In Situ Growth of Magnesium Oxide Nanoparticles on ITO Electrodes as Electrocatalysts for Detecting Bisphenol A in Thermal Paper
Abstract
1. Introduction
2. Results and Discussion
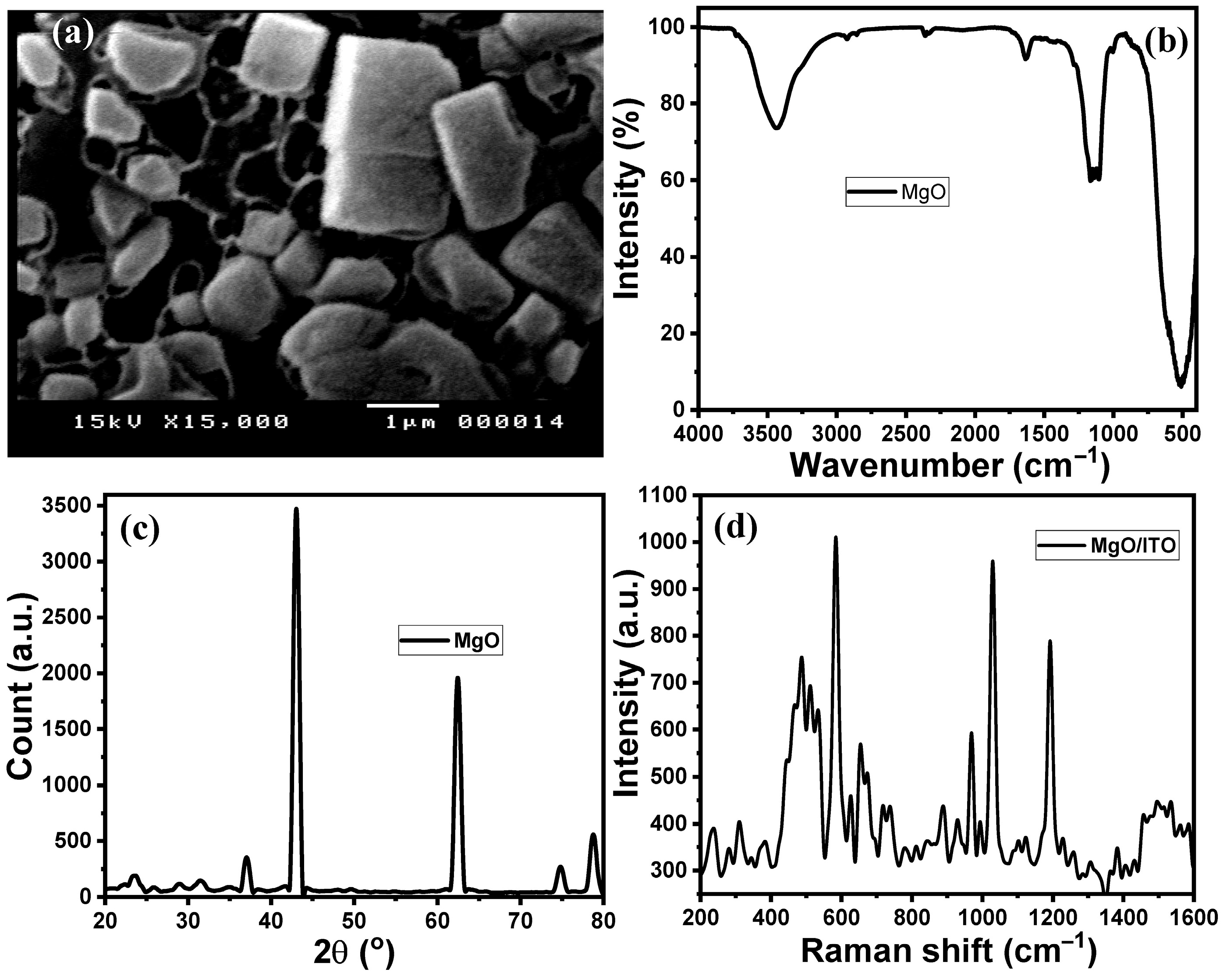
2.1. MgO NPs/ITO Electrodes’ Characterization
2.1.1. SEM Analysis
2.1.2. FTIR Analysis
2.1.3. XRD Analysis
2.1.4. Raman Spectroscopy Analysis
