Zr-SBA-15 Lewis Acid Catalyst: Activity in Meerwein Ponndorf Verley Reduction
Abstract
:1. Introduction
2. Results and Discussion
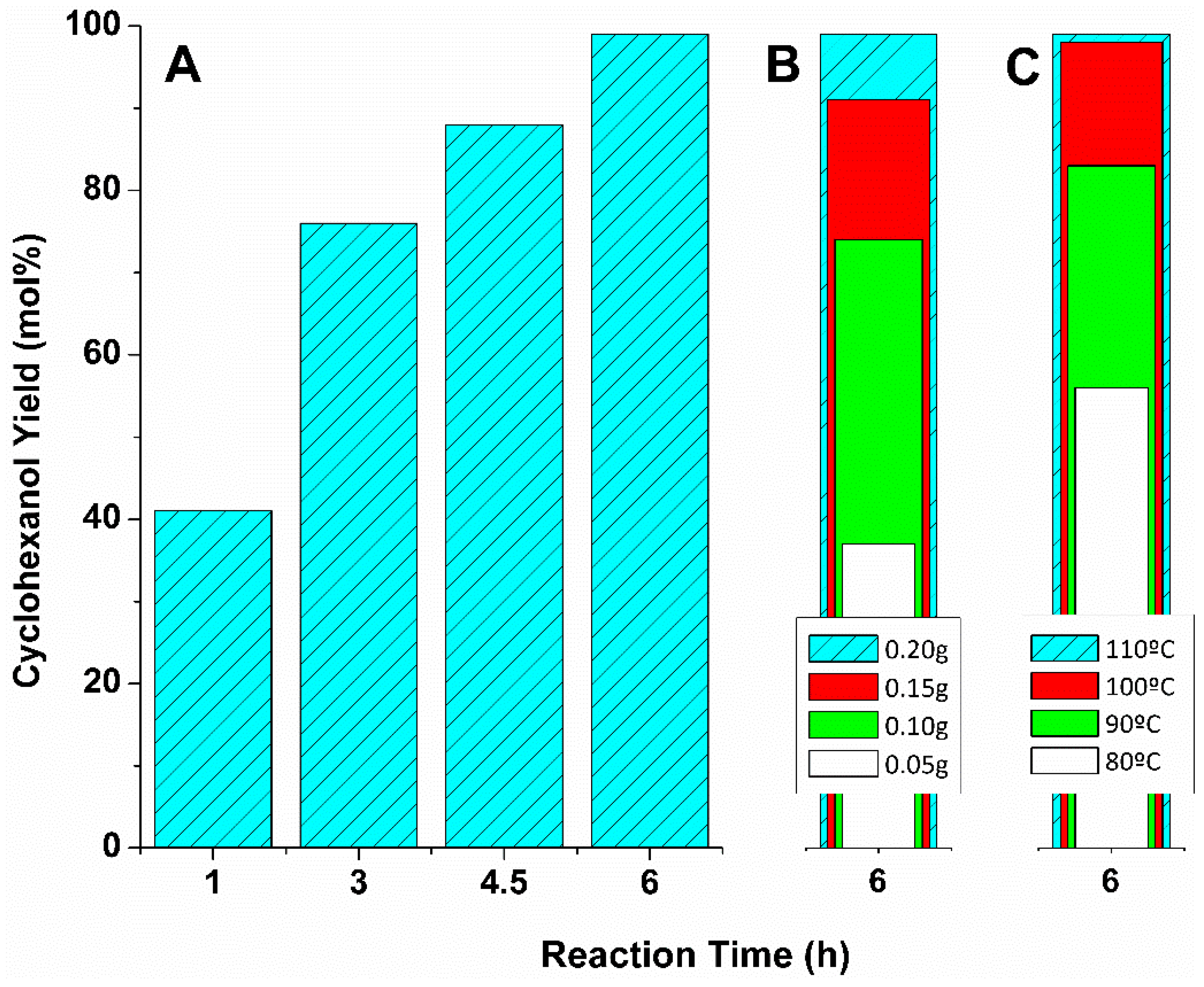
2.1. MPV Reduction of Cyclohexanone
| Entries | Sacrificing alcohol | Substrate | Product yield (mol %) |
|---|---|---|---|
| 1 | 2-Propanol | Cyclohexanone | 99 |
| 2 | 2-Butanol | Cyclohexanone | 97 |
| 3 | Cyclopentanol | Cyclohexanone | 24 |
| 4 | 2-methyl cyclohexanol | Cyclohexanone | 71 |
| 5 | 2-Propanol | 2-Butanone | 85 |
| 6 | 2-Propanol | Cyclopentanone | 21 |
| 7 | 2-Propanol | 2-methyl cyclohexanone | 8 |
| 8 | 2-Propanol | Ethyl levulinate | 42 a |
| 9 | 2-Propanol | Furfural | 24 |
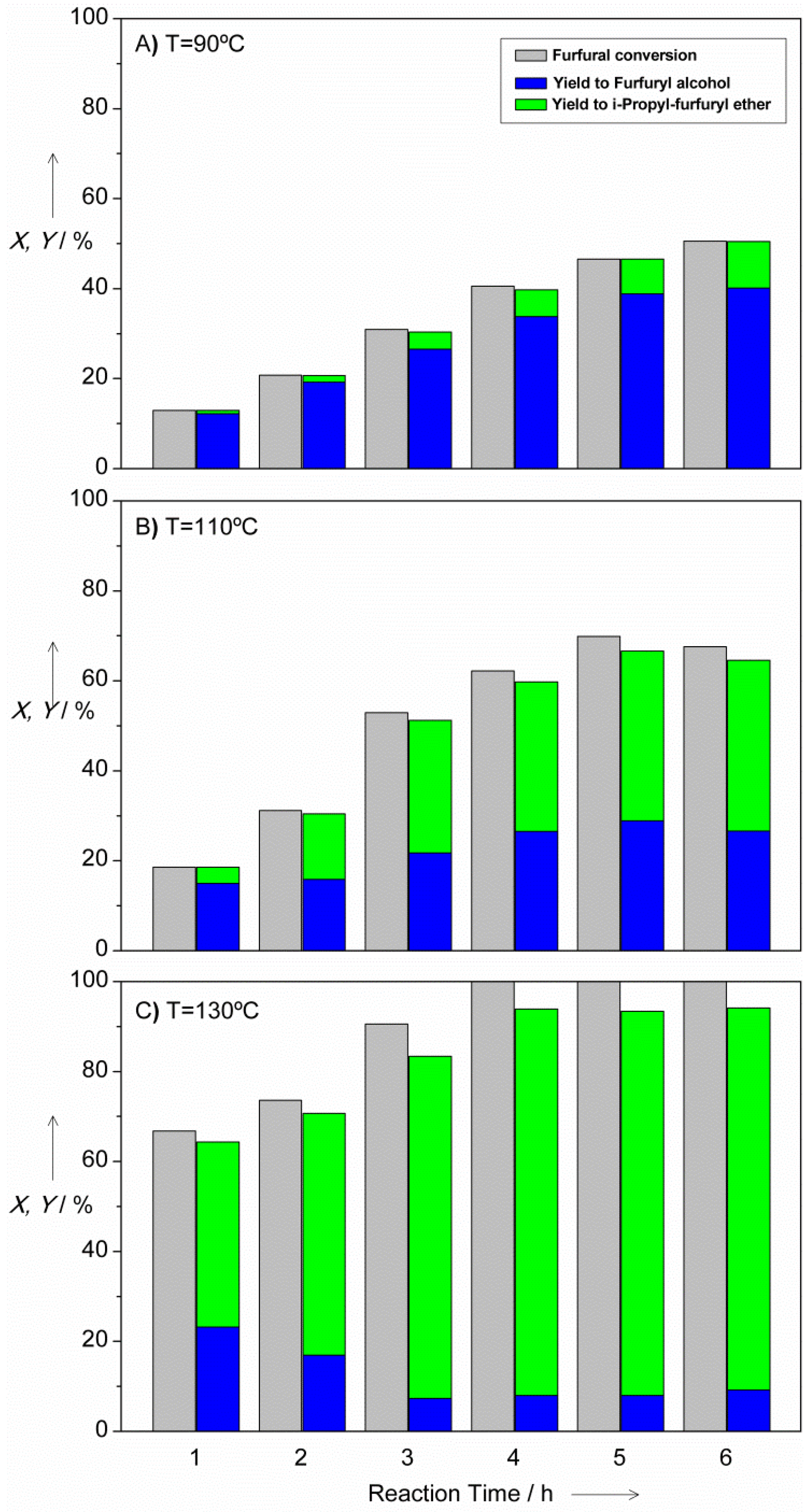
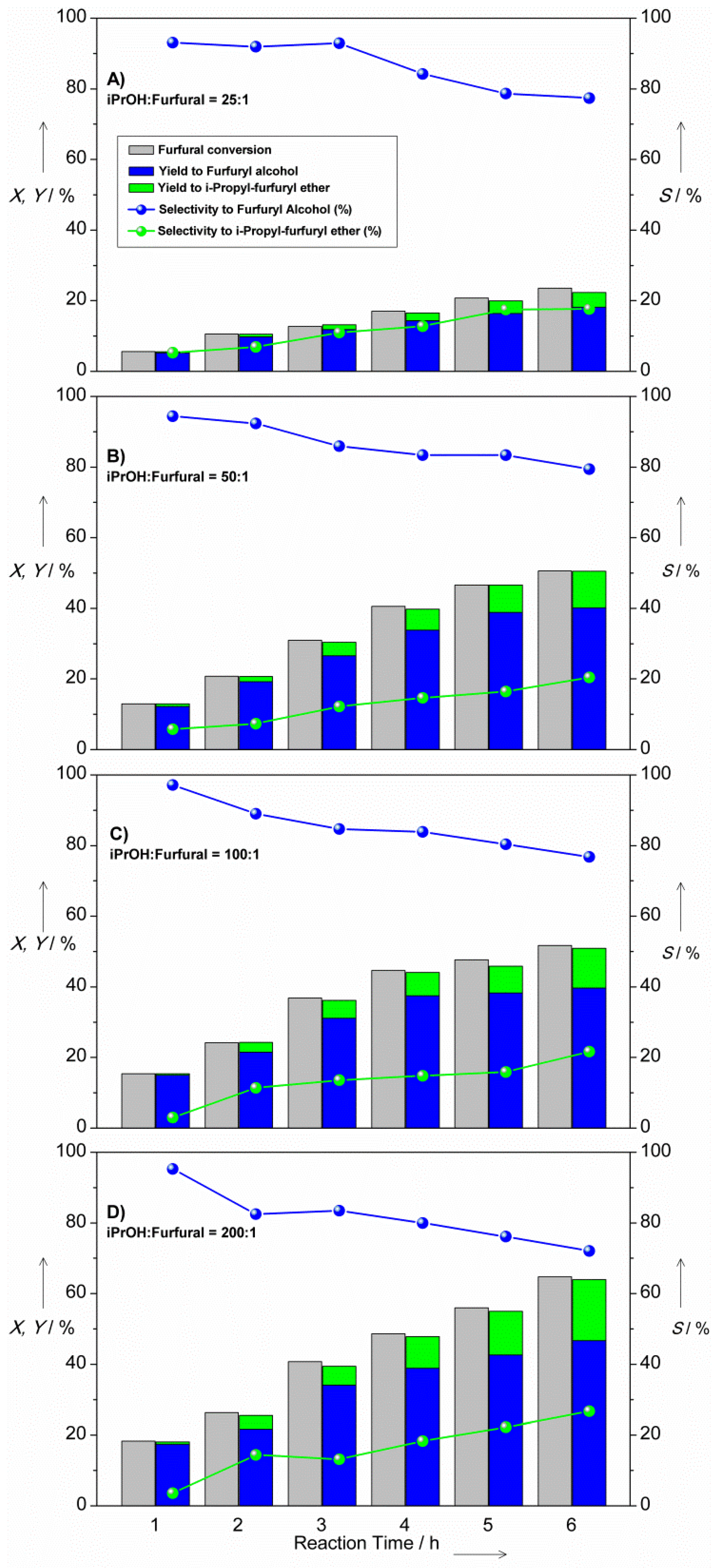
2.2. MPV Reduction of Furfural

3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis of Catalysts
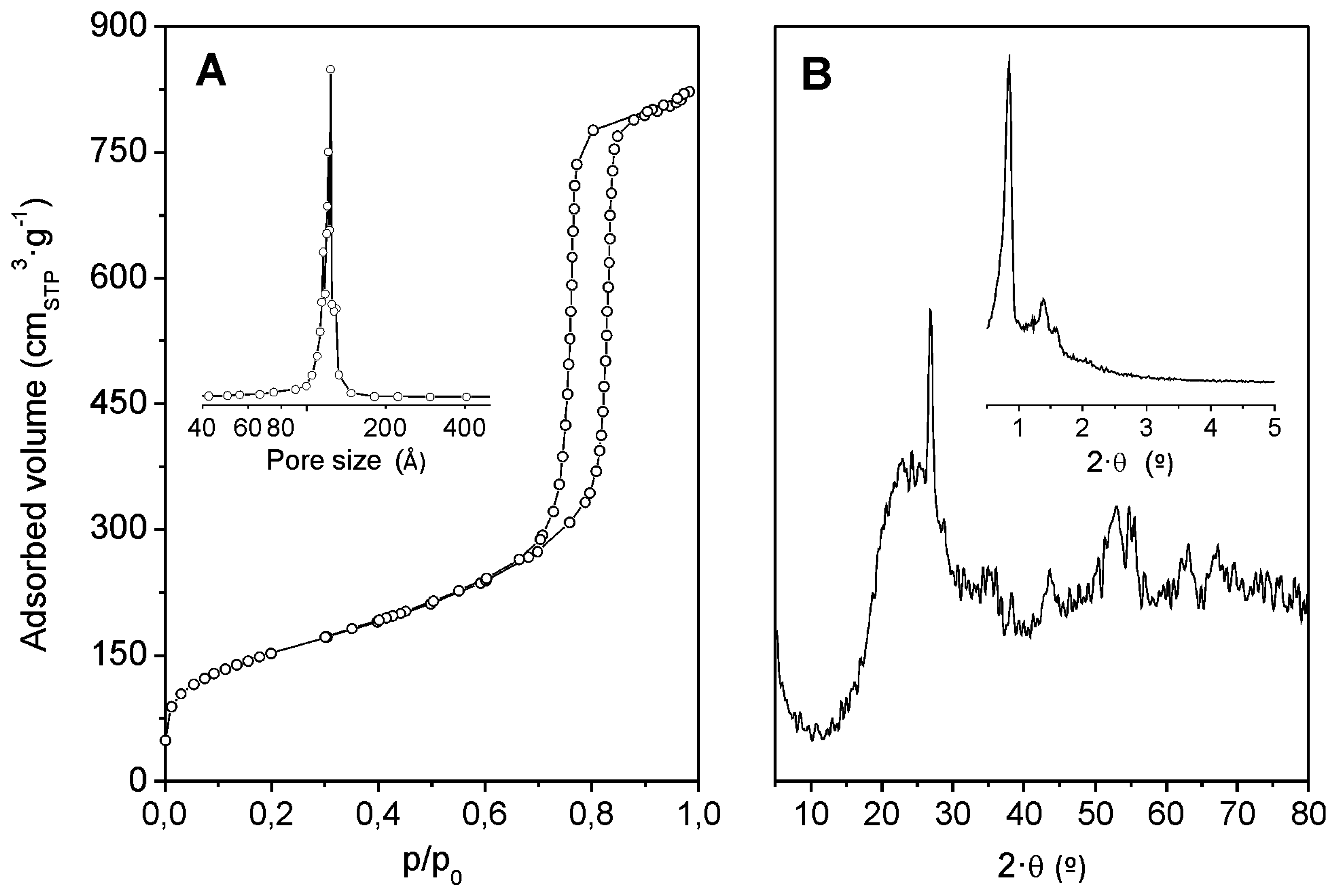
3.3. Catalyst Characterization
| Sample | SBET a (m2·g−1) | Vp b (cm3·g−1) | Dp c (Å) | a0 d (Å) | Zr e (%w/w) | Acid f (meq·g−1) |
|---|---|---|---|---|---|---|
| Zr-SBA | 553 | 1.26 | 123 | 135 | 8.3 | 0.38 |
3.4. MPV Catalytic Reaction Tests
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Meerwein, H.; Schmidt, R. Ein neues Verfahren zur Reduktion von Aldehyden und Ketonen. Liebigs Ann. 1925, 444, 221–238. (In German) [Google Scholar] [CrossRef]
- Verley, A. The exchange of functional groups between two molecules. The passage of ketones to alcohols and the reverse. Bull. Soc. Chim. Fr. 1925, 37, 871–874. [Google Scholar]
- Ponndorf, W.Z. Der reversible austausch der oxydationsstufen zwischen aldehyden oder ketonen einerseits und primären oder sekundären alkoholen anderseits. Angew. Chem. 1926, 39, 138–143. (In German) [Google Scholar] [CrossRef]
