Optimizing the Microscopic Structure of MIL-68(Al) by Co-Doping for Pollutant Removal and Mechanism
Abstract
1. Introduction
2. Results
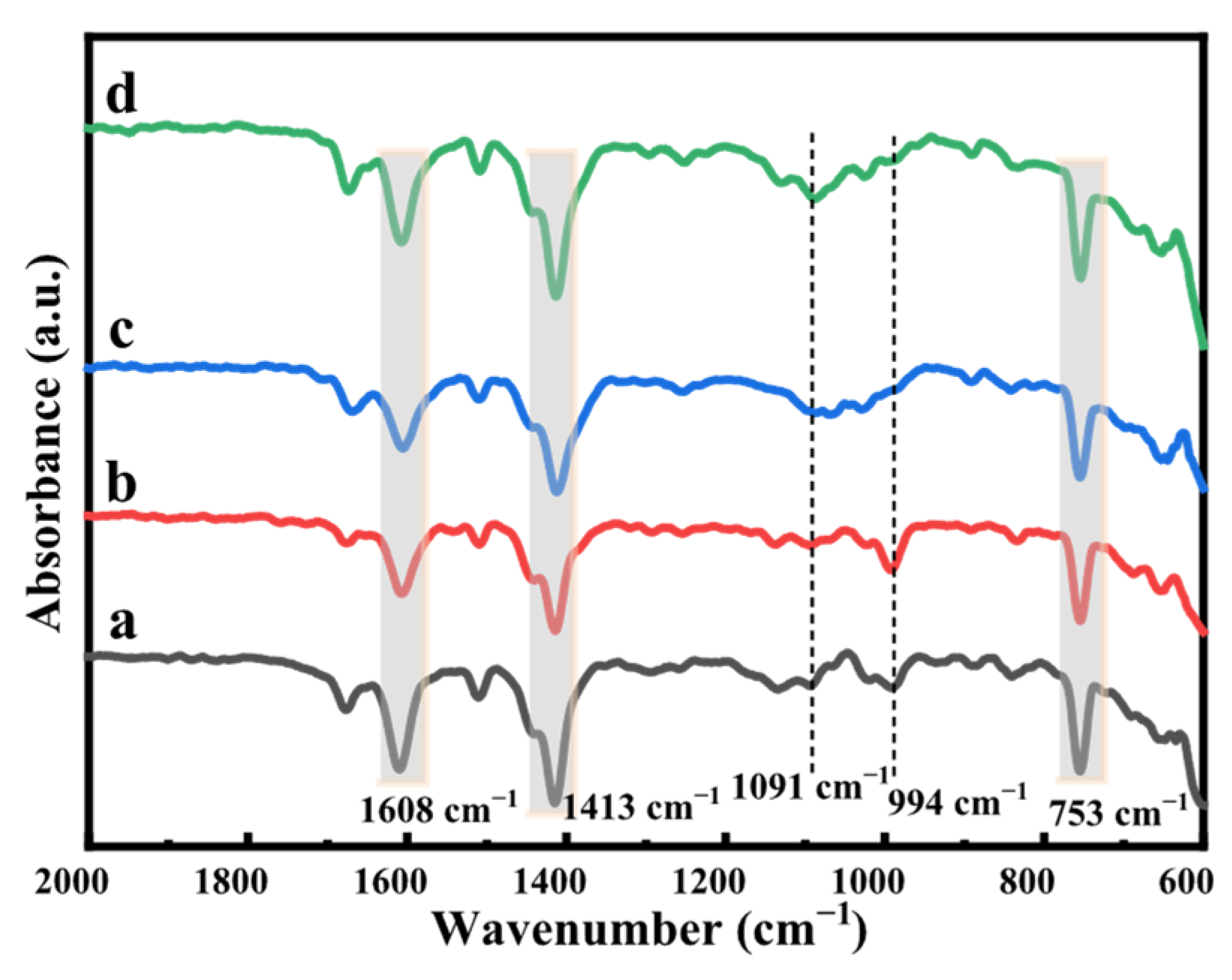
2.1. FT-IR Spectrum of MIL-68(Al)-Based Materials
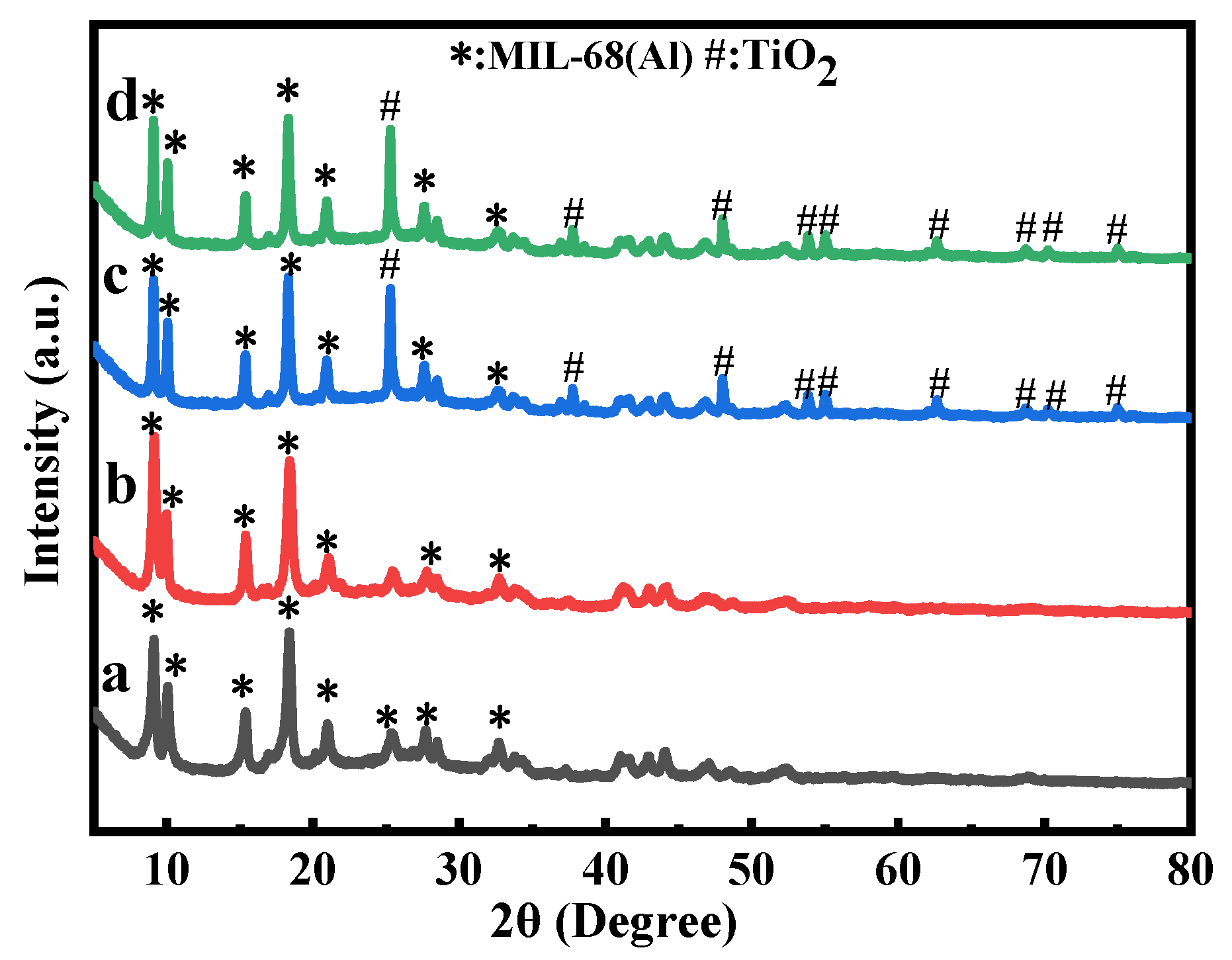
2.2. XRD of MIL-68(Al)-Based Materials
2.3. SEM and SEM Mapping of the MIL-68(Al)-Based Materials
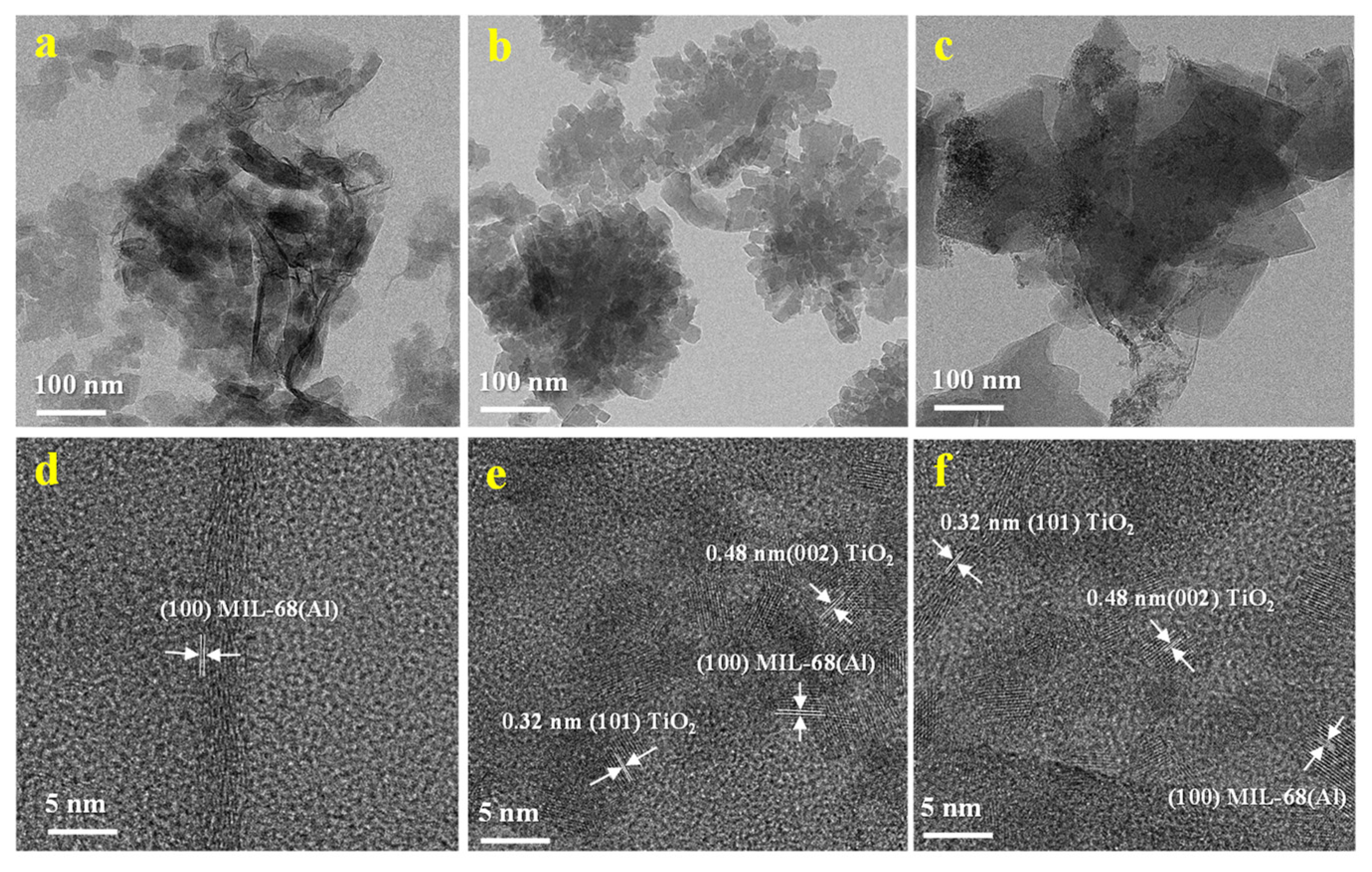
2.4. TEM of the MIL-68(Al)/GO/TiO2
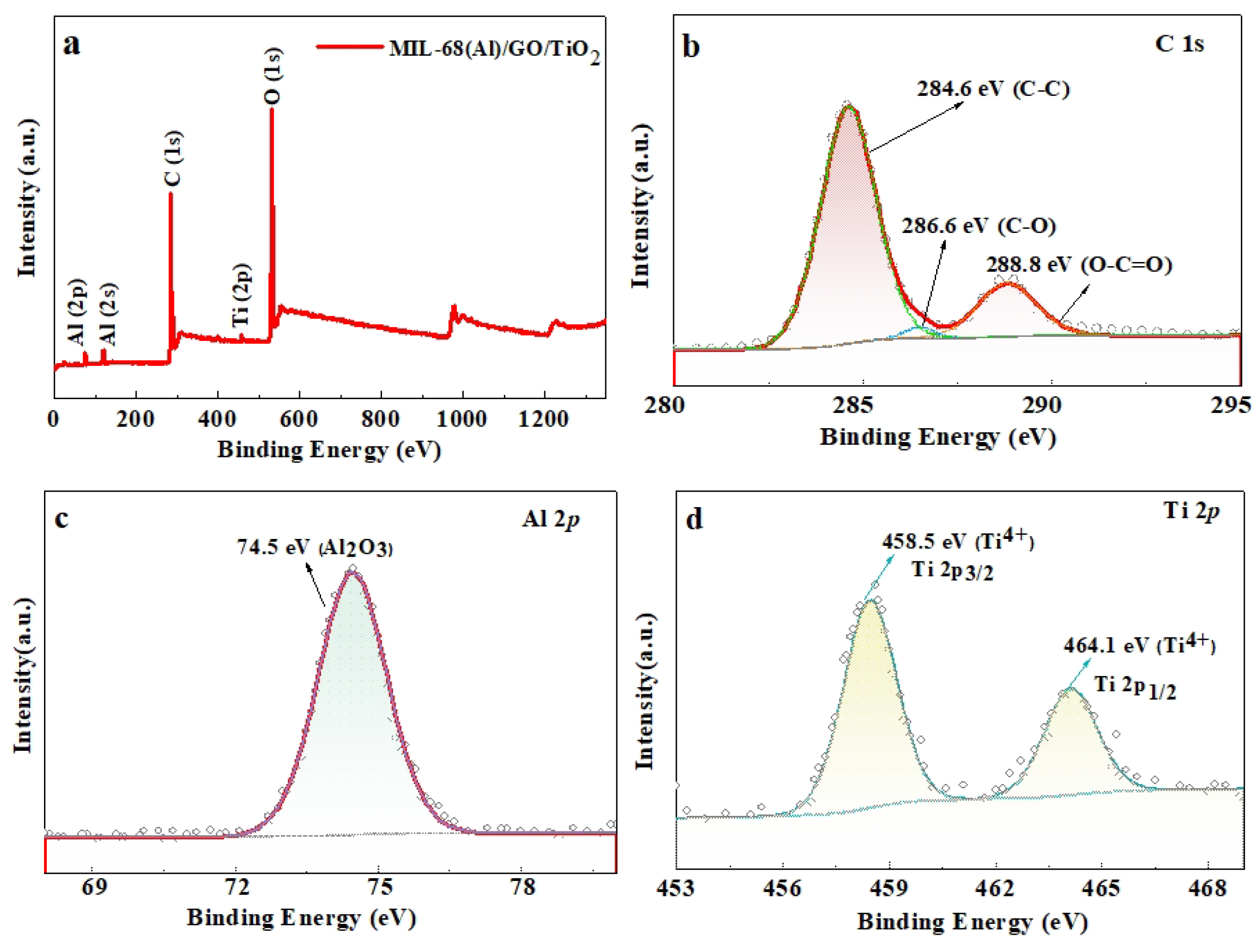
2.5. XPS of the MIL-68(Al)/GO/TiO2
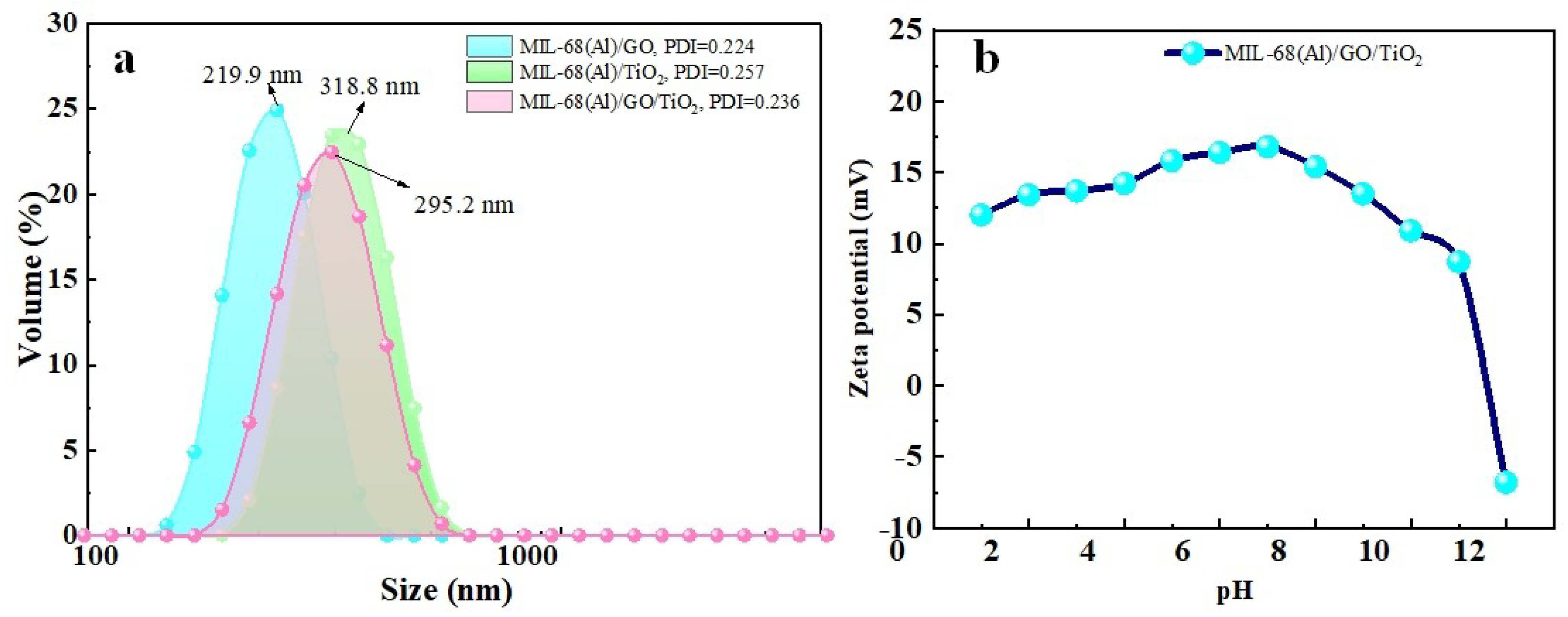
2.6. Particle Size Distribution and Zeta Potential of Materials
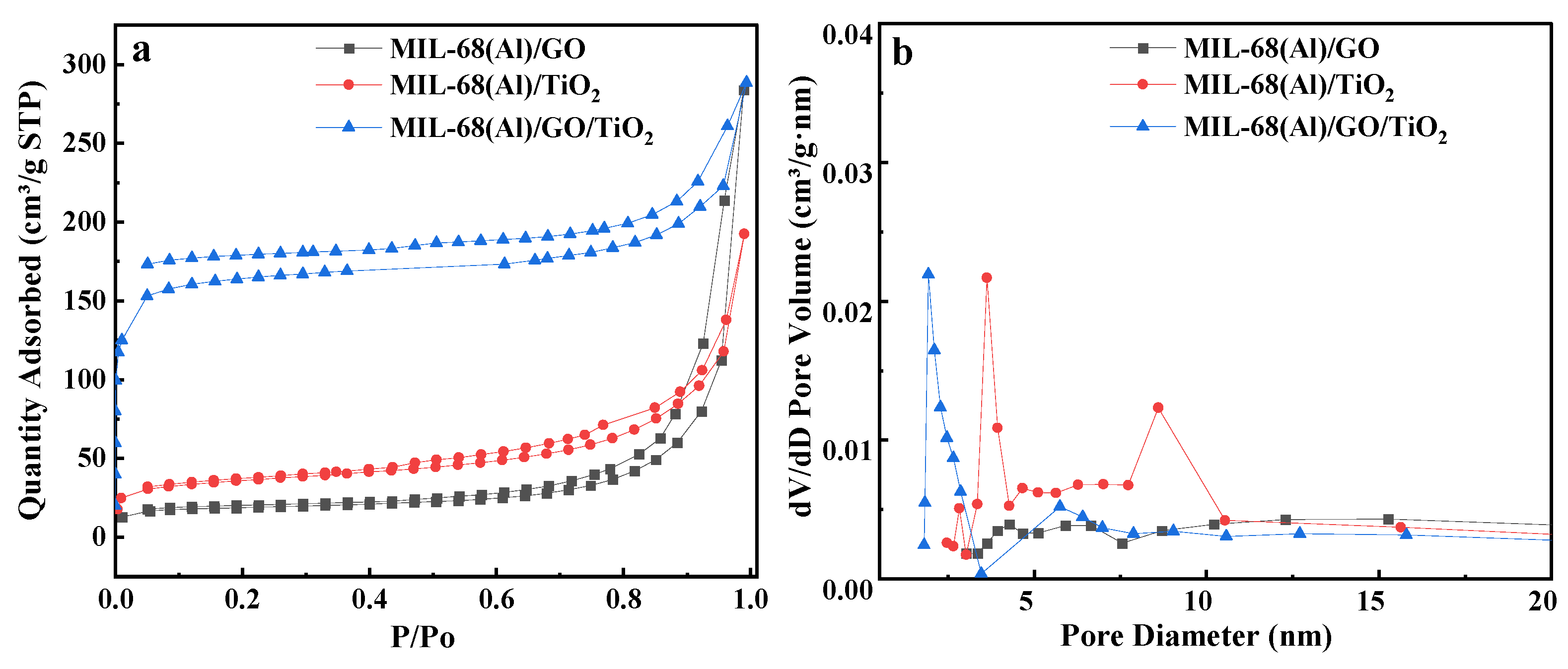
2.7. BET and Pore Diameter of the MIL-68(Al)-Based Materials
2.8. Adsorption Performance of MIL-68(Al)-Based Material
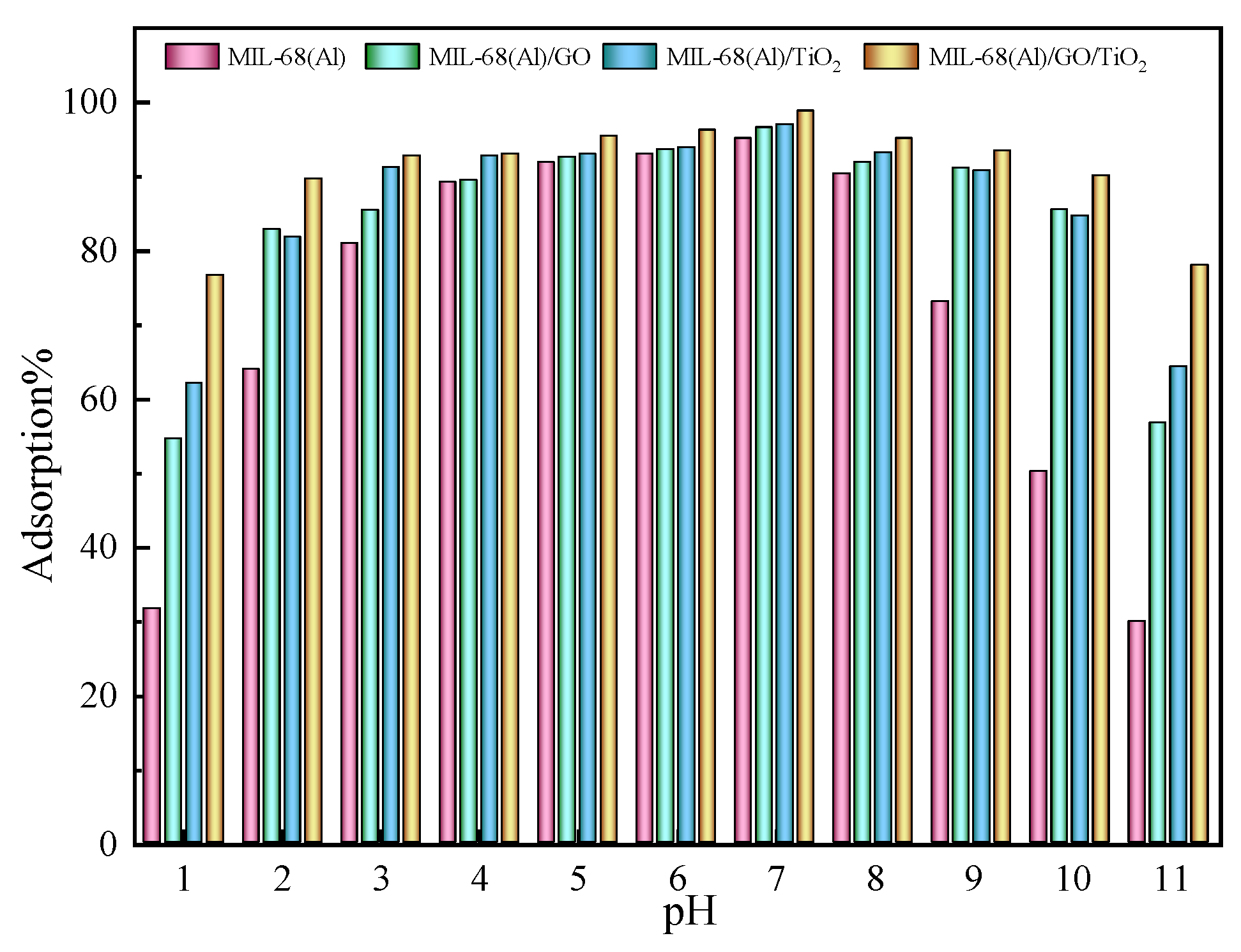
2.9. Effect of pH on MO Adsorption Efficiency
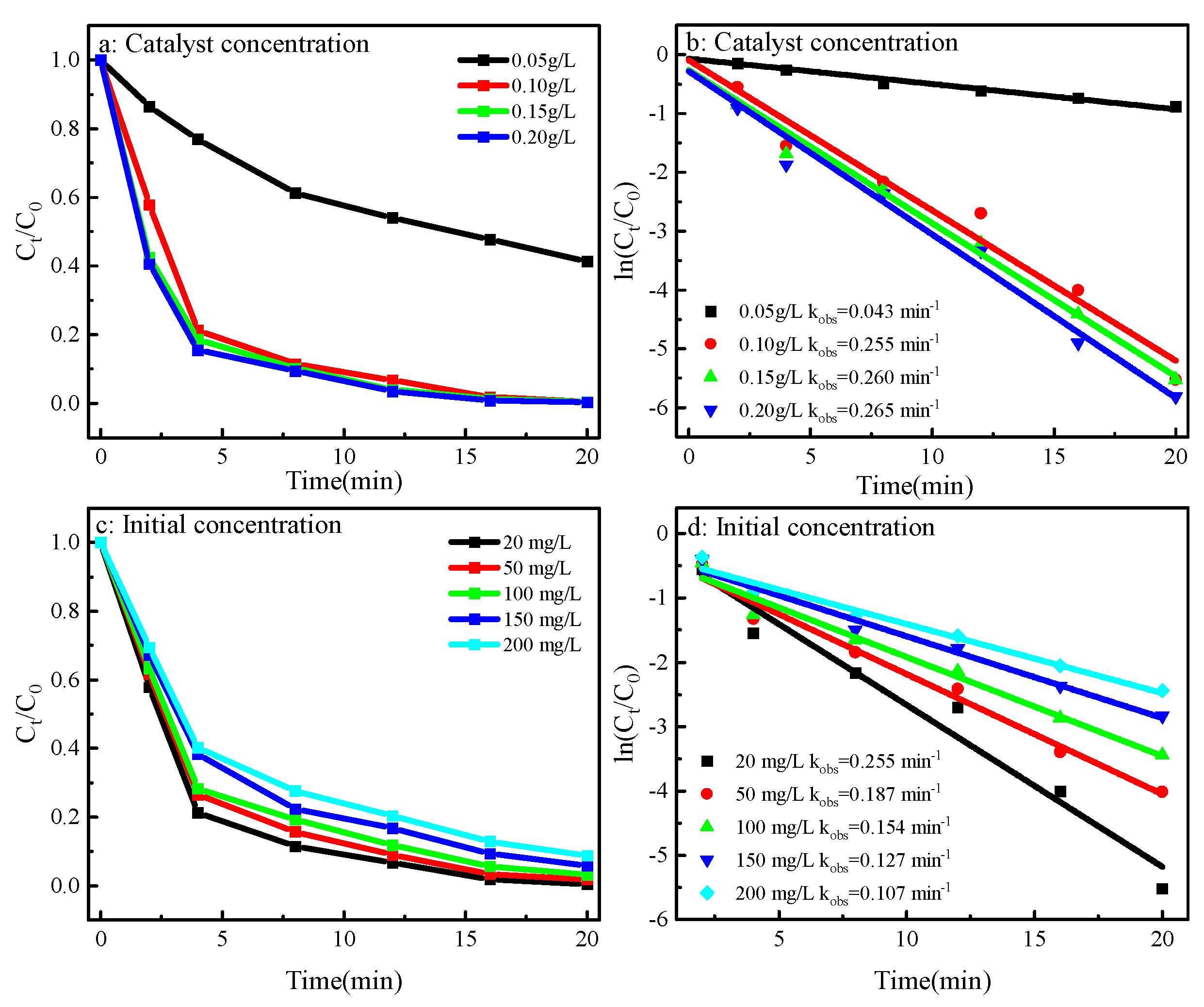
2.10. Influence of Catalyst Amount and Initial Concentration of MO
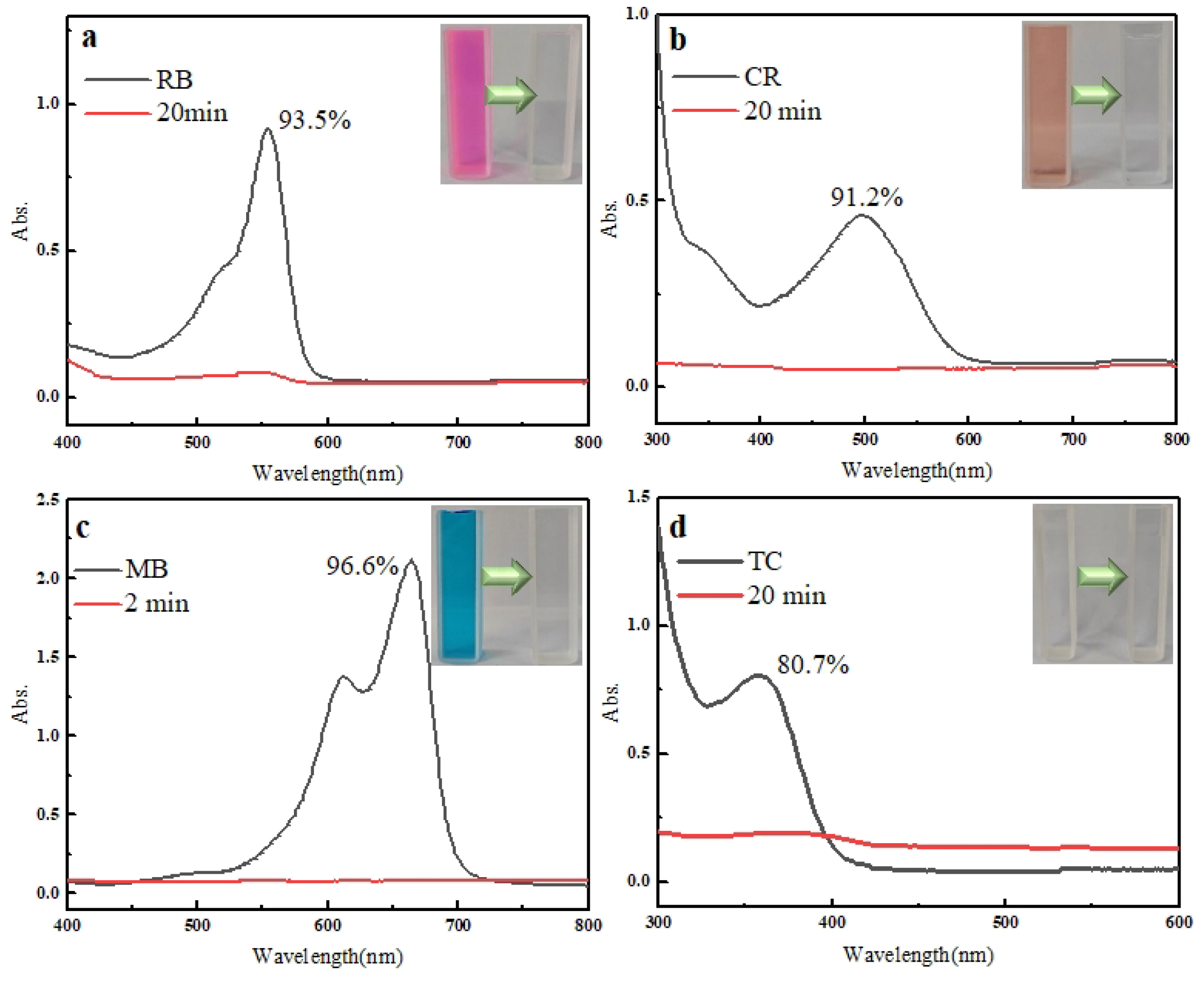
2.11. Adsorption Effects of Different Pollutants
2.12. Reuse of MIL-68(Al)/GO/TiO2 for MO
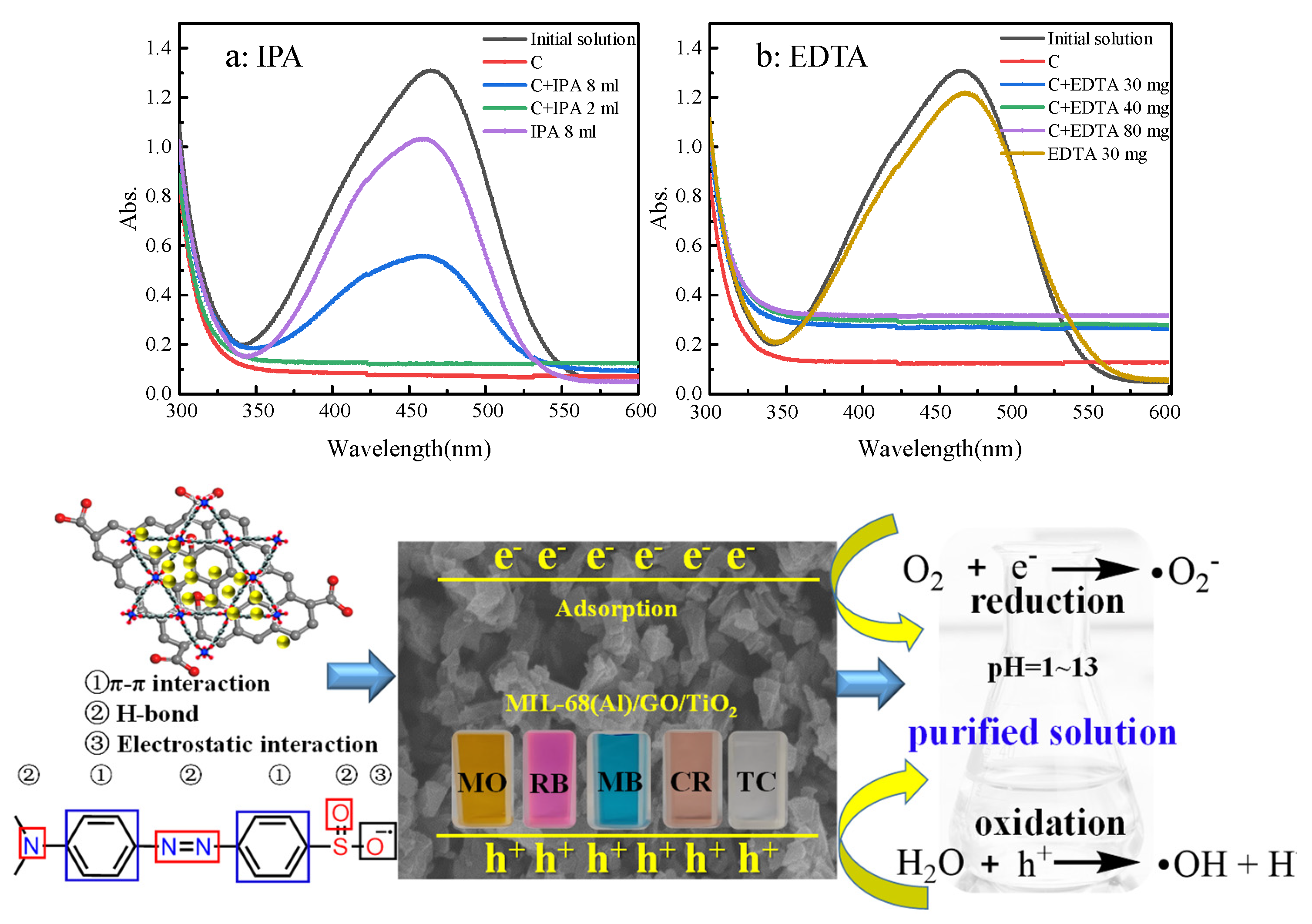
2.13. Adsorption Mechanism of MIL-68(Al)/GO/TiO2 for MO
3. Discussion
3.1. Comparison with Previously Reported Materials
3.2. Future Research
4. Materials and Methods
4.1. Materials
4.2. Analytical Methods
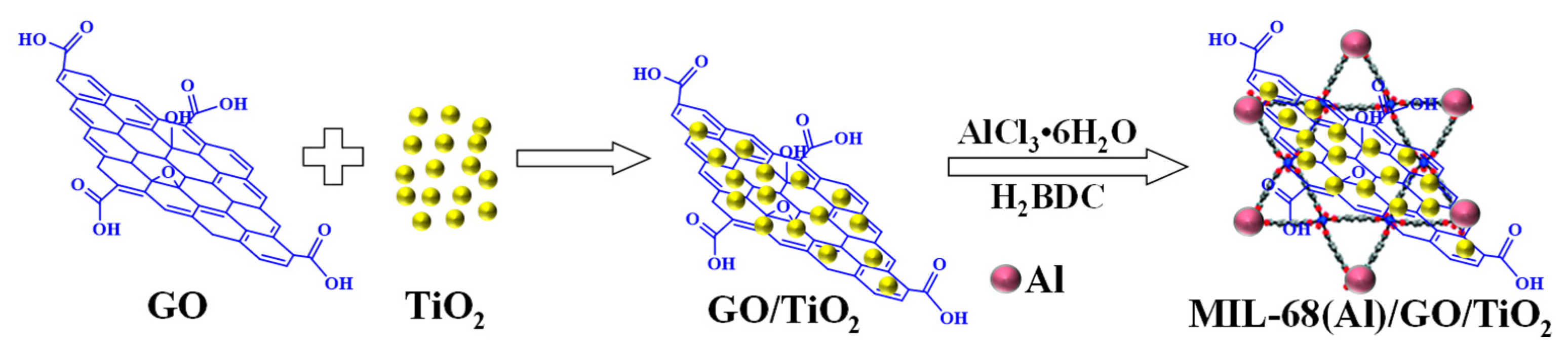
4.3. Preparation of Different Kinds of Catalysts
4.4. Catalytic Tests with Different MIL-68(Al)-Based Catalyst
4.5. Influence of Initial Concentration of MO
4.6. Adsorption of RB, CR, MB, and TC
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arutanti, O.; Sari, A.L.; Kartikowati, C.W.; Sari, A.A.; Arif, A.F. Design and application of homogeneous-structured TiO2/activated carbon nanocomposite for adsorption-photocatalytic degradation of MO. Water Air Soil Pollut. 2022, 233, 118. [Google Scholar] [CrossRef]
- Vasantham, A.; Thanigaimani, K.; Sudhakaran, R.; Mohan, S.; Arumugam, N.; Almansour, A.I.; Thenmozhi, P.A.; Mahalingam, S.M. High photo-recombination of Z-scheme h-BN@ZnO/TiO2 tube like nanocrystal towards debasement of toxic organic pollutants. Waste Biomass Valori 2024, 15, 6403–6414. [Google Scholar] [CrossRef]
- Wang, J.; Pi, H.; Zhao, P.; Zhou, N. Efficient removal of methyl orange and ciprofloxacin by reusable Eu-TiO2/PVDF membranes with adsorption and photocatalysis methods. RSC Adv. 2024, 14, 18432–18443. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Li, L.; Xu, Y.; Zuo, Y.; Li, G. Hybridization of brookite TiO2 with g-C3N4: A visible-light-driven photocatalyst for As3+ oxidation, MO degradation and water splitting for hydrogen evolution. J. Mater. Chem. A 2014, 2, 15774–15780. [Google Scholar] [CrossRef]
