Engineered NiCo2O4 Spinel Nanostructures for Enhanced Electrocatalytic Performance in Energy Storage and Non-Enzymatic Glucose Detection
Abstract
1. Introduction
2. Results and Discussion
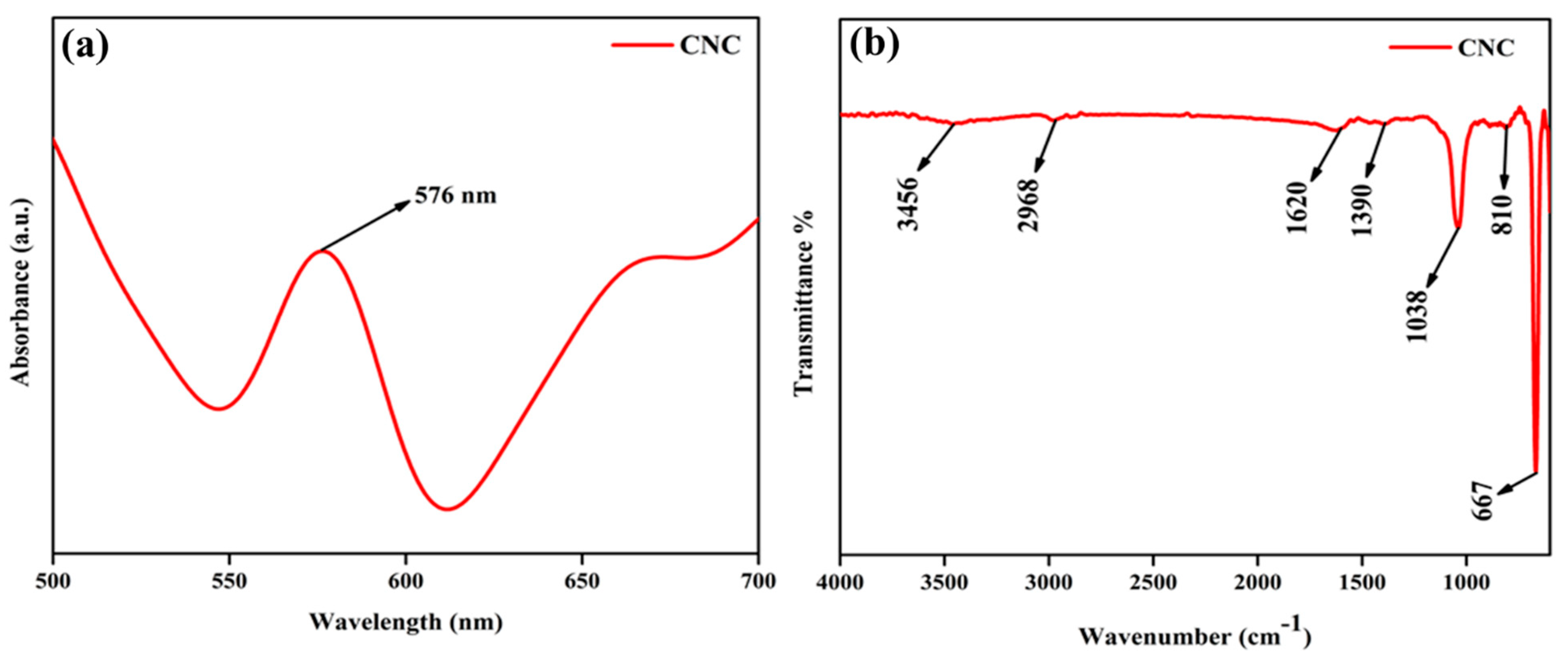
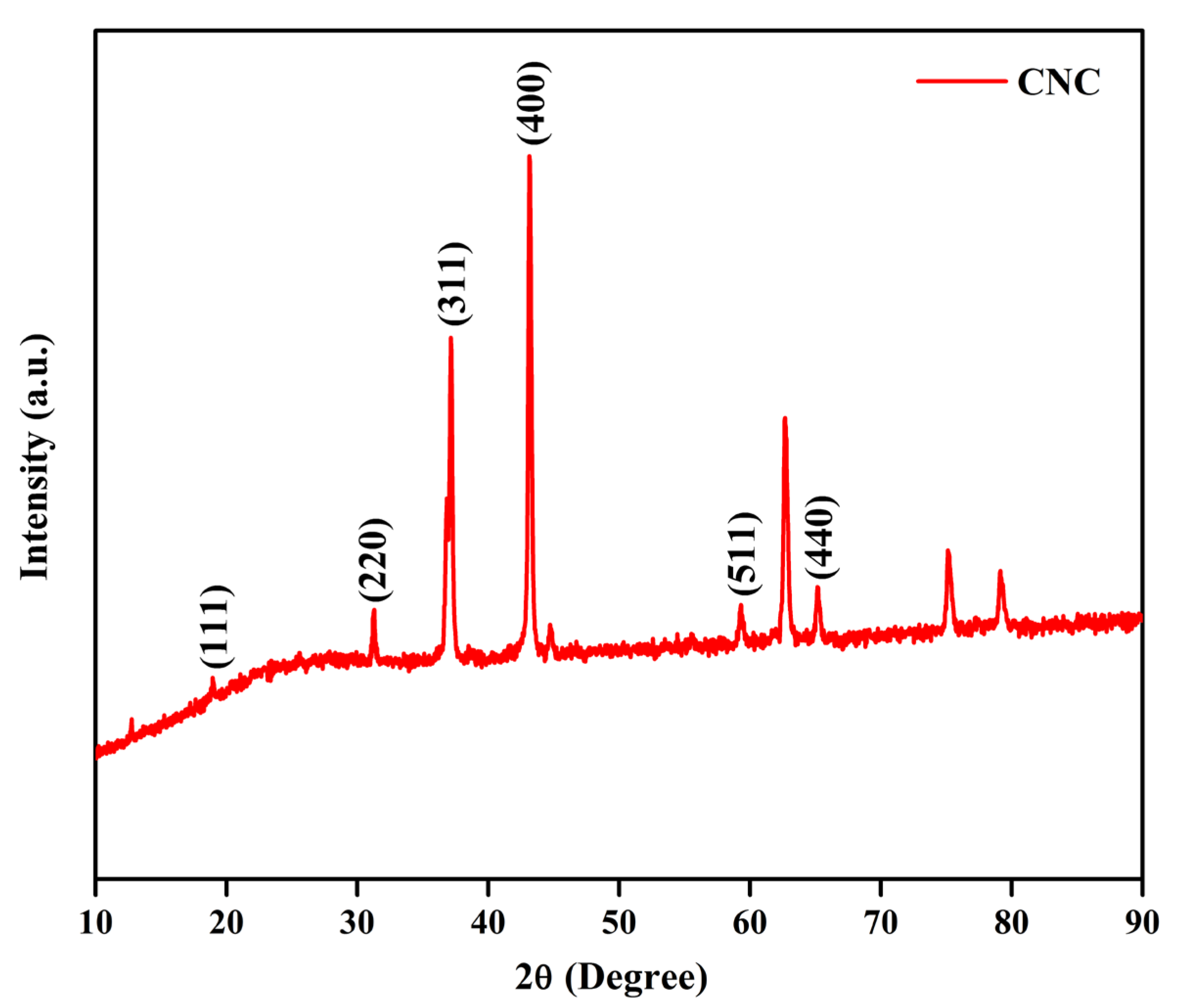
2.1. Structure, Optical, and Chemical Composition of the CNC
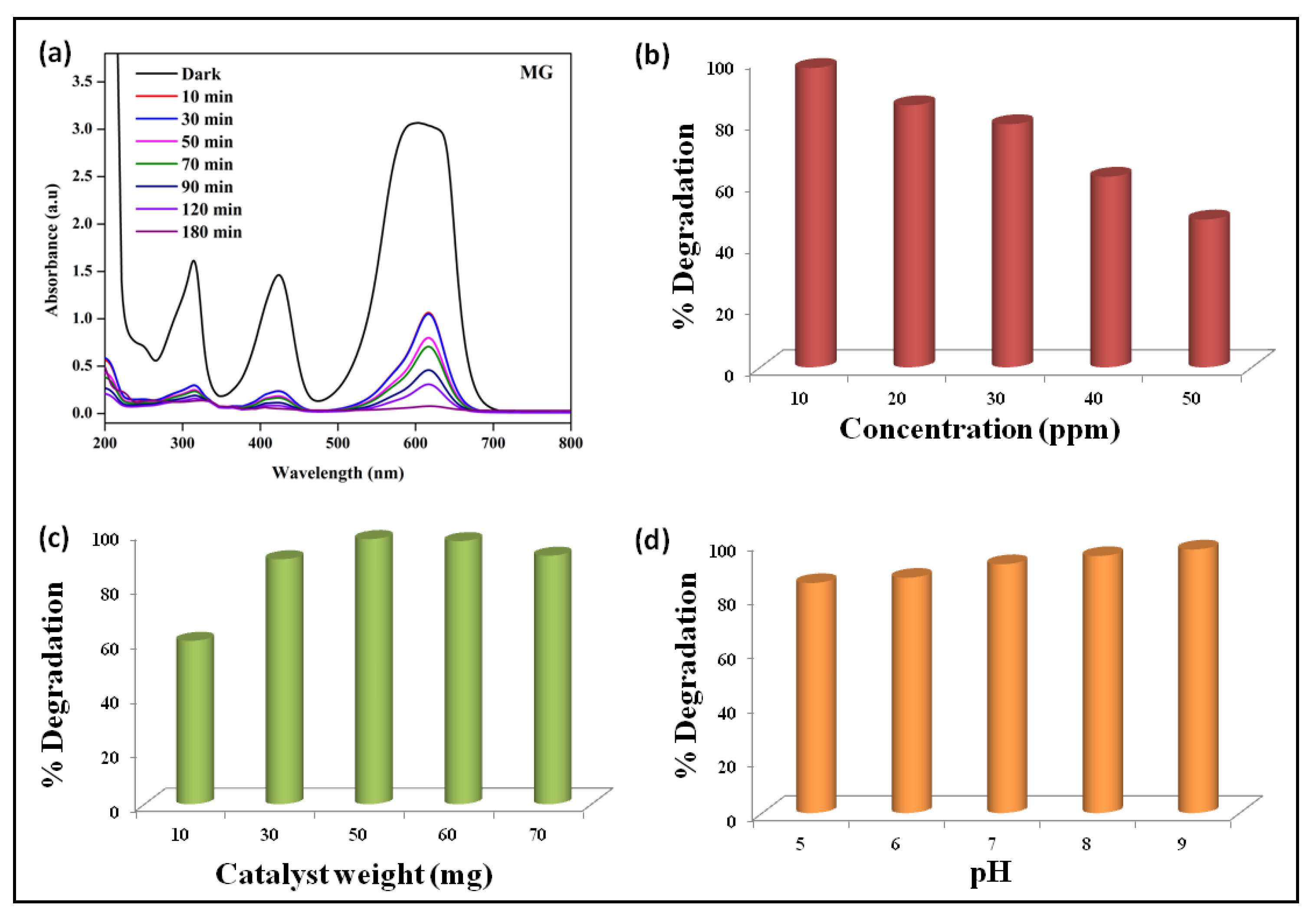
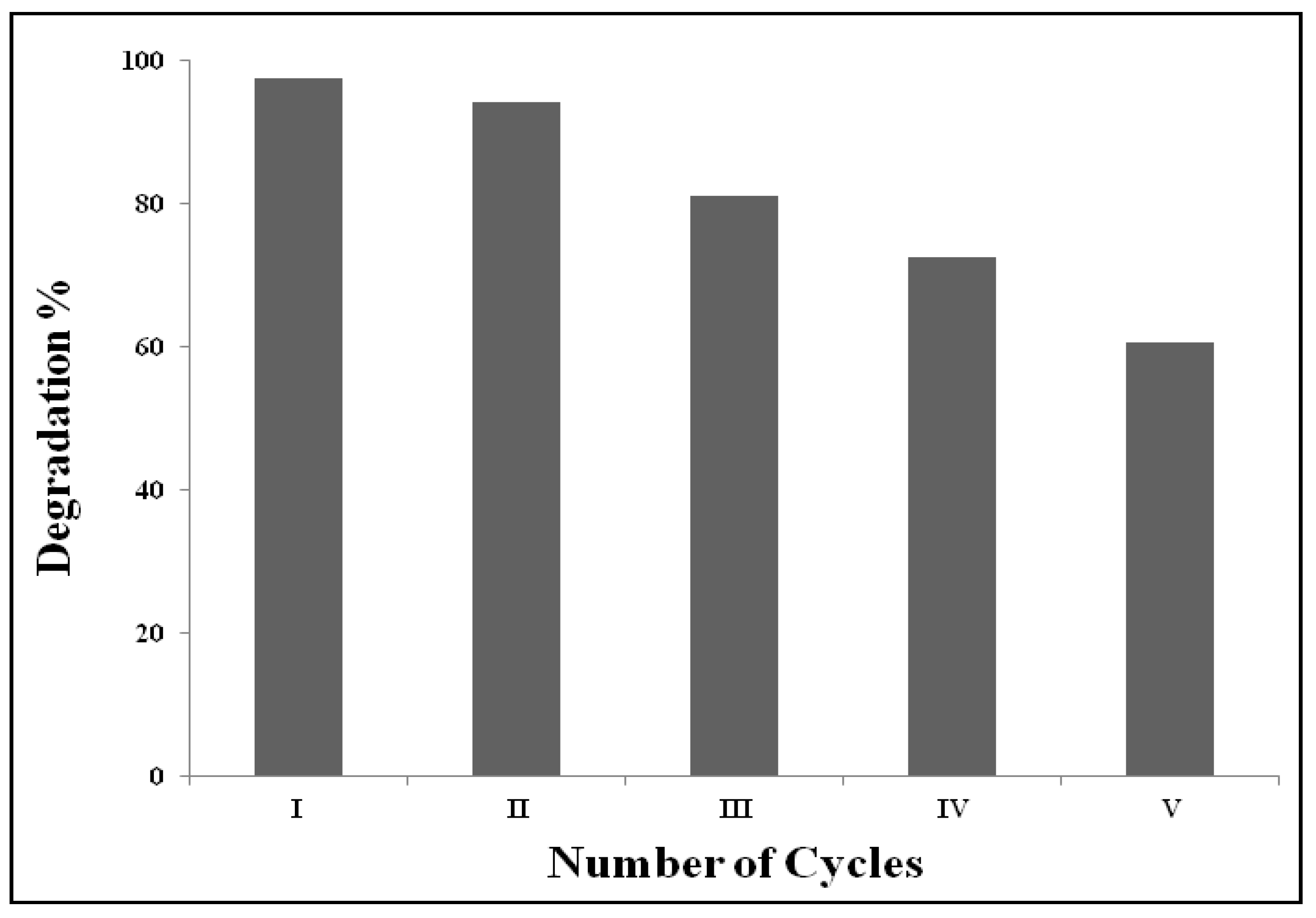
2.2. Photocatalytic Degradation of the MG
2.2.1. Effect of Concentration
2.2.2. Effect of Catalyst Weight
2.2.3. Effect of pH
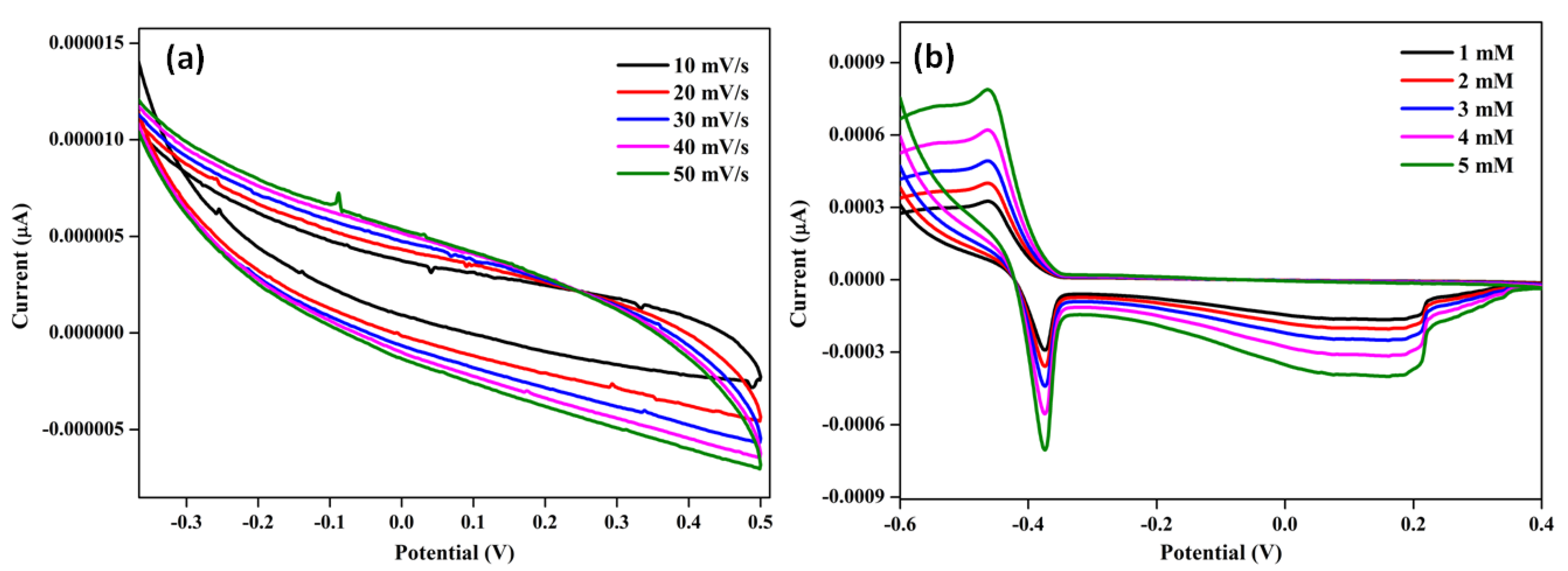
2.3. Glucose Sensing Performance of the CNC
3. Materials and Methods
3.1. Materials
3.2. Fabrication of the CNC
3.3. Methods
3.4. Photocatalytic Degradation of Malachite Green
3.5. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ansari, S.A.; Parveen, N.; Aljaafari, A.; Alshoaibi, A.; Alsulaim, G.M.; Alam, M.W.; Ansari, M.Z. Novel furfural-complexed approach to synthesizing carbon-Doped ZnO with breakthrough photocatalytic efficacy. J. Adv. Res. 2024, 73, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, E.M.; Amdeha, E. Enhanced photocatalytic degradation of malachite green dye by highly stable visible-light-responsive Fe-based tri-composite photocatalysts. Environ. Sci. Pollut. Res. 2022, 29, 69861–69874. [Google Scholar] [CrossRef]
