Effect of Iminodiacetic Acid-Modified Nieuwland Catalyst on the Acetylene Dimerization Reaction
Abstract
:1. Introduction
2. Results and Discussion
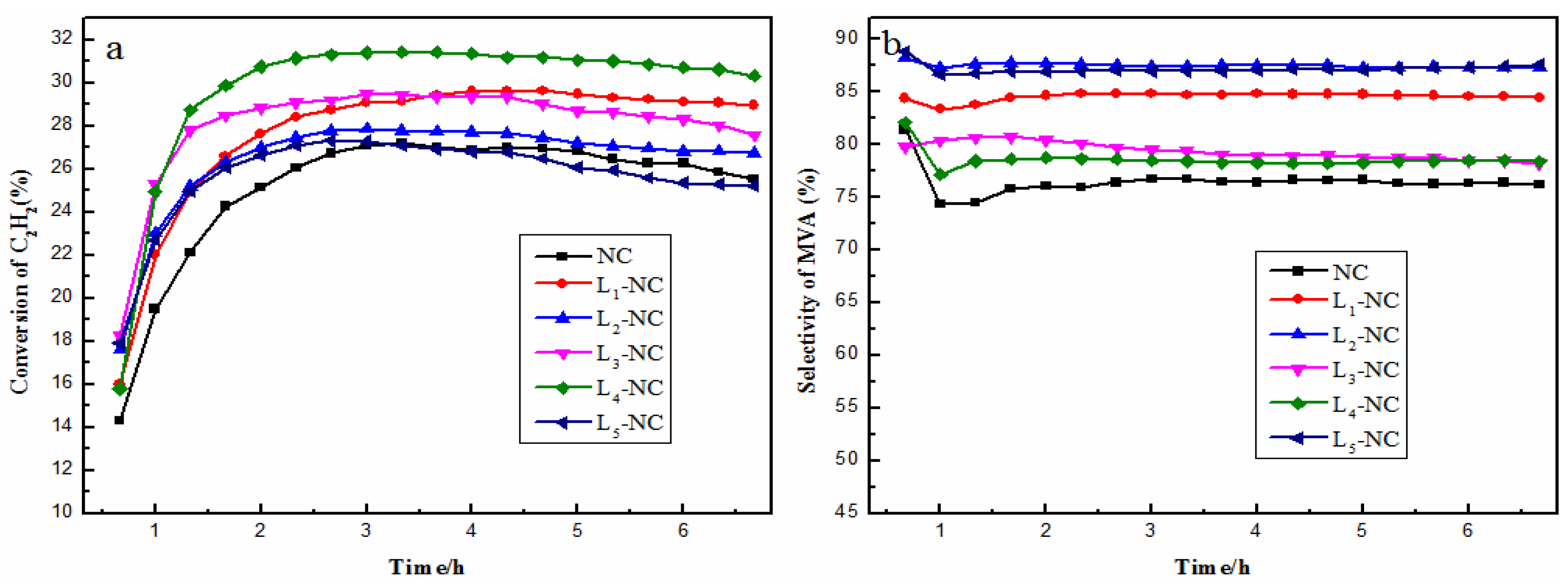
2.1. Catalytic Activities of NC and L-NC
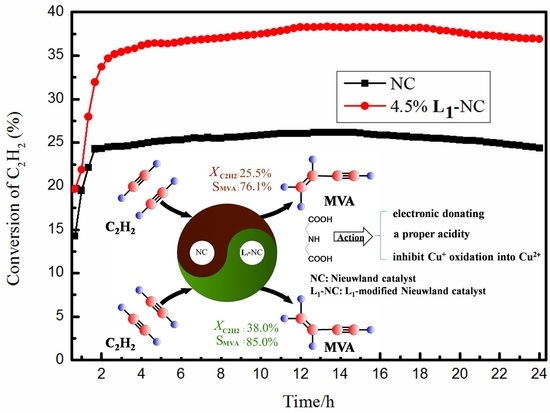
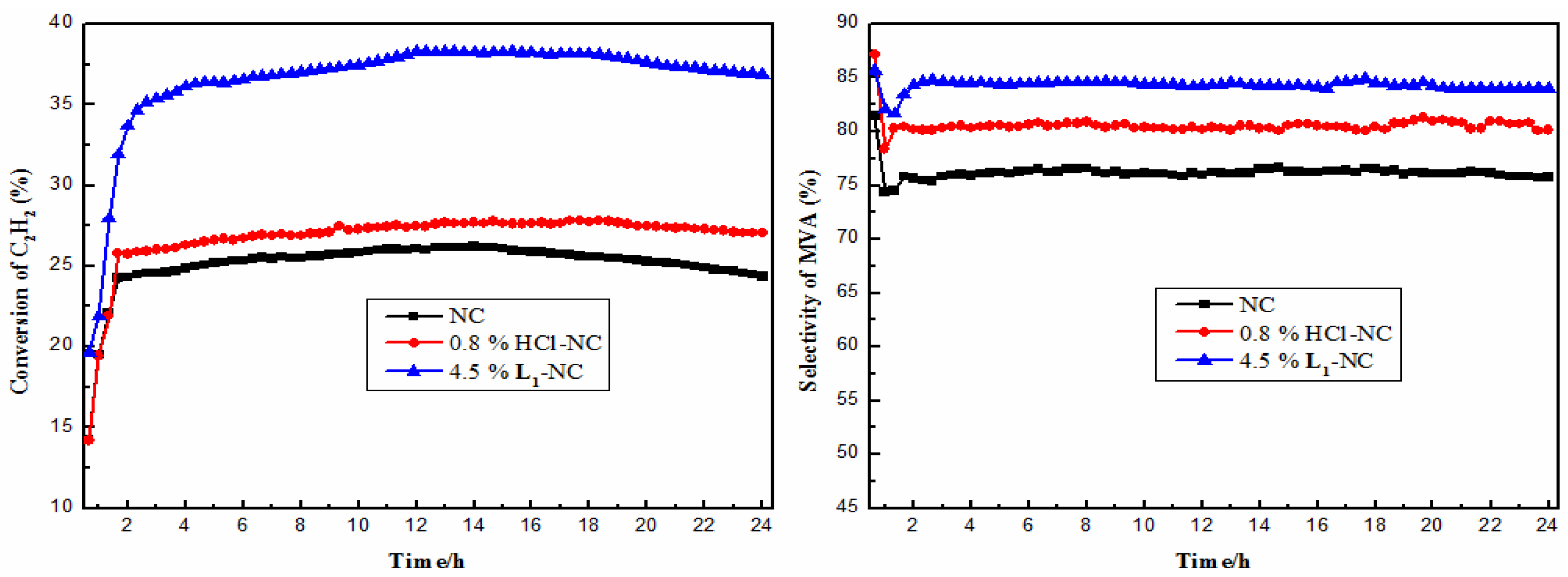
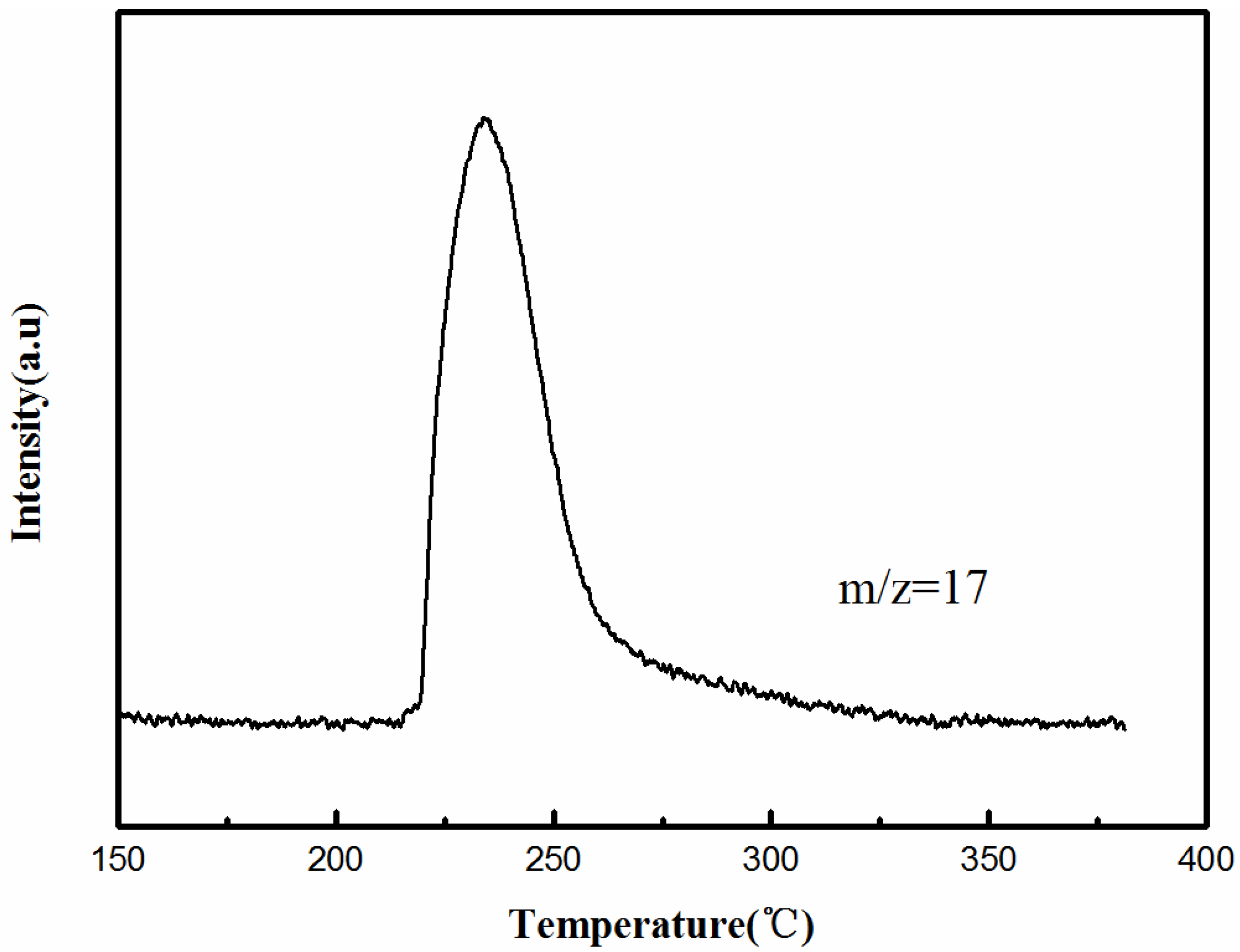
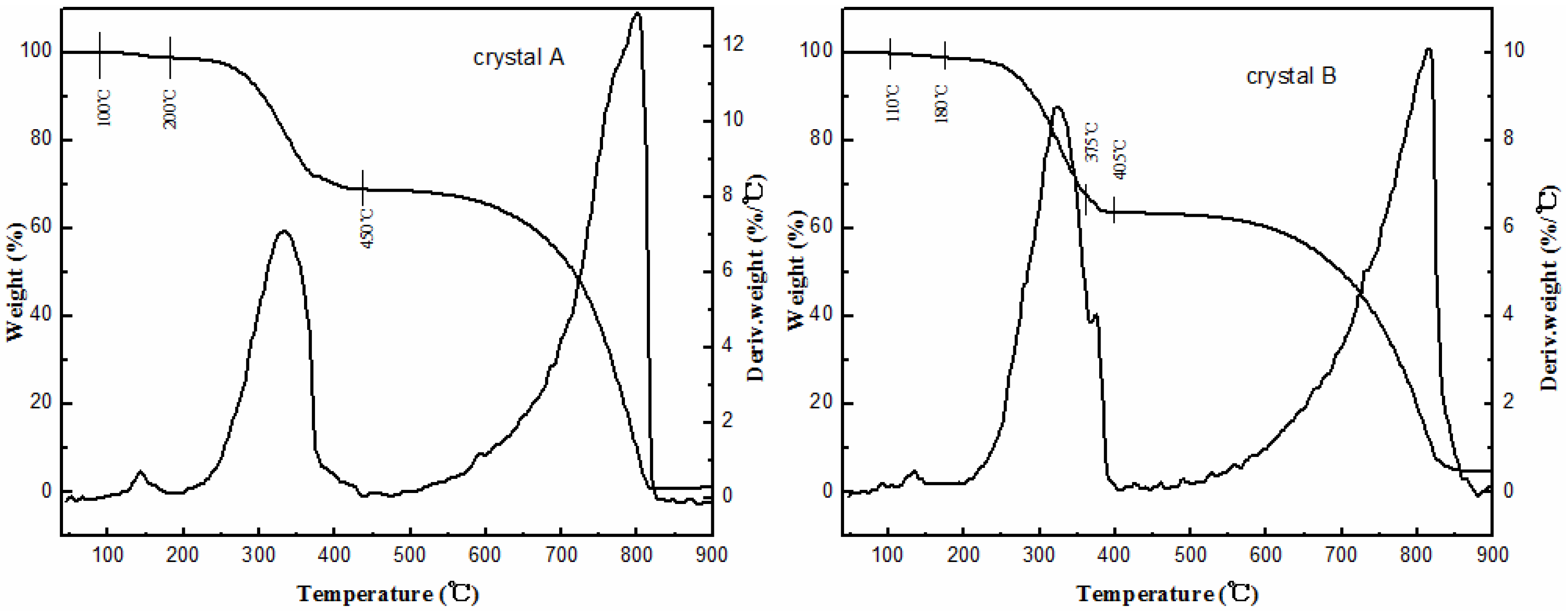
2.2. Stability Tests
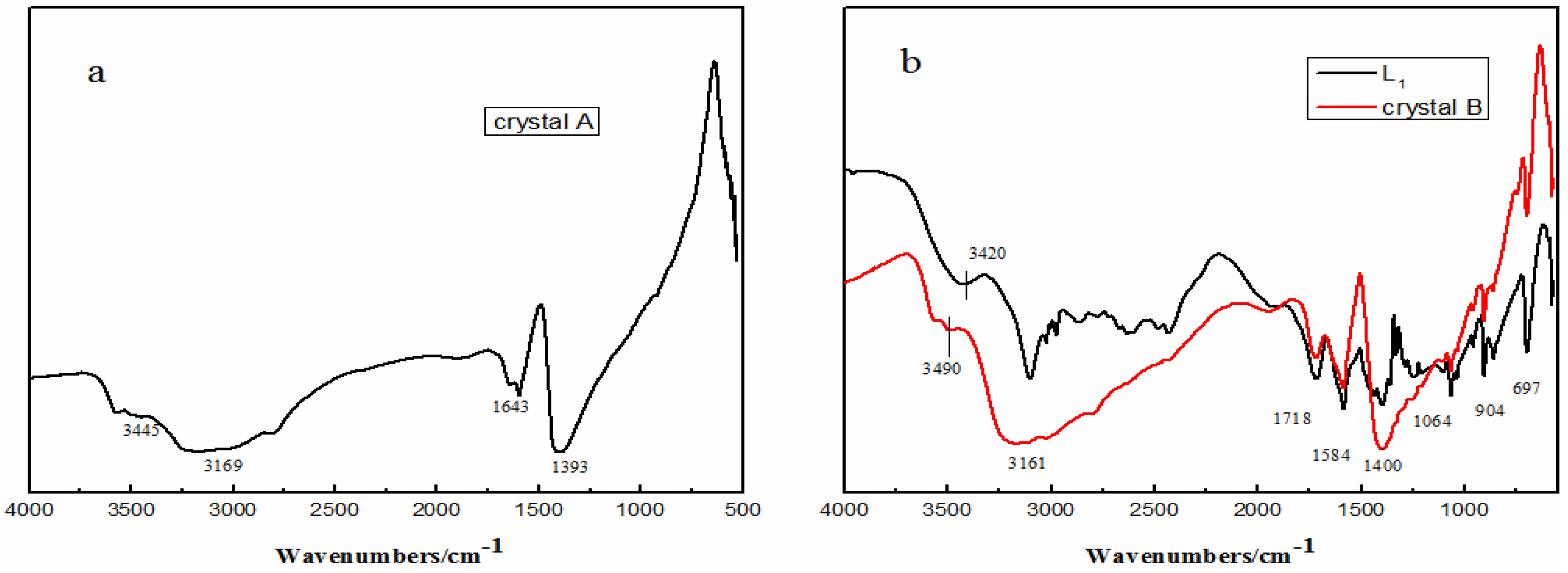
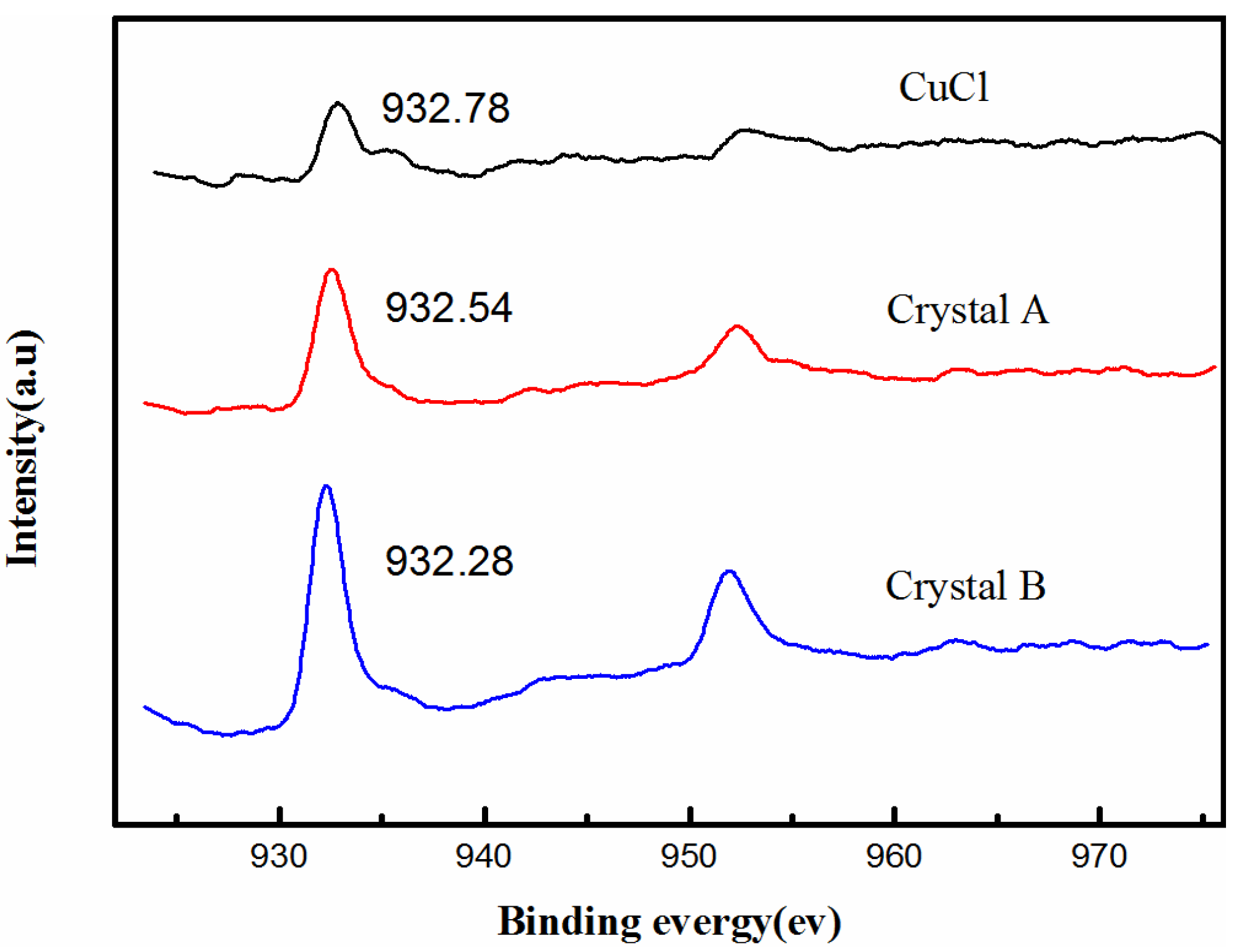
2.3. Structures of Crystals A and B
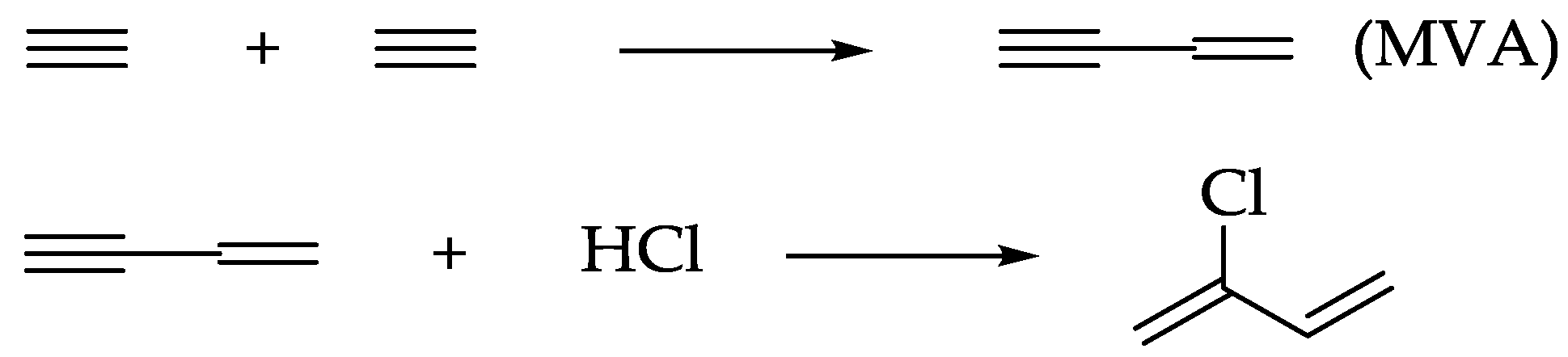
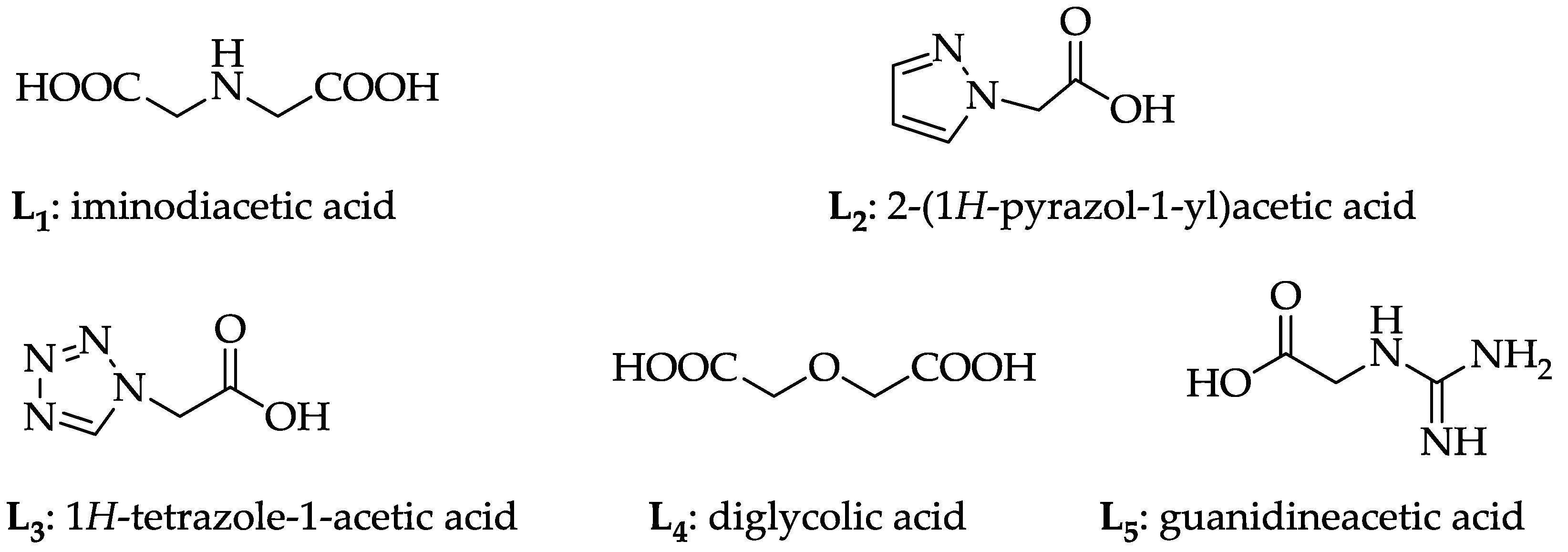
2.4. Action of the Ligand
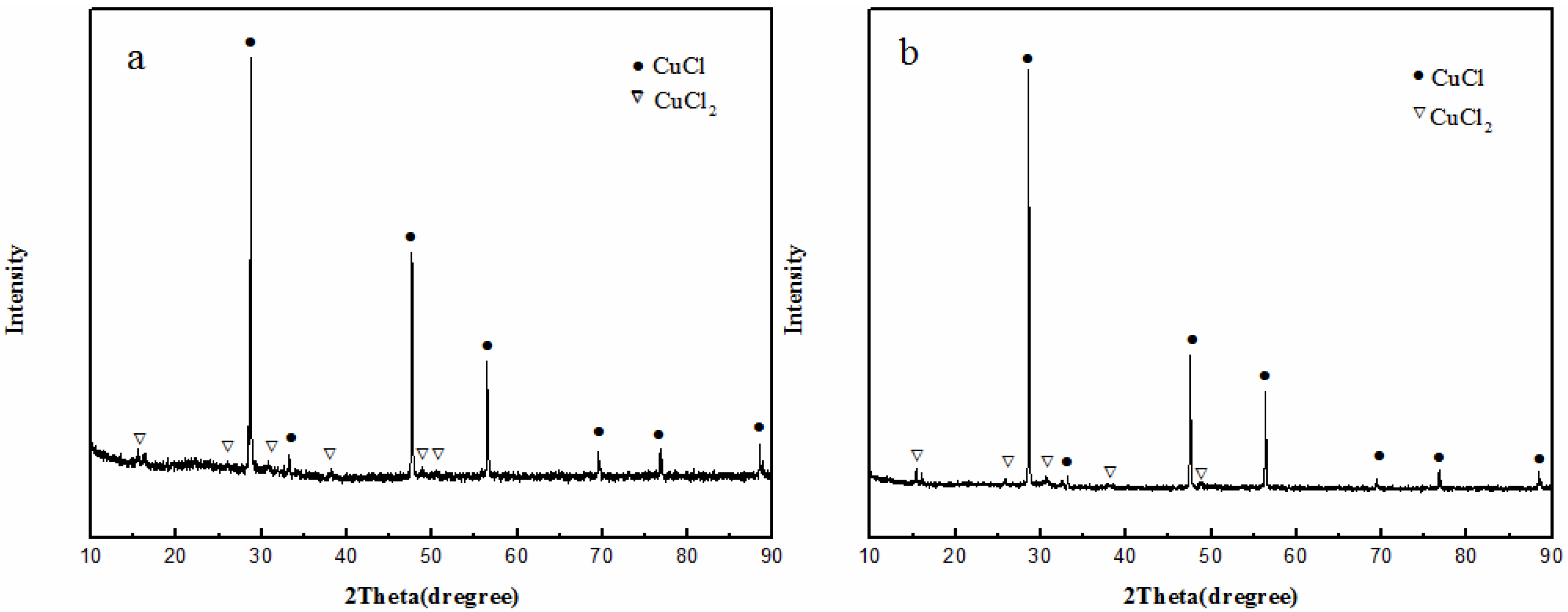
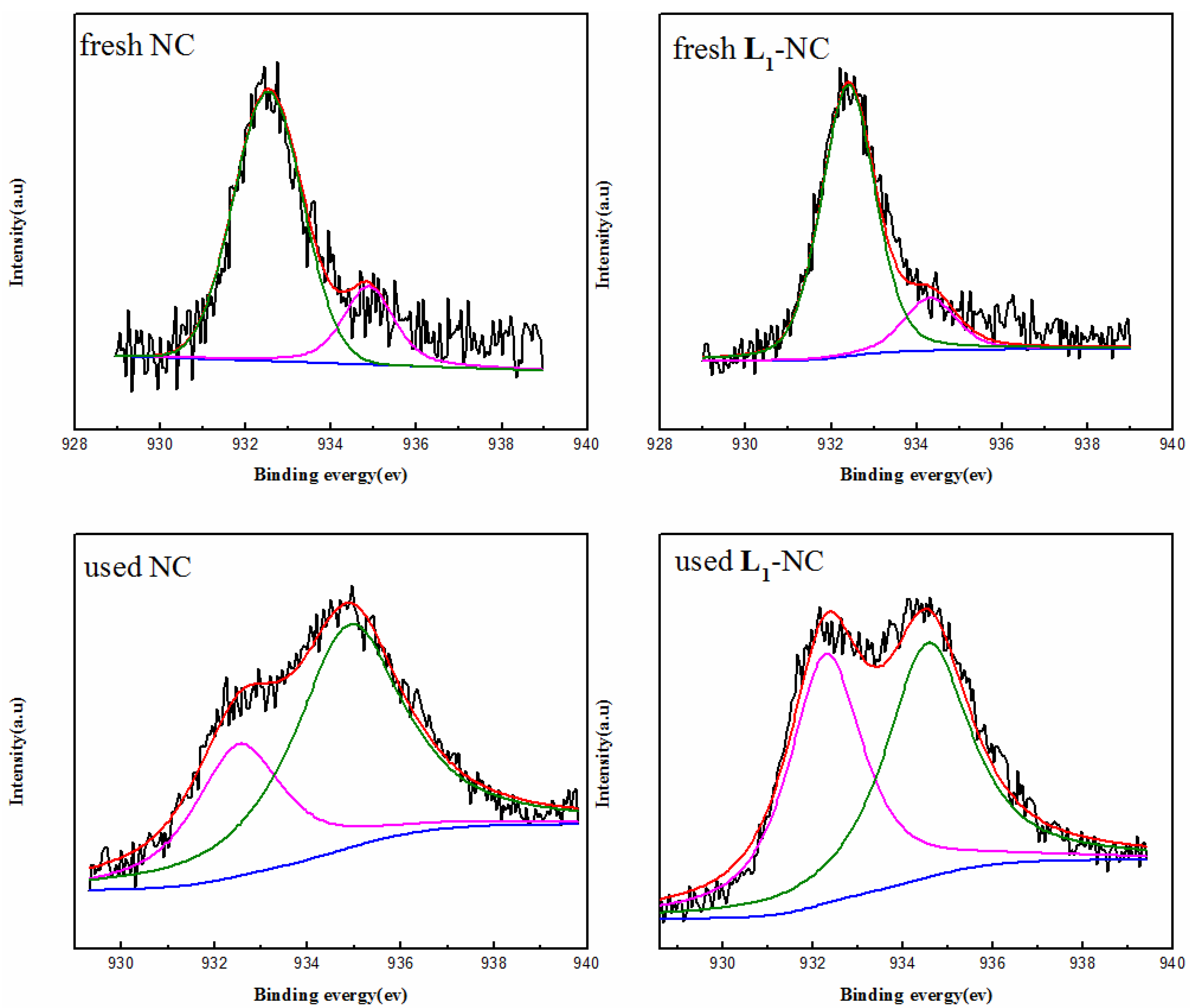
2.5. Relationship between the Valence of Copper and Catalytic Activity
3. Experimental
3.1. Materials and Catalyst Preparation
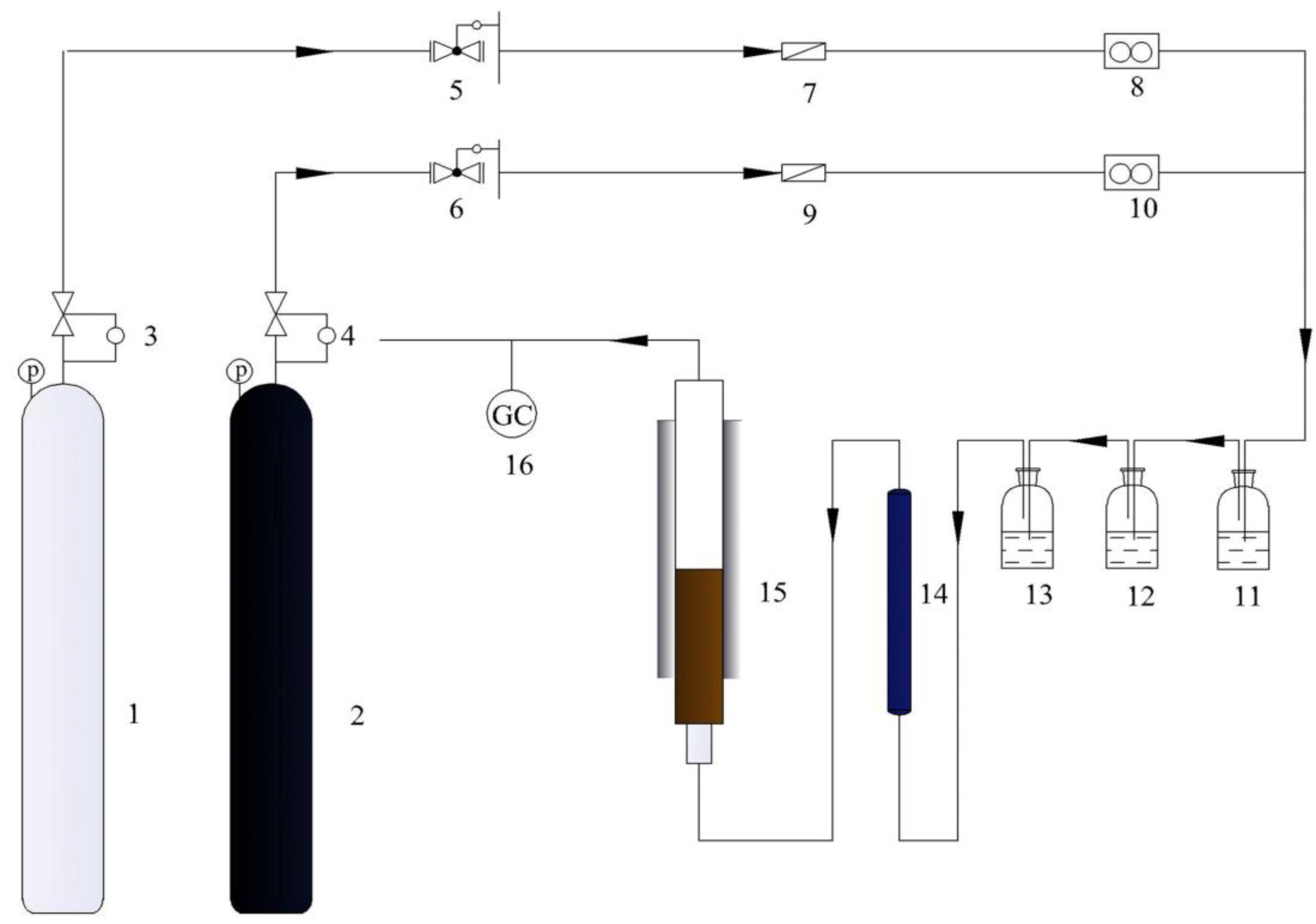
3.2. The Structure of the Reactor
3.3. Catalyst Preparation and the Dimerization Reaction of Acetylene
3.4. Analytical Methods
3.5. Separation of Crystals A and B from the NC and L1-NC Solutions
3.6. Treatment of the Used Catalyst Solutions
