Enhanced Oxygen Reduction Reaction Activity of Carbon-Supported Pt-Co Catalysts Prepared by Electroless Deposition and Galvanic Replacement
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Characterization
2.2. Electrochemical Characterization
3. Experimental
3.1. Synthesis of Pt-Co/C Catalyst
3.2. Material Characterization
4. Conclusions—Further Work
- (i)
- A low Co-content Pt-Co/C nanocatalyst (up to ca 1 wt.% Co and of a Pt ÷ Co atom ratio of 7:1) was successfully prepared by a generic, low-energy, and chemistry-intensive preparation route that involves electroless deposition Co-P onto C, followed by spontaneous partial galvanic replacement of Co and Co(OH)2 with Pt. Further work should aim at minimizing the precursor Co content as well as reclaiming/reusing the etched Co(II) species.)
- (ii)
- The used alternative method results in the formation of a Co-Pt core-shell structure. Further work should aim at testing the long-term performance of the catalyst to investigate whether this structure prevents gradual Co leaching during operation.
- (iii)
- Despite the presence of large catalyst aggregates and a relatively low surface area, the higher activity in Pt-Co/C may be attributed to its optimized composition and structure, facilitating efficient ORR as a result of the well-known modification of the electronic properties of Pt by less noble transition metals.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srinivasan, S. Fuel Cells: From Fundamentals to Applications; Springer Science: New York, NY, USA, 2006. [Google Scholar]
- Rimon, S.T.A.; Mourshed, M.; Kibria, M.G. Proton exchange membrane fuel cells: Advances in materials development, performance optimization, and future outlook. Energy Convers. Manag. X 2025, 27, 101102–101153. [Google Scholar] [CrossRef]