- Gadore, V.; Mishra, S.R.; Ahmaruzzaman, M. Green and environmentally sustainable fabrication of SnS2 quantum dots/chitosan nanocomposite for enhanced photocatalytic performance: Effect of process variables, and water matrices. J. Hazard. Mater. 2023, 444, 130301. [Google Scholar] [CrossRef]
- Hadi, Z.; Navarchian, A.H.; Rafienia, M. Synthesis of pH-responsive carboxymethyl chitosan for encapsulating tetracycline-HCl: Morphology, drug release behavior and antibacterial activity of microcapsules. J. Drug Deliv. Sci. Tec. 2023, 84, 104462. [Google Scholar] [CrossRef]
- Jiang, R.; Xiao, M.; Zhu, H.; Zhao, D.; Zang, X.; Fu, Y.; Zhu, J.; Wang, Q.; Liu, H. Sustainable chitosan-based materials as heterogeneous catalyst for application in wastewater treatment and water purification: An up-to-date review. Int. J. Biol. Macromol. 2024, 273, 133043. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Y.; Wang, M.; Chen, B.; Zhang, Y.; Sun, Y.; Chen, K.; Du, Q.; Wang, Y.; Pi, X.; et al. Efficient adsorption of Congo red by micro/nano MIL-88A (Fe, Al, Fe-Al)/chitosan composite sponge: Preparation, characterization, and adsorption mechanism. Int. J. Biol. Macromol. 2023, 239, 124157. [Google Scholar] [CrossRef]
- Han, B.; Gabriel, J.P. Thin-film nanocomposite (TFN) membrane technologies for the removal of emerging contaminants from wastewater. J. Clean. Prod. 2024, 480, 144043. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Wang, M.; Wang, X.; Cui, H.; Wei, J.; Li, X. The role of metal-organic frameworks in removing emerging contaminants in wastewater. J. Clean. Prod. 2023, 429, 139526. [Google Scholar] [CrossRef]
- Pesqueira, J.F.J.R.; Pereira, M.F.R.; Silva, A.M.T. Carbon-based composites in advanced wastewater treatment: A life cycle assessment of TiO2 and GO-TiO2 solar photocatalysis. J. Clean. Prod. 2024, 444, 140845. [Google Scholar] [CrossRef]
- Senkovska, I.; Bon, V.; Mosberger, A.; Wang, Y.; Kaskel, S. Adsorption and Separation by Flexible MOFs. Adv. Mater. 2025; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yin, B.H.; Huang, L.; Ding, L.; Lei, S.; Telfer, S.G.; Caro, J.; Wang, H. MOF membranes for gas separations. Prog. Mater. Sci. 2025, 151, 101432. [Google Scholar] [CrossRef]
- Wang, S.; Yu, Y.; Yu, J.; Wang, T.; Wang, P.; Li, Y.; Zhang, X.; Zhang, L.; Zhuang, H.; Chen, J. Encapsulation of metal oxide nanoparticles inside metal-organic frameworks via surfactant-assisted nanoconfined space. Nanotechnology 2020, 31, 255604. [Google Scholar] [CrossRef]
