 2011, 68, 200–205. [Google Scholar] [CrossRef]
- Ansorge-Schumacher, M.B.; Thum, O. Immobilised lipases in the cosmetics industry. Chem. Soc. Rev. 2013, 42, 6475–6490. [Google Scholar] [CrossRef]
- Adlercreutz, P. Immobilisation and application of lipases in organic media. Chem. Soc. Rev. 2013, 42, 6406–6436. [Google Scholar] [CrossRef]
- Kumar, A.; Dhar, K.; Kanwar, S.S.; Arora, P.K. Lipase catalysis in organic solvents: Advantages and applications. Biol. Proced. Online 2016, 18, 2. [Google Scholar] [CrossRef]
- Priyanka, P.; Tan, Y.Q.; Kinsella, G.K.; Henehan, G.T.; Ryan, B.J. Solvent stable microbial lipases: Current understanding and biotechnological applications. Biotechnol. Lett. 2019, 41, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Chakravorty, D.; Parameswaran, S.; Dubey, V.K.; Patra, S. Unraveling the rationale behind organic solvent stability of lipases. Appl. Biochem. Biotechnol. 2012, 167, 439–461. [Google Scholar] [CrossRef] [PubMed]
- Dwivedee, B.P.; Soni, S.; Sharma, M.; Bhaumik, J.; Laha, J.K.; Banerjee, U.C. Promiscuity of lipase-catalyzed reactions for organic synthesis: A recent update. ChemistrySelect 2018, 3, 2441–2466. [Google Scholar] [CrossRef]
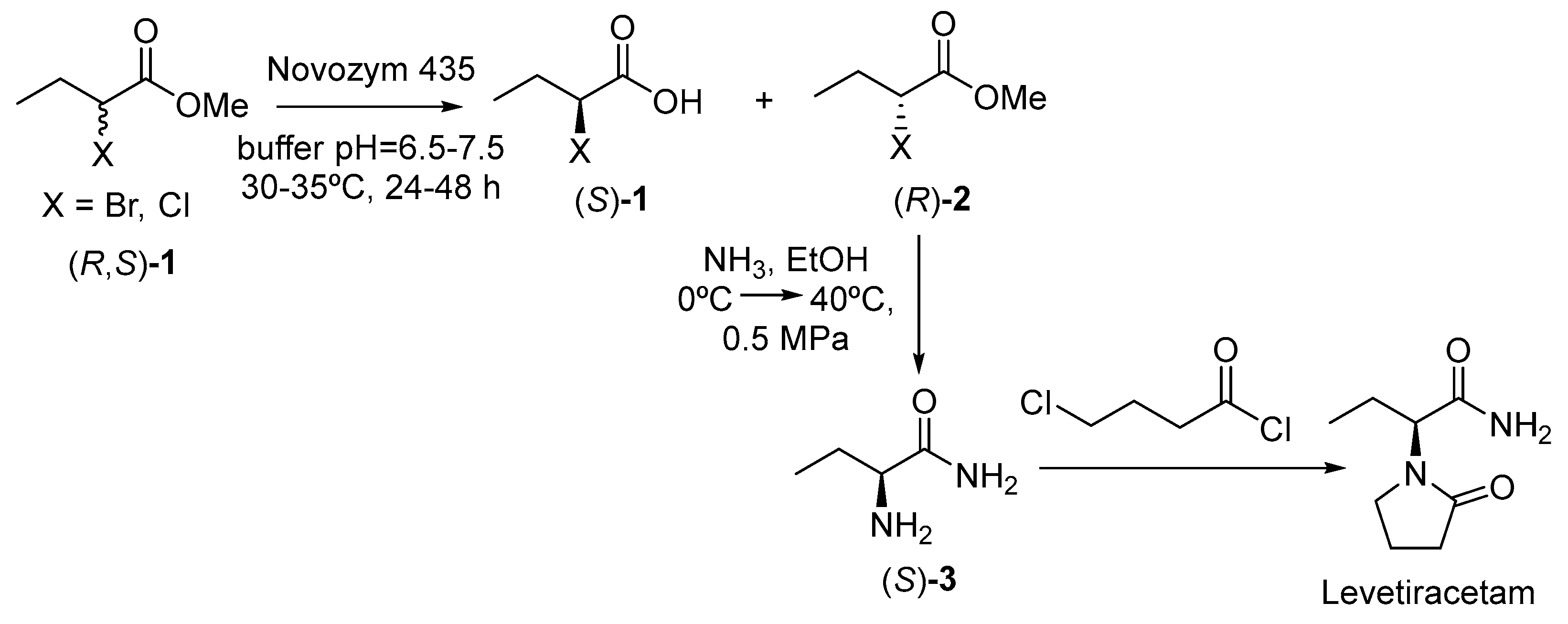
- Ji, Y.; Chen, B.; Qian, F.; He, Y.; Gao, X.; Hong, X. A Method for Preparing Levetiracetam. CN105063120B, 18 October 2015. [Google Scholar]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; dos Santos, J.C.S.; Rodrigues, R.C.; Berenguer-Murcia, A.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The perfect lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef]
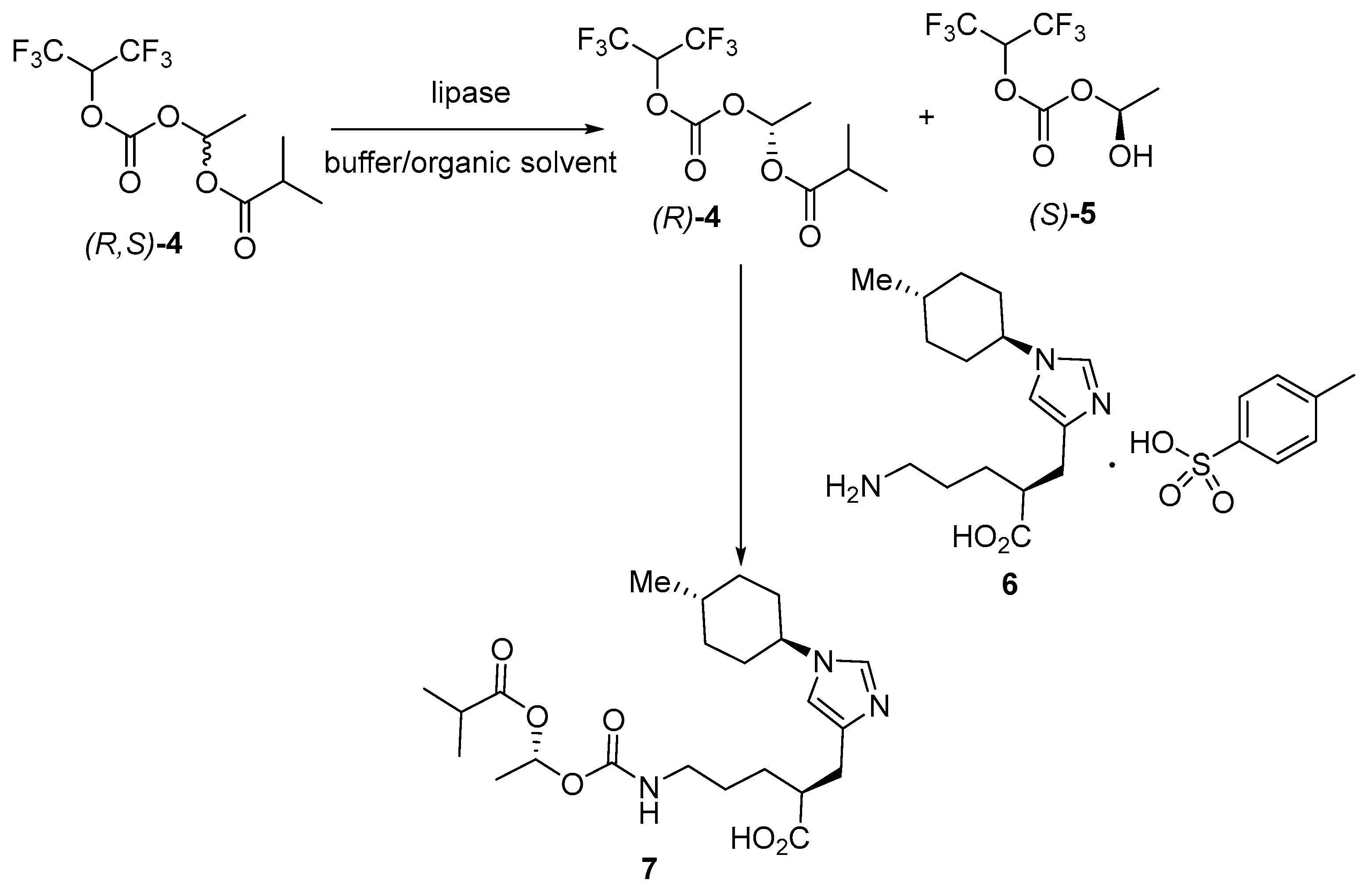
- Ueda, T.; Abe, Y. Novel Method for Producing 1-(acyloxy)alkyl Carbamate Derivatives. WO2016208709A1, 29 December 2016. [Google Scholar]
- Boffa, M.B.; Koschinsky, M.L. Curiouser and curiouser: Recent advances in measurement of thrombin-activatable fibirinolysis inhibitor (TAFI) and in understanding its molecular genetics, gene regulation, and biological roles. Clin. Biochem. 2007, 40, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Leurs, J.; Hendriks, D. Carboxypeptidase U (TAFla): A metallocarboxypeptidase with a distinct role in haemostasis and a possible risk factor for thrombotic disease. Thromb. Haemost. 2005, 94, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Colucci, M.; Semeraro, N. Thrombin activatable fibrinolysis inhibitor: At the nexus of fibrinolysis and inflammation. Thromb. Res. 2012, 129, 314–319. [Google Scholar] [CrossRef]
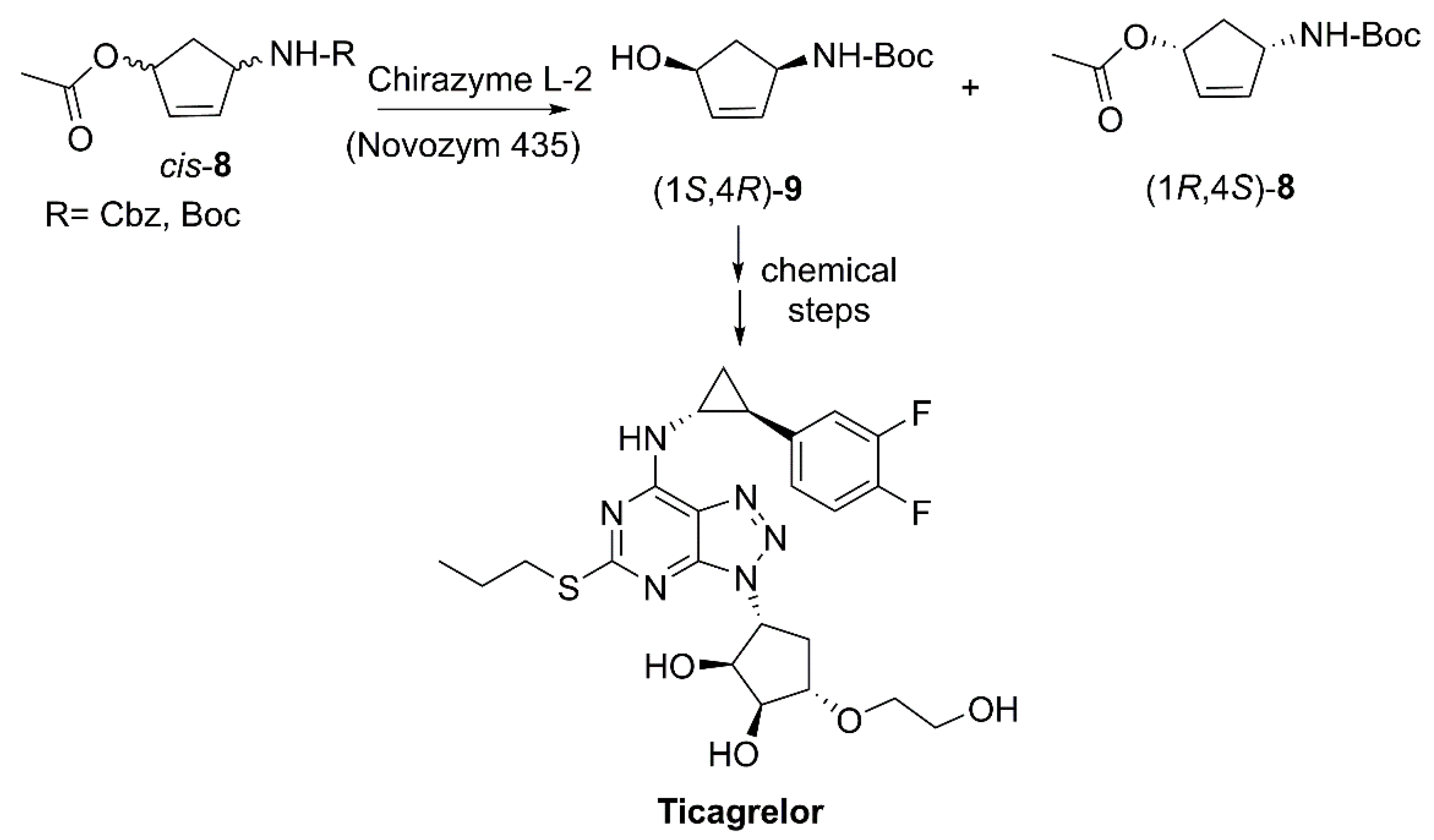
- Zheng, G.; Chen, Q. Method for producing ticagrelor chiral drug intermediates by using Candida antarctica lipase B. CN104164469A, 26 November 2014. [Google Scholar]
- Husted, S.; van Giezen, J.J.J. Ticagrelor: The first reversibly binding oral P2Y (12) receptor antagonist. Cardiovasc. Ther. 2009, 27, 259–274. [Google Scholar] [CrossRef]
- Danielak, D.; Karazniewicz-Lada, M.; Glowka, F. Ticagrelor in modern cardiology-an up-to-date review of most important aspects of ticagrelor pharmacotherapy. Expert Opin. Pharmacother. 2018, 19, 103–112. [Google Scholar] [CrossRef]
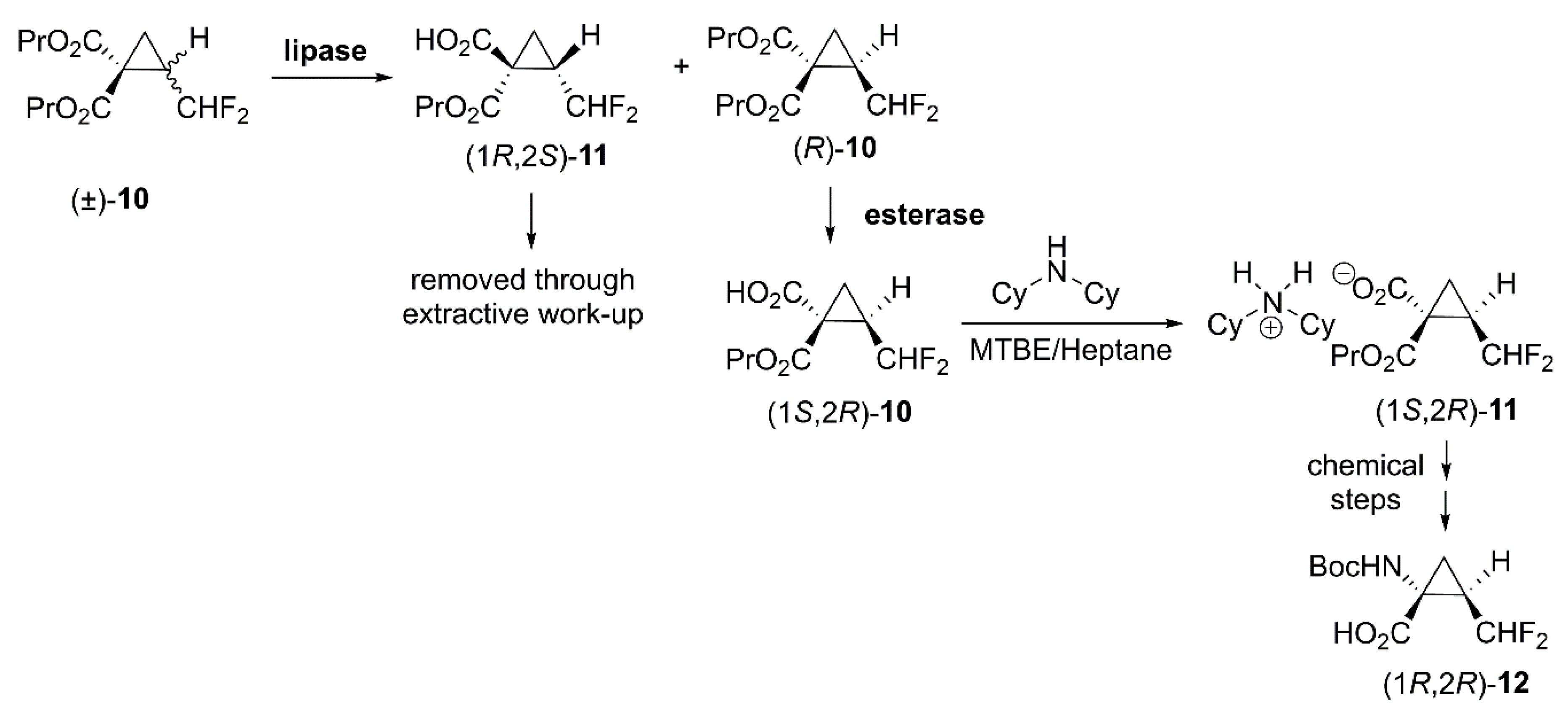
- Abrahamson, M.J.; Kielbus, A.B.; Riordan, W.T.; Hill, D.R.; Chemburkar, S.R.; Reddy, R.E.; Towne, T.B.; Mei, J.; Brown, G.J.; Mix, S. Enzymatic process for the preparation of (1S,2R)-2-(Difluoromethyl)-1-(propoxycarbonyl)cyclopropanecarboxylic Acid. US10316338B1, 11 June 2019. [Google Scholar]
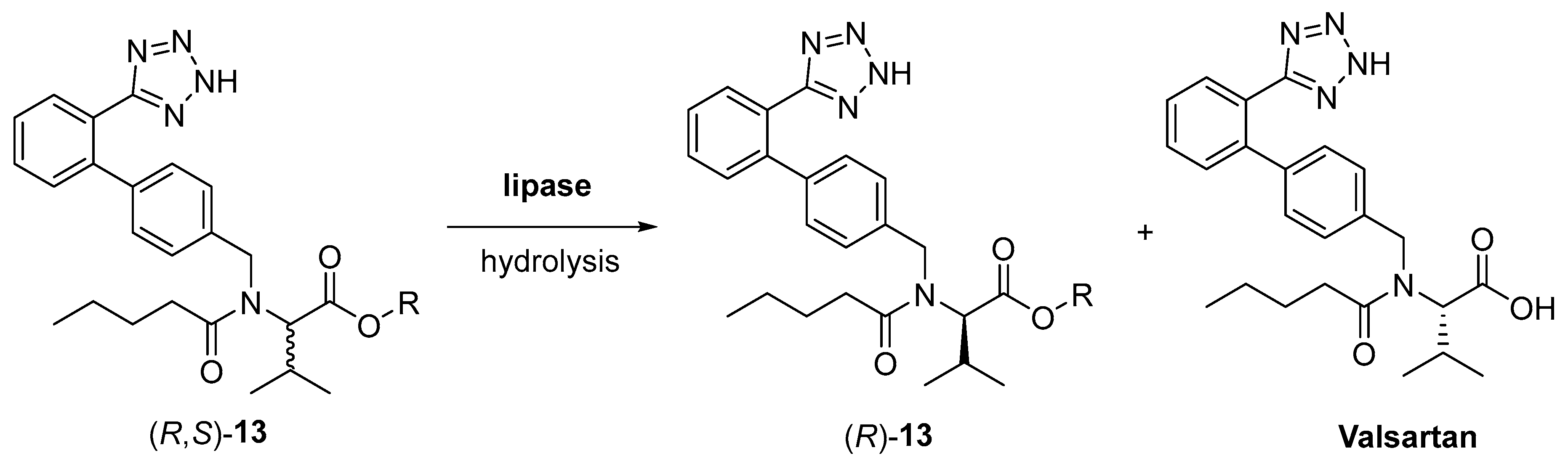
- Markham, A.; Goa, K.L. Valsartan-A review of its pharmacology and therapeutic use in essential hypertension. Drugs 1997, 54, 299–311. [Google Scholar] [CrossRef]
- Xia, J.; Chen, D. Method for Preparing L-Valsartan by Separating DL-Valsartan Ester with Lipase. CN105420338A, 23 March 2016. [Google Scholar]
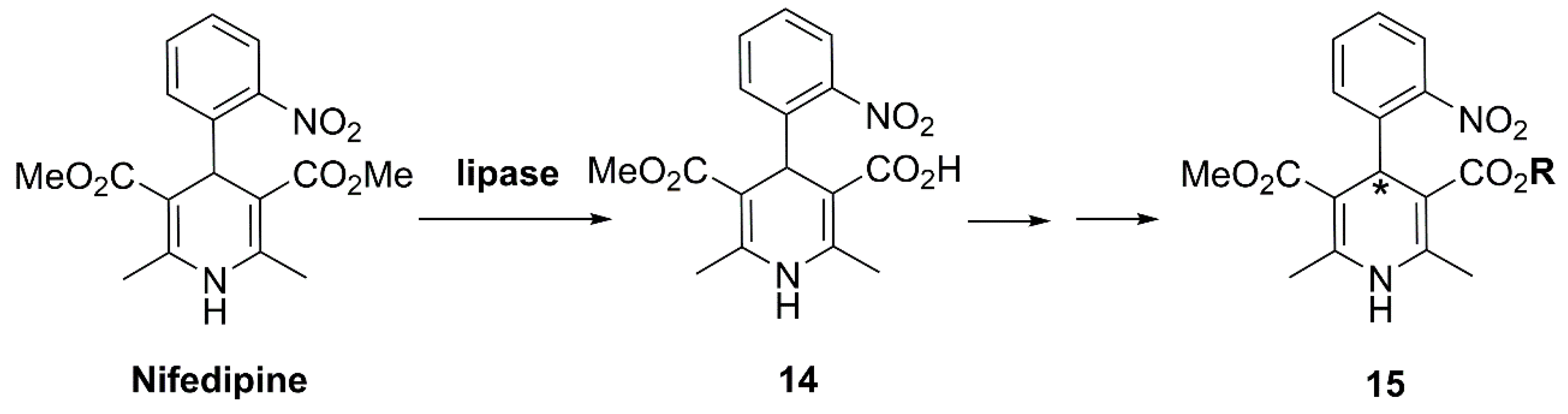
- Xin, J.; Sun, L.; Wang, Y.; Chen, L. Preparation Method of 1,4-Dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylic Acid Monomethyl Ester. CN106279000A, 4 January 2017. [Google Scholar]
- Curtis, T.M.; Scholfield, C.N. Nifedipine blocks Ca2+ store refilling through a pathway not involving L-type Ca2+ channels in rabbit arterioral smooth muscle. J. Physiol. 2001, 532, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A. Calcium Channel Blockers. In Hypertension: A Companion to Braunwald’s Heart Disease, 3rd ed.; Bakris, G.L., Sorrentino, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 242–253. [Google Scholar]
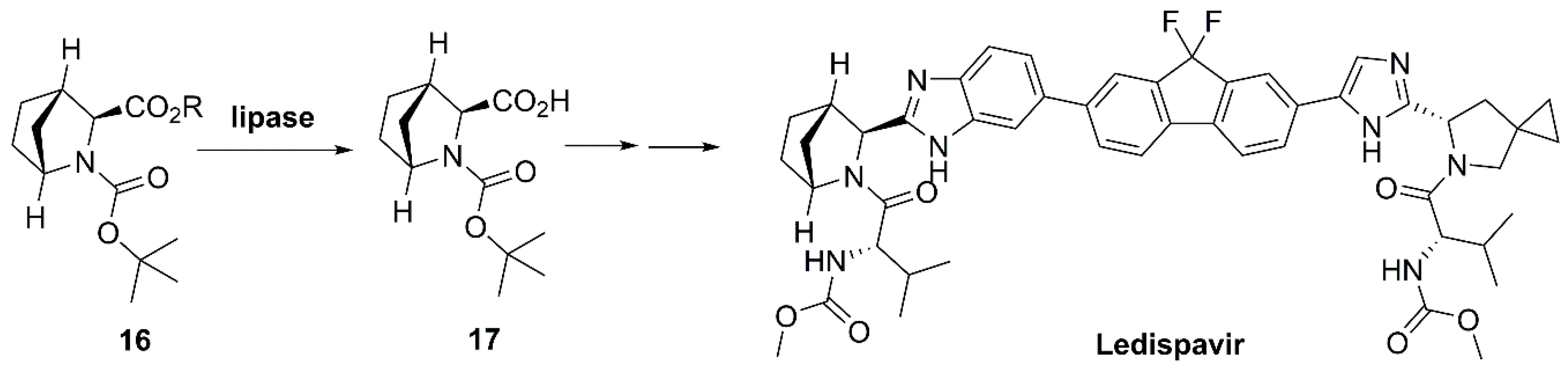
- Chen, X.; Sun, D.; Sun, Q.; He, S.; Wang, C.; Zhuang, M.; Yang, Y.; Xie, K.; Guo, P. Preparation Method of High-Purity Ledipasvir Intermediate (1R,3S,4S)-2-(tert-Butoxycarbonyl)-2-azabicyclo[2.2.1]heptane-3-carboxylic Acid. CN105461606A, 6 April 2016. [Google Scholar]
- Link, J.O.; Taylor, J.G.; Xu, L.H.; Mitchell, M.; Guo, H.Y.; Liu, H.T.; Kato, D.; Kirschberg, T.; Sun, J.Y.; Squires, N.; et al. Discovery of Ledipasvir (GS-5885): A potent, once-daily oral NS5A Inhibitor for the treatment of hepatitis C virus infection. J. Med. Chem. 2014, 57, 2033–2046. [Google Scholar] [CrossRef] [PubMed]
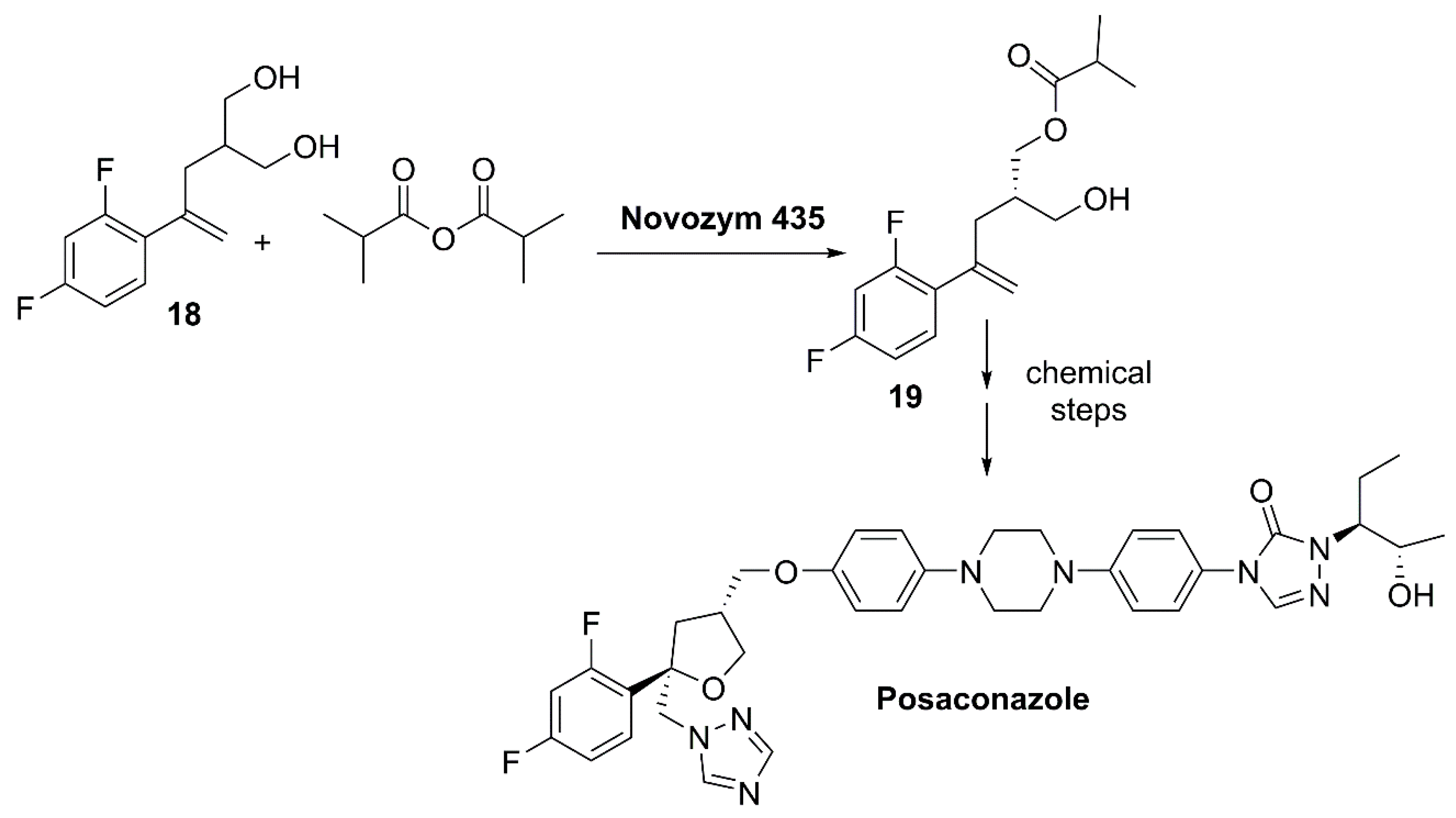
- Ku, L. Process for Synthesis of Posaconazole Intermediate 2-Methylpropionic Acid 4-(2,4-Difluorophenyl)-2-(2S)-hydroxymethyl-4-pentenyl Ester. CN105753693A, 13 July 2016. [Google Scholar]
- Saksena, A.K.; Girijavallabhan, V.M.; Lovey, R.G.; Pike, R.E.; Wang, H.Y.; Ganguly, A.K.; Morgan, B.; Zaks, A.; Puar, M.S. Highly stereoselective access to novel 2,2,4-trisubstituted tetrahydrofurans by halocyclization-Practical chemoenzymatic synthesis of SCH-51048, a broad-spectrum orally-active antifungal agent. Tetrahedron Lett. 1995, 36, 1787–1790. [Google Scholar] [CrossRef]
- Oberhuber, M.; Salchenegger, J.; De Souza, D.; Albert, M.; Wilhelm, T.; Langner, M.; Sturm, H.; Spitzenstaetter, H.P. Process for the Preparation of Chiral Triazolones. WO2011144653A1, 24 November 2011. [Google Scholar]
- Langner, M.; De Souza, D.; Pise, A.C.; Bhuta, S. Method for Preparation and Purification of Posaconazole and Posaconazole Intermediates. WO2011144657A1, 24 November 2011. [Google Scholar]
- Kunic Tesovic, B. Purification of Posaconazole Intermediates. EP2789610A1, 2014. [Google Scholar]
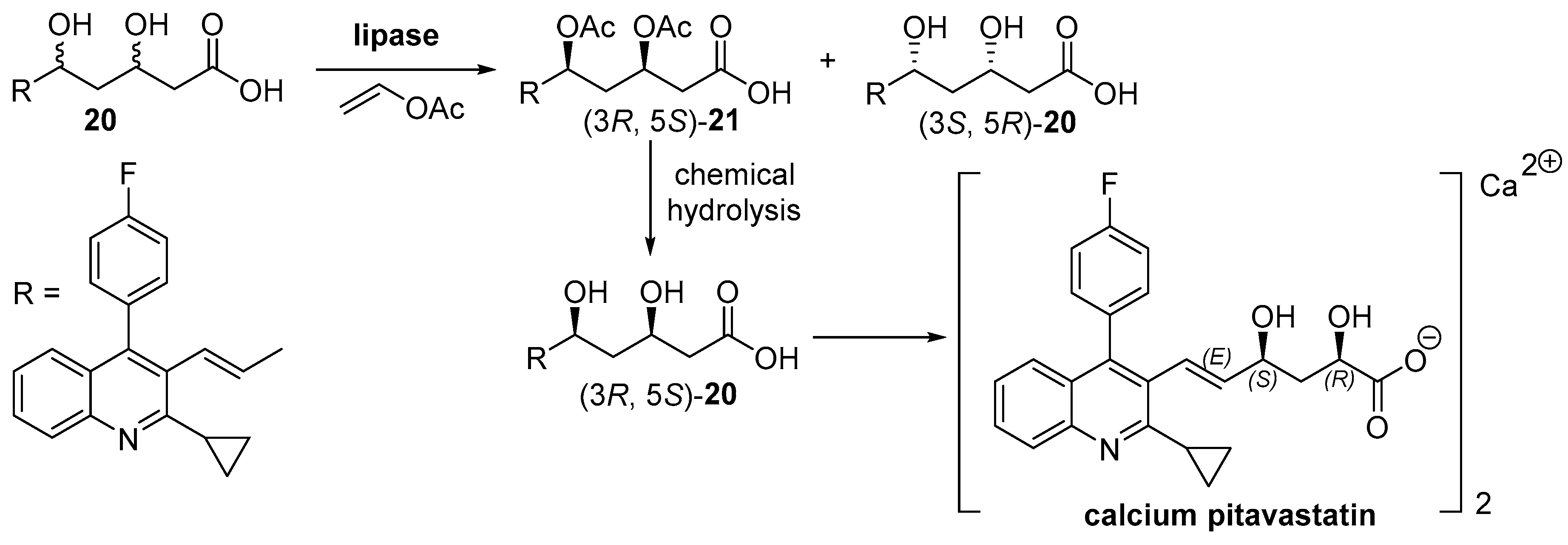
- Hoyos, P.; Pace, V.; Alcántara, A.R. Chiral building blocks for drugs synthesis via biotransformations. In Asymmetric Synthesis of Drugs and Natural Products; Nag, A., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 346–448. [Google Scholar]
- Hoyos, P.; Pace, V.; Alcántara, A.R. Biocatalyzed synthesis of statins: A sustainable strategy for the preparation of valuable drugs. Catalysts 2019, 9, 260. [Google Scholar] [CrossRef]
- Yu, C.; Zhai, M.; Wang, M.; Gong, X.; Zhang, T.; Zhai, H. Preparation Method of High-Purity Pitavastatin Calcium for Treating Hypercholesterolemia. CN103834705A, 15 October 2014. [Google Scholar]
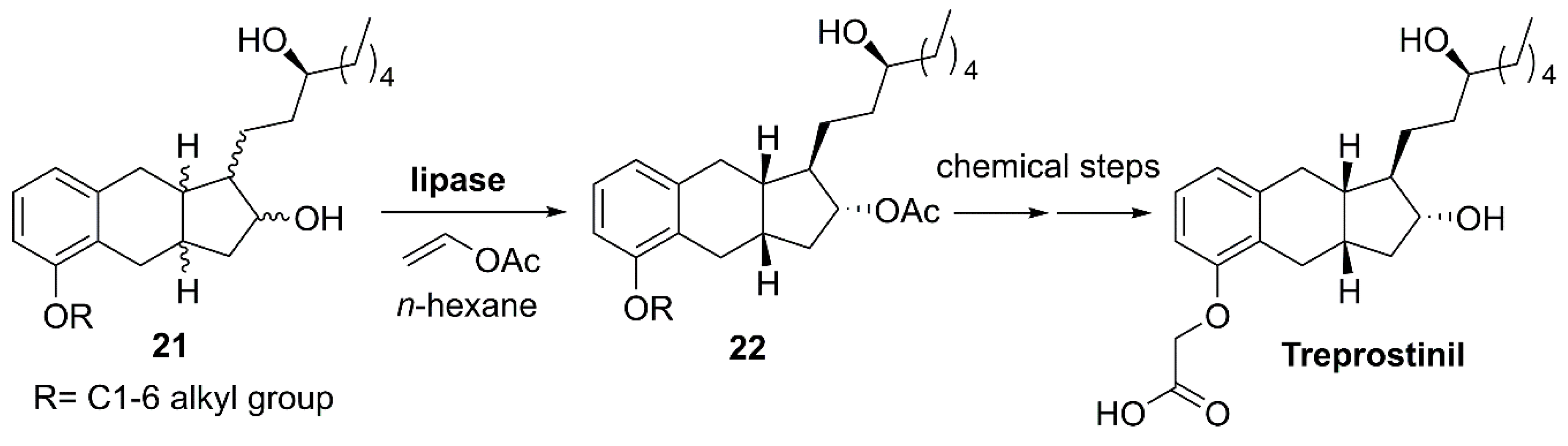
