Abstract
The laccase-incorporated nanoflower was fabricated and characterized by scanning electron microscope (SEM) and Fourier transform infrared spectroscopy (FTIR). SEM images indicate that the laccase-incorporated nanoflower has a high surface area, which may facilitate the mass transfer of the substrate and the product. FTIR spectrums identify the existence of laccase in the nanoflowers. The novel immobilized laccase was used for the synthesis of viniferin. The reaction conditions had been optimized and the laccase-incorporated nanoflower can show its maximum specific activity (16.3 µmol/g/h) under the optimal reaction conditions. The specific activity of the laccase in the nanoflowers is enhanced about 2.2-fold compared with free laccase in solution without copper (II) ions. Furthermore, the laccase in the nanoflowers shows an increase in specific activity of ~180% compared with free laccase in a solution containing high concentrations (similar to the concentration in the flower) of copper (II) ions. The results also indicate that the laccase in the nanoflowers retain 93.2% of its initial specific activity even after ten continuous batches.
1. Introduction
As a dimmer of resveratrol, viniferin has stronger antioxidant efficiency in vitro than resveratrol [1,2]. It can efficiently induce the apoptosis of leukemia B-cells, exhibit hepatoprotective effects activity in cultured rat hepatocytes, and inhibit human cytochrome P450 enzymes [3,4,5,6]. However, its further investigations are seriously limited due to its scarce availability. Viniferin can be synthesized via the chemical oxidation catalyzed by iron (III) chloride, thallium (III) nitrate (TTN), potassium hexacyanoferrate (III), or cerium (IV) sulfate [7,8]. Nevertheless, the poor selectivity of chemical methods may lead to some unwanted side-products. Compared with the chemical methods, the biocatalytic process catalyzed by laccase is a more effective and specific route [9]. For example, the laccases from Myceliophtora thermophyla and from Trametes pubescens have been used for the synthesis of viniferin and can give viniferin in 31% and 18% yields for 4 days, respectively [10]. The laccase from Trametes villosa can also present a specific activity of 5.8 μmol/mg/h in the synthesis of viniferin [11]. However, all these reported laccases exhibit poor specific activity in the synthesis of viniferin. Furthermore, the high cost of the laccases severely limits their industrial applications.
Enzyme immobilization can overcome these limitations [12,13,14,15]. Besides increasing the enzyme reusability and stability, enzyme immobilization can also greatly improve the enzyme activity [16,17,18]. The specific activity of the organophosphorus hydrolase can be enhanced about 2.0-fold by entrapping it into the HOOC-functionalized mesoporous silica [19,20,21]. M. miehei lipase adsorbed on hydrophobic support is even 20 times more active than its free enzyme [22]. The specific activity of encapsulated β-galactosidase from Aspergillus oryzae increases 1.8-fold compared with the free enzyme [23]. In 2012, a simple and versatile immobilization technology was reported by Zare and his colleagues to gain hybrid organic–inorganic nanoflower composed by various proteins and Cu3(PO4)2·3H2O [24]. Such hybrid organic–inorganic nanoflowers use copper (II) ions as the inorganic component and various proteins as the organic component. The growth of nanoflower is dominated by the coordination between nitrogen atoms of the amide groups in the protein backbone and copper ions. The laccase-Cu3(PO4)2·3H2O nanoflowers were also obtained through this method [24]. When the enzyme was used as the protein component of the hybrid nanoflower, the enhanced specific activity and stability could be obtained [25]. In our previous work, we had successfully adopted this method to prepare a novel immobilized lipase for resolution of (R,S)-2-pentanol in organic solvents [26] and found that the lipase in the nanoflowers can exhibit perfect specific activity and excellent reusability.
In this study, we prepared the laccase-incorporated nanoflower for efficiently synthesizing viniferin (Scheme 1). The fabricated laccase-incorporated nanoflower was characterized by a scanning electron microscope (SEM) and Fourier transform infrared spectroscopy (FTIR). The reaction conditions for the synthesis of viniferin catalyzed by the immobilized laccase had been optimized and the reusability of the immobilized laccase had also been investigated.
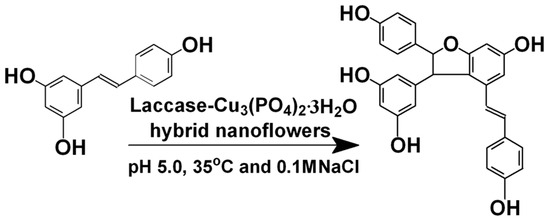
Scheme 1.
The synthesis catalyzed by laccase-incorporated nanoflowers.
2. Results and Discussion
2.1. Coupling Efficiency and Loading Efficiency
After the immobilization process was performed, the protein content in the pooled suspension and washing solution was measured by the Bradford method. Since no protein can be detected in the above-mentioned solution, the loading efficiency (%) is about 100%. The laccase loading amount in the nanoflowers is 200 mg laccase/g nanoflower. Moreover, the results from the ABTS assay demonstrate that the pooled suspension and washing solution only exhibit negligible oxidation activity. The laccase in the nanoflower shows 350% increase in activity (in terms of oxidizing ABTS) compared with free laccase in solution. Hence, the coupling efficiency (%) of laccase during the immobilization process is 350%. The significant increase in activity for laccase in the nanoflower is possibly due to the activation effect provided by copper (II) ions acted as the inorganic component in the nanoflowers [24].
