Synthesis of ZSM-5 Zeolite Using Coal Fly Ash as an Additive for the Methanol to Propylene (MTP) Reaction
Abstract
1. Introduction
2. Results and Discussion
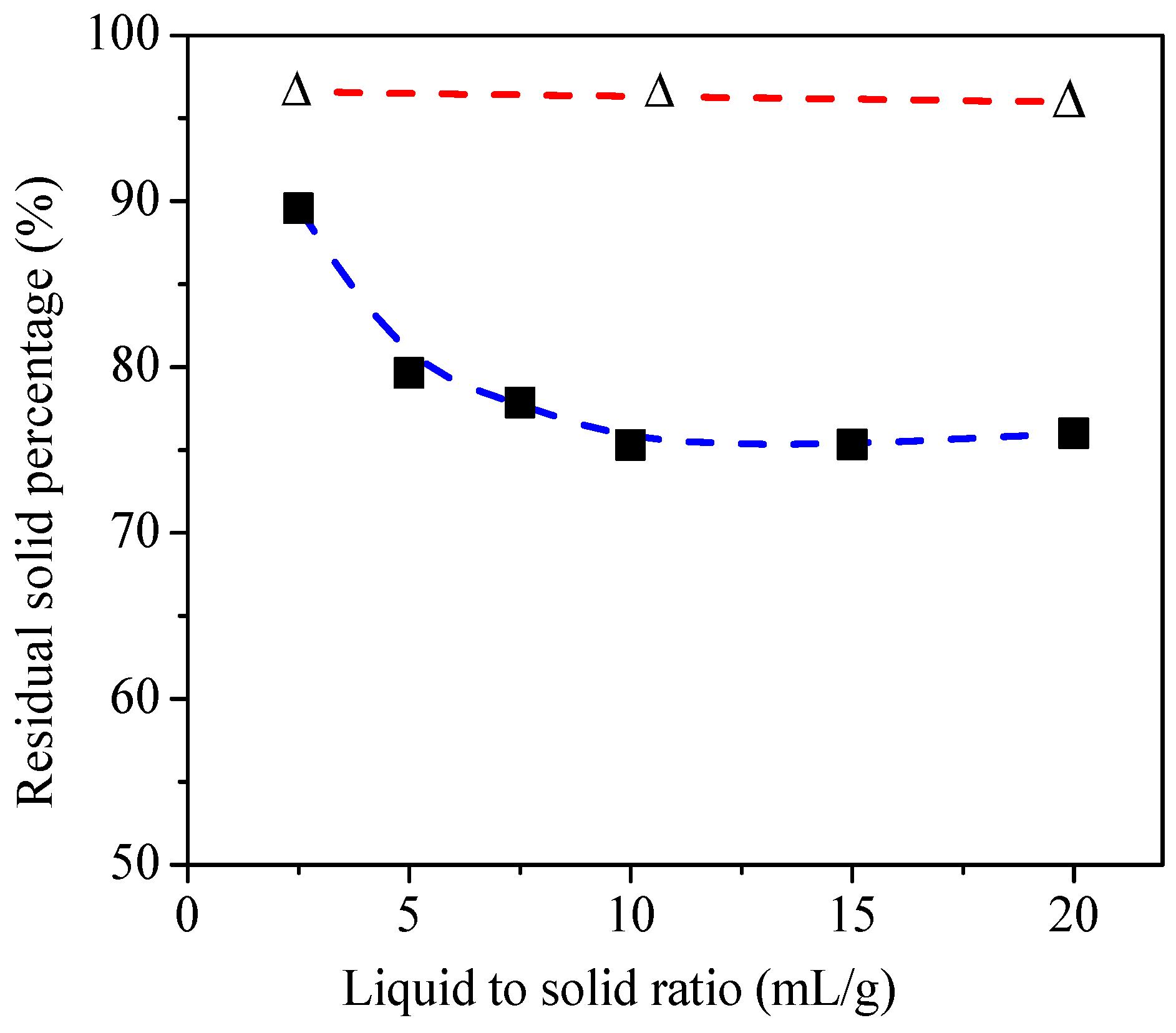
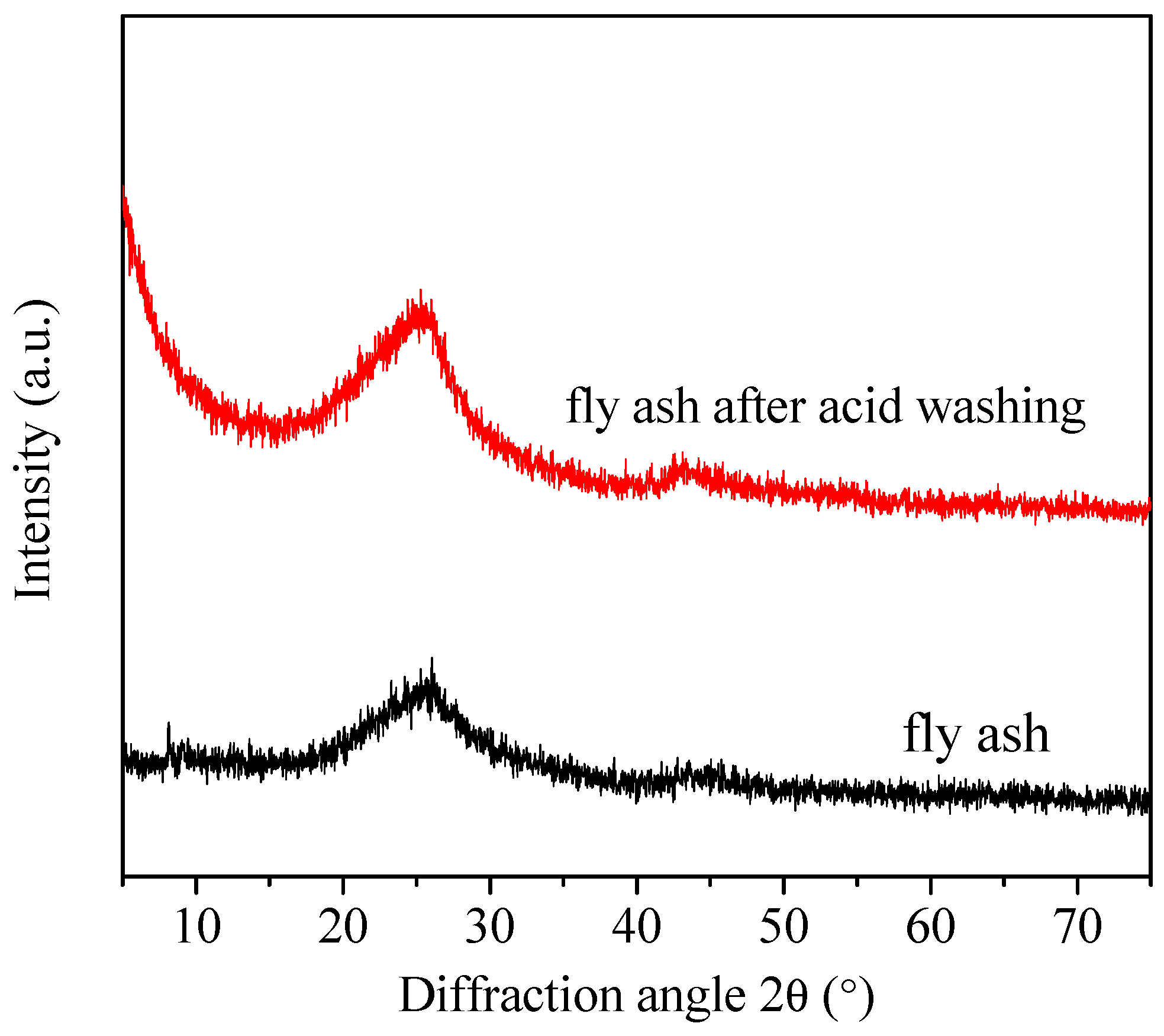
2.1. Fly Ash Pretreatment
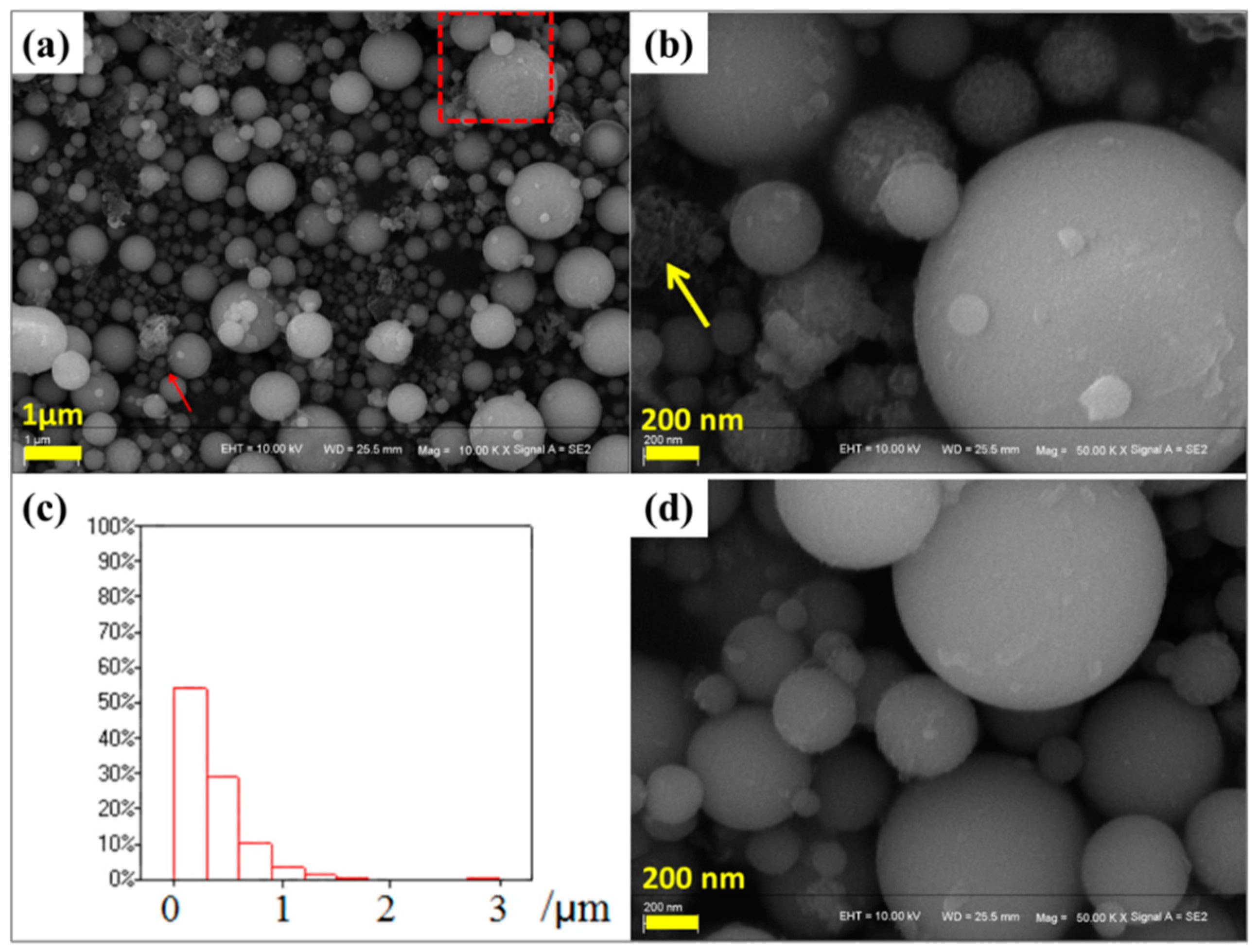
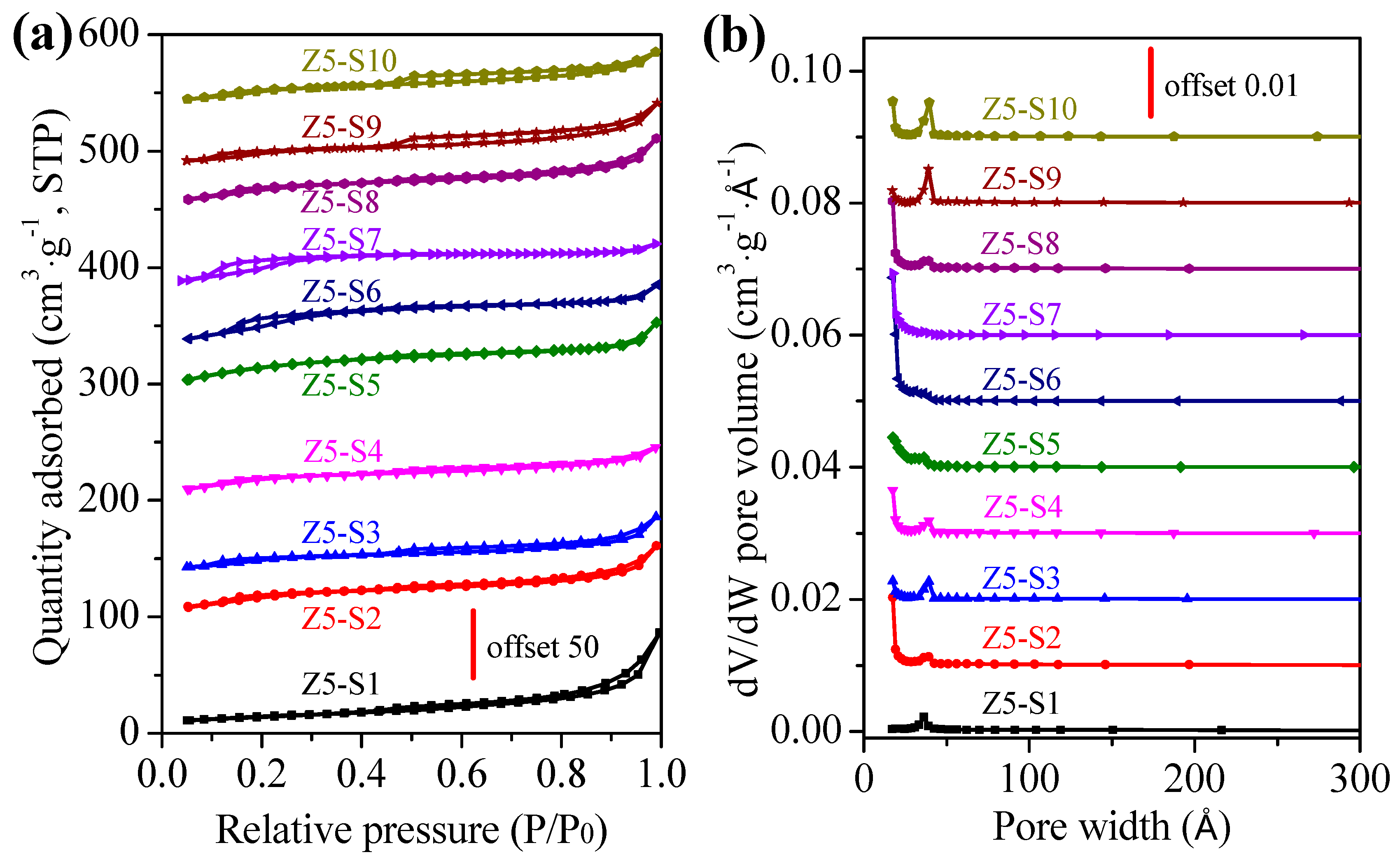
2.2. Physicochemical Properties of ZSM-5 Zeolites
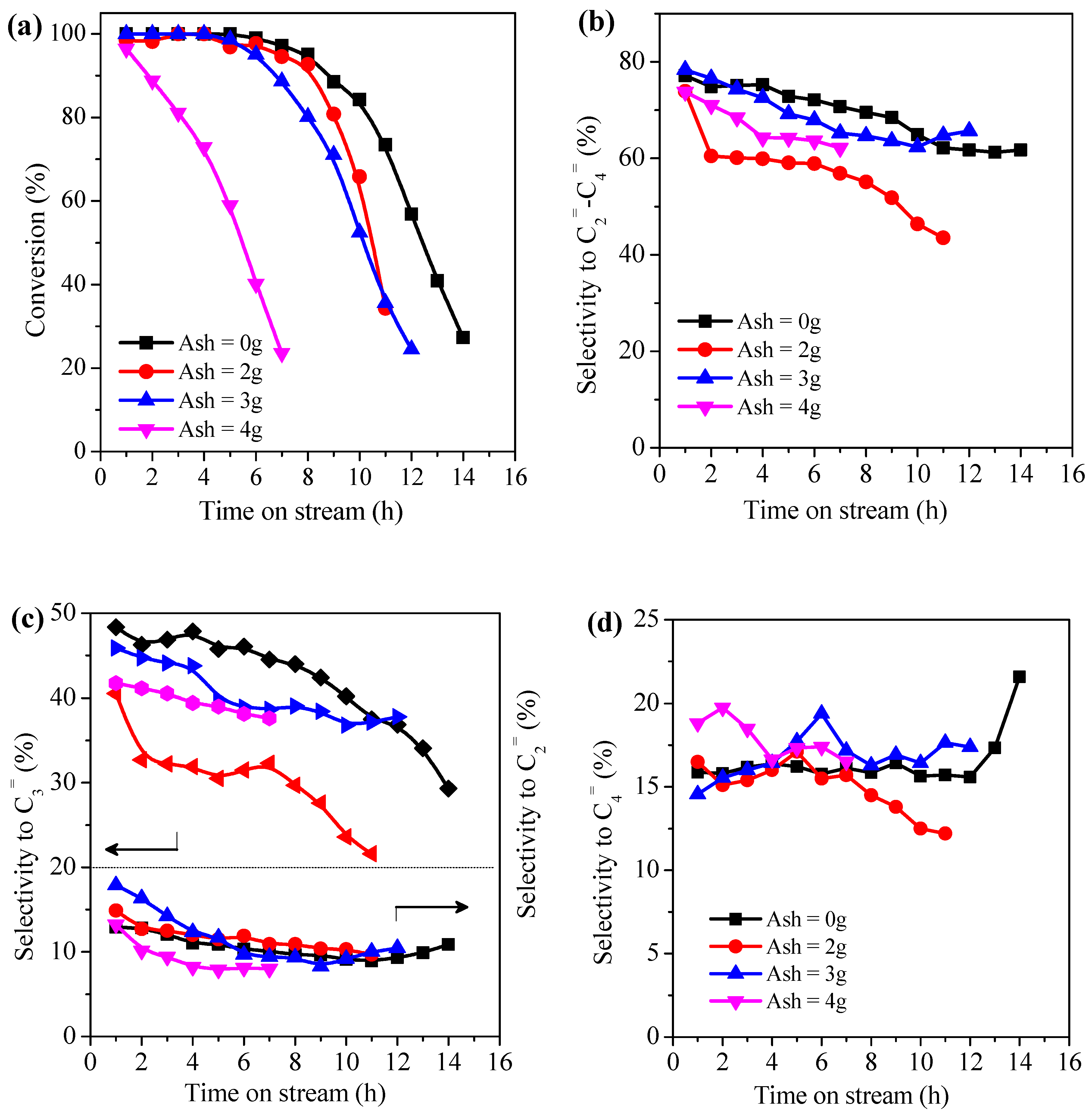
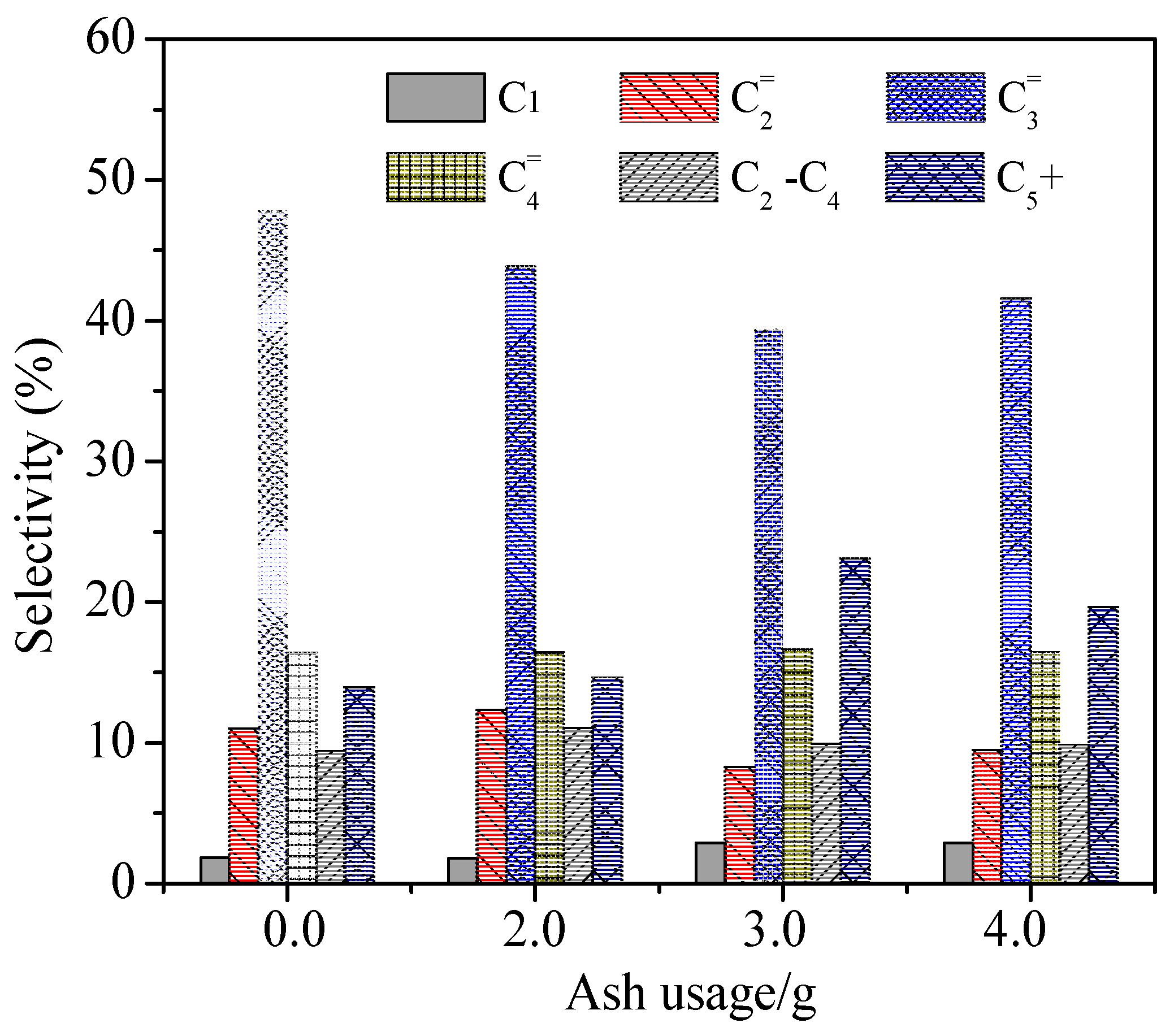
2.3. Catalytic Tests of As-Synthesized ZSM-5 Catalysts
3. Experimental Section
3.1. Raw Materials
3.2. Materials Synthesis
3.3. Characterization
3.4. Catalytic Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Belviso, C. State-of-the-art applications of fly ash from coal and biomass: A focus on zeolite synthesis processes and issues. Prog. Energy Combust. Sci. 2018, 65, 109–135. [Google Scholar] [CrossRef]
- Koukouzas, N.K.; Zeng, R.; Perdikatsis, V.; Xu, W.; Kakaras, E.K. Mineralogy and geochemistry of Greek and Chinese coal fly ash. Fuel 2006, 85, 2301–2309. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Vogt, E.T.C.; Weckhuysen, B.M. Fluid catalytic cracking: Recent developments on the grand old lady of zeolite catalysis. Chem. Soc. Rev. 2015, 44, 7342–7370. [Google Scholar] [CrossRef]
- Feng, R.; Qiao, K.; Wang, Y.-h.; Yan, Z.-f. Perspective on FCC catalyst in China. Appl. Petrochem. Res. 2013, 3, 63–70. [Google Scholar] [CrossRef][Green Version]
