Palladium-Catalyzed Intermolecular Oxidative Amination of Alkenes with Amines, Using Molecular Oxygen as Terminal Oxidant
Abstract
:1. Introduction
2. Results and Discussion
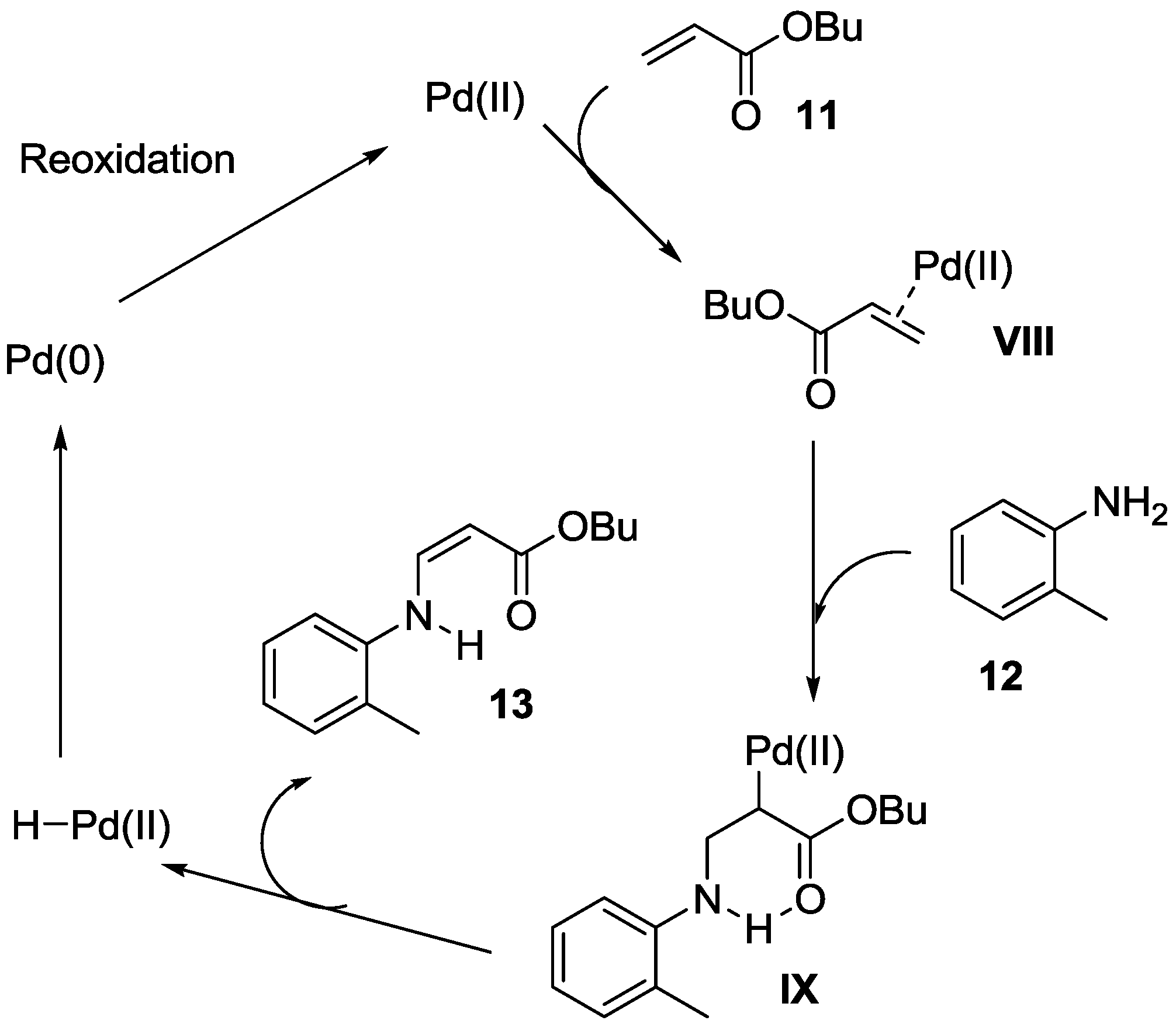
2.1. Oxidative Amination of Electron-Deficient Alkenes with Secondary Amines, Catalyzed by Pd(II)/NPMoV/O2 System

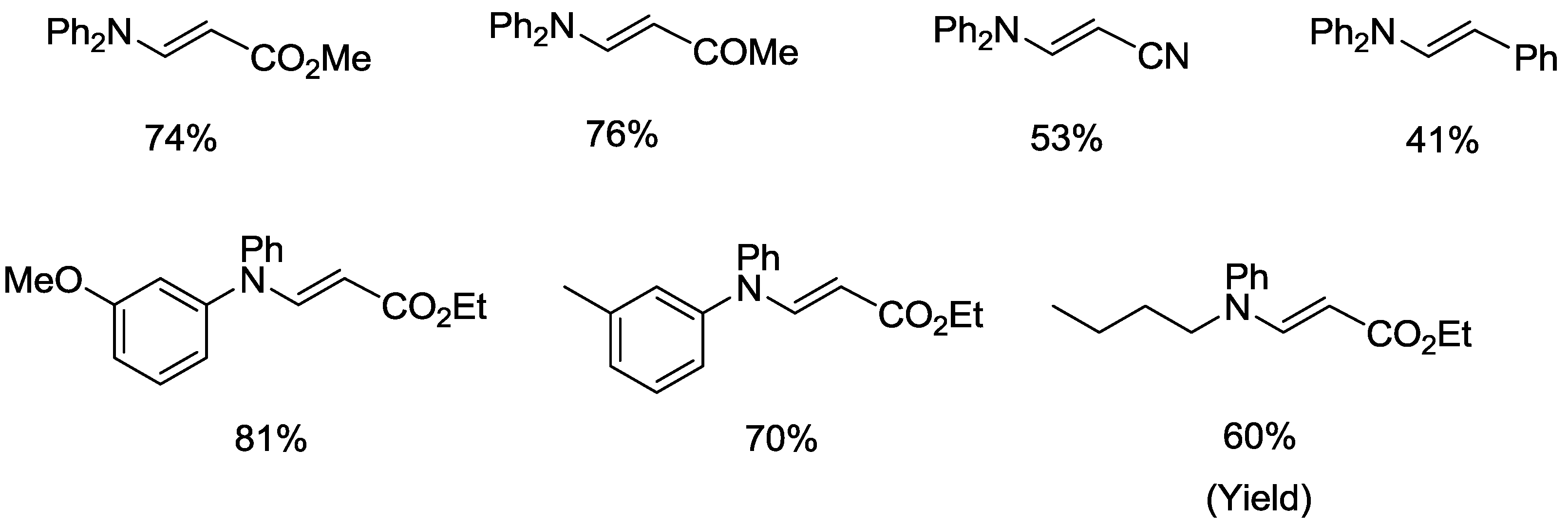


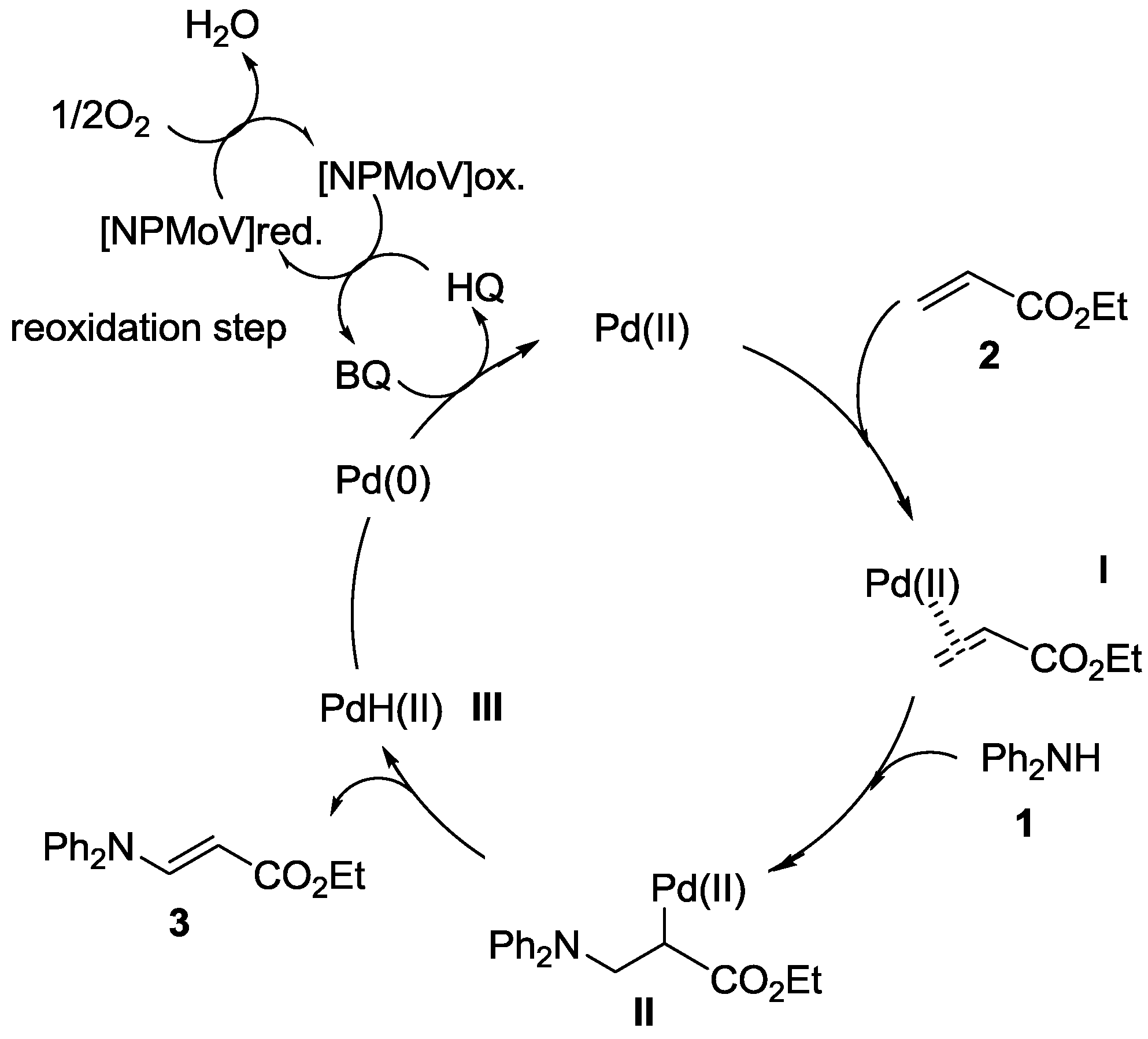
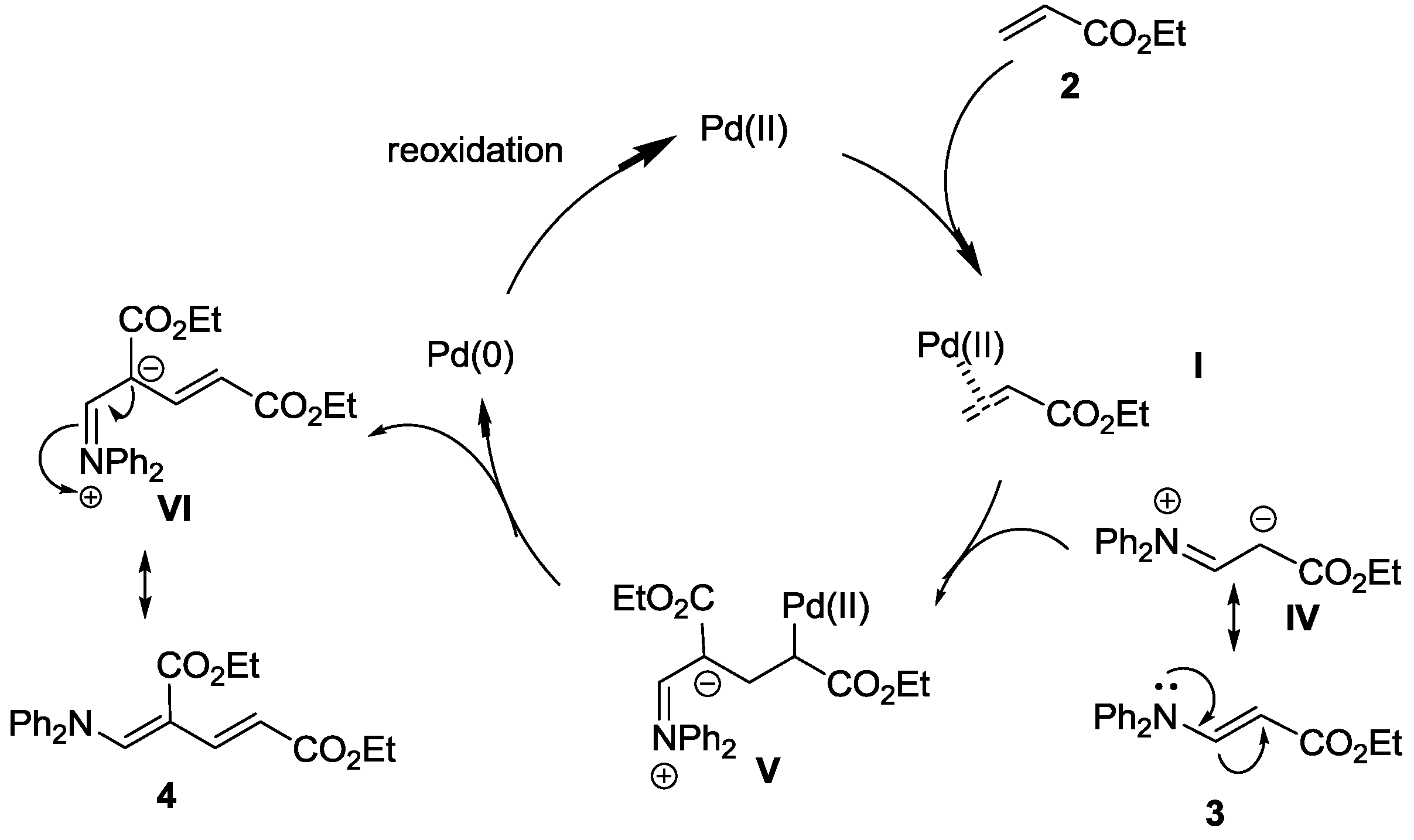
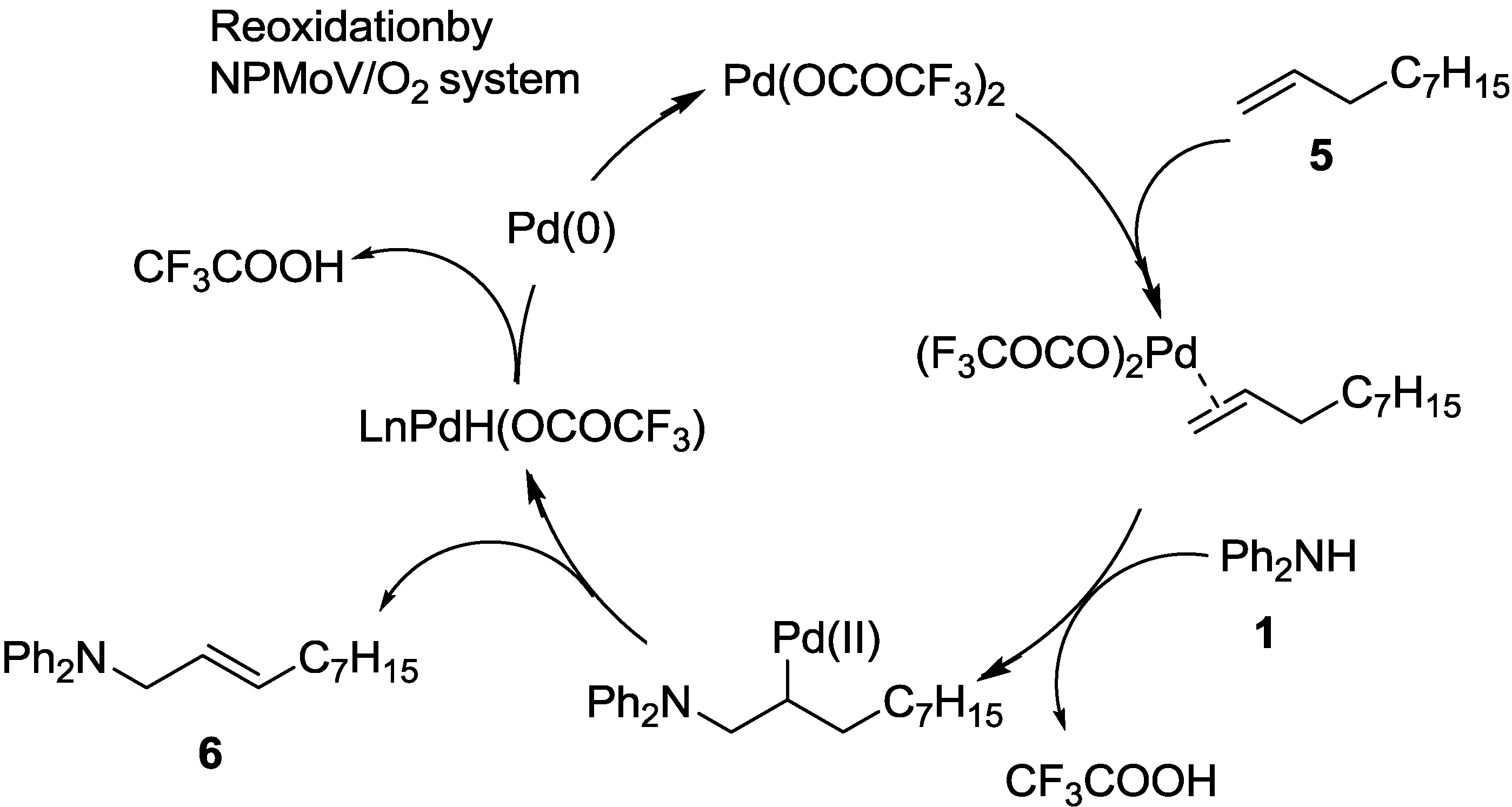
2.2. Oxidative Amination of Simple Alkenes with Secondary Amines, Catalyzed by Pd(II)/NPMoV/O2 System

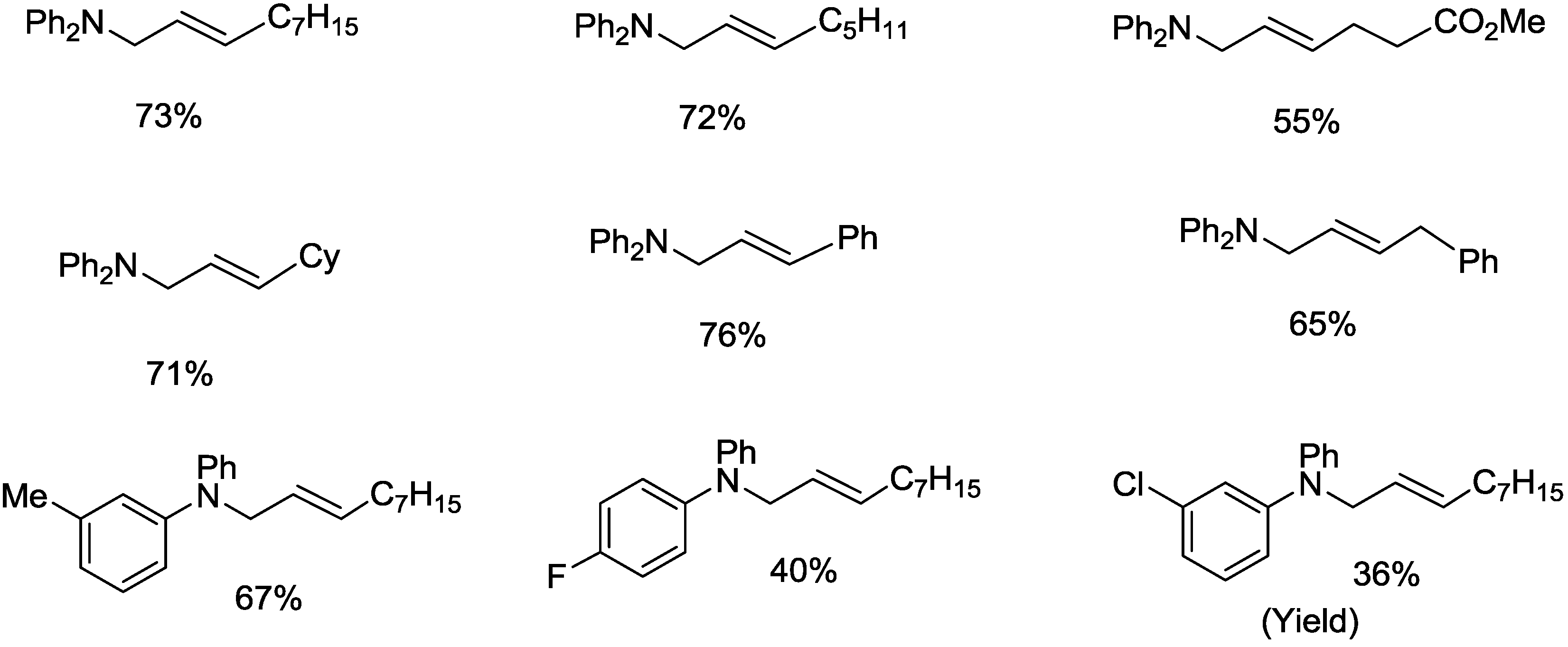


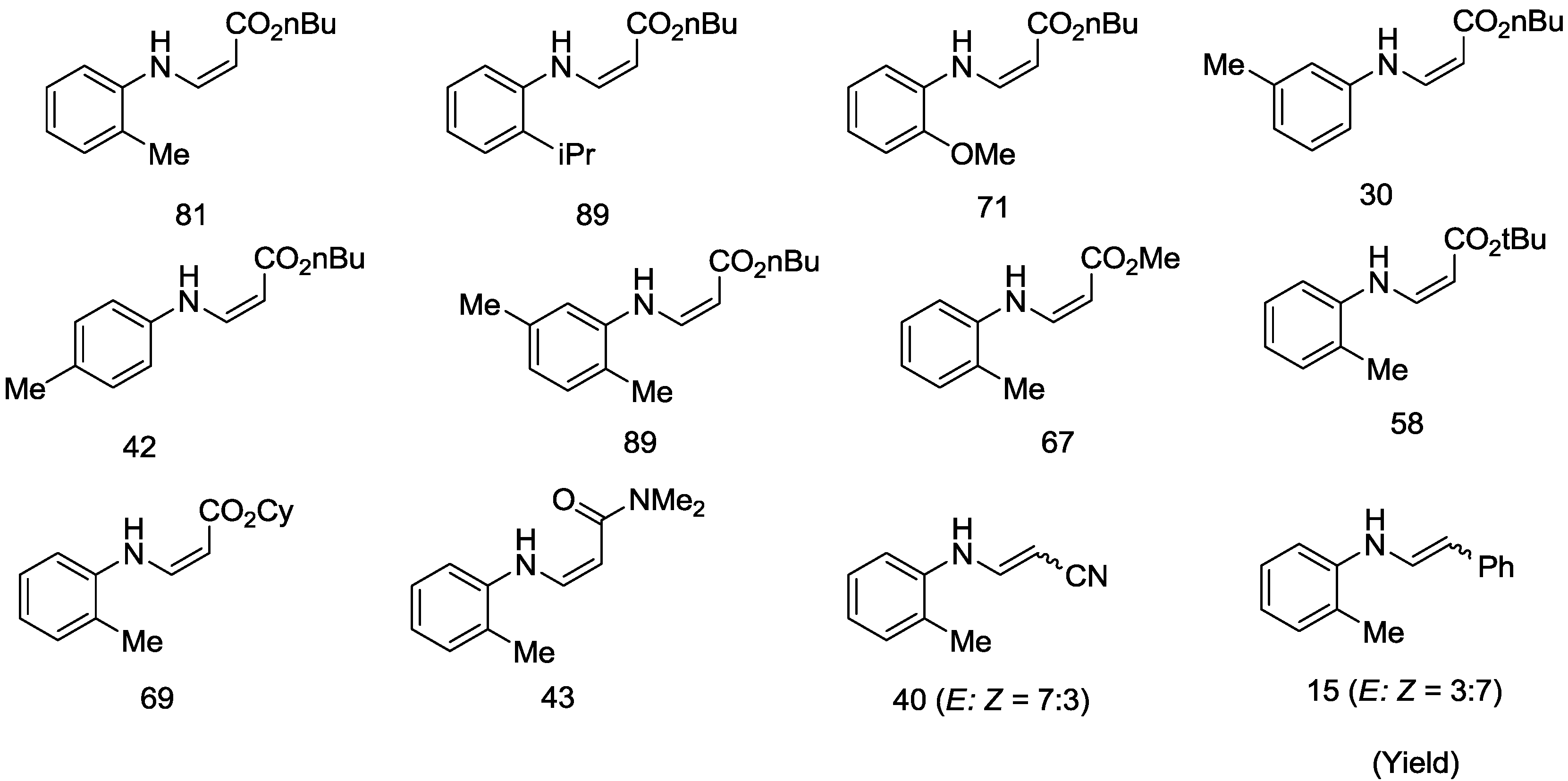
2.3. Palladium-Catalyzed Z-Selective Oxidative Amination of Ortho-Substituted Primary Anilines with Olefins under Open Air Atmosphere

3. Experimental Section
3.1. General
3.2. Reaction of 1 and 2 (Equation 1)
3.3. Reaction of 3 with 2 (Equation 3)
3.4. Reaction of 1 with 5 (Equation 4)
3.5. Reaction of 1 with 7 (Equation 5)
3.6. Reaction of 11 with 12 (Equation 6)
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Yu, N.; Chen, D.; Lee, K.C.L.; Lye, P.L.; Chang, J.W.W.; Deng, W.; Ng, M.C.Y.; Lu, T.; Khoo, M.L.; et al. Discovery of (2E)-3-{2-butyl-1-[2-(diethylamino)ethyl]-1H-benzimidazol-5-yl}-N-hydroxyacrylamide (SB939), an orally active histone deacetylase inhibitor with a superior preclinical profile. J. Med. Chem. 2011, 54, 4694–4720. [Google Scholar] [CrossRef]
- Wu, Q.-P.; Zhang, L.; Liang, M.; Sun, Z.; Xue, S. Sensitizers containing donor cascade and rhodanine-3-acetic acid moieties for dye-sensitized solar cells. Sol. Energy 2011, 85, 1–6. [Google Scholar] [CrossRef]
- Singh, T.S.; Moyon, N.S.; Mitra, S. Effect of solvent hydrogen bonding on the photophysical properties of intramolecular charge transfer probe trans-ethyl p-(dimethylamino)cinnamate and its derivative. Spectrochim. Acta A 2009, 73, 630–636. [Google Scholar] [CrossRef]
