Bio-Oil Steam Reforming over a Mining Residue Functionalized with Ni as Catalyst: Ni-UGSO
Abstract
:1. Introduction
2. Results and Discussion
2.1. Bio-Oil Properties
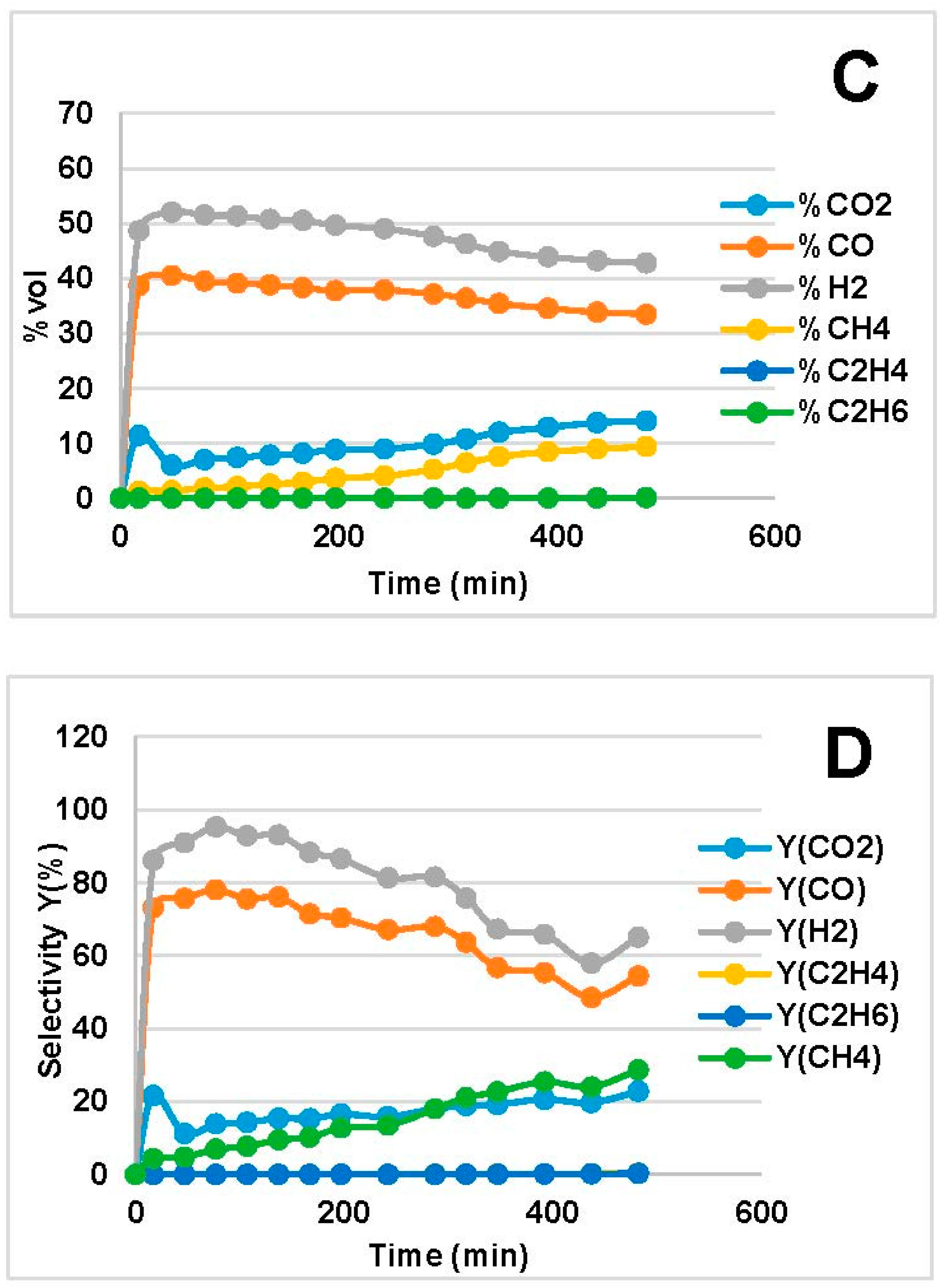
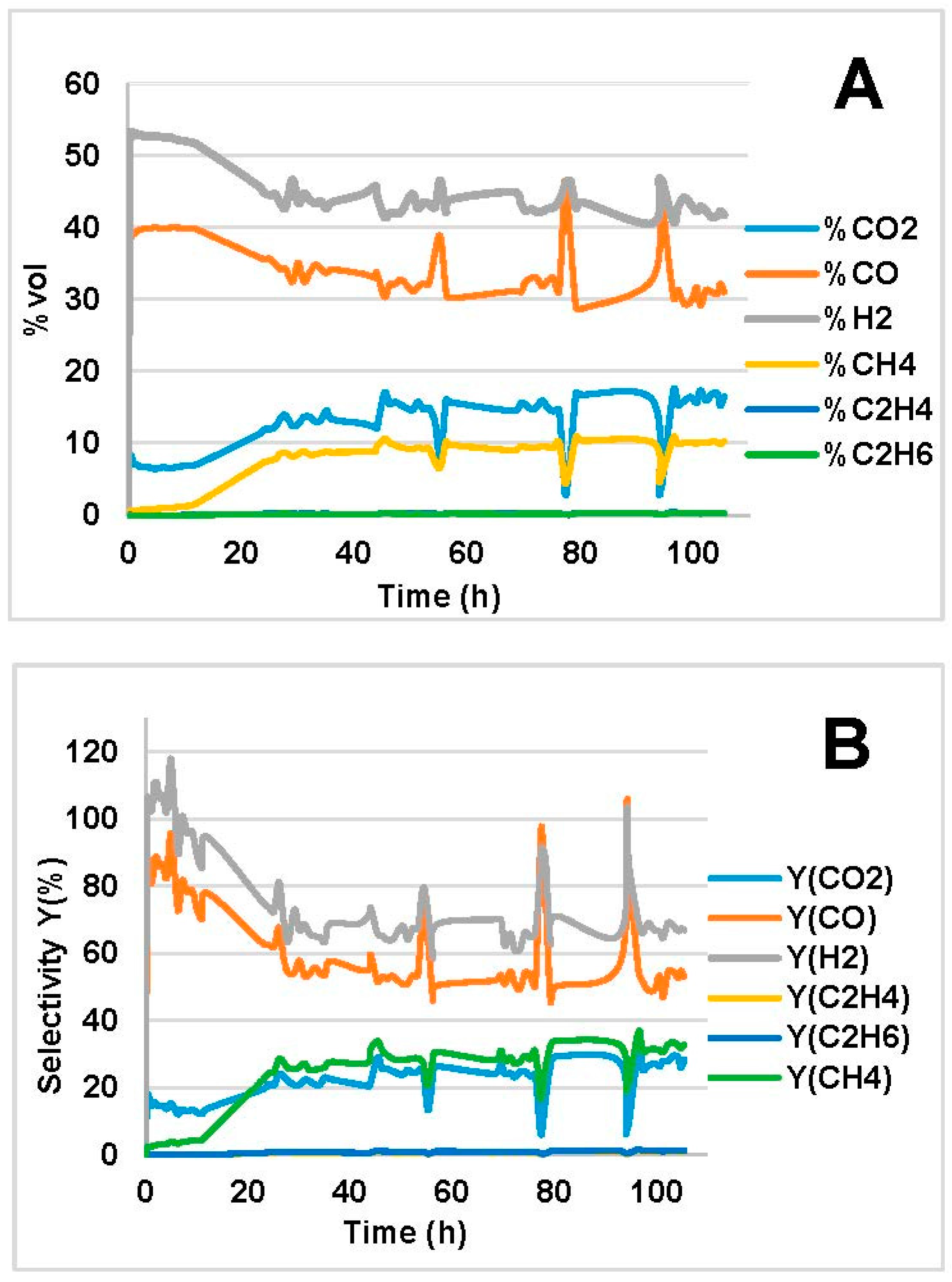
2.2. Steam Reforming Results
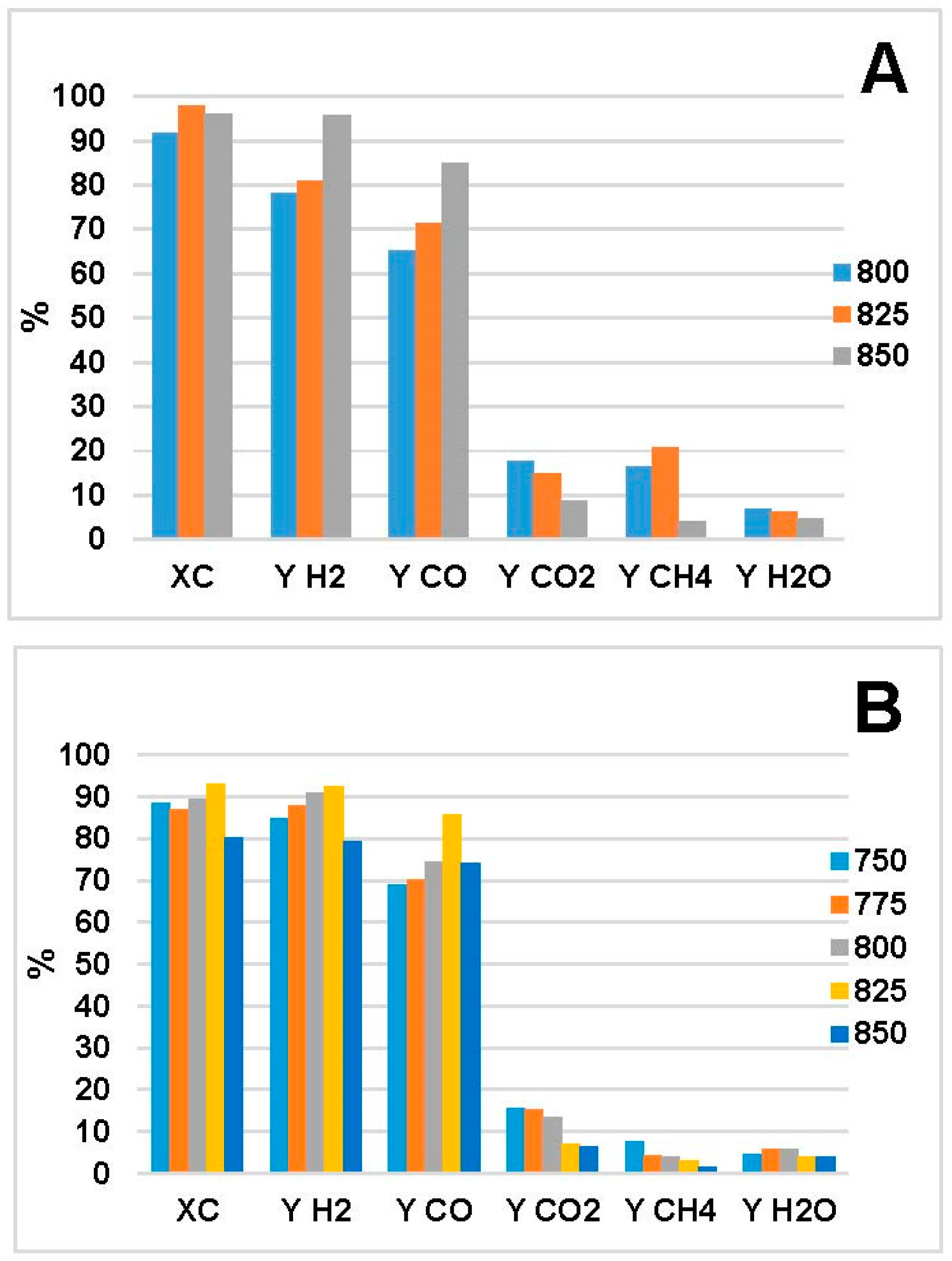
- Increased temperature evoked higher C conversion (XC), CO and H2 yields (, ) for both bio-oils. This was in agreement with the thermodynamic analysis of bio-oil model compounds studied by many authors [20,23,40] where it is found that the reaction is endothermic. The decrease in XC, and at 850 °C in Figure 1B is an outlier; we attribute that to experimental errors, which led to higher error in the mass balance than the average.
