Molecular Orientations Change Reaction Kinetics and Mechanism: A Review on Catalytic Alcohol Oxidation in Gas Phase and Liquid Phase on Size-Controlled Pt Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
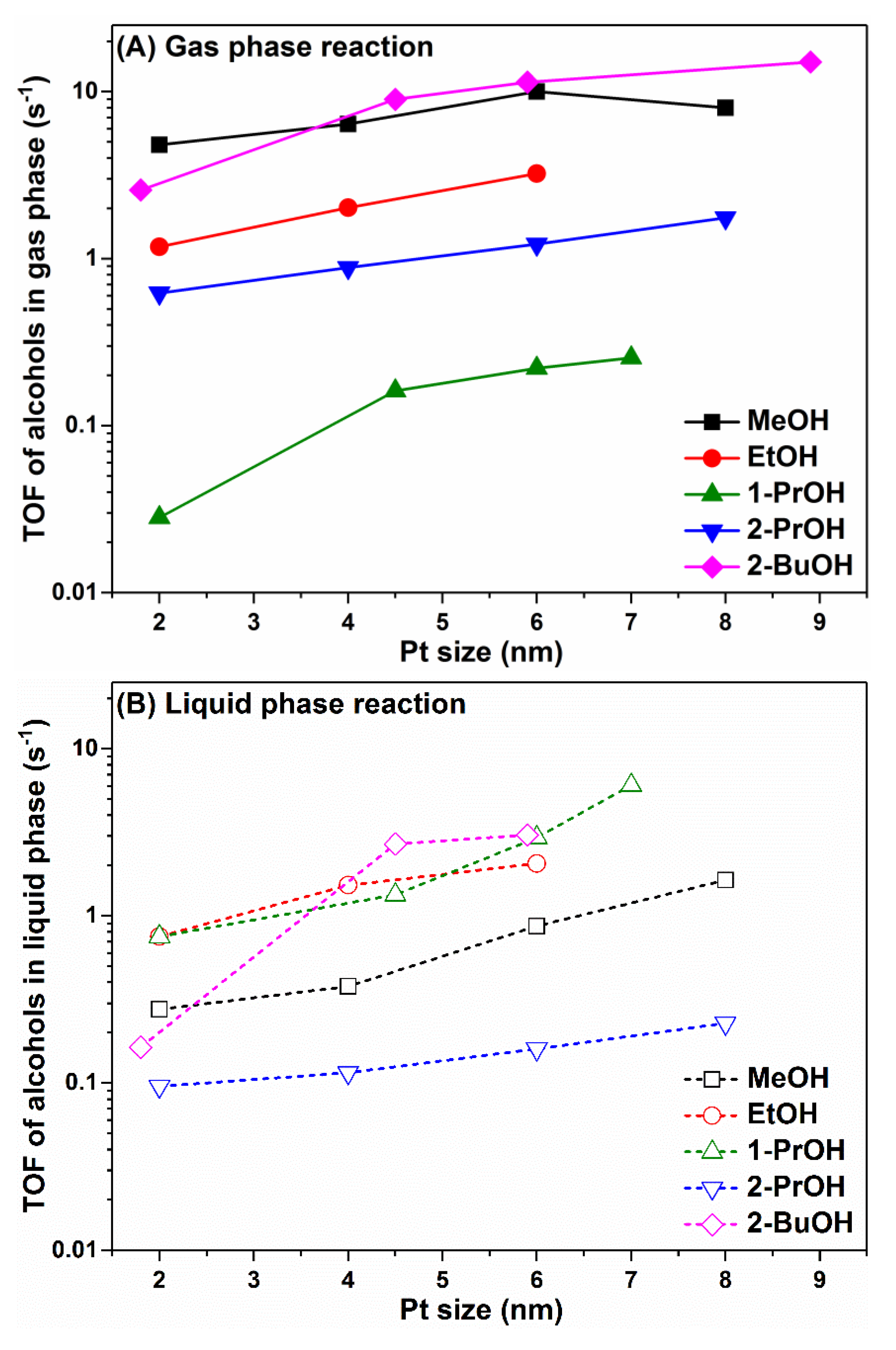
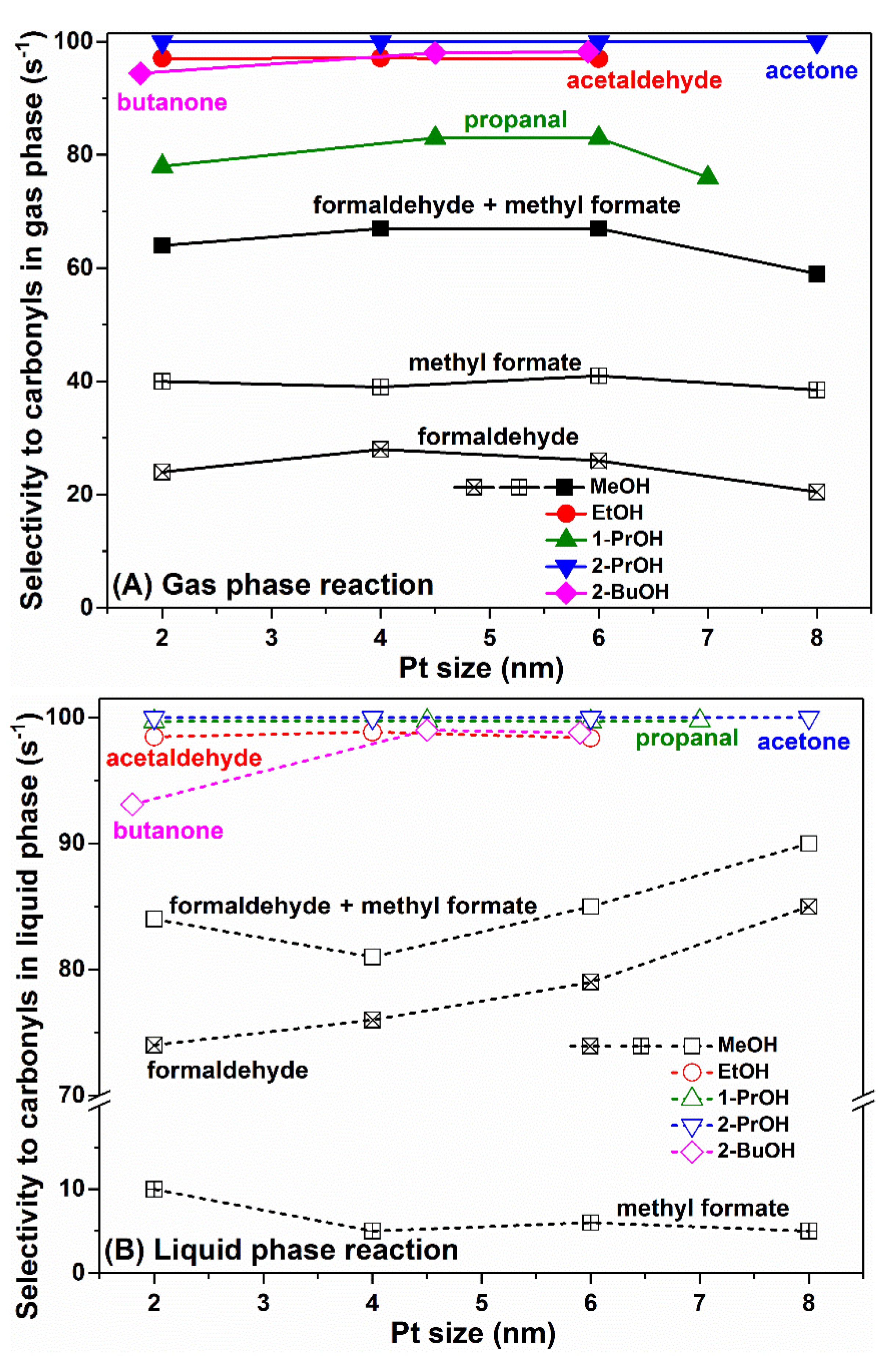
2.1. Turnover Rate Comparison for Alcohol Oxidation in Gas Phase and Liquid Phase
2.2. Size Effect of Pt Nanoparticles on Alcohol Oxidation in Gas Phase and Liquid Phase
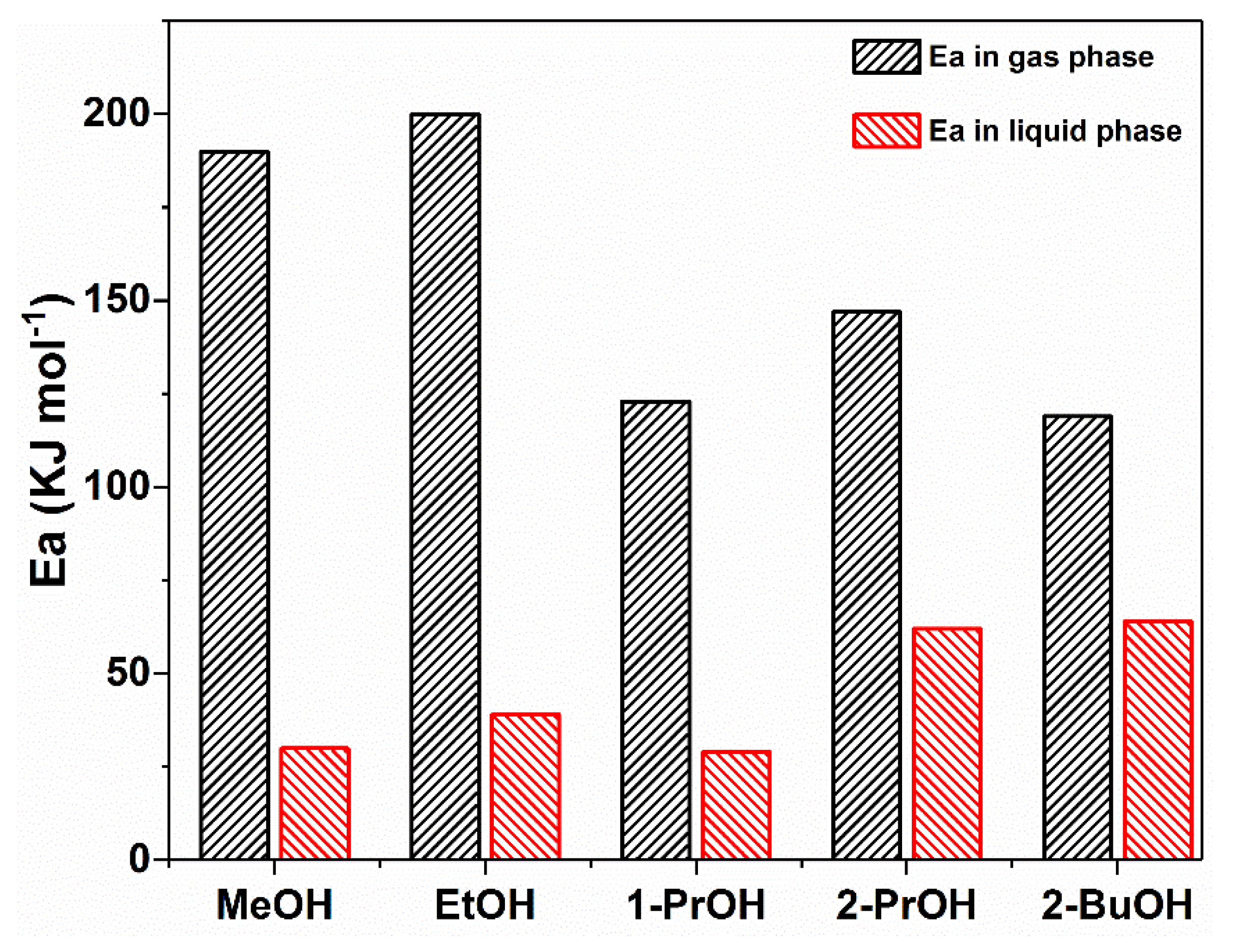
2.3. Different Activation Energies of Alcohol Oxidation in Gas Phase and Liquid Phase on Pt Nanoparticles
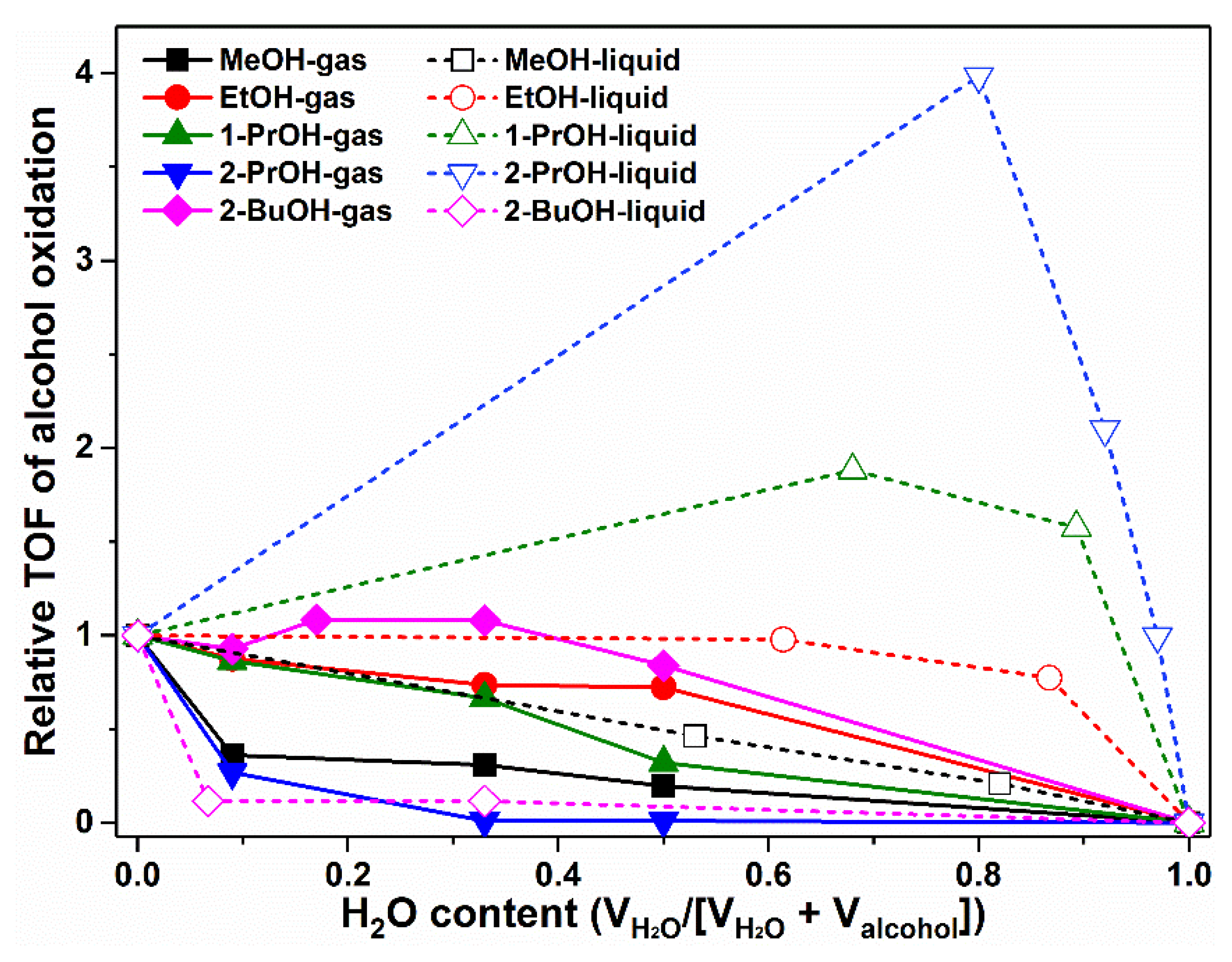
2.4. H2O Effect on Alcohol Oxidation in Gas Phase and Liquid Phase on Pt Nanoparticles
2.5. Case Study of 1-PrOH Oxidation Using SFG Spectra Analysis on Pt Thin Film and DFT Calculation in Gas and Liquid Phases
2.6. Case Study of 2-PrOH Oxidation Using SFG Spectra Analysis on Pt Nanoparticles and DFT Calculation in Gas Liquid Phases
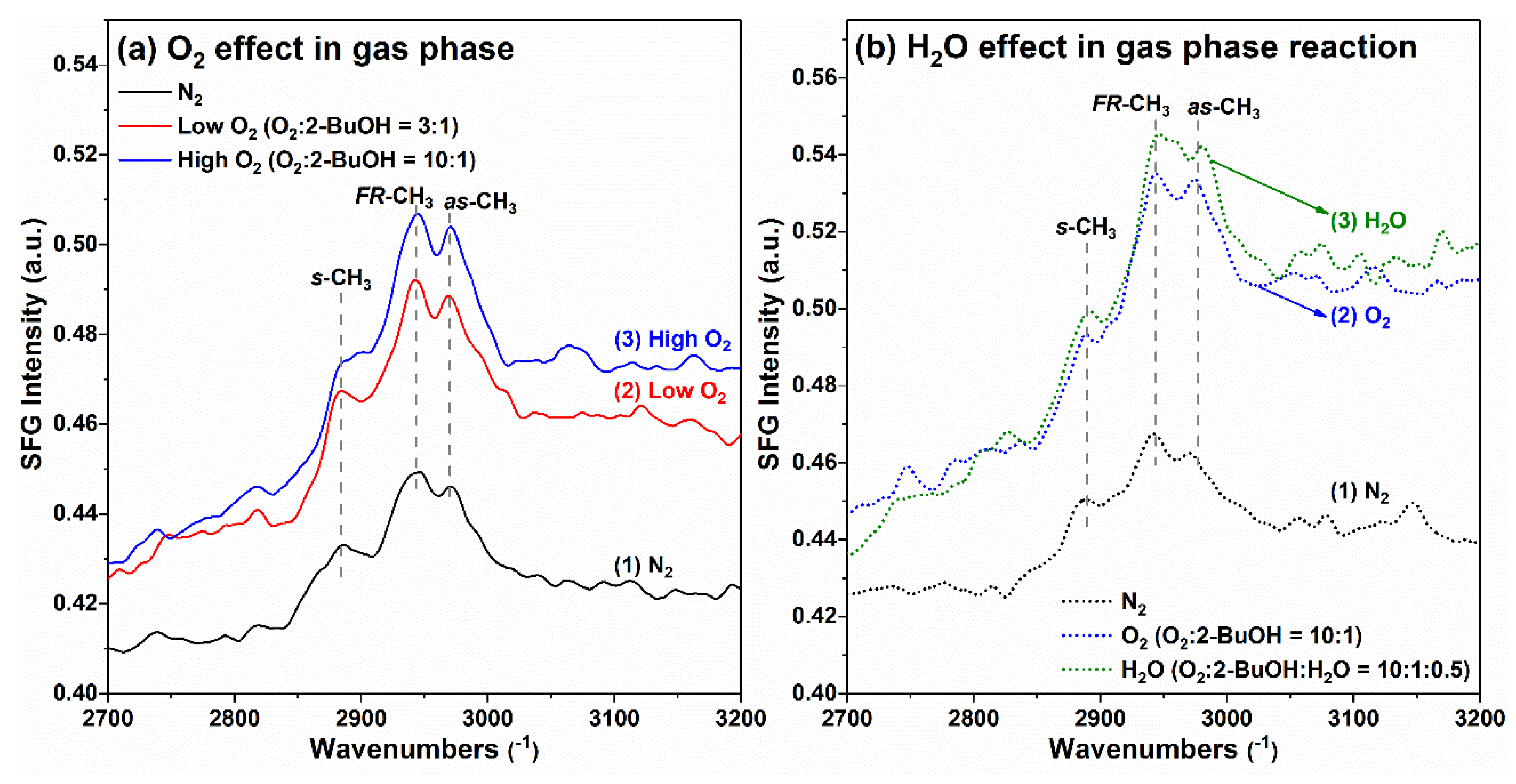
2.7. Case Study of 2-BuOH Oxidation Using SFG Spectra Analysis on Pt Thin Film in Gas Phase: O2 and H2O Effect
3. Materials and Methods
3.1. Pt Nanoparticle Synthesis and Encapulsation into Mesoporous Silica MCF-17
3.2. Catalytic Oxidation of Alcohols over Pt/MCF-17 Catalysts in Gas Phase and Liquid Phase
3.3. SFG Spectral Analysis for 1-PrOH, 2-PrOH and 2-BuOH Oxidation in Gas and Liquid Phases