- Torres, F.; Rubin, L.J. Treprostinil for the treatment of pulmonary arterial hypertension. Expert Rev. Cardiovasc. Ther. 2013, 11, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, R.M.; Rani, N.; Enache, L.A.; Rao, M.S.; Batra, H.; Guo, L.; Penmasta, R.A.; Staszewski, J.P.; Tuladhar, S.M.; Prakash, O.; et al. The intramolecular asymmetric Pauson-Khand cyclization as a novel and general stereoselective route to benzindene prostacyclins: Synthesis of UT-15 (treprostinil). J. Org. Chem. 2004, 69, 1890–1902. [Google Scholar] [CrossRef]
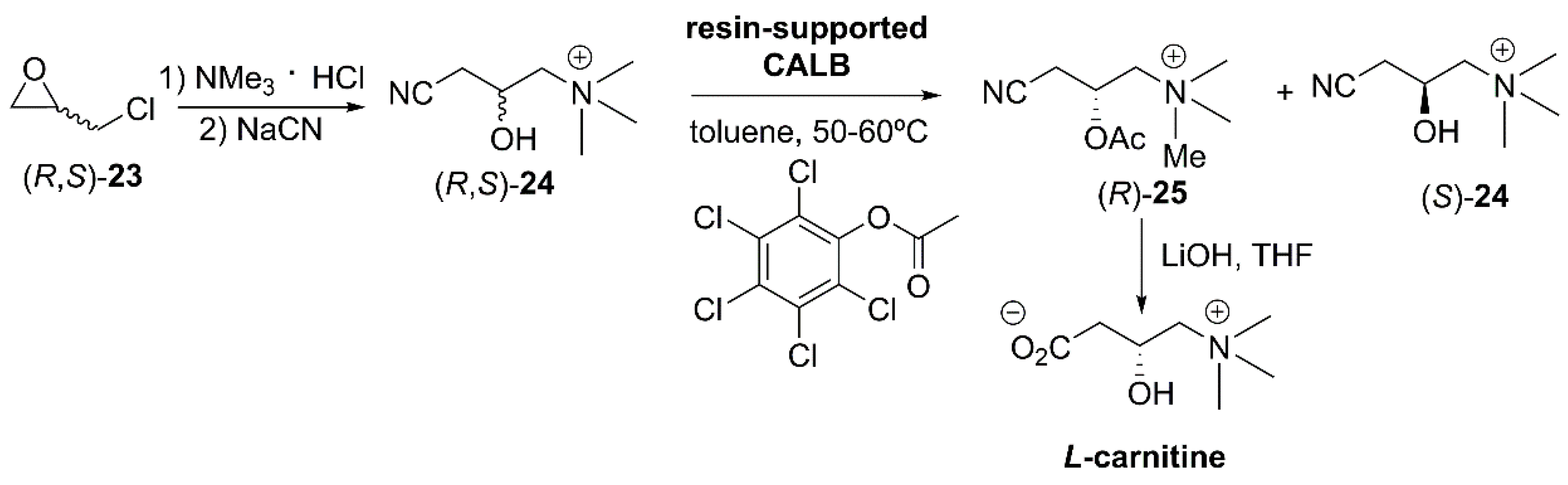
- Wang, Q.; Wang, T.; Sun, Y.; Wang, B.; You, B.; Pu, J.; Li, X.; Jiang, Y. Preparation of Levocarnitine. CN106748843A, 31 May 2017. [Google Scholar]
- Kraemer, W.J.; Volek, J.S.; Dunn-Lewis, C. L-Carnitine supplementation: Influence upon physiological function. Curr. Sports Med. Rep. 2008, 7, 218–223. [Google Scholar] [CrossRef]
- Bloomer, R.J.; Butawan, M.; Farney, T.M.; McAllister, M.J. An Overview of the Dietary Ingredient Carnitine; Academic Press Ltd.: London, UK, 2019; pp. 605–617. [Google Scholar]
- Gröger, H.J.A.M. Biocatalytic concepts for synthesizing amine bulk chemicals: Recent approaches towards linear and cyclic aliphatic primary amines and ω-substituted derivatives thereof. Appl. Microbiol. Biotechnol. 2019, 103, 83–95. [Google Scholar] [CrossRef]
- Kohls, H.; Steffen-Munsberg, F.; Höhne, M. Recent achievements in developing the biocatalytic toolbox for chiral amine synthesis. Curr. Opin. Chem. Biol. 2014, 19, 180–192. [Google Scholar] [CrossRef]
- Grogan, G. Synthesis of chiral amines using redox biocatalysis. Curr. Opin. Chem. Biol. 2018, 43, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Gomm, A.; O’Reilly, E. Transaminases for chiral amine synthesis. Curr. Opin. Chem. Biol. 2018, 43, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Ismail, H.; Lau, R.M.; van Rantwijk, F.; Sheldon, R.A. Fully enzymatic resolution of chiral amines: Acylation and deacylation in the presence of Candida antarctica lipase B. Adv. Synth. Catal. 2008, 350, 1511–1516. [Google Scholar] [CrossRef]
- Bhardwaj, K.K.; Gupta, R. Synthesis of chirally pure enantiomers by lipase. J. Oleo Sci. 2017, 66, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
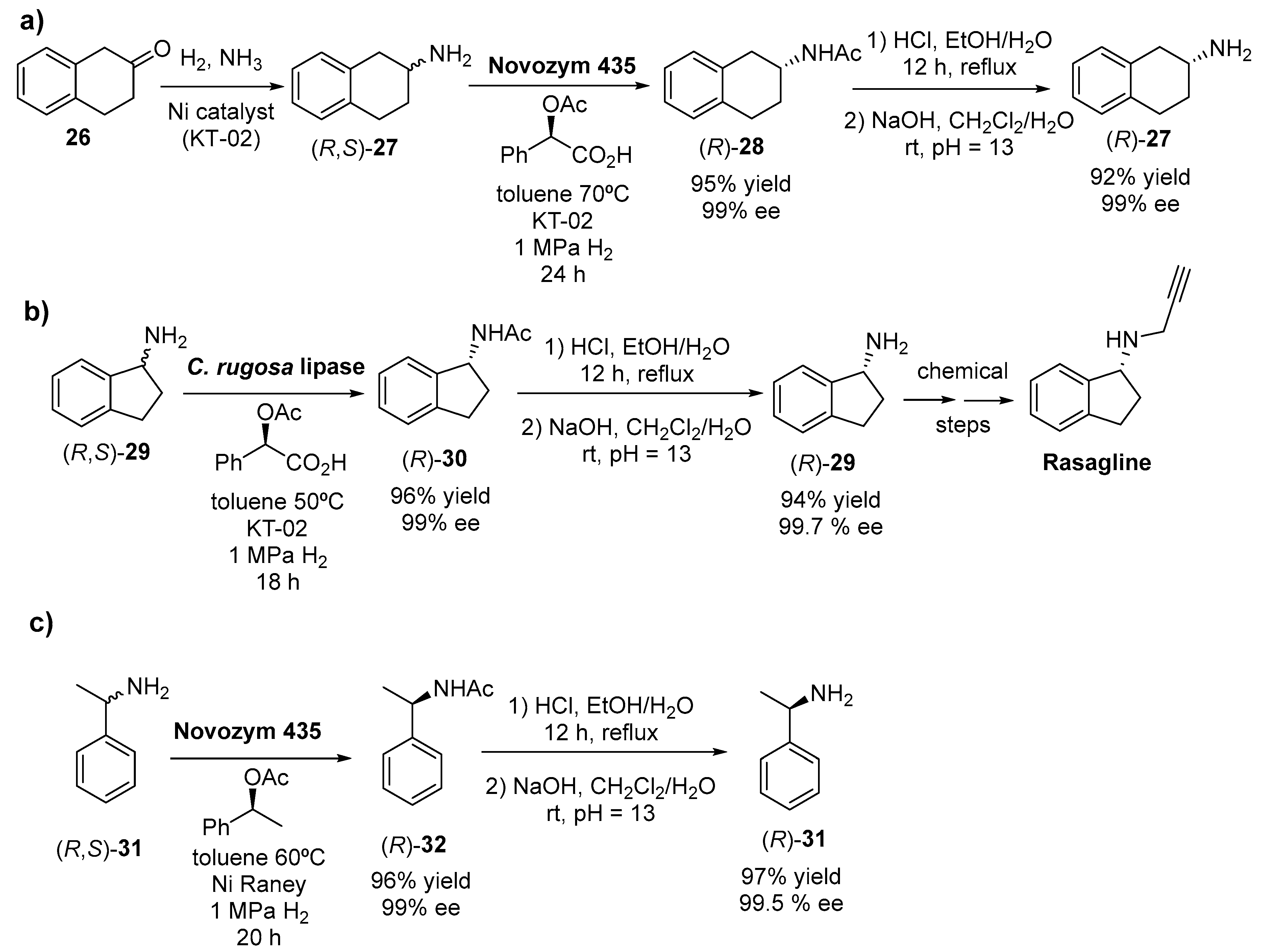
- Wang, J. Method for Preparation of (R)-2-Tetrahydronaphthylamine. CN104263801A, 7 January 2015. [Google Scholar]
- Bruinvels, J. Evidence for inhibition of reuptake of 5-hydroxytryptamine and noradrenaline by tetrahydronaphthylamine in rat brain. Br. J. Pharmacol. 1971, 42, 281–286. [Google Scholar] [CrossRef]
- Chen, Y. A Preparation Method of R-1-Aminoindane with Candida Rugosa Lipase as Biol. Resoln. Catalyzer. CN105063161A, 18 November 2015. [Google Scholar]
- Oldfield, V.; Keating, G.M.; Perry, C.M. Rasagiline-A review of its use in the management of Parkinson’s disease. Drugs 2007, 67, 1725–1747. [Google Scholar] [CrossRef] [PubMed]
- McCormack, P.L. Rasagiline: A Review of Its Use in the Treatment of Idiopathic Parkinson’s Disease. CNS Drugs 2014, 28, 1083–1097. [Google Scholar] [CrossRef]
- Chen, Y. A Resolution Method for Preparing Optically Pure R-1-phenylethylamine. CN104152525A, 19 November 2014. [Google Scholar]
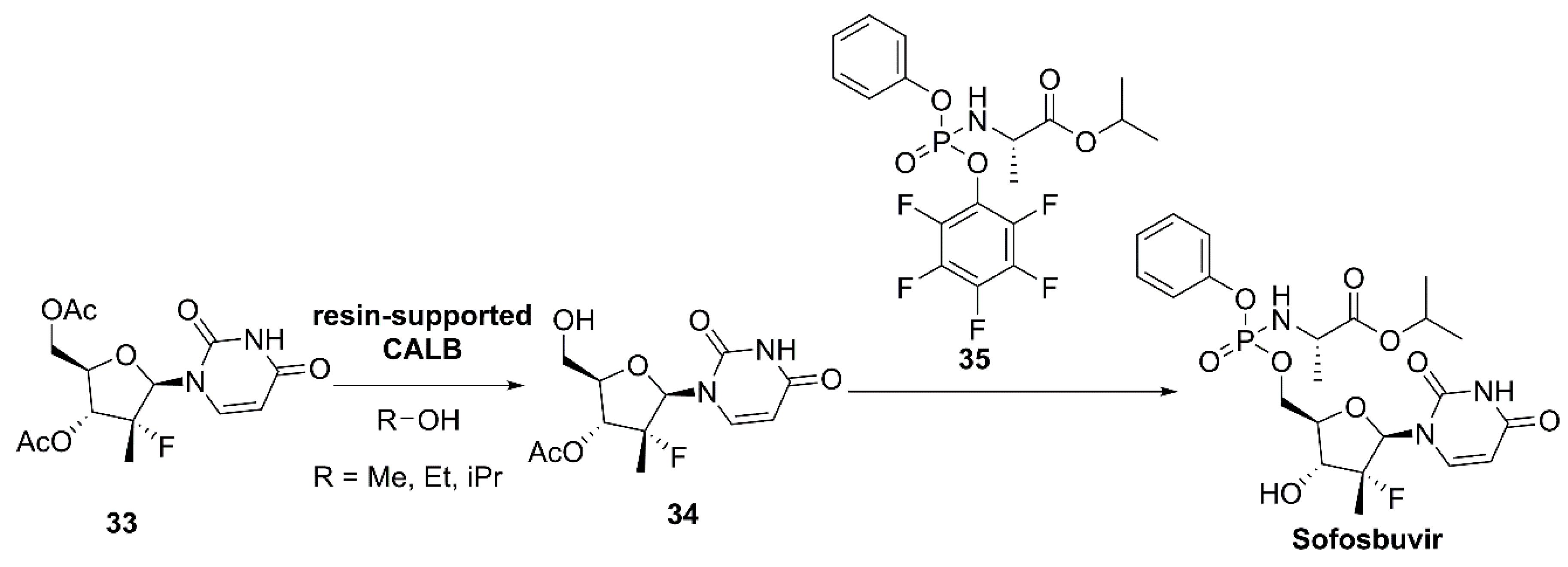
- Gaboardi, M.; Pallanza, G.; Baratella, M.; Castaldi, G.; Castaldi, M. Chemoenzymic Preparation of Sofosbuvir Involving a Lipase Catalyzed Regioselective Deacetylation. WO2017144423A1, 31 August 2017. [Google Scholar]
- Lawitz, E.; Mangia, A.; Wyles, D.; Rodriguez-Torres, M.; Hassanein, T.; Gordon, S.C.; Schultz, M.; Davis, M.N.; Kayali, Z.; Reddy, K.R.; et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N. Engl. J. Med. 2013, 368, 1878–1887. [Google Scholar] [CrossRef]
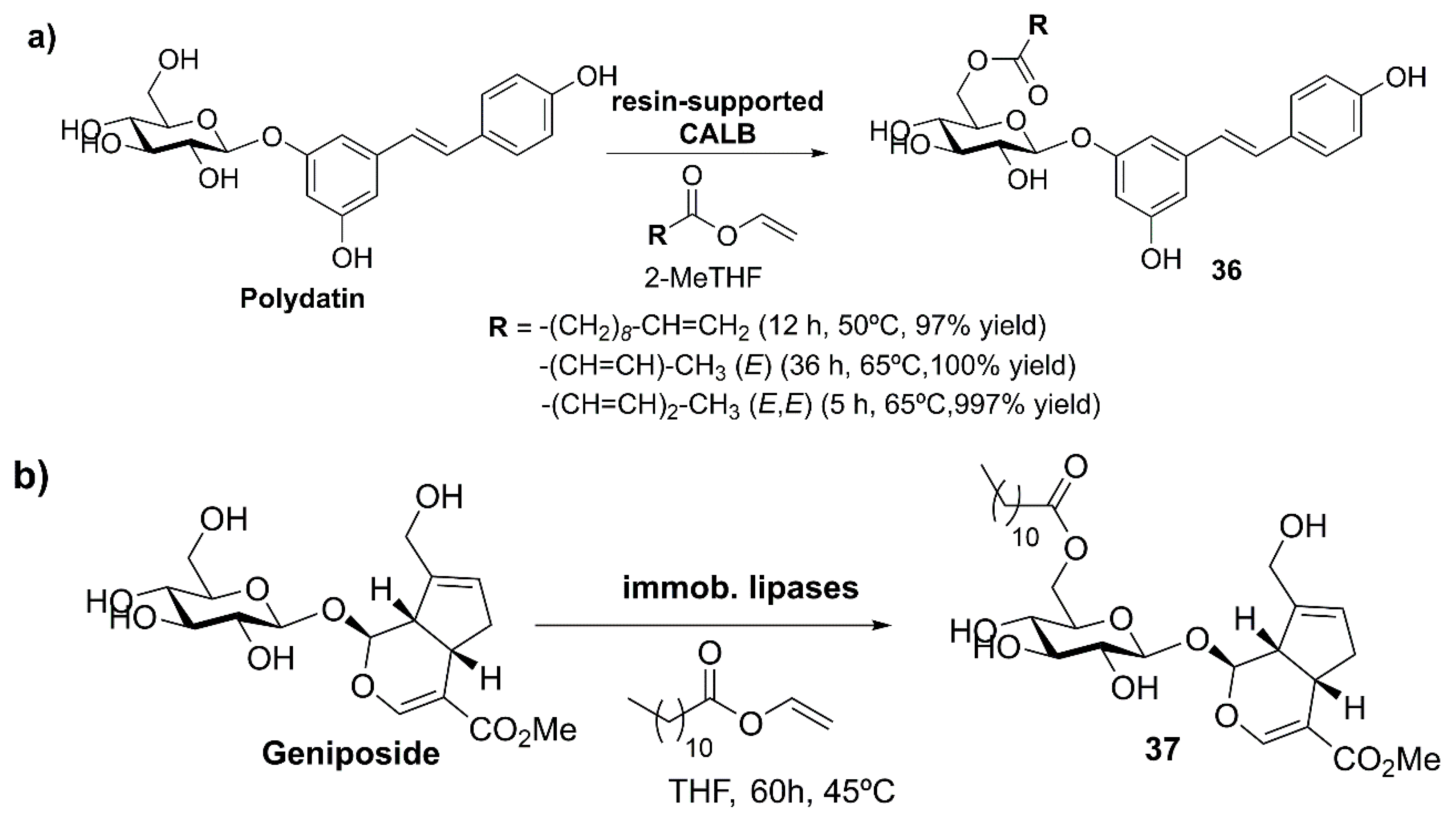
- Du, Q.H.; Peng, C.; Zhang, H. Polydatin: A review of pharmacology and pharmacokinetics. Pharm. Biol. 2013, 51, 1347–1354. [Google Scholar] [CrossRef]
- Sohretoglu, D.; Baran, M.Y.; Arroo, R.; Kuruuzum-Uz, A. Recent advances in chemistry, therapeutic properties and sources of polydatin. Phytochem. Rev. 2018, 17, 973–1005. [Google Scholar] [CrossRef]
- Wang, C.; Du, W.; Bi, Y.; Yuan, X.; Yang, R.; Ding, C.; Zhao, X.; Zhou, W. Preparation of Polydatin Ester Derivative and Application Thereof. CN105503970A, 20 April 2016. [Google Scholar]
- Alcántara, A.R.; Domínguez de Maria, P. Recent advances on the use of 2-methyltetrahydrofuran (2-MeTHF) in biotransformations. Curr. Green Chem. 2018, 5, 85–102. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Sun, W.; Wang, X. Metabolomics and proteomics annotate therapeutic mechanisms of geniposide. In Chinmedomics: The Integration of Serum Pharmacochemistry and Metabolomics to Elucidate the Scientific Value of Traditional Chinese Medicine; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 157–173. [Google Scholar]
- Yao, Z.; Lu, Y.; Ni, F.; Zhu, B.; Sun, Y. Method for Catalytic Synthesis of C-6’-Lauroyl Geniposide by Lipase. CN106282272A, 4 January 2017. [Google Scholar]
- Leong, X.Y.; Thanikachalam, P.V.; Pandey, M.; Ramamurthy, S. A systematic review of the protective role of swertiamarin in cardiac and metabolic diseases. Biomed. Pharmacother. 2016, 84, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Tyagi, R.K.; Tandel, N.; Garg, N.K.; Soni, N. The molecular targets of swertiamarin and its derivatives confer anti-diabetic and anti-hyperlipidemic effects. Curr. Drug Targets 2018, 19, 1958–1967. [Google Scholar] [CrossRef] [PubMed]
- Jun, C.; Xue-Ming, Z.; Chang-Xiao, L.; Tie-Jun, Z. Structure elucidation of metabolites of swertiamarin produced by Aspergillus niger. J. Mol. Struct. 2008, 878, 22–25. [Google Scholar] [CrossRef]
- Chang, J.; Li, Y.; Guo, J.; Yao, L. Preparation of 3-(3-acetyl-4-methylpyridine)-NHHP. CN106543193A, 29 March 2017. [Google Scholar]
- Paio, A.; Fogal, S.; Motterle, R. Enzymatic process for the preparation of testosterone and esters thereof. EP3064589A1, 2016. [Google Scholar]
- Wang, Z.; Yan, J.; Hong, H.; Lin, Y. Process for Preparation of (3S)-5-amino-3-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-5-oxo-pentanoic Acid. CN104356155A, 18 February 2015. [Google Scholar]
- Stensrud, K.; Venkitasubramanian, P. Esterification of 2,5-furan-dicarboxylic acid. US20150315166A1, 5 November 2015. [Google Scholar]
- Yamashita, M.; Kinsho, T. Chemoenzymic Preparation of (2R,12Z)-2-Benzoyloxy-12-heptadecene and (2S,12Z)-2-Hydroxy-12-heptadecene. US20160076063A1, 17 March 2016. [Google Scholar]
- Yuan, Z.; Wang, Z.; Lv, P.; Luo, W.; He, D.; Li, Z.; Fu, J.; Li, H.; Miao, C.; Yang, L. A Method for Preparing Kojic Acid Dipalmitate by Composite Enzymatic Method. CN105296554A, 3 February 2016. [Google Scholar]
- Li, M.; Li, Z. Enzymatic Resolution of Dl-Menthol with Ionic Liquid as Green Medium. CN104531823A, 22 April 2015. [Google Scholar]
- Antoniotti, S.; Filippi, J.J.; Notar Francesco, I.; Ramilijaona, J. Biotechnological Manufacture of Vetiveryl Esters. WO2016193208A1, 8 December 2016. [Google Scholar]