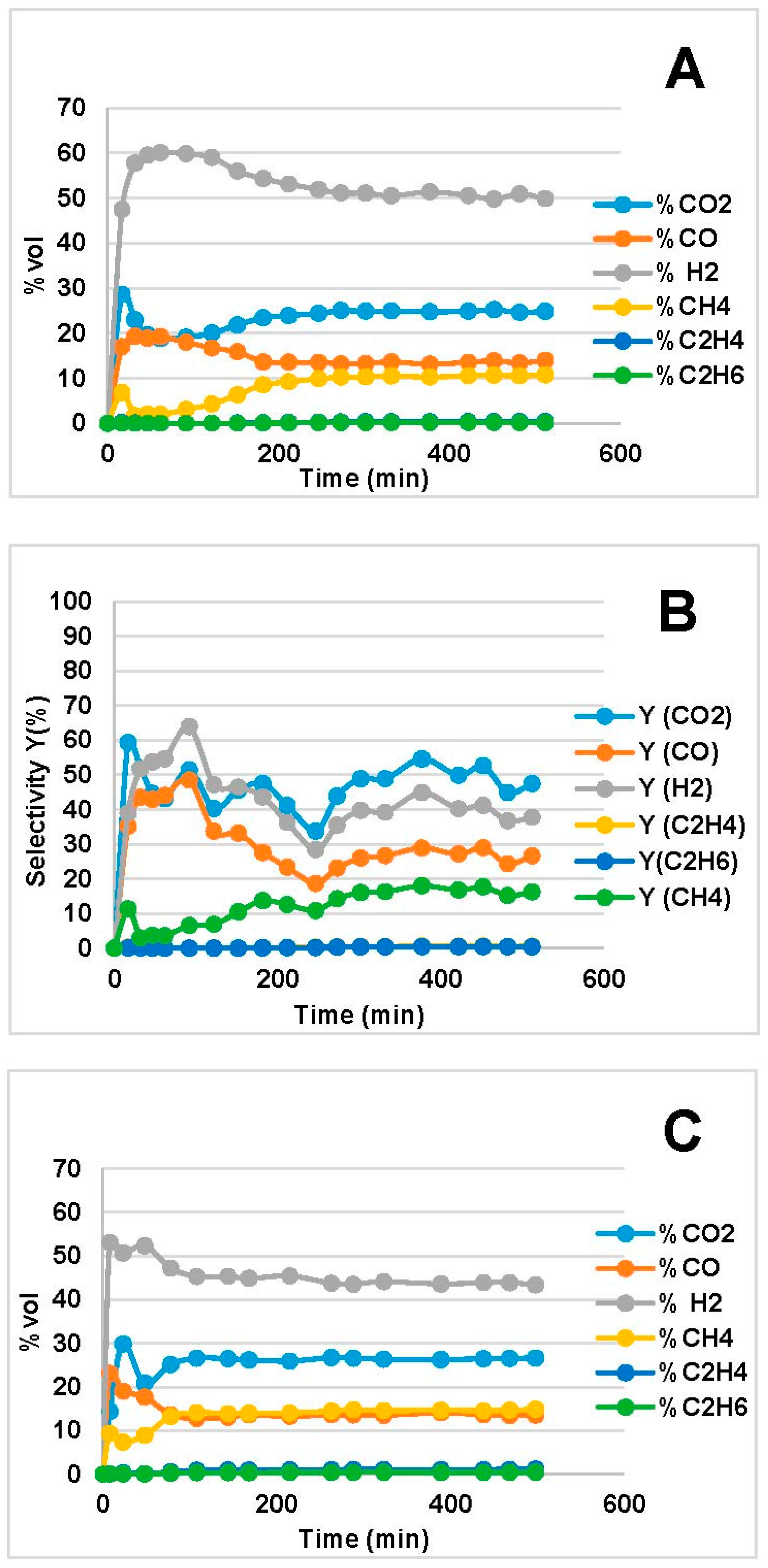
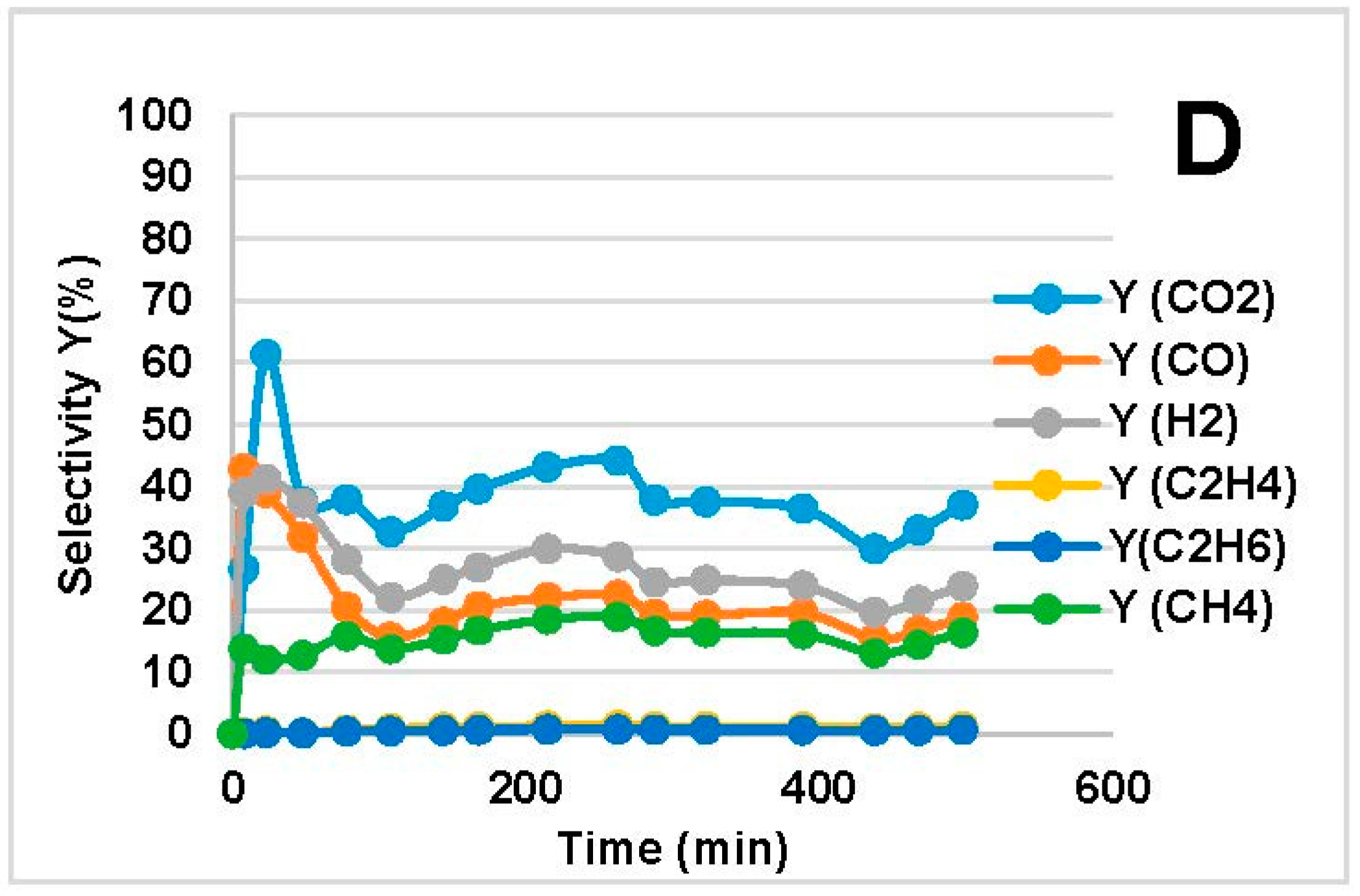
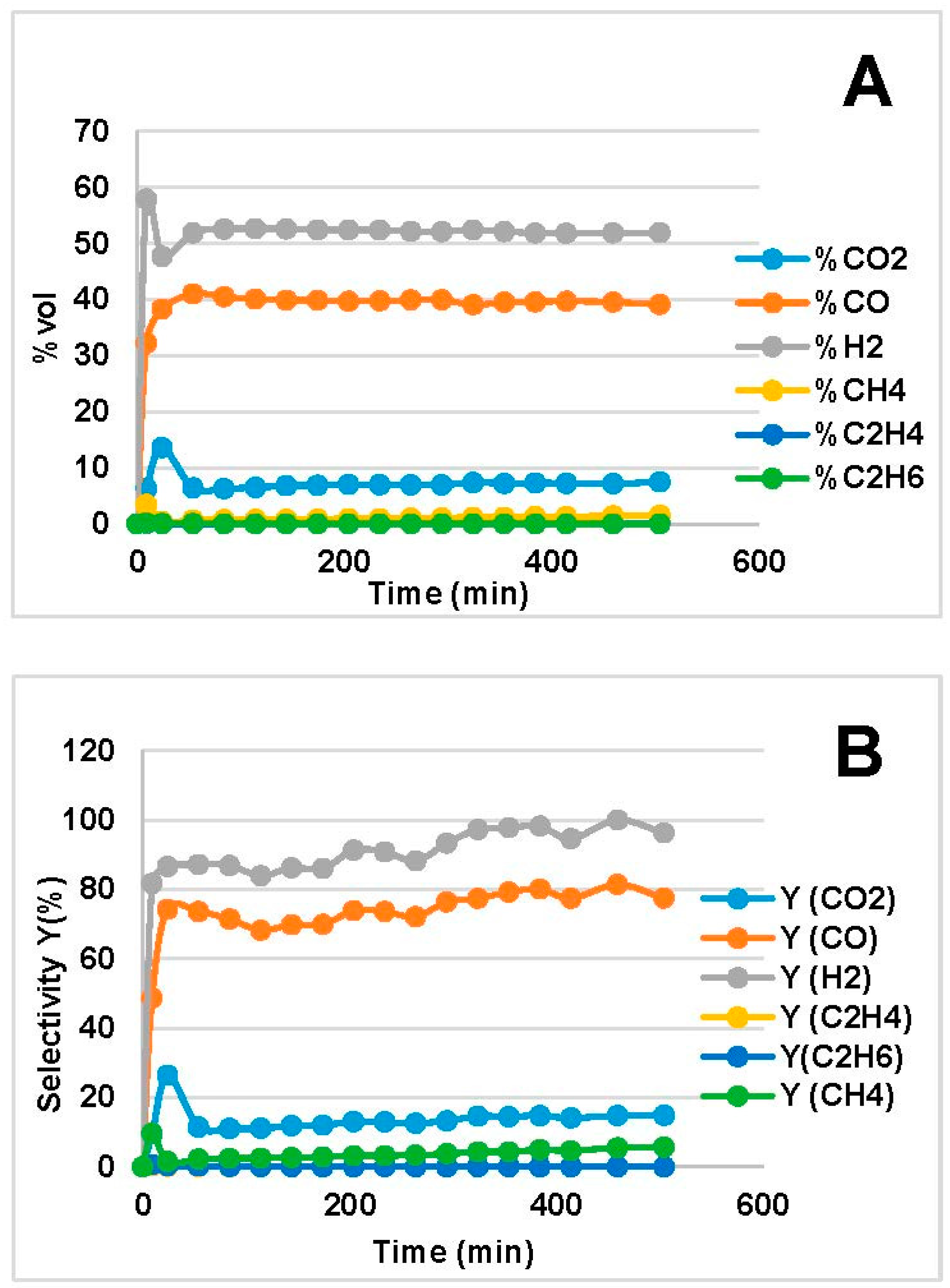
- CO and H2 were favored at low WHSV because of increased residence time over the catalyst bed. In the MemU bio-oil (Figure 3), we observe a decrease of CO2. A higher CO, H2 production through reforming means that there is a higher H2O consumption. If the increase of CO and H2 is equal, the WGS reaction (CO + H2O = CO2 + H2) will shift in such a way to compensate the H2O decrease; this means that the CO2 will decrease. This is the case for the MemU oil whose H2O/C is low; thus, the consumption of H2O by the reforming reaction has a higher impact on WGS. In the case of WU, we do not observe this behavior because the H2O/C is much higher.