- Cha, J.S. Recent developments in Meerwein-Ponndorf-Verley and related reactions for the reduction of organic functional groups using aluminium, boron and other metal reagents: A review. Org. Process Res. Dev. 2006, 10, 1032–1053. [Google Scholar] [CrossRef]
- Normand, M.; Kirillov, E.; Roisnel, T.; Carpentier, J.F. Meerwein-Ponndorf-Verley-type reduction processes in aluminium and indium isopropoxide complexes of imino-phenolate ligands. Organometallics 2012, 31, 5511–5519. [Google Scholar] [CrossRef]
- Uysal, B.; Aksu, Y.; Oksal, B.S. Chemoselective reduction of alpha, beta unsaturated aldehydes and ketones over mesoporous B(OiPr)3-MCM-41 catalyst via MPV reduction processes: Preparation, characterization and catalytic application. J. Porous Mater. 2013, 20, 115–127. [Google Scholar] [CrossRef]
- Kloetzing, R.J.; Krasovskiy, A.; Knochel, P. The Mg-Oppenauer oxidation as a mild method for the synthesis of aryl and metallocenyl ketones. Chem. Eur. J. 2007, 13, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Ooi, T.; Miura, T.; Takaya, K.; Ichikawa, H.; Maruoka, K. Zr(OBut)4 as an effective promoter for the Meerwein-Ponndorf-Verley alkynylation and cyanation of aldehydes: Development of new asymetric cyanohydrin synthyesis. Tetrahedron 2001, 57, 867–873. [Google Scholar] [CrossRef]
- Miñambres, J.F.; Marinas, A.; Marinas, J.M.; Urbano, F.J. Activity and deactivation of catalysts based on zirconium oxide modified with metal chlorides in the MPV reduction of crotonaldehyde. Appl. Catal. B 2013, 140–141, 386–395. [Google Scholar] [CrossRef]
