Preparation of Stable Cross-Linked Enzyme Aggregates (CLEAs) of a Ureibacillus thermosphaericus Esterase for Application in Malathion Removal from Wastewater
Abstract
:1. Introduction
2. Results and Discussion
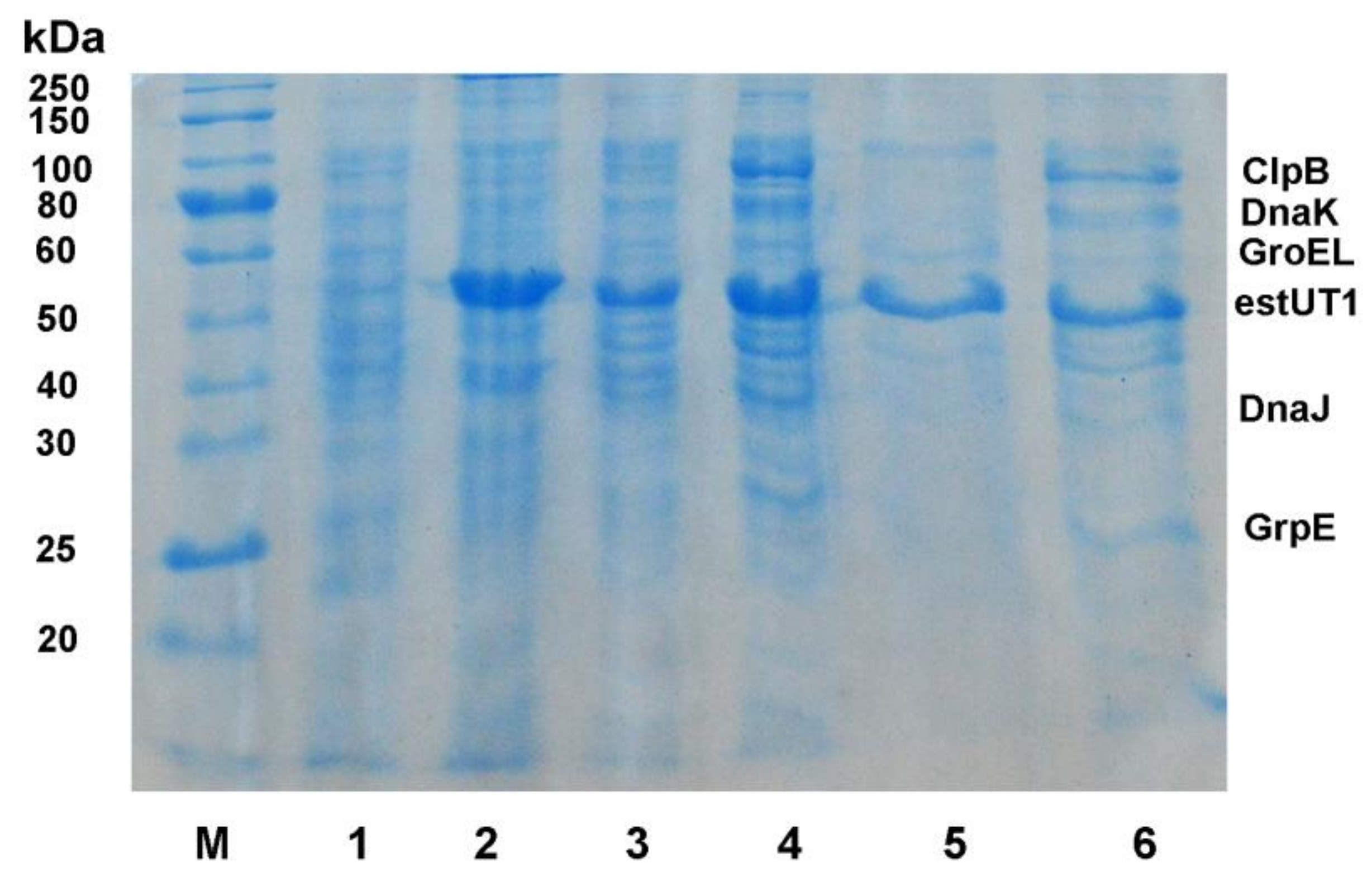
2.1. Chaperone Сo-Expression with estUT1
2.2. Preparation of CLEA-estUT1
2.2.1. Selection of Precipitant
2.2.2. Selection of Additives
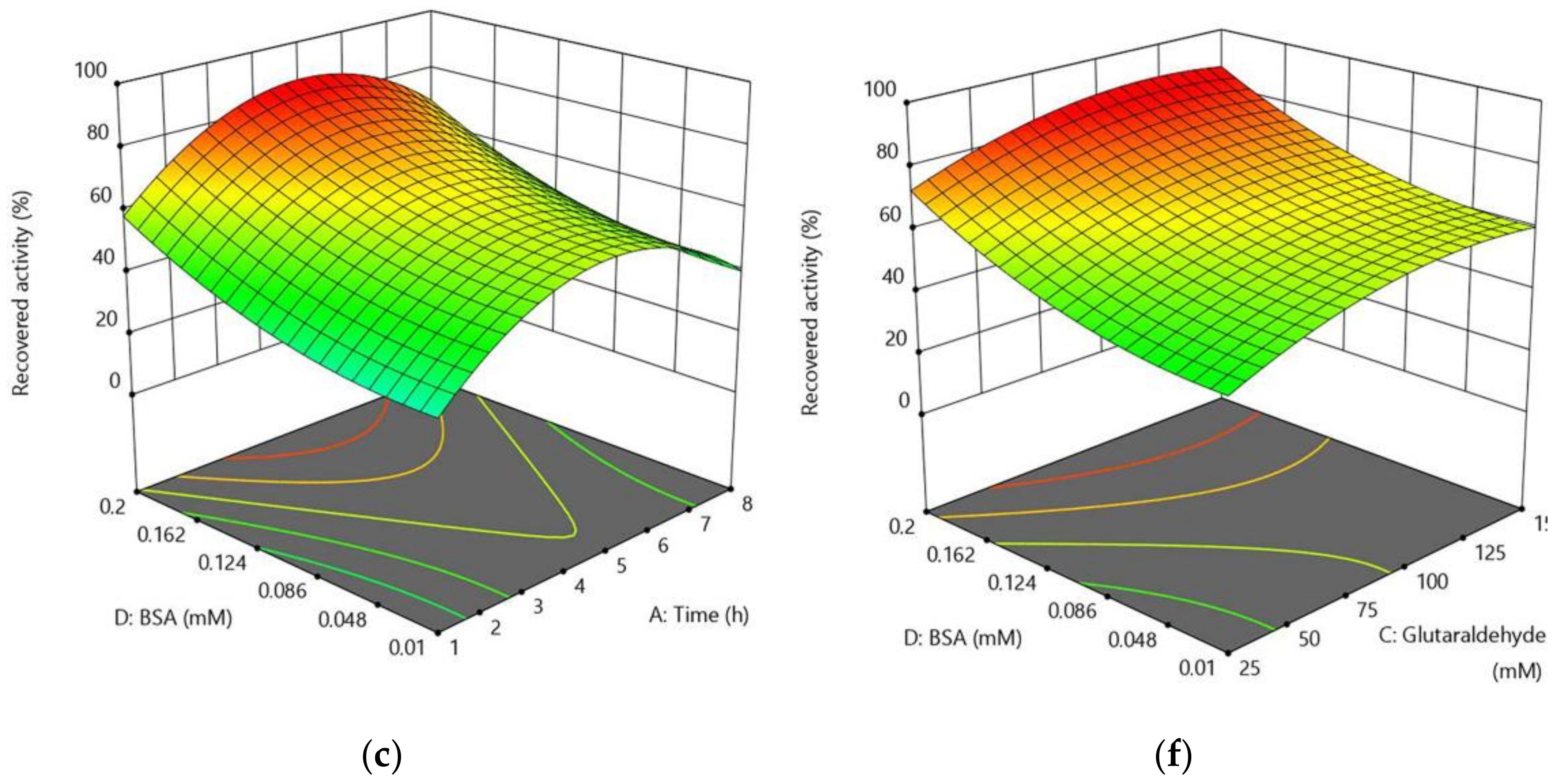
2.2.3. Optimization of CLEA-estUT1 Preparation
2.3. Study of CLEA-estUT1 Properties
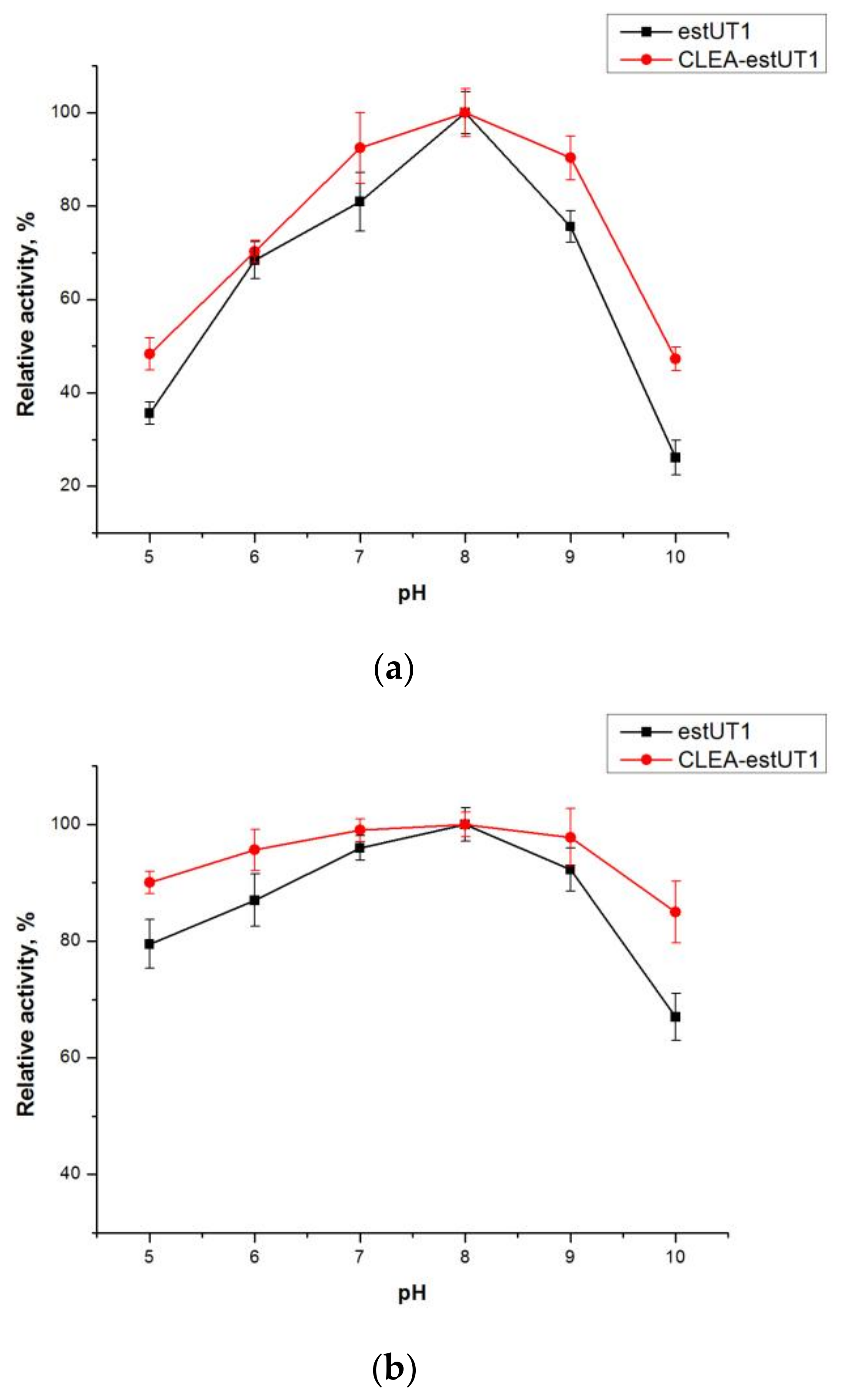
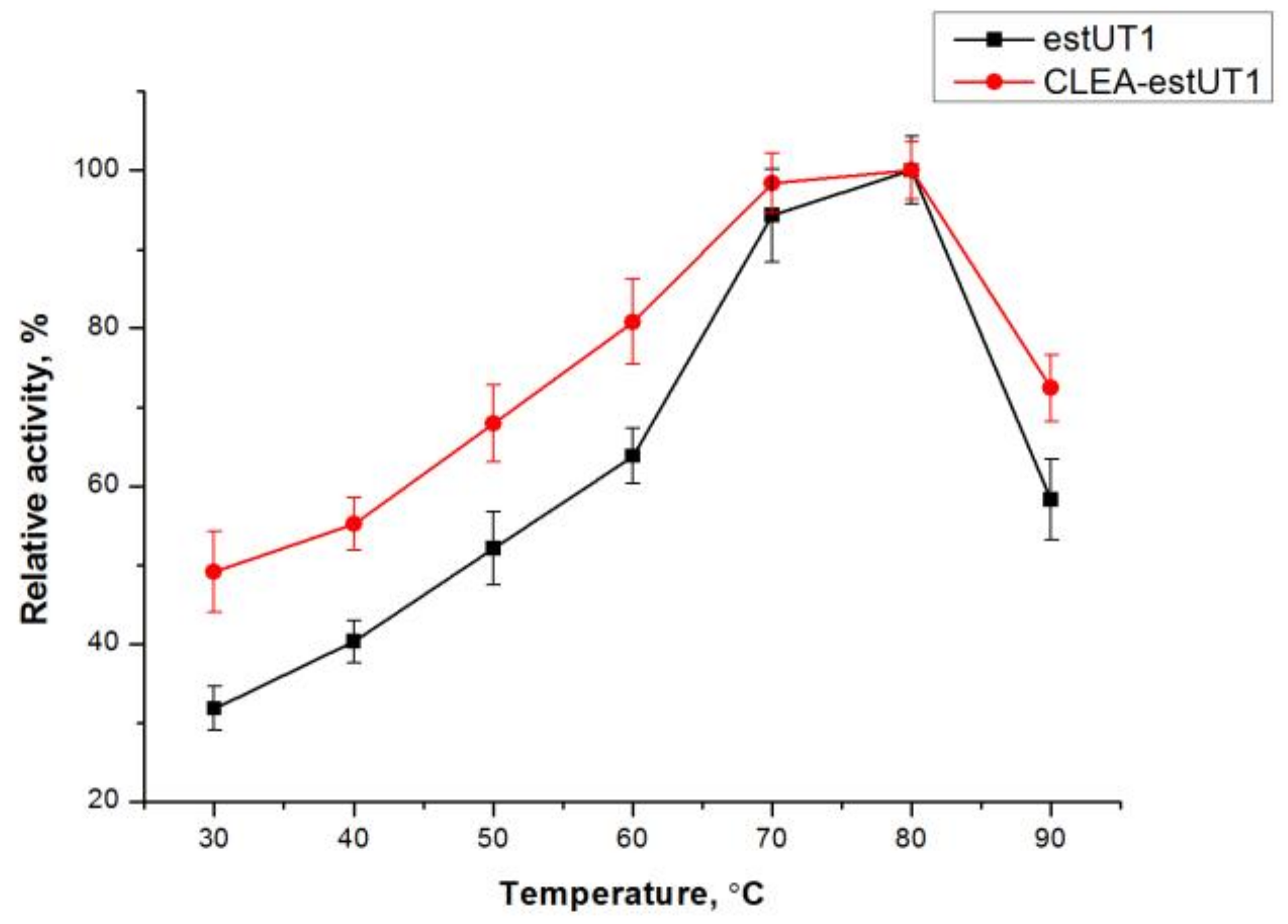
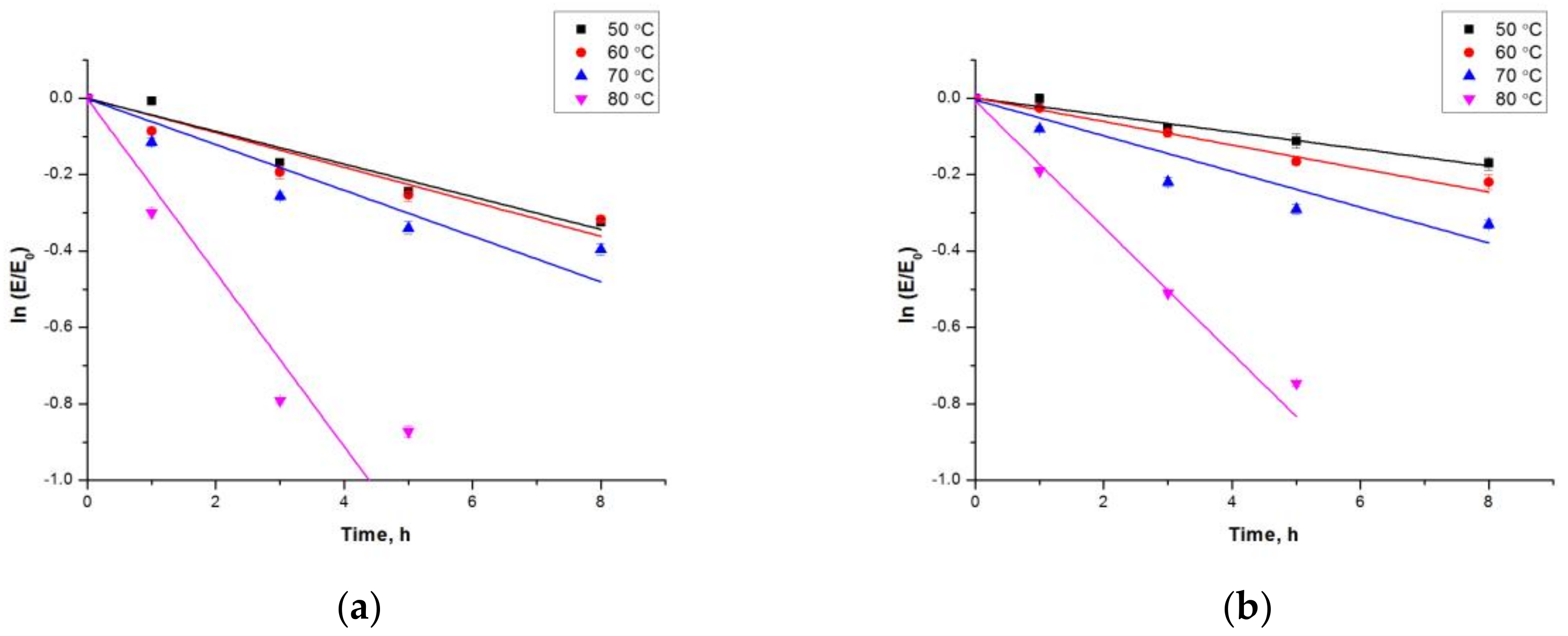
2.3.1. Effect of pH and Temperature on the Activity and Stability of Free estUT1 and CLEA-estUT1
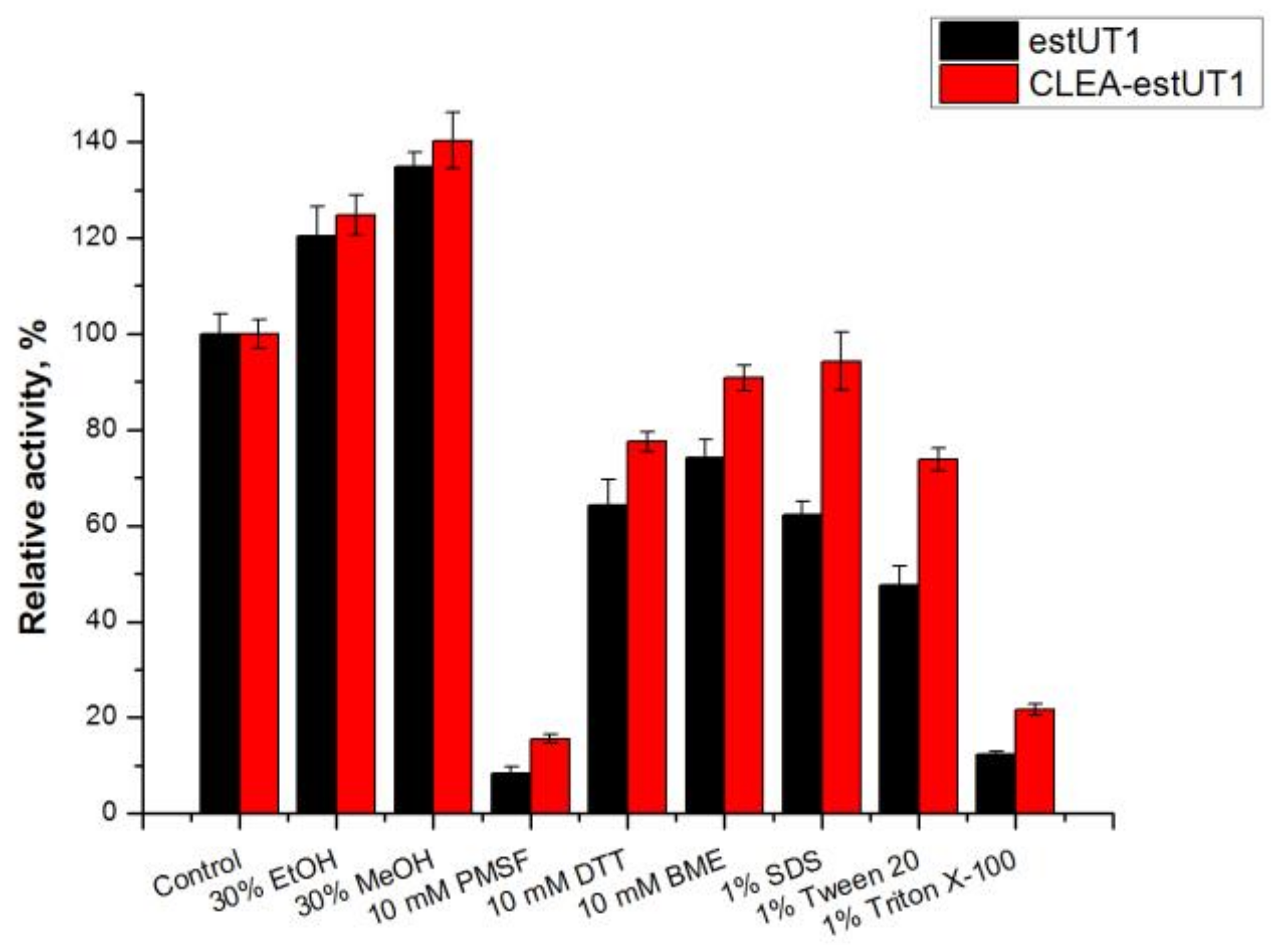
2.3.2. Effect of Chemicals on the Activity of Free estUT1 and CLEA-estUT1
2.3.3. Kinetic Studies of Free and Immobilized estUT1
2.3.4. Malathion Hydrolysis with CLEA-estUT1
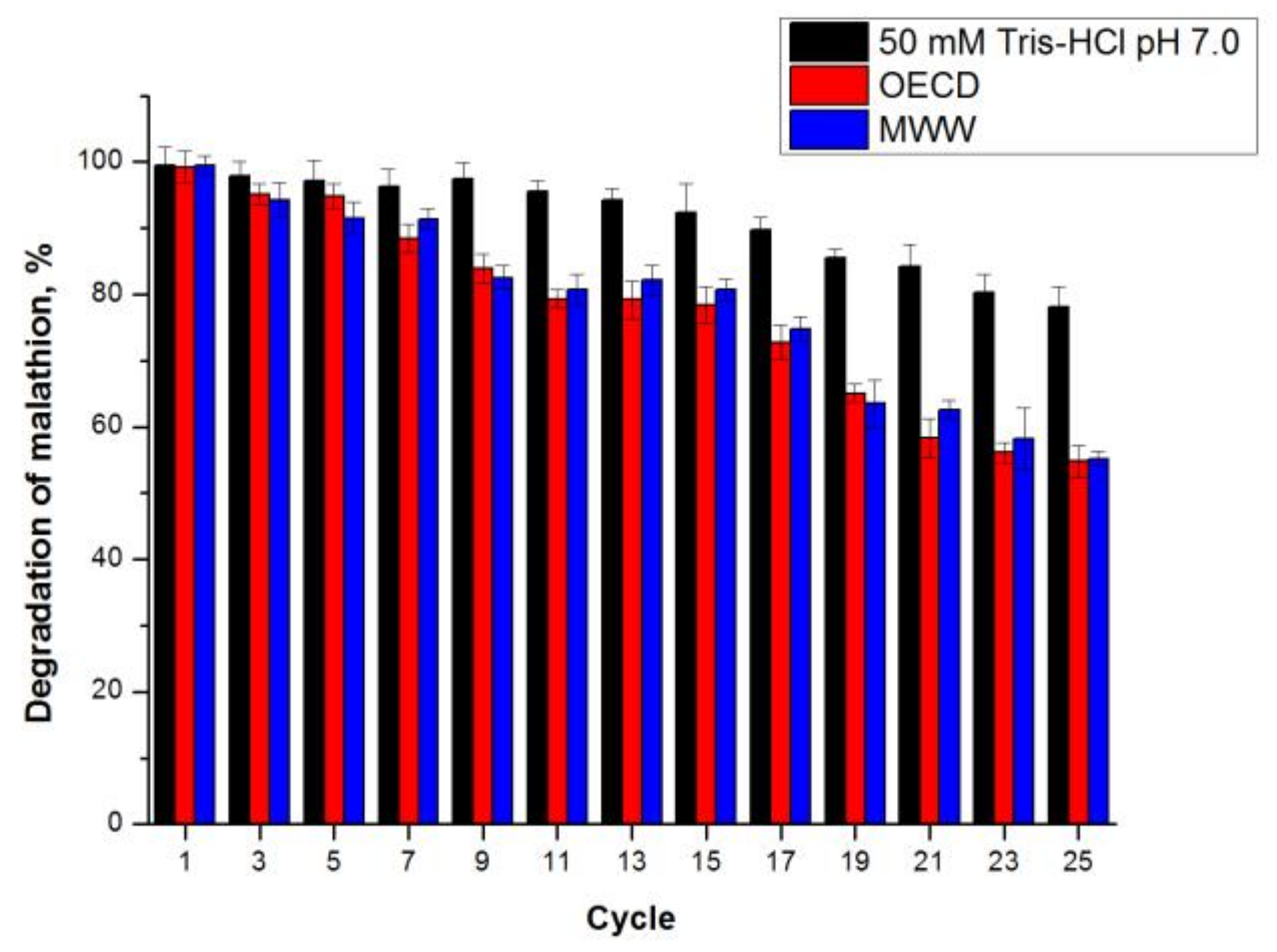
2.3.5. Comparison of Microbial Biodegradation and CLEA-estUT1 Hydrolysis of Malathion
3. Materials and Methods
3.1. Materials
3.2. Expression of Esterase estUT1
3.3. Co-Expression of Chaperones
3.4. Enzyme Activity Assay
3.5. Preparation of CLEA-estUT1
3.6. Selection of Precipitant
3.7. Selection of Various Additives
3.8. Optimization of CLEA-estUT1 Preparation
3.9. Properties of CLEA-estUT1 Biocatalyst
3.9.1. Effect of pH and Temperature
3.9.2. Effect of Chemicals
3.9.3. Kinetic Parameters
3.9.4. Operational Stability of CLEA-estUT1 during Hydrolysis of Malathion
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Firestone, J.A.; Smith-Weller, T.; Franklin, G.; Swanson, P.; Longstreth, W.T., Jr.; Checkoway, H. Pesticides and risk of parkinson disease: A population-based case-control study. Arch. Neurol. 2005, 62, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Alavanja, M.C.R.; Hoppin, J.A.; Kamel, F. Health effects of chronic pesticide exposure: Cancer and neurotoxicity. Ann. Rev. Public Health 2004, 25, 155–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pagilla, K. Treatment of malathion pesticide wastewater with nanofiltration and photo-fenton oxidation. Desalination 2010, 263, 36–44. [Google Scholar] [CrossRef]
- Shayeghi, M.; Dehghani, M.H.; Alimohammadi, M.; Goodini, K. Using ultraviolet irradiation for removal of malathion pesticide in water. J. Arthropod-Borne Dis. 2012, 6, 45–53. [Google Scholar] [PubMed]