- Xu, Z.; Zada, N.; Habib, F.; Ullah, H.; Hussain, K.; Ullah, N.; Bibi, M.; Bibi, M.; Ghani, H.; Khan, S.; et al. Enhanced photocatalytic degradation of mala-chite green dye using silver–manganese oxide nanoparticles. Molecules 2023, 28, 6241. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Chundawat, T.S.; Rawat, P.; Rao, G.K.; Vaya, D. Photocatalytic degradation of malachite green dye by ZnO and ZnO–β-cyclodextrin nanocomposite. Bull. Mater. Sci. 2021, 44, 1–8. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Clark, L.C., Jr.; Lyons, C. Electrode systems for continuos monitoring in cardio vascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef]
- Tonelli, D.; Gualandi, I.; Scavetta, E.; Mariani, F. Focus review on nanomaterial-based electrochemical sensing of glu-cose for health applications. Nanomaterials 2023, 13, 1883. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Salehi, R.; Arami, M.; Bahrami, H. Dye removal from colored textile wastewater using chitosan in binary systems. Desalination 2011, 267, 64–72. [Google Scholar] [CrossRef]
- Kumar, V.G.; Gokavarapu, S.D.; Rajeswari, A.; Dhas, T.S.; Karthick, V.; Kapadia, Z.; Shrestha, T.; Barathy, I.A.; Roy, A.; Sinha, S. Facile green synthesis of gold nanoparticles using leaf extract of antidiabetic potent Cassia auriculata. Colloid Surf. B. 2011, 87, 159–163. [Google Scholar] [CrossRef]
- Singh, J.; Dhaliwal, A.S. Plasmon-induced photocatalytic degradation of methylene blue dye using biosynthesized silver nanoparticles as photocatalyst. Environ. Technol. 2018, 41, 1520–1534. [Google Scholar] [CrossRef]
