Advances in Composite Photocatalysts for Efficient Degradation of Organic Pollutants: Strategies, Challenges, and Future Perspectives
Abstract
1. Introduction
2. Methodology
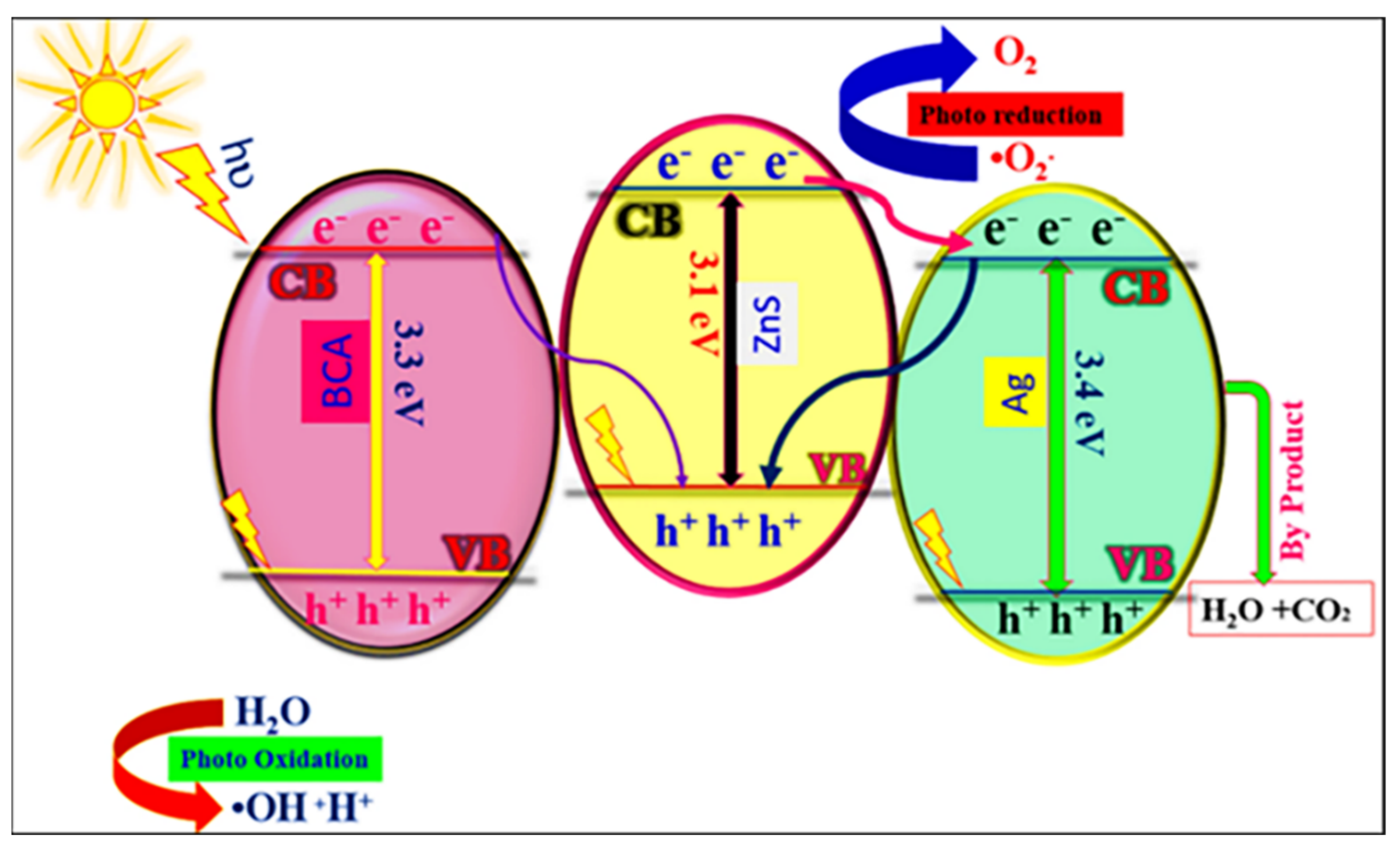
3. Mechanism of Photocatalysis
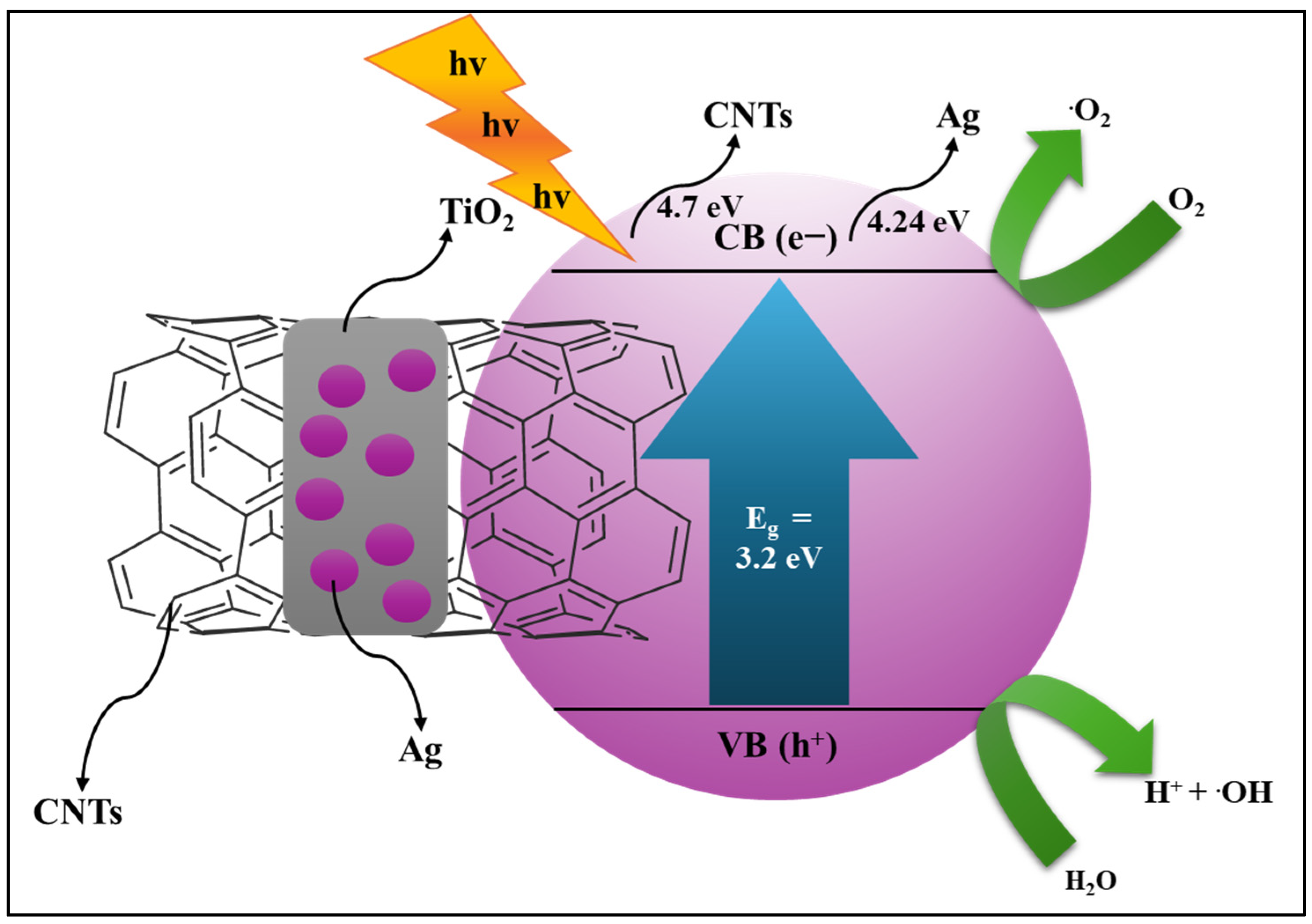
4. Carbon-Based Composites for Photocatalytic Degradation
5. Metal-Based Composites
| Metal-Based Composites | Synthetic Method | Degradation Efficiency | Band Gap | Ref. |
|---|---|---|---|---|
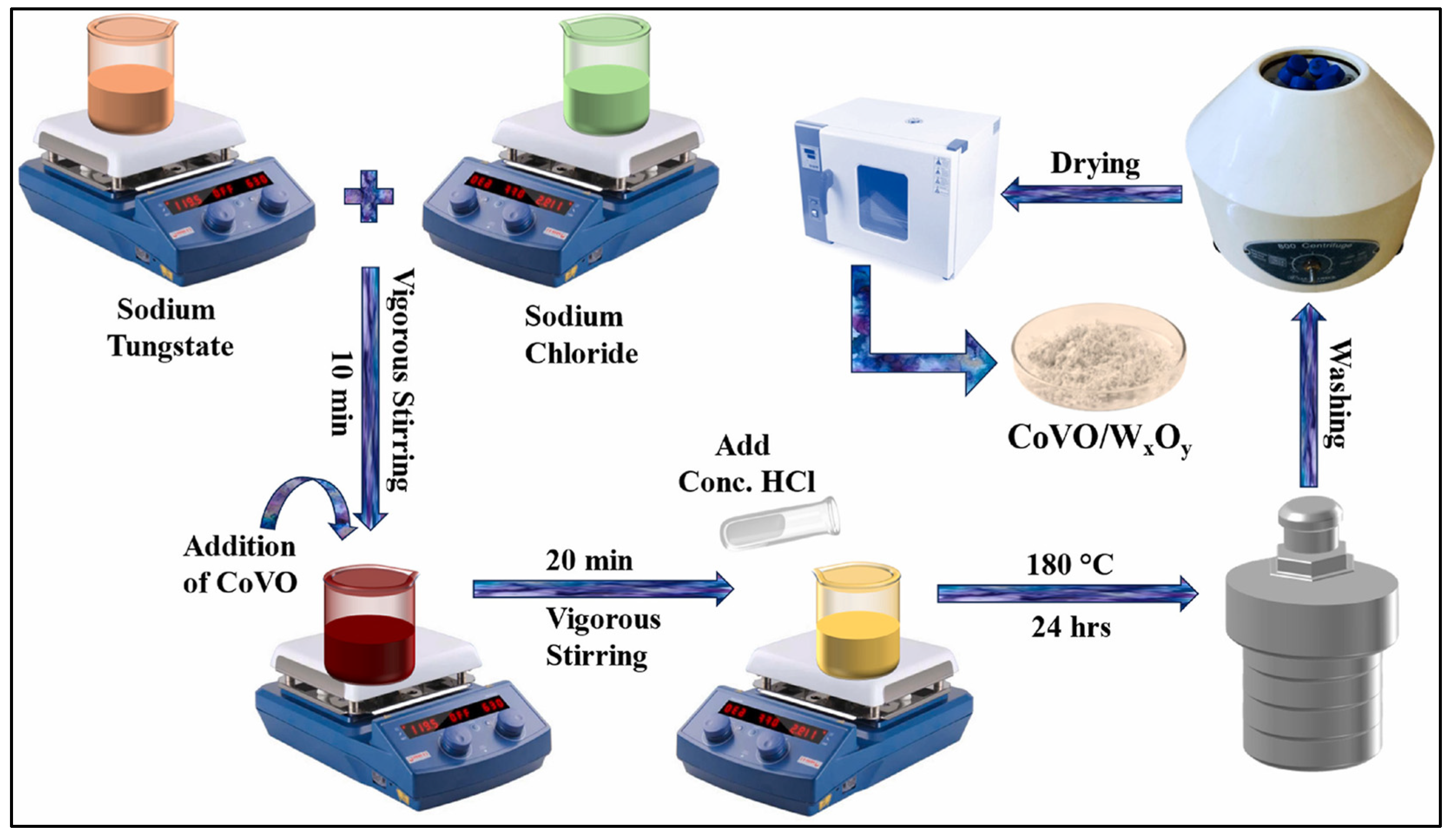
| CoVO/WxOy | Hydrothermal method | 96% | - | [109] |
| Cu2O-rich and Cu2O-poor | Electrodeposition | 48% for Cu2O-rich and 46% Cu2O-poor | ~2.0 eV | [110] |
| Cs3Bi2I9 | Hot-injection method | 98.2% | 4.9 eV, | [111] |
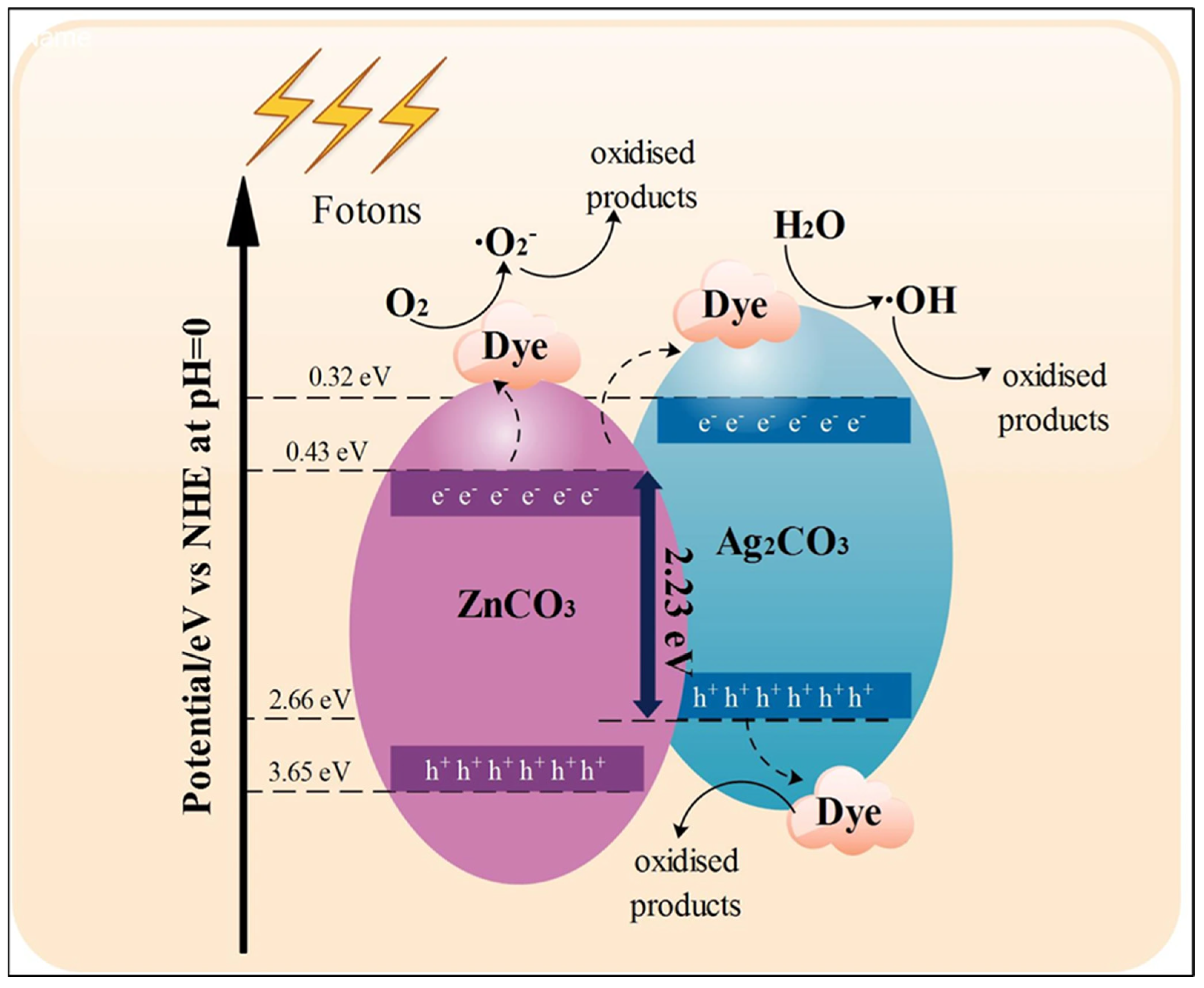
| Ag2CO3-ZnCO3 | Precipitation | 99.88% | 0.38 eV | [112] |
| CuO-WO3 | Co-precipitation | 92% | 2.1 to 2.4 eV | [113] |
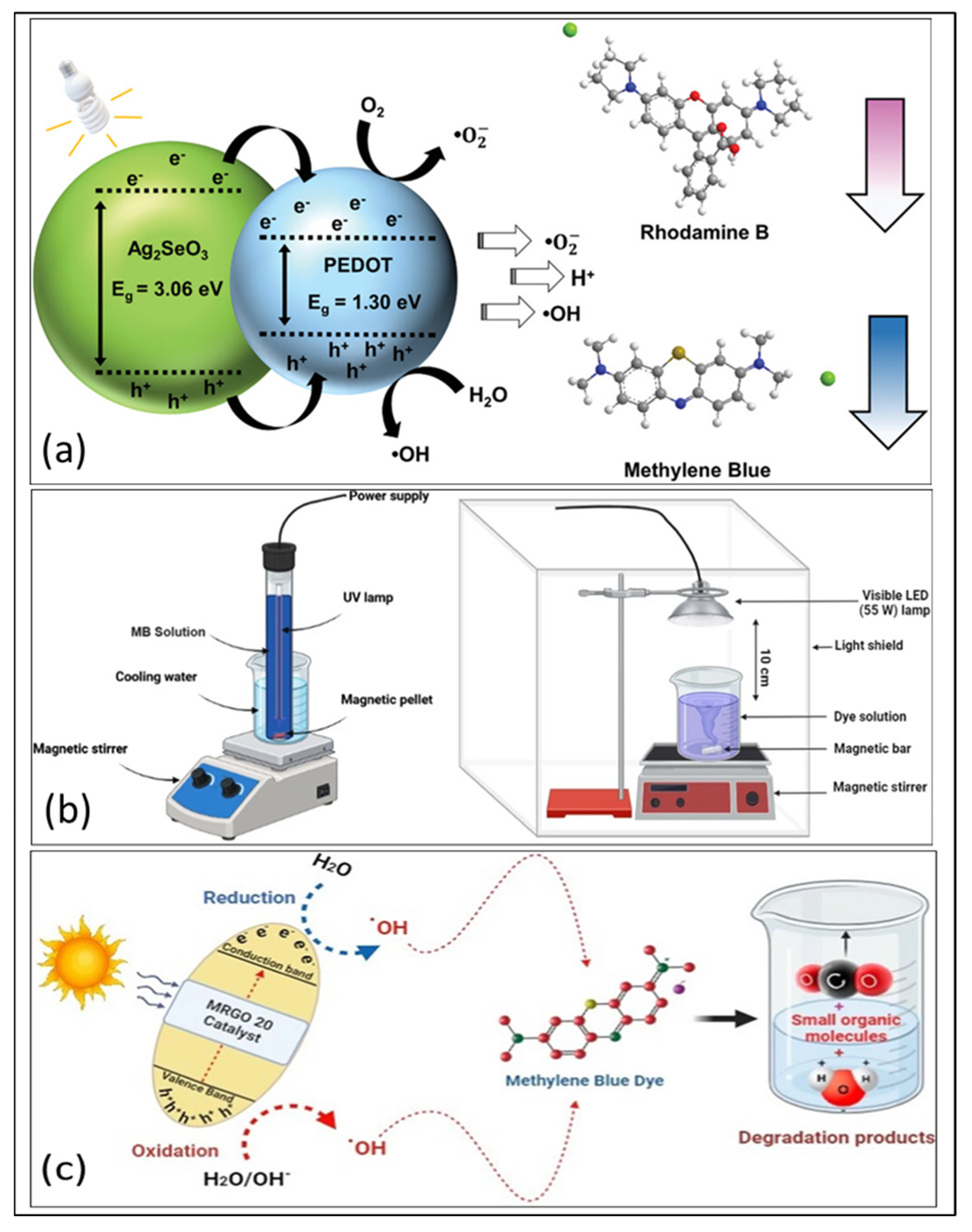
| PEDOT/Ag2SeO3 | In situ synthesis | 46.2% | 2.41 eV | [114] |
| RGO photocatalyst loaded with Zn0.5Cu0.5Fe2O4 | Co-precipitation | 95.2% | ~1.7 eV | [115] |
| MgFe2O4-TiO2 (MFO-TiO2 | Hydrothermal approach, followed by a calcination process | 99.53% | 3.2 and 3.0 eV | [120] |
| Diatomite-TiO2 composite | Impregnation method | 80% | 3 eV | [124] |
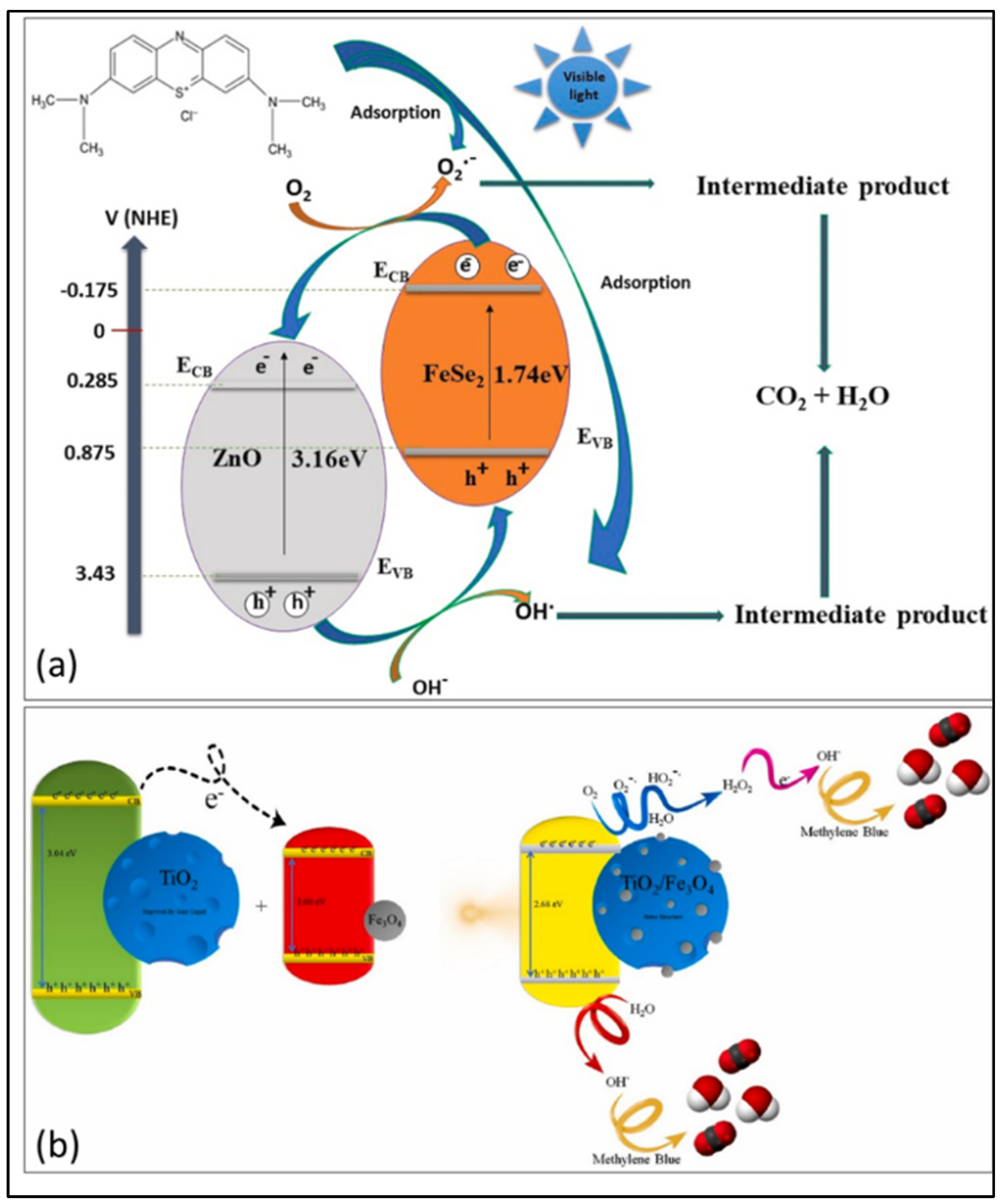
| FeSe2–ZnO | Cost-effective chemical method | 97% | 1.91 eV | [116] |
| TiO2/Fe3O4 | Sol-gel assisted method | 92% | - | [117] |
| TiO2-F-V-Mo | Sol-gel method | 0.0363% | 2.52 eV | [121] |
| TiO2-C@N | Sol hydrothermal method | 99.87% | - | [122] |
| kaolinite/TiO2 | Sol-gel method | 98% | 3.14 eV | [123] |
| TiO2/C-550 | Sol-gel method | 100% | 2.7 eV | [78] |
| B-doped g-C3N4/TiO2 | Co-precipitation | - | 1.5678 eV | [126] |
| (DM g. C3N4) TiO2 | Solvothermal | 97% | 2.23 eV | [130] |
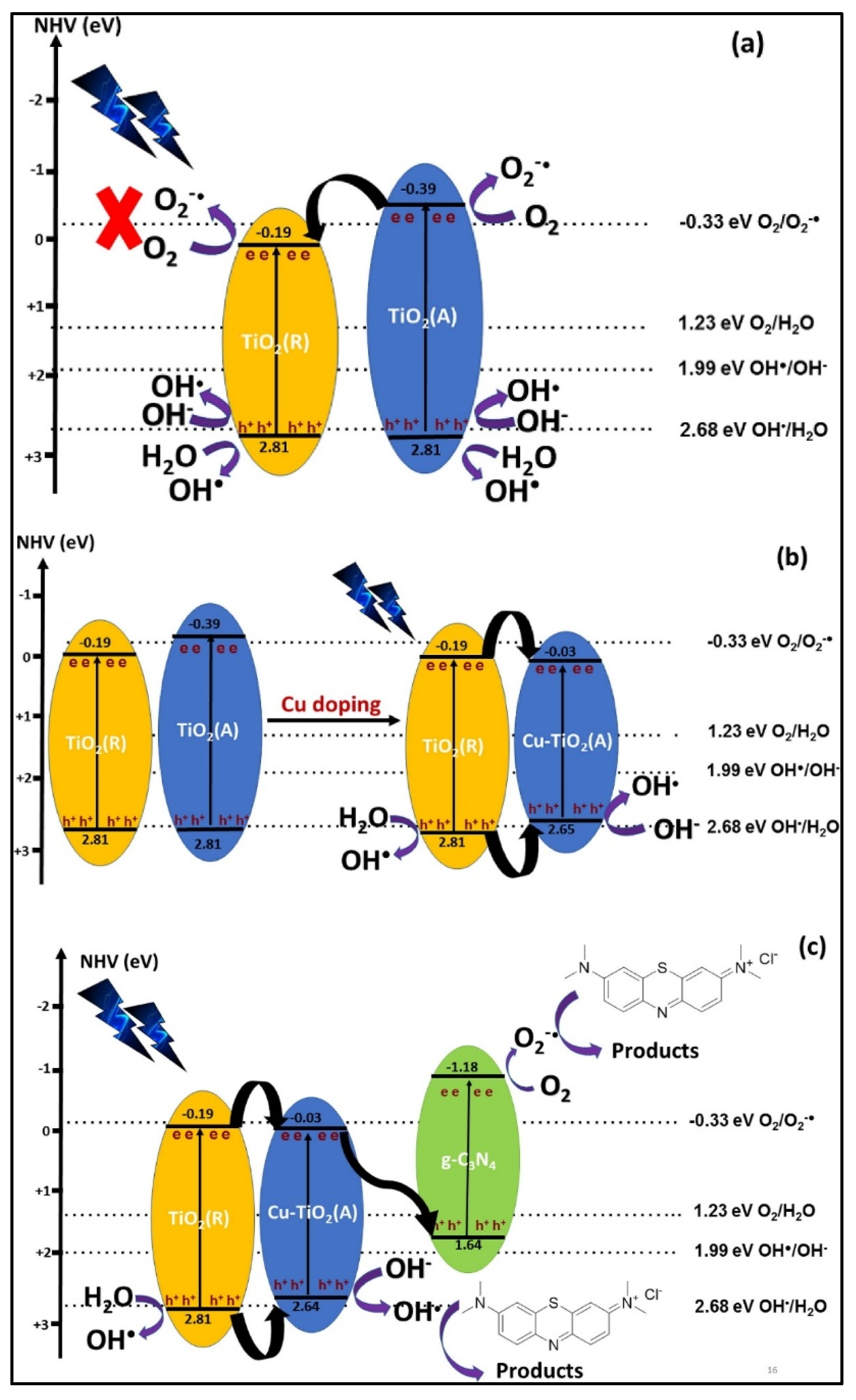
| Cu-TiO2/g-C3N4 | Hydrothermal method | - | 2.81 eV | [131] |
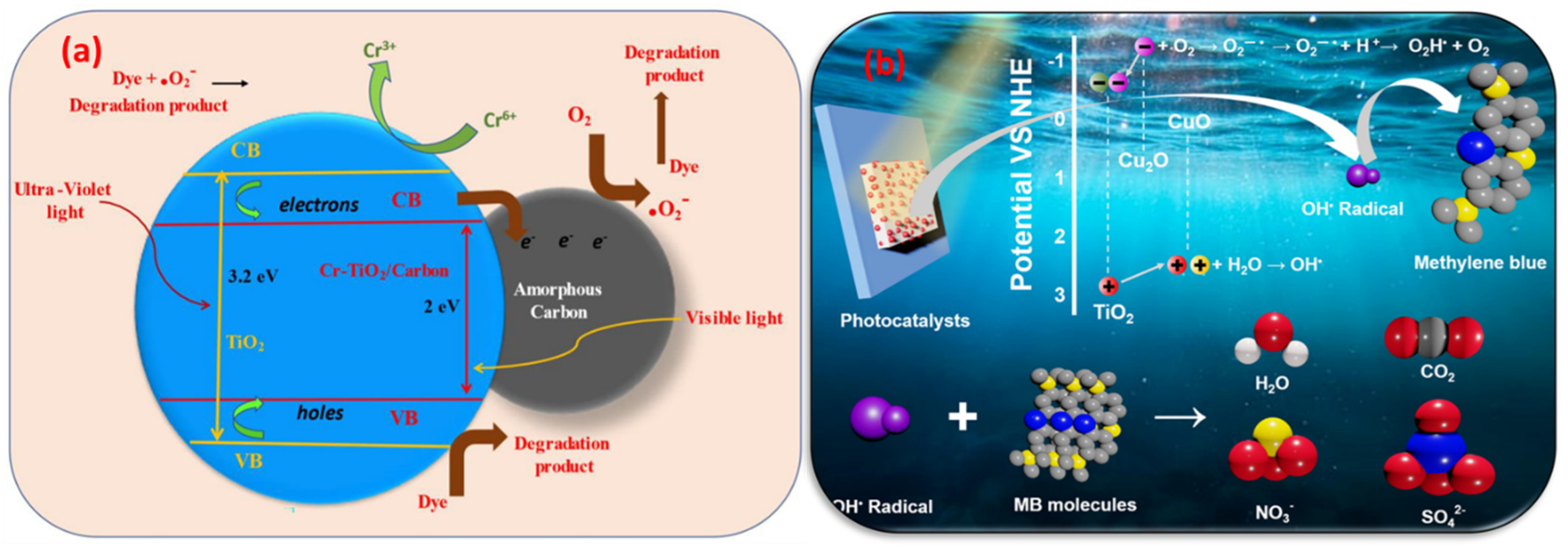
| Chromium-TiO2/carbon | Hydrothermal method | 90% | 3.2 eV | [128] |
| CoO/ZnO | Hydrothermal method | 67.5% | 3.44–3.14 eV | [132] |
| GO-ZnO@5Fe | Hydrothermal method | 100% | 2.58 | [140] |
| 2D/0D g-C3N4/TiO2 | Thermal polycondensation method | 100% | ~ 2.7 eV | [125] |
| ZnO/MNC | Co-precipitation method | 97.14% | - | [133] |
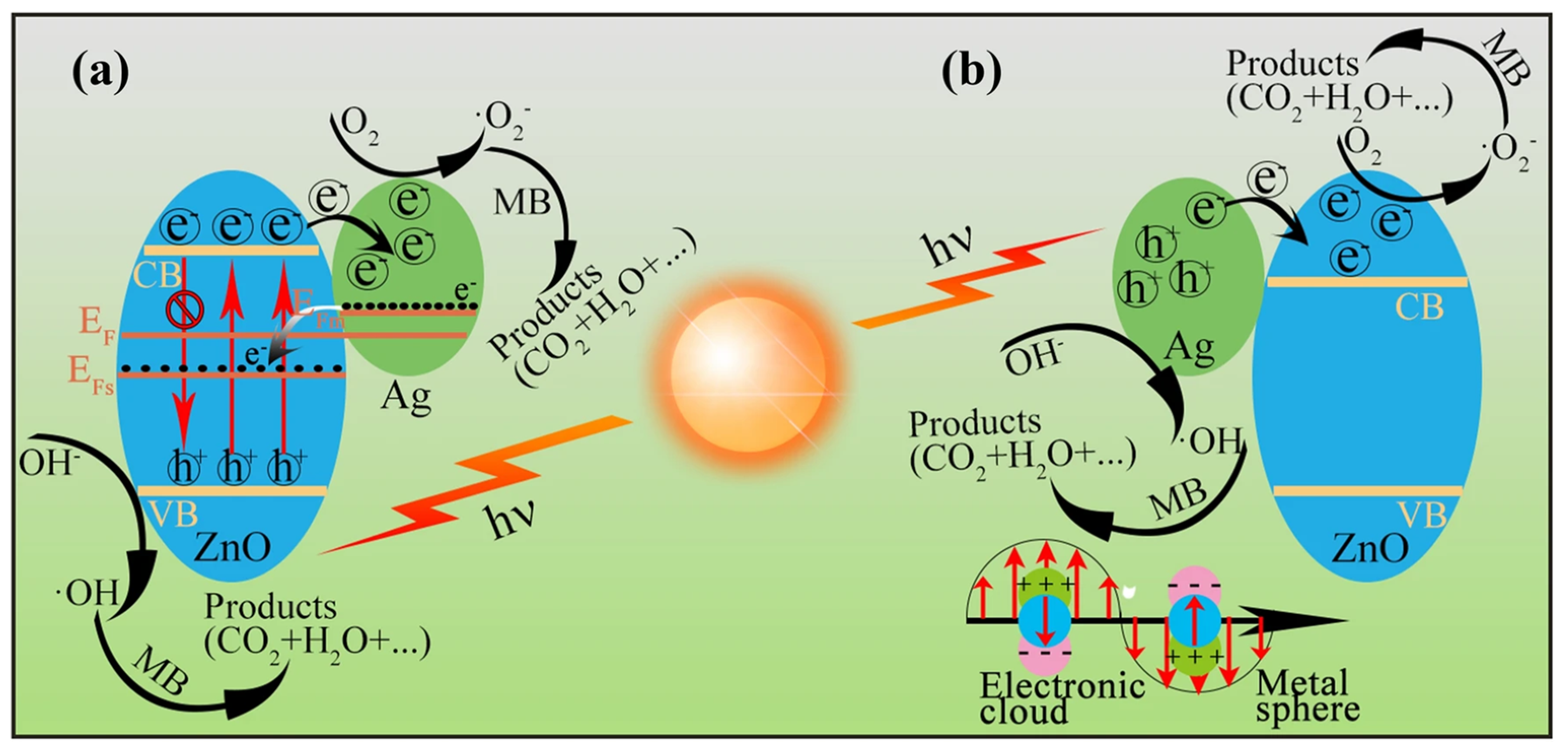
| Ag/ZnO | Hydrothermal method | 99.8%. | 3.16 eV | [138] |
| TiO2-cMDF | Carbonization method | 99% | 3.2 eV | [127] |
| TiO2/CuxO | Spin-coating technique | - | 1.70 eV | [129] |
| Fe3O4/ZnO | Solid state method | 88.5% | 3.39 eV | [137] |
| TiO2/C-800 | Calcination with a one-pot liquid phase reaction | 25.1% | - | [97] |
| Cu/ZnO | Incipient wetness impregnation | 99% | 3.37 eV | [134] |
| g-CN/ZnO | Pyrolysis method | 98% | - | [136] |
| α-Fe2O3-ZnO NC | Precipitation method | 56.9% | 2.73 eV | [139] |
| BiOI/C | Hydrothermal method | 79.6% | ~ 2.51 eV | [141] |
6. Metal–Organic-Framework-Based Composites
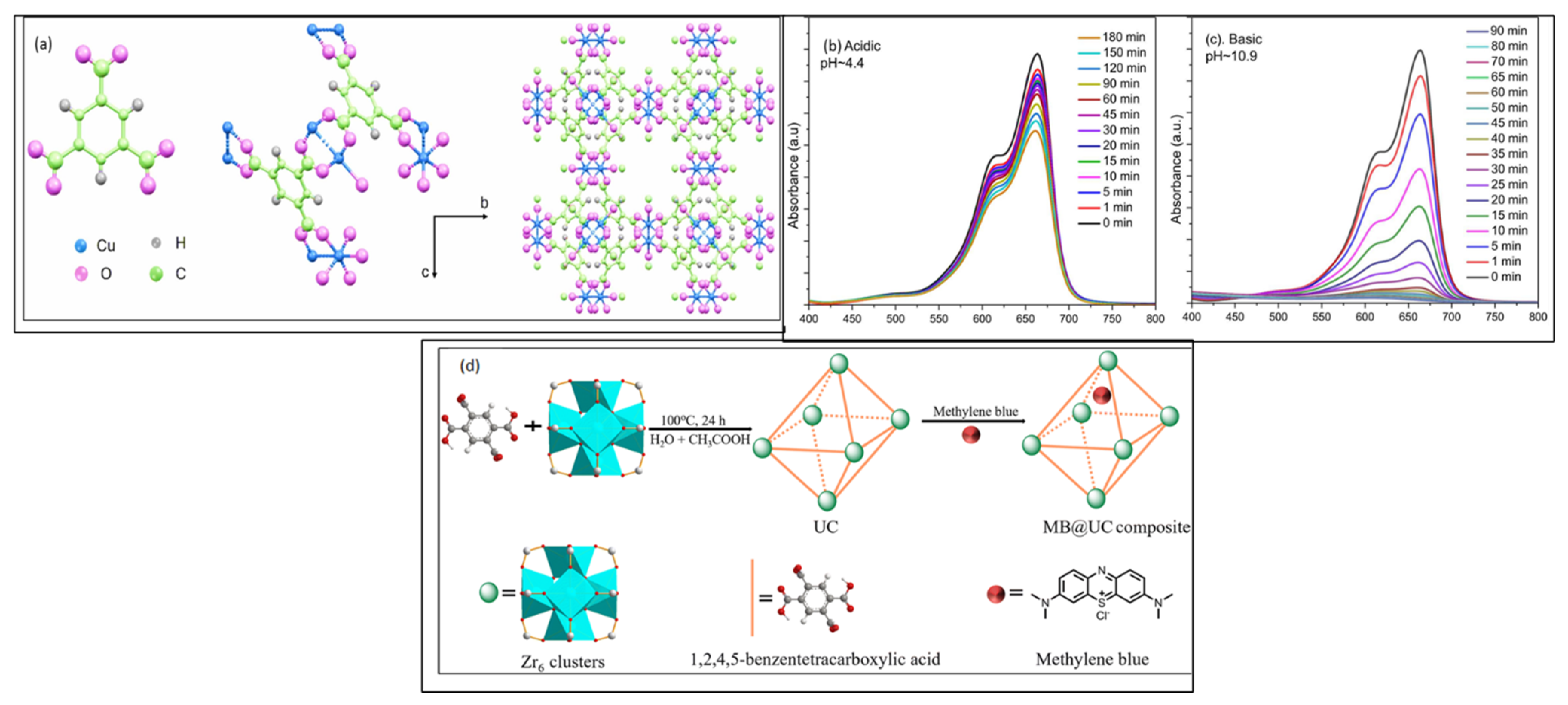
| MOF-Based Composites | Synthetic Method | Degradation Efficiency | Band Gap | Ref. |
|---|---|---|---|---|
| Bi2O3-ZnO/TiO2 MOF | Electrospinning | 98% | 2.9 eV | [148] |
| HKUST-1, a Cu-based MOF | Solvothermal method | 98.94% | 3.60 eV | [149] |
| MB-modified UiO-66-(COOH)2 MOF | Adsorption method | - | - | [150] |
| OM-PE@PbBrOH⊂ZIF-67 | Solvothermal method | 72% | 0.4 eV | [151] |
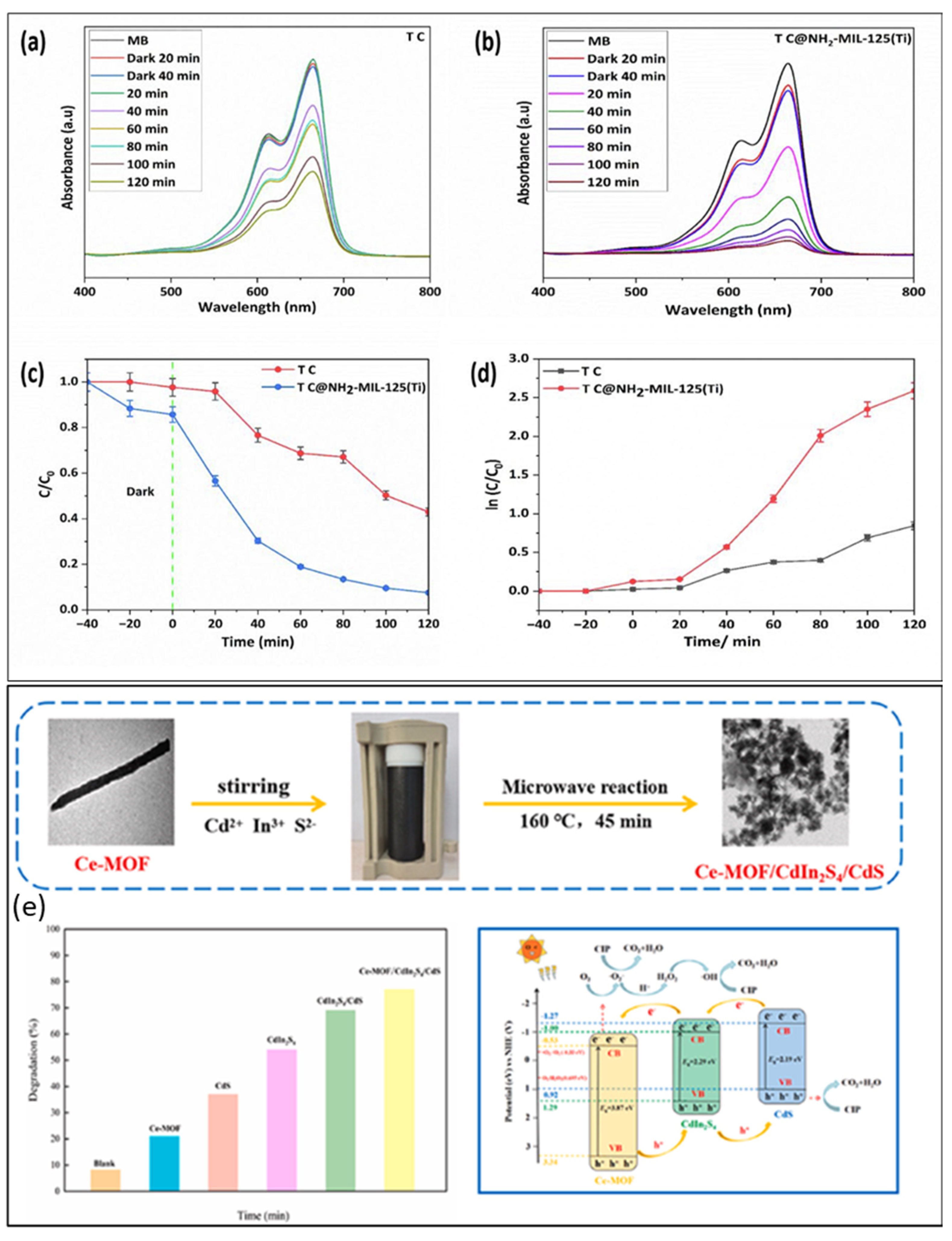
| Cu2O(TC)@NH2-MIL-125(Ti) | Solvothermal method | 74.88% | 1.69 eV | [152] |
| Ce-MOF/CdIn2S4/CdS | Hydrothermal method | 76.7% | 2.23 eV | [153] |
| Mo0.09@Ni-MOF | Solvothermal method | 84% | 1.83 eV | [154] |
| Cr-PTC single bond HIna/TiO2 | Solvothermal method | 88.55% | 2.02 eV | [155] |
| CuWO4@MIL-101(Fe) | Solvothermal method | 96.92% | - | [156] |
| Ni(20)-ZIF-8 | One-step room-temperature method | 93.22% | 4.40 eV | [157] |
| ZIF-8/POTS | Superhydrophobic method | 93.85% | 4.9 eV | [158] |
| MnMg-MOF | One-step cationic membrane electro-conversion | 88% | 0.37 eV | [159] |
| UNiMOF/Ti3C2 | Electrostatic self-assembly | 99.49% | 3.43 eV | [160] |
| ZIF-8/Ti3C2Tx | In situ growth | - | - | [163] |
| UiO-66/MXene | Solvothermal method | >97% | - | [161] |
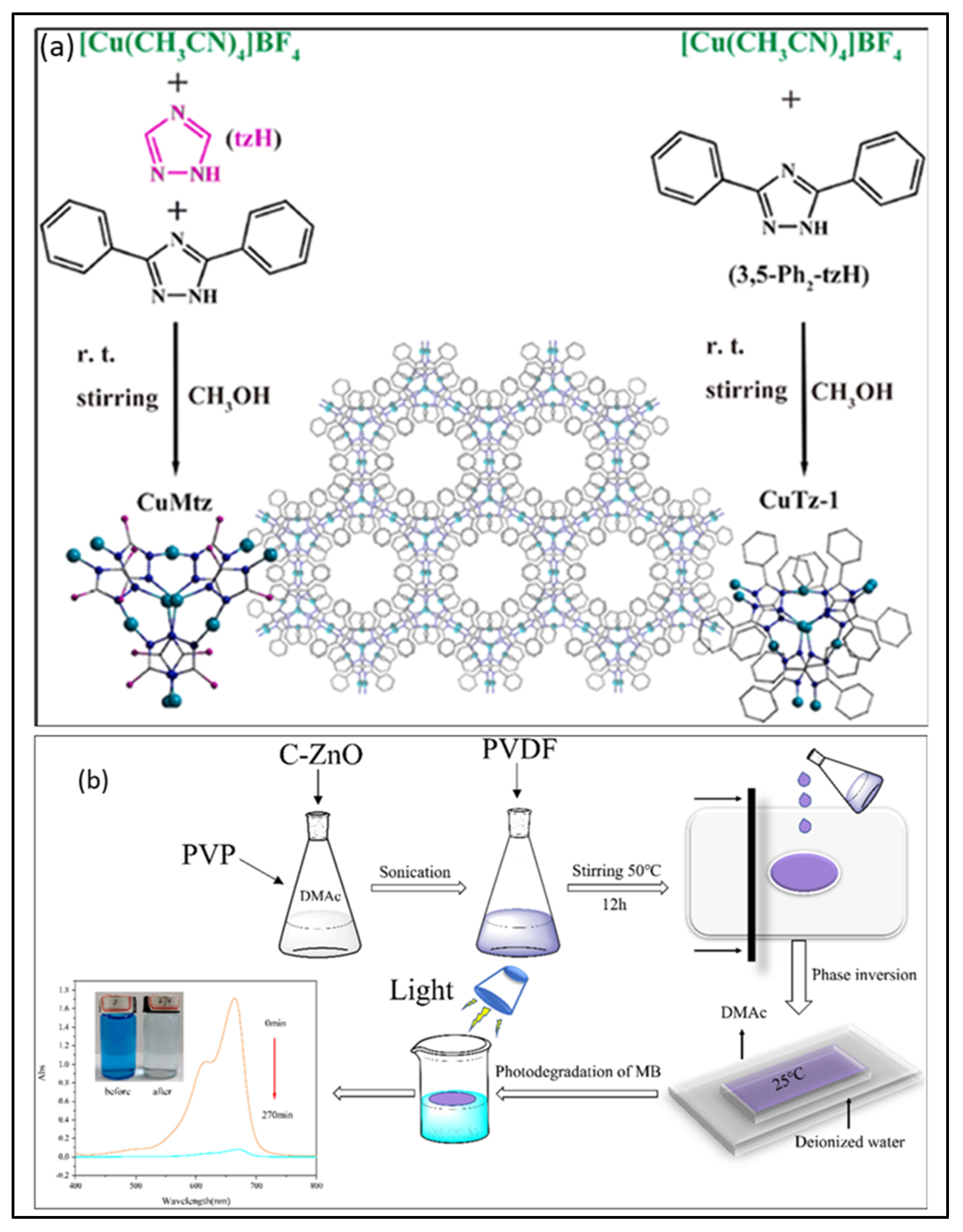
| CuTz-1 | One-pot economic synthesis | 73% | 1.72 eV | [162] |
| UIO-66-2OH (2,3) | Solvothermal method | 99.5% | 2.6 eV | [164] |
| Cd-MOF/CdS | In situ sulfurization | 91.9% | 2.9 eV | [165] |
| ZIF-8/ZnO | Phase inversion method | 95.01% | 3.40 eV | [166] |
| TiO2/Al2O3@Cu(BDC) | In situ incorporation of pre-synthesized precursors | - | 3.29 eV | [169] |
| MO@Co-MOF | Solvothermal method | 99.7% | 1.71 eV | [170] |
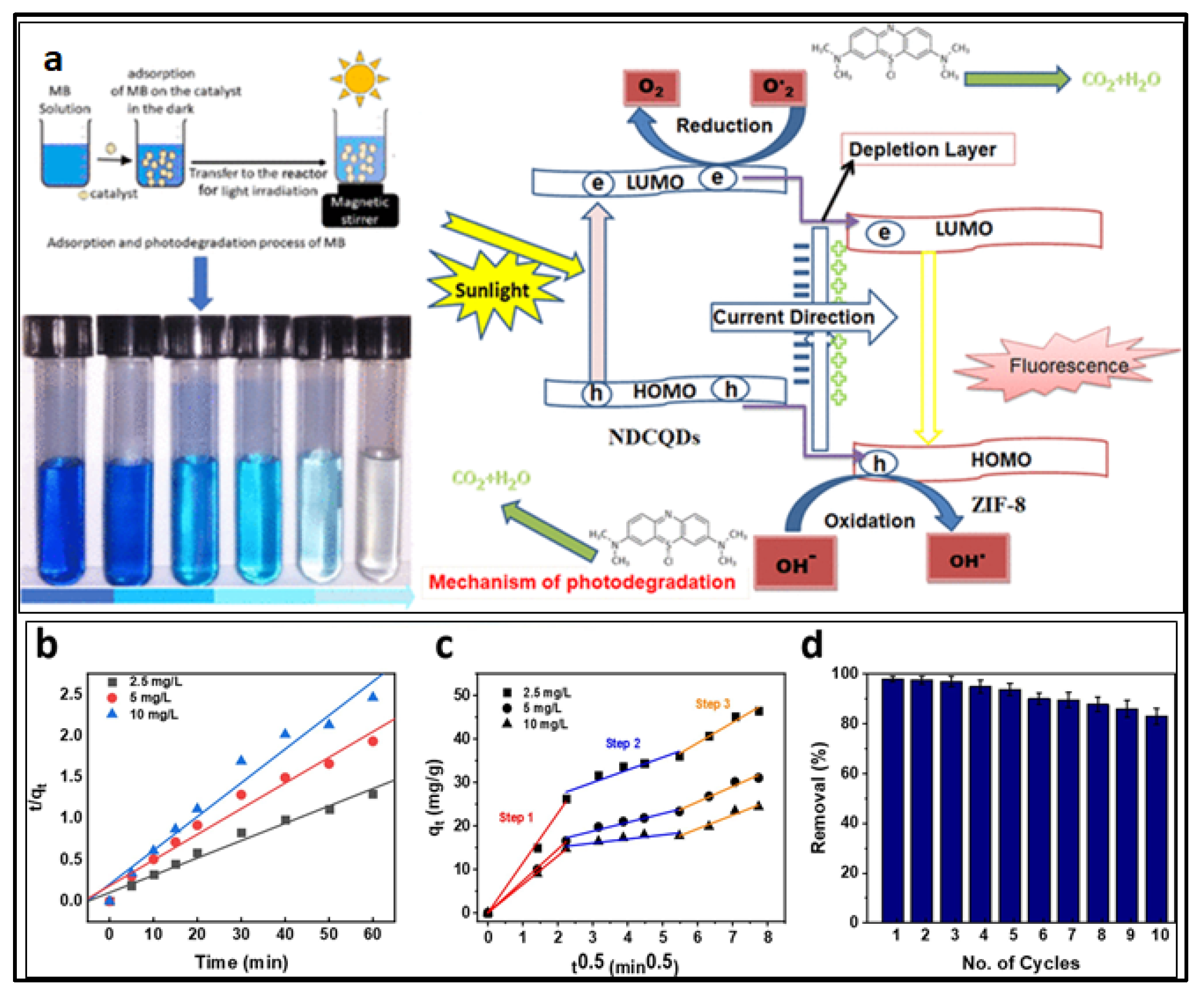
| NDCQDs/ZIF-8 | Hydrothermal method | 28% | 4.961 eV | [172] |
| CoFe2O4/SiO2/Cu-MOF | Sol-gel method | 98% | - | [173] |
| MOF-derived α-Fe2O3/ZnO | Calcination | 100% | 2.34 eV | [174] |
| MOF-5/GO | Hummer’s method | 92% | 3.5 eV | [175] |
6.1. Recovery and Reusability of Photocatalysts
6.2. Mineralization and Byproduct Formation
6.3. Challenges and Limitations
7. Conclusions
Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, S.; Feng, J.; Fan, M.; Pi, Y.; Hu, L.; Han, X.; Liu, M.; Sun, J.; Sun, J. Recent developments in heterogeneous photocatalytic water treatment using visible light-responsive photocatalysts: A review. RSC Adv. 2015, 5, 14610–14630. [Google Scholar] [CrossRef]
- Stojadinović, S.; Radić, N.; Tadić, N.; Vasilić, R.; Grbić, B. Enhanced ultraviolet light driven photocatalytic activity of ZnO particles incorporated by plasma electrolytic oxidation into Al2O3 coatings co-doped with Ce3+. Opt. Mater. 2020, 101, 109768. [Google Scholar] [CrossRef]
- Nasrabadi, T. An indexapproach tometallic pollution in riverwaters. Int. J. Environ. Res. 2015, 9, 385–394. [Google Scholar]
- Borker, P.; Salker, A. Photocatalytic degradation of textile azo dye over Ce1−xSnxO2 series. Mater. Sci. Eng. B 2006, 133, 55–60. [Google Scholar]
- Gupta, V.K.; Ali, I.; Saleh, T.A.; Nayak, A.; Agarwal, S. Chemical treatment technologies for waste-water recycling—An overview. RSC Adv. 2012, 2, 6380–6388. [Google Scholar] [CrossRef]
- Verma, N.; Chundawat, T.S.; Chandra, H.; Vaya, D. An efficient time reductive photocatalytic degradation of carcinogenic dyes by TiO2-GO nanocomposite. Mater. Res. Bull. 2023, 158, 112043. [Google Scholar] [CrossRef]
- Zhang, Q.-Q.; Ying, G.-G.; Pan, C.-G.; Liu, Y.-S.; Zhao, J.-L. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef]
- Rizzo, L.; Sannino, D.; Vaiano, V.; Sacco, O.; Scarpa, A.; Pietrogiacomi, D. Effect of solar simulated N-doped TiO2 photoca-talysis on the inactivation and antibiotic resistance of an E. coli strain in biologically treated urban wastewater. Appl. Catal. B Environ. 2014, 144, 369–378. [Google Scholar]
- Mudhoo, A.; Ramasamy, D.L.; Bhatnagar, A.; Usman, M.; Sillanpää, M. An analysis of the versatility and effectiveness of composts for sequestering heavy metal ions, dyes and xenobiotics from soils and aqueous milieus. Ecotoxicol. Environ. Saf. 2020, 197, 110587. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation ap-proaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar]
