Abstract
In this study, two simple Schiff base copper complexes [Cu(H2O)2(HL)]·2H2O (Complex 1) (H3L = 2-OH-4-(OH)-C6H2CH=NCH2CO2H) and [Cu(py)2(HL)] (Complex 2) (Py = pyridine) were initially achieved and authenticated by single-crystal X-ray structure analyses (SXRD), powder X-ray diffraction analyses (PXRD), FT-IR spectroscopy, and elemental analyses. The SXRD reveals that the Cu2+ center in Complex 1 exhibited a distorted square pyramidal geometry, which is constructed based on phenolate oxygen, water molecules, carboxylate oxygen, and imine nitrogen from a deprotonated H3L ligand in an NO4 fashion. The Cu2+ atom in Complex 2 had distorted square pyramidal geometry, and was coordinated with two pyridine molecules and one Gly-Schiff base ligand, exhibiting an N3O2 binding set. Additionally, the free water molecules in Complex 1 linked independent copper complexes by intermolecular hydrogen bond to form a 2D framework. However, the one-dimensional chain supramolecular structure of Complex 2 was formed by the intermolecular O–H…O hydrogen bonds. The oxygen reduction performance of the two complexes was analyzed by cyclic voltammetry (CV) and the rotating disk electrode (RDE) method. Both complexes could catalyze the conversion of oxygen to water through a predominant four-electron pathway, and the Cu–NxOy moieties might be the functional moieties for the catalytic activity. The catalytic pathways and underlying mechanisms are also discussed in detail, from which the structure–activity relationship of the complexes was obtained.
1. Introduction
In recent decades, studies on coordination compounds have been well developed, and the developed organometallic complexes have been widely used in many fields, such as the catalysts used in the chemical and petrochemical industries [1], the molecular recognition reagents used in analytical measurements [2], and the oxygen reduction catalysts used in fuel cells [3]. Most noticeably, many new fields have been developed, such as macrocyclic coordination compounds [4], supramolecular complexes [5], metal-organic frameworks [3], and functional complexes [5]. In addition, the application of these materials as the electrocatalysts used in fuel cells have attracted a great deal of attention from both the scientific and the industrial societies due to the possibilities of replacing the precious metal platinum as the cathodic catalyst [3,4,6], ultimately endowing upon the fuel cell, a type of clean energy device, more market competitiveness than other types of energy devices.
Pt free M–Nx/C containing materials has been considered as the alternative to Pt/C catalysts, and the studies on this field began to flourish since the discovery of the oxygen reduction reaction (ORR) capacity of some transition metal macrocycles as early as the 1960s [4]. The macrocycles used for the preparation of metal-macrocycles include phthalocyanine [7], porphyrin [8], and corrole [4], and the metal used for the complexes include Fe, Co, or Mn. The ORR activities of the metal-macrocycles can usually be enhanced by dispersing them on a variety of carbon materials with high surface areas due to the improved conductivity and the specific surface area [9]. In some studies, it has been shown that the types of central metal ions and macrocyclic compounds, as well as the peripherally substituted groups on macrocyclic compounds, greatly affect the electrocatalytic activity of the catalysts [10]. Gross Zeev summarized a variety of metallocorroles as ORR catalysts in a review and presented the effects of the metal center and the side substituent of the macrocycles on the performance of the electrocatalysts [10].
Metal organic frameworks (MOFs) have been considered as another type of organometallic complexes, which could be used as an ORR catalyst [3]. The innate advantages of the structural modification of MOFs render great potentiality for further improving the ORR activities of the materials, such as, the hierarchical structure, tunable molecular framework, the tailored channels, and the pores for finely regulating the reactions. However, the apparent drawback of MOFs is the unsatisfactory ORR activities caused by the low conductivity of this kind of material [3]. The MOFs/carbon composite, formed between MOFs and carbon materials in a certain way, especially by the hydrothermal process, could improve the conductivity of the catalyst and consequently lead to improved catalytic abilities in the composite catalysts [3].
As a transitional metal, copper ion has often been used as the coordination ion for the construction of organic Cu complexes, some of which have shown ORR activities, including the complexes formed between Cu and macrocyclic compounds, amino-alkyl ligands, substituted triazoles, and aromatic N-donor ligands [6]. The major concerns from the industrial society lie in the feasibility of integrating high performance with low cost, or the proper compromise between the two factors, consequently presenting a stunning opportunity for industrial applications. A simple organic complex, simple in structure and low in cost, can also facilitate study on the relationships between the property of the complex and its structure.
Schiff-base complexes have simpler structures than that of MOFs and have received intensive attention owing to their potentially diverse applications in many fields including ORR catalysts [11,12]. Some copper Schiff-based complexes could form adducts with dioxygen and may perform ORR activity via charge transfer under specific conditions [12]. The ORR performance of the complex is intimately related with the structure of the ligands and the electronic factors of the whole complex [6].
In the present study, two simple Schiff-base complexes were obtained by taking 2,4-dihydroxybenzaldehyde, pyridine, etc., as Schiff base ligands, which are available in mass quantities with low costs. The obtained complexes including Complexes 1 and 2 were characterized by SXRD, PXRD, FT-IR spectroscopy, and element analyses. Carbon powder was used as the support material for the preparation of the complex/carbon composite type ORR catalysts, as the method used for the preparation of MOFs/carbon composites [12]. The electrochemical and ORR performances of the complexes have been studied in detail, and the results show that the number of nitrogen atoms in the complexes (Cu–NxOy) is not a key factor in influencing the catalytic performance of the catalysts. When replacing water molecules with pyridine units in Complex 2, the ORR activity of Complex 2 was slightly different from that of Complex 1. The underlying mechanism is herein discussed.