- Alam, M.W.; Allag, N.; Utami, M.; Waheed-Ur-Rehman, M.; Al-Othoum, M.A.S.; Sadaf, S. Facile Green Synthesis of α-Bismuth Oxide Nanoparticles: Its Photocatalytic and Electrochemical Sensing of Glucose and Uric Acid in an Acidic Medium. J. Compos. Sci. 2024, 8, 47. [Google Scholar] [CrossRef]
- Hammad, A.S.; El-Bery, H.M.; El-Shazly, A.H.; Elkady, M.F. Effect of WO3 morphological structure on its photoelec-trochemical properties. Int. J. Electrochem. Sci. 2018, 13, 362–372. [Google Scholar] [CrossRef]
- Chandrasekar, S.; Nivetha, A.; Govindhan, T.; Palanisamy, S.; Chennappan, S.; Jayachandran, H.; Inbaraj, P. Multiprocessing Substrates Enhanced Ta2O5/NiCo2O4 Spinel Nanocomposites for Effective Electro-/Photocatalytic and Toxicological Effects via the Caenorhabditis elegans Model. Langmuir 2024, 40, 6077–6093. [Google Scholar] [CrossRef] [PubMed]
- Nivetha, A.; Prabha, I. Surfactant-Enhanced Nano Spinel Oxide for Applications in Catalysis, Dye Degradation and Antibacterial Activity. ChemistrySelect 2022, 7, e202202389. [Google Scholar] [CrossRef]
- Katubi, K.M.; Rasheed, A.; Ihsan, A.; Shaheen, B.; Alrowaili, Z.; Al-Buriahi, M.; Din, M.I.; Shakir, I.; Munir, S. Neodymium-doped nickel cobaltite reinforced with 2D MXene nanocomposite (Nd-NiCo2O4/MXene) for enhanced photocatalytic degradation of the organic pollutants. Opt. Mater. 2024, 152, 115390. [Google Scholar] [CrossRef]