- Guo, K.; Huang, W.; Zhu, N.; Hu, X.; Fang, Z.; Liu, Y. Method for Using Microreactor to Prepare Mercapto Functionalized Polylactone. CN105969816A, 28 September 2016. [Google Scholar]
- Jiang, L.; Fu, Q.; He, H.; Tang, S.; Gu, M. Enzymic Resolution of Racemic Leucine. CN103981248A, 13 August 2014. [Google Scholar]
- Perez-Sanchez, M.; Dominguez de Maria, P. Lipase Catalyzed in Situ Production of Acetaldehyde: A Controllable and Mild Strategy for Multi-Step Reactions. ChemCatChem 2012, 4, 617–619. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, W.; Wang, N. Preparation of 3,4-Dihydro-pyrimidin-2(1H)-one Derivative and Application thereof. CN106588782A, 26 April 2017. [Google Scholar]
- Kapoor, M.; Gupta, M.N. Lipase promiscuity and its biochemical applications. Process. Biochem. 2012, 47, 555–569. [Google Scholar] [CrossRef]
- Arora, B.; Mukherjee, J.; Gupta, M. Enzyme promiscuity: Using the dark side of enzyme specificity in white biotechnology. Sustain. Chem. Process. 2014, 2, 25. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, H.; Ding, Y.; Gu, M.; Li, H.; Ni, X. Preparation of 3-Substituted-2-Indolinone Compound with Lipase. CN104818305A, 5 August 2015. [Google Scholar]
- Hu, Y.; Liang, J.; Sun, A.; Zhang, Y.; Deng, D. A Method for Resolution of Methyl (±)-Mandelate with Esterase. CN104830944A, 12 August 2015. [Google Scholar]
- Hu, Y.; Liang, J.; Zhang, Y.; Sun, A.; Deng, D. Bacillus Esterase BSE01281 and Its Application in Resolution of (±)-1-Phenylethanol and (±)-Styralyl Acetate. CN104962533A, 7 October 2015. [Google Scholar]
- Hu, Y.; Wang, Y.; Zhang, Y. Esterase PHE1414 and Its Coding Gene, and Application as Catalyst in the Preparation of Thereof. CN105802935A, 27 July 2016. [Google Scholar]
- Zhan, C.-G.; Zheng, F.; Fang, L. High Activity Variants of Cocaine Esterase for Cocaine Hydrolysis in the Treatment of Overdose. US20160122732A1, 5 May 2016. [Google Scholar]
- Hou, H.; Wei, W.; Wang, M.; Cheng, J.; Wang, G.; Hu, B.; Li, J.; Yu, P. Enzymatic Preparation Method of 3-Deacetyl-7-Aminocephalosporanic Acid (D-7-Aca) from Cephalosporin C (Cpc) Sodium Salt by Utilizing Immobilized Cpc Acylase and Deacetylase. CN104480181A, 1 April 2015. [Google Scholar]
- Wong, K.; Short, J.M.; Burk, M.J.; Desantis, G.; Farwell, R. Nitrilases, Nucleic Acids Encoding Them and Methods for Making and Using Them. US9315792B2, 19 April 2016. [Google Scholar]
- Burk, M.; Chaplin, J.A.; Chi, E.; DeSantis, G.; Milan, A.; Robertson, D.; Short, J.M.; Weiner, D.P. Nitrilases. AU2013200739B2, 7 March 2013. [Google Scholar]
- Chaplin, J.A.; Weiner, D.P.; Milan, A.; Chi, E.; Short, J.M.; Madden, M.; Burk, M.; Robertson, D.; DeSantis, G. Nitrilases. EP2327767A1, 1 June 2011. [Google Scholar]
- Weiner, D.P.; Chi, E.; Chaplin, J.A.; Milan, A.; Short, J.M.; DeSantis, G.; Madden, M.; Burk, M.; Robertson, D.E. Nitrilases. US8906663B2, 9 December 2014. [Google Scholar]
- Vogel, A.; Schwarze, D.; Greiner-Stoeffele, T. Nitrilases. US8916364B2, 23 December 2014. [Google Scholar]
- Doenhoff, M.J.; Cioli, D.; Utzinger, J. Praziquantel: Mechanisms of action, resistance and new derivatives for schistosomiasis. Curr. Opin. Infect. Dis. 2008, 21, 659–667. [Google Scholar] [CrossRef]
- da Silva, V.B.R.; Boucherle, B.; El-Methni, J.; Hoffmann, B.; da Silva, A.L.; Fortune, A.; de Lima, M.D.A.; Thomas, A. Could we expect new praziquantel derivatives? A meta pharmacometrics/pharmacoinformatics analysis of all antischistosomal praziquantel derivatives found in the literature. SAR QSAR Environ. Res. 2019, 30, 383–401. [Google Scholar] [CrossRef]
- Qian, M. Enzymic Resolution Method for Preparing L-praziquantel. CN102911979A, 6 February 2013. [Google Scholar]
- Yao, P.; Wu, Q.; Yuan, J.; Han, C.; Feng, J.; Zhu, D.; Ma, Y. Method for Preparing Beta-Alanine by Enzymatic Hydrolysis of High Concentration of Beta-Amino Propionitrile. CN104195193A, 10 December 2014. [Google Scholar]
- Toth, C. Pregabalin: Latest safety evidence and clinical implications for the management of neuropathic pain. Ther. Adv. Drug Saf. 2014, 5, 38–56. [Google Scholar] [CrossRef] [PubMed]
- Gerardi, M.C.; Atzeni, F.; Batticciotto, A.; Di Franco, M.; Rizzi, M.; Sarzi-Puttini, P. The safety of pregabalin in the treatment of fibromyalgia. Expert Opin. Drug Saf. 2016, 15, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Mohile, S.S.; Yeranda, S.G.; Lunge, S.M.; Patel, R.M.; Gugale, S.B.; Thakur, R.M.; Mokal, R.A.; Gangopadhyay, A.K.; Nightingale, P.D. An improved green process for the preparation of pregabalin from isovaleraldehyde via enzymatic enantioselective hydrolysis of alkyl 3-cyano-5-methylhexanoate. WO2014072785A2, 15 May 2014. [Google Scholar]
- Mohile, S.S.; Yerande, S.G.; Lunge, S.M.; Patel, R.M.; Gugale, S.B.; Thakur, R.M.; Mokal, R.A.; Gangopadhyay, A.K.; Nightingale, P.D. An Improved Green Process for The Preparation of Pregabalin from Isovaleraldehyde Via Enzymatic Enantioselective Hydrolysis of Alkyl 3-Cyano-5-Methylhexanoate. IN2012MU03228A, 22 July 2016. [Google Scholar]
- Xue, Y.; Zheng, Y.; Xu, Z.; Liu, Z.; Wang, Y.; Su, X.; Jia, D. Method for Preparation of 1-Cyano-Cyclohexyl-Acetic Acid Using Nitrilase Engineering Bacteria. CN104212850A, 17 December 2014. [Google Scholar]
- Tardif, J.C.; Ford, I.; Tendera, M.; Bourassa, M.G.; Fox, K.; Initiative, I. Efficacy of ivabradine, a new selective I-f inhibitor, compared with atenolol in patients with chronic stable angina. Eur. Heart J. 2005, 26, 2529–2536. [Google Scholar] [CrossRef] [PubMed]
- Ide, T.; Ohtani, K.; Higo, T.; Tanaka, M.; Kawasaki, Y.; Tsutsui, H. Ivabradine for the treatment of cardiovascular diseases. Circ. J. 2019, 83, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Pedragosa-Moreau, S.; Lefoulon, F. Process for the Enzymatic Synthesis of (7s)-3,4-Dimethoxybicyclo [4.2.0] Octa-1,3,5-Triene-7-Carboxylic Acid and Application in the Synthesis of Ivabradine And Salts Thereof. US20140242644A1, 28 August 2014. [Google Scholar]
- Tao, J.; Tang, S.; Li, B.; Liu, G. Method for Preparing Monomethyl (R)-3-Hydroxyglutarate as Rosuvastatin Intermediate by Enzymolysis with Recombinant Nitrilase. CN103361386A, 23 October 2013. [Google Scholar]
- Tao, J.; Tang, S.; Li, B.; Liu, G. Method for Preparing Monomethyl (R)-3-Hydroxyglutarate as Rosuvastatin Intermediate by Enzymolysis with Recombinant Nitrilase. WO2014205917A1, 31 December 2014. [Google Scholar]
- Chen, L. Continuous Production Method of Hypolipemic Intermediate (R)-3-Hydroxyl Pentanedioic Acid Ethyl Ester. CN104372040A, 25 February 2015. [Google Scholar]
- Xu, P.; Yu, H.; Han, S.; Li, L. Preparation of Fosamprenavir Intermediate (2R,3S)-1,2-Epoxy-3-Tert-Butoxycarbonylamino-4-Phenylbutane. CN104803954A, 29 July 2015. [Google Scholar]
- Hu, L.; Xi, C.; Peng, D. Synthetic Method of Clopidogrel and Its Sulfate. CN107523594A, 29 December 2017. [Google Scholar]
- Xue, P.; Shuai, H.; Ma, Y.; Zhang, L.; Li, P. Preparation of (S)-2-Chlorophenylglycine Methyl Ester Single Enantiomers by Enzymic Resolution. CN104293875A, 21 January 2015. [Google Scholar]
