Recyclable TiO2–Fe3O4 Magnetic Composites for the Photocatalytic Degradation of Paracetamol: Comparative Effect of Pure Anatase and Mixed-Phase P25 TiO2
Abstract
1. Introduction
2. Results and Discussion
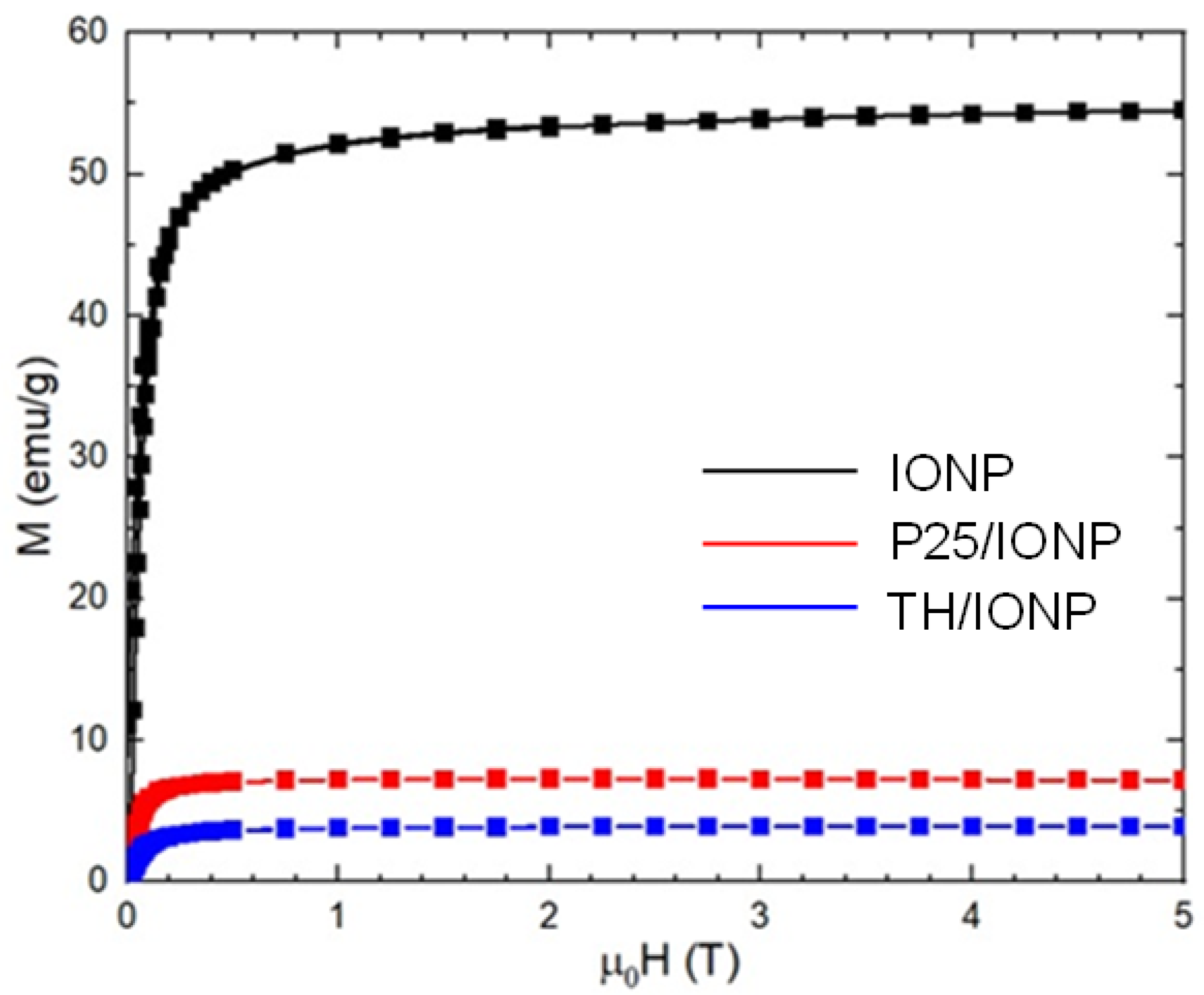
2.1. Structural and Optical Characterization of the TiO2 Support Materials and Their Composites
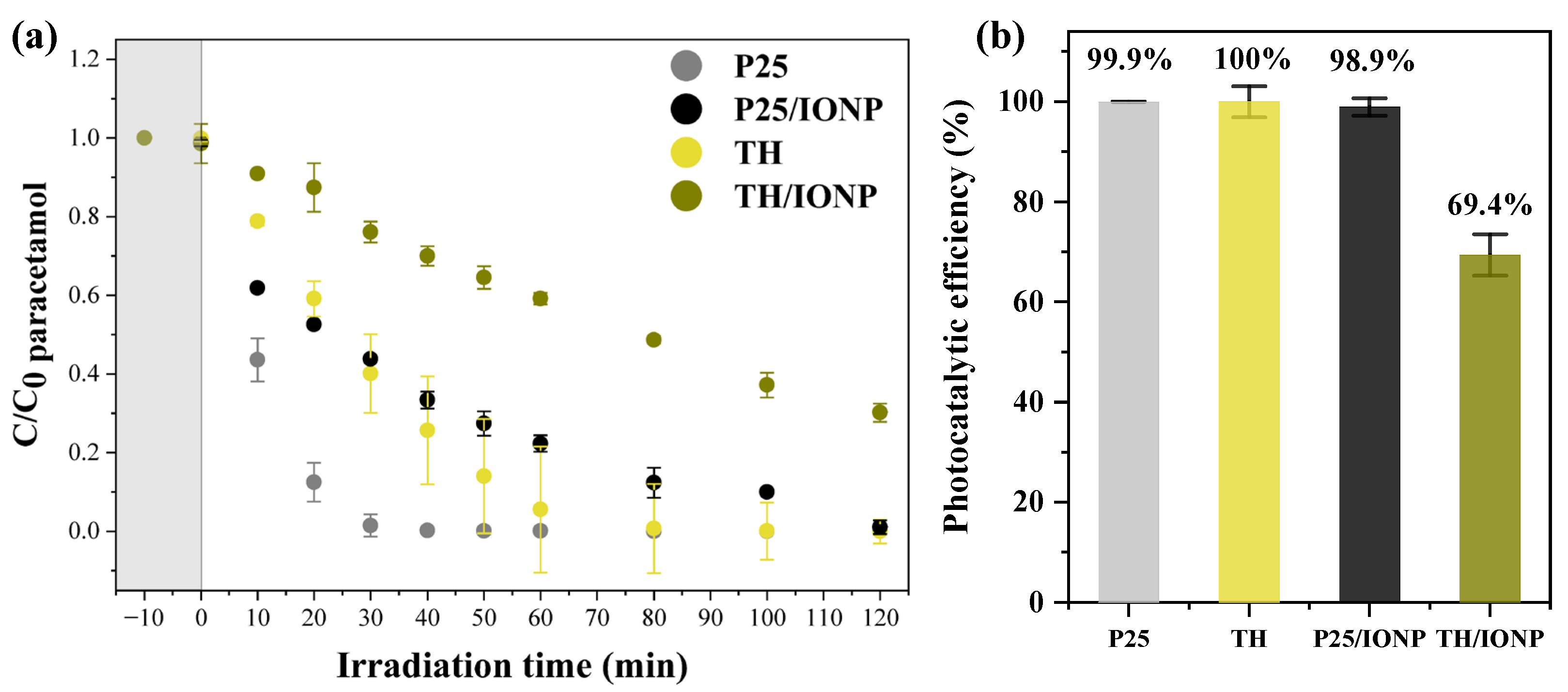
2.2. Investigation of the Photocatalytic Performance of the TiO2 Bases and Composites
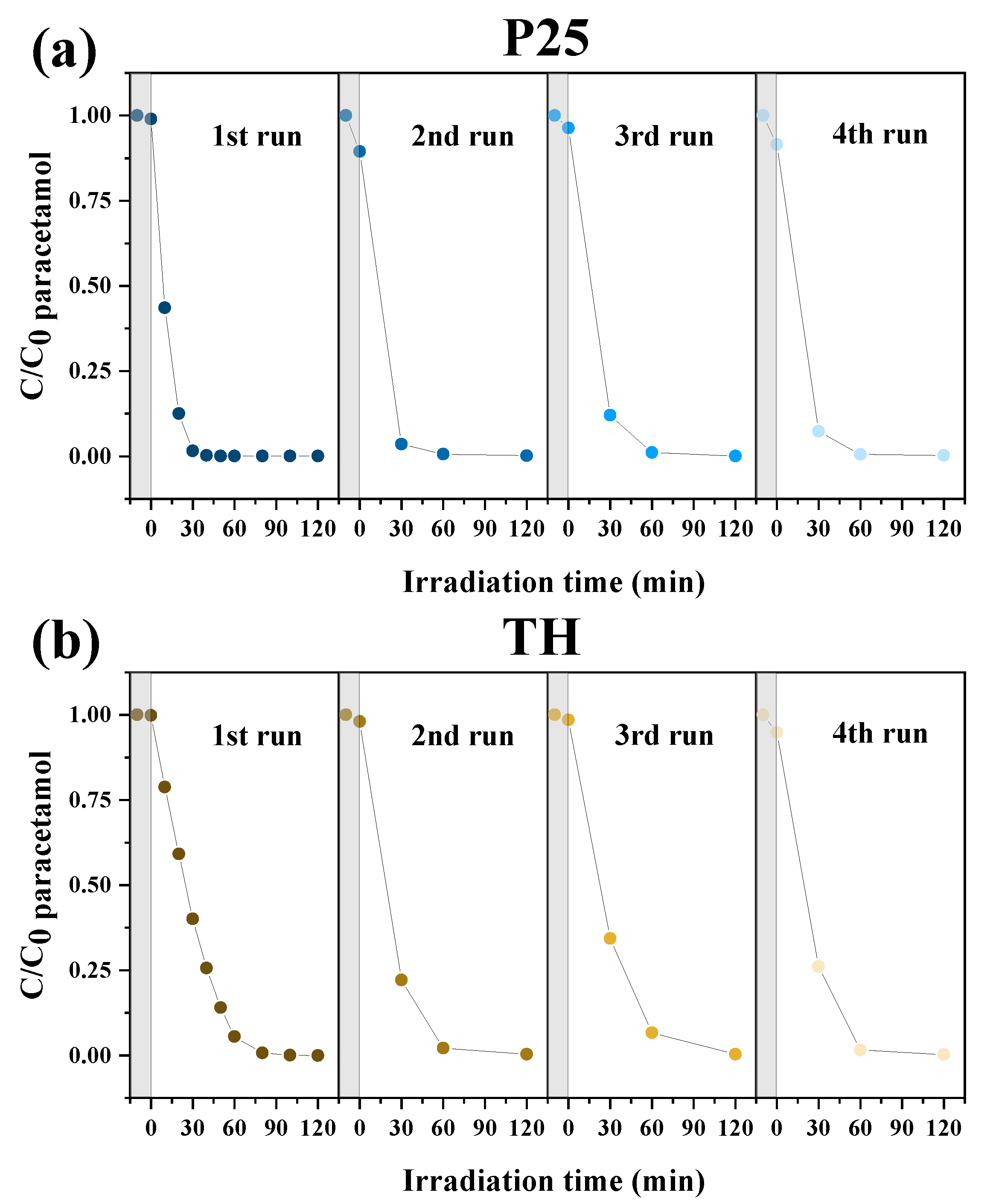
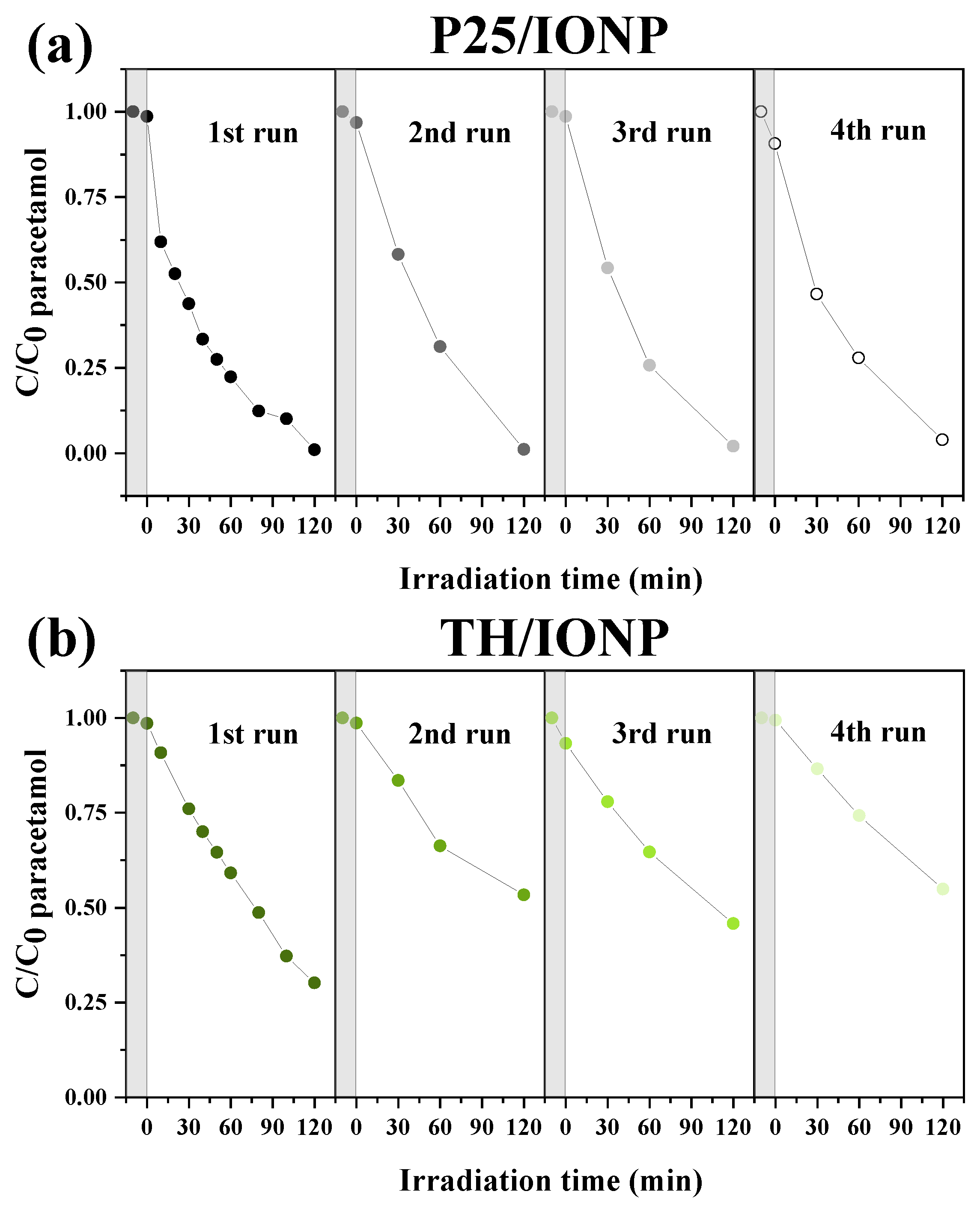
2.3. Recycling Tests of the Photocatalysts
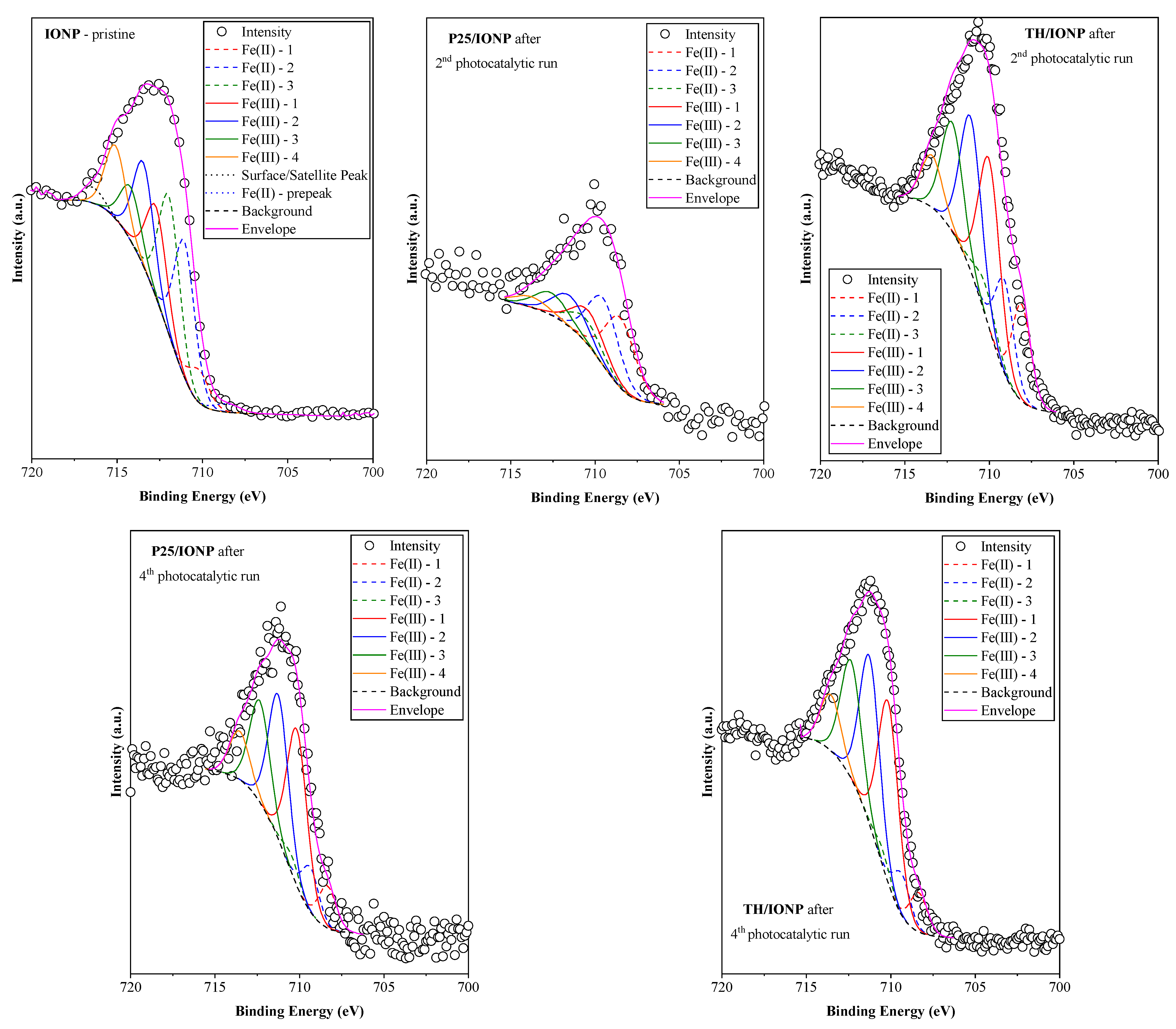
2.4. Characterization of Photocatalysts After Degradation Cycles
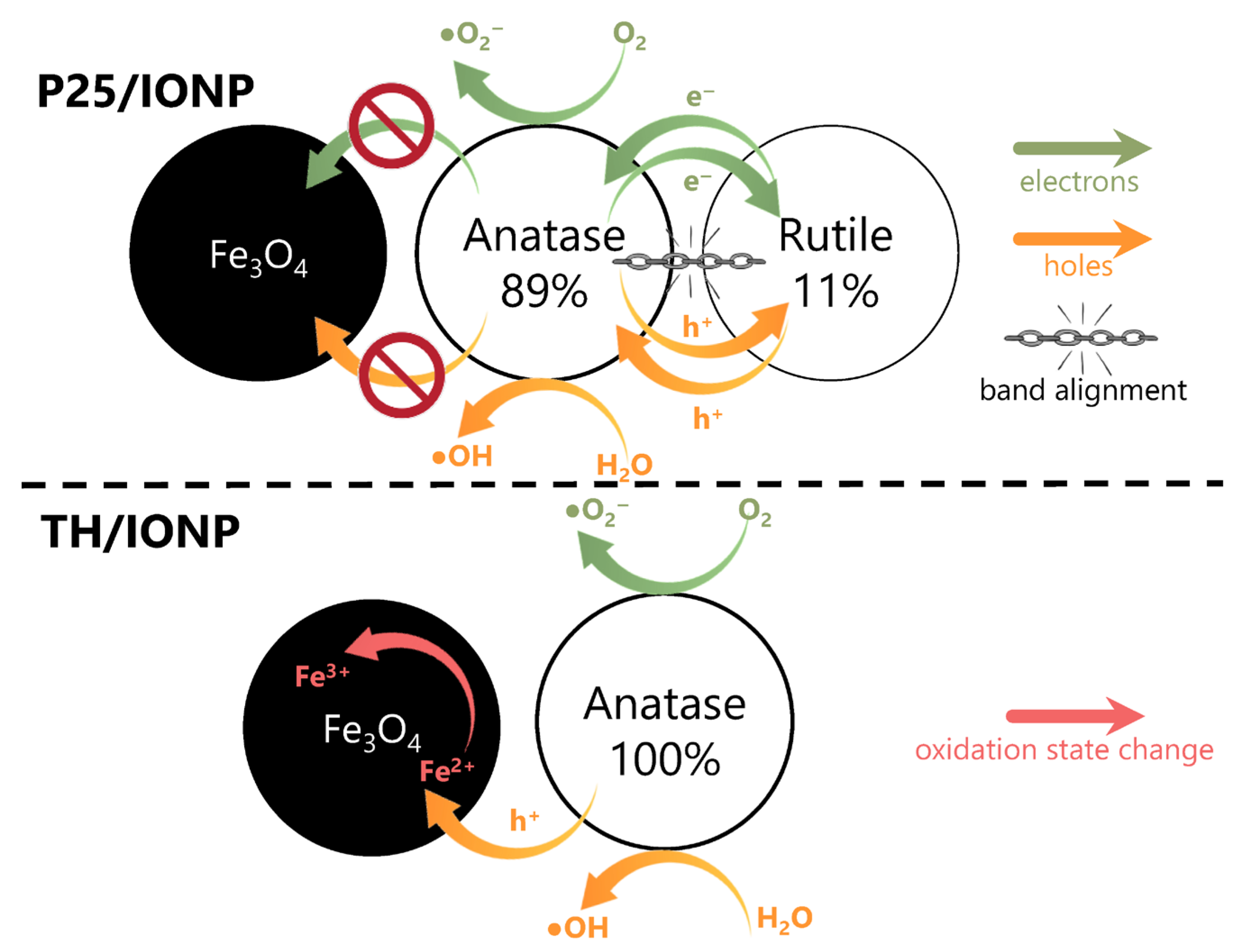
2.5. Linking Structural and Photocatalytic Data
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of Iron Oxide Nanoparticles
3.3. Synthesis of Anatase TiO2 Sample
3.4. Preparation of Magnetic Composites of Synthetic Anatase TiO2 and P25
3.5. Methods and Instrumentation
3.6. Photocatalytic Experiments
3.6.1. Preparation of Paracetamol Solution from Tablets
3.6.2. Method of Photocatalytic Activity Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Umair, M.; Kanwal, T.; Loddo, V.; Palmisano, L.; Bellardita, M. Review on Recent Advances in the Removal of Organic Drugs by Advanced Oxidation Processes. Catalysts 2023, 13, 1440. [Google Scholar] [CrossRef]
- Hegedus, M.; Lacina, P.; Plotěný, M.; Lev, J.; Kamenická, B.; Weidlich, T. Fast and efficient hydrodehalogenation of chlorinated benzenes in real wastewaters using Raney alloy. J. Water Process Eng. 2020, 38, 101645. [Google Scholar] [CrossRef]
- Cardoso, I.M.F.; da Silva, L.P.; da Silva, J.C.G.E. Nanomaterial-Based Advanced Oxidation/Reduction Processes for the Degradation of PFAS. Nanomaterials 2023, 13, 1668. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Xue, Q.; Liu, Y.; Liang, F.; Li, W.; Liu, T.; Zhuang, C.; Zhao, Z.; Li, S. Manipulating interfacial charge redistribution in Mn0.5Cd0.5S/N-rich C3N5 S-scheme heterojunction for high-performance photocatalytic removal of emerging contaminants. J. Mater. Sci. Technol. 2026, 243, 265–274. [Google Scholar] [CrossRef]
- Chi, S.W.; Din, A.T.M.; Abdullah, A.Z. Critical review on magnetic separable molecularly imprinted photocatalyst: Synthesis, reaction mechanism and outlook for practical applications. Mater. Sci. Semicond. Process 2025, 189, 109292. [Google Scholar] [CrossRef]