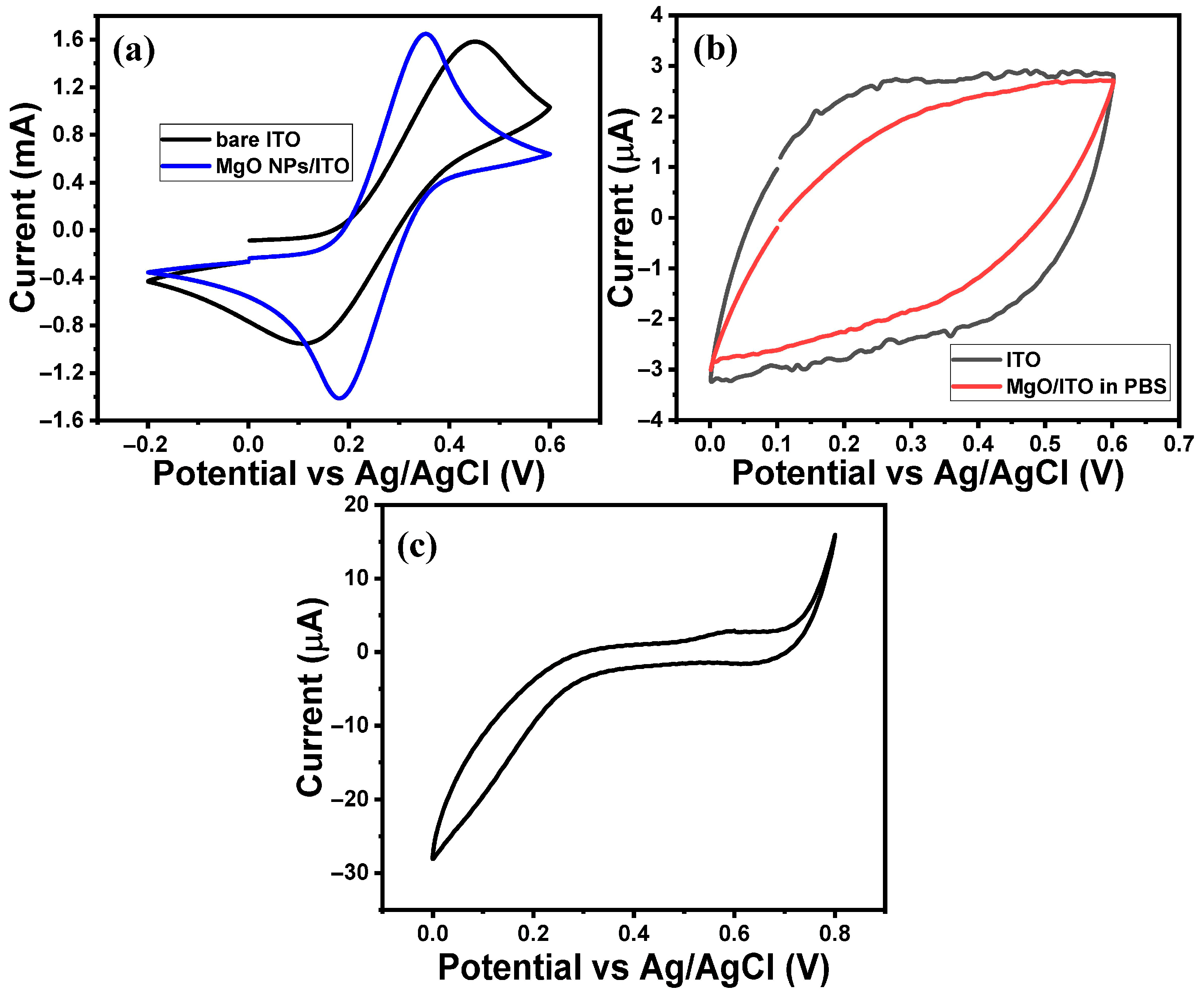
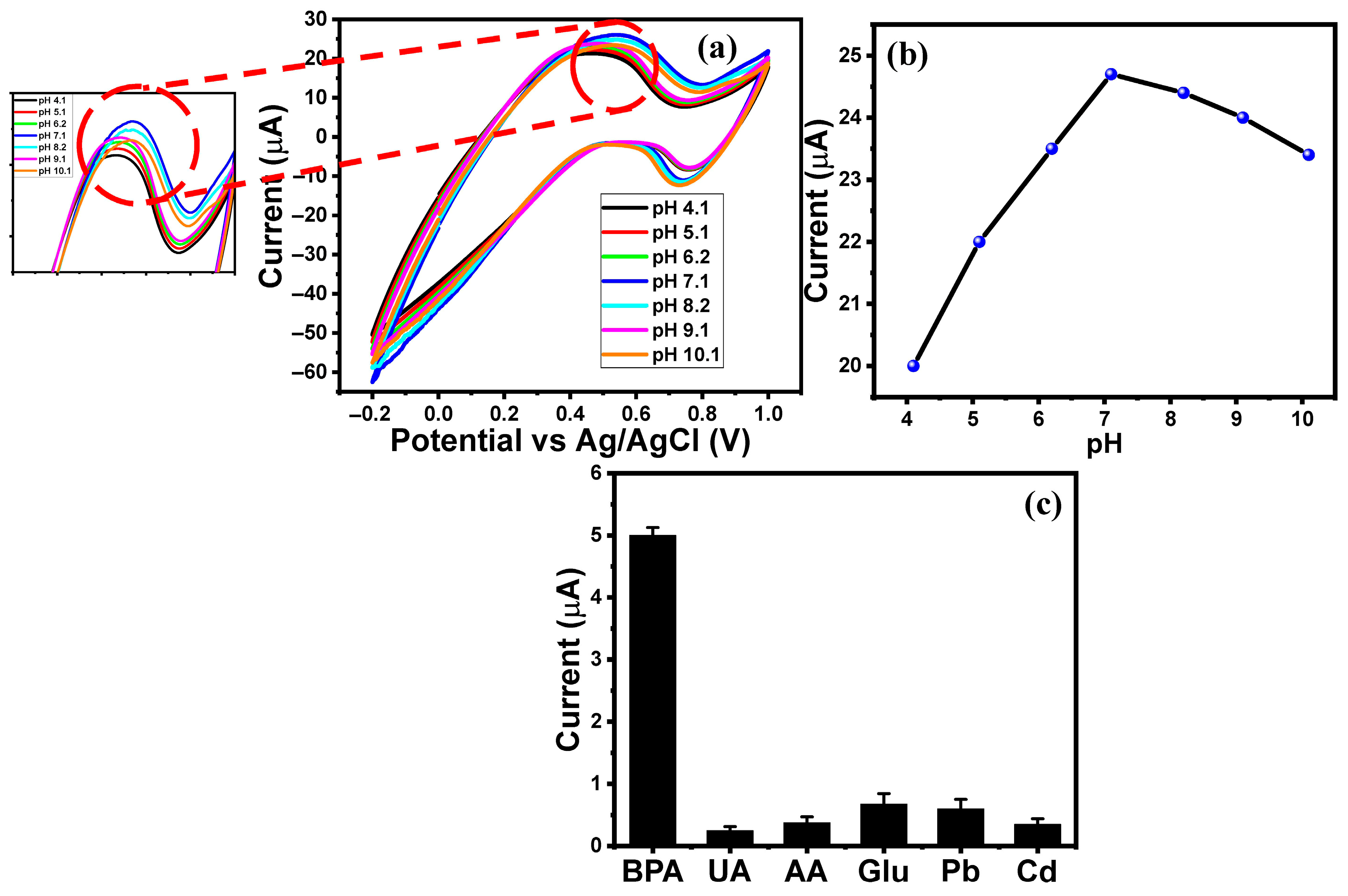
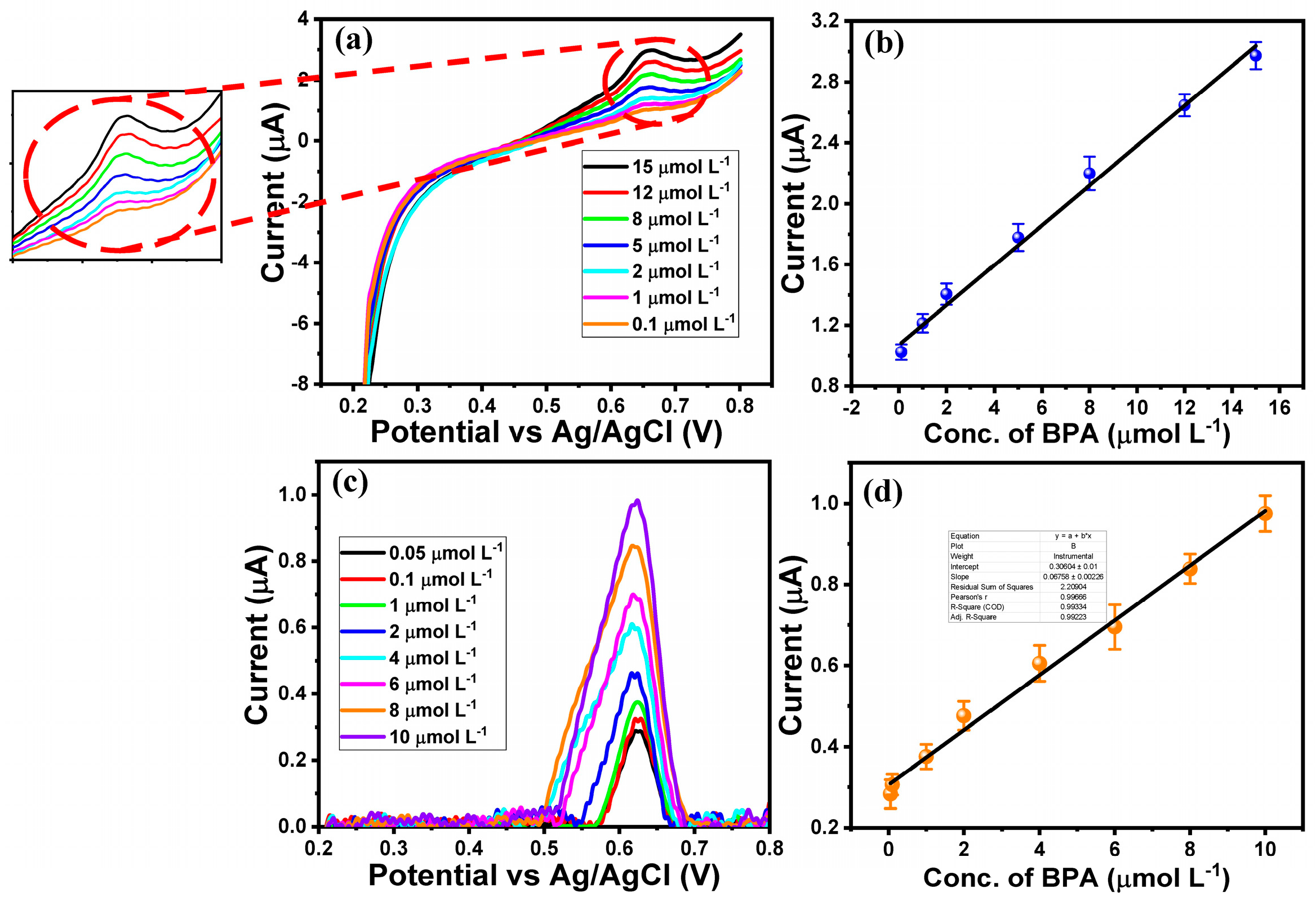
2.2. Electrochemical Performance of MgO/ITO Electrodes Towards BPA
2.3. Electrochemical Monitoring of BPA in Various Thermal Paper Samples
3. Materials and Methods
3.1. Materials
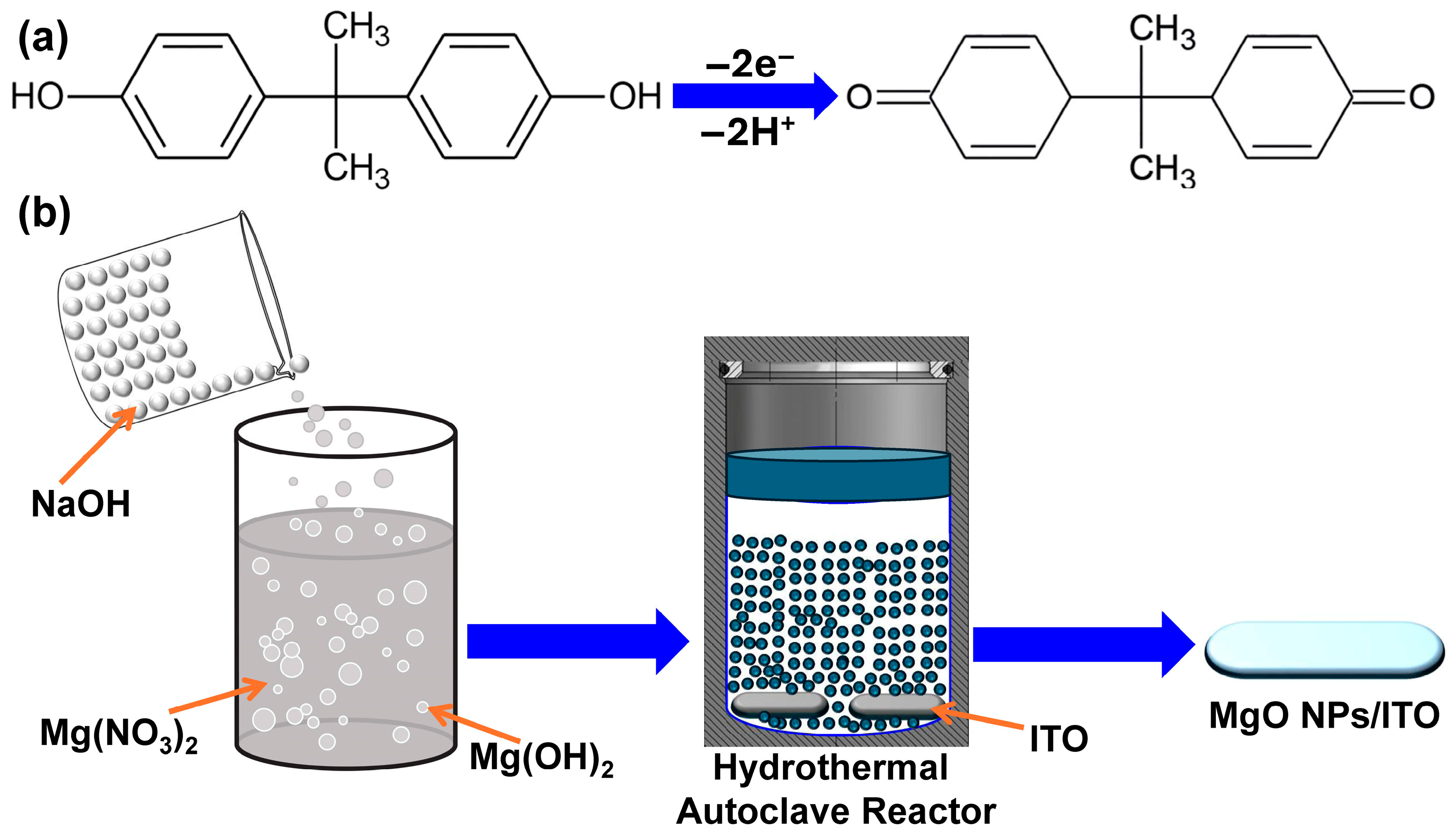
3.2. In Situ Fabrication of MgO/ITO Modified Electrodes
3.3. Sample Collection
3.4. Sample Extractions
3.5. Electrochemical Measurements
3.6. Instrumentation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Chemistry Council (ACC). Industrial & Business: Facts About BPA. 2022. Available online: https://www.factsaboutbpa.org/benefits-applications/industrial-business/ (accessed on 5 August 2025).