- Weckhuysen, B.M.; Yu, J. Recent advances in zeolite chemistry and catalysis. Chem. Soc. Rev. 2015, 44, 7022–7024. [Google Scholar] [CrossRef]
- Thomas, J.M.; Raja, R.; Lewis, D.W. Single-Site Heterogeneous Catalysts. Angew. Chem. Int. Ed. 2005, 44, 6456–6482. [Google Scholar] [CrossRef]
- Procopio, A.; Cravotto, G.; Oliverio, M.; Costanzo, P.; Nardi, M.; Paonessa, R. An eco-sustainable erbium(iii)-catalyzed method for formation/cleavage of O-tert-butoxy carbonates. Green Chem. 2011, 13, 436–443. [Google Scholar] [CrossRef]
- Procopio, A.; De Luca, G.; Nardi, M.; Oliverio, M.; Paonessa, R. General MW-assisted grafting of MCM-41: Study of the dependence on time dielectric heating and solvent. Green Chem. 2009, 11, 770–773. [Google Scholar] [CrossRef]
- Wang, F.-F.; Wu, H.-Z.; Ren, H.-F.; Liu, C.-L.; Xu, C.-L.; Dong, W.-S. Er/β-zeolite-catalyzed one-pot conversion of cellulose to lactic acid. J. Porous Mater. 2017, 24, 697–706. [Google Scholar] [CrossRef]
- Ballini, R.; Boscia, G.; Carloni, S.; Ciaralli, L.; Maggi, R.; Sartori, G. Zeolite HSZ-360 as a new reusable catalyst for the direct acetylation of alcohols and phenols under solventless conditions. Tetrahedron Lett. 1998, 39, 6049–6052. [Google Scholar] [CrossRef]
- Feng, R.; Wang, X.; Lin, J.; Li, Z.; Hou, K.; Yan, X.; Hu, X.; Yan, Z.; Rood, M.J. Two-stage glucose-assisted crystallization of ZSM-5 to improve methanol to propylene (MTP). Microporous Microporous Mater. 2017, 270, 57–66. [Google Scholar] [CrossRef]