- Hao, Y.; Xiao, Y.; Liu, X.; Ma, J.; Lu, Y.; Chang, Z.; Luo, D.; Li, L.; Feng, Q.; Xu, L.; et al. A Novel SnO2/ZnFe2O4 Magnetic Photocatalyst with Excellent Photocatalytic Performance in Rhodamine B Removal. Catalysts 2024, 14, 350. [Google Scholar] [CrossRef]
- Liang, Y.; Yin, Y.; Deng, Q.; Jiao, S.; Liang, X.; Huo, C.; Luo, Y. Efficient and easily recyclable photocatalytic reduction of Se(IV) from wastewater using stable TiO2/BiOBr/cloth: Mechanism insight and machine learning modeling. Sep. Purif. Technol. 2025, 352, 128021. [Google Scholar] [CrossRef]
- Ibrahim, I.; Elseman, A.M.; Sadek, H.; Eliwa, E.M.; Abusaif, M.S.; Kyriakos, P.; Belessiotis, G.V.; Mudgal, M.M.; Abdelbasir, S.M.; Elsayed, M.H.; et al. Membrane-Based Photocatalytic and Electrocatalytic Systems: A Review. Catalysts 2025, 15, 528. [Google Scholar] [CrossRef]
- Yu, Z.-F.; Yang, Y.; Zhuang, H.-F.; Shan, S.-D.; Beldean-Galea, M.-S.; Xue, Q.-Q.; Shen, X.-F.; Li, S.-J. In-situ growth of MIL-53 (Fe) on charcoal sponge as a highly efficient and recyclable photocatalyst for removal of Cr(VI). Rare Met. 2024, 43, 4344–4355. [Google Scholar] [CrossRef]
- Li, S.; Wu, F.; Lin, R.; Wang, J.; Li, C.; Li, Z.; Jiang, J.; Xiong, Y. Enabling photocatalytic hydrogen production over Fe-based MOFs by refining band structure with dye sensitization. Chem. Eng. J. 2022, 429, 132217. [Google Scholar] [CrossRef]
- Bamba, D.; Coulibaly, M.; Fort, C.I.; Coteţ, C.L.; Pap, Z.; Vajda, K.; Zoro, E.G.; Yao, N.A.; Danciu, V.; Robert, D. Synthesis and characterization of TiO 2 /C nanomaterials: Applications in water treatment. Phys. Status Solidi 2015, 252, 2503–2511. [Google Scholar] [CrossRef]
- Mendonça, T.A.P.; Giroto, A.S.; Chambi, J.T.; Cuffini, S.L.; Vieira, N.C.S.; Gonçalves, M. Efficient photocatalyst based on activated carbon/graphene oxide/TiO2 synthesized under acidic conditions for environmental remediation. J. Photochem. Photobiol. A Chem. 2025, 462, 116244. [Google Scholar] [CrossRef]
- Yang, X.-C.; Zhao, J.-T. Aerogel for Highly Efficient Photocatalytic Degradation. Gels 2024, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Baia, L.; Vulpoi, A.; Radu, T.; Karácsonyi, É.; Dombi, A.; Hernádi, K.; Danciu, V.; Simon, S.; Norén, K.; Canton, S.E.; et al. TiO2/WO3/Au nanoarchitectures’ photocatalytic activity “from degradation intermediates to catalysts’ structural peculiarities” Part II: Aerogel based composites—Fine details by spectroscopic means. Appl. Catal. B 2014, 148–149, 589–600. [Google Scholar] [CrossRef]
- Chen, F.; Zhao, J. Preparation and photocatalytic properties of a novel kind of loaded photocatalyst of TiO2/SiO2/γ-Fe2O3. Catal Lett. 1999, 58, 245–247. [Google Scholar] [CrossRef]
