Abstract
TiO2 photocatalysts are limited to ultraviolet light photo-activation, however, by coupling with NaYF4:(Yb,Er) they can utilize visible light activation, but with low efficiencies. In order to enhance visible light photo-activity, CuO nanoparticles were coupled with NaYF4:(Yb,Er) by mechanical ball-milling and coated with TiO2-sol. The coupling of CuO nanoparticles with NaYF4:(Yb,Er)/TiO2 caused the formation of a NaYF4:(Yb,Er)-CuO/TiO2 composite capable of visible absorption with a remarkably reduced band gap of ~2.5 eV. The NaYF4:(Yb,Er)-CuO/TiO2 composite in H2O2 showed the most efficient methylene blue (MB) degradation efficiency of more than 99.5% after exposure to visible light.
1. Introduction
TiO2, as a semiconductor, is well known for its chemical stability, non-toxicity and ultraviolet (UV) photo-activation [1,2]. This UV photo-activation limits TiO2 to partial utilization of the solar spectrum (3–5%) [3]. Several research studies have been conducted to reduce the TiO2 band gap and render it photo-active in the visible region [4,5]. These methods include doping with metal ions (Ag, Co, Fe, Cr, etc.) [6,7,8], non-metals (N, C, F, etc.) [9,10] and coupling TiO2 with metal oxides (SiO2, SnO2, WO3, etc.) [11,12,13] or phosphor materials (CaAl2O4:(Eu,Nd), NaYF4:(Yb,Er)) [14,15,16].
Interestingly, the coupling of TiO2 with upconversion phosphors can remarkably extend utilization of the whole solar spectrum [17]. The major advantage of upconversion phosphor is the ability to convert even low near-infrared (NIR) photons to higher energy photons in the UV and visible light range [18,19]. In this way, the NaYF4:(Yb, Er) upconversion phosphor characteristic emerges from Yb3+ ions which absorb the 980 nm wavelength spectrum and transfer energy to Er3+ ions which then emit visible light photons. Er3+ ions emit visible light at 450, 525, and 650 nm wavelengths due to transitions from its ground state 4I15/2 to 4F7/2, 4H11/2, 4F9/2 and 4G9/2, respectively [20,21,22]. For this reason, Yb3+ ions are termed sensitizers while Er3+ ions are activators. The outstanding photo-stability of upconversion phosphors has propelled their extensive research for dye sensitized solar cells, bio-imaging and UV-vis-NIR photocatalysis applications [19,23].
In a NaYF4:(Yb,Er)/TiO2 composite, NaYF4:(Yb,Er) phosphor can offer an efficient catalyst support and provide the interfacial energy band bending so that the TiO2 absorption edge can be extended to the visible region [14,22]. However, the visible light photocatalysis of a NaYF4:(Yb,Er)/TiO2 composite shows low efficiency. For example, complete photo-degradation of dye pollutants mixed with phosphor-TiO2 photocatalyst occurs after 10 h of exposure to a visible light source [17,24].
Photocatalysis efficiency depends on various factors which include intensity of illuminating light, temperature, concentration of dye pollutants and the amount of doping elements in the photocatalyst or the amount of photocatalyst in the reactor [25,26,27]. For instance, high photocatalyst loading in the reactor can significantly improve photocatalysis efficiency [28,29]. This is mainly because more photocatalyst particles increase the number of photo-active sites which enhance the reaction rate [30]. However, upconversion phosphors are synthesized with rare and scarce earth elements which are less cost competitive [31]. Hence, there is a need to synthesize a composite photocatalyst with high photocatalytic ability even at low catalyst loading in the photo-reactor.
One promising method to improve photocatalytic efficiencies involves the synthesis of multi-composites [10,32]. Recently, several upconversion phosphors of multi-composites were synthesized to produce core–shell structures with TiO2 and a third compound. For example, NaYF4:(Yb,Er)/TiO2 coupled with SiO2 has been reported. SiO2 in the NaYF4:(Yb,Er)/TiO2 composite can improve photovoltaic efficiency as well as facilitating energy transfer from phosphor to the TiO2 conduction band [33,34]. However, in this multi-composite, the photocatalytic efficiency is dependent on the thickness of the outer shell. Thus, a thick silica coating can hinder light absorption in the inner phosphor particles. The most important property of a photocatalyst is the ability to maintain chemical stability after a photo-degradation cycle. This photo-stability property is beneficial for recyclability [35,36]. Unfortunately, the core–shell structures are reported to be unstable during separation and centrifuge washing steps. Therefore, recyclability is limited for the core–shell photocatalyst [19].
Surprisingly, the coupling of NaYF4:(Yb,Er)/TiO2 with visible light-active CuO (1.2–2.0 eV) has not been extensively studied yet. Moreover, CuO is abundantly available, chemically stable and photo-active in H2O2 aqueous media [37]. It is important to note that H2O2 has been used to remove organic impurities with photocatalytic materials under light exposure [38,39,40]. The substantial advantage of coupling NaYF4:(Yb,Er) with TiO2 and CuO is to form composites with improved band structure for efficiently utilizing the broad solar spectrum in photocatalysis [22,33].
In this study, CuO nanoparticles were combined with the NaYF4:(Yb,Er)/TiO2 composite to enhance visible light absorption and photocatalytic activity. The effect of H2O2 on photocatalytic performance and the recyclability of NaYF4:(Yb,Er)-CuO/TiO2 photocatalyst were also examined.