3.7. Catalyst Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tachiyama, T.; Yoshida, M.; Aoyagia, T.; Fukuzumi, S. Mechanistic study on dimerization of acetylene with a Nieuwland catalyst. Appl. Organomet. Chem. 2008, 22, 205–210. [Google Scholar] [CrossRef]
- Rattanasom, N.; Kueseng, P.; Deeprasertkul, C. Improvement of the mechanical and thermal properties of silica-filled polychloroprene vulcanizates prepared from latex system. Appl. Polym. Sci. 2012, 124, 2657–2668. [Google Scholar] [CrossRef]
- Ismail, H.; Leong, H.C. Curing characteristics and mechanical properties of natural rubber/chloroprene rubber and epoxidized natural rubber/chloroprene rubber blends. Polym. Test. 2001, 20, 509–516. [Google Scholar] [CrossRef]
- Arutyunyan, R.M.; Pogosyan, V.S.; Simonyan, E.H.; Atoyants, A.L.; Djigardjian, E.M. In situ monitoring of the ambient air around the chloroprene rubber industrial plant using the Tradescantia-stamen-hair mutation assay. Mutat. Res. 1999, 426, 117–120. [Google Scholar] [CrossRef]
- Liu, J.G.; Han, M.H.; Zuo, Y.Z.; Wang, Z.W. Research progress of dimerization of acetylene to monovinylacetylene. Chem. Ind. Eng. Prog. 2011, 30, 942–947. [Google Scholar] [CrossRef]