- Kalidass, J.; Reji, M.; Sivasankar, T. Synthesis of Fe3O4 nanoparticles with enhanced properties via sonoelectrochemical approach: A comparative study with electrochemical and hydrothermal method. Chem. Eng. Process.—Process Intensif. 2024, 197, 109690. [Google Scholar] [CrossRef]
- Nguyen, M.D.; Tran, H.-V.; Xu, S.; Lee, T.R. Fe3O4 Nanoparticles: Structures, Synthesis, Magnetic Properties, Surface Functionalization, and Emerging Applications. Appl. Sci. 2021, 11, 11301. [Google Scholar] [CrossRef]
- Demortière, A.; Panissod, P.; Pichon, B.P.; Pourroy, G.; Guillon, D.; Donnio, B.; Bégin-Colin, S. Size-dependent properties of magnetic iron oxidenanocrystals. Nanoscale 2011, 3, 225–232. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, M.; Alhazmi, A.; Mohammad, A.; Khan, S.; Pal, D.B.; Haque, S.; Singh, R.; Mishra, P.K.; Gupta, V.K. Sustainable green approach to synthesize Fe3O4/α-Fe2O3 nanocomposite using waste pulp of Syzygium cumini and its application in functional stability of microbial cellulases. Sci. Rep. 2021, 11, 24371. [Google Scholar] [CrossRef]
- Nyirő-Kósa, I.; Rečnik, A.; Pósfai, M. Novel methods for the synthesis of magnetite nanoparticles with special morphologies and textured assemblages. J. Nanoparticle Res. 2012, 14, 1150. [Google Scholar] [CrossRef]
- López, J.; Rey, A.; Viñuelas-Zahinos, E.; Álvarez, P.M. Preparation of a new green magnetic Fe3O4 @TiO2-P25 photocatalyst for solar advanced oxidation processes in water. J. Environ. Chem. Eng. 2023, 11, 109999. [Google Scholar] [CrossRef]
- Li, Z.-D.; Wang, H.-L.; Wei, X.-N.; Liu, X.-Y.; Yang, Y.-F.; Jiang, W.-F. Preparation and photocatalytic performance of magnetic Fe3O4@TiO2 core–shell microspheres supported by silica aerogels from industrial fly ash. J. Alloys Compd. 2016, 659, 240–247. [Google Scholar] [CrossRef]
- Cendrowski, K.; Sikora, P.; Zielinska, B.; Horszczaruk, E.; Mijowska, E. Chemical and thermal stability of core-shelled magnetite nanoparticles and solid silica. Appl. Surf. Sci. 2017, 407, 391–397. [Google Scholar] [CrossRef]
- Korina, E.; Stoilova, O.; Manolova, N.; Rashkov, I. Polymer fibers with magnetic core decorated with titanium dioxide prospective for photocatalytic water treatment. J. Environ. Chem. Eng. 2018, 6, 2075–2084. [Google Scholar] [CrossRef]
- Wittmar, A.S.M.; Fu, Q.; Ulbricht, M. Photocatalytic and Magnetic Porous Cellulose-Based Nanocomposite Films Prepared by a Green Method. ACS Sustain. Chem. Eng. 2017, 5, 9858–9868. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO 2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A Chem. 2010, 216, 179–182. [Google Scholar] [CrossRef]
