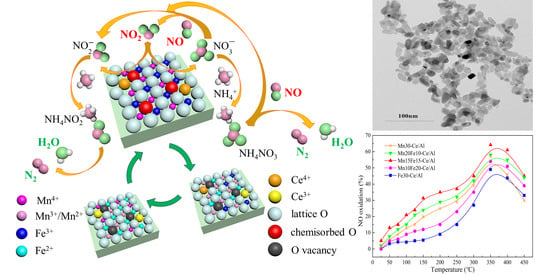
Structure–Activity Relationship Study of Mn/Fe Ratio Effects on Mn−Fe−Ce−Ox/γ-Al2O3 Nanocatalyst for NO Oxidation and Fast SCR Reaction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphological Characterization
2.1.1. Brunauer-Emmett-Teller (BET) Measurements
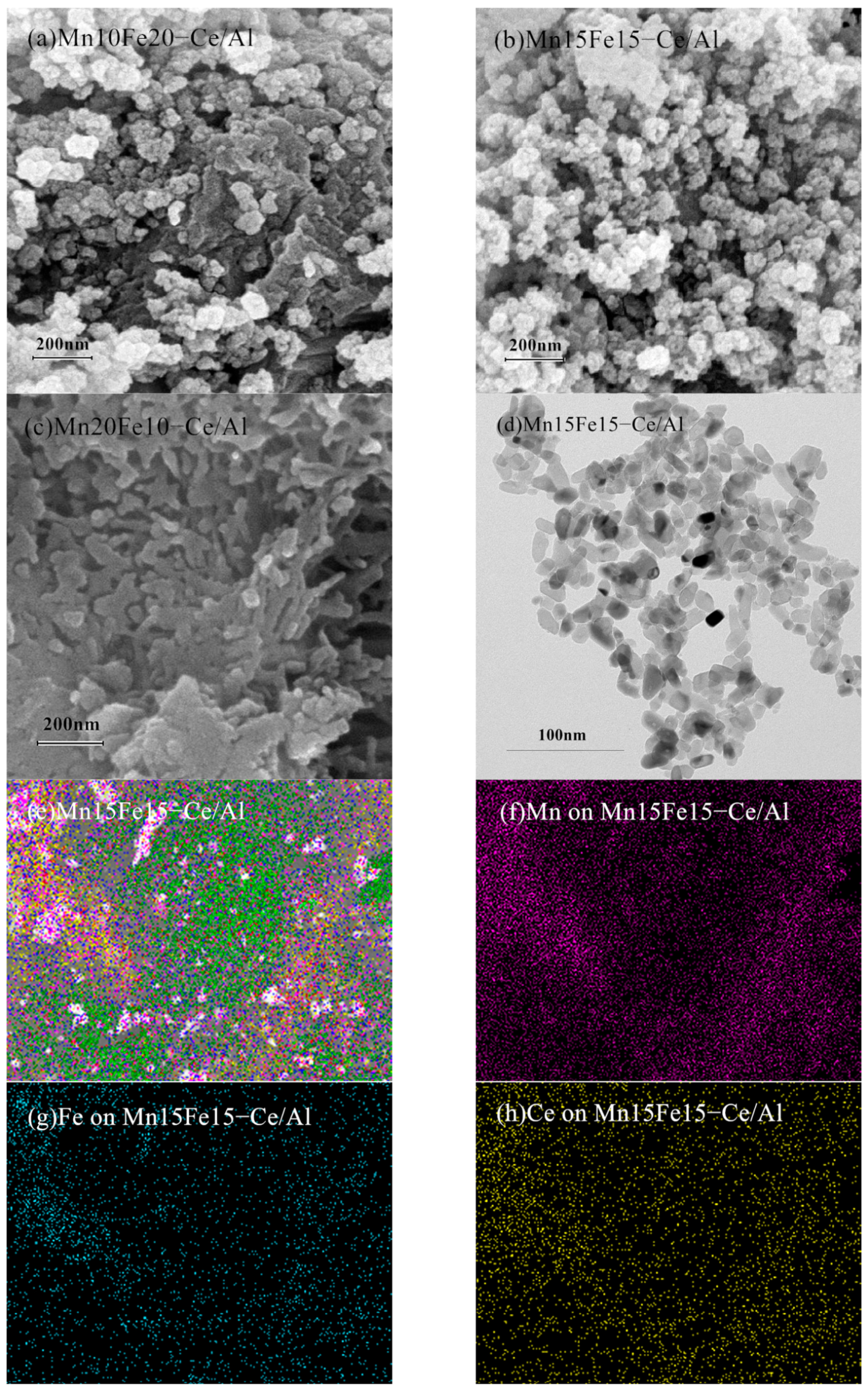
2.1.2. Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM) and Mapping Analysis
2.2. Structural Characterization
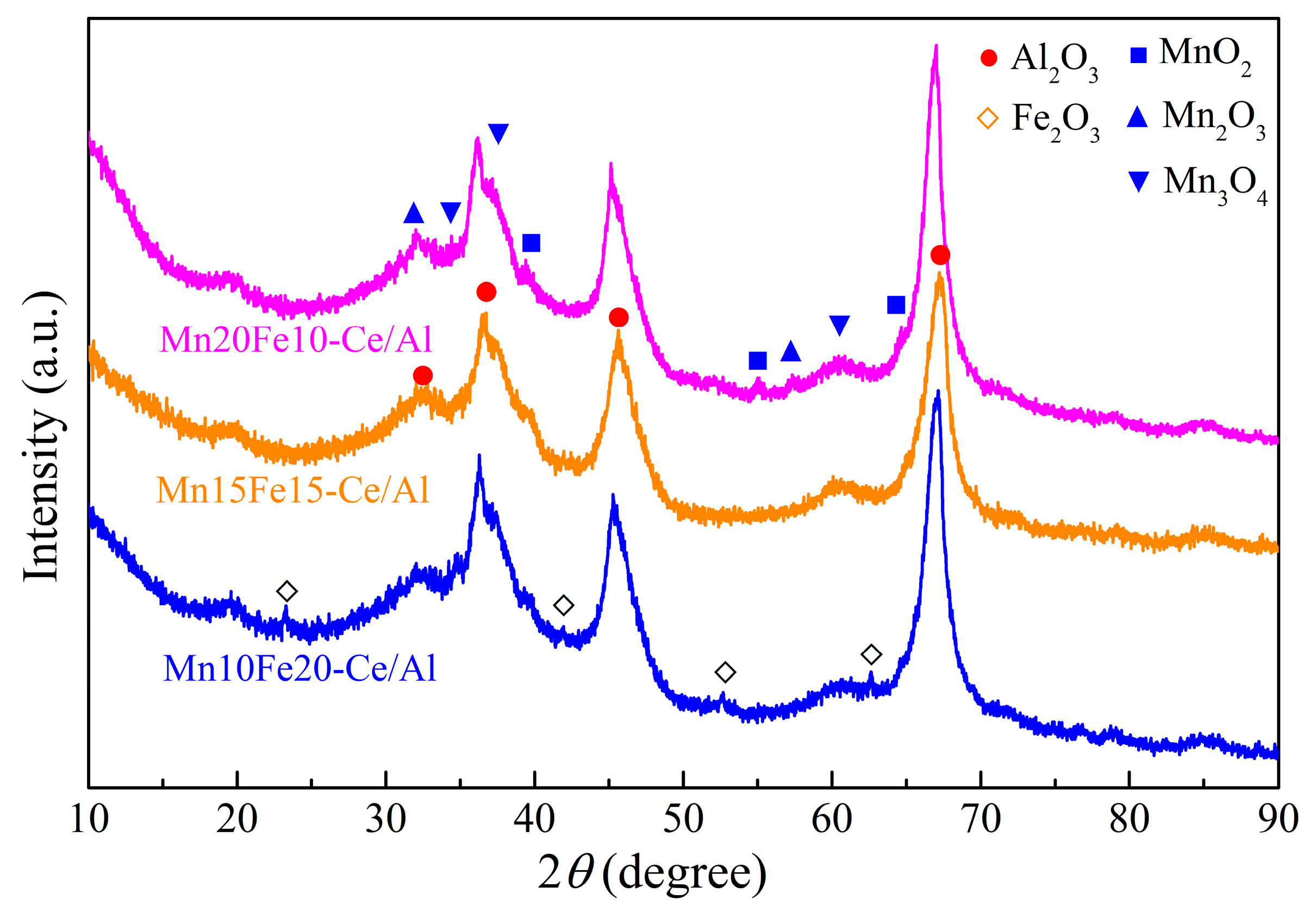
2.2.1. X-Ray Diffraction (XRD) Analysis
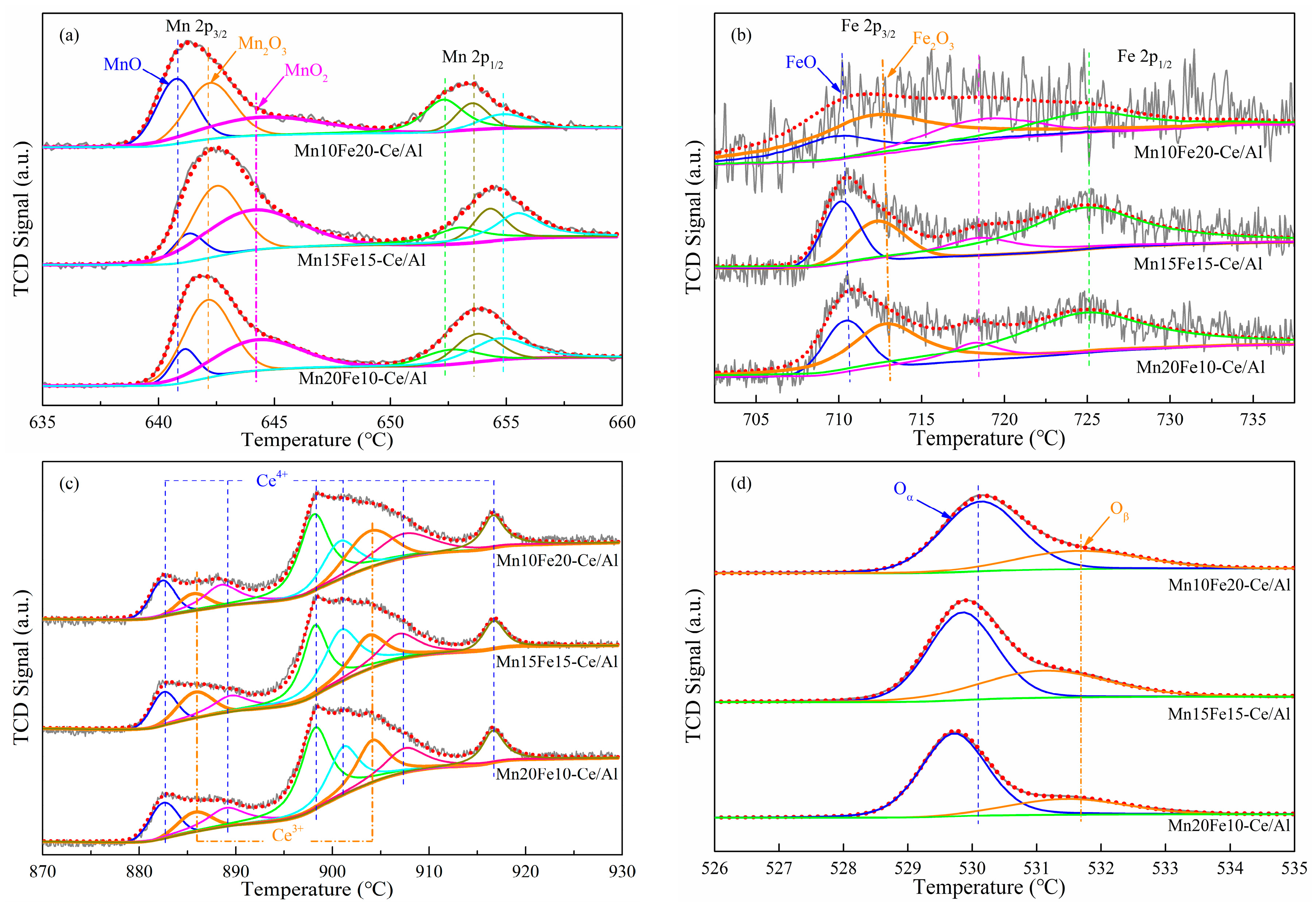
2.2.2. X-Ray Photoelectron Spectroscopy (XPS) Analysis
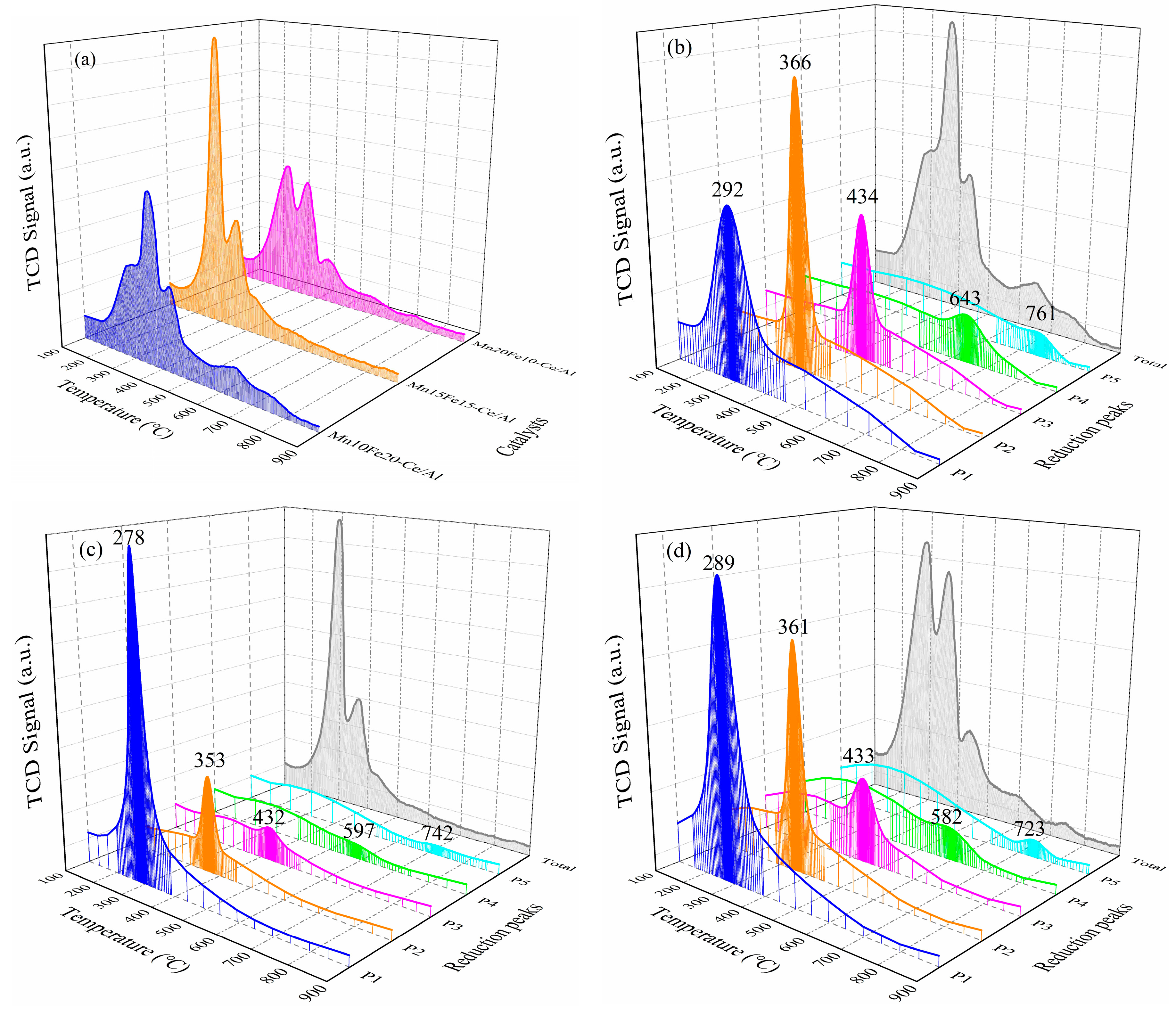
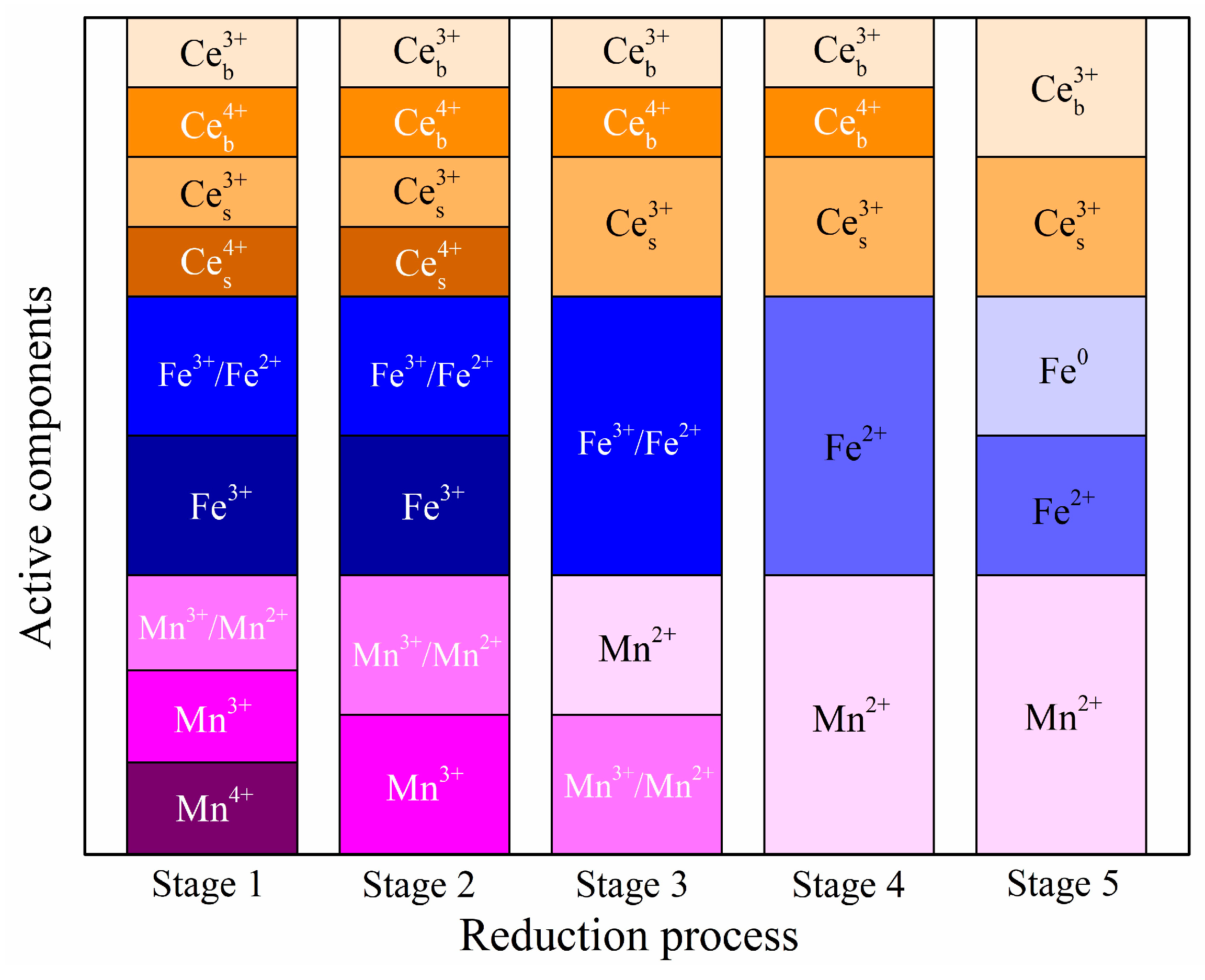
2.2.3. H2-Temperature-Programmed Reduction (H2-TPR) Analysis
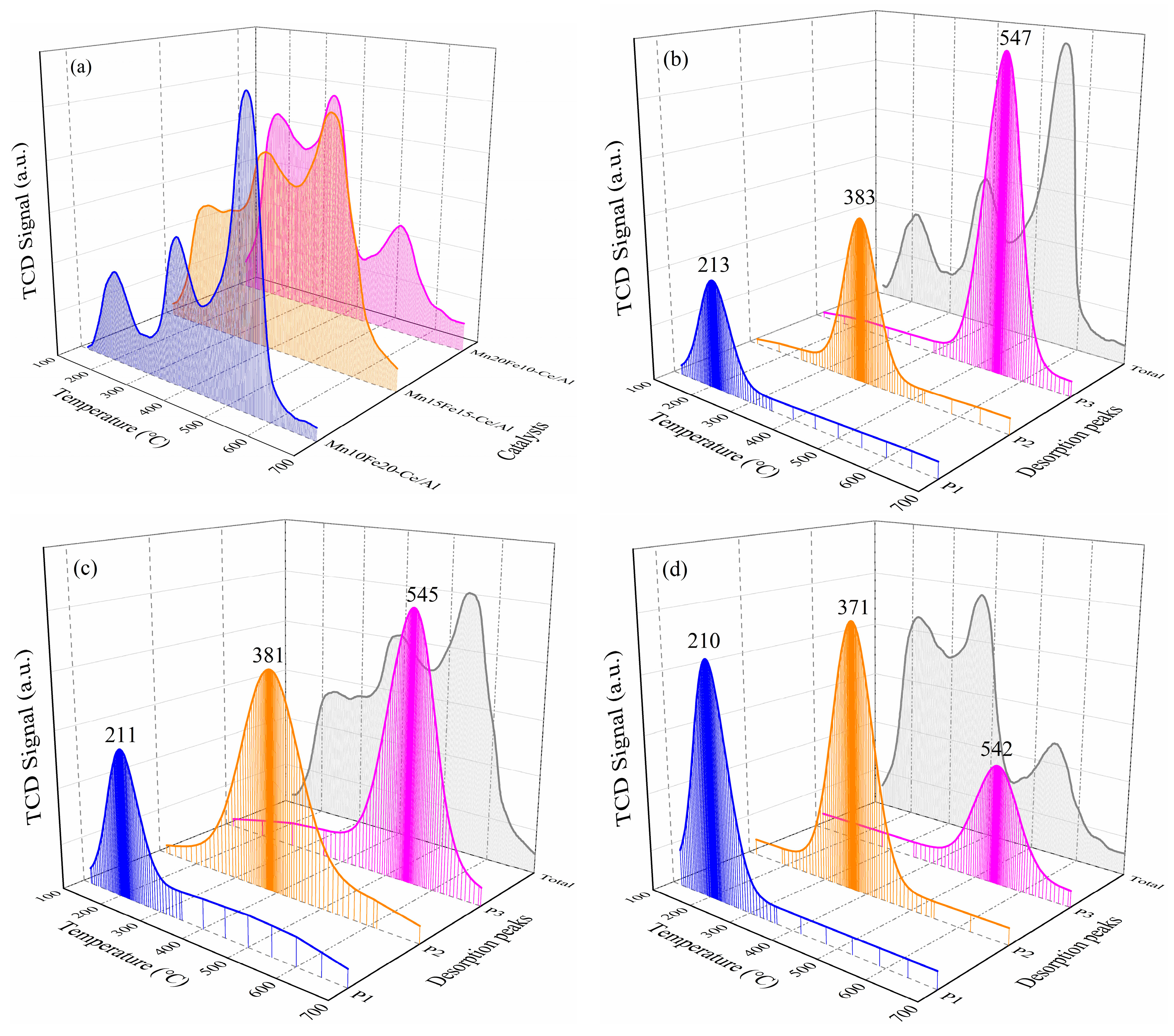
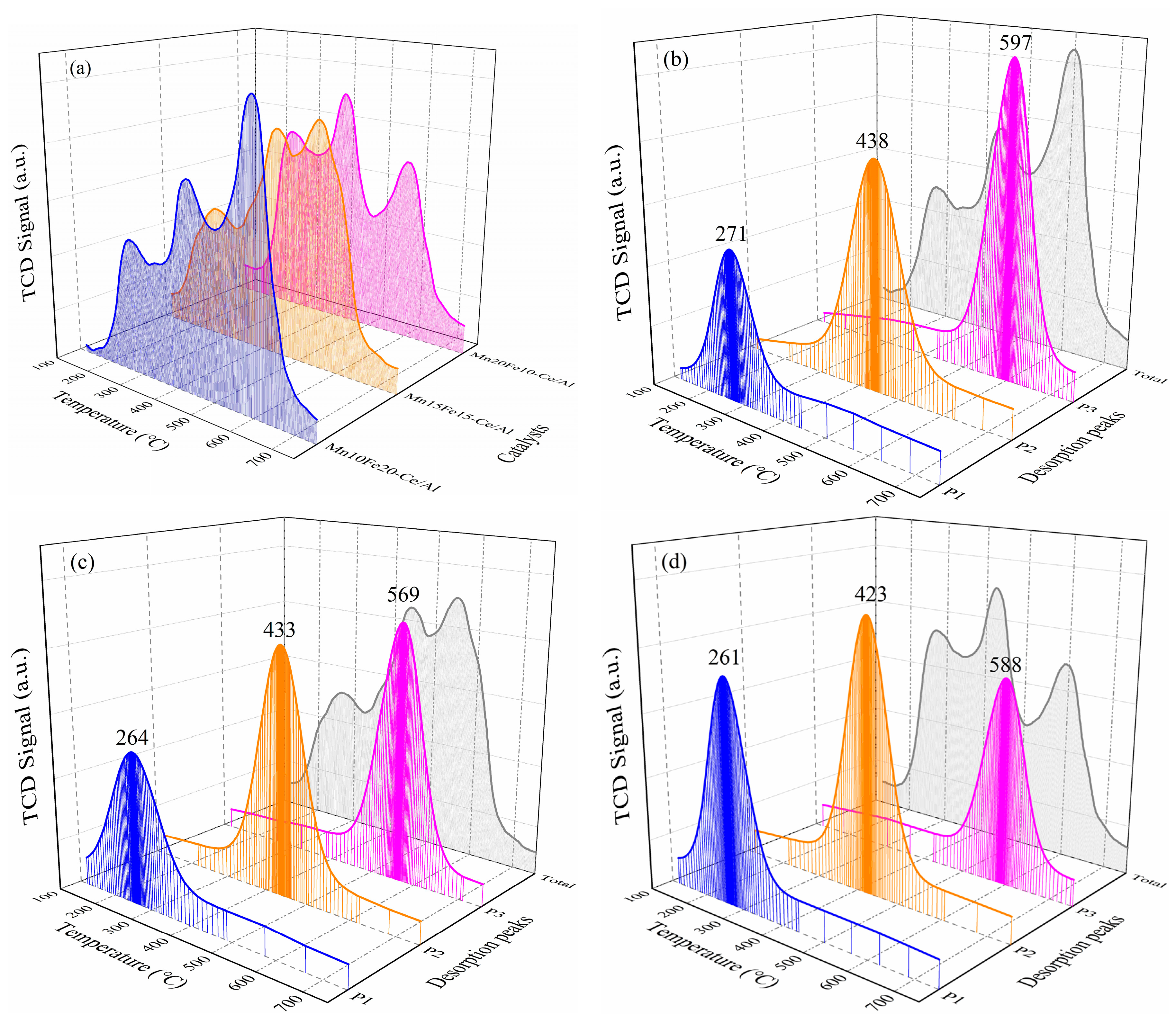
2.2.4. NH3-Temperature-Programmed Desorption (NH3-TPD) and NO-TPD Analysis
2.3. Fast SCR Activity of Mn−Fe−Ce−Ox/γ-Al2O3
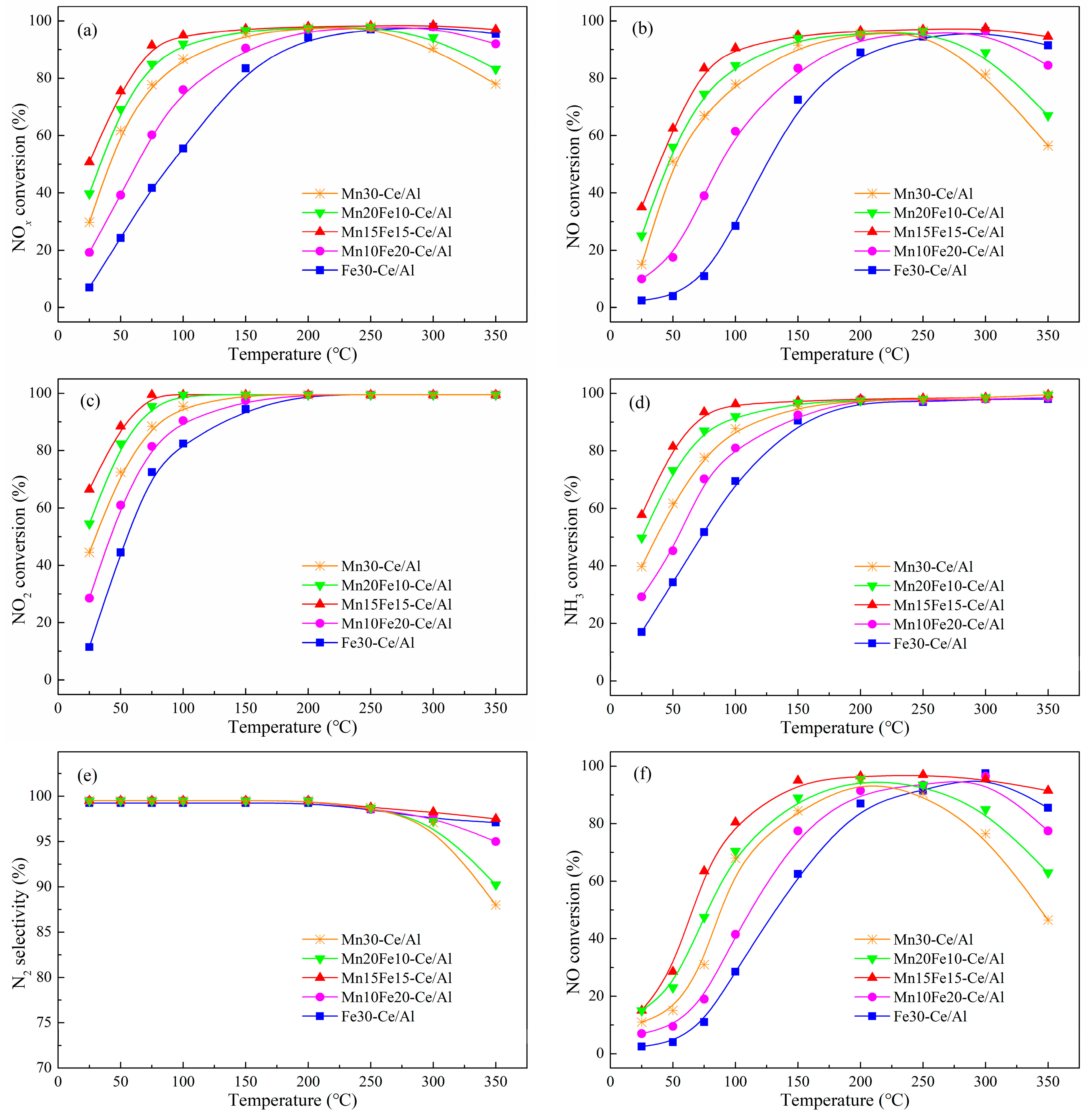
2.3.1. Effect of Mn/Fe Ratio on NO Oxidation of NO2
2.3.2. Effect of Mn/Fe Ratio on fast SCR Activity
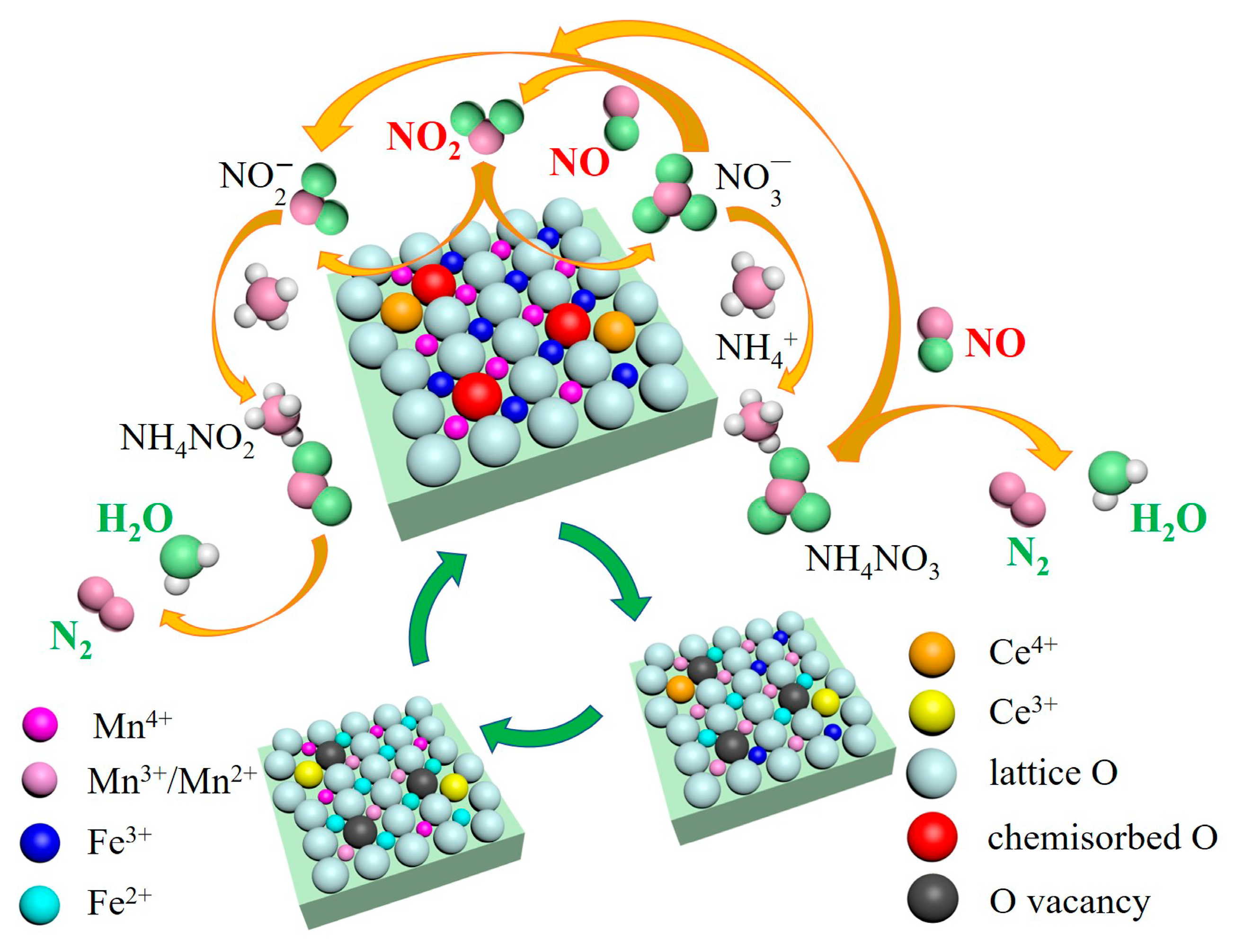
2.4. Reaction Mechanism Analysis
3. Materials and Methods
3.1. Catalysts’ Preparation
3.2. Catalysts’ Characterization
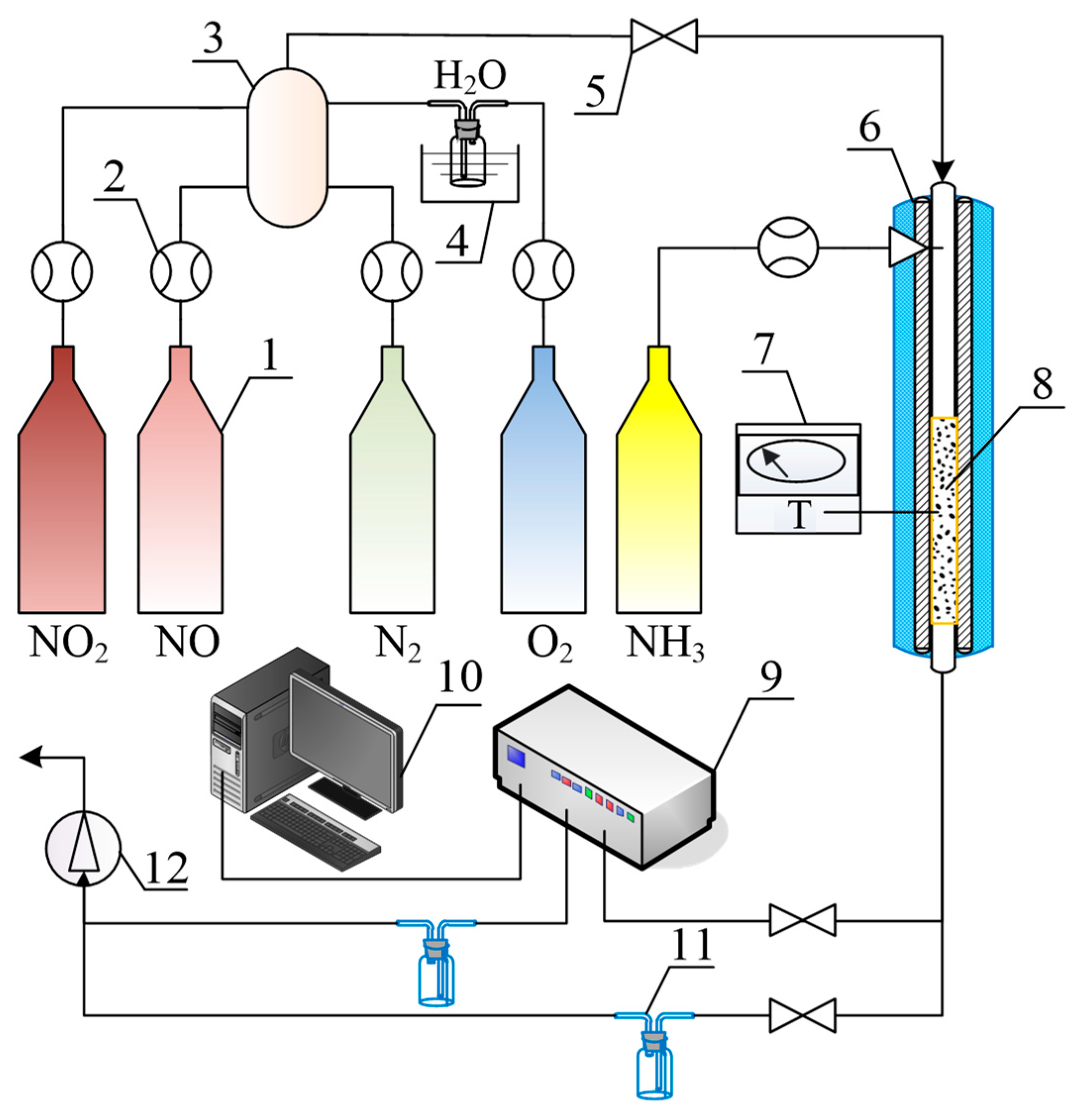
3.3. Catalytic Performance Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, H.; Ge, M.Y.; Wu, S.G.; Ye, B.C.; Su, Y.X. Iron based monolithic catalysts supported on Al2O3, SiO2, and TiO2: A comparison for NO reduction with propane. Fuel 2018, 220, 330–338. [Google Scholar] [CrossRef]
- Roy, S.; Hegde, M.S.; Madras, G. Catalysis for NOx abatement. Appl. Energy 2009, 86, 2283–2297. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, X.; Xie, A.; Luo, S.; Yao, C.; Li, X.; Zuo, S. V2O5-decorated Mn-Fe/attapulgite catalyst with high SO2 tolerance for SCR of NOx with NH3 at low temperature. Chem. Eng. J. 2017, 326, 1074–1085. [Google Scholar] [CrossRef]
- Bendrich, M.; Scheuer, A.; Hayes, R.E.; Votsmeier, M. Unified mechanistic model for Standard SCR, Fast SCR, and NO2 SCR over a copper chabazite catalyst. Appl. Catal. B Environ. 2018, 222, 76–87. [Google Scholar] [CrossRef]