- Alfonso Herrera, L.Á.; Camarillo Reyes, P.K.; Huerta Flores, A.M.; Martínez, L.T.; Rivera Villanueva, J.M. BDC-Zn MOF sensitization by MO/MB adsorption for photocatalytic hydrogen evolution under solar light. Mat. Sci. Semicon Proc. 2020, 109, 104950. [Google Scholar] [CrossRef]
- Hyoun Ahn, C.; Seok Yang, W.; Jae Kim, J.; Sudha Priyanga, G.; Thomas, T.; Deshpande, N.G.; Seong Lee, H.; Koun Cho, H. Design of hydrangea-type Co/Mo bimetal MOFs and MOF-derived Co/Mo2C embedded carbon composites for highly efficient oxygen evolution reaction. Chem. Eng. J. 2022, 435, 134815. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Wang, Y.; Ho, W.H.; Gu, Y.; Chuang, C.; Song, Y.; Kung, C. Electrochemical Evolution of Pore-Confined Metallic Molybdenum in a Metal–Organic Framework (MOF) for All-MOF-Based Pseudocapacitors. ACS Appl. Energ. Mater. 2020, 3, 6258–6267. [Google Scholar] [CrossRef]
- Zhang, M.; Lin, C.; Jiang, Z.; Tseng, W.J. Incorporation of polyoxometalate in La-BTC metal-organic frameworks for fast and selective removal of methylene blue dyes in water. J. Solid. State Chem. 2022, 316, 123562. [Google Scholar] [CrossRef]
- Zia, A.; Neupane, M.; McGlone, A.; He, R.; Xin, R.; Liu, Y.; Yan, Q.; Wang, J.; Li, L.; Cai, Z.; et al. Coupling metal-organic frameworks and wood-based carbon for water remediation. Nano Res. 2024, 17, 5661–5669. [Google Scholar] [CrossRef]
- Li, B.; Wen, H.M.; Yu, Y.; Cui, Y.; Zhou, W.; Chen, B.; Qian, G. Nanospace within metal-organic frameworks for gas storage and separation. Mater. Today Nano 2018, 2, 21–49. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Fan, W.; Sun, D. Flexible metal-organic frameworks for gas storage and separation. Dalton Trans. 2022, 51, 4608–4618. [Google Scholar] [CrossRef]
- Chen, F.; Wang, J.; Guo, L.; Huang, X.; Zhang, Z.; Yang, Q.; Yang, Y.; Ren, Q.; Bao, Z. Carbon dioxide capture in gallate-based metal-organic frameworks. Sep. Purif. Technol. 2022, 292, 121031. [Google Scholar] [CrossRef]
- Yang, L.; Qian, S.; Wang, X.; Cui, X.; Chen, B.; Xing, H. Energy-efficient separation alternatives: Metal-organic frameworks and membranes for hydrocarbon separation. Chem. Soc. Rev. 2020, 49, 5359–5406. [Google Scholar] [CrossRef]
- Jin, L.; Qian, X.; Wang, J.; Aslan, H.; Dong, M. MIL-68 (In) nano-rods for the removal of congo red dye from aqueous solution. J. Colloid. Interf. Sci. 2015, 453, 270–275. [Google Scholar] [CrossRef]
- Ke, F.; Qiu, L.; Yuan, Y.; Peng, F.; Jiang, X.; Xie, A.; Shen, Y.; Zhu, J. Thiol-functionalization of metal-organic framework by a facile coordination-based postsynthetic strategy and enhanced removal of Hg2+ from water. J. Hazard. Mater. 2011, 196, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, H.; Xu, L. Application of zeolite MCM-22 for basic dye removal from wastewater. J. Colloid. Interf. Sci. 2006, 295, 71–78. [Google Scholar] [CrossRef]