- Geed, S.R.; Kureel, M.K.; Shukla, A.K.; Singh, R.S.; Rai, B.N. Biodegradation of malathion and evaluation of kinetic parameters using three bacterial species. Resour. Effic. Technol. 2016, 2, S3–S11. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Ahn, J.-Y.; Moon, S.-H.; Lee, J. Biodegradation and detoxification of organophosphate insecticide, malathion by Fusarium oxysporum f. sp. pisi cutinase. Chemosphere 2005, 60, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, W.M.; Karam, M.A.; El-Shahat, R.M.; Adway, A.A. Biodegradation and utilization of organophosphorus pesticide malathion by cyanobacteria. BioMed Res. Int. 2014, 2014, 392682. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Zaffar, H.; Irshad, U.; Ahmad, R.; Khan, A.R.; Shah, M.M.; Bilal, M.; Iqbal, M.; Naqvi, T. Biodegradation of malathion by Bacillus licheniformis strain ML-1. Arch. Biol. Sci. 2016, 68, 9. [Google Scholar] [CrossRef]
- Goda, S.K.; Elsayed, I.E.; Khodair, T.A.; El-Sayed, W.; Mohamed, M.E. Screening for and isolation and identification of malathion-degrading bacteria: Cloning and sequencing a gene that potentially encodes the malathion-degrading enzyme, carboxylestrase in soil bacteria. Biodegradation 2010, 21, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Schofield, D.A.; DiNovo, A.A. Generation of a mutagenized organophosphorus hydrolase for the biodegradation of the organophosphate pesticides malathion and demeton-s. J. Appl. Microbiol. 2010, 109, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Xu, B.; Ding, J.; Liu, L.; Zhang, X.; Li, J.; Huang, Z. Heterologous expression and characterization of a malathion-hydrolyzing carboxylesterase from a thermophilic bacterium, Alicyclobacillus tengchongensis. Biotechnol. Lett. 2013, 35, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Samoylova, Y.V.; Piligaev, A.V.; Sorokina, K.N.; Rozanov, A.S.; Peltek, S.E.; Novikov, A.A.; Almyasheva, N.R.; Parmon, V.N. Application of the immobilized bacterial recombinant lipase from Geobacillus stearothermophilus G3 for the production of fatty acid methyl esters. Catal. Ind. 2016, 8, 187–193. [Google Scholar] [CrossRef]
- Samoylova, Y.V.; Piligaev, A.V.; Sorokina, K.N.; Parmon, V.N. Enzymatic interesterification of sunflower oil and hydrogenated soybean oil with the immobilized bacterial recombinant lipase from Geobacillus stearothermophilus G3. Catal. Ind. 2017, 9, 62–70. [Google Scholar] [CrossRef]
- Adhikari, S.; Chattopadhyay, P.; Ray, L. Biosorption of malathion by immobilized cells of Bacillus sp. S14. Chem. Spec. Bioavailabil. 2010, 22, 271–276. [Google Scholar] [CrossRef]
- Saida, F.; Uzan, M.; Odaert, B.; Bontems, F. Expression of highly toxic genes in E. coli: Special strategies and genetic tools. Curr. Protein Pept. Sci. 2006, 7, 47–56. [Google Scholar] [CrossRef] [PubMed]