- Ruggeri, M.P.; Grossale, A.; Nova, I.; Tronconi, E.; Jirglova, H.; Sobalik, Z. FTIR in situ mechanistic study of the NH3NO/NO2 “Fast SCR” reaction over a commercial Fe-ZSM-5 catalyst. Catal. Today 2012, 184, 107–114. [Google Scholar] [CrossRef]
- Koebel, M.; Elsener, M. Selective catalytic reduction of NO over commercial DeNOx-catalysts: Experimental determination of kinetic and thermodynamic parameters. Chem. Eng. Sci. 1998, 53, 657–669. [Google Scholar] [CrossRef]
- Gao, Y.; Luan, T.; Lu, T.; Cheng, K.; Xu, H. Performance of V2O5-WO3-MoO 3/TiO2 catalyst for selective catalytic reduction of NOx by NH3. Chin. J. Chem. Eng. 2013, 21, 1–7. [Google Scholar] [CrossRef]
- Li, G.; Wang, B.; Wang, H.; Ma, J.; Xu, W.Q.; Li, Y.; Han, Y.; Sun, Q. Fe and/or Mn oxides supported on fly ash-derived SBA-15 for low-temperature NH3-SCR. Catal. Commun. 2018, 108, 82–87. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, T.; Liu, H.; Chen, D.; Xu, B.; Qing, C. Low temperature SCR reaction over Nano-Structured Fe-Mn Oxides: Characterization, performance, and kinetic study. Appl. Surf. Sci. 2018, 457, 1116–1125. [Google Scholar] [CrossRef]
- Huang, T.-J.; Zhang, Y.-P.; Zhuang, K.; Lu, B.; Zhu, Y.-W.; Shen, K. Preparation of honeycombed holmium-modified Fe-Mn/TiO2 catalyst and its performance in the low temperature selective catalytic reduction of NOx. J. Fuel Chem. Technol. 2018, 46, 319–327. [Google Scholar] [CrossRef]
- Ke, Z.; Feng, Y.; Mingyuan, Z.; Jianming, D.; Xugen, W.; Jinli, Z.; Bin, D. Enhanced Low Temperature NO Reduction Performance via MnOx-Fe2O3/Vermiculite Monolithic Honeycomb Catalysts. Catalysts 2018, 8, 100. [Google Scholar] [CrossRef]
- Wang, X.; Wu, S.; Zou, W.; Yu, S.; Gui, K.; Dong, L. Fe-Mn/Al2O3 catalysts for low temperature selective catalytic reduction of NO with NH3. Chin. J. Catal. 2016, 37, 1314–1323. [Google Scholar] [CrossRef]
- Zhang, M.; Li, C.; Qu, L.; Fu, M.; Zeng, G.; Fan, C.; Ma, J.; Zhan, F. Catalytic oxidation of NO with O2 over FeMnOx/TiO2: Effect of iron and manganese oxides loading sequences and the catalytic mechanism study. Appl. Surf. Sci. 2014, 300, 58–65. [Google Scholar] [CrossRef]
- Fang, N.; Guo, J.; Shu, S.; Luo, H.; Chu, Y.; Li, J. Enhancement of low-temperature activity and sulfur resistance of Fe0.3Mn0.5Zr0.2 catalyst for NO removal by NH3-SCR. Chem. Eng. J. 2017, 325, 114–123. [Google Scholar] [CrossRef]
