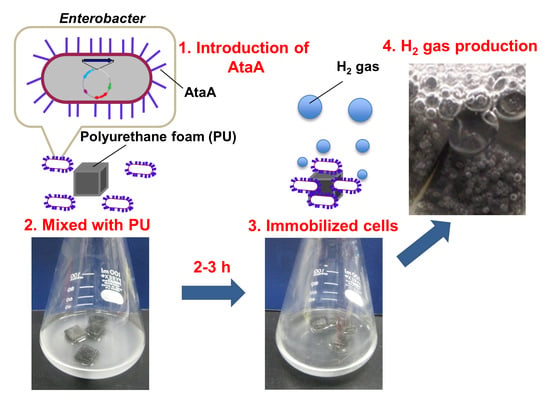
Immobilization of Enterobacter aerogenes by a Trimeric Autotransporter Adhesin, AtaA, and Its Application to Biohydrogen Production
Abstract
1. Introduction
2. Results
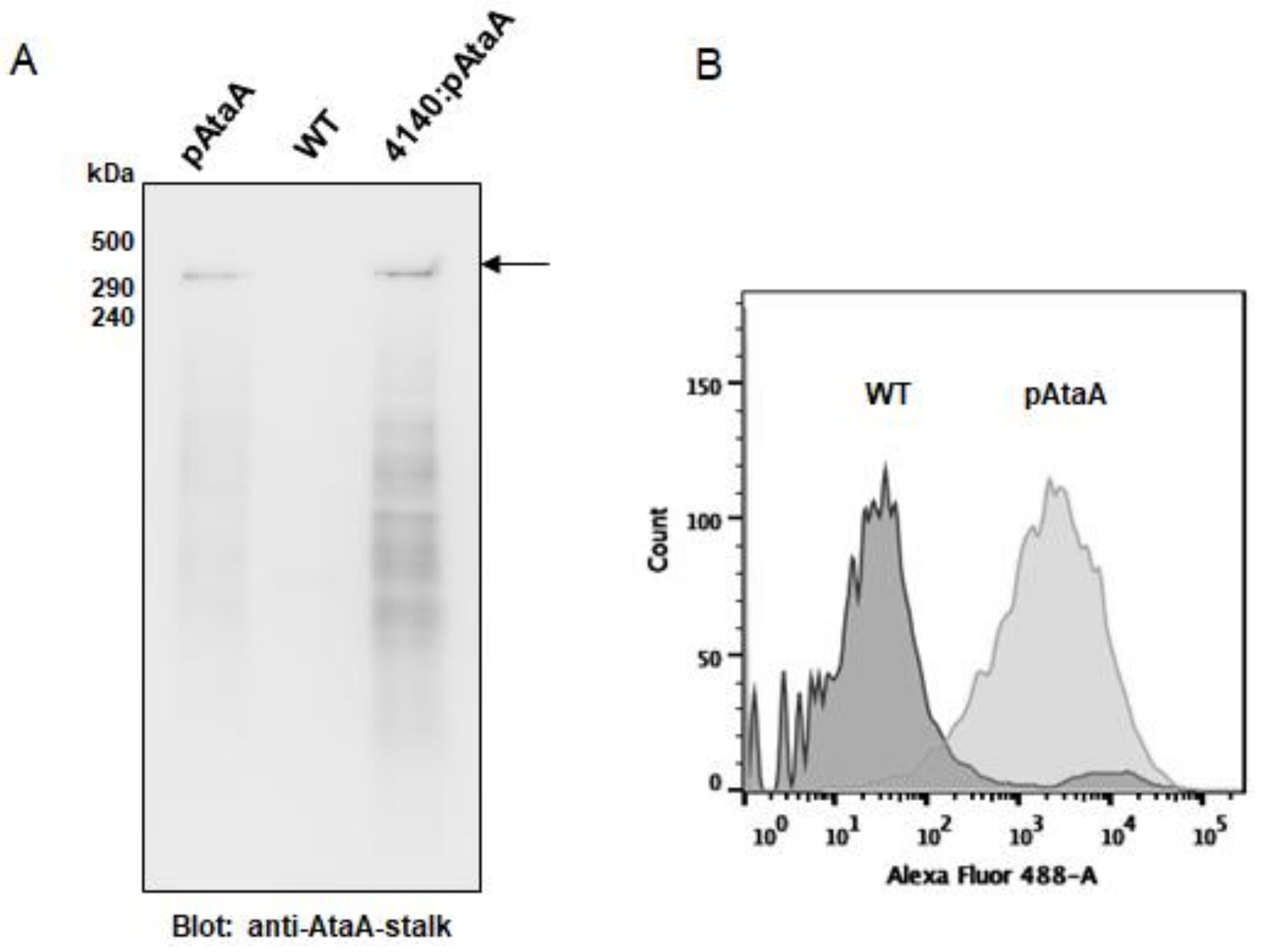
2.1. Production of AtaA on the Cell Surface of E. aerogenes
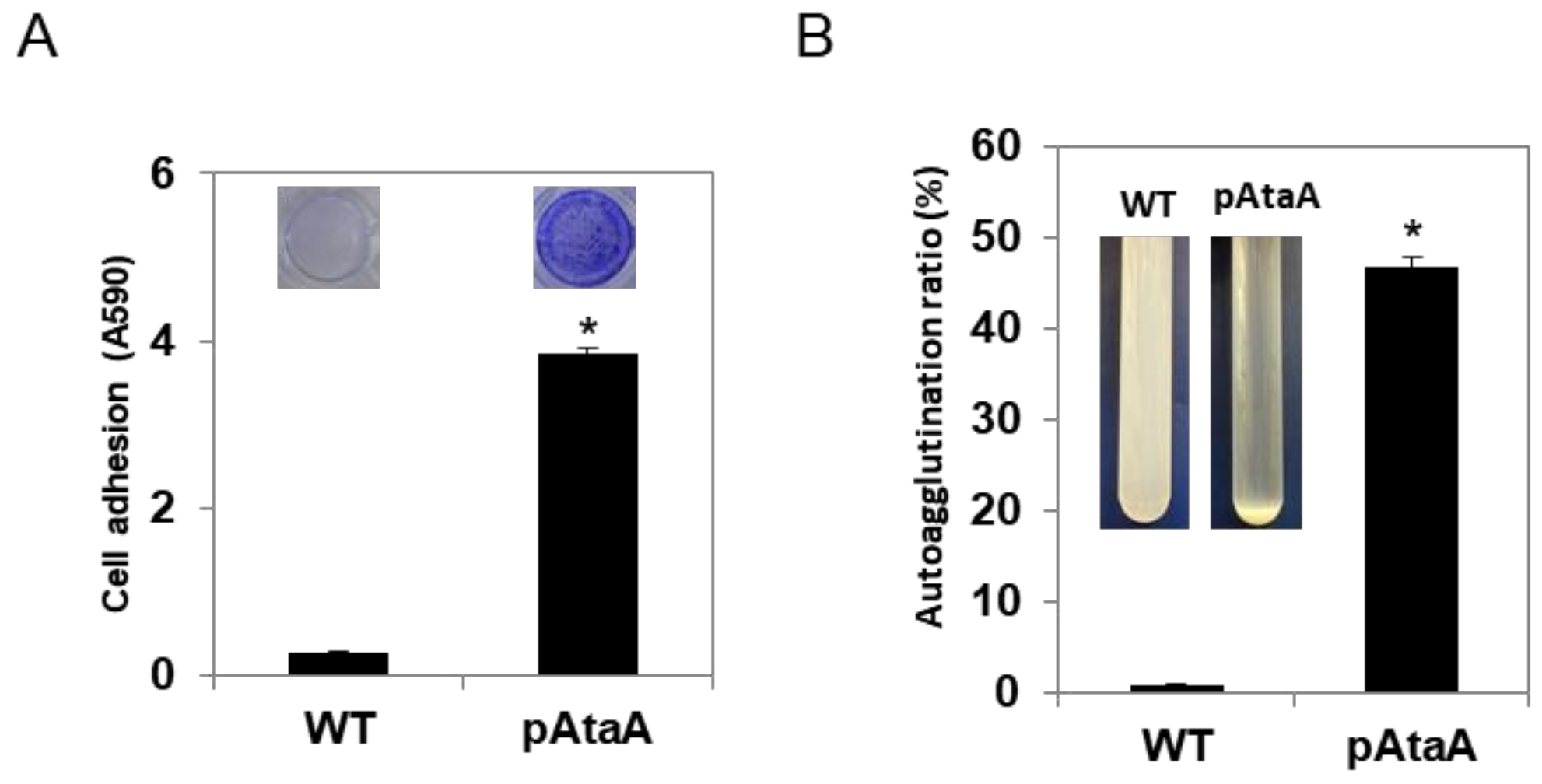
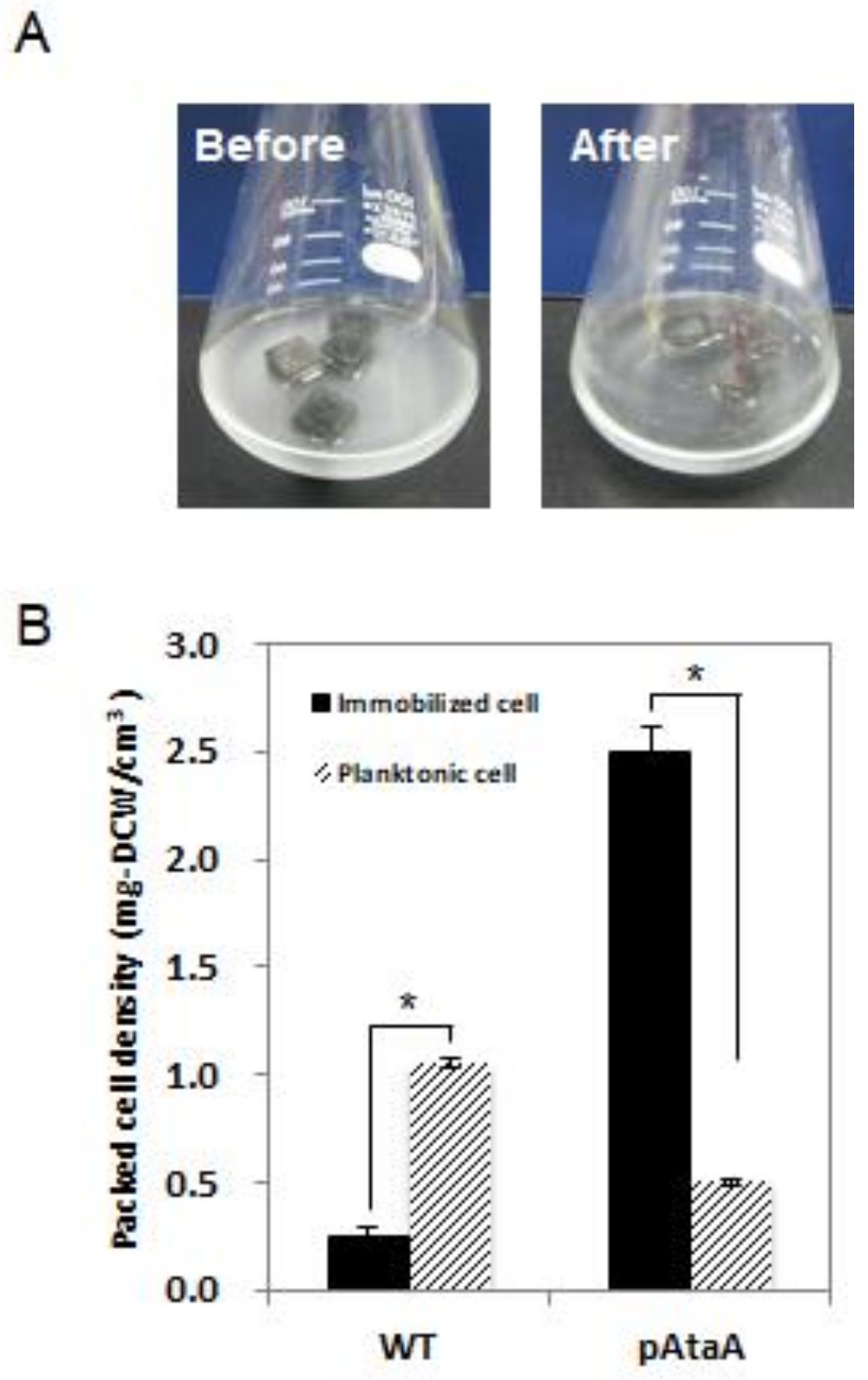
2.2. AtaA-Mediated Immobilization of E. aerogenes
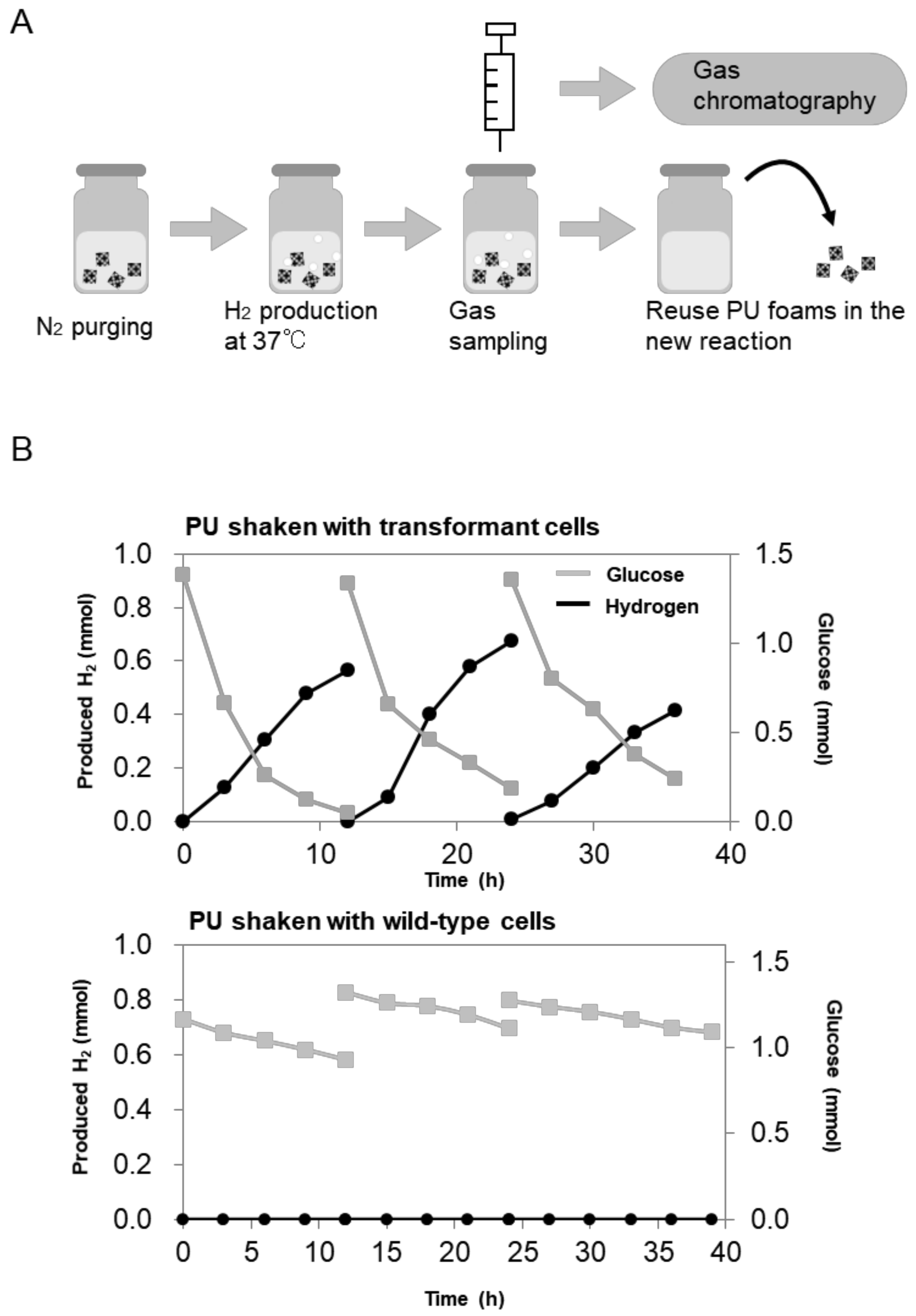
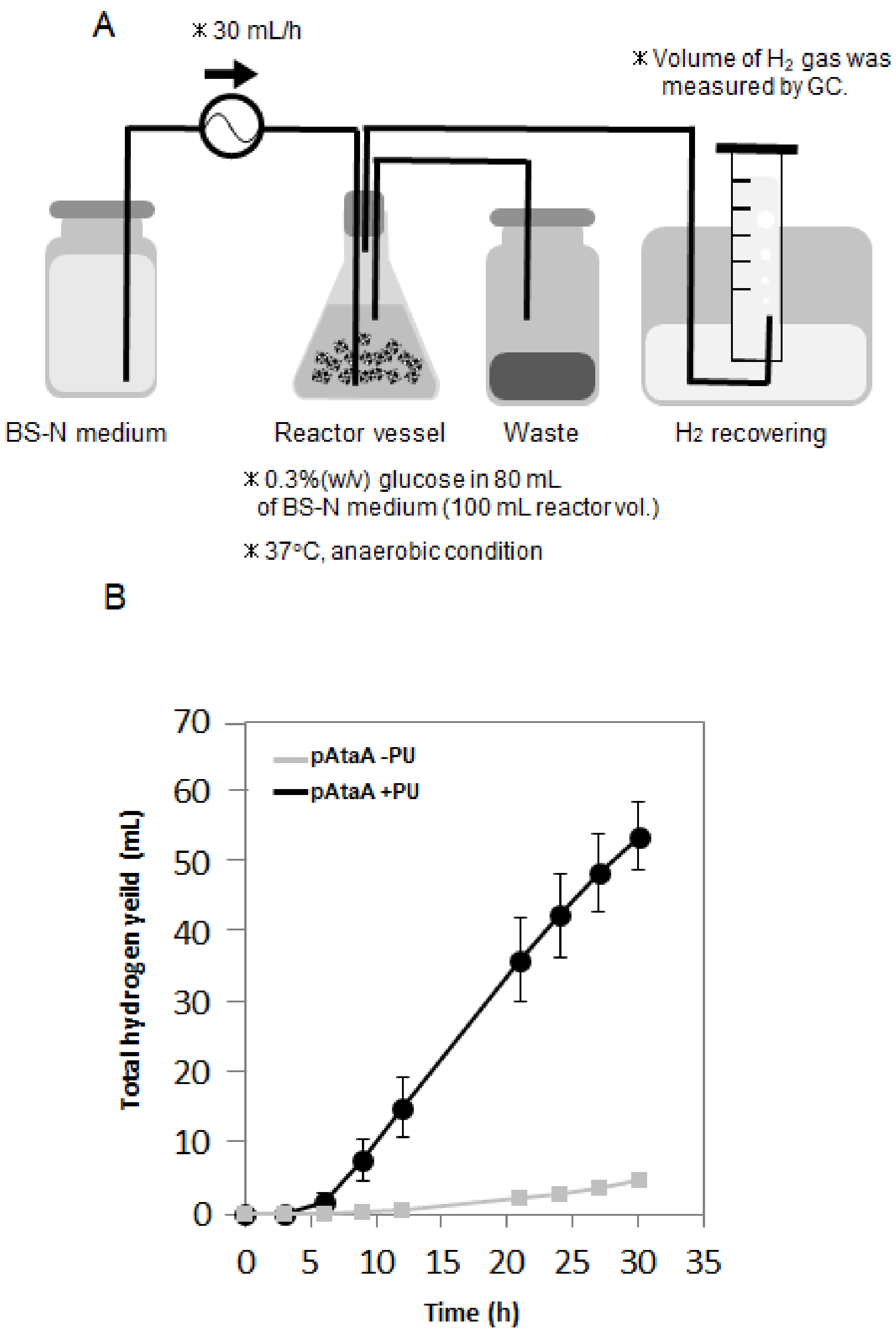
2.3. Repetitive and Continuous Hydrogen Production by Immobilized E. aerogenes
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Plasmid Construct, Bacterial Strains, and Culture Conditions
4.3. Transformation of E. aerogenes and Isolation of the Transformants
4.4. Analysis of AtaA Production by E. aerogenes Transformants
4.5. Cell Immobilization on Polyurethane
4.6. Hydrogen Production by the Immobilized Cells
4.7. Quantification of H2 Production by Gas Chromatography Coupled to a Thermal Conductivity Detector (GC–TCD)
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mudhoo, A.; Forster-Carneiro, T.; Sánchez, A. Biohydrogen production and bioprocess enhancement: A review. Crit. Rev. Biotechnol. 2011, 31, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Kalinci, Y.; Hepbasli, A.; Dincer, I. Biomass-based hydrogen production: A review and analysis. Int. J. Hydrog. Energy 2009, 34, 8799–8817. [Google Scholar] [CrossRef]
- Fang, H.H.P.; Liu, H.; Zhang, T. Bio-hydrogen production from wastewater. Water Sci. Technol. Water Supp. 2004, 4, 77–85. [Google Scholar]
- Kumar, N.; Das, D. Continuous hydrogen production by immobilized Enterobacter cloacae IIT-BT 08 using lignocellulosic materials as solid matrices. Enzyme Microb. Technol. 2001, 29, 280–287. [Google Scholar] [CrossRef]
