Immobilization of Chitosanases onto Magnetic Nanoparticles to Enhance Enzyme Performance
Abstract
1. Introduction
2. Results and Discussion
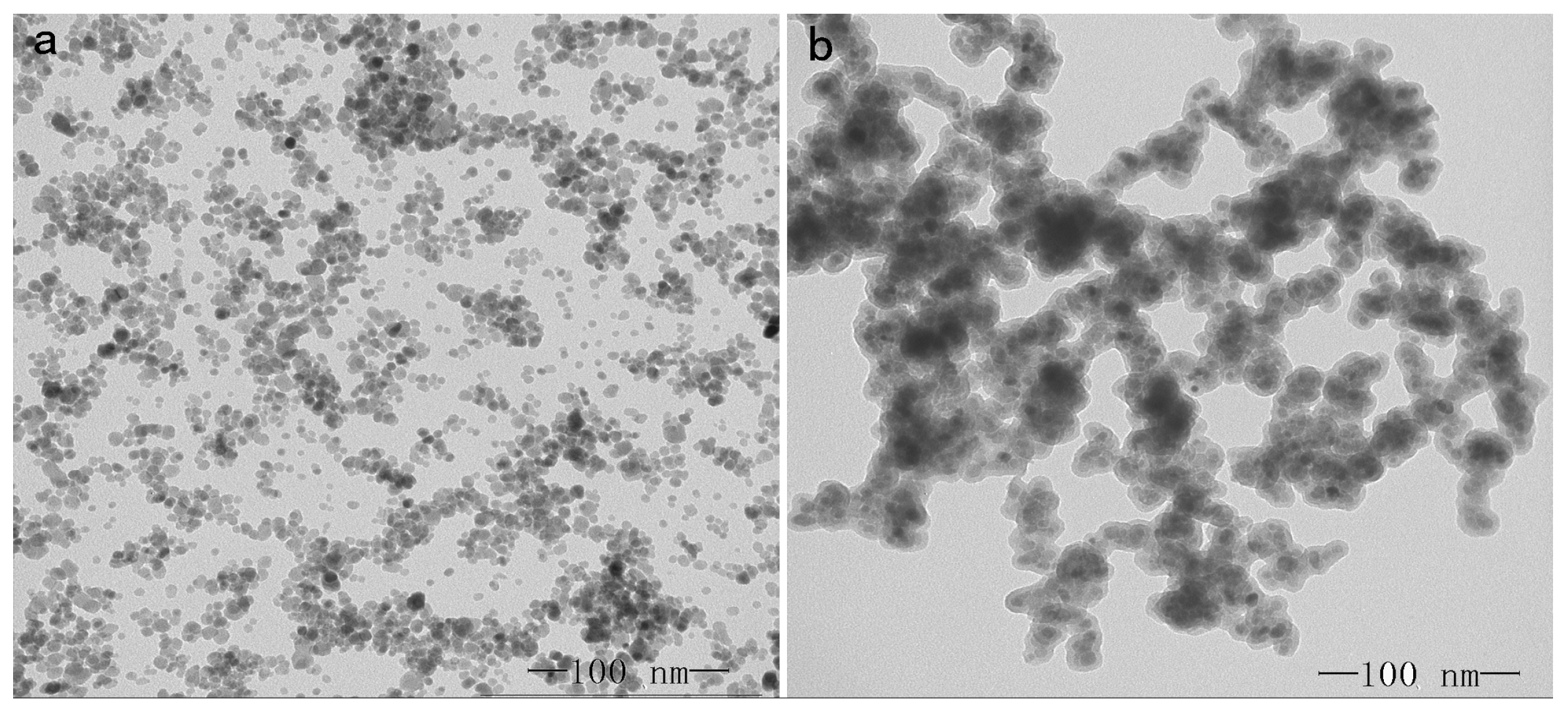
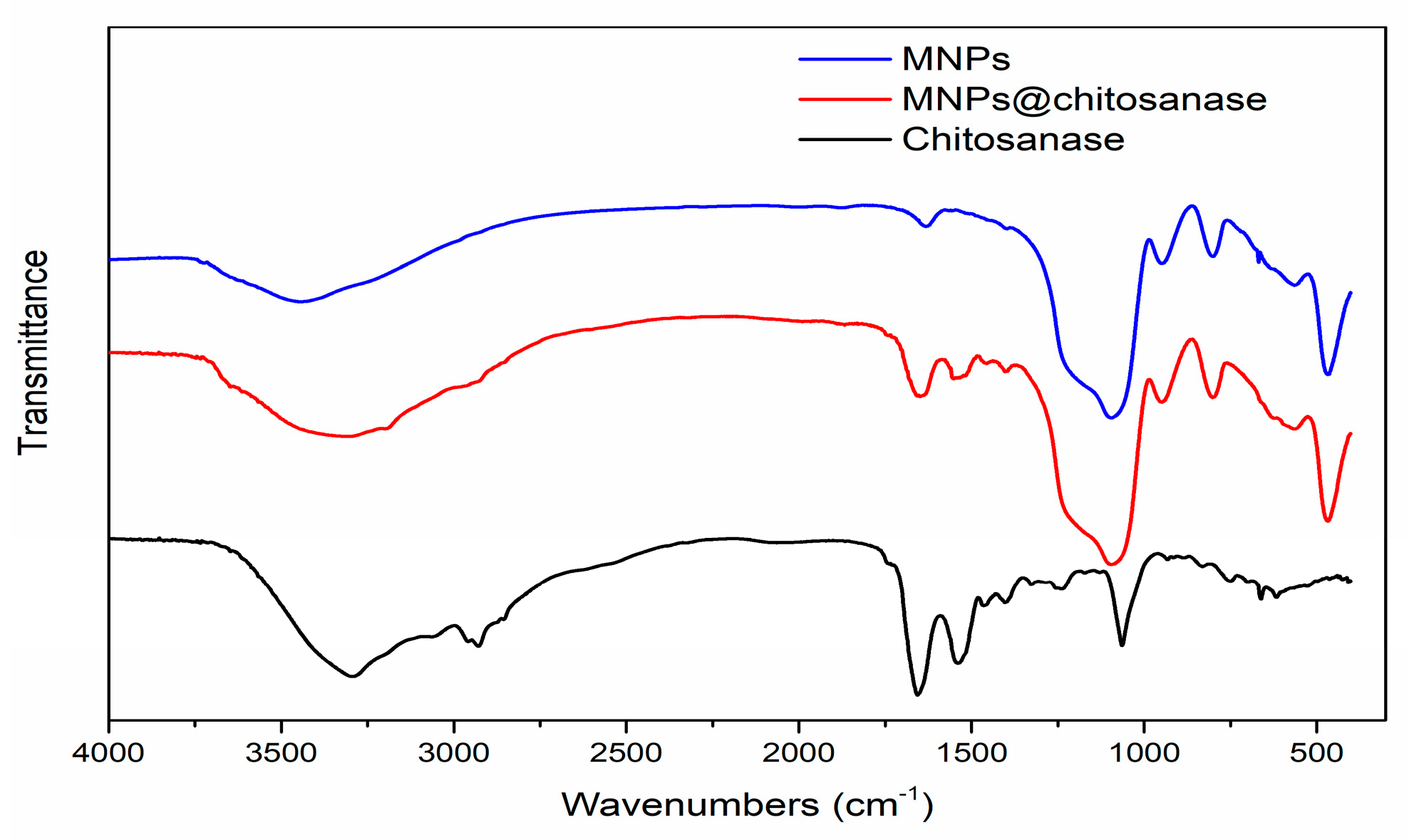
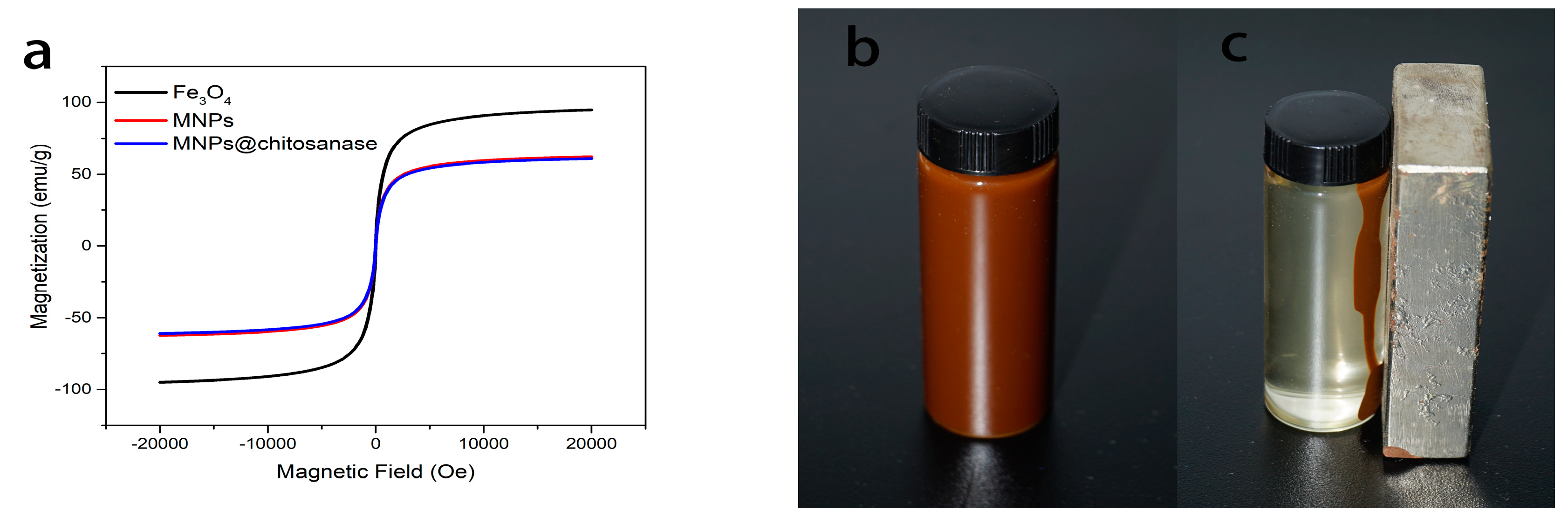
2.1. Characterization of Magnetic Nanoparticle
2.2. Characterization of Immobilized Chitosanases
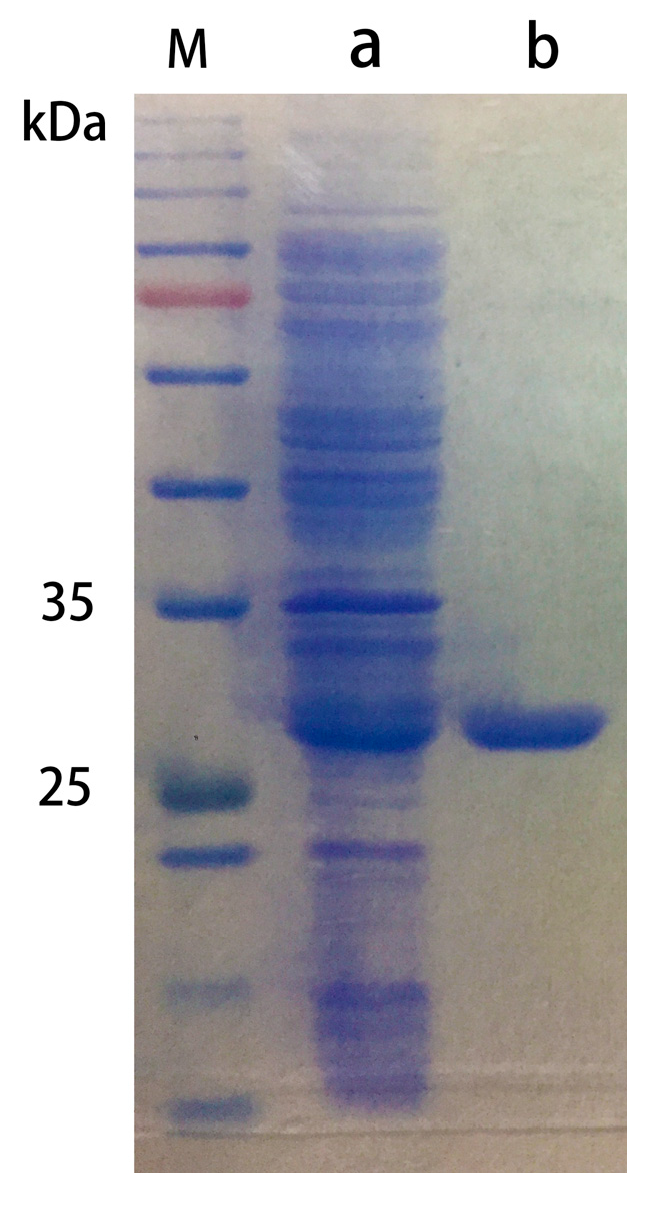
2.2.1. Purification of Chitosanase
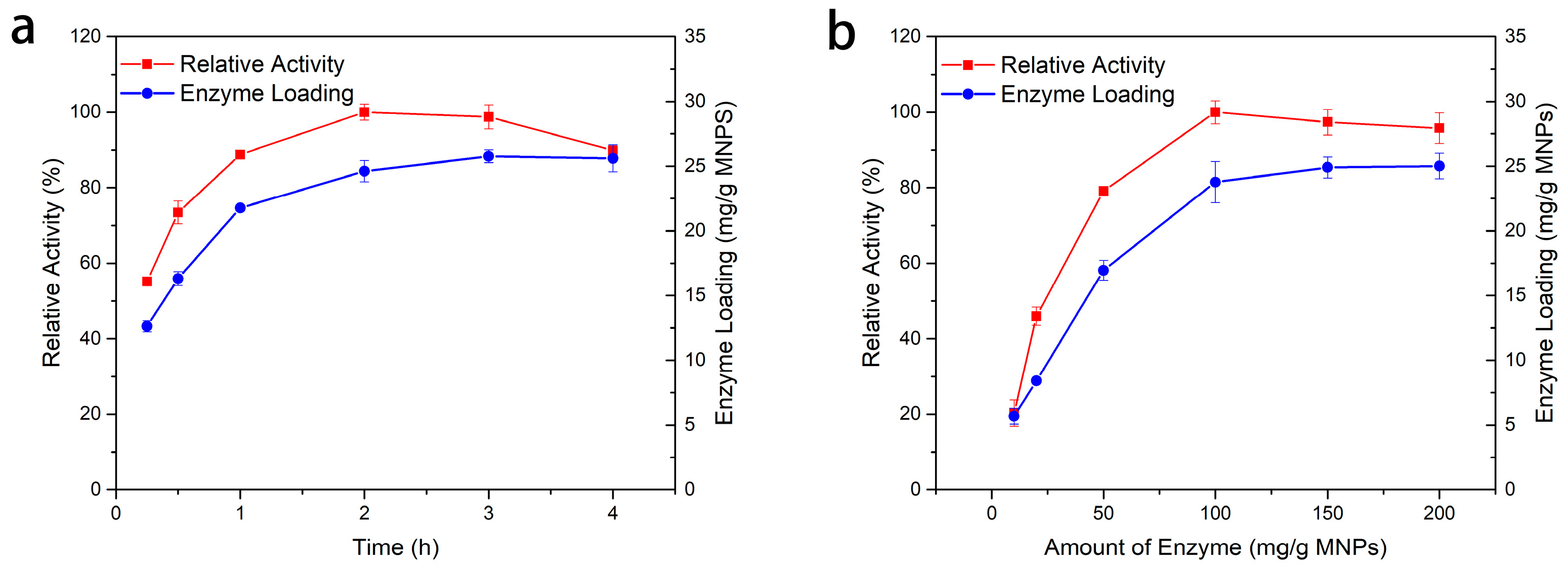
2.2.2. Optimum Conditions for Immobilization
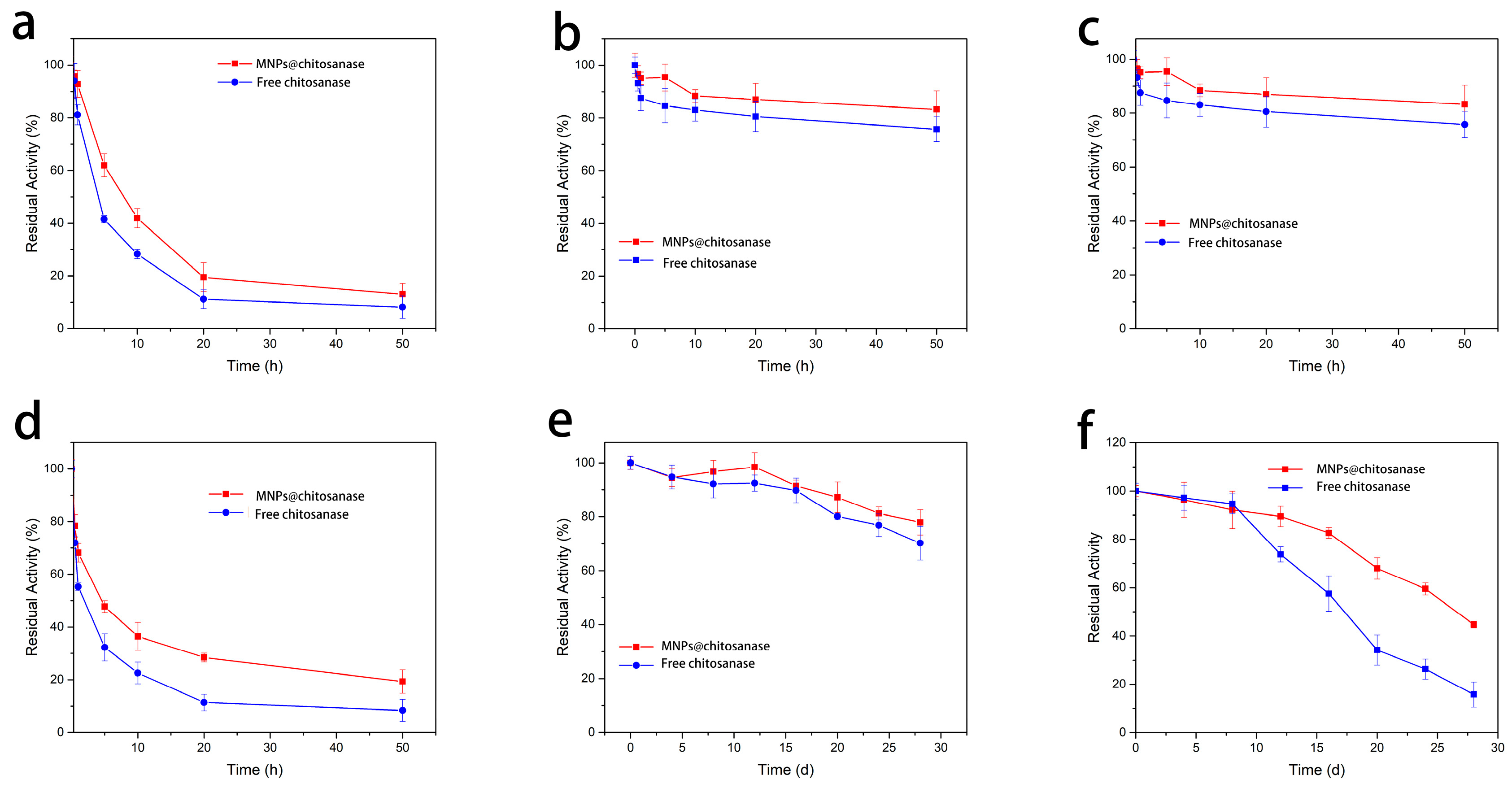
2.2.3. Stability and of Immobilized and Free Chitosanases
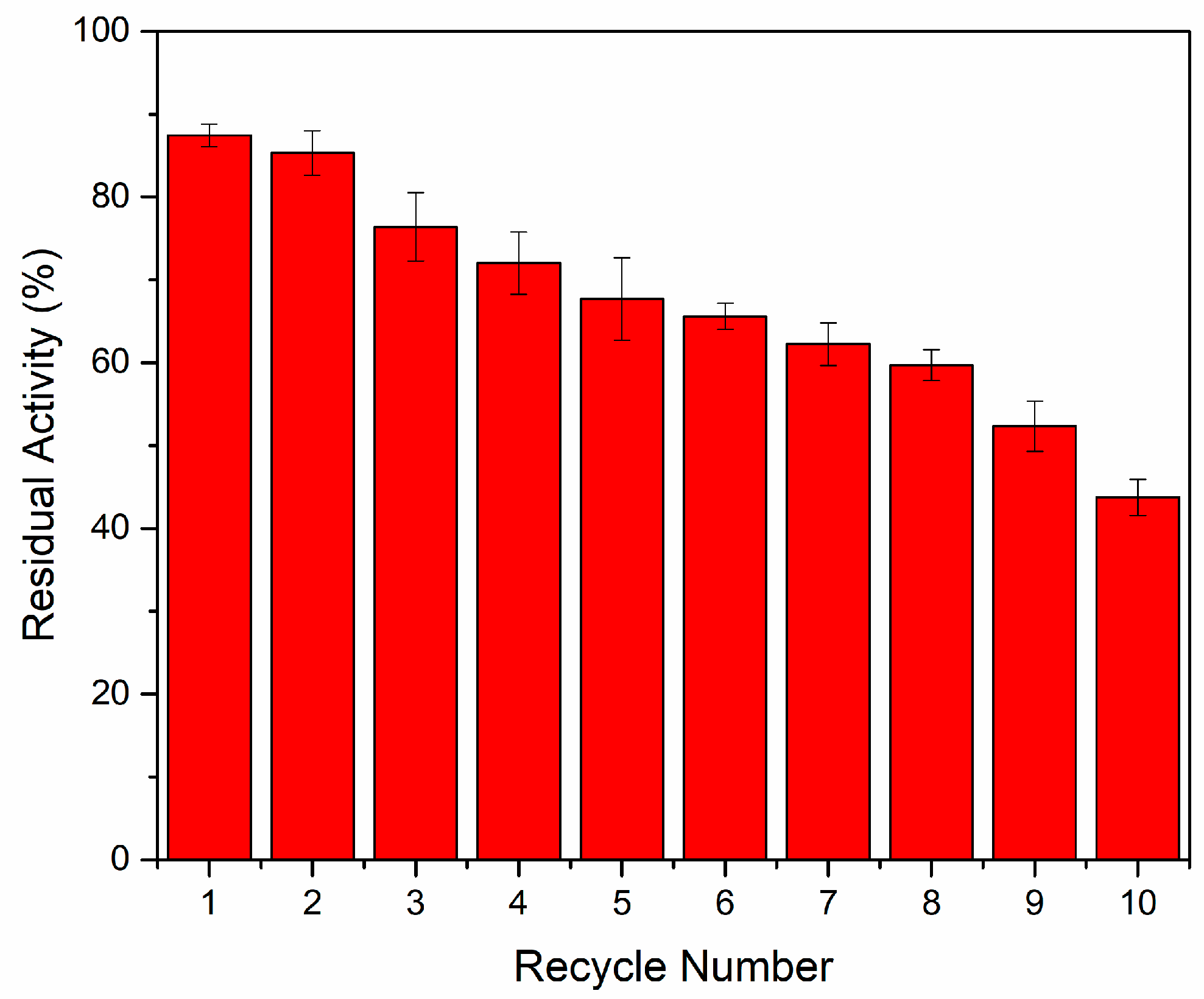
2.2.4. Reusability of Immobilized Chitosanases
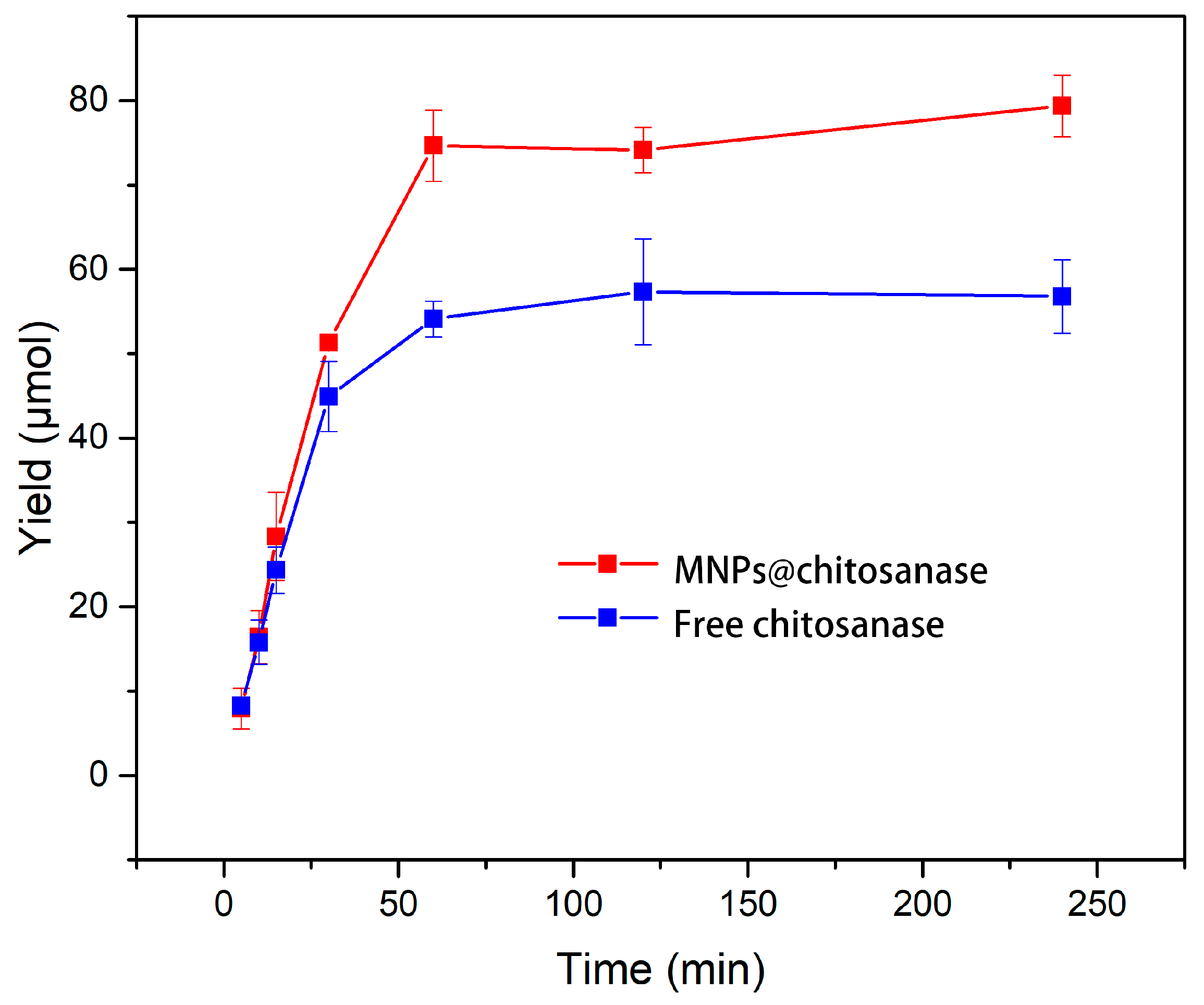
2.2.5. Catalytic Efficiency of Free and Immobilized Chitosanases
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Fe3O4 Nanoparticles
3.3. Preparation of Silica Coated Magnetite Nanoparticles
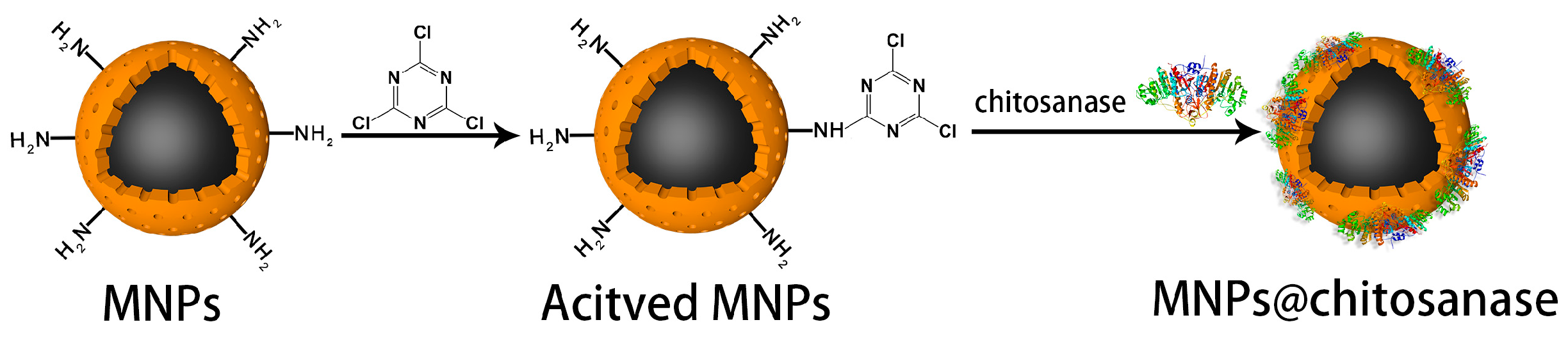
3.4. Nanoparticles Surface Modification
3.5. Purification of Chitosanase
3.6. Characterization
3.7. Chitosanase Immobilization
3.8. Assay of Chitosanase Activity
