Catalytic Performance of Fe(II)-Scorpionate Complexes towards Cyclohexane Oxidation in Organic, Ionic Liquid and/or Supercritical CO2 Media: A Comparative Study
Abstract
:1. Introduction
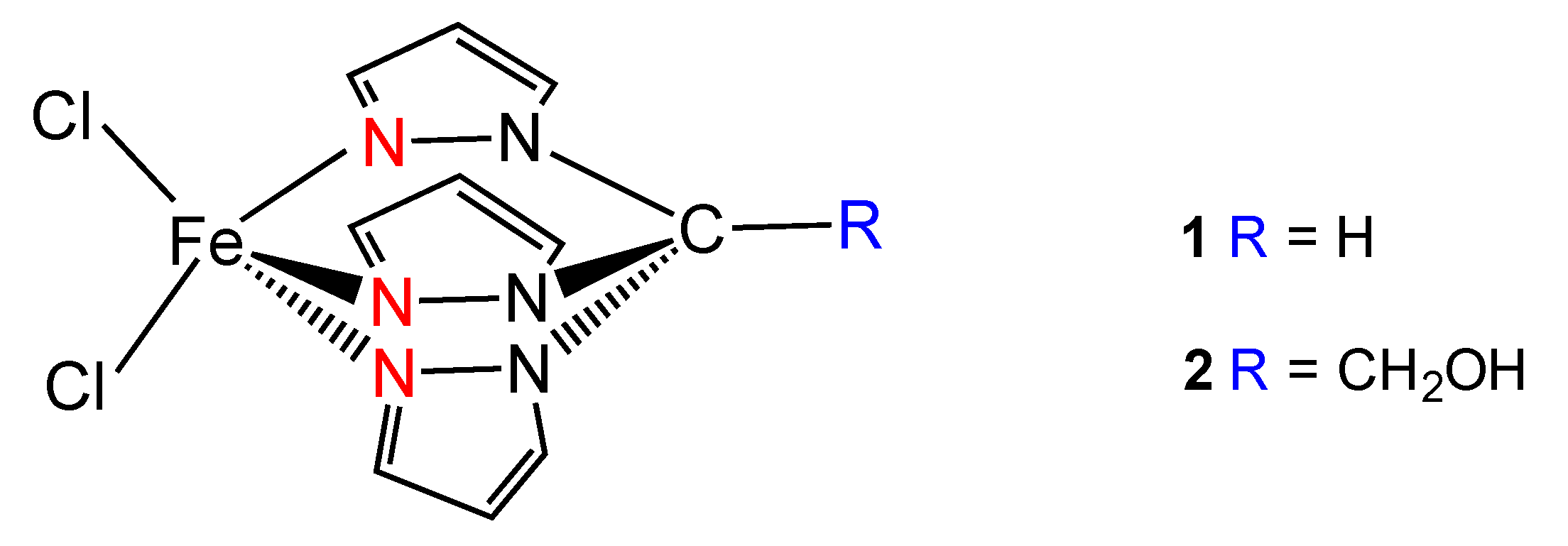
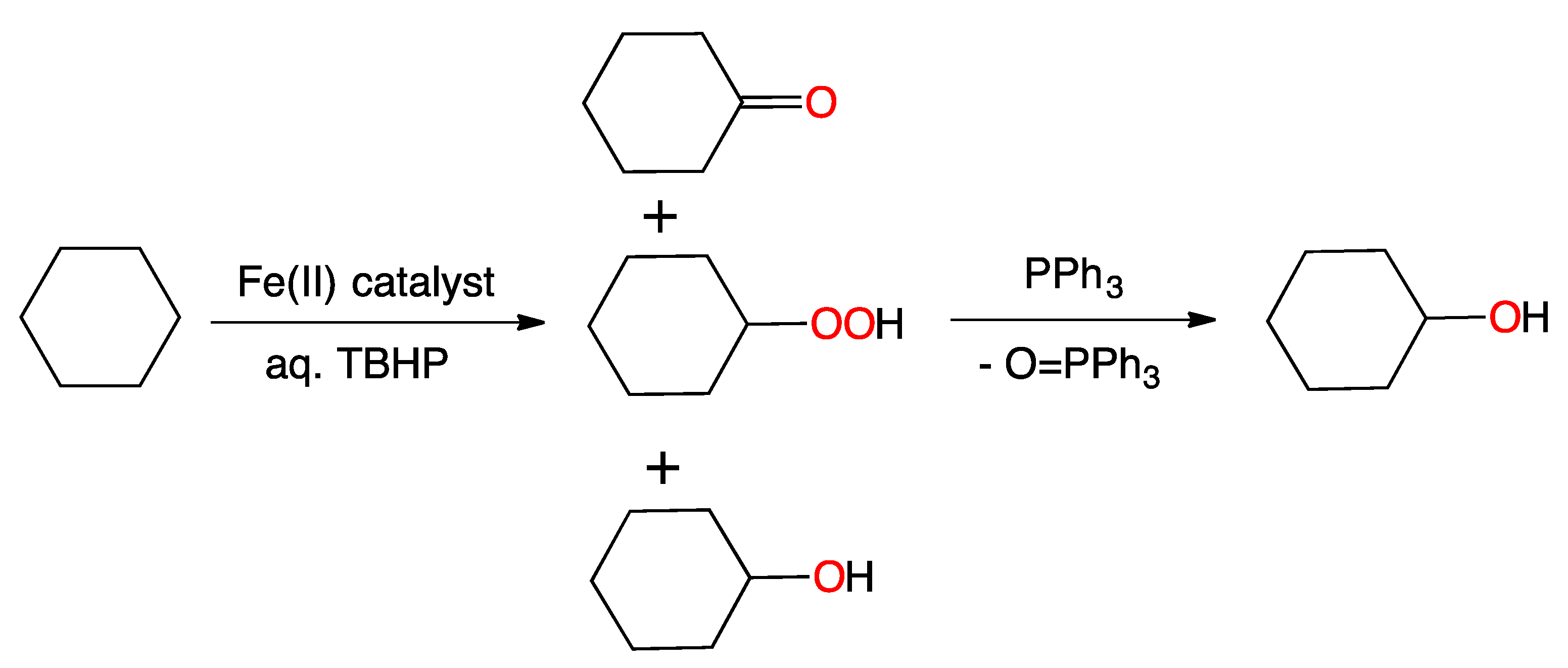
2. Results and Discussion
3. Materials and Methods
3.1. Procedure for the Catalytic Oxidations Using a Molecular or an Ionic Liquid Solvent
3.2. Procedure for the Catalytic Oxidations in scCO2 or in scCO2-IL
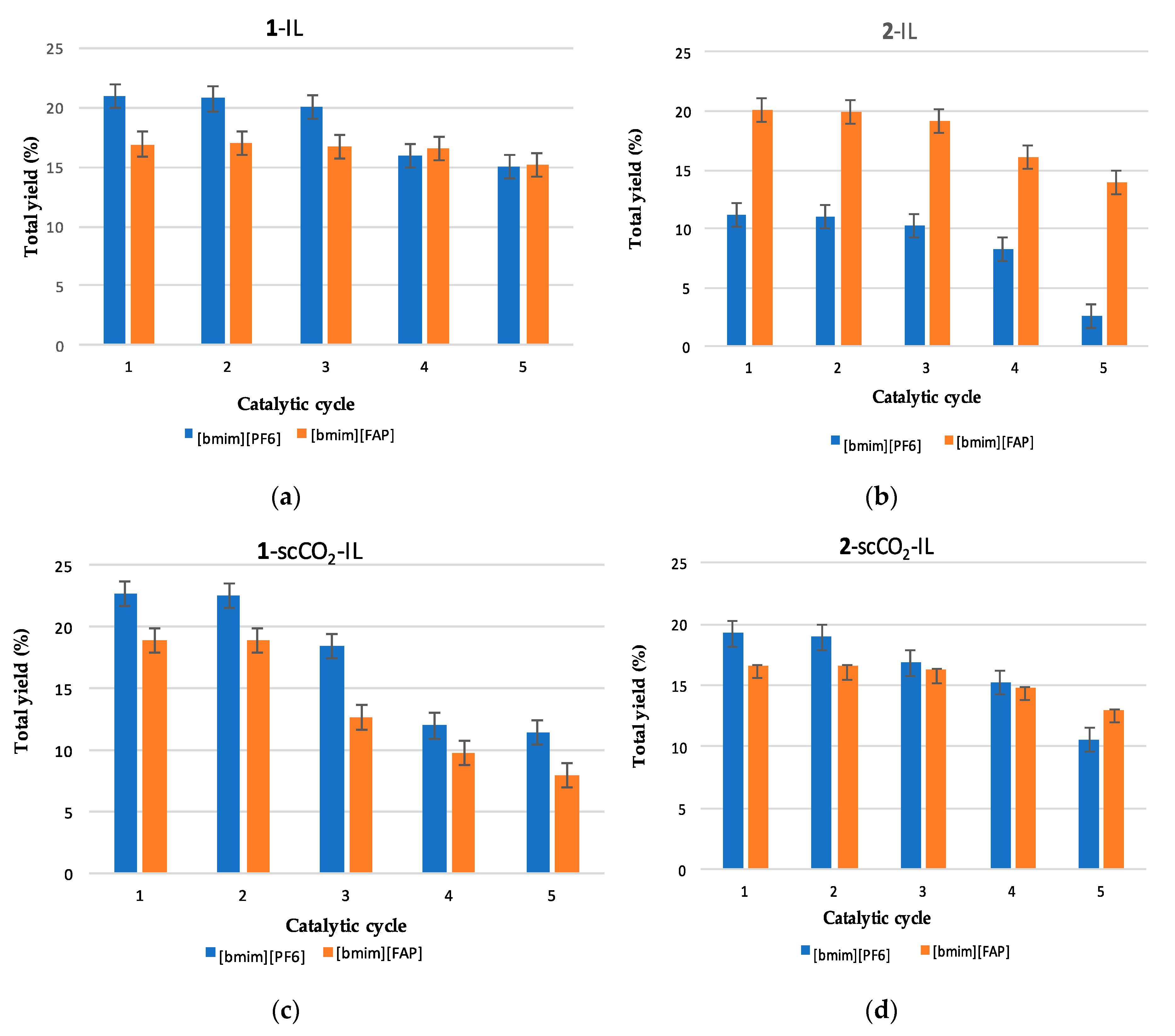
3.3. Catalyst Recycling
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Anastas, P.; Warner, J.C. Green Chemistry—Theory and Practice, 1st ed.; Oxford University Press: New York, NY, USA, 1998; p. 30. [Google Scholar]
- Muldoon, M. Chapter 4—Catalysis in ionic liquid-supercritical CO2. In Catalysis in Ionic Liquids: From Catalyst Synthesis to Application, 1st ed.; Hardacre, C., Pârvulescu, V., Eds.; Royal Society of Chemistry: London, UK, 2014; Volume 15, pp. 309–344. [Google Scholar]
- Zhou, L.; Akgerman, A. Catalytic oxidation of ethanol and acetaldehyde in supercritical carbon dioxide. Ind. Eng. Chem. Res. 1995, 34, 1588–1595. [Google Scholar] [CrossRef]
- Zhou, L.; Erkey, C.; Akgerman, A. Catalytic oxidation of toluene and tetralin in supercritical carbon dioxide. AIChE J. 1995, 41, 2122–2130. [Google Scholar] [CrossRef]
- Dooley, K.M.; Knopf, F.C. Oxidation catalysis in a supercritical fluid medium. Ind. Eng. Chem. Res. 1987, 26, 1910–1916. [Google Scholar] [CrossRef]
- Srinivas, P.; Mukhopadhyay, M. Oxidation of cyclohexane in supercritical carbon dioxide medium. Ind. Eng. Chem. Res. 1994, 33, 3118–3124. [Google Scholar] [CrossRef]
