Ag/CeO2 Composites for Catalytic Abatement of CO, Soot and VOCs
Abstract
1. Introduction
2. Topical Processes
2.1. CO Oxidation
2.2. Soot Oxidation
2.3. VOCs Abatement
3. Ag/CeO2 Composites: Insights from Theory
4. Emerging Applications
4.1. Photocatalysis
4.2. Electrocatalysis
5. Conclusions and Outlook
- the catalytic performance of Ag/CeO2 composites strongly depends on the preparation method that determines the morphology of both Ag and ceria nanoparticles, interfacial configuration and strength of metal–support interaction;
- active surface sites are formed at the Ag–CeO2 interface, with the interfacial O atoms exhibiting different reactivity as compared to other surface O atoms, while oxygen species over Ag particles are still of importance and participate in catalysis;
- positively charged Ag clusters facilitate the formation of surface oxygen vacancies over ceria support, while metal Ag nanoparticles promote the reduction of CeO2 nanocrystals and enhance their catalytic activity;
- an enhanced activity of Ag/CeO2 materials is caused by the highest surface oxygen vacancy concentration, high low-temperature reducibility as well as existence of lattice oxygen species and lattice defects formed with the participation of both silver and ceria;
- the role of impurities (such as alkali ions, carbon-containing species, etc., appeared on the surface and/or bulk of ceria during the preparation procedure and participating in transferring of electron density to O surface species) should be considered;
- redox properties are caused by coexistence and interplay between Ag+/Ag0 and Ce3+/Ce4+ pairs;
- high photocatalytic activity of Ag/CeO2 composites is caused by the ability of Ag nanoparticles to prolong the lifetime of photogenerated electron–hole pairs due to the effect of localized SPR and reduction of the recombination of free charges;
- enhanced electrocatalytic activity and good electrochemical stability of Ag/CeO2 composites are connected with strong interfacial interactions between Ag and CeO2 moieties that are caused by their specific morphology and architecture, which hinder the particulate agglomeration during the long-term electrocatalytic reaction.
Supplementary Materials
Supplementary File 1Acknowledgments
Conflicts of Interest
References
- Web Site of World Health Organization. Available online: http://www.who.int/mediacentre/factsheets/fs313/en/ (accessed on 2 May 2018).
- California Air Resources Board. Definitions of VOC and ROG; California Air Resources Board: Sacramento, CA, USA, 2004.
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)-A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Oh, S.-H.; Hoflund, G.B. Chemical state study of palladium powder and ceria-supported palladium during low-temperature CO oxidation. J. Phys. Chem. A 2006, 110, 7609–7613. [Google Scholar] [CrossRef] [PubMed]
- Slavinskaya, E.M.; Gulyaev, R.V.; Zadesenets, A.V.; Stonkus, O.A.; Zaikovskii, V.I.; Shubin, Y.V.; Korenev, S.V.; Boronin, A.I. Low-temperature CO oxidation by Pd/CeO2 catalysts synthesized using the coprecipitation method. Appl. Catal. B 2015, 166–167, 91–103. [Google Scholar] [CrossRef]
- Hinokuma, S.; Fujii, H.; Okamoto, M.; Ikeue, K.; Machida, M. Metallic Pd nanoparticles formed by Pd–O–Ce interaction: a reason for sintering-induced activation for CO oxidation. Chem. Mater. 2010, 22, 6183–6190. [Google Scholar] [CrossRef]
- Jin, M.; Park, J.-N.; Shon, J.K.; Kim, J.H.; Li, Z.; Park, Y.-K.; Kim, J.M. Low temperature CO oxidation over Pd catalysts supported on highly ordered mesoporous metal oxides. Catal. Today 2012, 185, 183–190. [Google Scholar] [CrossRef]
- Wu, J.; Zeng, L.; Cheng, D.; Chen, F.; Zhan, X.; Gong, J. Synthesis of Pd nanoparticles supported on CeO2 nanotubes for CO oxidation at low temperatures. Chin. J. Catal. 2016, 37, 83–90. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, X.F.; Meng, D.M.; Guo, Y.; Guo, Y.L.; Lu, G.Z. Effect of Ceria Crystal Plane on the Physicochemical and Catalytic Properties of Pd/Ceria for CO and Propane Oxidation. ACS Catal. 2016, 6, 2265–2279. [Google Scholar] [CrossRef]
- Si, G.; Yu, J.; Xiao, X.; Guo, X.; Huang, H.; Mao, D.; Lu, G. Boundary role of Nano-Pd catalyst supported on ceria and the approach of promoting the boundary effect. Mol. Catal. 2018, 444, 1–9. [Google Scholar] [CrossRef]
- Adijanto, L.; Sampath, A.; Yu, A.S.; Cargnello, M.; Fornasiero, P.; Gorte, R.J.; Vohs, J.M. Synthesis and Stability of Pd@CeO2 Core–Shell Catalyst Films in Solid Oxide Fuel Cell Anodes. ACS Catal. 2013, 3, 1801–1809. [Google Scholar] [CrossRef]
- Carlsson, P.A.; Skoglundh, M. Low-temperature oxidation of carbon monoxide and methane over alumina and ceria supported platinum catalysts. Appl. Catal. B. 2011, 101, 669–675. [Google Scholar] [CrossRef]
- Morfin, F.; Nguyen, T.S.; Rousset, J.L.; Piccolo, L. Synergy between hydrogen and ceria in Pt-catalyzed CO oxidation: An investigation on Pt–CeO2 catalysts synthesized by solution combustion. Appl. Catal. B 2016, 197, 2–13. [Google Scholar] [CrossRef]
- Lee, J.; Ryou, Y.; Kim, J.; Chan, X.; Kim, T.J.; Kim, D.H. Influence of the Defect Concentration of Ceria on the Pt Dispersion and the CO Oxidation Activity of Pt/CeO2. J. Phys. Chem. C 2018, 122, 4972–4983. [Google Scholar] [CrossRef]
- Peng, R.; Li, S.; Sun, X.; Ren, Q.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Size effect of Pt nanoparticles on the catalytic oxidation of toluene over Pt/CeO2 catalysts. Appl. Catal. B 2018, 220, 462–470. [Google Scholar] [CrossRef]
- Bera, P.; Gayen, A.; Hegde, M.S.; Lalla, N.P.; Spadaro, L.; Frusteri, F.; Arena, F. Promoting Effect of CeO2 in Combustion Synthesized Pt/CeO2 Catalyst for CO Oxidation. J. Phys. Chem. B 2003, 107, 6122–6130. [Google Scholar] [CrossRef]
- Zhang, R.; Lu, K.; Zong, L.; Tong, S.; Wang, X.; Feng, G. Gold supported on ceria nanotubes for CO oxidation. Appl. Surf. Sci. 2017, 416, 183–190. [Google Scholar] [CrossRef]
- Zhang, R.; Lu, K.; Zong, L.; Tong, S.; Wang, X.; Zhou, J.; Lu, Z.-H.; Feng, G. CO Oxidation Activity at Room Temperature over Au/CeO2 Catalysts: Disclosure of Induction Period and Humidity Effect. Mol. Catal. 2017, 442, 173–180. [Google Scholar] [CrossRef]
- Centeno, M.A.; Reina, T.R.; Ivanova, S.; Laguna, O.H.; Odriozola, J.A. Au/CeO2 Catalysts: Structure and CO Oxidation Activity. Catalysts 2016, 6, 158. [Google Scholar] [CrossRef]
- El-Moemen, A.A.; Abdel-Mageed, A.M.; Bansmann, J.; Parlinska-Wojtan, M.; Behm, R.J.; Kučerová, G. Deactivation of Au/CeO2 catalysts during CO oxidation: Influence of pretreatment and reaction conditions. J. Catal. 2016, 341, 160–179. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Mallesham, B.; Reddy, P.S.; Großmann, D.; Grünert, W.; Reddy, B.M. Nano-Au/CeO2 catalysts for CO oxidation: Influence of dopants (Fe, La and Zr) on the physicochemical properties and catalytic activity. Appl. Catal. B 2014, 144, 900–908. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.-S.; Chen, B.; Zhu, X.; Shi, C.; Zhu, A.-M. CO Oxidation Activity at Room Temperature over Au/CeO2 Catalysts: Disclosure of Induction Period and Humidity Effect. ACS Catal. 2014, 4, 3481–3489. [Google Scholar] [CrossRef]