2. Discussion
2.1. SXRD Analysis
The crystal structure and polyhedral geometry of Complexes 1–2 are shown in Figure 1, Figure 2, Figure 3 and Figure 4. Crystallographic data and selected bond distances and bond angles for Complexes 1 and 2 are listed in Table 1 and Table 2, respectively. As the Schiff base copper Complexes 1 and 2 have similar structure motifs, only the structure of Complex 1 is described in detail here. Complex 1 was prepared via the reaction of 4-hydroxysalicylaldehyde with glycine, and the molecular structure of the copper complex was determined by SXRD. SXRD analysis revealed that the copper (II) atom is five-coordinated in the form of a distorted square pyramidal geometry (Figure 3 and Figure 4). The basal plane of the square pyramid is occupied by the phenolic oxygen atom (O1), the carboxyl oxygen (O3), the nitrogen atom (N1) of the imine group, and one terminal O atom (O5) of coordinated water molecule, and the apical O atom (O6) of a neighboring molecule is used to complete the coordination. The Cu−O and Cu−N bond distances [Cu1-O1 = 1.916(5) Å; Cu1-O3 = 1.964(6) Å; Cu1-N1 = 1.923(7) Å; Cu1-O5 = 1.971(6) Å] lie within the acceptable range and are comparable to those in other related complexes [CuL2(ClO4)](ClO)4; where HL2 = 5-methyl-3-(5-hydroxyhexahydro-2-pyrimidyl)pyrazole [13] and [Cu(L1)] (H2L1) = 2,3-bis((2-hydroxybenzylidene)amino)-2,3-butenedinitrile) [14]. The apical Cu1-O6 bond length is 2.378(7) Å, which is a little longer than that of the similar Schiff base copper complexes [15]. It is noted that the copper (II) atom, the phenolic oxygen atom and carboxyl oxygen atom is nearly linear, which shows the bent coordination mode with the metal atom (the bond angles of O1-Cu1-O3, N1-Cu1-O3, and O1-Cu1-N1 are 178.6(2)°, 83.3(3)°, and 95.3(3)°, respectively). Meanwhile, the phenyl ring (C1–C6) and the C1/C6/C7/N1/O1/Cu1 chelating ring are almost coplanar, with a small dihedral angle of 1.47(2)°.
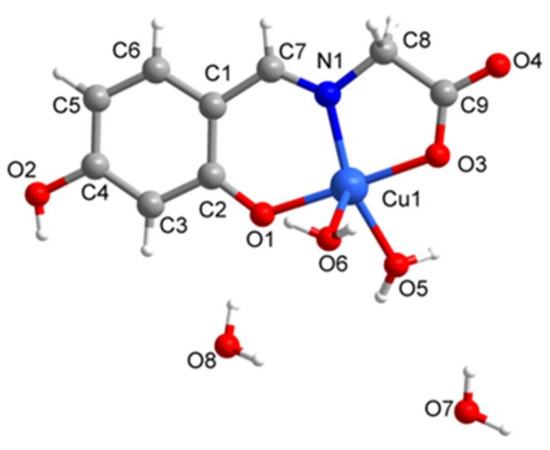
Figure 1.
The crystal structure of Complex 1.
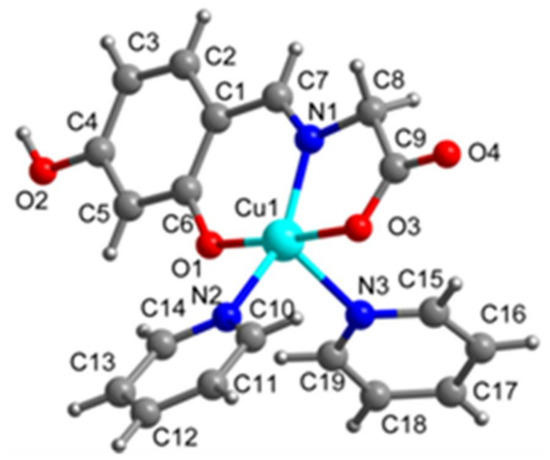
Figure 2.
The crystal structure of Complex 2.
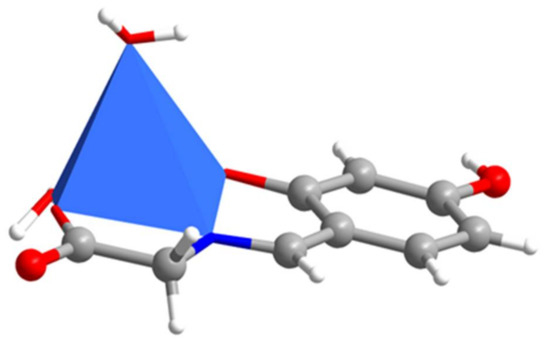
Figure 3.
The polyhedron of Complex 1.
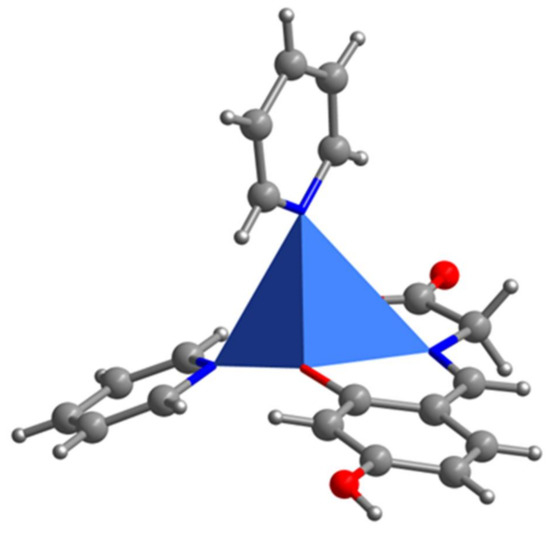
Figure 4.
The polyhedron of Complex 2.

Table 1.
Crystallographic data for Complexes 1 and 2. GOOF: Goodness of fit test; CCDC: Cambridge Crystallographic Data Centre.

Table 2.
Selected bond distances (Å) and bond angles (°) for Complexes 1 and 2.
Strikingly, in Complex 1, weak O–H…O hydrogen-bonding interactions of the phenolic O–H groups with the uncoordinated carboxylate O atoms of the adjacent molecules [O2…O4 = 2.665 Å] are important to stabilize a one-dimensional polymeric chain. Moreover, these 1D chains are further supported by the intermolecular O–H…O hydrogen bonds between free water molecules and the copper (II) complexes [O1…O8 = 2.779 Å; O4…O6 = 2.796 Å; O3…O7 = 2.802 Å] (Figure 5a). Finally, a 2D supramolecular framework (Figure 5b) can be formed by intermolecular hydrogen-bonding interactions (O6…O8…O7) arising from a short water chain from the free water and coordinated water molecules [O6…O8 = 2.772 Å; O8…O7 = 2.803 Å].