- Losch, P.; Pinar, A.B.; Willinger, M.G.; Soukup, K.; Chavan, S.; Vincent, B.; Pale, P.; Louis, B. H-ZSM-5 zeolite model crystals: Structure-diffusion-activity relationship in methanol-to-olefins catalysis. J. Catal. 2017, 345, 11–23. [Google Scholar] [CrossRef]
- Xue, T.; Li, S.; Wu, H.; Wu, P.; He, M. Eco-friendly and cost-effective synthesis of ZSM-5 aggregates with hierarchical porosity. Ind. Eng. Chem. Res. 2017, 56, 13535–13542. [Google Scholar] [CrossRef]
- Ojumu, T.V.; Du Plessis, P.W.; Petrik, L.F. Synthesis of zeolite A from coal fly ash using ultrasonic treatment—A replacement for fusion step. Ultrason. Sonochem. 2016, 31, 342–349. [Google Scholar] [CrossRef]
- Srinivasan, A.; Grutzeck, M.W. The adsorption of SO2 by zeolites synthesized from fly ash. Environ. Sci. Technol. 1999, 33, 1464–1469. [Google Scholar] [CrossRef]
- Cardoso, A.M.; Horn, M.B.; Ferret, L.S.; Azevedo, C.M.N.; Pires, M. Integrated synthesis of zeolites 4A and Na–P1 using coal fly ash for application in the formulation of detergents and swine wastewater treatment. J. Hazard. Mater. 2015, 287, 69–77. [Google Scholar] [CrossRef]
- Chen, Y.; Cong, S.; Wang, Q.; Han, H.; Lu, J.; Kang, Y.; Kang, W.; Wang, H.; Han, S.; Song, H.; et al. Optimization of crystal growth of sub-micron ZSM-5 zeolite prepared by using Al(OH)3 extracted from fly ash as an aluminum source. J. Hazard. Mater. 2018, 349, 18–26. [Google Scholar] [CrossRef]