- Jiménez-Sanchidrián, C.; Hidalgo, J.M.; Ruiz, J.R. Reduction of heterocyclic carboxaldehydes via Meerwein-Ponndorf-Verley reaction. Appl. Catal. A 2006, 303, 23–28. [Google Scholar] [CrossRef]
- Ruiz, J.R.; Jiménez-Sanchidrián, C. Heterogeneous catalysis in the Meerwein-Ponndorf-Verley reduction of carbonyl compounds. Curr. Org. Chem. 2007, 11, 1113–1125. [Google Scholar] [CrossRef]
- Liu, S.H.; Jaenicke, S.; Chuah, G.K. Hydrous zirconia as a selective catalyst for the Meerwein-Ponndorf-Verley reduction of cinnamaldehyde. J. Catal. 2002, 206, 321–330. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, S.H.; Jaenicke, S.; Chuah, G.K. Zirconia catalysts in Meerwein-Ponndorf-Verley reduction of citral. Catal. Today 2004, 97, 249–255. [Google Scholar] [CrossRef]
- Battilocchio, C.; Hawkins, J.M.; Ley, S.V. A mild and efficient flow procedure for the transfer hydrogenation of ketones and aldehydes using hydrous zirconia. Org. Lett. 2003, 15, 2278–2281. [Google Scholar]
- Zhu, Y.; Chuah, G.-K.; Jaenicke, S. Chemo- and regioselective Meerwein-Ponndorf-Verley and Oppenauer reactions catalysed by Al-free Zr-zeolite beta. J. Catal. 2004, 227, 1–10. [Google Scholar]
- Zhu, Y.; Chuah, G.K.; Jaenicke, S. Selective Meerwein-Ponndorf-Verley reduction of α, β-unsaturated aldehydes over Zr-zeolite beta. J. Catal. 2006, 241, 25–33. [Google Scholar] [CrossRef]
- Boronat, M.; Corma, A.; Renz, M. Mechanism of the Meerwein-Ponndorf-Verley-Oppenauer (MPVO) redox equilibrium on Sn- and Zr-beta zeolite catalysts. J. Phys. Chem. 2006, 110, 21168–21174. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Niah, W.; Jaenicke, S.; Chuah, G.-K. A tandem cyclization and hydrogenation of (±)-citronellal to menthol over bifunctional Ni/Zr-beta and mixed Zr-beta and Ni/MCM-41. J. Catal. 2007, 248, 1–10. [Google Scholar] [CrossRef]