3.4. DFT Calculation of Molecular Orientation of 1-PrOH and 2-PrOH on Pt Surface in Gas Phase and Liquid Phase
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- DiCosimo, R.; Whitesides, G.M. Oxidation of 2-propanol to acetone by dioxygen on a platinized electrode under open-circuit conditions. J. Phys. Chem. 1989, 93, 768–775. [Google Scholar] [CrossRef]
- Zhao, X.; Yin, M.; Ma, L.; Liang, L.; Liu, C.; Liao, J.; Lu, T.; Xing, W. Recent advances in catalysts for direct methanol fuel cells. Energy Environ. Sci. 2011, 4, 2736–2753. [Google Scholar] [CrossRef]
- Besson, M.; Gallezot, P. Selective oxidation of alcohols and aldehydes on metal catalysts. Catal. Today 2000, 57, 127–141. [Google Scholar] [CrossRef]
- Gauthier, E.; Benziger, J.B. Gas management and multiphase flow in direct alcohol fuel cells. Electrochim. Acta 2014, 128, 238–247. [Google Scholar] [CrossRef]
- Gomes, J.F.; Bergamaski, K.; Pinto, M.F.S.; Miranda, P.B. Reaction intermediates of ethanol electro-oxidation on platinum investigated by sfg spectroscopy. J. Catal. 2013, 302, 67–82. [Google Scholar] [CrossRef]
- Mallat, T.; Baiker, A. Oxidation of alcohols with molecular oxygen on solid catalysts. Chem. Rev. 2004, 104, 3037–3058. [Google Scholar] [CrossRef] [PubMed]
- Jelemensky, L.; Kuster, B.F.M.; Marin, G.B. Multiple steady-states for the oxidation of aqueous ethanol with oxygen on a carbon supported platinum catalyst. Catal. Lett. 1994, 30, 269–277. [Google Scholar] [CrossRef]