- Soongprasit, K.; Sricharoenchaikul, V.; Atong, D. Pyrolysis of Millettia (Pongamia) pinnata waste for bio-oil production using a fly ash derived ZSM-5 catalyst. J. Anal. Appl. Pyrolysis 2019, 139, 239–249. [Google Scholar] [CrossRef]
- Vichaphund, S.; Wimuktiwan, P.; Sricharoenchaikul, V.; Atong, D. In situ catalytic pyrolysis of Jatropha wastes using ZSM-5 from hydrothermal alkaline fusion of fly ash. J. Anal. Appl. Pyrolysis 2019, 139, 156–166. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, Y.; Ren, Q.; Wang, Y.; Lyu, Q. Impact of residual carbon on ash fusibility of semi-char from an industrial circulating fluidized bed gasifier. Energy Fuel 2019, 33, 531–540. [Google Scholar] [CrossRef]
- Li, F.; Liu, Q.; Li, M.; Fang, Y. Understanding fly-ash formation during fluidized-bed gasification of high-silicon-aluminum coal based on its characteristics. Energy 2018, 150, 142–152. [Google Scholar] [CrossRef]
- Zhou, J.; Teng, J.; Ren, L.; Wang, Y.; Liu, Z.; Liu, W.; Yang, W.; Xie, Z. Full-crystalline hierarchical monolithic ZSM-5 zeolites as superiorly active and long-lived practical catalysts in methanol-to-hydrocarbons reaction. J. Catal. 2016, 340, 166–176. [Google Scholar] [CrossRef]
- Sotomayor, F.J.; Cychosz, K.A.; Thommes, M. Characterization of micro/mesoporous materials by physisorption: Concepts and case studies. Acc. Mater. Surf. Res. 2018, 3, 34–50. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Janssen, A.H.; Schmidt, I.; Jacobsen, C.J.H.; Koster, A.J.; de Jong, K.P. Exploratory study of mesopore templating with carbon during zeolite synthesis. Microporous Microporous Mater. 2003, 65, 59–75. [Google Scholar] [CrossRef]
