Abstract
Reaction of the bulky bi-phenols 2,2′-RCH[4,6-(t-Bu)2C6H2OH]2 (R = Me L1MeH2, Ph L1PhH2) with the bis(imido) molybdenum(VI) tert-butoxides [Mo(NR1)(NR2)(Ot-Bu)2] (R1 = R2 = 2,6-C6H3-i-Pr2; R1 = t-Bu, R2 = C6F5) afforded, following the successive removal of tert-butanol, the complexes [Mo(NC6H3i-Pr2-2,6)2L1Me] (1), [Mo(NC6H3i-Pr2-2,6)2L1Ph] (2) and [Mo(Nt-Bu)(μ-NC6F5)(L1Me)]2 (3). Similar use of the tri-phenol 2,6-bis(3,5-di-tert -butyl-2-hydroxybenzyl)-4-methylphenol (L2H3) with [Mo(NC6H3i-Pr2-2,6)2(Ot-Bu)2] afforded the oxo-bridged product [Mo(NC6H3i-Pr2-2,6)(NCMe)(μ-O)L2H]2 (4), whilst use of the tetra-phenols α,α,α′,α′-tetrakis(3,5-di-tert-butyl-2-hydroxyphenyl)-p- or -m-xylene L3pH4/L3mH4 led to {[Mo(NC6H3i-Pr2-2,6)2]2(μ-L3p)} (5) or {[Mo(NC6H3i-Pr2-2,6)2]2(μ-L3m)} (6), respectively. Similar use of [Mo(NC6F5)2(Ot-Bu)2] with L3pH4 afforded, after work-up, the complex {[Mo(NC6F5)(Ot-Bu)2]2(μ-L3p)}·6MeCN (7·6MeCN). Molecular structures of 1, 2·CH2Cl2, 3, 4·6MeCN, 6·2C6H14, and 7·6MeCN are reported and these complexes have been screened for their ability to ring open polymerize (ROP) ε-caprolactone; for comparative studies the precursor complex [Mo(NC6H3i-Pr2-2,6)2Cl2(DME)] (DME = 1,2-dimethoxyethane) has also been screened. Results revealed that good activity is only achievable at temperatures of ≥100 °C over periods of 1 h or more. Polymer polydispersities were narrow, but observed molecular weights (Mn) were much lower than calculated values.
1. Introduction
There remains significant academic interest in the design of new catalyst systems capable of producing biodegradable polymers [1,2,3]. This interest stems in part from issues relating to the inertness of polyethylene and subsequent landfill issues, but also from the potential for such biodegradable polymers to be employed in other areas such as the biomedical arena. This is typified by polycaprolactone (PCL), which has found application in tissue engineering and possesses drug permeability [4]. The formation of PCL via the ring opening polymerization (ROP) of ε-caprolactone using metal complexes as initiators, usually in the form of alkoxides, is a favoured synthetic route for PCL formation. However, despite the continued interest in such systems, the catalysts deployed in ROP tend to be either based on main group species, primarily of aluminum, or are based on a select number of transition metals, lanthanides or, more recently, systems utilizing alkali/alkaline earth metals. By contrast, the more earth-abundant metals, have received far less attention. We were attracted to the potential use of molybdenum for the ROP of cyclic esters given its excellent track record over the last couple of decades in ring opening metathesis polymerization (ROMP), as well as its low cost and toxicity. Such ROMP studies have revealed the ability of the molybdenum complexes to promote living polymerizations, and to tolerate a wide range of functionalities, which bodes well for the proposed studies herein [5]. Central to such chemistry has been the use of high valent bis(imido) species, due to their ease of preparation and facile modification. The variety of precursor anilines available means that there is much scope for controlling both the steric and electronic properties of the resultant imido group at the metal, which in turn can influence both the catalytic activity and properties of the polymer products. With this in mind, we recently reported the use of molybdenum chelate complexes derived from the oxydianiline [(2-NH2C6H4)2O], and found that that for the ROP of ε-caprolactone, conversion rates were good (>90%) at high temperatures (100 °C) [6]. As part of that study, a siloxide complex was also isolated and was found to be active without the need for the addition of external alcohol; for the chloride species the addition of benzyl alcohol was necessary to generate an alkoxide. Previous use of molybdenum species in the ROP of cyclic esters is somewhat limited [7,8,9,10,11,12]. Given this, we have now extended our studies to high-valent molybdenum imido phenolate chemistry, where again the expectation is that the addition of an external alcohol would not be necessary for ROP activity. We report the use of bulky di-phenols in combination with bulky organoimido groups which allows for the isolation of mono-nuclear four coordinate complexes, whilst variation of the imido group can lead to bridged di-nuclear species. The use of tri- and tetra-phenolates has also been explored, and in the case of the latter, in the form of the para and meta pro-ligands, the possibility of possible cooperative effects has been investigated. The complexes prepared/screened herein are shown in Scheme 1.
