Effect of the Cr2O3 Promoter on Pt/WO3-ZrO2 Catalysts for n-Heptane Isomerization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Cr2O3 Loading on Pt/ WO3/ZrO2
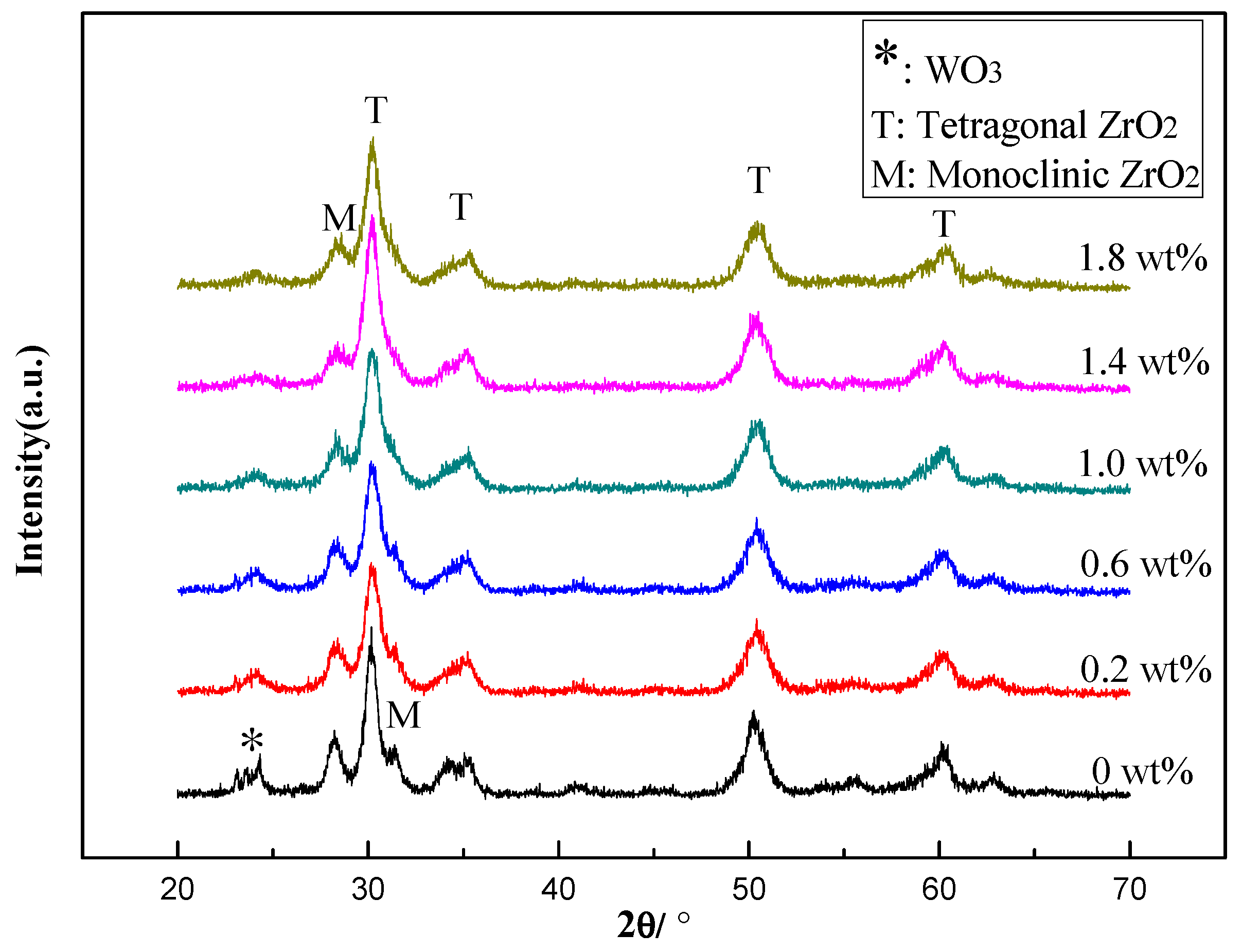
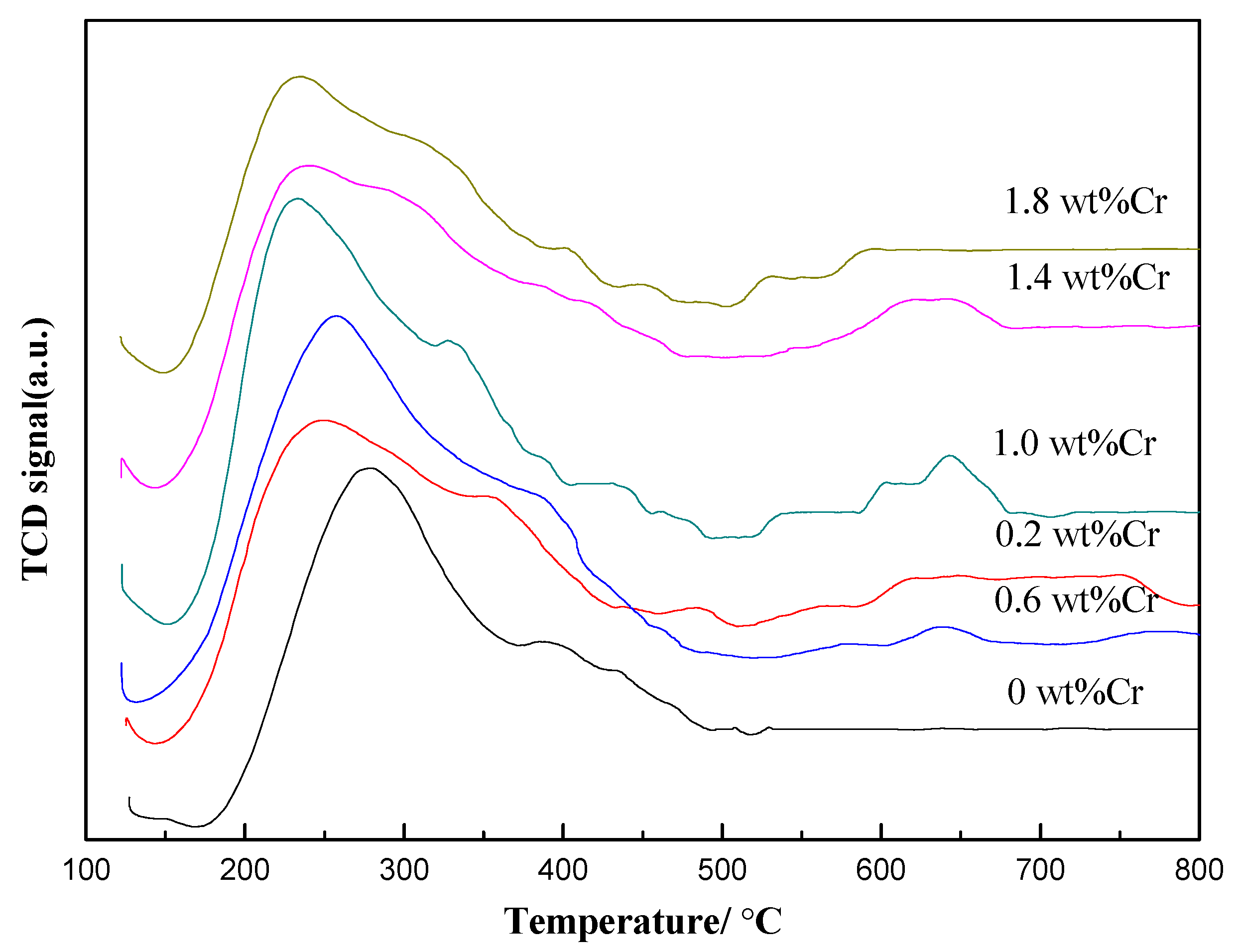
2.1.1. Characterization of the Catalysts
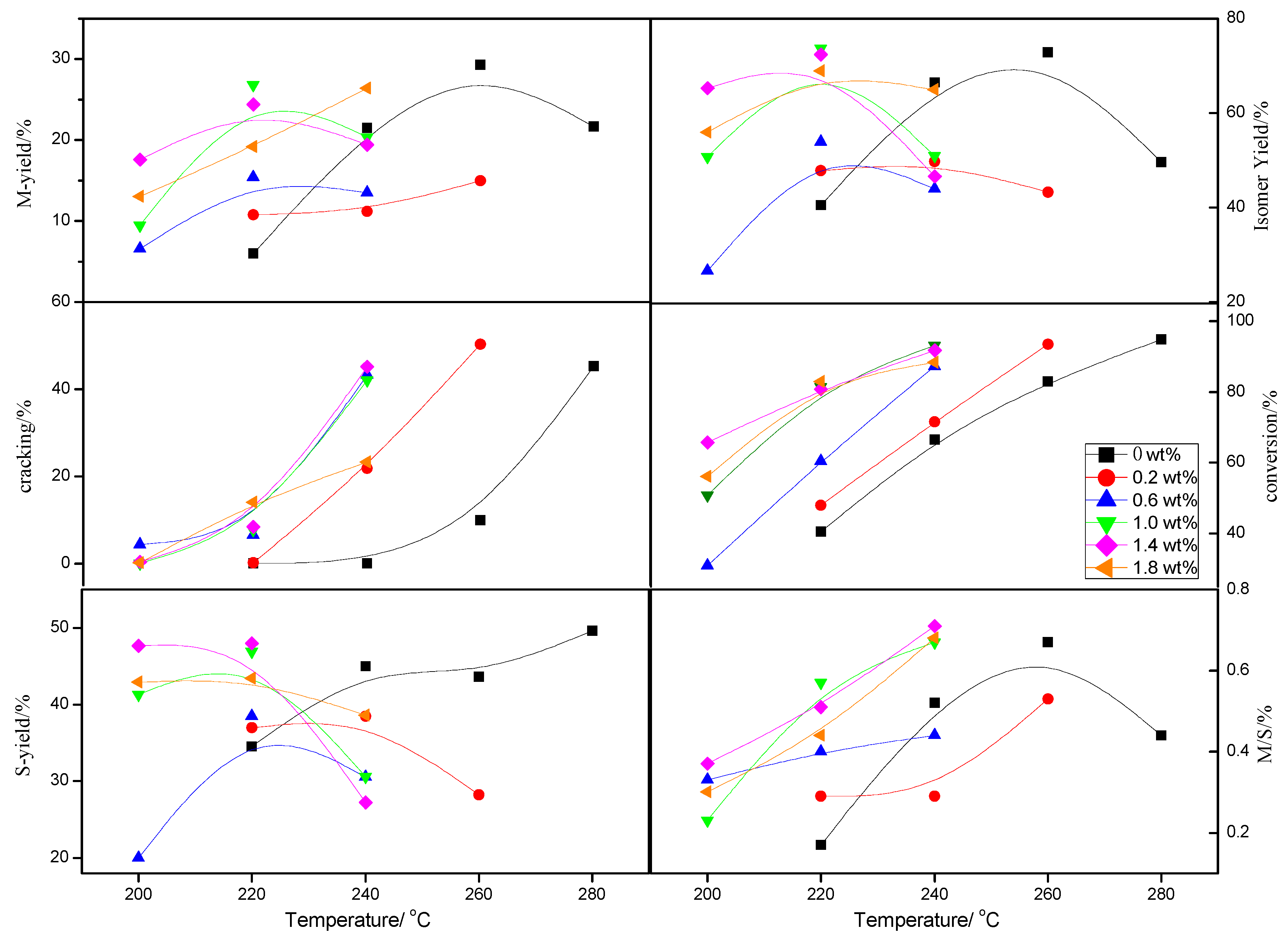
2.1.2. Catalytic Activity of the Catalysts
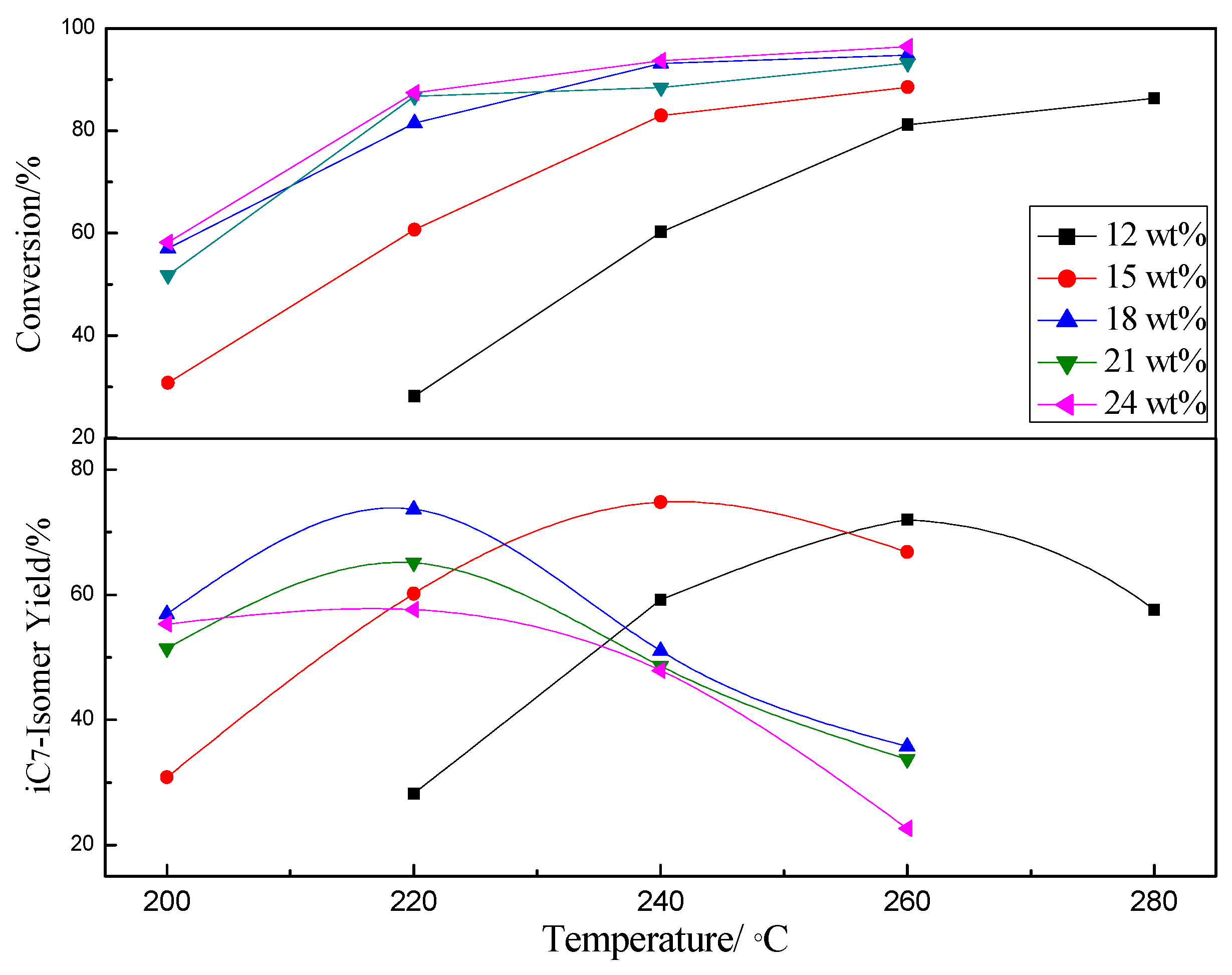
2.2. Effect of WO3 on Pt/WZ
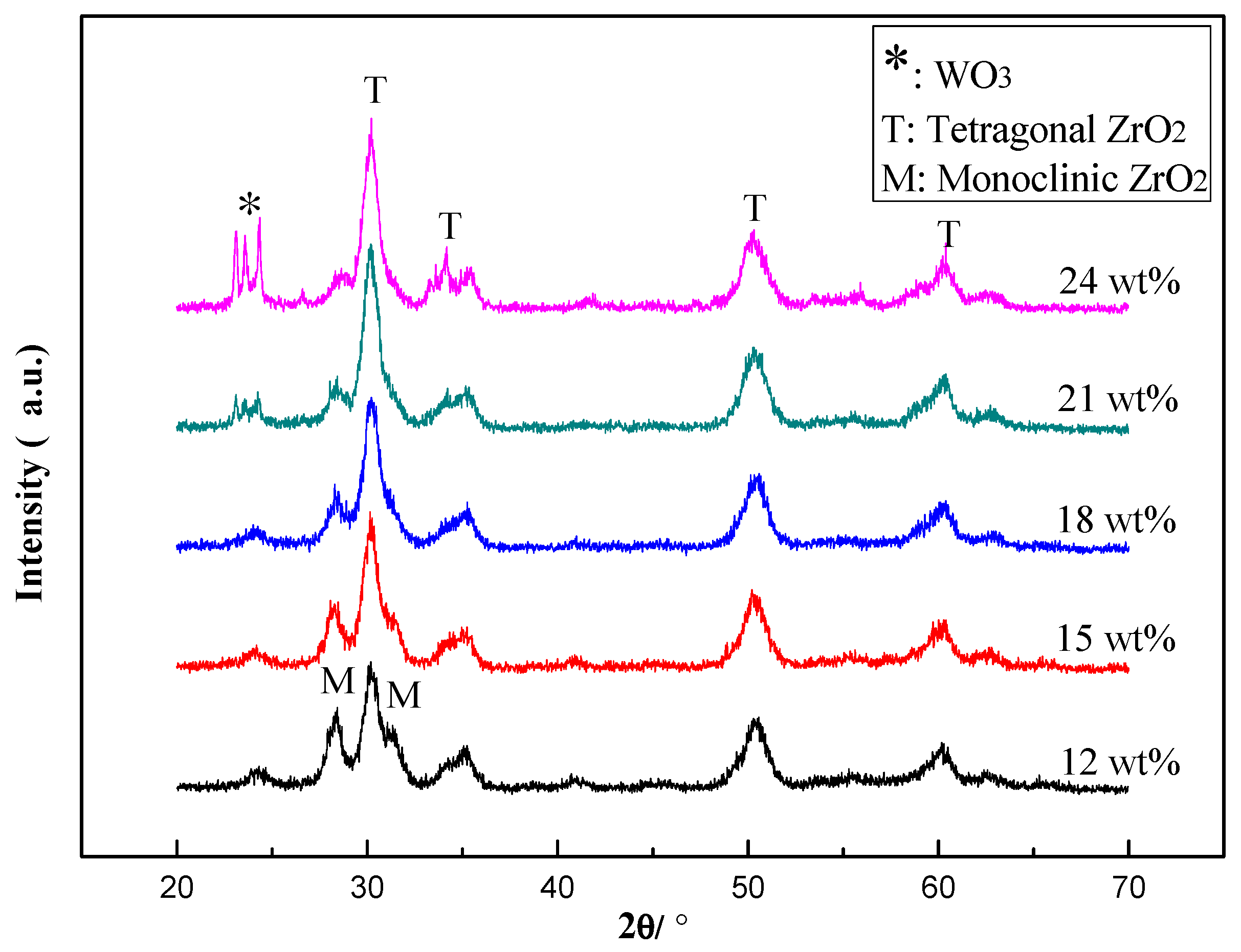
2.2.1. Characterization of the Catalysts
2.2.2. Catalytic Activity of the Catalysts
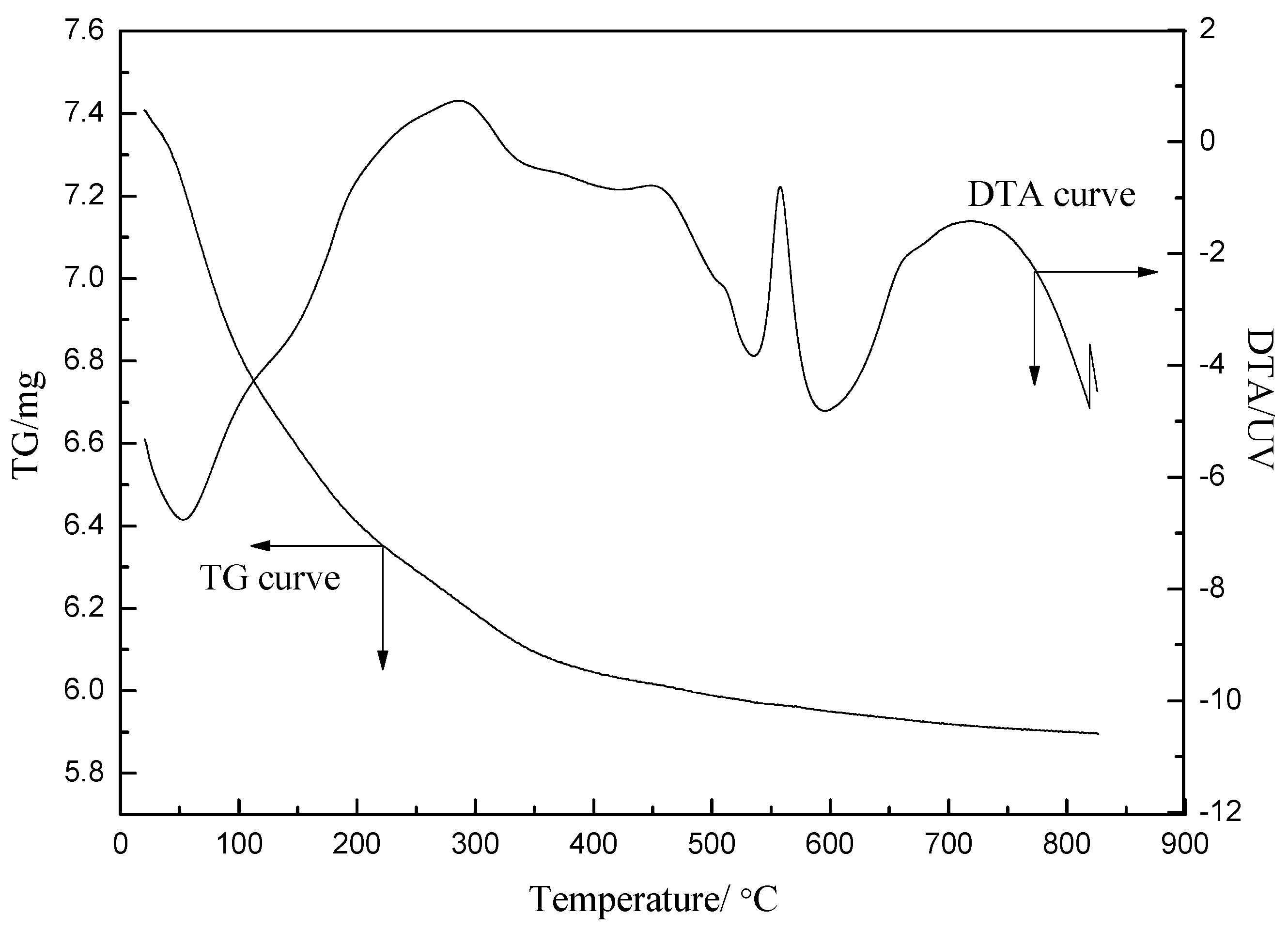
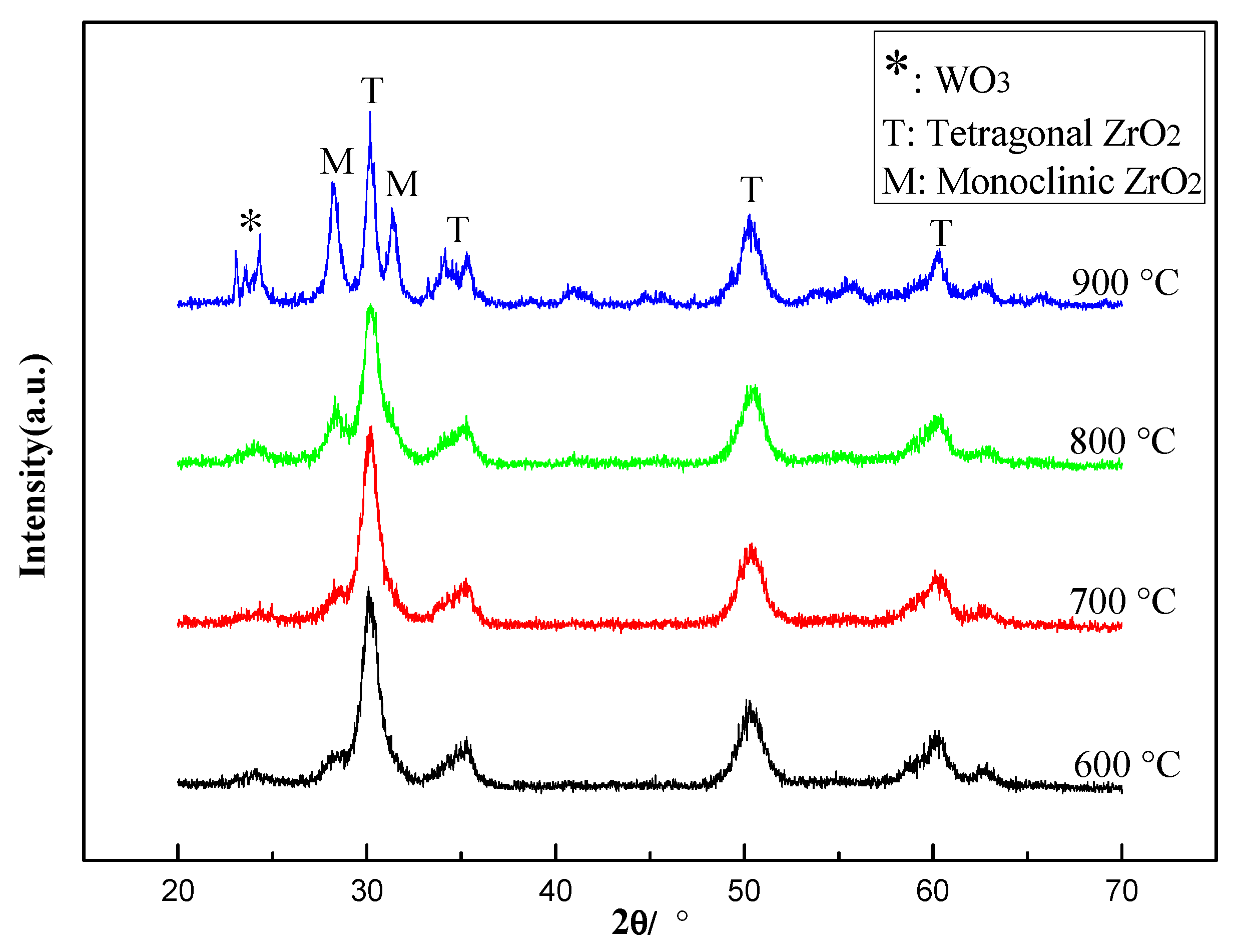
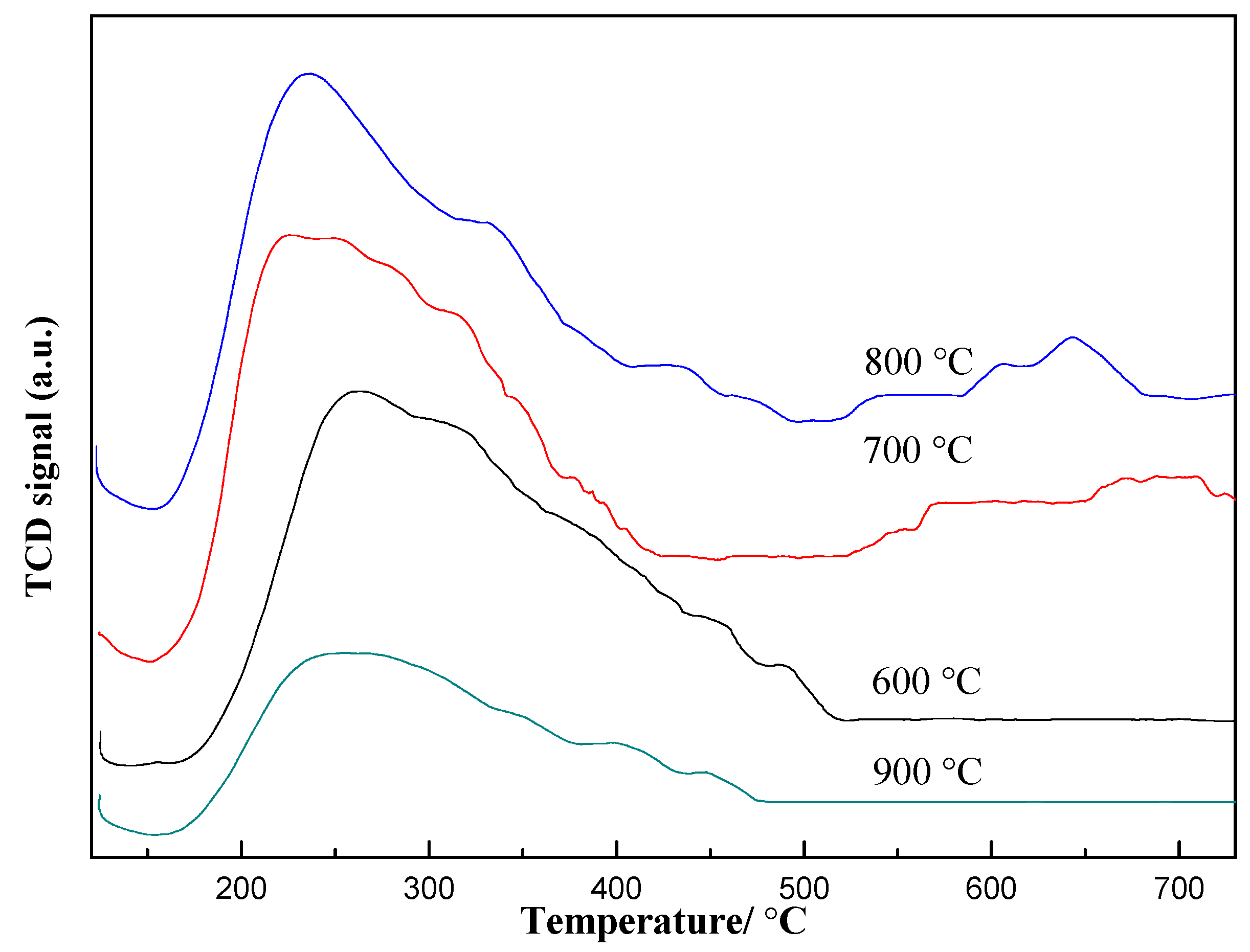
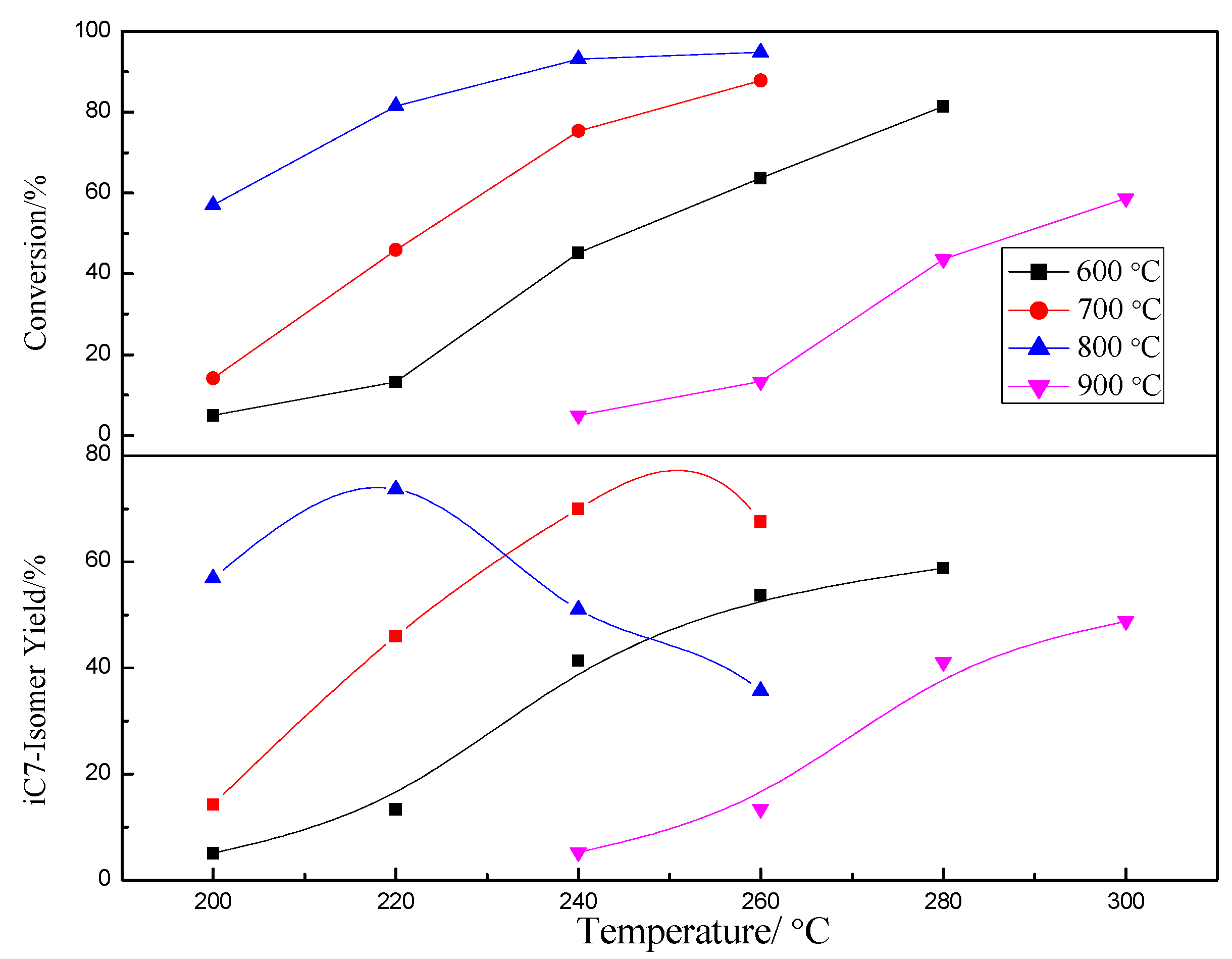
2.3. Effect of Calcination Temperature on Catalyst Activity
2.3.1. Characterization of the Catalysts
2.3.2. Catalytic Activity of the Catalysts
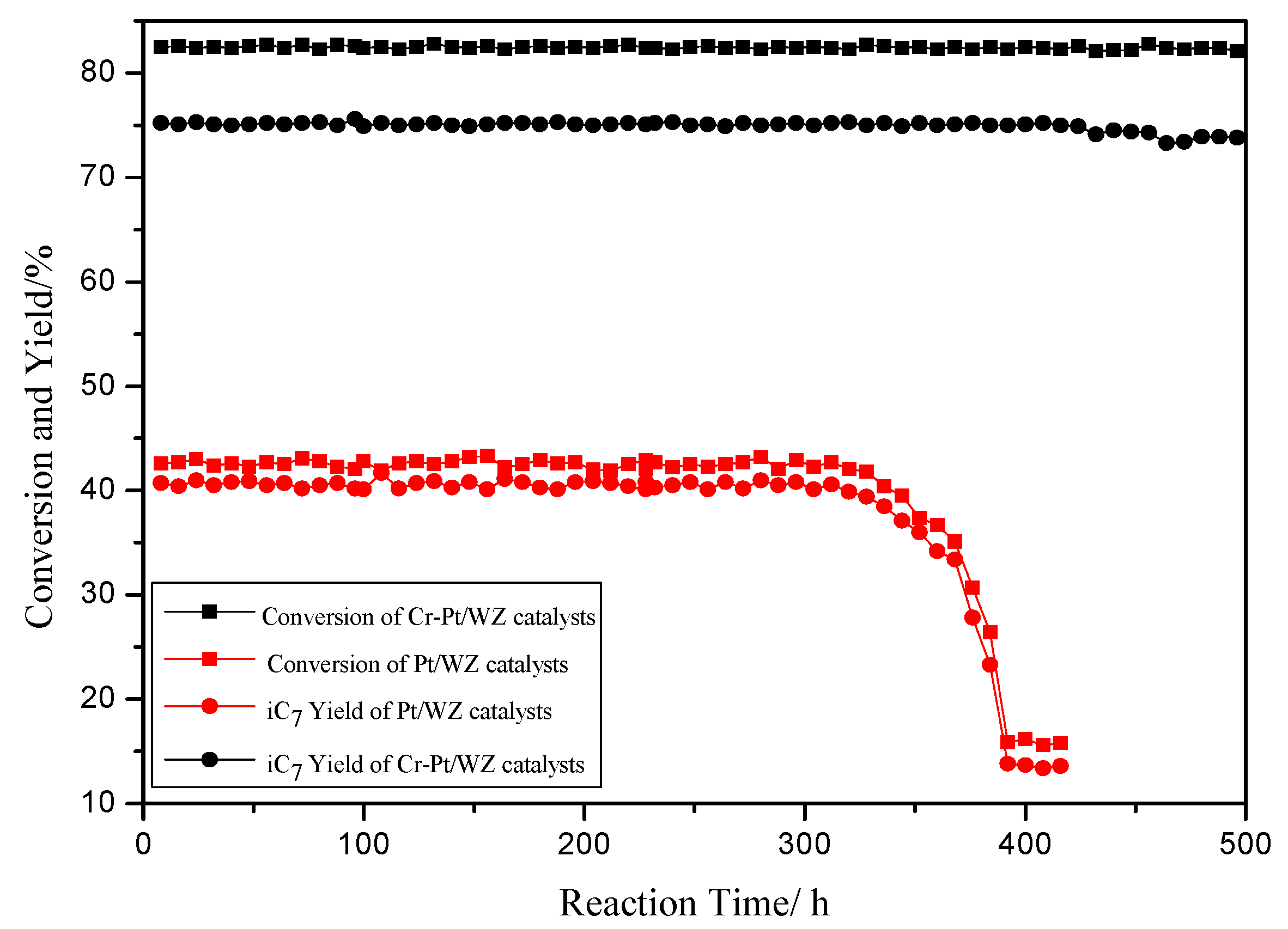
2.4. Catalyst Stability Study
2.5. Application of Catalyst in the Industrial Raw Material Isomerization
3. Experimental
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Tests and Product Analysis
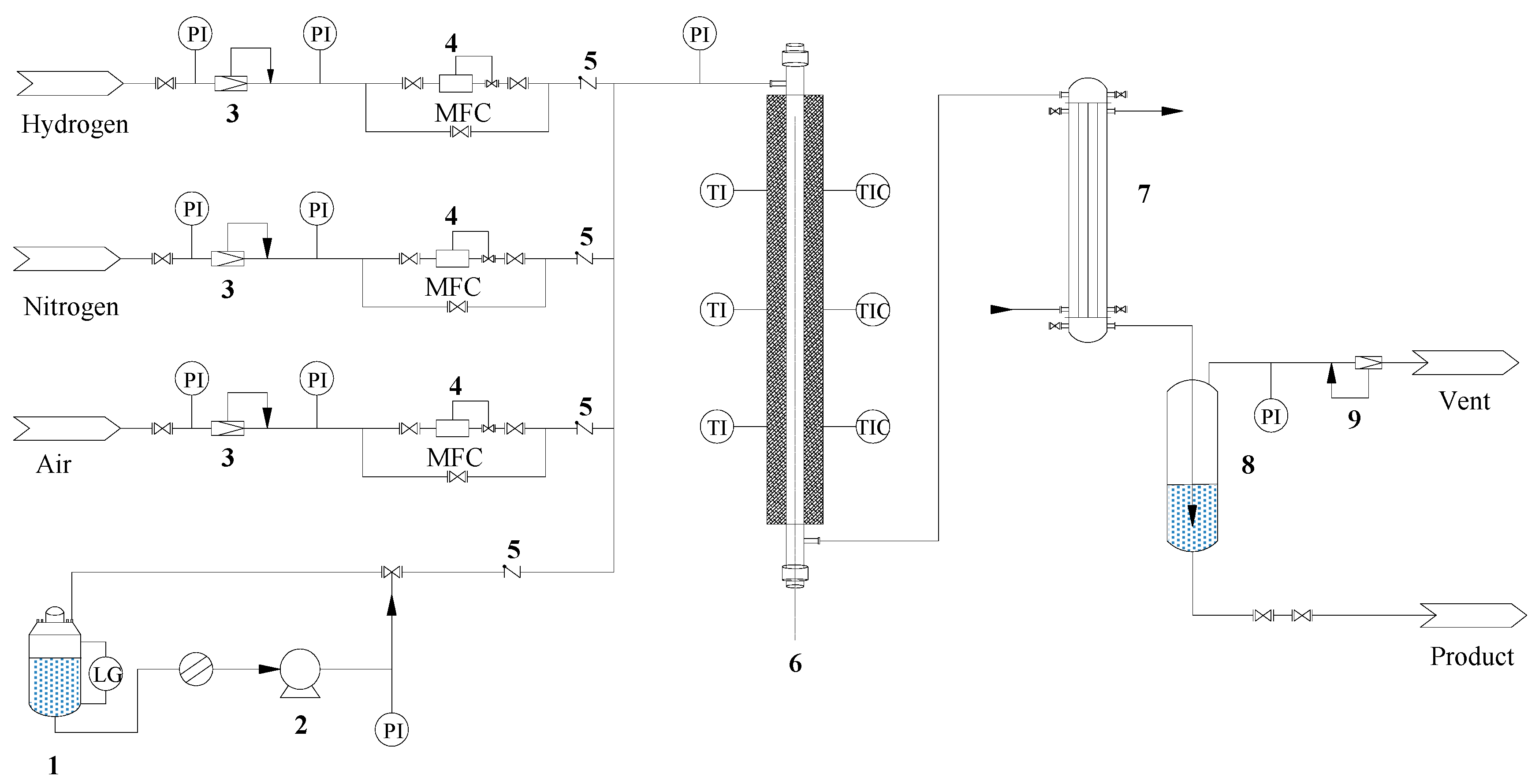
3.3.1. Catalytic Tests