- Mukhopadhyay, M.; Srinivas, P. Multicomponent solubilities of reactants and products of cyclohexane oxidation in supercritical carbon dioxide. Ind. Eng. Chem. Res. 1996, 35, 4713–4717. [Google Scholar] [CrossRef]
- Srinivas, P.; Mukhopadhyay, M. Influence of the thermodynamic state on cyclohexane oxidation kinetics in carbon dioxide medium. Ind. Eng. Chem. Res. 1997, 36, 2066–2074. [Google Scholar] [CrossRef]
- Hou, Z.; Han, B.; Gao, L.; Liu, Z.; Yang, G. Selective oxidation of cyclohexane in compressed CO2 and in liquid solvents over MnAPO-5 molecular sieve. Green Chem. 2002, 4, 426–430. [Google Scholar] [CrossRef]
- Medina-Gonzalez, Y.; Camy, S.; Condoret, J.S. ScCO2/green solvents: Biphasic promising systems for cleaner chemicals manufacturing. ACS Sustain. Chem. Eng. 2014, 2, 2623–2636. [Google Scholar] [CrossRef]
- Sahle-Demessie, E.; Gonzalez, M.A.; Enriquez, J.; Zhao, Q. Selective oxidation in supercritical carbon dioxide using clean oxidants. Ind. Eng. Chem. Res. 2000, 39, 4858–4864. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Kuznetsov, M.L.; Pombeiro, A.J.L. Tuning Cyclohexane Oxidation: Combination of microwave irradiation and ionic liquid with the C-scorpionate [FeCl2(Tpm)] catalyst. Organomet 2017, 36, 192–198. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Hazra, S.; Pombeiro, A.J.L. Catalytic oxidation of cyclohexane with hydrogen peroxide and a tetracopper(II) complex in an ionic liquid. C. R. Chim. 2015, 18, 758–765. [Google Scholar] [CrossRef]
- Freire, M.G.; Cláudio, A.F.M.; Araújo, J.M.M.; Coutinho, J.A.P.; Marrucho, I.M.; Canongia Lopes, J.N.; Rebelo, L.P.N. Aqueous biphasic systems: A boost brought about by using ionic liquids. Chem. Soc. Rev. 2012, 41, 4966–4995. [Google Scholar] [CrossRef] [PubMed]
- Pârvulescu, V.I.; Hardacre, C. Catalysis in ionic liquids. Chem. Rev. 2007, 107, 2615–2665. [Google Scholar] [CrossRef] [PubMed]