Scheme 1.
Complexes 1–8 prepared and screened for ring opening polymerization (ROP) herein.
2. Results and Discussion
2.1. Di-Phenolate Compounds
The interaction of [Mo(NC6H3i-Pr2-2,6)2(Ot-Bu)2] (formed in situ from [Mo(NC6H3i-Pr2-2,6)2Cl2(dme)] and a slight excess of LiOt-Bu) and the di-phenol 2,2′-CH3CH[4,6-(t-Bu)2C6H2OH]2 (L1MeH2) in diethyl ether readily gives multigram quantities of [Mo(NC6H3i-Pr2-2,6)2L1Me] (1) in good yield (ca. 70%). Stoichiometrically 1 is formed via the loss of two molecules of tert-butanol, which can be removed during the reaction by removing volatiles in-vacuo and then adding more solvent (diethyl ether) and repeating the process several times. Small golden-yellow prisms of 1 suitable for an X-ray structure determination using synchrotron radiation were grown from a saturated heptane solution at ambient temperature. The molecular structure is shown in Figure 1 with bond lengths and angles given in the caption; crystallographic data is presented in Table 1. There is one molecule in the asymmetric unit. The space group is chiral and essentially a single enantiomer has crystallized out. The geometry about the Mo atom is essentially tetrahedral with distortions from ideal varying from 104.93(12) to 119.03(10)°. The two Mo–N distances are similar [1.746(3) and 1.760(3) Å], however the corresponding Mo–N–C angles are somewhat different [171.5(2) versus 158.7(3)°]; the latter is at the lower limit anticipated for a linear imido group. A related complex [Mo(NPh)2(edtc)2] (edtc = S2CNEt2) has been shown to contain one bent (139.4(4)°) and one more linear (169.4(4)°) imido ligand [13,14]. The eight membered ring chelate of 1 adopts a flattened chair-like conformation in stark contrast to the boat-like conformations found previously in complexes containing the related bi-phenol ligand 2,2′-CH2(4-Me,6-t-BuC6H2OH)2 [15,16,17,18,19,20,21]; the “bite angle” of the chelate is 119.03(10)°. In the IR spectrum of 1 the OH band of the free ligand (ca. 3486 cm−1) has disappeared, but there are new bands in the region 750–850 cm−1 assignable to vMo-O. In the 1H NMR spectrum (see experimental), there is a significant shift in the position of the CH bridge proton resonance; such sensitivity to coordination environment has been noted previously [15].
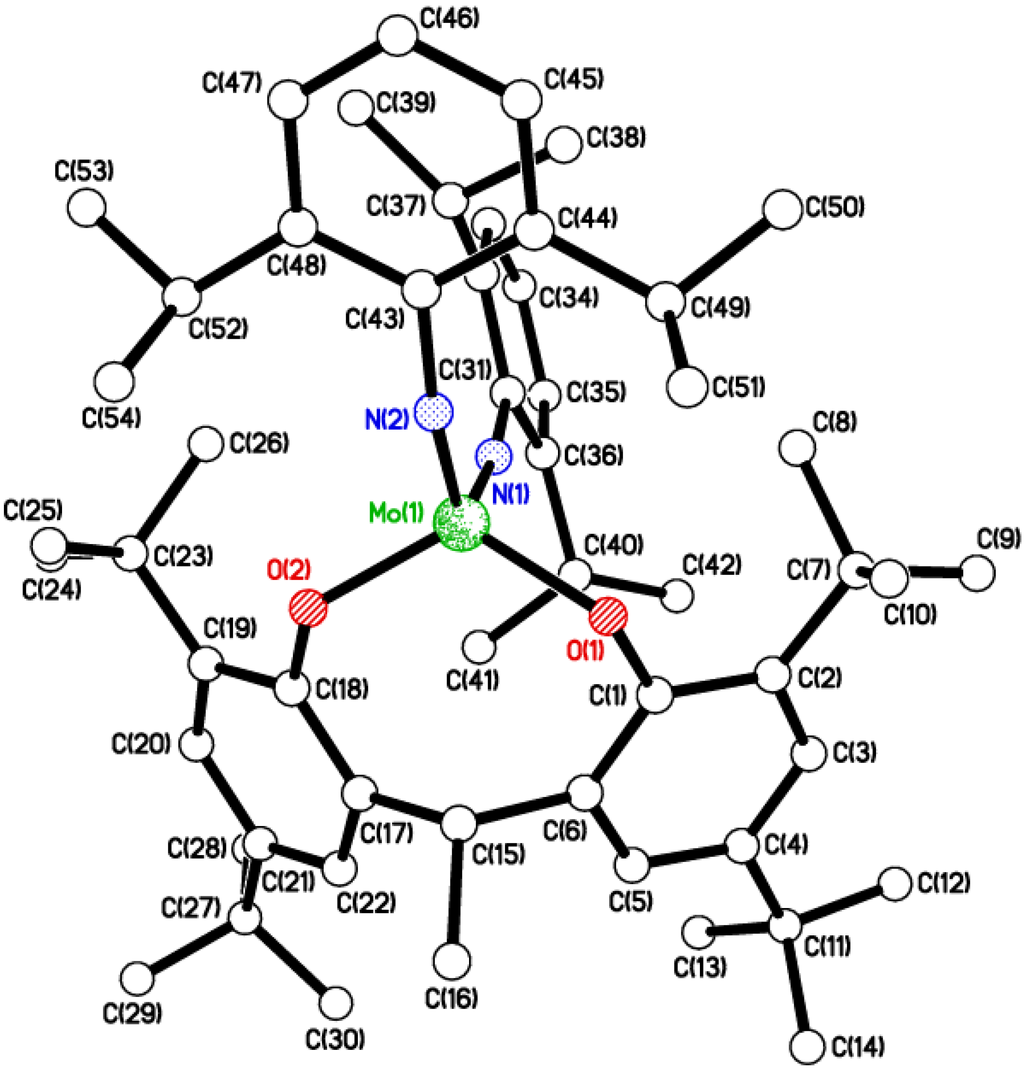
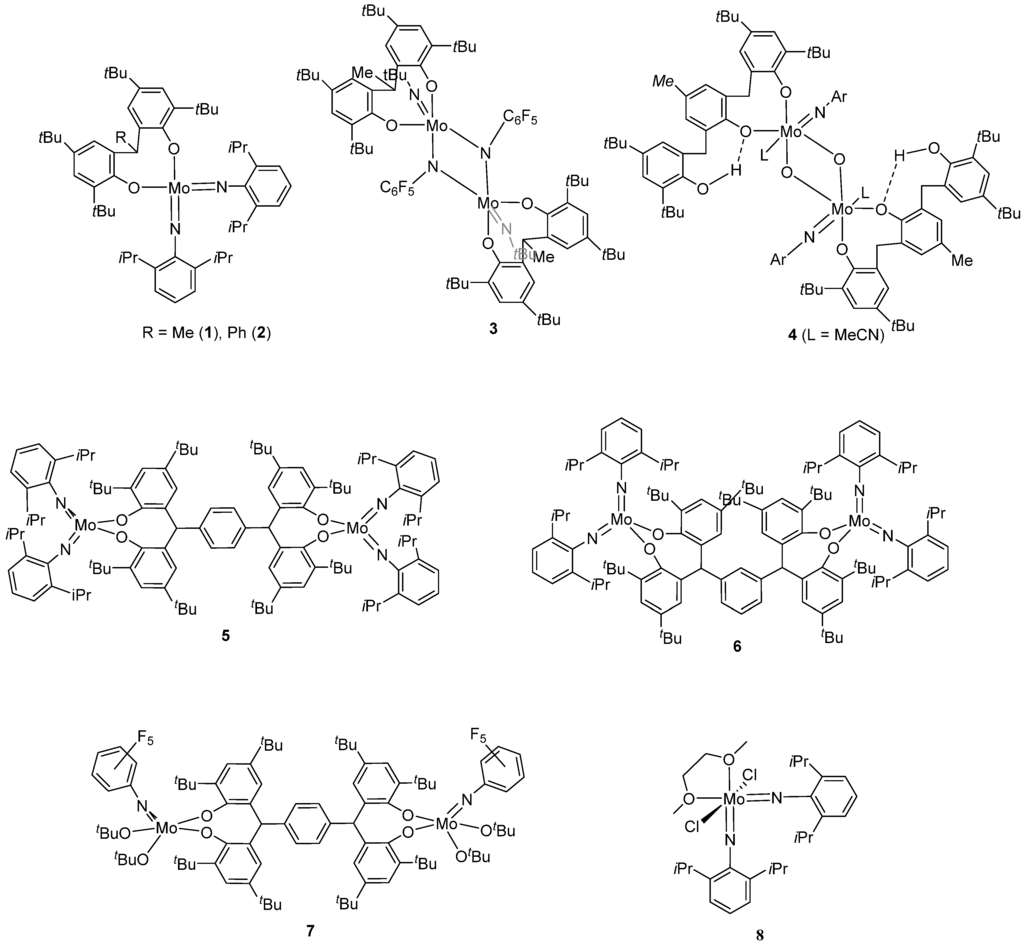
Figure 1.
Molecular structure of [Mo(NC6H3i-Pr2-2,6)2L1Me] (1), showing the atom numbering scheme. Selected bond lengths (Å) and angles (°): Mo(1)–N(1) 1.746(3), Mo(1)–N(2) 1.760(3), Mo(1)–O(1) 1.929(2), Mo(1)–O(2) 1.921(2); N(1)–Mo(1)–N(2) 113.30(14), N(1)–Mo(1)–O(2) 106.91(12), N(2)–Mo(1)–O(2) 104.93(12), N(1)–Mo(1)–O(1) 106.91(12), N(2)–Mo(1)–O(1) 106.00(12), Mo(1)–N(1)–C(31) 171.5(2), Mo(1)–N(2)–C(43) 158.7(3).
Similar use of the di-phenol 2,2′-C6H5CH[4,6-(t-Bu)2C6H2OH]2 (L1PhH2) led to the related complex [Mo(NC6H3i-Pr2-2,6)2L1Ph] (2), again in good yield (ca. 70%). In the 1H NMR spectrum, the CH bridge proton is found at δ 4.89 ppm (cf. 5.16 ppm in 1). Crystals of 2·CH2Cl2 suitable for X-ray analysis were grown from a saturated dichloromethane solution at 0 °C. The molecular structure is shown in Figure 2 with bond lengths and angles given in caption; crystallographic data is presented in Table 1.
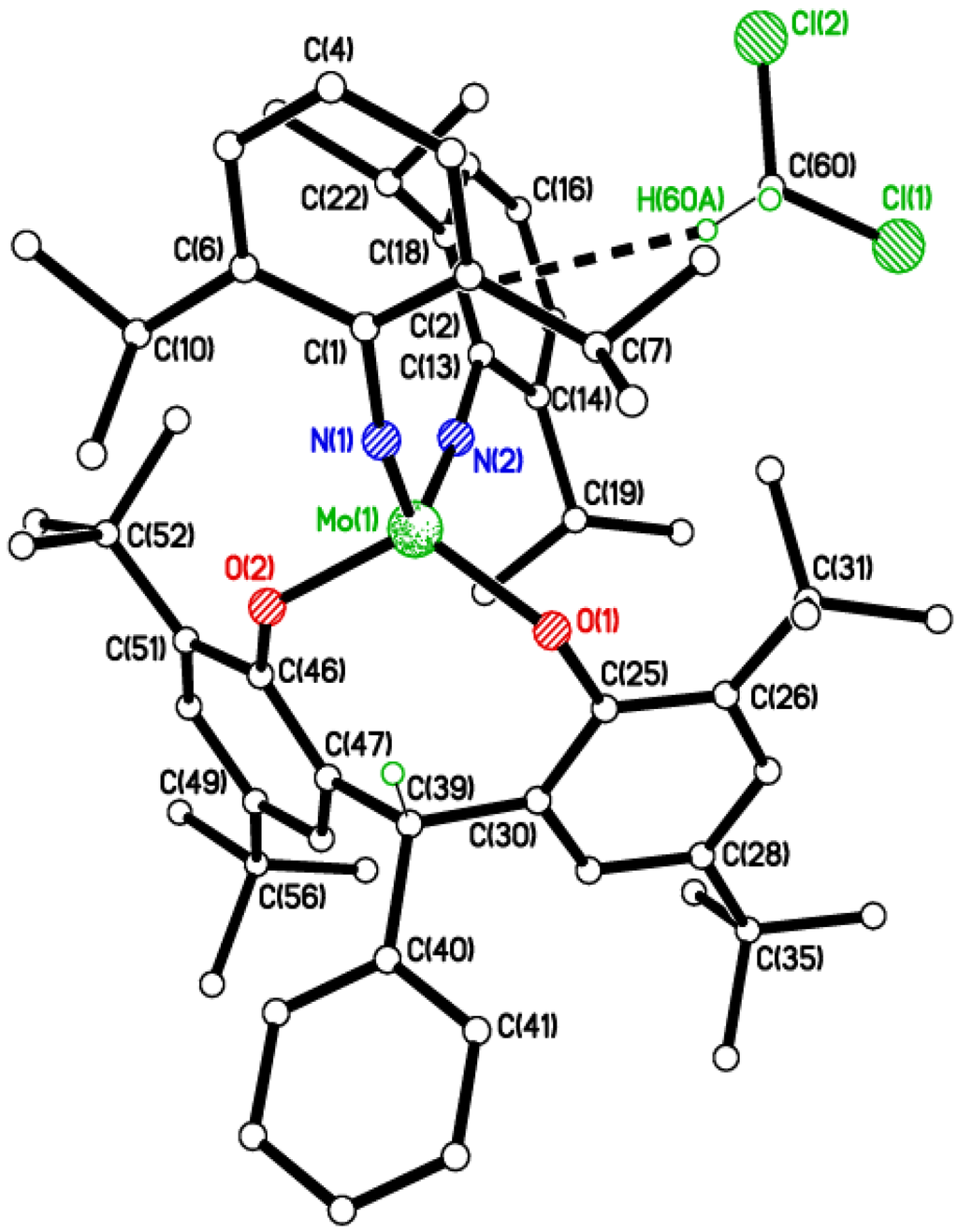
Figure 2.
Crystal structure of [Mo(NC6H3i-Pr2-2,6)2L1Ph]·CH2Cl2 (2·CH2Cl2), showing the atom numbering scheme. Selected bond lengths (Å) and angles (°): Mo(1)–N(1) 1.7610(17), Mo(1)–N(2) 1.7476(17), Mo(1)–O(1) 1.9280(14), Mo(1)–O(2) 1.921(2); N(1)–Mo(1)–N(2) 112.38(8), N(1)–Mo(1)–O(2) 104.24(7), N(2)–Mo(1)–O(2) 108.10(7) (7), N(1)–Mo(1)–O(1) 104.79(7), N(2)–Mo(1)–O(1) 107.78(7), O(1)–Mo(1)–O(2) 119.59(6), Mo(1)–N(1)–C(1) 157.23(14), Mo(1)–N(2)–C(13) 172.31(14).
As for 1, the metal centre has a distorted tetrahedral geometry, as seen in the bond angles for N(1)–Mo(1)–O(2) 104.24(7) and O(1)–Mo(1)–O(2) 119.59(6)°. Again, as in 1, the two imido groups are somewhat different, with Mo–N–C angles of 172.30(15) and 157.26(15)°. The eight membered ring chelate again adopts a flattened chair-like conformation; the “bite angle” of the chelate is 119.59(6)°. One solvent molecule of crystallization (dichloromethane) forms a C–H π interaction at 2.584(4) Å to the ring centroid C(13)–C(18). In contrast to the previous use of L1MeH2 [15,16,17,18,19,20,21], L1PhH2 has been relatively unexplored. Indeed, a search of the CSD revealed only one example (in titanium chemistry) [22,23].