- Jang, K.-B.; Park, K.R.; Kim, K.M.; Hyun, S.-K.; Jeon, J.; Song, Y.S.; Park, S.-K.; Moon, K.-I.; Ahn, C.; Lim, S.-C.; et al. Synthesis of NiCo2O4 Nanostructures and Their Electrochemial Properties for Glucose Detection. Nanomaterials 2020, 11, 55. [Google Scholar] [CrossRef]
- Sahoo, L.; Mohapatra, N. Tailoring magnetic properties of α-MnO2@NiCo2O4 core/shell nanostructure. J. Mater. Sci. Mater. Electron. 2024, 35, 1–16. [Google Scholar] [CrossRef]
- Kumar, M.S.; Yasoda, K.Y.; Kothurkar, N.K.; Batabyal, S.K. Simple synthesis route of glycine-assisted PANi-NiCo2O4 porous powder for electrochemical application. Ionics 2019, 25, 4499–4507. [Google Scholar] [CrossRef]
- Sivashanmugam, G.; Anbusrinivasan, P. CTAB-templated synthesis and characterization of nanorod-shaped NiCo2O4 crystals for supercapacitor application. Chem. Pap. 2019, 74, 1309–1319. [Google Scholar] [CrossRef]
- Nivetha, A.; Sakthivel, C.; Hemalatha, J.; Senthamil, C.; Prabha, I. Fabrication of a bifunctionalyzed Calotropis gigantea inspired Ag–Cu–Co trimetal oxide for the remediation of methylene blue, and its larvicidal and antibacterial applications. New J. Chem. 2023, 47, 12375–12392. [Google Scholar] [CrossRef]
- Anandan, K.; Rajendran, V. Structural, optical and magnetic properties of well-dispersed NiO nanoparticles synthesized by CTAB assisted solvothermal process. Int. J. Nanosci. Nanotechnol. 2012, 2, 24–29. [Google Scholar]