- De Marco, A. Strategies for successful recombinant expression of disulfide bond-dependent proteins in Escherichia coli. Microb. Cell Fact. 2009, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant protein expression in Escherichia coli: Advances and challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef] [PubMed]
- Valero, F. Heterologous expression systems for lipases: A review. In Lipases and Phospholipases: Methods and Protocols; Sandoval, G., Ed.; Humana Press: Totowa, NJ, USA, 2012; pp. 161–178. [Google Scholar]
- Shuo-shuo, C.; Xue-zheng, L.; Ji-hong, S. Effects of co-expression of molecular chaperones on heterologous soluble expression of the cold-active lipase lip-948. Protein Expr. Purif. 2011, 77, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Tiwari, M.K.; Singh, R.; Lee, J.-K. From protein engineering to immobilization: Promising strategies for the upgrade of industrial enzymes. Int. J. Mol. Sci. 2013, 14, 1232–1277. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Kim, H. Transesterification using the cross-linked enzyme aggregate of Photobacterium lipolyticum lipase M37. J. Microbiol. Biotechnol. 2011, 21, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Tükel, S.S.; Hürrem, F.; Yildirim, D.; Alptekin, Ö. Preparation of crosslinked enzyme aggregates (CLEA) of catalase and its characterization. J. Mol. Catal. B Enzym. 2013, 97, 252–257. [Google Scholar] [CrossRef]
- Morales, A.; Barbosa, O.; Rueda, N.; Fonseca, Z.; Torres, R.; Rodrigues, R.C.; Ortiz, C.; Fernandez-Lafuente, R. Optimization and characterization of CLEAs of the very thermostable dimeric peroxidase from Roystonea regia. RSC Adv. 2015, 5, 53047–53053. [Google Scholar] [CrossRef]
- Jang, E.; Ryu, B.; Kim, T. Identification, characterization, and immobilization of an organic solvent-stable alkaline hydrolase (PA27) from Pseudomonas aeruginosa MH38. Molecules 2014, 19, 14396. [Google Scholar] [CrossRef] [PubMed]
- Kumari, V.; Shah, S.; Gupta, M.N. Preparation of biodiesel by lipase-catalyzed transesterification of high free fatty acid containing oil from Madhuca indica. Energy Fuels 2007, 21, 368–372. [Google Scholar] [CrossRef]
- Piligaev, A.V.; Sorokina, K.N.; Samoylova, Y.V.; Parmon, V.N. Lipid production by microalga Micractinium sp. IC-76 in a flat panel photobioreactor and its transesterification with cross-linked enzyme aggregates of Burkholderia cepacia lipase. Energy Convers. Manag. 2018, 156, 1–9. [Google Scholar] [CrossRef]