Use of the mixed-imido precursor [Mo(Nt-Bu)(NC6F5)(Ot-Bu)2] with L1MeH2 led, following work-up, to the orange complex [Mo(Nt-Bu)(NC6F5)L1Me]2 (3), which was readily crystallized from a saturated acetonitrile solution on prolonged standing (2 days) at ambient temperature. The molecular structure (Figure 3) revealed that half of the complex comprises the asymmetric unit. The molecule lies on a centre of symmetry i, and possesses asymmetric imido (C6F5) bridges, the latter arising given the differing trans environments. The terminal tert-butylimido groups are near linear [Mo–N(1)–C(1) 178.0(2)°]. There is literature precedent for bending of C6F5N groups in preference to tert-butylimido groups when present in the same complex, which is attributed to the more electron-releasing nature of the latter [14]. Furthermore, bridging arylimido groups have been structurally characterized in a complex also containing linear, terminal tert-butylimido ligation [24]. The eight membered ring chelates each adopt a flattened chair-like conformation; the “bite angle” of the chelates are 117.20(9)°.
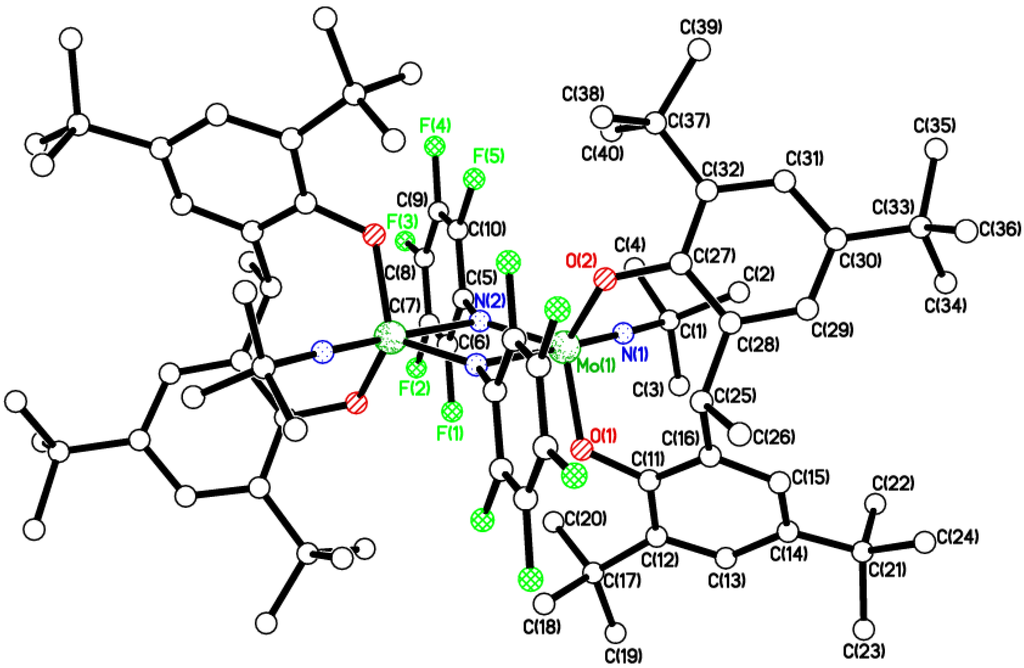
Figure 3.
Molecular structure of [Mo(Nt-Bu)(NC6F5)L1Me]2 (3), showing the atom numbering scheme. Selected bond lengths (Å) and angles (°): Mo(1)–N(1) 1.721(2), Mo(1)–N(2) 1.856(2), Mo(1)–O(1) 1.9324(19), Mo(1)–O(2) 1.9260(19); N(1)–Mo(1)–N(2) 101.29(11), N(1)–Mo(1)–O(2) 98.61(10), N(2)–Mo(1)–O(2) 116.77(9), N(1)–Mo(1)–O(1) 98.73(10), N(2)–Mo(1)–O(1) 117.94(10), O(1)–Mo(1)–O(2) 117.20(9), Mo(1)–O(1)–C(11) 122.77(18), Mo(1)–O(2)–C(27) 123.83(16), Mo(1)–N(1)–C(1) 178.0(2).
2.2. Tri-Phenolate Compound
When the tri-phenol 2,6-bis(3,5-di-tert-butyl-2-hydroxybenzyl)-4-methylphenol (L2H3) [25] was reacted with [Mo(NC6H3i-Pr2-2,6)2(Ot-Bu)2], the oxo-bridged complex [Mo(NC6H3i-Pr2-2,6)(NCMe) (μ-O)L2H]2·6MeCN (4·6MeCN) was isolated from a saturated acetonitrile solution on prolonged standing at ambient temperature. The presence of the oxo bridges was thought to be the result of fortuitous hydrolysis (also resulting in the elimination of aniline). The molecular structure of 4 is shown in Figure 4, with selected bond lengths and angles given in the caption. Half of the complex and three acetonitrile molecules comprise the asymmetric unit. The molecule resides on a centre of symmetry i, and possesses asymmetric oxo bridges. Each molybdenum centre exhibits a distorted octahedral environment, for example Mo(1) is 0.3349(6) Å out of the O4 plane. Of the three acetonitrile molecules of crystallization, two lie in clefts of the phenol/di-phenolate ligand, namely those solvent molecules containing N(4) and N(5); the other containing N(3) lies between molecules of 4. The bonding mode of the tri-phenol derived ligand in 4 is reminiscent of that observed for the tungsten(VI) complex [W(eg)2LaH] (eg = 1,2-ethanediolato, LaH = doubly deprotonated form of 2,6-bis(3,5-dimethyl -2-hydroxybenzyl)-4-t-butylphenol) and the niobium complexes [NbCl3(NCMe)LbH] and [NbCl(NCMe)La/bH]2 (LbH = doubly deprotonated form of 2,6-bis(4-methyl-6-t-butylsalicyl) -4-t-butylphenol [26,27]. The eight membered ring chelates adopts a boat-like conformation; the “bite angle” of the chelate is 93.43(7)°, which is much smaller than observed in 1–3 (ca. 119°) due to the higher coordination number of 6 as opposed to 4 or 5. In solution however, the 1H NMR spectrum (in CDCl3, C6D6 or CD3CN) of 4 contained only two resonances for the tert-butyl groups, which is not consistent with the unsymmetrical nature of the tri-phenol derived ligand observed in the solid state.
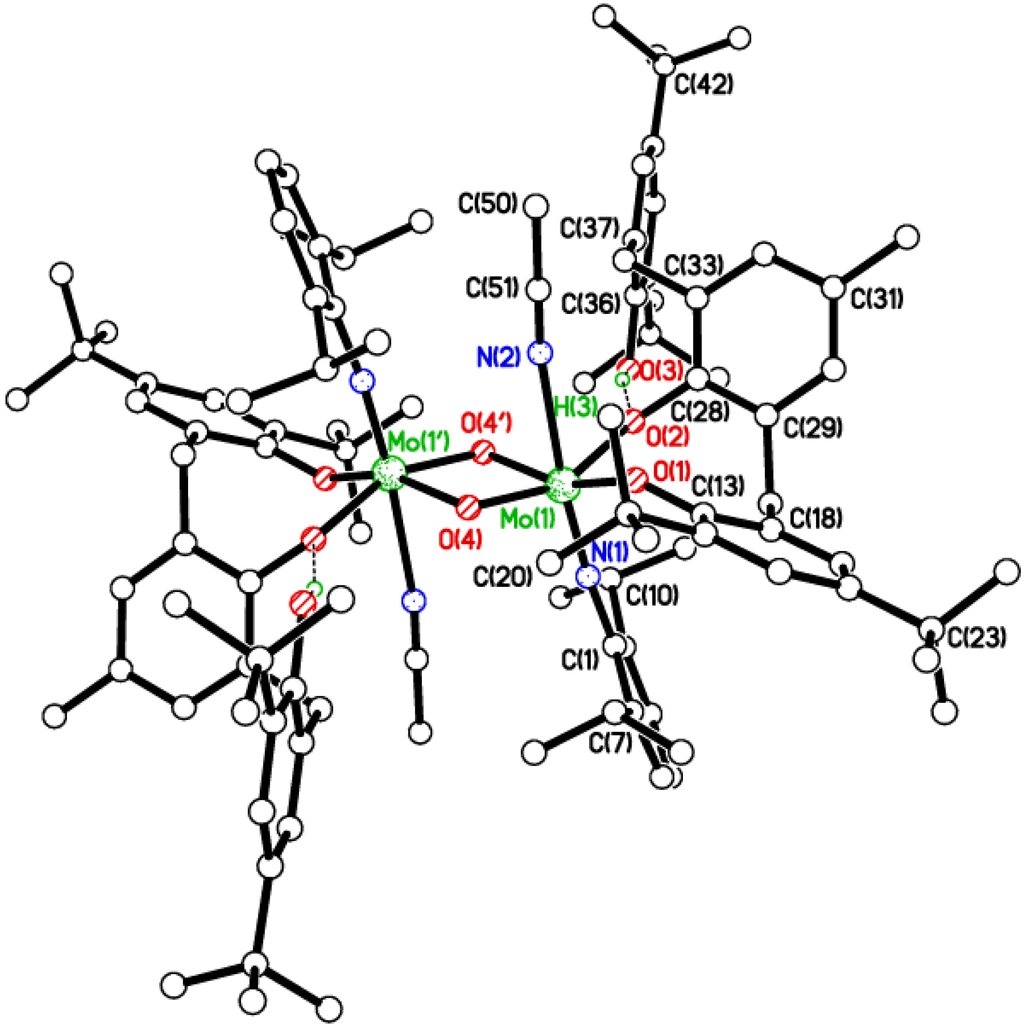
Figure 4.
Molecular structure of [Mo(NC6H3i-Pr2-2,6)(NCMe)(μ-O)L2H]2 (4), showing the atom numbering scheme. Six MeCN molecules of crystallization and most H atoms omitted for clarity. Selected bond lengths (Å) and angles (°): Mo(1)–O(1) 1.9072(16), Mo(1)–O(2) 2.0257(16), Mo(1)–O(4) 1.8688(16), Mo(1)–N(1) 1.728(2), Mo(1)–N(2) 2.400(2); O(1)–Mo(1)–O(2) 93.43(7), Mo(1)–O(1)–C(13) 145.46(15), Mo(1)–O(2)–C(28) 127.49(14), Mo(1)–N(1)–C(1) 174.59(17), Mo(1)-O(4)—Mo(1i) 100.56(7), Mo(1)–N(1)–C(1) 174.59(17).