- The amount of H2O and O in the two bio-oils had a significant impact on SR performance. Comparison of bio-oil MemU (Figure 1) and bio-oil WU (Figure 2) showed that the former gave better results in terms of H2 selectivity (up to 94%) and CO selectivity (up to 84%), while selectivity was maximum 43% and 36%, respectively, for bio-oil WU SR. It appeared that a ratio of O/C ≈ 1 (bio-oil MemU) was good enough for SR with Ni-UGSO compared to O/C ≈ 3 (bio-oil WU), which suggested that the catalyst was more active at low H2O or O content.
2.3. Catalyst Characterization
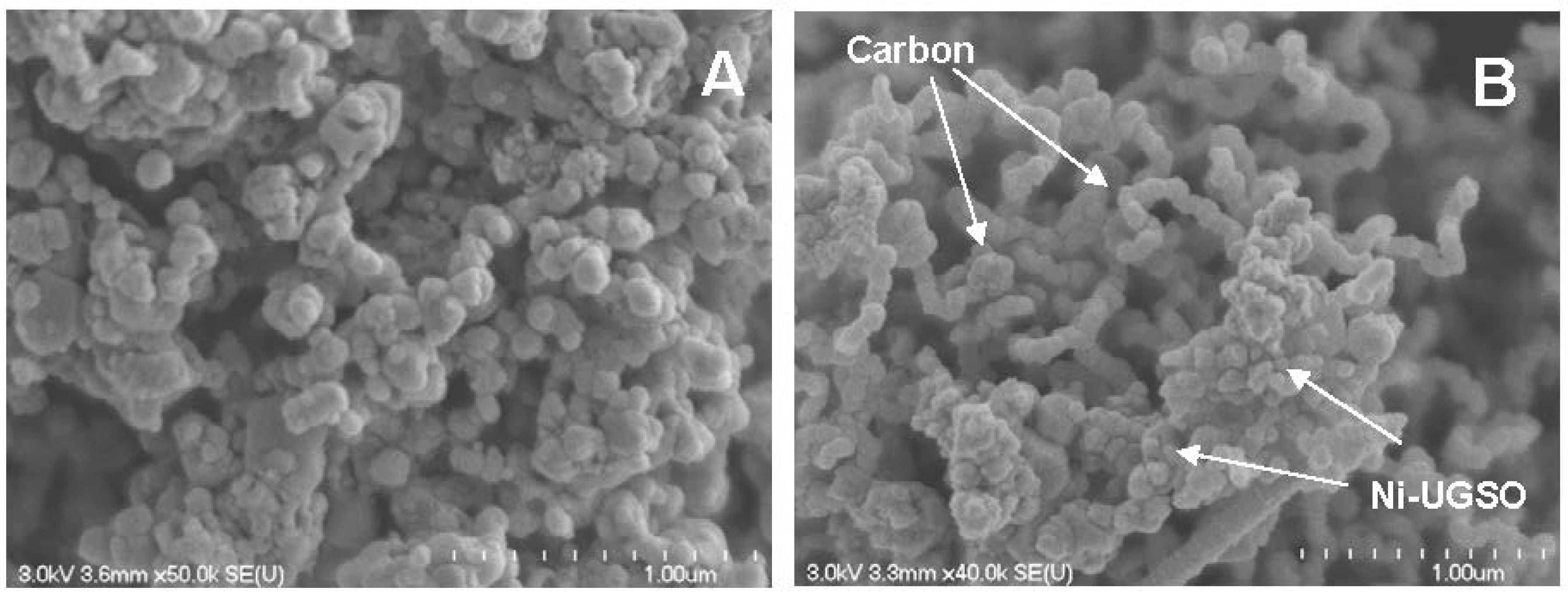
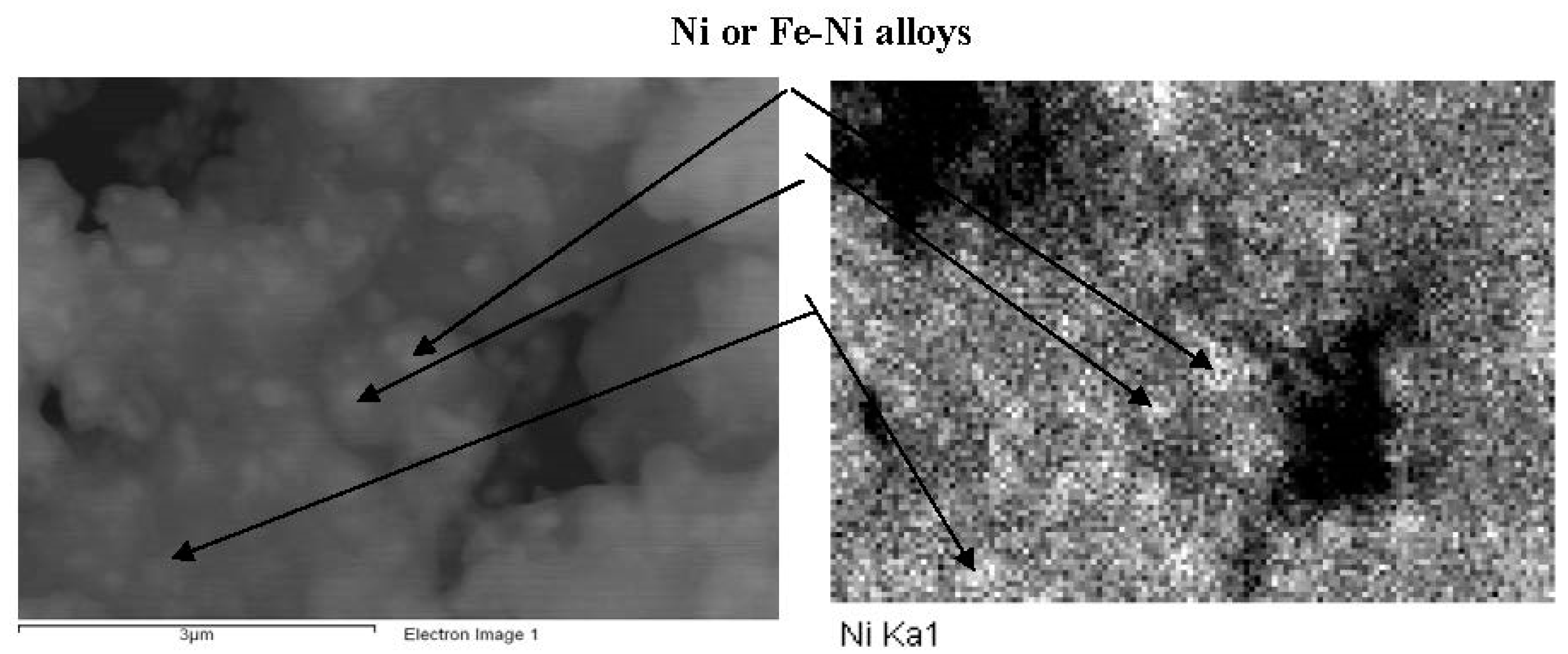
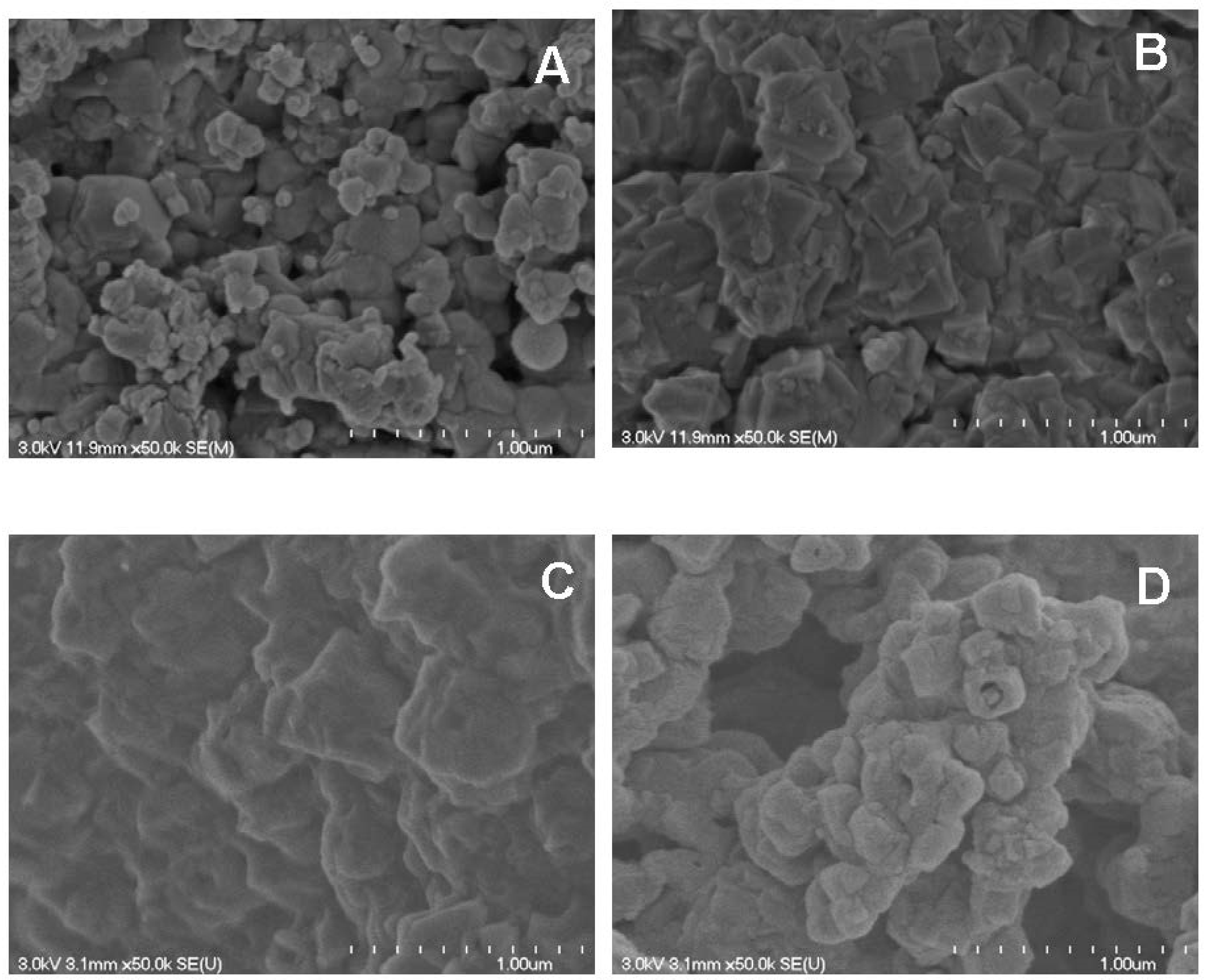
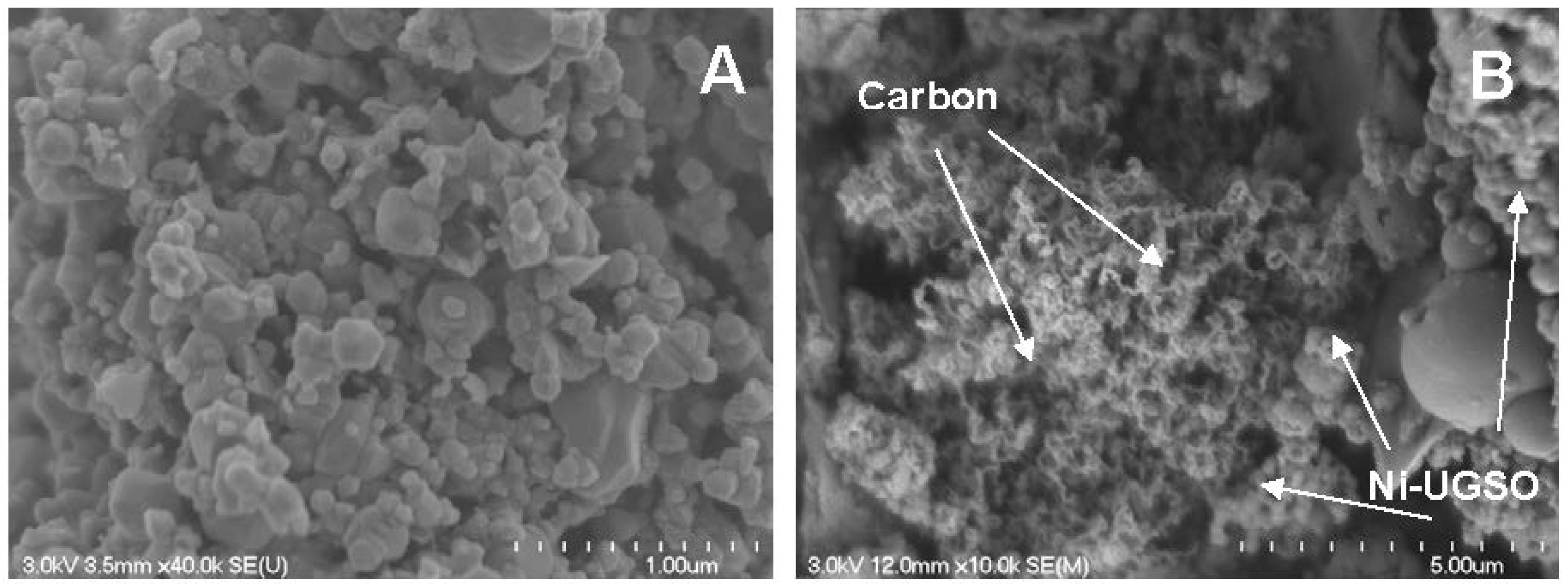
2.3.1. Scanning Electron Microscopy
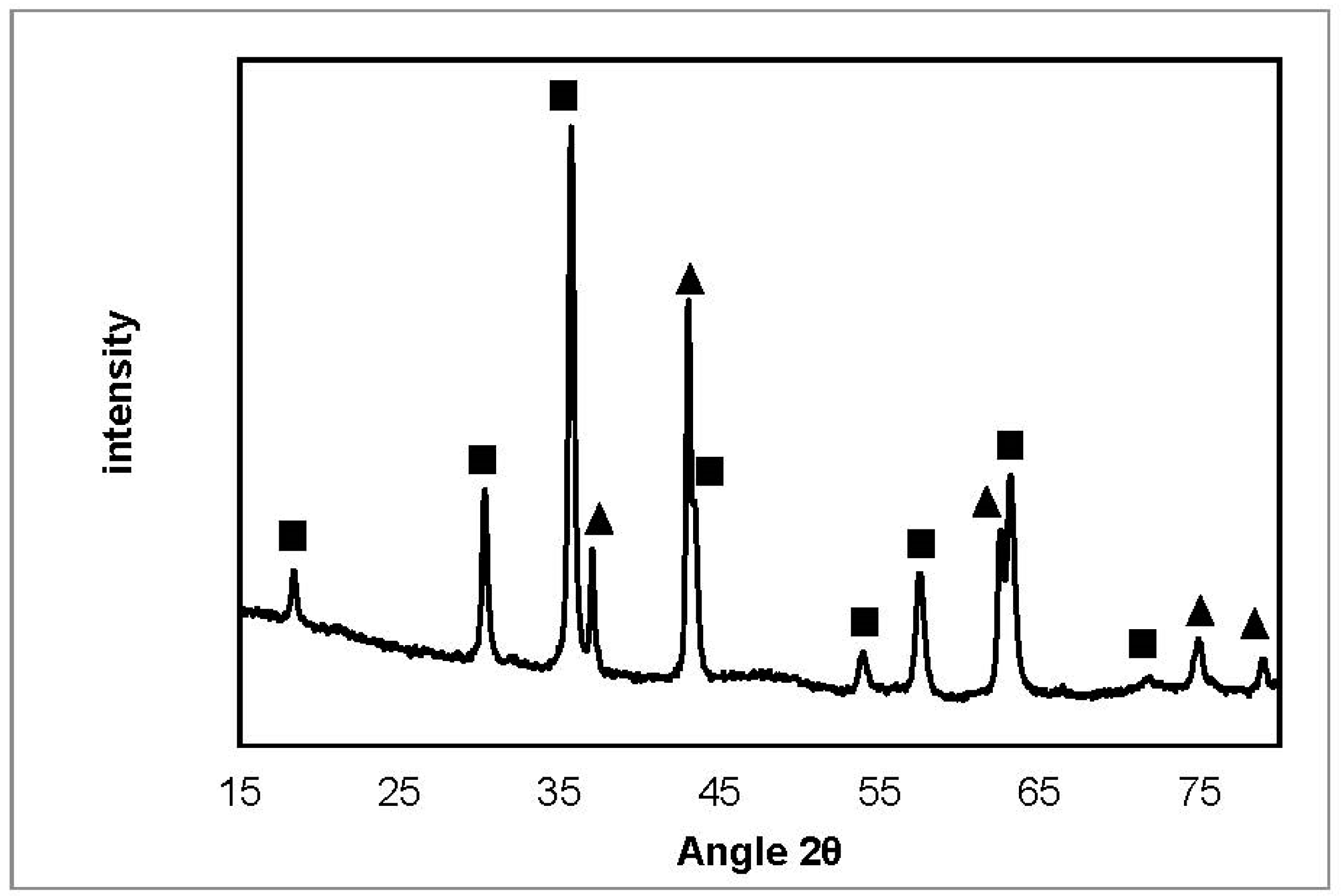
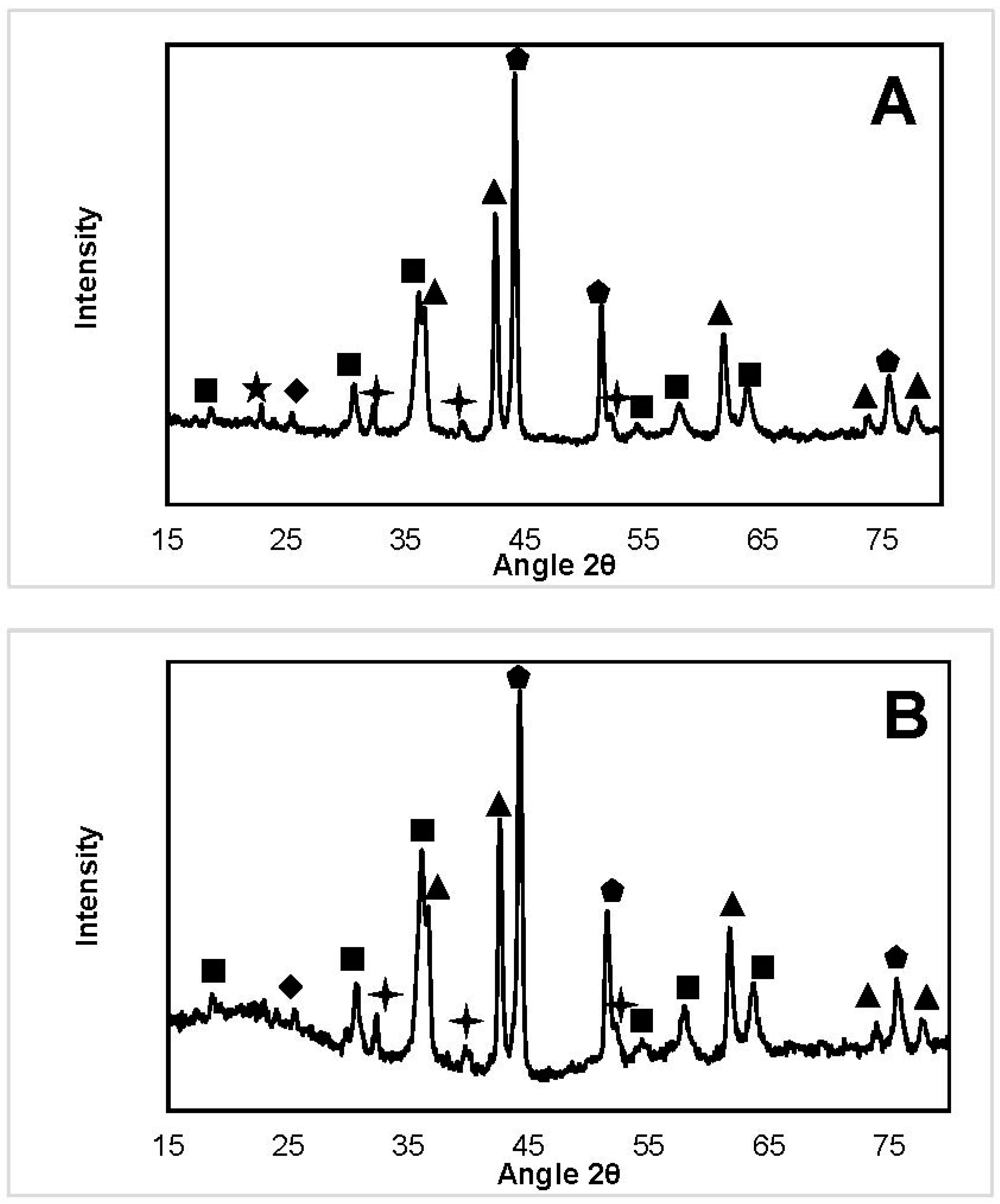
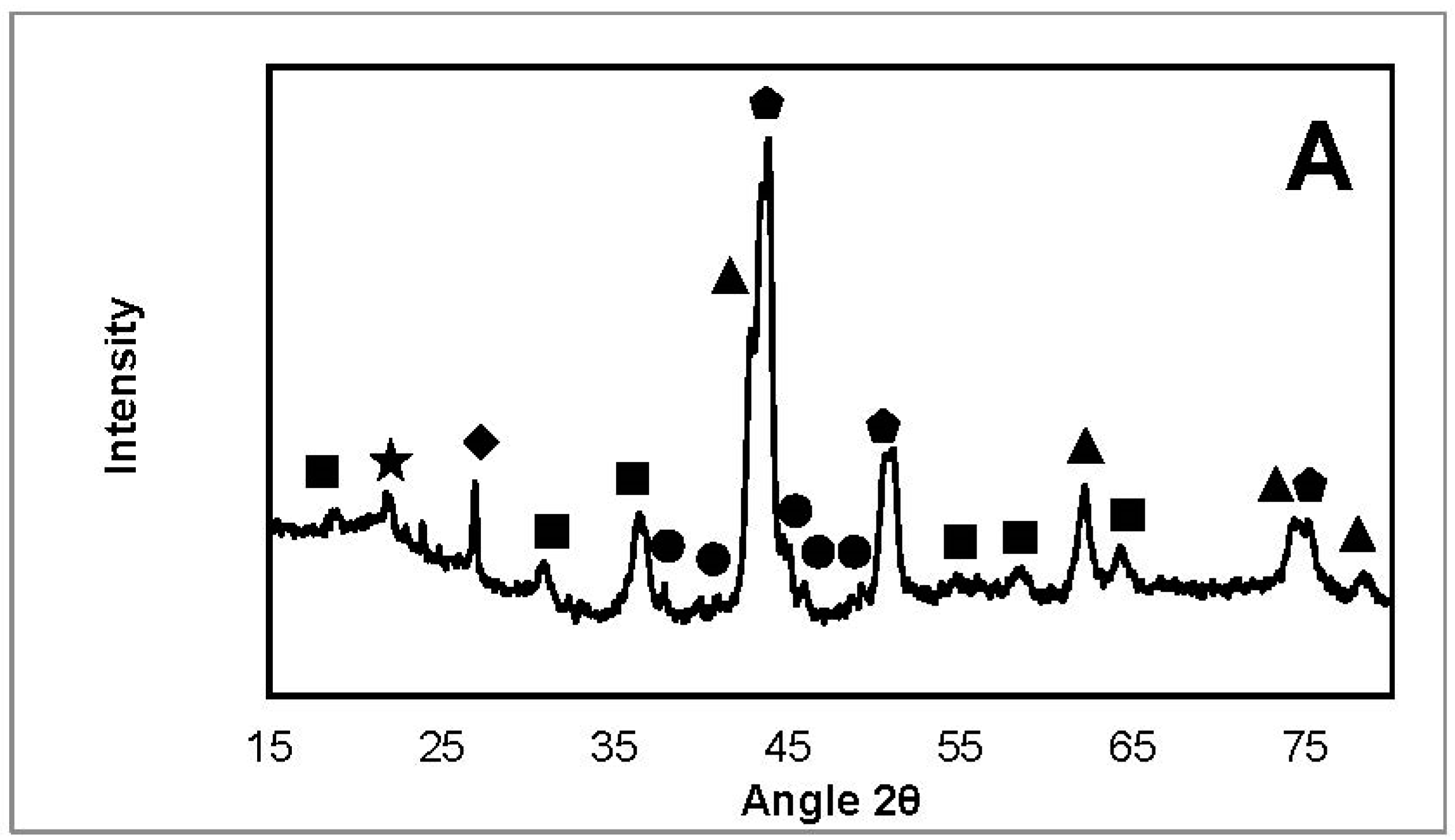
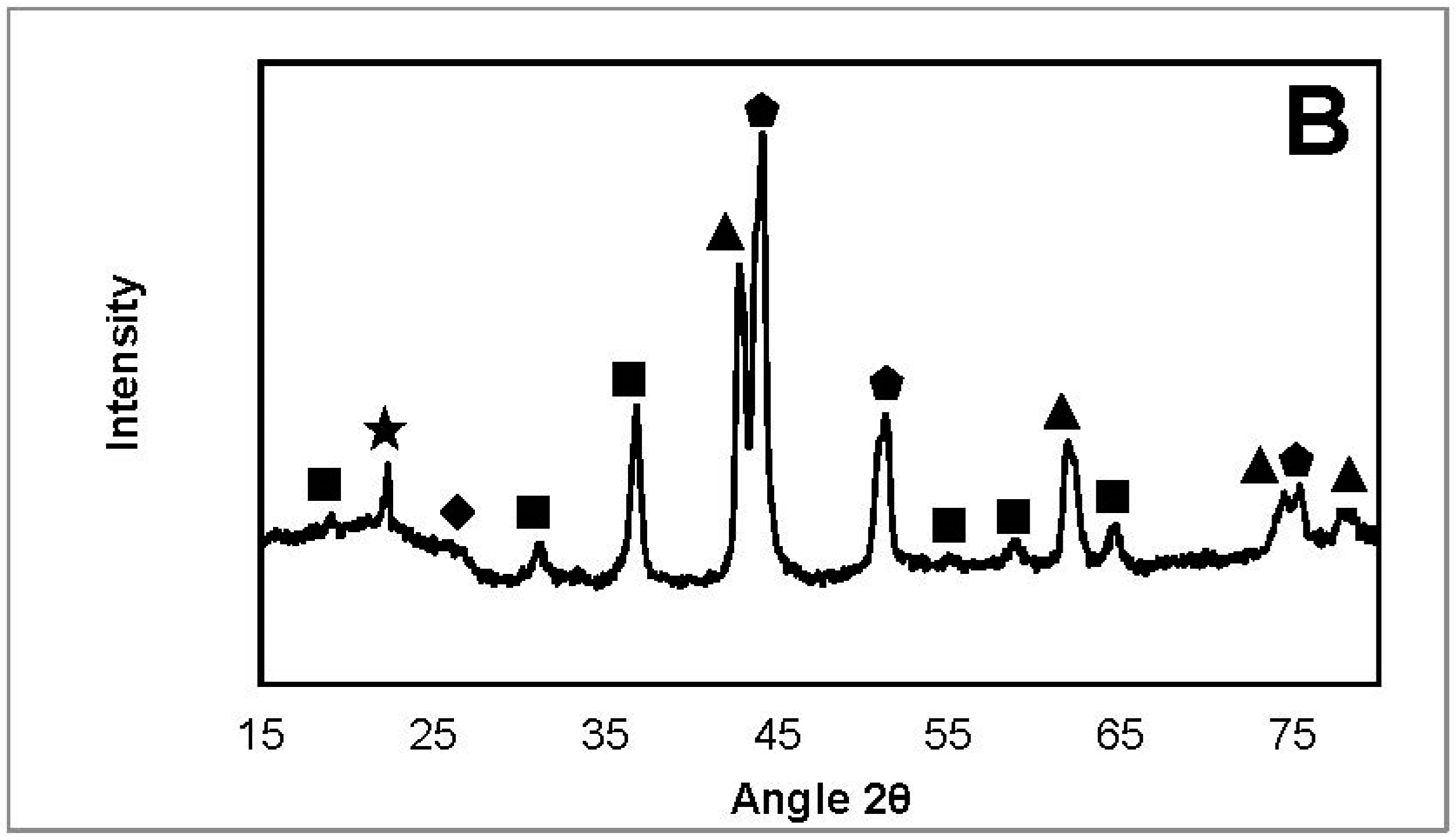
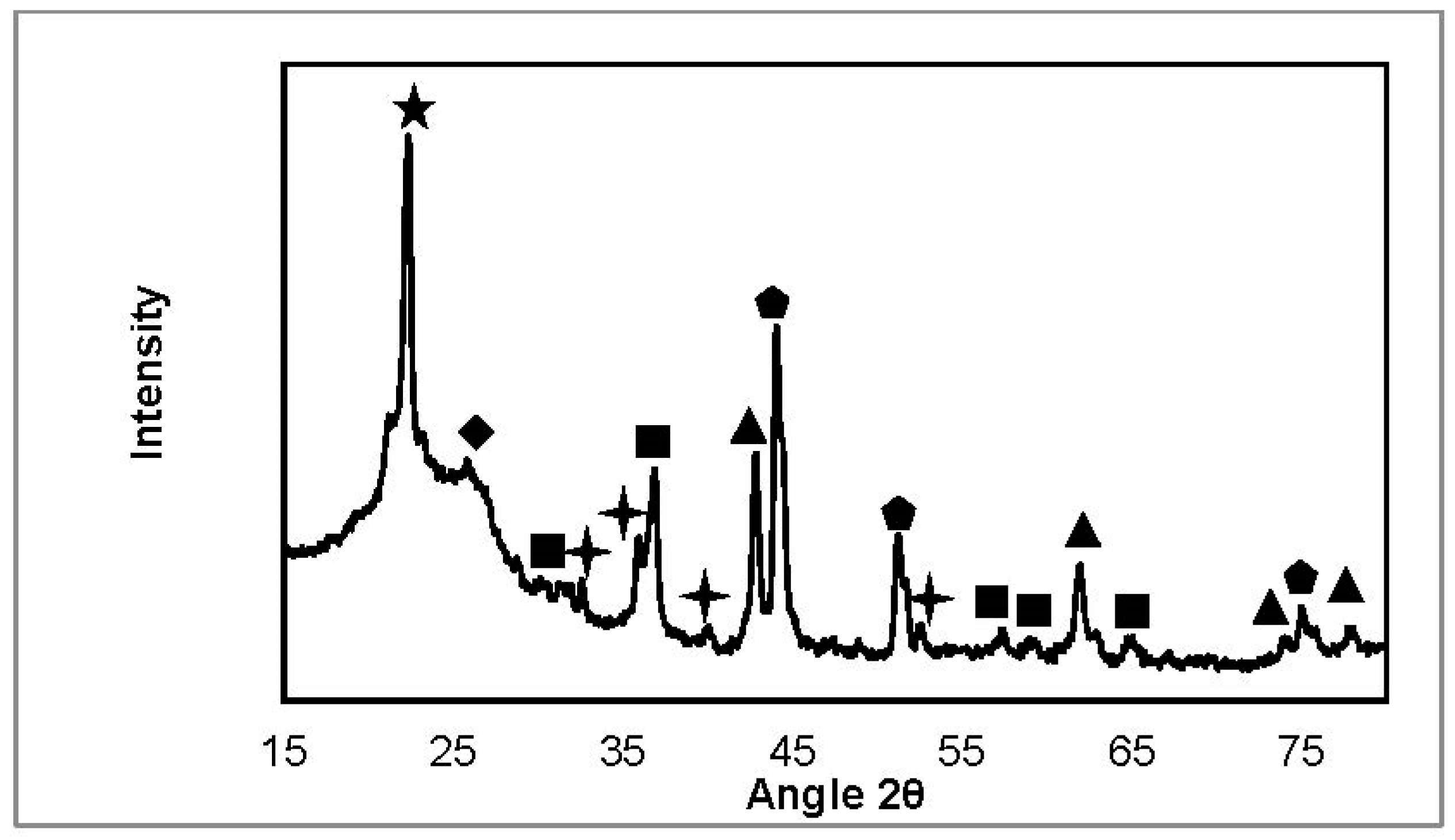