- Gita, S.; Hussan, A.; Choudhury, T. Impact of textile dyes waste on aquatic environments and its treatment. Environ. Ecol. 2017, 35, 2349–2353. [Google Scholar]
- Padhi, B. Pollution due to synthetic dyes toxicity & carcinogenicity studies and remediation. Int. J. Environ. Sci. 2012, 3, 940. [Google Scholar]
- Mohan, D.; Shukla, S.P. Hazardous consequences of textile mill effluents on soil and their remediation approaches. Clean. Eng. Technol. 2022, 7, 100434. [Google Scholar] [CrossRef]
- Muraro, P.C.L.; Mortari, S.R.; Vizzotto, B.S.; Chuy, G.; Dos Santos, C.; Brum, L.F.W.; da Silva, W.L. Iron oxide nanocatalyst with titanium and silver nanoparticles: Synthesis, characterization and photocatalytic activity on the degradation of Rhodamine B dye. Sci. Rep. 2020, 10, 3055. [Google Scholar] [CrossRef]
- Zollinger, H. Color Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Tang, W.Z.; An, H. UV/TiO2 photocatalytic oxidation of commercial dyes in aqueous solutions. Chemosphere 1995, 31, 4157–4170. [Google Scholar] [CrossRef]
- Prado, A.G.; Bolzon, L.B.; Pedroso, C.P.; Moura, A.O.; Costa, L.L. Nb2O5 as efficient and recyclable photocatalyst for indigo carmine degradation. Appl. Catal. B Environ. 2008, 82, 219–224. [Google Scholar] [CrossRef]
- Zinicovscaia, I. Conventional methods of wastewater treatment. In Cyanobacteria for Bioremediation of Wastewaters; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 17–25. [Google Scholar]
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Arslan, I.; Balcioglu, I.A. Advanced oxidation of raw and biotreated textile industry wastewater with O3, H2O2/UV-C and their sequential application. J. Chem. Technol. Biotechnol. 2001, 76, 53–60. [Google Scholar]
- Chakrabarti, S.; Dutta, B.K. Photocatalytic degradation of model textile dyes in wastewater using ZnO as semiconductor cat-alyst. J. Hazard. Mater. 2004, 112, 269–278. [Google Scholar] [CrossRef]
- Reddy, M.P.; Venugopal, A.; Subrahmanyam, M. Hydroxyapatite photocatalytic degradation of calmagite (an azo dye) in aqueous suspension. Appl. Catal. B Environ. 2007, 69, 164–170. [Google Scholar] [CrossRef]
- Stylidi, M.; Kondarides, D.I.; Verykios, X.E. Visible light-induced photocatalytic degradation of Acid Orange 7 in aqueous TiO2 suspensions. Appl. Catal. B Environ. 2004, 47, 189–201. [Google Scholar]
- Wang, F.; Min, S.; Han, Y.; Feng, L. Visible-light-induced photocatalytic degradation of methylene blue with polyani-line-sensitized TiO2 composite photocatalysts. Superlattices Microstruct. 2010, 48, 170–180. [Google Scholar] [CrossRef]
- Chahar, D.; Kumar, D.; Thakur, P.; Thakur, A. Visible light induced photocatalytic degradation of methylene blue dye by using Mg doped Co-Zn nanoferrites. Mater. Res. Bull. 2023, 162, 112205. [Google Scholar] [CrossRef]
- Jiang, G.; Lin, Z.; Chen, C.; Zhu, L.; Chang, Q.; Wang, N.; Wei, W.; Tang, H. TiO2 nanoparticles assembled on graphene oxide nanosheets with high photocatalytic activity for removal of pollutants. Carbon 2011, 49, 2693–2701. [Google Scholar] [CrossRef]
- Fernández, C.; Larrechi, M.S.; Callao, M.P. An analytical overview of processes for removing organic dyes from wastewater effluents. TrAC Trends Anal. Chem. 2010, 29, 1202–1211. [Google Scholar] [CrossRef]
- Fouad, K.; Bassyouni, M.; Alalm, M.G.; Saleh, M.Y. Recent developments in recalcitrant organic pollutants degradation using immobilized photocatalysts. Appl. Phys. A 2021, 127, 612. [Google Scholar] [CrossRef]
- Bhatia, D.; Sharma, N.R.; Singh, J.; Kanwar, R.S. Biological methods for textile dye removal from wastewater: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1836–1876. [Google Scholar] [CrossRef]
- Chen, B.; Bu, Y.; Yang, J.; Nian, W.; Hao, S. Methods for total organic halogen (TOX) analysis in water: Past, present, and future. Chem. Eng. J. 2020, 399, 125675. [Google Scholar] [CrossRef]
- Cairone, S.; Hegab, H.M.; Khalil, H.; Nassar, L.; Wadi, V.S.; Naddeo, V.; Hasan, S.W. Novel eco-friendly polylactic acid nanocomposite integrated membrane system for sustainable wastewater treatment: Performance evaluation and antifouling analysis. Sci. Total Environ. 2024, 912, 168715. [Google Scholar] [CrossRef]
- El Messaoudi, N.; El Khomri, M.; El Mouden, A.; Bouich, A.; Jada, A.; Lacherai, A.; Iqbal, H.M.; Mulla, S.I.; Kumar, V.; Amé-rico-Pinheiro, J.H.P. Regeneration and reusability of non-conventional low-cost adsorbents to remove dyes from wastewaters in multiple consecutive adsorption–desorption cycles: A review. Biomass Convers. Biorefinery 2024, 14, 11739–11756. [Google Scholar] [CrossRef]
- Ang, W.L.; Mohammad, A.W. State of the art and sustainability of natural coagulants in water and wastewater treatment. J. Clean. Prod. 2020, 262, 121267. [Google Scholar] [CrossRef]
- dos Santos, J.D.; Veit, M.T.; Juchen, P.T.; da Cunha Gonçalves, G.; Palacio, S.M.; Fagundes-Klen, M. Use of different coagulants for cassava processing wastewater treatment. J. Environ. Chem. Eng. 2018, 6, 1821–1827. [Google Scholar] [CrossRef]
- Moghaddas, S.M.T.H.; Elahi, B.; Darroudi, M.; Javanbakht, V. Green synthesis of hexagonal-shaped zinc oxide nanosheets using mucilage from flaxseed for removal of methylene blue from aqueous solution. J. Mol. Liq. 2019, 296, 111834. [Google Scholar] [CrossRef]
- Qi, Q.; Zhang, T.; Yu, Q.; Wang, R.; Zeng, Y.; Liu, L.; Yang, H. Properties of humidity sensing ZnO nanorods-base sensor fab-ricated by screen-printing. Sens. Actuators B Chem. 2008, 133, 638–643. [Google Scholar] [CrossRef]
- Wang, C.; Liu, H.; Qu, Y. TiO2-based photocatalytic process for purification of polluted water: Bridging fundamentals to applications. J. Nanomater. 2013, 2013, 319637. [Google Scholar] [CrossRef]
- Anwar, D.I.; Mulyadi, D. Synthesis of Fe-TiO2 composite as a photocatalyst for degradation of methylene blue. Procedia Chem. 2015, 17, 49–54. [Google Scholar]
- Xia, S.-J.; Liu, F.-X.; Ni, Z.-M.; Shi, W.; Xue, J.-L.; Qian, P.-P. Ti-based layered double hydroxides: Efficient photocatalysts for azo dyes degradation under visible light. Appl. Catal. B Environ. 2014, 144, 570–579. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, L.; Wang, H.; Wang, W.; Zhang, L. TiO2/graphene porous composite and its photocatalytic degradation of methylene blue. Mater. Des. 2016, 108, 632–639. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Karthikeyan, D.; Lee, Y.R. Facile synthesis of zinc oxide nanoparticles decorated graphene oxide composite via simple solvothermal route and their photocatalytic activity on methylene blue degradation. J. Photochem. Photobiol. B Biol. 2016, 162, 500–510. [Google Scholar] [CrossRef]
- Lv, T.; Pan, L.; Liu, X.; Lu, T.; Zhu, G.; Sun, Z. Enhanced photocatalytic degradation of methylene blue by ZnO-reduced graphene oxide composite synthesized via microwave-assisted reaction. J. Alloys Compd. 2011, 509, 10086–10091. [Google Scholar] [CrossRef]