- Tanisho, S.; Ishiwata, Y. Continuous hydrogen production from molasses by the bacterium Enterobacter aerogenes. Int. J. Hydrog. Energy 1994, 19, 807–812. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, Y.; Chen, Y.; Zhu, S.; Shen, S. Hydrogen production and wastewater treatment in a microbial electrolysis cell with a biocathode. Water Environ. Res. 2014, 86, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Miyake, J. Hydrogen Production Using Photosynthetic Bacteria. In Microbial Production; Anazawa, H., Shimizu, S., Eds.; Springer: Tokyo, Japan, 2014; pp. 263–281. ISBN 978-4-431-54606-1. [Google Scholar]
- Logan, B.E.; Call, D.; Cheng, S.; Hamelers, H.V.; Sleutels, T.H.; Jeremiasse, A.W.; Rozendal, R.A. Microbial electrolysis cells for high yield hydrogen gas production from organic matter. Environ. Sci. Technol. 2008, 42, 8630–8640. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-J.; Show, K.-Y.; Su, A. Dark fermentation on biohydrogen production: Pure culture. Bioresour. Technol. 2011, 102, 8393–8402. [Google Scholar] [CrossRef] [PubMed]
- Tanisho, S.; Wakao, N.; Kosako, Y. Biological hydrogen production by Enterobacter aerogenes. J. Chem. Eng. Jpn. 1983, 16, 529–530. [Google Scholar] [CrossRef][Green Version]
- Ito, T.; Nakashimada, Y.; Kakizono, T.; Nishio, N. High-yield production of hydrogen by Enterobacter aerogenes mutants with decreased a-acetolactate synthase activity. J. Biosci. Bioeng. 2004, 97, 227–232. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, J.; Liu, Z.; Ren, Y.; Li, G. Hydrogen production from the monomeric sugars hydrolyzed from hemicellulose by Enterobacter aerogenes. Renew. Energy 2009, 34, 2774–2779. [Google Scholar] [CrossRef]