2.3. Tetra-Phenolate Compounds
The synthetic methodology was then extended to the relatively unexplored tetra-phenols α,α,α′,α′-tetrakis(3,5-di-tert-butyl-2-hydroxyphenyl)-p-xylene L3pH4 and α,α,α′,α′-tetrakis(3,5-di-tert -butyl-2-hydroxyphenyl)-m--xylene L3mH4 [28]. Treatment of either L3pH4 or L3mH4 with [Mo(NC6H3i -Pr2-2,6)2(Ot-Bu)2] afforded tetra-imido complexes, namely {[Mo(NC6H3i-Pr2-2,6)2]2(μ-L3p)} (5) and {[Mo(NC6H3i-Pr2-2,6)2]2(μ-L3m)} (6), respectively in moderate to good yield. Red plate-like crystals of 6·2C6H14 suitable for an X-ray structure determination were obtained on recrystallization from a saturated hexane solution at 0 °C. The molecular structure of 6 is shown in Figure 5 with selected bond lengths and angles given in the caption; crystallographic data are given in Table 1. The molecule lies on a 2-fold axis that passes through the vector C(32)–C(33).

Table 1.
Crystallographic data for the complexes 1, 2·CH2Cl2, 3, 4·6MeCN, 6·2C6H14, 7·6MeCN and 8.
| Compound | 1 | 2·CH2Cl2 | 3 | 4·6MeCN | 6·2C6H14 | 7·6MeCN | 8 |
|---|---|---|---|---|---|---|---|
| Formula | C54H78MoN2O2 | C59H80MoN2O2·CH2Cl2 | C80H106F10Mo2N4O4 | C102H140Mo2N4O8·6(C2H3N) | C112H154Mo2N4O4·2(C6H14) | C92H122F10Mo2N2O8·6(C2H3N) | C28H44Cl2MoN2O2 |
| Formula weight | 883.12 | 1030.11 | 1569.56 | 1988.37 | 1984.61 | 2012.11 | 607.49 |
| Crystal system | Triclinic | Triclinic | Monoclinic | Triclinic | Monoclinic | Triclinic | Triclinic |
| Space group | P1 | P-1 | P21/n | P-1 | C2/c | Pī | Pī |
| Unit cell dimensions | |||||||
| a (Å) | 10.0435(8) | 11.7691(7) | 14.2727(8) | 13.0178(8) | 40.815(3) | 18.7522(10) | 10.0491(10) |
| b (Å) | 10.3143(8) | 14.1045(9) | 15.4944(8) | 13.4853(8) | 17.0938(11) | 22.6704(12) | 10.6022(11) |
| c (Å) | 12.8862(11) | 18.0657(12) | 18.0242(10) | 16.9177(10) | 16.3488(11) | 25.8469(18) | 15.6133(19) |
| α (º) | 74.5019(14) | 83.543(5) | - | 106.1133(10) | 90 | 79.933(6) | 92.902(9) |
| β (º) | 87.3130(14) | 81.066(5) | 98.4505(10) | 96.4972(10) | 94.8200(10) | 81.162(6) | 90.577(9) |
| γ (º) | 78.6415(14) | 74.829(5) | - | 92.7062(10) | 90 | 81.434(6) | 112.932(8) |
| V (Å3) | 1261.15(18) | 2851.1(3) | 3942.7(4) | 2825.3(3) | 11365.9(13) | 10605.9(11) | 1529.2(3) |
| Z | 1 | 2 | 2 | 1 | 4 | 4 | 2 |
| Temperature (K) | 150(2) | 150(2) | 150(2) | 150(2) | 100(2) | 100(2) | 150(2) |
| Wavelength (Å) | 0.6861 | 0.71073 | 0.71073 | 0.71073 | 0.71073 | 0.71073 | 0.71073 |
| Calculated density (g·cm−3) | 1.163 | 1.200 | 1.322 | 1.169 | 1.160 | 1.260 | 1.319 |
| Absorption coefficient (mm−1) | 0.27 | 0.37 | 0.39 | 0.28 | 0.27 | 0.31 | 0.63 |
| Transmission factors (min./max.) | 0.960 and 0.995 | 0.912 and 0.857 | 0.958 and 0.985 | 0.936 and 0.970 | 1.000 and 0.634 | 0.976 and 0.994 | 0.981 and 0.855 |
| Crystal size (mm3) | 0.15 × 0.12 × 0.02 | 0.50 × 0.45 × 0.45 | 0.11 × 0.07 × 0.04 | 0.24 × 0.15 × 0.11 | 0.18 × 0.09 × 0.04 | 0.08 × 0.05 × 0.02 | 0.40 × 0.38 × 0.06 |
| θ (max) (°) | 29.2 | 29.3 | 28.9 | 27.5 | 25.0 | 26.4 | |
| Reflections measured | 13007 | 30297 | 24317 | 25167 | 67463 | 101749 | 11706 |
| Unique reflections | 11751 | 15207 | 9273 | 13076 | 12964 | 36898 | 6145 |
| Rint | 0.031 | 0.0572 | 0.048 | 0.027 | 0.0710 | 0.182 | 0.0965 |
| Reflections with F2 > 2σ(F2) | 11618 | 11245 | 6286 | 10128 | 9692 | 14715 | 3429 |
| Number of parameters | 554 | 615 | 498 | 659 | 566 | 2420 | 326 |
| R1 (F2 > 2σ(F2)) | 0.038 | 0.042 | 0.044 | 0.043 | 0.044 | 0.084 | 0.063 |
| wR2 (all data) | 0.094 | 0.102 | 0.110 | 0.107 | 0.126 | 0.212 | 0.156 |
| GOOF, S | 1.01 | 0.91 | 1.03 | 1.03 | 1.02 | 0.86 | 0.89 |
| Largest difference peak and hole (e Å−3) | 0.75 and −0.49 | 1.11 and −1.31 | 0.46 and −0.46 | 0.70 and −0.54 | 0.67 and −0.47 | 0.88 and −2.06 | 0.55 and −0.95 |
Each molybdenum centre is four coordinate and exhibits a distorted tetrahedral geometry with angles in the range 105.30(7) to 115.06(5)°, the largest angle being associated with the chelate. Each eight membered ring chelate adopts a flattened chair-like conformation. The imido groups are categorized as linear, with that at N(1) lying to the lower end of the range associated with linearity [Mo–N(1)–C(34) 156.29(16)°]. The difference between the imido angles here in 6 is ca. 13.6°, which compares favorably with that in 1 (ca. 13.0°) and is slightly smaller than that observed in 2 (ca. 15.0°). There is tendency in these complexes for the shorter Mo–N bond length to be associated with the larger Mo–N–C angle; a similar situation has been observed in molybdenum and tungsten imido alkylidene chemistry [29,30,31,32]. The distance between Mo centers in this system is 10.588 Å.
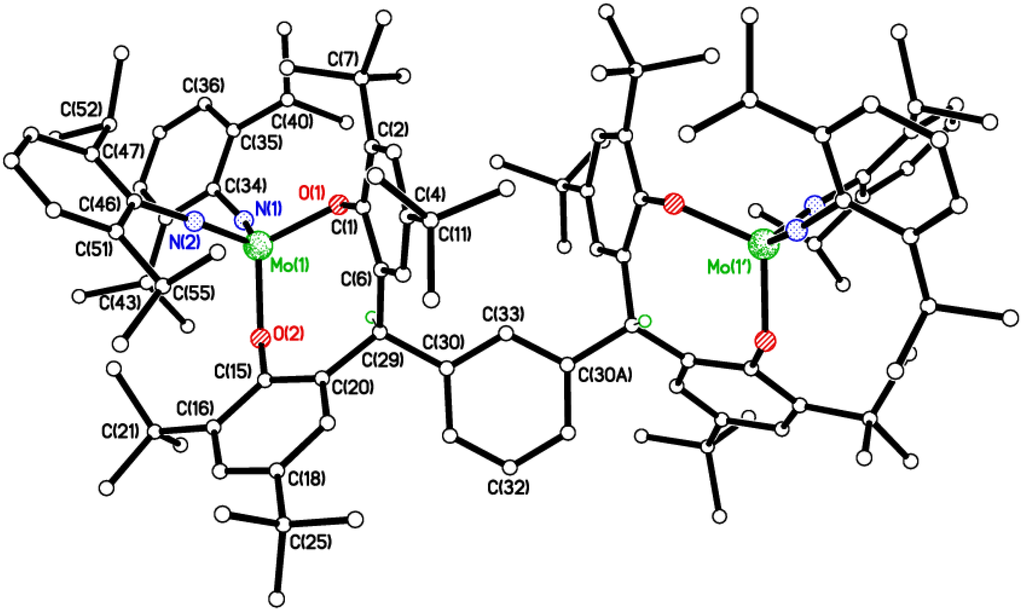
Figure 5.
Molecular structure of {[Mo(NC6H3i-Pr2-2,6)2]2(μ-L3m)} (6), showing the atom numbering scheme. H atoms and two disordered hexane molecules of crystallization have been omitted for clarity. Selected bond lengths (Å) and angles (°): Mo(1)–N(1) 1.7608(17), Mo(1)–N(2) 1.7490(19), Mo(1)–O(1) 1.9315(14), Mo(1)–O(2) 1.9294(13); N(1)–Mo(1)–N(2) 111.43(8), N(1)–Mo(1)–O(2) 106.83(7), N(2)–Mo(1)–O(2) 108.33(7), N(1)–Mo(1)–O(1) 105.30(7), N(2)–Mo(1)–O(1) 109.87(7), O(1)–Mo(1)–O(2) 115.06(5), Mo(1)–N(1)–C(34) 156.29(16), Mo(1)–N(2)–C(46) 169.98(15).
Surprisingly, when the imido precursor employed was [Mo(NC6F5)2(Ot-Bu)2] with L3pH4, the reaction proceeded via loss of aniline rather than alcohol, which must be due to the differing electronics associated with the C6F5 group. Crystals of {[Mo(NC6F5)(Ot-Bu)2]2(μ-L3p)}·6MeCN (7·6MeCN) were grown from a saturated acetonitrile solution. The molecular structure is shown in Figure 6, with selected bond lengths and angles given in the caption. Each molybdenum centre in 7 is five coordinate, bound by the chelate, one imido group and two tert-butoxide ligands. There are two similar metal complexes and twelve acetonitrile molecules in the asymmetric unit. The geometries at the metal can best be described as distorted trigonal pyramidal with the imido group and one of the chelate phenoxide oxygen atoms occupying axial positions. The imido group is slightly bent [Mo(1)–N(1)–C(65) 161.3(5)°] though is still considered linear. However, the two tert-butoxides are clearly bent [Mo(1)–O(5)–C(71) 142.8(5), Mo(1)–O(6)–C(75) 138.6(5)°] with slightly different bond lengths [Mo–O(5) 1.821(5), Mo(1)–O(6) 1.884(5)]; neither alkoxide is required to act as a three electron donor to attain an overall eighteen electron count. Chisholm and coworkers have noted a correlation between M–OR bond distances and M–O–C angles [33].