- Shah, S.; Gupta, M.N. Kinetic resolution of (±)-1-phenylethanol in [Bmim][PF6] using high activity preparations of lipases. Bioorgan. Med. Chem. Lett. 2007, 17, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Samoylova, Y.V.; Sorokina, K.N.; Romanenko, M.V.; Parmon, V.N. Cloning, expression and characterization of the esterase estUT1 from Ureibacillus thermosphaericus which belongs to a new lipase family XVIII. Extremophiles 2018, 22, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Mogk, A.; Mayer, M.P.; Deuerling, E. Mechanisms of Protein Folding: Molecular Chaperones and Their Application in Biotechnology. Chembiochem 2002, 3, 807–814. [Google Scholar] [CrossRef]
- Schlieker, C.; Bukau, B.; Mogk, A. Prevention and reversion of protein aggregation by molecular chaperones in the E. coli cytosol: Implications for their applicability in biotechnology. J. Biotechnol. 2002, 96, 13–21. [Google Scholar] [CrossRef]
- Mogk, A.; Tomoyasu, T.; Goloubinoff, P.; Rüdiger, S.; Röder, D.; Langen, H.; Bukau, B. Identification of thermolabile Escherichia coli proteins: Prevention and reversion of aggregation by DnaK and ClpB. EMBO J. 1999, 18, 6934–6949. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, H.P.; Mortensen, K.K. Soluble expression of recombinant proteins in the cytoplasm of Escherichia coli. Microb. Cell Fact. 2005, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- De Marco, A.; Deuerling, E.; Mogk, A.; Tomoyasu, T.; Bukau, B. Chaperone-based procedure to increase yields of soluble recombinant proteins produced in E. coli. BMC Biotechnol. 2007, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Hanefeld, U.; Gardossi, L.; Magner, E. Understanding enzyme immobilisation. Chem. Soc. Rev. 2009, 38, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Khanahmadi, S.; Yusof, F.; Amid, A.; Mahmod, S.S.; Mahat, M.K. Optimized preparation and characterization of CLEA-lipase from cocoa pod husk. J. Biotechnol. 2015, 202, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.-W.; Yu, H.-L.; Li, C.-X.; Pan, J.; Xu, J.-H. Immobilization of Bacillus subtilis esterase by simple cross-linking for enzymatic resolution of dl-menthyl acetate. J. Mol. Catal. B Enzym. 2011, 70, 138–143. [Google Scholar] [CrossRef]
- Nadar, S.S.; Muley, A.B.; Ladole, M.R.; Joshi, P.U. Macromolecular cross-linked enzyme aggregates (M-CLEAs) of α-amylase. Int. J. Biol. Macromol. 2016, 84, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Mahmod, S.S.; Yusof, F.; Jami, M.S.; Khanahmadi, S.; Shah, H. Development of an immobilized biocatalyst with lipase and protease activities as a multipurpose cross-linked enzyme aggregate (multi-CLEA). Process Biochem. 2015, 50, 2144–2157. [Google Scholar] [CrossRef]