- Tang, P.; Ding, Y.; Chen, H.; Wang, Y. Preparation of TiO2/Fe3O4 composite by sol-gel method and its photocatalytic activity for removal of Rhodamine B from water. Ferroelectrics 2020, 562, 66–72. [Google Scholar] [CrossRef]
- Kubiak, A. Comparative study of TiO2–Fe3O4 photocatalysts synthesized by conventional and microwave methods for metronidazole removal. Sci. Rep. 2023, 13, 12075. [Google Scholar] [CrossRef]
- Madima, N.; Kefeni, K.K.; Mishra, S.B.; Mishra, A.K.; Kuvarega, A.T. Fabrication of magnetic recoverable Fe3O4/TiO2 heterostructure for photocatalytic degradation of rhodamine B dye. Inorg. Chem. Commun. 2022, 145, 109966. [Google Scholar] [CrossRef]
- Lendzion-Bieluń, Z.; Wojciechowska, A.; Grzechulska-Damszel, J.; Narkiewicz, U.; Śniadecki, Z.; Idzikowski, B. Effective processes of phenol degradation on Fe3O4–TiO2 nanostructured magnetic photocatalyst. J. Phys. Chem. Solids 2020, 136, 109178. [Google Scholar] [CrossRef]
- Kubiak, A.; Kubacka, M.; Gabała, E.; Dobrowolska, A.; Synoradzki, K.; Siwińska-Ciesielczyk, K.; Czaczyk, K.; Jesionowski, T. Hydrothermally Assisted Fabrication of TiO2-Fe3O4 Composite Materials and Their Antibacterial Activity. Materials 2020, 13, 4715. [Google Scholar] [CrossRef]
- Harifi, T.; Montazer, M. A novel magnetic reusable nanocomposite with enhanced photocatalytic activities for dye degradation. Sep. Purif. Technol. 2014, 134, 210–219. [Google Scholar] [CrossRef]
- Chang, J.; Zhang, Q.; Liu, Y.; Shi, Y.; Qin, Z. Preparation of Fe3O4/TiO2 magnetic photocatalyst for photocatalytic degradation of phenol. J. Mater. Sci. Mater. Electron. 2018, 29, 8258–8266. [Google Scholar] [CrossRef]
- Pandey, B.; Pandey, A.K.; Bhardwaj, L.; Dubey, S.K. Biodegradation of acetaminophen: Current knowledge and future directions with mechanistic insights from omics. Chemosphere 2025, 372, 144096. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, H.; Rong, C.; Li, A.; Hua, X.; Dong, D.; Liang, D.; Liu, H. Photocatalytic Degradation of Acetaminophen in Aqueous Environments: A Mini Review. Toxics 2023, 11, 604. [Google Scholar] [CrossRef]
- Imran, M.; Riaz, S.; Naseem, S. Synthesis and Characterization of Titania Nanoparticles by Sol-gel Technique. Mater. Today Proc. 2015, 2, 5455–5461. [Google Scholar] [CrossRef]
- Nalbandian, L.; Patrikiadou, E.; Zaspalis, V.; Patrikidou, A.; Hatzidaki, E.; Papandreou, C.N. Magnetic Nanoparticles in Medical Diagnostic Applications: Synthesis, Characterization and Proteins Conjugation. Curr. Nanosci. 2016, 12, 455–468. [Google Scholar] [CrossRef]