- Nishiwaki, K.; Kobayashi, M.; Takeuchi, T.; Matuoto, K.; Osakada, K. Nieuwland catalysts: Investigation of structure in the solid state and in solution and performance in the dimerization of acetylene. J. Mol. Catal. A Chem. 2001, 175, 73–81. [Google Scholar] [CrossRef]
- Tokita, Y.; Okamoto, A.; Nishiwaki, K.; Kobayashi, M.; Nakamura, E. Kinetics of copper(I)-catalyzed dimerization and hydration of acetylene in water. Bull. Chem. Soc. Jpn. 2004, 77, 1395–1399. [Google Scholar] [CrossRef]
- Tachiyama, T.; Yoshida, M.; Aoyagi, T.; Fukuzumi, S. Deuterium kinetic isotope effects and H/D exchange in dimerization of acetylene with a Nieuwland catalyst in aqueous media. J. Phys. Org. Chem. 2008, 21, 510–515. [Google Scholar] [CrossRef]
- Liu, H.Y.; Xie, J.W.; Liu, P.; Dai, B. Effect of Cu+/Cu2+ Ratio on the Catalytic Behavior of Anhydrous Nieuwland Catalyst during Dimerization of Acetylene. Catalysts 2016, 6, 120. [Google Scholar] [CrossRef]
- Liu, J.G.; Han, M.H.; Wang, Z.W. Studies on the catalytic performance of the Nieuwland catalyst and anhydrous catalyst in the dimerization of acetylene to monovinylacetylene. Adv. Mater. Res. 2012, 550–553, 312–316. [Google Scholar] [CrossRef]