- Argun, H.; Kargi, F. Bio-hydrogen production by different operational modes of dark and photo-fermentation: An overview. Int. J. Hydrog. Energy 2011, 36, 7443–7459. [Google Scholar] [CrossRef]
- Bagai, R.; Madamwar, D. Prolonged evolution of photohydrogen by intermittent supply of nitrogen using a combined system of Phormidium valderianum, Halobacterium halobium, and Escherichia coli. Int. J. Hydrog. Energy 1998, 23, 545–550. [Google Scholar] [CrossRef]
- Chang, J.-S.; Lee, K.-S.; Lin, P.-J. Biohydrogen production with fixed-bed bioreactors. Int. J. Hydrog. Energy 2002, 27, 1167–1174. [Google Scholar] [CrossRef]
- Liu, Z.D.; Lv, F.X.; Zheng, H.; Zhang, C.; Wei, F.; Xing, X.H. Enhanced hydrogen production in a UASB reactor by retaining microbial consortium onto carbon nanotubes (CNTs). Int. J. Hydrog. Energy 2012, 37, 10619–10626. [Google Scholar] [CrossRef]
- Tanisho, S.; Ishiwata, Y. Continuous hydrogen production from molasses by fermentation using urethane foam as a support of flocks. Int. J. Hydrog. Energy 1995, 20, 541–545. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Lin, C.-N.; Chang, J.-S.; Lee, K.-S.; Lin, P.-J. Microbial hydrogen production with immobilized sewage sludge. Biotechnol. Prog. 2002, 18, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, H.; Maeda, Y.; Hirose, J.; Hayashi, S.; Takasaki, Y. H2 production by immobilized cells of Clostridium butyricum on porous glass beads. Biotechnol. Tech. 1997, 11, 431–433. [Google Scholar] [CrossRef]
- Ishikawa, M.; Nakatani, H.; Hori, K. AtaA, a new member of the trimeric autotransporter adhesins from Acinetobacter sp. Tol 5 mediating high adhesiveness to various abiotic surfaces. PLoS ONE 2012, 7, e48830. [Google Scholar] [CrossRef] [PubMed]