- Kumar, V.B.; Collin, A.; Sankar, M.G.; Subramanian, K. Fuel cell technologies in the automotive sector: A focus on proton exchange membrane and Alkaline fuel cells. Green Technol. Sustain. 2025, 3, 100218–100236. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Wilkinson, D.P.; Wang, H. Progress in preparation of non-noble electrocatalysts for PEM fuel cell reactions. J. Power Sources 2006, 156, 171–182. [Google Scholar] [CrossRef]
- Gewirth, A.A.; Thorum, M.S. Electroreduction of dioxygen for fuel-cell applications: Materials and challenges. Inorg. Chem. 2010, 49, 3557–3566. [Google Scholar] [CrossRef]
- Norskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jonsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Z.; Yu, J.; Wang, H.; Qin, Y.; Xing, L.; Du, L. Research Progress of Pt-Based Catalysts toward Cathodic Oxygen Reduction Reactions for Proton Exchange Membrane Fuel Cells. Catalysts 2024, 14, 569. [Google Scholar] [CrossRef]
- Zhan, F.; Huang, L.; Luo, Y.; Chen, M.; Tan, R.; Liu, X.; Liu, G.; Feng, Z. Recent advances on support materials for enhanced Pt-based catalysts: Applications in oxygen reduction reactions for electrochemical energy storage. J. Mater. Sci. 2025, 60, 2199–2223. [Google Scholar] [CrossRef]
- Antolini, E.; Zignani, S.; Santos, S.; Gonzalez, E. Palladium-based electrodes: A way to reduce platinum content in polymer electrolyte membrane fuel cells. Electrochim. Acta 2011, 56, 2299–2305. [Google Scholar] [CrossRef]
- Huang, L.; Xu, H.-C.; Jing, B.; Li, Q.-X.; Yi, W.; Sun, S.-G. Progress of Pt-Based Catalysts in Proton-Exchange Membrane Fuel Cells. J. Electrochem. 2022, 28, 2108061–2108078. [Google Scholar]
- Lee, S.-Y.; Kim, M.-K.; Son, U.-H.; Han, S.; Lee, S.; Joh, H.-I. Recent progress of low-loaded platinum on well-functionalized carbon electrocatalysts for oxygen reduction reaction. Carbon Lett. 2025, 35, 107–127. [Google Scholar] [CrossRef]
- Mohd Nor, M.A.A.; Abdul Rani, M.A.A.; Loh, K.S.; Choo, T.F.; Lim, K.L.; Osman, S.H.; Loy, A.C.M.; Wong, W.Y. Maximizing oxygen reduction reaction performance with minimal Pt: A review of high mass activity Pt-M catalysts. J. Power Sources 2025, 654, 237869–237892. [Google Scholar] [CrossRef]