- Boningari, T.; Pappas, D.K.; Ettireddy, P.R.; Kotrba, A.; Smirniotis, P.G. Influence of SiO2 on M/TiO2 (M = Cu, Mn, and Ce) Formulations for Low-Temperature Selective Catalytic Reduction of NOx with NH3: Surface Properties and Key Components in Relation to the Activity of NOx Reduction. Ind. Eng. Chem. Res. 2015, 54, 2261–2273. [Google Scholar] [CrossRef]
- France, L.J.; Yang, Q.; Li, W.; Chen, Z.; Guang, J.; Guo, D.; Wang, L.; Li, X. Ceria modified FeMnOx—Enhanced performance and sulphur resistance for low-temperature SCR of NOx. Appl. Catal. B Environ. 2017, 206, 203–215. [Google Scholar] [CrossRef]
- Cao, L.; Chen, L.; Wu, X.; Ran, R.; Xu, T.; Chen, Z.; Weng, D. TRA and DRIFTS studies of the fast SCR reaction over CeO2/TiO2 catalyst at low temperatures. Appl. Catal. A Gen. 2018, 557, 46–54. [Google Scholar] [CrossRef]
- Panahi, P.N.; Delahay, G.; Mousavi, S.M. Activity of gamma-Al2O3-based Mn, Cu, and Co oxide nanocatalysts for selective catalytic reduction of nitric oxide with ammonia. Turk. J. Chem. 2017, 41, 272–281. [Google Scholar] [CrossRef]
- Zhao, W.; Tang, Y.; Wan, Y.; Li, L.; Yao, S.; Li, X.; Gu, J.; Li, Y.; Shi, J. Promotion effects of SiO2 or/and Al2O3 doped CeO2/TiO2 catalysts for selective catalytic reduction of NO by NH3. J. Hazard. Mater. 2014, 278, 350–359. [Google Scholar] [CrossRef]
- Xu, H.; Liu, S.; Wang, Y.; Lin, Q.; Lin, C.; Lan, L.; Wang, Q.; Chen, Y. Promotional effect of Al2O3 on WO3/CeO2-ZrO2 monolithic catalyst for selective catalytic reduction of nitrogen oxides with ammonia after hydrothermal aging treatment. Appl. Surf. Sci. 2018, 427, 656–669. [Google Scholar] [CrossRef]
- Gao, Y.; Luan, T.; Zhang, W.; Li, H. The promotional effects of cerium on the catalytic properties of Al2O3-supported MnFeOx for NO oxidation and fast SCR reaction. Res. Chem. Intermed. 2018, in press. [Google Scholar] [CrossRef]
- Wang, C.; Yu, F.; Zhu, M.; Wang, X.; Dan, J.; Zhang, J.; Cao, P.; Dai, B. Microspherical MnO2-CeO2-Al2O3 mixed oxide for monolithic honeycomb catalyst and application in selective catalytic reduction of NOx with NH3 at 50–150 °C. Chem. Eng. J. 2018, 346, 182–192. [Google Scholar] [CrossRef]
