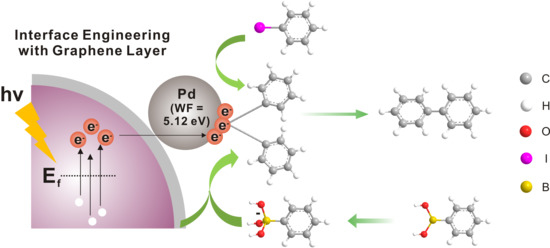
Interface-Controlled Pd Nanodot-Au Nanoparticle Colloids for Efficient Visible-Light-Induced Photocatalytic Suzuki-Miyaura Coupling Reaction
Abstract
1. Introduction
2. Results and Discussion
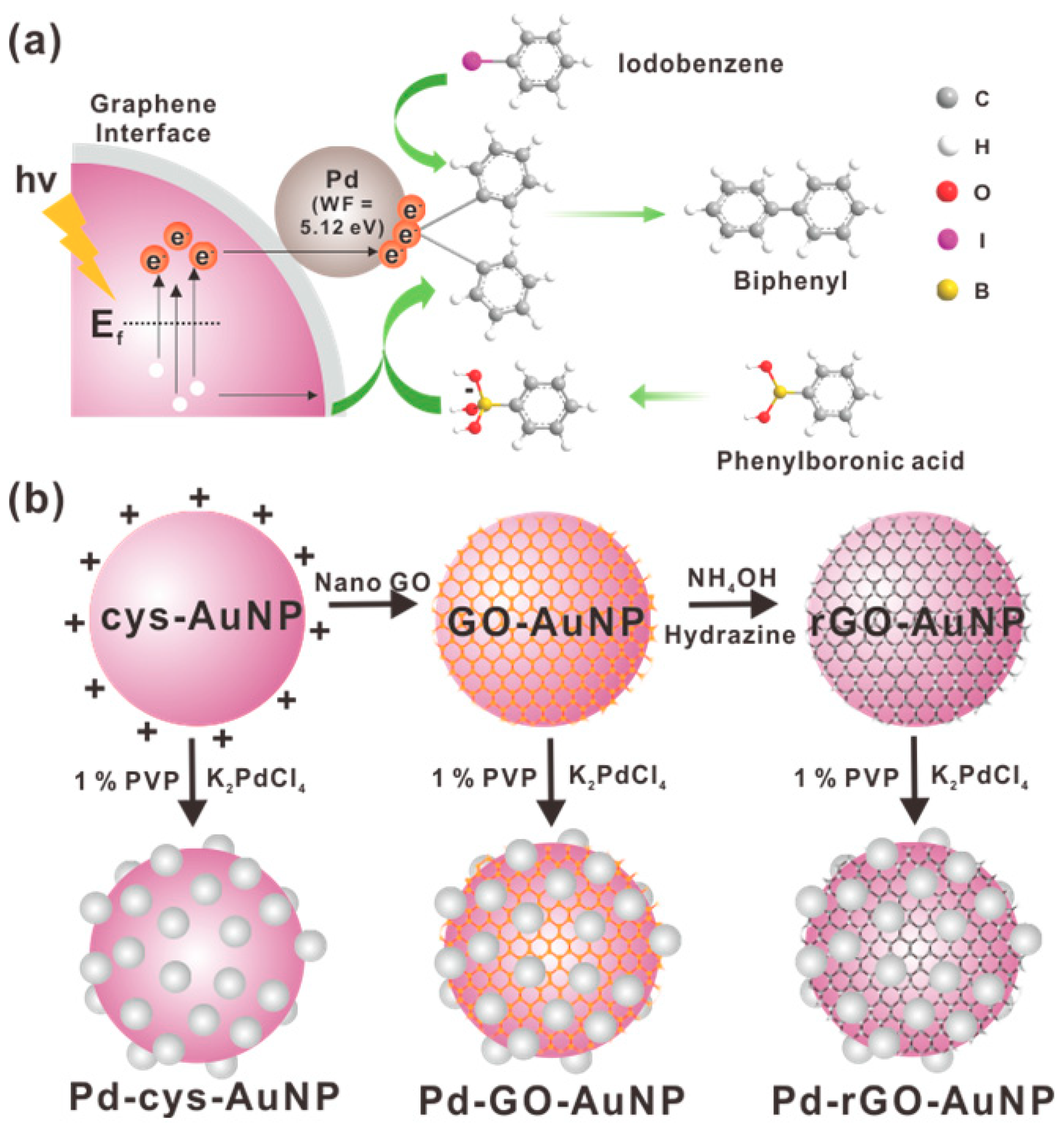
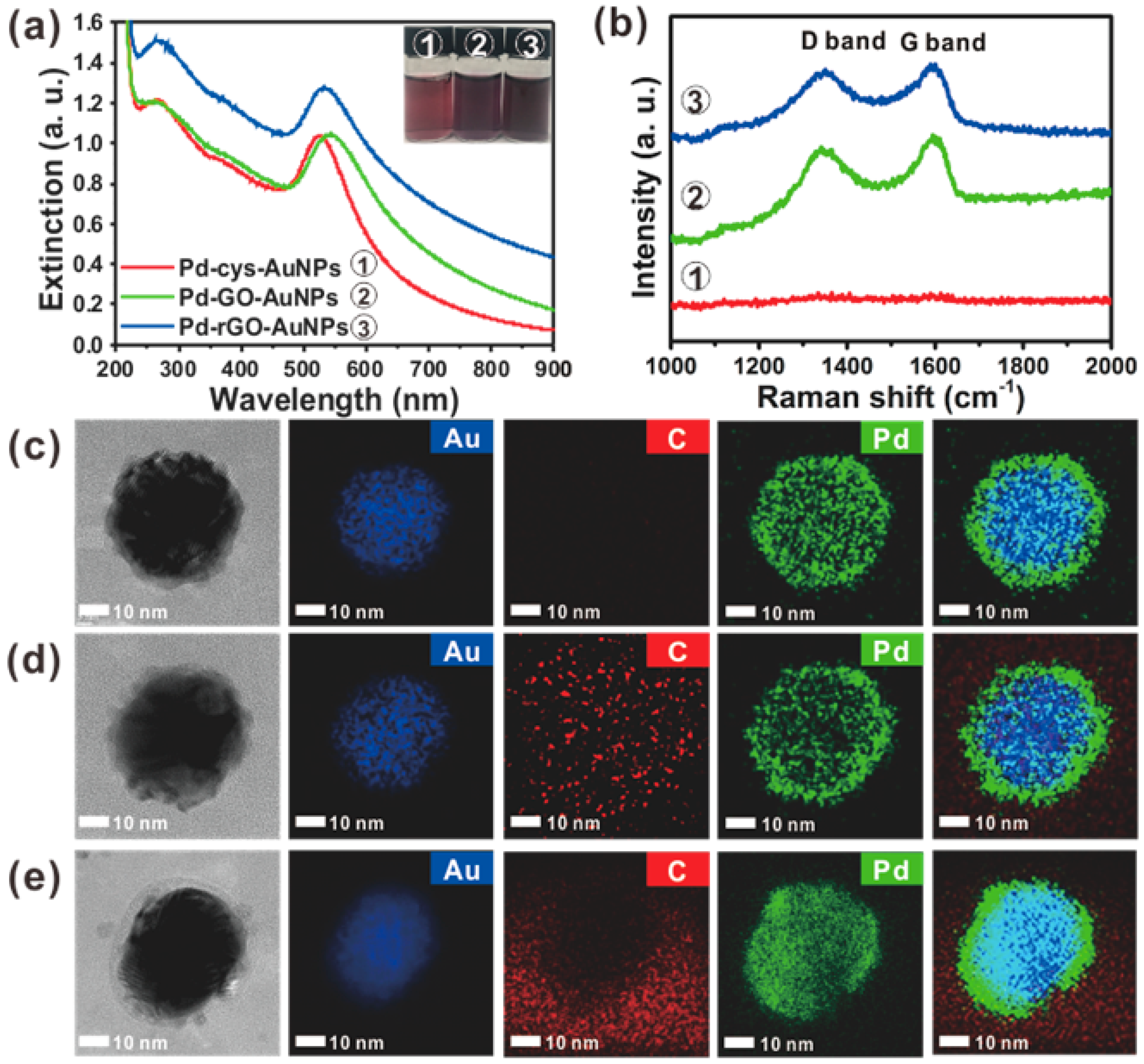
2.1. Synthesis and Characterization of Pd-Modified-Au Nanoparticles with Graphene Interface
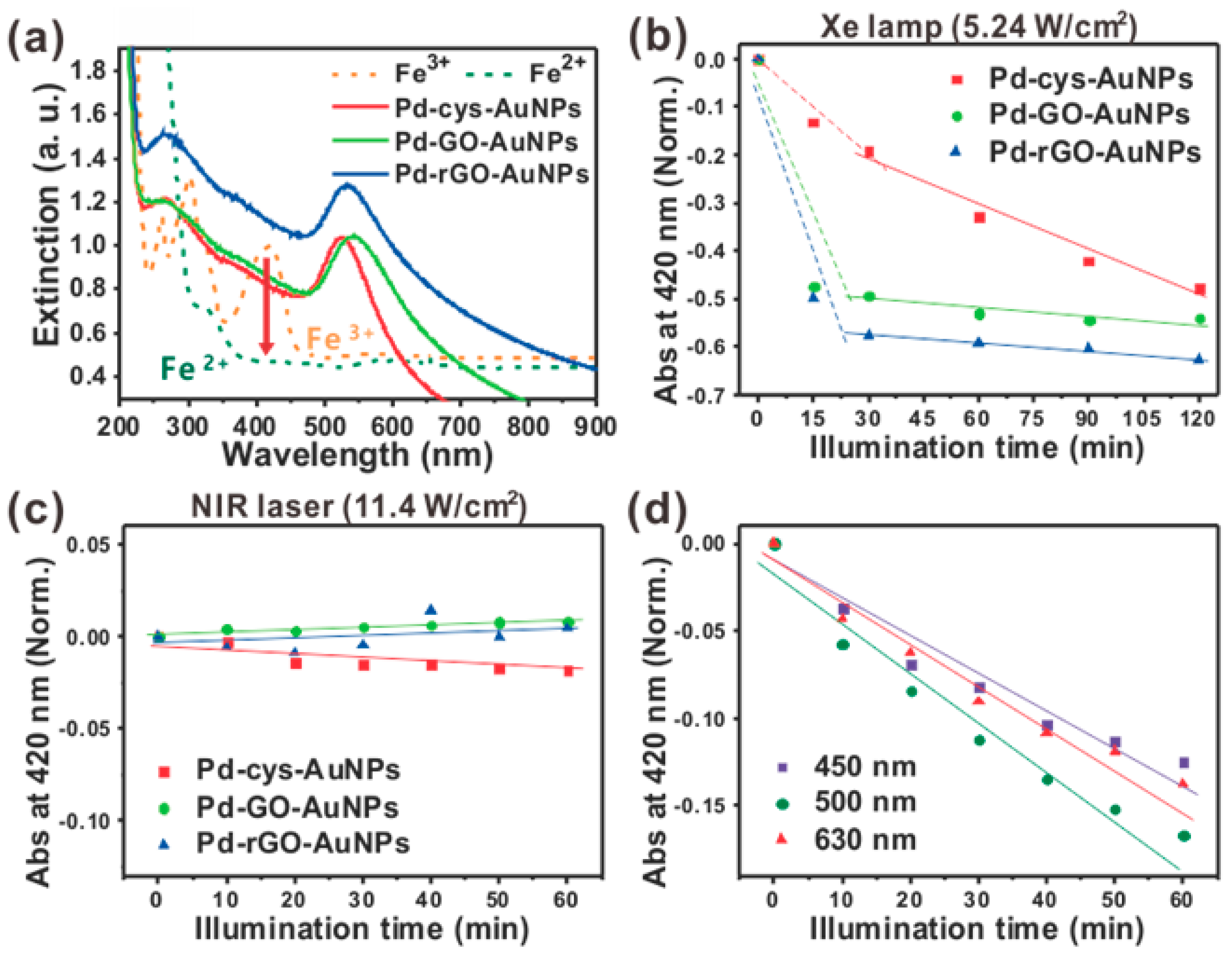
2.2. Time-Dependent Fe3+ Reduction Study with Visible Light and Near-Infrared Light Source
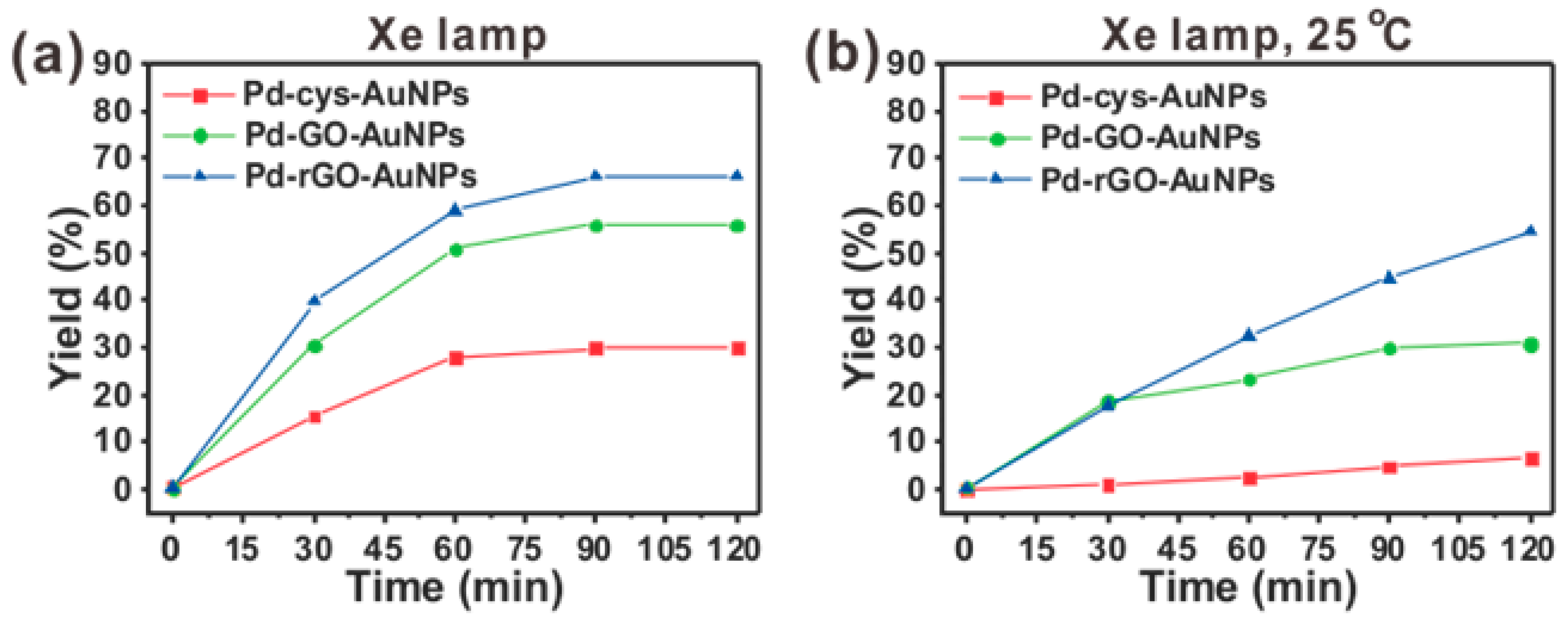
2.3. Photocatalytic Suzuki-Miyaura Cross-Coupling Reactions at Various Conditions
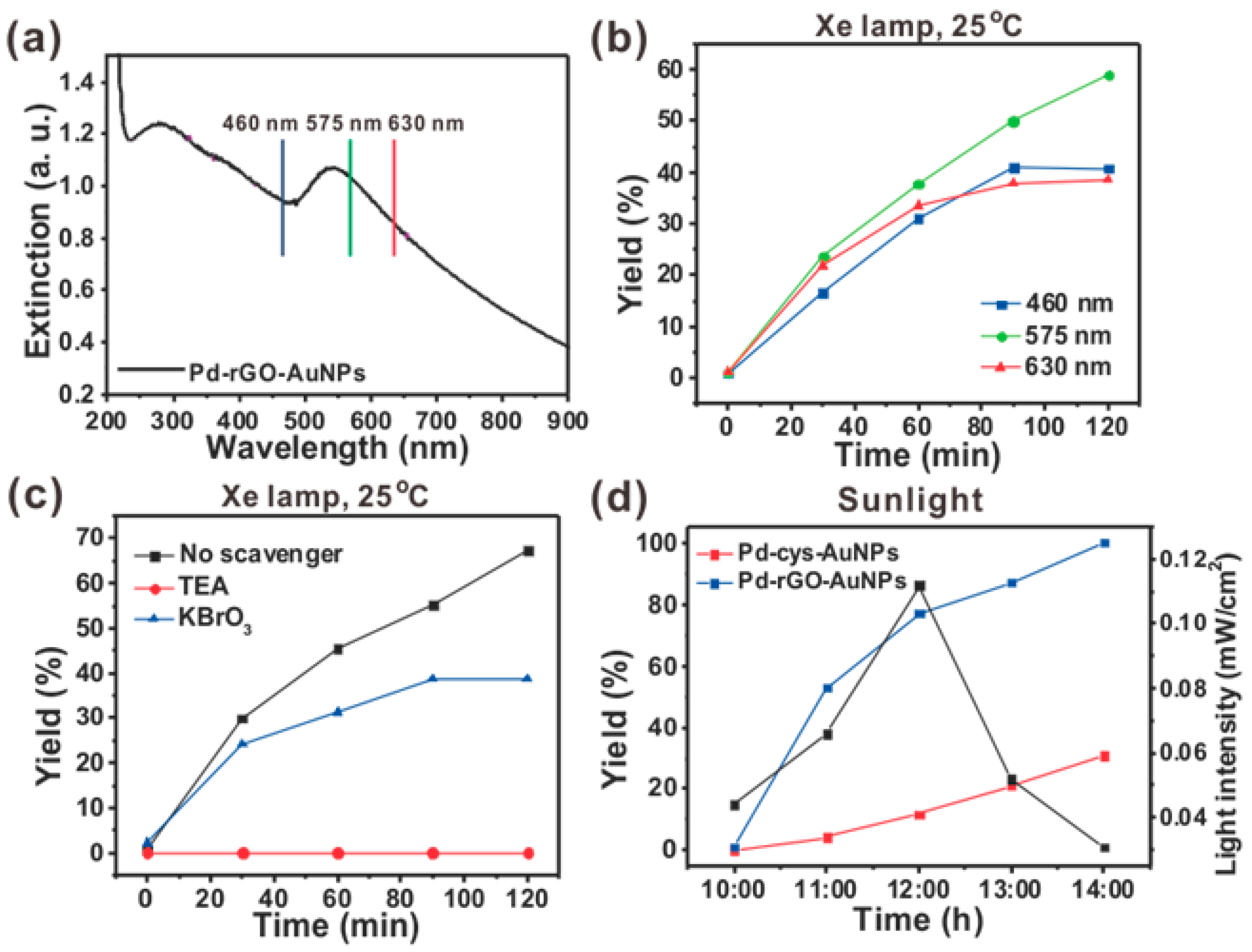
2.4. Suzuki-Miyaura Cross-Coupling Reaction under Three Different Wavelengths
2.5. Mechanism Study
2.6. Suzuki-Miyaura Cross-Coupling Reaction under Sunlight
2.7. Photocatalytic Performance of Pd-rGO AuNPs for Various Aryl Halides and Substituted Phenyobronic Acid
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Cysteamine-Modified AuNPs (cys-AuNPs)
3.3. Preparation of Nanosized Graphene Oxide (GO)
3.4. Preparation of GO-Coated or rGO-Coated Gold Nanoparticles
3.5. Synthesis of Pd-Nanodot-Modified cys-AuNPs (Pd-cys-AuNPs), Pd-Nanodot-Modified GO-AuNPs (Pd-GO-AuNPs), and Pd-Nanodot-Modified rGO-AuNPs (Pd-rGO-AuNPs)
3.6. Experimental Conditions for Kinetic Study