- Zhao, Z.L.; Liu, Y.M.; Wu, H.H.; Li, X.H.; He, M.Y.; Wu, P. Hydrothermal synthesis of mesoporous zirconosilicate with enhanced textural and catalytic properties with the aid of amphiphilic organosilane. Microporous Mesoporous Mater. 2009, 123, 324–330. [Google Scholar] [CrossRef]
- Zhang, B.; Tang, M.; Yuan, J.; Wu, L. Support effect in Meerwein-Ponndorf-Verley reduction of benzaldehyde over supported zirconia catalysts. Chin. J. Catal. 2012, 33, 914–922. [Google Scholar] [CrossRef]
- De Bruyn, M.; Limbourg, M.; Denayer, J.; Baron, G.V.; Parvulescu, V.; Grobet, P.J.; de Vos, D.E.; Jacobs, P.A. Mesoporous Zr and Hf catalysts for chemoselective MPV reductions of unsaturated ketones. Appl. Catal. A 2003, 254, 189–201. [Google Scholar] [CrossRef]
- Zhu, Y.; Jaenicke, S.; Chuah, G.-K. Supported zirconium propoxide—A versatile heterogeneous catalyst for the Meerwein-Ponndorf-Verley reduction. J. Catal. 2003, 218, 396–404. [Google Scholar] [CrossRef]
- Ramanathan, A.; Castro Villalobos, M.C.; Kwakernaak, C.; Telalovic, S.; Hanefeld, U. Zr-TUD-1: A Lewis acidic, three dimensional, mesoporous, zirconium-containing catalyst. Chem. Eur. J. 2008, 14, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Telalovic, S.; Ramanathan, A.; Fei Ng, J.; Maheswari, R.; Kwakernaak, C.; Soulimani, F.; Bouwer, H.C.; Chuah, G.-K.; Weckuysen, B.M.; Hanefeld, U. On the synergistic catalytic properties of bimetallic mesoporous materials containing aluminium and zirconium: The Prins cyclisation of citronellal. Chem. Eur. J. 2011, 17, 2077–2088. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, J.; Melero, J.A.; Bautista, L.F.; Morales, G.; Sánchez-Vázquez, R.; Andreola, M.T.; Lizarraga-Fernandez, A. Zr-SBA-15 as an efficient acid catalyst for FAME production from crude palm oil. Catal. Today 2011, 167, 46–55. [Google Scholar] [CrossRef]
- Iglesias, J.; Gracia, M.D.; Luque, R.; Romero, A.A.; Melero, J.A. Maximizing the accesibility of active species in weakly acidic Zr-SBA-15 materials. ChemCatChem 2012, 4, 379–386. [Google Scholar] [CrossRef]
- Melero, J.A.; Bautista, L.F.; Iglesias, J.; Morales, G.; Sánchez-Vázquez, R. Zr-SBA-15 acid catalyst: Optimization of the synthesis and reaction conditions for biodiesel production from low-grade oils and fats. Catal. Today 2012, 195, 44–53. [Google Scholar] [CrossRef]
- Iglesias, J.; Melero, J.A.; Bautista, L.F.; Morales, G.; Sánchez-Vázquez, R. Continuous production of biodiesel from low grade feedstock in presence of Zr-SBA-15: Catalyst performance and resistance against deactivation. Catal. Today 2014, 234, 174–181. [Google Scholar] [CrossRef]
- Klomp, D.; Maschmeyer, T.; Hanefeld, U.; Peters, J.A. Mechanism of homogeneously and heterogeneously catalysed Meerwein-Ponndorf-Verley-Oppenauer reactions for the racemisation of secondary alcohols. Chem. Eur. J. 2004, 10, 2088–2094. [Google Scholar] [CrossRef] [PubMed]
- Klomp, D.; Djanashvili, K.; Svennun, N.C.; Chantapariyavat, N.; Wong, C.S.; Vilela, F.; Maschmeyer, T.; Peters, J.A.; Hanefeld, U. Combined epimerisation and acylation: Meerwein-Ponndorf-Verley-Oppenauer catalysts in action. Org. Biomol. Chem. 2005, 3, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Orozco, L.M.; Renz, M. From MOFs to zeolites: Zirconium sites for epoxide rearrangement. New J. Chem. 2013, 37, 3496–3502. [Google Scholar] [CrossRef]