- Basumatary, S.F.; Patir, K.; Das, B.; Saikia, P.; Brahma, S.; Basumatary, B.; Nath, B.; Basumatary, B.; Basumatary, S. Production of renewable biodiesel using metal organic frameworks based materials as efficient heterogeneous catalysts. J. Clean. Prod. 2022, 358, 131955. [Google Scholar] [CrossRef]
- Liu, D.; Jiang, P.; Li, X.; Liu, J.; Zhou, L.; Wang, X.; Zhou, F. 3D printing of metal-organic frameworks decorated hierarchical porous ceramics for high-efficiency catalytic degradation. Chem. Eng. J. 2020, 397, 125392. [Google Scholar] [CrossRef]
- Liu, Y.; Howarth, A.J.; Vermeulen, N.A.; Moon, S.; Hupp, J.T.; Farha, O.K. Catalytic degradation of chemical warfare agents and their simulants by metal-organic frameworks. Coordin Chem. Rev. 2017, 346, 101–111. [Google Scholar] [CrossRef]
- Nasser Abdelhamid, H.; Mathew, A.P. Cellulose-zeolitic imidazolate frameworks (CelloZIFs) for multifunctional environmental remediation: Adsorption and catalytic degradation. Chem. Eng. J. 2021, 426, 131733. [Google Scholar] [CrossRef]
- Vellingiri, K.; Philip, L.; Kim, K. Metal-organic frameworks as media for the catalytic degradation of chemical warfare agents. Coordin Chem. Rev. 2017, 353, 159–179. [Google Scholar] [CrossRef]
- Channab, B.; El Ouardi, M.; Ait Layachi, O.; Marrane, S.E.; El Idrissi, A.; BaQais, A.; Ait Ahsaine, H. Recent trends on MIL-Fe metal-organic frameworks: Synthesis approaches, structural insights, and applications in organic pollutant adsorption and photocatalytic degradation. Environmental Science. Nano 2023, 1, 2957–2988. [Google Scholar] [CrossRef]
- Fang, Z.; Liu, Y.; Qi, J.; Xu, Z.; Qi, T.; Wang, L. Establishing a high-speed electron transfer channel via CuS/MIL-Fe heterojunction catalyst for photo-Fenton degradation of acetaminophen. Appl. Catal. B Environ. 2023, 320, 121979. [Google Scholar] [CrossRef]
- Guo, X.; Li, X.; Huang, Y.; Peng, Y.; Hu, T.; Chen, Y.; Cheng, J. The photocatalytic degradation performance and mechanism of rod-like hollow derivatives from Co-doped MIL-68(In) under visible light. J. Solid. State Chem. 2024, 333, 124589. [Google Scholar] [CrossRef]
- Rahmani, M.; Abbasi, A.; Hosseini, M. Fabrication of visible light responsive nitrogen-doped carbon quantum dots/MIL-101(Cr) composites for enhanced photodegradation of Rhodamine B. J. Photoch Photobiolo A 2023, 445, 115019. [Google Scholar] [CrossRef]
- Rezaei, S.; Landarani-Isfahani, A.; Moghadam, M.; Tangestaninejad, S.; Mirkhani, V.; Mohammadpoor-Baltork, I. Mono- and multifold C-C coupling reactions catalyzed by a palladium complex encapsulated in MIL-Cr as a three dimensional nano reactor. RSC Adv. 2016, 6, 92463–92472. [Google Scholar] [CrossRef]