- Hallett, J.P.; Welton, T. Room-temperature ionic liquids: Solvents for synthesis and catalysis. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Giernoth, R. Homogeneous Catalysis in Ionic Liquids. In Topics Current Chemistry, 1st ed.; Bargon, J., Ed.; Springer: Berlin, Germany, 2007; Volume 276, pp. 1–23. [Google Scholar]
- Ribeiro, A.P.C.; Karabach, Y.Y.; Martins, L.M.D.R.S.; Gamal, A.; da Silva, M.F.C.G.; Pombeiro, A.J.L. Nickel (II)-2-amino-4-alkoxy-1, 3, 5-triazapentadienate complexes as catalysts for Heck and Henry reactions. RSC Adv. 2016, 6, 29159–29163. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Ribeiro, A.P.C.; Pombeiro, A.J.L. Highly selective cyclohexane oxidation catalyzed by ferrocene in ionic liquid medium. Lett. Org. Chem. 2017, 14. [Google Scholar] [CrossRef]
- Tiago, G.A.O.; Ribeiro, A.; Mahmudov, K.T.; da Silva, M.F.C.G.; Branco, L.C.; Pombeiro, A.J.L. Mononuclear copper(II) complexes of arylhydrazone of 1H-Indene-1,3(2H)-dione as catalysts for the oxidation of 1-phenylethanol in ionic liquid medium. RSC Adv. 2016, 6, 83412–83420. [Google Scholar] [CrossRef]
- Hazra, S.; Ribeiro, A.P.C.; da Silva, M.F.C.G.; de Castro, C.A.N.; Pombeiro, A.J.L. Syntheses and crystal structures of benzene-sulfonate and -carboxylate copper polymers and their application to oxidation of cyclohexane in ionic liquid under mild conditions. Dalton Trans. 2016, 45, 13957–13968. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.M.D.R.S.; Pombeiro, A.J.L. Water-soluble C-scorpionate complexes: Catalytic and biological applications. Eur. J. Inorg. Chem. 2016, 15–16, 2236–2252. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Pombeiro, A.J.L. Tris(pyrazol-1yl)methane metal complexes for catalytic mild oxidative functionalizations of alkanes, alkenes and ketones. Coord. Chem. Rev. 2014, 265, 74–88. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S. C-homoscorpionate oxidation catalysts—Electrochemical and catalytic activity. Catalysts 2017, 7, 12. [Google Scholar] [CrossRef]