- Ignatchenko, A.V. Density functional theory study of carboxylic acids adsorption and enolization on monoclinic zirconia surfaces. J. Phys. Chem. C 2011, 115, 16012–16018. [Google Scholar] [CrossRef]
- Liu, S.H.; Chuah, G.K.; Jaenicke, S. Liquid-phase oppenauer oxidation of primary allylic and benzylic alcohols to corresponding aldehydes by solid zirconia catalysts. J. Mol. Catal. A 2004, 220, 267–274. [Google Scholar] [CrossRef]
- Mauriello, F.; Armandi, M.; Bonelli, B.; Onida, B.; Garrone, E. H-bonding of furan and its hydrogenated derivatives with the isolated hydroxyl of amorphous silica: An IR spectroscopy and thermodynamic study. J. Phys. Chem. C 2010, 114, 18233–18239. [Google Scholar] [CrossRef]
- Nagaraja, B.M.; Padmasri, A.H.; David Raju, B.; Rama Rao, K.S. Vapor phase selective hydrogenation of furfural to furfuryl alcohol over Cu-MgO coprecipitated catalysts. J. Mol. Catal. A 2007, 265, 90–97. [Google Scholar] [CrossRef]
- Neves, P.; Antunes, M.M.; Russo, P.A.; Abrantes, J.P.; Lima, S.; Fernandes, A.; Pillinger, M.; Rocha, S.M.; Ribeiro, M.F.; Valente, A.A. Production of biomass-derived furanic ethers and levulinate esters using heterogeneous acid catalysts. Green. Chem. 2013, 15, 3367–3376. [Google Scholar] [CrossRef]
- Neves, P.; Russo, P.A.; Fernandes, A.; Antunes, M.M.; Farinha, J.; Pillinger, M.; Ribeiro, M.F.; Castanheiro, J.E.; Valente, A.A. Mesoporous zirconia-based mixed oxides as versatile acid catalysts for producing bio-additives from furfuryl alcohol and glycerol. Appl. Catal. A 2014, 487, 148–157. [Google Scholar] [CrossRef]