- Ghalamchi, L.; Rasoulifard, M.H. Immobilization of Fe3O4/TiO2 nanocomposite thin layer on the glass tubes in a component parabolic collector for the treatment of DR23. Int. J. Environ. Sci. Technol. 2019, 16, 7509–7522. [Google Scholar] [CrossRef]
- Younis, A.B.; Milosavljevic, V.; Fialova, T.; Smerkova, K.; Michalkova, H.; Svec, P.; Antal, P.; Kopel, P.; Adam, V.; Zurek, L.; et al. Synthesis and characterization of TiO2 nanoparticles combined with geraniol and their synergistic antibacterial activity. BMC Microbiol. 2023, 23, 207. [Google Scholar] [CrossRef]
- Thamir, A.D.; Haider, A.J.; Ali, G.A. Preparation of NanostructureTiO2 at Different Temperatures by Pulsed Laser Deposition as Solar Cell. Eng. Technol. J. 2016, 34, 193–204. [Google Scholar] [CrossRef]
- Anselmi, C.; Mosconi, E.; Pastore, M.; Ronca, E.; De Angelis, F. Adsorption of organic dyes on TiO2 surfaces in dye-sensitized solar cells: Interplay of theory and experiment. Phys. Chem. Chem. Phys. 2012, 14, 15963. [Google Scholar] [CrossRef] [PubMed]
- Kurtan, U.; Topkaya, R.; Baykal, A.; Toprak, M.S. Temperature dependent magnetic properties of CoFe2O4/CTAB nanocomposite synthesized by sol–gel auto-combustion technique. Ceram. Int. 2013, 39, 6551–6558. [Google Scholar] [CrossRef]
- Stoner, E.C.; Wohlfarth, E.P. A mechanism of magnetic hysteresis in heterogeneous alloys, Philosophical Transactions of the Royal Society of London. Series A. Math. Phys. Sci. 1948, 240, 599–642. [Google Scholar] [CrossRef]
- Alzoubi, G.M.; Masadeh, A.S.; Shatnawi, M.T.M. Investigation of the structural, morphological, and magnetic properties of small crystalline Co–Cu ferrite nanoparticles in the single-domain regime. AIP Adv. 2022, 12. [Google Scholar] [CrossRef]
- Muthukrishnan, S.; Vidya, R.; Sjåstad, A.O. Band gap engineering of anatase TiO2 by ambipolar doping: A first principles study. Mater. Chem. Phys. 2023, 299, 127467. [Google Scholar] [CrossRef]
- Shi, H.; Lin, Y.; Jiang, Z.; Su, Y.; Ding, X.; Zhang, X.; Zhu, H.; Zhang, R. Enhanced optical absorption and photocatalytic activity of anatase TiO2 through C Nd-codoped: A DFT+ U calculations. J. Phys. Chem. Solids 2017, 109, 70–77. [Google Scholar] [CrossRef]
- Hossain, M.K.; Mortuza, A.A.; Sen, S.K.; Basher, M.K.; Ashraf, M.W.; Tayyaba, S.; Mia, M.N.H.; Uddin, M.J. A comparative study on the influence of pure anatase and Degussa-P25 TiO2 nanomaterials on the structural and optical properties of dye sensitized solar cell (DSSC) photoanode. Optik 2018, 171, 507–516. [Google Scholar] [CrossRef]