- Li, H.-F.; Zhang, N.; Chen, P.; Luo, M.-F.; Lu, J.-Q. High surface area Au/CeO2 catalysts for low temperature formaldehyde oxidation. Appl. Catal. B 2011, 110, 279–285. [Google Scholar] [CrossRef]
- Satsuma, A.; Yanagihara, M.; Ohyama, J.; Shimizu, K. Oxidation of CO over Ru/ceria prepared by self-dispersion of Ru metal powder into nano-sized particle. Catal. Today 2013, 201, 62–67. [Google Scholar] [CrossRef]
- Vargas, E.; Simakov, A.; Rangel, R.; Castillon, F. CO oxidation over Ce–Ru–O catalysts. In Proceedings of the 20th North American Catalysis Meeting, Houston, TX, USA, 17–22 June 2007. [Google Scholar]
- Asadullah, M.; Fujimoto, K.; Tomishige, K. Catalytic Performance of Rh/CeO2 in the Gasification of Cellulose to Synthesis Gas at Low Temperature. Ind. Eng. Chem. Res. 2001, 40, 5894–5900. [Google Scholar] [CrossRef]
- Li, K.; Wang, X.; Zhou, Z.; Wu, X.; Weng, D. Oxygen Storage Capacity of Pt-, Pd-, Rh/CeO2-Based Oxide Catalyst. J. Rare Earths 2007, 25, 6–10. [Google Scholar]
- Kurnatowska, M.; Kepinski, L. Structure and thermal stability of nanocrystalline Ce1−xRhxO2−y in reducing and oxidizing atmosphere. Mater. Res. Bull. 2013, 48, 852–862. [Google Scholar] [CrossRef]
- Zhao, Y.; Teng, B.-T.; Wen, X.-D.; Zhao, Y.; Zhao, L.-H.; Luo, M.-F. A theoretical evaluation and comparison of MxCe1−xO2−δ (M = Au, Pd, Pt, and Rh) catalysts. Catal. Commun. 2012, 27, 63–68. [Google Scholar] [CrossRef]
- Li, Y.; Cai, Y.; Xing, X.; Chen, N.; Deng, D.; Wang, Y. Catalytic activity for CO oxidation of Cu–CeO2 composite nanoparticles synthesized by a hydrothermal method. Anal. Methods 2015, 7, 3238–3245. [Google Scholar] [CrossRef]
- Sundar, R.S.; Deevi, S. CO oxidation activity of Cu–CeO2 nano-composite catalysts prepared by laser vaporization and controlled condensation. J. Nanopart. Res. 2006, 8, 497–509. [Google Scholar] [CrossRef]
- Xu, X.; Li, J.; Hao, Z. CeO2-Co3O4 Catalysts for CO Oxidation. J. Rare Earths 2006, 24, 172–176. [Google Scholar] [CrossRef]
- Quiroz, J.; Giraudon, J.-M.; Gervasini, A.; Dujardin, C.; Lancelot, C.; Trentesaux, M.; Lamonier, J.-F. Total Oxidation of Formaldehyde over MnOx-CeO2 Catalysts: The Effect of Acid Treatment. ACS Catal. 2015, 5, 2260–2269. [Google Scholar] [CrossRef]
- Huang, H.; Xu, Y.; Feng, Q.; Leung, D.Y. Low temperature catalytic oxidation of volatile organic compounds: A review. Catal. Sci. Technol. 2015, 5, 2649–2669. [Google Scholar] [CrossRef]
- Vodyankina, O.V.; Blokhina, A.S.; Kurzina, I.A.; Sobolev, V.I.; Koltunov, K.Y.; Chukhlomina, L.N.; Dvilis, E.S. Selective oxidation of alcohols over Ag-containing Si3N4 catalysts. Catal. Today 2013, 203, 127–132. [Google Scholar] [CrossRef]
- Mamontov, G.V.; Grabchenko, M.V.; Sobolev, V.I.; Zaikovskii, V.I.; Vodyankina, O.V. Ethanol dehydrogenation over Ag-CeO2/SiO2 catalyst: Role of Ag-CeO2 interface. Appl. Catal. A 2016, 528, 161–167. [Google Scholar] [CrossRef]
- Dutov, V.V.; Mamontov, G.V.; Sobolev, V.I.; Vodyankina, O.V. Silica-supported silver-containing OMS-2 catalysts for ethanol oxidative dehydrogenation. Catal. Today 2016, 278, 164–173. [Google Scholar] [CrossRef]
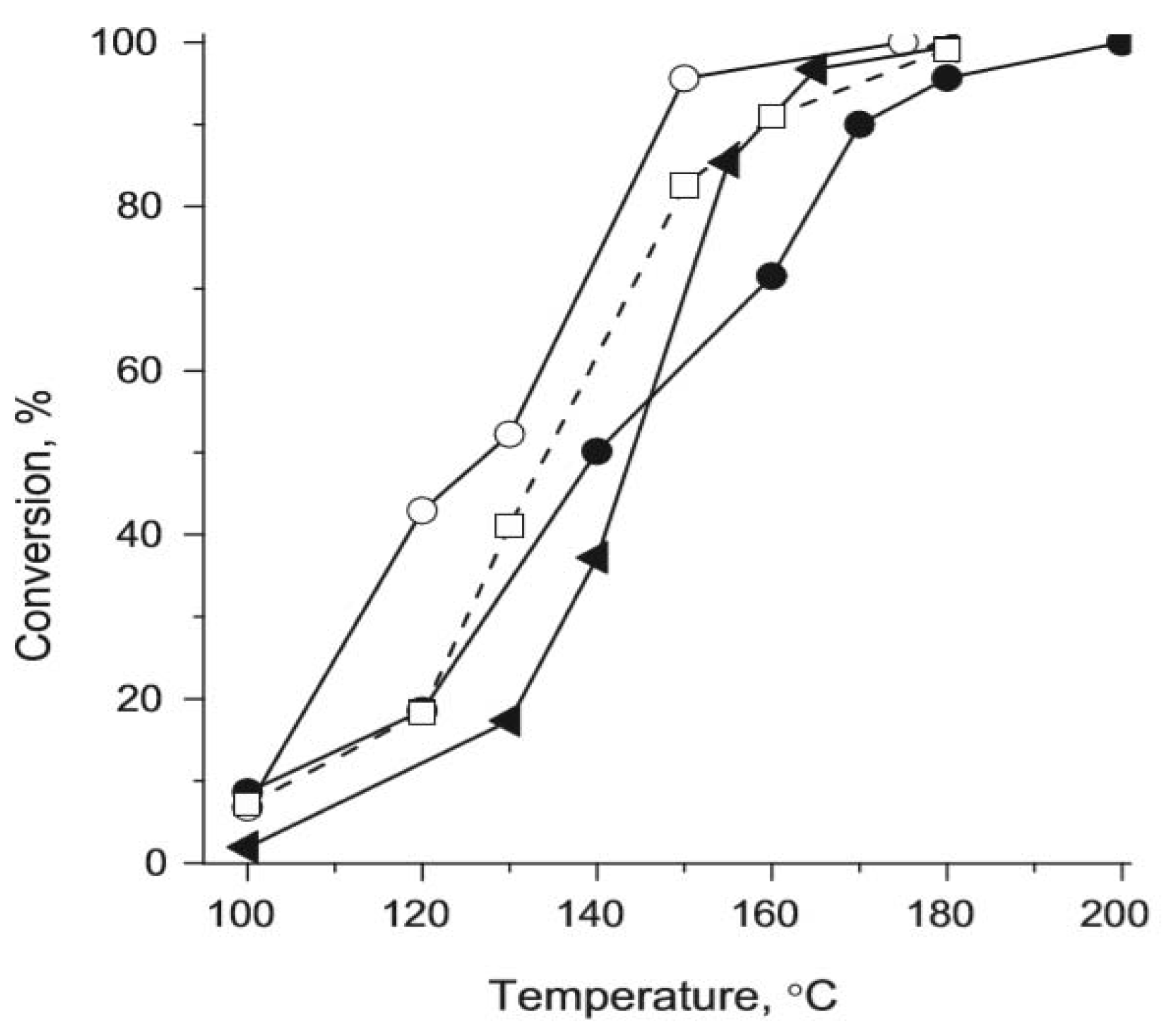
- Mamontov, G.V.; Gorbunova, A.S.; Vyshegorodtseva, E.V.; Zaikovskii, V.I.; Vodyankina, O.V. Selective oxidation of CO in the presence of propylene over Ag/MCM-41 catalyst. Catal. Today 2018. [Google Scholar] [CrossRef]
- Pan, C.-J.; Tsai, M.-C.; Su, W.-N.; Rick, J.; Akalework, N.G.; Agegnehu, A.K.; Cheng, S.-Y.; Hwang, B.-J. Tuning/exploiting Strong Metal-Support Interaction (SMSI) in Heterogeneous Catalysis. J. Taiwan Inst. Chem. E 2017, 74, 154–186. [Google Scholar] [CrossRef]
- Ma, L.; Wang, D.; Li, J.; Bai, B.; Fu, L.; Li, Y. Ag/CeO2 nanospheres: Efficient catalysts for formaldehyde oxidation. Appl. Catal. B 2014, 148, 36–43. [Google Scholar] [CrossRef]
- Skaf, M.; Aouad, S.; Hany, S.; Cousin, R.; Abi-Aadand, E.; Aboukais, A. Physicochemical characterization and catalytic performance of 10% Ag/CeO2 catalysts prepared by impregnation and deposition-precipitation. J. Catal. 2014, 320, 137–146. [Google Scholar] [CrossRef]
- Qu, Z.; Yu, F.; Zhang, X.; Wang, Y.; Gao, J. Support effects on the structure and catalytic activity of mesoporous Ag/CeO2 catalysts for CO oxidation. Chem. Eng. J. 2013, 229, 522–532. [Google Scholar] [CrossRef]
- Chang, S.; Li, M.; Hua, Q.; Zhang, L.; Ma, Y.; Ye, B.; Huang, W. Shape-dependent interplay between oxygen vacancies and Ag–CeO2 interaction in Ag/CeO2 catalysts and their influence on the catalytic activity. J. Catal. 2012, 293, 195–204. [Google Scholar] [CrossRef]
- Kayama, T.; Yamazaki, K.; Shinjoh, H. Nanostructured Ceria−Silver Synthesized in a One-Pot Redox Reaction Catalyzes Carbon Oxidation. J. Am. Chem. Soc. 2010, 132, 13154–13155. [Google Scholar]
- Shimizu, K.; Kawachi, H.; Satsuma, A. Study of active sites and mechanism for soot oxidation by silver-loaded ceria catalyst. Appl. Catal. B Environ. 2010, 96, 169–175. [Google Scholar] [CrossRef]
- Aneggi, E.; Llorca, J.; de Leitenburg, C.; Dolcetti, G.; Trovarelli, A. Soot Combustion Over Silver-Supported Catalysts. Appl. Catal. B Environ. 2009, 91, 489–498. [Google Scholar] [CrossRef]
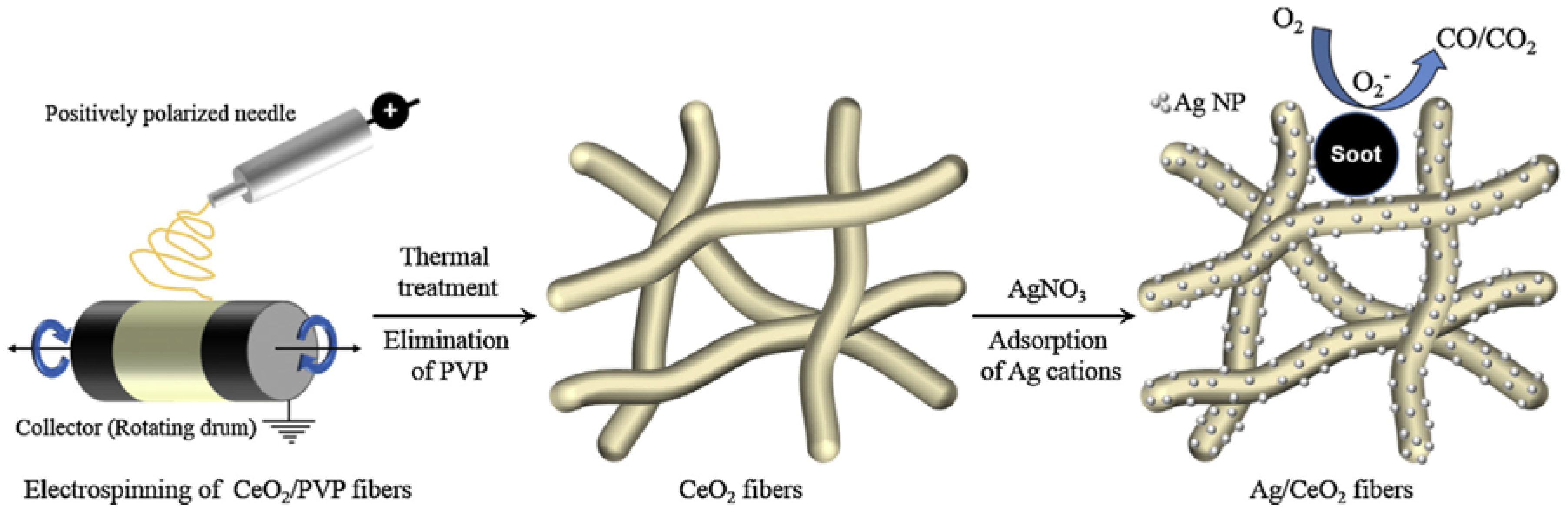
- Lee, C.; Park, J.; Shul, Y.-G.; Einaga, H.; Teraoka, Y. Ag supported on electrospun macro-structure CeO2 fibrous mats for diesel soot oxidation. Appl. Catal. B Environ. 2015, 174, 185–192. [Google Scholar] [CrossRef]
- Machida, M.; Murata, Y.; Kishikawa, K.; Zhang, D.; Ikeue, K. On the Reasons for High Activity of CeO2 Catalyst for Soot Oxidation. Chem. Mater. 2008, 20, 4489–4494. [Google Scholar] [CrossRef]
- Yamazaki, K.; Kayama, T.; Dong, F.; Shinjoh, H. A mechanistic study on soot oxidation over CeO2-Ag catalyst with ‘rice-ball’ morphology. J. Catal. 2011, 282, 289–298. [Google Scholar] [CrossRef]
- Leng, Q.; Yang, D.; Yang, Q.; Hu, C.; Kang, Y.; Wang, M.; Hashim, M. Building novel Ag/CeO2 heterostructure for enhancing photocatalytic activity. Mater. Res. Bull. 2015, 65, 266–272. [Google Scholar] [CrossRef]
- Li, G.; Lu, F.; Wei, X.; Song, X.; Sun, Z.; Yang, Z.; Yang, S. Nanoporous Ag–CeO2 ribbons prepared by chemical dealloying and their electrocatalytic properties. J. Mater. Chem. 2013, 1, 4974–4981. [Google Scholar] [CrossRef]
- Zhang, X.; Li, G.; Song, X.; Yang, S.; Sun, Z. Three-dimensional architecture of Ag/CeO2 nanorod composites prepared by dealloying and their electrocatalytic performance. RSC Adv. 2017, 7, 32442–32451. [Google Scholar] [CrossRef]
- Imamura, S.; Yamada, H.; Utani, K. Combustion activity of Ag/CeO2 composite catalyst. Appl. Catal. A Gen. 2000, 192, 221–226. [Google Scholar] [CrossRef]
- Scirè, S.; Riccobene, P.M.; Crisafulli, C. Ceria supported group IB metal catalysts for the combustion of volatile organic compounds and the preferential oxidation of CO. Appl. Catal. B Environ. 2010, 101, 109–117. [Google Scholar] [CrossRef]