- Zheng, Y.G.; Yin, H.H.; Yu, D.F.; Chen, X.; Tang, X.L.; Zhang, X.J.; Xue, Y.P.; Wang, Y.J.; Liu, Z.Q. Recent advances in biotechnological applications of alcohol dehydrogenases. Appl. Microbiol. Biotechnol. 2017, 101, 987–1001. [Google Scholar] [CrossRef]
- Zheng, G.-W.; Ni, Y.; Xu, J.-H. Biocatalytic Processes for the Synthesis of Chiral Alcohols. In Applied Biocatalysis: From Fundamental Science to Industrial Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 219–250. [Google Scholar]
- Kratzer, R.; Woodley, J.M.; Nidetzky, B. Rules for biocatalyst and reaction engineering to implement effective, NAD(P)H-dependent, whole cell bioreductions. Biotechnol. Adv. 2015, 33, 1641–1652. [Google Scholar] [CrossRef] [PubMed]
- An, J.H.; Nie, Y.; Xu, Y. Structural insights into alcohol dehydrogenases catalyzing asymmetric reductions. Crit. Rev. Biotechnol. 2019, 39, 366–379. [Google Scholar] [CrossRef]
- Chen, B.S.; de Souza, F.Z.R. Enzymatic synthesis of enantiopure alcohols: Current state and perspectives. RSC Adv. 2019, 9, 2102–2115. [Google Scholar] [CrossRef]
- Uppada, V.; Bhaduri, S.; Noronha, S.B. Cofactor regeneration-an important aspect of biocatalysis. Curr. Sci. 2014, 106, 946–957. [Google Scholar]
- Kara, S.; Schrittwieser, J.H.; Hollmann, F. Strategies for Cofactor Regeneration in Biocatalyzed Reductions; Blackwell Science Publ: Oxford, UK, 2014; pp. 209–238. [Google Scholar]
- Berenguer-Murcia, A.; Fernandez-Lafuente, R. New Trends in the Recycling of NAD(P)H for the Design of Sustainable Asymmetric Reductions Catalyzed by Dehydrogenases. Curr. Org. Chem. 2010, 14, 1000–1021. [Google Scholar] [CrossRef]
- Catapano, A.L. Ezetimibe: A selective inhibitor of cholesterol absorption. Eur. Heart J. Suppl. 2001, 3, E6–E10. [Google Scholar] [CrossRef]
- Bruckert, E.; Giral, P.; Tellier, P. Perspectives in cholesterol-lowering therapy-The role of ezetimibe, a new selective inhibitor of intestinal cholesterol absorption. Circulation 2003, 107, 3124–3128. [Google Scholar] [CrossRef] [PubMed]
- Kosoglou, T.; Statkevich, P.; Johnson-Levonas, A.O.; Paolini, J.F.; Bergman, A.J.; Alton, K.B. Ezetimibe-A review of its metabolism, pharmacokinetics and drug interactions. Clin. Pharmacokinet 2005, 44, 467–494. [Google Scholar] [CrossRef] [PubMed]
- Mundorff, E.; De Vries, E. Ketoreductase Polypeptides for The Stereoselective Production of (4S)-3[(5S)-5(4-Fluorophenyl)-5-hydroxypentanoyl]-4-phenyl-1,3-oxazolidin-2-one. WO2010025085A2, 4 March 2010. [Google Scholar]
- Crowe, M.A.; Alvizo, O.; Behrouzian, B.; Bong, Y.K.; Collier, S.J.; Gohel, A.; Mavinahalli, J.; Modukuru, N.; Mundorff, E.; Smith, D.; et al. Genetically Engineered Carbonyl Reductase for Ezetimibe Synthesis. WO2011140219A1, 10 November 2011. [Google Scholar]
- Luo, Y.; Ding, S.; Qu, X.; Sun, C. Intermediates Used for Synthesis of Ezetimibe, and Their Preparation Method and Application. CN104860980A, 26 August 2015. [Google Scholar]
- Feng, W.; Wang, J. Preparation of Immobilized Ketoreductase or Carbonyl Reductase for Production of Ezetimibe Intermediate with Higher Efficiency and Reduced Wastes. CN105754983A, 13 July 2016. [Google Scholar]
- Wu, J.; Chen, S.; He, X.; Yang, L.; Xu, G. Engineering Bacterium and Method for Preparation of Tert-butyl (3r,5s)-6-chloro-3,5-dihydroxy-hexanoate. CN104087546A, 8 October 2014. [Google Scholar]
- De Lucchi, O.; Tartaggia, S.; Ferrari, C.; Galvagni, M.; Pontini, M.; Fogal, S.; Motterle, R.; Moreno, R.M.; Comely, A. Process for Preparation of Intermediates for the Synthesis of Statins. WO2014128022A1, 28 August 2014. [Google Scholar]
- Hong, H.; Chen, C.; Li, J.; Shen, L.; Guo, L.; Tian, H. Process for Preparation of Chiral Intermediates for Statin Drugs. WO2015168998A1, 12 November 2015. [Google Scholar]
- Scott, J.P.; Peters-Golden, M. Antileukotriene agents for the treatment of lung disease. Am. J. Respir. Crit. Care Med. 2013, 188, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Matsuse, H.; Kohno, S. Leukotriene receptor antagonists pranlukast and montelukast for treating asthma. Expert Opin. Pharmacother. 2014, 15, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Aslani, A.; Beigi, M. Design, formulation, and physicochemical evaluation of montelukast orally disintegrating tablet. Int. J. Prev. Med. 2016, 7, 8. [Google Scholar] [CrossRef]
- Song, J.; Zhang, C.; Long, Z.; Liu, X.; Cai, S. Process for Preparation of Montelukast Sodium Intermediate. CN103936671A, 23 July 2014. [Google Scholar]
- Lou, X.; Wang, X. Method for Preparation of Montelukast Sodium Intermediate. CN104326976A, 4 February 2015. [Google Scholar]
- Lattanzi, S.; Brigo, F.; Cagnetti, C.; Verrotti, A.; Zaccara, G.; Silvestrini, M. Eslicarbazepine acetate in the treatment of adults with partial-onset epilepsy: An evidence-based review of efficacy, safety and place in therapy. Core Evid. 2018, 13, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Gohel, A.; Smith, D.; Wong, B.; Sukumaran, J.; Yeo, W.L.; Collier, S.J.; Novick, S. Biocatalytic Process for Preparing Eslicarbazepine and Analogs Thereof. US20140199735A1, 17 July 2014. [Google Scholar]
- Schena, G.; Caplan, M.J. Everything You Always Wanted to Know about (3)-AR * (* But Were Afraid to Ask). Cells 2019, 8, 357. [Google Scholar] [CrossRef]
- Morriello, G.J.; Wendt, H.R.; Bansal, A.; Di Salvo, J.; Feighner, S.; He, J.F.; Hurley, A.L.; Hreniuk, D.L.; Salituro, G.M.; Reddy, M.V.; et al. Design of a novel pyrrolidine scaffold utilized in the discovery of potent and selective human beta(3) adrenergic receptor agonists. Bioorg. Med. Chem. Lett. 2011, 21, 1865–1870. [Google Scholar] [CrossRef]
- Chung, J.Y.L.; Campos, K.; Cleator, E.; Dunn, R.F.; Gibson, A.; Hoerrner, R.S.; Keen, S.; Lieberman, D.; Liu, Z.; Lynch, J.; et al. Process for Making Beta 3 Agonists and Intermediates. US20140242645A1, 28 August 2014. [Google Scholar]
- Xu, F.; Chung, J.Y.L.; Moore, J.C.; Liu, Z.Q.; Yoshikawa, N.; Hoerrner, R.S.; Lee, J.; Royzen, M.; Cleator, E.; Gibson, A.G.; et al. Asymmetric synthesis of cis-2,5-disubstituted pyrrolidine, the core scaffold of beta (3)-AR agonists. Org. Lett. 2013, 15, 1342–1345. [Google Scholar] [CrossRef]
- Krug, A.W.; Ziegler, C.G.; Bornstein, S.R. DHEA and DHEA-S, and their functions in the brain and adrenal medulla. In Neuroactive Steroids in Brain Function, Behavior and Neuropsychiatric Disorders: Novel Strategies for Research and Treatment; Ritsner, M.S., Weizman, A., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 227–239. [Google Scholar]
- Fryszkowska, A.; Quirmbach, M.S.; Gorantla, S.S.C.; Alieti, S.R.; Poreddy, S.R.; Dinne, N.K.R.; Timmanna, U.; Dahanukar, V. Processes for the Preparation of Dehydroepiandrosterone and Its Intermediates. WO2014188353A2, 27 November 2014. [Google Scholar]
- Xie, X.; Huang, X.; Zhang, J.; Zhang, R. Biological Preparation Method of Dehydroepiandrosterone. CN105695551A, 22 June 2016. [Google Scholar]
- Tao, J.; Le, Y. A Biological Preparation Method of (1R, 2S)-N-pyrrolidinyl Norephedrine. CN104805148A, 29 July 2015. [Google Scholar]