- Kirk-Othmer. Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons: New York, NY, USA, 2014. [Google Scholar]
- Elvers, B. Ullmann’s Encyclopedia of Industrial Chemistry, 6th ed.; Ullmann, F., Hawkins, S., Schula, G., Gerhartz, W., Russey, W.E., Elvers, B., Eds.; Wiley-VCH: Weinheim, Germany, 1999–2016; Volume 11, pp. 41–49. [Google Scholar]
- Clerici, M.G.; Ricci, M.; Strukul, G. Metal-Catalysis in Industrial Organic Processes; Chiusoli, G.P., Maitlis, P.M., Eds.; Royal Society of Chemistry: Cambridge, UK, 2006. [Google Scholar]
- Silva, T.F.S.; Alegria, E.C.B.A.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Scorpionate vanadium, iron and copper complexes as selective catalysts for the peroxydative oxidation of cyclohexane under mild conditions. Adv. Synth. Catal. 2008, 350, 706–716. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Alegria, E.C.B.A.; Martins, L.M.D.R.S.; Duarte, T.A.G.; Pombeiro, A.J.L. C-scorpionate iron(II) complexes as highly selective catalysts for the hydrocarboxylation of cyclohexane. Adv. Synth. Catal. 2017. under review. [Google Scholar]
- Hou, Z.; Han, B.; Gao, L.; Jiang, T.; Liu, Z.; Chang, Y.; Zhang, X.; He, J. Wacker oxidation of 1-hexene in 1-n-butyl-3-methylimidazolium hexafluorophosphate ([bmim][PF6]), supercritical (SC) CO2, and SC CO2/[bmim][PF6] mixed solvent. New J. Chem. 2002, 26, 1246–1248. [Google Scholar] [CrossRef]