3.3.2. Product Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guo, X. Development of clean fuels: The Development of Gasoline Standards and Emission Regulations and the Trend of Clean Gasoline Production Technology in China. Pet. Prod. Appl. Res. 2012, 30, 4–13. [Google Scholar]
- Vallero, D. Chapter 28—Air quality status and trends. In Fundamentals of Air Pollution; Elsevier: New York, NY, USA, 2014; pp. 755–785. [Google Scholar]
- Nastaran, P.; Mohammad, H.P.; Nasibeh, P. Effect of WOx Promoter on Pt/ZrO2-HMS Catalysts for n-Heptane Isomerization: Catalytic Performance and Kinetics Study. Chin. Chem. Lett. 2017, 28, 546–552. [Google Scholar]
- Shkurenok, V.A.; Smolikov, M.D.; Yablokova, S.S.; Kiryanov, D.I.; Belyi, A.S.; Paukshtis, E.A.; Leonteva, N.N.; Gulyaeva, T.I.; Shilova, A.V.; Drozdov, V.A. Pt/WO3/ZrO2 Catalysts for n-Heptane Isomerization. Procedia Eng. 2015, 113, 62–67. [Google Scholar] [CrossRef]
- Khurshid, M.; Al-Khattaf, S.S. n-Heptane isomerization over Pt/WO3-ZrO2: A kinetic study. Appl. Catal. A Gener. 2009, 368, 56–64. [Google Scholar] [CrossRef]