2.2. SEM
Figure 1A,B reveal that the samples consist of large quantities of flower-like nanoparticles with diameters in the range of 7~10 µm. The morphology of the nanoflowers closely resembles the shape of the marigold in nature (the inset of Figure 1A). As shown in Figure 1C, the nanoflowers have hierarchical structures with high surface-to-volume ratios. Figure 1D demonstrates that disordered fragments are formed without laccase. These results suggest that laccase plays a crucial role in the fabrication of the nanoflowers.
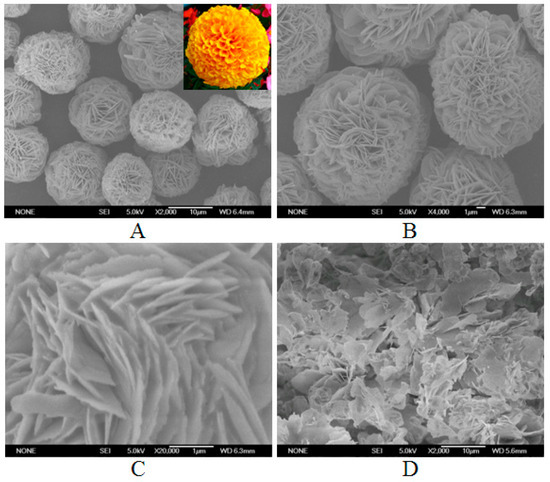
Figure 1.
Scanning electron microscope (SEM) of the samples in the presence or absence of laccase. (A–C) laccase-incorporated nanoflowers with different enlargement factors. Inset of (A) the image of marigold in nature. (D) Cu3(PO4)2·3H2O matrices without laccase.
2.3. FTIR
Cu3(PO4)2·3H2O, laccase and laccase-incorporated nanoflower were characterized by FTIR spectrum in the region of 400–4000 cm−1 to certify the presence of laccase in the nanoflowers (Figure 2). The vibration bands of PO43− can be seen at 1053 cm−1 and 556 cm−1 (Curve 1 and Curve 3 in Figure 2) [27]. The amide I and II bands of the laccase can be observed at 1646 cm−1 and 1533 cm−1 (Curve 2 and Curve 3 in Figure 2) [28]. The amide I band at 1646 cm−1 derives mainly from the C=O stretching vibrations of the peptide linkages in laccase. The amide II at 1533 cm−1 is primarily attributed to in-plane NH bending vibration and CN stretching vibration in laccase [29]. These results verify existence of laccase in the nanoflowers.
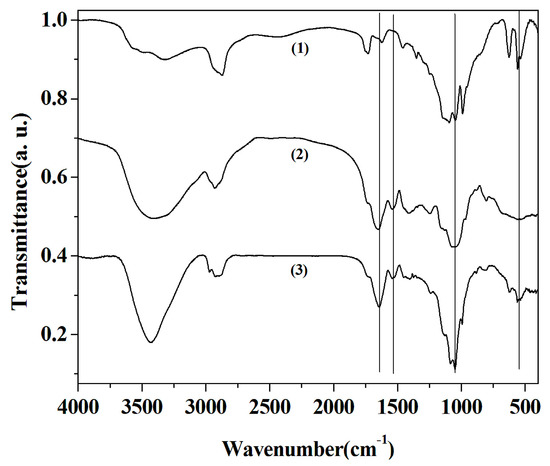
Figure 2.
Fourier transform infrared spectroscopy (FTIR) spectrum of Cu3(PO4)2·3H2O matrices without laccase (Curve 1), free laccase (Curve 2) and laccase-incorporated nanoflower (Curve 3).
2.4. EDS Spectrum
The EDS spectrum of the laccase-incorporated nanoflower indicates that the sample consists mainly of C, N, Cu, P, and oxygen (Figure 3b). In contrast, only the signals of Cu, P, and oxygen can be observed in the energy-dispersive X-ray spectroscopy (EDS) spectrum of the Cu3(PO4)2·3H2O matrices (Figure 3a). The atom ratio of Cu/P is approximately 3:2 in both samples. The signals for C and N in the EDS spectrum of the laccase-incorporated nanoflower shall be ascribed to the contribution of laccase. Hence, the EDS spectrum of the laccase-incorporated nanoflower reveals that laccase was successfully incorporated into the nanoflowers.
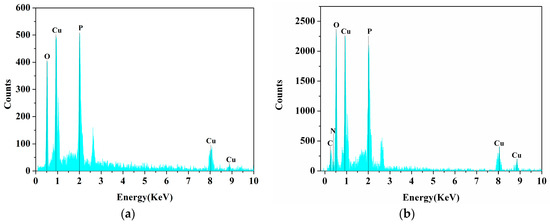
Figure 3.
Energy-dispersive X-ray spectroscopy (EDS) spectrum of Cu3(PO4)2·3H2O matrices without laccase (a) and laccase-incorporated nanoflower (b).