- Groen, J.C.; Pérez-Ramírez, J. Critical appraisal of mesopore characterization by adsorption analysis. Appl. Catal. A 2004, 268, 121–125. [Google Scholar] [CrossRef]
- Feng, R.; Hu, X.; Yan, X.; Yan, Z.; Rood, M.J. A high surface area mesoporous γ-Al2O3 with tailoring texture by glucose template for ethanol dehydration to ethylene. Microporous Microporous Mater. 2017, 241, 89–97. [Google Scholar] [CrossRef]
- Feng, R.; Liu, S.; Bai, P.; Qiao, K.; Wang, Y.; Al-Megren, H.A.; Rood, M.J.; Yan, Z. Preparation and Characterization of γ-Al2O3 with rich Brönsted acid sites and its application in the fluid catalytic cracking process. J. Phys. Chem. C 2014, 118, 6226–6234. [Google Scholar] [CrossRef]
- Feng, R.; Yan, X.; Hu, X.; Qiao, K.; Yan, Z.; Rood, M.J. High performance of H3BO3 modified USY and equilibrium catalyst with tailored acid sites in catalytic cracking. Microporous Microporous Mater. 2017, 243, 319–330. [Google Scholar] [CrossRef]
- Wan, Z.; Li, G.K.; Wang, C.; Yang, H.; Zhang, D. Relating coke formation and characteristics to deactivation of ZSM-5 zeolite in methanol to gasoline conversion. Appl. Catal. A 2018, 549, 141–151. [Google Scholar] [CrossRef]
- Feng, R.; Yan, X.; Hu, X.; Yan, Z.; Lin, J.; Li, Z.; Hou, K.; Rood, M.J. Surface dealumination of micro-sized ZSM-5 for improving propylene selectivity and catalyst lifetime in methanol to propylene (MTP) reaction. Catal. Commun. 2018, 109, 1–5. [Google Scholar] [CrossRef]