3.9. Stability and Reusability of Immobilized Chitosanases
3.10. Catalytic Efficiency of Free and Immobilized Chitosanases
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, S.K.; Rajapakse, N. Enzymatic production and biological activities of chitosan oligosaccharides (COS): A review. Carbohydr. Polym. 2005, 62, 357–368. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, E.J.; Piao, Z.; Yun, Y.C.; Shin, Y.C. Purification and characterization of chitosanase from Bacillus sp. strain KCTC 0377BP and its application for the production of chitosan oligosaccharides. Appl. Environ. Microbiol. 2004, 70, 4522–4531. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Mao, S.; Guan, Y.; Luo, L.; Luo, L.; Pan, Y. Effects of dietary chitosan oligosaccharides and Bacillus coagulans on the growth, innate immunity and resistance of koi (Cyprinus carpio koi). Aquaculture 2012, 342–343, 36–41. [Google Scholar] [CrossRef]
- Lu, Y.; Slomberg, D.L.; Schoenfisch, M.H. Nitric oxide-releasing chitosan oligosaccharides as antibacterial agents. Biomaterials 2014, 35, 1716–1724. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Yang, X.; Wang, J.; Li, Y.; Yu, H.; Zhang, Y.; Liu, G. Advances in characterisation and biological activities of chitosan and chitosan oligosaccharides. Food Chem. 2016, 190, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Mikami, T.; Okawa, Y.; Tokoro, A.; Suzuki, S.; Suzuki, M. Antitumor effect of hexa-N-acetylchitohexaose and chitohexaose. Carbohydr. Res. 1986, 151, 403–408. [Google Scholar] [CrossRef]
- Pires, C.T.; Vilela, J.A.; Airoldi, C. The effect of chitin alkaline deacetylation at different condition on particle properties. Procedia Chem. 2014, 9, 220–225. [Google Scholar] [CrossRef]
- Pareek, N.; Vivekanand, V.; Saroj, S.; Sharma, A.K.; Singh, R.P. Purification and characterization of chitin deacetylase from Penicillium oxalicum, SAEM-51. Carbohydr. Polym. 2012, 87, 1091–1097. [Google Scholar] [CrossRef]
- Naqvi, S.; Cord-Landwehr, S.; Singh, R.; Bernard, F.; Kolkenbrock, S.; EI Gueddari, N.E.; Moerschbacher, B.M. A recombinant fungal chitin deacetylase produces fully defined chitosan oligomers with novel patterns of acetylation. Appl. Environ. Microbiol. 2016, 82, 6645–6655. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, N.; Tewari, R.; Hoondal, G.S. Biotechnological aspects of chitinolytic enzymes: A review. Appl. Microbiol. Biotechnol. 