- Liu, J.G.; Han, M.H.; Wang, Z.W. Effect of solvent on catalytic performance of anhydrous catalyst in acetylene dimerization to monovinylacetylene. J. Energy Chem. 2013, 22, 599–604. [Google Scholar] [CrossRef]
- Liu, H.Y.; Xie, J.W.; Liu, P.; Liu, Z.Y.; Dai, B. Study on anhydrous catalyst in acetylene dimerization to monovinylacetylene. J. Shihezi Univ. 2015, 33, 622–627. [Google Scholar] [CrossRef]
- Han, M.H.; Liu, J.G.; Zuo, Y.Z.; Wang, Z.W. Nieuwland Catalyst Preparation Method and Application. CN Patent 103285931 A, 27 February 2012. [Google Scholar]
- Han, M.H.; Liu, J.G.; Zuo, Y.Z.; Wang, Z.W. Monovinylacetylene Product and Preparation Method of Monovinylacetylene. CN Patent 103288572 A, 11 September 2013. [Google Scholar]
- Tao, C.Y.; Du, J.; Fan, X.; Liu, Z.H.; Xiao, C.C.; Ma, J.X.; Xue, H.T.; Shen, H.N.; Sun, D.G.; Liu, R.L. A Method of Preparing Hydrous Catalyst of Synthesizing Monovinylacetylene. CN Patent 101940953 A, 12 January 2011. [Google Scholar]