- Qin, C.; Dang, X.; Huang, J.; Teng, J.; Huang, X. Plasma-catalytic oxidation of adsorbed toluene on Ag–Mn/γ-Al2O3: Comparison of gas flow-through and gas circulation treatment. Chem. Eng. J. 2016, 299, 85–92. [Google Scholar] [CrossRef]
- Fu, H.; Sun, S.; Yang, X.; Li, W.; An, X.; Zhang, H.; Dong, Y.; Jiang, X.; Yu, A. A facile coating method to construct uniform porous α-Fe2O3@TiO2 core-shell nanostructures with enhanced solar light photocatalytic activity. Powder Technol. 2018, 328, 389–396. [Google Scholar] [CrossRef]
- Kapteijn, F.; Singoredjo, L.; Andreini, A.; Moulijn, J.A. Activity and selectivity of pure manganese oxides in the selective catalytic reduction of nitric oxide with ammonia. Appl. Catal. B Environ. 1994, 3, 173–189. [Google Scholar] [CrossRef]
- Pérez Vélez, R.; Ellmers, I.; Huang, H.; Bentrup, U.; Schünemann, V.; Grünert, W.; Brückner, A. Identifying active sites for fast NH3-SCR of NO/NO2 mixtures over Fe-ZSM-5 by operando EPR and UV–vis spectroscopy. J. Catal. 2014, 316, 103–111. [Google Scholar] [CrossRef]
- Schwidder, M.; Heikens, S.; De Toni, A.; Geisler, S.; Berndt, M.; Brückner, A.; Grünert, W. The role of NO2 in the selective catalytic reduction of nitrogen oxides over Fe-ZSM-5 catalysts: Active sites for the conversion of NO and of NO/NO2 mixtures. J. Catal. 2008, 259, 96–103. [Google Scholar] [CrossRef]
- Wang, C.; Yu, F.; Zhu, M.; Tang, C.; Dong, L.; Dai, B. Synthesis of Both Powdered and Preformed MnOx-CeO2-Al2O3 Catalysts by Self-Propagating High-Temperature Synthesis for the Selective Catalytic Reduction of NOx with NH3. ACS Omega 2018, 3, 5692–5703. [Google Scholar] [CrossRef]
- Gao, G.; Shi, J.-W.; Liu, C.; Gao, C.; Fan, Z.; Niu, C. Mn/CeO2 catalysts for SCR of NOx with NH3: Comparative study on the effect of supports on low-temperature catalytic activity. Appl. Surf. Sci. 2017, 411, 338–346. [Google Scholar] [CrossRef]
- Wang, T.; Wan, Z.; Yang, X.; Zhang, X.; Niu, X.; Sun, B. Promotional effect of iron modification on the catalytic properties of Mn-Fe/ZSM-5 catalysts in the Fast SCR reaction. Fuel Process. Technol. 2018, 169, 112–121. [Google Scholar] [CrossRef]