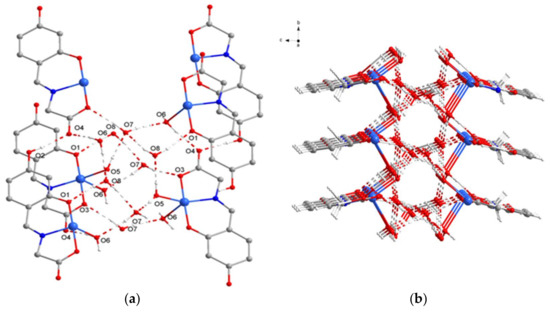
Figure 5.
(a) The intermolecular O–H…O hydrogen bonds; (b) Representation of the 2D topologies of Complex 1. Hydrogen bonds are shown as dashed lines.
As depicted in Figure 2, the crystal structure of Complex 2 consists of one centrosymmetric copper(II) atom, which is five-coordinated in the form of a slightly distorted square pyramidal geometry (Figure 4). The corresponding bond lengths of Cu–O and Cu–N are in agreement with those of the previously reported copper complexes [16]. The supramolecular one-dimensional chain structure of Complex 2 is formed by an intermolecular interaction of an O–H...O hydrogen bond and the discrete copper (II) unit (Figure 6).
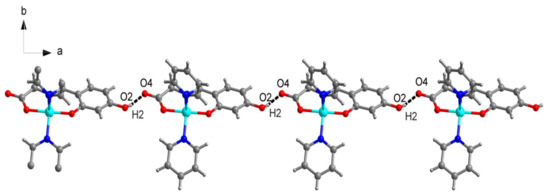
Figure 6.
A representation of the one-dimensional chains of Complex 2 via the a–b plane.
2.2. Infrared Spectroscopy (IR) Analysis
Fourier transform infrared spectroscopy (FT-IR) spectroscopy can provide valuable information for the analysis of Schiff base complexes [17]. As shown in Figure 7 and Figure 8, strong peaks were observed at 1625 and 1538 cm−1 for Complex 1 and at 1631 cm−1 and 1594 cm−1 for Complex 2 due to imine νC=N stretching. The appearance of two peaks for the imine groups in Complexes 1 and 2 indicates the nature of Schiff base ligands [18].
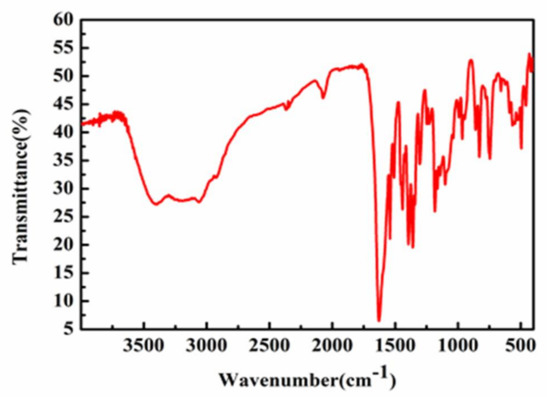
Figure 7.
The IR spectrum of Complex 1.
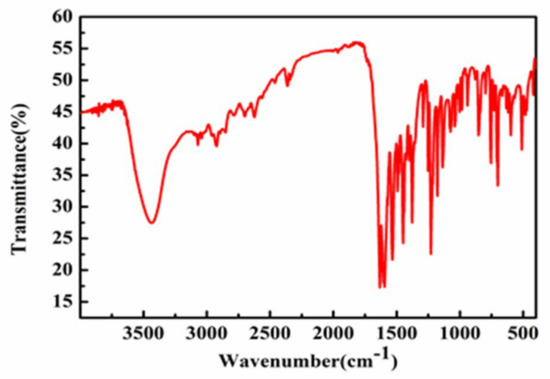
Figure 8.
The IR spectrum of Complex 2.
On complexation, strong bands were observed at around 1362 cm−1 and 1228 cm−1 in the complexes, indicating copper–nitrogen bond formation [19]. This case was further supported by the appearance of a medium-intensity band at around 493 cm−1 and 500 cm−1 in Complexes 1 and 2, respectively, due to Cu–N stretching vibrations modes [20].
2.3. Polycrystal Powder X-ray Diffraction (PXRD) Analysis
The experimental and simulated PXRD patterns of Complexes 1 and 2 are shown in Figure 9 and Figure 10. These peaks positions were in good agreement with each other, indicating the phase purity of Complexes 1 and 2.
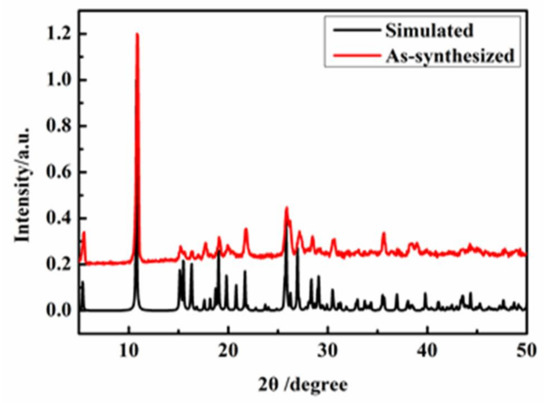
Figure 9.
The PXRD spectrum of Complex 1.
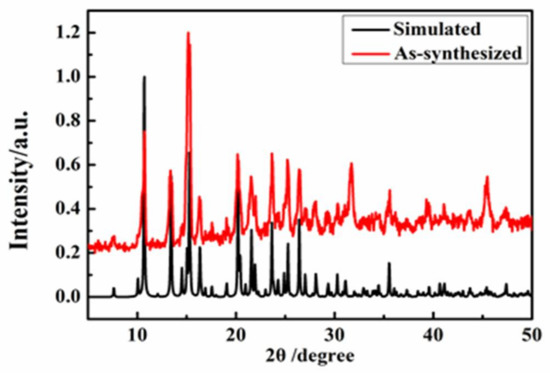
Figure 10.
The PXRD spectrum of Complex 2.