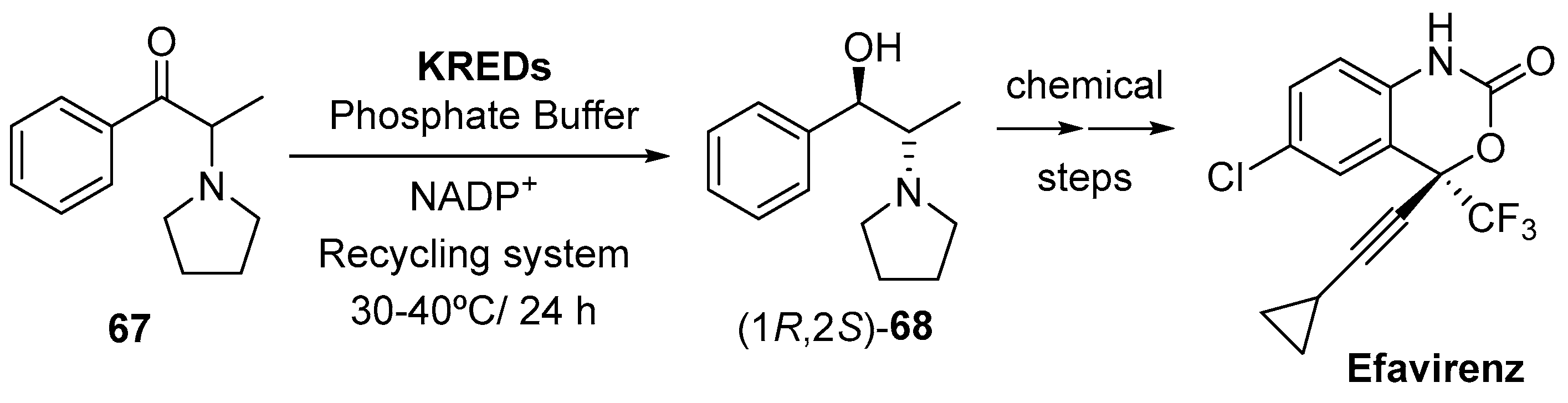
- Yasuda, N.; Tan, L. Efavirenz, a non-nucleoside reverse transcriptase inhibitor (NNRTI), and a previous structurally related development candidate. In Art of Process Chemistry; Yasuda, N., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; pp. 1–43. [Google Scholar]
- Costa, C.C.P.; Boechat, N.; da Silva, F.C.; Rosario, S.L.; Bezerra, T.C.; Bastos, M.M. The Efavirenz: Structure-activity relantionship and synthesis methods. Rev. Virtual Quim. 2015, 7, 1347–1370. [Google Scholar] [CrossRef]
- Jin, Y.; Yao, Y.; Han, G. Enzymatic Method for Preparation of (S)-3-Piperidinol and Its Derivatives with High Chiral Purity from N-3-Piperidone and Its Derivatives. CN103898178A, 2 July 2014. [Google Scholar]
- Wu, Z.; Ren, Z.; Liu, Y. Preparation of Thienyl Chiral Alcohol Compound as Intermediate for Synthesis of Duloxetine by Biocatalysis in the Presence of Carbonyl Reductase. CN104830924A, 12 August 2015. [Google Scholar]
- Carter, N.J.; McCormack, P.L. Duloxetine. CNS Drugs 2009, 23, 523–541. [Google Scholar] [CrossRef] [PubMed]
- Detke, M.J.; Lu, Y.L.; Goldstein, D.J.; Hayes, J.R.; Demitrack, M.A. Duloxetine, 60 mg once daily, for major depressive disorder: A randomized double-blind placebo-controlled trial. J. Clin. Psychiatry 2002, 63, 308–315. [Google Scholar] [CrossRef] [PubMed]
- De Donatis, D.; Florio, V.; Forceili, S.; Saria, A.; Mercolini, L.; Serretti, A.; Conca, A. Duloxetine plasma level and antidepressant response. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 92, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Savile, C.; Gruber, J.M.; Mundorff, E.; Huisman, G.W.; Collier, S.J. Ketoreductase Polypeptides for the Stereospecific Production of (S)-3-aryl-3-hydroxypropanamines from 3-aryl-3-ketopropanamines. WO2010025238A2, 4 March 2010. [Google Scholar]
- Nie, Y.; Xu, Y.; Wang, Y. Method for Asymmetric Synthesis of Duloxetine Intermediate with Carbonyl Reductase. CN105803013A, 27 July 2016. [Google Scholar]
- Zhang, Z.J.; Pan, J.; Ma, B.D.; Xu, J.H. Efficient biocatalytic synthesis of chiral chemicals. In Bioreactor Engineering Research and Industrial Applications; Springer Science and Business Media Deutschland GmbH: Berlin, Germany, 2016; Volume 155, pp. 55–106. [Google Scholar]
- Xie, X.; Huang, X.; Zhang, J.; Zhang, R. Method for Preparing Ethyl (R)-2-hydroxy-4-phenylbutyrate. CN105732373A, 6 July 2016. [Google Scholar]
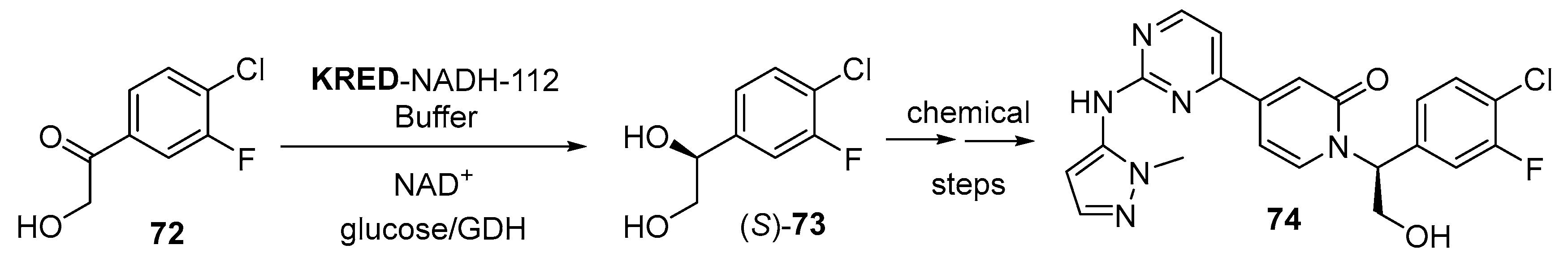
- Lin, J.; Chestakova, A.; Gu, W.; Iding, H.; Li, J.; Linghu, X.; Meier, P.; Sha, C.; Stults, J.; Wang, Y.; et al. Process for the Manufacturing of a Pyrazolylaminopyrimidine Derivative. WO2015154674A1, 15 October 2015. [Google Scholar]
- Blake, J.F.; Burkard, M.; Chan, J.; Chen, H.F.; Chou, K.J.; Diaz, D.; Dudley, D.A.; Gaudino, J.J.; Gould, S.E.; Grina, J.; et al. Discovery of (S)-1-(1-(4-Chloro-3-fluorophenyl)-2-hydroxyethyl)-4-(2-((1-methyl-1H-py razol-5-yl)amino)pyrimidin-4-yl)pyridin-2(1H)-one (GDC-0994), an extracellular signal-regulated kinase 1/2 (ERK1/2) inhibitor in early clinical development. J. Med. Chem. 2016, 59, 5650–5660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.K.; Peyear, T.; Patmanidis, I.; Greathouse, D.V.; Marrink, S.J.; Andersen, O.S.; Ingolfsson, H.I. Fluorinated Alcohols’ Effects on Lipid Bilayer Properties. Biophys. J. 2018, 115, 679–689. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, W.; Liang, X. Preparation of (S)-1,1,1-Trifluoroisopropanol by Enzymic Resolution. CN104894169A, 9 Septenber 2015. [Google Scholar]
- Bong, Y.K.; Vogel, M.; Collier, S.J.; Mitchell, V.; Mavinahalli, J. Polypeptides for a Ketoreductase-Mediated Stereoselective Route to Alpha Chloroalcohols. US8796002B2, 5 August 2014. [Google Scholar]
- Mangas-Sanchez, J.; France, S.P.; Montgomery, S.L.; Aleku, G.A.; Man, H.; Sharma, M.; Ramsden, J.I.; Grogan, G.; Turner, N.J. Imine reductases (IREDs). Curr. Opin. Chem. Biol. 2017, 37, 19–25. [Google Scholar] [CrossRef]
- Grogan, G.; Turner, N.J. InspIRED by nature: NADPH-dependent imine reductases (IREDs) as catalysts for the preparation of chiral amines. Chem. Eur. J. 2016, 22, 1900–1907. [Google Scholar] [CrossRef]
- Maugeri, Z.; Rother, D. Application of Imine Reductases (IREDs) in Micro-Aqueous Reaction Systems. Adv. Synth. Catal. 2016, 358, 2745–2750. [Google Scholar] [CrossRef]
- Maugeri, Z.; Rother, D. Reductive amination of ketones catalyzed by whole cell biocatalysts containing imine reductases (IREDs). J. Biotechnol. 2017, 258, 167–170. [Google Scholar] [CrossRef]
- Lenz, M.; Borlinghaus, N.; Weinmann, L.; Nestl, B.M. Recent advances in imine reductase-catalyzed reactions. World, J. Microbiol. Biotechnol. 2017, 33, 10. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, S.C.; Brzezniak, A.; France, S.P.; Ramsden, J.I.; Mangas-Sanchez, J.; Montgomery, S.L.; Heath, R.S.; Turner, N.J. Imine reductases, reductive aminases, and amine oxidases for the synthesis of chiral amines: Discovery, characterization, and synthetic applications. In Enzymes in Synthetic Biology; Scrutton, N., Ed.; Elsevier Academic Press Inc.: San Diego, CA, USA, 2018; Volume 608, pp. 131–149. [Google Scholar]
- Gamenara, D.; Domínguez de María, P. Enantioselective imine reduction catalyzed by imine reductases and artificial metalloenzymes. Org. Biomol. Chem. 2014, 12, 2989–2992. [Google Scholar] [CrossRef] [PubMed]
- Velikogne, S.; Resch, V.; Dertnig, C.; Schrittwieser, J.H.; Kroutil, W. Sequence-based in-silico discovery, characterisation, and biocatalytic application of a set of imine reductases. ChemCatChem 2018, 10, 3236–3246. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.D.; Grogan, G.; Bommarius, A.; Yun, H. Oxidoreductase-catalyzed synthesis of chiral amines. ACS Catal. 2018, 8, 10985–11015. [Google Scholar] [CrossRef]
- Agard, N.J.; Alvizo, O.; Mayo, M.A.; Minor, S.N.; Riggins, J.N.; Moore, J.C. Variants of the Opine Dehydrogenase of Arthrobacter C1 for Use in the Reductive Amination of Ketones and Amines. US20150132807A1, 14 May 2015. [Google Scholar]
- Chen, H.; Moore, J.; Collier, S.J.; Smith, D.; Nazor, J.; Hughes, G.; Janey, J.; Huisman, G.; Novick, S.; Agard, N.; et al. Engineered Imine Reductases and Methods for the Reductive Amination of Ketone and Amine Compounds. WO2013170050A1, 14 November 2013. [Google Scholar]
- Debarge, S.; Erdman, D.T.; O’Neill, P.M.; Kumar, R.; Karmilowicz, M.J. Process and Intermediates for the Preparation of Pregabalin. WO2014155291A1, 2 October 2014. [Google Scholar]
- Turner, N.J. Enantioselective oxidation of C-O and C-N bonds using oxidases. Chem. Rev. 2011, 111, 4073–4087. [Google Scholar] [CrossRef] [PubMed]
- Hollmann, F.; Arends, I.; Buehler, K.; Schallmey, A.; Buhler, B. Enzyme-mediated oxidations for the chemist. Green Chem. 2011, 13, 226–265. [Google Scholar] [CrossRef]
- Romano, D.; Villa, R.; Molinari, F. Preparative biotransformations: Oxidation of alcohols. ChemCatChem 2012, 4, 739–749. [Google Scholar] [CrossRef]