- Huang, B.; Yu, D.; Sheng, Z.; Yang, L. Novel CeO2@TiO2 core–shell nanostructure catalyst for selective catalytic reduction of NOx with NH3. J. Environ. Sci. 2017, 55, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Boningari, T.; Somogyvari, A.; Smirniotis, P.G. Ce-Based Catalysts for the Selective Catalytic Reduction of NOx in the Presence of Excess Oxygen and Simulated Diesel Engine Exhaust Conditions. Ind. Eng. Chem. Res. 2017, 56, 5483–5494. [Google Scholar] [CrossRef]
- Yao, X.; Zhao, R.; Chen, L.; Du, J.; Tao, C.; Yang, F.; Dong, L. Selective catalytic reduction of NOx by NH3 over CeO2 supported on TiO2: Comparison of anatase, brookite, and rutile. Appl. Catal. B Environ. 2017, 208, 82–93. [Google Scholar] [CrossRef]
- Yang, S.; Liao, Y.; Xiong, S.; Qi, F.; Dang, H.; Xiao, X.; Li, J. N2 Selectivity of NO Reduction by NH3 over MnOx-CeO2: Mechanism and Key Factors. J. Phys. Chem. C 2014, 118, 21500–21508. [Google Scholar] [CrossRef]
- Wang, B.; Chi, C.; Xu, M.; Wang, C.; Meng, D. Plasma-catalytic removal of toluene over CeO2-MnOx catalysts in an atmosphere dielectric barrier discharge. Chem. Eng. J. 2017, 322, 679–692. [Google Scholar] [CrossRef]
- Xiong, Y.; Tang, C.; Yao, X.; Zhang, L.; Li, L.; Wang, X.; Deng, Y.; Gao, F.; Dong, L. Effect of metal ions doping (M = Ti4+, Sn4+) on the catalytic performance of MnOx/CeO2 catalyst for low temperature selective catalytic reduction of NO with NH3. Appl. Catal. A Gen. 2015, 495, 206–216. [Google Scholar] [CrossRef]
- Yao, X.; Ma, K.; Zou, W.; He, S.; An, J.; Yang, F.; Dong, L. Influence of preparation methods on the physicochemical properties and catalytic performance of MnOx-CeO2 catalysts for NH3-SCR at low temperature. Chin. J. Catal. 2017, 38, 146–159. [Google Scholar] [CrossRef]
- Zhu, X.; Tu, X.; Mei, D.; Zheng, C.; Zhou, J.; Gao, X.; Luo, Z.; Ni, M.; Cen, K. Investigation of hybrid plasma-catalytic removal of acetone over CuO/γ-Al2O3 catalysts using response surface method. Chemosphere 2016, 155, 9–17. [Google Scholar] [CrossRef]
- Stanciulescu, M.; Caravaggio, G.; Dobri, A.; Moir, J.; Burich, R.; Charland, J.P.; Bulsink, P. Low-temperature selective catalytic reduction of NOx with NH3 over Mn-containing catalysts. Appl. Catal. B Environ. 2012, 123–124, 229–240. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, F.; Li, H.; Yang, Q.; Wang, L.; Li, X. Low-Temperature Selective Catalytic Reduction of NOx with NH3 over Fe–Mn Mixed-Oxide Catalysts Containing Fe3Mn3O8 Phase. Ind. Eng. Chem. Res. 2012, 51, 202–212. [Google Scholar] [CrossRef]