- Ciriminna, R.; Pandarus, V.; Béland, F.; Xu, Y.-J.; Pagliaro, M. Heterogeneously catalyzed alcohol oxidation for the fine chemical industry. Org. Process Res. Dev. 2015, 19, 1554–1558. [Google Scholar] [CrossRef]
- Tatsumi, H.; Liu, F.; Han, H.-L.; Carl, L.M.; Sapi, A.; Somorjai, G.A. Alcohol oxidation at platinum–gas and platinum–liquid interfaces: The effect of platinum nanoparticle size, water coadsorption, and alcohol concentration. J. Phys. Chem. C 2017, 121, 7365–7371. [Google Scholar] [CrossRef]
- Davis, S.E.; Ide, M.S.; Davis, R.J. Selective oxidation of alcohols and aldehydes over supported metal nanoparticles. Green Chem. 2013, 15, 17–45. [Google Scholar] [CrossRef]
- Feng, J.; Ma, C.; Miedziak, P.J.; Edwards, J.K.; Brett, G.L.; Li, D.; Du, Y.; Morgan, D.J.; Hutchings, G.J. Au-pd nanoalloys supported on mg-al mixed metal oxides as a multifunctional catalyst for solvent-free oxidation of benzyl alcohol. Dalton Trans. 2013, 42, 14498–14508. [Google Scholar] [CrossRef] [PubMed]
- Papes Filho, A.C.; Maciel Filho, R. Hybrid training approach for artificial neural networks using genetic algorithms for rate of reaction estimation: Application to industrial methanol oxidation to formaldehyde on silver catalyst. Chem. Eng. J. 2010, 157, 501–508. [Google Scholar] [CrossRef]