- Lal, S.; Asha, P.; Divyarani, K.; Raghu, M.; Devi, V.A.; Alharthi, F.A.; Nabgan, W.; Sreenivasa, S.; Kumar, S.; Jeon, B.-H.; et al. Enhancing photocatalytic, photoelectrochemical hydrogen evolution, and dye degradation using p-type NiCo2O4 spinel photocatalyst synthesized via tapioca leaf extract mediated process. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132262. [Google Scholar] [CrossRef]
- Naz, A.; Bibi, I.; Majid, F.; Dahshan, A.; Jilani, K.; Taj, B.; Ghafoor, A.; Nazeer, Z.; Alzahrani, F.M.; Iqbal, M. Cu and Fe doped NiCo2O4/g-C3N4 nanocomposite ferroelectric, magnetic, dielectric and optical properties: Visible light-driven photocatalytic degradation of RhB and CR dyes. Diam. Relat. Mater. 2023, 141, 110592. [Google Scholar] [CrossRef]
- Sirach, R.; Dave, P.N. Artificial neural network modelling and experimental investigations of malachite green adsorption on novel carboxymethyl cellulose/β-cyclodextrin/nickel cobaltite composite. Heliyon 2024, 10, e33820. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Yi, J.; Liang, S.; Zhang, F.; Zhang, Y.; Lv, S.-W. Architecting an indirect Z-scheme NiCo2O4@CdS–Ag photocatalytic system with enhanced charge transfer for high-efficiency degradation of emerging pollutants. Environ. Res. 2022, 208, 112739. [Google Scholar] [CrossRef]
- Shafique, M.; Iqbal, T.; Mahmood, H.; Khan, M.A.; Naeem, M.; Ahmed, I.; Ahmad, P. Surfactant-assisted synthesis of NiCo2O4/NiO nanocomposite by facile atmospheric pressure microplasma electrochemical process with photocatalytic applications. J. Mater. Sci. Mater. Electron. 