- Fiorenza, R.; Crisafulli, C.; Condorelli, G.G.; Lupo, F.; Scirè, S. Au–Ag/CeO2 and Au–Cu/CeO2 Catalysts for Volatile Organic Compounds Oxidation and CO Preferential Oxidation. Catal. Lett. 2015, 145, 1691–1702. [Google Scholar] [CrossRef]
- Wang, L.; He, H.; Yu, Y.; Sun, L.; Liu, S.; Zhang, C.; He, L. Morphology-dependent bactericidal activities of Ag/CeO2 catalysts against Escherichia coli. J. Inorg. Biochem. 2014, 135, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Beuhler, R.J.; Rao, R.M.; Hrbek, J.; White, M.G. Study of the Partial Oxidation of Methanol to Formaldehyde on a Polycrystalline Ag Foil. J. Phys. Chem. B 2001, 105, 5950–5956. [Google Scholar] [CrossRef]
- Mamontov, G.V.; Magaev, O.V.; Knyazev, A.S.; Vodyankina, O.V. Influence of phosphate addition on activity of Ag and Cu catalysts for partial oxidation of alcohols. Catal. Today 2013, 203, 122–126. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Grinter, D.C.; Liu, Z.; Palomino, R.M.; Senanayake, S.D. Ceria-based model catalysts: Fundamental studies on the importance of the metal-ceria interface in CO oxidation, the water-gas shift, CO2 hydrogenation, and methane and alcohol reforming. Chem. Soc. Rev. 2017, 46, 1824–1841. [Google Scholar] [CrossRef] [PubMed]
- Zagaynov, I.V.; Naumkin, A.V.; Grigoriev, Y.V. Perspective intermediate temperature ceria based catalysts for CO oxidation. Appl. Catal. B Environ. 2018, 236, 171–175. [Google Scholar] [CrossRef]
- Slavinskaya, E.M.; Stadnichenko, A.I.; Muravyov, V.V.; Kardash, T.Y.; Derevyannikova, E.A.; Zaikovskii, V.I.; Stonkus, O.A.; Lapin, I.N.; Svetlichnyi, V.A.; Boronin, A.I. Transformation of a Pt–CeO2 Mechanical Mixture of Pulsed-Laser-Ablated Nanoparticles to a Highly Active Catalyst for Carbon Monoxide Oxidation. ChemCatChem 2018, 10, 2232–2247. [Google Scholar] [CrossRef]
- Park, Y.; Kim, S.K.; Pradhan, D.; Sohn, Y. Thermal H2-treatment effects on CO/CO2 conversion over Pd-doped CeO2 comparison with Au and Ag-doped CeO2. Reac. Kinet. Mech. Cat. 2014, 113, 85–100. [Google Scholar] [CrossRef]
- Park, Y.; Na, Y.; Pradhan, Y.; Sohn, Y. Liquid-Phase Ethanol Oxidation and Gas-Phase CO Oxidation Reactions over M Doped (M = Ag, Au, Pd, and Ni) and MM’ Codoped CeO2 Nanoparticles. J. Catal. 2016, 2176576. [Google Scholar] [CrossRef]
- Dutov, V.V.; Mamontov, G.V.; Zaikovskii, V.I.; Liotta, L.F.; Vodyankina, O.V. Low-temperature CO oxidation over Ag/SiO2 catalysts: Effect of OH/Ag ratio. Appl. Catal. B 2018, 221, 598–609. [Google Scholar] [CrossRef]
- Dutov, V.V.; Mamontov, G.V.; Zaikovskii, V.I.; Vodyankina, O.V. The effect of support pretreatment on activity of Ag/SiO2 catalysts in low-temperature CO oxidation. Catal. Today 2016, 278, 150–156. [Google Scholar] [CrossRef]
- Afanasev, D.S.; Yakovina, O.A.; Kuznetsova, N.I.; Lisitsyn, A.S. High activity in CO oxidation of Ag nanoparticles supported on fumed silica. Catal. Commun. 2012, 22, 43–47. [Google Scholar] [CrossRef]
- Liu, H.; Ma, D.; Blackley, R.A.; Zhou, W.; Bao, X. Highly active mesostructured silica hosted silver catalysts for CO oxidation using the one-pot synthesis approach. Chem. Commun. 2008, 2677–2678. [Google Scholar] [CrossRef] [PubMed]
- Mamontov, G.V.; Dutov, V.V.; Sobolev, V.I.; Vodyankina, O.V. Effect of transition metal oxide additives on the activity of an Ag/SiO2 catalyst in carbon monoxide oxidation. Kinet. Catal. 2013, 54, 487–491. [Google Scholar] [CrossRef]
- Zhang, D.; Du, X.; Shi, L.; Gao, R. Shape-controlled synthesis and catalytic application of ceria nanomaterials. Dalton Trans. 2012, 41, 14455–14475. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.-X.; Sun, L.-D.; Zhang, Y.-W.; Si, R.; Feng, W.; Zhang, H.-P.; Liu, H.-C.; Yan, C.-H. Shape-Selective Synthesis and Oxygen Storage Behavior of Ceria Nanopolyhedra, Nanorods, and Nanocubes. J. Phys. Chem. B. 2005, 109, 24380–24385. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, M.; Overbury, S.H. On the structure dependence of CO oxidation over CeO2 nanocrystals with well-defined surface planes. J. Catal. 2012, 285, 61–73. [Google Scholar] [CrossRef]
- Kang, Y.; Sun, M.; Li, A. Studies of the Catalytic Oxidation of CO Over Ag/CeO2 Catalyst. Catal. Lett. 2012, 142, 1498–1504. [Google Scholar] [CrossRef]
- Li, G.; Zhang, X.; Feng, W.; Fang, X.; Liu, J. Nanoporous CeO2–Ag catalysts prepared by etching the CeO2/CuO/Ag2O mixed oxides for CO oxidation. Corros. Sci. 2018, 134, 140–148. [Google Scholar] [CrossRef]
- Liang, X.; Xiao, J.; Chen, B.; Li, Y. Catalytically Stable and Active CeO2 Mesoporous Spheres. Inorg. Chem. 2010, 49, 8188–8190. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Tang, Z. Noble metal nanoparticle@metal oxide core/yolk–shell nanostructures as catalysts: recent progress and perspective. Nanoscale 2014, 6, 3995–4011. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Arandiyan, H.; Scott, J.; Bagheri, A.; Dai, H.; Amal, R. Recent Advances in Ordered Meso/macroporous Metal Oxides for Heterogeneous Catalysis: A Review. J. Mater. Chem. A 2017, 5, 8825–8846. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Huang, X.; Li, G. Fabrication of Ag–CeO2 core–shell nanospheres with enhanced catalytic performance due to strengthening of the interfacial interactions. J. Mater. Chem. 2012, 22, 10480–10487. [Google Scholar] [CrossRef]
- Badri, A.; Binet, C.; Lavalley, J.-C. An FTIR study of surface ceria hydroxy groups during a redox process with H2. J. Chem. Soc. Faraday Trans. 1996, 92, 4669–4673. [Google Scholar] [CrossRef]
- Grabchenko, M.V.; Mamontov, G.V.; Zaikovskii, V.I.; La Parola., V.; Liotta, L.F.; Vodyankina, O.V. Design of Ag-CeO2/SiO2 catalyst for oxidative dehydrogenation of ethanol: Control of Ag–CeO2 interfacial interaction. Catal. Today 2018. [Google Scholar] [CrossRef]
- Grabchenko, M.V.; Mamontov, G.V.; Zaikovskii, V.I.; Vodyankina, O.V. Effect of the metal−support interaction in Ag/CeO2 catalysts on their activity in ethanol oxidation. Kinet. Catal. 2017, 58, 642–648. [Google Scholar] [CrossRef]
- Mitsudome, T.; Mikami, Y.; Matoba, M.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Design of a silver-cerium dioxide core-shell nanocomposite catalyst for chemoselective reduction reactions. Angew. Chem. Int. Ed. 2012, 51, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Ma, S.; Zhan, S. Superior photocatalytic disinfection effect of Ag-3D ordered mesoporous CeO2 under visible light. Appl. Catal. B 2018, 224, 27–37. [Google Scholar] [CrossRef]
- Stanmore, B.R.; Brilhac, J.F.; Gilot, P. The oxidation of soot: a review of experiments, mechanisms and models. Carbon 2001, 39, 2247–2268. [Google Scholar] [CrossRef]
- Neyertz, C.A.; Banus, E.D.; Mir’o, E.E.; Querini, C.A. Potassium-promoted Ce0.65Zr0.35O2 monolithic catalysts for diesel soot combustion. Chem. Eng. J. 2014, 248, 394–405. [Google Scholar] [CrossRef]
- Fino, D.; Bensaid, S.; Piumetti, M.; Russo, N. A review on the catalyticcombustion of soot in diesel particulate filters for automotive applications:from powder catalysts to structured reactors. Appl. Catal. A 2016, 509, 75–96. [Google Scholar] [CrossRef]