- Blanchard, L.A.; Hancu, D.; Beckman, E.J.; Brennecke, J.F. Green processing using ionic liquids and CO2. Nature 1999, 399, 28–29. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Martins, A.; Alegria, E.C.B.A.; Carvalho, A.P.; Pombeiro, A.J.L. Efficient cyclohexane oxidation with hydrogen peroxide catalysed by a C-scorpionate iron(II) complex immobilized on desilicated MOR zeolite. Appl. Catal. A Gen. 2013, 464–465, 43–50. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; de Peixoto Almeida, M.; Carabineiro, S.A.C.; Figueiredo, J.L.; Pombeiro, A.J.L. Heterogenisation of a C-scorpionate Fe(II) complex in carbon materials for cyclohexane oxidation with hydrogen peroxide. ChemCatChem 2013, 5, 3847–3856. [Google Scholar] [CrossRef]
- Pombeiro, A.J.L.; Martins, L.M.D.R.S.; Ribeiro, A.P. Process for the Microwave-assisted Conversion of Cycloalkanes to the Corresponding Alcohol-Ketone Mixtures, with Hydrogen Peroxide, and Using a Scorpionate Chloro-Complex of Iron(II) as Catalyst. PT 107797, 25 July 2014. [Google Scholar]
- Pombeiro, A.J.L.; Martins, L.M.D.R.S.; Alegria, E.C.B.A.; Silva, T.F.S. Scorpionate Chloro-Complexes of Iron and Vanadium and their Application as Catalysts for the Partial Oxidation, under Mild and Environmentally Tolerable Conditions, of Cyclohexane to Cyclohexanol and Cyclohexanone. PT 104153, 4 July 2008. [Google Scholar]
- Pombeiro, A.J.L. (Ed.) Toward functionalization of alkanes under environmentally benign conditions. In Advances in Organometallic Chemistry and Catalysis; John Wiley & Sons: Hoboken, NJ, USA, 2013; Chapter 2; pp. 15–25. [Google Scholar]
- Kerler, B.; Martin, A.; Jans, A.; Baerns, M. Partial oxidation of propane in compressed carbon dioxide using CoOx/SiO2 catalysts. Appl. Catal. A Gen. 2001, 220, 243–252. [Google Scholar] [CrossRef]
- Ionic Liquids Product Range for Your Innovations; Merck Catalog; Merck KGaA: Darmstadt, Germany, 2014.