- Cao, F.; Ding, R.; Rui, Z.; Wang, X.; Meng, Z.; Zhang, B.; Dong, W.; Li, J.; Liu, J.; Jiang, X. Advances in Low Pt Loading Membrane Electrode Assembly for Proton Exchange Membrane Fuel Cells. Molecules 2023, 28, 773. [Google Scholar] [CrossRef]
- Geboes, B.; Mintsouli, I.; Wouters, B.; Georgieva, J.; Kakaroglou, A.; Sotiropoulos, S.; Valova, E.; Armyanov, S.; Hubin, A.; Breugelmans, T. Surface and electrochemical characterisation of a Pt-Cu/C nano-structured electrocatalyst, prepared by galvanic displacement. Appl. Catal. B Environ. 2014, 150–151, 249–256. [Google Scholar] [CrossRef]
- Shao, M.; Chang, Q.; Dodelet, J.-P.; Chenitz, R. Recent Advances in Electrocatalysts for Oxygen Reduction Reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Zhao, Y.; Wang, F. Composition-controlled synthesis of carbon-supported Pt–Co alloy nanoparticles and the origin of their ORR activity enhancement. Phys. Chem. Chem. Phys. 2014, 16, 19298–19306. [Google Scholar] [CrossRef] [PubMed]
- Salgado, J.R.C.; Antolini, E.; Gonzalez, E.R. Structure and Activity of Carbon-Supported Pt-Co Electrocatalysts for Oxygen Reduction. Phys. Chem. B 2004, 108, 17767–17774. [Google Scholar] [CrossRef]
- Weber, P.; Weber, D.J.; Dosche, C.; Oezaslan, M. Durable Pt-Based Core–Shell Catalysts with Metallic and Oxidized Co Species for Boosting the Oxygen Reduction Reaction. ACS Catal. 2022, 12, 6394–6408. [Google Scholar] [CrossRef]
- Shen, J.-F.; Hu, S.-N.; Tian, N.; Li, M.-Y.; Yang, S.-L.; Tian, S.-Y.; Chen, M.-S.; Zhou, Z.-Y.; Sun, S.-G. P-Doping Strategy Increasing the Durability of PtCo Nanoparticles for the Oxygen Reduction Reaction. ACS Sustain. Chem. Eng. 2023, 11, 11660–11667. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Mun, B.S.; Arenz, M.; Mayrhofer, K.J.J.; Lucas, C.A.; Wang, G.; Ross, P.N.; Markovic, N.M. Trends in Electrocatalysis on Extended and Nanoscale Pt-Bimetallic Alloy Surfaces. Nat. Mater. 2007, 6, 241–247. [Google Scholar] [CrossRef]