- Neeft, P.A.; Makkee, M.; Moulijn, J.A. Catalysts for the oxidation of soot from diesel exhaust gases. I. An exploratory study. Appl. Catal. B 1996, 8, 57–78. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Hikosaka, R. Analyzing Loose Contact Oxidation of Diesel Engine Soot and Ag/CeO2 Catalyst Using Nonlinear Regression Analysis. Bull. Chem. React. Eng. Catal. 2017, 12, 14–23. [Google Scholar] [CrossRef]
- Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D. Nanostructured ceria-based catalysts for soot combustion: Investigations on the surface sensitivity. Appl. Catal. B 2015, 165, 742–751. [Google Scholar] [CrossRef]
- Hernández-Giménez, A.M.; Castelló, D.L.; Bueno-López, A. Diesel soot combustion catalysts: Review of active phases. Chem. Pap. 2014, 68, 1154–1168. [Google Scholar] [CrossRef]
- Shen, Q.; Wu, M.; Wang, H.; He, C.; Hao, Z.; Wei, W.; Sun, Y. Facile synthesis of catalytically active CeO2 for soot combustion. Catal. Sci. Technol. 2015, 5, 1941–1952. [Google Scholar] [CrossRef]
- Gnanamani, M.K.; Jacobs, G.; Martinelli, M.; Shafer, W.D.; Hopps, S.D.; Thomas, G.A.; Davis, B.H. Dehydration of 1,5-Pentanediol over Na-Doped CeO2 Catalysts. ChemCatChem 2018, 10, 1148–1154. [Google Scholar] [CrossRef]
- Miceli, P.; Bensaid, S.; Russo, N.; Fino, D. CeO2-based catalysts with engineered morphologies for soot oxidation to enhance soot-catalyst contact. Nanoscale Res. Lett. 2014, 9, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Guillén-Hurtado, N.; Bueno-López, A.; García-García, A. Catalytic performances of ceria and ceria-zirconia materials for the combustion of diesel soot under NOx/O2 and O2. Importance of the cerium precursor salt. Appl. Catal. A 2012, 437–438, 166–172. [Google Scholar] [CrossRef]
- Bensaid, S.; Russo, N.; Fino, D. CeO2 catalysts with fibrous morphology for soot oxidation: The importance of the soot-catalyst contact conditions. Catal. Today 2013, 216, 57–63. [Google Scholar] [CrossRef]
- Atribak, I.; Such-Basáñez, I.; Bueno-López, A.; García-García, A. Comparison of the catalytic activity of MO2 (M = Ti, Zr, Ce) for soot oxidation under NOx/O2. J. Catal. 2007, 250, 75–84. [Google Scholar] [CrossRef]
- Zhang, W.; Niu, X.; Chen, L.; Yuan, F.; Zhu, Y. Soot Combustion over Nanostructured Ceria with Different Morphologies. Sci. Rep. 2016, 6, 29062. [Google Scholar] [CrossRef] [PubMed]
- Aneggi, E.; Wiater, D.; de Leitenburg, C.; Llorca, J.; Trovarelli, A. Shape-Dependent Activity of Ceria in Soot Combustion. ACS Catal. 2014, 4, 172–181. [Google Scholar] [CrossRef]
- Kaspar, J.; Fornasiero, P.; Graziani, M. Use of CeO2-based oxides in the three-way catalysis. Catal. Today 1999, 50, 285–298. [Google Scholar] [CrossRef]
- Mukherjee, D.; Rao, B.G.; Reddy, B.M. CO and soot oxidation activity of doped ceria: Influence of dopants. Appl. Catal. B 2016, 197, 105–115. [Google Scholar] [CrossRef]
- Mukherjeea, D.; Reddy, B.M. Noble metal-free CeO2-based mixed oxides for CO and soot oxidation. Catal. Today 2018, 309, 227–235. [Google Scholar] [CrossRef]
- Kong, D.; Wang, G.; Pan, Y.; Hu, S.; Hou, J.; Pan, H.; Campbell, C.T.; Zhu, J. Growth, Structure, and Stability of Ag on CeO2(111): Synchrotron Radiation Photoemission Studies. J. Phys. Chem. C 2011, 115, 6715–6725. [Google Scholar] [CrossRef]
- Liu, S.; Wu, X.; Weng, D.; . Ran, R. Ceria-based catalysts for soot oxidation: A review. J. Rare Earth 2015, 33, 567–590. [Google Scholar] [CrossRef]
- Severin, N.; Kirstein, S.; Sokolov, I.M.; Rabe, J.P. Rapid trench channeling of graphenes with catalytic silver nanoparticles. Nano Lett. 2009, 9, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, K.; Sakakibara, Y.; Dong, F.; Shinjoh, H. The remote oxidation of soot separated by ash deposits viasilver–ceria composite catalysts. Appl. Catal. A 2014, 476, 113–120. [Google Scholar] [CrossRef]
- Liu, S.; Wu, X.; Liu, W.; Chen, W.; Ran, R.; Li, M.; Weng, D. Soot oxidation over CeO2 and Ag/CeO2: Factors determining the catalyst activity and stability during reaction. J. Catal. 2016, 337, 188–198. [Google Scholar] [CrossRef]
- Wu, S.; Yang, Y.; Lu, C.; Ma, Y.; Yuan, S.; Qian, G. Soot oxidation over CeO2 or Ag/CeO2: influences of bulk oxygen vacancies and surface oxygen vacancies on activity and stability of catalyst. Eur. J. Inorg. Chem. 2018, 2018, 2944–2951. [Google Scholar] [CrossRef]
- Piumetti, M.; van der Linden, B.; Makkee, M.; Miceli, P.; Fino, D.; Russo, N.; Bensaid, S. Contact dynamics for a solid–solid reaction mediated by gas-phase oxygen: Study on the soot oxidation over ceria-based catalysts. Appl. Catal. B 2016, 199, 96–107. [Google Scholar] [CrossRef]
- Shangguan, W.F.; Teraoka, Y.; Kagawa, S. Kinetics of soot–O2, soot–NO and soot–O2–NO reactions over spinel-type CuFe2O4 catalyst. Appl. Catal. B 1997, 12, 237–247. [Google Scholar] [CrossRef]
- Aneggi, E.; Leitenburg, C.; Trovarelli, A. On the role of lattice/surface oxygen in ceria-zirconia catalysts for diesel soot combustion. Catal. Today 2012, 181, 108–115. [Google Scholar] [CrossRef]
- Bueno-López, A. Diesel soot combustion ceria catalysts. Appl. Catal. B 2014, 146, 1–11. [Google Scholar] [CrossRef]
- Bueno-López, A.; Krishna, K.; Makkee, M.; Moulijn, J.A. Enhanced soot oxidation by lattice oxygen via La3+–doped CeO2. J. Catal. 2005, 230, 237–248. [Google Scholar] [CrossRef]
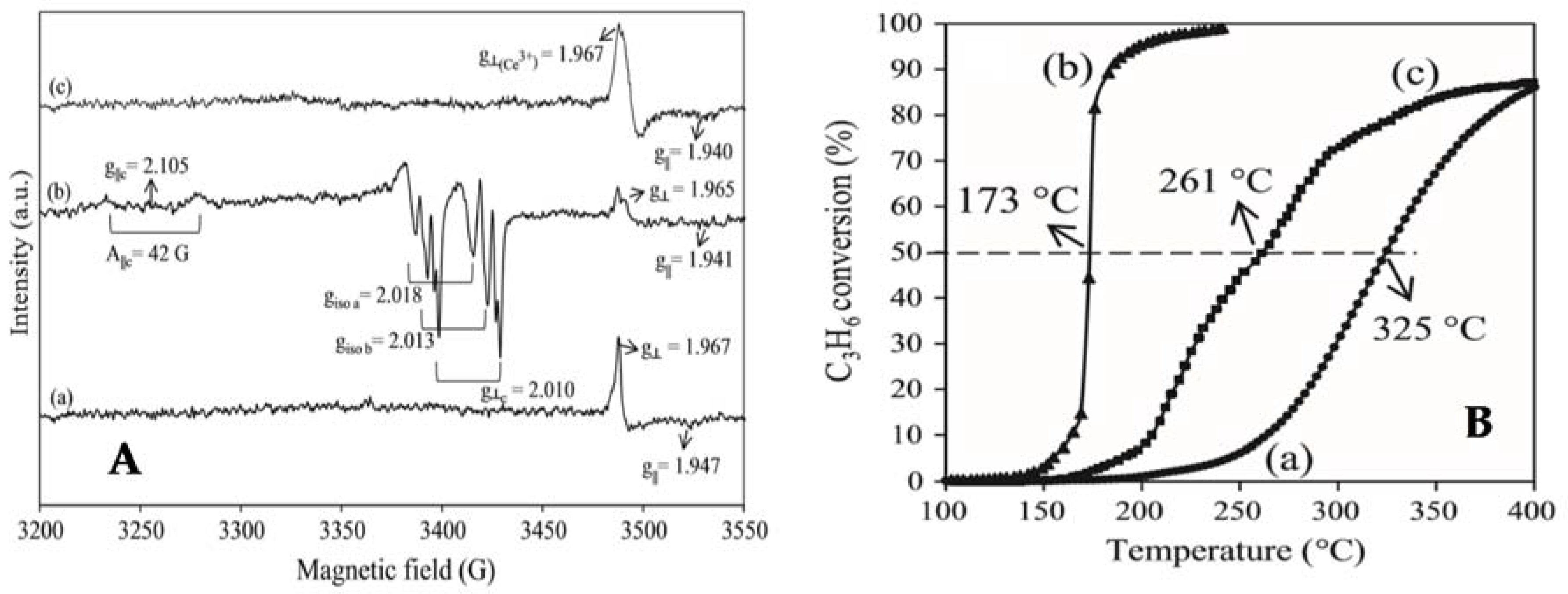
- Wang, H.; Liu, S.; Zhao, Z.; Zou, X.; Liu, M.; Liu, W.; Wu, X.; Weng, D. Activation and deactivation of Ag/CeO2 during soot oxidation: influences of interfacial ceria reduction. Catal. Sci. Technol. 2017, 7, 2129–2139. [Google Scholar] [CrossRef]
- Hosokawa, S.; Taniguchi, M.; Utani, K.; Kanai, H.; Imamura, S. Affinity order among noble metals and CeO2. Appl. Catal. A. 2005, 289, 115–120. [Google Scholar] [CrossRef]
- Nagai, Y.; Hirabayashi, T.; Dohmae, K.; Takagi, N.; Minami, T.; Shinjoh, H.; Matsumoto, S. Sintering inhibition mechanism of platinum supported on ceria-based oxide and Ptoxide–support interaction. J. Catal. 2006, 242, 103–109. [Google Scholar] [CrossRef]
- Nagai, Y.; Dohmae, K.; Ikeda, Y.; Takagi, N.; Hara, N.; Tanabe, T.; Guilera, G.; Pascarelli, S.; Newton, M.A.; Takahashi, N.; et al. In situ observation of platinum sintering on ceria-based oxide for autoexhaust catalysts using Turbo-XAS. Catal. Today 2011, 175, 133–140. [Google Scholar] [CrossRef]
- Malhautier, L.; Quijano, G.; Avezac, M.; Rocher, J.; Fanlo, J.L. Kinetic characterization of toluene biodegradation by Rhodococcus erythropolis: towards a rationale for microflora enhancement in bioreactors devoted to air treatment. Chem. Eng. J. 2014, 247, 199–204. [Google Scholar] [CrossRef]
- Li, L.; Liu, S.; Liu, J. Surface modification of coconut shell based activated carbon for the improvement of hydrophobic VOC removal. J. Hazard. Mater. 2011, 192, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Thévenet, F.; Sivachandiran, L.; Guaitella, O.; Barakat, C.; Rousseau, A. Plasma–catalyst coupling for volatile organic compound removal and indoor air treatment: a review. J. Phys. D Appl. Phys. 2014, 47, 224011. [Google Scholar] [CrossRef]