3.7. Experimental Setup for Suzuki-Miyaura Cross-Coupling Reactions
3.8. Suzuki-Miyaura Cross-Coupling Reaction with Sunlight
3.9. Analytical Conditions to Monitor the Suzuki C-C Coupling Reaction
3.10. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Molnár, A.R.D. Efficient, selective, and recyclable palladium catalysts in carbon–carbon coupling reactions. Chem. Rev. 2011, 111, 2251–2320. [Google Scholar] [CrossRef] [PubMed]
- Glasspoole, B.W.; Crudden, C.M. Cross-coupling: The final frontier. Nat. Chem. 2011, 3, 912–913. [Google Scholar] [CrossRef] [PubMed]
- Fihri, A.; Bouhrara, M.; Nekoueishahraki, B.; Basset, J.-M.; Polshettiwar, V. Nanocatalysts for Suzuki cross-coupling reactions. Chem. Soc. Rev. 2011, 40, 5181–5203. [Google Scholar] [CrossRef] [PubMed]
- Miyaura, N.; Yamada, K.; Suzuki, A. A new stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides. Tetrahedron Lett. 1979, 20, 3437–3440. [Google Scholar] [CrossRef]
- Ngnie, G.; Dedzo, G.K.; Detellier, C. Synthesis and catalytic application of palladium nanoparticles supported on kaolinite-based nanohybrid materials. Dalton Trans. 2016, 45, 9065–9072. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.R.; Woo, H.; Jang, S.; Cheon, J.Y.; Kim, C.; Park, J.; Park, K.H.; Joo, S.H. Ordered mesoporous carbon supported colloidal Pd nanoparticle based model catalysts for Suzuki coupling reactions: Impact of organic capping agents. ChemCatChem 2012, 4, 1587–1594. [Google Scholar] [CrossRef]