- Tao, C.Y.; Du, J.; Fan, X.; Liu, Z.H.; Xiao, C.C.; Ma, J.X.; Xue, H.T.; Shen, H.N.; Sun, D.G.; Liu, R.L. An Improved Water System Nieuwland Catalyst and Its Application. CN Patent 101733150 A, 18 December 2009. [Google Scholar]
- Tao, C.Y.; Du, J.; Fan, X.; Liu, Z.H.; Sun, D.G.; Shen, H.N.; Liu, R.L.; Zuo, Z.H.; Xiao, C.C.; Zhen, X.X. A Method of Preparing Catalyst of Synthesizing Monovinylacetylene and Application. CN Patent 101786022 A, 28 July 2010. [Google Scholar]
- Liu, J.G.; Zuo, Y.Z.; Han, M.H.; Wang, Z.W.; Wang, D.Z. Stability improvement of the Nieuwland catalyst in the dimerization of acetylene to monovinylacetylene. J. Nat. Gas Chem. 2012, 21, 495–500. [Google Scholar] [CrossRef]
- Liu, J.G.; Zuo, Y.Z.; Han, M.H.; Wang, Z.W. Improvement of anhydrous catalyst stability in acetylene dimerization by regulating acidity. J. Chem. Technol. Biotechnol. 2013, 88, 408–414. [Google Scholar] [CrossRef]
- Liu, Z.H.; Yu, Y.L.; Tao, C.Y.; Du, J.; Liu, R.L.; Fan, X.; Peng, M.; Sun, D.G.; Zuo, Z.H.; Ma, J.X. A Method of Dimerization of Acetylene to Monovinylacetylene. CN Patent 102775266 A, 14 November 2012. [Google Scholar]