- Hino, M.; Arata, K. Synthesis of Solid Superacid of Tungsten Oxide Supported on Zirconia and Its Catalytic Action for Reactions of Butane and Pentane. J. Chem. Soc. Chem. Commun. 1988, 18, 407–425. [Google Scholar] [CrossRef]
- Chen, X.R.; Chen, C.L.; Xu, N.P.; Mou, C.Y. Al- and Ga-promoted WO3/ZrO2, Strong Solid Acid Catalysts and Their Catalytic Activities in n-Butane Isomerization. Catal. Today 2004, 9, 129–134. [Google Scholar] [CrossRef]
- Kuznetsova, L.I.; Kazbanova, A.V.; Kuznetsov, P.N.; Tarasova, L.S. Activity of the Pt/WO42-/ZrO2 catalyst in hydroisomerization reaction of n-heptane-benzene mixture. Petrol. Chem. 2015, 55, 57–62. [Google Scholar] [CrossRef]
- Jermy, B.R.; Khurshid, M.; Al-Daous, M.A.; Hattori, H.; Al-Khattaf, S.S. Optimizing Preparative Conditions for Tungstated Zirconia Modified with Platinum as Catalyst for Heptane Isomerization. Catal. Today 2011, 164, 148–153. [Google Scholar] [CrossRef]
- Shakun, A.N.; Fedorova, M.L. A Method for Isomerization of Light Gasoline Fractions Containing C7-C8 Paraffinic Hydrocarbons. Russia Patent 2,408,659, 20 July 2009. [Google Scholar]