- Beccalli, E.M.; Broggini, G.; Martinelli, M.; Sottocornola, S. C-C, C-O, C-N bond formation on sp2 carbon by palladium(II)-catalyzed reactions involving oxidant agents. Chem. Rev. 2007, 107, 5318–5365. [Google Scholar] [CrossRef]
- Bras, J.L.; Muzart, J. Intermolecular dehydrogenative Heck reactions. Chem. Rev. 2011, 111, 1170–1214. [Google Scholar] [CrossRef]
- Vasseur, A.; Muzart, J.; Bras, J.L. Dehydrogenative Heck reaction of furans and thiophenes with styrenes under mild conditions and influence of the oxidizing agent on the reaction rate. Chem. Eur. J. 2011, 17, 12556–12560. [Google Scholar] [CrossRef]
- Wencel-Delord, J.; Dröge, T.; Liu, F.; Glorius, F. Towards mild metal-catalyzed C-H bond activation. Chem. Soc. Rev. 2011, 40, 4740–4761. [Google Scholar] [CrossRef]
- Besset, T.; Kuhl, N.; Patureau, F.W.; Glorius, F. RhIII-catalyzed oxidative olefination of vinylic C-H Bonds: efficient and selective access to di-unsaturated α-amino acid derivatives and other linear 1,3-butadienes. Chem. Eur. J. 2011, 17, 7167–7171. [Google Scholar] [CrossRef]
- Patureau, F.W.; Besset, T.; Glorius, F. Rhodium-catalyzed oxidative olefination of C-H bonds in acetophenones and benzamides. Angew. Chem. Int. Ed. 2011, 50, 1064–1067. [Google Scholar] [CrossRef]
- Stahl, S.S. Palladium oxidase catalysis. Selective oxidation of organic chemicals by direct dioxygen-coupled turnover. Angew. Chem. Int. Ed. 2004, 43, 3400–3420. [Google Scholar] [CrossRef]
- Kotov, V.; Scarborough, C.C.; Stahl, S.S. Palladium-catalyzed aerobic oxidative amination of alkenes: Development of intra- and intermolecular aza-Wacker reactions. Inorg. Chem. 2007, 46, 1910–1923. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Tan, J.; Wang, Z. A facile access to pyrroles from amino acids via an aza-Wacker cyclization. J. Org. Chem. 2008, 73, 5180–5182. [Google Scholar] [CrossRef]
- Liu, G.; Stahl, S.S. Two-faced reactivity of alkenes: cis- versus trans-aminopalladation in aerobic Pd-catalyzed intramolecular aza-Wacker reactions. J. Am. Chem. Soc. 2007, 129, 6328–6335. [Google Scholar]