- Slot Thierry, K.; Eisenberg, D.; van Noordenne, D.; Jungbacker, P.; Rothenberg, G. Cooperative catalysis for selective alcohol oxidation with molecular oxygen. Chem. Eur. J. 2016, 22, 12307–12311. [Google Scholar] [CrossRef] [PubMed]
- Vinod, C.P.; Wilson, K.; Lee Adam, F. Recent advances in the heterogeneously catalysed aerobic selective oxidation of alcohols. J. Chem. Technol. Biotechnol. 2011, 86, 161–171. [Google Scholar] [CrossRef]
- Kopylovich, M.N.; Ribeiro, A.P.C.; Alegria, E.C.B.A.; Martins, N.M.R.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Chapter Three-Catalytic oxidation of alcohols: Recent advances. In Advances in Organometallic Chemistry; Pérez, P.J., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 63, pp. 91–174. [Google Scholar]
- Enache, D.I.; Edwards, J.K.; Landon, P.; Solsona-Espriu, B.; Carley, A.F.; Herzing, A.A.; Watanabe, M.; Kiely, C.J.; Knight, D.W.; Hutchings, G.J. Solvent-free oxidation of primary alcohols to aldehydes using Au-Pd/TiO2 catalysts. Science 2006, 311, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.S.; Kaur, H.; Shah, D. Selective oxidation of alcohols by supported gold nanoparticles: Recent advances. RSC Adv. 2016, 6, 28688–28727. [Google Scholar] [CrossRef]
- Mallat, T.; Baiker, A. Oxidation of alcohols with molecular oxygen on platinum metal catalysts in aqueous solutions. Catal. Today 1994, 19, 247–283. [Google Scholar] [CrossRef]
- Wang, H.; An, K.; Sapi, A.; Liu, F.; Somorjai, G.A. Effects of nanoparticle size and metal/support interactions in pt-catalyzed methanol oxidation reactions in gas and liquid phases. Catal. Lett. 2014, 144, 1930–1938. [Google Scholar] [CrossRef]