2021, 32, 17865–17875. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Yang, H.; Sui, T.; Tang, F.; Mu, J.; Chang, Z. Enhanced interface charge transfer through heterostructure cou-pling of NiO/NiCo2O4 and carbon layer for photocatalysis. J. Phys. Chem. Solids 2025, 197, 112404. [Google Scholar] [CrossRef]
- Avinash, B.; Ravikumar, C.; Kumar, M.A.; Nagaswarupa, H.; Santosh, M.; Bhatt, A.S.; Kuznetsov, D. Nano CuO: Electrochemical sensor for the determination of paracetamol and d-glucose. J. Phys. Chem. Solids 2019, 134, 193–200. [Google Scholar] [CrossRef]
- Zhao, J.; Zheng, C.; Gao, J.; Gui, J.; Deng, L.; Wang, Y.; Xu, R. Co3O4 nanoparticles embedded in laser-induced graphene for a flexible and highly sensitive enzyme-free glucose biosensor. Sens. Actuator B-Chem. 2021, 347, 130653. [Google Scholar] [CrossRef]
- Wang, M.; Liu, F.; Zhang, Z.; Meng, E.; Gong, F.; Li, F. Co3O4 nanoparticles as a noninvasive electrochemical sensor for glucose detection in saliva. Nano 2021, 16, 2150009. [Google Scholar] [CrossRef]
- Hussein, B.A.; Tsegaye, A.A.; Shifera, G.; Taddesse, A.M. A sensitive non-enzymatic electrochemical glucose sensor based on a ZnO/Co3O4/reduced graphene oxide nanocomposite. Sens. Diagn 2022, 2, 347–360. [Google Scholar] [CrossRef]
- Ahamad, N.; Banerjee, S.; Wei, C.-C.; Lu, K.-C.; Khedulkar, A.P.; Jian, W.-B.; Mahmood, S.; Chu, C.-W.; Lin, H.-C. Flexible Non-Enzymatic Glucose Sensors: One-Step Green Synthesis of NiO Nanoporous Films via an Electro-Exploding Wire Technique. ACS Appl. Mater. Interfaces 2024, 16, 64494–64504. [Google Scholar] [CrossRef]
