Synergistic Degradation of Dye Wastewaters Using Binary or Ternary Oxide Systems with Immobilized Laccase
Abstract
:1. Introduction
2. Results and Discussion
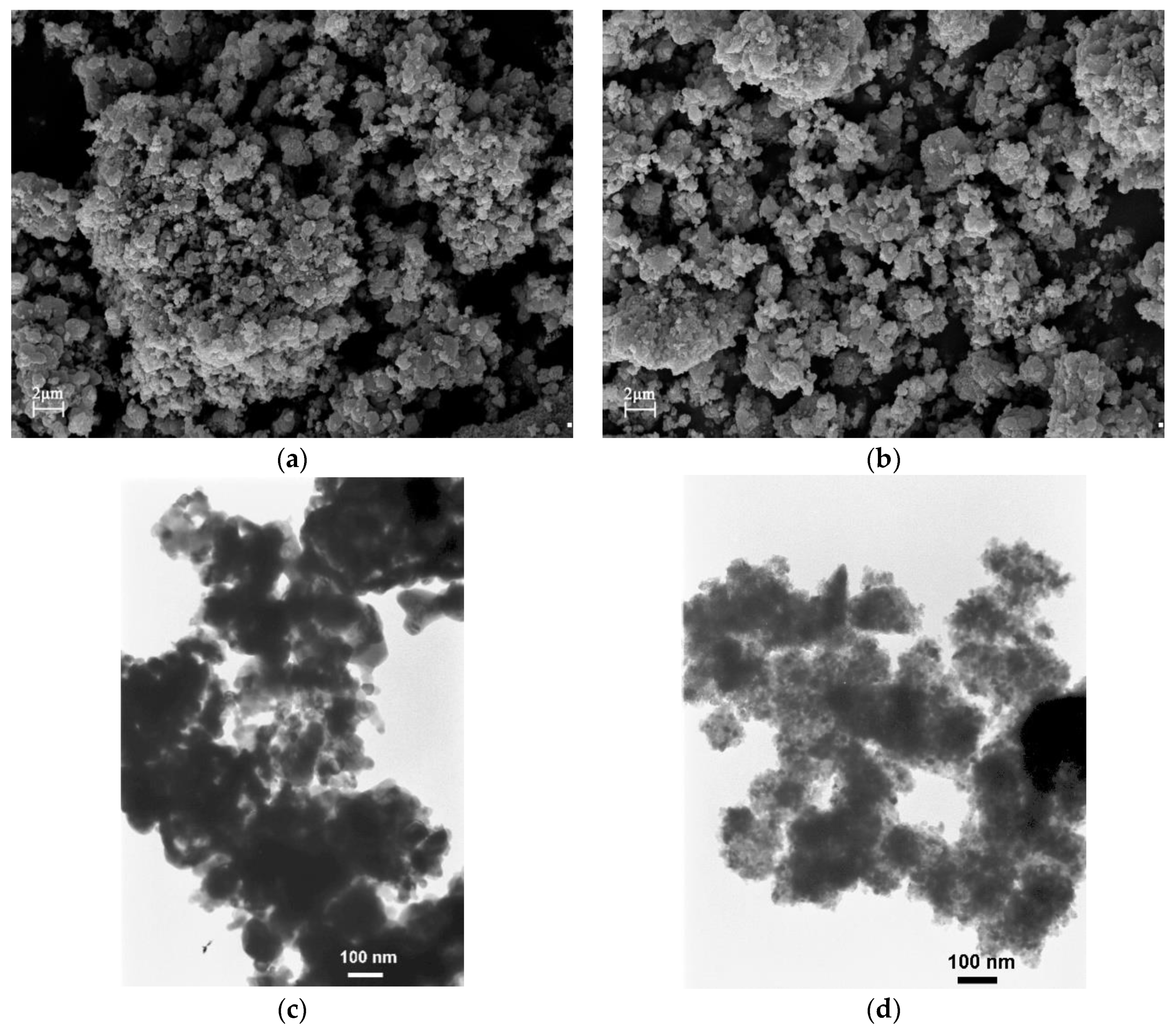
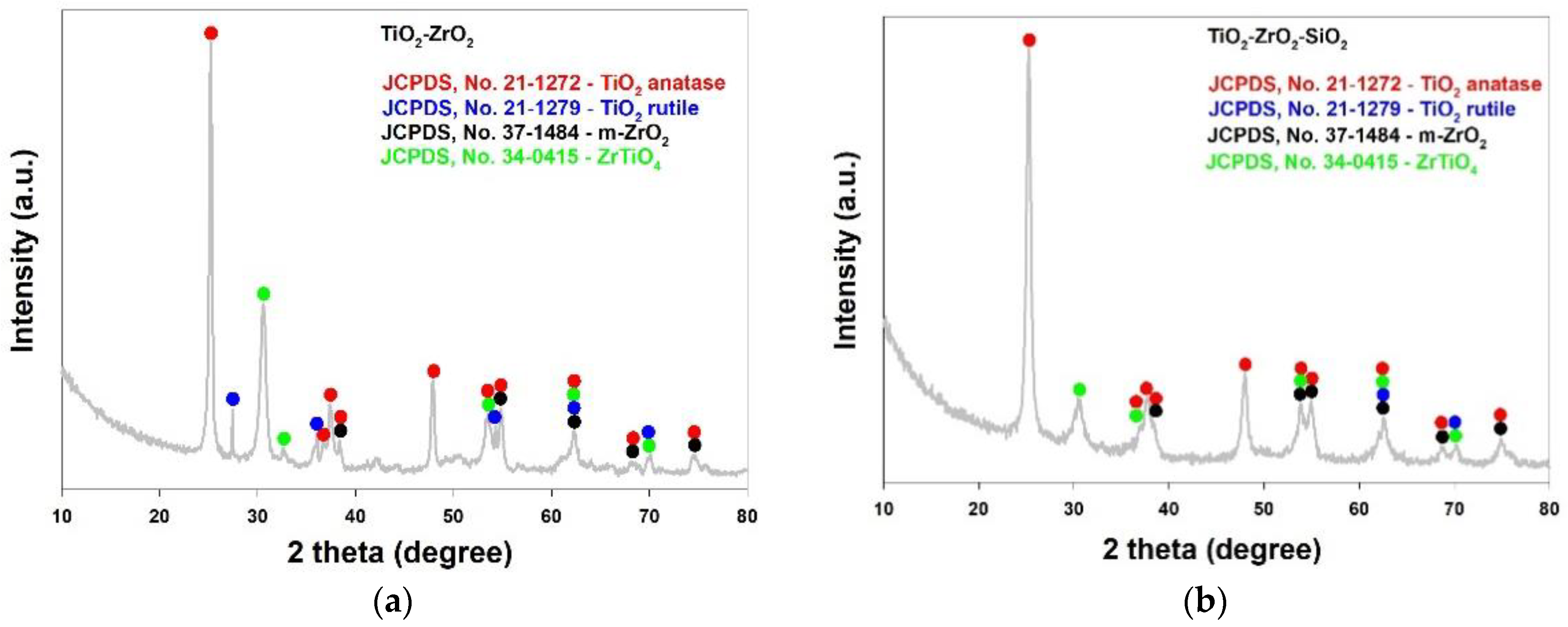
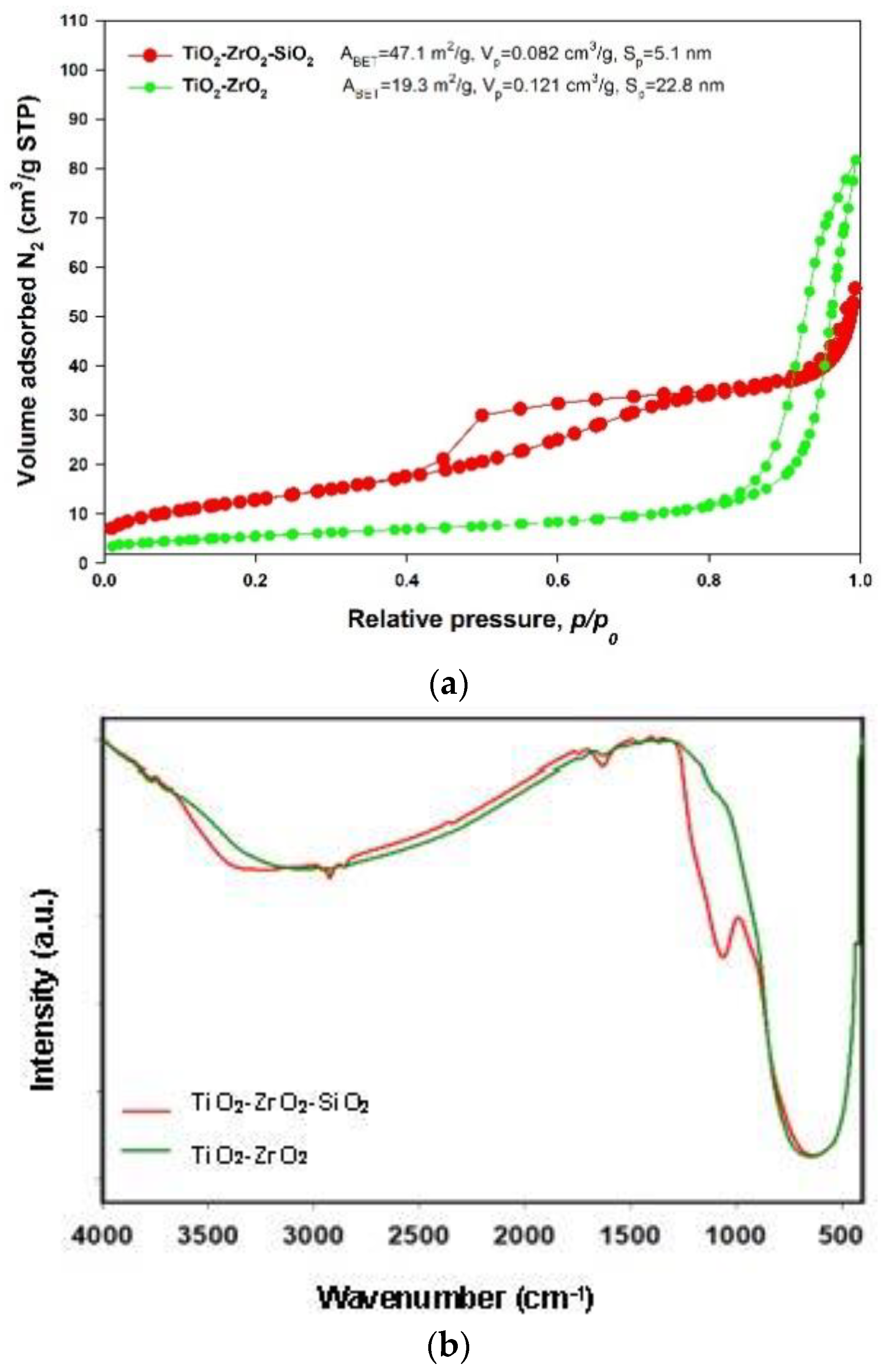
2.1. Supports Characterization
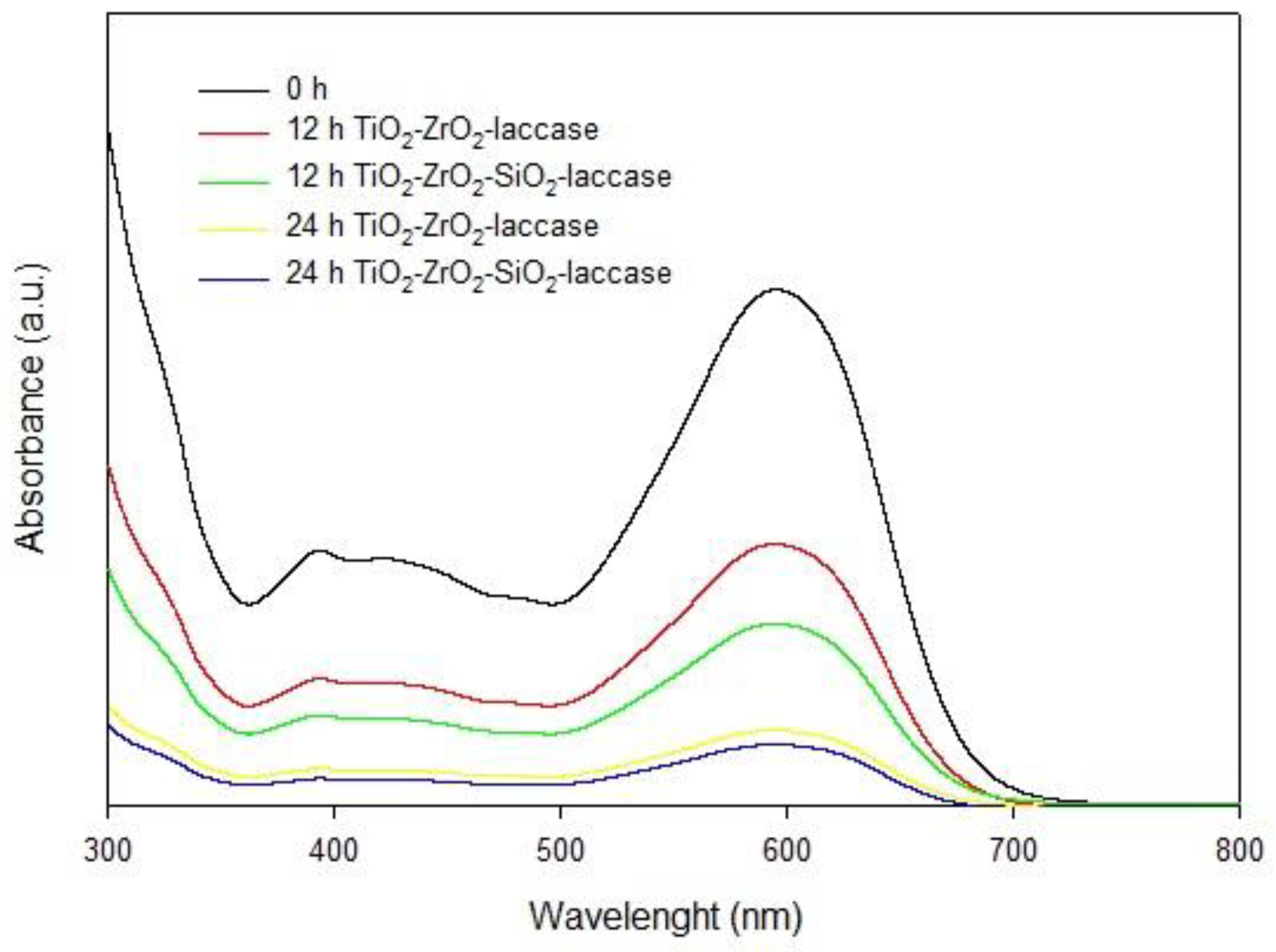
2.2. Characterization of the Biocatalytic Systems Obtained
2.3. Dye Removal
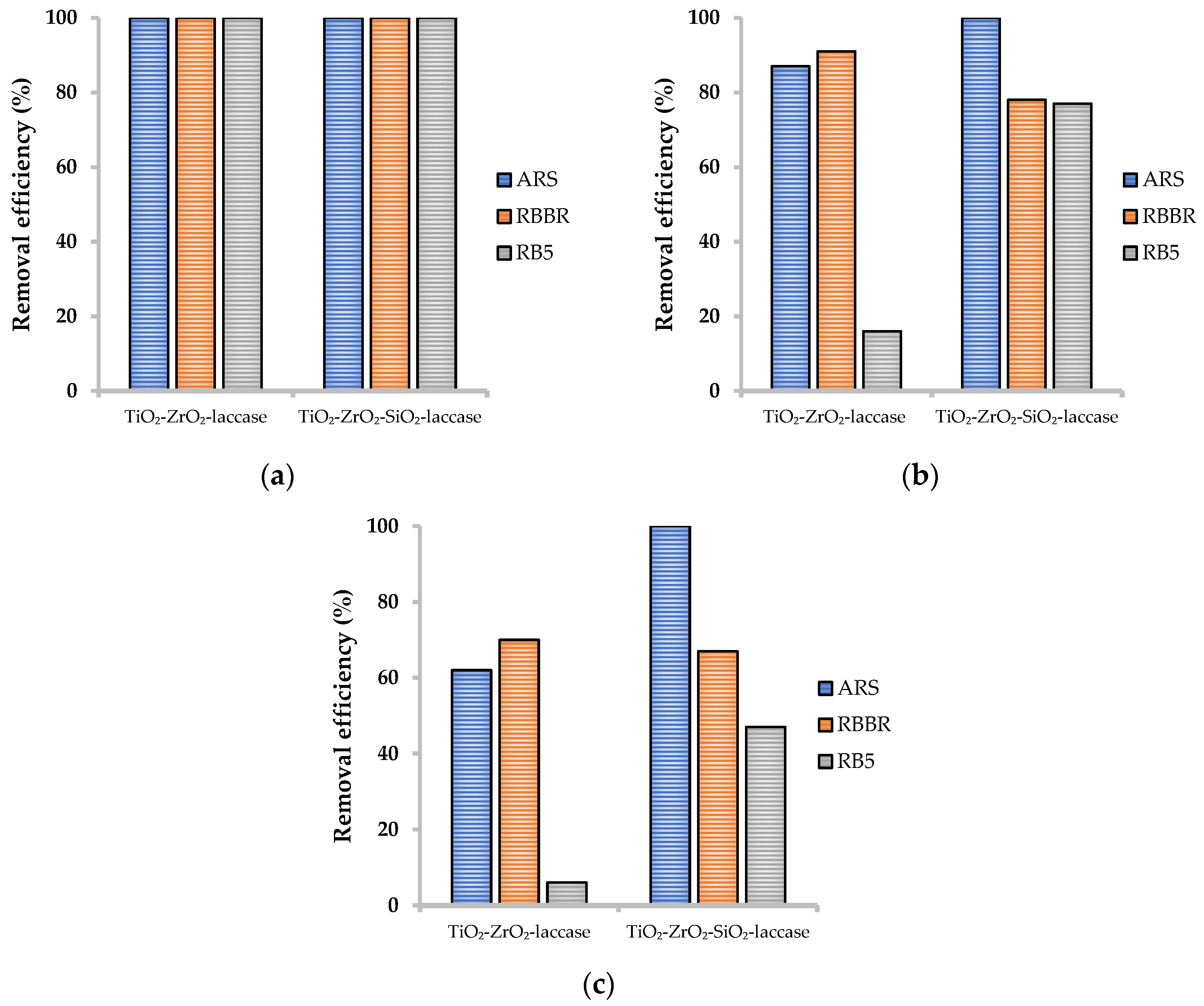
2.3.1. Dye Removal in the Presence of Oxide Materials with Inactivated Enzyme
2.3.2. Effect of a Concentration of Dyes Solution on Removal Efficiencies
2.3.3. Effect of pH of Dyes Solution on Removal Efficiencies
2.3.4. Effect of Temperature of Dyes Solution on Removal Efficiencies
2.3.5. Removal of Dyes from Dye Mixtures
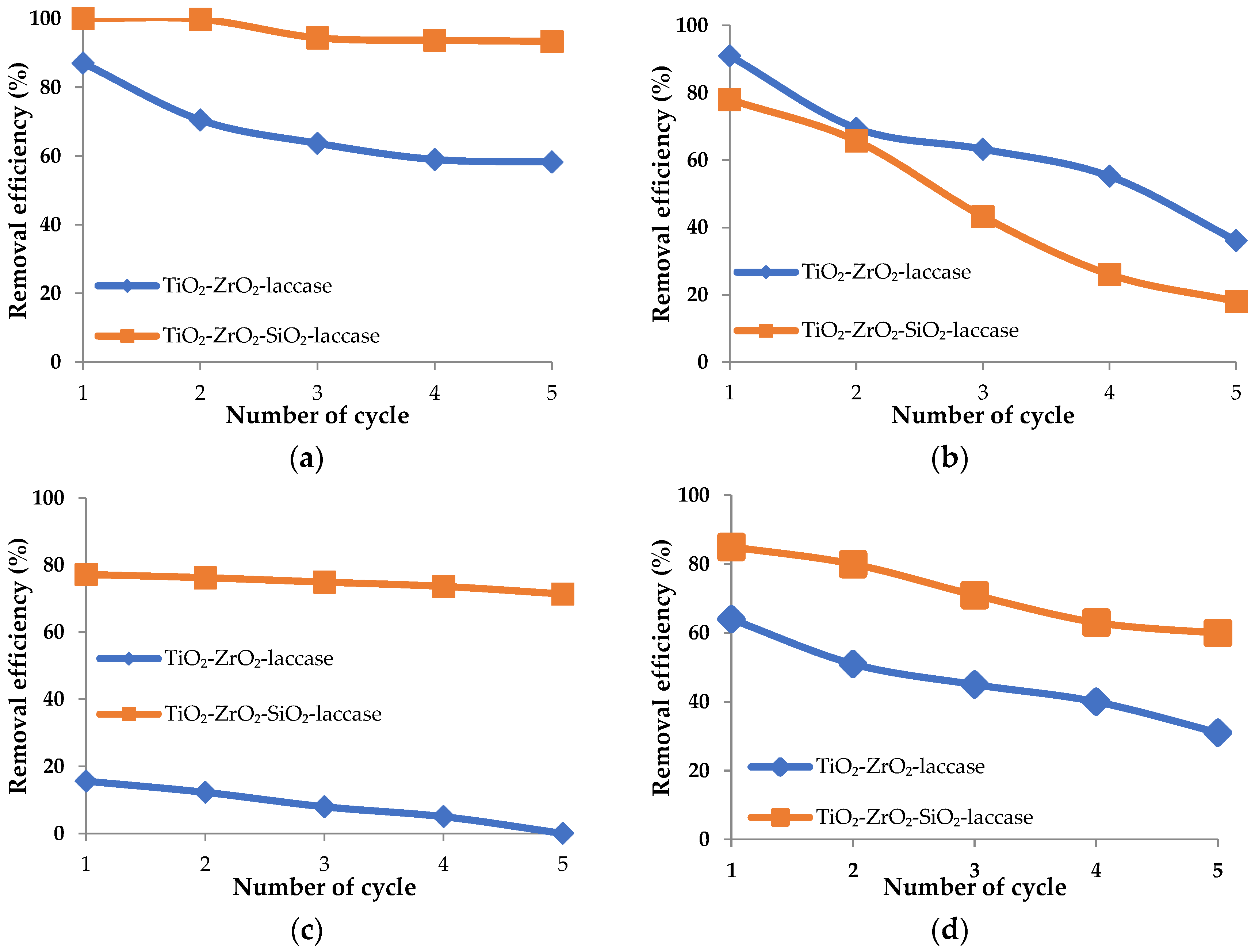
2.3.6. Reusability of the Biocatalytic Systems Obtained
2.3.7. COD Determination
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Synthesis of TiO2-ZrO2 and TiO2-ZrO2-SiO2 Oxide Systems
3.3. Laccase Immobilization
3.4. Storage Stability and Kinetic Measurements of Free and Immobilized Laccase
3.5. Dyes Degradation Experiments
3.6. Analytical Techniques
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mohammad, N.M.; Salehi, R. Dye removal from colored textile wastewater using chitosan in binary systems. Desalination 2011, 267, 64–72. [Google Scholar] [CrossRef]
- Jabeen, U.; Shah, S.M.; Khan, S.U. Photo catalytic degradation of Alizarin Red S using ZnS and cadmium doped ZnS nanoparticles under unfiltered sunlight. Surf. Interfaces 2017, 6, 40–49. [Google Scholar] [CrossRef]
- Kausar, A.; Iqbal, M.; Javed, A.; Aftab, K.; Nazli, Z.; Bhatti, H.N.; Nouren, S. Dyes adsorption using clay and modified clay: A review. J. Mol. Liq. 2018, 256, 395–407. [Google Scholar] [CrossRef]
- Anjaneyulu, Y.; Chary, N.S.; Raj, D.S.S. Decolourization of industrial effluents—Available methods and emerging technologies—A review. Rev. Environ. Sci. Biotechnol. 2005, 4, 245–273. [Google Scholar] [CrossRef]