- Cao, F.; Su, S.; Xiang, J.; Wang, P.; Hu, S.; Sun, L.; Zhang, A. The activity and mechanism study of Fe–Mn–Ce/γ-Al2O3 catalyst for low temperature selective catalytic reduction of NO with NH3. Fuel 2015, 139, 232–239. [Google Scholar] [CrossRef]
- Wu, S.; Yao, X.; Zhang, L.; Cao, Y.; Zou, W.; Li, L.; Ma, K.; Tang, C.; Gao, F.; Dong, L. Improved low temperature NH3-SCR performance of FeMnTiOx mixed oxide with CTAB-assisted synthesis. Chem. Commun. 2015, 51, 3470–3473. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhang, X.; Su, D.; Wang, Z.; Chang, J.; Ma, C. NO reduction by CO over copper catalyst supported on mixed CeO2 and Fe2O3: Catalyst design and activity test. Appl. Catal. B Environ. 2018, 239, 485–501. [Google Scholar] [CrossRef]
- Jin, R.; Liu, Y.; Wu, Z.; Wang, H.; Gu, T. Low-temperature selective catalytic reduction of NO with NH3 over MnCe oxides supported on TiO2 and Al2O3: A comparative study. Chemosphere 2010, 78, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Grossale, A.; Nova, I.; Tronconi, E. Ammonia blocking of the “Fast SCR” reactivity over a commercial Fe-zeolite catalyst for Diesel exhaust aftertreatment. J. Catal. 2009, 265, 141–147. [Google Scholar] [CrossRef]
- Nova, I.; Ciardelli, C.; Tronconi, E.; Chatterjee, D.; Bandl-Konrad, B. NH3-SCR of NO over a V-based catalyst: Low-T redox kinetics with NH3 inhibition. AIChE J. 2006, 52, 3222–3233. [Google Scholar] [CrossRef]
- Gao, C.; Shi, J.-W.; Fan, Z.; Wang, B.; Wang, Y.; He, C.; Wang, X.; Li, J.; Niu, C. “Fast SCR” reaction over Sm-modified MnOx-TiO2 for promoting reduction of NOx with NH3. Appl. Catal. A Gen. 2018, 564, 102–112. [Google Scholar] [CrossRef]
- Grossale, A.; Nova, I.; Tronconi, E.; Chatterjee, D.; Weibel, M. The chemistry of the NO/NO2–NH3 “fast” SCR reaction over Fe-ZSM5 investigated by transient reaction analysis. J. Catal. 2008, 256, 312–322. [Google Scholar] [CrossRef]