2.5. Optimizing the Reaction Conditions for Synthesis of Viniferin
The pH, temperature, and ionic strength are three critical factors that may affect the enzyme performance of laccase [30]. Therefore, these factors were surveyed for obtaining the optimal reaction conditions.
2.5.1. pH
A pH variation ranging from pH 3.0 to 8.0 was tested to investigate its effect on the specific activities of free laccase and laccase in the nanoflowers. It can be found that immobilized laccase gives its maximal activity at pH 5.0, while the free laccase exhibits its highest activity at pH 4.0 (Figure 4). As a result, pH 4 and pH 5 were chosen as the optimum pH for free laccase and immobilized laccase, respectively. The discrepancy of optimum pH for free and immobilized laccase might be attributed to the distortions on laccase conformation caused by the changes in its microenvironment. Immobilized laccase maintains 92% of its maximal activity at pH 3.0, while free laccase retains 49% of its highest activity. At pH 8.0, immobilized laccase loses 12% of its maximal activity, whereas the free one suffers the loss of 89% of its highest activity. Furthermore, pH stability assay demonstrate that laccase in the nanoflower is less sensitive to pH change compared with the free one (see Supplementary Materials, Section S1). The SEM images in Figure S3a–c confirm that the morphologies of the nanoflowers at different pH levels do not change (see Supplementary Materials Section S4), suggesting that the hierarchical structures of the nanoflowers still exist at different pH levels.
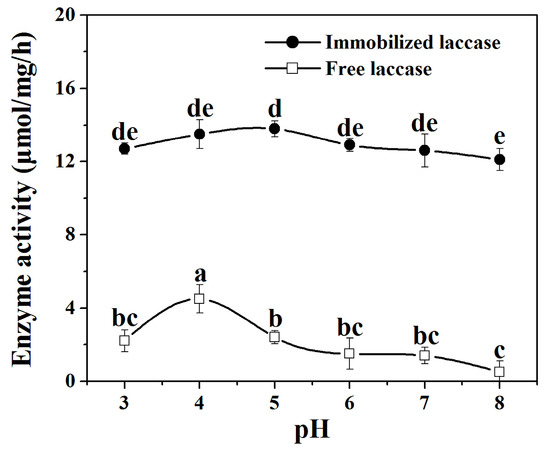
Figure 4.
Effect of pH on the enzyme activity of laccase and laccase in the nanoflowers. Reaction conditions: trans-resveratrol 2 mmol, n-butanol (30 mL), Na2HPO4–sodium citrate buffer (15 mL) (pH 3–8), immobilized laccase or free laccase (enzyme content: 5 mg), reaction temperature: 45 °C. Post hoc all pairwise comparisons for the specific activities of free or immobilized laccase at different pH values were performed using Tukey’s test (p < 0.05). Means with the same letter are not significantly different. Letters (a, b, c, d, e) identify different statistically significant groups (Tukey’s test, p < 0.05).
2.5.2. Temperature
The effect of temperature on the specific activities of free laccase and immobilized laccase was assessed in the range of 5–65 °C. Figure 5 shows that both of the free laccase and the immobilized laccase exhibit the highest specific activity at 35 °C. Accordingly, all remaining experiments were performed at 35 °C for both immobilized laccase and free laccase. At 5 °C, laccase in the nanoflowers presents 76% of the highest activity, whereas free laccase merely manifests 38% of the highest activity. At 65 °C, immobilized laccase only lost 23% of its maximal activity, while free laccase misses 64% of its maximal activity. The thermal stability assay also reveals that the specific activity of laccase in the nanoflower decreases at a slower rate than that of the free one with the increase of the incubation time (see Supplementary Materials, Section S2), which implies the immobilization of laccase in the nanoflower might preserve the tertiary structure of the protein and protect the laccase from disassembling the active center caused by the diminution of non-covalent forces at higher temperatures [31,32,33]. The SEM images in Figure S3d–f verify that no significant change in the morphologies of the nanoflowers at different temperatures occurs (see Supplementary Materials, Section S4), indicating that the hierarchical structures of the nanoflowers cannot be damaged at different temperatures.
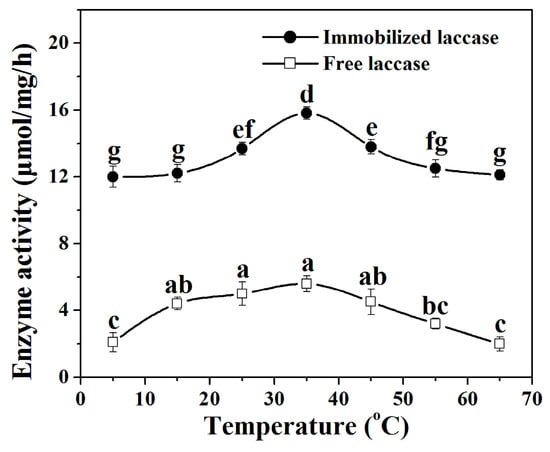
Figure 5.