- Faraco, V.; Pezzella, C.; Miele, A.; Giardina, P.; Sannia, G. Bio-remediation of colored industrial wastewaters by the white-rot fungi Phanerochaete chrysosporium and Pleurotus ostreatus and their enzymes. Biodegradation 2009, 20, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Hautphenne, C.; Pennickx, M.; Debaste, F. Product formation from phenolic compounds removal by laccases: A review. Environ. Technol. 2016, 5, 250–266. [Google Scholar] [CrossRef]
- He, K.; Chen, G.; Zeng, G.; Chen, A.; Huang, A.; Shi, J.; Huang, T.; Peng, M.; Hu, L. Three-dimensional graphene supported catalysts for organic dyes degradation. Appl. Catal. B 2018, 228, 19–28. [Google Scholar] [CrossRef]
- Ramírez-Montoya, L.A.; Hernández-Montoya, V.; Montes-Morán, M.A.; Jáuregui-Rincón, J.; Cervantes, F.J. Decolorization of dyes with different molecular properties using free and immobilized laccases from Trametes versicolor. J. Mol. Liq. 2015, 212, 30–37. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Khataee, A.R.; Kasiri, M.B. Photocatalytic degradation of organic dyes in the presence of nanostructured titanium dioxide: Influence of the chemical structure of dyes. J. Mol. Catal. A Chem. 2010, 328, 8–26. [Google Scholar] [CrossRef]
- Iqbal, M.; Nisar, J.; Adil, M.; Abbas, M.; Riaz, M.; Tahir, M.A.; Younus, M.; Shahid, M. Mutagenicity and cytotoxicity evaluation of photo-catalytically treated petroleum refinery wastewater using an array of bioassays. Chemosphere 2017, 168, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, A.; Bhatti, H.N.; Iqbal, M.; Noreen, S. Basic dye adsorption onto clay/MnFe2O4 composite: A mechanistic study. Water Environ. Res. 2017, 89, 301–311. [Google Scholar] [CrossRef] [PubMed]
- El-Zahar, A.A.; Awwad, N.S.; El-Katori, E.E. Removal of bromophenol blue dye from industrial waste water by synthesizing polymer-clay composite. J. Mol. Liq. 2014, 199, 454–461. [Google Scholar] [CrossRef]