- Arora, S.S.; Bhan, A. The critical role of methanol pressure in controlling its transfer dehydrogenation and the corresponding effect on propylene-to-ethylene ratio during methanol-to-hydrocarbons catalysis on H-ZSM-5. J. Catal. 2017, 356, 300–306. [Google Scholar] [CrossRef]
- Tian, P.; Wei, Y.; Ye, M.; Liu, Z. Methanol to olefins (MTO): From fundamentals to commercialization. ACS Catal. 2015, 5, 1922–1938. [Google Scholar] [CrossRef]
- Vandichel, M.; Lesthaeghe, D.; Van der Mynsbrugge, J.; Waroquier, M.; Van Speybroeck, V. Assembly of cyclic hydrocarbons from ethene and propene in acid zeolite catalysis to produce active catalytic sites for MTO conversion. J. Catal. 2010, 271, 67–78. [Google Scholar] [CrossRef][Green Version]
- An, H.; Zhang, F.; Guan, Z.; Liu, X.; Fan, F.; Li, C. Investigating the coke formation mechanism of H-ZSM-5 during methanol dehydration using operando UV–Raman spectroscopy. ACS Catal. 2018, 8, 9207–9215. [Google Scholar] [CrossRef]
- Emeis, C.A. Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
- Feng, R.; Yan, X.; Hu, X.; Wang, Y.; Li, Z.; Hou, K.; Lin, J. Hierarchical ZSM-5 zeolite designed by combining desilication and dealumination with related study of n-heptane cracking performance. J. Porous Mater. 2018, 25, 1743–1756. [Google Scholar] [CrossRef]
- Jiang, L.; Li, C.; Xu, M.; Xing, A.; Feng, R.; Wu, J. Investigation on and industrial application of degrading of methanol feed in methanol to propylene process. Chin. J. Chem. Eng. 2018, 26, 2102–2111. [Google Scholar] [CrossRef]