- Yoshiyuki, T.; Ayako, S.; Takako, S.; Tatsuya, T.; Kazuhisa, S. Why does a color-developing phenomenon occur on thermal paper comprising of a fluoran dye and a color developer molecule? Bull. Chem. Soc. Jpn. 2002, 75, 2225–2231. [Google Scholar] [CrossRef]
- Bernier, M.R.; Vandenberg, L.N. Handling of thermal paper: Implications for dermal exposure to Bisphenol A and its alternatives. PLoS ONE 2017, 12, e0178449. [Google Scholar] [CrossRef] [PubMed]
- Toxic Impressions: BPA in Thermal Paper, a New Report by Toxics Link. IPEN. 2017. Available online: https://ipen.org/news/toxic-impressions-bpa-thermal-paper-new-report-toxics-link (accessed on 5 August 2025).
- Vom Saal, F.S.; Vandenberg, L.N. Update on the Health Effects of Bisphenol A: Overwhelming Evidence of Harm. Endocrinology 2021, 162, bqaa171. [Google Scholar] [CrossRef]
- Mendum, T.; Stoler, E.; Van Benschoten, H.; Warner, J.C. Concentration of bisphenol A in thermal paper. Green Chem. Lett. Rev. 2011, 4, 81–86. [Google Scholar] [CrossRef]
- EFSA Press Release. April 2023. Available online: https://www.efsa.europa.eu/en/news/bisphenol-food-health-risk (accessed on 5 August 2025).
- Available online: https://www.ehn.org/how-much-bpa-in-our-bodies-2641524955.html (accessed on 5 August 2025).
- Taylor, J.A.; vom Saal, F.S.; Welshons, W.V.; Drury, B.; Rottinghaus, G.; Hunt, P.A.; Toutain, P.L.; Laffont, C.M.; VandeVoort, C.A. Similarity of bisphenol A pharmacokinetics in rhesus monkeys and mice: Relevance for human exposure. Environ. Health Perspect. 2011, 119, 422–430. [Google Scholar] [CrossRef]
- El-Said, W.A.; Abdelshakour, M.; Choi, J.-H.; Choi, J.-W. Application of conducting polymer nanostructures to electrochemical biosensors. Molecules 2020, 25, 307. [Google Scholar] [CrossRef]
- Frankowski, R.; Zgoła-Grzeskowiak, A.; Grzeskowiak, T.; Sojka, K. The presence of bisphenol A in the thermal paper in the face of changing European regulations—A comparative global research. Environ. Pollut. 2020, 265, 114879. [Google Scholar] [CrossRef]
- Reale, E.; Vernez, D.; Hopf, N.B. Skin Absorption of Bisphenol A and Its Alternatives in Thermal Paper. Ann. Work. Expo. Health 2021, 65, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Lunder, S.; Andrews, D.; Houlihan, J. Synthetic Estrogen BPA Coats Cash Register Receipts. 2010. Available online: http://www.ewg.org/bpa-in-store-receipts (accessed on 13 May 2011).
- Balakrishnan, B.; Henare, K.; Thorstensen, E.B.; Ponnampalam, A.P.; Mitchell, M.D. Transfer of bisphenol A across the human placenta. Am. J. Obs. Gynecol. 2010, 202, 393.e1–393.e7. [Google Scholar] [CrossRef]
- vom Saal, F.S.; Hughes, C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ. Health Perspect. 2005, 113, 926–933. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Goldman, L.R. Children’s vulnerability to toxic chemicals: A challenge and opportunity to strengthen health and environmental policy. Health Aff. 2011, 30, 842–850. [Google Scholar] [CrossRef]
- Schwartz, A.W.; Landrigan, P.J. Bisphenol A in thermal paper receipts: An opportunity for evidence-based prevention. Environ. Health Perspect. 2012, 120, A14–A15. [Google Scholar] [CrossRef]
- European Union (EU). Commission regulation 2016/2235 of 12 December 2016 amending annex XVII to regulation (EC) No 1907/2006 of the European parliament and of the council concerning the registration, evaluation, authorisation and restriction of chemicals (REACH) as regards bisphenol A. Off. J. Eur. Union 2016, L337, 3–5. [Google Scholar]
- Molina-Molina, J.M.; Jiménez-Díaz, I.; Fernández, M.F.; Rodriguez-Carrillo, A.; Peinado, F.M.; Mustieles, V.; Barouki, R.; Piccoli, C.; Olea, N.; Freire, C. Determination of bisphenol A and bisphenol S concentrations and assessment of estrogen- and anti-androgen-like activities in thermal paper receipts from Brazil, France, and Spain. Environ. Res. 2019, 170, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Karakilic, V.; Soulsbury, K. Bisphenol A (BPA) in Thermal Paper Used for Receipts. BCIT Environ. Public. Health 2016, 205, 1–10. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Li, Q.; Meteku, B.E.; Zhao, R.; Cui, B.; Li, X.; Zeng, J. Electro-enhanced solid-phase microextraction of bisphenol A from thermal papers using a three-dimensional graphene coated fiber. J. Chromatogr. A 2019, 1585, 27–33. [Google Scholar] [CrossRef]
- Castro, G.; Rodrı’guez, I.; Ramil, M.; Cela, R. Direct analysis in real time accurate mass spectrometry determination of bisphenol A in thermal printing paper. Talanta 2019, 205, 120086. [Google Scholar] [CrossRef]
- Yalcin, M.S.; Gecgel, C.; Battal, D. Determination of Bisphenol A in thermal paper receipts. JOTCSA 2016, 3, 167–174. [Google Scholar] [CrossRef][Green Version]