- Dong, T.; Zhao, L.; Huang, Y.; Tan, X. Preparation of cross-linked aggregates of aminoacylase from Aspergillus melleus by using bovine serum albumin as an inert additive. Bioresour. Technol. 2010, 101, 6569–6571. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Beg, Q.; Lorenz, P. Bacterial alkaline proteases: Molecular approaches and industrial applications. Appl. Microbiol. Biotechnol. 2002, 59, 15–32. [Google Scholar] [PubMed]
- Torres, M.P.G.; Foresti, M.L.; Ferreira, M.L. Effect of different parameters on the hydrolytic activity of cross-linked enzyme aggregates (CLEAs) of lipase from Thermomyces lanuginosa. Biochem. Eng. J. 2013, 72, 18–23. [Google Scholar] [CrossRef]
- Kartal, F.; Kilinc, A. Crosslinked aggregates of Rhizopus oryzae lipase as industrial biocatalysts: Preparation, optimization, characterization, and application for enantioselective resolution reactions. Biotechnol. Prog. 2012, 28, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Talekar, S.; Waingade, S.; Gaikwad, V.; Patil, S.; Nagavekar, N. Preparation and characterization of cross linked enzyme aggregates (CLEAs) of Bacillus amyloliquefaciens alpha amylase. J. Biochem. Technol. 2012, 3, 349–353. [Google Scholar]
- Yu, C.-Y.; Li, X.-F.; Lou, W.-Y.; Zong, M.-H. Cross-linked enzyme aggregates of mung bean epoxide hydrolases: A highly active, stable and recyclable biocatalyst for asymmetric hydrolysis of epoxides. J. Biotechnol. 2013, 166, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.; Ryu, B.H.; Doohun Kim, T. Identification, characterization, immobilization of a novel type hydrolase (LmH) from Listeria monocytogenes. Int. J. Biol. Macromol. 2015, 72, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.J.; Jones, M.N. The biodegradation of surfactants in the environment. Biochim. Biophys. Acta (BBA) Biomembr. 2000, 1508, 235–251. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Manzoor, U. A review of removal of pollutants from water/wastewater using different types of nanomaterials. Adv. Mater. Sci. Eng. 2014, 2014, 825910. [Google Scholar] [CrossRef]
- Xu, D.-Y.; Yang, Y.; Yang, Z. Activity and stability of cross-linked tyrosinase aggregates in aqueous and nonaqueous media. J. Biotechnol. 2011, 152, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Mahmod, S.S.; Yusof, F.; Jami, M.S.; Khanahmadi, S. Optimizing the preparation conditions and characterization of a stable and recyclable cross-linked enzyme aggregate (CLEA)-protease. Bioresour. Bioprocess. 2016, 3, 3. [Google Scholar] [CrossRef]