- Alam, M.W.; Al Qahtani, H.S.; Souayeh, B.; Ahmed, W.; Albalawi, H.; Farhan, M.; Abuzir, A.; Naeem, S. Novel Copper-Zinc-Manganese Ternary Metal Oxide Nanocomposite as Heterogeneous Catalyst for Glucose Sensor and Antibacterial Activity. Antioxidants 2022, 11, 1064. [Google Scholar] [CrossRef]


| S. No | Material | Pollutant | Efficiency and Time | Ref. |
|---|---|---|---|---|
| 1 | Cu- and Fe-doped NiCo2O4/g-C3N4 | Rhodamine B and Congo Red | 89% and 120 min | [23] |
| 2 | carboxymethyl cellulose/β-cyclodextrin/NiCo2O4 | Bisphenol-A Malachite Green Congo Red | <40% and 170 min 90% and 170 min No adsorption | [24] |
| 3 | NiCo2O4@CdS–Ag | Ofloxacin | 99.14% | [25] |
| 4 | NiCo2O4/NiO | Rhodamine B | 83.66% and 120 min | [26] |
| 5 | NiO/NiCo2O4 | Rhodamine B and Methylene Blue | 94.75% and 120 min 93.55% and 120 min | [27] |
| 6 | CNC | Malachite Green | 97.62% and 180 min | Present Work |
| S. No | Catalyst | Sample | Efficiency | Ref. |
|---|---|---|---|---|
| 1 | Graphene/Co3O4 | Enzyme-free glucose | 214 µA mM−1 cm−2 at 0.49 s | [29] |
| 2 | Co3O4 | Glucose in saliva | 2495.79 µA mM−1 cm−2 | [30] |
| 3 | ZnO/Co3O4/Graphene oxide | Enzyme-free glucose | 1551.38 µA mM−1 cm−2 at 3 s | [31] |
| 4 | NiO nanoporous film | Enzyme-free glucose | 1202 µA mM−1 cm−2 | [32] |