2.3.2. X-ray Diffraction
2.3.3. TGA Analysis
2.3.4. BET Measurement
3. Materials and Methods
3.1. Bio-Oils
3.2. Catalyst Preparation and Characterization
3.2.1. UGSO
3.2.2. Ni-UGSO
3.3. Experiments and Calculations
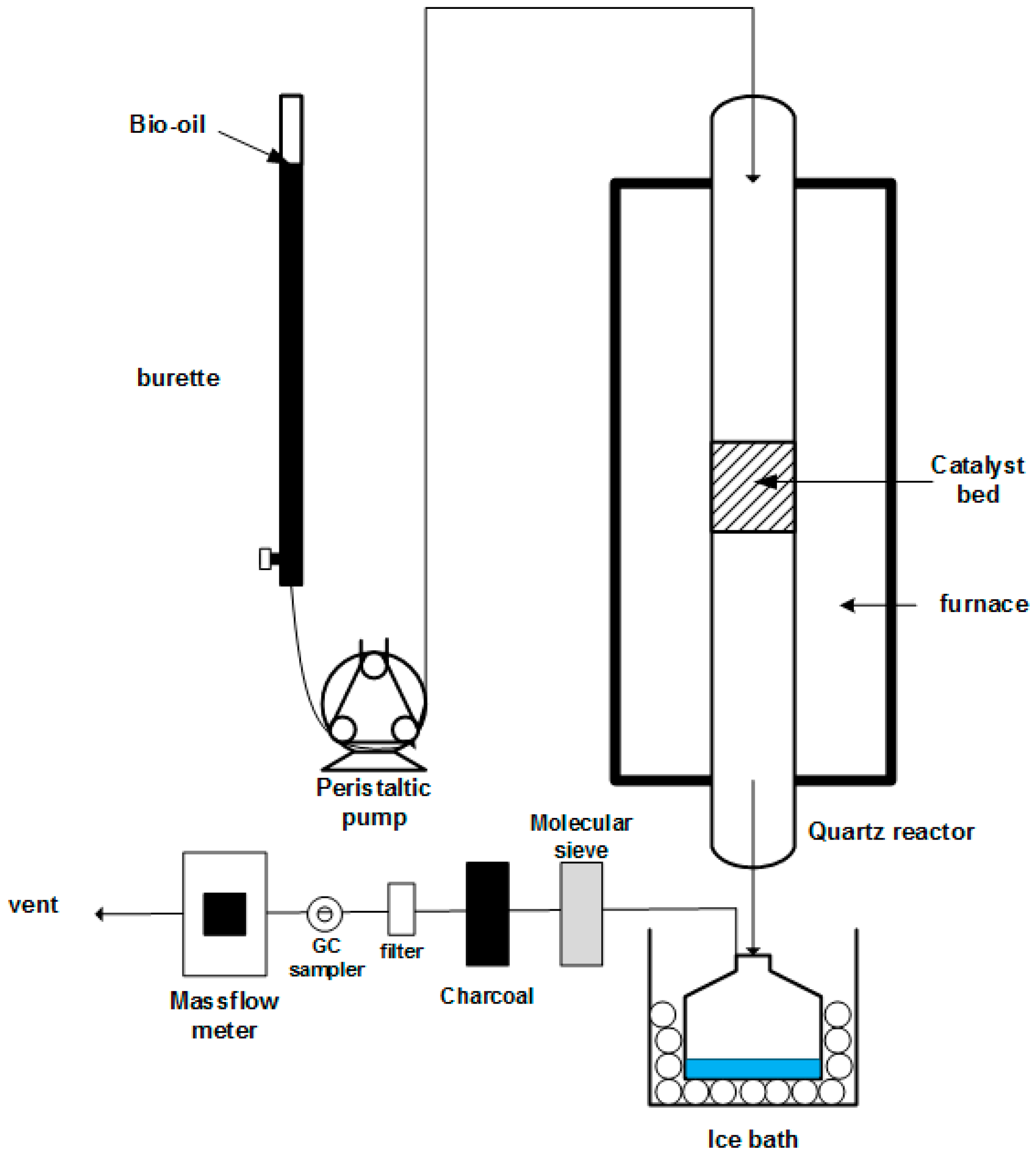
3.3.1. Experimental Set-Up
3.3.2. Test Conditions
3.3.3. Calculations
- The injection flowrate of the bio-oil was adjusted to ~0.1 mL/min; the amounts of C, H and O injected were calculated by multiplying mass flowrate, and the mass fraction of each element was determined by elemental analysis.
- Concentration measurements of gases by GC are reported in Table 7: the interval between each measurement was 30 min.
- The flowrate of the produced gas was measured by massflow meter and recorded.
- Using the ideal gas equation, we calculated the molar flow of each gas at 1 atm and 20 °C, and, by multiplying the stoichiometric number, we got the amount of each element at each time t, and we then summed it to obtain the total (Table 8).