Effect of temperature on the activity of free laccase and laccase in the nanoflowers. Reaction conditions: trans-resveratrol 2 mmol, n-butanol (30 mL), Na2HPO4–sodium citrate buffer (15 mL) (pH 5.0 for immobilized laccase, pH 4.0 for free laccase), immobilized laccase or free laccase (enzyme content: 5 mg), reaction temperature: 5–65 °C. Post hoc all pairwise comparisons for the specific activities of free or immobilized laccase at different temperatures were performed using Tukey’s test (p < 0.05). Means with the same letter are not significantly different. Letters (a, b, c, d, e, f, g) identify different statistically significant groups (Tukey’s test, p < 0.05).
2.5.3. Ionic Strength
The effect of ionic strength on the specific activity of immobilized laccase and free laccase was evaluated at 0.0~1.0 M NaCl. The results shows that the specific activities of immobilized laccases almost keep constant with the increase of NaCl concentration from 0.0 to 0.25 M, and then slowly decreasing with the further increase of NaCl concentration in the range 0.25~1.0 M (Figure 6). Thus, 0.1 M NaCl was performed for immobilized laccase in the remaining experiments. Therefore, 0.25 M NaCl was determined as the optimal reaction condition for free laccase. The experimental results suggest that laccase in the nanoflowers display better stability to resist the change of ionic strength compared with the free one.
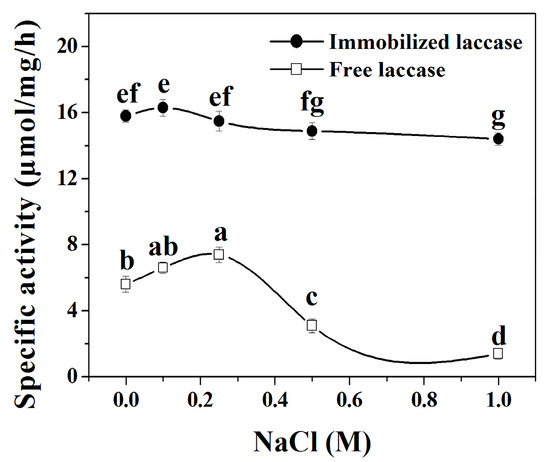
Figure 6.
Effect of NaCl concentration on the activity of laccase and laccase in the nanoflowers. Reaction conditions: trans-resveratrol 2 mmol, n-butanol (30 mL), Na2HPO4–sodium citrate buffer (15 mL) containing 0.0–1.0 M NaCl (pH 5.0 for immobilized laccase, pH 4.0 for free laccase), immobilized laccase or free laccase (enzyme content: 5 mg), reaction temperature: 35 °C. Post hoc all pairwise comparisons for the specific activities of free or immobilized laccase at different concentrations of NaCl were performed using Tukey’s test (p < 0.05). Means with the same letter are not significantly different. Letters (a, b, c, d, e, f, g) identify different statistically significant groups (Tukey’s test, p < 0.05).
2.6. Comparison of Specific Activity
Since copper is a component of the active site of laccases, the activity of laccase in the solution can be enhanced by adding copper (II) ions. As can be seen in Table 1, laccase in the nanoflowers shows a 2.2-fold increase in specific activity as compared with laccase in buffer solutions without copper (II) ions. The improvement of specific activity implies that copper ion (II) in the nanoflowers can help to enhance laccase activity in a manner similar to that in solution [34]. The specific activity of laccase in the nanoflowers is 1.8 times than that of the case in buffer solutions containing copper (II) ions. The enhancement of activation effect in the nanoflowers is possibly attributed to the cooperative effects of the nanoscale-entrapped laccase molecules. The specific activity of free laccase does not change after adding copper phosphate crystals into buffer solutions. The copper phosphate crystals without the laccase cannot catalyze the formation of viniferin. Laccase in the nanoflowers gives 59% isolated yield after the synthesis reaction was performed for 3 days. In general, the enzyme copper could bind to the functional groups (–NH2, –OH) of the substrate forming an inactive complex, while Cu2+ ions in enzyme copper could also form complexes with substrates through their functional groups, thus preventing the formation of an inactive complex [35]. Zare et al. addressed that the formation of nanoflowers depended on the appearance of the Cu(II)-protein complex, which provided a location for nucleation of the primary crystals [24]. Hence, we speculate that, in this experiment, the Cu(II) ions that originated from the Cu(II)-laccase complex located in the nanoflower might raise the number of resveratrol molecules forming active complexes with the laccase, causing the increase of the specific activity of laccase in the nanoflower. Moreover, the results also show that the difference of the specific activities in immobilized laccase at 4 and 25 °C is much lower than that of the free one (see Supplementary Materials, Table S1), verifying that the slight diffusion limitation of the substrate and the product in the nanoflower occurs during the reaction. In addition, the average velocity of the accumulation of viniferin catalyzed by laccase in the nanoflowers (78.67 μmol/mg/day) is about 20~30 times higher than Luca Forti et al.′s results [10].

Table 1.
Comparison of specific activity of free and immobilized laccase in different conditions.
2.7. Reusability
The reusability of the immobilized laccase was studied (Table 2). It can be found that immobilized laccase can retain 93.2% of its initial specific activity even after 10 recycles. The results suggest that immobilized laccase possess excellent reusability, which may facilitate the synthesis of viniferin during a continuous operation. The slight decrease of laccase activity might ascribe to the little leakage of laccase caused by the soaking, separating, and washing processes used in the reaction cycles [36,37].