- Destaillats, H.; Sleiman, M.; Sullivan, D.P.; Jacquiod, C.; Sablayrolles, J.; Molins, L. Key parameters influencing the performance of photocatalytic oxidation (PCO) air purification under realistic indoor conditions. Appl. Catal. B 2012, 128, 159–170. [Google Scholar] [CrossRef]
- Yuan, M.H.; Chang, C.Y.; Shie, J.L.; Chang, C.C.; Chen, J.H.; Tsai, W.T. Destruction of naphthalene via ozone-catalytic oxidation process over Pt/Al2O3 catalyst. J. Hazard. Mater. 2010, 175, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Liotta, L.F. Catalytic oxidation of volatile organic compounds on supported noble metals. Appl. Catal. B 2010, 100, 403–412. [Google Scholar] [CrossRef]
- Everaert, K.; Baeyens, J. Catalytic combustion of volatile organic compounds. J. Hazard. Mater. 2004, 109, 113–139. [Google Scholar] [CrossRef] [PubMed]
- Armor, J. N. Environmental catalysis. Appl. Catal. B 1992, 1, 221–256. [Google Scholar] [CrossRef]
- Spivey, J.J. Complete catalytic oxidation of volatile organics. Ind. Eng. Chem. Res. 1987, 26, 2165–2180. [Google Scholar] [CrossRef]
- Ordóñez, S.; Bello, L.; Sastre, H.; Rosal, R.; Dıez, F.V. Kinetics of the deep oxidation of benzene, toluene, n-hexane and their binary mixtures over a platinum on γ-alumina catalyst. Appl. Catal. B 2002, 38, 139–149. [Google Scholar] [CrossRef]
- Huang, H.; Hu, P.; Huang, H.; Chen, J.; Ye, X.; Leung, D.Y. Highly dispersed and active supported Pt nanoparticles for gaseous formaldehyde oxidation: Influence of particle size. Chem. Eng. J. 2014, 252, 320–326. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.; He, H. Complete oxidation of o-xylene over Pd/Al2O3 catalyst at low temperature. Catal. Today 2008, 139, 15–23. [Google Scholar] [CrossRef]
- Qi, J.; Chen, J.; Li, G.; Li, S.; Gao, Y.; Tang, Z. Facile synthesis of core–shell Au@ CeO2 nanocomposites with remarkably enhanced catalytic activity for CO oxidation. Energy Environ. Sci. 2012, 5, 8937–8941. [Google Scholar] [CrossRef]
- Hosseini, M.; Barakat, T.; Cousin, R.; Aboukaïs, A.; Su, B.L.; De Weireld, G.; Siffert, S. Catalytic performance of core–shell and alloy Pd–Au nanoparticles for total oxidation of VOC: the effect of metal deposition. Appl. Catal. B 2012, 111, 218–224. [Google Scholar] [CrossRef]
- Xu, R.; Wang, X.; Wang, D.; Zhou, K.; Li, Y. Surface structure effects in nanocrystal MnO2 and Ag/MnO2 catalytic oxidation of CO. J. Catal. 2006, 237, 426–430. [Google Scholar] [CrossRef]
- Bai, B.; Arandiyan, H.; Li, J. Comparison of the performance for oxidation of formaldehyde on nano-Co3O4, 2D-Co3O4, and 3D-Co3O4 catalysts. Appl. Catal. B 2013, 142, 677–683. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and catalytic applications of CeO2–based materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef] [PubMed]
- Hanafiah, M.A.K.M.; Hussin, Z.M.; Ariff, N.F.M.; Ngah, W.S.W.; Ibrahim, S.C. Monosodium glutamate functionalized chitosan beads for adsorption of precious cerium ion. Adv. Mater. Res. 2014, 970, 198–203. [Google Scholar] [CrossRef]
- Min, C.K. Nanostructured Pt/MnO2 Catalysts and Their Performance for Oxygen Reduction Reaction in Air Cathode Microbial Fuel Cell. Ph.D. Thesis, University Malaysia Pahang, Pekan, Pahang, Malaysia, June 2014. [Google Scholar]
- Abdel-Mageed, A.M.; Kučerová, G.; El-Moemen, A.A.; Bansmann, J.; Widmann, D.; Behm, R.J. Geometric and electronic structure of Au on Au/CeO2 catalysts during the CO oxidation: Deactivation by reaction induced particle growth. J. Phys. Conf. Ser. 2016, 712, 012044. [Google Scholar] [CrossRef]
- Tan, H.; Wang, J.; Yu, S.; Zhou, K. Support morphology-dependent catalytic activity of Pd/CeO2 for formaldehyde oxidation. Environ. Sci. Technol. 2015, 49, 8675–8682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Zhang, Y.; Chen, M.; Wang, L.; Zhang, C.; He, H. Effect of support on the activity of Ag-based catalysts for formaldehyde oxidation. Sci. Rep. 2015, 5, 12950. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, L. Highly efficient formaldehyde elimination over meso-structured M/CeO2 (M=Pd, Pt, Au and Ag) catalyst under ambient conditions. RSC Adv. 2015, 5, 36428–36433. [Google Scholar] [CrossRef]
- Yu, L.; Peng, R.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Ag supported on CeO2 with different morphologies for the catalytic oxidation of HCHO. Chem. Eng. J. 2018, 334, 2480–2487. [Google Scholar] [CrossRef]
- Imamura, S.; Uchihori, D.; Utani, K.; Ito, T. Oxidative decomposition of formaldehyde on silver-cerium composite oxide catalyst. Catal. Lett. 1994, 24, 377–384. [Google Scholar] [CrossRef]
- Benaissa, S.; Chérif-Aouali, L.; Siffert, S.; Aboukais, A.; Cousin, R.; Bengueddach, A. New Nanosilver/Ceria Catalyst for Atmospheric Pollution Treatment. Nano 2015, 10, 1550043. [Google Scholar] [CrossRef]
- Aboukais, A.; Skaf, M.; Hany, S.; Cousin, R.; Aouad, S.; Labaki, M.; Abi-Aad, E. A comparative study of Cu, Ag and Au doped CeO2 in the total oxidation of volatile organic compounds (VOCs). Mater. Chem. Phys. 2016, 177, 570–576. [Google Scholar] [CrossRef]
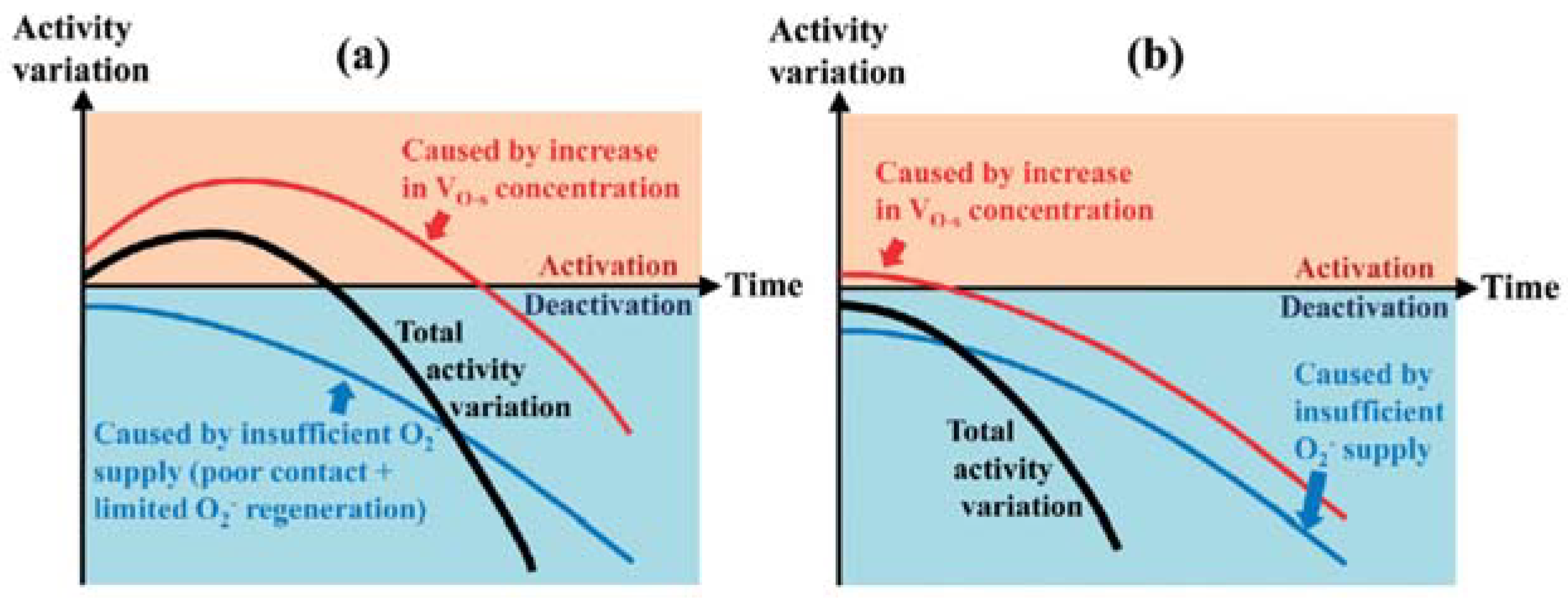
- Liu, M.; Wu, X.; Liu, S.; Gao, Y.; Chen, Z.; Ma, Y.; Weng, D. Study of Ag/CeO2 catalysts for naphthalene oxidation: Balancing the oxygen availability and oxygen regeneration capacity. Appl. Catal. B 2017, 219, 231–240. [Google Scholar] [CrossRef]
- Yang, H.; Deng, J.; Liu, Y.; Xie, S.; Wu, Z.; Dai, H. Preparation and catalytic performance of Ag, Au, Pd or Pt nanoparticles supported on 3DOM CeO2–Al2O3 for toluene oxidation. J. Mol. Catal. A Chem. 2016, 414, 9–18. [Google Scholar] [CrossRef]
- Kharlamova, T.; Mamontov, G.; Salaev, M.; Zaikovskii, V.; Popova, G.; Sobolev, V.; Vodyankina, O. Silica-supported silver catalysts modified by cerium/manganese oxides for total oxidation of formaldehyde. Appl. Catal. A 2013, 467, 519–529. [Google Scholar] [CrossRef]
- Mullins, D.R. The surface chemistry of cerium oxide. Surf. Sci. Rep. 2015, 70, 42–85. [Google Scholar] [CrossRef]
- Nolan, N. Surface Effects in the Reactivity of Ceria: A First Principles Perspective. Catal. Mater. Well-Defined Struct. 2015, 159–192. [Google Scholar]
- Plata, J.J.; Márquez, A.M.; Fdez Sanz, J. Improving the density functional theory+U description of CeO2 by including the contribution of the O 2p electrons. J. Chem. Phys. 2012, 136, 041101. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, S.M.; Viñes, F.; Nilius, N.; Shaikhutdinov, S.; Neyman, K.M. Absolute surface step energies: Accurate theoretical methods applied to ceria nanoislands. J. Phys. Chem. Lett. 2012, 3, 1956–1961. [Google Scholar] [CrossRef]
- Nolan, M. Hybrid density functional theory description of oxygen vacancies in the CeO2 (110) and (100) surfaces. Chem. Phys. Lett. 2010, 499, 126–130. [Google Scholar] [CrossRef]
- Paier, J.; Penschke, C.; Sauer, J. Oxygen defects and surface chemistry of ceria: Quantum chemical studies compared to experiment. Chem. Rev. 2013, 113, 3949–3985. [Google Scholar] [CrossRef] [PubMed]