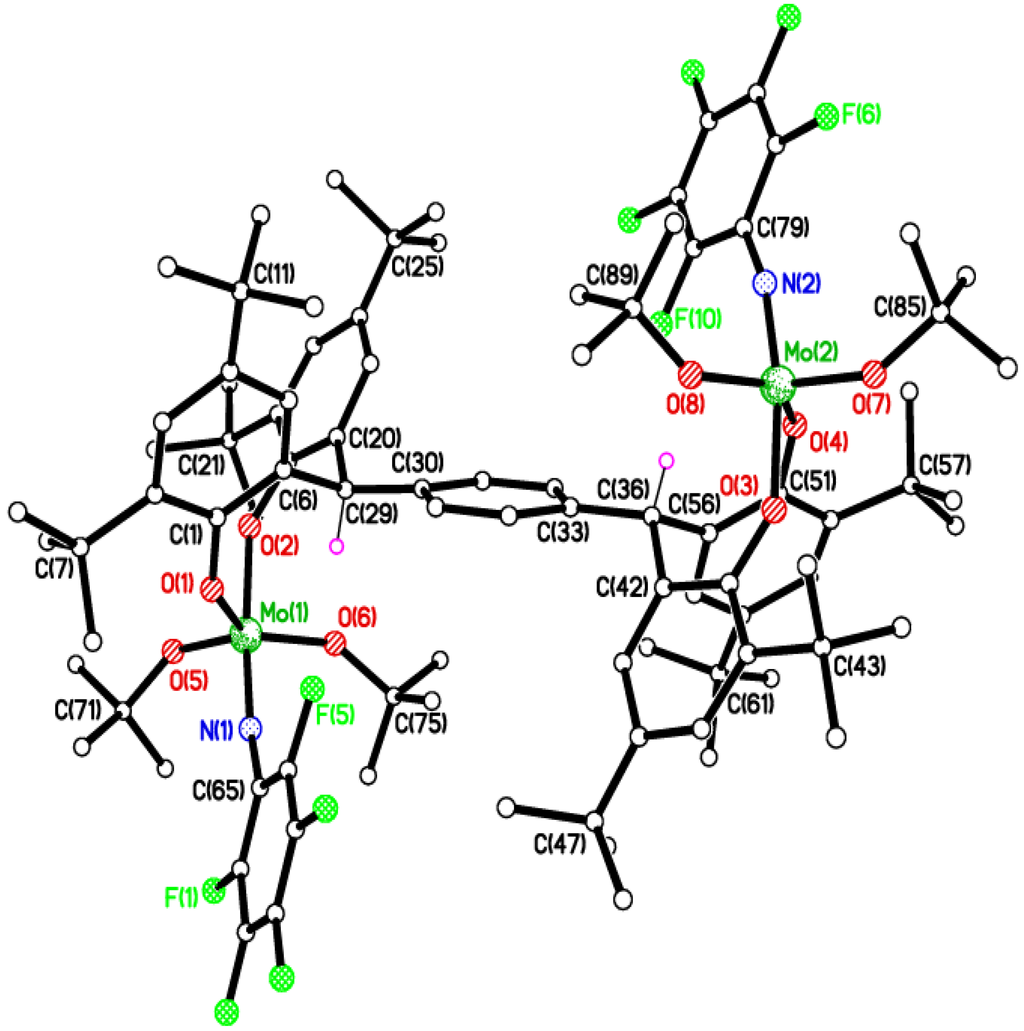
Figure 6.
Molecular structure of {[Mo(NC6F5)(Ot-Bu)2]2(μ-L3p)}·6MeCN (7·6MeCN), showing the atom numbering scheme for one of the two similar molecules in the asymmetric unit. Most H atoms and solvent molecules of crystallization omitted for clarity. Selected bond lengths (Å) and angles (°): Mo(1)–O(1) 1.921(5), Mo(1)–O(2) 1.945(5), Mo(1)–N(1) 1.766(6), Mo(1)–O(5) 1.821(5), Mo(1)–O(6) 1.884(5); Mo(1)–N(1)–C(65) 161.3(5), Mo(1)–O(1)–C(1) 141.1(5), Mo(1)–O(2)–(15) 146.6(4), O(1)–Mo(1)–O(2) 85.3(2), Mo(1)–O(5)–C(71) 142.8(5), Mo(1)–O(6)–C(75) 138.6(5), O(5)–Mo(1)–O(6) 112.6(2), N(1)–Mo(1)–O(2) 168.6(2) Mo(1)–N(1)–C(65) 161.3(5), Mo(2)–N(2)–C(79) 157.9(6).
The molecular structure of the complex [Mo(NC6H3i-Pr2-2,6)2Cl2(dme)] (8) has also been determined and is given in the (see ESI, Figure S1, Tables S1 and S2). A number of such mononuclear bis(imido) dichloro molybdenum(VI) complexes have been structurally characterized; a search of the CSD revealed 14 hits [22].
2.4. Polymerization Screening
Complexes 1–8 have been screened for their ability to act as catalysts for the ring opening polymerization (ROP) of ε-caprolactone and the results are presented in Table 2. At temperatures below 80 °C, the systems were inactive. At 80 °C, the systems utilizing 1, 2, 6 and 7 exhibited moderate activity with conversions of about 45%–50%, whilst the combination of 8/BnOH exhibited good conversion (ca. 85%). At 100 °C, there was little or no activity for reaction times of less than one hour. In most cases, excellent conversions were achieved over 6 h, and little was gained by prolonging the reaction time beyond this point. Although the complexes 1–7 are phenolates (and 7 also a tert-butoxide), we have screened them both in the presence and absence of benzyl alcohol (BnOH) to monitor if this is beneficial or not. The presence or absence of BnOH had little effect on %conversion or control (eg runs 30 v 31 and 39 v 40), though depending on the temperature there was either an increase or decrease in the observed molecular weight (Mn). All systems produced polyesters with narrow dispersities with unimodal characteristics (Mw/Mn 1.08 to 1.72); those at the higher end of the range were associated with increases in the CL:cat:BnOH ratio (runs 17, 19 and 20) and are perhaps indicative of some transesterification reactions occurring under such conditions. Such ratio changes (for 3) however led to little change in the %conversion.
In terms of structure-activity relationships, in the case of 1 versus 2, the presence of the bulkier phenyl group in the bridge of the di-phenol appears to have only a slight effect with 1 exhibiting a better conversion at 100 °C over 6 h (99% cf. 95%). The bridging imido complex 3 exhibits activity on a par with 1 containing the same di-phenolate ligand. Analysis of the results for the tetra-phenolate systems 5 to 7 indicates that at 100 °C over various reaction times, the meta system out-performs the para system (i.e., 5 v 6; runs 24 v 29, 26 v 32, 27 v 33), which is tentatively assigned to the closer proximity of the metal centers in 6 and thus an enhanced cooperative effect. A comparison of the use of different imido group in the meta system (6 v 7) is not possible given the different structures adopted, however it is evident that meta system 7 is comparable with 6 over 6 or 12 h and is superior over shorter reaction periods (runs 35 and 36). There appears to be no advantage in having two metals present over one (5, 6 cf. 1, 2). Interestingly, the bis(imido) dichloride complex 8, in the presence of BnOH, also exhibits excellent conversions at 100 °C when employed for 3 h or more.
In general, the observed polymer molecular weights were lower than expected, which indicates that in most cases, there were significant trans-esterification reactions occurring. Such a trend has been noted previously when using molybdenum-based species [6,7,8,9,10,11,12]. The MALDI-ToF spectra of the resultant PCL revealed (see ESI Figure S2) a major series of peaks with separation 114 g·mol−1 (i.e., the monomer) with evidence of a secondary minor set of peaks resulting from hydrolysis under ionization conditions. Examination of the 1H NMR spectrum (see ESI Figure S3) of the same samples revealed peaks at δ 5.10 and 3.65 assigned to benzyl ester and hydroxymethylene end groups. Interestingly for 3, a plot of number average molecular weight (Mn) and monomer conversion was approximately linear, which was suggestive of a well-controlled polymerization, this despite the apparent trans-esterification processes present (see ESI Figure S4).
Comparison of these systems with other molybdenum-based catalysts reveals that it is typical for high temperatures (≥80 °C) to be employed to achieve activity. Neutral chelate complexes derived from the oxydianiline [(2-NH2C6H4)2O] can achieve good conversion rates (>90%) at high temperatures (100 °C) over 12h; the tetra-nuclear siloxide complex [Mo4Cl3(NtBu)3(OSiMe3)(μ4-O)(L)2(L′)2] (where L = (2-NC6H4)O, L′ = (2-NH2C6H4)(2-NC6H4)O) performed best achieving a conversions >90% over 1 h [6]. Of the other Mo-based systems known, bis(salicylaldehydato)dioxomolydenum operates effectively at 110 °C in mesitylene, whilst ammonium decamolybdate functions as a melt at 150 °C [9,12]. As observed herein, such molybdenum systems are susceptible to trans-esterification processes.

Table 2.