- Olsen, M.H.N.; Salomao, G.C.; Drago, V.; Femandes, C.; Horn, A.; Cardozo, L.; Antunes, O.A.C. Oxidation of cyclohexane in supercritical carbon dioxide catalyzed by iron tetraphenylporphyrin. J. Supercrit. Fluids 2005, 34, 119–124. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Willauer, H.D.; Swatloski, R.P.; Visser, A.E.; Rogers, R.D. Room temperature ionic liquids as novel media for ‘clean’ liquid–liquid extraction. Chem. Commun. 1998, 1765–1766. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kozlov, Y.N.; Nizova, G.V.; Süss-Fink, G.; Stanislas, S.; Kitaygorodskiy, A.; Kulikova, V.S. Oxidations by the reagent “O2–H2O2–vanadium derivative–pyrazine-2-carboxylic acid”. Part 12.1 Main features, kinetics and mechanism of alkane hydroperoxidation. J. Chem. Soc. Perkin Trans. 2001, 2, 1351–1371. [Google Scholar] [CrossRef]
- Fernandes, R.R.; Lasri, J.; da Silva, M.F.C.G.; Palavra, A.M.F.; da Silva, J.A.L.; da Silva, J.J.R.F.; Pombeiro, A.J.L. Oxadiazoline and Ketoimine Palladium(II) complexes as highly efficient catalysts for Suzuki–Miyaura cross-coupling reactions in supercritical carbon dioxide. Adv. Synth. Catal. 2011, 353, 1153–1160. [Google Scholar] [CrossRef]
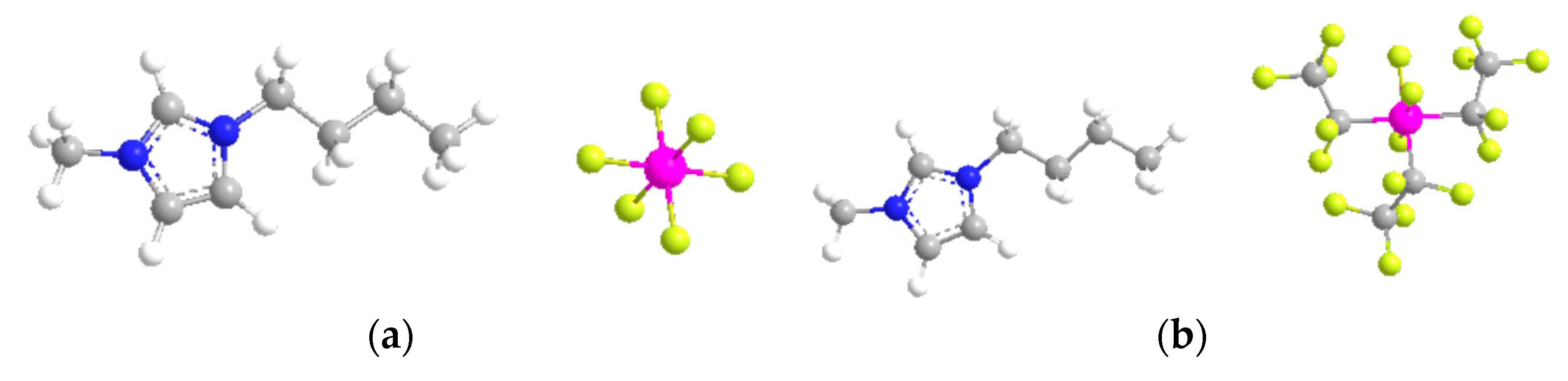
| Entry | Time | Yield (%) b | Total TON c | Total TOF d | Selectivity towards Cyclo-Hexanone e (%) | ||
|---|---|---|---|---|---|---|---|
| (h) | Cyclo-Hexanol | Cyclo-Hexanone | Total | (h−1) | |||
| CH3CN | |||||||
| 1 | 1 | 1.5 | 1.6 | 3.1 | 15.5 | 15.5 | 52 |
| 2 | 3 | 2.3 | 2.8 | 5.1 | 25.5 | 8.5 | 55 |
| 3 | 6 | 2.9 | 3.2 | 6.1 | 30.5 | 5.1 | 52 |
| [bmim][PF6] | |||||||
| 4 | 1 | 4.3 | 8.4 | 12.7 | 63.5 | 63.5 | 66 |
| 5 | 3 | 5.9 | 11.9 | 17.8 | 89.0 | 29.7 | 67 |
| 6 | 6 | 8.3 | 12.6 | 20.9 | 104.5 | 17.4 | 60 |
| [bmim][FAP] | |||||||
| 7 | 1 | 2.9 | 6.3 | 9.2 | 46.0 | 230.0 | 68 |
| 8 | 3 | 3.9 | 9.2 | 13.1 | 65.5 | 21.8 | 70 |
| 9 | 6 | 4.1 | 12.8 | 16.9 | 84.5 | 14.1 | 76 |
| scCO2 f | |||||||
| 10 | 1 | 2.3 | 2.5 | 4.8 | 24.0 | 120.0 | 52 |
| 11 | 3 | 4.8 | 6.3 | 11.1 | 55.5 | 18.5 | 57 |
| 12 | 6 | 6.1 | 8.2 | 14.3 | 71.5 | 11.9 | 57 |
| Entry | Time | Yield (%) b | Total TON c | Total TOF d | Selectivity towards Cyclo-Hexanone e (%) | ||
|---|---|---|---|---|---|---|---|
| (h) | Cyclo-Hexanol | Cyclo-Hexanone | Total | (h−1) | |||
| CH3CN | |||||||
| 1 | 1 | 0.8 | 1.2 | 2.0 | 10.0 | 10 | 60 |
| 2 | 3 | 1.1 | 1.5 | 2.6 | 13.0 | 4.3 | 58 |