- Selvam, P.P.; Preethi, S.; Basakaralingam, P.; Thinakaran, N.; Sivasamy, A.; Sivanesan, S. Removal of rhodamine B from aqueous solution by adsorption onto sodium montmorillonite. J. Hazard. Mater. 2008, 155, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Prigione, V.; Tigini, V.; Pezzella, C.; Anastasi, A.; Sannia, G.; Varese, G.C. Decolourisation and detoxification of textile effluents by fungal biosorption. Water Res. 2008, 42, 2911–2920. [Google Scholar] [CrossRef] [PubMed]
- Chairin, T.; Nitheranont, T.; Watanebe, A.; Asada, Y.; Khanongnuch, C.; Lumyong, S. Biodegradation of bisphenol A and decolorization of synthetic dyes by laccase from white-rot fungus, Trametes polyzona. Appl. Biochem. Biotechnol. 2013, 169, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Markiton, M.; Boncel, S.; Janas, D.; Chrobok, A. Highly active nanobiocatalyst from lipase noncovalently immobilized on multiwalled carbon nanotubes from Baeyer-Villiger synthesis of lactones. ACS Sustain. Chem. Eng. 2017, 5, 1685–1691. [Google Scholar] [CrossRef]
- Roy, U.; Sengupta, S.; Banerjee, P.; Das, P.; Bhowal, A.; Datta, S. Assessment on the decolourization of textile dye (Reactive Yellow) using Pseudomonas sp. immobilized on fly ash: Response surface methodology optimization and toxicity evaluation. J. Environ. Manag. 2018, 223, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Wangpradit, R.; Chitprasert, P. Chitosan-coated Lentinus polychrous Lév.: Integrated biosorption and biodegradation systems for decolorization of anionic reactive dyes. Int. Biodeterior. Biodegrad. 2014, 93, 168–176. [Google Scholar] [CrossRef]
- Rani, M.; Shanker, U.; Chaurasia, A.K. Catalytic potential of laccase immobilized on transition metal oxides nanomaterials: Degradation of Alizarin Red S dye. J. Environ. Chem. Eng. 2017, 5, 2730–2739. [Google Scholar] [CrossRef]
- Xu, H.M.; Sun, X.F.; Wang, S.Y.; Song, C.; Wang, S.G. Development of laccase/graphene oxide membrane for enhanced synthetic dyes separation and degradation. Sep. Purif. Technol. 2018, 204, 255–260. [Google Scholar] [CrossRef]