- Geens, T.; Goeyens, L.; Kannan, K.; Neels, H.; Covaci, A. Levels of bisphenol-A in thermal paper receipts from Belgium and estimation of human exposure. Sci. Total Environ. 2012, 435–436, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.; Barbato, F.; Grumetto, L. Monitoring of bisphenol A and bisphenol S in thermal paper receipts from the Italian market and estimated transdermal human intake: A pilot study. Sci. Total Environ. 2017, 599–600, 68–75. [Google Scholar] [CrossRef]
- Manfo, F.P.; Jubendradass, R.; Nantia, E.A.; Moundipa, P.F.; Mathur, P.P. Adverse effects of bisphenol A on male reproductive function. Rev. Environ. Contam. Toxicol. 2014, 228, 57–82. [Google Scholar]
- Ohore, O.E.; Zhang, S. Endocrine disrupting effects of bisphenol A exposure and recent advances on its removal by water treatment systems. A review. Sci. Afr. 2019, 5, e00135. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, J.; Wu, C. Determination of Endocrine Disruption Potential of Bisphenol A Alternatives in Food Contact Materials Using In Vitro Assays: State of the Art and Future Challenges. J. Agric. Food Chem. 2019, 67, 12613–12625. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Chen, S.; Shi, T.; Li, C.; Wang, Y.; Zhang, H. Competitive Plasmonic Biomimetic Enzyme-Linked Immunosorbent Assay for Sensitive Detection of Bisphenol A. Food Chem. 2021, 344, 128602. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Wan, S.; Lin, C.; Lin, X.; Xie, Z. Towards online specific recognition and sensitive analysis of bisphenol A by using AuNPs@aptamer hybrid-silica affinity monolithic column with LC-MS. Talanta 2020, 219, 121275. [Google Scholar] [CrossRef]
- Martín-Pozo, L.; Martín-Bueno, J.; Moscoso-Ruiz, I.; Zafra-Gómez, A. Chapter 18—Methods of bisphenol A detection by gas chromatography and mass spectrometry (GC-Ms) in human breast milk and foodstuff. In Emerging Contaminants in the Environment; Sarma, H., Dominguez, D.C., Lee, W.-Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 465–493. [Google Scholar]
- Owczarek, K.; Waraksa, E.; Kłodzi’nska, E.; Zrobok, Y.; Ozimek, M.; Racho’n, D.; Kudłak, B.; Wasik, A.; Mazerska, Z. Validated GC–MS method for determination of bisphenol A and its five analogues in dietary and nutritional supplements. Microchem. J. 2022, 180, 107643. [Google Scholar] [CrossRef]
- Hou, C.; Zhao, L.; Geng, F.; Wang, D.; Guo, L.-H. Donor/acceptor nanoparticle pair-based singlet oxygen channeling homogenous chemiluminescence immunoassay for quantitative determination of bisphenol A. Anal. Bioanal. Chem. 2016, 408, 8795–8804. [Google Scholar] [CrossRef]
- Hosseinzadeh, L.; Khoshroo, A.; Adib, K.; Rahimi-Nasrabadi, M.; Ahmadi, F. Determination of Homocysteine Using a Dopamine-Functionalized Graphene Composite. Microchem. J. 2021, 165, 106124. [Google Scholar] [CrossRef]
- Sobhani-Nasab, A.; Hoseinpour, S.M.; Rahimi-Nasrabadi, M.; Pourmasoud, S.; Eghbali-Arani, M.; Ahmadi, F.J. Synthesis of Fe3O4/CdWO4/carbon dots heterostructure with excellent visible light photocatalytic stability and activity for degradation of 4-nitrophenol and organic pollutant. Mater. Sci. Mater. Electron. 2021, 32, 26998–27013. [Google Scholar] [CrossRef]
- Hafezi, M.; Rostami, M.; Hosseini, A.; Rahimi-Nasrabadi, M.; Fasihi-Ramandi, M.; Badiei, A.; Ahmadi, F.J. Cur-loaded ZnFe2O4@ mZnO@ N-GQDs biocompatible nano-carriers for smart and controlled targeted drug delivery with pH-triggered and ultrasound irradiation. Mol. Liq. 2021, 322, 114875. [Google Scholar] [CrossRef]
- Rostami, M.; Nasab, A.S.; Fasihi-Ramandi, M.; Badiei, A.; Rahimi-Nasrabadi, M.; Ahmadi, F. The ZnFe2O4@mZnO–N/RGO nano-composite as a carrier and an intelligent releaser drug with dual pH-and ultrasound-triggered control. New J. Chem. 2021, 45, 4280. [Google Scholar] [CrossRef]
- Sohouli, E.; Ghalkhani, M.; Zargar, T.; Joseph, Y.; Rahimi-Nasrabadi, M.; Ahmadi, F.; Plonska-Brzezinska, M.E.; Ehrlich, H. A new electrochemical aptasensor based on gold/nitrogen-doped carbon nano-onions for the detection of Staphylococcus aureus. Electrochim. Acta 2021, 403, 139633. [Google Scholar] [CrossRef]
- Ghanbari, M.H.; Khoshroo, A.; Sobati, H.; Ganjali, M.R.; Rahimi-Nasrabadi, M.; Ahmadi, F. An electrochemical sensor based on poly (L-Cysteine)@ AuNPs@ reduced graphene oxide nanocomposite for determination of levofloxacin. Microchem. J. 2019, 147, 198. [Google Scholar] [CrossRef]
- Khoshroo, A.; Sadrjavadi, K.; Taran, M.; Fattahi, A. Electrochemical system designed on a copper tape platform as a nonenzymatic glucose sensor. Sens. Actuators B 2020, 325, 128778. [Google Scholar] [CrossRef]
- Khoshroo, A.; Hosseinzadeh, L.; Adib, K.; Rahimi-Nasrabadi, M.; Ahmadi, F. Earlier diagnoses of acute leukemia by a sandwich type of electrochemical aptasensor based on copper sulfide-graphene composite. Anal. Chim. Acta 2021, 1146, 1–10. [Google Scholar] [CrossRef]
- Zhang, C.; Yi, Y.; Zhou, Y.; Zhou, R.; Zhu, G. Homogeneous electroanalysis coupled with colorimetry dual-mode sensing of silver ion in water based on target-inhibited peroxidase activity of Pt@ZIF-8 nanozyme. Microchem. J. 2024, 197, 109839. [Google Scholar] [CrossRef]