- Koh, S.; Leisch, J.; Toney, M.F.; Strasser, P. Structure-Activity-Stability Relationships of Pt-Co Alloy Electrocatalysts in Gas-Diffusion Electrode Layers. J. Phys. Chem. C 2007, 111, 3744–3752. [Google Scholar] [CrossRef]
- Oezaslan, M.; Strasser, P. Activity of dealloyed PtCo3 and PtCu3 nanoparticle electrocatalyst for oxygen reduction reaction in polymer electrolyte membrane fuel cell. J. Power Sources 2011, 196, 5240–5249. [Google Scholar] [CrossRef]
- Wang, X.X.; Hwang, S.; Pan, Y.T.; Chen, K.; He, Y.; Karakalos, S.; Zhang, H.; Spendelow, J.S.; Su, D.; Wu, G. Ordered Pt3Co intermetallic nanoparticles derived from metal-organic frameworks for oxygen reduction. Nano Lett. 2018, 18, 4163–4171. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yu, X.; Hou, J.; Xiang, Z. Secondary reduction strategy synthesis of Pt–Co nanoparticle catalysts towards boosting the activity of proton exchange membrane fuel cells. Particuology 2023, 79, 18–26. [Google Scholar] [CrossRef]
- Antolini, E. Platinum-based ternary catalysts for low temperature fuel cells: Part I. Preparation methods and structural characteristics. Appl. Catal. B Environ. 2007, 74, 324–336. [Google Scholar] [CrossRef]
- Antolini, E. Carbon supports for low-temperature fuel cell catalysts. Appl. Catal. B Environ. 2009, 88, 1–24. [Google Scholar] [CrossRef]
- Brankovic, S.R.; Wang, J.X.; Adžić, R.R. Metal monolayer deposition by replacement of metal adlayers on electrode surfaces. Surf. Sci. Lett. 2001, 474, L173–L179. [Google Scholar] [CrossRef]
- Van Brussel, M.; Kokkinidis, G.; Vandendael, I.; Buess-Herman, C. High performance gold-supported platinum electrocatalyst for oxygen reduction. Electrochem. Commun. 2002, 4, 808–813. [Google Scholar] [CrossRef]