- Yang, G.; Zhang, W. Regioselective Pd-catalyzed aerobic aza-Wacker cyclization for preparation of isoindolinones and isoquinolin-1(2H)-ones. Org. Lett. 2012, 14, 268–271. [Google Scholar] [CrossRef]
- Liwosz, T.W.; Chemler, S.R. Copper-catalyzed oxidative amination and allylic amination of alkenes. Chem. Eur. J. 2013, 19, 12771–12777. [Google Scholar] [CrossRef]
- Jiménez, M.V.; Pérez-Torrente, J.J.; Bartolomé, M.I.; Lahoz, F.J.; Oro, L.A. Rational design of efficient rhodium catalysts for the anti-markovnikov oxidative amination of styrene. Chem. Commun. 2010, 46, 5322–5324. [Google Scholar]
- Jiménez, M.V.; Bartolomé, M.I.; Pérez-Torrente, J.J.; Gómez, D.; Modrego, F.J.; Oro, L.A. Mechanistic studies on the catalytic oxidative amination of alkenes by rhodium (I) complexes with hemilabile phosphines. ChemCatChem 2013, 5, 263–276. [Google Scholar] [CrossRef]
- Jiménez, M.V.; Bartolomé, M.I.; Pérez-Torrente, J.J.; Lahoz, F.J.; Oro, L.A. Rhodium(I) complexes with hemilabile phosphines: Rational design for efficient oxidative amination catalysts. ChemCatChem 2012, 4, 1298–1310. [Google Scholar] [CrossRef]
- Timokhin, V.I.; Anastasi, N.R.; Stahl, S.S. Dioxygen-coupled oxidative amination of styrene. J. Am. Chem. Soc. 2003, 125, 12996–12997. [Google Scholar]
- Brice, J.L.; Harang, J.E.; Timokhin, V.I.; Anastasi, N.R.; Stahl, S.S. Aerobic oxidative amination of unactivated alkenes catalyzed by palladium. J. Am. Chem. Soc. 2005, 127, 2868–2869. [Google Scholar]
- Timokhin, V.I.; Stahl, S.S. Bronsted base-modulated regioselectivity in the aerobic oxidative amination of styrene catalyzed by palladium. J. Am. Chem. Soc. 2005, 127, 17888–17893. [Google Scholar] [CrossRef]
- Scarborough, C.C.; Stahl, S.S. Synthesis of pyrrolidines via palladium(II)-catalyzed aerobic oxidative carboamination of butyl vinyl ether and styrenes with allyl tosylamides. Org. Lett. 2006, 8, 3251–3254. [Google Scholar] [CrossRef]