- Wu, S.K.; Liu, J.; Li, Z. Organic synthesis via oxidative cascade biocatalysis. Synlett 2016, 27, 2644–2658. [Google Scholar]
- Dong, J.J.; Fernandez-Fueyo, E.; Hollmann, F.; Paul, C.E.; Pesic, M.; Schmidt, S.; Wang, Y.H.; Younes, S.; Zhang, W.Y. Biocatalytic oxidation reactions: A chemist’s perspective. Angew. Chem.Int. Ed. 2018, 57, 9238–9261. [Google Scholar] [CrossRef]
- Liu, J.; Wu, S.K.; Li, Z. Recent advances in enzymatic oxidation of alcohols. Curr. Opin. Chem. Biol. 2018, 43, 77–86. [Google Scholar] [CrossRef]
- Schulz, S.; Girhard, M.; Urlacher, V.B. Biocatalysis: Key to Selective Oxidations. ChemCatChem 2012, 4, 1889–1895. [Google Scholar] [CrossRef]
- Gamenara, D.; Seoane, G.; Méndez, P.S.; Domínguez de de María, P. Redox Biocatalysis: Fundamentals and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Wang, Y.H.; Lan, D.M.; Durrani, R.; Hollmann, F. Peroxygenases en route to becoming dream catalysts. What are the opportunities and challenges? Curr. Opin. Chem. Biol. 2017, 37, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Van Rantwijk, F.; Sheldon, R.A. Selective oxygen transfer catalysed by heme peroxidases: Synthetic and mechanistic aspects. Curr. Opin. Biotechnol. 2000, 11, 554–564. [Google Scholar] [CrossRef]
- Hofrichter, M.; Ullrich, R. Oxidations catalyzed by fungal peroxygenases. Curr. Opin. Chem. Biol. 2014, 19, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Urlacher, V.B.; Girhard, M. Cytochrome P450 monooxygenases: An update on perspectives for synthetic application. Trends Biotechnol. 2012, 30, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Fasan, R. Tuning P450 enzymes as oxidation catalysts. ACS Catal. 2012, 2, 647–666. [Google Scholar] [CrossRef]
- Bucko, M.; Gemeiner, P.; Schenkmayerova, A.; Krajcovic, T.; Rudroff, F.; Mihovilovic, M.D. Baeyer-Villiger oxidations: Biotechnological approach. Appl. Microbiol. Biotechnol. 2016, 100, 6585–6599. [Google Scholar] [CrossRef] [PubMed]
- Bondani, P.B.; Fraaije, M.W.; de Gonzalo, G. Recent developments in flavin-based catalysis: Enzymatic sulfoxidations. In Green Biocatalysis; Patel, R.N., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 149–164. [Google Scholar]
- Urlacher, V.B.; Girhard, M. Cytochrome P450 monooxygenases in biotechnology and synthetic biology. Trends Biotechnol. 2019, 37, 882–897. [Google Scholar] [CrossRef]
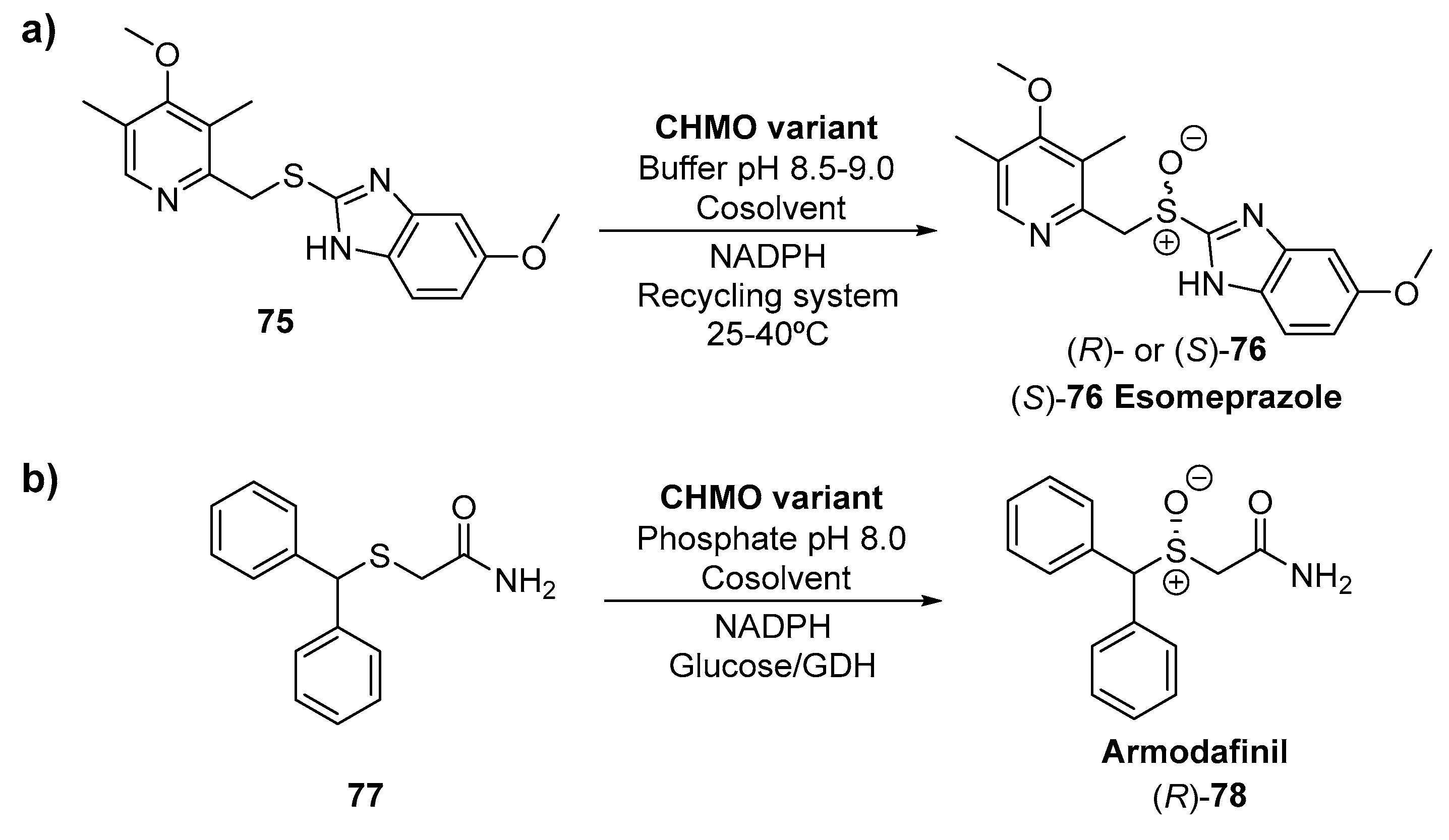
- Strand, D.S.; Kim, D.; Peura, D.A. 25 Years of proton pump inhibitors: A comprehensive review. Gut Liver 2017, 11, 27–37. [Google Scholar] [CrossRef]
- Bong, Y.K.; Clay, M.D.; Collier, S.J.; Mijts, B.; Vogel, M.; Zhang, X.; Zhu, J.; Nazor, J.; Smith, D.; Song, S. Synthesis of Prazole Compounds. US8895271B2, 25 November 2014. [Google Scholar]
- Kayser, M.M.; Clouthier, C.M. New bioorganic reagents: Evolved cyclohexanone monooxygenases—Why is it more selective? J. Org. Chem. 2006, 71, 8424–8430. [Google Scholar] [CrossRef]
- Ang, E.L.; Clay, M.D.; Behrouzian, B.; Eberhard, E.; Collier, S.J.; Fu Fan, J.; Smith, D.; Song, S.; Alvizo, O.; Widegren, M.; et al. Chemoenzymic Synthesis of Armodafinil Using Genetically Modified Acinetobacter Cyclohexanone Monooxygenase. WO2012078800A2, 14 June 2012. [Google Scholar]
- Phillips, J.B.; Simmons, R.G.; Arnold, R.D. A single dose of armodafinil significantly promotes vigilance 11 h post-dose. Mil. Med. 2011, 176, 833–839. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Garnock-Jones, K.P.; Dhillon, S.; Scott, L.J. Armodafinil. CNS Drugs 2009, 23, 793–803. [Google Scholar] [CrossRef] [PubMed]
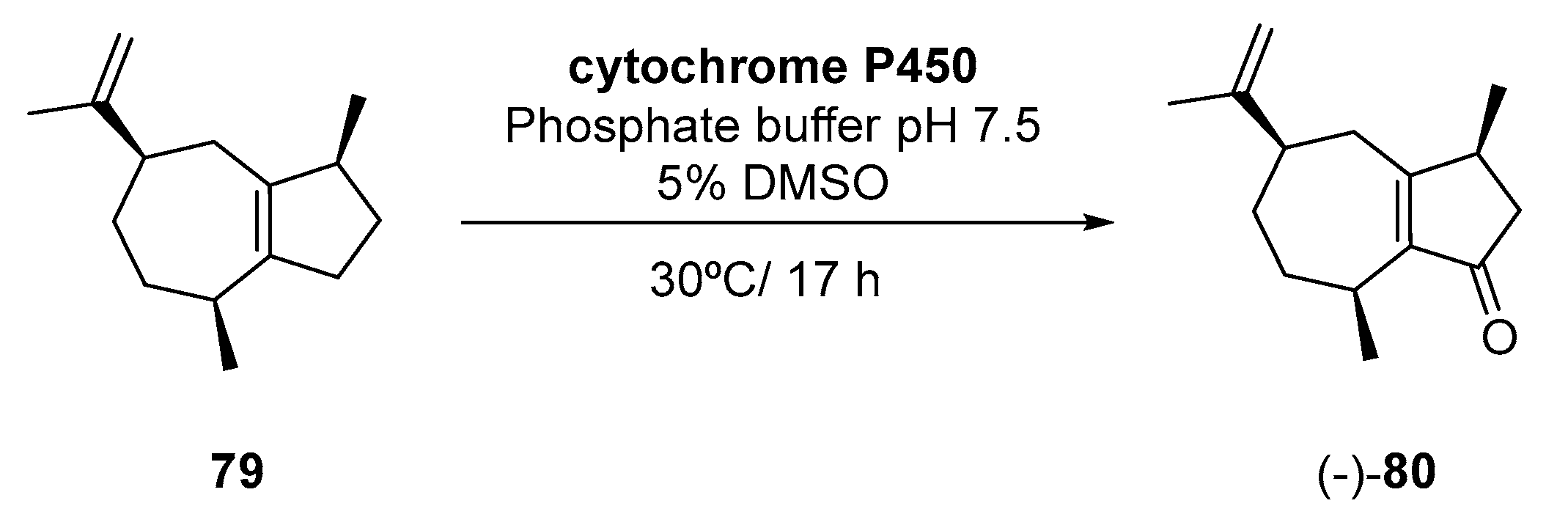
- Kino, K.; Furuya, T. Production of (−)-rotundone from α-guaiene catalyzed by cytochrome P450 enzymes. EP3255151A2, 13 December 2017. [Google Scholar]
- Wood, C.; Siebert, T.E.; Parker, M.; Capone, D.L.; Elsey, G.M.; Pollnitz, A.P.; Eggers, M.; Meier, M.; Vossing, T.; Widder, S.; et al. From wine to pepper: Rotundone, an obscure sesquiterpene, is a potent spicy aroma compound. J. Agric. Food Chem. 2008, 56, 3738–3744. [Google Scholar] [CrossRef] [PubMed]
- Pecyna, M.J.; Schnorr, K.M.; Ullrich, R.; Scheibner, K.; Kluge, M.G.; Hofrichter, M. Fungal Peroxygenases and Use for Regioselective Oxidation of N-Heterocycles. WO2008119780A2, 9 October 2008. [Google Scholar]
- Steffen-Munsberg, F.; Vickers, C.; Kohls, H.; Land, H.; Mallin, H.; Nobili, A.; Skalden, L.; van den Bergh, T.; Joosten, H.J.; Berglund, P.; et al. Bioinformatic analysis of a PLP-dependent enzyme superfamily suitable for biocatalytic applications. Biotechnol. Adv. 2015, 33, 566–604. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.D.; Grogan, G.; Bommarius, A.; Yun, H. Recent Advances in omega-transaminase-mediated biocatalysis for the enantioselective synthesis of chiral amines. Catalysts 2018, 8, 254. [Google Scholar] [CrossRef]
- Bornscheuer, U.; Hanlon, S.P.; Iding, H.; Pavlidis, I.; Spurr, P.; Steffen Weiss, M.; Wirz, B. Variants of a Bacterial Transaminase with Increased Catalytic Activity for the Synthesis of Chiral Amines. WO2016166120A1, 20 October 2016. [Google Scholar]
- Dhawan, I.K.; Miller, G.; Zhang, X. Transaminase Polypeptides with Improved Thermostability and Enantioselectivity for the Synthesis of Chiral Amines. WO2010081053A2, 15 July 2010. [Google Scholar]
- Hughes, G.; Devine, P.N.; Fleitz, F.J.; Grau, B.T.; Limanto, J.; Savile, C.; Mundorff, E. Engineering of Arthrobacter Transaminase Variants, and Use for Biocatalytic Asymmetric Preparation of Chiral Amines from Prochiral Ketones. WO2011005477A1, 13 January 2011. [Google Scholar]
- Savile, C.; Mundorff, E.; Moore, J.C.; Devine, P.N.; Janey, J.M. Construction of Arthrobacter KNK168 Transaminase Variants for Biocatalytic Manufacture of Sitagliptin. WO2010099501A2, 2 September 2010. [Google Scholar]
- Schuermann, M.; Peeters, W.P.H.; Smeets, N.H.J.; Schwab, H.; Steiner, K.; Lypetska, K.M.; Strohmeier, G. Stereoselective Transamination Catalyzed by Novel R-Selective Transaminases. WO2012007548A1, 19 January 2012. [Google Scholar]
- Hong, H.; Gao, F.; Li, Y.; Zhang, Y.; Li, S. Sequence of Transaminase Mutants and Its Use for Synthesizing R configuration Chiral Amine. CN104328094A, 4 February 2015. [Google Scholar]
- Tao, R.; Zhu, F.; Shen, Q.; Sun, L.; Shen, Z.; Jiang, Y.; Yang, C. Transaminase for Producing Antiepileptic Drug Levetiracetam Intermediate L-2-aminobutyric acid. CN105441403A, 30 March 2016. [Google Scholar]
- Wang, H.; Xie, Y.; Wang, J.; Fan, H.; Wei, D. S type ω-transaminase ATA-W12 and Gene and Application Thereof. CN106520719A, 22 March 2017. [Google Scholar]
- Shin, J.-S.; Park, E.-S.; Han, S.-W. Omega-transaminase Bacterial Mutants with Activity Improvements Toward Ketones and Methods for Producing Optically Pure Amines. US20160298092A1, 13 October 2016. [Google Scholar]
- Shin, J.S.; Park, E.S. Production of Optically Active Amines by Using Omega Transaminase Derived from Ochrobactrum Anthropi and Devoid of Substrate and Product Inhibition. KR2014123166A, 22 October 2014. [Google Scholar]