- Hori, K.; Yamashita, S.; Ishii, S.; Kitagawa, M.; Tanji, Y.; Unno, H. Isolation, characterization and application to off-gas treatment of toluene-degrading bacteria. J. Chem. Eng. Jpn. 2001, 34, 1120–1126. [Google Scholar] [CrossRef]
- Hori, K.; Ishikawa, M.; Yamada, M.; Higuchi, A.; Ishikawa, Y.; Ebi, H. Production of peritrichate bacterionanofibers and their proteinaceous components by Acinetobacter sp. Tol 5 cells affected by growth substrates. J. Biosci. Bioeng. 2011, 111, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Unno, H.; Miyata, S.; Hori, K. Effect of cell appendages on the adhesion properties of a highly adhesive bacterium, Acinetobacter sp. Tol 5. Biosci. Biotechnol. Biochem. 2006, 70, 2635–2640. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ishikawa, M.; Shigemori, K.; Suzuki, A.; Hori, K. Evaluation of adhesiveness of Acinetobacter sp. Tol 5 to abiotic surfaces. J. Biosci. Bioeng. 2012, 113, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Koki, J.; Unno, H.; Hori, K. Two morphological types of cell appendages on a strongly adhesive bacterium, Acinetobacter sp. Strain Tol 5. Appl. Environ. Microbiol. 2004, 70, 5026–5029. [Google Scholar] [CrossRef] [PubMed]
- Hoiczyk, E.; Roggenkamp, A.; Reichenbecher, M.; Lupas, A.; Heesemann, J. Structure and sequence analysis of Yersinia YadA and Moraxella UspAs reveal a novel class of adhesins. EMBO J. 2000, 19, 5989–5999. [Google Scholar] [CrossRef] [PubMed]