2. Results and Discussion
Figure 1 shows x-ray diffraction (XRD) patterns for NaYF4:(Yb,Er), CuO, NaYF4:(Yb,Er)-CuO and NaYF4:(Yb,Er)-CuO/TiO2 composite. The pure phosphor was referenced to the characteristic peaks of JCPDS No. 77-2042 while the CuO nanoparticles were referenced to JCPDS No. 045-0937. With increasing annealing temperature from 350 °C to 550 °C (from Figure 1c–f), the NaYF4:(Yb,Er) phosphor main peaks occurred at (111), (200), (220) and (311) were diminishing. While CuO characteristic peaks corresponding to (002), (111), (202) and (020) emerged along the NaYF4:(Yb,Er)-CuO composites. Besides NaYF4:(Yb,Er) and CuO characteristic peaks, new peaks occurred at 17°, 29°, 30°, 39°, 43°, 53° and 77° and are suggested to be (hexagonal) β-NaYF4. These diffraction peaks corresponding to β-NaYF4 are indexed in JCPDS No. 28-1192 as (100), (110), (101), (111), (201), (300) and (302) respectively. The thermal treatment may have induced the phase transition from α to β-NaYF4. Furthermore, due to the co-existence of three phases (cubic NaYF4, hexagonal NaYF4 and CuO), it is expected that intermetallic compounds are formed during thermal treatment. Although no other Cu phases were identified at different annealing conditions, a 450 °C annealing temperature is known for pure CuO phases with band gaps between 1.2 eV and 2 eV [41]. Therefore, NaYF4:(Yb,Er)-CuO composites annealed at 450 °C were utilized for TiO2 coating. TiO2 peaks could not be observed in the NaYF4:(Yb,Er)-CuO/TiO2 composite because its amount was beyond the XRD detection limit.
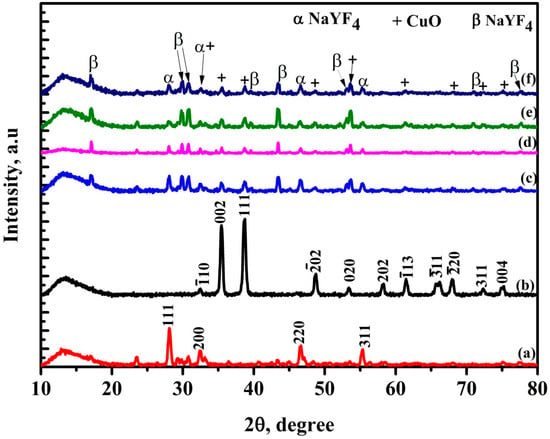
Figure 1.
XRD spectra of (a) NaYF4:(Yb,Er), (b) CuO, (c) NaYF4:(Yb,Er)-CuO at 350 °C, (d) NaYF4:(Yb,Er)-CuO at 450 °C, (e) NaYF4:(Yb,Er)-CuO at 550 °C, and (f) NaYF4:(Yb,Er)-CuO/TiO2 composite at 450 °C.
Figure 2 shows SEM images of (a) NaYF4:(Yb,Er), (b) CuO, (c) NaYF4:(Yb,Er)-CuO, (d) NaYF4:(Yb,Er)-CuO/TiO2. The prepared NaYF4:(Yb,Er) phosphor has agglomerated particles as shown in Figure 2a around 2 µm in diameter. However, the CuO particles in Figure 2b are dense spherical particles. In Figure 2c, NaYF4:(Yb,Er)-CuO composite powders consist of particles of sizes below 1 µm. It is evident that mechanical ball-milling might reduce the size of NaYF4:(Yb,Er) phosphor agglomerates. The NaYF4:(Yb,Er)-CuO/TiO2 composite in Figure 2d consists of agglomerates with dense particles of nanometer range, due to TiO2 coating. Figure 2e shows the particle size distribution for the NaYF4:(Yb,Er)-CuO/TiO2 composite. The particle sizes are ranging between 0.8 and 4 µm. The EDS spectra for the NaYF4:(Yb,Er)-CuO/TiO2 composite confirmed the presence of all elements, as shown in Figure 2f.
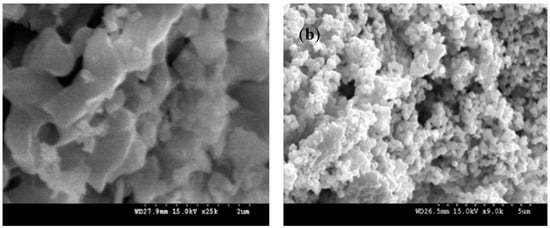
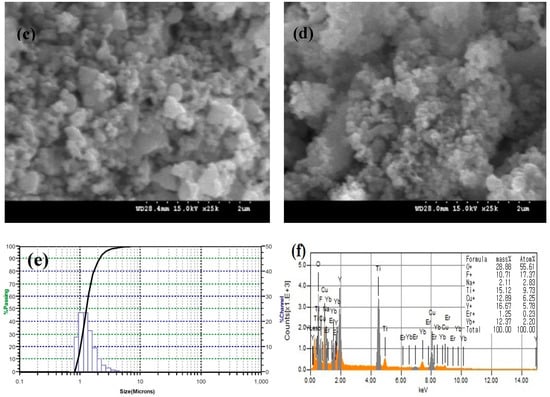
Figure 2.
SEM images of (a) NaYF4:(Yb,Er), ×25k, (b) CuO, ×9k, (c) NaYF4:(Yb,Er)-CuO, ×25k, (d) NaYF4:(Yb,Er)-CuO/TiO2, x25k, (e) particle size distribution of NaYF4:(Yb,Er)-CuO/TiO2 composite and (f) EDS spectra for the NaYF4:(Yb,Er)-CuO/TiO2 composite.
Figure 3 shows N2 adsorption-desorption isotherms for the NaYF4:(Yb,Er)/TiO2 and NaYF4:(Yb,Er)-CuO/TiO2 photocatalyst composites. The specific surface areas in the table insert in Figure 3 are 15.9 m2/g and 12.4 m2/g for NaYF4:(Yb,Er)/TiO2 and NaYF4:(Yb,Er)-CuO/TiO2, respectively, while the pore size and adsorbed volume are 8.93 nm and 0.0355 cm3/g for NaYF4:(Yb,Er)/TiO2 and 13.3 nm and 0.0413 cm3/g for NaYF4:(Yb,Er)-CuO/TiO2, respectively. The specific surface area of pure NaYF4:(Yb,Er) in other previous research is 11.5 m2/g [22]. The differences in the specific surface area, pore volume and diameter are dependent on the synthesis variables and changes in crystalline composition of the photocatalysts. Therefore, the low surface area and high volume adsorbed/pore diameter observed in the NaYF4:(Yb,Er)-CuO/TiO2 composite confirms the change in crystallinity with CuO nanoparticles coupling.