- Truppo, M.D.; Janey, J.M.; Hughes, G. Immobilization of Transaminases on Resin Carriers for Use in the Manufacture of Sitagliptin. WO2012177527A1, 27 December 2012. [Google Scholar]
- Huang, Q.; Zhang, X.; Xiang, S. One Kind of Immobilization Method of Transaminase. CN104774830A, 15 July 2015. [Google Scholar]
- Truppo, M.D.; Journet, M.; Strotman, H.; McMullen, J.P.; Grosser, S.T. Immobilized Transaminases for the Preparation of Sitagliptin by Stereospecific Amination of Prositagliptin Ketone. WO2014133928A1, 4 September 2014. [Google Scholar]
- Aroda, V.R.; Henry, R.R.; Han, J.; Huang, W.Y.; DeYoung, M.B.; Darsow, T.; Hoogwerf, B.J. Efficacy of GLP-1 Receptor Agonists and DPP-4 Inhibitors: Meta-Analysis and Systematic Review. Clin. Ther. 2012, 34, 1247–1258. [Google Scholar] [CrossRef]
- Luo, Y.; Ding, S.; Qu, X.; Guo, F. Immobilized Transaminase and Its Application in Synthesizing Sitagliptin Intermediate. CN104805069A, 29 July 2015. [Google Scholar]
- Luo, Y.; Ding, S.; Qu, X. Immobilized Transaminase and Its Application in Synthesis of Sitagliptin Intermediate. CN105219745A, 6 January 2016. [Google Scholar]
- Luo, Y.; Ding, S.; Qu, X. Mycobacterium Vanbaalenii Transaminase and Its Application in Preparing Sitagliptin Intermediate. CN105018440A, 4 November 2015. [Google Scholar]
- Savile, C.K.; Janey, J.M.; Mundorff, E.C.; Moore, J.C.; Tam, S.; Jarvis, W.R.; Colbeck, J.C.; Krebber, A.; Fleitz, F.J.; Brands, J.; et al. Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture. Science 2010, 329, 305–309. [Google Scholar] [CrossRef]
- Robins, K.; Bornscheuer, U.; Hoehne, M. Process for Preparation of Optically Active N-protected 3-Aminopyrrolidine or Optically Active N-protected 3-aminopiperidine and the Corresponding Ketones by Optical Resolution of the Racemic Amine Mixtures Employing a Bacterial Omega-Transaminase. WO2008028654A1, 13 March 2008. [Google Scholar]
- Robins, K.; Bornscheuer, U.; Hoehne, M. Preparation of Optically Active Chiral Amines Using a Stereoselective transaminase. EP1818411A1, 15 August 2007. [Google Scholar]
- Lin, Y.; Hong, H.; Yan, J.; Wang, Z. Preparation of 3-Aminopyrrolidine Compounds and Their Application. CN104262225A, 7 January 2015. [Google Scholar]
- Stass, H.; Dalhoff, A.; Kubitza, D.; Schuhly, U. Pharmacokinetics, safety, and tolerability of ascending single doses of moxifloxacin, a new 8-methoxy quinolone, administered to healthy subjects. Antimicrob. Agents Chemother. 1998, 42, 2060–2065. [Google Scholar] [CrossRef]
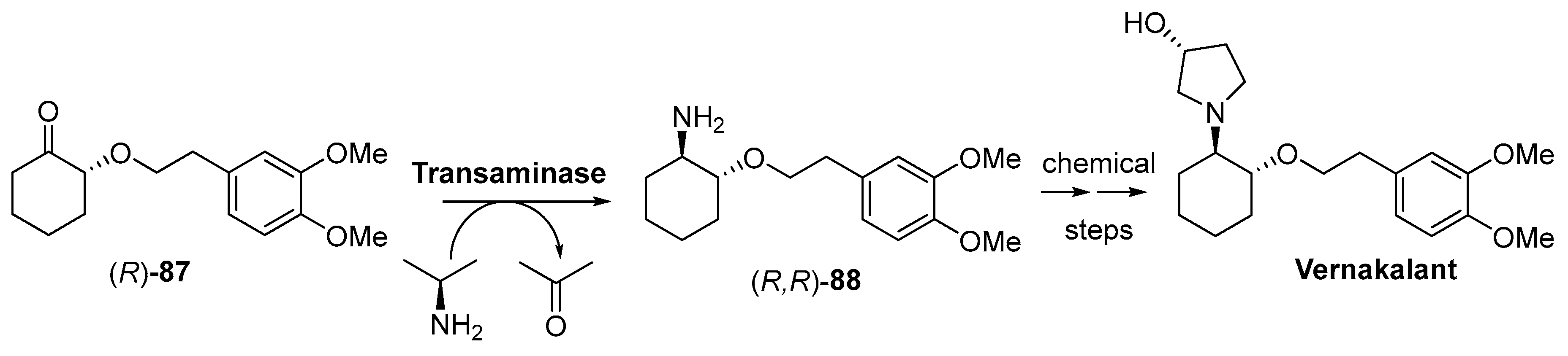
- Limanto, J.; Beutner, G.; Grau, B.; Janey, J.; Klapars, A.; Ashley, E.R.; Strotman, H.R.; Truppo, M.D.; Hughes, G.; Cabirol, F.; et al. Biocatalysts and Methods for the Synthesis of (1R,2R)-2-(3,4-dimethoxyphenethoxy)cyclohexanamine. US8932836B2, 13 January 2015. [Google Scholar]
- Kossaify, A. Vernakalant in atrial fibrillation: A relatively new weapon in the armamentarium against an old enemy. Drug Target. Insights 2019, 13, 7. [Google Scholar] [CrossRef]
- Hall, A.J.M.; Mitchell, A.R.J. Introducing Vernakalant into clinical practice. Arrhythm. Electrophysiol. Rev. 2019, 8, 70–74. [Google Scholar] [CrossRef]
- Cabirol, F.; Gohel, A.; Oh, S.H.; Smith, D.; Wong, B.; LaLonde, J. Biocatalysts and Methods for the Synthesis of (S)-3-(1-aminoethyl)-phenol. WO2011159910A2, 22 December 2011. [Google Scholar]
- Cabirol, F.; Chen, H.; Gohel, A.; Salim, P.; Smith, D.; Janey, J.; Kosjek, B.; Tang, W.L.; Hsieh, H.; Pham, S. Biocatalysts and Methods for the Synthesis of Substituted Lactams. US9109209B2, 18 August 2015. [Google Scholar]
- Limanto, J.; Beutner, G.L.; Yin, J.; Klapars, A.; Ashley, E.R.; Strotman, H.R.; Truppo, M.D.; Chung, C.K.; Hughes, G.; Liu, Z.; et al. Process for Preparing Aminocyclohexyl Ether Compounds. US9006460B2, 14 April 2015. [Google Scholar]
- Austad, B.; Bahadoor, A.; Belani, J.D.; Janardanannair, S.; Johannes, C.W.; Keaney, G.F.; Lo, C.K.; Wallerstein, S.L. Enzymatic Transamination of Cyclopamine Analogs. WO2011017551A1, 10 February 2011. [Google Scholar]
- Crowe, M.; Foulkes, M.; Francese, G.; Grimler, D.; Kuesters, E.; Laumen, K.; Li, Y.; Lin, C.; Nazor, J.; Ruch, T.; et al. Chemical Process for Preparing Spiroindolones and Intermediates Thereof. WO2013139987A1, 26 September 2013. [Google Scholar]
- Sieber, V.; Grammann, K.; Ruehmann, B.; Haas, T.; Pfeffer, J.; Doderer, K.; Rollmann, C.; Skerra, A.; Rausch, C.; Lerchner, A. Enzymic transamination of multicyclic dianhydro diuloses. WO2010089171A2, 12 August 2010. [Google Scholar]
- Tao, J.; Liang, X.; Le, Y. Method for Preparation of (S)-2-amino-1-butanol by Biological Process. CN105177071A, 23 December 2015. [Google Scholar]
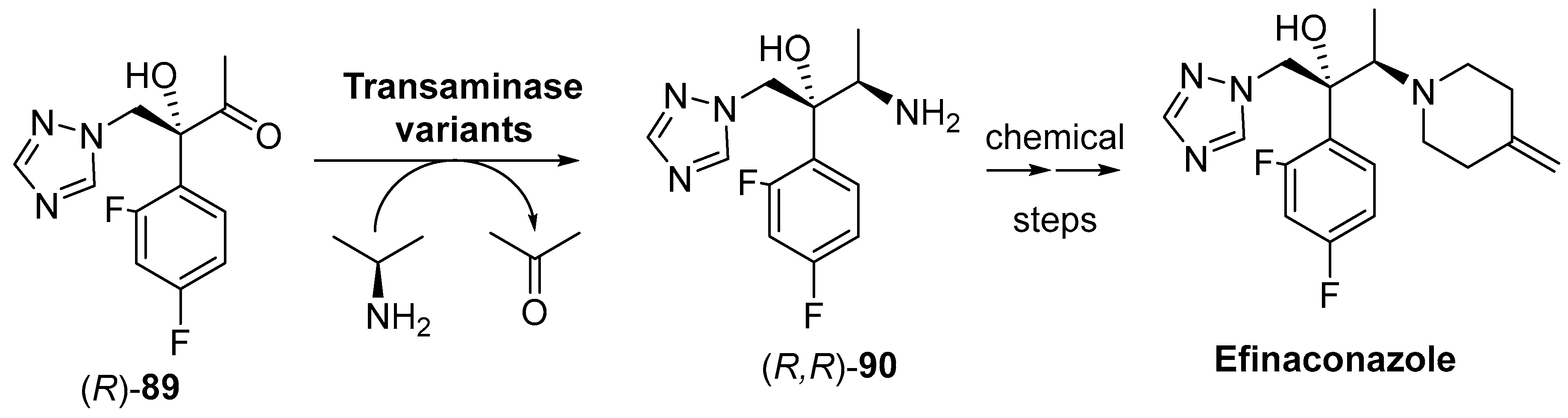
- Qu, D.; Huang, Y. Synthesis Method of Efinaconazole Intermediate. CN105039450A, 11 November 2015. [Google Scholar]
- Tatsumi, Y.; Nagashima, M.; Shibanushi, T.; Iwata, A.; Kangawa, Y.; Inui, F.; Siu, W.J.J.; Pillai, R.; Nishiyama, Y. Mechanism of action of efinaconazole, a novel triazole antifungal agent. Antimicrob. Agents Chemother. 2013, 57, 2405–2409. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Cernea, M. How effective is efinaconazole in the management of onychomycosis? Expert Opin. Pharmacother. 2016, 17, 611–618. [Google Scholar] [CrossRef] [PubMed]
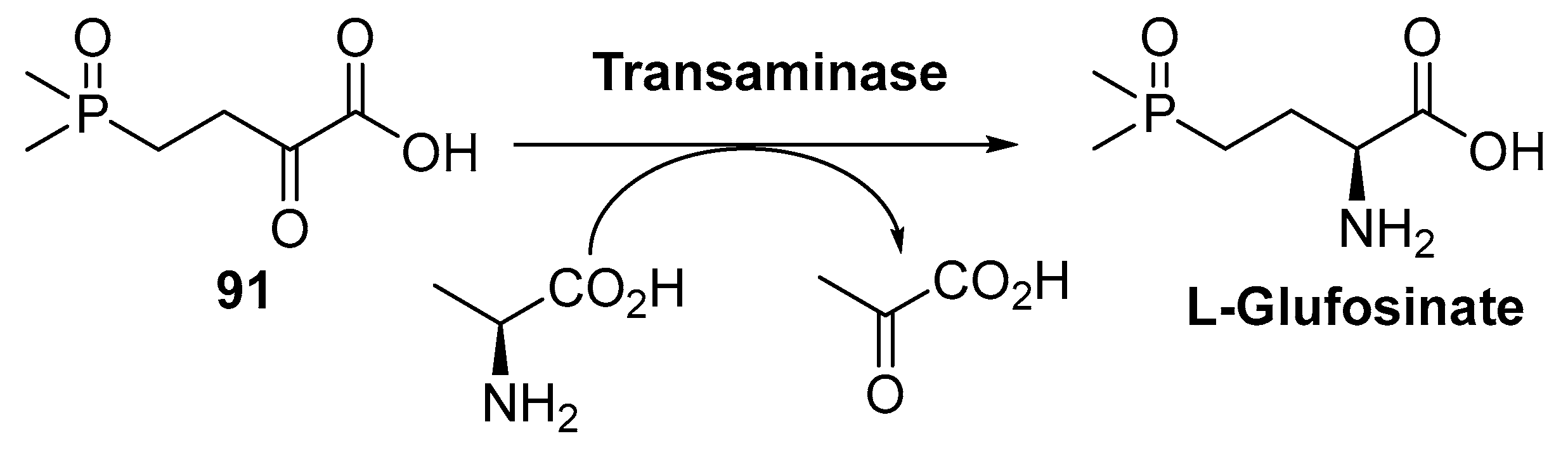
- Occhipinti, A.; Berlicki, L.; Giberti, S.; Dziedziola, G.; Kafarski, P.; Forlani, G. Effectiveness and mode of action of phosphonate inhibitors of plant glutamine synthetase. Pest Manag. Sci. 2010, 66, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhou, H.; Meng, L.; Liu, S.; Wei, Y.; Gu, S.; Yuan, X.; Fang, H.; Liu, Y.; Xu, G.; et al. A Production Method of L-glufosinate ammonium. CN105603015A, 25 May 2016. [Google Scholar]
- Green, B.M.; Gradley, M.L. Two-step Enzymic Synthesis of L-glufosinate from Racemic Mixture. US20170253897A1, 7 September 2017. [Google Scholar]
- Bulger, P.G.; Kosjek, B.; Rivera, N. Biocatalytic Transamination Process for the Enantioselective Syntheses of 3-Arylpiperidine, Key Intermediate in the Prepn. of Poly (adp-ribose) Polymerase Inhibitors. WO2014088984A1, 12 June 2014. [Google Scholar]
- Kim, M.H.; Han, G.S. Method for Producing D-alanine Using Fusion Enzyme. KR2017132446A, 4 December 2017. [Google Scholar]
- Yun, D.H.; Lee, H.S. Enzymatic Production Method of Optically Active Beta-Amino Acids Including Intermediate for the Synthesis of Sitagliptin. KR101565439B1, 18 November 2015. [Google Scholar]
- Shin, J.S.; Park, E.S.; Dong, J.Y. Simultaneous Production of High-Purity Optically Active Alkylamine and Aryl Alkylamine Using Transaminase. KR2014108422A, 11 September 2014. [Google Scholar]
- Shin, J.-S.; Park, E.-S.; Malik, M.S. Method for Preparing Optically Active Amine Compound Using Deracemization. WO2014092496A1, 19 June 2014. [Google Scholar]
- Huisman, G.W.; Agard, N.J.; Mijts, B.; Vroom, J.; Zhang, X. Robust Variants of a Phenylalanine Ammonia Lyase with Increased Catalytic Activity for Therapeutic Use. WO2014172541A2, 23 October 2014. [Google Scholar]