- Singh, N.; Basu, S.; Vankelecom, I.F.J.; Balakrishnan, M. Covalently immobilized laccase for decolourization of glucose-glycine maillard products as colourant of distillery wastewater. Appl. Biochem. Biotechnol. 2015, 177, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. Developments in support materials for immobilization of oxidoreductases: A comprehensive review. Adv. Colloid Interface Sci. 2018, 258, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Jin, X.; Long, N.; Zhang, R. Improved biodegradation of synthetic azo dye by horseradish peroxidase cross-linked on nano-composite support. Int. J. Biol. Macromol. 2017, 95, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Hu, Y.; Guo, C.; Huang, W.; Liu, C.Z. Enhanced phenol degradation in coking wastewater by immobilized laccase on magnetic mesoporous silica nanoparticles in a magnetically stabilized fluidized bed. Bioresour. Technol. 2012, 110, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Siwińska-Stefańska, K.; Kurc, B. A composite TiO2-ZrO2-SiO2 oxide system as a high-perfomance anode material for lithium-ion batteries. J. Electrochem. Soc. 2017, 164, A728–A734. [Google Scholar] [CrossRef]
- Adamczyk, A. The influence of ZrO2 precursor type on the structure of ZrO2-TiO2-SiO2 gels and selected thin films. J. Mol. Struct. 2018, 1171, 706–716. [Google Scholar] [CrossRef]
- Ilk, S.; Demircan, D.; Saglam, S.; Saglam, N.; Rzayev, Z.M.O. Immobilization of laccase onto a porous nanocomposite: Application for textile dye degradation. Turk. J. Chem. 2016, 40, 262–276. [Google Scholar] [CrossRef]
- Zdarta, J.; Antecka, K.; Jędrzak, A.; Synoradzki, K.; Łuczak, M.; Jesionowski, T. Biopolymers conjugated with magnetite as support materials for trypsin immobilization and protein digestion. Colloids Surf. B Biointerfaces 2018, 169, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Arica, M.Y.; Senel, S.; Alaeddinoglu, N.G.; Patir, S.; Denizli, A. Invertase immobilized on spacer-arm attached poly(hydroxyethyl metacrylate) membrane: Preparation and properties. J. Appl. Polym. Sci. 2000, 75, 1685–1692. [Google Scholar] [CrossRef]