- We weighed solid C deposited on the reactor wall and the liquid produced in the condenser, considering that it was composed 100% of H2O. In this example, mC = 1.63 g (0.135 mol) and mH2O = 3.43 g (nH = 0.38 mol, nO = 0.19 mol).
- We calculated the yield at each time t and for each product with Equations (6)–(9) (Table 9).
- Then, we calculated relative error between input and output and we got (Table 10).
4. Conclusions
- The catalyst was activated quickly (without a pre-reduction step).
- It was efficient at H2O/C, being 2–5 times lower than those used in industrial H2 production.
- No severe deactivation was observed in all tests, even after C formation.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Lehto, J.; Oasmaa, A.; Solantausta, Y.; Kytö, M.; Chiaramonti, D. Fuel Oil Quality and Combustion of Fast Pyrolysis Bio-Oils; VTT Publication: Helsinki, Finland, 2013; p. 79. [Google Scholar]
- Abdullah, H.; Wu, H. Bioslurry as a fuel. 4. Preparation of bioslurry fuels from biochar and the bio-oil-rich fractions after bio-oil/biodiesel extraction. Energy Fuels 2011, 25, 1759–1771. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Bonini, M.; Fratini, E.; Tondi, G.; Gartner, K.; Bridgwater, A.V.; Grimm, H.P.; Soldaini, I.; Webster, A.; Baglioni, P. Development of emulsions from biomass pyrolysis liquid and diesel and their use in engines—Part 1: Emulsion production. Biomass Bioenergy 2003, 25, 85–99. [Google Scholar] [CrossRef]
- Elliott, D.C. Historical developments in hydroprocessing bio-oils. Energy Fuels 2007, 21, 1792–1815. [Google Scholar] [CrossRef]
- Czernik, S.; Bridgwater, A.V. Overview of applications of biomass fast pyrolysis oil. Energy Fuels 2004, 18, 590–598. [Google Scholar] [CrossRef]
- Diebold, J.P. A Review of the Chemical and Physical Mechanisms of the Storage Stability of Fast Pyrolysis Bio-Oils; U.S. Department of Energy: Oak Ridge, TN, USA, 2000.