2.4. Electrochemical Analysis
Figure 11a presents the CV curves of Complex 1, which was subjected to nitrogen-saturated and oxygen-saturated supporting electrolytes, respectively, and the ORR activity of the complex was determined with the discrepancy in current amplitudes at a certain voltage potential [21]. In the N2 atmosphere, a pair of irreversible and apparent redox peaks was observed at 0.32 and 0.60 V, and three tiny reduction peaks were observed at −0.06 V, 0.16 V, and 0.44 V in the backward scan. In the O2 atmosphere, the CV curve of Complex 1 changed substantially. A pair of pseudo-reversible peaks was found at 0.34 and 0.56 V, which originated from the original peaks at 0.32 and 0.60 V. The introduction of oxygen to the medium greatly enhanced the magnitude of both the oxidative and reductive peaks. At the same time, the potential of the reduction peak increased, whereas the potential of the oxidative peak decreased. It was noticed that the redox peaks at 0.34 and 0.56 V were similar to those of the standard electrode potential of Cu+/Cu2+ [21]. Therefore, the oxygen reduction process was accompanied by a change in the valence of copper ions, which confirmed the involvement of copper ions in the ORR process of the complex. The ORR process of some copper complexes with substituted 1,10-phenanthroline has been studied by some groups, and a mono-Cu reaction mechanism was proposed [22]. In the ORR process of the mononuclear copper complexes (similar to the complexes reported in this study), the copper ion center was considered to form an adduct with dioxygen via charge transfer, and the Cu–O2 complex was then reduced and protonated in subsequent steps, leading to the reduction of oxygen to water by a four-electron pathway [23]. Based on the mechanism, the ORR pathway for Complex 1 can be expressed as follows:
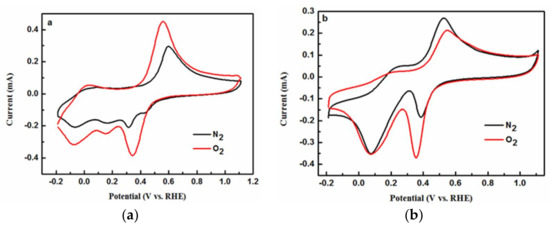
Figure 11.
Cyclic voltammetry (CV) of Complex 1 in PBS saturated with N2 and O2 (a), CV of Complex 2 in PBS saturated N2 and O2 (b). Scan rate: 100 mV/s.
The reduction of copper center Cu2+ in Complex 1 to Cu+ occurred at the reduction peak at 0.34 V and simultaneously gave out a coordination site for oxygen molecules (Equation (1)). A binding of oxygen molecule led to the formation of a Cu2+-superoxide intermediate (Equation (2)). The formation of this intermediate has been experimentally verified in the ORR process of other mononuclear copper complexes with multidentate N-donor ligands [24,25]. Limited by experimental conditions, the identification of the intermediate products was not involved in this study. Followed the binding reaction shown in Equation (2), further reduction of the intermediate complex led to the formation of Cu2+-peroxide species (Equation (3)), which might occur at 0.15 V in the CV of Complex 1 under an oxygen-saturated phosphate buffer solution (PBS) solution. The protonation reaction of the above intermediate occurs as shown in Equation (4) in the presence of water (Equation (4)). The final reduction and protonation reactions (Equation (5)) followed the reactions in Equation (4), and led to the release of water and the recovery of Complex 1, which might occur at −0.07 V.
The CV curves of Complex 2 were also conducted in two gas saturated media. In the nitrogen-saturated medium, two pairs of redox peaks were found and located at 0.38 and 0.53 V as well as 0.07 and 0.28 V, respectively. In the oxygen-saturated medium, two pairs of similar redox peaks were also found for Complex 2, at 0.36 and 0.56 V as well as 0.07 and 0.21 V, respectively. The replacement of nitrogen gas by oxygen gas also led to a significant increase in the reduction peak current at 0.36 V, which also suggested the catalytic ability of Complex 2 with respect to oxygen. The proposed pathway for the reduction of oxygen under the catalytic action of Complex 2 can be expressed as follows:
In this reduction process (Equation (6)), the Cu2+ center is reduced to Cu+, which is accompanied by the formation of Cu2+-superoxide intermediate (Equation (7)). The CV curve of Complex 1 was distinct from that of Complex 2 in that there was an intensified and broad reduction peak centered at 0.07 V for the latter. Moreover, the reduction peak was nearly unchanged from the nitrogen bubbled testing medium to the oxygen-bubbled testing medium. The origin of the reduction peak at 0.07 V under a nitrogen-saturated condition might be the negative-ionization of the pyridine ligand, which is stabilized by the positively charged Cu2+ center ion in Complex 2. The ligands of H2O molecule in Complex 1 have no aromatic properties or negative-ionization properties, which led to the absence of any apparent reduction peak at 0.07 V for Complex 1. As the CV scan was conducted towards further negative potentials from 0.36 to −0.2 V, the reduction of Cu2+-superoxide to Cu2+-peroxide (Equation (8)), and the subsequent protonation (Equation (9)) and reduction reactions (Equation (10)), might occur in the same way as that of Complex 1. The corresponding reduction peaks (as shown in Figure 11a) for these reactions might be overlaid by the negative-ionization peak of Complex 2 and do not appear in Figure 11b.
In order to quantitatively characterize the ORR activity of the copper complexes, their linear sweep voltammetry (LSV) curves were taken by employing a rotating disk electrode (RDE) electrode [26]. The tests were taken in an O2-saturated 0.1 M PBS buffer, and the rotation rates of the electrode were set between 400 and 1600 rpm. The magnitudes of the current densities increased with the increase in rotation speeds. The two complexes had no limiting current platform as owned by the commercial Pt/C catalyst (Figure 12c), and their current densities were also somewhat lower than that of the Pt/C catalyst at the same potential. The ORR onset potential (Eonset) is an important parameter for evaluating the performance of the catalysts, and the data were obtained in the LSV curves as shown in Figure 12. The Eonset values were 0.45 V, 0.58 V, and 0.89 V, respectively, for Complex 1, Complex 2, and Pt/C, respectively, with the latter having the highest Eonset value. In addition, a slightly higher Eonset value was found for Complex 2 compared with that of Complex 1, which might have resulted from the stabilization effect of pyridine due to its aromatic property. The delocalization of the conjugated ligand (such as pyridine in Complex 2) might enhance the stabilities of the intermediates produced from the complexes during the electrocatalytic process, which is conducive to the ORR performance of the complexes and to a high Eonset value. The water molecules do not have aromatic properties, which might account for the lower Eonset value of Complex 1 compared with that of Complex 2. The data presented in Figure 12 indicated that the ORR activities of the two complexes are inferior to that of the Pt/C to some extent.