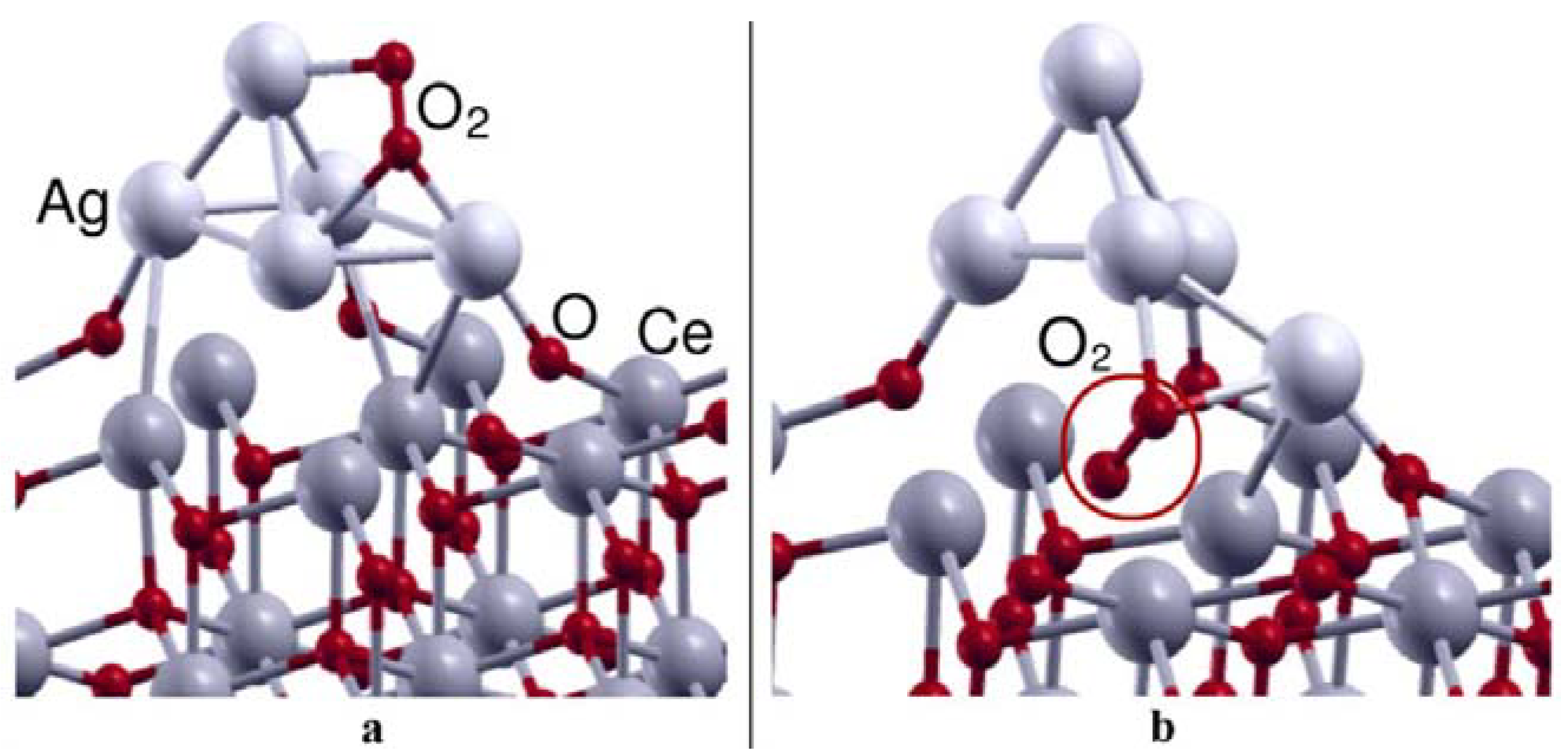
- Preda, G.; Pacchioni, G. Formation of oxygen active species in Ag-modified CeO2 catalyst for soot oxidation: A DFT study. Catal. Today 2011, 177, 31–38. [Google Scholar] [CrossRef]
- Zhu, K.-J.; Liu, J.; Yang, Y.-J.; Xu, Y.-X.; Teng, B.-T.; Wen, X.-D.; Fan, M. A method to explore the quantitative interactions between metal and ceria for M/CeO2 catalysts. Surf. Sci. 2018, 669, 79–86. [Google Scholar] [CrossRef]
- Tereshchuk, P.; Freire, R.L.H.; Ungureanu, C.G.; Seminovski, Y.; Kiejnac, A.; Da Silva, J.L.F. The role of charge transfer in the oxidation state change of Ce atoms in the TM13–CeO2(111) systems (TM = Pd, Ag, Pt, Au): A DFT + U investigation. Phys. Chem. Chem. Phys. 2015, 17, 13520–13530. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, M.J.; Tereshchuk, P.; Da Silva, J.L.F. Theoretical Investigation of Small Transition-Metal Clusters Supported on the CeO2 (111) Surface. J. Phys. Chem. C 2014, 118, 21438–21446. [Google Scholar] [CrossRef]
- Chen, L.-J.; Tang, Y.; Cui, L.; Ouyang, C.; Shi, S. Charge transfer and formation of Ce3+ upon adsorption of metal atom M (M = Cu, Ag, Au) on CeO2 (100) surface. J. Power Sources 2013, 234, 69–81. [Google Scholar] [CrossRef]
- Luches, P.; Pagliuca, F.; Valeri, S.; Illas, F.; Preda, G.; Pacchioni, G. Nature of Ag Islands and Nanoparticles on the CeO2 (111) Surface. J. Phys. Chem. C 2012, 116, 1122–1132. [Google Scholar] [CrossRef]
- Cui, L.; Tang, Y.; Zhang, H.; Hector, L.G., Jr.; Ouyang, C.; Shi, S.; Lib, H.; Chen, L. First-principles investigation of transition metal atom M (M = Cu, Ag, Au) adsorption on CeO2 (110). Phys. Chem. Chem. Phys. 2012, 14, 1923–1933. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, H.; Cui, L.; Ouyang, C.; Shi, S.; Tang, W.; Li, H.; Chen, L. Electronic states of metal (Cu, Ag, Au) atom on CeO2 (111) surface: The role of local structural distortion. J. Power Sources 2012, 197, 28–37. [Google Scholar]
- Branda, M.M.; Hernández, N.C.; Sanz, J.F.; Illas, F. Density functional theory study of the interaction of Cu, Ag, and Au atoms with the regular CeO2 (111) surface. J. Phys. Chem. C 2010, 114, 1934–1941. [Google Scholar] [CrossRef]
- Hay, P.J.; Martin, R.L.; Uddin, J.; Scuseria, G.E. Theoretical study of CeO2 and Ce2O3 using a screened hybrid density functional. J. Chem. Phys. 2006, 125, 034712. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.L.F.; Ganduglia-Pirovano, M.V.; Sauer, J.; Bayer, V.; Kresse, G. Hybrid functionals applied to rare-earth oxides: The example of ceria. Phys. Rev. B 2007, 75, 045121. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, X.; Lu, Z.; Li, S.; Hermansson, K. Oxygen vacancy pairs on CeO2 (110): A DFT + U study. Phys. Lett. A 2009, 373, 2786–2792. [Google Scholar] [CrossRef]
- Dudarev, S.L.; Botton, G.A.; Savrasov, S.Y.; Humphreys, C.J.; Sutton, A.P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys. Rev. B 1998, 57, 1505–1509. [Google Scholar] [CrossRef]
- Branda, M.M.; Castellani, N.J.; Grau-Crespo, R.; de Leeuw, N.H.; Hernandez, N.C.; Sanz, J.F.; Neyman, K.M.; Illas, F.J. On the difficulties of present theoretical models to predict the oxidation state of atomic Au adsorbed on regular sites of CeO2(111). J. Chem. Phys. 2009, 131, 094702. [Google Scholar]
- Trovarelli, A.; Fornasiero, P. Catalysis by Ceria and Related Materials, 2nd ed.; Imperial College Press: London, UK, 2013. [Google Scholar]
- Wen, X.-J.; Niu, C.-G.; Zhang, L.; Liang, C.; Zeng, G.-M. A novel Ag2O/CeO2 heterojunction photocatalysts for photocatalytic degradation of enrofloxacin: possible degradation pathways, mineralization activity and an in depth mechanism insight. Appl. Catal. B 2018, 221, 701–714. [Google Scholar] [CrossRef]
- Xie, S.; Wang, Z.; Cheng, F.; Zhang, P.; Mai, W.; Tong, Y. Ceria and ceria-based nanostructured materials for photoenergy applications. Nano Energy 2017, 34, 313–337. [Google Scholar] [CrossRef]
- Channei, D.; Inceesungvorn, B.; Wetchakun, N.; Ukritnukun, S.; Nattestad, A.; Chen, J.; Phanichphant, S. Photocatalytic degradation of methyl orange by CeO2 and Fe–doped CeO2 films under visible light irradiation. Sci. Rep. 2014, 4, 5757. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Koshy, P.; Chen, W.-F.; Qi, S.; Sorrell, C.C. Photocatalytic materials and technologies for air purification. J. Hazard. Mater. 2017, 325, 340–366. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, Q.; Kong, M.; Shi, W.; Huang, J.; Tang, J.; Zhao, X. Coupling oxygen ion conduction to photocatalysis in mesoporous nanorod-like ceria significantly improves photocatalytic efficiency. J. Phys. Chem. C 2011, 115, 14050–14057. [Google Scholar] [CrossRef]
- Sabari Arul, N.; Mangalaraj, D.; Whan Kim, T. Photocatalytic degradation mechanisms of self-assembled rose-flower-like CeO2 hierarchical nanostructures. Appl. Phys. Lett. 2013, 102, 223115. [Google Scholar] [CrossRef]
- Ko, J.W.; Kim, J.H.; Park, C.B. Synthesis of visible light-active CeO2 sheets via mussel-inspired CaCO3 mineralization. J. Mater. Chem. A 2013, 1, 241–245. [Google Scholar] [CrossRef]
- Huang, Y.; Long, B.; Tang, M.; Rui, Z.; Balogun, M.-S.; Tong, Y.; Ji, H. Bifunctional catalytic material: An ultrastable and high-performance surface defect CeO2 nanosheets for formaldehyde thermal oxidation and photocatalytic oxidation. Appl. Catal. B 2016, 181, 779–787. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, Q.; Yuan, S.; Zhang, M.; Ohno, T. Morphology control and photocatalytic characterization of yttrium-doped hedgehog-like CeO2. Appl. Catal. B 2015, 164, 120–127. [Google Scholar] [CrossRef]
- Ji, Y.; Ferronato, C.; Salvador, A.; Yang, X.; Chovelon, J.M. Degradation of ciprofloxacin and sulfamethoxazole by ferrous-activated persulfate: implications for remediation of groundwater contaminated by antibiotics. Sci. Total Environ. 2014, 472, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.F.; Ma, Z.L.; Huang, W.Q.; Tian, Y.; Jiao, C.; Yang, Z.M.; Wan, Z.; Pan, A. Ag3PO4 Semiconductor Photocatalyst: Possibilities and Challenges. J. Nanomater. 2013, 8, 371356. [Google Scholar]
- Yang, Z.-M.; Huang, G.-F.; Huang, W.-Q.; Wei, J.-M.; Yan, X.-G.; Liu, Y.-Y.; Jiao, C.; Wan, Z.; Pan, A. Novel Ag3PO4/CeO2 composite with high efficiency and stability for photocatalytic applications. J. Mater. Chem. A 2014, 2, 1750–1756. [Google Scholar] [CrossRef]
- Wu, C. Synthesis of Ag2CO3/CeO2 microcomposite with visible light-driven photocatalytic activity. Mater. Lett. 2015, 152, 76–78. [Google Scholar] [CrossRef]
- Wen, X.-J.; Niu, C.-G.; Huang, D.-W.; Zhang, L.; Liang, C.; Zeng, G.-M. Study of the photocatalytic degradation pathway of norfloxacin and mineralization activity using a novel ternary Ag/AgCl-CeO2 photocatalyst. J. Catal. 2017, 355, 73–86. [Google Scholar] [CrossRef]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Hashimoto, K.; Kominami, H. Gold and copper nanoparticles supported on cerium(IV) oxide a photocatalyst mineralizing organic acids under red light irradiation. ChemCatChem 2011, 3, 1619–1623. [Google Scholar] [CrossRef]