Table 2.
Reusability of immobilized laccase.
3. Materials and Methods
3.1. Chemicals
Laccase (EC 1.1.3.4) from Botrytis sp. (15 EU/mg, one enzyme unit (EU) is defined as the amount of enzyme required to oxidize 1 µmol of 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) sodium salt (ABTS) per minute at pH 4.5 and 37 °C) was obtained from Wuxi enzyme preparation plant (Wuxi, China). The ABTS was purchased from Sigma–Aldrich (St. Louis, MO, USA). trans-Resveratrol (R5010, 99%) was purchased from Fluka (Buchs, Switzerland). Viniferin was purchased from Actichem (Montauban, France). KBr (spectral grade) was obtained from BDH Co. (Poole, UK). All other chemicals and reagents were of analytical grade. All aqueous solutions were prepared with Milli-Q water.
3.2. Preparation of Laccase-Incorporated Nanoflower
The preparation of laccase-incorporated nanoflower was achieved according to the previous work with a slight modification [26]. At first, 60 mL of a 1 mg/mL laccase solution (900 EU) was added to 3 L of phosphate buffer (50 mmol/L, pH 7.4), followed by the addition of 20 mL of CuSO4 solution (120 mmol/L). Herein, the optimal concentration of laccase (0.02 mg/mL) was used to achieve the laccase-incorporated nanoflowers with the best specific activity for synthesizing viniferin. Then, the mixture was incubated at 25 °C for three days. Blue product could be found at the bottom of the flask. Finally, the blue product was collected by centrifugation (12,000 rpm for 20 min) and washed by the de-ionized water for three times. The protein concentration in the supernatant was quantified using the Bradford protein assay with BSA as a standard [38]. The loading efficiency (%) of laccase was calculated by the formula below:
L = loading efficiency (%), W1 = the total amount of laccase added to the immobilization system, and W2 = the total amount of laccase recovered in the pooled supernatant and washing fractions.
The coupling efficiency (%) was calculated according to the following formula:
Bound activity = the activity of immobilized laccase, Applied activity = the activity of laccase added to the immobilization system, and Unbound activity = the activity of laccase came from the pooled supernatant and washing fractions. The activity was determined using ABTS as a substrate at pH 4.5 and 37 °C. The detailed procedure of ABTS assay is as follows: a 0.1 M solution of ABTS (0.3 mL) in 0.01 M acetate buffer (pH = 4.5) was diluted with 0.01 M acetate buffer (2.6 mL, pH = 4.5) and treated with a solution of laccase in the same buffer (0.1mL). The change in absorption was followed via UV/Vis-spectroscopy (λ = 414 nm).
3.3. Characterization of Laccase-Incorporated Nanoflower
The morphologies of the samples were observed by a JSM-6700F electron microscope (JEOL, Tokyo, Japan) with an acceleration voltage of 30 kV. The FTIR spectrums of the samples were surveyed using Nicolet 5700 FTIR spectrometer with a resolution of 4 cm−1 through the KBr method. The EDS spectra (JEOL JSM-6700F, Tokyo, Japan) were also used to analyze the composition of the samples.
3.4. Screening the Reaction Conditions
The optimization of the reaction conditions was implemented using trans-resveratrol as a substrate. Effect of pH on the specific activity of laccase and laccase in the nanoflowers was investigated in the pH range 3~8. Effect of temperature on the specific activity of free laccase and laccase in the nanoflowers was measured at different temperatures (5~65 °C). The effect of NaCl concentration on the specific activity of laccase and laccase in the nanoflowers was assessed at different NaCl concentrations (0.0~1.0 M). The specific activity (μmol/mg/h) was defined as the amount (in micromoles) of trans-resveratrol converted per hour per milligram of enzyme. The average velocity (μmol/mg/day) of the accumulation of viniferin during the synthesis reaction was defined as the amount (in micromoles) of viniferin produced per day per milligram of enzyme. The experiments were performed in triplicate, and all data were obtained based on the average values. The curves were obtained by a spline fit connecting sequential data points. The SEM images of laccase incorporated nanoflowers at different reaction conditions were recorded after the reaction.
3.5. Synthesis of Viniferin under Optimum Conditions
For immobilized laccase, the reaction mixture was composed by trans-resveratrol (2 mmol), 30 mL of n-butanol, and 15 mL of Na2HPO4–sodium citrate buffer solution containing 0.1 M NaCl (pH 5.0). Immobilized laccase (25 mg) was added into the reaction mixture, and the reaction was performed at 35 °C. For free laccase, the reaction mixture was made up of trans-resveratrol (2 mmol), 30 mL of n-butanol, and 15 mL of Na2HPO4–sodium citrate buffer solution containing 0.25 M NaCl (pH 4.0). Laccase (5 mg) was added into the reaction mixture for triggering the reaction at 35 °C. The reaction mixture was incubated at 35 °C for 3 days. A 200 µL aliquot of the mixture was withdrawn with an interval of 1 hour and then centrifuged at 4500× g for 3 min. A 2 µL aliquot was taken from the suspension and was analyzed by gas chromatography (GC-2014) with flame ionization detector (FID).