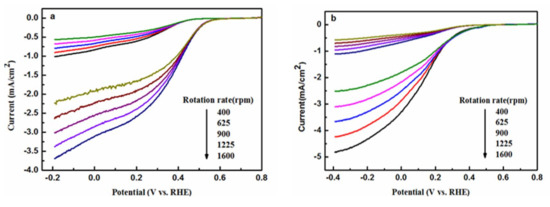
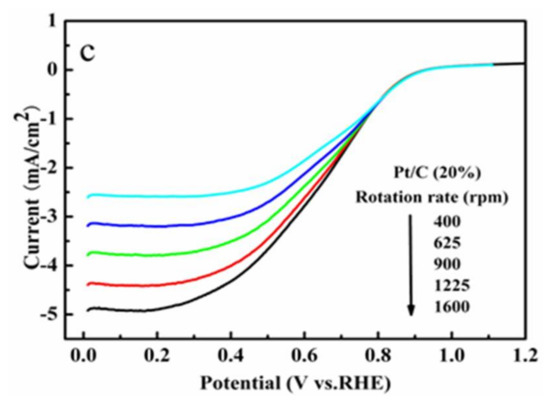
Figure 12.
Polarization curves of Complex 1/C (a) and Complex 2/C (b) at various rotating rates in PBS solution saturated with O2 and N2, and of Pt/C (c) at various rotating rates in PBS solution saturated with O2.
To obtain the electron transfer numbers (n) involved in the reduction of oxygen, the Koutecky–Levich (K-L) equation was utilized to plot the relationship between j−1 and ω−1. The K-L equation can be expressed as follows:
where Jk refers to the kinetic current densities, Jdiff represents the diffusion-limiting current densities, n is the number of the electrons involved in the ORR process, and ω is the angular velocity of the rotating electrode. Other parameters including F, Do, Co and η are all constant values and have been used in our previous reports [27,28].
1/J = 1/Jk + 1/Jdiff = 1/Jk + 1/Bω1/2
B = 0.62nFCoDo2/3v−1/6
As shown in Figure 13, there is an approximate linear relationship between the above two parameters, suggesting a first-order reaction towards the rotation rate in the electrolyte [27]. The n value involved in the reduction of per oxygen molecules of Complex 1 was about 3.7 at a certain range of potentials (Figure 13a), similarly, the n values of Complex 2 were averaged to be 3.6 (Figure 13b), slightly lower than that of Complex 1. According to previous reports, the functional moieties for ORR activity in metal complexes is M–Nx, and the value for x might play a role in determining the capabilities of the catalysts [4]. It was interesting in the present study that the number of the nitrogen atoms chelated with copper ion was not a determining factor influencing the ORR activities of the complex since the ORR performance of Complex 2 was somewhat inferior to that of Complex 1 in terms of n values, although Complex 2 has more nitrogen numbers than that of the latter one. Compared with pyridine molecule, the H2O molecule has a smaller bulk volume, and the copper atom in Complex 2 has a smaller steric hindrance effect than the copper atom in Complex 2. A small steric effect is conducive to the binding of oxygen to the complexes in the ORR process, as well as the formation of the intermediated products (as shown in Equations (1)–(10)), which might account for the slightly higher n values of Complex 1 compared with that of Complex 2.
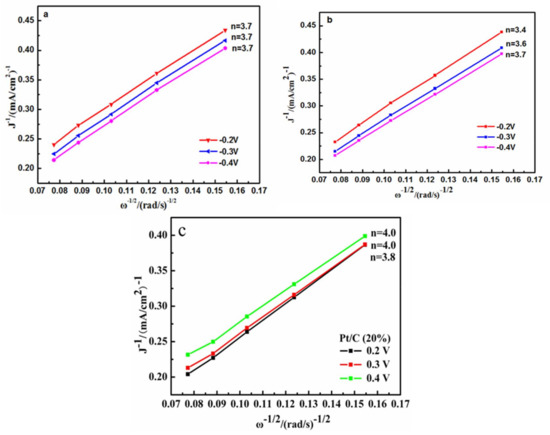
Figure 13.
Koutecky–Levich (K-L) plots and electron transfer numbers of Complex 1/C (a), Complex 2/C (b), and Pt/C (c) at −0.2 V, −0.3 V, and −0.4 V vs. RHE.
In consideration of the Eonset and electron transfer number (ETN) values, the metal-complex-based catalysts have ORR activities somewhat lower than that of the commercially available Pt catalysts. Table 3 also lists the ORR activities of other copper complexes formed between copper ion (II) and nitrogen containing ligands, from which it was found that the selection of nitrogen-containing ligands has an apparent influence on the Eonset and ETN values. It was a pity that the copper complexes reported in this study were found to have Eonset values lower than those of other complexes listed in Table 3. The copper complexes reported in this study, like other complexes listed in Table 3, had ETNs of about 4 at pH 7.

Table 3.
Oxygen reduction reaction (ORR) abilities of some M–Nx-containing non-noble catalysts reported in recent years. ETN: Electron transfer number; PBS: phosphate buffer solution; RHE: reversible hydrogen electrode.
Considering the low price of these non-platinum catalysts, they may have important application prospects in certain fuel cells. Macrocycle-based materials have been used as cathodic catalysts for microbial fuel cells [12], which operate in neutral aqueous solutions, and deactivation of catalysts in direct methanol fuel cells does not appear to be affected by such phenomena as methanol crossover or carbon monoxide poisoning [12]. In all, the simple copper complexes reported in this study show satisfactory ORR activities in neutral aqueous solutions and might be used as competitive cathodic catalysts in microbial fuel cells due to their low preparation costs.
Continuous CV sans were undertaken for up to 600 cycles for the evaluation of the stability of the complexes in the ORR process. The reduction in the peak currents were recorded at the 600th scan, and the losses were 15.71% (at 0.34 V vs. RHE) and 9.76% (at 0.36 V vs. RHE) compared with the 1st scans, which showed that the cycle stability of Complex 2/C was slightly better than that of Complex 1/C (Figure 14). The aromatic properties of pyridine as the ligand was helpful to stabilize the charged intermediates formed during the redox process of the complexes, which could account for the higher stability of Complex 2/C than that of Complex 1/C. In general, both of the complexes had moderate redox stabilities, as considering the performances of other complexes reported previously [29,30,31,32].