Ring opening polymerization of ɛ-CL using complexes 1–8.
| Entry | Cat. | CL:Mo:BnOH | Temp./°C | Time/h | Conversion (%) a | Mn,GPC b | Mn,Cal c | PDI d |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 250:1:1 | 80 | 0.5 | - | - | - | - |
| 2 | 1 | 250:1:1 | 100 | 0.5 | - | - | - | - |
| 3 | 1 | 250:1:1 | 100 | 1 | 70 | 4.62 | 20.08 | 1.41 |
| 4 | 1 | 250:1:0 | 80 | 3 | 45 | 3.77 | 12.84 | 1.17 |
| 5 | 1 | 250:1:1 | 100 | 3 | 86 | 5.53 | 24.64 | 1.22 |
| 6 | 1 | 250:1:1 | 100 | 6 | 99 | 6.40 | 28.35 | 1.40 |
| 7 | 1 | 250:1:1 | 100 | 12 | 99.5 | 10.67 | 28.50 | 1.40 |
| 8 | 2 | 250:1:1 | 100 | 0.5 | - | - | - | - |
| 9 | 2 | 250:1:1 | 100 | 1 | 96 | 5.23 | 27.50 | 1.29 |
| 10 | 2 | 250:1:0 | 80 | 3 | 51 | 2.19 | 14.55 | 1.19 |
| 11 | 2 | 250:1:1 | 100 | 3 | 89 | 7.14 | 25.50 | 1.25 |
| 12 | 2 | 250:1:1 | 100 | 6 | 95 | 7.24 | 27.21 | 1.22 |
| 13 | 2 | 250:1:1 | 100 | 12 | 99 | 10.33 | 28.35 | 1.57 |
| 14 | 3 | 250:1:0 | 80 | 3 | - | - | - | - |
| 15 | 3 | 250:1:1 | 100 | 3 | 55 | 2.14 | 15.80 | 1.11 |
| 16 | 3 | 250:1:1 | 100 | 6 | 99 | 6.74 | 28.35 | 1.17 |
| 17 | 3 | 125:1:1 | 100 | 12 | 99 | 8.51 | 14.23 | 1.60 |
| 18 | 3 | 250:1:1 | 100 | 12 | 99 | 9.73 | 28.35 | 1.46 |
| 19 | 3 | 500:1:1 | 100 | 12 | 99.5 | 13.00 | 56.89 | 1.72 |
| 20 | 3 | 1000:1:1 | 100 | 12 | 98 | 16.14 | 111.96 | 1.72 |
| 21 | 4 | 250:1:1 | 100 | 12 | 99 | 7.80 | 28.35 | 1.25 |
| 22 | 5 | 250:1:0 | 80 | 3 | - | 0.68 | - | 1.22 |
| 23 | 5 | 250:1:1 | 30 | 24 | - | - | - | - |
| 24 | 5 | 250:1:1 | 100 | 1 | 45 | 0.822 | 12.94 | 1.23 |
| 25 | 5 | 250:1:1 | 100 | 3 | 98 | 2.84 | 28.07 | 1.23 |
| 26 | 5 | 250:1:1 | 100 | 6 | 93 | 3.13 | 26.64 | 1.17 |
| 27 | 5 | 250:1:1 | 100 | 12 | 98 | 4.39 | 28.07 | 1.17 |
| 28 | 6 | 200:1:1 | 100 | 0.5 | - | - | - | - |
| 29 | 6 | 200:1:1 | 100 | 1 | 68 | 5.43 | 19.51 | 1.13 |
| 30 | 6 | 250:1:0 | 80 | 3 | 50 | 1.93 | 14.26 | 1.24 |
| 31 | 6 | 250:1:1 | 80 | 3 | 48 | 3.75 | 13.80 | 1.22 |
| 32 | 6 | 250:1:1 | 100 | 6 | 100 | 7.15 | 28.64 | 1.34 |
| 33 | 6 | 250:1:1 | 100 | 12 | 100 | 7.27 | 28.64 | 1.26 |
| 34 | 7 | 250:1:0 | 80 | 3 | - | - | - | - |
| 35 | 7 | 250:1:1 | 100 | 0.5 | - | - | - | - |
| 36 | 7 | 250:1:0 | 100 | 1 | 95 | 2.14 | 27.10 | 1.11 |
| 37 | 7 | 250:1:0 | 100 | 3 | 98 | 2.25 | 27.96 | 1.08 |
| 38 | 7 | 250:1:0 | 100 | 6 | 99.5 | 8.79 | 28.39 | 1.33 |
| 39 | 7 | 250:1:0 | 100 | 12 | 99 | 9.11 | 28.24 | 1.37 |
| 40 | 7 | 250:1:1 | 100 | 12 | 99 | 6.10 | 28.35 | 1.46 |
| 41 | 8 | 250:1:1 | 80 | 3 | 85 | 2.64 | 24.36 | 1.21 |
| 42 | 8 | 250:1:1 | 100 | 3 | 99 | 5.97 | 28.35 | 1.19 |
| 43 | 8 | 250:1:1 | 100 | 6 | 98 | 8.21 | 28.07 | 1.36 |
| 44 | 8 | 250:1:1 | 100 | 12 | 100 | 14.32 | 28.64 | 1.46 |
a By 1H NMR analysis; b × 10−3, obtained from GPC analysis times 0.56 × 10−3; c F.W. (Monomer).[M]/[BnOH](conversion)10−3; d from GPC.
3. Experimental Section
3.1. General
All manipulations were carried out under an atmosphere of dry nitrogen using conventional Schlenk and cannula techniques or in a conventional nitrogen-filled glove box. Diethylether, toluene and acetonitrile were refluxed over sodium and benzophenone. Dichloromethane and acetonitrile were refluxed over calcium hydride. All solvents were distilled and degassed prior to use. IR spectra (nujol mulls) were recorded on a Nicolet iS5 FT IR spectrometer, Thermo Fisher Scientific, Madison, USA; 1H NMR spectra were recorded at room temperature on a JNM LA-400 spectrometer, JEOL, Tokyo, Japan or a JNM ECP400 spectrometer, JEOL, Tokyo, Japan. The 1H NMR spectra were calibrated against the residual protio impurity of the deuterated solvent. Elemental analyses were performed by the elemental analysis service at the London Metropolitan University. The pro-ligands 2,6-bis(3,5-di-tert-butyl-2-hydroxybenzyl)-4-methylphenol (L2H3), α,α,α′,α′-tetrakis(3,5-di-tert-butyl -2-hydroxyphenyl)-p-xylene (L3pH4),α,α,α′,α′-tetra(3,5-di-tert-butyl-2-hydroxyphenyl–m-)xylene -meta-tetraphenol (L3mH4) and 2,2′-C6H5CH[4,6-(t-Bu)2C6H2OH]2 (L1PhH2) were prepared as described in the literature [23,27]. The complex [Mo(NC6H3i-Pr2-2,6)2Cl2(dme)] was prepared as described in the literature [34]. Pro-ligand 2,2′-CH3CH[4,6-(t-Bu)2C6H2OH]2 (L1MeH2) was purchased commercially and dried in-vacuo prior to use.
3.2. Synthesis of [Mo(NC6H3i-Pr2-2,6)2L1Me] (1)
A mixture of [Mo(NC6H3i-Pr2-2,6)2(Ot-Bu)2] (0.94 g, 1.6 mmol) and L1MeH2 (0.70 g, 1.6 mmol) were stirred in diethyl ether (ca. 20 mL) for 1 h. The volatiles were removed under reduced pressure and the residue taken-up in diethyl ether (ca. 20 mL). This cycle was repeated three times before the residue was extracted with hot heptane (ca. 30 mL). Yellow prisms of the product were deposited on cooling to room temperature. Yield 0.62 g, 44%. Further crops of 1 can be obtained from the mother-liquor; overall yield 70%. Found: C, 72.89; H, 9.01; N, 3.05. MoN2O2C54H78 requires C, 73.44; H, 8.90; N, 3.17. IR: 2369w, 1587w, 1322m, 1262s, 1221s, 1153m, 1130s, 1105s, 1020s, 982m, 932w, 901w, 882w, 827s, 752s, 730m, 691w, 630w, 608w, 578m. 537w, 455w. 1H NMR (CDCl3, 400 MHz): δ 7.64 (d, 2H, 3JHH = 2.4 Hz, arylH), 7.17–6.66 (several m, 8H, arylH), 5.16 (q, 1H, JHH = 6.8 Hz, CH), 3.72 (sept, 2H, 3JHH = 6.8 Hz, CH(CH3)2), 2.75 (sept(br), 2H, 3JHH = 6.8 Hz, CH(CH3)2), 1.66 (d, 3H, JHH = 6.8 Hz, CH3), 1.28 (s, 18H, (CH3)3C), 1.24 (d, 12H, JHH = 7.2 Hz, (CH3)2CH), 1.19 (s, 18H, (CH3)3C), 0.3 (d, 12H, 3JHH = 6.8 Hz, (CH3)2CH). M.S. (EI+): 884 (MH+), 707 (MH+ − ArNH2).
3.3. Synthesis of [Mo(NC6H3i-Pr2-2,6)2L1Ph] (2)
The compounds [Mo(NC6H3i-Pr2-2,6)2(Ot-Bu)2] (1.00 g, 1.6 mmol) and L1PhH2 (0.84 g, 1.6 mmol) in pentane (30 cm3) were refluxed for 12 h. On cooling, the volatiles were removed under reduced pressure and the residue was taken up in CH2Cl2 (20 cm3). Orange-yellow prisms of the product were deposited upon standing at room temperature (2 days). Yield 0.85 g, 47%. Further crops of 2 can be obtained on concentrating and cooling of the mother liquor, overall yield ca. 70%. Found: C, 75.02; H, 8.50; N, 2.95, C59H80MoN2O2 (sample dried in-vacuo for 2 h) requires C, 74.97; H, 8.53; N, 2.96%. 1H NMR (C6D6, 400 MHz): δ 7.83 (d, 2H, JHH = 2.4 Hz, arylH, 7.43–6.78 (several m, 8H, arylH), 4.89 (s, 1H, CH), 4.07 (sept, 2H, JHH = 6.8 Hz, CH(CH3)2), 3.20 (sept(br), 2H, CH(CH3)2, 1.46 (s, 18H, (CH3)3C), 1.15 (d, 12H, JHH = 6.8 Hz, (CH3)2CH), 1.33(s, 18H, (CH3)3C), 0.65 (d, 12H, JHH = 6.8 Hz, (CH3)2CH). IR: 2350w, 1567w, 1323m, 1262s, 1221s, 1152m, 1120s, 1100s, 1036s, 979m, 933w, 910w, 882w, 828s, 756s, 749m, 705w, 664w, 607w, 574m. M.S. (EI+): 945 (M+).