- Kominami, H.; Tanaka, A.; Hashimoto, K. Mineralization of organic acids in aqueous suspensions of gold nanoparticles supported on cerium(IV) oxide powder under visible light irradiation. Chem. Commun. 2010, 46, 1287–1289. [Google Scholar] [CrossRef] [PubMed]
- Liu, I.-T.; Hon, M.-H.; Kuan, C.-Y.; Teoh, L.-G. Structure and optical properties of Ag/CeO2 nanocomposites. Appl. Phys. A 2013, 111, 1181–1186. [Google Scholar] [CrossRef]
- Liu, T.; Li, B.; Wang, Y.; Ge, Z.; Shi, J. Facile Synthesis of Ag/CeO2 Mesoporous Composites with Enhanced Visible Light Photocatalytic Properties. Asian J. Chem. 2014, 26, 1355–1357. [Google Scholar]
- Hao, Y.; Li, L.; Liu, D.; Yu, H.; Zhou, Q. The synergy of SPR effect and Z-scheme of Ag on enhanced photocatalytic performance of 3DOM Ag/CeO2-ZrO2 composite. Mol. Catal. 2018, 447, 37–46. [Google Scholar] [CrossRef]
- Saravanan, R.; Agarwal, S.; Gupta, V.K.; Khan, M.M.; Gracia, F.; Mosquera, E.; Narayanan, V.; Stephen, A. Line defect Ce3+ induced Ag/CeO2/ZnO nanostructure for visible-light photocatalytic activity. J. Photochem. Photobiol. A 2018, 353, 499–506. [Google Scholar] [CrossRef]
- Mittal, M.; Gupta, A.; Pandey, O.P. Role of oxygen vacancies in Ag/Au doped CeO2 nanoparticles for fast photocatalysis. Sol. Energy 2018, 165, 206–216. [Google Scholar] [CrossRef]
- Barsuk, D.; Zadick, A.; Chatenet, M.; Georgarakis, K.; Panagiotopoulos, N.T.; Champion, Y.; Moreira Jorge, A., Jr. Nanoporous silver for electrocatalysis application in alkaline fuel cells. Mater. Des. 2016, 111, 528–536. [Google Scholar] [CrossRef]
- Lu, Q.; Rosen, J.; Zhou, Y.; Hutchings, G.S.; Kimmel, Y.C.; Chen, J.G.; Jiao, F. A selective and efficient electrocatalyst for carbon dioxide reduction. Nat. Commun. 2014, 5, 3242. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Macia, L.; Smyth, M.R.; Killard, A.J. Evaluation of a silver-based electrocatalyst for the determination of hydrogen peroxide formed via enzymatic oxidation. Talanta 2012, 99, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Sreeremya, T.S.; Krishnan, A.; Remani, K.C.; Patil, K.R.; Brougham, D.F.; Ghosh, S. Shape-selective oriented cerium oxide nanocrystals permit assessment of the effect of the exposed facets on catalytic activity and oxygen storage capacity. ACS Appl. Mater. Interfaces 2015, 7, 8545–8555. [Google Scholar] [CrossRef] [PubMed]
- Meher, S.K.; Rao, G.R. Novel nanostructured CeO2 as efficient catalyst for energy and environmental applications. J. Chem. Sci. 2014, 126, 361–372. [Google Scholar] [CrossRef]
- Melchionna, M.; Fornasiero, P. The role of ceria-based nanostructured materials in energy applications. Mater. Today 2014, 17, 349–357. [Google Scholar] [CrossRef]
- Li, G.; Zhang, X.; Wang, L.; Song, X.; Sun, Z. Promoting Effect of Au on the Nanoporous Ag/CeO2 Composites Prepared by Dealloying for Borohydride Electro-Oxidation. J. Electrochem. Soc. 2013, 160, 1116–1122. [Google Scholar] [CrossRef]
- Sun, S.; Xue, Y.; Wang, Q.; Li, S.; Huang, H.; Miao, H.; Liu, Z. Electrocatalytic activity of silver decorated ceria microspheres for the oxygen reduction reaction and their application in aluminium–air batteries. Chem. Commun. 2017, 53, 7921–7924. [Google Scholar] [CrossRef] [PubMed]
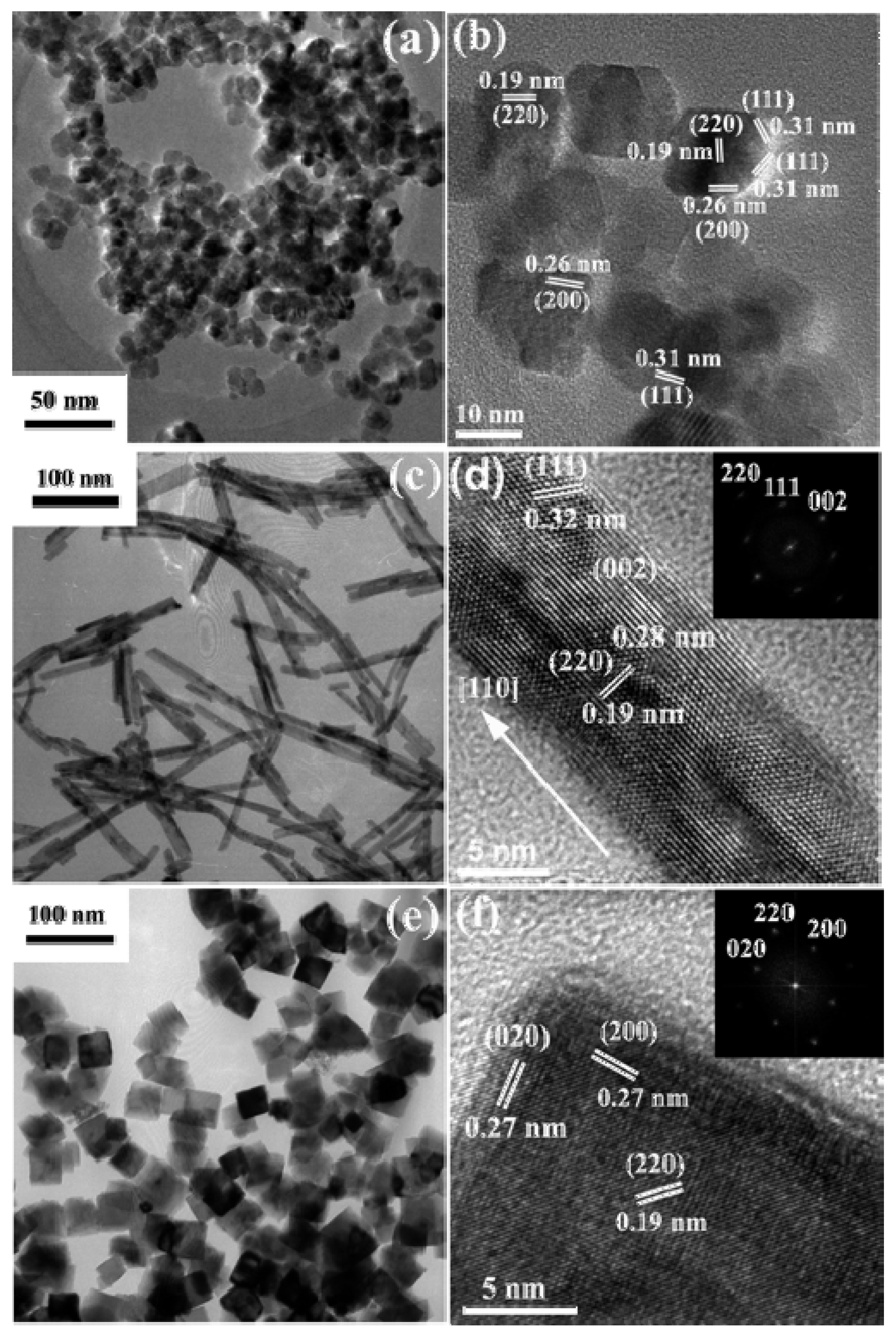
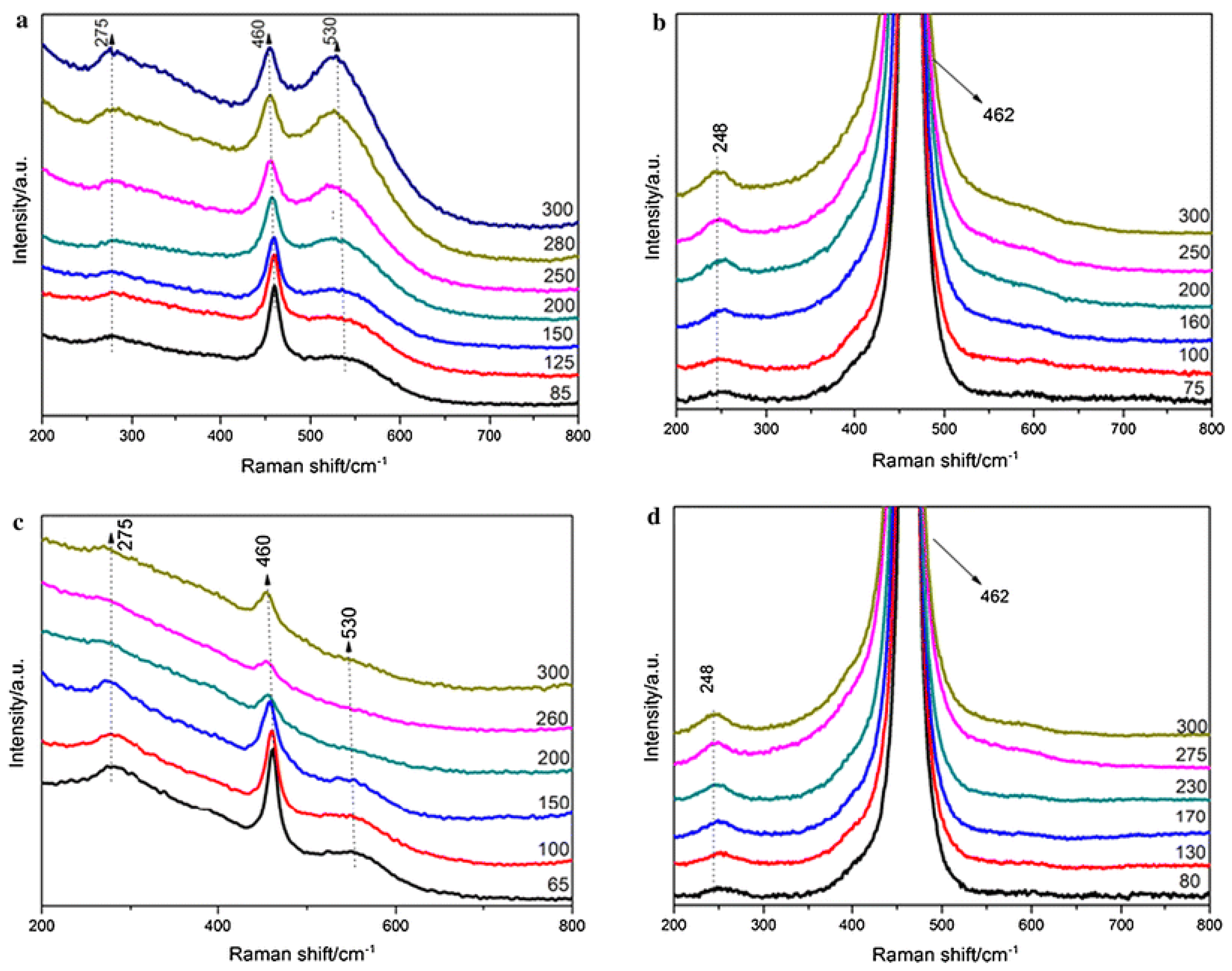
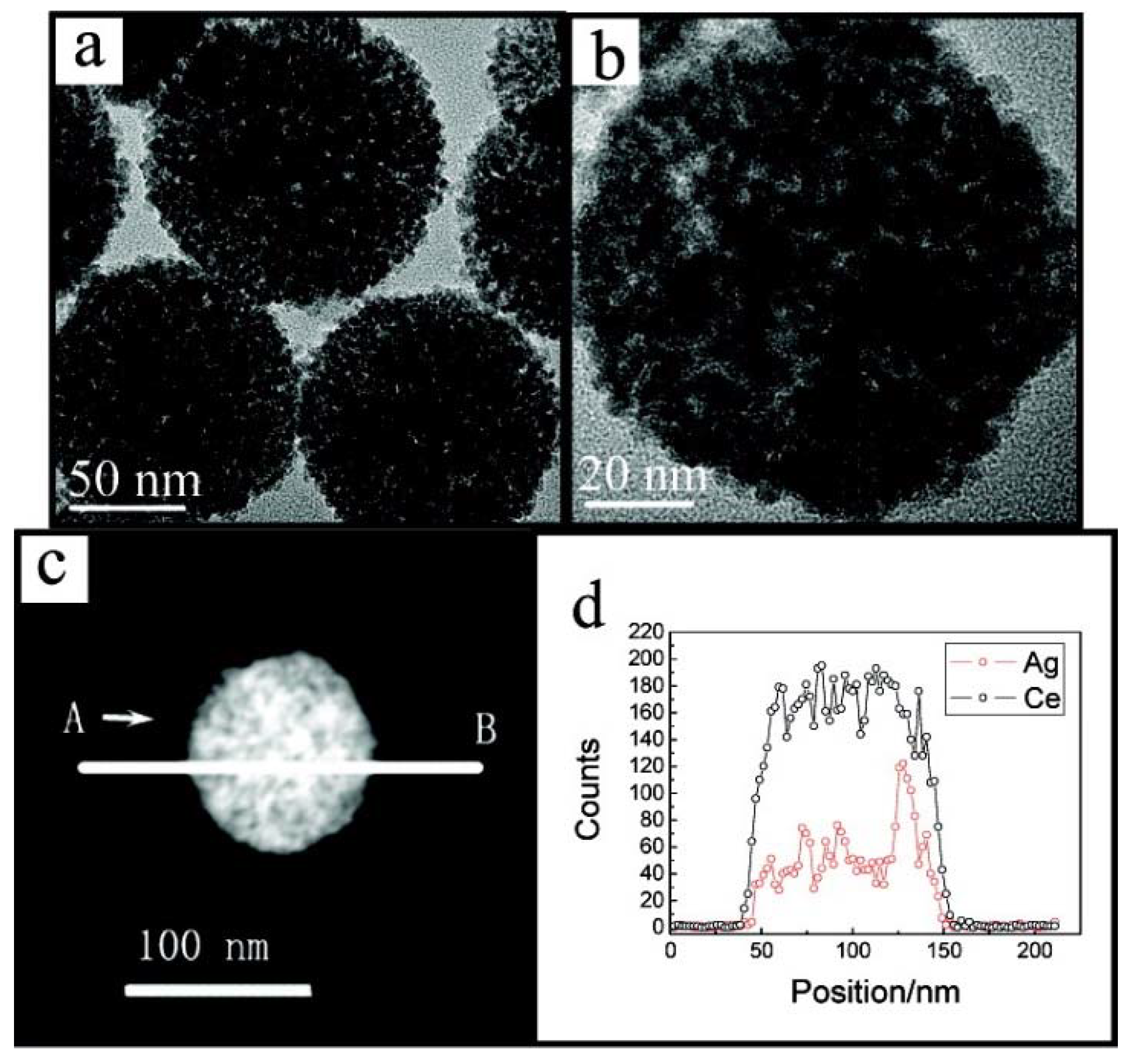
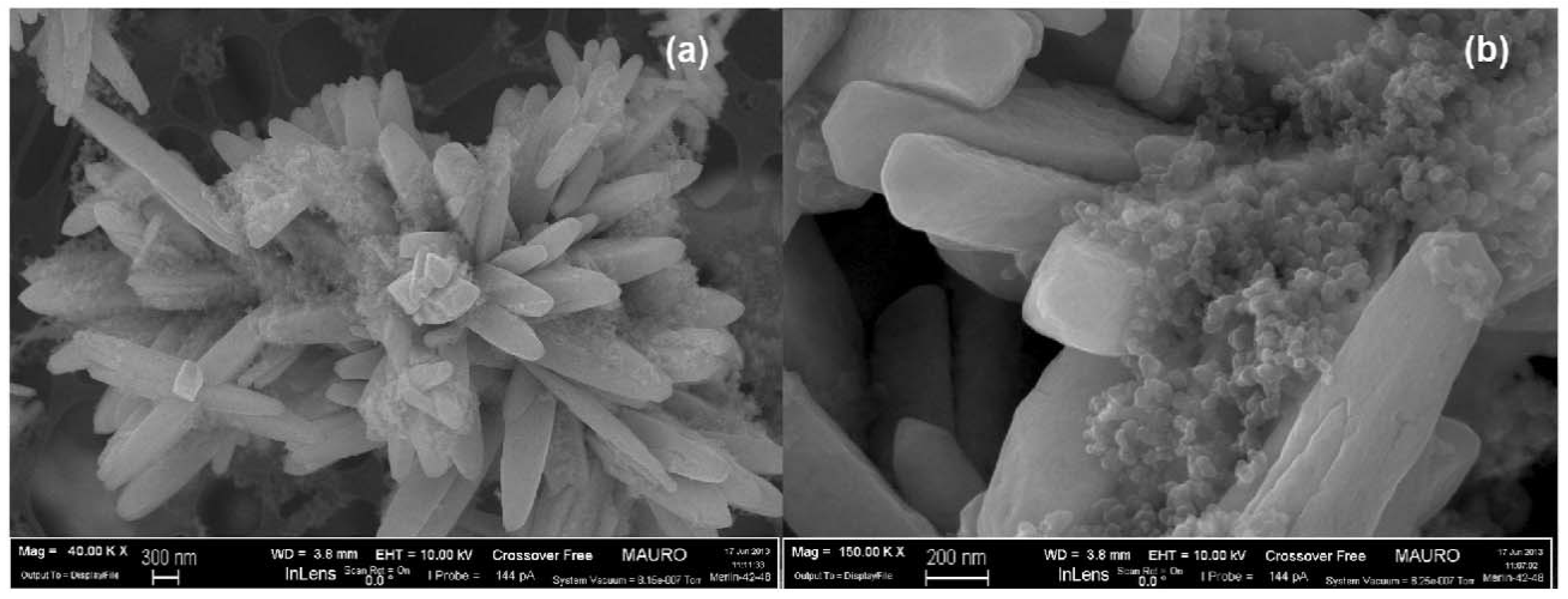
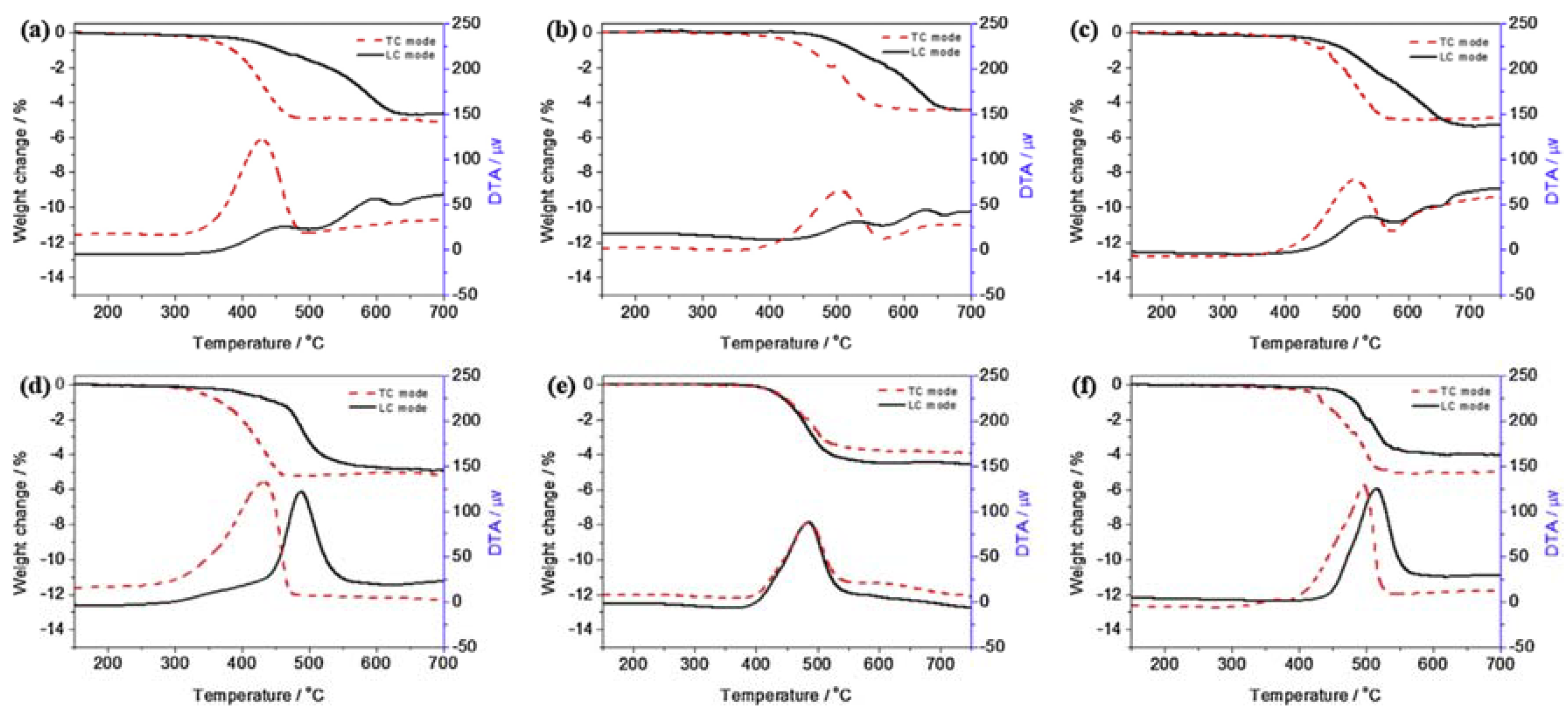
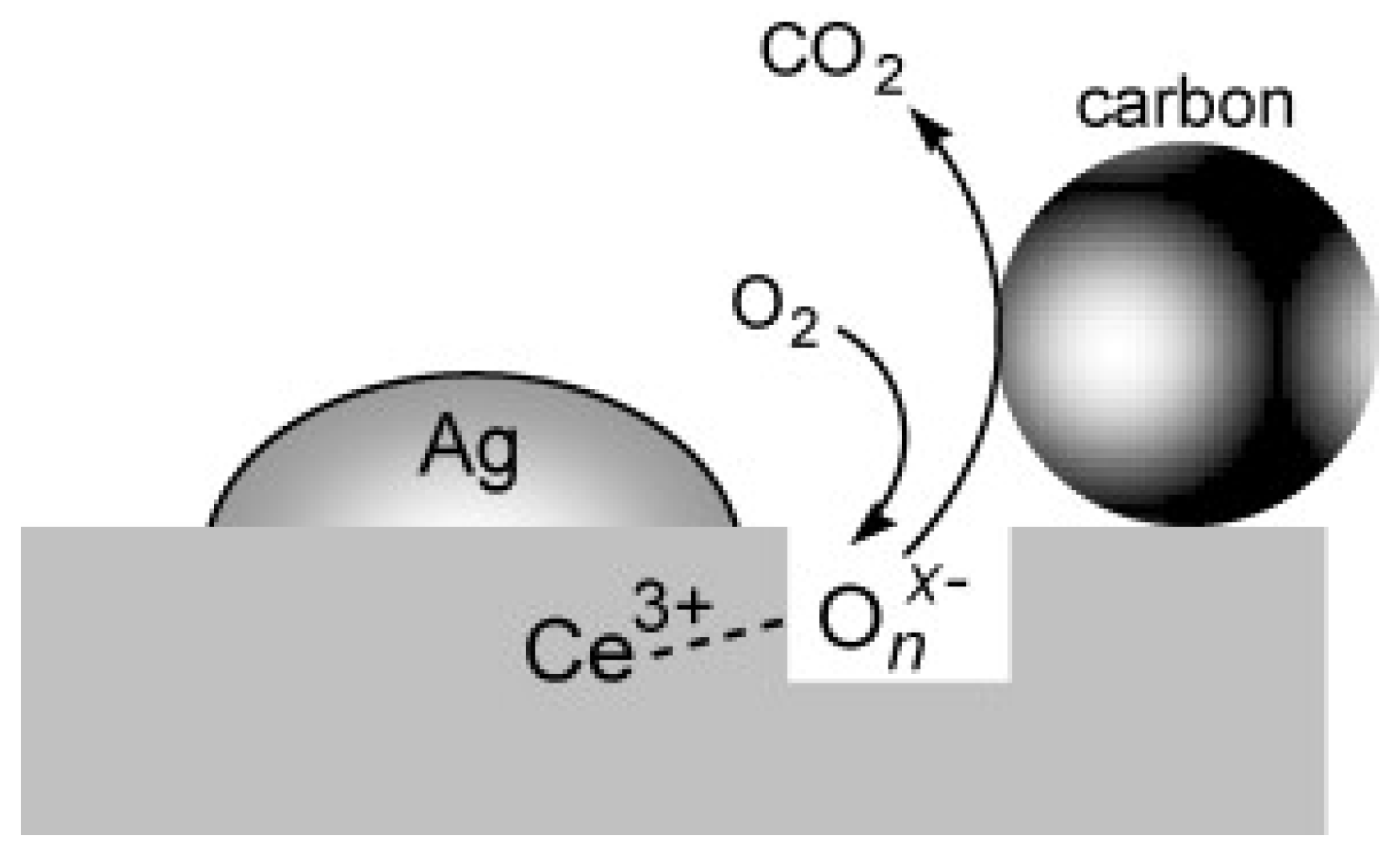
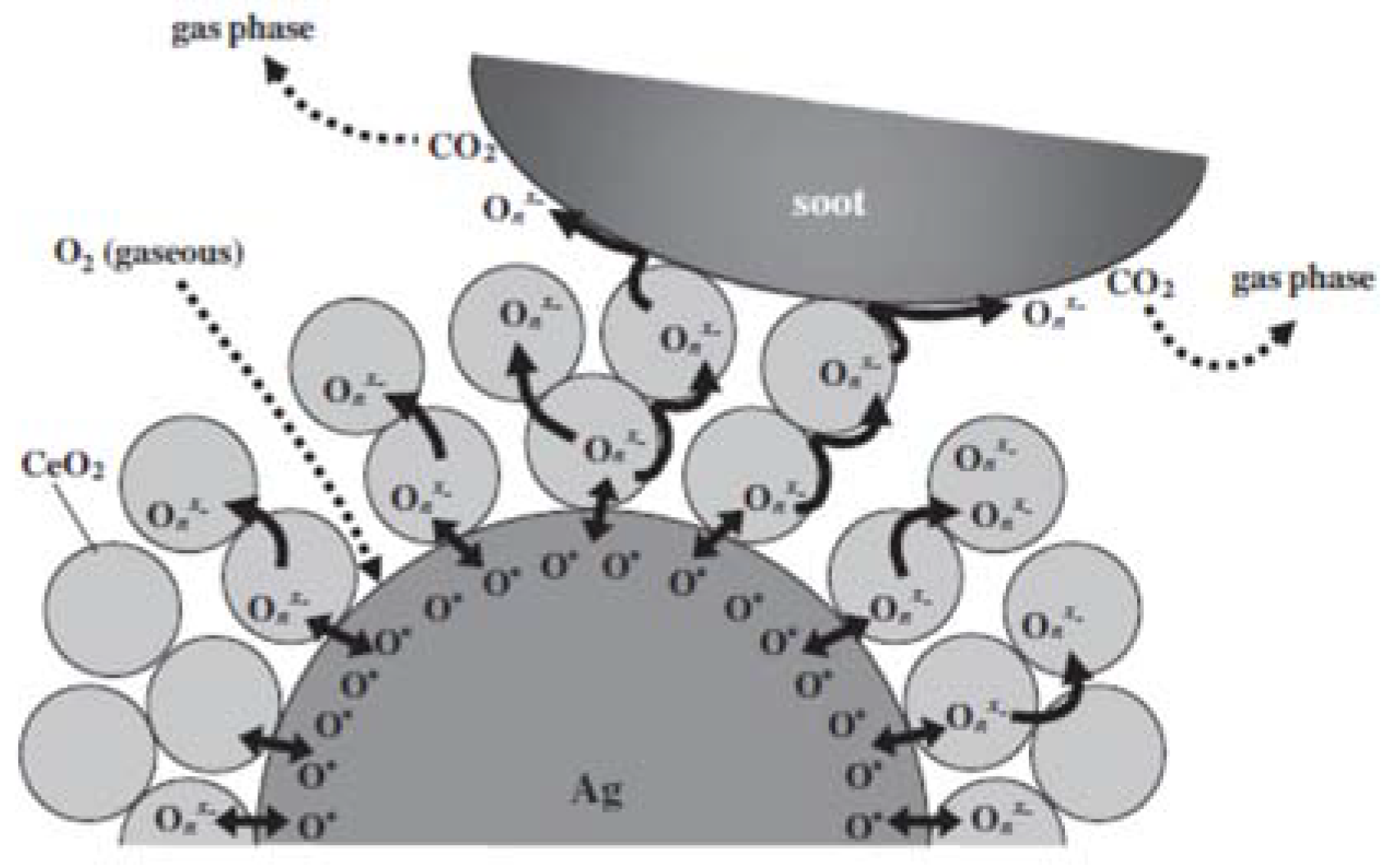
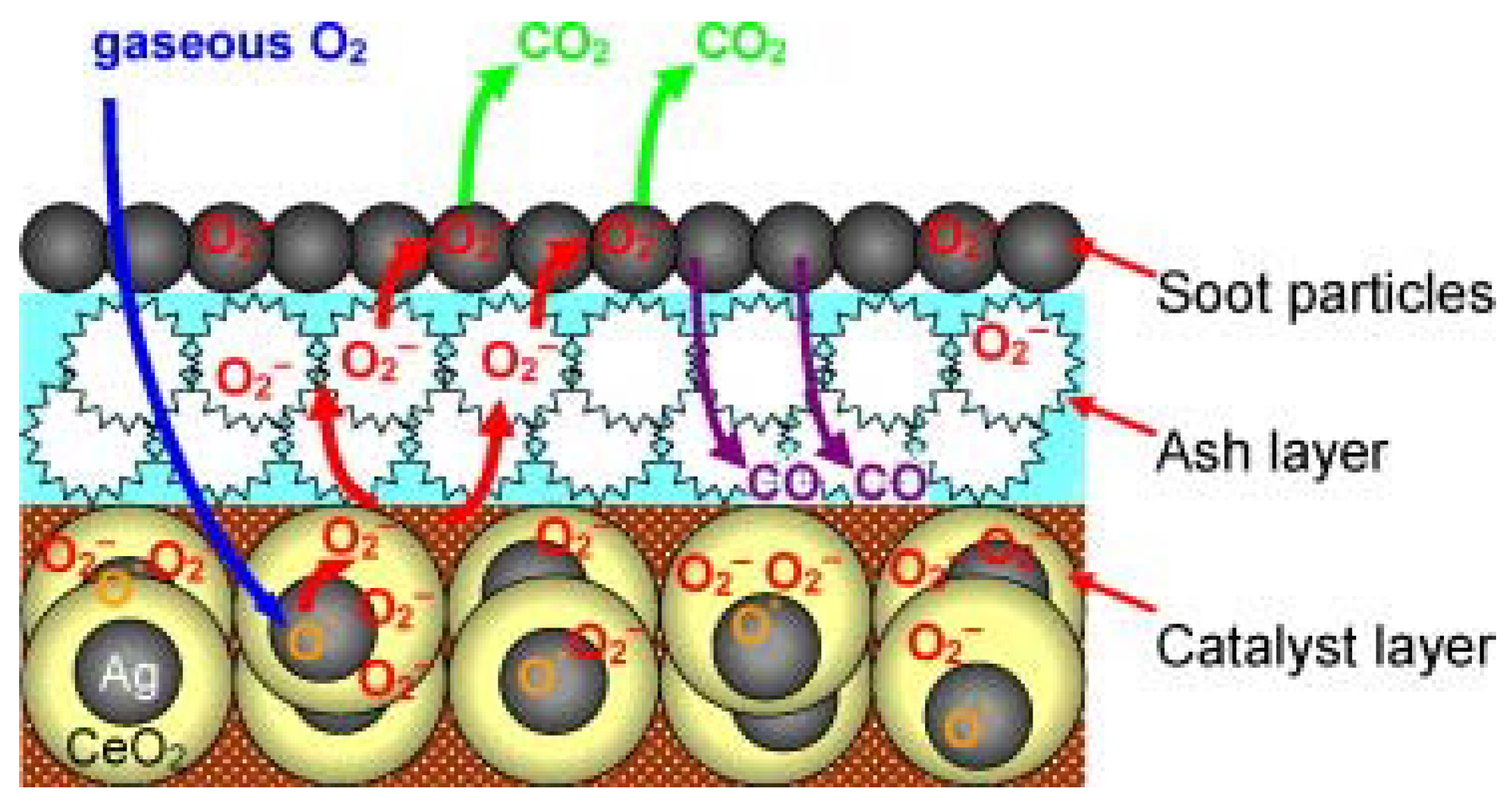
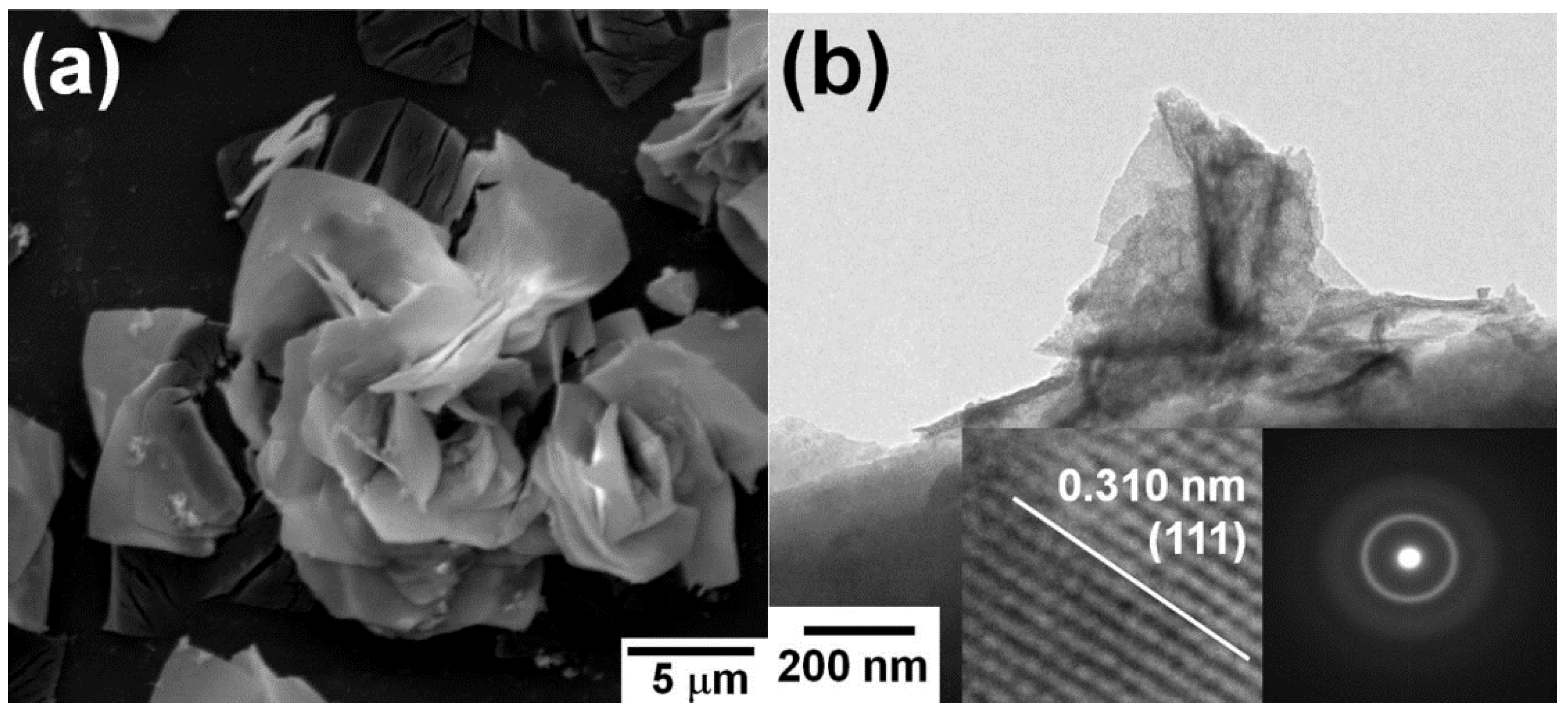
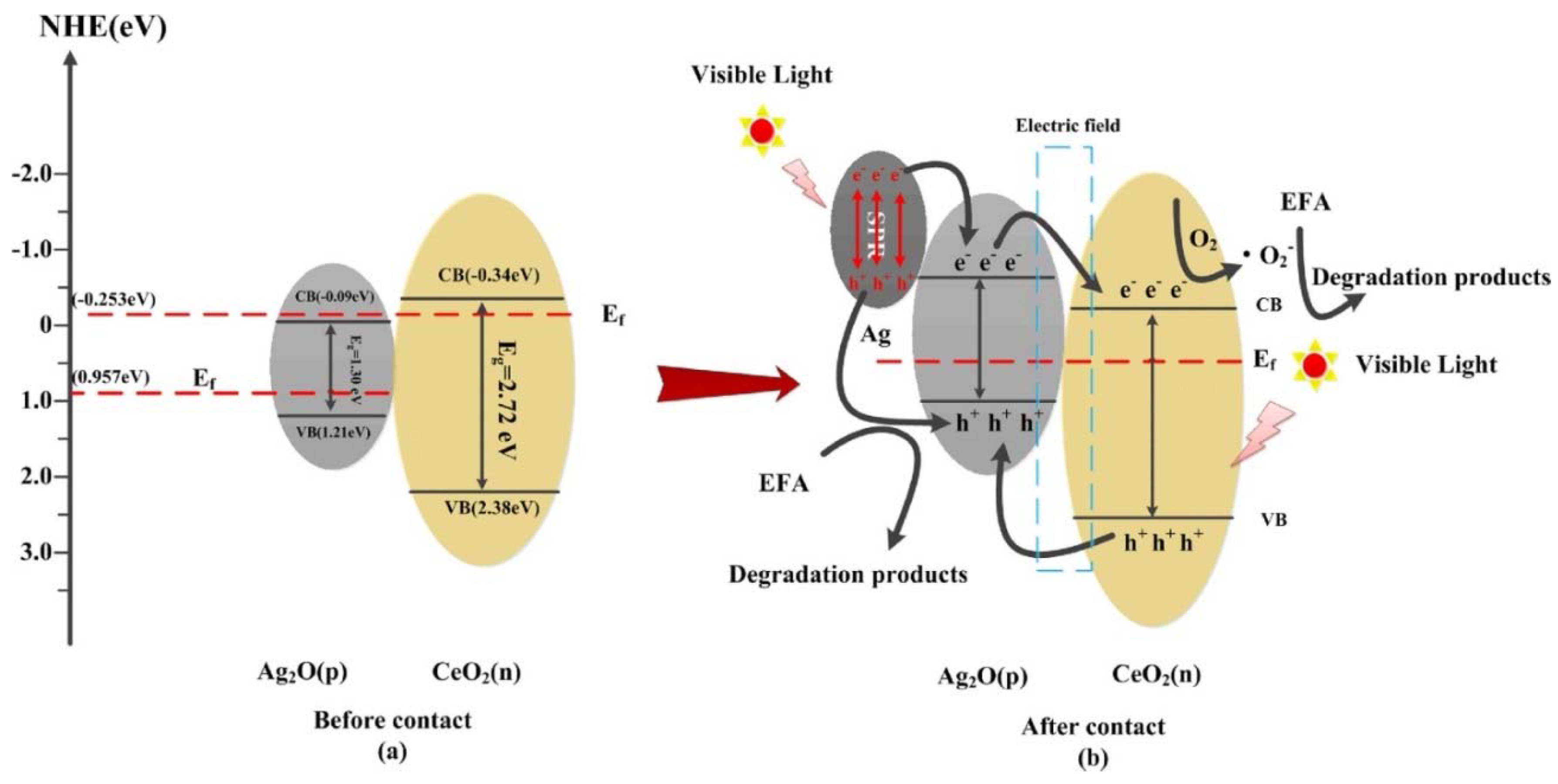
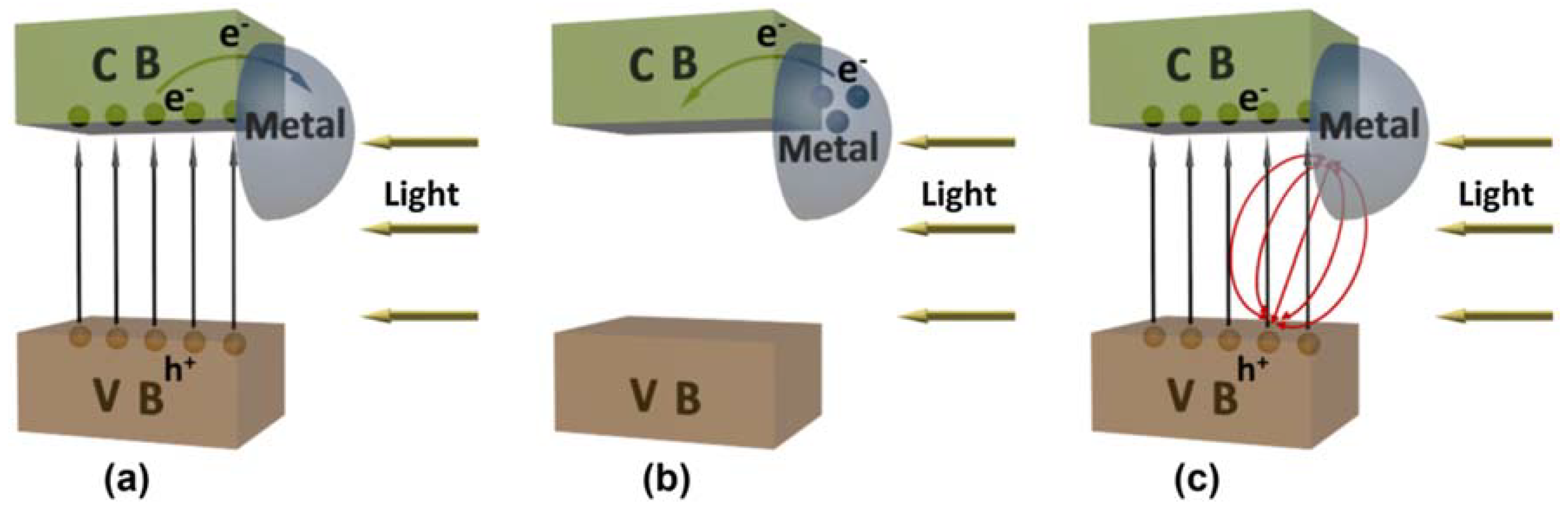
| Catalyst | Preparation Method | CeO2 Morphology | SBET, m2/g | Particle Size, nm | Ce3+/Ce4+ Ratio | Catalyst/Soot Ratio | Contact Mode | Reaction Conditions | T10, °C | T50, °C | T90/Tmax, °C | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ag | CeO2 | ||||||||||||
| CeO2-NC | hydrothermal | nanocubes | 11 | - | 100 | 0.45 | 4:1 (mass.) | TC | 1% O2/N2 500 mL/min, isothermal reactions at 300 °C and 350 °C | - | 430 | - | [105] |
| CeO2-NP | thermal decomposition | irregular shaped | 71 | - | 15 | 0.57 | - | 458 | - | ||||
| CeO2-Sp | hydrothermal | spindles | 79 | - | 25 | 0.53 | - | 527 | - | ||||
| CeO2-30 | precipitation at 30 °C | irregular shaped | 49 | - | 11 | 0.52 | 4:1 (mass.) | LC | 20% O2/80% N2 | - | - | 598 | [106] |
| CeO2-50 | precipitation at 50 °C | 41 | - | 15 | 0.51 | - | 542 | - | |||||
| CeO2-70 | precipitation at 70 °C | 49 | - | 15 | 0.50 | - | 542 | - | |||||
| Ce-R | hydrothermal | nanorods | 80 | - | 250 nm × 2 μm | Ce3+ 25.1 at. % | 9:1 (mass.) | LC | 10 vol%O2/N2 | 356 | 500 | 554 | [96] |
| TC | 286 | 368 | 400 | ||||||||||
| Ce-P | solvothermal | irregular shaped | 88 | - | 30–40 | Ce3+ 16.5 at. % | LC | 413 | 521 | 573 | |||
| TC | 320 | 433 | 474 | ||||||||||
| Ce-F | solvothermal | flakes | 62 | - | 25 | Ce3+ 19.1 at. % | LC | 433 | 554 | 622 | |||
| TC | 306 | 383 | 440 | ||||||||||
| Ce-SAS | hydrothermal route in a batch stirred-tank reactor | SA stars | 124 | - | 10 | N/A | 45:5 (mass.) | LC | 50% air/ 50% N2 constant 100 mL min−1 | 450 | 560 | 610 | [107] |
| TC | 385 | 415 | 505 | ||||||||||
| Ce-NC | hydrothermal | nanocubes | 4 | - | 54 | N/A | 45:5 (mass.) | LC | 420 | 465 | 575 | [93] | |
| TC | 370 | 385 | 430 | ||||||||||
| Ce-ND | thermal decomposition | irregular shaped | 72 | - | 7–35 | N/A | 45:5 (mass.) | LC | 50% air/ 50% N2 100 mL min−1 | 475 | 530 | 600 | |
| TC | 360 | 390 | 498 | ||||||||||
| Ce-NC | hydrothermal | nanocubes | 4 | - | 54 | Ce3+ 27.6 at. % | 45:5 (mass.) | LC | 10% of O2/N2 at rate of 100 cm3 min−1 | 417 | 477 | 584 | [88] |
| TC | 396 | 400 | 425 | ||||||||||
| Ce-NR | hydrothermal | nanorods | 4 | - | 43 | Ce3+ 25.5 at. % | LC | 429 | 536 | 623 | |||
| TC | 381 | 416 | 455 | ||||||||||
| Ce-M | improved grafting | mesoporous | 75 | - | 5 | Ce3+ 25.5 at. % | LC | 398 | 538 | 604 | |||
| TC | 374 | 464 | 510 | ||||||||||
| Ce-SCS | solution combustion | mesoporous | 69 | - | 35 | Ce3+ 36.1 at. % | LC | 436 | 580 | 633 | |||
| TC | 392 | 476 | 558 | ||||||||||
| CeO2-CP1-F | co-precipitation | irregular shaped | 52.6 | - | 8.46 | Ce3+ 21.71 at. % | 45:5 (mass.) | LC | 5% O2/Ar, 200 mLmin−1 | - | - | 545 | [90] |
| CeO2-CP2-F | modified co-precipitation HNO3/Ce(NO3)3 = 0.5 (mol) | 22.7 | - | 7.87 | Ce3+ 12.77 at. % | - | - | 530 | |||||
| CeO2-CP3-F | modified co-precipitation HNO3/Ce(NO3)3 = 1 (mol) | 24.6 | - | 6.05 | Ce3+ 11.90 at. % | - | - | 480 | |||||
| CeO2-CP4-F | modified co-precipitation HNO3/Ce(NO3)3 = 2 (mol) | irregular shaped | 30.13 | - | 6.07 | Ce3+ 10.58 at. % | 45:5 (mass.) | LC | 5% O2/Ar, 200 mLmin−1 | - | - | 465 | [90] |
| CeO2-CP4-A | CeO2-CP4 calcined at 750 °C for 6 h | irregular shaped | 1.80 | - | 47.18 | Ce3+ 15.60 at. % | - | - | 440 | ||||
| CeO2-S-F | solid combustion | 77.1 | - | 9.63 | Ce3+ 26.58 at. % | - | - | 540 | |||||
| CeO2-CA-F | citric acid sol–gel | 45.0 | - | 9.68 | Ce3+ 30.76 at. % | - | - | 560 | |||||