- Ross-Medgaarden, E.I.; Knowles, W.V.; Kim, T.; Wong, M.S.; Zhou, W.; Kiely, C.J.; Wachs, I.E. New insights into the nature of the acidic catalytic active sites present in ZrO2-supported tungsten oxide catalysts. J. Catal. 2008, 256, 108–125. [Google Scholar] [CrossRef]
- Saur, O.; Bensitel, M.; Saad, A.B.M.; Lavalley, J.C. ChemInform Abstract: Structure and stability of sulfated alumina and titania. ChemInform 1986, 17. [Google Scholar] [CrossRef]
- Zhou, W.; Ross-Medgaarden, E.I.; Knowles, W.V.; Wong, M.S.; Wachs, I.E.; Kiely, C.J. Identification of active Zr-WO(x) clusters on a ZrO2 support for solid acid catalysts. Nat. Chem. 2009, 1, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Santiesteban, J.G.; Vartuli, J.C.; Han, S.; Bastian, R.D.; Chang, C.D. Influence of the preparative method on the activity of highly acidic WOx/ZrO2 and the relative acid activity compared with zeolites. J. Catal. 1997, 168, 431–441. [Google Scholar] [CrossRef]
- Scheithauer, M.; And RK, G.; Knözinger, H. Genesis and structure of WOx/ZrO2 solid acid catalysts. Langmuir 1998, 14, 3019–3029. [Google Scholar] [CrossRef]
- Nie, Y.; Shang, S.; Xu, X.; Hua, W.; Yue, Y.; Gao, Z. In2O3-doped Pt/WO3/ZrO2, As a Novel Efficient Catalyst for Hydroisomerization of n-Heptane. Appl. Catal. A Gener. 2012, 433–434, 69–74. [Google Scholar] [CrossRef]