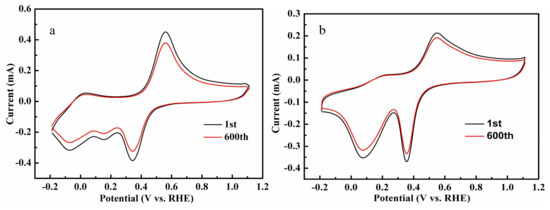
Figure 14.
Continuous cyclic voltammetry (CV) measurements of Complex 1/C (a) and Complex 2/C (b) in PBS saturated with O2, Scan rate: 100 mV/s.
3. Materials and Experimental
3.1. Materials
2,4-Dihydroxybenzaldehyde, glycine, NaOH, copper chloride, MeOH, NaH2PO4·2H2O, K3[Fe(CN)6], Na2HPO4·12H2O, and isopropanol (99.5%) were all of analytical grade and purchased from Aladdin Co., Ltd. Nafion (5 wt %, Dupont, Wilmington, DE, USA), and carbon (Vulcan XC-72, (Dupont, Wilmington, DE, USA) was obtained from the Nanjing Hui Yu Energy Technology Co., Ltd. (Nanjing, China). Pure grade O2 or N2 was used for saturating the solutions, which were prepared by using ultrapure.
3.2. Synthesis
3.2.1. Synthesis Procedure of Complex 1
2,4-Dihydroxybenzaldehyde (1 mmol) and glycine (1 mmol) and 20 mL of EtOH-H2O (5:1) were successively dosed to a 50 mL round-bottom flask equipped with magnetic stir bars. The reaction mixture was stirred at 80 °C for 4 h, and NaOH (1 mmol) and copper chloride (1 mmol) were added to the above solution. After vigorous stirring for another 2 h at 80 °C, the solution was cooled and filtered. Upon slow evaporation of the deep green colored solution at room temperature, the blue crystals of Complex 1 were formed after a few days. Yield: 67%. Anal. calcd. (%) (found) for C9H15NO8Cu: C, 32.88 (31.96); H, 4.60 (4.53); N, 4.26 (4.14); Cu, 19.33 (19.28). IR (KBr, cm−1): 3068 (m), 1630(s), 1592 (m), 1393 (m), 1359 (s), 1305 (m), 1248 (m), 1183(s), 1101 (m), 1036 (m), 965 (m), 830 (s), 746 (s), 566 (m), 496 (m).
3.2.2. Synthesis Procedure of Complex 2
According to our previous related work [33], 2,4-dihydroxybenzaldehyde (1 mmol) was dissolved in 30 mL of EtOH and subsequently mixed with glycine (1 mmol) and NaOH (1 mmol). The mixture was refluxed for 3 h. Afterward, the mixture was cooled to room temperature. To this mixture, copper chloride (1 mmol) was added, and the mixture was further stirred for another 2 h. Finally, the green solid formed was filtered and washed with ethanol (5 mL × 3) and then dried in air. The green solid was dissolved with pyridine, and stirred for 30 min, filtered, and left at room temperature. The green crystals of Complex 2 were formed after a few days and collected by filtration. Yield: 64%. Anal. calcd. (%) (Found) for C19H17CuN3O4: C, 55.00 (55.17); H, 4.13 (4.25); N, 10.13 (10.09). IR spectrum, ν (cm−1): 3070 (m), 2618 (m), 1631 (s), 1594 (m), 1533 (m), 1448 (m), 1228 (s), 1178 (m), 1074 (s), 851 (s), 754 (s), 708 (m), 595 (s), 500 (m).
3.3. Physical Characterization of the Crystals
Single-crystal X-ray diffraction data for the two Schiff base copper Complexes 1 and 2 were conducted on a Bruker-AXS CCD diffractometer (Bruker AXS, Karlsruhe, Germany) equipped with a graphite-monochromated Mo Ka radiation (λ = 0.71073 Å) at 298 K. All absorption corrections were applied using a multi-scan technique. The structures were solved by the direct method and refined through full-matrix least-squares techniques method on F2 using the SHELXTL 97 crystallographic software package. The FT-IR spectra were recorded with KBr as pellets in the range 4000–400 cm−1 on Nicolet 170 SXFT/IR spectrometer. The powder X-ray diffraction pattern was collected using a Rigaku D/max-2550 diffractometer (Rigaku Corporation, Tokyo, Japan) with Cu Kα radiation.
3.4. Preparation of the Catalysts Modified Electrode
The complex crystals were fully ground to a fine powder, which was then used to prepare the catalyst slurry with Vulcan X-72 carbon powder. A solution that consisted of 200 µL of isopropanol, 5 µL of nafion solution, and 600 µL of H2O was prepared in advance for the dispersion of the catalysts. Exactly 1.6 mg of the Vulcan X-72 carbon powder was added to 805 µL of the above solution, and the obtained slurry was conducted with continuous ultrasonic dispersion for 30 min. Afterward, 1.6 mg of the complex was added to the above suspension, and the mixture was subjected to another hour of ultrasonic dispersion, and the resultant catalyst slurry was finally obtained. Before the fabrication of each of the catalyst composite, the working electrode (WE) was cleaned with Al2O3 (0.3 µm) slurry to ensure the electrode met test needs [34,35].
3.5. Electrochemical Measurements
Gamry Instruments Reference 3000 potentiostat (Gamry, Warminster, Pennsylvania, USA) was used for all of the electrochemical measurements. A glassy carbon electrode with a diameter of 5 mm was used as the WE, and a platinum ring made from Pt wire with a diameter of 1 mm was used as the counter electrode. In addition, an Ag/AgCl (saturated KCl) electrode was used as the reference electrode. The combination of the RDE 710 Rotating apparatus (Pine) with WE electrode was utilized for the linear sweep voltammetry (LSV) experiments. The medium used for testing, or the support electrolyte is 0.1 M phosphate buffer solution (PBS, pH = 7.0). The electrolyte was either bubbled with oxygen or with nitrogen until saturated prior to each tests. According to requirements, the electrolyte solution could be saturated with nitrogen or oxygen gas. Repeated CV measurements up to 20 cycles (between 0.2 and 1.1 V vs. RHE) were conducted for the activation of the electrolyte before the evaluation of the ORR activities of the catalyst composite.