- Saleh, R.; Taufik, A. Degradation of methylene blue and congo-red dyes using Fenton, photo-Fenton, sono-Fenton, and sonophoto-Fenton methods in the presence of iron (II, III) oxide/zinc oxide/graphene (Fe3O4/ZnO/graphene) composites. Sep. Purif. Technol. 2019, 210, 563–573. [Google Scholar] [CrossRef]
- Syed, N.; Feng, Y.; Fahad, R.; Huang, J.; Mahar, F.K. Carbon-based composite nanofibers for photocatalytic degradation of methylene blue dye under visible light. Polym. Adv. Technol. 2023, 34, 2286–2297. [Google Scholar] [CrossRef]
- Asokan, J.; Kumar, P.; Arjunan, G.; Shalini, M.G. Photocatalytic Performance of Spinel Ferrites and their Carbon-Based Composites for Environmental Pollutant Degradation. J. Clust. Sci. 2025, 36, 42. [Google Scholar] [CrossRef]
- Ma, B.; Hu, W.; Zhou, Y.; Li, Y.; Zhang, Y.; Zhou, M. Synthesis of MOF@ COF composites and study on dye adsorption prop-erties. Inorg. Chem. Commun. 2025, 172, 113475. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Wu, J.; Jiang, Z.; Zhang, X.; Meng, F. The Application of Multifunctional Metal–Organic Frameworks for the Detection, Adsorption, and Degradation of Contaminants in an Aquatic Environment. Molecules 2025, 30, 1336. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, M.; Chinnuraj, I.P.; Rajendran, R.; Rojviroon, T.; Rojviroon, O.; Thangavelu, P.; Sirivithayapakorn, S. Development of eco-friendly SrTiO3/multiwalled carbon nanotube (STO/MWCNT) composite with enhanced performance for photocatalytic applications in environment remediation and energy storage. Diam. Relat. Mater. 2025, 155, 112254. [Google Scholar] [CrossRef]
- Sirusy, S.; Ashrafi, H.; Akhond, M. Synthesis and Utilization of rGO/Ultrathin Nanotube Bi5O7I for Photodegradation of Methylene Blue and Photoreduction of Cr6+ to Cr3+ toward Detoxification of Water. ACS Appl. Nano Mater. 2025, 8, 3927–3941. [Google Scholar] [CrossRef]
- Arya, K.; Kumar, A.; Sharma, A.; Thakur, K.; Kumar, R.; Mehta, S.K.; Singh, S.; Kumar, V.; Kataria, R. Light-assisted synergistic effect of Zn-MOF@ rGO nanocomposite for methylene blue degradation and toxicity analysis to water reclamation. Inorg. Chem. Commun. 2025, 173, 113768. [Google Scholar] [CrossRef]
- Mazarji, M.; Mahmoodi, N.M.; Bidhendi, G.N.; Li, A.; Li, M.; James, A.; Mahmoodi, B.; Pan, J. Synthesis, characterization, and enhanced photocatalytic dye degradation: Optimizing graphene-based ZnO-CdSe nanocomposites via response surface methodology. J. Alloys Compd. 2025, 1010, 177999. [Google Scholar] [CrossRef]
- Wang, F.; Li, Q.; Xu, D. Recent progress in semiconductor-based nanocomposite photocatalysts for solar-to-chemical energy conversion. Adv. Energy Mater. 2017, 7, 1700529. [Google Scholar] [CrossRef]
- Majeed, A.; Ibrahim, A.H.; Al-Rawi, S.S.; Iqbal, M.A.; Kashif, M.; Yousif, M.; Abidin, Z.U.; Ali, S.; Arbaz, M.; Hussain, S.A. Green organo-photooxidative method for the degradation of methylene blue dye. ACS Omega 2024, 9, 12069–12083. [Google Scholar] [CrossRef] [PubMed]
- Tranfield, D.; Denyer, D.; Smart, P. Towards a methodology for developing evidence-informed management knowledge by means of systematic review. Br. J. Manag. 2003, 14, 207–222. [Google Scholar] [CrossRef]
- Centobelli, P.; Cerchione, R.; Esposito, E. Environmental sustainability in the service industry of transportation and logistics service providers: Systematic literature review and research directions. Transp. Res. Part D Transp. Environ. 2017, 53, 454–470. [Google Scholar] [CrossRef]
- Humayun, M.; Ullah, H.; Usman, M.; Habibi-Yangjeh, A.; Tahir, A.A.; Wang, C.; Luo, W. Perovskite-type lanthanum ferrite based photocatalysts: Preparation, properties, and applications. J. Energy Chem. 2022, 66, 314–338. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Liu, H.; Liu, C.; Wan, Y.; Long, Y.; Cai, Z. Recent advances and applications of semiconductor photo-catalytic technology. Appl. Sci. 2019, 9, 2489. [Google Scholar] [CrossRef]
- Lettieri, S.; Pavone, M.; Fioravanti, A.; Santamaria Amato, L.; Maddalena, P. Charge carrier processes and optical properties in TiO2 and TiO2-based heterojunction photocatalysts: A review. Materials 2021, 14, 1645. [Google Scholar] [CrossRef]
- Kumar, S.G.; Devi, L.G. Review on modified TiO2 photocatalysis under UV/visible light: Selected results and related mecha-nisms on interfacial charge carrier transfer dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef]
- Zhang, L.; Ran, J.; Qiao, S.-Z.; Jaroniec, M. Characterization of semiconductor photocatalysts. Chem. Soc. Rev. 2019, 48, 5184–5206. [Google Scholar] [CrossRef]
- Sharma, E.; Thakur, V.; Sangar, S.; Singh, K. Recent progress on heterostructures of photocatalysts for environmental reme-diation. Mater. Today Proc. 2020, 32, 584–593. [Google Scholar] [CrossRef]
- Sheng, H.; Li, Q.; Ma, W.; Ji, H.; Chen, C.; Zhao, J. Photocatalytic degradation of organic pollutants on surface anionized TiO2: Common effect of anions for high hole-availability by water. Appl. Catal. B Environ. 2013, 138, 212–218. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Arora, I.; Chawla, H.; Chandra, A.; Sagadevan, S.; Garg, S. Advances in the strategies for enhancing the photocatalytic activity of TiO2: Conversion from UV-light active to visible-light active photocatalyst. Inorg. Chem. Commun. 2022, 143, 109700. [Google Scholar] [CrossRef]
- Djurišić, A.B.; He, Y.; Ng, A. Visible-light photocatalysts: Prospects and challenges. Apl. Mater. 2020, 8, 030903. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Carbon-based sustainable nanomaterials for water treatment: State-of-art and future perspectives. Chemosphere 2021, 263, 128005. [Google Scholar] [CrossRef] [PubMed]
- Ayanda, O.S.; Mmuoegbulam, A.O.; Okezie, O.; Durumin Iya, N.I.; Mohammed, S.a.E.; James, P.H.; Muhammad, A.B.; Unimke, A.A.; Alim, S.A.; Yahaya, S.M. Recent progress in carbon-based nanomaterials: Critical review. J. Nanoparticle Res. 2024, 26, 106. [Google Scholar] [CrossRef]
- Feng, S.; Chen, T.; Liu, Z.; Shi, J.; Yue, X.; Li, Y. Z-scheme CdS/CQDs/g-C3N4 composites with visible-near-infrared light re-sponse for efficient photocatalytic organic pollutant degradation. Sci. Total Environ. 2020, 704, 135404. [Google Scholar] [CrossRef] [PubMed]
- Abimannan, G.; Sengodan, P.; Ravichandran, S.; Mary Anjalin, F.; Kumar, K.R.; Maadeswaran, P. Structural, optical, mor-phological and charge transfer properties of CeO2/MWCNTs nanocomposite and their photocatalytic activity of organic dye degradation. J. Sol-Gel Sci. Technol. 2023, 105, 625–636. [Google Scholar]
- Jiang, G.; Zheng, X.; Wang, Y.; Li, T.; Sun, X. Photo-degradation of methylene blue by multi-walled carbon nanotubes/TiO2 composites. Powder Technol. 2011, 207, 465–469. [Google Scholar] [CrossRef]
- Ye, X.; Wang, Z.; Wang, Q.; Chen, D.; Lin, Y.; Liu, S. Enhanced photocatalytic activity of ternary multilayered Ag/TiO2/CNT composites for methylene blue degradation. Micro Nano Lett. 2019, 14, 771–776. [Google Scholar] [CrossRef]