- Liu, G.; Stahl, S.S. Highly regioselective Pd-catalyzed intermolecular aminoacetoxylation of alkenes and evidence for cis-aminopalladation and S(N)2 C-O bond formation. J. Am. Chem. Soc. 2006, 128, 7179–7181. [Google Scholar] [CrossRef]
- Rogers, M.M.; Kotov, V.; Chatwichien, J.; Stahl, S.S. Palladium-catalyzed oxidative amination of alkenes: improved catalyst reoxidation enables the use of alkene as the limiting reagent. Org. Lett. 2007, 9, 4331–4334. [Google Scholar] [CrossRef]
- Wu, L.; Qiu, S.; Liu, G. Bronsted base-modulated regioselective pd-catalyzed intramolecular aerobic oxidative amination of alkenes: Formation of seven-membered amides and evidence for allylic C-H activation. Org. Lett. 2009, 11, 2707–2710. [Google Scholar] [CrossRef]
- Liu, G.; Yin, G.; Wu, L. Palladium-catalyzed intermolecular aerobic oxidative amination of terminal alkenes: Efficient synthesis of linear allylamine derivatives. Angew. Chem. Int. Ed. 2008, 47, 4733–4736. [Google Scholar] [CrossRef]
- Reed, S.A.; White, M.C. Catalytic intermolecular linear allylic C-H amination via heterobimetallic catalysis. J. Am. Chem. Soc. 2008, 130, 3316–3318. [Google Scholar] [CrossRef]
- Paradine, S.M.; White, M.C. Iron-catalyzed intramolecular allylic C-H amination. J. Am. Chem. Soc. 2012, 134, 2036–2039. [Google Scholar] [CrossRef]
- Reed, S.A.; Mazzotti, A.R.; White, M.C. A catalytic, Bronsted base strategy for intermolecular allylic C-H amination. J. Am. Chem. Soc. 2009, 131, 11701–11706. [Google Scholar]
- Fraunhoffer, K.J.; White, M.C. syn-1,2-Amino alcohols via diastereoselective allylic C-H amination. J. Am. Chem. Soc. 2007, 129, 7274–7276. [Google Scholar] [CrossRef]
- Jiang, C.; Covell, D.J.; Stepan, A.F.; Plummer, M.S.; White, M.C. Sequential allylic C-H amination/vinylic C-H arylation: A strategy for unnatural amino acid synthesis from α-olefins. Org. Lett. 2012, 14, 1386–1389. [Google Scholar]
- Rice, G.T.; White, M.C. Allylic C-H amination for the preparation of syn-1,3-amino alcohol motifs. J. Am. Chem. Soc. 2009, 131, 11707–11711. [Google Scholar] [CrossRef]