3.6. Reusability
After each cycle, the reaction mixture was centrifuged at 12,000 rpm for 3 min. The obtained precipitation was washed with Na2HPO4-sodium citrate buffer (0.1 M, pH 7.5) to remove any residual substrate or product and then kept overnight in a vacuum oven for a complete drying. After centrifugation, washing and drying, the powder was used for the next cycle under the same conditions. The residual activity of the recycled enzyme was compared with the specific activity of the first cycle (100%).
3.7. HPLC Analytical Procedure
The reaction was monitored by HPLC (SPD-M20A, Japan) equipped with a Thermo C18 column (250 × 4.6 mm, Agilent, CA, USA), a ternary pump (DGU-20A3, Kyoto, Japan), and an autosampler (SIL-20A HT, Kyoto, Japan). The elution was performed with a flow rate of 1.0 mL/min at 30 °C, and UV detection was at 310 nm. The samples were measured by a gradient elution system, and the eluent was a mixture of methanol/water (containing 0.1% of acetic acid). First, 30% of methanol (v/v) was applied for 5 min. A gradient from 30% to 50% of methanol (v/v) was performed in 5 min, and 50% methanol elution was maintained for 40 min. Finally, a gradient from 50% to 30% of methanol (v/v) was applied for 10 min. trans-Resveratrol and purified viniferin were dissolved in methanol and then filtered through a 0.2 μm syringe filter prior to injection. High-performance liquid chromatography (HPLC) peaks of trans-resveratrol and viniferin were identified using retention times and by comparing UV-vis spectra with standards. The HPLC analysis shows that viniferin is the only one product during the reaction if this is compared with commercial viniferin standard. Under these conditions, the retention times and absorbance maxima of trans-resveratrol and purified viniferin are 19.3 min (306 nm) and 25.1 min (324 nm), respectively. The structure of the product was characterized by 1 H nuclear magnetic resonance (NMR) spectra using a Varian Unity-500 spectrometer (see supporting information, Section S3).
4. Conclusions
In this study, the laccase-incorporated nanoflower was successfully prepared and used for the synthesis of viniferin. The specific activity of the laccase in the nanoflowers is about 2.2 times that of free laccase in solution without copper (II) ions. The specific activity of the laccase in the nanoflowers has an increase in specific activity of ~180% compared with free laccase in solution containing copper (II) ions. Furthermore, the immobilized laccase exhibit excellent reusability, high pH stability and thermo-stability. In this work, the size of enzyme-incorporated nanoflowers is 7~10 µm. Although a high surface area of the nanoflowers results in an increased mass transfer for the substrate and the product, the enzyme located in the interior of the nanoflowers still suffer from the slight diffusion limitation of the substrate and the product compared with the counterpart located in the exterior of the nanoflowers. We speculate that the catalytic activity of enzyme in the nanoflowers may be further improved when the size of enzyme-incorporated nanoflowers reaches several hundreds of nanometers. In the future, the preparation of the enzyme-incorporated nanoflowers with a smaller diameter shall be attempted to completely overcome the mass transfer limitations of the product and the substrate in the nanoflowers.
Supplementary Materials
The following are available online at www.mdpi.com/2073-4344/7/6/188/s1, Figure S1: pH stability of free or immobilized laccase tested at different pH values using resveratrol as substrate; Figure S2: Thermal stability at 45 °C (a), 55 °C (b) and 65 °C (c) of free laccase and immobilized laccase tested in different time intervals using resveratrol as substrate; Figure S3: SEM images of laccase-incorporated nanoflower at 35 °C: (a) pH = 3, (b) pH = 5, and (c) pH = 8 or different temperatures at pH 5.0: (d) 5 °C, (e) 35 °C, and (f) 65 °C; Table S1. The specific activity of free and immobilized laccase at 4 °C and 25 °C.
Acknowledgments
The authors are grateful for the financial support from the National High Technology Research and Development Program of China (“863” Program, 2012AA022202B), the PhD Scientific Research Start-up Fund Project of Jilin Agricultural University (201607), the 13th Five-Year scientific research Planning Project of the Education Department of Jilin Province (JJKH20170305KJ) and the Jilin Province Innovation Platform of Straw Comprehensive Utilization Technology (Technology and Innovation Platform of Colleges and Universities of Jilin Province (2014) C-1)).