- Klapiszewski, Ł.; Zdarta, J.; Jesionowski, T. Titania/lignin hybrid materials as a novel support for α-amylase immobilization: A comprehensive study. Colloids Surf. B Biointerfaces 2018, 162, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Chi, C.; Li, F.; Zhang, B. Laccase-polyacrylonitrile nanofibrous membrane: Highly immobilized, stable, reusable, and efficacious for 2,4,6-trichlorophenol removal. ACS Appl. Mater. Interfaces 2013, 5, 12554–12560. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Antecka, K.; Frankowski, R.; Zgoła-Grześkowiak, A.; Ehrlich, H.; Jesionowski, T. The effect of operational parameters on the biodegradation of bisphenols by Trametes versicolor laccase immobilized on Hippospongia communis spongin scaffolds. Sci. Total Environ. 2018, 615, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Vignesh Kumar, T.H.; Sivasankar, V.; Fayoud, N.; Oualid, H.A.; Sundramoorthy, A.K. Synthesis and characterization of coral-like hierarchical MgO incorporated fly ash composite for the effective adsorption of azo dye from aqueous solution. Appl. Surf. Sci. 2018, 449, 719–728. [Google Scholar] [CrossRef]
- Jesionowski, T.; Przybylska, A.; Kurc, B.; Ciesielczyk, F. Hybrid pigments preparation via adsorption of C.I. Mordant Red 3 on both unmodified and aminosilane-functionalised silica supports. Dyes Pigments 2011, 89, 127–136. [Google Scholar] [CrossRef]
- Miyamoto, M.; Hamajiama, A.; Oumi, Y.; Uemiya, S. Effect of basicity of metal doped ZrO2 supports on hydrogen production reactions. Int. J. Hydrogen Energy 2018, 43, 730–738. [Google Scholar] [CrossRef]
- Kamaz, M.; Rocha, P.; Sengupta, A.; Qian, X.; Wickramasinghe, R.S. Efficient removal of chemically toxic dyes using microorganism from activated sludge: Understanding sorption mechanism, kinetics, and associated thermodynamics. Sep. Sci. Technol. 2018, 53, 1760–1776. [Google Scholar] [CrossRef]
- Jesionowski, T. Synthesis of organic-inorganic hybrids via adsorption of dye on an aminosilane-functionalised silica surface. Dyes Pigments 2002, 55, 133–141. [Google Scholar] [CrossRef]
- Li, W.X.; Sun, H.Y.; Zhang, R.F. Immobilization of laccase on a novel ZnO/SiO2 nano-composited support for dye decolorization. In Proceedings of the 2015 Global Conference on Polymer and Composite Materials, Materials Science and Engineering, Beijing, China, 16–18 May 2015; Volume 87. [Google Scholar] [CrossRef]