- Qi, X.; Rice, G.T.; Lall, M.S.; Plummer, M.S.; White, M.C. Diversification of a β-lactam pharmacophore via allylic C-H amination: Accelerating effect of Lewis acid co-catalyst. Tetrahedron 2010, 66, 4816–4826. [Google Scholar]
- van Benthem, R.A.T.M.; Hiemstra, H.; Longarela, G.R.; Speckamp, W.N. Formamide as a superior nitrogen nucleophile in palladium(II) mediated synthesis of imidazolidines. Tetrahedron Lett. 1994, 35, 9281–9284. [Google Scholar]
- Rönn, M.; Bäckvall, J.-E.; Andersson, P.G. Palladium(II)-catalyzed cyclization using molecular oxygen as reoxydant. Tetrahedron Lett. 1995, 36, 7749–7752. [Google Scholar] [CrossRef]
- Larock, R.C.; Hightower, T.R.; Hasvold, L.A.; Peterson, K.P. Palladium(II)-catalyzed cyclization of olefinic tosylamides. J. Org. Chem. 1996, 61, 3584–3585. [Google Scholar] [CrossRef]
- Fix, S.R.; Brice, J.L.; Stahl, S.S. Efficient intramolecular oxidative amination of olefins through direct dioxygen-coupled palladium catalysis. Angew. Chem. Int. Ed. 2002, 41, 164–166. [Google Scholar] [CrossRef]
- Trend, R.M.; Ramtohul, Y.K.; Ferreira, E.M.; Stoltz, B.M. Palladium-catalyzed oxidative wacker cyclizations in nonpolar organic solvents with molecular oxygen: A stepping stone to asymmetric aerobic cyclizations. Angew. Chem. Int. Ed. 2003, 42, 2892–2895. [Google Scholar] [CrossRef]
- Bozell, J.J.; Hegedus, L.S. Palladium-assisted functionalization of olefins: A new amination of electron-deficient olefins. J. Org. Chem. 1981, 46, 2561–2563. [Google Scholar] [CrossRef]
- Beller, M.; Eichberger, M.; Trauthwein, H. Anti-Markovnikov functionalization of unsaturated compounds. 1. Anti-Markovnikov functionalization of olefins: Rhodium-catalyzed oxidative amination of styrenes. Angew. Chem. Int. Ed. 1997, 36, 2225–2227. [Google Scholar]
- Tillack, A.; Trauthwein, H.; Hartung, C.G.; Eichberger, M.; Pitter, S.; Jansen, A.; Beller, M. Anti-Markovnikov reactions. 8. Rhodium-catalyzed amination of aromatic olefins. Monatsh. Chem. 2000, 131, 1327–1334. [Google Scholar] [CrossRef]