- Diebold, J.P.; Czernik, S. Additives to Lower and Stabilize the Viscosity of Pyrolysis Oils during Storage. Energy Fuels 1997, 11, 1081–1091. [Google Scholar] [CrossRef]
- Czernik, S.; French, R. Distributed production of hydrogen by auto-thermal reforming of fast pyrolysis bio-oil. Int. J. Hydrogen Energy 2014, 39, 744–750. [Google Scholar] [CrossRef]
- Wang, D.; Czernik, S.; Chornet, E. Production of hydrogen from biomass by catalytic steam reforming of fast pyrolysis oils. Energy Fuels 1998, 12, 19–24. [Google Scholar] [CrossRef]
- Czernik, S.; Evans, R.; French, R. Hydrogen from biomass-production by steam reforming of biomass pyrolysis oil. Catal. Today 2007, 129, 265–268. [Google Scholar] [CrossRef]
- Czernik, S.; French, R.; Feik, C.; Chornet, E. Hydrogen by Catalytic Steam Reforming of Liquid Byproducts from Biomass Thermoconversion Processes. Ind. Eng. Chem. Res. 2002, 41, 4209–4215. [Google Scholar] [CrossRef]
- Wright, M.M.; Daugaard, D.E.; Satrio, J.A.; Brown, R.C. Techno-economic analysis of biomass fast pyrolysis to transportation fuels. Fuel 2010, 89 (Suppl. 1), S2–S10. [Google Scholar] [CrossRef]
- Wang, D.; Czernik, S.; Montane, D.; Mann, M.; Chornet, E. Biomass to Hydrogen via Fast Pyrolysis and Catalytic Steam Reforming of the Pyrolysis Oil or Its Fractions. Ind. Eng. Chem. Res. 1997, 36, 1507–1518. [Google Scholar] [CrossRef]
- Garcia, L.; French, R.; Czernik, S.; Chornet, E. Catalytic steam reforming of bio-oils for the production of hydrogen: Effects of catalyst composition. Appl. Catal. A Gen. 2000, 201, 225–239. [Google Scholar] [CrossRef]
- Rennard, D.; French, R.; Czernik, S.; Josephson, T.; Schmidt, L. Production of synthesis gas by partial oxidation and steam reforming of biomass pyrolysis oils. Int. J. Hydrogen Energy 2010, 35, 4048–4059. [Google Scholar] [CrossRef]
- Marda, J.R.; DiBenedetto, J.; McKibben, S.; Evans, R.J.; Czernik, S.; French, R.J.; Dean, A.M. Non-catalytic partial oxidation of bio-oil to synthesis gas for distributed hydrogen production. Int. J. Hydrogen Energy 2009, 34, 8519–8534. [Google Scholar] [CrossRef]
- Hu, X.; Lu, G. Bio-oil steam reforming, partial oxidation or oxidative steam reforming coupled with bio-oil dry reforming to eliminate CO2 emission. Int. J. Hydrogen Energy 2010, 35, 7169–7176. [Google Scholar] [CrossRef]
- Xie, H.; Yu, Q.; Zuo, Z.; Han, Z.; Yao, X.; Qin, Q. Hydrogen production via sorption-enhanced catalytic steam reforming of bio-oil. Int. J. Hydrogen Energy 2016, 41, 2345–2353. [Google Scholar] [CrossRef]
- Seyedeyn-azad, F.; Abedi, J.; Sampouri, S. Catalytic Steam Reforming of Aqueous Phase of Bio-Oil over Ni-Based Alumina-Supported Catalysts. Ind. Eng. Chem. Res. 2014, 53, 17937–17944. [Google Scholar] [CrossRef]
- Gao, N.; Han, Y.; Quan, C.; Wu, C. Promoting hydrogen-rich syngas production from catalytic reforming of biomass pyrolysis oil on nanosized nickel-ceramic catalysts. Appl. Therm. Eng. 2017, 125, 297–305. [Google Scholar] [CrossRef]
- Quan, C.; Xu, S.; Zhou, C. Steam reforming of bio-oil from coconut shell pyrolysis over Fe/olivine catalyst. Energy Convers. Manag. 2017, 141, 40–47. [Google Scholar] [CrossRef]
- Remón, J.; Broust, F.; Volle, G.; García, L.; Arauzo, J. Hydrogen production from pine and poplar bio-oils by catalytic steam reforming. Influence of the bio-oil composition on the process. Int. J. Hydrogen Energy 2015, 40, 5593–5608. [Google Scholar] [CrossRef]
- Vagia, E.C.; Lemonidou, A.A. Thermodynamic analysis of hydrogen production via steam reforming of selected components of aqueous bio-oil fraction. Int. J. Hydrogen Energy 2007, 32, 212–223. [Google Scholar] [CrossRef]
- Vagia, E.C.; Lemonidou, A.A. Thermodynamic analysis of hydrogen production via autothermal steam reforming of selected components of aqueous bio-oil fraction. Int. J. Hydrogen Energy 2008, 33, 2489–2500. [Google Scholar] [CrossRef]
- González-Gil, R.; Chamorro-Burgos, I.; Herrera, C.; Larrubia, M.A.; Laborde, M.; Marino, F.; Alemany, L.J. Production of hydrogen by catalytic steam reforming of oxygenated model compounds on Ni-modified supported catalysts. Simulation and experimental study. Int. J. Hydrogen Energy 2015, 40, 11217–11227. [Google Scholar] [CrossRef]
- Trane-Restrup, R.; Jensen, A.D. Steam reforming of cyclic model compounds of bio-oil over Ni-based catalysts: Product distribution and carbon formation. Appl. Catal. B Environ. 2015, 165, 117–127. [Google Scholar] [CrossRef]
- Abatzoglou, N.; Fauteux-Lefebvre, C. Review of catalytic syngas production through steam or dry reforming and partial oxidation of studied liquid compounds. Wiley Interdiscip. Rev. Energy Environ. 2016, 5, 169–187. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R.; Sehested, J.; Noerskov, J.K. Hydrogen and Synthesis Gas by Steam- and CO2 Reforming. Adv. Catal. 2003, 34, 17. [Google Scholar] [CrossRef]
- Bengaard, H.S.; Nørskov, J.K.; Sehested, J.; Clausen, B.S.; Nielsen, L.P.; Molenbroek, A.M.; Rostrup-Nielsen, J.R. Steam Reforming and Graphite Formation on Ni Catalysts. J. Catal. 2002, 209, 365–384. [Google Scholar] [CrossRef]
- Navarro, R.M.; Guil-Lopez, R.; Gonzalez-Carballo, J.M.; Cubero, A.; Ismail, A.A.; Al-Sayari, S.A.; Fierro, J.L.G. Bimetallic MNi/Al2O3-La catalysts (M = Pt, Cu) for acetone steam reforming: Role of M on catalyst structure and activity. Appl. Catal. A Gen. 2014, 474, 168–177. [Google Scholar] [CrossRef]
- Christensen, K.O.; Chen, D.; Lødeng, R.; Holmen, A. Effect of supports and Ni crystal size on carbon formation and sintering during steam methane reforming. Appl. Catal. A Gen. 2006, 314, 9–22. [Google Scholar] [CrossRef]
- Fauteux-Lefebvre, C.; Abatzoglou, N.; Blanchard, J.; Gitzhofer, F. Steam reforming of liquid hydrocarbons over a nickel-alumina spinel catalyst. J. Power Sources 2010, 195, 3275–3283. [Google Scholar] [CrossRef]
- Fauteux-Lefebvre, C.; Abatzoglou, N.; Braidy, N.; Achouri, I.E. Diesel steam reforming with a nickel-alumina spinel catalyst for solid oxide fuel cell application. J. Power Sources 2011, 196, 7673–7680. [Google Scholar] [CrossRef]
- Achouri, I.E.; Abatzoglou, N.; Braidy, N.; Bastien, S. New insights on the role of YSZ in a NiAl2O4/Al2O3-YSZ catalyst. Appl. Catal. A Gen. 2015, 497, 42–50. [Google Scholar] [CrossRef]
- Abatzoglou, N.; Chamoumi, M. Process for Producing Catalysts from Mining Residue and Catalysts Produced Therefrom. WO 2017/011906 A1, 26 January 2017. [Google Scholar]
- Chamoumi, M.; Abatzoglou, N.; Blanchard, J.; Iliuta, M.-C.; Larachi, F. Dry reforming of methane with a new catalyst derived from a negative value mining residue spinellized with nickel. Catal. Today 2017, 2, 86–98. [Google Scholar] [CrossRef]
- Chamoumi, M. Nouvelle Génération de Catalyseur Supportés par Valorisation d’un Résidu D’enrichessement (Procédé UGS) D’une Scorie de TiO2: Le Catalyseur Ni-UGSO Appliqué au Reformage de Méthane; Université de Sherbrooke: Sherbrooke, QC, Canada, 2017. [Google Scholar]
- Arregi, A.; Lopez, G.; Amutio, M.; Barbarias, I.; Bilbao, J.; Olazar, M. Hydrogen production from biomass by continuous fast pyrolysis and in-line steam reforming. RSC Adv. 2016, 6, 25975–25985. [Google Scholar] [CrossRef]
- Resende, K.A.; Ávila-Neto, C.N.; Rabelo-Neto, R.C.; Noronha, F.B.; Hori, C.E. Thermodynamic analysis and reaction routes of steam reforming of bio-oil aqueous fraction. Renew. Energy 2015, 80, 166–176. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R. Catalytic Steam Reforming; Springer: Berlin, Germany, 1984. [Google Scholar]
- Sehested, J.; Gelten, J.A.P.; Helveg, S. Sintering of nickel catalysts: Effects of time, atmosphere, temperature, nickel-carrier interactions, and dopants. Appl. Catal. A Gen. 2006, 309, 237–246. [Google Scholar] [CrossRef]
- Braidy, N.; Bastien, S.; Blanchard, J.; Fauteux-lefebvre, C.; Achouri, I.E.; Abatzoglou, N. Activation mechanism and microstructural evolution of a YSZ/Ni-alumina catalyst for dry reforming of methane. Catal. Today 2017, 291, 99–105. [Google Scholar] [CrossRef]
- Lea-Langton, A.; Zin, R.M.; Dupont, V.; Twigg, M.V. Biomass pyrolysis oils for hydrogen production using chemical looping reforming. Int. J. Hydrogen Energy 2012, 37, 2037–2043. [Google Scholar] [CrossRef]