2006, 71, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Thadathil, N.; Velappan, S.P. Recent developments in chitosanase research and its biotechnological applications: A review. Food Chem. 2014, 150, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Enzyme Immobilization: The quest for optimum performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Trevan, M.D. Enzyme immobilization by adsorption. In New Protein Techniques; Walker, J.M., Ed.; Humana Press: New York, NY, USA, 1988; Volume 3, pp. 481–489. ISBN 978-1-59259-490-0. [Google Scholar]
- Zhou, Z.; Hartmann, M. Recent progress in biocatalysis with enzymes immobilized on mesoporous hosts. Top. Catal. 2012, 55, 1081–1100. [Google Scholar] [CrossRef]
- Ranjbakhsh, E.; Bordbar, A.K.; Abbasi, M.; Khosropour, A.R.; Shams, E. Enhancement of stability and catalytic activity of immobilized lipase on silica-coated modified magnetite nanoparticles. Chem. Eng. J. 2012, 179, 272–276. [Google Scholar] [CrossRef]
- Straathof, A.J.; Panke, S.; Schmid, A. The production of fine chemicals by biotransformations. Curr. Opin. Biotechnol. 2012, 13, 548–556. [Google Scholar] [CrossRef]
- Vaghari, H.; Jafarizadeh-Malmiri, H.; Mohammadlou, M.; Berenjian, A.; Anarjan, N.; Jafari, N.; Nasiri, S. Application of magnetic nanoparticles in smart enzyme immobilization. Biotechnol. Lett. 2016, 38, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.A.; Park, H.J.; Driscoll, A.J. Enzyme nanoparticle fabrication: Magnetic nanoparticle synthesis and enzyme immobilization. In Enzyme Stabilization and Immobilization; Humana Press: New York, NY, USA, 2011; pp. 183–191. ISBN 978-1-60761-895-9. [Google Scholar]
- Liao, M.H.; Chen, D.H. Immobilization of yeast alcohol dehydrogenase on magnetic nanoparticles for improving its stability. Biotechnol. Lett. 2001, 23, 1723–1727. [Google Scholar] [CrossRef]
- Hu, B.; Pan, J.; Yu, H.L.; Liu, J.W.; Xu, J.H. Immobilization of Serratia marcescens lipase onto amino-functionalized magnetic nanoparticles for repeated use in enzymatic synthesis of Diltiazem intermediate. Process Biochem. 2009, 44, 1019–1024. [Google Scholar] [CrossRef]
- Xie, W.; Ma, N. Enzymatic transesterification of soybean oil by using immobilized lipase on magnetic nano-particles. Biomass Bioenergy 2010, 34, 890–896. [Google Scholar] [CrossRef]