- Liu, X.; Yao, Y.; Ying, Y.; Ping, J. Recent advances in nanomaterial-enabled screen-printed electrochemical sensors for heavy metal detection. TrAC Trends Anal. Chem. 2019, 115, 187–202. [Google Scholar] [CrossRef]
- Dhaffouli, A.; Salazar-Carballo, P.A.; Carinelli, S.; Holzinger, M.; Barhoumi, H. Improved electrochemical sensor using functionalized silica nanoparticles (SiO2-APTES) for high selectivity detection of lead ions. Mater. Chem. Phys. 2024, 318, 129253. [Google Scholar] [CrossRef]
- Baluta, S.; Meloni, F.; Halicka, K.; Szyszka, A.; Zucca, A.; Pilo, M.I.; Cabaj, J. Differential pulse voltammetry and chronoamperometry as analytical tools for epinephrine detection using a tyrosinase-based electrochemical biosensor. RSC Adv. 2022, 12, 25342–25353. [Google Scholar] [CrossRef]
- Richardson, S.D.; Kimura, S.Y. Water Analysis: Emerging Contaminants and Current Issues. Anal. Chem. 2020, 92, 473–505. [Google Scholar] [CrossRef]
- Moulahi, N.; Echabaane, M.; Chaabene, M.; Baouab, M.H.V.; Ben Chaabane, R. Impedimetric Sensor for Iron (III) Detection Based on Small Molecule (E)-2-((Phenylimino)Methyl) Phenol-Modified Platinum Electrode. J. Iran. Chem. Soc. 2023, 20, 1427–1438. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Eslami, V.; Khoshroo, A. Nickel nitride nanoparticles as efficient electrocatalyst for effective electro-oxidation of ethanol and methanol in alkaline media. Mater. Sci. Eng. B 2018, 229, 201–205. [Google Scholar] [CrossRef]
- Mehmandoust, M.; Erk, N.; Karaman, O.; Karimi, F.; Bijad, M.; Karaman, C. Three-dimensional porous reduced graphene oxide decorated with carbon quantum dots and platinum nanoparticles for highly selective determination of azo dye compound tartrazine. Food Chem. Toxicol. 2021, 158, 112698. [Google Scholar] [CrossRef]
- Huang, N.; Liu, M.; Li, H.; Zhang, Y.; Yao, S. Synergetic Signal Amplification Based on Electrochemical Reduced Graphene Oxide-Ferrocene Derivative Hybrid and Gold Nanoparticles as an Ultra-Sensitive Detection Platform for Bisphenol A. Anal. Chim. Acta 2015, 853, 249–257. [Google Scholar] [CrossRef]
- He, S.; Xia, H.; Chang, F. Enzyme free electrochemical determination of bisphenol A using screen-printed electrode modified by graphdiyne and carbon nanotubes. Microchem. J. 2022, 182, 107858. [Google Scholar] [CrossRef]
- Shen, M.; Li, W.; Chen, F.; Chen, L.; Chen, Y.; Chen, S.; Ren, S.; Han, D. A ratiometric electrochemical sensor for bisphenol A detection based on Ag@Fe3O4-rGO composite. Microchem. J. 2023, 186, 108315. [Google Scholar] [CrossRef]
- Ma, J.; Yuan, J.; Xu, Y.; Jiang, Y.; Bai, W.; Zheng, J. Ultrasensitive electrochemical determination of bisphenol A in food samples based on a strategy for activity enhancement of enzyme: Layer-by-layer self-assembly of tyrosinase between two-dimensional porphyrin metal–organic framework nanofilms. Chem. Eng. J. 2022, 446, 137001. [Google Scholar] [CrossRef]
- Verma, D.; Yadav, A.K.; Mukherjee, M.D.; Solanki, P.R. Fabrication of a sensitive electrochemical sensor platform using reduced graphene oxide-molybdenum trioxide nanocomposite for BPA detection: An endocrine disruptor. J. Environ. Chem. Eng. 2021, 9, 105504. [Google Scholar] [CrossRef]
- Rejab, F.; Dardouri, N.E.; Rouis, A.; Echabaane, M.; Nasri, H.; Lakard, B.; Halima, H.B.; Jaffrezic-Renault, N. A Selective Electrochemical Sensor for Bisphenol A Detection Based on Cadmium (II) (bromophenyl)porphyrin and Gold Nanoparticles. Micromachines 2024, 15, 1508. [Google Scholar] [CrossRef] [PubMed]
- Luis-Sunga, M.; Carinelli, S.; García, G.; González-Mora, J.L.; Salazar-Carballo, P.A. Electrochemical Detection of Bisphenol A Based on Gold Nanoparticles/Multi-Walled Carbon Nanotubes: Applications on Glassy Carbon and Screen Printed Electrodes. Sensors 2024, 24, 2570. [Google Scholar] [CrossRef] [PubMed]
- Fouad, D.M.; El-Said, W.A.; Mohamed, M.B. Spectroscopic characterization of magnetic Fe3O4@Au core shell nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. Source Preview 2015, 140, 392–397. [Google Scholar] [CrossRef]
- El-Said, W.A.; Alsulmi, A.; Alshitari, W. Hydrothermal synthesis of Mn3O4 nanorods modified indium tin oxide electrode as an efficient nanocatalyst towards direct urea electrooxidation. PLoS ONE 2022, 17, e0272586. [Google Scholar] [CrossRef]
- Khan, F.; Akhtar, N.; Jalal, N.; Hussain, I.; Szmigielski, R.; Hayat, M.Q.; Ahmad, H.B.; El-Said, W.A.; Yang, M.; Janjua, H.A. Carbon-dot wrapped ZnO nanoparticle-based photoelectrochemical sensor for selective monitoring of H2O2 released from cancer cells. Microchim. Acta 2019, 186, 127. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; El-Said, W.A.; Choi, J.-W. Highly sensitive electrochemical detection of potential cytotoxicity of CdSe/ZnS quantum dots using neural cell chip. Biosens. Bioelectron. Source Preview 2012, 32, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.M.; El-Kady, M.F.; Atta, N.F.; Galal, A. Gold Nanoparticles Decorated Graphene as a High Performance Sensor for Determination of Trace Hydrazine Levels in Water. Electroanalysis 2018, 30, 1757–1766. [Google Scholar] [CrossRef]