- Linke, D.; Riess, T.; Autenrieth, I.B.; Lupas, A.; Kempf, V.A.J. Trimeric autotransporter adhesins: Variable structure, common function. Trends Microbiol. 2006, 14, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Hori, K.; Ohara, Y.; Ishikawa, M.; Nakatani, H. Effectiveness of direct immobilization of bacterial cells onto material surfaces using the bacterionanofiber protein AtaA. Appl. Microbiol. Biotechnol. 2015, 99, 5025–5032. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Shigemori, K.; Hori, K. Application of the adhesive bacterionanofiber AtaA to a novel microbial immobilization method for the production of indigo as a model chemical. Biotechnol. Bioeng. 2014, 111, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.H.; Jun, B.H.; Yanagida, M.; Tanji, Y.; Unno, H. Effect of C/N values on microbial simultaneous removal of carbonaceous and nitrogenous substances in wastewater by single continuous-flow fluidized-bed bioreactor containing porous carrier particles. Biochem. Eng. J. 2000, 5, 29–37. [Google Scholar] [CrossRef]
- Guo, W.; Ngo, H.-H.; Dharmawan, F.; Palmer, C.G. Roles of polyurethane foam in aerobic moving and fixed bed bioreactors. Bioresour. Technol. 2010, 101, 1435–1439. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Wang, Y.; Wang, T.; Zheng, H.; Chu, L.; Zhang, C.; Chen, H.; Kong, X.; Xing, X.-H. Effects of packing rates of cubic-shaped polyurethane foam carriers on the microbial community and the removal of organics and nitrogen in moving bed biofilm reactors. Bioresour. Technol. 2012, 117, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, S.; Ohara, Y.; Nakatani, H.; Hori, K. Reversible bacterial immobilization based on the salt-dependent adhesion of the bacterionanofiber protein AtaA. Microb. Cell Fact. 2017, 16. [Google Scholar] [CrossRef] [PubMed]