- Papadimitriou, S.; Tegou, A.; Pavlidou, E.; Kokkinidis, G.; Sotiropoulos, S. Methanol oxidation at platinized lead coatings prepared by a two-step electrodeposition–electroless deposition process on glassy carbon and platinum substrates. Electrochim. Acta 2007, 52, 6254–6260. [Google Scholar] [CrossRef]
- Tegou, A.; Papadimitriou, S.; Armyanov, S.; Valova, E.; Kokkinidis, G.; Sotiropoulos, S. Oxygen reduction at platinum- and gold-coated iron, cobalt, nickel and lead deposits on glassy carbon substrates. J. Electroanal. Chem. 2008, 623, 187–196. [Google Scholar] [CrossRef]
- Tegou, A.; Papadimitriou, S.; Kokkinidis, G.; Sotiropoulos, S. A rotating disc electrode study of oxygen reduction at platinised nickel and cobalt coatings. J. Solid State Electrochem. 2010, 14, 175–184. [Google Scholar] [CrossRef]
- Papadimitriou, S.; Armyanov, S.; Valova, E.; Hubin, A.; Steenhaut, O.; Pavlidou, E.; Kokkinidis, G.; Sotiropoulos, S. Methanol Oxidation at Pt−Cu, Pt−Ni, and Pt−Co Electrode Coatings Prepared by a Galvanic Replacement Process. J. Phys. Chem. C 2010, 114, 5217–5223. [Google Scholar] [CrossRef]
- Mintsouli, I.; Georgieva, J.; Valova, E.; Armyanov, S.; Kakaroglou, A.; Hubin, A.; Steenhaut, O.; Dille, J.; Papaderakis, A.; Kokkinidis, G.; et al. Pt–Ni carbon-supported catalysts for methanol oxidation prepared by Ni electroless deposition and its galvanic replacement by Pt. J. Solid State Electrochem. 2013, 17, 435–443. [Google Scholar] [CrossRef]
- Mintsouli, I.; Georgieva, J.; Armyanov, S.; Valova, E.; Avdeev, G.; Hubin, A.; Steenhaut, O.; Dille, J.; Tsiplakides, D.; Balomenou, S.; et al. Pt-Cu electrocatalysts for methanol oxidation prepared by partial galvanic replacement of Cu/carbon powder precursors. Appl. Catal. B Environ. 2013, 136–137, 160–167. [Google Scholar] [CrossRef]
- Georgieva, J.; Valova, E.; Mintsouli, I.; Sotiropoulos, S.; Armyanov, S.; Kakaroglou, A.; Hubin, A.; Steenhaut, O.; Dille, J. Carbon-supported Pt(Cu) electrocatalysts for methanol oxidation prepared by Cu electroless deposition and its galvanic replacement by Pt. J. Appl. Electrochem. 2014, 44, 215–224. [Google Scholar] [CrossRef]