- Han, M.H.; Liu, J.G.; Zuo, Y.Z.; Wang, Z.W. The Method and Application of Anhydrous Nieuwland Catalyst during Dimerization of Acetylene. CN Patent 103285925 A, 11 September 2013. [Google Scholar]
- Liu, Z.H.; Yu, Y.L.; Tao, C.Y.; Fan, X. Acetylene dimerization catalyzed by LaCl3-modified Nieuwland catalyst. CIESC J. 2014, 65, 1260–1266. [Google Scholar] [CrossRef]
- Liu, Z.H.; Yu, Y.L.; Tao, C.Y.; Du, J.; Liu, R.L.; Fan, X.; Peng, M.; Sun, D.G.; Zuo, Z.H.; Ma, J.X. A Method of Synthesizing Monovinylacetylene in Aqueous Solution. CN Patent 102731241 A, 17 October 2012. [Google Scholar]
- Du, J.; Liu, Z.H.; Tao, C.Y. A Method of Synthesizing Monovinylacetylene. CN Patent 102964198 A, 13 March 2013. [Google Scholar]
- Zhang, Y.K.; Jia, Z.K.; Zhen, B.; Han, M.H. Investigation of the acetylene dimerization catalyzed by Nieuwland catalyst. CIESC J. 2016, 67, 294–299. [Google Scholar] [CrossRef]
- Lu, J.L.; Xie, J.W.; Liu, H.Y.; Liu, P.; Liu, Z.Y.; Dai, B. Strontium chloride modified Nieuwland catalyst in the dimerization of acetylene to monovinylacetylene. Asian J. Chem. 2014, 26, 8211–8214. [Google Scholar] [CrossRef]