- Sapi, A.; Liu, F.; Cai, X.; Thompson, C.M.; Wang, H.; An, K.; Krier, J.M.; Somorjai, G.A. Comparing the catalytic oxidation of ethanol at the solid–gas and solid–liquid interfaces over size-controlled pt nanoparticles: Striking differences in kinetics and mechanism. Nano Lett. 2014, 14, 6727–6730. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Han, H.-L.; Carl, L.M.; Zherebetskyy, D.; An, K.; Wang, L.-W.; Somorjai, G.A. Catalytic 1-propanol oxidation on size-controlled platinum nanoparticles at solid-gas and solid-liquid interfaces: Significant differences in kinetics and mechanisms. J. Phys. Chem. C 2018. [Google Scholar] [CrossRef]
- Wang, H.; Sapi, A.; Thompson, C.M.; Liu, F.; Zherebetskyy, D.; Krier, J.M.; Carl, L.M.; Cai, X.; Wang, L.-W.; Somorjai, G.A. Dramatically different kinetics and mechanism at solid/liquid and solid/gas interfaces for catalytic isopropanol oxidation over size-controlled platinum nanoparticles. J. Am. Chem. Soc. 2014, 136, 10515–10520. [Google Scholar] [CrossRef] [PubMed]
- Saturated Vapor Pressure. Available online: http://ddbonline.ddbst.com/AntoineCalculation/AntoineCalculationCGI.exe (accessed on 1 February 2018).
- NIST Chemistry WebBook. Available online: https://webbook.nist.gov/cgi/inchi?ID=C71238&Mask=4&Type=ANTOINE&Plot=on (accessed on 1 February 2018).