- Compeán-Jasso, M.E.; Ruiz, F.; Martínez, J.R.; Herrera-Gómez, A. Magnetic properties of magnetite nanoparticles synthesized by forced hydrolysis. Mater. Lett. 2008, 62, 4248–4250. [Google Scholar] [CrossRef]
- Mirza, I.M.; Ali, K.; Sarfraz, A.K.; Ali, A.; Ul Haq, A. A study of dielectric, optical and magnetic characteristics of maghemite nanocrystallites. Mater. Chem. Phys. 2015, 164, 183–187. [Google Scholar] [CrossRef]
- Bui, H.T.; Im, S.M.; Kim, K.; Kim, W.; Lee, H. Photocatalytic degradation of phenolic compounds of defect engineered Fe3O4: An alternative approach to solar activation via ligand-to-metal charge transfer. Appl. Surf. Sci. 2020, 509, 144853. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Akbar, A.; Riaz, S.; Bashir, M.; Naseem, S. Effect of Fe3+/Fe2+ Ratio on Superparamagnetic Behavior of Spin Coated Iron Oxide Thin Films. IEEE Trans. Magn. 2014, 50, 1–4. [Google Scholar] [CrossRef]
- Žerjav, G.; Žižek, K.; Zavašnik, J.; Pintar, A. Brookite vs. rutile vs. anatase: What`s behind their various photocatalytic activities? J. Environ. Chem. Eng. 2022, 10, 107722. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Abdullah, M.A.B.; Chin, L.Y. TiO2/PKSAC functionalized with Fe3O4 for efficient concurrent removal of heavy metal ions from water. Colloid. Interface Sci. Commun. 2021, 40, 100353. [Google Scholar] [CrossRef]
- Komaraiah, D.; Radha, E.; Sivakumar, J.; Reddy, M.V.R.; Sayanna, R. Influence of Fe3+ ion doping on the luminescence emission behavior and photocatalytic activity of Fe3+, Eu3+-codoped TiO2 thin films. J. Alloys Compd. 2021, 868, 159109. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, L.; Xu, C.; Zhang, W.; Chen, M.; Xu, Q.; Diao, G. A novel method for facile preparation of recoverable Fe3O4@TiO2 core-shell nanospheres and their advanced photocatalytic application. Chem. Phys. Lett. 2020, 761, 138073. [Google Scholar] [CrossRef]
- Hossain, M.S.; Ahmed, S. Easy and green synthesis of TiO2 (Anatase and Rutile): Estimation of crystallite size using Scherrer equation, Williamson-Hall plot, Monshi-Scherrer Model, size-strain plot, Halder- Wagner Model. Results Mater. 2023, 20, 100492. [Google Scholar] [CrossRef]
- Landi, S.; Segundo, I.R.; Freitas, E.; Vasilevskiy, M.; Carneiro, J.; Tavares, C.J. Use and misuse of the Kubelka-Munk function to obtain the band gap energy from diffuse reflectance measurements. Solid. State Commun. 2022, 341, 114573. [Google Scholar] [CrossRef]