| 5 | CNC | Enzyme-free glucose | 159 µA mM−1 0.38 mM | Present Work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nivetha, A.; Rakesh, S.S.; Chidambaram, P.P.; Al Naim, A.F.; Parveen, N.; Alagarswamy, S.; Ansari, S.A.; Alam, M.W. Engineered NiCo2O4 Spinel Nanostructures for Enhanced Electrocatalytic Performance in Energy Storage and Non-Enzymatic Glucose Detection. Catalysts 2025, 15, 899. https://doi.org/10.3390/catal15090899
Nivetha A, Rakesh SS, Chidambaram PP, Al Naim AF, Parveen N, Alagarswamy S, Ansari SA, Alam MW. Engineered NiCo2O4 Spinel Nanostructures for Enhanced Electrocatalytic Performance in Energy Storage and Non-Enzymatic Glucose Detection. Catalysts. 2025; 15(9):899. https://doi.org/10.3390/catal15090899
Chicago/Turabian StyleNivetha, Ambikapathi, Srirangarayan Subramanian Rakesh, Prabu P. Chidambaram, Abdullah F. Al Naim, Nazish Parveen, Senthil Alagarswamy, Sajid Ali Ansari, and Mir Waqas Alam. 2025. "Engineered NiCo2O4 Spinel Nanostructures for Enhanced Electrocatalytic Performance in Energy Storage and Non-Enzymatic Glucose Detection" Catalysts 15, no. 9: 899. https://doi.org/10.3390/catal15090899
APA StyleNivetha, A., Rakesh, S. S., Chidambaram, P. P., Al Naim, A. F., Parveen, N., Alagarswamy, S., Ansari, S. A., & Alam, M. W. (2025). Engineered NiCo2O4 Spinel Nanostructures for Enhanced Electrocatalytic Performance in Energy Storage and Non-Enzymatic Glucose Detection. Catalysts, 15(9), 899. https://doi.org/10.3390/catal15090899