- Kemme, H.R.; Kreps, S.I. Vapor pressure of primary n-alkyl chlorides and alcohols. J. Chem. Eng. Data 1969, 14, 98–102. [Google Scholar] [CrossRef]
- Kobayashi, H.; Higashimoto, S. DFT study on the reaction mechanisms behind the catalytic oxidation of benzyl alcohol into benzaldehyde by O2 over anatase TiO2 surfaces with hydroxyl groups: Role of visible-light irradiation. Appl. Catal. B Environ. 2015, 170–171, 135–143. [Google Scholar] [CrossRef]
- Welty, J.R.; Wicks, C.E.; Wilson, R.E.; Rorrer, G.L. Fundamentals of Momentum, Heat, and Mass Transfer 5th Edition; John Wiley & Sons, Inc.: New York, NY, USA, 2007; ISBN 2900470128687. [Google Scholar]
- Ferrell, R.T.; Himmelblau, D.M. Diffusion coefficients of nitrogen and oxygen in water. J. Chem. Eng. Data 1967, 12, 111–115. [Google Scholar] [CrossRef]
- Alger, D.B. The water solubility of 2-butanol: A widespread error. J. Chem. Educ. 1991, 68, 939. [Google Scholar] [CrossRef]
- Mullen, G.M.; Zhang, L.; Evans, E.J.; Yan, T.; Henkelman, G.; Mullins, C.B. Oxygen and hydroxyl species induce multiple reaction pathways for the partial oxidation of allyl alcohol on gold. J. Am. Chem. Soc. 2014, 136, 6489–6498. [Google Scholar] [CrossRef] [PubMed]