- Magne, O.S. Use of nanoparticles as catalysts in organic synthesis-cross-coupling reactions. Curr. Org. Chem. 2014, 18, 312–326. [Google Scholar]
- Mora, M.; Jimenez-Sanchidrian, C.; Rafael Ruiz, J. Recent advances in the heterogeneous palladium-catalysed Suzuki cross-coupling reaction. Curr. Org. Chem. 2012, 16, 1128–1150. [Google Scholar] [CrossRef]
- Clavero, C. Plasmon-induced hot-electron generation at nanoparticle/metal-oxide interfaces for photovoltaic and photocatalytic devices. Nat. Photonics 2014, 8, 95–103. [Google Scholar] [CrossRef]
- Shaw, M.H.; Twilton, J.; MacMillan, D.W.C. Photoredox catalysis in organic chemistry. J. Org. Chem. 2016, 81, 6898–6926. [Google Scholar] [CrossRef] [PubMed]
- Nicewicz, D.A.; MacMillan, D.W. Merging photoredox catalysis with organocatalysis: The direct asymmetric alkylation of aldehydes. Science 2008, 322, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Tucker, J.W.; Stephenson, C.R.J. Shining light on photoredox catalysis: Theory and synthetic applications. J. Org. Chem. 2012, 77, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Martina, K.; Manzoli, M.; Gaudino, E.C.; Cravotto, G. Eco-friendly physical activation methods for Suzuki–Miyaura reactions. Catalysts 2017, 7, 98. [Google Scholar] [CrossRef]