- Scarborough, C.C.; Bergant, A.; Sazama, G.T.; Guzei, I.A.; Spencer, L.C.; Stahl, S.S. Synthesis of PdII complexes bearing an enantiomerically resolved seven-membered N-heterocyclic carbene ligand and initial studies of their use in asymmetric Wacker-type oxidative cyclization reactions. Tetrahedron 2009, 65, 508–5092. [Google Scholar]
- Yu, H.; Fu, Y.; Guo, Q.; Lin, Z. Theoretical Investigations on Mechanisms of Pd(OAc)2-Catalyzed Intramolecular Diaminations in the Presence of Bases and Oxidants. Organometallics 2009, 28, 4507–4512. [Google Scholar] [CrossRef]
- Ohashi, S.; Sakaguchi, S.; Ishii, Y. Carboxylation of anisole derivatives with CO and O2 catalyzed by Pd(OAc)2 and molybdovanadophosphates. Chem. Commun. 2005, 486–488. [Google Scholar] [CrossRef]
- Yamada, S.; Sakaguchi, S.; Ishii, Y. Carboxylation and hydroxylation of biphenyl by the Pd/molybdovanadophosphoric acid/dioxygen stsyem. J. Mol. Catal. A 2007, 262, 48–51. [Google Scholar] [CrossRef]
- Yamada, S.; Ohashi, S.; Obora, Y.; Sakaguchi, S.; Ishii, Y. Carboxylation of benzene with CO and O2 catalyzed by Pd(OAc)2 combined with molybdovanadophosphates. J. Mol. Catal. 2008, 282, 22–27. [Google Scholar] [CrossRef]
- Yokota, T.; Tani, M.; Sakaguchi, S.; Ishii, Y. Direct coupling of benzene with olefin catalyzed by Pd(OAc)2 combined with heteropolyoxometalate under dioxygen. J. Am. Chem. Soc. 2003, 125, 1476–1477. [Google Scholar] [CrossRef]
- Tani, M.; Sakaguchi, S.; Ishii, Y. Pd(OAc)2-Catalyzed oxidative coupling reaction of benzenes with olefins in the presence of molybdovanadophosphoric acid under atmospheric dioxygen and air. J. Org. Chem. 2004, 69, 1221–1226. [Google Scholar] [CrossRef]
- Yamada, T.; Sakaguchi, S.; Ishii, Y. Oxidative coupling of benzenes with α,β-unsaturated aldehydes by the Pd(OAc)2/molybdovanadophosphoric acid/O2 system. J. Org. Chem. 2005, 70, 5471–5474. [Google Scholar] [CrossRef]