- Mullen, G.M.; Zhang, L.; Evans, E.J.; Yan, T.; Henkelman, G.; Mullins, C.B. Control of selectivity in allylic alcohol oxidation on gold surfaces: The role of oxygen adatoms and hydroxyl species. Phys. Chem. Chem. Phys. 2015, 17, 4730–4738. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.M.; Carl, L.M.; Somorjai, G.A. Sum frequency generation study of the interfacial layer in liquid-phase heterogeneously catalyzed oxidation of 2-propanol on platinum: Effect of the concentrations of water and 2-propanol at the interface. J. Phys. Chem. C 2013, 117, 26077–26083. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Klimeš, J.; Bowler, D.R.; Michaelides, A. Van der waals density functionals applied to solids. Phys. Rev. B 2011, 83, 195131. [Google Scholar] [CrossRef]
- Román-Pérez, G.; Soler, J.M. Efficient implementation of a van der waals density functional: Application to double-wall carbon nanotubes. Phys. Rev. Lett. 2009, 103, 096102. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef]

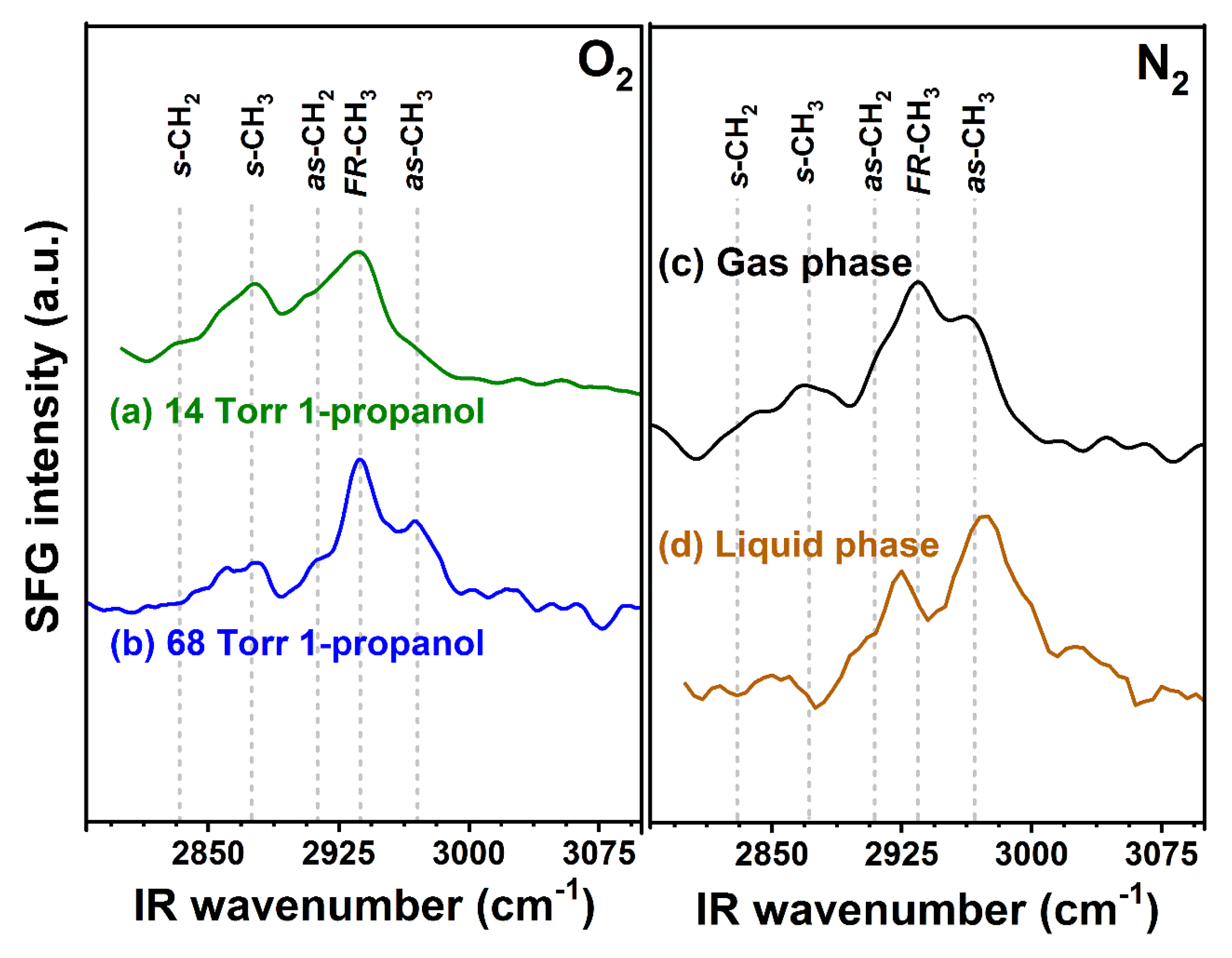
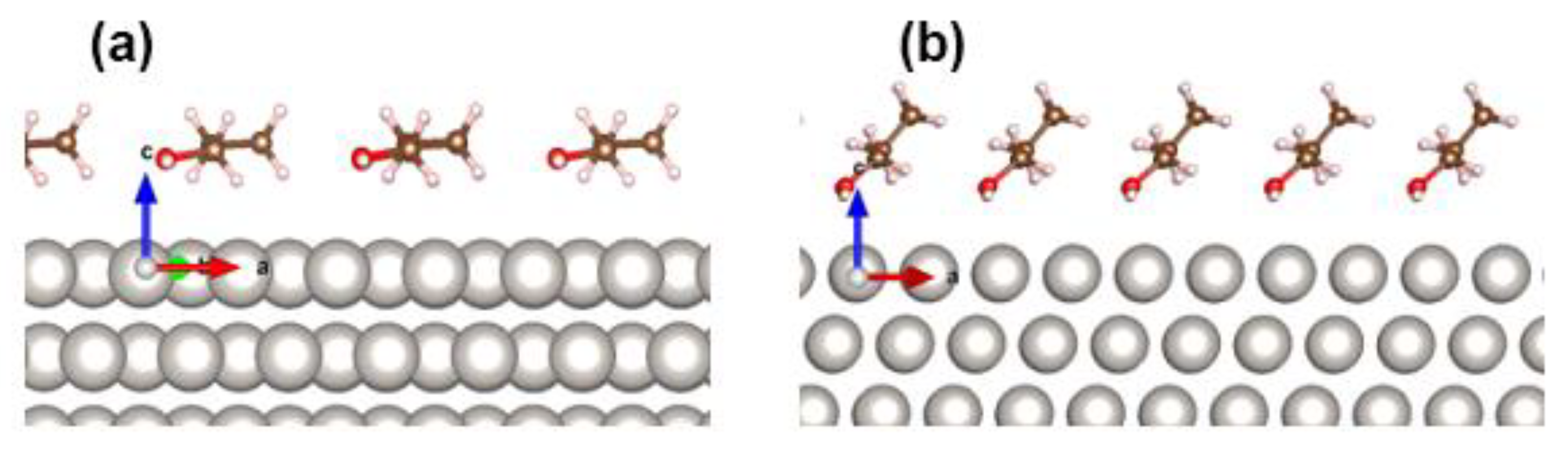
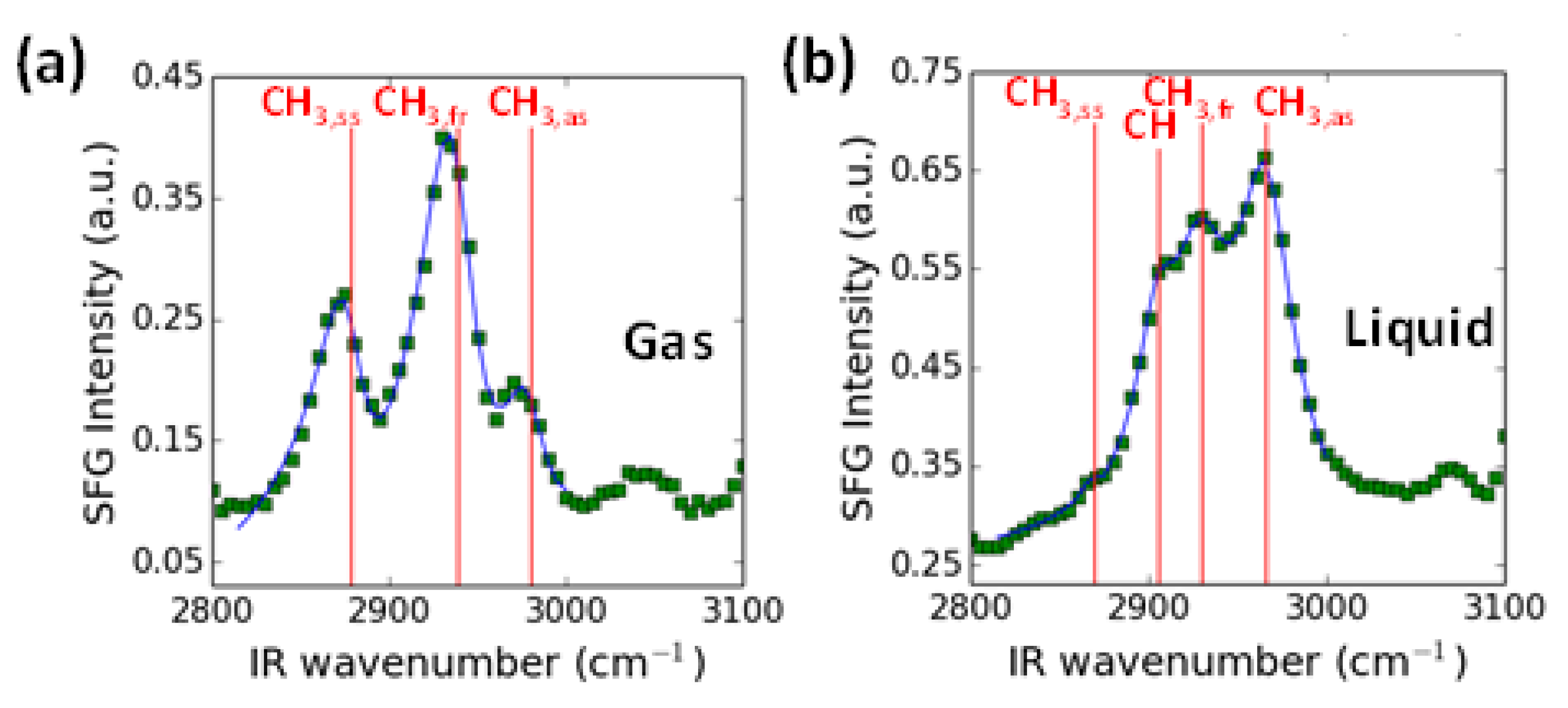
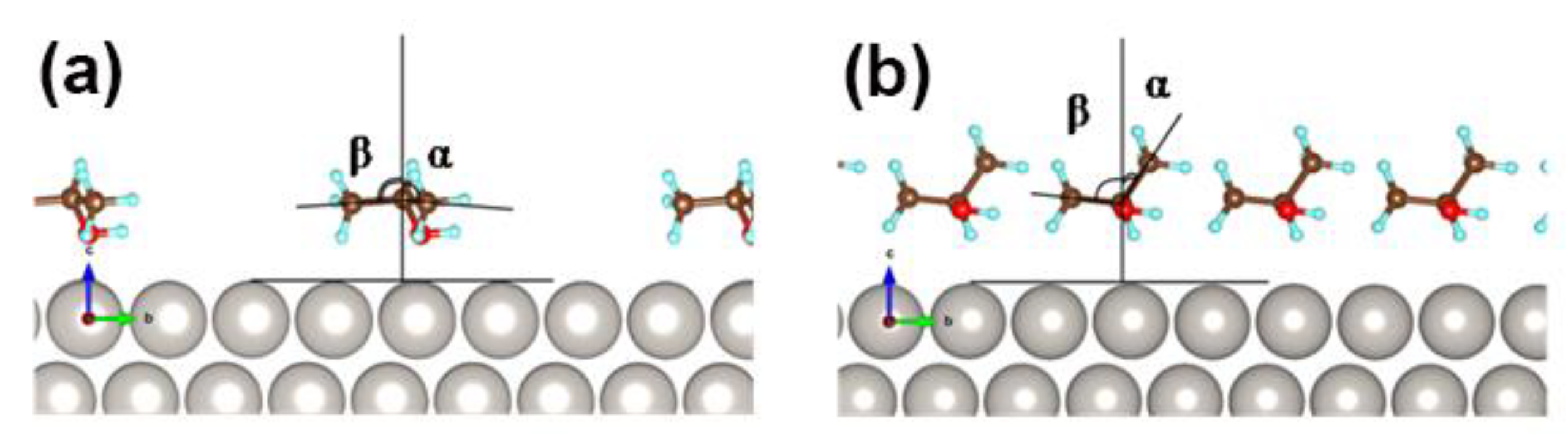
| Concentration (molecules/nm2) | α (°) | Halc-Pt (nm) |
|---|---|---|
| 0.94 | 6 | 0.317 |
| 3.75 | 41 | 0.261 |
| Concentration (molecules/nm2) | α (°) | β (°) | α-H-Pt (nm) |
|---|---|---|---|
| 0.94 | 86 | 86 | 0.445 |