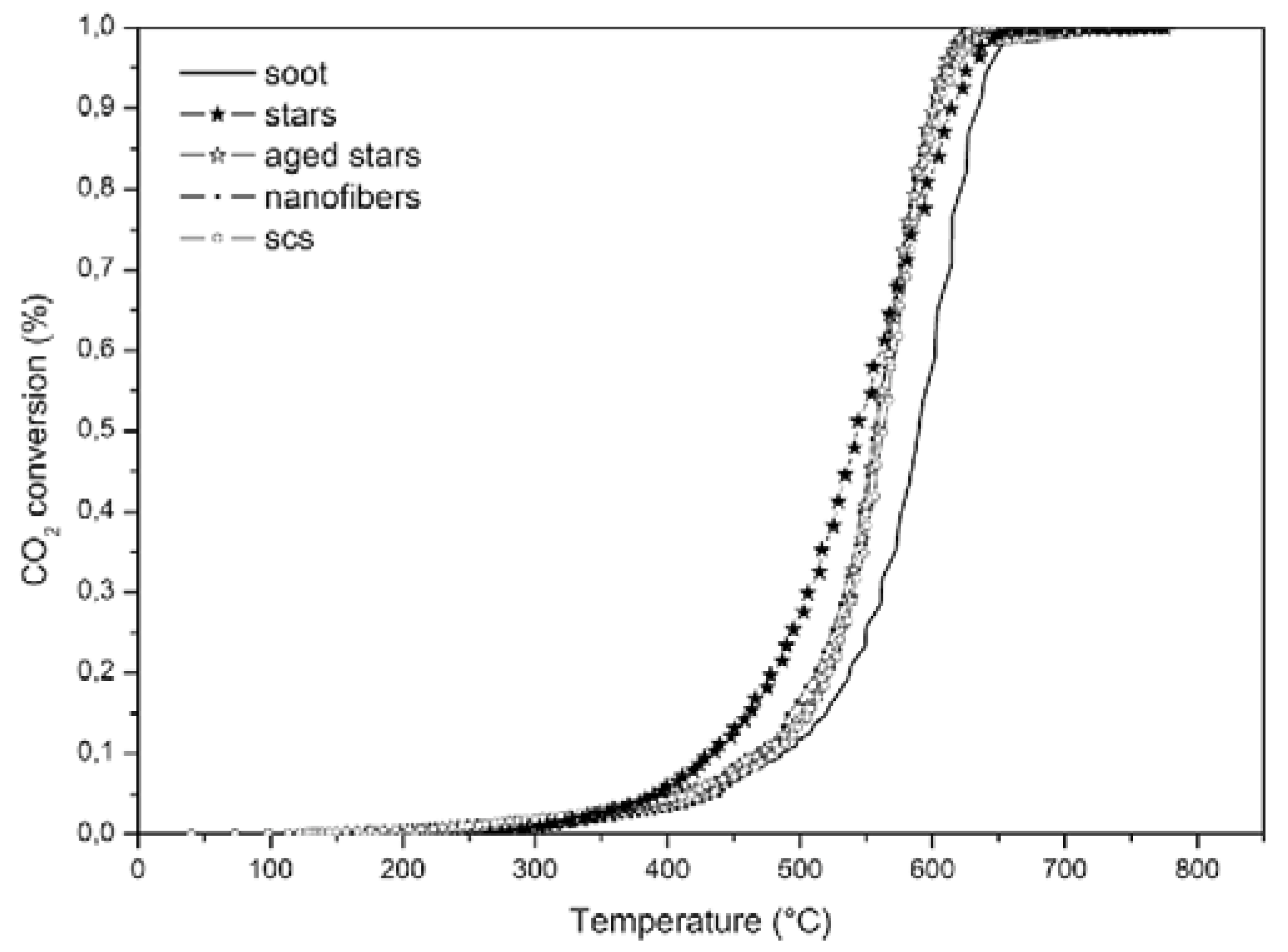
| CeO2-500 | electrospinning with calcination at 500 °C | nanofibers | 20.4 | - | 241–253 | N/A | 95:5 (mass.) | LC | 21% O2 and 79% N2, 100 mL/min | 596 | [47] | ||
| TC | - | - | 429 | ||||||||||
| CeO2-800 | electrospinning calcination at 800 °C | 3.45 | - | 241–253 | N/A | LC | - | - | 633 | ||||
| TC | - | - | 504 | ||||||||||
| CeO2-1000 | electrospinning calcinations at 1000 °C | 3.40 | - | 241–253 | N/A | LC | - | - | 639 | ||||
| TC | |||||||||||||
| - | - | 513 | |||||||||||
| CeO2 | precipitation | irregular-shaped | 45 | - | N/A | N/A | 20:1 (mass.) | TC | 10% O2/N2 10 °C min−1 | - | - | 393 | [48] |
| CeO2 | precipitation/ripening | nanofibers | 4 | - | 72 | N/A | 45:5 (mass.) | LC | 10% O2/N2 | 480 | 555 | 560 | [92] |
| TC | 383 | 439 | 445 | ||||||||||
| CeO2 | solution combustion | uncontrolled nanopowders | 31 | - | 45 | N/A | LC | 483 | 562 | 562 | |||
| TC | 358 | 411 | 417 | ||||||||||
| CeO2 | hydrothermal | three-dimensional SA stars | 105 | - | 9 | N/A | 45:5 (mass.) | LC | 10% O2/N2 | 435 | 543 | 552 | [92] |
| TC | 354 | 410 | 403 | ||||||||||
| CeO2 | SA stars aged 5 h at 600 °C | Aged SA stars | 50 | - | 15 | N/A | LC | 473 | 559 | 559 | |||
| TC | 381 | 453 | 465 | ||||||||||
| AgCe-NC | incipient wetness impregnation (Ag-5 wt. %) | nanocubes | 10 | 1.5–3.5 | 100 | 0.34 | 4:1 (mass.) | TC | 1% O2/N2 500 mL/min 100,000 h−1, isothermal reactions at 300 °C | - | 376 | - | [105] |
| AgCe-NP | incipient wetness impregnation (Ag-5wt. %) | irregular shaped | 64 | 1.5–3.5 | 16 | 0.52 | - | 389 | - | ||||
| AgCe-Sp | incipient wetness impregnation (Ag-5 wt. %) | spindles | 69 | 1.5–3.5 | 27 | 0.37 | - | 411 | - | ||||
| Ag/CeO2-30 | incipient wetness impregnation (Ag-5 wt. %) | irregular shaped | 37 | 5 | 15 | 0.29 | 4:1 (mass.) | LC | 1% O2/N2 after 4 cycles | 522 | 606 | 691 | [106] |
| Ag/CeO2-50 | 33 | 8 | 20 | 0.27 | 488 | 596 | 660 | ||||||
| Ag/CeO2-70 | 37 | 8 | 15 | 0.23 | 504 | 602 | 675 | ||||||
| Ag/CeO2-500 | electrospinning calcination at 500 °C (Ag-4.5 wt. %) | nanofibers | 5.07 | 10 | 241–253 | N/A | 95:5 (mass.) | LC | 21% O2, 79% N2, 100 mL/min | - | - | 481 | [47] |
| TC | - | - | 429 | ||||||||||
| Ag/CeO2-800 | electrospinning calcination at 800 °C (Ag-4.5 wt. %) | 3.07 | 10 | 241–253 | N/A | LC | - | - | 485 | ||||
| TC | - | - | 484 | ||||||||||
| Ag/CeO2-1000 | electrospinning calcinations at 1000 °C (Ag-4.5 wt. %) | 2.74 | 10 | 241–253 | N/A | LC | - | - | 514 | ||||
| TC | - | - | 496 | ||||||||||
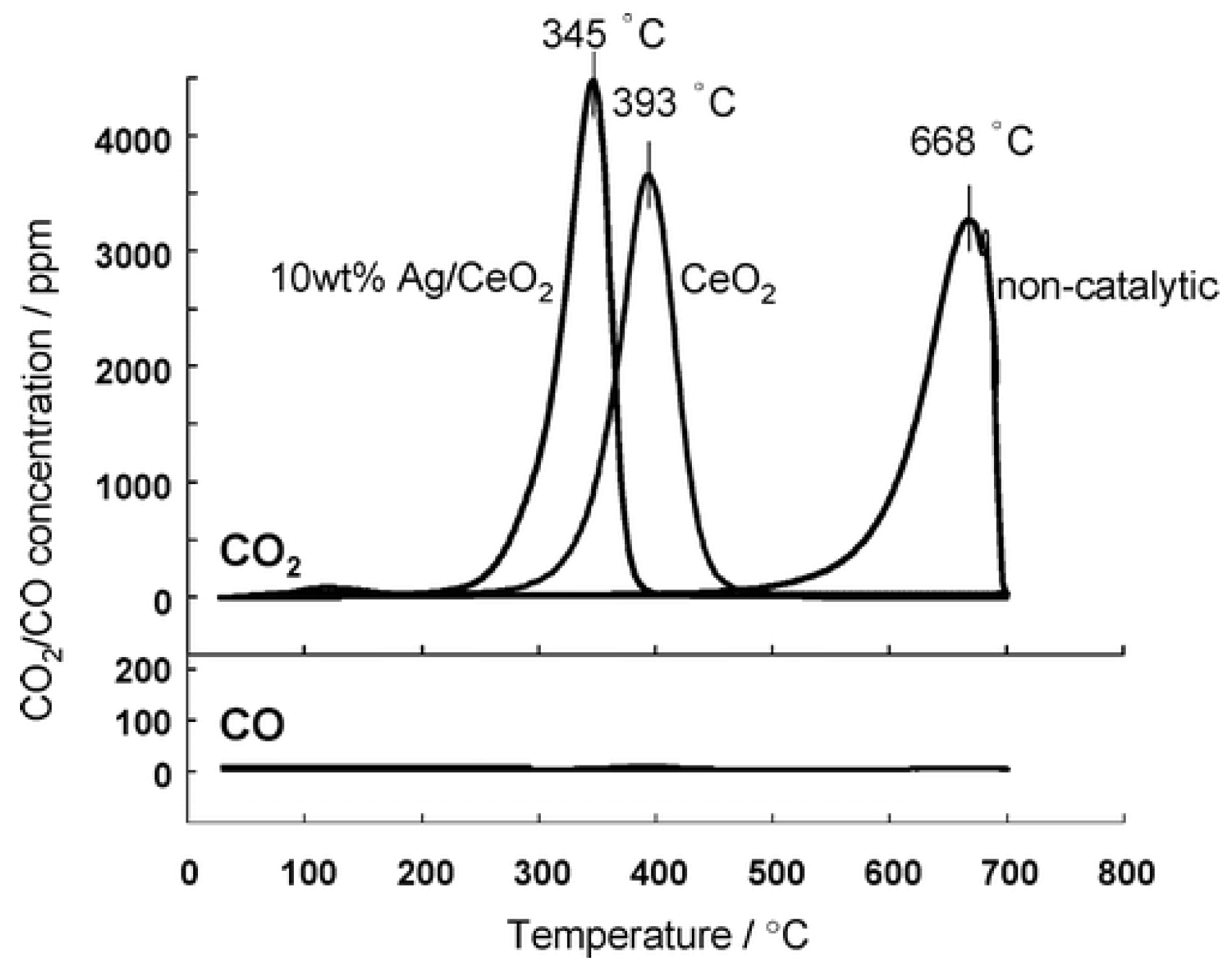
| Ag/CeO2 | incipient wetness impregnation (Ag-10wt. %) | irregular shaped | N/A | N/A | N/A | N/A | 20:1 (mass.) | TC | 10% O2/N2 10 °C·min−1 | - | - | 345 | [48] |
| CeO2–Ag | co-precipitation (Ag-39 wt. %) | rice-ball | 14.7 | 36 | 16 | N/A | 19:1 (mass.) | LC | 10% O2/He at 50 mL/min | - | - | 376 | [49] |
| TC | 315 | ||||||||||||
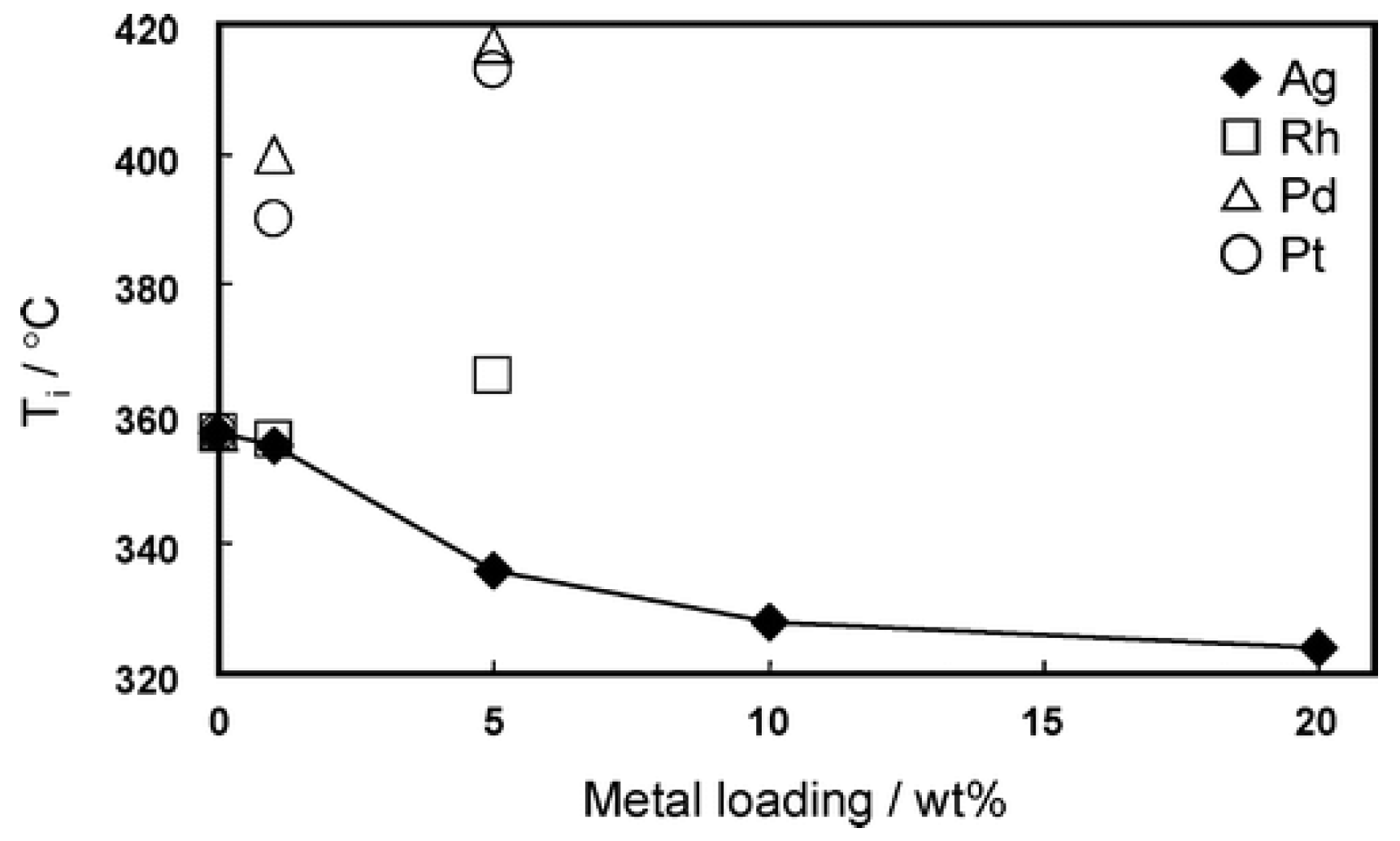
| Ag(39)/CeO2 | impregnation (Ag-39 wt. %) | irregular shaped | 30.1 | 89 | 21 | N/A | LC | - | - | 563 | |||
| TC | 381 | ||||||||||||
| Ag(10)/CeO2 | impregnation (Ag-10 wt. %) | 52.0 | 60 | 20 | N/A | LC | - | - | 526 | ||||
| TC | 362 | ||||||||||||
| Ag(3.2)/CeO2 | impregnation (Ag-3.2 wt. %) | 59.2 | 28 | 20 | N/A | LC | - | - | 550 | ||||
| TC | 371 | ||||||||||||
| Ag(1.9)/CeO2 | impregnation (Ag-1.9 wt. %) | 70.0 | 20 | 20 | N/A | LC | - | - | 596 | ||||
| TC | 414 | ||||||||||||
| Ag(0.95)/CeO2 | impregnation (Ag-0.95 wt. %) | 78.1 | n.d | 20 | N/A | LC | - | - | 610 | ||||
| TC | 466 | ||||||||||||
| Type of VOC | Preparation Method | Loading of Ag, wt. % | TVOC conv., °C | S, m2/g | Mean Ag NP Diameter (nm) | Reaction Conditions | TOF × 103, s−1 | T, °C | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|
| CH2O | CP | 61.3 28.4 15 7.69 | 80%: 150 | 40.5 to 84.4 | N/A | 1 mL of catalyst, CH2O: 0.42%, methanol: 0.074%, H2O: 19.9%, N2: 62.7%, O2: 16.9% GHSV = 21,000 h−1 Trange: 423–573 K | - | - | [140] | |
| CH2O | WI | 8 | 100%: 125 | 113.7 | N/A | 110 ppm of CH2O 20% O2, N2 balance GHSV = 100,000 mL (gcat·h)−1 Kinetic studies: 1400 ppm of CH2O. GHSV = 302,000 mL (gcat·h)−1 | 6.8 | 100 | [137] | |
| CH2O | WI | 1 | 100%: 100 | 70.8 | <3 | 50 mg of catalyst 600 ppm CH2O 20.0 vol% O2, N2 balance GHSV = 120,000–360,000 h−1 | 1.8 | 100 | [138] | |
| CH2O | HT WI | 2 | 100%: 110 | HT: 125.4. WI: 55.5 | nanospheres HT: 14.8 WI: 2.4 | 50 mg of catalyst powder mixed with quartz sand 810 ppm of CH2O 20% O2, N2 balance GHSV = 84,000 h−1 | 5.0 | 110 | [40] | |
| CH2O | HT WI | 5 | 100%: 110 (nr) 50%: nr: 74 np: 89 nc: 108 | nr: 128.46 np: 104.74 nc: 72.63 | np: 4.0 nr: 6.0 ± 2.0 nm and 50.0–100.0 nm | 50 mg of catalyst 810 ppm of CH2O GHSV = 84,000 h−1 contact time was 0.34 s Trange: 30–240 | 1.9 * TOFAg nr: 71.0 np: 46.0 nc: 31.0 | 100 | [139] | |
| propylene | WI DP | 10 | 50%: WI: 173 DP: 261 | WI:92 DP:84 | N/A | 100 mg of fine catalyst powder, air and 6000 ppm of C3H6, reactive flow of 100 mL min−1 | WI: 2.2 DP: 0.13 | 170 | [41] | |
| propylene | WI DPU IRC | 4 | 50%: WI: 221 DPU: 260 IRC: 200 | WI: 149 DPU: 123 IRC: 99 | N/A | 200 mg of catalyst 6000 ppm of C3H6 a total flow of 100 mL/min Trange: 60–400 | WI: 0.27 DPU: 0.22 IRC: 0.14 | 170 | [141] | |
| propylene | WI DP | 2.14 | 50%: WI: 220 DP: 245 | WI: 98 DP: 118 | N/A | 100 mg of fine catalyst powder 6000 ppm of C3H6 a total flow of 100 mL min−1 Trange: 100–400 | WI: 0.8 DP: 0.5 | 170 | [142] | |
| toluene | 50%: WI: 240 | 2000 ppm of C7H8 | 0.34 | 170 | ||||||
| toluene | DP CP | 4.8 4.7 | 50%: DP: 265 CP: 260 | DP: 112 CP: 130 | DP: 7.1–6.7 CP: <4–3.3 | 0.7 vol.% VOC 10 vol.% O2 He balance GHSV = 7.6 × 10−3 molVOC h−1 gcat −1 | - | - | [54] | |
| methanol | 50%: DP: 131 CP: 113 | - | - | |||||||
| acetone | 50%: DP: 225 CP: 220 | - | - | |||||||
| naphthalene | WI | 1 | 100%: 240 50%: 175 (1 wt. % Ag) | 143 | 8.7 | 120 ppm naphthalene 10% O2, N2 balance total gas flow rate was 400 mL/min GHSV = 175,000 h−1 Trange: 160–300 | 1.5 | 170 | [143] | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabchenko, M.V.; Mikheeva, N.N.; Mamontov, G.V.; Salaev, M.A.; Liotta, L.F.; Vodyankina, O.V. Ag/CeO2 Composites for Catalytic Abatement of CO, Soot and VOCs. Catalysts 2018, 8, 285. https://doi.org/10.3390/catal8070285
Grabchenko MV, Mikheeva NN, Mamontov GV, Salaev MA, Liotta LF, Vodyankina OV. Ag/CeO2 Composites for Catalytic Abatement of CO, Soot and VOCs. Catalysts. 2018; 8(7):285. https://doi.org/10.3390/catal8070285
Chicago/Turabian StyleGrabchenko, M. V., N. N. Mikheeva, G. V. Mamontov, M. A. Salaev, L. F. Liotta, and O. V. Vodyankina. 2018. "Ag/CeO2 Composites for Catalytic Abatement of CO, Soot and VOCs" Catalysts 8, no. 7: 285. https://doi.org/10.3390/catal8070285
APA StyleGrabchenko, M. V., Mikheeva, N. N., Mamontov, G. V., Salaev, M. A., Liotta, L. F., & Vodyankina, O. V. (2018). Ag/CeO2 Composites for Catalytic Abatement of CO, Soot and VOCs. Catalysts, 8(7), 285. https://doi.org/10.3390/catal8070285