Author Contributions
Zhuofu Wu and Heng Li performed the synthesis of the nanoflower and wrote the paper; Xuejun Zhu and Lei Wang measured the specific activities of the nanoflower; Shuai Li and Zhi Wang analyzed the data; Zhengqiang Li and Guang Chen conceived and designed the experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zghonda, N.; Yoshida, S.; Araki, M.; Kusunoki, M.; Mliki, A.; Ghorbel, A.; Miyazaki, H. Greater effectiveness of ε-viniferin in red wine than its monomer resveratrol for inhibiting vascular smooth muscle cell proliferation and migration. Biosci. Biotechnol. Biochem. 2011, 75, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Zghonda, N.; Yoshida, S.; Ezaki, S.; Otake, Y.; Murakami, C.; Mliki, A.; Ghorbel, A.; Miyazaki, H. Ε-viniferin is more effective than its monomer resveratrol in improving the functions of vascular endothelial cells and the heart. Biosci. Biotechnol. Biochem. 2012, 76, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Billard, C.; Izard, J.-C.; Roman, V.; Kern, C.; Mathiot, C.; Mentz, F.; Kolb, J.-P. Comparative antiproliferative and apoptotic effects of resveratrol, ϵ-viniferin and vine-shots derived polyphenols (Vineatrols) on chronic B lymphocytic leukemia cells and normal human lymphocytes. Leuk. Lymphoma 2002, 43, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Quiney, C.; Dauzonne, D.; Kern, C.; Fourneron, J.-D.; Izard, J.-C.; Mohammad, R.M.; Kolb, J.-P.; Billard, C. Flavones and polyphenols inhibit the NO pathway during apoptosis of leukemia B-cells. Leuk. Res. 2004, 28, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Oshima, Y.; Namao, K.; Kamijou, A.; Matsuoka, S.; Nakano, M.; Terao, K.; Ohizumi, Y. Powerful hepatoprotective and hepatotoxic plant oligostilbenes, isolated from the oriental medicinal plant Vitis coignetiae (Vitaceae). Experientia 1995, 51, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Piver, B.; Berthou, F.; Dreano, Y.; Lucas, D. Differential inhibition of human cytochrome P450 enzymes by ε-viniferin, the dimer of resveratrol: Comparison with resveratrol and polyphenols from alcoholized beverages. Life Sci. 2003, 73, 1199–1213. [Google Scholar] [CrossRef]
- Takaya, Y.; Terashima, K.; Ito, J.; He, Y.-H.; Tateoka, M.; Yamaguchi, N.; Niwa, M. Biomimic transformation of resveratrol. Tetrahedron 2005, 61, 10285–10290. [Google Scholar] [CrossRef]
- Yao, C.S.; Lin, M.; Wang, Y.H. Synthesis of the active stilbenoids by photooxidation reaction of trans-ϵ-viniferin. Chin. J. Chem. 2004, 22, 1350–1355. [Google Scholar] [CrossRef]
- Pezet, R. Purification and characterization of a 32-kDa laccase-like stilbene oxidase produced by Botrytis cinerea pers.: Fr. FEMS Microbiol. Lett. 1998, 167, 203–208. [Google Scholar] [CrossRef]
- Nicotra, S.; Cramarossa, M.R.; Mucci, A.; Pagnoni, U.M.; Riva, S.; Forti, L. Biotransformation of resveratrol: Synthesis of trans-dehydrodimers catalyzed by laccases from Myceliophtora thermophyla and from Trametes pubescens. Tetrahedron 2004, 60, 595–600. [Google Scholar] [CrossRef]
- Zhang, H.; Xun, E.; Wang, J.; Chen, G.; Cheng, T.; Wang, Z.; Ji, T.; Wang, L. Immobilization of laccase for oxidative coupling of trans-resveratrol and its derivatives. Int. J. Mol. Sci. 2012, 13, 5998–6008. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Klapiszewski, L.; Jedrzak, A.; Nowicki, M.; Moszynski, D.; Jesionowski, T. Lipase B from Candida antarctica immobilized on a silica-lignin matrix as a stable and reusable biocatalytic system. Catalysts 2016, 7, 14. [Google Scholar] [CrossRef]
- Rodriguez-Colinas, B.; Fernandez-Arrojo, L.; Santos-Moriano, P.; Ballesteros, A.O.; Plou, F.J. Continuous packed bed reactor with immobilized β-galactosidase for production of galactooligosaccharides (GOS). Catalysts 2016, 6, 189. [Google Scholar] [CrossRef]
- Chen, P.-C.; Qian, Y.-C.; Fang, F.; Zhu, X.-Y.; Huang, X.-J. Adsorption and activity of lipase on polyphosphazene-modified polypropylene membrane surface. Catalysts 2016, 6, 174. [Google Scholar] [CrossRef]
- Ziegler-Borowska, M.; Chelminiak-Dudkiewicz, D.; Siódmiak, T.; Sikora, A.; Wegrzynowska-Drzymalska, K.; Skopinska-Wisniewska, J.; Kaczmarek, H.; Marszałł, M. Chitosan–collagen coated magnetic nanoparticles for lipase immobilization—New type of “enzyme friendly” polymer shell crosslinking with squaric acid. Catalysts 2017, 7, 26. [Google Scholar] [CrossRef]