- Mintsouli, I.; Georgieva, J.; Papaderakis, A.; Armyanov, S.; Valova, E.; Khomenko, V.; Balomenou, S.; Tsiplakides, D.; Sotiropoulos, S. Methanol oxidation at platinized copper particles prepared by galvanic replacement. J. Electrochem. Sci. Eng. 2016, 6, 17–28. [Google Scholar] [CrossRef]
- Li, F.; Zhu, Y.; Ueno, H.; Deng, T. An Electrochemically Prepared Mixed Phase of Cobalt Hydroxide/Oxyhydroxide as a Cathode for Aqueous Zinc Ion Batteries. Inorganics 2023, 11, 400. [Google Scholar] [CrossRef]
- Chetty, R.; Kundu, S.; Xia, W.; Bron, M.; Schuhmann, W.; Chirila, V.; Brandl, W.; Reinecke, T.; Muhler, M. PtRu nanoparticles supported on nitrogen-doped multiwalled carbon nanotubes as catalyst for methanol electrooxidation. Electrochim. Acta 2009, 54, 4208–4215. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Kocha, S.K.; Sompalli, B.; Wagner, F.T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B Environ. 2005, 56, 9–35. [Google Scholar] [CrossRef]
- Papaderakis, A.; Matouli, I.; Spyridou, O.N.; Grammenos, A.O.; Banti, A.; Touni, A.; Pliatsikas, N.; Panagiotis Patsalas, P.; Sotiropoulos, S. Ternary IrO2-Pt-Ni deposits prepared by galvanic replacement as bifunctional oxygen catalysts. J. Electroanal. Chem. 2020, 877, 114499–114510. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, Z.; Sun, J.; Chen, S.; Zhang, J.; Zhang, S. Precise Pt-skin manipulation of strain and ligand effects for oxygen reduction. eScience 2025, 5, 100396–100408. [Google Scholar] [CrossRef]
- Jeon, M.K.; Zhang, Y.; McGinn, P.J. A comparative study of PtCo, PtCr, and PtCoCr catalysts for oxygen electro-reduction reaction. Electrochim. Acta 2010, 55, 5318–5325. [Google Scholar] [CrossRef]
- He, Q.; Mukerjee, S. Electrocatalysis of oxygen reduction on carbon-supported PtCo catalysts prepared by water-in-oil micro-emulsion. Electrochim. Acta 2010, 55, 1709–1719. [Google Scholar] [CrossRef]