- Zhang, H.; Zeng, D.; Liu, Z. The law of approach to saturation in ferromagnets originating from the magnetocrystalline anisotropy. J. Magn. Magn. Mater. 2010, 322, 2375–2380. [Google Scholar] [CrossRef]
- Fairley, N.; Fernandez, V.; Richard-Plouet, M.; Guillot-Deudon, C.; Walton, J.; Smith, E.; Flahaut, D.; Greiner, M.; Biesinger, M.; Tougaard, S.; et al. Systematic and collaborative approach to problem solving using X-ray photoelectron spectroscopy. Appl. Surf. Sci. Adv. 2021, 5, 100112. [Google Scholar] [CrossRef]
- Haavik, C.; Stølen, S.; Fjellvåg, H.; Hanfland, M.; Häusermann, D. Equation of state of magnetite and its high-pressure modification: Thermodynamics of the Fe-O system at high pressure. American Mineralogist 2000, 85, 514–523. [Google Scholar] [CrossRef]
- Kim, W.; Suh, C.-Y.; Cho, S.-W.; Roh, K.-M.; Kwon, H.; Song, K.; Shon, I.-J. A new method for the identification and quantification of magnetite–maghemite mixture using conventional X-ray diffraction technique. Talanta 2012, 94, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.; Bonifacio, C.S.; Majidi, H.; van Benthem, K. Stabilization of metal(II)oxides on the nanoscale. Mater. Res. Lett. 2020, 8, 41–47. [Google Scholar] [CrossRef]
- Testa-Anta, M.; Ramos-Docampo, M.A.; Comesaña-Hermo, M.; Rivas-Murias, B.; Salgueiriño, V. Raman spectroscopy to unravel the magnetic properties of iron oxide nanocrystals for bio-related applications. Nanoscale Adv. 2019, 1, 2086–2103. [Google Scholar] [CrossRef]
- Drummond, A.L.; Feitoza, N.C.; Duarte, G.C.; Sales, M.J.A.; Silva, L.P.; Chaker, J.A.; Bakuzis, A.F.; Sousa, M.H. Reducing Size-Dispersion in One-Pot Aqueous Synthesis of Maghemite Nanoparticles. J. Nanosci. Nanotechnol. 2012, 12, 8061–8066. [Google Scholar] [CrossRef]
- Khasawneh, O.F.S.; Palaniandy, P.; Ahmadipour, M.; Mohammadi, H.; Hamdan, M.R.B. Removal of acetaminophen using Fe2O3-TiO2 nanocomposites by photocatalysis under simulated solar irradiation: Optimization study. J. Environ. Chem. Eng. 2021, 9, 104921. [Google Scholar] [CrossRef]
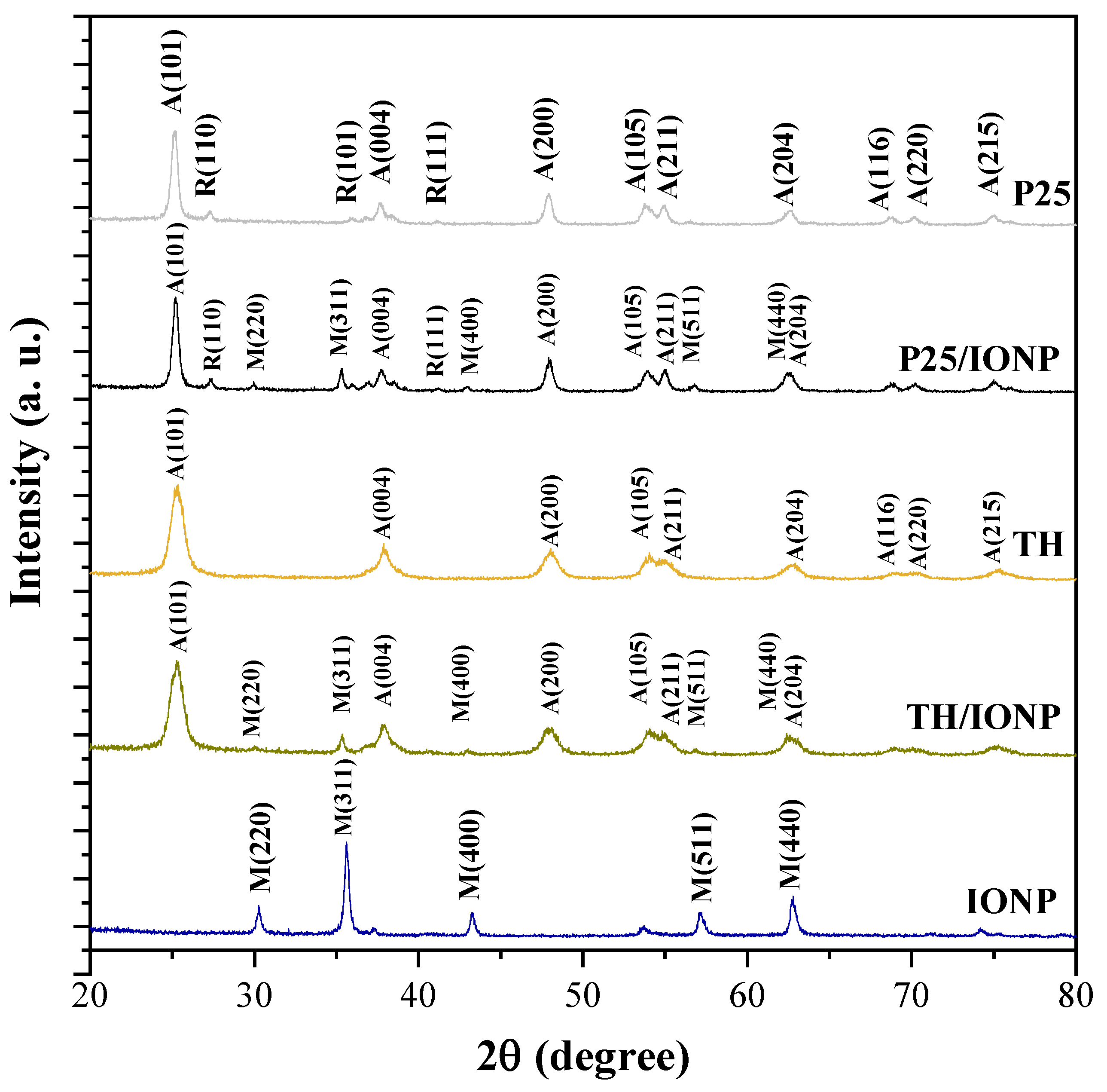

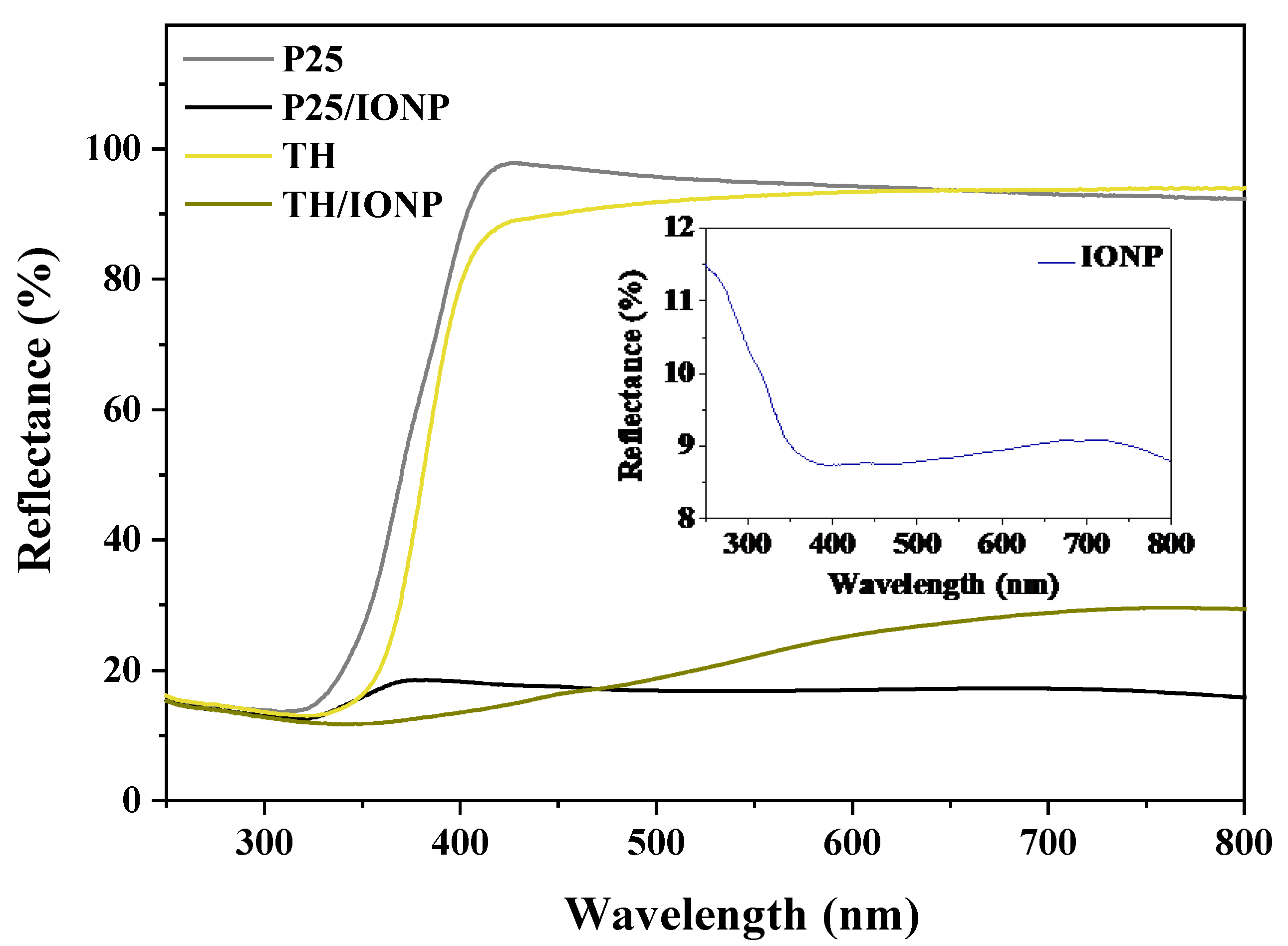
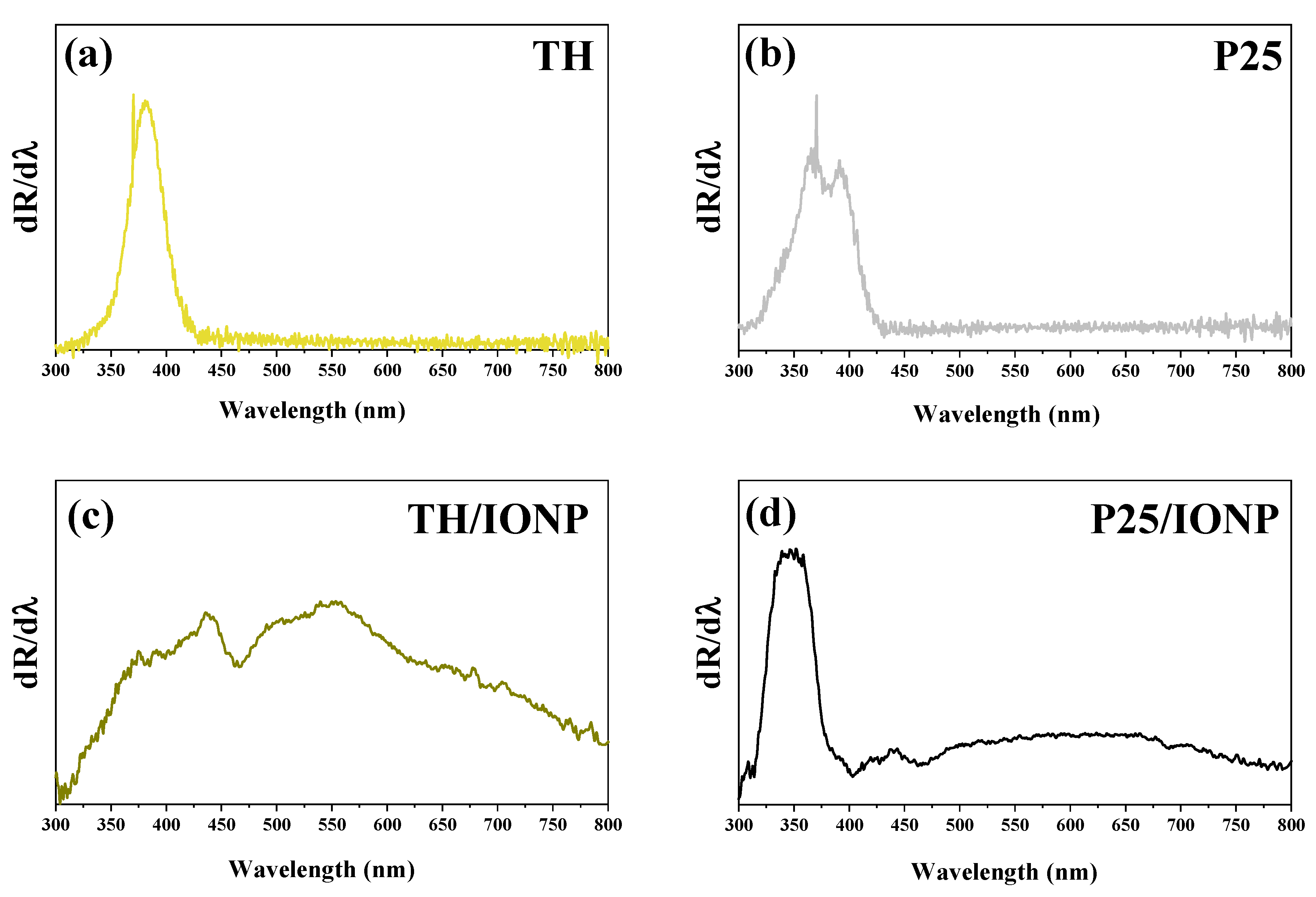

| Sample Name |
NaOH
Concentration [M] |
Synthesis
Temperature [°C] | Hydrothermal Treatment [°C] | Crystal Phase | Primary Crystallite Size [nm] |
|---|---|---|---|---|---|
| IONP_0.8_75 | 0.8 | 75 | - | magnetite | 46.4 |
| P25 | - | 25 | - | anatase | 24 |
| rutile | 36 | ||||
| TH | - | 25 | 180 | anatase | 9.2 |
| P25/IONP | 0.8 | 75 | - | anatase | 25 |
| rutile | 41 | ||||
| magnetite | 65.2 | ||||
| TH/IONP | 0.8 | 75 | - | anatase | 9.9 |
| magnetite | 52.3 |