- Sasai, Y.; Kanno, H.; Doi, N.; Yamauchi, Y.; Kuzuya, M.; Kondo, S.-I. Synthesis and characterization of highly stabilized polymer–trypsin conjugates with autolysis resistance. Catalysts 2016, 7, 4. [Google Scholar] [CrossRef]
- Belhacene, K.; Elagli, A.; Vivien, C.; Treizebré, A.; Dhulster, P.; Supiot, P.; Froidevaux, R. Investigation of the effect of plasma polymerized siloxane coating for enzyme immobilization and microfluidic device conception. Catalysts 2016, 6, 209. [Google Scholar] [CrossRef]
- Liu, W.; Li, R.; Liu, D.; Feng, W. Enhancing the enzymatic activity of a heme-dependent peroxidase through genetic modification. Catalysts 2016, 6, 166. [Google Scholar] [CrossRef]
- Lei, C.; Soares, T.A.; Shin, Y.; Liu, J.; Ackerman, E.J. Enzyme specific activity in functionalized nanoporous supports. Nanotechnology 2008, 19, 125102. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-X.; Dill, K.A. Stabilization of proteins in confined spaces. Biochemistry 2001, 40, 11289–11293. [Google Scholar] [CrossRef] [PubMed]
- Minton, A.P. The influence of macromolecular crowding and macromolecular confinement on biochemical reactions in physiological media. J. Biol. Chem. 2001, 276, 10577–10580. [Google Scholar] [CrossRef] [PubMed]
- Palomo, J.M.; Muñoz, G.; Fernández-Lorente, G.; Mateo, C.; Fernández-Lafuente, R.; Guisán, J.M. Interfacial adsorption of lipases on very hydrophobic support (octadecyl–sepabeads): Immobilization, hyperactivation and stabilization of the open form of lipases. J. Mol. Catal. B Enzym. 2002, 19, 279–286. [Google Scholar] [CrossRef]
- Wu, Z.; Dong, M.; Lu, M.; Li, Z. Encapsulation of β-galactosidase from Aspergillus oryzae based on “fish-in-net” approach with molecular imprinting technique. J. Mol. Catal. B Enzym. 2010, 63, 75–80. [Google Scholar] [CrossRef]
- Ge, J.; Lei, J.; Zare, R.N. Protein-inorganic hybrid nanoflowers. Nat. Nanotechnol. 2012, 7, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.; Otari, S.V.; Kang, Y.C.; Lee, J.-K. Protein–inorganic hybrid system for efficient his-tagged enzymes immobilization and its application in L-xylulose production. RSC Adv. 2017, 7, 3488–3494. [Google Scholar] [CrossRef]
- Wu, Z.; Li, X.; Li, F.; Yue, H.; He, C.; Xie, F.; Wang, Z. Enantioselective transesterification of (R,S)-2-pentanol catalyzed by a new flower-like nanobioreactor. RSC Adv. 2014, 4, 33998–34002. [Google Scholar] [CrossRef]
- Cho, I.S.; Kim, D.W.; Lee, S.; Kwak, C.H.; Bae, S.T.; Noh, J.H.; Yoon, S.H.; Jung, H.S.; Kim, D.W.; Hong, K.S. Synthesis of Cu2PO4OH hierarchical superstructures with photocatalytic activity in visible light. Adv. Funct. Mater. 2008, 18, 2154–2162. [Google Scholar] [CrossRef]
- Yang, W.-J.; Griffiths, P.R.; Byler, D.M.; Susi, H. Protein conformation by infrared spectroscopy: Resolution enhancement by fourier self-deconvolution. Appl. Spectrosc. 1985, 39, 282–287. [Google Scholar] [CrossRef]
- Krimm, S.; Bandekar, J. Vibrational spectroscopy and conformation of peptides, polypeptides, and proteins. Adv. Protein Chem. 1986, 38, 181–364. [Google Scholar] [PubMed]
- Laidler, K.J.; Bunting, P.S. The Chemical Kinetics of Enzyme Action, 2nd ed.; Clarendon Press Oxford: Oxford, UK, 1973; p. 63. [Google Scholar]
- Murphy, K.P. Stabilization of protein structure. In Protein Structure, Stability, and Folding; Springer: New York, NY, USA, 2001; pp. 1–16. [Google Scholar]
- Xu, L.; Ke, C.; Huang, Y.; Yan, Y. Immobilized Aspergillus niger lipase with SiO2 nanoparticles in sol-gel materials. Catalysts 2016, 6, 149. [Google Scholar] [CrossRef]
- Rivero, C.W.; Palomo, J.M. Covalent immobilization of Candida rugosa lipase at alkaline pH and their application in the regioselective deprotection of per-O-acetylated thymidine. Catalysts 2016, 6, 115. [Google Scholar] [CrossRef]
- Murugesan, K.; Kim, Y.-M.; Jeon, J.-R.; Chang, Y.-S. Effect of metal ions on reactive dye decolorization by laccase from Ganoderma lucidum. J. Hazard. Mater. 2009, 168, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Peisach, J.; Levine, W.G. A comparison of the enzymic activities of pig ceruloplasmin and Rhus vernicifera laccase. J. Biol. Chem. 1965, 240, 2284–2289. [Google Scholar] [PubMed]
- Yabuki, S. How to lengthen the long-term stability of enzyme membranes: Trends and strategies. Catalysts 2017, 7, 36. [Google Scholar] [CrossRef]
- Alnoch, R.C.; Rodrigues de Melo, R.; Palomo, J.M.; Maltempi de Souza, E.; Krieger, N.; Mateo, C. New tailor-made alkyl-aldehyde bifunctional supports for lipase immobilization. Catalysts 2016, 6, 191. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).