- Iwasaki, M.; Shinjoh, H. A comparative study of “standard”, “fast” and “NO2” SCR reactions over Fe/zeolite catalyst. Appl. Catal. A Gen. 2010, 390, 71–77. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Wang, P.; Hu, W.; Zhang, S.; Shi, Q.; Zhan, S. Low-temperature selective catalytic reduction of NOx with NH3 over MnFeOx nanorods. Chem. Eng. J. 2017, 330, 213–222. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Ablikim, W.; Wang, J.; Chang, H.; Ma, L.; Xu, J.; Ge, M.; Arandiyan, H. CeO2–WO3 Mixed Oxides for the Selective Catalytic Reduction of NOx by NH3 Over a Wide Temperature Range. Catal. Lett. 2011, 141, 1859–1864. [Google Scholar] [CrossRef]
- Liu, Z.; Yi, Y.; Zhang, S.; Zhu, T.; Zhu, J.; Wang, J. Selective catalytic reduction of NOx with NH3 over Mn-Ce mixed oxide catalyst at low temperatures. Catal. Today 2013, 216, 76–81. [Google Scholar] [CrossRef]
- Yang, S.; Li, J.; Wang, C.; Chen, J.; Ma, L.; Chang, H.; Chen, L.; Peng, Y.; Yan, N. Fe-Ti spinel for the selective catalytic reduction of NO with NH3: Mechanism and structure-activity relationship. Appl. Catal. B Environ. 2012, 117–118, 73–80. [Google Scholar] [CrossRef]

| Samples | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| Fe30−Ce/Al | 58.2 | 0.33 | 28.76 |
| Mn10Fe20−Ce/Al | 77.4 | 0.56 | 23.84 |
| Mn15Fe15−Ce/Al | 122.7 | 0.73 | 18.06 |
| Mn20Fe10−Ce/Al | 88.2 | 0.54 | 33.60 |
| Mn30−Ce/Al | 73.3 | 0.50 | 37.99 |
| Samples | Binding Energy (eV)/Atomic Composition (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mn | Fe | Ce | O | ||||||
| Mn2+ 2p3/2 | Mn3+ 2p3/2 | Mn4+ 2p3/2 | Fe2+ 2p3/2 | Fe3+ 2p3/2 | Ce3+ 3d5/2 | Ce4+ 3d5/2 | Oα 1s | Oβ 1s | |
| Mn10Fe20−Ce/Al | 640.8/33.9 | 642.1/36.2 | 644.1/29.9 | 709.8/28.1 | 712.0/71.9 | 885.819.4 | 882.6/80.6 | 530.3/76.6 | 531.7/23.4 |
| Mn15Fe15−Ce/Al | 641.3/6.8 | 642.5/39.4 | 644.4/53.8 | 709.1/52.4 | 711.3/47.6 | 885.5/33.8 | 882.4/66.2 | 529.7/62.6 | 531.2/37.4 |
| Mn20Fe10−Ce/Al | 641.1/12.7 | 642.247.2 | 644.2/40.1 | 709.4/42.7 | 711.8/57.3 | 885.7/23.8 | 882.5/76.2 | 529.4/70.2 | 531.3/29.8 |
| Samples | Temperature (°C)/H2 Consumption (mmol/g) | |||||
|---|---|---|---|---|---|---|
| Peak 1 | Peak 2 | Peak 3 | Peak 4 | Peak 5 | Total | |
| Mn10Fe20−Ce/Al | 292/1.45 | 366/2.30 | 434/0.80 | 643/0.27 | 761/0.11 | --/4.93 |
| Mn15Fe15−Ce/Al | 278/4.86 | 353/0.85 | 432/0.16 | 597/0.14 | 742/0.10 | --/6.11 |