- Knight, M.W.; Wang, Y.; Urban, A.S.; Sobhani, A.; Zheng, B.Y.; Nordlander, P.; Halas, N.J. Embedding plasmonic nanostructure diodes enhances hot electron emission. Nano Lett. 2013, 13, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Atwater, H.A.; Polman, A. Plasmonics for improved photovoltaic devices. Nat. Mater. 2010, 9, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Tachikawa, T.; Majima, T. Single-particle study of Pt-modified Au nanorods for plasmon-enhanced hydrogen generation in visible to near-infrared region. J. Am. Chem. Soc. 2014, 136, 6870–6873. [Google Scholar] [CrossRef] [PubMed]
- Brongersma, M.L.; Halas, N.J.; Nordlander, P. Plasmon-induced hot carrier science and technology. Nat. Nanotechnol. 2015, 10, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Kawashima, M.; Yamashita, H. Visible-light-enhanced Suzuki–Miyaura coupling reaction by cooperative photocatalysis with an Ru–Pd bimetallic complex. Chem. Commun. 2014, 50, 14501–14503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chang, C.; Huang, Z.; Ma, Y.; Gao, W.; Li, J.; Qu, Y. Visible-Light-Activated Suzuki–Miyaura Coupling Reactions of Aryl Chlorides over the Multifunctional Pd/Au/Porous Nanorods of CeO2 Catalysts. ACS Catal. 2015, 5, 6481–6488. [Google Scholar] [CrossRef]