- Chao, C.; Guan, H.; Zhang, J.; Liu, Y.; Zhao, Y.; Zhang, B. Immobilization of laccase onto porous vinyl alcohol/halloysite hybrid beads for dye removal. Water Sci. Technol. 2018, 77, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Rangelov, S.; Nicell, J.A. A model of the transient kinetics of laccase-catalyzed oxidation of phenol at micromolar concentrations. Biochem. Eng. J. 2015, 99, 1–15. [Google Scholar] [CrossRef]
- Kim, Y.J.; Nicell, J.A. Impact of reaction conditions on the laccase-catalyzed conversion of bisphenol A. Bioresour. Technol. 2006, 97, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Reda, F.M.; Hassan, N.S.; El-Moghazy, A.N. Decolorization of synthetic dyes by free and immobilized laccases from newly isolated strain Brevibacterium halotolerans N11 (KY883983). Biocatal. Agric. Biotechnol. 2018, 15, 138–145. [Google Scholar] [CrossRef]
- Zdarta, J.; Jędrzak, A.; Klapiszewski, Ł.; Jesionowski, T. Immobilization of cellulase on a functional inorganic–organic hybrid support: Stability and kinetic study. Catalysts 2017, 7, 374. [Google Scholar] [CrossRef]
- Ramírez-Montoya, L.A.; Hernández-Montoya, V.; Montes-Morán, M.A.; Cervantes, F.J. Correlation between mesopore volume of carbon supports and the immobilization of laccase from Trametes versicolor for the decolorization of Acid Orange 7. J. Environ. Manag. 2015, 162, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, K.; Faramarzi, M.A.; Mahvi, A.H.; Gholami, M.; Esrafili, A.; Forootanfar, H.; Farzadkia, M. Elimination and detoxification of sulfathiaziole and sulfamethoxazole assisted by laccase immobilized on porous silica beads. Int. Biodeterior. Biodegrad. 2015, 97, 107–114. [Google Scholar] [CrossRef]
- Sadighi, A.; Faramarzi, M.A. Congo Red decolorization by immobilized laccase through chitosan nanoparticles on the glass beads. J. Taiwan Inst. Chem. Eng. 2013, 44, 156–162. [Google Scholar] [CrossRef]