3.4. Synthesis of [Mo(Nt-Bu)(μ-NC6F5)(L1Me)]2 (3)
As for 1, but using [Mo(NC6F5)(Nt-Bu)Cl2(dme)] (1.00 g, 2.02 mmol), LiOt-Bu (0.33 g, 4.1 mmol) and 2,2′-CH3CH[4,6-(t-Bu)2C6H2OH]2 (0.89 g, 2.0 mmol). Extraction into acetonitrile or dichloromethane and standing at ambient temperature for 2 days afforded 3 as yellow/orange prisms in ca. 30%–35% yield. Found: C, 59.59; H, 6.73; N, 3.31. C80H106F10Mo2N4O4·½CH2Cl2 requires C, 59.97; H, 6.69; N, 3.48. IR: 1506s, 1495s, 1485vs, 1406w, 1374w, 1361w, 1311w, 1293w, 1261m, 1243m, 1217s, 1185m, 1164m, 1150m, 1127m, 1101m, 1023s, 991s, 904w, 876w, 860w, 831m, 802m, 765w, 760m, 750m, 727m, 692w, 663w, 634w, 600m, 574s. 1H NMR (CDCl3): δ 7.25 (d, 2H, JHH obsc by solvent = 2.3 Hz, arylH), 7.20 (d, 4H, JHH = 2.3 Hz, arylH), 7.03 (d, 1H, JHH = 2.5 Hz, arylH), 6.99 (d, 1H, JHH = 2.5 Hz, arylH),4.45 (q, 2H, JHH = 5.4 Hz, CH), 1.69 (d, 3H, JHH = 5.4 Hz, CH3), 1.36 (s, 36H, (CH3)3C), 1.30 (s, 36H, (CH3)3C), 0.07 (s, 18H, imido (CH3)3C). 19F NMR (С6D6): δ −163.25 (m, 4F,o-F),−165.82(m,4F,m-F), −174.58 (m, 2F, p-F). M.S. (Electrospray): 1387.5 (M+ − C6F5NH2), 1241 (M+ − C6F5NH2 − 2t-BuNH2).
3.5. Synthesis of [Mo(NC6H3i-Pr2-2,6)(NCMe)(μ-O)L2H]2 (4)
As for 1, but using [Mo(NC6H3i-Pr2-2,6)2(Ot-Bu)2] (1.00 g, 1.6 mmol) and L2H3 (0.87 g, 1.6 mmol). The product was crystallized from a saturated acetonitrile solution on prolonged standing at −20 °C. Yield 1.13 g, 71%. Found: C, 69.33; H, 8.08; N, 3.45. C102 H140 Mo2 N4 O8 (sample dried in-vacuo–5MeCN) requires C, 70.05; H, 8.08; N, 3.93. IR: 2726w, 2681w, 1658w, 1570w, 1463s, 1377s, 1306w, 1261s, 1232w, 1201w, 1093bs, 1020bs, 920w, 876w, 859w, 800s, 721w, 660w, 617w. 1H NMR (CDCl3): δ 8.04–6.64 (overlapping m, 18H, arylH), 5.45 (bm, 4H, CH2), 3.86 (br s, 4H, CH2), 2.74 (br sept, 4H, CH(CH3)2), 2.23 (s, 6H, CH3), 2.01(s, 6H, CH3CN), 1.42 (s, 36H, (CH3)3C), 1.27 (overlapping signals, 60H, (CH3)3C + CH(CH3)2); OH not observed. M.S. (MALDI): 1505.7 (M+ − 7MeCN − ArNH2 − H2O).
3.6. Synthesis of {[Mo(NC6H3i-Pr2-2,6)]2(μ-L3p)} (5)
To the compounds [Mo(NC6H3i-Pr2-2,6)2(Ot-Bu)2] (0.84 g, 2.2 mmol) and L3pH4 (1.00 g, 1.09 mmol) was added diethyl ether (30 mL). After stirring for 10 mins, the volatiles were removed in-vacuo, and the process was repeated three times. The residue was then extracted into acetonitrile solution and on prolonged standing at −20 °C crystals of 5 were formed. Yield 0.79 g, 40%. Found: C, 73.78; H, 9.06; N, 2.79%. C112H154Mo2N4O4 requires C, 74.12; H, 8.49; N, 3.09%. IR: 2725w, 2359w, 2340w, 1620w, 1568w, 1461s, 1377s, 1324w, 1261w, 1221m, 1152w, 1099m, 1021w, 933w, 909s, 871s, 799s, 753w, 742w, 722w. 1H NMR (C6D6, 400 MHz): δ 7.80–6.89 (several m, 24H, arylH), 5.50 (s, 1H, CH), 4.90 (s, 1H, CH), 2.64 (sept, 8H, JHH = 6.8 Hz CH(CH3)2), 1.51 (s, 38H, (CH3)3C), 1.21 (s, 38H, (CH3)3C), 1.15 (d, 24H, JHH = 6.8 Hz, (CH3)2CH), 1.13 (d, 24H, JHH = 6.8 Hz, (CH3)2CH). M.S. (MALDI): 1126 (M+ − 3H2NC6H3i-Pr2-2,6 – t-Bu – Mo).
3.7. Synthesis of {[Mo(NC6H3i-Pr2-2,6))]2(μ-L3m)} (6)
As for 5, but using [Mo(NC6H3i-Pr2-2,6))2(Ot-Bu)2] (1.26 g, 3.27 mmol) and L3mH4 (1.50 g, 1.63 mmol). Yellow prisms of the product 6 were deposited on cooling to room temperature. Yield 1.40 g, 47.7%. Further crops can be obtained from the mother-liquor; overall yield 70%. Found: C, 73.94; H, 8.51; N, 2.88%. C112H154Mo2N4O4 (sample dried in-vacuo for 12 h, -2C6H14) requires C, 74.12; H, 8.49; N, 3.09%. IR: 2345w, 1541w, 1321w, 1281w, 1220m, 1139m, 1053w, 981m, 930s, 903s, 852s, 760w, 747m. 1H NMR (CDCl3, 400 MHz): δ 7.31–6.63 (several m, 24H, arylH), 5.56 (s, 1H, CH), 4.71 (s, 1H, CH), 2.95 (sept, 8H, JHH 6.6 Hz CH(CH3)2), 1.35 (s, 38H, (CH3)3C), 1.28 (d, 24H, JHH = 6.6 Hz, (CH3)2CH), 1.26 (d, 24H, JHH = 6.6 Hz, (CH3)2CH), 1.14 (s, 38H, (CH3)3C). M.S. (Electrospray): 1152 (M+ − 3ArNH2 − 2t-Bu).
3.8. Synthesis of {[Mo(NC6F5)(Ot-Bu)2]2(μ-L3p)} (7)
As for 5, but using [Mo(NC6F5)2(Ot-Bu)2] (1.32 g, 2.18 mmol) with L3pH4 (1.00 g, 1.09 mmol) affording 7 as orange colored prisms (1.23 g, 56%) on recrystallization from acetonitrile or dichloromethane. Found: C, 57.18; H, 6.40; N, 1.68. C92H122Mo2F10N2O8·2½CH2Cl2 requires C, 57.40; H, 6.47; N, 1.42%. IR: 2726w, 2360w, 2341w, 1590w, 1571w, 1463s, 1377s, 1324w, 1261s, 1236w, 1214w, 1199w, 1150w, 1102m, 1021m, 958w, 914w, 870m, 760w, 721m. 1H NMR (CDCl3): δ 7.26–6.45 (overlapping m, 12H, arylH), 5.61 (s, 1H, CH), 4.72 (s, 1H, CH), 1.41 (s, 36H, (CH3)3C), 1.36 (s, 18H, (CH3)3CO), 1.14 (s, 18H, (CH3)3CO), 1.09 (s, 36H, (CH3)3C). 19F NMR (С6D6): δ − 163.29 (m, 4F, o-F), −165.82 (m, 4F, m-F), −172.83 (m, 2F, p-F). M.S. (Electrospray): 1583 (M+ − C6F5NH2), 1509 (M+ − C6F5NH2 − t-BuOH), 1435 (M+ − C6F5NH2 − 2t-BuOH), 1361 (M+ − C6F5NH2 − 3t-BuOH), 1287 (M+ − C6F5NH2 − 4t-BuOH).
3.9. Procedure for ROP
Typical polymerization procedures in the presence of one equivalent of benzyl alcohol (Table 1, run 1) are as follows. A toluene solution of 1 (0.010 mmol, in 1.0 mL toluene) and BnOH (0.010 mmol) were added into a Schlenk tube in the glove-box at room temperature. The solution was stirred for 2 min, and then ε-caprolactone (2.5 mmol) along with 1.5 mL toluene was added to the solution. The reaction mixture was then placed into an oil bath pre-heated to the required temperature, and the solution was stirred for the prescribed time. The polymerization mixture was then quenched by addition of an excess of glacial acetic acid (0.2 mL), and the resultant solution was then poured into methanol (200 mL). The resultant polymer was then collected on filter paper and was dried in vacuo.
3.10. Crystallography
X-ray diffraction data for 1 were collected using synchrotron radiation at Daresbury Laboratory, Station 9.8, using silcon 111 monochromated radiation and a Bruker 1K CCD detector. X-ray diffraction data for 2 and 8 were collected using a Stoe & Cie GmbH, Darmstadt, Germany. Diffraction data for 3 and 4·6MeCN were collected on a Bruker AXS GmbH, Karlsruhe, Germany. Diffraction data for 6·2C6H14 and 7·6MeCN were collected on a Rigaku Corp., Tokyo, Japan. All data collections except that for 1 utilised monochromated Mo-Kα radiation and ω-scans. Standard procedures were employed for the integration and processing of the data. Crystals were coated in a thin film of perfluoropolyether oil and mounted at the tip of a glass fibre (MiTeGen mount for 6·2C6H14 and 7·6MeCN) located on a goniometer. All data sets were collected from crystals at low temperature using an Oxford Cryosystems, Long Hanborough, Oxfordshire, UK. Crystal structures were solved using routine automatic direct methods implemented within SHELXS-97 [35] or iterative charge-flipping methods (SHELXT) [36]. Completion of structures was achieved by performing least squares refinement against all unique F2 values using SHELXL-2014 [36]. All non-H atoms were refined with anisotropic displacement parameters. Hydrogen atoms were placed using a riding model except for H(3) in 4·6MeCN for which the coordinates were freely refined. The Platon SQUEEZE routine was used to model regions of disordered hexane solvent of crystallisation (2 molecules per complex) in 6·2C6H14 and MeCN (2.5 per complex) in 7·6MeCN [37].
CCDC 1425489-1425495 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
4. Conclusions
In summary, a new family of molybdenum(VI) phenolate complexes have been structurally characterized and exploited as catalysts for the ring opening polymerization of ε-caprolactone. The ROP proceeded in a controlled fashion in terms of polydispersity, but polymer molecular weights (Mn) were lower than calculated values; MALDI-ToF spectra indicated a degree of trans-esterification was taking place. The ROP process using these Mo-based catalysts required high temperature (≥100 °C) and prolonged reaction times (≥1 h). The ROP results were suggestive of some structure-activity relationships, for example it was beneficial to employ a ligand set derived from a meta tetra-phenol rather than a para tetra-phenol, presumably as this brought the metals into closer proximity. However, there seemed to be no advantage gained by employing a complex containing more than one molybdenum centre.