- Rubio-Caballero, J.M.; Saravanamurugan, S.; Marieles-Torres, P.; Riisager, A. Acetalization of furfural with zeolites under benign reaction conditions. Catal. Today 2014, 234, 233–236. [Google Scholar] [CrossRef]
- Tang, X.; Hu, L.; Sun, Y.; Zhao, G.; Hao, W.; Lin, L. Conversion of biomass-derived ethyl levulinate into γ-valerolactone via hydrogen transfer from supercritical ethanol over a ZrO2 catalyst. RSC Adv. 2013, 3, 10277–10284. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iglesias, J.; Melero, J.A.; Morales, G.; Moreno, J.; Segura, Y.; Paniagua, M.; Cambra, A.; Hernández, B. Zr-SBA-15 Lewis Acid Catalyst: Activity in Meerwein Ponndorf Verley Reduction. Catalysts 2015, 5, 1911-1927. https://doi.org/10.3390/catal5041911
Iglesias J, Melero JA, Morales G, Moreno J, Segura Y, Paniagua M, Cambra A, Hernández B. Zr-SBA-15 Lewis Acid Catalyst: Activity in Meerwein Ponndorf Verley Reduction. Catalysts. 2015; 5(4):1911-1927. https://doi.org/10.3390/catal5041911
Chicago/Turabian StyleIglesias, Jose, Juan Antonio Melero, Gabriel Morales, Jovita Moreno, Yolanda Segura, Marta Paniagua, Alberto Cambra, and Blanca Hernández. 2015. "Zr-SBA-15 Lewis Acid Catalyst: Activity in Meerwein Ponndorf Verley Reduction" Catalysts 5, no. 4: 1911-1927. https://doi.org/10.3390/catal5041911
APA StyleIglesias, J., Melero, J. A., Morales, G., Moreno, J., Segura, Y., Paniagua, M., Cambra, A., & Hernández, B. (2015). Zr-SBA-15 Lewis Acid Catalyst: Activity in Meerwein Ponndorf Verley Reduction. Catalysts, 5(4), 1911-1927. https://doi.org/10.3390/catal5041911