| Sample | SiO2 | Al2O3 | Fe2O3 | CaO | K2O | Residual Carbon | SiO2/Al2O3 Molar Ratio |
|---|---|---|---|---|---|---|---|
| As received fly ash | 36.74 | 21.91 | 5.47 | 5.68 | 1.32 | 28.51 | 2.96 |
| After acid washing | 44.98 | 10.05 | 2.34 | 2.40 | 0.70 | 39.64 | 7.91 |
| Item | Preparation Conditions | RC a/% | SiO2/Al2O3 Ratio b | SBET/m2·g−1 | Volume/cm3·g−1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fly Ash/g | TEOS/mol | Na2O/SiO2 ratio | Total c | Smeso | Smicro | Total d | Vmeso | Vmicro | |||
| Z5-S1 | 4.0 | 0.0 | 0.055 | - | 5.63 | 36.0 | 30.2 | 5.8 | 0.009 | 0.009 | - |
| Z5-S2 | 4.0 | 0.017 | 0.055 | 31.81 | 9.54 | 145.1 | 44.5 | 100.6 | 0.010 | 0.001 | 0.010 |
| Z5-S3 | 4.0 | 0.033 | 0.055 | 42.24 | 12.29 | 158.7 | 48.3 | 110.4 | 0.084 | 0.011 | 0.073 |
| Z5-S4 | 4.0 | 0.067 | 0.055 | 52.94 | 17.42 | 217.1 | 66.8 | 150.3 | 0.071 | 0.002 | 0.069 |
| Z5-S5 | 0.0 | 0.067 | 0.055 | 100.0 | 72.67 | 358.5 | 88.8 | 269.7 | 0.095 | 0.010 | 0.085 |
| Z5-S6 | 2.0 | 0.067 | 0.055 | 99.83 | 72.66 | 328.8 | 69.8 | 259.0 | 0.104 | 0.012 | 0.092 |
| Z5-S7 | 3.0 | 0.067 | 0.055 | 93.83 | 73.33 | 326.8 | 75.9 | 250.9 | 0.077 | 0.004 | 0.073 |
| Z5-S8 | 4.0 | 0.067 | 0.028 | 49.88 | 18.33 | 215.3 | 84.6 | 130.7 | 0.101 | 0.016 | 0.045 |
| Z5-S9 | 4.0 | 0.067 | 0.084 | 34.48 | 15.77 | 157.0 | 52.8 | 97.9 | 0.096 | 0.056 | 0.040 |