| 3.75 | 38 | 84 | 0.257 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Wang, H.; Sapi, A.; Tatsumi, H.; Zherebetskyy, D.; Han, H.-L.; Carl, L.M.; Somorjai, G.A. Molecular Orientations Change Reaction Kinetics and Mechanism: A Review on Catalytic Alcohol Oxidation in Gas Phase and Liquid Phase on Size-Controlled Pt Nanoparticles. Catalysts 2018, 8, 226. https://doi.org/10.3390/catal8060226
Liu F, Wang H, Sapi A, Tatsumi H, Zherebetskyy D, Han H-L, Carl LM, Somorjai GA. Molecular Orientations Change Reaction Kinetics and Mechanism: A Review on Catalytic Alcohol Oxidation in Gas Phase and Liquid Phase on Size-Controlled Pt Nanoparticles. Catalysts. 2018; 8(6):226. https://doi.org/10.3390/catal8060226
Chicago/Turabian StyleLiu, Fudong, Hailiang Wang, Andras Sapi, Hironori Tatsumi, Danylo Zherebetskyy, Hui-Ling Han, Lindsay M. Carl, and Gabor A. Somorjai. 2018. "Molecular Orientations Change Reaction Kinetics and Mechanism: A Review on Catalytic Alcohol Oxidation in Gas Phase and Liquid Phase on Size-Controlled Pt Nanoparticles" Catalysts 8, no. 6: 226. https://doi.org/10.3390/catal8060226
APA StyleLiu, F., Wang, H., Sapi, A., Tatsumi, H., Zherebetskyy, D., Han, H.-L., Carl, L. M., & Somorjai, G. A. (2018). Molecular Orientations Change Reaction Kinetics and Mechanism: A Review on Catalytic Alcohol Oxidation in Gas Phase and Liquid Phase on Size-Controlled Pt Nanoparticles. Catalysts, 8(6), 226. https://doi.org/10.3390/catal8060226