- Thai, K.; Choi, J.; Franzin, C.M.; Marassi, F.M. Bcl-xl as a fusion protein for the high-level expression of membrane-associated proteins. Protein Sci. 2005, 14, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Diefenderfer, C.; Lee, J.; Mlyanarski, S.; Guo, Y.; Glover, K.J. Reliable expression and purification of highly insoluble transmembrane domains. Anal. Biochem. 2009, 384, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Laage, R.; Langosch, D. Strategies for prokaryotic expression of eukaryotic membrane proteins. Traffic 2001, 2, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Satar, I.; Ghasemi, M.; Aljlil, S.A.; Isahak, W.N.R.W.; Abdalla, A.M.; Alam, J.; Daud, W.R.W.; Yarmo, M.A.; Akbarzadeh, O. Production of hydrogen by Enterobacter aerogenes in an immobilized cell reactor. Int. J. Hydrog. Energy 2017, 42, 9024–9030. [Google Scholar] [CrossRef]
- Yokoi, H.; Tokushige, T.; Hirose, J.; Hayashi, S.; Takasaki, Y. Hydrogen production by immobilized cells of aciduric Enterobacter aerogenes strain HO-39. J. Ferment. Bioeng. 1997, 83, 481–484. [Google Scholar] [CrossRef]
- Palazzi, E.; Fabiano, B.; Perego, P. Process development of continuous hydrogen production by Enterobacter aerogenes in a packed column reactor. Bioprocess Eng. 2000, 22, 205–213. [Google Scholar] [CrossRef]
- Lu, Y.; Lai, Q.; Zhang, C.; Zhao, H.; Xing, X.-H. Alteration of energy metabolism in Enterobacter aerogenes by external addition of pyrophosphates and overexpression of polyphosphate kinase for enhanced hydrogen production. J. Chem. Technol. Biotechnol. 2012, 87, 996–1003. [Google Scholar] [CrossRef]
- Zhang, C.; Lv, F.-X.; Xing, X.-H. Bioengineering of the Enterobacter aerogenes strain for biohydrogen production. Bioresour. Technol. 2011, 102, 8344–8349. [Google Scholar] [CrossRef] [PubMed]