- Liang, W.Q.; Wang, Z.Y.; Li, H.; Wu, P.C.; Hu, J.M.; Luo, N.; Cao, L.X.; Liu, Y.H. Purification and characterization of a novel pyrethroid hydrolase from Aspergillus niger ZD11. J. Agric. Food Chem. 2005, 53, 7415–7420. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, Z.K.; Ahmed, M.A.; Fetyan, N.A.; Elnagdy, S.M. Isolation and molecular characterisation of malathion-degrading bacterial strains from waste water in Egypt. J. Adv. Res. 2010, 1, 145–149. [Google Scholar] [CrossRef]
- Singh, B.; Kaur, J.; Singh, K. Biodegradation of malathion by Brevibacillus sp. strain KB2 and Bacillus cereus strain PU. World J. Microbiol. Biotechnol. 2012, 28, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Eom, G.T.; Song, J.K.; Ahn, J.H.; Seo, Y.S.; Rhee, J.S. Enhancement of the efficiency of secretion of heterologous lipase in Escherichia coli by directed evolution of the abc transporter system. Appl. Environ. Microbiol. 2005, 71, 3468–3474. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Park, S.; Kim, Y.H.; Won, K.; Lee, S.H. Immobilization of formate dehydrogenase from Candida boidinii through cross-linked enzyme aggregates. J. Mol. Catal. B Enzym. 2013, 97, 209–214. [Google Scholar] [CrossRef]
- Lawton, J.M.; Doonan, S. Thermal inactivation and chaperonin-mediated renaturation of mitochondrial aspartate aminotransferase. Biochem. J. 1998, 334, 219. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, S.; Lu, X.; Rao, S.; Kang, Z.; Li, J.; Wang, M.; Chen, J. Thermal inactivation of a recombinant lipoxygenase from Pseudomonas aeruginosa BBE in the absence and presence of additives. J. Sci. Food Agric. 2014, 94, 1753–1757. [Google Scholar] [CrossRef] [PubMed]
- OECD. Guidelines for Testing of Chemicals Simulation Tests-Aerobic Sewage Treatment; Technical Report; Organisation for Economic Co-Operation and Development (OECD): Paris, France, 1996; pp. 19–142. [Google Scholar]
- Piligaev, A.V.; Sorokina, K.N.; Shashkov, M.V.; Parmon, V.N. Screening and comparative metabolic profiling of high lipid content microalgae strains for application in wastewater treatment. Bioresour. Technol. 2018, 250, 538–547. [Google Scholar] [CrossRef] [PubMed]

| Precipitant Type | Recovered Activity, % |
|---|---|
| Acetone | 30.3 ± 2.8 |
| tert-Butanol | 34.5 ± 4.7 |
| Isopropanol | 8.7 ± 1.1 |
| Ethanol | 6.2 ± 0.4 |
| Ammonium sulfate (50%, w/v) | 58.4 ± 4.1 |
| Additives | Relative Activity, % |
|---|---|
| Control 1 | 100.0 ± 4.7 |
| BSA | 154.2 ± 16.5 |
| SDS | 109.7 ± 8.4 |
| Run | Time, h | Ammonium Sulfate, % (w/v) | Glutaraldehyde, mM | BSA, mM | Recovered Activity, % |
|---|---|---|---|---|---|
| 1 | 4.5 | 55 | 25 | 0.105 | 45.1 |