- Abbas, A.; Amin, H.M.A. Silver nanoparticles modified electrodes for electroanalysis: An updated review and a perspective. Microchem. J. 2022, 175, 107166. [Google Scholar] [CrossRef]
- dos Santos, W.T.P.; Amin, H.M.A.; Compton, R.G. A nano-carbon electrode optimized for adsorptive stripping voltammetry: Application to detection of the stimulant selegiline in authentic saliva. Sens. Actuators B Chem. 2019, 279, 433–439. [Google Scholar] [CrossRef]
- Gurule, A.C.; Gaikwad, S.S.; Kajale, D.D.; Shinde, V.S.; Jadhav, G.R.; Gaikwad, V.B. Synthesis of magnesium oxide nanoparticles via hydrothermal and sol-gel methods: Charaterization and their application for H2S and NO2 gas sensing. J. Indian Chem. Soc. 2025, 102, 101496. [Google Scholar] [CrossRef]
- Desai, P.; Darji, V.; Deshpande, M.P.; Chaki, S.H.; Sutariya, P.G.; Soni, H.; Hirpara, B. Dielectric performance of nanostructured magnesium oxide and effect of cobalt substitution. Mater. Today Commun. 2024, 38, 108022. [Google Scholar] [CrossRef]
- Sutradhar, N.; Sinhamahapatra, A.; Pahari, S.K.; Pal, P.; Bajaj, H.C.; Mukhopadhyay, I.; Panda, A.B. Fabrication of Highly Sensitive and Stable Hydroxylamine Electrochemical Sensor Based on Gold Nanoparticles and Metal–Metalloporphyrin Framework Modified Electrode. ACS Appl. Mater. Interfaces 2016, 8, 18173–18181. [Google Scholar] [CrossRef]
- Nourozi, B.; Aminian, A.; Fili, N.; Zangeneh, Y.; Boochani, A.; Darabi, P. The electronic and optical properties of MgO monolayer: Based on GGA-mBJ. Results Phys. 2019, 12, 2038–2043. [Google Scholar] [CrossRef]
- Manjunatha, K.G.; Swamy, B.E.K.; Madhuchandra, H.D.; Gururaj, K.J.; Vishnumurthy, K.A. Synthesis and characterization of MgO nanoparticle and their surfactant modified carbon paste electrode sensing for paracetamol. Sens. Int. 2021, 2, 100127. [Google Scholar] [CrossRef]
- Manjunatha, K.G.; Swamy, B.E.K.; Jayaprakash, G.K.; Vishnumurthy, K.A. Synthesis of MgO Nanoparticle Surfactant Free Modified Carbon Paste Electrode: A Voltammetric Study. Anal. Bioanal. Electrochem. 2022, 14, 921–933. [Google Scholar]
- Amir, A.; Faisal, M.; Hussain, M.A.; Haq, E.U.; Raza, K.; Rehman, Z.U. A facile synthesis of nano-magnesia by ultrasonication assisted co-precipitation method for antibacterial activity, in: MATEC Web of Conferences. EDP Sci. 2024, 398, 01037. [Google Scholar] [CrossRef]
- Toprak, B.Ç.; Efkere, H.I.; Aydın, S.Ş.; Tataroğlu, A.; Özçelik, S. Structural, morphological, optical and electrical characterization of MgO thin films grown by sputtering technique on different substrates. J. Mater. Sci. Mater. Electron. 2024, 35, 1389. [Google Scholar] [CrossRef]
- Azhdar, B. Influence of fuel-to-oxidizer ratio, potential of hydrogen and annealing temperature on the structural and optical properties of nanocrystalline MgO powders synthesized by the hydrothermal method. J. Exp. Nanosci. 2023, 18, 2276278. [Google Scholar] [CrossRef]
- Mei, S.; Jiang, F.; Liu, N.; Feng, Z.; Zheng, Y.; Yang, W.; Zhang, N. Sol-gel synthesis of magnesium oxide nanoparticles and their evaluation as a therapeutic agent for the treatment of osteoarthritis. Nanomedicine 2024, 19, 1867–1878. [Google Scholar] [CrossRef]
- Krishna, B.V.; Rao, P.T.; Lakshmi, B.D.; Vasudha, K.; Basha, S.E.; Kumar, B.P.; Kiran, P.S.S.; Chandra, K.S.; Ramachandra, R.K. Green fabrication of tinospora cordifolia-derived MgO nanoparticles: Potential for diabatic control and oxidant protection. Next Mater. 2024, 3, 100171. [Google Scholar] [CrossRef]
- Kumari, S.V.G.; Pakshirajan, K.; Pugazhenthi, G. Synthesis and characterization of MgO nanostructures: A comparative study on the effect of preparation route. Mater. Chem. Phys. 2023, 294, 127036. [Google Scholar] [CrossRef]
- Lone, G.A.; Nazir, N.; Balal, M.; Ikram, M. Thin film deposition of undoped and Nidoped cobalt ferrite on MgO (110) substrates using pulsed laser deposition technique. Thin Solid. Film. 2024, 791, 140243. [Google Scholar] [CrossRef]
- Sharma, M.; Jeevanandam, P.J. Synthesis of magnesium oxide particles with stacks of plates morphology. Alloys Compd. 2011, 509, 7881–7885. [Google Scholar] [CrossRef]
- Ahmada, K.; Mobin, S.M. High surface area 3D-MgO flowers as the modifier for the working electrode for efficient detection of 4-chlorophenol. Nanoscale Adv. 2019, 1, 719. [Google Scholar] [CrossRef] [PubMed]
- Pilarska, A.A.; Klapiszewski, Ł.; Jesionowski, T. Recent development in the synthesis, modification, and application of Mg(OH)2 and MgO: A review. Powder Technol. 2017, 319, 373–407. [Google Scholar] [CrossRef]
- Dong, X.X.; Li, M.Y.; Feng, N.N.; Sun, Y.M.; Yang, C.; Xu, Z.L. A nanoporous MgO-based nonenzymatic electrochemical sensor for rapid screening of hydrogen peroxide in milk. RSC Adv. 2015, 5, 86485–86489. [Google Scholar] [CrossRef]