- Yu, Y.; Jimmy, C.Y.; Chan, C.-Y.; Che, Y.-K.; Zhao, J.-C.; Ding, L.; Ge, W.-K.; Wong, P.-K. Enhancement of adsorption and photocatalytic activity of TiO2 by using carbon nanotubes for the treatment of azo dye. Appl. Catal. B Environ. 2005, 61, 1–11. [Google Scholar] [CrossRef]
- Mashkoor, F.; Nasar, A. Inamuddin Carbon nanotube-based adsorbents for the removal of dyes from waters: A review. Environ. Chem. Lett. 2020, 18, 605–629. [Google Scholar] [CrossRef]
- Pan, H.; Tianyang, L.; Keting, L.; Xu, L. Preparation and properties of high-efficiency lignin-carbon based photocatalytic composites for the degradation of dye wastewater under visible light. J. Wood Chem. Technol. 2025, 45, 11–22. [Google Scholar] [CrossRef]
- Bai, H.; Xiong, R.; Wang, N.; Tian, M.; Zhao, J.; Tang, F.; Jiang, J. Synergistic effects of rare-metal ytterbium doping on TiO2/g-C3N5 heterostructures for enhanced photocatalytic degradation of methylene blue. Inorg. Chem. Commun. 2025, 175, 114159. [Google Scholar] [CrossRef]
- Yadav, P.R.; Bhagat, P.R. Metal Free Innovative Approach for the Removal of Methylene Blue from Simulated Water Sample by Novel Porphyrin Based Photocatalyst. Opt. Mater. 2025, 160, 116757. [Google Scholar] [CrossRef]
- Song, Y.-J.; Li, H.-C.; Xiong, Z.-W.; Cheng, L.; Du, M.; Liu, Z.-Q.; Li, J.; Li, D.-Q. TiO2/carbon composites from waste sawdust for methylene blue photodegradation. Diam. Relat. Mater. 2023, 136, 109918. [Google Scholar] [CrossRef]
- Sriramoju, J.B.; Muniyappa, M.; Marilingaiah, N.R.; Sabbanahalli, C.; Shetty, M.; Mudike, R.; Chitrabanu, C.P.; Shivaramu, P.D.; Nagaraju, G.; Rangappa, K.S.; et al. Carbon-based TiO2−x heterostructure nanocomposites for enhanced photocatalytic degradation of dye molecules. Ceram. Int. 2021, 47, 10314–10321. [Google Scholar] [CrossRef]
- Ma, B.; Zhu, J.; Xu, Y.; Zhang, L.; Liu, D.; Chen, C.; Sun, B. Photocatalytic degradation of methylene blue using ZnO modified with nano-biochar derived from bacterial cellulose. Ceram. Int. 2025, 51, 14948–14956. [Google Scholar] [CrossRef]
- Ullah, S.; Kumar, O.P.; Ali, R.; Riaz, N.N.; Ahmad, A.; Tufail, M.K.; Muhammad, N.; Rehman, A.u.; Shah, S.S.A.; Nazir, M.A. Biomass Derived Hybrid Activated Carbon/Iron Oxide Composite for Photodegradation of Methylene Blue Upon Visible Light. ChemistrySelect 2025, 10, e202405028. [Google Scholar] [CrossRef]
- Pourali, S.; Amrollahi, R.; Alamolhoda, S.; Masoudpanah, S. In situ synthesis of ZnO/g-C3N4 based composites for photo-degradation of methylene blue under visible light. Sci. Rep. 2025, 15, 462. [Google Scholar] [CrossRef]
- Elias, M.; Alam, R.; Khatun, S.; Hossain, M.S.; Shah, S.S.; Aziz, M.A.; Uddin, M.N.; Hossain, M.A. Hydrothermal synthesis of carboxylated functionalized jute stick carbon and reduced graphene oxide based ZnO nanocomposite photocatalysts: A comparative study. J. Ind. Eng. Chem. 2025, 143, 424–436. [Google Scholar] [CrossRef]
- Gindose, T.G.; Atisme, T.B.; Hailegebreal, T.D.; Zereffa, E.A. ZnO Modified g-C3N4–MnO2 Composite for Photodegradation of Methylene Blue. ChemistrySelect 2025, 10, e202403418. [Google Scholar] [CrossRef]
- Shanmugam, P.; Parasuraman, B.; Mandlimath, T.R.; Smith, S.M.; Thangavelu, P.; Tangjaideborisu, Y.; Nakorn, P.N.; Boonyuen, S. Synergistic effect of boron and sulphur co-doping g-C3N4 nanosheet/Ag2S heterojunctions for high-performance visible light-driven photocatalytic methylene blue. Inorg. Chem. Commun. 2025, 174, 113912. [Google Scholar] [CrossRef]
- Tahir, S.; Zahid, M.; Hanif, M.; Bhatti, I.; Naqvi, S.; Bhatti, H.; Jilani, A.; Alshareef, S.; El-Sharnouby, M.; Shahid, I. The syner-gistic effect of g-C3N4/GO/CuFe2O4 for efficient sunlight-driven photocatalytic degradation of methylene blue. Int. J. Environ. Sci. Technol. 2025, 22, 4829–4846. [Google Scholar] [CrossRef]
- Shanmugam, P.; Parasuraman, B.; Boonyuen, S.; Thangavelu, P.; AlSalhi, M.S.; Zheng, A.L.T.; Viji, A. Hydrothermal synthesis and photocatalytic application of ZnS-Ag composites based on biomass-derived carbon aerogel for the visible light degradation of methylene blue. Environ. Geochem. Health 2024, 46, 92. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Xu, D.; Xie, Y.; Liu, F.; Wang, W.; Shao, H.; Ma, Q.; Yu, H.; Yu, W.; Dong, X. Tri-functional aerogel photocatalyst with an S-scheme heterojunction for the efficient removal of dyes and antibiotic and hydrogen generation. J. Colloid Interface Sci. 2022, 628, 614–626. [Google Scholar] [CrossRef]
- Zhang, X.; Sathiyaseelan, A.; Zhang, L.; Lu, Y.; Jin, T.; Wang, M.-H. Zirconium and cerium dioxide fabricated activated car-bon-based nanocomposites for enhanced adsorption and photocatalytic removal of methylene blue and tetracycline hydro-chloride. Environ. Res. 2024, 261, 119720. [Google Scholar]
- Ji, X.; Tan, Z.; Yang, H.; Shi, Z.; Yang, J.; Alhadhrami, A.; Zhang, J.; Mersal, G.A.; El-Bahy, Z.M.; Guo, Z. Sustainable bamboo charcoal based nanocomposite catalysts for rapid adsorption and photo-Fenton degradation of toxic dyes. Sustain. Mater. Technol. 2024, 41, e01080. [Google Scholar] [CrossRef]
- Shinde, V.; Tanwade, P.; Katayama, T.; Furube, A.; Sathe, B.; Koinkar, P. Ternary composite WS2/GO/Au synthesized from laser ablation and hydrothermal method for photo-and electro-chemical degradation of methylene blue dye. Surf. Interfaces 2024, 46, 104067. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Ahmed, M.A.; Mohamed, A.A. Fabrication of NiO/g-C3N4 Z-scheme heterojunction for enhanced photocata-lytic degradation of methylene blue dye. Opt. Mater. 2024, 151, 115339. [Google Scholar] [CrossRef]
- Ragupathi, H.; Mariadhas, J.; Venkatesh, K.; Inbanathan, S.; Choe, Y. Decorating 2D graphene oxides sheets with spherical shaped Fe3O4 for the applications of supercapacitors and sunlight induced sonophotocatalytic degradation of methylene blue dye. Colloids Surf. A Physicochem. Eng. Asp. 2024, 683, 132927. [Google Scholar] [CrossRef]
- Anum, A.; Nazir, M.A.; Shah, S.S.A.; Elnaggar, A.Y.; Mahmoud, M.; El-Bahy, S.M.; Malik, M.; Wattoo, M.A.; Rehman, A.u. Advanced Nix/MoSx/MOF-2@ g-C3N4 carbon nanostructures for the effective eradication of the Methylene blue dye. Fuller. Nanotub. Carbon Nanostructures 2024, 32, 1103–1114. [Google Scholar] [CrossRef]
- Ramírez-Aparicio, J.; Samaniego-Benítez, J.E.; Murillo-Tovar, M.A.; Benítez-Benítez, J.L.; Muñoz-Sandoval, E.; Gar-cía-Betancourt, M.L. Removal and surface photocatalytic degradation of methylene blue on carbon nanostructures. Diam. Relat. Mater. 2021, 119, 108544. [Google Scholar] [CrossRef]
- Ahmad, A.; Jini, D.; Aravind, M.; Parvathiraja, C.; Ali, R.; Kiyani, M.Z.; Alothman, A. A novel study on synthesis of egg shell based activated carbon for degradation of methylene blue via photocatalysis. Arab. J. Chem. 2020, 13, 8717–8722. [Google Scholar] [CrossRef]
- Cai, J.; Hu, S.; Xiang, J.; Zhang, H.; Men, D. The effect of graphitized carbon on the adsorption and photocatalytic degradation of methylene blue over TiO2/C composites. RSC Adv. 2020, 10, 40830–40842. [Google Scholar] [CrossRef]