- Xiao, L.; Qin, L.Z.; Chen, X.R.; Chen, C.L. Kinetic Study of n-Heptane Isomerization over Pt/Ga2O3/WO3/ZrO2 Catalyst. J. Chem. Eng. Chin. Univ. 2009, 23, 417–422. [Google Scholar]
- Hua, W.; Sommer, J. Alumina-doped Pt/WOx/ZrO2, Catalysts for n-Heptane Isomerization. Appl. Catal. A Gen. 2002, 232, 129–135. [Google Scholar] [CrossRef]
- Reddy, B.M.; Reddy, V.R. Influence of SO42−, Cr2O3, MoO3, and WO3 on the stability of ZrO2-tetragonal phase. J. Mater. Sci. Lett. 2000, 19, 763–765. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, J.M.; Yao, Y.N.; Hua, W.M.; Miao, C.X. Studies on WO3/ZrO2 and MoO3/ZrO2 Solid Superacid Sytems. Chem. Res. Chin. Univ. 1995, 1, 111–115. [Google Scholar]
- Afanasiev, P.; Geantet, C.; Vedrine, J.C. Influence of preparation method on the acidity of MoO3(WO3)/ZrO2 catalyst. J. Chem. Soc. Faraday Trans. 1994, 90, 193–202. [Google Scholar] [CrossRef]
- Zhao, B.Y.; Xu, X.P.; Gao, J.M.; Ma, H.R.; Tang, Y.Q. The effect of the preparation methods on the structure of WO3/ZrO2. Acta Phys.-Chim. Sin. 1995, 11, 979–984. [Google Scholar]