- Lei, L.; Liu, X.; Li, Y.; Cui, Y.; Yang, Y.; Qin, G. Study on synthesis of poly(GMA)-grafted Fe3O4/SiOX magnetic nanoparticles using atom transfer radical polymerization and their application for lipase immobilization. Mater. Chem. Phys. 2011, 125, 866–871. [Google Scholar] [CrossRef]
- Mrówczyński, R.; Nan, A.; Liebscher, J. Magnetic nanoparticle-supported organocatalysts—An efficient way of recycling and reuse. RSC Adv. 2014, 4, 5927–5952. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Liu, Z.; Huang, W.C.; Xue, C.; Mao, X. Co-immobilization of β-agarase and α-neoagarobiose hydrolase for enhancing the production of 3,6-anhydro-l-galactose. J. Agric. Food Chem. 2018, 66, 7087–7095. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Kong, W.; Zhou, L.; He, Y.; Ma, L.; Wang, Y.; Yin, L.; Jiang, Y. Monodisperse core-shell magnetic organosilica nanoflowers with radial wrinkle for lipase immobilization. Chem. Eng. J. 2017, 309, 70–79. [Google Scholar] [CrossRef]
- Shareghi, B.; Farhadian, S.; Zamani, N.; Salavati-Niasari, M.; Gholamrezaei, S. Stability and enzyme activity of lysozyme in the presence of Fe3O4 nanoparticles. Monatshefte Chem. 2016, 147, 465–471. [Google Scholar] [CrossRef]