4. Conclusions
In this work, two mononuclear copper Schiff base complexes were synthesized and used as catalysts for ORR. The two complexes had simple and similar structures, but the difference is that the water molecules used as ligands in Complex 1 are replaced by pyridine molecules in Complex 2. The structures of the two complexes were identified by X-ray crystallography and X-ray diffraction. Electrochemical measurements such as CV and LSV showed that both catalysts showed ORR catalyst activities and catalyzed the conversion of oxygen to water by nearly a four-electron pathway. The results showed that Cu–NxOy units, especially the copper ion, played a crucial role in the ORR process, and the number of nitrogen atoms in the above units had limited effects on the ORR activities of the catalysts. Relative to the H2O molecule, the aromatic N-donor ligands are preferable to give a higher Eonset value for the resultant complex due to the stabilization effect of the ligands on the intermediated products produced in the ORR process. The bulk volume represents another factor influencing the ORR activities of the complexes, and a small bulk volume of the ligands is beneficial to increase the n values of the resultant complexes. The conclusion of the present study has some interest for the design of metal complex-based ORR catalysts.
Acknowledgments
The work was financially supported by the National Natural Science Foundation of China (51473074, 21401094, 21601079).
Author Contributions
Zhe Ma conducted instrumental analysis and drafted the first edition of the manuscript. Ya Chu synthesized the ligand and prepared the complexes. Chonggang Fu conducted the electrochemical measurements. Hongmei Du analyzed electrochemical data. Xianqiang Huang guided the synthesis of the complex compounds and identification of the crystal structures. Jinsheng Zhao edited the manuscript and polished the language of the manuscript.
Conflicts of Interest
The authors declare no conflict interest.
References
- Kumar, R.; Yadav, A.; Mahiya, K.; Mathur, P. Copper (II) complexes with box or flower type morphology: Sustainability versus perishability upon catalytic recycling. Inorg. Chim. Acta 2016, 450, 279–284. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Yuan, S.; Day, G.; Wang, X.; Yang, X.Y.; Zhou, H.C. Luminescent sensors based on metal-organic frameworks. Coodination Chem. Rev. 2018, 354, 28–45. [Google Scholar] [CrossRef]
- Fan, T.Y.; Yin, F.X.; Wang, H.; He, X.B.; Li, G.R. A metal-organic-framework/carbon composite with enhanced bifunctional electrocatalytic activities towards oxygen reduction/evolution reactions. Int. J. Hydrogen Energy 2017, 42, 17376–17385. [Google Scholar] [CrossRef]
- Levy, N.; Mahammed, A.; Friedman, A.; Gavriel, B.; Gross, Z.; Elbaz, L. Metallocorroles as non-precious metal electrocatalysts for highly efficient oxygen reduction in alkaline media. ChemCatChem 2016, 8, 2832–2837. [Google Scholar] [CrossRef]
- Hossain, M.D.; Sato, T.; Higuchi, M. A green copper-based metallo-supramolecular polymer: Synthesis, structure, and electrochromic properties. Chem. Asian J. 2013, 8, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Thorseth, M.A.; Tornow, C.E.; Tse, E.C.M.; Gewirth, A.A. Cu complexes that catalyze the oxygen reduction reaction. Coordination Chem. Rev. 2013, 257, 130–139. [Google Scholar] [CrossRef]
- Burkitt, R.; Whiffen, T.R.; Yu, E.H. Iron phthalocyanine and MnOx composite catalysts for microbial fuel cell applications. Appl. Catal. B Environ. 2016, 181, 279–288. [Google Scholar] [CrossRef]
- Liu, B.C.; Brückner, C.; Lei, Y.; Cheng, Y.; Santoro, C.; Li, B.K. Cobalt porphyrin-based material as methanol tolerant cathode in single chamber microbial fuel cells. J. Power Source 2014, 257, 246–253. [Google Scholar] [CrossRef]
- Arul, A.; Pak, H.; Moon, K.U.; Christy, M.; Oh, M.Y.; Nahm, K.S. Metallomacrocyclic-carbon complex: A study of bifunctional electrocatalytic activity for oxygen reduction and oxygen evolution reactions and their lithium-oxygen battery applications. Appl. Catal. B Environ. 2018, 220, 488–496. [Google Scholar] [CrossRef]
- Mahammed, A.; Gross, Z. Metallocorroles as electrocatalysts for the oxygen reduction reaction (ORR). Isr. J. Chem. 2016, 56, 756–762. [Google Scholar] [CrossRef]
- Choi, Y.K.; Park, J.K.; Jeon, S. Electrocatalytic reduction of dioxygen at carbon electrodes modified with Co.(II)-Schiff base complexes in various pH solutions. Electroanalysis 1999, 11, 134–138. [Google Scholar] [CrossRef]
- Ghasemi, M.; Wan Daud, W.R.; Rahimnejad, M.; Rezayi, M.; Fatemi, A.; Jafari, Y.; Somalu, M.R.; Manzour, A. Copper-phthalocyanine and nickel nanoparticles as novel cathode catalysts in microbial fuel cells. Int. J. Hydrogen Energy 2013, 38, 9533–9540. [Google Scholar] [CrossRef]
- Pal, S.; Barik, A.K.; Gupta, S.; Hazra, A.; Kar, S.K.; Peng, S.M. Copper(II) mediated anion dependent formation of Schiff base complexes. Inorg. Chem. 2005, 44, 3880–3889. [Google Scholar] [CrossRef] [PubMed]
- Maclachlan, M.J.; Park, M.K.; Thompson, L.K. Coordination compounds of Schiff-base ligands derived from diaminomaleonitrile (dmn): Mononuclear, dinuclear, and macrocyclic derivatives. Inorg. Chem. 1996, 35, 5492–5499. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, R.; Palaniandavar, M.; Halcrow, M.A. Copper (II) complexes of sterically hindered Schiff base ligands: Synthesis, structure, spectra and electrochemistry. Polyhedron 2006, 25, 1077–1088. [Google Scholar] [CrossRef]