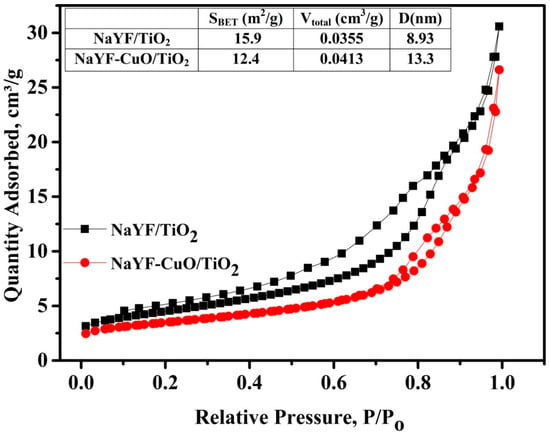
Figure 3.
N2 adsorption-desorption isotherms of NaYF4:(Yb,Er)/TiO2 and NaYF4:(Yb,Er)-CuO/TiO2. NaYF represents NaYF4:(Yb,Er).
Figure 4a shows the UV-vis absorption spectra for NaYF4:(Yb,Er), CuO and NaYF4:(Yb,Er)-CuO/TiO2 samples vs. wavelength. The NaYF4:(Yb,Er)-CuO/TiO2 composite exhibited similar absorption characteristics to CuO, but with an extended UV absorption edge at 380 nm. This absorption edge is related to both TiO2 nanoparticles and NaYF4:(Yb,Er). The enlarged UV-vis absorption spectra in Figure 4b clearly shows absorption peaks at 380 nm, 522 nm and 655 nm which are characteristic absorption peaks for NaYF4:(Yb,Er) upconversion phosphors. These Er3+ absorption peaks were originated from the ground states 4I15/2 to 4G9/2, 4F7/2 and 4S3/2, respectively [42]. However, in the TiO2 coated NaYF4:(Yb,Er) phosphor, the 380 nm peak is not observed because it corresponds to the TiO2 absorption range. The estimated band energies for CuO, NaYF4:(Yb,Er)/TiO2 and NaYF4:(Yb,Er)-CuO/TiO2 are 1.5 eV, 3.18 eV and 2.5 eV, respectively, as shown in Figure 4c. The remarkable band gap reduction in NaYF4:(Yb,Er)-CuO/TiO2 typically signifies the composites can be photo-activated with both UV and visible light for photocatalysis reactions.
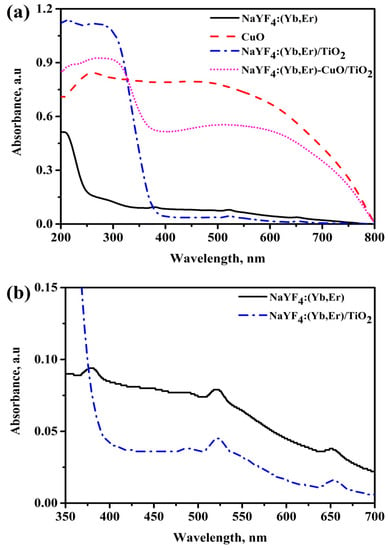
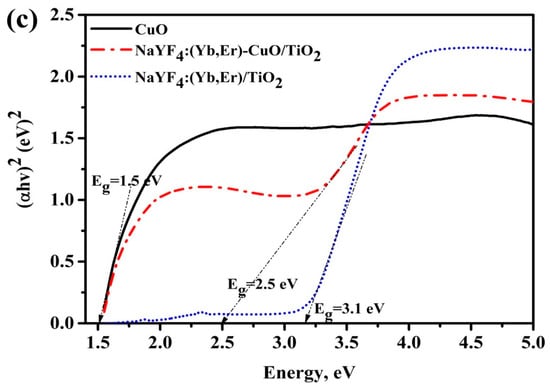
Figure 4.
UV-vis diffuse reflectance spectra for (a) NaYF4:(Yb,Er), CuO and NaYF4:(Yb,Er)-CuO/TiO2 composite; (b) enlargement spectra at 350–700 nm wavelength axis in (a) and (c) Kubelka-Munk plots with the band gap energies.
Figure 5a shows the photoluminescence spectra of NaYF4:(Yb,Er), NaYF4:(Yb,Er)-CuO and NaYF4:(Yb,Er)-CuO/TiO2. The intensity of the photoluminescence (PL) peaks denotes the extent of energy transfer efficiency [18]. Pure NaYF4:(Yb,Er) phosphor has visible light emission peaks at 450 nm, 545 nm, 660 nm and a near infrared peak at 825 nm. However, the emission peaks diminish with coupling NaYF4:(Yb,Er) with CuO nanoparticles, TiO2 coating on NaYF4:(Yb,Er) phosphor and in NaYF4:(Yb,Er)-CuO composite. Although energy transfer efficiency is evaluated by the decrease in peak intensities, in TiO2 coated samples, the peak reduction is generally referenced to light emission hindrance by TiO2 nanoparticles [14]. However, the overall peak suppression can be explained by considering the band structure of the NaYF4:(Yb,Er)-CuO/TiO2 composite. Figure 5c shows the schematic band structure of the NaYF4:(Yb,Er)-CuO/TiO2 composite and the energy transfer processes labelled (A), (B), (C) and (D). Firstly, if process (A) is considered as excitation of NaYF4:(Yb,Er) phosphor with 980 nm illumination, because of its luminescent characteristics, energy photons in the visible and NIR are emitted. Thus, as seen in Figure 5a, emission peaks were observed at 450 nm, 545 nm, 660 nm and 825 nm. Secondly, if process (B) is considered as the energy transfer route for light photons emitted from NaYF4:(Yb,Er), the visible light peaks are absorbed by CuO. However, the emission peaks are suppressed at (C) in the NaYF4:(Yb,Er)-CuO composite. Finally, when considering light photons emitted by NaYF4:(Yb,Er) phosphor at (B) are transferred to TiO2 at (C) via CuO, energy losses are inevitable at (D). The reasons for peak suppression being that energy is lost through multi-phonon vibrations and the blockage of phosphor emission centers by TiO2 and CuO nanoparticles [17].