- Arroyo, M.; Sánchez-Montero, J.M.; Sinisterra, J.V. Thermal stabilization of immobilized lipase B from Candida antarctica, on different supports: Effect of water activity on enzymatic activity in organic media. Enzyme Microb. Technol. 1999, 24, 3–12. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Barbosa, O.; Torres, R.; Ortiz, C.; Berenguer-Murcia, Á.; Rodrigues, R.C.; Fernandez-Lafuente, R. Heterofunctional supports in enzyme immobilization: From traditional immobilization protocols to opportunities in tuning enzyme properties. Biomacromolecules 2013, 14, 2433–2462. [Google Scholar] [CrossRef] [PubMed]
- Khosroshahi, M.E.; Ghazanfari, L. Synthesis and functionalization of SiO2, coated Fe3O4, nanoparticles with amine groups based on self-assembly. Mater. Sci. Eng. C 2012, 32, 1043–1049. [Google Scholar] [CrossRef]
- Jang, J.H.; Lim, H.B. Characterization and analytical application of surface modified magnetic nanoparticles. Microchem. J. 2010, 94, 148–158. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B. Conjugated polymer as a signal amplifier for novel silica nanoparticle-based fluoroimmunoassay. Biosens. Bioelectron. 2009, 24, 3293–3298. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Batule, B.S.; Park, K.S.; Kim, M.I.; Park, H.G. Ultrafast sonochemical synthesis of protein-inorganic nanoflowers. Int. J. Nanomed. 2015, 10, 137. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Guo, N.; Huang, W.; Zhang, Z.; Mao, X. Immobilization of Chitosanases onto Magnetic Nanoparticles to Enhance Enzyme Performance. Catalysts 2018, 8, 401. https://doi.org/10.3390/catal8090401
Wang W, Guo N, Huang W, Zhang Z, Mao X. Immobilization of Chitosanases onto Magnetic Nanoparticles to Enhance Enzyme Performance. Catalysts. 2018; 8(9):401. https://doi.org/10.3390/catal8090401
Chicago/Turabian StyleWang, Wei, Na Guo, Wencan Huang, Zhaohui Zhang, and Xiangzhao Mao. 2018. "Immobilization of Chitosanases onto Magnetic Nanoparticles to Enhance Enzyme Performance" Catalysts 8, no. 9: 401. https://doi.org/10.3390/catal8090401
APA StyleWang, W., Guo, N., Huang, W., Zhang, Z., & Mao, X. (2018). Immobilization of Chitosanases onto Magnetic Nanoparticles to Enhance Enzyme Performance. Catalysts, 8(9), 401. https://doi.org/10.3390/catal8090401