- Wu, S.; You, X.; Yang, C.; Cheng, J. Adsorption behavior of methyl orange onto an aluminum-based metal organic framework, MIL-68(Al). Water Sci. Technol. 2017, 75, 2800–2810. [Google Scholar] [CrossRef]
- Yang, C.; Wu, S.; Cheng, J.; Chen, Y. Indium-based metal-organic framework/graphite oxide composite as an efficient adsorbent in the adsorption of rhodamine B from aqueous solution. J. Alloy Compd. 2016, 687, 804–812. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, W.; Shi, J.; Zhao, Z.; Xia, Q.; Li, Y.; Wang, H.; Li, Z. A novel MOF/graphene oxide composite GrO@MIL-101 with high adsorption capacity for acetone. J. Mater. Chem. A 2014, 2, 4722–4730. [Google Scholar] [CrossRef]
- Compton, O.C.; Nguyen, S.T. Graphene oxide, highly reduced graphene oxide, and graphene: Versatile building blocks for carbon-based materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef]
- Meshkat, S.; Kaliaguine, S.; Rodrigue, D. Mixed matrix membranes based on amine and non-amine MIL-53(Al) in Pebax® MH-1657 for CO2 separation. Sep. Purif. Technol. 2018, 200, 177–190. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, G.; Li, L.; Wang, X.; Li, N.; Zhao, R.; Lin, J. Facile fabrication of MIL-96 as coating fiber for solid-phase microextraction of trihalomethanes and halonitromethanes in water samples. Chem. Eng. J. 2018, 350, 240–247. [Google Scholar] [CrossRef]
- Lei, M.; Wang, C.; Guo, J.; Zhang, G.; Zhu, L.; Zhou, Q.; Tang, H. In-situ preparation of magnetic palladium-iron oxide/graphene oxide and its efficient catalytic reduction and dechlorination of pentachlorobenzene. Sep. Purif. Technol. 2025, 362, 131638. [Google Scholar] [CrossRef]
- Li, C.; Liu, G.; Gao, C.; Yang, R.; Zakharov, O.; Hu, Y.; Yan, Y.; Geng, Y. Atomic-scale understanding of graphene oxide lubrication-assisted grinding of GaN crystals. Int. J. Mech. Sci. 2025, 286, 109934. [Google Scholar] [CrossRef]
- Rathore, H.K.; Das, C.; Biswas, G.; Kumar, M.; Sankhala, S.K.; Sarkar, D. Cerium oxide nanoparticles decorated on graphene oxide nanosheets as battery-type cathode material for supercapacitors. J. Colloid. Interf. Sci. 2025, 685, 280–290. [Google Scholar] [CrossRef]
- Wu, S.; Yu, L.; Xiao, F.; You, X.; Yang, C.; Cheng, J. Synthesis of aluminum-based MOF/graphite oxide composite and enhanced removal of methyl orange. J. Alloy Compd. 2017, 724, 625–632. [Google Scholar] [CrossRef]
- Li, C.; Sun, L.; Niu, J.; Reka, A.A.; Feng, P.; Garcia, H. Core-shell Bi-containing spheres and TiO2 nanoparticles co-loaded on kaolinite as an efficient photocatalyst for methyl orange degradation. Catal. Commun. 2023, 175, 106609. [Google Scholar] [CrossRef]