- Barton, D.G.; Shtein, M.; Wilson, R.D.; Soled, S.L.; Iglesia, E. Structure and electronic properties of solid acids based on tungsten oxide nanostructures. J. Phys. Chem. B 1999, 103, 630–640. [Google Scholar] [CrossRef]
- Yori, J.C.; Parera, J.M. Influence of the crystalline structure of ZrO2, on the metallic properties of Pt in Pt/WO3-ZrO2, catalysts. Catal. Lett. 2000, 65, 205–208. [Google Scholar] [CrossRef]


| Sample | SBET/(m2∙g−1) | VP/(cm3∙g−1) | dP/nm |
|---|---|---|---|
| Pt/WZ | 77.06 | 0.153 | 6.54 |
| 0.2 wt% Cr-Pt/WZ | 80.94 | 0.161 | 6.53 |
| 0.6 wt% Cr-Pt/WZ | 105.26 | 0.168 | 6.14 |
| 1.0 wt% Cr-Pt/WZ | 103.5 | 0.177 | 6.85 |
| 1.4 wt% Cr-Pt/WZ | 92.94 | 0.163 | 5.84 |
| 1.8 wt% Cr-Pt/WZ | 89.94 | 0.161 | 5.83 |
| Sample | SBET/(m2∙g−1) | VP/(cm3∙g−1) | dP/(nm) |
|---|---|---|---|
| Cr-Pt/12 wt%WZ | 102.8 | 0.180 | 6.86 |
| Cr-Pt/15 wt%WZ | 128.3 | 0.182 | 6.90 |
| Cr-Pt/18 wt%WZ | 103.5 | 0.177 | 6.85 |
| Cr-Pt/21 wt%WZ | 89.54 | 0.166 | 6.42 |
| Cr-Pt/24 wt%WZ | 75.21 | 0.124 | 6.57 |
| Calcination Temperature/(°C) | SBET/(m2∙g−1) | VP/(cm3∙g−1) | dP/(nm) |
|---|---|---|---|
| 600 | 129.8 | 0.246 | 5.81 |
| 700 | 107.9 | 0.189 | 6.20 |
| 800 | 103.5 | 0.177 | 6.85 |