- Yamada, T.; Sakakura, A.; Sakaguchi, S.; Obora, Y.; Ishii, Y. Oxidative arylation of ethylene with benzene catalyzed by Pd(OAc)2/heteropoly acid/O2 system. New J. Chem. 2008, 32, 738–742. [Google Scholar] [CrossRef]
- Obora, Y.; Okabe, Y.; Ishii, Y. Direct oxidative coupling of benzenes with acrylonitriles to cinnamonitriles catalyzed by Pd(OAc)2/HPMoV/O2 system. Org. Biomol. Chem. 2010, 8, 4071–4073. [Google Scholar] [CrossRef]
- Mizuta, Y.; Obora, Y.; Shimizu, Y.; Ishii, Y. para-Selective aerobic oxidative C-H olefination of aminobenzenes catalyzed by palladium/molybdovanadophosphoric acid/2,4,6-trimethylbenzoic acid system. ChemCatChem 2012, 4, 187–191. [Google Scholar] [CrossRef]
- Yokota, T.; Sakaguchi, S.; Ishii, Y. Oxidation of unsaturated hydrocarbons by Pd(OAc)2 using a molybdovanadophosphate/dioxygen system. J. Jpn. Petrol. Inst. 2003, 46, 15–27. [Google Scholar] [CrossRef]
- Yokota, T.; Fujibayashi, S.; Nishiyama, Y.; Sakaguchi, S.; Ishii, Y. Molybdovanadophosphate (NPMoV)/hydroquinone/O2 system as an efficient reoxidation system in palladium-catalyzed oxidation of alkenes. J. Mol. Catal. A 1996, 114, 113–122. [Google Scholar] [CrossRef]
- Yokota, T.; Sakakura, A.; Tani, M.; Sakaguchi, S.; Ishii, Y. Selective Wacker-type oxidation of terminal alkenes and dienes using the Pd(II)/molybdovanadophosphate (NPMoV)/O2 system. Teterahedron Lett. 2002, 43, 8887–8891. [Google Scholar] [CrossRef]
- Yokota, T.; Sakaguchi, S.; Ishii, Y. Oxidative carbomethoxylation of alkenes using a Pd(II)/molybdovanadophosphate (NPMoV) system under carbon monoxide and air. J. Org. Chem. 2002, 67, 5005–5008. [Google Scholar] [CrossRef]
- Obora, Y.; Shimizu, Y.; Ishii, Y. Intermolecular oxidative amination of olefins with amines catalyzed by the Pd(II)/NPMoV/O2 system. Org. Lett. 2009, 11, 5058–5061. [Google Scholar] [CrossRef]
- Shimizu, Y.; Obora, Y.; Ishii, Y. Intermolecular aerobic oxidative allylic amination of simple alkenes with diarylamines catalyzed by the Pd(OCOCF3)2/NPMoV/O2 sysyem. Org. Lett. 2010, 12, 1372–1374. [Google Scholar] [CrossRef]