- Jeon, M.K.; McGinn, P.J. Co-alloying effect of Co and Cr with Pt for oxygen electro-reduction reaction. Electrochim. Acta 2012, 64, 147–153. [Google Scholar] [CrossRef]
- Shinozaki, K.; Zack, J.W.; Pylypenko, S.; Richards, R.M.; Pivovar, B.S.; Kocha, S.S. Benchmarking the oxygen reduction reaction activity of Pt-based catalysts using standardized rotating disk electrode methods. Int. J. Hydrogen Energy 2015, 40, 16820–16830. [Google Scholar] [CrossRef]
- Cai, Y.; Gao, P.; Wang, F.; Zhu, H. Carbon supported chemically ordered nanoparicles with stable Pt shell and their superior catalysis toward the oxygen reduction reaction. Electrochim. Acta 2017, 245, 924–933. [Google Scholar] [CrossRef]
- Gunnarson, A.; De Bellis, J.; Imhof, T.; Pfänder, N.; Ledendecker, M.; Schüth, F. Facile Solid-State Synthesis of Supported PtNi and PtCo Bimetallic Nanoparticles for the Oxygen Reduction Reaction. Chem. Mater. 2023, 35, 2006–2015. [Google Scholar] [CrossRef]
- Xu, W.-C.; Zhang, Z.-M.; Yang, C.-H.; Zhao, K.-M.; Wang, Y.; Tian, N.; Zhou, Z.-Y.; Sun, S.-G. Promotion mechanism of PtCo intermetallic ordered alloys in oxygen reduction reaction and its application in fuel cells. Electrochem. Commun. 2023, 152, 107516–107522. [Google Scholar] [CrossRef]
- Yin, S.; Song, Y.; Liu, H.; Cui, J.; Liu, Z.; Li, Y.; Xue, T.; Tang, W.; Zhang, D.; Li, H.; et al. Well-Defined PtCo@Pt Core-Shell Nanodendrite Electrocatalyst for Highly Durable Oxygen Reduction Reaction. Small 2025, 21, 2410080. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Sasaki, K.; Adzic, R.R. Electrocatalysts for methanol oxidation with ultra low content of Pt and Ru. Electrochem. Commun. 2009, 11, 1135–1138. [Google Scholar] [CrossRef]
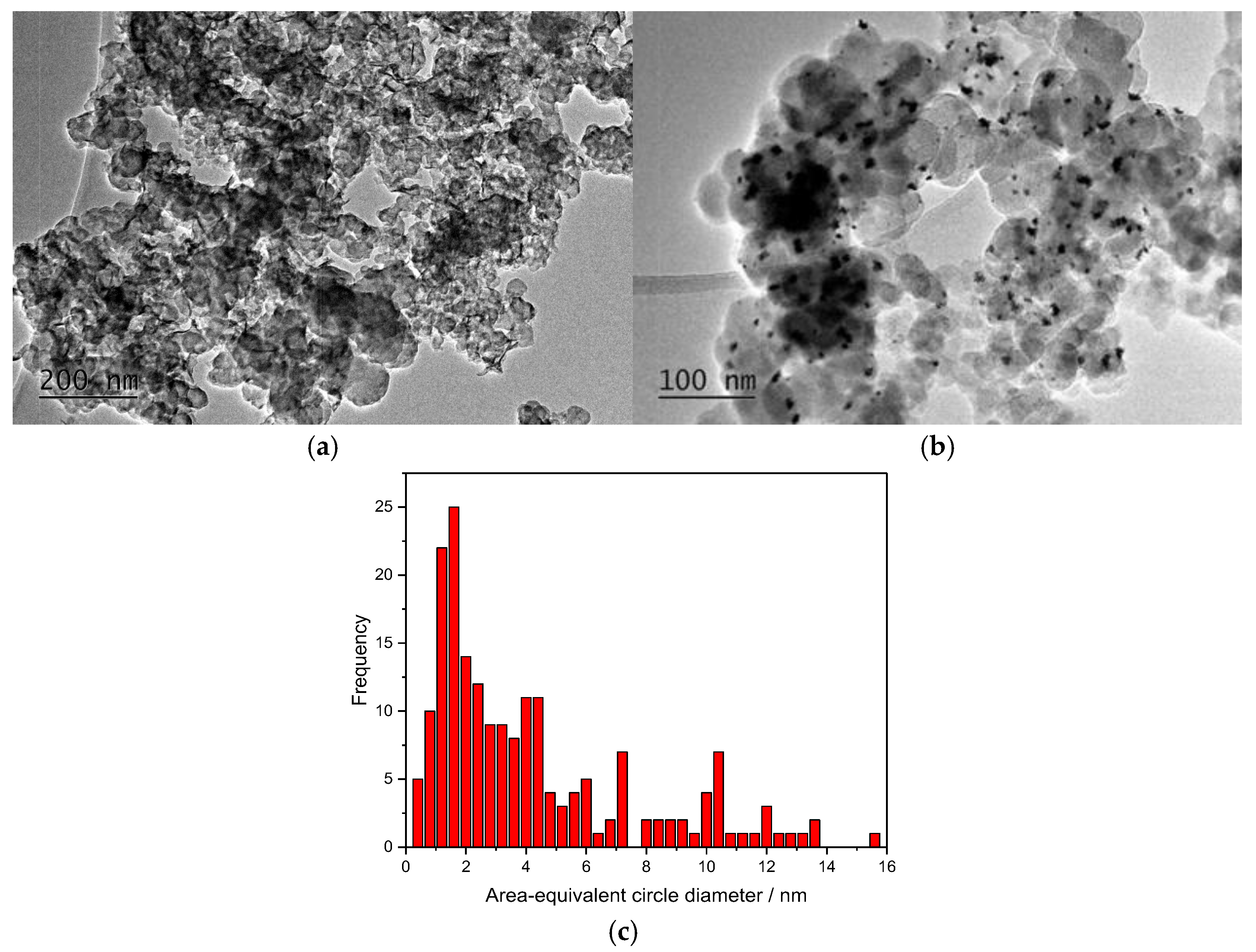
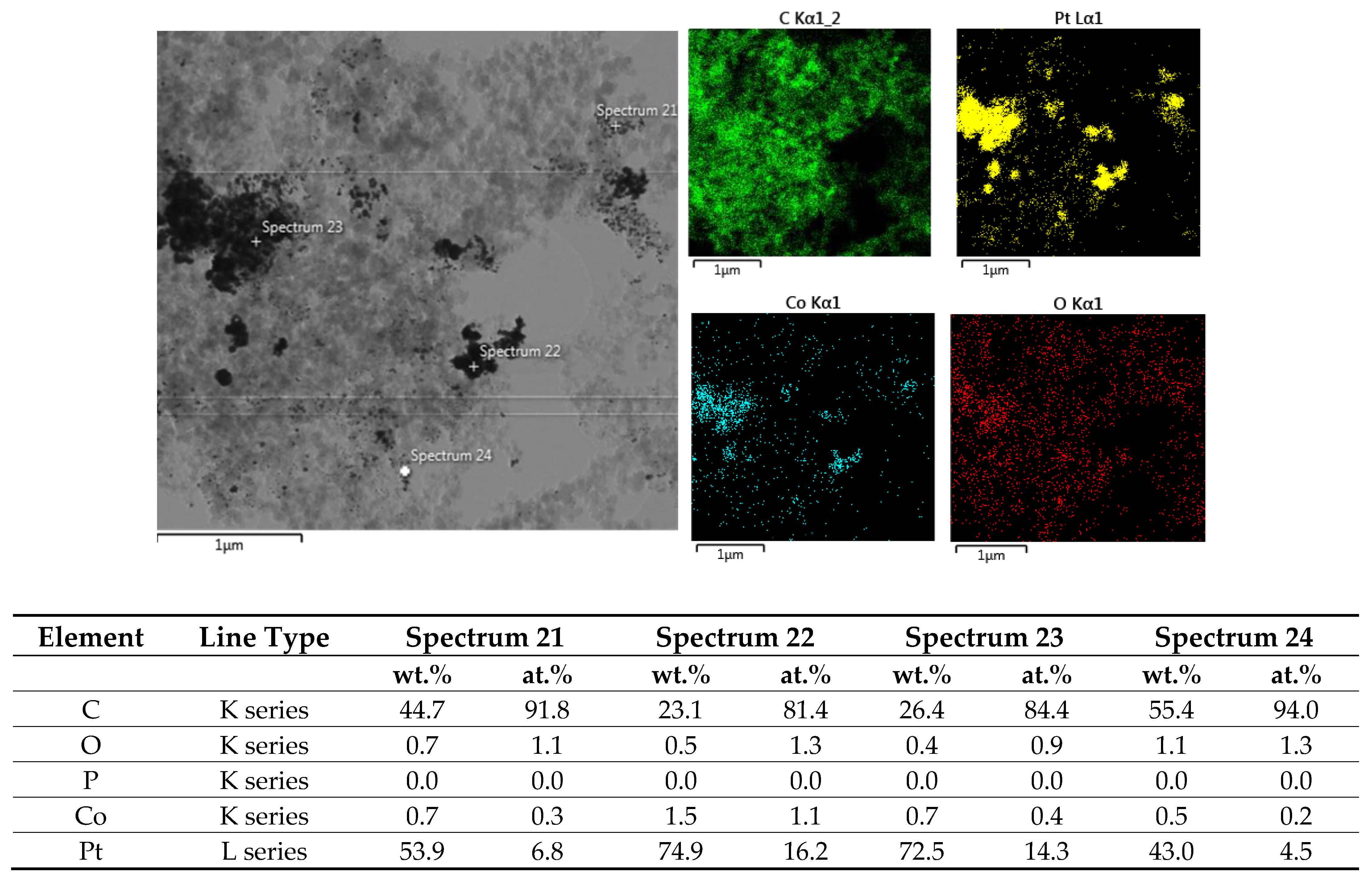
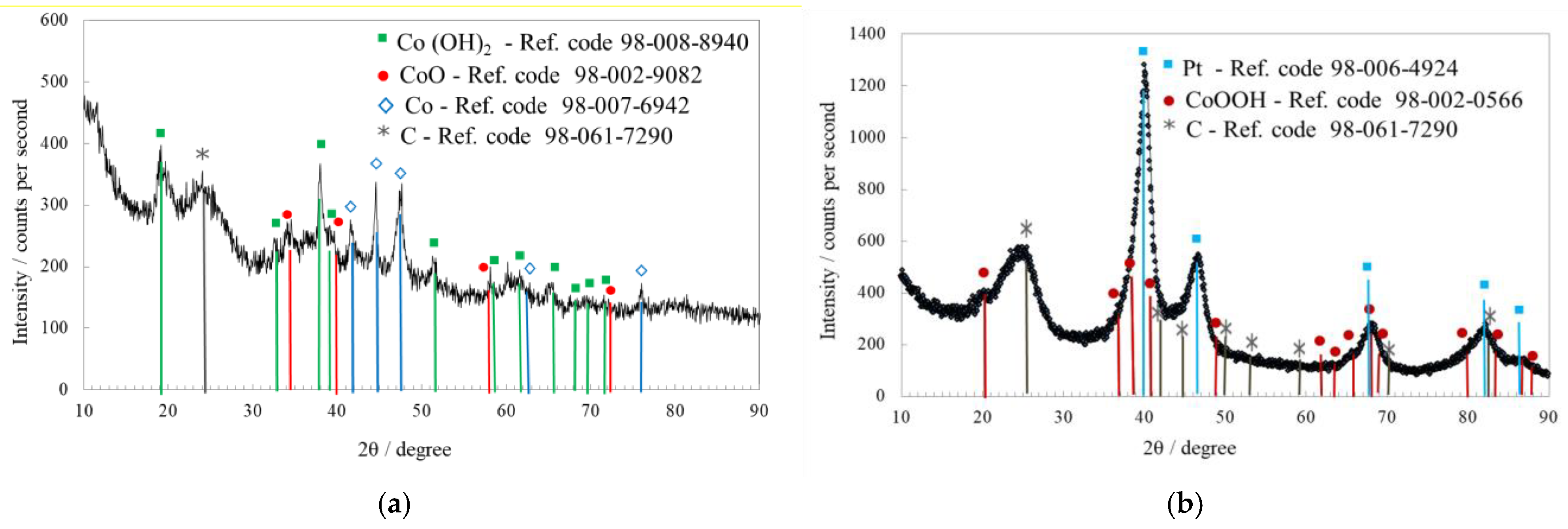
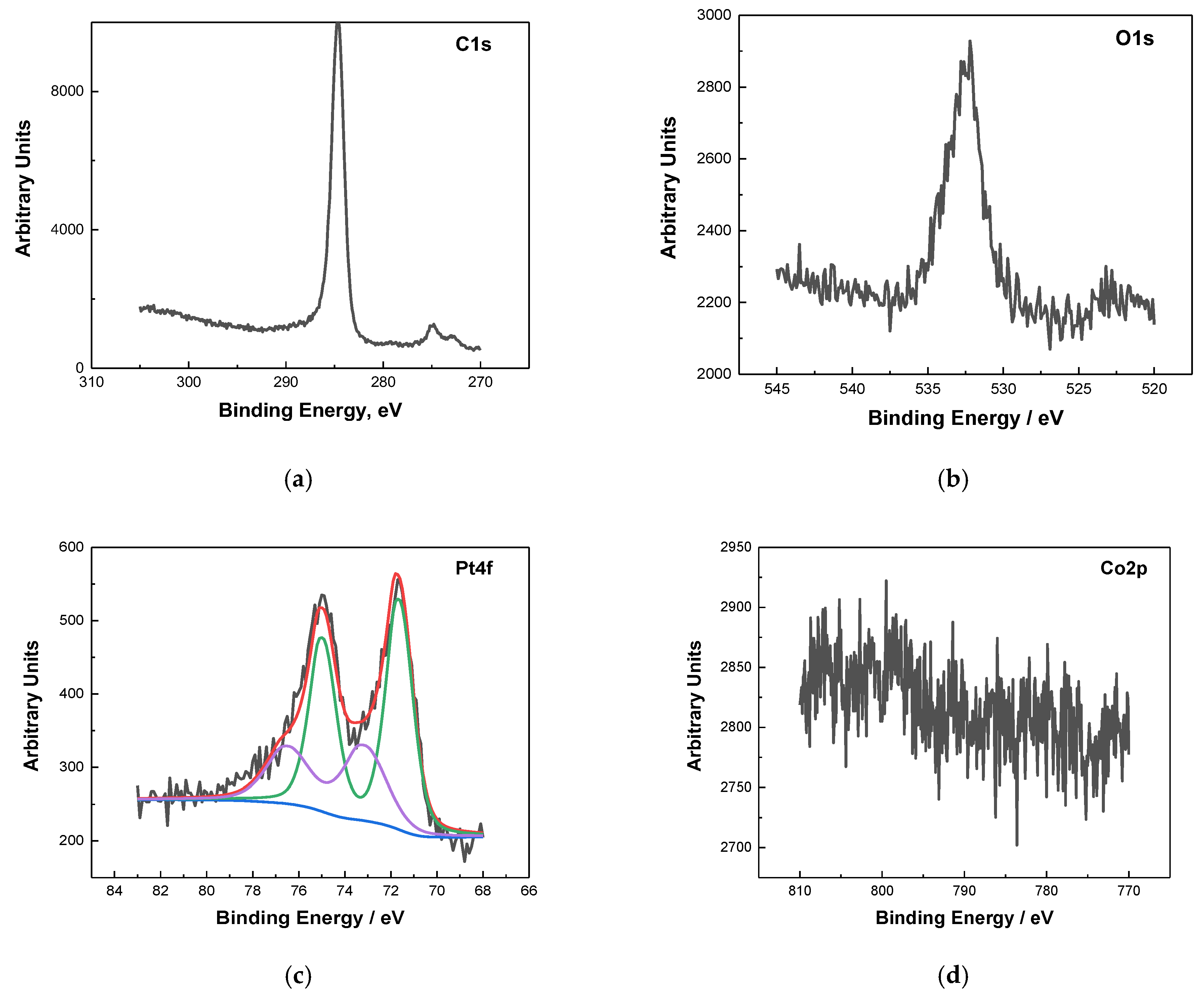
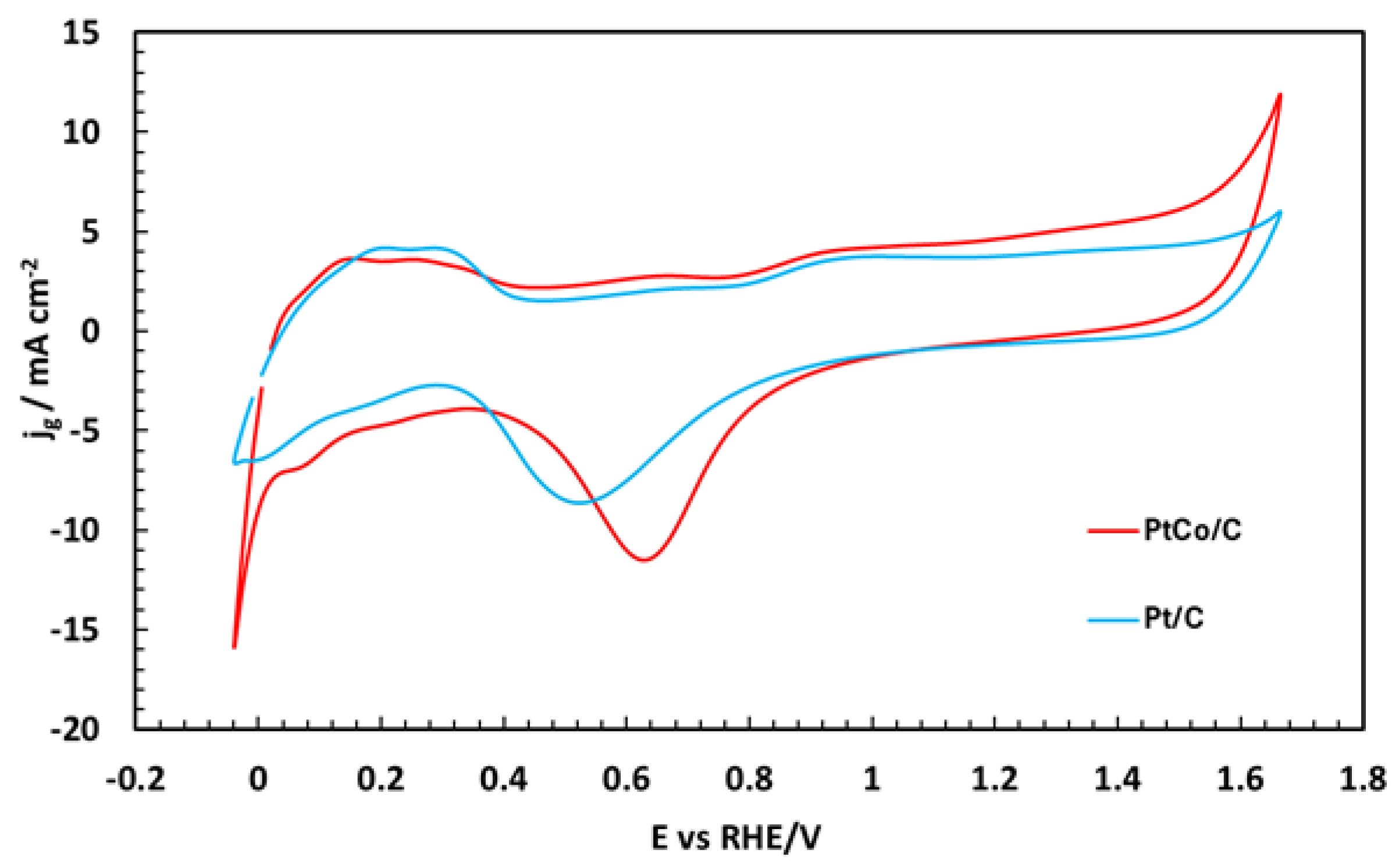
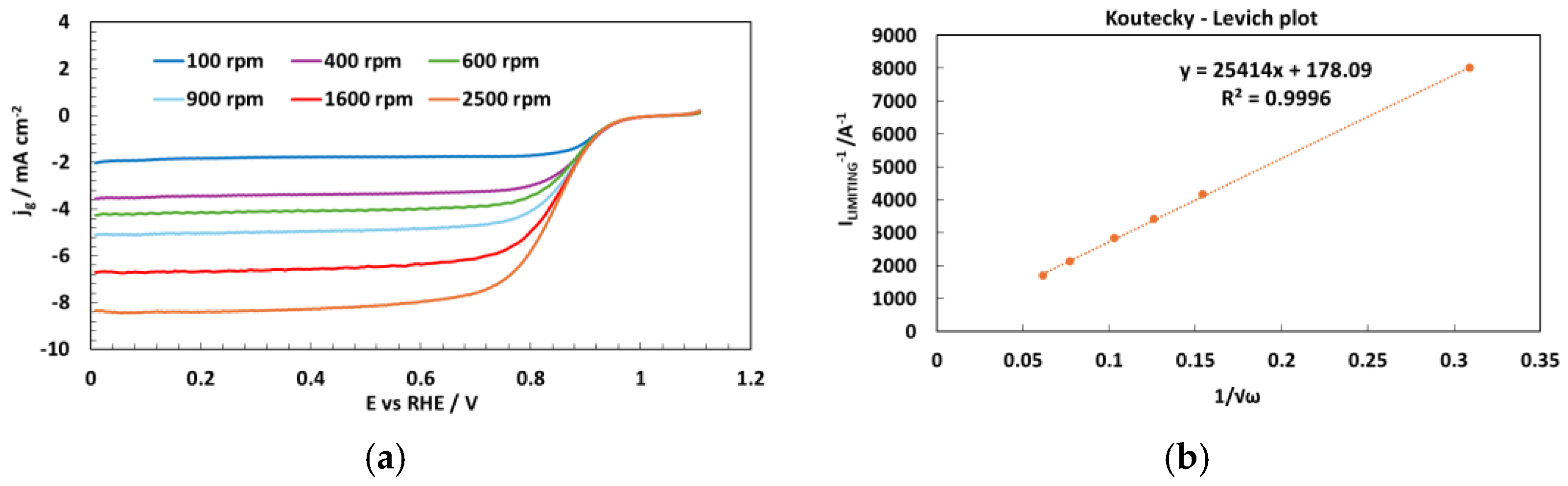

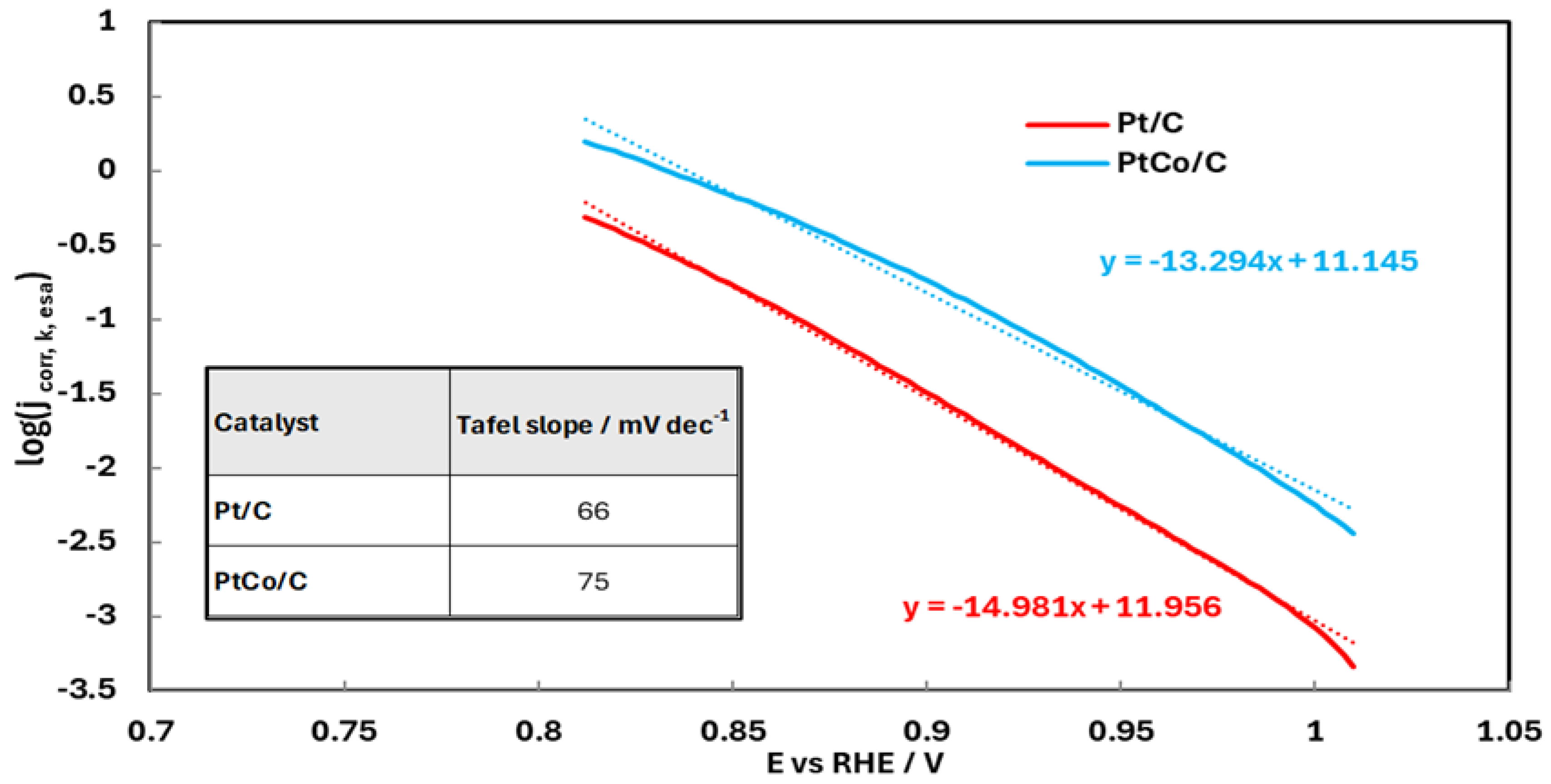