| 2 | 1 | 80 | 25 | 0.2 | 42.0 |
| 3 | 8 | 30 | 25 | 0.01 | 7.5 |
| 4 | 4.5 | 55 | 87.5 | 0.105 | 72.4 |
| 5 | 1 | 80 | 25 | 0.01 | 9.7 |
| 6 | 4.5 | 55 | 87.5 | 0.105 | 71.4 |
| 7 | 4.5 | 55 | 87.5 | 0.2 | 85.4 |
| 8 | 8 | 80 | 25 | 0.01 | 19.8 |
| 9 | 1 | 30 | 150 | 0.01 | 21.8 |
| 10 | 4.5 | 55 | 87.5 | 0.105 | 74.1 |
| 11 | 1 | 30 | 150 | 0.2 | 38.7 |
| 12 | 1 | 80 | 150 | 0.2 | 50.4 |
| 13 | 4.5 | 55 | 87.5 | 0.105 | 65.1 |
| 14 | 8 | 55 | 87.5 | 0.105 | 40.4 |
| 15 | 1 | 30 | 25 | 0.2 | 29.6 |
| 16 | 1 | 80 | 150 | 0.01 | 18.9 |
| 17 | 4.5 | 30 | 87.5 | 0.105 | 43.2 |
| 18 | 4.5 | 80 | 87.5 | 0.105 | 60.3 |
| 19 | 8 | 30 | 150 | 0.01 | 18.2 |
| 20 | 8 | 80 | 150 | 0.01 | 47.2 |
| 21 | 1 | 30 | 25 | 0.01 | 5.3 |
| 22 | 4.5 | 55 | 87.5 | 0.105 | 65.7 |
| 23 | 8 | 30 | 25 | 0.2 | 32.1 |
| 24 | 1 | 55 | 87.5 | 0.105 | 37.2 |
| 25 | 4.5 | 55 | 87.5 | 0.01 | 54.2 |
| 26 | 8 | 30 | 150 | 0.2 | 52.4 |
| 27 | 4.5 | 55 | 87.5 | 0.105 | 70.6 |
| 28 | 8 | 80 | 25 | 0.2 | 51.3 |
| 29 | 8 | 80 | 150 | 0.2 | 70.0 |
| 30 | 4.5 | 55 | 150 | 0.105 | 65.0 |
| Source | Sum of Squares | F-Value | p-Value |
|---|---|---|---|
| Model | 13,771.01 | 33.36 | <0.0001 |
| Time, A | 403.56 | 13.69 | 0.0021 |
| Ammonium sulfate, B | 808.96 | 27.44 | 0.0001 |
| Glutaraldehyde, C | 1090.45 | 36.98 | <0.0001 |
| BSA, D | 3449.76 | 117.00 | <0.0001 |
| AB | 172.27 | 5.84 | 0.0288 |
| AC | 71.83 | 2.44 | 0.1394 |
| AD | 4.00 | 0.14 | 0.7178 |
| BC | 3.28 | 0.11 | 0.7435 |
| BD | 20.48 | 0.69 | 0.4177 |
| CD | 3.59 | 0.12 | 0.7319 |
| A2 | 1451.57 | 49.23 | <0.0001 |
| B2 | 296.06 | 10.04 | 0.0064 |
| C2 | 142.83 | 4.84 | 0.0438 |
| D2 | 138.84 | 4.71 | 0.0465 |
| Lack of fit | 375.04 | 2.79 | 0.1345 |
| Time, h | Ammonium Sulfate, % (w/v) | Glutaraldehyde, mM | BSA, mM | Recovered Activity, % | |
|---|---|---|---|---|---|
| Experimental | Predicted | ||||
| 5.1 | 65.1 | 120.6 | 0.2 | 90.6 ± 2.7 | 91.3 |
| Temperature, °C | ki, h−1 | t1/2, h | ||
|---|---|---|---|---|
| estUT1 | CLEA-estUT1 | estUT1 | CLEA-estUT1 | |
| 50 | 4.3 × 10−2 | 2.2 × 10−2 | 16.1 | 31.3 |
| 60 | 4.5 × 10−2 | 3.1 × 10−2 | 15.4 | 22.5 |
| 70 | 6.0 × 10−2 | 4.7 × 10−2 | 11.5 | 14.8 |
| 80 | 22.7 × 10−2 | 16.5 × 10−2 | 3.1 | 4.2 |
| Substrate | Km, μM | kcat, s−1 | kcat/Km, s−1 mM−1 | |||
|---|---|---|---|---|---|---|
| estUT1 | CLEA-estUT1 | estUT1 | CLEA-estUT1 | estUT1 | CLEA-estUT1 | |
| pNPC2 | 96.0 ± 5.3 | 176.7 ± 8.5 | 27.0 ± 0.7 | 31.1 ± 1.2 | 281.3 ± 8.7 | 176.1 ± 2.2 |
| pNPC4 | 550.0 ± 78.1 | 710.7 ± 64.7 | 39.2 ± 1.2 | 41.8 ± 0.4 | 72.1 ± 8.8 | 59.1 ± 5.6 |
| pNPC8 | 2113.3 ± 180.4 | 2490.0 ± 141.8 | 54.1 ± 2.0 | 56.9 ± 1.2 | 25.2 ± 1.3 | 22.9 ± 1.1 |