- Goyal, Y.; Kumar, M.; Gayen, K. Metabolic engineering for enhanced hydrogen production: A review. Can. J. Microbiol. 2012, 59, 59–78. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Hori, K. A new simple method for introducing an unmarked mutation into a large gene of non-competent gram-negative bacteria by FLP/FRT recombination. BMC Microbiol. 2013, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.; Priefer, U.; Pühler, A. A broad host range mobilization system for in vivo genetic engineering: Transposon mutagenesis in gram negative bacteria. Nat. Biotechnol. 1983, 1, 784–791. [Google Scholar] [CrossRef]
- Simmons, J.S. A culture medium for differentiating organisms of typhoid-colon aerogenes groups and for isolation of certain fungi. J. Infect. Dis. 1926, 39, 209–214. [Google Scholar] [CrossRef]
| Biocatalyst | Immobilization Method | Reactor Type | Feed Stock | Production Rate | Conversion Rate | Advantage/ Disadvantage | Reference |
|---|---|---|---|---|---|---|---|
| Enterobacter aerogenes IAM1183 | Adhesion of AtaA protein on polyurethane foam | CSTR in Erlenmeyer flask | Glucose | 42 mL·h−1·L−1 (1.87 mmol·h−1·L−1) | 0.6 mol/mol glucose (Batch) | Immediate and direct immobilization (2–3 h)/ Low production rate | This study |
| Enterobacter aerogenes ATCC 13048 | Gel entrupment in alginate gel | Packed-bed tubular column reactor | Glucose | n/d | 9.44 mmol/g glucose (1.7 mol/mol glucose) | High conversion rate/ Complex immobilization processes | [37] |
| Enterobacter aerogenes HO-39 | Adsorptionon porous glass beads | Packed-bed column reactor | Glucose | 850 mL·h−1·L−1 (37 mmol·h−1·L−1) | 0.73 mol/mol glucose | High production rate/ complex immobilization processes | [38] |