| 900 | 73.2 | 0.120 | 5.20 |
| CNum | N-P | I-P | O | N | A | Total |
|---|---|---|---|---|---|---|
| C-5 | 0.25 | 0.18 | 0.00 | 0.36 | 0.00 | 0.79 |
| C-6 | 4.55 | 4.04 | 0.04 | 2.82 | 0.02 | 11.47 |
| C-7 | 9.92 | 14.40 | 0.62 | 1.33 | 0.11 | 26.38 |
| C-8 | 11.39 | 16.87 | 0.00 | 2.46 | 0.61 | 31.33 |
| C-9 | 8.95 | 8.51 | 0.00 | 3.25 | 0.10 | 20.81 |
| C-10 | 1.84 | 5.16 | 0.00 | 0.36 | 0.08 | 7.44 |
| C-11 | 0.26 | 1.34 | 0.00 | 0.00 | 0.00 | 1.60 |
| CNum | N-P | I-P | O | N | A | Total |
|---|---|---|---|---|---|---|
| C-3 | 0.37 | 0.00 | 0.00 | 0.00 | 0.00 | 0.37 |
| C-4 | 1.55 | 6.72 | 0.00 | 0.00 | 0.00 | 8.27 |
| C-5 | 2.11 | 10.93 | 0.00 | 0.00 | 0.00 | 13.04 |
| C-6 | 6.04 | 14.36 | 0.00 | 1.55 | 0.00 | 21.95 |
| C-7 | 7.14 | 32.84 | 0.00 | 3.19 | 0.01 | 43.18 |
| C-8 | 1.32 | 6.21 | 0.00 | 3.21 | 0.08 | 10.82 |
| C-9 | 0.32 | 0.79 | 0.00 | 1.18 | 0.00 | 2.29 |
| C-10 | 0.03 | 0.08 | 0.00 | 0.00 | 0.00 | 0.11 |
| CNum | N-P | I-P | O | N | A | Total |
|---|---|---|---|---|---|---|
| C-3 | 0.22 | 0.00 | 0.00 | 0.00 | 0.00 | 0.22 |
| C-4 | 1.16 | 4.76 | 0.00 | 0.00 | 0.00 | 5.92 |
| C-5 | 1.91 | 9.96 | 0.00 | 2.01 | 0.00 | 13.88 |