- Lu, J.L.; Liu, H.Y.; Xie, J.W.; Liu, P.; Liu, Z.Y.; Dai, B. Study on catalytic activity of zinc(II)-copper(I) collaborative bimetallic catalysis in acetylene dimerization reaction. J. Shihezi Univ. 2014, 32, 213–217. [Google Scholar] [CrossRef]
- Lu, J.L.; Liu, H.Y.; Xie, J.W.; Liu, P.; Dai, B.; Liu, Z.Y. Effect of polyethylene glycol/Nieuwland catalyst on acetylene dimerization reaction. Chem. Eng. 2015, 43, 60–64. [Google Scholar] [CrossRef]

| Catalysts | Acetylene Conversion (%) | MVA Selectivity (%) | Yield (%) |
|---|---|---|---|
| NC | 26.4 | 76.4 | 20.1 |
| L1-NC | 29.3 | 84.6 | 24.8 |
| L2-NC | 27.3 | 87.5 | 23.8 |
| L3-NC | 28.8 | 79.4 | 22.9 |
| L4-NC | 29.7 | 78.5 | 23.3 |
| L5-NC | 26.3 | 87.1 | 22.9 |
| L1 (%) | Acetylene Conversion (%) | MVA Selectivity (%) | Yield (%) |
|---|---|---|---|
| 0 | 26.4 | 76.4 | 20.1 |
| 1.5 | 29.7 | 82.3 | 24.4 |
| 3.0 | 29.2 | 84.7 | 24.7 |
| 4.5 | 38.0 | 84.2 | 32.0 |
| 6.0 | 32.5 | 85.5 | 27.8 |
| 7.5 | 31.0 | 86.0 | 26.7 |
| Catalysts | Acetylene Conversion (%) | MVA Selectivity (%) | Yield (%) |
|---|---|---|---|
| NC | 25.5 | 76.1 | 19.4 |
| HCl-NC | 27.2 | 80.5 | 21.9 |
| L1-NC | 37.5 | 84.4 | 31.7 |