- Marpongahtun; Andriayani; Muis, Y.; Gea, S.; Amaturrahim, S.A.; Attaurrazaq, B.; Daulay, A.; Salmiati, S. Biomass-derived N-doped carbon dots/TiO2 for visible-light-induced degradation of methyl orange in wastewater. Chem. Afr. 2024, 7, 3319–3328. [Google Scholar] [CrossRef]
- Palliyalil, S.; Chola, R.K.V.; Vigneshwaran, S.; Poovathumkuzhi, N.C.; Chelaveettil, B.M.; Meenakshi, S. Ternary system of TiO2 confined chitosan-polyaniline heterostructure photocatalyst for the degradation of anionic and cationic dyes. Environ. Technol. Innov. 2022, 28, 102586. [Google Scholar] [CrossRef]
- Yue, X.; Hou, Y.; Cao, C.; Cao, X.; Song, H.; Jiang, Y.; Zhou, L.; He, Y.; Zheng, X.; Ma, L.; et al. Visible-light-driven photobiocatalytic synthesis of chiral sulfoxides over immobilized chloroperoxidase on magnetic yolk-shell Fe3O4@Cu-H-TiO2 photocatalyst. J. Clean. Prod. 2024, 478, 143992. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, H.; Yang, M. TiO2 nanoparticles supported on PMMA nanofibers for photocatalytic degradation of methyl orange. J. Colloid. Interf. Sci. 2017, 508, 500–507. [Google Scholar] [CrossRef]
- Ramasubbu, V.; Ram Kumar, P.; Chellapandi, T.; Madhumitha, G.; Mothi, E.M.; Shajan, X.S. Zn(II) porphyrin sensitized (TiO2@Cd-MOF) nanocomposite aerogel as novel photocatalyst for the effective degradation of methyl orange (MO) dye. Opt. Mater. 2022, 132, 112558. [Google Scholar] [CrossRef]
- Sun, L.; Que, Z.; Ruan, T.; Yuan, Z.; Gong, W.; Mei, S.; Chen, Z.; Liu, Y. The synthesis of Ag/TiO2 via the DC magnetron sputtering method and its application in the photocatalytic degradation of methyl orange in Na2SO4 Solution. Appl. Sci. 2024, 14, 4014. [Google Scholar] [CrossRef]
- Yu, L.; Cao, W.; Wu, S.; Yang, C.; Cheng, J. Removal of tetracycline from aqueous solution by MOF/graphite oxide pellets: Preparation, characteristic, adsorption performance and mechanism. Ecotox Environ. Safe 2018, 164, 289–296. [Google Scholar] [CrossRef]
- Hua, T.; Li, D.; Li, X.; Lin, J.; Niu, J.; Cheng, J.; Zhou, X.; Hu, Y. Synthesis of mesoporous-structured MIL-68(Al)/MCM-41-NH2 for methyl orange adsorption: Optimization and Selectivity. Environ. Res. 2022, 215, 114433. [Google Scholar] [CrossRef]
- Luo, Z.; Chen, H.; Wu, S.; Yang, C.; Cheng, J. Enhanced removal of bisphenol A from aqueous solution by aluminum-based MOF/sodium alginate-chitosan composite beads. Chemosphere 2019, 237, 124493. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, W.; Yao, Q.; Huang, L.; Chen, L.; Boury, B.; Chen, Z. Zn-free MOFs like MIL-53(Al) and MIL-125(Ti) for the preparation of defect-rich, ultrafine ZnO nanosheets with high photocatalytic performance. Appl. Catal. B Environ. 2019, 244, 719–731. [Google Scholar] [CrossRef]