| Sample Name | Ms (emu/g) | μ0Hc (mT) | Mr (emu/g) | Mr/Ms |
|---|---|---|---|---|
| IONP_0.8_75 | 54.2 (2) | 14 | 9.38 | 0.17 |
| P25/IONP | 7.51 (1) | 15 | 1.97 | 0.26 |
| TH/IONP | 4.22 (7) | 14 | 1.1 | 0.26 |
| Photocatalyst | Cycle | Adsorption (%) | Paracetamol Degradation Efficiency (%) | kapp (min−1) | R2 |
|---|---|---|---|---|---|
| P25 | 1 | 1.0 | 99.9 | 0.132 | 0.9566 |
| 2 | 10.5 | 99.9 | 0.082 | 0.9677 | |
| 3 | 3.7 | 99.9 | 0.075 | 0.9983 | |
| 4 | 8.4 | 99.7 | 0.085 | 0.9999 | |
| TH | 1 | 0.1 | 100.0 | 0.046 | 0.9470 |
| 2 | 1.9 | 99.7 | 0.064 | 0.9838 | |
| 3 | 1.5 | 99.6 | 0.045 | 0.9842 | |
| 4 | 5.2 | 99.7 | 0.068 | 0.9563 | |
| P25/IONP | 1 | 1.4 | 98.9 | 0.023 | 0.9799 |
| 2 | 3.2 | 98.8 | 0.019 | 0.9966 | |
| 3 | 1.4 | 97.9 | 0.022 | 0.9961 | |
| 4 | 9.3 | 95.7 | 0.020 | 0.9944 | |
| TH/IONP | 1 | 1.4 | 69.4 | 0.009 | 0.9998 |
| 2 | 1.3 | 45.9 | 0.007 | 0.9911 | |
| 3 | 6.7 | 50.9 | 0.006 | 0.9999 | |
| 4 | 0.6 | 44.8 | 0.005 | 0.9991 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saszet, K.; Guliman, S.; Szalma, L.; Székely, I.; Tetean, R.; Todea, M.; Szamosvölgyi, Á.; Mureșan-Pop, M.; Barbu-Tudoran, L.; Magyari, K.; et al. Recyclable TiO2–Fe3O4 Magnetic Composites for the Photocatalytic Degradation of Paracetamol: Comparative Effect of Pure Anatase and Mixed-Phase P25 TiO2. Catalysts 2025, 15, 839. https://doi.org/10.3390/catal15090839
Saszet K, Guliman S, Szalma L, Székely I, Tetean R, Todea M, Szamosvölgyi Á, Mureșan-Pop M, Barbu-Tudoran L, Magyari K, et al. Recyclable TiO2–Fe3O4 Magnetic Composites for the Photocatalytic Degradation of Paracetamol: Comparative Effect of Pure Anatase and Mixed-Phase P25 TiO2. Catalysts. 2025; 15(9):839. https://doi.org/10.3390/catal15090839
Chicago/Turabian StyleSaszet, Kata, Simona Guliman, Lilla Szalma, István Székely, Romulus Tetean, Milica Todea, Ákos Szamosvölgyi, Marieta Mureșan-Pop, Lucian Barbu-Tudoran, Klára Magyari, and et al. 2025. "Recyclable TiO2–Fe3O4 Magnetic Composites for the Photocatalytic Degradation of Paracetamol: Comparative Effect of Pure Anatase and Mixed-Phase P25 TiO2" Catalysts 15, no. 9: 839. https://doi.org/10.3390/catal15090839
APA StyleSaszet, K., Guliman, S., Szalma, L., Székely, I., Tetean, R., Todea, M., Szamosvölgyi, Á., Mureșan-Pop, M., Barbu-Tudoran, L., Magyari, K., Pop, L.-C., Pap, Z., & Baia, L. (2025). Recyclable TiO2–Fe3O4 Magnetic Composites for the Photocatalytic Degradation of Paracetamol: Comparative Effect of Pure Anatase and Mixed-Phase P25 TiO2. Catalysts, 15(9), 839. https://doi.org/10.3390/catal15090839