- Singh, N.; Singh, P.K.; Singh, M.; Tandon, P.; Singh, S.K.; Singh, S. Fabrication and characterization of polyaniline, polyaniline/MgO (30%), and polyaniline/MgO (40%) nanocomposites for their employment in LPG sensing at room temperature. J. Mater. Sci. Mater. Electron. 2019, 30, 4487–4498. [Google Scholar] [CrossRef]
- Abinaya, S.; Kavitha, H.P. Magnesium Oxide Nanoparticles: Effective Antilarvicidal and Antibacterial Agents. ACS Omega 2023, 8, 5225–5233. [Google Scholar] [CrossRef] [PubMed]
- Dekermenjian, M.; Ruediger, A.P.; Merlen, A. Raman spectroscopy investigation of magnesium oxide nanoparticles. RSC Adv. 2023, 13, 26683–26689. [Google Scholar] [CrossRef]
- Athar, T.; Hakeem, A.; Ahmed, W. Synthesis of MgO Nanopowder via Non Aqueous Sol–Gel Method. Adv. Sci. Lett. 2012, 5, 27–29. [Google Scholar] [CrossRef]
- Muhaymin, A.; Mohamed, H.E.A.; Hkiri, K.; Safdar, A.; Azizi, S. Green synthesis of magnesium oxide nanoparticles using Hyphaene thebaica extract and their photocatalytic activities. Sci. Rep. 2024, 14, 20135. [Google Scholar] [CrossRef] [PubMed]
- Hema, M. Synthesis of hierarchical structured MgO by sol-gel method. Nano Bull. 2013, 2, 130106. [Google Scholar]
- Rezaei, M.; Khajenoori, M.; Nematollahi, B. Synthesis of high surface area nanocrystalline MgO by pluronic P123 triblock copolymer surfactant. Powder Technol. 2011, 205, 112–116. [Google Scholar] [CrossRef]
- Geler-Kremer, J.; Posadas, A.B.; Demkov, A.A. Preparation of clean MgO surface by oxygen plasma: Comparison with standard substrate cleaning procedures. J. Vac. Sci. Technol. B 2020, 38, 062201. [Google Scholar] [CrossRef]
- Fonoll-Rubio, R.; Placidi, M.; Hoelscher, T.; Thomere, A.; Li-Kao, Z.J.; Guc, M.; Izquierdo-Roca, V.; Scheer, R.; Pérez-Rodríguez, A. Characterization of the Stability of Indium Tin Oxide and Functional Layers for Semitransparent Back-Contact Applications on Cu(in,Ga)Se2 Solar Cells. Sol. RRL 2022, 6, 2101071. [Google Scholar] [CrossRef]
- Atta, N.F.; Ahmed, R.A.; Amin, H.M.A.; Galal, A. Monodispersed Gold Nanoparticles Decorated Carbon Nanotubes as an Enhanced Sensing Platform for Nanomolar Detection of Tramadol. Electroanalysis 2012, 24, 2135–2146. [Google Scholar] [CrossRef]
- Jaballah, M.B.; Messaoud, N.B.; Dridi, C. Development of cost-effective and sustainable sensing nanoplatform based on green AgNPs for the determination of BPA in water. J. Mater. Sci. 2022, 33, 6981–6998. [Google Scholar] [CrossRef]
- He, L.; Yang, Y.; Kim, J.; Yao, L.; Dong, X.; Li, T.; Piao, Y. Multi-layered enzyme coating on highly conductive magnetic biochar nanoparticles for bisphenol A sensing in water. Chem. Eng. J. 2020, 384, 123276. [Google Scholar] [CrossRef]
- Maximiano, L.V.; Correa, L.B.; Gomes-da-Silva, N.C.; da Costa, L.S.; Da Silva, M.G.P.; Chaves, A.V.; Franco, M.L.; Fechine, P.B.A.; de Menezes, A.S.; Santos-Oliveira, R.; et al. Magnesium whitlockite nanoparticles: Hydrothermal synthesis, anti-inflammatory and anti-cancer potential. Colloids Surf. B Biointerfaces 2024, 239, 113931. [Google Scholar] [CrossRef]
- Semerjian, L.; Alawadhi, N.; Nazer, K. Detection of bisphenol A in thermal paper receipts and assessment of human exposure: A case study from Sharjah, United Arab Emirates. PLoS ONE 2023, 18, e0283675. [Google Scholar] [CrossRef]

| Sensor | Linear Range (μmol L−1) | LOD (nmol L−1) | Reference |
|---|---|---|---|
| Ag@Fe3O4-rGO/GCE | 0.1–10 | 22 | [52] |
| Tyr@Cu–TCPP/GCE | 0.0035–18.9 | 1.2 | [53] |
| rGO/MoO3NPs/ITO | 0.82–76 | 0.12 | [54] |
| AgNPs/f-MWCNT/GCE | 0.3–0.8 | 220 | [91] |
| ML-TYR/Mag-BCNPs-COOH/MGCE | 0.01–1.01 | 2.8 | [92] |
| MgO NPs/ITO | 0.05–10 | 1.13 | This work |
| Sample Number | BPA Concentration (μmol L−1) |
|---|---|
| 1 | 0.047 |
| 2 | 0 |
| 3 | 0.019 |
| 4 | 0.04 |
| 5 | 0.0148 |
| 6 | 1.33 |
| 7 | 0.0139 |
| 8 | 0.0229 |
| 9 | 0.141 |
| 10 | 0.0189 |
| 11 | 0 |
| 12 | 0.68 |
| 13 | 0.835 |
| 14 | 0 |
| 15 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhdhar, A.; El-Said, W.A. In Situ Growth of Magnesium Oxide Nanoparticles on ITO Electrodes as Electrocatalysts for Detecting Bisphenol A in Thermal Paper. Catalysts 2025, 15, 901. https://doi.org/10.3390/catal15090901
Akhdhar A, El-Said WA. In Situ Growth of Magnesium Oxide Nanoparticles on ITO Electrodes as Electrocatalysts for Detecting Bisphenol A in Thermal Paper. Catalysts. 2025; 15(9):901. https://doi.org/10.3390/catal15090901
Chicago/Turabian StyleAkhdhar, Abdullah, and Waleed A. El-Said. 2025. "In Situ Growth of Magnesium Oxide Nanoparticles on ITO Electrodes as Electrocatalysts for Detecting Bisphenol A in Thermal Paper" Catalysts 15, no. 9: 901. https://doi.org/10.3390/catal15090901
APA StyleAkhdhar, A., & El-Said, W. A. (2025). In Situ Growth of Magnesium Oxide Nanoparticles on ITO Electrodes as Electrocatalysts for Detecting Bisphenol A in Thermal Paper. Catalysts, 15(9), 901. https://doi.org/10.3390/catal15090901