- Wu, L.; Chen, Y.; Li, Y.; Meng, Q.; Duan, T. Functionally integrated g-C3N4@ wood-derived carbon with an orderly inter-connected porous structure. Appl. Surf. Sci. 2021, 540, 148440. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Mani, S.; Perumal, S.; Vinodh, R.; Thirunavukkarasu, S.; Lee, Y.R. Facile synthesis of a novel nitrogen-doped carbon dot adorned zinc oxide composite for photodegradation of methylene blue. Dalton Trans. 2020, 49, 17725–17736. [Google Scholar] [CrossRef]
- Dai, Z.; Ren, P.; Cao, Q.; Gao, X.; He, W.; Xiao, Y.; Jin, Y.; Ren, F. Synthesis of TiO2@ lignin based carbon nanofibers composite materials with highly efficient photocatalytic to methylene blue dye. J. Polym. Res. 2020, 27, 1–12. [Google Scholar] [CrossRef]
- Nawaz, A.; Goudarzi, S.; Saravanan, P.; Zarrin, H. Z-scheme induced g-C3N4/WS2 heterojunction photocatalyst with im-proved electron mobility for enhanced solar photocatalysis. Sol. Energy 2021, 228, 53–67. [Google Scholar] [CrossRef]
- Karimi, M.A.; Atashkadi, M.; Ranjbar, M.; Habibi-Yangjeh, A. Novel visible-light-driven photocatalyst of NiO/Cd/g-C3N4 for enhanced degradation of methylene blue. Arab. J. Chem. 2020, 13, 5810–5820. [Google Scholar] [CrossRef]
- Jayaprakash, R.; Dineshbabu, N.; Selvaraj, S.; Vignesh, S.; Arun, T.; Ravichandran, K. Hydrothermally constructed and visi-ble-light activated efficient NiO/ZnO/g-C3N4 ternary nanocomposites for methylene blue dye degradation and antibacterial applications. Inorg. Chem. Commun. 2024, 159, 111643. [Google Scholar] [CrossRef]
- Utomo, W.P.; Afifah, P.A.I.; Rozafia, A.I.; Mahardika, A.A.; Santoso, E.; Liu, R.; Hartanto, D. Modulation of particle size and morphology of zinc oxide in graphitic carbon nitride/zinc oxide composites for enhanced photocatalytic degradation of methylene blue. Surf. Interfaces 2024, 46, 104017. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, P.; Li, X.; Luo, M.; Zhang, W.; Chen, M.; Zhou, X. Green preparation of palm powder-derived carbon dots co-doped with sulfur/chlorine and their application in visible-light photocatalysis. Spectrochim. Acta Part A Mol. Biomol. Spec-trosc. 2020, 227, 117659. [Google Scholar] [CrossRef]
- Son, B.T.; Long, N.V.; Hang, N.T.N. The development of biomass-derived carbon-based photocatalysts for the visi-ble-light-driven photodegradation of pollutants: A comprehensive review. RSC Adv. 2021, 11, 30574–30596. [Google Scholar] [CrossRef]
- Guo, M.; Hu, Y.; Wang, R.; Yu, H.; Sun, L. Molecularly imprinted polymer-based photocatalyst for highly selective degradation of methylene blue. Environ. Res. 2021, 194, 110684. [Google Scholar] [CrossRef]
- Ghorai, K.; Panda, A.; Bhattacharjee, M.; Mandal, D.; Hossain, A.; Bera, P.; Seikh, M.M.; Gayen, A. Facile synthesis of CuCr2O4/CeO2 nanocomposite: A new Fenton like catalyst with domestic LED light assisted improved photocatalytic activity for the degradation of RhB, MB and MO dyes. Appl. Surf. Sci. 2021, 536, 147604. [Google Scholar] [CrossRef]
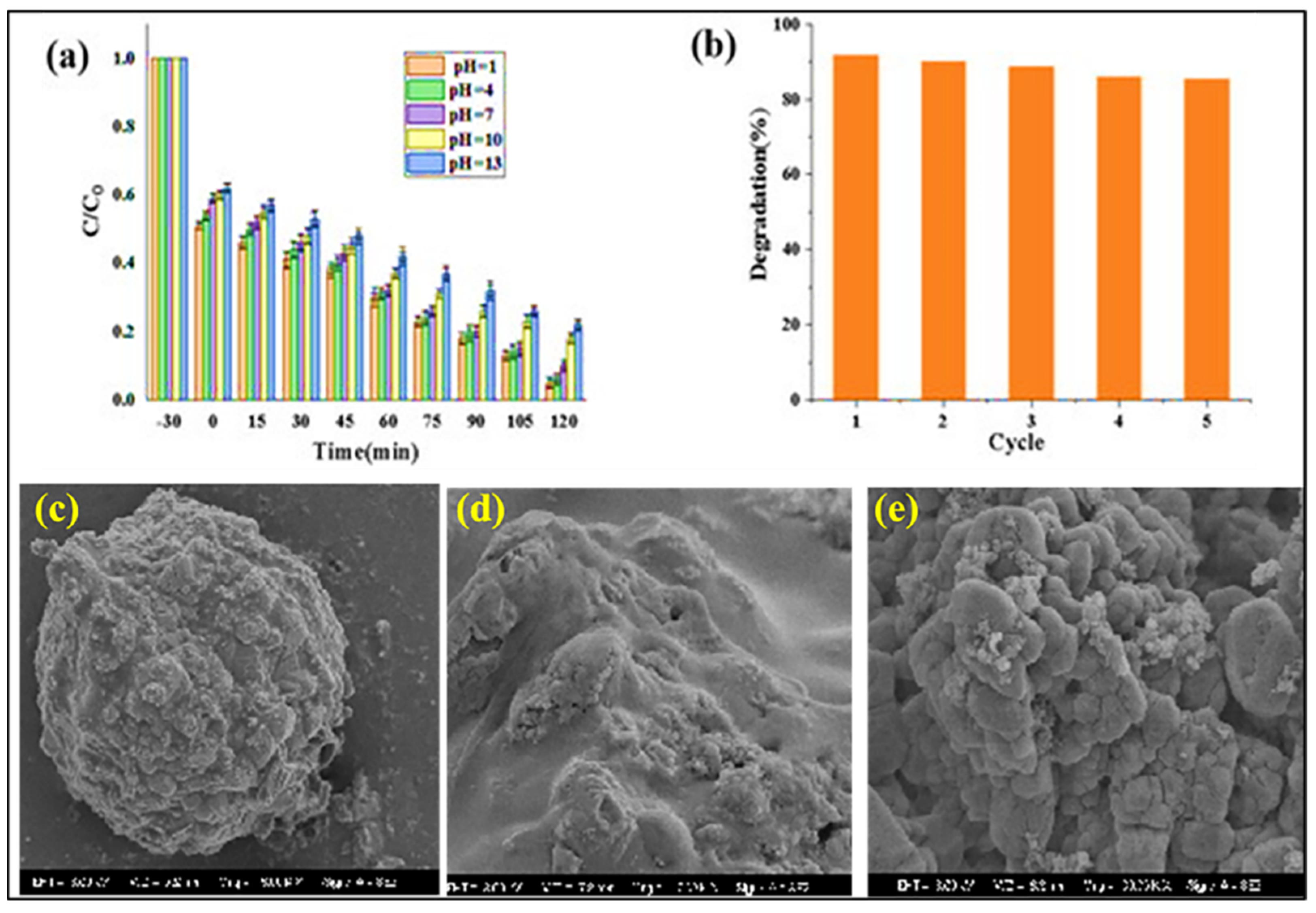
- Zubair, A.; Nawaz, F.; Fareed, I.; Khan, M.D.; Ali, Z.; Nawaz, M.; Anam, H.S.; Tahir, M.; Butt, F.K. Novel CoVO/WxOy composites for methylene blue photodegradation and electrocatalytic applications. Mater. Sci. Semicond. Process. 2025, 186, 109104. [Google Scholar] [CrossRef]
- Maulana, M.A.R.; TriGoutomo, B.; Mahmud, A. The effect of Phase Composition on the Photocatalytic Activity of Cu2O/CuO/Cu Composites for Methylene Blue Photodegradation. Chem. Mater. 2025, 4, 1–8. [Google Scholar] [CrossRef]
- Tang, T.; Zhang, H.; Wang, H.; Dou, X.; Wen, J.; Jiang, L. Composite ZIF-8 with Cs3Bi2I9 to Enhance the Photodegradation Ability on Methylene Blue. Molecules 2025, 30, 1413. [Google Scholar] [CrossRef] [PubMed]
- Długosz, O.; Chlebowska, Z.; Banach, M. Preparation of Materials Based on Metal Carbonate Nanoparticles for Photodegra-dation of Organic Pollutants. J. Clust. Sci. 2025, 36, 55. [Google Scholar] [CrossRef]
- Urooj, N.; Arshad, M.; Arshad, R.; Intisar, A.; Batool, M.; Bashir, F.; Kousar, R. Remarkable visible light actuated photodeg-radation of methylene blue using copper oxide/tungsten oxide heterojunction composites. Mater. Sci. Eng. B 2025, 315, 118079. [Google Scholar] [CrossRef]
- Rethnakumaran, A.V.; Menamparambath, M.M. In Situ Generation of Poly (3, 4-ethylenedioxythiophene)/Ag2SeO3 Nano-hybrids at Hexane/Water Interface for Photodegradation of Organic Dyes. Macromol. Mater. Eng. 2025, 310, 2400409. [Google Scholar] [CrossRef]
- Abuzeyad, O.H.; El-Khawaga, A.M.; Tantawy, H.; Gobara, M.; Elsayed, M.A. Merits photocatalytic activity of rGO/zinc copper ferrite magnetic nanocatalyst for photodegradation of methylene blue (MB) dye. Discov. Nano 2025, 20, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Awais, M.; Hussain, R.; Shah, A.; Alajmi, M.F.; Maryam, R.; Hussain, A.; Khan, S.U.; ur Rahman, S. Design of novel FeSe2/ZnO composites and their application in photodegradation of methylene blue. Phys. B Condens. Matter 2024, 685, 416048. [Google Scholar] [CrossRef]
- Borhani, M.N.; Tavakoli, A.; Mollaei, A.M.; Borhani, T.N. Visible light photodegradation of methylene blue by ionic liquid based TiO2/Fe3O4 nanophotocatalysts. Opt. Mater. 2024, 154, 115627. [Google Scholar] [CrossRef]
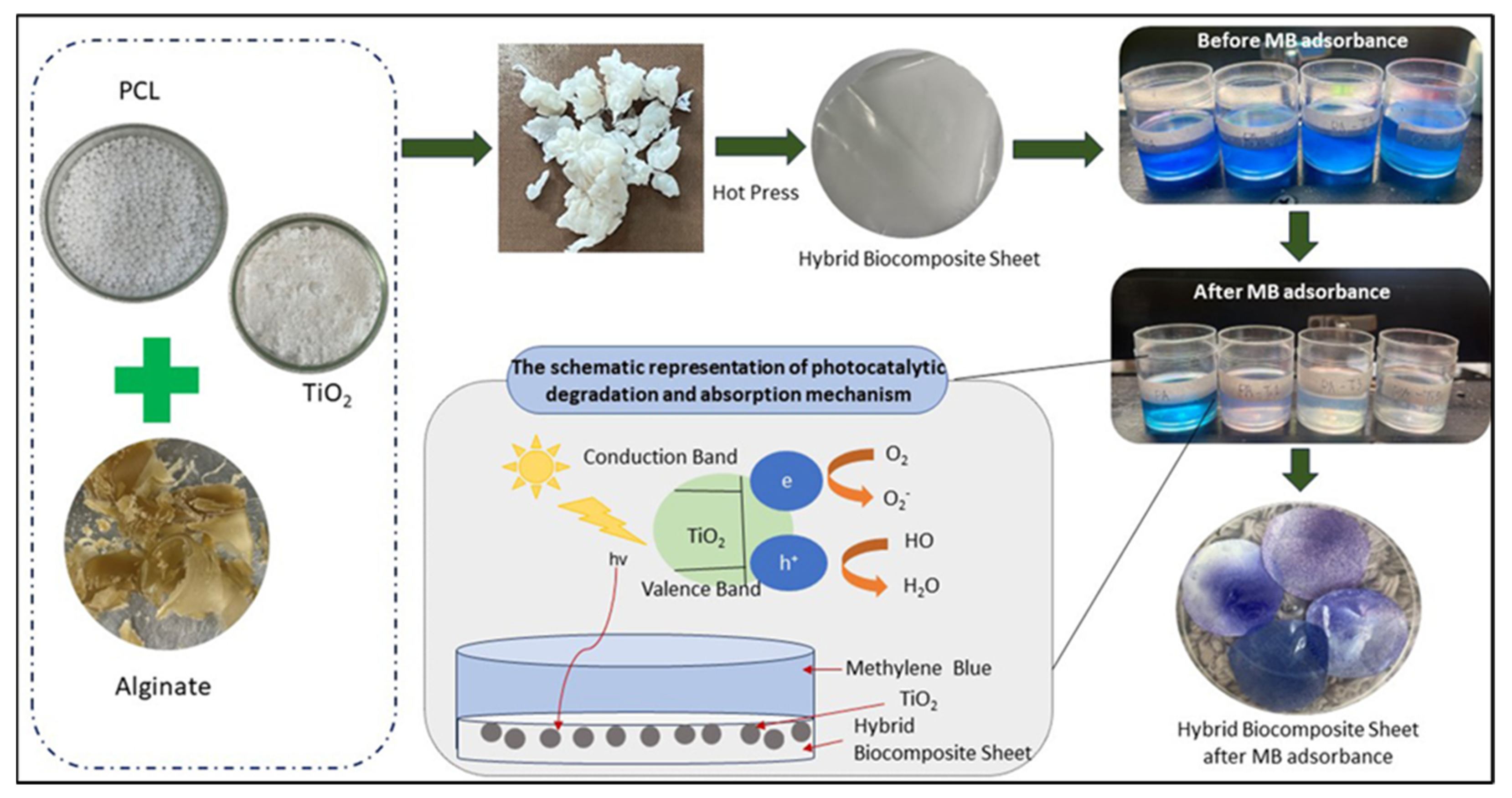
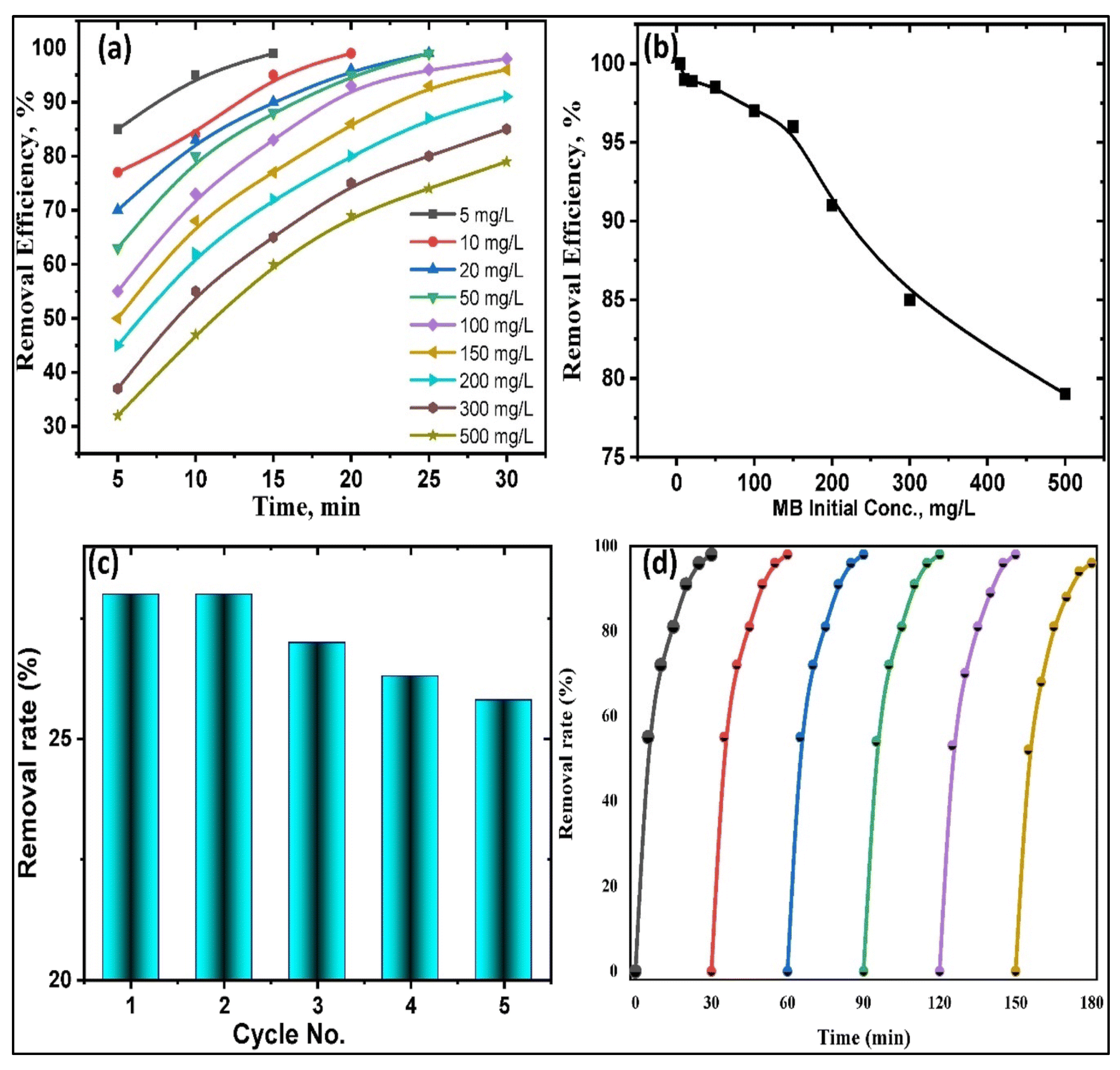
- Bukit, B.F.; Pratama, A.W.; Frida, E.; Sedayu, B.B.; Fransiska, D.; Purnomo, D.; Rochima, E.; Rahmawati, I.; Suhartana, S.; Syamani, F.A. Eco-friendly alginate/PCL-TiO2 hybrid biocomposites: Preparation, properties, and methylene blue photodeg-radation. South Afr. J. Chem. Eng. 2025, 51, 254–264. [Google Scholar]
- Agalya, K.; Vijayakumar, S.; Vidhya, E.; Prathipkumar, S.; Mythili, R.; Devanesan, S.; AlSalhi, M.S.; Kim, W. Fabrication of PVA/TiO2 Composites Via Green Synthesis and Assessment of their Photodegradation and Anti-Germ Capabilities. Waste Biomass Valorization 2024, 15, 6441–6451. [Google Scholar] [CrossRef]
- Hieu, N.H.; Hai, N.D.; Dat, N.M.; Nam, N.T.H.; Giang, N.T.H.; Cong, C.Q.; Huong, L.M. Chitosan-mediated coupling of MgFe2O4–TiO2 nanocomposites for efficient methylene blue photodegradation. ACS Appl. Nano Mater. 2024, 7, 6583–6595. [Google Scholar] [CrossRef]
- Kassas, A.; Dhaini, B.; Zahwa, I.; Zayyat, R.; Shaito, A.; Hussein, B.; Mouyane, M.; Bernard, J.; Houivet, D.; Toufaily, J. Sun-light-activated composite TiO2-FV-Mo materials for photodegradation of the organic pollutant methylene blue. Heliyon 2024, 10, e40489. [Google Scholar] [CrossRef]
- Onwubiko, V.; Matsushita, Y.; Elshehy, E.A.; El-Khouly, M.E. Facile synthesis of TiO2–carbon composite doped nitrogen for efficient photodegradation of noxious methylene blue dye. RSC Adv. 2024, 14, 34298–34310. [Google Scholar] [CrossRef]
- Fendi, K.; Bouzidi, N.; Boudraa, R.; Saidani, A.; Manseri, A.; Quesada, D.E.; Hai, T.N.; Bollinger, J.-C.; Salvestrini, S.; Kebir, M.; et al. Testing of kaolinite/TiO2 nanocomposites for methylene blue removal: Photodegradation and mechanism. Int. J. Chem. React. Eng. 2024, 22, 1493–1508. [Google Scholar] [CrossRef]