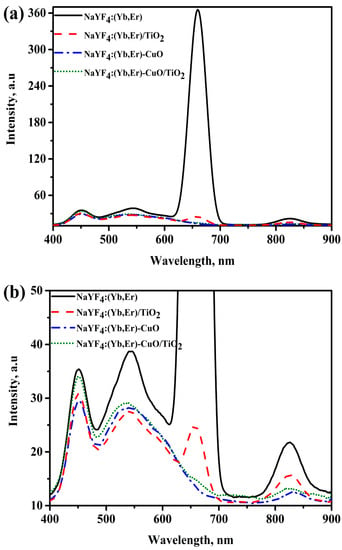
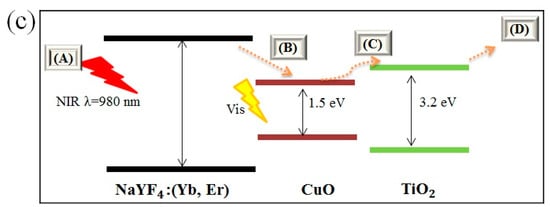
Figure 5.
Photoluminescence spectra of (a) NaYF4:(Yb,Er), NaYF4:(Yb,Er)-CuO and NaYF4:(Yb,Er)-CuO/TiO2; (b) enlargement spectra at 10 to 50 intensity axes and (c) schematic band structure and energy transfer routes in the NaYF4:(Yb,Er)-CuO/TiO2 composites.
Figure 6a shows the degradation efficiency of the methylene blue (MB) solution with H2O2 only (no photocatalyst), without and with H2O2 for NaYF4:(Yb,Er)/TiO2 and NaYF4:(Yb,Er)-CuO/TiO2 under visible light for 1 h. The NaYF4:(Yb,Er)-CuO/TiO2 composites exhibit the fastest degradation efficiency, whereby 60% of the MB solution is degraded within 1 h. The photocatalytic reaction of NaYF4:(Yb,Er)/TiO2 without H2O2 oxidant molecules is very slow, but with hydrogen peroxide the reaction is enhanced. This is attributed to the unique role of hydrogen peroxide decomposing in the presence of a photocatalyst and light to give hydroxyl and superoxide ions which attack and degrade MB-dye molecules [39]. The photocatalytic degradation of MB solution with NaYF4:(Yb,Er)-CuO/TiO2 follows first order reaction kinetics as shown in Figure 6b. The rate constant for NaYF4:(Yb,Er)-CuO/TiO2 is 0.008 min−1. Whilst for NaYF4:(Yb,Er)/TiO2 without and with H2O2 is 0.0015 min−1 and 0.0043 min−1, respectively. Accordingly from the rate constants, the degradation with NaYF4:(Yb,Er)-CuO/TiO2 is five times and almost two times faster than in NaYF4:(Yb,Er)/TiO2 without and with H2O2, respectively.
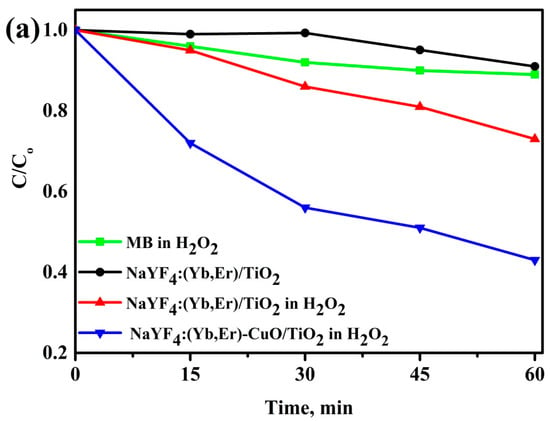
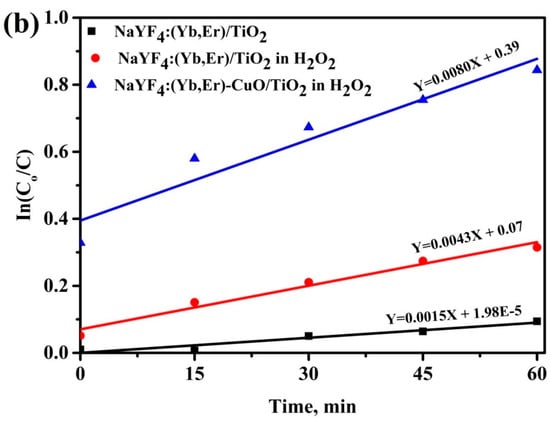
Figure 6.
Degradation efficiency of methylene blue (MB) solutions mixed with (a) NaYF4:(Yb,Er)-CuO/TiO2 composites and (b) rate kinetics for the samples in (a).
Figure 7a shows the photo-degradation efficiency of MB solution with and without H2O2 for three samples: NaYF4:(Yb,Er)/TiO2, NaYF4:(Yb,Er)-CuO and NaYF4:(Yb,Er)-CuO/TiO2 under visible light. Clearly, the NaYF4:(Yb,Er)-CuO/TiO2 composite in H2O2 has the most efficient photo-activity by decomposing the MB solution more than 99.5% after exposure to visible light. This photocatalytic activity has higher efficiency as compared to only TiO2-coated phosphor in our previous work [16] and other research [33,43]. The coupling of CuO nanoparticles with NaYF4:(Yb, Er)/TiO2 caused the formation of visible light-active nanocomposites. Thus, the NaYF4:(Yb, Er)-CuO/TiO2 nanocomposites and H2O2 oxidant molecules enhanced photo-activation and separation of electron-hole pairs for improved photocatalytic activity. Figure 7b shows the recyclability of the NaYF4:(Yb,Er)-CuO/TiO2 composite under visible light for three cycles. After the first run, the photocatalytic degradation efficiency of the MB solution decreased from almost 100% to 80% and almost 60% in the second and third cycle, respectively. The noticeable difference in photocatalyst efficiency is due to several factors relating to surface-adsorption characteristics, such as Cu leaching [44] and the possible loss of TiO2 nanoparticles [43]. Specifically, the centrifugal process and several washing steps during material preparation may result in a loss of TiO2 nanoparticles.