| Z5-S10 | 4.0 | 0.067 | 0.114 | 29.01 | 19.96 | 166.8 | 70.0 | 96.8 | 0.081 | 0.066 | 0.015 |
| Items | Acid Amount (µmol∙g−1) a | Acid Amount (µmol∙g−1) b | |||||
|---|---|---|---|---|---|---|---|
| Total | Weak c | Medium Strong d | Strong e | Total | Brönsted Acid Sites | Lewis Acid Sites | |
| S5 | 197.4 | 91.7 | 61.1 | 44.6 | 117.8 | 6.5 | 111.3 |
| S6 | 238.6 | 89.8 | 51.4 | 97.4 | 124.9 | 7.5 | 117.4 |
| S7 | 302.9 | 121.4 | 57.2 | 124.3 | 64.4 | 8.9 | 55.5 |
| S4 | 440.5 | 227.1 | 127.9 | 85.5 | 58.8 | 10.8 | 48.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, R.; Chen, K.; Yan, X.; Hu, X.; Zhang, Y.; Wu, J. Synthesis of ZSM-5 Zeolite Using Coal Fly Ash as an Additive for the Methanol to Propylene (MTP) Reaction. Catalysts 2019, 9, 788. https://doi.org/10.3390/catal9100788
Feng R, Chen K, Yan X, Hu X, Zhang Y, Wu J. Synthesis of ZSM-5 Zeolite Using Coal Fly Ash as an Additive for the Methanol to Propylene (MTP) Reaction. Catalysts. 2019; 9(10):788. https://doi.org/10.3390/catal9100788
Chicago/Turabian StyleFeng, Rui, Kening Chen, Xinlong Yan, Xiaoyan Hu, Yixin Zhang, and Jianjun Wu. 2019. "Synthesis of ZSM-5 Zeolite Using Coal Fly Ash as an Additive for the Methanol to Propylene (MTP) Reaction" Catalysts 9, no. 10: 788. https://doi.org/10.3390/catal9100788
APA StyleFeng, R., Chen, K., Yan, X., Hu, X., Zhang, Y., & Wu, J. (2019). Synthesis of ZSM-5 Zeolite Using Coal Fly Ash as an Additive for the Methanol to Propylene (MTP) Reaction. Catalysts, 9(10), 788. https://doi.org/10.3390/catal9100788