- Coba, A.J.O.; Briceño, S.; Vizuete, K.; Debut, A.; González, G. Diatomite with TiO2 nanoparticles for the photocatalytic deg-radation of methylene blue. Carbon Trends 2025, 19, 100488. [Google Scholar] [CrossRef]
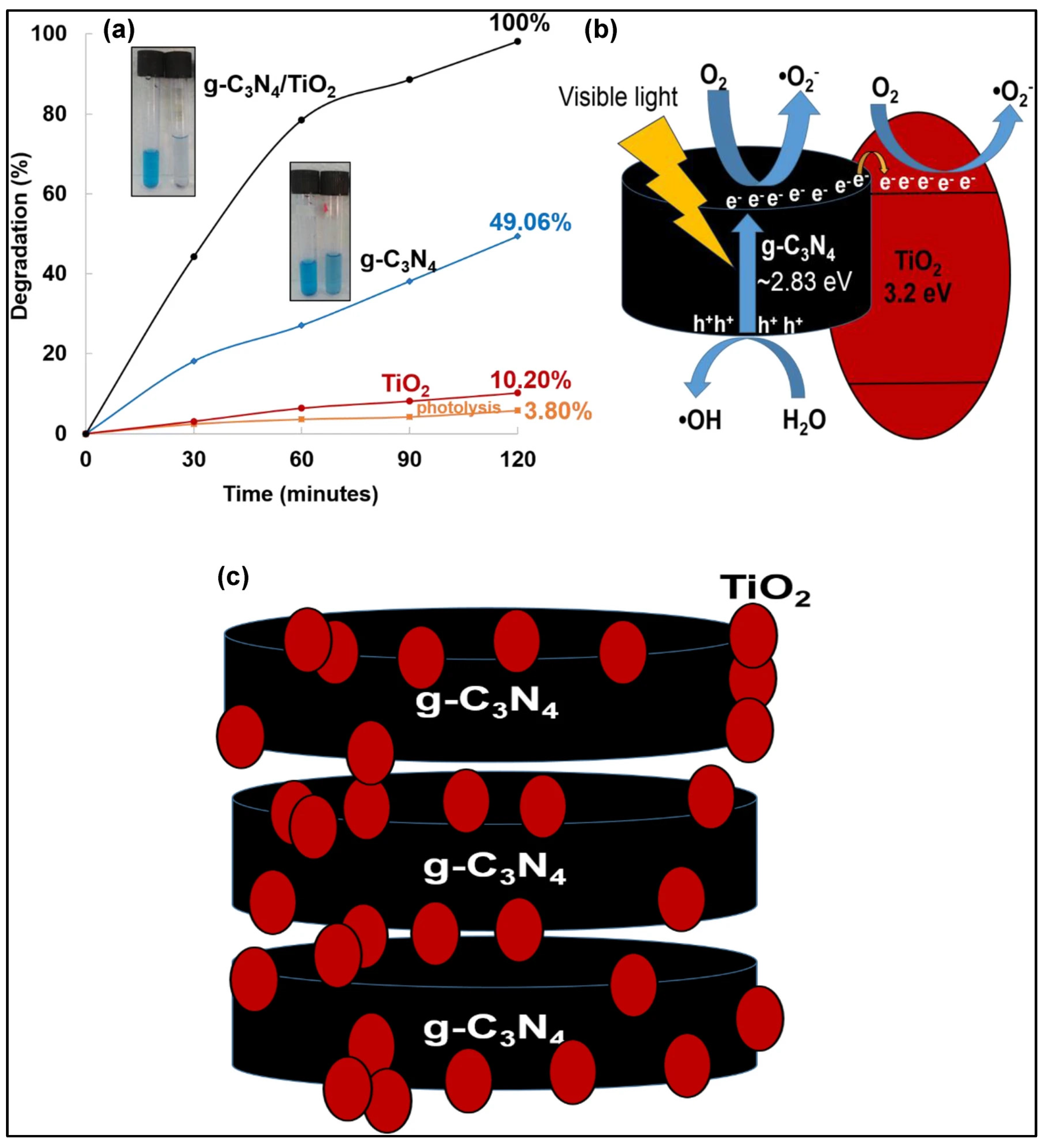
- Razali, M.H.; Md Fauzi, M.A.F.; Mohd Azam, B.; Yusoff, M. g-C3N4/TiO2 nanocomposite photocatalyst for methylene blue photodegradation under visible light. Appl. Nanosci. 2022, 12, 3197–3206. [Google Scholar] [CrossRef]
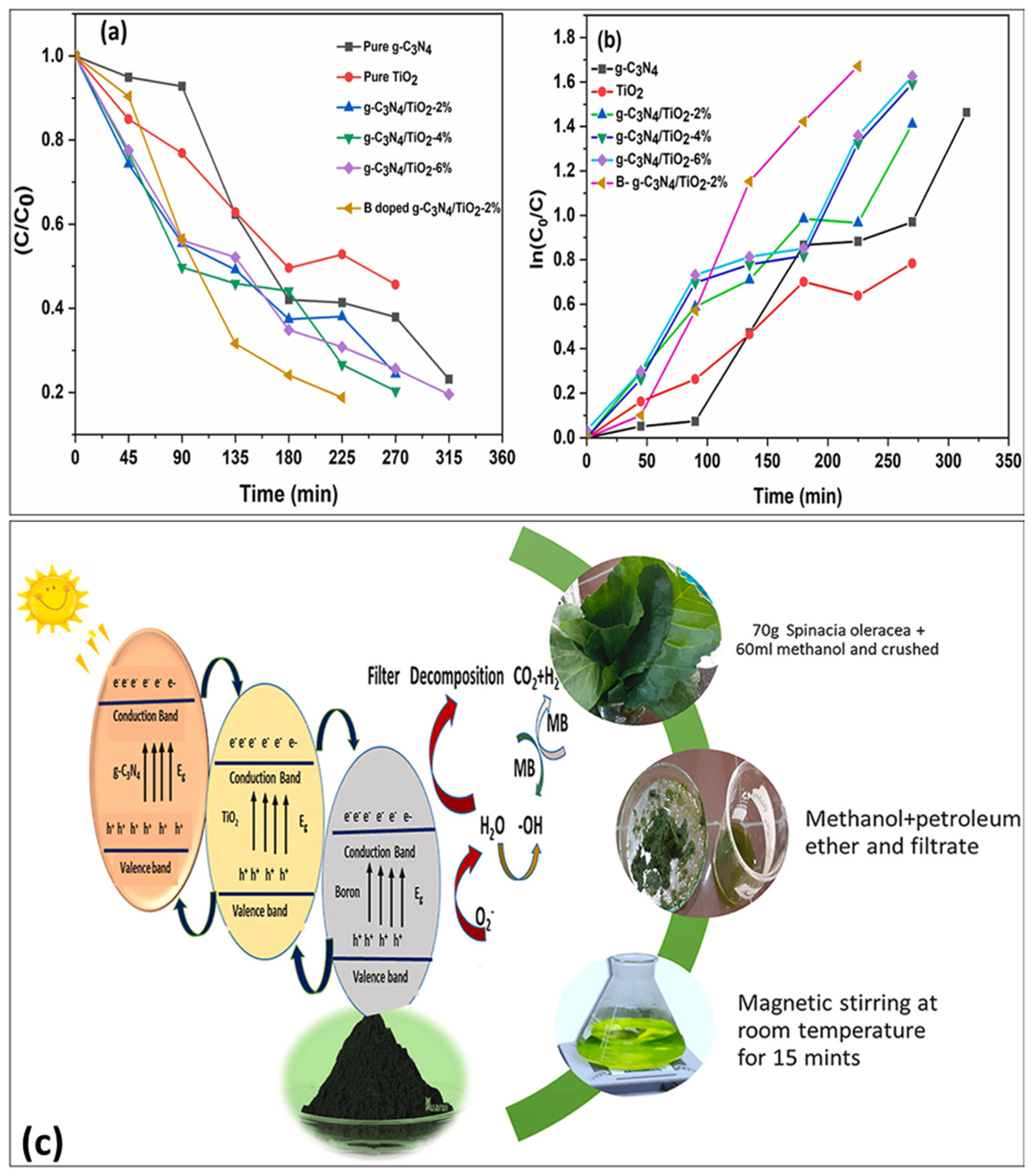
- Faryad, S.; Azhar, U.; Tahir, M.B.; Ali, W.; Arif, M.; Sagir, M. Spinach-derived boron-doped g-C3N4/TiO2 composites for efficient photo-degradation of methylene blue dye. Chemosphere 2023, 320, 138002. [Google Scholar] [CrossRef] [PubMed]
- Pe III, J.A.; Mun, S.P.; Lee, M. TiO2-carbonized medium-density fiberboard for the photodegradation of methylene blue. Wood Sci. Technol. 2021, 55, 1109–1122. [Google Scholar] [CrossRef]
- Padwal, Y.; Panchang, R.; Fouad, H.; Terashima, C.; Chauhan, R.; Gosavi, S.W. Unveiling the mechanism of enhanced meth-ylene blue degradation using chromium-TiO2/carbon nanocomposite photocatalyst. J. Solid State Electrochem. 2023, 27, 3557–3568. [Google Scholar] [CrossRef]
- Yoo, H.; Kim, J.H. Photoactive TiO2/CuxO composite films for photocatalytic degradation of methylene blue pollutant mole-cules. Adv. Powder Technol. 2021, 32, 1287–1293. [Google Scholar] [CrossRef]
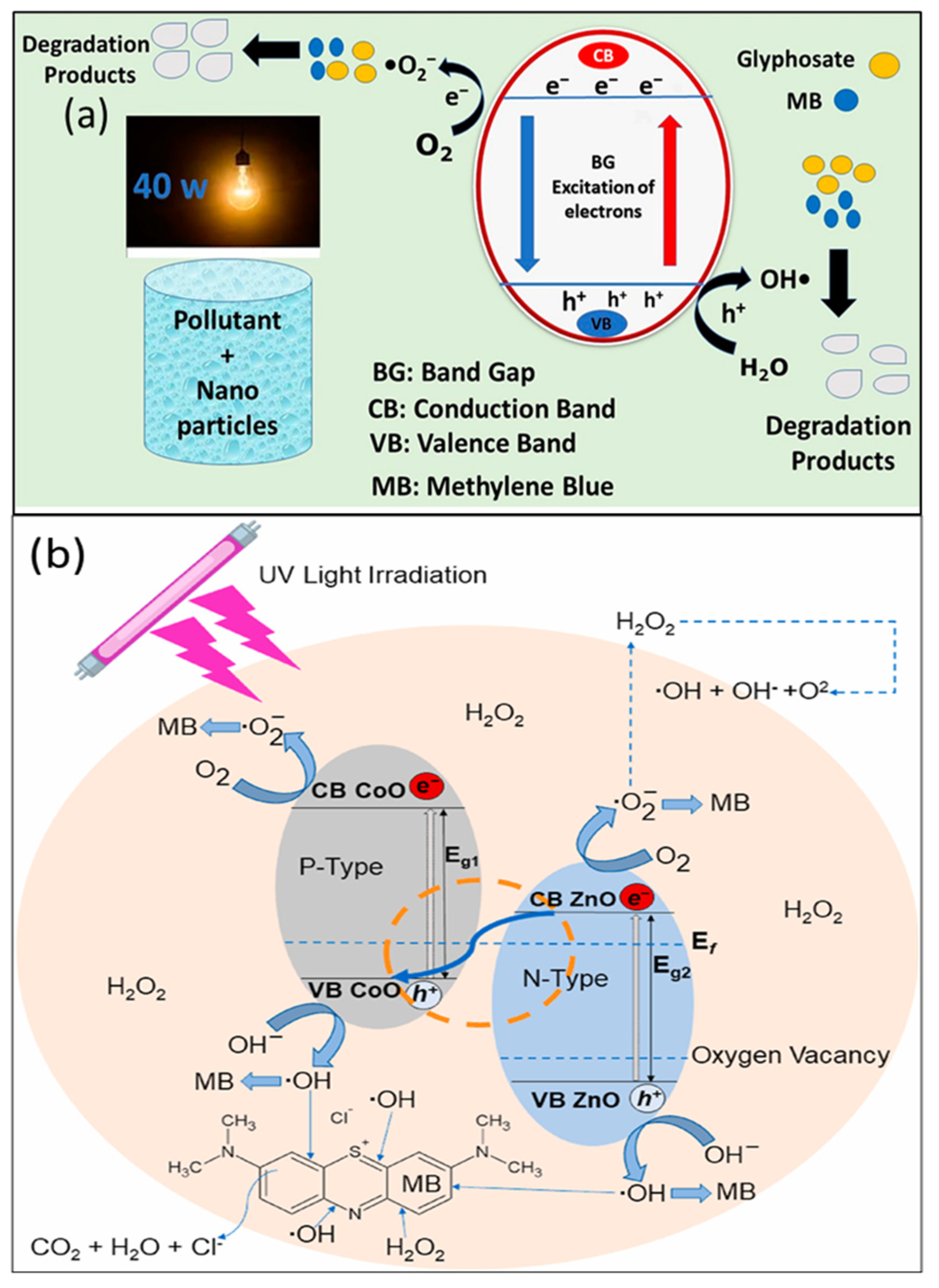
- Aziz, K.; Naz, A.; Manzoor, S.; Khan, M.I.; Shanableh, A.; Fernandez Garcia, J. Visible light photodegradation of glyphosate and methylene blue using defect-modified graphitic carbon nitride decorated with Ag/TiO2. Catalysts 2023, 13, 1087. [Google Scholar] [CrossRef]
- Liyanaarachchi, H.; Thambiliyagodage, C.; Liyanaarachchi, C.; Samarakoon, U. Efficient photocatalysis of Cu doped TiO2/g-C3N4 for the photodegradation of methylene blue. Arab. J. Chem. 2023, 16, 104749. [Google Scholar] [CrossRef]
- Rini, N.P.; Istiqomah, N.I.; Suharyadi, E. Enhancing photodegradation of methylene blue and reusability using CoO/ZnO composite nanoparticles. Case Stud. Chem. Environ. Eng. 2023, 7, 100301. [Google Scholar] [CrossRef]
- Ngullie, R.C.; Bhuvaneswari, K.; Shanmugam, P.; Boonyuen, S.; Smith, S.M.; Sathishkumar, M. Magnetically recoverable bi-omass-derived carbon-aerogel supported ZnO (ZnO/MNC) composites for the photodegradation of methylene blue. Catalysts 2022, 12, 1073. [Google Scholar] [CrossRef]
- Acedo-Mendoza, A.; Infantes-Molina, A.; Vargas-Hernández, D.; Chávez-Sánchez, C.; Rodríguez-Castellón, E.; Tánori-Córdova, J. Photodegradation of methylene blue and methyl orange with CuO supported on ZnO photocatalysts: The effect of copper loading and reaction temperature. Mater. Sci. Semicond. Process. 2020, 119, 105257. [Google Scholar] [CrossRef]
- Bekru, A.G.; Tufa, L.T.; Zelekew, O.A.; Goddati, M.; Lee, J.; Sabir, F.K. Green synthesis of a CuO–ZnO nanocomposite for efficient photodegradation of methylene blue and reduction of 4-nitrophenol. ACS Omega 2022, 7, 30908–30919. [Google Scholar] [CrossRef]
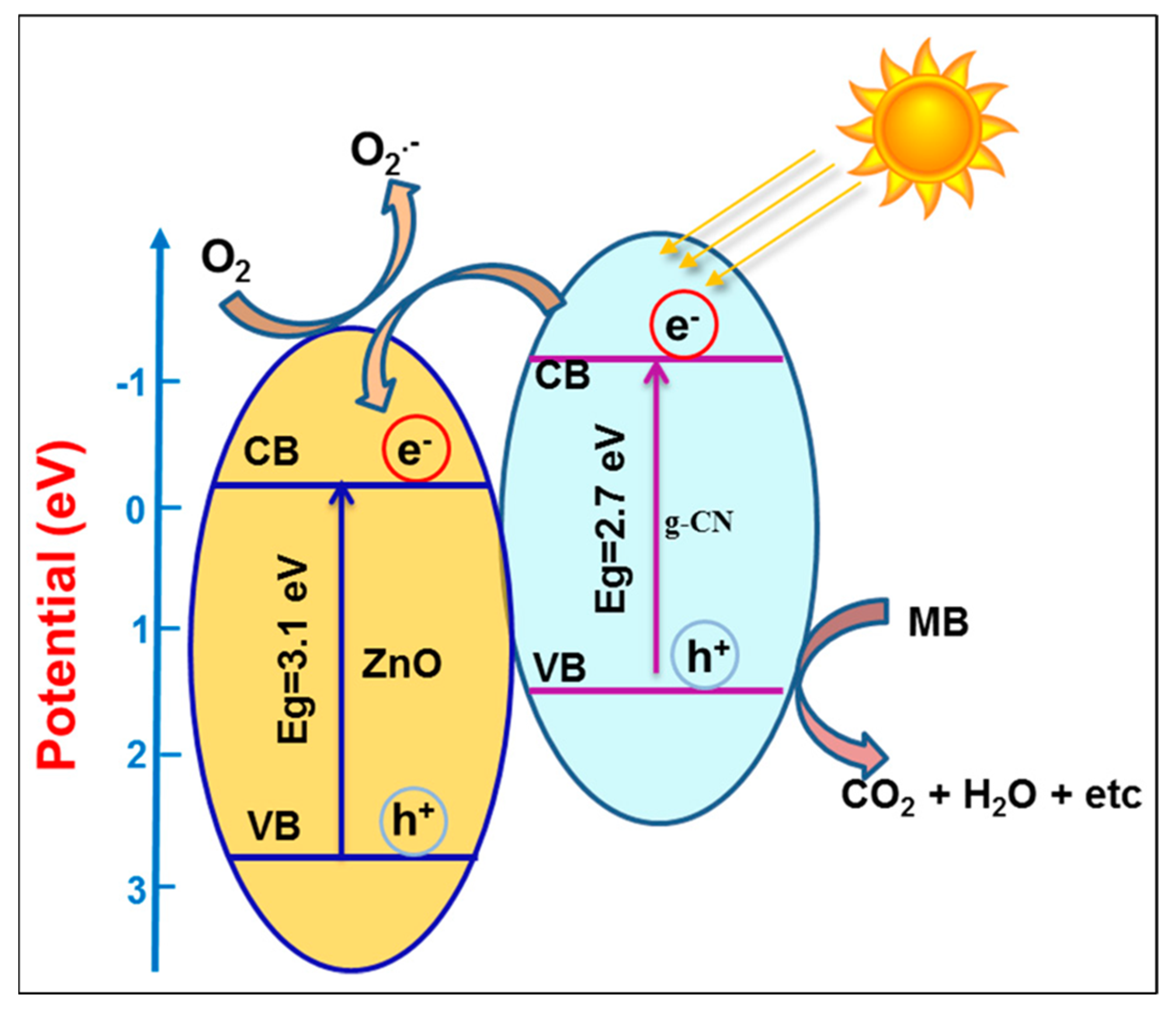
- Ngullie, R.C.; Alaswad, S.O.; Bhuvaneswari, K.; Shanmugam, P.; Pazhanivel, T.; Arunachalam, P. Synthesis and characteri-zation of efficient ZnO/g-C3N4 nanocomposites photocatalyst for photocatalytic degradation of methylene blue. Coatings 2020, 10, 500. [Google Scholar] [CrossRef]
- Elshypany, R.; Selim, H.; Zakaria, K.; Moustafa, A.H.; Sadeek, S.A.; Sharaa, S.; Raynaud, P.; Nada, A.A. Elaboration of Fe3O4/ZnO nanocomposite with highly performance photocatalytic activity for degradation methylene blue under visible light irradiation. Environ. Technol. Innov. 2021, 23, 101710. [Google Scholar] [CrossRef]
- Guo, Y.; Fu, X.; Liu, R.; Chu, M.; Tian, W. Efficient green photocatalyst of Ag/ZnO nanoparticles for methylene blue photo-degradation. J. Mater. Sci. Mater. Electron. 2022, 33, 1–13. [Google Scholar] [CrossRef]
- Noruozi, A.; Nezamzadeh-Ejhieh, A. Preparation, characterization, and investigation of the catalytic property of α-Fe2O3-ZnO nanoparticles in the photodegradation and mineralization of methylene blue. Chem. Phys. Lett. 2020, 752, 137587. [Google Scholar] [CrossRef]
- Sahoo, S.; Bhuyan, M.; kumar Sahu, A.; Alagarsamy, P.; Sahoo, D. Photodegradation of methylene blue by met-al-nanoparticles-modulated-graphene-based composites: An efficient way of sewage water management. Solid State Sci. 2023, 142, 107255. [Google Scholar] [CrossRef]
- Yang, J.; Yao, X.; Wu, H.; Li, D.; Liang, J.; Tang, X.; Zhu, Z. Fabrication of biocarbon modified BiOI heterostructures for enhanced organic pollutants photodegradation activity. J. Mater. Sci. Mater. Electron. 2025, 36, 1–12. [Google Scholar] [CrossRef]
- Saini, I.; Singh, V.; Hamad, S.; Ram, S. Recent development in bimetallic metal organic frameworks as photocatalytic material. Inorg. Chem. Commun. 2024, 160, 111897. [Google Scholar] [CrossRef]
- Mohammadnejad, M.; Nekoo, N.M.; Alizadeh, S.; Sadeghi, S.; Geranmayeh, S. Enhanced removal of organic dyes from aqueous solutions by new magnetic HKUST-1: Facile strategy for synthesis. Sci. Rep. 2023, 13, 17981. [Google Scholar] [CrossRef]
- Zhang, J.; Su, C.; Xie, X.; Liu, P.; Huq, M.E. Enhanced visible light photocatalytic degradation of dyes in aqueous solution activated by HKUST-1: Performance and mechanism. RSC Adv. 2020, 10, 37028–37034. [Google Scholar] [CrossRef]
- Gupta, H.; Saini, I.; Singh, V.; Singh, V.; Yarramaneni, S.; Grover, P. Fast decomposition of organic contaminant in wastewater using Zn and Mn bimetallic metal organic frameworks. Polyhedron 2024, 261, 117116. [Google Scholar] [CrossRef]
- Gupta, H.; Saini, I.; Singh, V.; Singh, V.; Mishra, B. Enhancing photocatalytic activity via formation of heterojunctions intro-duced through postmetalation of metal organic frameworks with silver ions. Phys. Scr. 2024, 99, 1059a1053. [Google Scholar] [CrossRef]
- Van Tran, T.; Nguyen, D.T.C.; Nguyen, T.T.; Le, H.T.; Van Nguyen, C.; Nguyen, T.D. Metal-organic framework HKUST-1-based Cu/Cu2O/CuO@ C porous composite: Rapid synthesis and uptake application in antibiotics remediation. J. Water Process Eng. 2020, 36, 101319. [Google Scholar] [CrossRef]
- Saffari, M.; Ghorbanloo, M.; Morsali, A.; Gan, Y.; Su, Y. Metal Organic Framework-Derived Bi2O3-ZnO/TiO2 Nanofibers Catalysts for Enhanced Photodegradation of Methylene Blue. Catal. Lett. 2025, 155, 132. [Google Scholar] [CrossRef]
- Singh, V.; Singh, V.; Thakur, O.; Kumar, A.; Yarramaneni, S. Study of Structural Changes Induced in Copper-based Metal Organic Framework during Photocatalytic Dye Degradation of Methylene Blue using Vibrational Spectroscopy. J. Alloys Compd. 2025, 1022, 180073. [Google Scholar]