| Sample | Co wt.% | P wt.% | Pt wt.% |
|---|---|---|---|
| Co-P/C | 24 | 0.4 | |
| Pt-Co/C | 0.7 | 0 | 17 |
| Catalyst | Initial Co wt.% | Pt wt.% | Co wt.% | Pt mass Specific Area, m2 g−1 | Jm/mA mg−1 | Jesa/mA cm−2 |
|---|---|---|---|---|---|---|
| η = −330 mV | ||||||
| Pt/C | 0 | 20 | 0 | 31 | 9.93 | 0.032 |
| Pt-Co/C | 28 | 17 | 0.7 | 19 | 36.6 | 0.182 |
| η = −380 mV | ||||||
| Pt/C | 0 | 20 | 0 | 31 | 50.6 | 0.165 |
| Pt-Co/C | 28 | 17 | 0.7 | 19 | 133 | 0.661 |
| Reference | Jesa Enhancement Factor | Jm Enhancement Factor |
|---|---|---|
| [18] | 12 | 4 |
| [21] | 3 | - |
| [22] | 5 | 3 |
| [42] (annealed) | 7 (3) | 0.5 (1) |
| [43] | 5 | 1 |
| [44] (annealed) | 2 (3) | 1 (0.5) |
| [45] | 2 | 1 |
| [46] | 2.5 | 3 |
| [47] | 4 | 7 |
| [48] | 9 | 6 |
| [49] | 14 | 3 |
| [this work] | 7 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banti, A.; Avramova, I.; Sotiropoulos, S.; Georgieva, J. Enhanced Oxygen Reduction Reaction Activity of Carbon-Supported Pt-Co Catalysts Prepared by Electroless Deposition and Galvanic Replacement. Catalysts 2025, 15, 895. https://doi.org/10.3390/catal15090895
Banti A, Avramova I, Sotiropoulos S, Georgieva J. Enhanced Oxygen Reduction Reaction Activity of Carbon-Supported Pt-Co Catalysts Prepared by Electroless Deposition and Galvanic Replacement. Catalysts. 2025; 15(9):895. https://doi.org/10.3390/catal15090895
Chicago/Turabian StyleBanti, Angeliki, Ivalina Avramova, Sotiris Sotiropoulos, and Jenia Georgieva. 2025. "Enhanced Oxygen Reduction Reaction Activity of Carbon-Supported Pt-Co Catalysts Prepared by Electroless Deposition and Galvanic Replacement" Catalysts 15, no. 9: 895. https://doi.org/10.3390/catal15090895
APA StyleBanti, A., Avramova, I., Sotiropoulos, S., & Georgieva, J. (2025). Enhanced Oxygen Reduction Reaction Activity of Carbon-Supported Pt-Co Catalysts Prepared by Electroless Deposition and Galvanic Replacement. Catalysts, 15(9), 895. https://doi.org/10.3390/catal15090895