- Cao, J.; He, P.; Mohammed, M.A.; Zhao, X.; Young, R.J.; Derby, B.; Kinloch, I.A.; Dryfe, R.A.W. Two-step electrochemical intercalation and oxidation of graphite for the mass production of graphene oxide. J. Am. Chem. Soc. 2017, 139, 17446–17456. [Google Scholar] [CrossRef]
- Li, W.; He, Y.; Bao, W.B.; Bao, H.L.; Li, D.Y.; Zhang, C.L.; Wang, M. Novel TiO2/GO/M-MMT nano-heterostructured composites exhibiting high photocatalytic activity. Front. Chem. 2023, 11, 1113186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Li, X.; Zhao, X.; Anning, C.; Crittenden, J.; Lyu, X. Photocatalytic water splitting of ternary graphene-like photocatalyst for the photocatalytic hydrogen production. Front. Env. Sci. Eng. 2020, 14, 69. [Google Scholar] [CrossRef]
- Gong, R.; Ye, J.; Dai, W.; Yan, X.; Hu, J.; Hu, X.; Li, S.; Huang, H. Adsorptive removal of methyl orange and methylene blue from aqueous solution with finger-citron-residue-based activated carbon. Ind. Eng. Chem. Res. 2013, 52, 14297–14303. [Google Scholar] [CrossRef]
- Xie, L.; Liu, D.; Huang, H.; Yang, Q.; Zhong, C. Efficient capture of nitrobenzene from waste water using metal-organic frameworks. Chem. Eng. J. 2014, 246, 142–149. [Google Scholar] [CrossRef]
- Lin, C.; Gao, Y.; Zhang, J.; Xue, D.; Fang, H.; Tian, J.; Zhou, C.; Zhang, C.; Li, Y.; Li, H. GO/TiO2 composites as a highly active photocatalyst for the degradation of methyl orange. J. Mater. Res. 2020, 35, 1307–1315. [Google Scholar] [CrossRef]
- Zuo, L.; Zhang, T.; Zhang, Z.; Hou, J.; Liu, G.; Du, J.; Li, L. A 3D binuclear salen-based multifunctional MOF: Degradation of MO dye and highly selective sensing of Fe3+. Inorg. Chem. Commun. 2019, 99, 113–118. [Google Scholar] [CrossRef]
- Hussain, A.; Maqsood, S.; Ji, R.; Zhang, Q.; Farooq, M.U.; Boota, M.; Umer, M.; Hashim, M.; Naeem, H.; Toor, Z.S.; et al. Investigation of transition metal-doped graphitic carbon nitride for MO dye degradation. Diam. Relat. Mater. 2023, 132, 109648. [Google Scholar] [CrossRef]
- Meraj, U.; Laiq, E.; Bushra, R.; Qurtulen; Ahmad, M.; Gupta, S.; Mujahid, M. Hydrothermal synthesis of okra waste-derived CQDs-CNTs nanocomposites: Potent catalysts for pollutant degradation of RhB and MO dyes and its interaction with lysozyme protein. Biomass Convers. Biorefinery 2025, 15, 20493. [Google Scholar] [CrossRef]
- Peng, W.; Cai, L.; Lu, Y.; Zhang, Y. Preparation of Mn-Co-MCM-41 molecular sieve with thermosensitive template and its degradation performance for rhodamine B. Catalyats 2023, 13, 991. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, W.; Zhu, Z.; Zhang, L.; Peng, W. Temperature-sensitive template for preparation of ZnO/CeO2 composite photocatalytic materials and its catalytic performance. Molecules 2024, 29, 3589. [Google Scholar] [CrossRef]