- Zhou, J.; Zhou, Q.; Sun, H.; Li, X.; Chen, A.; Chen, J.; Chu, C. Selective detoxification of a sulfur mustard simulant in air by a methylene blue-functionalized metal–organic framework. Dalton Trans. 2025, 54, 1827–1837. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Luo, L.; Tawiah, B.; Liu, C.; Hua, J.; Ming, Y.; Xin, J.H.; Wong, W.-Y.; Fei, B. Water-Stable Mapbbr3@ Pbbroh Qds Confined by Metal–Organic Framework for Photodegradation of Wastewater. J. Colloid Interface Sci. 2025, 700, 138581. [Google Scholar] [CrossRef]
- Sakeerali, C.; Heo, S.M.; Kim, C.W. Preparation of core–shell structured Cu2O@ NH2-MIL-125 (Ti) MOF and efficient photo-catalytic degradation of methylene blue. Chem. Phys. 2025, 591, 112602. [Google Scholar] [CrossRef]
- Zheng, H.; Ma, F.; Ling, M.; Yang, Z.; Ji, R.; Jiang, Y.; Li, L.; Zhang, W. The multicomponent heterostructure flower spherical Ce-MOF/CdIn2S4/CdS for ciprofloxacin photodegradation via effective electron transfer. J. Solid State Chem. 2025, 344, 125210. [Google Scholar] [CrossRef]
- Liaqat, R.; Jamshaid, M.; Abo-Dief, H.M.; Ali, S.E.; El-Bahy, Z.M.; Fiaz, M.; Wattoo, M.A.; ur Rehman, A. Mo@ Ni-MOF nanocomposite: A promising photocatalyst for photodegradation of Methylene blue. J. Mol. Struct. 2024, 1315, 139011. [Google Scholar] [CrossRef]
- Adawiah, A.; Fitriani, S.; Andriyani, L.; Azizah, Y.N.; Wahyudin, W.; Sukandar, D.; Zulys, A. Optimization of methylene blue photodegradation by Cr-PTCHIna/TiO2 composite using box-behnken design application. Sustain. Chem. Clim. Action 2024, 5, 100047. [Google Scholar] [CrossRef]
- Khan, M.; Ahmed, M.M.; Akhtar, M.N.; Sajid, M.; Riaz, N.N.; Asif, M.; Kashif, M.; Shabbir, B.; Ahmad, K.; Saeed, M. Fabri-cation of CuWO4@ MIL-101 (Fe) nanocomposite for efficient OER and photodegradation of methylene blue. Heliyon 2024, 10, e40546. [Google Scholar] [CrossRef] [PubMed]
- Zulfa, L.L.; Hidayat, A.R.P.; Utomo, W.P.; Subagyo, R.; Kusumawati, E.N.; Kusumawati, Y.; Hartanto, D.; Widyastuti, W.; Ediati, R. Facile synthesis of Ni-ZIF-8 with improved photodegradation performance for methylene blue. Case Stud. Chem. Environ. Eng. 2024, 10, 100828. [Google Scholar] [CrossRef]
- Li, M.; Xiao, W.; Yin, Z.; Chen, Y.; Luo, Y.; Hong, Z.; Xue, M. Construction of a robust MOF-based superhydrophobic composite coating with the excellent performance in antifouling, drag reduction, and organic photodegradation. Prog. Org. Coat. 2024, 186, 108086. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, T.; Dou, Z.; Yang, H. MnMg-MOF material photo-Fenton reaction degradation of methylene blue. Mater. Sci. Semicond. Process. 2024, 171, 108021. [Google Scholar] [CrossRef]
- Cheng, L.; Tang, Y.; Xie, M.; Sun, Y.; Liu, H. 2D ultrathin NiMOF decorated by Ti3C2 MXene for highly improved photocatalytic performance. J. Alloys Compd. 2021, 864, 158913. [Google Scholar] [CrossRef]
- Kashif, S.; Akram, S.; Murtaza, M.; Amjad, A.; Shah, S.S.A.; Waseem, A. Development of MOF-MXene composite for the removal of dyes and antibiotic. Diam. Relat. Mater. 2023, 136, 110023. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, T.; Guo, P.; Zhang, W.; Yang, G. High removal of methyl blue over copper mixed-triazolate MOF by both adsorption and photodegradation from aqueous solution. Microporous Mesoporous Mater. 2024, 369, 113053. [Google Scholar] [CrossRef]
- Gu, C.; Weng, W.; Lu, C.; Tan, P.; Jiang, Y.; Zhang, Q.; Liu, X.; Sun, L. Decorating MXene with tiny ZIF-8 nanoparticles: An effective approach to construct composites for water pollutant removal. Chin. J. Chem. Eng. 2022, 42, 42–48. [Google Scholar] [CrossRef]
- Li, S.; Yang, S.; Liang, G.; Yan, M.; Wei, C.; Lu, Y. Regulation and photocatalytic degradation mechanism of a hydroxyl modified UiO-66 type metal organic framework. RSC Adv. 2023, 13, 5273–5282. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.; Zhang, Y.; Zheng, J.; Ge, S.; Lin, J.; Pan, D.; Naik, N.; Guo, Z. In-situ constructing visible light CdS/Cd-MOF photo-catalyst with enhanced photodegradation of methylene blue. Particuology 2022, 69, 111–122. [Google Scholar] [CrossRef]
- Tang, T.; Li, C.; He, W.; Hong, W.; Zhu, H.; Liu, G.; Yu, Y.; Lei, C. Preparation of MOF-derived C-ZnO/PVDF composites membrane for the degradation of methylene blue under UV-light irradiation. J. Alloys Compd. 2022, 894, 162559. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, Q.; Al-Enizi, A.M.; Nafady, A.; Ma, S. Recent advances in MOF-based photocatalysis: Environmental remedi-ation under visible light. Inorg. Chem. Front. 2020, 7, 300–339. [Google Scholar] [CrossRef]
- Brahmi, C.; Benltifa, M.; Vaulot, C.; Michelin, L.; Dumur, F.; Millange, F.; Frigoli, M.; Airoudj, A.; Morlet-Savary, F.; Bousselmi, L. New hybrid MOF/polymer composites for the photodegradation of organic dyes. Eur. Polym. J. 2021, 154, 110560. [Google Scholar] [CrossRef]
- Jatoi, Y.F.; Fiaz, M.; Athar, M. Synthesis of efficient TiO2/Al2O3@ Cu (BDC) composite for water splitting and photodegradation of methylene blue. J. Aust. Ceram. Soc. 2021, 57, 489–496. [Google Scholar] [CrossRef]
- El-Fawal, E.M.; Abd El Salam, H. Deposition of dyes on Cobalt-based metal-organic framework (Co-MOF) composites with promoted achievement photocatalytic degradation of an anionic dye (EBT) under visible light irradiation. Int. J. Environ. Anal. Chem. 2023, 103, 106–122. [Google Scholar] [CrossRef]
- Hariganesh, S.; Vadivel, S.; Maruthamani, D.; Kumaravel, M.; Paul, B.; Balasubramanian, N.; Vijayaraghavan, T. Facile large scale synthesis of CuCr2O4/CuO nanocomposite using MOF route for photocatalytic degradation of methylene blue and tet-racycline under visible light. Appl. Organomet. Chem. 2020, 34, e5365. [Google Scholar] [CrossRef]
- Si, Y.; Li, X.; Yang, G.; Mie, X.; Ge, L. Fabrication of a novel core–shell CQDs@ ZIF-8 composite with enhanced photocatalytic activity. J. Mater. Sci. 2020, 55, 13049–13061. [Google Scholar] [CrossRef]
- Saemian, T.; Sadr, M.H.; Yaraki, M.T.; Gharagozlou, M.; Soltani, B. Synthesis and characterization of CoFe2O4/SiO2/Cu-MOF for degradation of methylene blue through catalytic sono-Fenton-like reaction. Inorg. Chem. Commun. 2022, 138, 109305. [Google Scholar] [CrossRef]
- Zulfa, L.L.; Ediati, R.; Hidayat, A.R.P.; Subagyo, R.; Faaizatunnisa, N.; Kusumawati, Y.; Hartanto, D.; Widiastuti, N.; Utomo, W.P.; Santoso, M. Synergistic effect of modified pore and heterojunction of MOF-derived α-Fe2O3/ZnO for superior photo-catalytic degradation of methylene blue. RSC Adv. 2023, 13, 3818–3834. [Google Scholar] [CrossRef]
- Bouider, B.; Haffad, S.; Bouakaz, B.S.; Berd, M.; Ouhnia, S.; Habi, A. MOF-5/Graphene oxide composite photocatalyst for enhanced photocatalytic activity of methylene blue degradation under solar light. J. Inorg. Organomet. Polym. Mater. 2023, 33, 4001–4011. [Google Scholar] [CrossRef]
- Gholamrezapor, E.; Eslami, A. Photocatalytic degradation of methylene blue in aqueous solution by magnetic ZnMn2O4@ copper porphyrin nanocomposite. J. Iran. Chem. Soc. 2022, 19, 1071–1080. [Google Scholar] [CrossRef]