- Zheng, B.Y.; Zhao, H.; Manjavacas, A.; McClain, M.; Nordlander, P.; Halas, N.J. Distinguishing between plasmon-induced and photoexcited carriers in a device geometry. Nat. Commun. 2015, 6, 7797. [Google Scholar] [CrossRef] [PubMed]
- Hoggard, A.; Wang, L.-Y.; Ma, L.; Fang, Y.; You, G.; Olson, J.; Liu, Z.; Chang, W.-S.; Ajayan, P.M.; Link, S. Using the plasmon linewidth to calculate the time and efficiency of electron transfer between gold nanorods and graphene. ACS Nano 2013, 7, 11209–11217. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.W.; Sobhani, H.; Nordlander, P.; Halas, N.J. Photodetection with active optical antennas. Science 2011, 332, 702–704. [Google Scholar] [CrossRef] [PubMed]
- DuChene, J.S.; Sweeny, B.C.; Johnston-Peck, A.C.; Su, D.; Stach, E.A.; Wei, W.D. Prolonged hot electron dynamics in plasmonic-metal/semiconductor heterostructures with implications for solar photocatalysis. Angew. Chem. Int. Ed. 2014, 53, 7887–7891. [Google Scholar] [CrossRef] [PubMed]
- Mubeen, S.; Lee, J.; Singh, N.; Kramer, S.; Stucky, G.D.; Moskovits, M. An autonomous photosynthetic device in which all charge carriers derive from surface plasmons. Nat. Nanotechnol. 2013, 8, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Lee, A.; Lee, T.; Lim, M.; Lim, D.-K. Ultrafast and efficient transport of hot plasmonic electrons by graphene for Pt free, highly efficient visible-light responsive photocatalyst. Nano Lett. 2016, 16, 1760–1767. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; Jang, C.; Xiao, S.; Ishigami, M.; Fuhrer, M.S. Intrinsic and extrinsic performance limits of graphene devices on SiO2. Nat. Nanotechnol. 2008, 3, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Nieboer, E.; Mcbryde, W.A.E. Free-energy relationships in coordination chemistry. III. A Comprehensive. Can. J. Chem. 1972, 51, 2512–2524. [Google Scholar] [CrossRef]
- Kumar, D.; Kaur, S.; Lim, D.-K. Plasmon-assisted and visible-light induced graphene oxide reduction and efficient fluorescence quenching. Chem. Commun. 2014, 50, 13481–13484. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Dumett Torres, D.; Jain, P.K. Activation Energies of Plasmonic Catalysts. Nano Lett. 2016, 16, 3399–3407. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.-W.; El-Sayed, M.A. Plasmonic field effect on the hexacyanoferrate (III)-thiosulfate electron transfer catalytic reaction on gold nanoparticles: Electromagnetic or thermal? J. Phys. Chem. C 2009, 113, 19585–19590. [Google Scholar] [CrossRef]
- Adleman, J.R.; Boyd, D.A.; Goodwin, D.G.; Psaltis, D. Heterogenous catalysis mediated by plasmon heating. Nano Lett. 2009, 9, 4417–4423. [Google Scholar] [CrossRef] [PubMed]