- Kang, J.H.; Kim, T.; Choi, J.; Park, J.; Kim, Y.S.; Chang, M.S.; Jung, H.; Park, K.T.; Yang, S.J.; Park, C.R. Hidden Second Oxidation Step of Hummers Method. Chem. Mater. 2016, 28, 756. [Google Scholar] [CrossRef]
- Bi, J.; Bao, K.; Zhao, M.; Zha, Y.; Chen, T.; Huang, C.; Lv, Z. Novel phenol-functionalized hypercrosslinked polymer for efficient tetracycline and iodine adsorption. Appl. Surf. Sci. 2025, 688, 162385. [Google Scholar] [CrossRef]
- Espargaró, A.; Llabrés, S.; Saupe, S.J.; Curutchet, C.; Luque, F.J.; Sabaté, R. On the binding of congo red to amyloid fibrils. Angew. Chem. Int. Ed. 2020, 59, 8104. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhu, J.; Xu, Y.; Zhang, L.; Liu, D.; Chen, C.; Sun, B. Photocatalytic adsorption of methylene blue using ZnO modified with nano-biochar derived from bacterial cellulose. Ceram. Int. 2025, 51, 948–956. [Google Scholar] [CrossRef]
- Xu, L.; Ge, Y.; Zhou, X.; Xing, M.; Wu, X.; Wang, Y. Study on the sonocatalytic removal of tetracycline by an type-II heterojunction CuS/FeWO4. J. Alloys Compd. 2025, 1015, 178826. [Google Scholar] [CrossRef]



| Catalyst | MO (mg L−1) | Time (min) | Removal (%) | Reference |
|---|---|---|---|---|
| Ag/TiO2 | 10 | 55 | 100 | [53] |
| N-CDs/TiO2 | 100 ppm | 20 | 62.5 | [48] |
| GO/TiO2 | 20 | 240 | 90.0 | [63] |
| Salen-based MOF | 20 | 250 | 87.9 | [64] |
| Activated carbon | 400 | 240 | 94.1 | [61] |
| Zn-doped g-C3N4 | 20 | 150 | 90.0 | [65] |
| CQD/CNTs | 10 | 40 | 99.1 | [66] |
| MIL-68(Al)/GO/TiO2 | 20–200 | 20 | 99.7 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, W.; Yang, W.; Wang, M.; Zhang, L.; Liu, X.; Zhang, Y. Optimizing the Microscopic Structure of MIL-68(Al) by Co-Doping for Pollutant Removal and Mechanism. Catalysts 2025, 15, 900. https://doi.org/10.3390/catal15090900
Peng W, Yang W, Wang M, Zhang L, Liu X, Zhang Y. Optimizing the Microscopic Structure of MIL-68(Al) by Co-Doping for Pollutant Removal and Mechanism. Catalysts. 2025; 15(9):900. https://doi.org/10.3390/catal15090900
Chicago/Turabian StylePeng, Wenju, Wenjie Yang, Meng Wang, Lin Zhang, Xianxiang Liu, and Yaoyao Zhang. 2025. "Optimizing the Microscopic Structure of MIL-68(Al) by Co-Doping for Pollutant Removal and Mechanism" Catalysts 15, no. 9: 900. https://doi.org/10.3390/catal15090900
APA StylePeng, W., Yang, W., Wang, M., Zhang, L., Liu, X., & Zhang, Y. (2025). Optimizing the Microscopic Structure of MIL-68(Al) by Co-Doping for Pollutant Removal and Mechanism. Catalysts, 15(9), 900. https://doi.org/10.3390/catal15090900