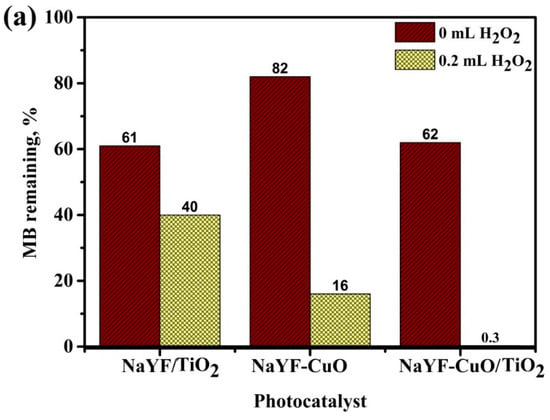
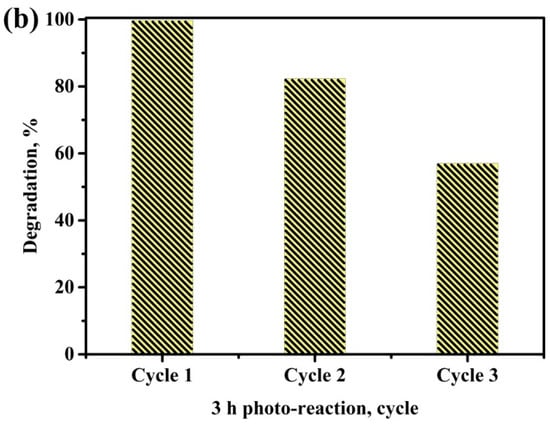
Figure 7.
Photo-degradation of the MB solution (a) with and without H2O2 for NaYF4:(Yb,Er)/TiO2, NaYF4:(Yb,Er)-CuO and NaYF4:(Yb,Er)-CuO/TiO2 under visible light for 3 h (NaYF represents NaYF4:(Yb,Er)) and (b) the recyclability of the NaYF4:(Yb,Er)-CuO/TiO2 composite.
The overall photocatalytic activity can be influenced by the chemical composition, specific surface area, optical property of the photocatalyst and the presence of H2O2 oxidant molecules [39]. Firstly, the change in compositional crystallinity by coupling NaYF4:(Yb,Er)/TiO2 with CuO evidently improved the photocatalytic activity. Moreover, the co-existence of CuO, α-NaYF4 and β-NaYF4 phases as confirmed by XRD further increases the TiO2 absorption edge towards visible light. Secondly, it is well known that the high specific surface area is related to the enhancement of photocatalytic activity. Nevertheless, the case is unique in the NaYF4:(Yb,Er)/TiO2 and NaYF4:(Yb,Er)-CuO/TiO2 photocatalyst composites. Thus, NaYF4:(Yb,Er)/TiO2 showed higher specific area (15.9 m2/g), but exhibited lower photocatalytic activity than the NaYF4:(Yb,Er)-CuO/TiO2 (12.4 m2/g) [45]. The explanation for this discrepancy is regardless of the specific surface area; the dominating property is the existence of a visible light-active CuO compound. Therefore, with CuO nanoparticle coupling (as the third compound in the NaYF4:(Yb,Er)-CuO/TiO2 composite) visible light absorption by composites is improved and these composites have more efficient charge transfer properties than only TiO2 coated phosphor [33]. Thirdly, the photoactivity is low without H2O2 oxidant molecules in the photo-reaction mixture of TiO2 coated composites (NaYF4:(Yb, Er)/TiO2 and NaYF4:(Yb,Er)-CuO/TiO2) and NaYF4:(Yb,Er)-CuO as exhibited in Figure 7a. Although photo-degradation up to 40% is observed in the TiO2-coated composites, the NaYF4:(Yb,Er)-CuO degraded only 20% of MB solution. This is due to the higher oxidizing ability of TiO2 as compared to CuO [46]. In contrast, the photocatalyst mixture with H2O2 oxidant molecules showed an improvement in photocatalytic activity of the NaYF4:(Yb,Er)/TiO2, NaYF4:(Yb,Er)-CuO and NaYF4:(Yb,Er)-CuO/TiO2. Thus, the H2O2 oxidant was photo-degraded to produce hydroxyl and super oxide ions which might degrade the MB-dye solution. The superior oxidizing power of H2O2 molecules has been utilized in several research studies to enhance the photocatalytic activity of pure CuO and its composite materials [47]. Finally, the major advantage of coupling CuO and TiO2 nanoparticles with phosphor is the inevitable alteration of the NaYF4:(Yb,Er)-CuO/TiO2 composites band structure, as observed in both visible light absorption and band gap estimations in Figure 4. The NaYF4:(Yb,Er) can also facilitate the electron-hole charge separation process which enhances photocatalytic efficiencies [17]. Additionally, the phosphor crystalline surface not only supports the nanoparticles, but it also facilitates energy transfer within the composite system as described in Figure 5c. Accordingly, the composites can also be utilized efficiently in solar harvesting systems.