- Gomes Silva, C.U.; Juárez, R.; Marino, T.; Molinari, R.; García, H. Influence of excitation wavelength (UV or visible light) on the photocatalytic activity of titania containing gold nanoparticles for the generation of hydrogen or oxygen from water. J. Am. Chem. Soc. 2010, 133, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Naya, S.-I.; Inoue, A.; Tada, H. Self-assembled heterosupramolecular visible light photocatalyst consisting of gold nanoparticle-loaded titanium (IV) dioxide and surfactant. J. Am. Chem. Soc. 2010, 132, 6292–6293. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.H.; Kang, E.; Park, H.; Han, T.; Lee, C.-H.; Lim, D.-K. Pd-nanodot decorated MoS2 nanosheets as a highly efficient photocatalyst for the visible-light-induced Suzuki–Miyaura coupling reaction. J. Mater. Chem. A 2017, 5, 24965–24971. [Google Scholar] [CrossRef]
- Moreno-Mañas, M.; Pérez, M.; Pleixats, R. Palladium-catalyzed Suzuki-type self-coupling of arylboronic acids. A mechanistic study. J. Org. Chem. 1996, 61, 2346–2351. [Google Scholar] [CrossRef]
- Lim, D.-K.; Barhoumi, A.; Wylie, R.G.; Reznor, G.; Langer, R.S.; Kohane, D.S. Enhanced photothermal effect of plasmonic nanoparticles coated with reduced graphene oxide. Nano Lett. 2013, 13, 4075–4079. [Google Scholar] [CrossRef] [PubMed]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-H.; Baar, M.; Blechert, S.; Antonietti, M. Facilitating room-temperature Suzuki coupling reaction with light: Mott-Schottky photocatalyst for CC-coupling. Sci. Rep. 2013, 3, 1743. [Google Scholar] [CrossRef]