| C-6 | 6.58 | 12.96 | 0.00 | 1.70 | 0.00 | 21.24 |
| C-7 | 8.05 | 32.79 | 0.00 | 3.91 | 0.01 | 44.76 |
| C-8 | 1.35 | 6.46 | 0.00 | 3.54 | 0.09 | 11.44 |
| C-9 | 0.26 | 0.89 | 0.00 | 1.30 | 0.00 | 2.45 |
| C-10 | 0.02 | 0.06 | 0.00 | 0.00 | 0.00 | 0.08 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, G.; Zhang, R.; Zhao, Q.; Yang, S.; Jin, H.; Guo, X. Effect of the Cr2O3 Promoter on Pt/WO3-ZrO2 Catalysts for n-Heptane Isomerization. Catalysts 2018, 8, 522. https://doi.org/10.3390/catal8110522
He G, Zhang R, Zhao Q, Yang S, Jin H, Guo X. Effect of the Cr2O3 Promoter on Pt/WO3-ZrO2 Catalysts for n-Heptane Isomerization. Catalysts. 2018; 8(11):522. https://doi.org/10.3390/catal8110522
Chicago/Turabian StyleHe, Guangxiang, Rongrong Zhang, Qian Zhao, Suohe Yang, Haibo Jin, and Xiaoyan Guo. 2018. "Effect of the Cr2O3 Promoter on Pt/WO3-ZrO2 Catalysts for n-Heptane Isomerization" Catalysts 8, no. 11: 522. https://doi.org/10.3390/catal8110522
APA StyleHe, G., Zhang, R., Zhao, Q., Yang, S., Jin, H., & Guo, X. (2018). Effect of the Cr2O3 Promoter on Pt/WO3-ZrO2 Catalysts for n-Heptane Isomerization. Catalysts, 8(11), 522. https://doi.org/10.3390/catal8110522