| 3 | 6 | 1.3 | 2.1 | 3.4 | 17.0 | 2.8 | 62 |
| [bmim][PF6] | |||||||
| 4 | 1 | 3.3 | 5.7 | 9.0 | 45.0 | 45 | 63 |
| 5 | 3 | 6.5 | 8.3 | 14.8 | 74.0 | 24.7 | 56 |
| 6 | 6 | 7.5 | 9.4 | 16.9 | 84.5 | 14.1 | 56 |
| [bmim][FAP] | |||||||
| 7 | 1 | 1.4 | 3.4 | 4.8 | 24.0 | 24 | 71 |
| 8 | 3 | 1.9 | 5.1 | 7.0 | 35.0 | 11.7 | 73 |
| 9 | 6 | 2.5 | 8.6 | 11.1 | 55.5 | 9.3 | 77 |
| scCO2 f | |||||||
| 10 | 1 | 3.4 | 4.9 | 8.3 | 41.5 | 41.5 | 59 |
| 11 | 3 | 4.5 | 5.1 | 9.6 | 48.0 | 16.0 | 53 |
| 12 | 6 | 5.3 | 7.5 | 12.8 | 64.0 | 10.7 | 59 |
| Entry | Catalyst | Yield (%) b | Total TON c | Total TOF d | Selectivity towards Cyclo-Hexanone e (%) | ||
|---|---|---|---|---|---|---|---|
| Cyclo-Hexanone | Cyclo-Hexanol | Total | (h−1) | ||||
| scCO2-[bmim][PF6] | |||||||
| 1 | 1 | 17.6 | 1.3 | 18.9 | 95 | 94.5 | 93 |
| 2 | 2 | 15.9 | 0.7 | 16.6 | 83 | 41.5 | 96 |
| scCO2-[bmim][FAP] | |||||||
| 3 | 1 | 19.5 | 3.2 | 22.7 | 114 | 113.5 | 86 |
| 4 | 2 | 16.7 | 2.5 | 19.2 | 96 | 48.0 | 87 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Alegria, E.C.B.A.; Matias, I.A.S.; Duarte, T.A.G.; Pombeiro, A.J.L. Catalytic Performance of Fe(II)-Scorpionate Complexes towards Cyclohexane Oxidation in Organic, Ionic Liquid and/or Supercritical CO2 Media: A Comparative Study. Catalysts 2017, 7, 230. https://doi.org/10.3390/catal7080230
Ribeiro APC, Martins LMDRS, Alegria ECBA, Matias IAS, Duarte TAG, Pombeiro AJL. Catalytic Performance of Fe(II)-Scorpionate Complexes towards Cyclohexane Oxidation in Organic, Ionic Liquid and/or Supercritical CO2 Media: A Comparative Study. Catalysts. 2017; 7(8):230. https://doi.org/10.3390/catal7080230
Chicago/Turabian StyleRibeiro, Ana P. C., Luísa M. D. R. S. Martins, Elisabete C. B. A. Alegria, Inês A. S. Matias, Tiago A. G. Duarte, and Armando J. L. Pombeiro. 2017. "Catalytic Performance of Fe(II)-Scorpionate Complexes towards Cyclohexane Oxidation in Organic, Ionic Liquid and/or Supercritical CO2 Media: A Comparative Study" Catalysts 7, no. 8: 230. https://doi.org/10.3390/catal7080230
APA StyleRibeiro, A. P. C., Martins, L. M. D. R. S., Alegria, E. C. B. A., Matias, I. A. S., Duarte, T. A. G., & Pombeiro, A. J. L. (2017). Catalytic Performance of Fe(II)-Scorpionate Complexes towards Cyclohexane Oxidation in Organic, Ionic Liquid and/or Supercritical CO2 Media: A Comparative Study. Catalysts, 7(8), 230. https://doi.org/10.3390/catal7080230