- Scheuermann, G.M.; Rumi, L.; Steurer, P.; Bannwarth, W.; Mülhaupt, R. Palladium nanoparticles on graphite oxide and its functionalized graphene derivatives as highly active catalysts for the Suzuki-Miyaura coupling reaction. J. Am. Chem. Soc. 2009, 131, 8262–8270. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, E.; Shin, H.H.; Lim, D.-K. Interface-Controlled Pd Nanodot-Au Nanoparticle Colloids for Efficient Visible-Light-Induced Photocatalytic Suzuki-Miyaura Coupling Reaction. Catalysts 2018, 8, 463. https://doi.org/10.3390/catal8100463
Kang E, Shin HH, Lim D-K. Interface-Controlled Pd Nanodot-Au Nanoparticle Colloids for Efficient Visible-Light-Induced Photocatalytic Suzuki-Miyaura Coupling Reaction. Catalysts. 2018; 8(10):463. https://doi.org/10.3390/catal8100463
Chicago/Turabian StyleKang, Eunmi, Hyeon Ho Shin, and Dong-Kwon Lim. 2018. "Interface-Controlled Pd Nanodot-Au Nanoparticle Colloids for Efficient Visible-Light-Induced Photocatalytic Suzuki-Miyaura Coupling Reaction" Catalysts 8, no. 10: 463. https://doi.org/10.3390/catal8100463
APA StyleKang, E., Shin, H. H., & Lim, D.-K. (2018). Interface-Controlled Pd Nanodot-Au Nanoparticle Colloids for Efficient Visible-Light-Induced Photocatalytic Suzuki-Miyaura Coupling Reaction. Catalysts, 8(10), 463. https://doi.org/10.3390/catal8100463