3. Materials and Methods
NaYF4:(Yb, Er) phosphor was synthesized by solution combustion as described in our previous work [16]. A yttrium rare earth nitrate solution consisting of Y2O3 (99.99%, Daejung Chemicals & Metals Co., Ltd., Siheung-si, Gyeonggi-do, Korea), Yb2O3 (99.9%, Wako Pure Chemical Industries Ltd., Osaka, Japan) and Er2O3 (99.9%, Sigma Aldrich, Shanghai, China) with 0.77:0.2:0.03 molar ratios, respectively, was prepared under magnetic stirring in nitric acid. The fuel mixture of Na2SiF6 (98.0%, Duksan Pure Chemical Co., Ltd., Ansansi, Kyunggido, Korea), CO(NH2)2 (99.0%, Samchun Pure Chemical Co., Ltd., Pyeongtaek, Gyeonggi-do, Korea) and NH4HF2 (95.0%, Duksan Pure Chemical Co., Ltd., Ansansi, Kyunggido, Korea) was added to the rare earth yttrium nitrate solution and placed in a muffle furnace (SK1700-B30, Thermotechno Co., Siheung-si, Gyeonggi-do, Korea) for combustion at 650 °C for 5 min. Simultaneously, 4.5 g of Cu(NO3)2∙3H2O (99.5%, Junsei Chemical Co., Ltd., Tokyo, Japan) was dissolved in 100 mL of distilled water, following by a 1 mL acetic acid (99.0%, Duksan Pure Chemical Co., Ltd., Ansansi, Kyunggido, Korea) addition and magnetic stirring for 1 h at 100 °C. Then, 8 M NaOH (Duksan Pure Chemical Co., Ltd., Ansansi, Kyunggido, Korea) was added to obtain a black CuO precipitate followed by filtering, washing with distilled water and annealing at 500 °C for 4 h. Then, NaYF4:(Yb, Er)-CuO composites at 80:20 wt.% ratio were mixed with ball milling and annealed at 450 °C. Finally, NaYF4:(Yb, Er)-CuO composites were coated with 0.4 M TiO2-sol prepared from titanium (IV) n-butoxide (99.0%, Acros Organics, Morris, NJ, USA), 50 mL of ethyl alcohol (99.9%, Duksan Pure Chemical Co., Ltd., Ansansi, Kyunggido, Korea) and 10 mL of distilled water at 50 °C. The TiO2-coated composites were dried at 100 °C in an electric oven for 12 h and calcined at 450 °C for 2 h.
The synthesized samples of NaYF4:(Yb,Er)-CuO/TiO2 were characterized for crystallinity by a X-ray diffraction (XRD, D8 Discover, Bruker AXS GmbH, Karlsruhe, Germany) with Cu Kα radiation. The morphology of the powder samples was analyzed by scanning electron microscope (SEM, Hitachi S-4300, Hitachi Ltd., Tokyo, Japan). The chemical composition of the composite sample was examined by energy dispersive spectroscopy (EDS, JEOL, JP/JSM-6330F, Kyoto, Japan). The particle size analysis of the NaYF4:(Yb,Er)-CuO/TiO2 composite was obtained by a particle size analyzer (Microtrac, S-3500, Largo, FL, USA). The specific surface area of the photocatalyst was determined by the Brunauer-Emmett-Teller (BET) method on an N2 based chemisorption-physisorption micropore analyzer (3flex Micromeritics, Norcross, GA, USA). The photoluminescence (PL) spectra were obtained by a fluorescence spectrophotometer (F-4200, Hitachi, Tokyo, Japan) with an excitation slit of 5 nm and 10 nm emission slit. UV-vis diffuse reflectance spectra were observed using an UV-vis spectrophotometer (UV-1601, Shimadzu, Kyoto, Japan).
The photocatalytic activity was examined by monitoring the UV-vis absorbance of 2 ppm methylene blue (MB, 95.0%, Duksan Pure Chemical Co., Ltd., Ansansi, Kyunggido, Korea) solution under 200 W (clear, Ilkwang Co., Ltd., Dalseogu, Daegu, Korea) visible light irradiation with a 410 nm filter on top of beaker (UV L41 (W) 82 mm, Kenko Zeta, Tokina Co., Ltd., Tokyo, Japan). Prior to irradiation, photocatalyst powders weighing 100 mg were dispersed in a 100 mL beaker of MB solution. Then, the photo-reaction mixture was magnetically stirred for 30 min in darkness to achieve adsorption-desorption equilibria. For comparisons, photo-degradation efficiency was examined with 0.2 mL and without H2O2 (28.0%, Duksan Pure Chemical Co., Ltd., Ansansi, Kyunggido, Korea) oxidant molecules. The degradation rate of MB solution mixed with photocatalyst composites was studied by sampling a small amount of solution photo-degraded at 15 min intervals for 1 h. Then, the photocatalyst-mixed solution was further exposed to visible light for 2 h to achieve a 3 h degradation cycle. After the first 3 h cycle, the photocatalyst-mixed solution was centrifugated and washed with ethanol in water to remove adsorbed dye. Then, the photocatalyst powders were dried in an electric oven for 100 °C for 2 h. The recyclability test was carried out for 2 more cycles with the collected NaYF4:(Yb,Er)-CuO/TiO2 powders using the described photoactivity measurement. The photo-reaction beaker was placed in an ice-water bath to avoid temperature rise during the experiment. Photocatalyst concentration was maintained at 1 mg/mL of MB solution with 0.2 mL H2O2 oxidant. The photo-degraded MB solution was centrifugated and examined for absorption by an UV-vis-NIR spectrophotometer (UV-3150, Shimadzu, Kyoto, Japan).
4. Conclusions
A visible light photo-active NaYF4:(Yb,Er)-CuO/TiO2 composite has been synthesized. The increase in annealing temperature enhanced the crystallinity of the NaYF4:(Yb,Er)-CuO/TiO2 composite. The combination of NaYF4:(Yb,Er), CuO and TiO2 greatly enhances UV-vis optical properties and photocatalytic degradation efficiency for MB solution under visible light irradiation. The NaYF4:(Yb,Er)-CuO/TiO2 photocatalyst exhibited some recyclability of up to 60% in the third cycle, which could be beneficial for possible application in photocatalyst systems.
Author Contributions
Supervision, J.-S.K.; writing—original draft, S.M.; writing—review and editing, S.-C.J.