- Song, P.; Wen, D.; Guo, Z.X.; Korakianitis, T. Oxidation investigation of nickel nanoparticles. Phys. Chem. Chem. Phys. 2008, 10, 5057. [Google Scholar] [CrossRef] [PubMed]
- Papari, S.; Hawboldt, K.; Helleur, R. Production and Characterization of Pyrolysis Oil from Sawmill Residues in an Auger Reactor. Ind. Eng. Chem. Res. 2017, 56, 1920–1925. [Google Scholar] [CrossRef]
- Gooty, A.T.; Li, D.; Berruti, F.; Briens, C. Kraft-lignin pyrolysis and fractional condensation of its bio-oil vapors. J. Anal. Appl. Pyrolysis 2014, 106, 33–40. [Google Scholar] [CrossRef]
- Venderbosch, R.H.; Ardiyanti, A.R.; Wildschut, J.; Oasmaa, A.; Heeres, H.J. Stabilization of biomass-derived pyrolysis oils. J. Chem. Technol. Biotechnol. 2010, 85, 674–686. [Google Scholar] [CrossRef]
- Guéguin, M.; Cardarelli, F. Chemistry and Mineralogy of Titania-Rich Slags. Part 1—Hemo-Ilmenite, Sulphate, and Upgraded Titania Slags. Miner. Process. Extr. Metall. Rev. 2007, 28, 1–58. [Google Scholar] [CrossRef]
- Chamoumi, M.; Abatzoglou, N. NiFe2O4 production from α-Fe2O3 via improved solid state reaction: Application as catalyst in CH4 dry reforming. Can. J. Chem. Eng. 2016, 94, 1801–1808. [Google Scholar] [CrossRef]



| Properties | Bio-Oil MemU | Bio-Oil WU |
|---|---|---|
| Density | 1.187 | 1.087 |
| % C * | 39.4 | 18.8 |
| % H * | 7.0 | 7.9 |
| % O * | 53.6 | 73.3 |
| % H2O * | 34.3 | 52.3 |
| pH | 2.4 | 2.9 |
| H2O/C ** | 0.60 | 1.85 |
| O/C ** | 1.02 | 2.92 |
| Catalyst | Feedstock | Temperature (°C) | H2O/C (mol/mol) | Space Velocity (h−1) | Reactor | TOS (h) | YH2 (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| Ni-UGSO | RBO MemU | 750–850 | 0.6 | 1.7–6.6 (W) | Fixed bed | 8.3 | 78–95 | This work |
| RBO WU | 1.9 | 1.8–7.1 (W) | 16–43 | |||||
| C11-NK | BOAq | 800–850 | 7–9 | 0.96–2.7 (W) | Fluidized bed | 4–90 | 77–89 | [12] |
| Ni-MgO/Al2O3 | BOAq | 850 | 3.2–4.2 | 30 (W) | Fixed bed | 1 | 12–61 | [20] |
| Ni/ceramic foam | RBO | 500–800 | 2.6 | 1.5–4 (W) | Fixed bed | 0.5–2 | 54.5–93.5 | [21] |
| ICI 46-1/4 | BOAq | 700–75 | 5–35 | 760–1130 (G) | Fixed bed | 1–6 | 76–100 | [10] |
| UCI G-91 | ||||||||
| Ce-Ni/Co/Al2O3 + CO2 sorbent | RBO | 650–850 | 9–15 | 0.08–0.23 (L) | Fixed bed | 0.5 | 65–85 | [19] |
| Ni/Al2O3 | BOAq | 600 | 0.5–3.5 | 1.5–3.8 (W) | Fixed bed (CLR) | 0.6–1.7 | 59–83 | [44] |
| G90LDP | RBO | 550–700 | 3.9–9.7 | 2–24 (W) ** | Fluidized bed | - | 20–95 | [39] |
| T = 800 °C WHSV = 2.0 g/gcat/h Δt = 105 h | Bio-Oil MemU | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Period (h) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) |
| 0–12 | 96.2 | 100 | 81.6 | 14.1 | 0.87 | 52.6 | 39.7 | 6.8 | 0.9 |
| 30–105 | 96.7 | 66.8 | 52.7 | 25.0 | 6.9 | 43.1 | 32.2 | 14.8 | 9.4 |
 | NiFeAlO4, MgAl2O4, FeAl2O4 |  | SiO2 (from quartz wool) |
 | MgO, NiO, NiO-MgO |  | Fe3C |
 | Ni, FeNi, Fe0.5Ni0.5, FeNi3 |  | Fe2O3 |
 | Carbone |
| Sample | S (m2/g) | Pore Volume (cm3/g) |
|---|---|---|
| Fresh catalyst | 6.70 | 0.0202 |
| Used catalyst (MemU) | 10.49 | 0.0241 |
| Used catalyst (WU) | 8.98 | 0.0144 |
| Reactant | Catalyst Weight | Temperature (°C) | Injection Flowrate | TOS |
|---|---|---|---|---|
| Bio-oil WU Bio-oil MemU | 1 g and 4 g | 750, 775, 800, 825, 850 | ~0.1 mL/min | ~500 min |
| Bio-oil MemU | 4 g | 800 | ~0.1 mL/min | 105 h |
| Time (min) | Concentration (% mol) | |||||
|---|---|---|---|---|---|---|
| CO2 | CO | H2 | C2H4 | C2H6 | CH4 | |
| 0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 9 | 6.27 | 32.22 | 57.88 | 0.08 | 0.13 | 3.42 |
| 24 | 13.60 | 38.25 | 47.65 | 0.03 | 0.05 | 0.41 |
| 54 | 6.39 | 41.00 | 51.89 | 0.01 | 0.03 | 0.66 |
| 84 | 6.26 | 40.46 | 52.52 | 0.01 | 0.02 | 0.74 |
| 114 | 6.49 | 40.07 | 52.65 | 0.00 | 0.01 | 0.78 |
| 144 | 6.77 | 39.85 | 52.56 | 0.00 | 0.01 | 0.81 |
| 174 | 6.86 | 39.87 | 52.40 | 0.00 | 0.01 | 0.86 |
| 204 | 7.00 | 39.67 | 52.40 | 0.00 | 0.01 | 0.92 |
| 234 | 6.96 | 39.74 | 52.37 | 0.00 | 0.00 | 0.92 |
| 264 | 6.93 | 39.91 | 52.15 | 0.00 | 0.00 | 1.00 |
| 294 | 6.93 | 39.92 | 52.10 | 0.00 | 0.00 | 1.04 |
| 324 | 7.35 | 39.05 | 52.42 | 0.00 | 0.00 | 1.17 |
| 354 | 7.22 | 39.51 | 52.11 | 0.00 | 0.00 | 1.15 |
| 384 | 7.29 | 39.57 | 51.84 | 0.01 | 0.00 | 1.29 |
| 414 | 7.21 | 39.69 | 51.82 | 0.01 | 0.01 | 1.27 |
| 459 | 7.17 | 39.52 | 51.87 | 0.01 | 0.01 | 1.42 |
| 504 | 7.46 | 39.09 | 51.90 | 0.01 | 0.01 | 1.53 |
| Time (min) | Number of Moles | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C in | H in | CO2 | CO | H2 | C2H4 | C2H6 | CH4 | ||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 9 | 0.0347 | 0.0742 | 0.0033 | 0.0169 | 0.0303 | 0.0000 | 0.0001 | 0.0018 | |
| 24 | 0.0579 | 0.1237 | 0.0153 | 0.0430 | 0.0535 | 0.0000 | 0.0001 | 0.0005 | |
| 54 | 0.1158 | 0.2474 | 0.0133 | 0.0852 | 0.1078 | 0.0000 | 0.0001 | 0.0014 | |
| 84 | 0.1158 | 0.2474 | 0.0128 | 0.0828 | 0.1075 | 0.0000 | 0.0000 | 0.0015 | |
| 114 | 0.1158 | 0.2474 | 0.0128 | 0.0790 | 0.1038 | 0.0000 | 0.0000 | 0.0015 | |
| 144 | 0.1158 | 0.2474 | 0.0137 | 0.0809 | 0.1067 | 0.0000 | 0.0000 | 0.0016 | |
| 174 | 0.1158 | 0.2474 | 0.0139 | 0.0810 | 0.1064 | 0.0000 | 0.0000 | 0.0017 | |
| 204 | 0.1158 | 0.2474 | 0.0151 | 0.0855 | 0.1129 | 0.0000 | 0.0000 | 0.0020 | |
| 234 | 0.1158 | 0.2474 | 0.0149 | 0.0852 | 0.1123 | 0.0000 | 0.0000 | 0.0020 | |
| 264 | 0.1158 | 0.2474 | 0.0145 | 0.0835 | 0.1092 | 0.0000 | 0.0000 | 0.0021 | |
| 294 | 0.1158 | 0.2474 | 0.0153 | 0.0884 | 0.1154 | 0.0000 | 0.0000 | 0.0023 | |
| 324 | 0.1158 | 0.2474 | 0.0169 | 0.0896 | 0.1203 | 0.0000 | 0.0000 | 0.0027 | |
| 354 | 0.1158 | 0.2474 | 0.0168 | 0.0917 | 0.1210 | 0.0000 | 0.0000 | 0.0027 | |
| 384 | 0.1158 | 0.2474 | 0.0171 | 0.0928 | 0.1215 | 0.0000 | 0.0000 | 0.0030 | |
| 414 | 0.1158 | 0.2474 | 0.0163 | 0.0897 | 0.1171 | 0.0000 | 0.0000 | 0.0029 | |
| 459 | 0.1737 | 0.3711 | 0.0256 | 0.1414 | 0.1855 | 0.0000 | 0.0000 | 0.0051 | |
| 504 | 0.1737 | 0.3711 | 0.0257 | 0.1346 | 0.1787 | 0.0000 | 0.0000 | 0.0053 | |
| Element | Total Element Produced per Molecule | Total | |||||||
| C | 1.9456 | 0.2633 | 1.4511 | 0.0005 | 0.0008 | 0.0400 | 1.7558 | ||
| H | 4.1564 | 3.8200 | 0.0011 | 0.0025 | 0.1602 | 3.9837 | |||
| O | 0.5266 | 1.4511 | 1.9778 | ||||||
| Time (min) | Yield (%) | |||||
|---|---|---|---|---|---|---|
| CO2 | CO | H2 | C2H4 | C2H6 | CH4 | |
| 0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.5 | 9.7 |
| 9 | 9.5 | 48.6 | 8.7 | 0.1 | 0.3 | 1.5 |
| 24 | 26.4 | 74.2 | 86.5 | 0.0 | 0.2 | 2.2 |
| 54 | 11.5 | 73.6 | 87.2 | 0.0 | 0.1 | 2.4 |
| 84 | 11.1 | 71.5 | 86.9 | 0.0 | 0.1 | 2.5 |
| 114 | 11.1 | 68.2 | 83.9 | 0.0 | 0.0 | 2.7 |
| 144 | 11.9 | 69.8 | 86.2 | 0.0 | 0.0 | 2.8 |
| 174 | 12.0 | 69.9 | 86.0 | 0.0 | 0.0 | 3.2 |
| 204 | 13.0 | 73.8 | 91.3 | 0.0 | 0.0 | 3.2 |
| 234 | 12.9 | 73.6 | 90.8 | 0.0 | 0.0 | 3.4 |
| 264 | 12.5 | 72.1 | 88.2 | 0.0 | 0.0 | 3.7 |
| 294 | 13.3 | 76.4 | 93.3 | 0.0 | 0.0 | 4.3 |
| 324 | 14.6 | 77.4 | 97.3 | 0.0 | 0.0 | 4.3 |
| 354 | 14.5 | 79.2 | 97.8 | 0.0 | 0.0 | 4.9 |
| 384 | 14.8 | 80.1 | 98.2 | 0.0 | 0.0 | 4.6 |
| 414 | 14.1 | 77.4 | 94.6 | 0.0 | 0.0 | 5.5 |
| 459 | 14.8 | 81.4 | 100.0 | 0.0 | 0.0 | 5.7 |
| 504 | 14.8 | 77.5 | 96.3 | 0.2 | 0.5 | 9.7 |
| C in (mol) | C out (mol) | Error (%) |
| 1.96 | 1.89 | 3.73 |
| H in (mol) | H out (mol) | Error (%) |
| 4.20 | 4.36 | 3.98 |
| O in (mol) | O out (mol) | Error (%) |
| 2.00 | 2.17 | 8.25 |
| Bio-Oil in (g) | Bio-Oil out (g) | Error (%) |
| 59.82 | 61.76 | 3.23 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bali, A.; Blanchard, J.; Chamoumi, M.; Abatzoglou, N. Bio-Oil Steam Reforming over a Mining Residue Functionalized with Ni as Catalyst: Ni-UGSO. Catalysts 2018, 8, 1. https://doi.org/10.3390/catal8010001
Bali A, Blanchard J, Chamoumi M, Abatzoglou N. Bio-Oil Steam Reforming over a Mining Residue Functionalized with Ni as Catalyst: Ni-UGSO. Catalysts. 2018; 8(1):1. https://doi.org/10.3390/catal8010001
Chicago/Turabian StyleBali, Amine, Jasmin Blanchard, Mostafa Chamoumi, and Nicolas Abatzoglou. 2018. "Bio-Oil Steam Reforming over a Mining Residue Functionalized with Ni as Catalyst: Ni-UGSO" Catalysts 8, no. 1: 1. https://doi.org/10.3390/catal8010001
APA StyleBali, A., Blanchard, J., Chamoumi, M., & Abatzoglou, N. (2018). Bio-Oil Steam Reforming over a Mining Residue Functionalized with Ni as Catalyst: Ni-UGSO. Catalysts, 8(1), 1. https://doi.org/10.3390/catal8010001