- Roy, P.; Manassero, M. Tetranuclear copper (II)-Schiff-base complexes as active catalysts for oxidation of cyclohexane and toluene. Dalton Trans. 2010, 39, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.M.; Roy, A.S.; Mondal, P.; Mubarak, M.; Mondal, S.; Hossain, D. Synthesis, catalytic oxidation and antimicrobial activity of copper (II) Schiff base complex. J. Mol. Catal. A Chem. 2011, 336, 106–114. [Google Scholar] [CrossRef]
- Golcu, A.; Tumer, M.; Demirelli, H.; Wheatley, R.A. Cd(II) and Cu(II) complexes of polydentate Schiff base ligands: Synthesis, characterization, properties and biological activity. Inorg. Chim. Acta 2005, 358, 1785–1797. [Google Scholar] [CrossRef]
- Samanta, B.; Chakraborty, J.; Choudhury, C.R.; Dey, S.K.; Dey, D.K.; Batten, S.R. New Cu(II) complexes with polydentate chelating Schiff base ligands: Synthesis, structures, characterisations and biochemical activity studies. Chin. J. Struct. Chem. 2007, 18, 33–41. [Google Scholar] [CrossRef]
- Sundaravel, K.; Suresh, E.; Palaniandavar, M. Synthesis, structures, spectral and electrochemical properties of copper (II) complexes of sterically hindered Schiff base ligands. Inorg. Chim. Acta 2009, 362, 199–207. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, X.L.; Wang, M.; Kong, L.Q; Zhao, J.S. 1,10-phenanthroline metal complex covalently bonding to poly-(pyrrole-3-carboxylic acid)-coated carbon: An efficient electrocatalyst for oxygen reduction. Electrochim. Acta 2015, 180, 86–95. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Ottenwaelder, X.; Stack, T.D.P.; Chidsey, C.E.D. Kinetic and mechanistic studies of the electrocatalytic reduction of O2 to H2O with mononuclear Cu complexes of substituted 1,10-phenanthrolines. J. Phys. Chem. A 2007, 111, 12641–12650. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.B.; Anson, F.C. Mechanistic aspects of the electroreduction of dioxygen as catalyzed by copper-phenathroline complexes adsorbed on graphite electrode. Inorg. Chem. 1994, 33, 5003–5009. [Google Scholar] [CrossRef]
- Schatz, M.; Raab, V.; Foxon, S.P.; Brehm, G.; Schneider, S.; Reiher, M.; Holthausen, M.C.; Sundermeyer, J.; Schindler, S. Combined spectroscopic and theoretical evidence for a persistent end-on copper superoxo complex. Angew. Chem. Int. Ed. 2004, 43, 4360–4363. [Google Scholar] [CrossRef] [PubMed]
- Aboelella, N.W.; Lewis, E.A.; Reynolds, A.M.; Brennessel, W.W.; Cramer, C.J.; Tolman, W.B. Snapshots of dioxygen activation by copper: The structure of a 1:1 Cu/O2 adduct and its use in syntheses of asymmetric bis (μ-oxo) complexes. J. Am. Chem. Soc. 2002, 124, 10660–10661. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhu, Q.; Xu, A.W. Noble-metal-free Fe-N/C catalyst for highly efficient oxygen reduction reaction under both alkaline and acidic conditions. J. Am. Chem. Soc. 2014, 136, 11027–11033. [Google Scholar] [CrossRef] [PubMed]
- He, H.Y.; Wang, M.; Zhao, J.S.; Zhang, Y. Poly(10,12-bis(4-hexylthiophen-2-yl)thieno[3′,4′:5,6]pyrazino[2,3-f][1,10]-phenanthroline)-copper(II) complex(II) complex as an efficient electrocatalyst for oxygen reduction. Chem. Eng. J. 2017, 316, 680–691. [Google Scholar] [CrossRef]
- Yang, T.T.; Wang, M.; Ju, X.P.; Zhao, J.S.; Fu, C.G. The efficient oxygen reduction catalysts based on the Non-Noble metal and conducting polymers. Int. J. Electrochem. Sci. 2017, 12, 12125–12139. [Google Scholar] [CrossRef]
- Thorseth, M.A.; Letko, C.S.; Tse, E.C.M.; Rauchfuss, T.B.; Gewirth, A.A. Ligand effects on the overpotential for dioxygen reduction by tris(2-pyridylmethyl) amine derivatives. Inorg. Chem. 2013, 52, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Iwase, K.; Yoshioka, T.; Nakanishi, S.; Hashimoto, K.; Kamiya, K. Copper-modified covalent trizazine frameworks as non-noble-metal electrocatalysts for oxygen reduction. Angew. Chem. Int. Ed. 2015, 54, 11068–11072. [Google Scholar] [CrossRef] [PubMed]
- Thorum, M.S.; Yadav, J.; Gewirth, A.A. Oxygen reduction activity of a copper complex of 3,5-diamino-1,2,4-triazole supported on carbon black. Angew. Chem. Int. Ed. 2009, 48, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Oyaizu, N.; Shimazu, K.; Yagi, I. Oxygen reduction reaction catalyzed by self-assembled monolayers of copper-based electrocatalysts on a polycrystalline gold surface. J. Phys. Chem. C 2016, 120, 15814–15822. [Google Scholar] [CrossRef]
- Li, C.J.; Yan, C.X.; Yang, X.X.; Ren, Y.H.; Xue, Z.C.; Cui, C.S. Two 2,4-dihydroxybenzaldehydeglycine Schiff base complexes: Syntheses, structures and selective oxidation catalytic properties for alcohols. Chin. J. Inorg. Chem. 2016, 32, 891–898. [Google Scholar]
- Zhao, Y.Y.; Chu, Y.; Ju, X.P.; Zhao, J.S.; Kong, L.Q.; Zhang, Y. Carbon-supported copper-based nitrogen-containing supramolecule as an efficient oxygen reduction reaction catalyst in neutral medium. Catalysts 2018, 8, 53. [Google Scholar] [CrossRef]
- He, H.Y.; Wang, M.; Zhang, Y.; Zhao, J.S. Soluble conjugated polymer enriched with pyridinic nitrogen and its application as high-performance catalyst for oxygen reduction. J. Solid State Electrochem. 2017, 21, 1639–1651. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).