Funding
This research work was supported by a grant (17-CTAP-C133057-01) from the Ministry of Land, Infrastructure, and Transport of the Korean government.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Henderson, M.A. A surface science perspective on TiO2 photocatalysis. Surf. Sci. Rep. 2011, 66, 185–297. [Google Scholar] [CrossRef]
- Litter, M.I. Heterogeneous photocatalysis: Transition metal ions in photocatalytic systems. Appl. Catal. B Environ. 1999, 23, 89–114. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Yadav, H.M.; Kim, J.S. Solvothermal synthesis of anatase TiO2-graphene oxide nanocomposites and their photocatalytic performance. J. Alloys Compd. 2016, 688, 123–129. [Google Scholar] [CrossRef]
- Luna, A.L.; Valenzuela, M.A.; Justin, C.C.; Vázquez, P.; Rodriguez, J.L.; Avendaño, J.R.; Alfaro, S.; Tirado, S.; Garduño, A.; Rosa, J.M.D. Photocatalytic degradation of gallic acid over CuO-TiO2 composites under UV/Vis LEDs irradiation. Appl. Catal. A Gen. 2016, 521, 140–141. [Google Scholar] [CrossRef]
- Gupta, K.; Singh, R.P.; Pandey, A.; Pandey, A. Photocatalytic antibacterial performance of TiO2 and Ag-doped TiO2 against S. Aureus. P. Aeruginosa and E. Coli. Beilstein J. Nanotechnol. 2013, 4, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Matsuoka, M.; Ueshima, M.; Anpo, M. Recent developments in titanium oxide-based photocatalysts. Appl. Catal. A Gen. 2007, 325, 1–14. [Google Scholar] [CrossRef]
- Kubacka, A.; Colón, G.; García, M.F. Cationic (V, Mo, Nb, W) doping of TiO2-anatase: A real alternative for visible light-driven photocatalysts. Catal. Today 2009, 143, 286–292. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Selli, E. Doping TiO2 with p-block elements: Effects on photocatalytic activity. J. Photochem. Photobiol. C Photochem. Rev. 2013, 14, 13–28. [Google Scholar] [CrossRef]
- Dahl, M.; Liu, Y.; Yin, Y. Composite Titanium Dioxide Nanomaterials. Am. Chem. Soc. 2014, 114, 9853–9889. [Google Scholar] [CrossRef] [PubMed]
- Araque, D.G.; Peña, P.A.; Ortega, D.R.; Rojas, L.L.; Gómez, R. SnO2-TiO2 structures and the effect of CuO, CoO metal oxide on photocatalytic hydrogen production. J. Chem. Technol. Biotechnol. 2017, 92, 1531–1539. [Google Scholar] [CrossRef]
- Lafond, V.; Mutin, P.H.; Vioux, A. Non-hydrolytic sol-gel routes based on alkyl halide elimination: Toward better mixed oxide catalysts and new supports application to the preparation of a SiO2-TiO2 epoxidation catalyst. J. Mol. Catal. A Chem. 2002, 182–183, 81–88. [Google Scholar] [CrossRef]
- Pan, J.H.; Sun, D.D.; Lee, W.I. Preparation of periodically organized mesoporous bicomponent TiO2 and SnO2-based thin films by controlling the hydrolytic kinetics of inorganic precursors during EISA process. Mater. Lett. 2011, 65, 2836–2840. [Google Scholar] [CrossRef]
- Kim, J.-S.; Sung, H.-J.; Kim, B.-J. Photocatalytic characteristics for the nanocrystalline TiO2 on the Ag-doped CaAl2O4:(Eu,Nd) phosphor. Appl. Surf. Sci. 2015, 334, 151–156. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L. Lanthanide-doped nanomaterials for luminescence detection and imaging. TrAC Trends Anal. Chem. 2014, 62, 123–134. [Google Scholar] [CrossRef]
- Mavengere, S.; Yadav, H.M.; Kim, J.-S. Photocatalytic properties of nanocrystalline TiO2 coupled with up-conversion phosphors. J. Ceram. Sci. Tech. 2017, 8, 67–72. [Google Scholar]
- Xu, D.-X.; Lian, Z.-W.; Fu, M.-L.; Yuan, B.; Shi, J.-W.; Cui, H.-J. Advanced near-infrared-driven photocatalyst: Fabrication, characterization, and photocatalytic performance of β-NaYF4:Yb3+,Tm3+@TiO2 core@shell microcrystals. Appl. Catal. B Environ. 2013, 142, 377–386. [Google Scholar] [CrossRef]
- Hsu, C.H.; Lu, C.H. Microwave-hydrothermally synthesized (Sr1−x−yCexTby)Si2O2- Si2O2-δ N2+μ phosphors: Efficient energy transfer, structural refinement and photoluminescence properties. J. Mater. Chem. 2011, 21, 2932–2939. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, Y.; Jiang, Y.; Zhang, M.; Sun, W.; Zhao, X.-Z. Coupling effects of Au-decorated core-shell β-NaYF4:Er/Yb@SiO2 microprisms in dye-sensitized solar cells: Plasmon resonance versus upconversion. Electrochim. Acta. 2015, 180, 394–400. [Google Scholar] [CrossRef]
- Schietinger, S.; Menezes, L.D.S.; Lauritzen, B.; Benson, O. Observation of size dependence in multicolor upconversion in single Yb3+, Er3+ Codoped NaYF4 nanocrystals. Nano Lett. 2009, 9, 2477–2481. [Google Scholar] [CrossRef] [PubMed]
- Bednarkiewicz, A.; Wawrzynczyk, D.; Nyk, M.; Samoć, M. Tuning red-green-white up-conversion color in nano NaYF4:Er/Yb phosphor. J. Rare Earths 2011, 29, 1152–1156. [Google Scholar] [CrossRef]
- Wu, X.; Yin, S.; Dong, Q.; Liu, B.; Wang, Y.; Sekino, T.; Lee, S.W.; Sato, T. UV, visible and near-infrared lights induced NOx destruction activity of (Yb,Er)-NaYF4/C-TiO2 composite. Sci. Rep. 2013, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xiao, L.; Luo, Q.; Li, X.; An, J.; Duan, Y. Highly efficient visible light TiO2 photocatalyst prepared by sol-gel method at temperatures lower than 300 °C. J. Hazard. Mater. 2011, 192, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Di, W.; Zhai, X.; Yang, R.; Qin, W. NIR-responsive photocatalytic activity and mechanism of NaYF4:Yb,Tm@TiO2 core-shell nanoparticles. ACS Catal. 2013, 3, 405–412. [Google Scholar] [CrossRef]