- Wu, B.; Szymanski, W.; Crismaru, C.G.; Feringa, B.L.; Janssen, D.B. C-N Lyases Catalyzing Addition of Ammonia, Amines, and Amides to C=C and C=O Bonds. In Enzyme Catalysis in Organic Synthesis, 3rd ed.; Drauz, K., Groeger, H., May, O., Eds.; Jonh Wiley and Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 749–778. [Google Scholar]
- Huisman, G.W.; Agard, N.J.; Elgart, D.; Zhang, X. Tyrosine: Ammonia Lyase Variants Stable at Acid pH and Resistant to Proteolysis and Their Use in Treatment of Metabolic Disease. WO2015161019A1, 22 October 2015. [Google Scholar]
- Weiner, D.; Varvak, A.; Richardson, T.; Podar, M.; Burke, E.; Healey, S. Lyase Enzymes, Nucleic Acids Encoding Them and Methods for Their Production and Commercial Use. WO2006099207A2, 21 September 2006. [Google Scholar]
- Bruehlmann, F.; Fourage, L.; Wahler, D. Engineering of Modified Guava 13-hydroperoxide Lyase Variants with Improved Enzymic Activity for Use in the Production of Aldehydes and Alcohols. WO2009001304A1, 31 December 2008. [Google Scholar]
- Asano, Y.; Dadashipour Lakmehsar, M.; Ishida, Y. Purification and characterization of novel (R)-hydroxynitrile lyase. WO2015133462A1, 11 September 2015. [Google Scholar]
- Eggert, T.; Andexer, J. An (R)-hydroxynitrile Lyase from a Non-Cyanogenic Member of the Brassicaceae for Use in Stereoselective Addition. WO2008067807A2, 12 June 2008. [Google Scholar]
- Glieder, A. R-Hydroxynitrile Lyase Random Variants and Their Use for Preparing Optically Pure, Sterically Hindered Cyanohydrins. WO2008071695A1, 19 June 2008. [Google Scholar]
- Xiao, Y.; Yang, W.; Chen, M.; Gong, B.; Yan, Y.; Tan, C. Levodopa Crystal Powder and Preparation Method Thereof. CN106117072A, 16 November 2016. [Google Scholar]
- De Deurwaerdère, P.; Di Giovanni, G.; Millan, M.J. Expanding the repertoire of L-DOPA’s actions: A comprehensive review of its functional neurochemistry. Prog. Neurobiol. 2017, 151, 57–100. [Google Scholar] [CrossRef] [PubMed]
- Jenner, P. Molecular mechanisms of L-DOPA-induced dyskinesia. Nat. Rev. Neurosci. 2008, 9, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Cools, R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson’s disease. Neurosci. Biobehav. Rev. 2006, 30, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Rother, D.; Pohl, M.; Sehl, T.; Baraibar, A.G. Enzymic Twostep Process for the Production of Cathine. DE102013009631A1, 11 December 2014. [Google Scholar]
- Rother, D.; Pohl, M.; Sehl, T.; Baraibar, A.G. Process for Producing Cathine. WO2014198247A1, 18 December 2014. [Google Scholar]
- Hauner, H.; Hastreiter, L.; Werdier, D.; Chen-Stute, A.; Scholze, J.; Bluher, M. Efficacy and safety of cathine (nor-pseudoephedrine) in the treatment of obesity: A randomized dose-finding study. Obes. Facts 2017, 10, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Alsufyani, H.A.; Docherty, J.R. Cathine and MHA act as indirect sympathomimetics to produce cardiovascular actions. Br. J. Pharmacol. 2019, 176, 3036. [Google Scholar]
- Clapés, P. Enzymatic C-C Bond Formation. In Organic Synthesis Using Biocatalysis; Goswami, A., Stewart, J.D., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 285–337. [Google Scholar]
- Brovetto, M.; Gamenara, D.; Mendez, P.S.; Seoane, G.A. C-C Bond-forming lyases in organic synthesis. Chem. Rev. 2011, 111, 4346–4403. [Google Scholar] [CrossRef]
- Sehl, T.; Hailes, H.C.; Ward, J.M.; Menyes, U.; Pohl, M.; Rother, D. Efficient 2-step biocatalytic strategies for the synthesis of all nor(pseudo) ephedrine isomers. Green Chem. 2014, 16, 3341–3348. [Google Scholar] [CrossRef]
- Sehl, T.; Hailes, H.C.; Ward, J.M.; Wardenga, R.; von Lieres, E.; Offermann, H.; Westphal, R.; Pohl, M.; Rother, D. Two steps in one pot: Enzyme cascade for the synthesis of nor(pseudo)ephedrine from inexpensive starting materials. Angew. Chem. Int. Ed. 2013, 52, 6772–6775. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, V.; Sehl, T.; Frindi-Wosch, I.; Simon, R.C.; Kroutil, W.; Rother, D. Methoxamine Synthesis in a Biocatalytic 1-Pot 2-Step Cascade Approach. ACS Catal. 2019, 9, 7380–7388. [Google Scholar] [CrossRef]
- Mrak, P.; Zohar, T.; Oslaj, M.; Kopitar, G. Enzymatic Synthesis of Statins and Intermediates Thereof. WO2012095244A2, 19 July 2012. [Google Scholar]
- Casar, Z.; Mesar, T.; Kopitar, G.; Mrak, P.; Oslaj, M. Synthesis of Statins from (4R,6S)-6-(dialkoxymethyl) tetrahydro-2H-pyran-2,4-diol. WO2008119810A2, 9 October 2008. [Google Scholar]
- Cluzeau, J.; Casar, Z.; Mrak, P.; Oslaj, M.; Kopitar, G. Process for the Production of ((2S,4R)-4,6-dihydroxytetrahydro-2H-pyran-2-yl)methyl Carboxylates and Its Further use to Synthesisze Statins. WO2009092702A2, 20 July 2009. [Google Scholar]
- Bauer, D.W.; Hu, S.; O’Neill, P.M.; Watson, T.J.N. Process for Preparing Chiral Compounds Using 2-Deoxyribose-5-phosphate aldolase. WO2009019561A2, 12 February 2009. [Google Scholar]
- Matsumoto, K.; Kazuno, Y.; Higashimura, N.; Ohshima, T.; Sakuraba, H. Enzymic Preparation of Chiral Hydroxyaldehydes. WO2005098012A1, 20 October 2005. [Google Scholar]
- Zhu, G.; Qi, X.; Li, B.; Wang, C.; Zhang, H.; Liu, Y.; Pan, X. Chemoenzymic Synthesis of Atorvastatin Calcium Side Chain with High Chiral Purity. CN103060396A, 12 September 2013. [Google Scholar]
- Oh, D.G.; Lee, S.H.; Hong, S.H. Method for Producing Tagatose from Fructose and Enzyme Composition Therefor. KR1480422B1, 5 February 2015. [Google Scholar]
- Oh, D.K.; Hong, S.H.; Lee, S.H. Method for Producing Tagatose from Fructose and Enzyme Composition Therefor. WO2015016544A1, 5 February 2015. [Google Scholar]
- Xu, G.; Zeng, H.; Zhao, S.; Chen, M. Method for Producing L-alpha-aminobutyric acid with High Optical Activity by Biological Catalysis. CN104774881A, 15 July 2015. [Google Scholar]
- Kroutil, W.; Busto, E.; Simon, R. Method for Producing p-vinylphenols. AT516155A4, 15 March 2016. [Google Scholar]
- Kroutil, W.; Busto, E.; Simon, R. Method for Producing p-vinylphenols. WO2016141397A1, 15 September 2016. [Google Scholar]




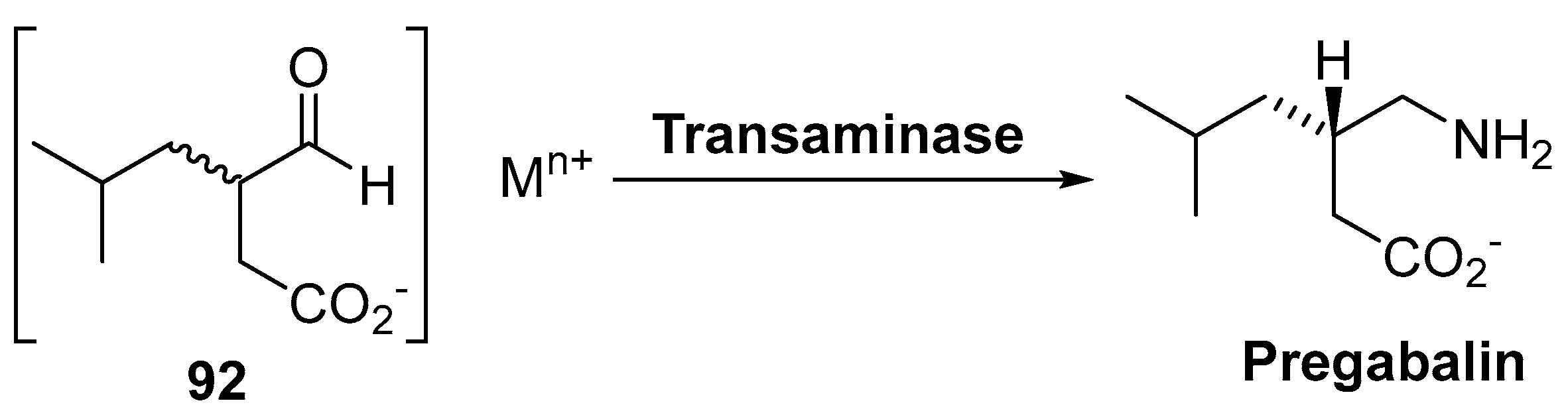
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de María, P.D.; de Gonzalo, G.; Alcántara, A.R. Biocatalysis as Useful Tool in Asymmetric Synthesis: An Assessment of Recently Granted Patents (2014–2019). Catalysts 2019, 9, 802. https://doi.org/10.3390/catal9100802
de María PD, de Gonzalo G, Alcántara AR. Biocatalysis as Useful Tool in Asymmetric Synthesis: An Assessment of Recently Granted Patents (2014–2019). Catalysts. 2019; 9(10):802. https://doi.org/10.3390/catal9100802
Chicago/Turabian Stylede María, Pablo Domínguez, Gonzalo de Gonzalo, and Andrés R. Alcántara. 2019. "Biocatalysis as Useful Tool in Asymmetric Synthesis: An Assessment of Recently Granted Patents (2014–2019)" Catalysts 9, no. 10: 802. https://doi.org/10.3390/catal9100802
APA Stylede María, P. D., de Gonzalo, G., & Alcántara, A. R. (2019). Biocatalysis as Useful Tool in Asymmetric Synthesis: An Assessment of Recently Granted Patents (2014–2019). Catalysts, 9(10), 802. https://doi.org/10.3390/catal9100802