| Enterobacter cloacae IIT-BT 08 | Biofilm formation on Lignocellulosic agroresidues | Packed-bed reactor | Glucose | 75.6 mmol·h−1·L−1 | n/d | High production rate/ Long setup time (20 h) | [4] |
| Enterobacter aerogenes NCIMB 10102 | Biofilm formation on synthetic sponge | Packed-bed column reactor | Glucose | 10.2 mmol·h−1·L−1 | 1.36 to 3.02 mmol/mmol glucose | High production and conversion rate/ Long setup time | [39] |
| Enterobacter aerogenes E.82005 | Biofilm formation on polyurethane foam | Hand-made fermenter | Molasses from a sugar refinery | 13 mmol·h−1·L-culture−1 | 1.5–3.5 mol/mol sugar | High production and conversion rate/ Long setup time (14 days) | [17] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakatani, H.; Ding, N.; Ohara, Y.; Hori, K. Immobilization of Enterobacter aerogenes by a Trimeric Autotransporter Adhesin, AtaA, and Its Application to Biohydrogen Production. Catalysts 2018, 8, 159. https://doi.org/10.3390/catal8040159
Nakatani H, Ding N, Ohara Y, Hori K. Immobilization of Enterobacter aerogenes by a Trimeric Autotransporter Adhesin, AtaA, and Its Application to Biohydrogen Production. Catalysts. 2018; 8(4):159. https://doi.org/10.3390/catal8040159
Chicago/Turabian StyleNakatani, Hajime, Nan Ding, Yuki Ohara, and Katsutoshi Hori. 2018. "Immobilization of Enterobacter aerogenes by a Trimeric Autotransporter Adhesin, AtaA, and Its Application to Biohydrogen Production" Catalysts 8, no. 4: 159. https://doi.org/10.3390/catal8040159
APA StyleNakatani, H., Ding, N., Ohara, Y., & Hori, K. (2018). Immobilization of Enterobacter aerogenes by a Trimeric Autotransporter Adhesin, AtaA, and Its Application to Biohydrogen Production. Catalysts, 8(4), 159. https://doi.org/10.3390/catal8040159