- Mizuta, Y.; Yasuda, K.; Obora, Y. Palladium-catalyzed Z-selective oxidative amination of ortho-substituted primary anilines with olefins under an open air atmosphere. J. Org. Chem. 2013, 78, 6332–6337. [Google Scholar] [CrossRef]
- Obora, Y.; Ishii, Y. Pd(II)/HPMoV-catalyzed direct oxidative coupling reaction of benzenes with olefins. Molecules 2010, 15, 1487–1500. [Google Scholar] [CrossRef]
- Obora, Y.; Ogawa, Y.; Imai, Y.; Kawamura, T.; Tsuji, Y. Palladium complex catalyzed acylation of allylic esters with acylsilanes. J. Am. Chem. Soc. 2001, 123, 10489–10493. [Google Scholar] [CrossRef]
- Hegedus, L.S.; Åkermark, B.; Zetterberg, K.; Olsson, L.F. Palladium-assisted amination of olefins. A mechanistic study. J. Am. Chem. Soc. 1984, 106, 7122–7126. [Google Scholar] [CrossRef]
- Lee, J.M.; Ahn, D.-S.; Jung, D.Y.; Lee, J.; Do, Y.; Kim, S.K.; Chang, S. Hydrogen-bond-directed highly stereoselective synthesis of Z-enamides via Pd-catalyzed oxidative amidation of conjugated olefins. J. Am. Chem. Soc. 2006, 128, 12954–12962. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Obora, Y.; Ishii, Y. Palladium-Catalyzed Intermolecular Oxidative Amination of Alkenes with Amines, Using Molecular Oxygen as Terminal Oxidant. Catalysts 2013, 3, 794-810. https://doi.org/10.3390/catal3040794
Obora Y, Ishii Y. Palladium-Catalyzed Intermolecular Oxidative Amination of Alkenes with Amines, Using Molecular Oxygen as Terminal Oxidant. Catalysts. 2013; 3(4):794-810. https://doi.org/10.3390/catal3040794
Chicago/Turabian StyleObora, Yasushi, and Yasutaka Ishii. 2013. "Palladium-Catalyzed Intermolecular Oxidative Amination of Alkenes with Amines, Using Molecular Oxygen as Terminal Oxidant" Catalysts 3, no. 4: 794-810. https://doi.org/10.3390/catal3040794
APA StyleObora, Y., & Ishii, Y. (2013). Palladium-Catalyzed Intermolecular Oxidative Amination of Alkenes with Amines, Using Molecular Oxygen as Terminal Oxidant. Catalysts, 3(4), 794-810. https://doi.org/10.3390/catal3040794