| Mn20Fe10−Ce/Al | 289/2.92 | 361/2.13 | 433/0.51 | 582/0.28 | 723/0.13 | --/5.97 |
| Samples | Temperature (°C)/NH3 Composition (mmol/g) | Temperature (°C)/NO Composition (mmol/g) | ||||||
|---|---|---|---|---|---|---|---|---|
| Peak 1 | Peak 2 | Peak 3 | Total | Peak 1 | Peak 2 | Peak 3 | Total | |
| Mn10Fe20−Ce/Al | 213/0.16 | 383/0.32 | 547/0.76 | --/1.24 | 271/0.32 | 438/0.56 | 597/0.70 | --/1.58 |
| Mn15Fe15−Ce/Al | 211/0.38 | 381/0.68 | 545/0.55 | --/1.61 | 264/0.44 | 433/0.67 | 569/0.64 | --/1.75 |
| Mn20Fe10−Ce/Al | 210/0.56 | 371/0.71 | 542/0.29 | --/1.56 | 261/0.59 | 423/0.69 | 588/0.39 | --/1.67 |
| Samples | Mn | Fe | Ce | Mn:Fe | |||
|---|---|---|---|---|---|---|---|
| Mass Fraction (%) | Molar Fraction (%) | Mass Fraction (%) | Molar Fraction (%) | Mass Fraction (%) | Molar Fraction (%) | MOLAR RATIO | |
| Fe30−Ce/Al | 0 | 0 | 30.0 | 93.8 | 5.0 | 6.2 | -- |
| Mn10Fe20−Ce/Al | 10.0 | 31.3 | 20.0 | 62.5 | 5.0 | 6.2 | 0.5:1 |
| Mn15Fe15−Ce/Al | 15.0 | 47.0 | 15.0 | 46.8 | 5.0 | 6.2 | 1:1 |
| Mn20Fe10−Ce/Al | 20.0 | 62.6 | 10.0 | 31.2 | 5.0 | 6.2 | 2:1 |
| Mn30−Ce/Al | 30.0 | 93.9 | 0 | 0 | 5.0 | 6.1 | -- |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Luan, T.; Zhang, M.; Zhang, W.; Feng, W. Structure–Activity Relationship Study of Mn/Fe Ratio Effects on Mn−Fe−Ce−Ox/γ-Al2O3 Nanocatalyst for NO Oxidation and Fast SCR Reaction. Catalysts 2018, 8, 642. https://doi.org/10.3390/catal8120642
Gao Y, Luan T, Zhang M, Zhang W, Feng W. Structure–Activity Relationship Study of Mn/Fe Ratio Effects on Mn−Fe−Ce−Ox/γ-Al2O3 Nanocatalyst for NO Oxidation and Fast SCR Reaction. Catalysts. 2018; 8(12):642. https://doi.org/10.3390/catal8120642
Chicago/Turabian StyleGao, Yan, Tao Luan, Mingyang Zhang, Wenke Zhang, and Wenchen Feng. 2018. "Structure–Activity Relationship Study of Mn/Fe Ratio Effects on Mn−Fe−Ce−Ox/γ-Al2O3 Nanocatalyst for NO Oxidation and Fast SCR Reaction" Catalysts 8, no. 12: 642. https://doi.org/10.3390/catal8120642
APA StyleGao, Y., Luan, T., Zhang, M., Zhang, W., & Feng, W. (2018). Structure–Activity Relationship Study of Mn/Fe Ratio Effects on Mn−Fe−Ce−Ox/γ-Al2O3 Nanocatalyst for NO Oxidation and Fast SCR Reaction. Catalysts, 8(12), 642. https://doi.org/10.3390/catal8120642