- Fernández-Fernández, M.; Sanromán, M.A.; Moldes, D. Recent developments and applications of immobilized laccase. Biotechnol. Adv. 2013, 31, 1808–1825. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.F.; Meng, G.; Cui, B.K.; Si, J.; Dai, Y.C. Chitosan crosslinked with genipin as supporting matrix for biodegradation of synthetic dyes: Laccase immobilization and characterization. Chem. Eng. Res. Des. 2018, 132, 664–676. [Google Scholar] [CrossRef]
- Alinsafi, A.; da Motta, M.; Le Bonte, S.; Pons, M.N.; Benhammou, A. Effect of variability on the treatment of textile dyeing wastewater by activated sludge. Dyes Pigments 2006, 69, 31–39. [Google Scholar] [CrossRef]
- Paz, A.; Carballo, J.; Perez, M.J.; Domínguez, J.M. Biological treatment of model dyes and textile wastewaters. Chemosphere 2017, 181, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]

| Kinetic Parameters and Immobilization Data | Free Laccase | TiO2-ZrO2-Laccase | TiO2-ZrO2-SiO2-Laccase |
|---|---|---|---|
| Km (mM) | 0.057 ± 0.004 | 0.108 ± 0.016 | 0.124 ± 0.019 |
| Vmax (U/mg) | 0.046 ± 0.006 | 0.036 ± 0.002 | 0.029 ± 0.003 |
| Amount of enzyme (mg/g) | - | 83 ± 3.2 | 96 ± 2.7 |
| Immobilization yield (%) | - | 83 ± 3.4 | 96 ± 2.2 |
| Dye Concentration (mg/L) | Sorption Efficiency (%) | |||||
|---|---|---|---|---|---|---|
| ARS | RBBR | RB5 | ||||
| TiO2-ZrO2-Laccase | TiO2-ZrO2-SiO2-Laccase | TiO2-ZrO2-Laccase | TiO2-ZrO2-SiO2-Laccase | TiO2-ZrO2-Laccase | TiO2-ZrO2-SiO2-Laccase | |
| 1 | 74 | 100 | 76 | 46 | 24 | 37 |
| 5 | 28 | 51 | 48 | 48 | 15 | 23 |
| 10 | 0 | 25 | 46 | 28 | 6 | 12 |
| pH | Removal Efficiency (%) | |||||
|---|---|---|---|---|---|---|
| ARS | RBBR | RB5 | ||||
| TiO2-ZrO2-Laccase | TiO2-ZrO2-SiO2-Laccase | TiO2-ZrO2-Laccase | TiO2-ZrO2-SiO2-Laccase | TiO2-ZrO2-Laccase | TiO2-ZrO2-SiO2-Laccase | |
| 4 | 90 | 100 | 87 | 75 | 11 | 76 |
| 5 | 87 | 100 | 91 | 78 | 16 | 77 |
| 6 | 86 | 100 | 90 | 76 | 6 | 75 |
| Temp. (°C) | Removal Efficiency (%) | |||||
|---|---|---|---|---|---|---|
| ARS | RBBR | RB5 | ||||
| TiO2-ZrO2-Laccase | TiO2-ZrO2-SiO2-Laccase | TiO2-ZrO2-Laccase | TiO2-ZrO2-SiO2-Laccase | TiO2-ZrO2-Laccase | TiO2-ZrO2-SiO2-Laccase | |
| 5 | 74 | 87 | 70 | 62 | 7 | 42 |