- Tabaei, H.S.M.; Kazemeini, M.; Fattahi, M. Preparation and characterization of visible light sensitive nano titanium dioxide photocatalyst. Sci. Iran. 2012, 19, 1626–1631. [Google Scholar] [CrossRef]
- Ma, L.; Jia, I.; Guo, X.; Xiang, L. Current status and perspective of rare earth catalytic materials and catalysis. Chin. J. Catal. 2014, 35, 108–119. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Z.; Li, F.; Huang, Y.; Hu, X.; Huang, H.; Peng, R.; Zhai, X.; Fu, Z.; Lu, Y. Multifunctional single-phase photocatalysts: Extended near infrared photoactivity and reliable magnetic recyclability. Sci. Rep. 2015, 5, 15511. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, W.; Ni, Y.; Lu, C.; Xu, Z. Different upconversion properties of β-NaYF4:Yb3+, Tm3+/Er3+ in Affecting the near-infrared-driven photocatalytic activity of high-reactive TiO2. ACS Appl. Mater. Interfaces. 2014, 6, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.M.; Kolekar, T.V.; Pawar, S.H.; Kim, J.-S. Enhanced photocatalytic inactivation of bacteria on Fe-containing TiO2 nanoparticles under fluorescent light. J. Mater. Sci. Mater. Med. 2016, 27, 57. [Google Scholar] [CrossRef] [PubMed]
- Reszczyńska, J.; Grzyb, T.; Sobczak, J.W.; Lisowski, W.; Gazda, M.; Ohtani, B.; Zaleska, A. Lanthanide co-doped TiO2: The effect of metal type and amount on surface properties and photocatalytic activity. Appl. Surf. Sci. 2014, 307, 333–345. [Google Scholar] [CrossRef]
- Deboer, M.A.; Lammertsma, K. Scarcity of rare earth elements. ChemSusChem 2013, 6, 2045–2055. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Han, Y.; Lim, C.S.; Lu, Y.; Wang, J.; Xu, J.; Chen, H.; Zhang, C.; Hong, M.; Liu, X. Simultaneous phase and size control of upconversion nanocrystals through lanthanide doping. Nature 2010, 463, 1061–1065. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Di, W.; Chen, C.; Liu, C.; Wang, X.; Qin, W. Enhanced near-infrared photocatalysis of NaYF4:Yb, Tm/CdS/TiO2 composites. Dalt. Trans. 2014, 43, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Luoshan, M.; Bai, L.; Bu, C.; Liu, X.; Zhu, Y.; Guo, K.; Jiang, R.; Li, M.; Zhao, X. Surface plasmon resonance enhanced multi-shell-modified upconversion NaYF4:Yb3+, Er3+@SiO2@Au@TiO2 crystallites for dye-sensitized solar cells. J. Power Sources 2016, 307, 468–473. [Google Scholar] [CrossRef]
- Zhang, H.; Millington, K.R.; Wang, X. The photostability of wool doped with photocatalytic titanium dioxide nanoparticles. Polym. Degrad. Stab. 2009, 94, 278–283. [Google Scholar] [CrossRef]
- Luo, L.; Li, Y.; Hou, J.; Yang, Y. Visible photocatalysis and photostability of Ag3PO4 photocatalyst. Appl. Surf. Sci. 2014, 319, 332–338. [Google Scholar] [CrossRef]
- Deng, X.; Wang, C.; Shao, M.; Xu, X.; Huang, J. Low-temperature solution synthesis of CuO/Cu2O nanostructures for enhanced photocatalytic activity with added H2O2: Synergistic effect and mechanism insight. RSC Adv. 2017, 7, 4329–4338. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Liu, Y. Promotion of multi-electron transfer for enhanced photocatalysis: A review focused on oxygen reduction reaction. Appl. Surf. Sci. 2015, 358, 28–45. [Google Scholar] [CrossRef]
- Sahel, K.; Elsellami, L.; Mirali, I.; Dappozze, F.; Bouhent, M.; Guillard, C. Hydrogen peroxide and photocatalysis. Appl. Catal. B Environ. 2016, 188, 106–112. [Google Scholar] [CrossRef]
- Ratnasamy, P.; Srinivas, D.; Knözinger, H. Active sites and reactive intermediates in titanium silicate molecular sieves. Adv. Catal. 2004, 48, 1–169. [Google Scholar]
- Dubale, A.A.; Pan, C.; Tamirat, A.G.; Chen, H.-M.; Su, W.-N.; Chen, C.-H.; Rick, J.; Ayele, D.W.; Aragaw, B.A.; Lee, J.-F.; et al. Heterostructured Cu2O/CuO decorated with nickel as a highly efficient photocathode for photoelectrochemical water reduction. J. Mater. Chem. A 2015, 3, 12482–12499. [Google Scholar] [CrossRef]
- Singh, V.; Haritha, P.; Venkatramu, V.; Kim, S.H. Efficient visible upconversion luminescence in Er3+ and Er3+/Yb3+ co-doped Y2O3 phosphors obtained by solution combustion reaction. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 126, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Long, J.; Fan, Z.; Du, M.; Xiong, S.; Zhao, D.; Ji, F.; He, Q.; Zeng, Y.; Xu, X. Synthesis and photocatalytic activity of hexagonal phase NaYF4:Ho3+@TiO2 core–shell microcrystals. CrystEngComm 2016, 18, 6471–6482. [Google Scholar] [CrossRef]
- Gombac, V.; Sordelli, L.; Montini, T.; Delgado, J.J.; Adamski, A.; Adami, G.; Cargnello, M.; Bernal, S.; Fornasiero, P. CuOx−TiO2 Photocatalysts for H2 production from ethanol and glycerol solutions. J. Phys. Chem. A 2010, 114, 3916–3925. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ji, G.; Dastageer, M.A.; Zhu, L.; Wang, J.; Zhang, B.; Chang, X.; Gondal, M.A. Highly-active direct Z-scheme Si/TiO2 photocatalyst for boosted CO2 reduction into value-added methanol. RSC Adv. 2014, 4, 56961–56969. [Google Scholar] [CrossRef]
- Paschoalino, F.C.S.; Paschoalino, M.P.; Jordão, E.; Jardim, W.F. Evaluation of TiO2, ZnO, CuO and Ga2O3 on the photocatalytic degradation of phenol using an annular-flow photocatalytic reactor. Open J. Phys. Chem. 2012, 2, 135–140. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, Q.; Zhao, Q.; Ma, L.; Ding, M.; Xu, X. Effects of architectures and H2O2 additions on the photocatalytic performance of hierarchical Cu2O nanostructures. Nanoscale Res. Lett. 2015, 10, 2–10. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).