| Catalyst | Area%, Binding Energy (eV) | |||
|---|---|---|---|---|
| Cu+ | Cu2+ | |||
| Fresh | Used | Fresh | Used | |
| NC | 79.09 (932.53) | 32.85 (932.51) | 20.91 (934.87) | 67.15 (934.88) |
| L1-NC | 83.14 (932.31) | 48.50 (932.30) | 16.86 (934.51) | 51.50 (934.56) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, Y.; Luo, J.; Xie, J.; Dai, B. Effect of Iminodiacetic Acid-Modified Nieuwland Catalyst on the Acetylene Dimerization Reaction. Catalysts 2017, 7, 394. https://doi.org/10.3390/catal7120394
You Y, Luo J, Xie J, Dai B. Effect of Iminodiacetic Acid-Modified Nieuwland Catalyst on the Acetylene Dimerization Reaction. Catalysts. 2017; 7(12):394. https://doi.org/10.3390/catal7120394
Chicago/Turabian StyleYou, Yanhe, Juan Luo, Jianwei Xie, and Bin Dai. 2017. "Effect of Iminodiacetic Acid-Modified Nieuwland Catalyst on the Acetylene Dimerization Reaction" Catalysts 7, no. 12: 394. https://doi.org/10.3390/catal7120394
APA StyleYou, Y., Luo, J., Xie, J., & Dai, B. (2017). Effect of Iminodiacetic Acid-Modified Nieuwland Catalyst on the Acetylene Dimerization Reaction. Catalysts, 7(12), 394. https://doi.org/10.3390/catal7120394