| Malathion | 19.5 ± 1.1 | 29.6 ± 3.3 | 16.3 ± 0.3 | 15.2 ± 1.0 | 835.5 ± 58.7 | 515.4 ± 22.8 |
| Biocatalyst | Experimental Condition | Removal of Malathion, % | Reference |
|---|---|---|---|
| CLEA-estUT1 (U. thermosphaericus) | Malathion concentration = 27.5 mg L−1 Temperature = 37 °C Time = 14 h pH = 7.0 rpm = 250 | 99.4 ± 2.8 | This study |
| CLEA-estUT1 (U. thermosphaericus) | Malathion concentration = 27.5 mg L−1 Temperature = 37 °C Time = 14 h Municipal wastewater (MWW) rpm = 250 | 99.5 ± 1.4 | This study |
| Bacillus sp. S14 immobilized in 3% calcium alginate | Malathion concentration = 50 mg L−1 Temperature = 25 °C Time = 8 h pH = 7.0 rpm = 120 | 64.4 | [15] |
| B. licheniformis strain ML-1 | Malathion concentration = 25 mg L−1 Temperature = 32 °C Time = 5 days pH = 7.5 rpm = 250 | 78 | [8] |
| Carboxylesterase D1CarE5 (A. tengchongensis) | Malathion concentration = 5 mg L−1 Temperature = 37 °C Time = 100 min pH = 7.0 | 89 | [11] |
| Bacillus thuringiensis | Malathion concentration = 250 mg L−1 Temperature = 30 °C Time = 3 days | 50 | [53] |
| Bacillus cereus strain PU | Malathion concentration = 72.5 mg L−1 Temperature = 30 °C Time = 7 days pH = 7.0 | 49.31 | [54] |
| P. putida | Malathion concentration = 125 mg L−1 Temperature = 30 °C Time = 7 days pH = 7.0 rpm = 150 | 72 | [5] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samoylova, Y.V.; Sorokina, K.N.; Piligaev, A.V.; Parmon, V.N. Preparation of Stable Cross-Linked Enzyme Aggregates (CLEAs) of a Ureibacillus thermosphaericus Esterase for Application in Malathion Removal from Wastewater. Catalysts 2018, 8, 154. https://doi.org/10.3390/catal8040154
Samoylova YV, Sorokina KN, Piligaev AV, Parmon VN. Preparation of Stable Cross-Linked Enzyme Aggregates (CLEAs) of a Ureibacillus thermosphaericus Esterase for Application in Malathion Removal from Wastewater. Catalysts. 2018; 8(4):154. https://doi.org/10.3390/catal8040154
Chicago/Turabian StyleSamoylova, Yuliya V., Ksenia N. Sorokina, Alexander V. Piligaev, and Valentin N. Parmon. 2018. "Preparation of Stable Cross-Linked Enzyme Aggregates (CLEAs) of a Ureibacillus thermosphaericus Esterase for Application in Malathion Removal from Wastewater" Catalysts 8, no. 4: 154. https://doi.org/10.3390/catal8040154
APA StyleSamoylova, Y. V., Sorokina, K. N., Piligaev, A. V., & Parmon, V. N. (2018). Preparation of Stable Cross-Linked Enzyme Aggregates (CLEAs) of a Ureibacillus thermosphaericus Esterase for Application in Malathion Removal from Wastewater. Catalysts, 8(4), 154. https://doi.org/10.3390/catal8040154