Supplementary Files
Supplementary File 1Author Contributions
Yahya Al-Khafaji performed some of the synthetic work and carried out the ROP catalysis; Timothy J. Prior and Mark R.J. Elsegood collected and analyzed the crystallographic data and produced the crystallographic figures; Carl Redshaw performed some of the synthetic work and conceived and designed the experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef] [PubMed]
- Arbaoui, A.; Redshaw, C. Metal catalysts for ε-caprolactone polymerization. Polym. Chem. 2010, 1, 801–826. [Google Scholar] [CrossRef]
- Sisson, A.L.; Ekinci, D.; Lendlein, A. The contemporary role of ε-caprolactone chemistry to create advanced polymer architectures. Polymer 2013, 54, 4333–4350. [Google Scholar] [CrossRef]
- Drumright, R.E.; Gruber, P.R.; Henton, D.E. Polylactic Acid Technology. Adv. Mater. 2000, 12, 1841–1846. [Google Scholar] [CrossRef]
- Schrock, R.R. Recent advances in olefin metathesis by molybdenum and tungsten imido alkylidene complexes. J. Mol. Catal. A 2004, 213, 21–30. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, K.-Q.; Redshaw, C.; Elsegood, M.R.J. Molybdenum complexes derived from the oxydianiline [(2-NH2C6H4)2O]: Synthesis, characterization and ε-caprolactone ROP capability. Dalton Trans. 2015, 44, 13133–13140. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.M.; Nakanishi, M.; Kimura, M. Process for the Preparation of Lactone Polyesters. US Patent DE2947978, 15 September 1981. [Google Scholar]
- Báez, J.E.; Martῐnez-Rosales, M.; Martῐnez-Richa, A. Ring-opening polymerization of lactones catalyzed by decamolybdate anion. Polymer 2003, 44, 6767–6772. [Google Scholar] [CrossRef]
- Báez, J.E.; Martῐnez-Richa, A. Synthesis and characterization of poly(ε-caprolactone) and copolyesters by catalysis with molybdenum compounds: Polymers with acid-functional asymmetric telechelic architecture. Polymer 2005, 46, 12118–12129. [Google Scholar] [CrossRef]
- Báez, J.E.; Marcos-Fernández, A.; Martῐnez-Richa, A. One-step route to α-hydroxyl-ω-(carboxylic acid) polylactones using catalysis by decamolybdate anion. Macromolecules 2005, 38, 1599–1608. [Google Scholar] [CrossRef]
- Mahha, Y.; Atlamsani, A.; Blais, J.-C.; Tessier, M.; Brégeault, J.M.; Salles, L. Oligomerization of ε-caprolactone and δ-valerolactone using heteropolyacid initiators and vanadium or molybdenum complexes. J. Mol. Catal. A 2005, 234, 63–73. [Google Scholar] [CrossRef]
- Maruta, Y.; Abiko, A. Bis(salicyldehydato)dioxomolybdenum complexes: Catalysis for ring opening polymerization. Polym. Bull. 2014, 71, 1413–1440. [Google Scholar] [CrossRef]
- Haymore, B.L.; Maatta, E.A.; Wentworth, R.A.D. A Bisphenylnitrene Complex of Molybdenum with a Bent Nitrene Ligand. Preparation and Structure of cis-Mo(NC6F5)2(S2CN(C2H5)2)2. J. Am. Chem. Soc. 1979, 101, 2063–2068. [Google Scholar] [CrossRef]
- Gibson, V.C.; Redshaw, C.; Clegg, W.; Elsegood, M.R.J. “Electronic bending” of imido ligands and the effect on the coordination mode of a tridentate ancillary ligand. Polyhedron 2007, 26, 3161–3167. [Google Scholar] [CrossRef]
- Floriani, C.; Corazza, F.; Lesueur, W.; Chiesi-Villa, A.; Guastini, C. A sensitive spectroscopic probe for monitoring changes in the coordination sphere of titanium: Eight-membered dioxatitanacycles and their organometallic derivatives. Angew. Chem. Int. 1989, 28, 66–67. [Google Scholar] [CrossRef]
- Toscano, P.J.; Schermerhorn, E.J.; Dettelbacher, C.; Macherone, D. Monomeric four-coordinate vanadium(V) oxo complexes containing a labile ligand: Synthesis and X-ray structural characterization of [(C23H30O2)V(O)Cl]. Chem. Commun. 1991, 933–934. [Google Scholar] [CrossRef]
- Corazza, F.; Floriani, C.; Chiesi-Villa, A.; Guastini, C. Titanium(IV) and zirconium(IV) tetrahydroborate moieties supported by a phenoxo ligand. Inorg. Chem. 1991, 30, 145–148. [Google Scholar] [CrossRef]
- Okuda, J.; Fokken, S.; Kaug, H.-C.; Massa, W. Synthesis and characterization of mononuclear titanium complexes containing a bis(phenoxyl) ligand derived from 2,2′-methylene-bis (6-tert-butyl-4-methylphenol). Chem. Ber. 1995, 128, 221–227. [Google Scholar] [CrossRef]
- Chisholm, M.H.; Huang, J.-H.; Huffmann, J.C.; Streib, W.E.; Tiedtke, D. Synthesis and structural characterization of 2,2′-methylene-bis(6-t-butyl-4-methyl-phenoxide) complexes of titanium, zirconium and tantalum. Polyhedron 1997, 16, 2941–2949. [Google Scholar] [CrossRef]
- Chisholm, M.H.; Huang, J.-H.; Huffmann, J.C.; Parkin, I.P. Dinuclear (d3-d3) diolate complexes of molybdenum and tungsten. 2. Derivatives of 2,2′-methylenebis(6-tert-butyl-4-methylphenoxide). Direct observation of the conversion of bridged to chelate isomers (M = Mo) and reversible carbon-hydrogen bond oxidative addition (M = W). Inorg. Chem. 1997, 36, 1642–1651. [Google Scholar] [PubMed]
- Mulford, D.R.; Fanwick, P.E.; Rothwell, I.P. Group 4 and 5 metal derivatives of 2,2′-methylene -bis(6-phenylphenoxide). Polyhedron 2000, 19, 35–42. [Google Scholar] [CrossRef]
- Allen, F.H. The Cambridge Structural Database: A quarter of a million crystal structures and rising. Acta. Crystallogr. Sect. B 2002, 58, 380–388. [Google Scholar] [CrossRef]
- Maestri, A.G.; Brown, S.N. Titanatranes derailed: Static and dynamic triethanolamine slippage induced by polyphenoxide chelation. Inorg. Chem. 2004, 43, 6995–7004. [Google Scholar] [CrossRef] [PubMed]
- Redshaw, C.; Gibson, V.C.; Elsegood, M.R.J.; Clegg, W. New coordination modes at molybdenum for 2-diphenylphosphinoaniline derived ligands. Chem. Commun. 2007, 1951–1953. [Google Scholar] [CrossRef]
- Gordon, B.W.F.; Scott, M.J. Linked aryloxides ligands: Synthesis and characterization of alkali metal clusters of a partial calix[n]arene. Inorg. Chim. Acta 2000, 297, 206–216. [Google Scholar] [CrossRef]
- Matsuo, T.; Kawaguchi, H. Synthesis and structures of Niobium(V) complexes stabilized by linear-linked aryloxide trimers. Inorg. Chem. 2002, 41, 6090–6098. [Google Scholar] [CrossRef] [PubMed]
- Redshaw, C.; Humphrey, S.M. Complexes of tungsten (IV, VI) derived from linked aryloxide ligands. Polyhedron 2006, 25, 1946–1954. [Google Scholar] [CrossRef]
- Tang, L.; Wasserman, E.P.; Neithamer, D.R.; Krystosek, R.D.; Cheng, Y.; Price, P.C.; He, Y.; Emge, T.J. Highly active catalysts for the ring opening polymerization of ethylene oxide and propylene oxide based on products of alkylaluminum compounds with bulky tetraphenol ligands. Macromolecules 2008, 41, 7306–7315. [Google Scholar] [CrossRef]
- Cundari, T.R.; Gordon, M.S. Principal Resonance Contributors to High-Valent Transition-Metal Alkylidene Complexes. J. Am. Chem. Soc. 1991, 113, 5231–5243. [Google Scholar] [CrossRef]
- Fox, H.H.; Schofield, M.H.; Schrock, R.R. Electronic Structure of Mo(VI) Alkylidene Complexes and an Examination of Reactive Intermediates Using the SCF-Xα-SW Method. Organometallics 1994, 13, 2804–2815. [Google Scholar] [CrossRef]
- Poater, A.; Solans-Monfort, X.; Clot, E.; Copéret, C.; Eisenstein, O. DFT calculations of d° M(NR)(CHtBu)(X)(Y) (M = Mo, W; R = CPh3, 2,6-iPr2-C6H3; X and Y = CH2tBu, OtBu, OSi(OtBu)3) olefin metathesis catalysts: Structural, spectroscopic and electronic properties. Dalton Trans. 2006, 25, 3077–3087. [Google Scholar] [CrossRef] [PubMed]
- Oskam, J.H.; Fox, H.H.; Yap, K.B.; McConville, D.H.; O’Dell, R.; Lichtenstein, B.J.; Schrock, R.R. Ligand variation in alkylidene complexes of the type Mo(CHR)(NR′)(OR′′)2. J. Organomet. Chem. 1993, 459, 185–198. [Google Scholar] [CrossRef]
- Chisholm, M.H.; Cotton, F.A.; Folting, K.; Huffman, J.C.; Raterman, A.L.; Shamshoum, E.S. Insertion reactions of hexaalkoxydimolybdenum and –ditungsten compounds with organic isocyanates. Syntheses and structures of W2(OCMe3)4[N(C6H5)C(O)OCMe3]2 and Mo2(O-i-Pr)4 [N(C6H5)C(O)O-i-Pr]. Inorg. Chem. 1984, 23, 4423–4427. [Google Scholar] [CrossRef]
- Dyer, P.W.; Gibson, V.C.; Howard, J.A.K.; Whittle, B.; Wilson, C. Four coordinate bis(imido) alkene complexes of molybdenum(IV): Relatives of the zirconocene family. J. Chem. Soc. Chem. Commun. 1992, 1666–1668. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, C71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Van der Sluis, P.; Spek, A.L. BYPASS: An effective method for the refinement of crystal structures containing disordered solvent regions. Acta Crystallogr. A 1990, A46, 194–201. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).