| 25 | 87 | 100 | 91 | 78 | 16 | 77 |
| 50 | 83 | 91 | 82 | 66 | 7 | 49 |
| Solution | Biocatalytic System | COD Reduction Efficiency (%) |
|---|---|---|
| ARS | TiO2-ZrO2-laccase | 78.58 |
| TiO2-ZrO2-SiO2-laccase | 84.66 | |
| RBBR | TiO2-ZrO2-laccase | 71.52 |
| TiO2-ZrO2-SiO2-laccase | 64.34 | |
| RB5 | TiO2-ZrO2-laccase | 20.35 |
| TiO2-ZrO2-SiO2-laccase | 87.65 | |
| Dyes mixture | TiO2-ZrO2-laccase | 89.40 |
| TiO2-ZrO2-SiO2-laccase | 93.84 |
| Name | Molecular Formula | Chemical Structure | Molecular Weight (g/mol) | λmax (nm) * |
|---|---|---|---|---|
| Alizarin Red S (ARS) | C14H7NaO7S | 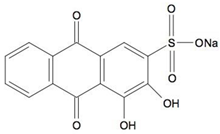 | 342.26 | 422 |
| Remazol Brilliant Blue R (RBBR) | C22H16N2Na2O11S3 | 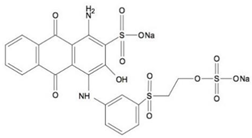 | 626.54 | 590 |
| Reactive Black 5 (RB5) | C26H21N5Na4O19S6 |  | 991.82 | 600 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antecka, K.; Zdarta, J.; Siwińska-Stefańska, K.; Sztuk, G.; Jankowska, E.; Oleskowicz-Popiel, P.; Jesionowski, T. Synergistic Degradation of Dye Wastewaters Using Binary or Ternary Oxide Systems with Immobilized Laccase. Catalysts 2018, 8, 402. https://doi.org/10.3390/catal8090402
Antecka K, Zdarta J, Siwińska-Stefańska K, Sztuk G, Jankowska E, Oleskowicz-Popiel P, Jesionowski T. Synergistic Degradation of Dye Wastewaters Using Binary or Ternary Oxide Systems with Immobilized Laccase. Catalysts. 2018; 8(9):402. https://doi.org/10.3390/catal8090402
Chicago/Turabian StyleAntecka, Katarzyna, Jakub Zdarta, Katarzyna Siwińska-Stefańska, Grzegorz Sztuk, Ewelina Jankowska, Piotr Oleskowicz-Popiel, and Teofil Jesionowski. 2018. "Synergistic Degradation of Dye Wastewaters Using Binary or Ternary Oxide Systems with Immobilized Laccase" Catalysts 8, no. 9: 402. https://doi.org/10.3390/catal8090402
APA StyleAntecka, K., Zdarta, J., Siwińska-Stefańska, K., Sztuk, G., Jankowska, E., Oleskowicz-Popiel, P., & Jesionowski, T. (2018). Synergistic Degradation of Dye Wastewaters Using Binary or Ternary Oxide Systems with Immobilized Laccase. Catalysts, 8(9), 402. https://doi.org/10.3390/catal8090402