- Ziaadini, F.; Mostafavi, A.; Shamspur, T.; Fathirad, F. Photocatalytic degradation of methylene blue from aqueous solution using Fe3O4@ SiO2@ CeO2 core-shell magnetic nanostructure as an effective catalyst. Adv. Environ. Technol. 2019, 5, 127–132. [Google Scholar]
- Al-Sharabi, M.; Baiocco, D.; Lobel, B.T.; Cayre, O.J.; Zhang, Z.; Routh, A.F. Magnetic zinc oxide/silica microbeads for the photocatalytic degradation of azo dyes. Colloids Surf. A Physicochem. Eng. Asp. 2024, 695, 134169. [Google Scholar] [CrossRef]
- Kumar, A.P.; Ahmed, F.; Kumar, S.; Anuradha, G.; Harish, K.; Kumar, B.P. Synthesis of magnetically recoverable Ru/Fe3O4 nanocomposite for efficient photocatalytic degradation of methylene blue. J. Clust. Sci. 2022, 33, 853–865. [Google Scholar] [CrossRef]
- Light, S. Gadolinium Nanoparticle Decorated Multiwalled Carbon. Ph.D. Thesis, University of Johannesburg, Johannesburg, South Africa, 2012. [Google Scholar]
- Gangadhar, A.; Abhilash Mavinakere, R.; Jagadish, K.; Srikantaswamy, S. Photo-catalytic dye degradation of methylene blue by using ZrO2/MWCNT nanocomposites. Water Pract. Technol. 2021, 16, 1265–1276. [Google Scholar] [CrossRef]
- Arias, M.-C.; Aguilar, C.; Piza, M.; Zarazua, E.; Anguebes, F.; Anguebes, F.; Anguebes, F.; Anguebes, F.; Cordova, V. Removal of the methylene blue dye (MB) with catalysts of Au-TiO2: Kinetic and degradation pathway. Mod. Res. Catal. 2021, 10, 1–14. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.; Sain, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Iervolino, G.; Zammit, I.; Vaiano, V.; Rizzo, L. Limitations and prospects for wastewater treatment by UV and visible-light-active heterogeneous photocatalysis: A critical review. Heterog. Photocatal. Recent Adv. 2019, 378, 225–264. [Google Scholar]
- Younis, S.A.; Kim, K.-H. Heterogeneous photocatalysis scalability for environmental remediation: Opportunities and challenges. Catalysts 2020, 10, 1109. [Google Scholar] [CrossRef]
- Alalm, M.G.; Djellabi, R.; Meroni, D.; Pirola, C.; Bianchi, C.L.; Boffito, D.C. Toward scaling-up photocatalytic process for multiphase environmental applications. Catalysts 2021, 11, 562. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef]
- Sudhaik, A.; Raizada, P.; Rangabhashiyam, S.; Singh, A.; Nguyen, V.-H.; Van Le, Q.; Khan, A.A.P.; Hu, C.; Huang, C.-W.; Ahamad, T.; et al. Copper sulfides based photocatalysts for degradation of environmental pollution hazards: A review on the recent catalyst design concepts and future perspectives. Surf. Interfaces 2022, 33, 102182. [Google Scholar] [CrossRef]
- Zhang, N.; Xing, Z.; Li, Z.; Zhou, W. Sulfur vacancy engineering of metal sulfide photocatalysts for solar energy conversion. Chem Catal. 2023, 3, 100375. [Google Scholar] [CrossRef]
- Kumari, H.; Sonia; Suman; Ranga, R.; Chahal, S.; Devi, S.; Sharma, S.; Kumar, S.; Kumar, P.; Kumar, S.; et al. A review on photocatalysis used for wastewater treatment: Dye degradation. Water Air Soil Pollut. 2023, 234, 349. [Google Scholar] [CrossRef]
- Ahuja, T.; Brighu, U.; Saxena, K. Recent advances in photocatalytic materials and their applications for treatment of wastewater: A review. J. Water Process Eng. 2023, 53, 103759. [Google Scholar] [CrossRef]
- Chalatsi-Diamanti, P.; Isari, E.A.; Grilla, E.; Kokkinos, P.; Kalavrouziotis, I.K. Recent prospects, challenges and advancements of photocatalysis as a wastewater treatment method. Water Emerg. Contam. Nanoplastics 2025, 4, 12. [Google Scholar] [CrossRef]
- Qudsieh, I.Y.; Ali, M.A.; Maafa, I.M. Effect of water matrix on photocatalytic degradation of organic pollutants in water: A literature review. Rev. Chem. Eng. 2025, 41, 539–573. [Google Scholar] [CrossRef]
- Adenuga, D.O.; Tichapondwa, S.M.; Chirwa, E.M. Influence of wastewater matrix on the visible light degradation of phenol using AgCl/Bi24O31Cl10 photocatalyst. Environ. Sci. Pollut. Res. 2023, 30, 98922–98933. [Google Scholar] [CrossRef] [PubMed]
- Azzaz, A.A.; Jellali, S.; Hamed, N.B.H.; El Jery, A.; Khezami, L.; Assadi, A.A.; Amrane, A. Photocatalytic treatment of wastewater containing simultaneous organic and inorganic pollution: Competition and operating parameters effects. Catalysts 2021, 11, 855. [Google Scholar] [CrossRef]


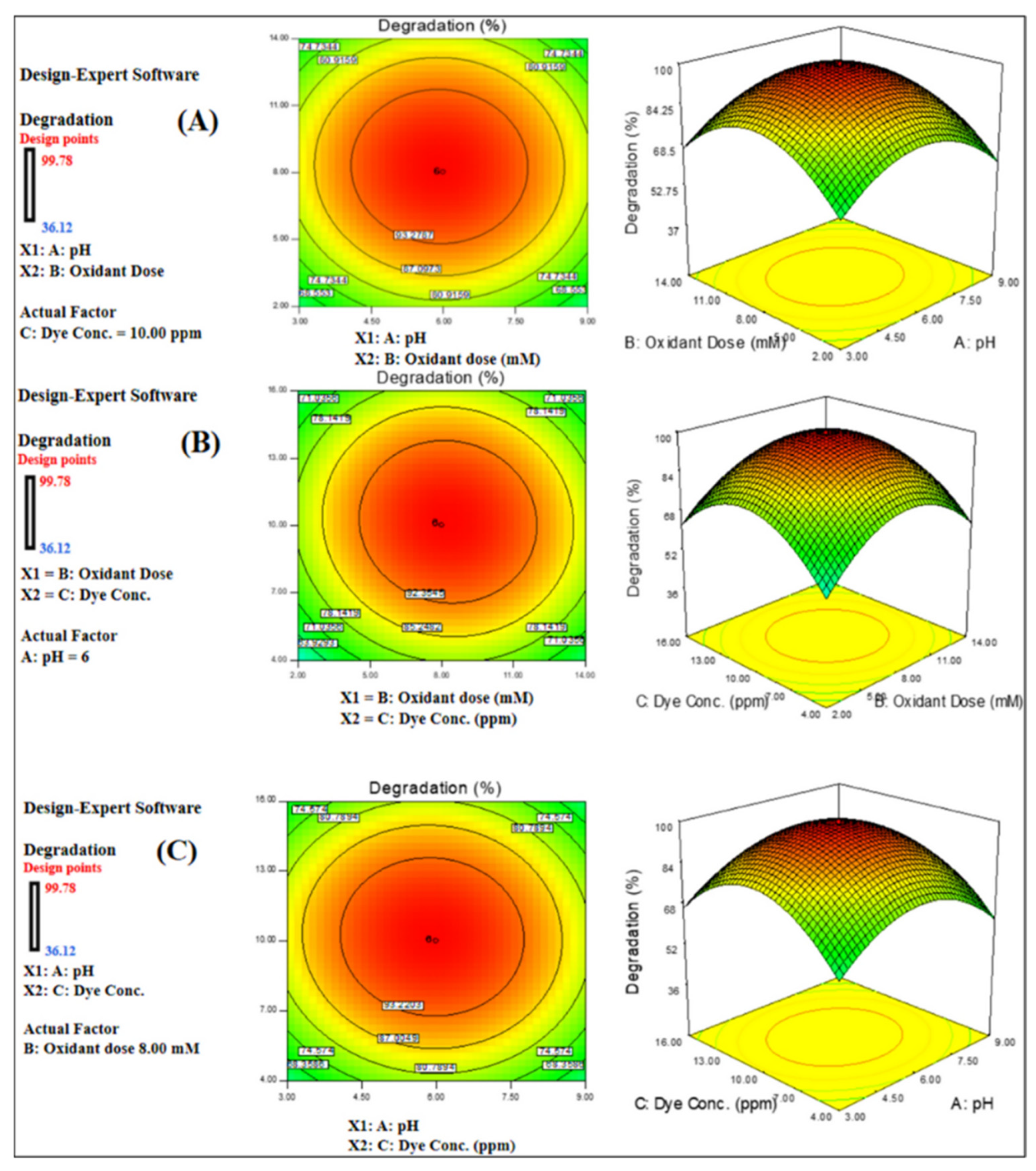


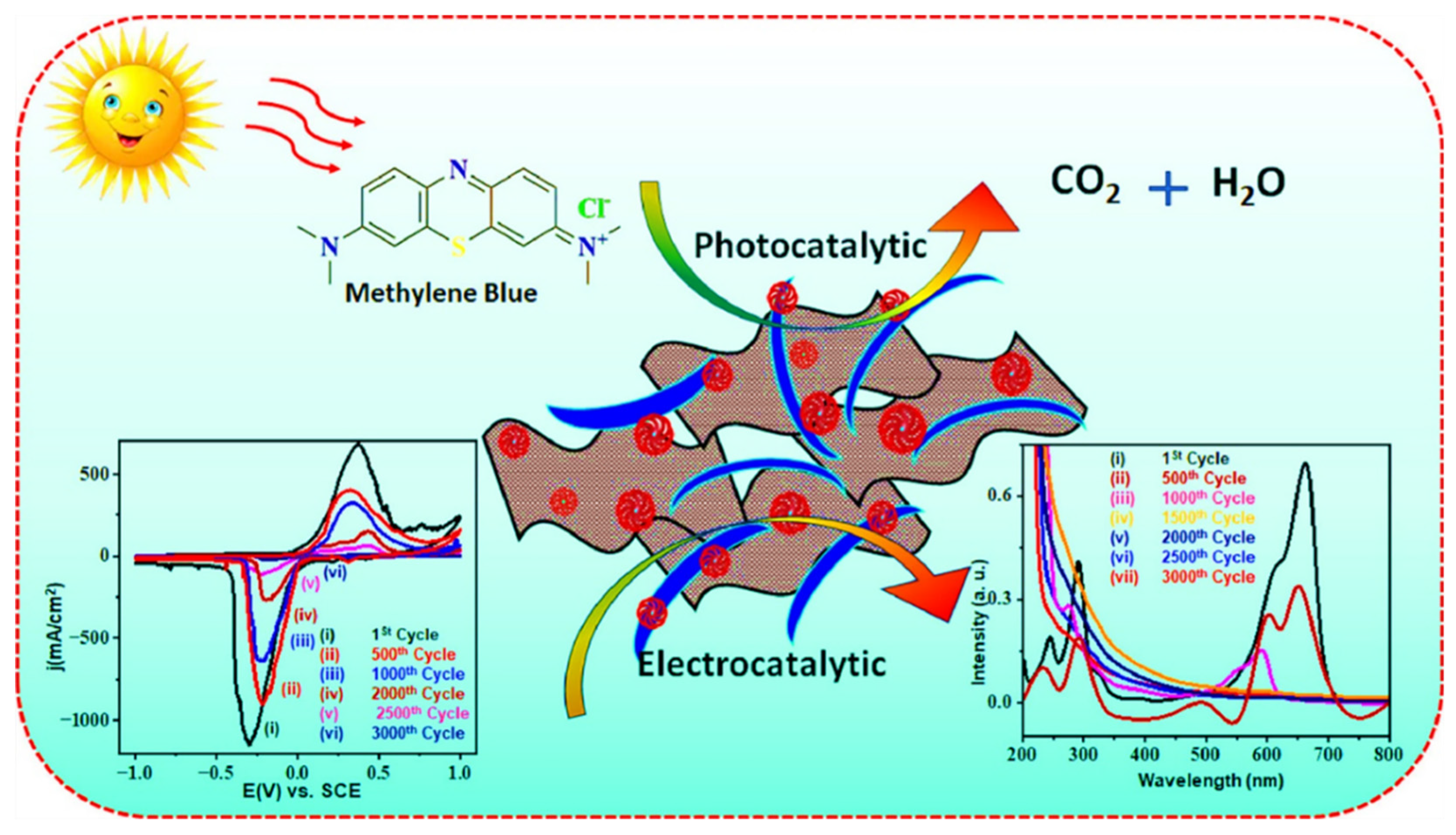
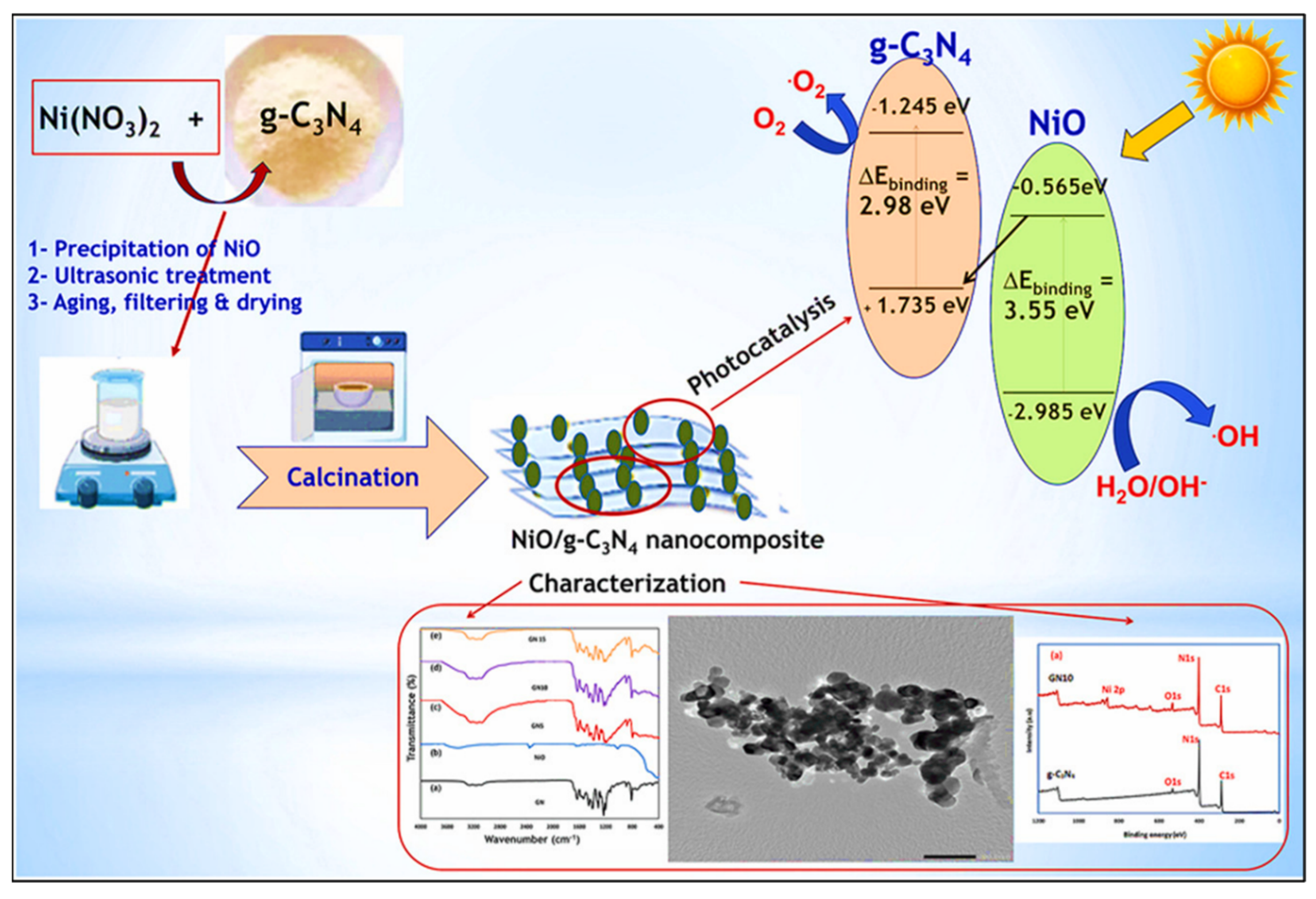
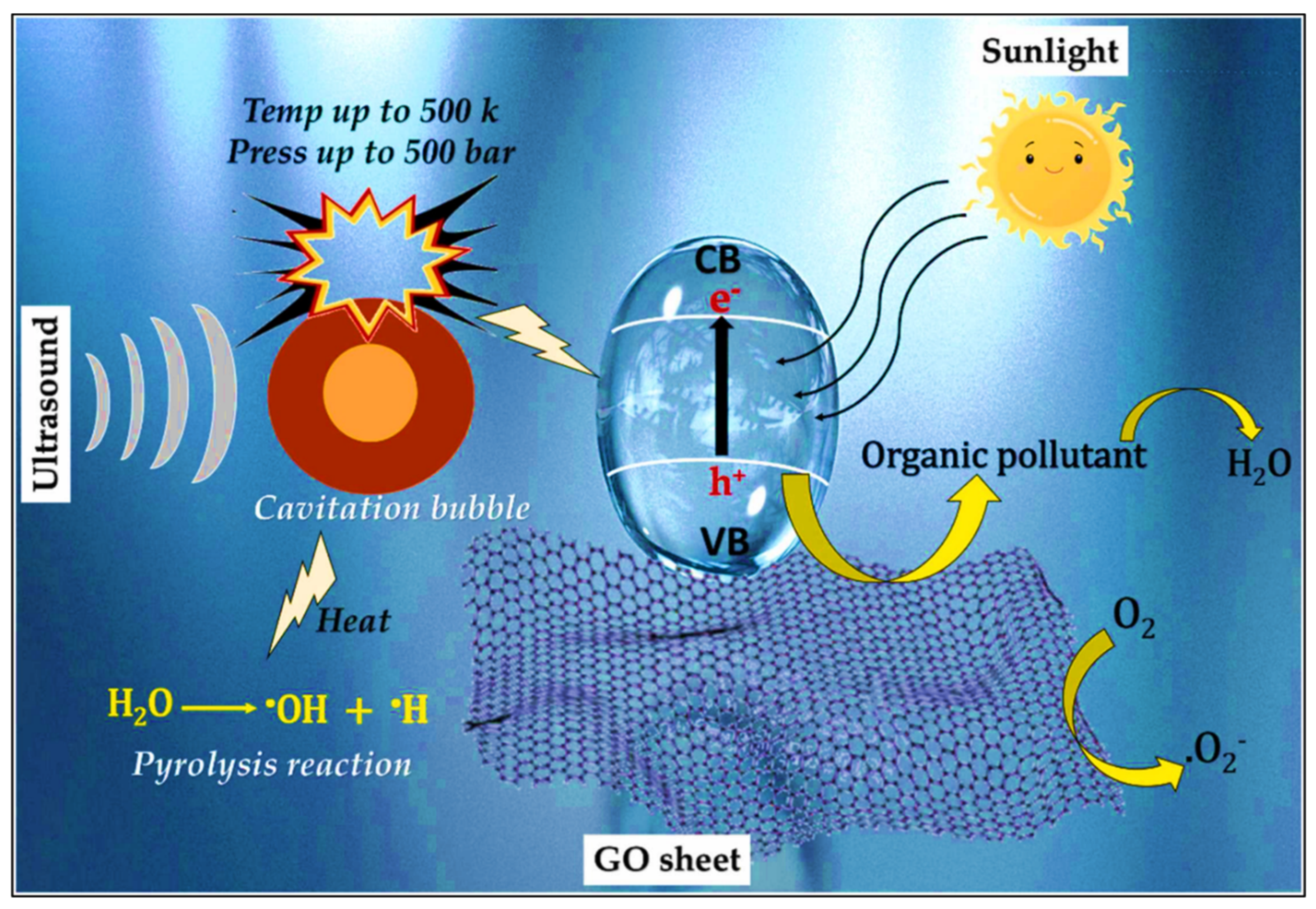
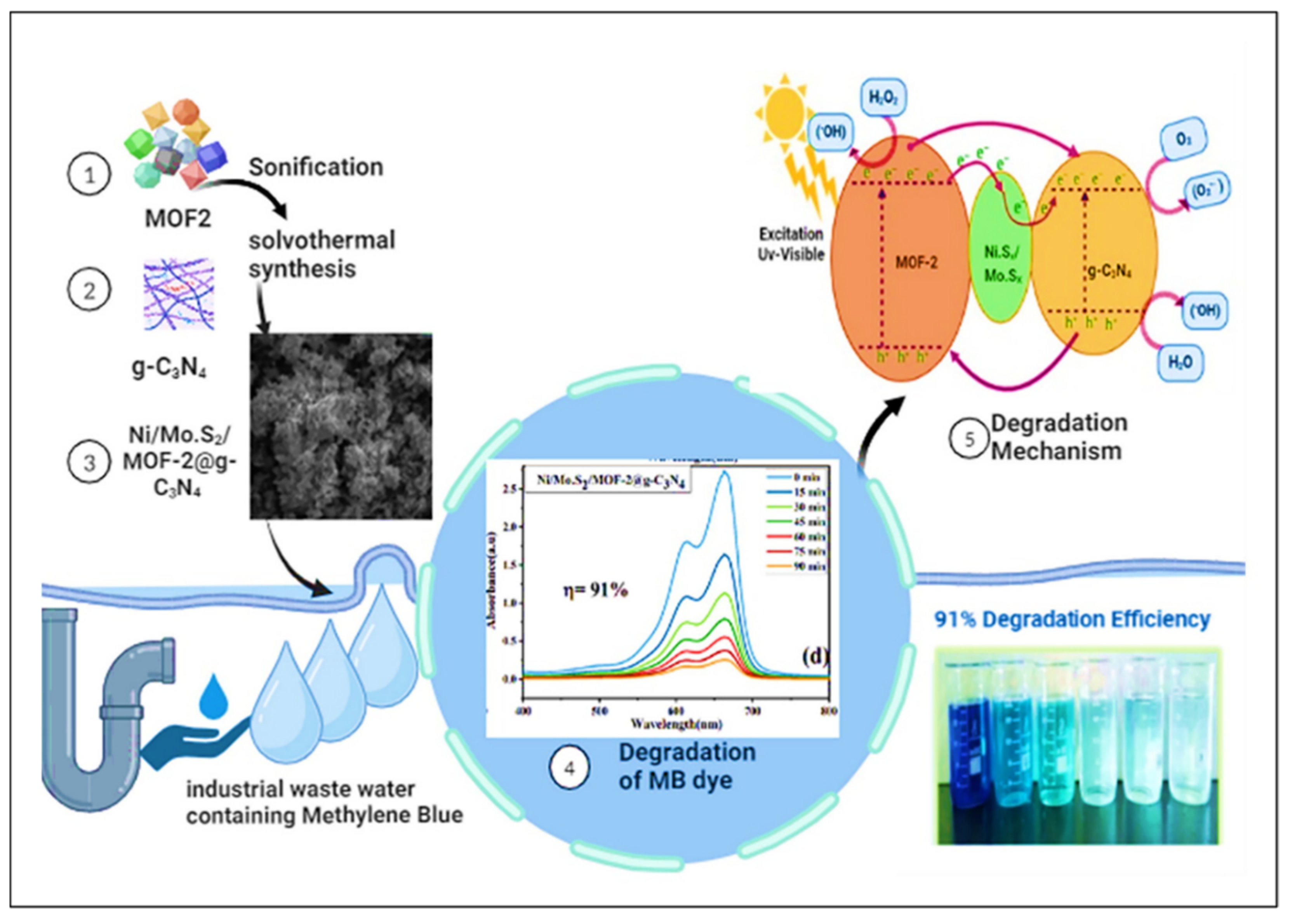
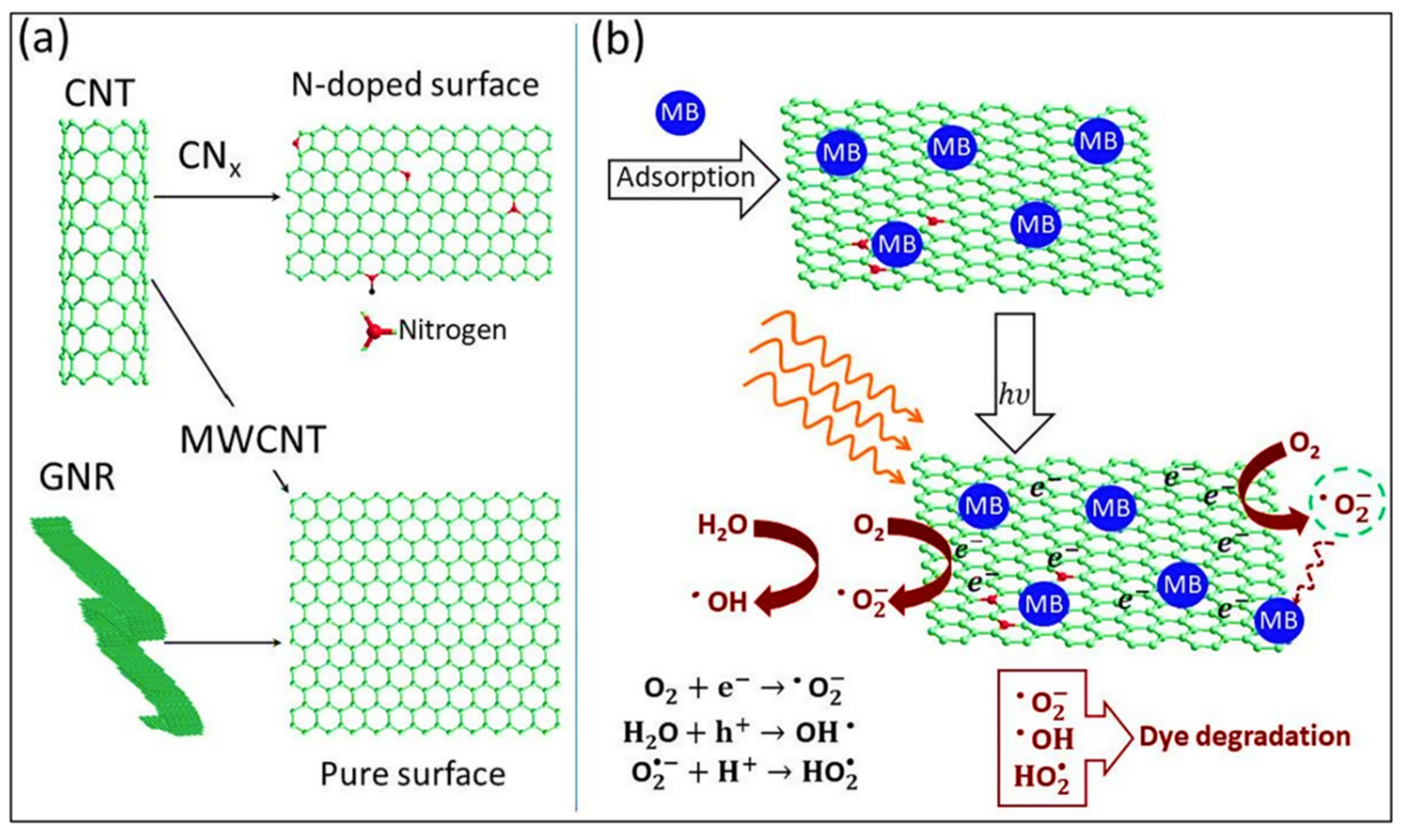
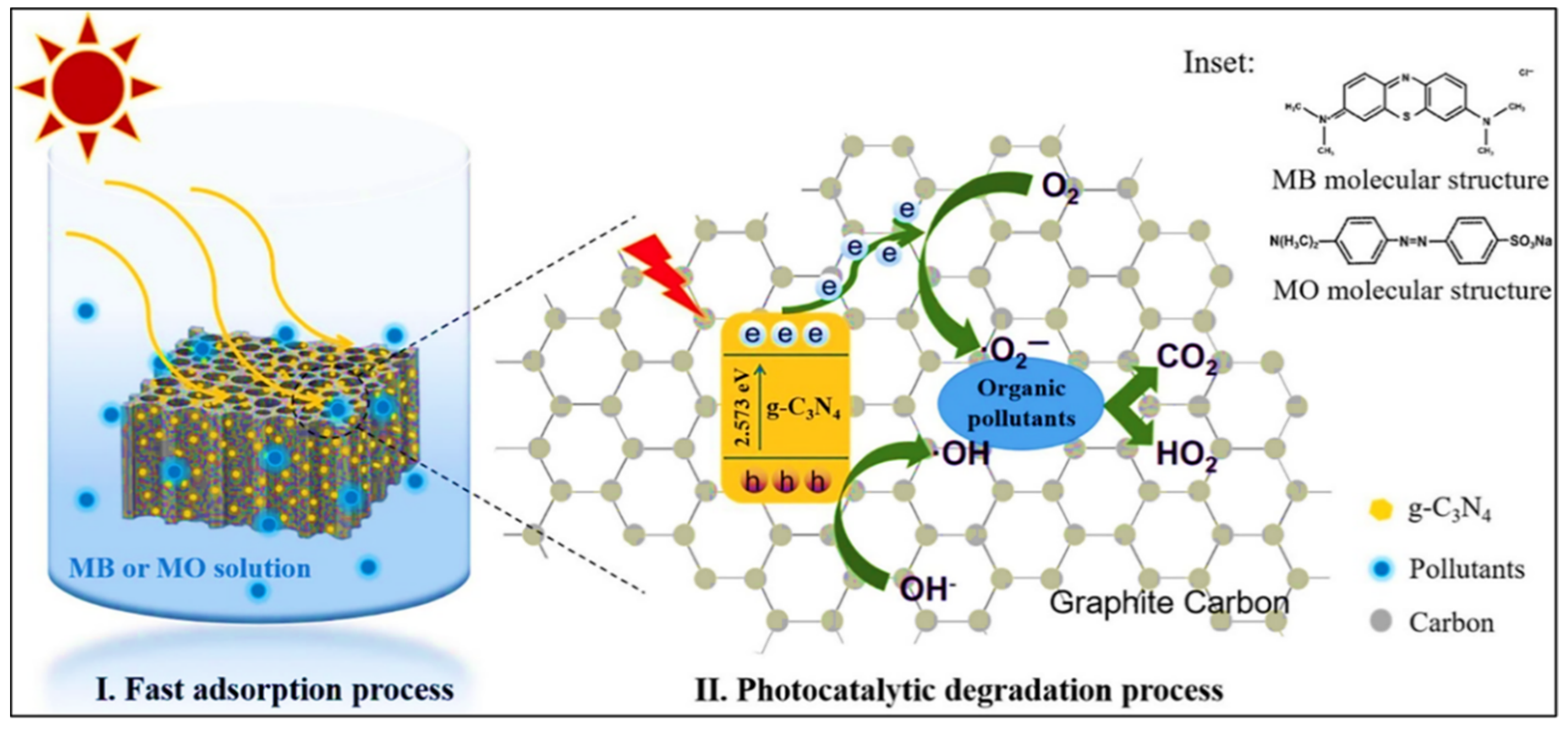
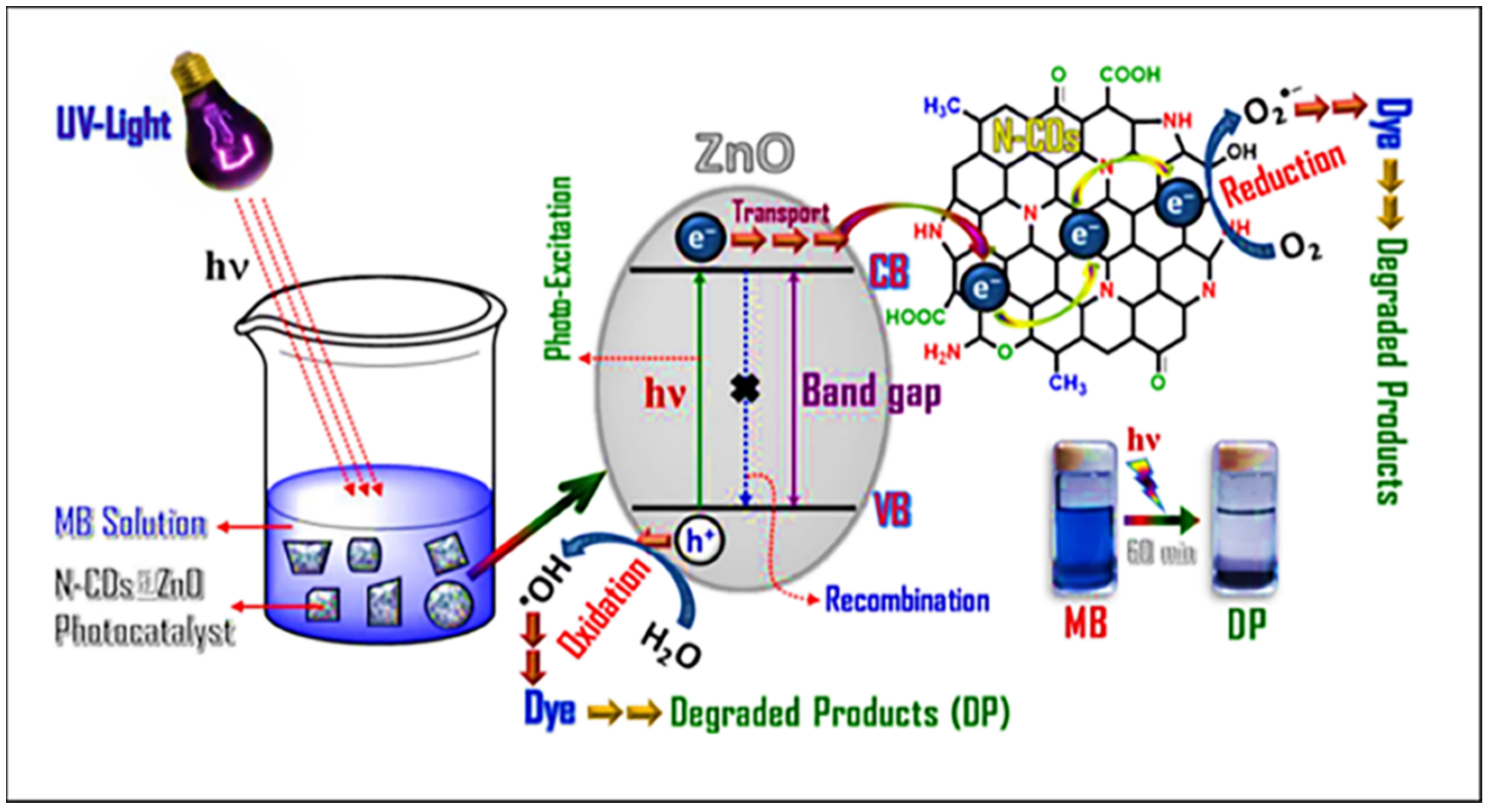
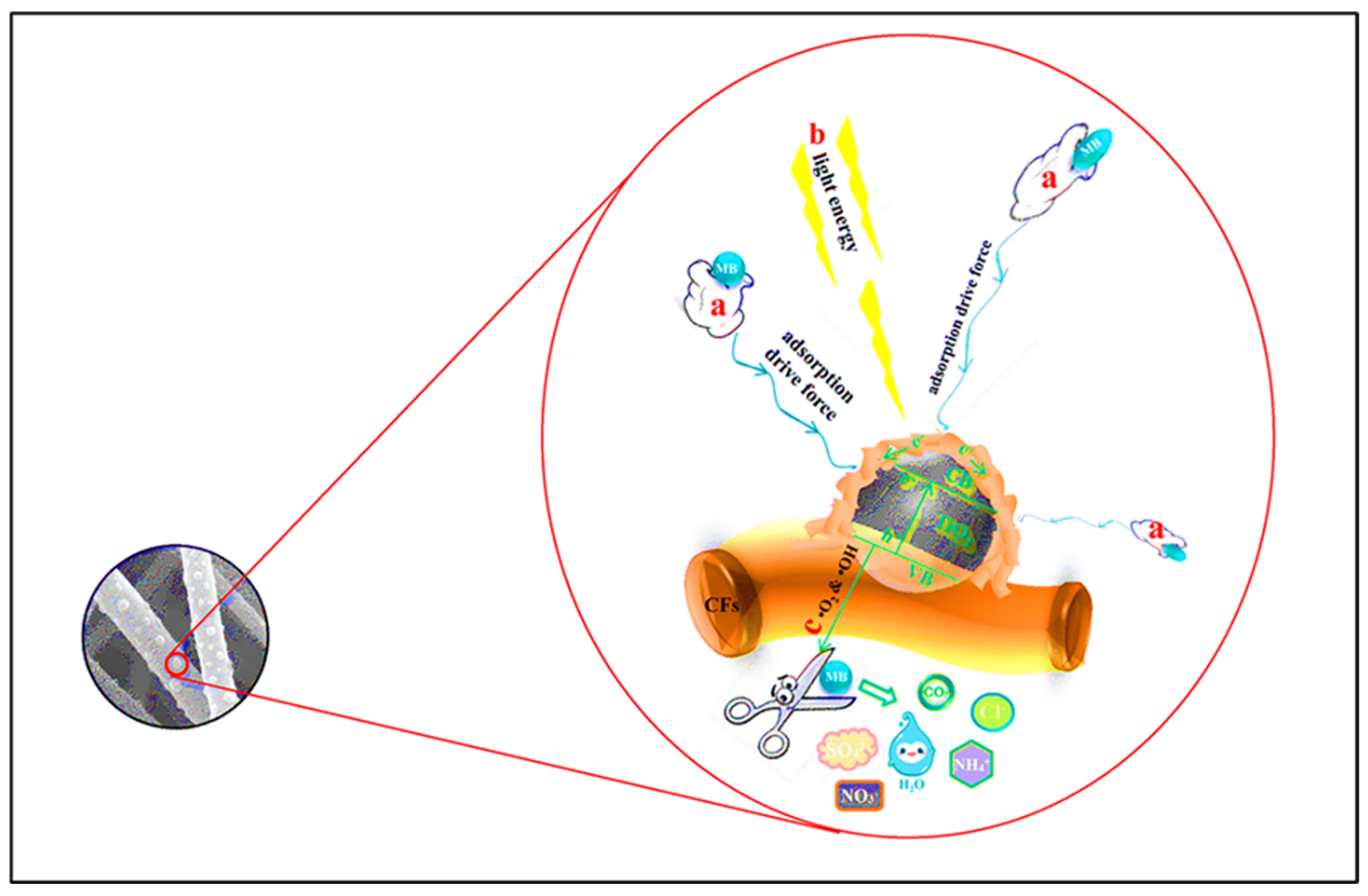




| Sr. No. | Treatment Methods | Description | Advantages | Limitations | Refs. |
|---|---|---|---|---|---|
| 1 | Photocatalysis | Utilizes light-activated catalysts. | Environmentally friendly, cost-effective, high energy saving, minimal secondary pollutants, and the easiest catalyst loading. | Difficulty in regeneration and recovery. | [29] |
| 2 | Biological | Uses microorganisms to degrade the material. | Cost-effective and excellent reduction of color. | Not applicable to highly concentrated organic waste, difficult to control, and has low efficiency in dye degradation. | [30] |
| 3 | Chemical precipitation | Use of various chemicals, such as lime or aluminum sulfate, to convert dissolved material into a solid substance. | Energy consumption is low and effective in removing organic halogens. | Excessive use of chemicals (such as lime, oxidants, and H2S) and the development of sludge. | [31] |
| 4 | Membrane filtration | Use of a semi-permeable membrane to eliminate the contaminants. | Rapid and eco-friendly method. | High operational cost and membrane fouling reduce efficiency. | [32] |
| 5 | Adsorption | Pollutants adhere to porous materials. | Simple and cost-effective. | The generation of secondary waste and adsorbent saturation requires regeneration. | [33] |
| 6 | Coagulation/flocculation | Use of coagulants such as alum, potash alum, and polyaluminum chloride for the removal of materials. | Simple and good for removing pollutants. | Requires a high dose of chemicals, the formation of massive sludge, and larger particles. | [34,35] |
| Photocatalysts | Synthesis Method | Band Gap | Efficiency | Ref. |
|---|---|---|---|---|
| GO/PAN/CQD | Hydrothermal process | 1.79 eV | 100% | [45] |
| CdS/CQDs/g-C3N4 | Calcination process | 2.68 eV | 86% | [69] |
| MWCNTs/TiO2 | Liquid phase deposition method | 2.8–2.95 eV | 90% | [70] |
| Ag/TiO2/CNT | Sonochemical method | 3.2 eV | 98% | [72] |
| Lignin-based carbon/cadmium sulfide composite | In situ precipitation method | 2.38 eV | 91.7% | [75] |
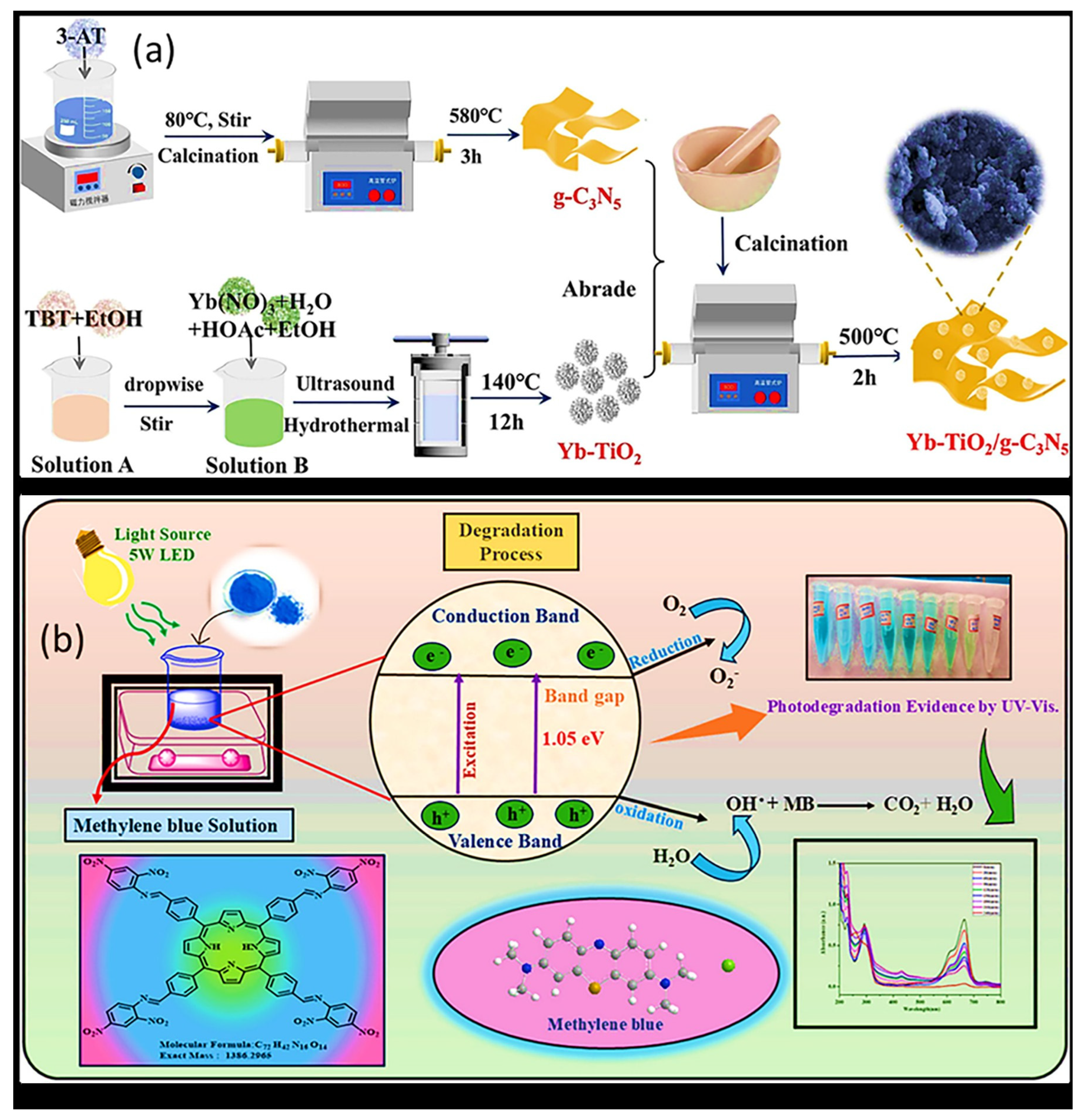
| Yb-TiO2/g-C3N5 | Hydrothermal process | 2.77 eV | 96.57% | [76] |
| PcDNPIMC | Adler–Longo method | - | 95.5% | [77] |
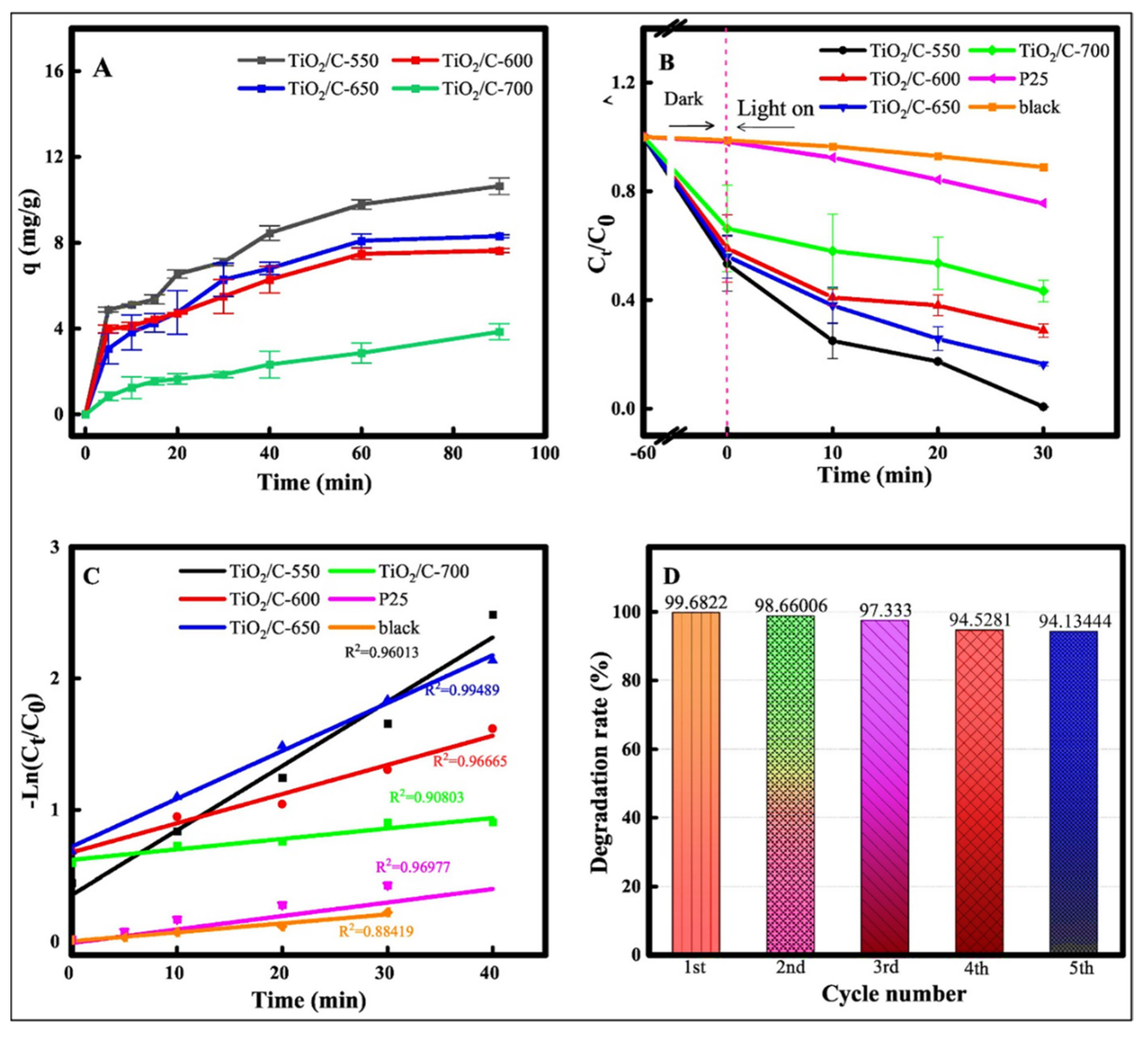
| TiO2/C-550 | Sol-gel method | 2.7 eV | 100% | [78] |
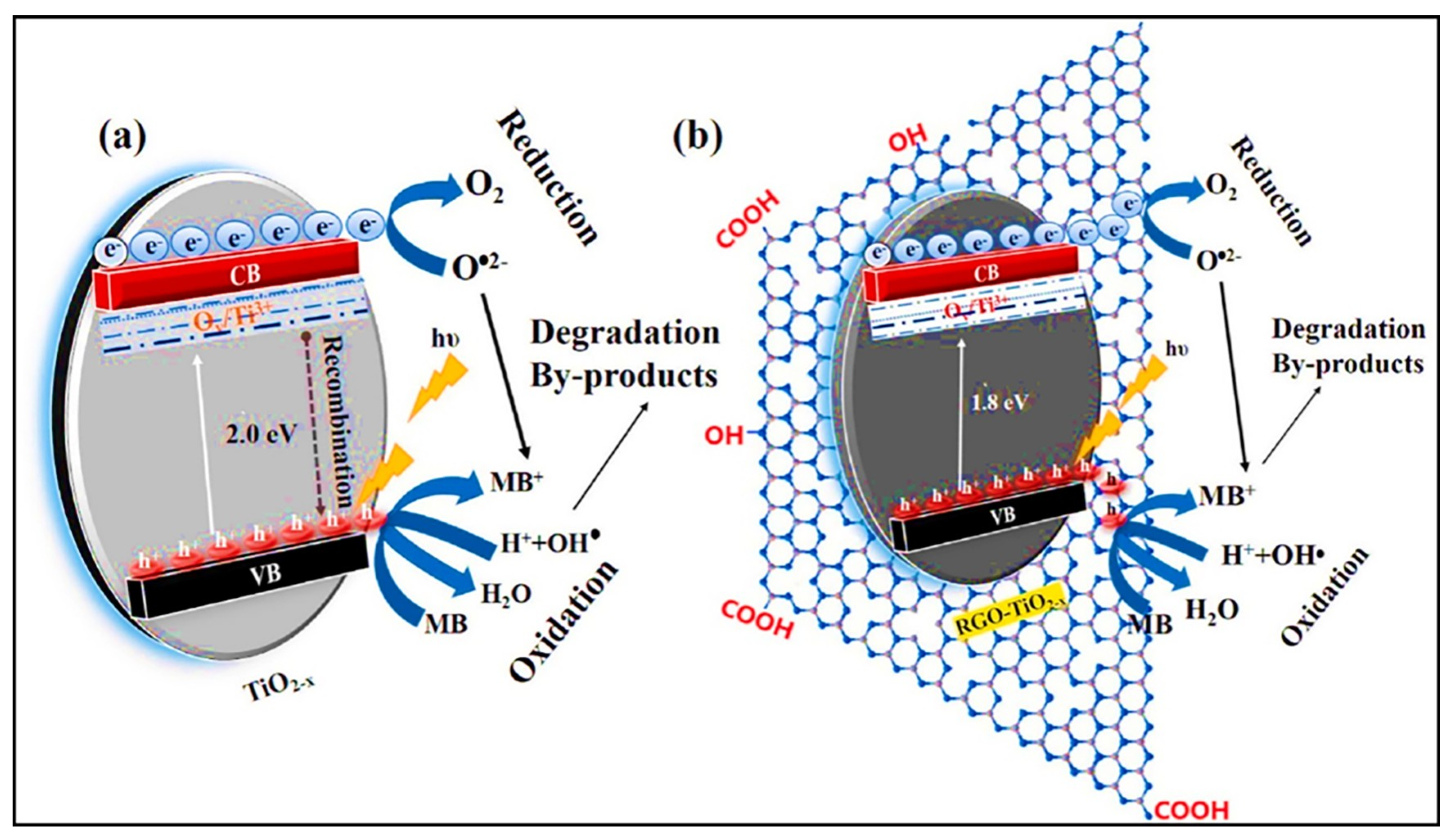
| RGO-TiO2−x | Hydrothermal process | 1.8 eV | - | [79] |
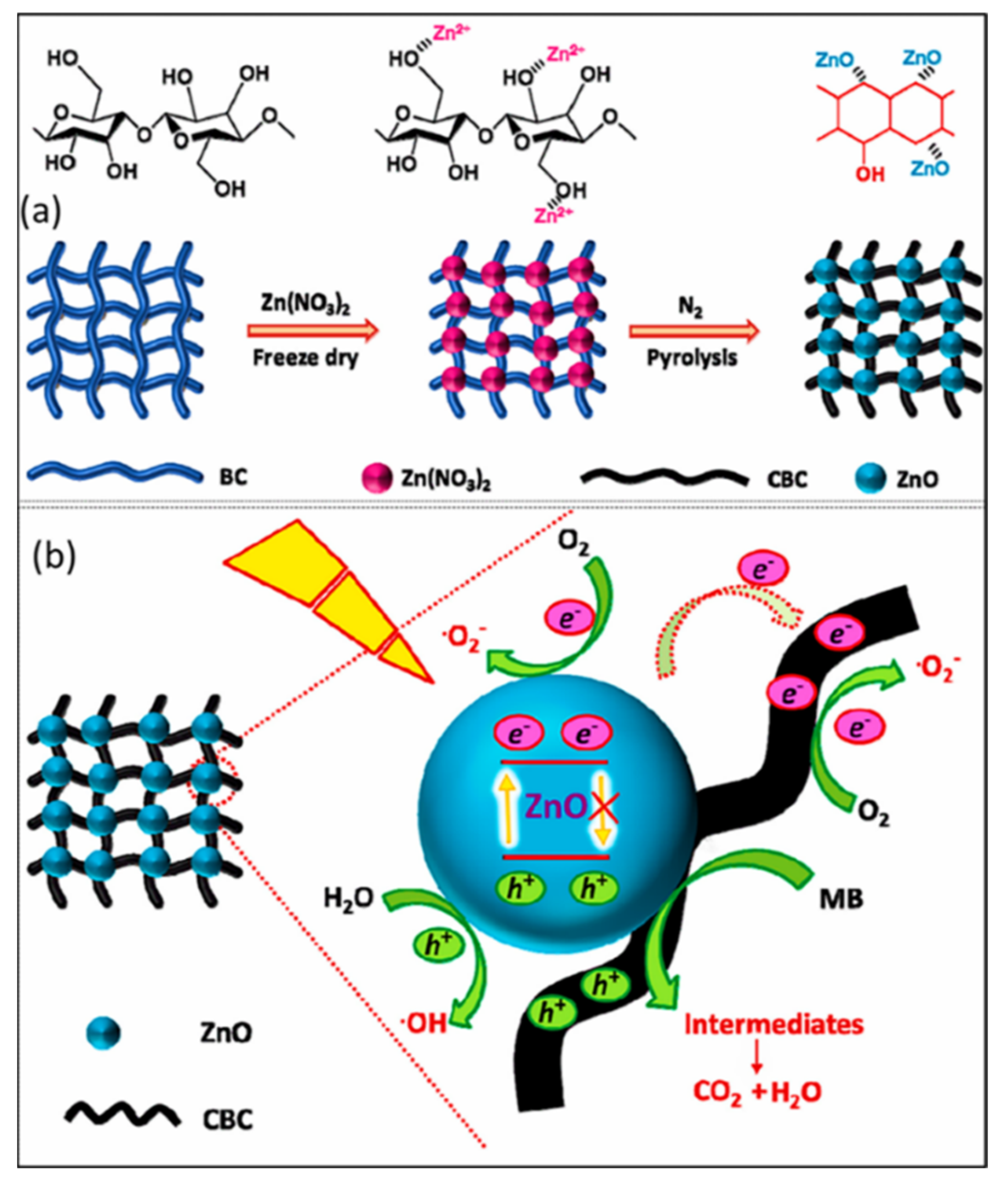
| ZnO@CBC | Impregnation–Pyrolysis method | ~3.20 eV | 99.6% | [80] |
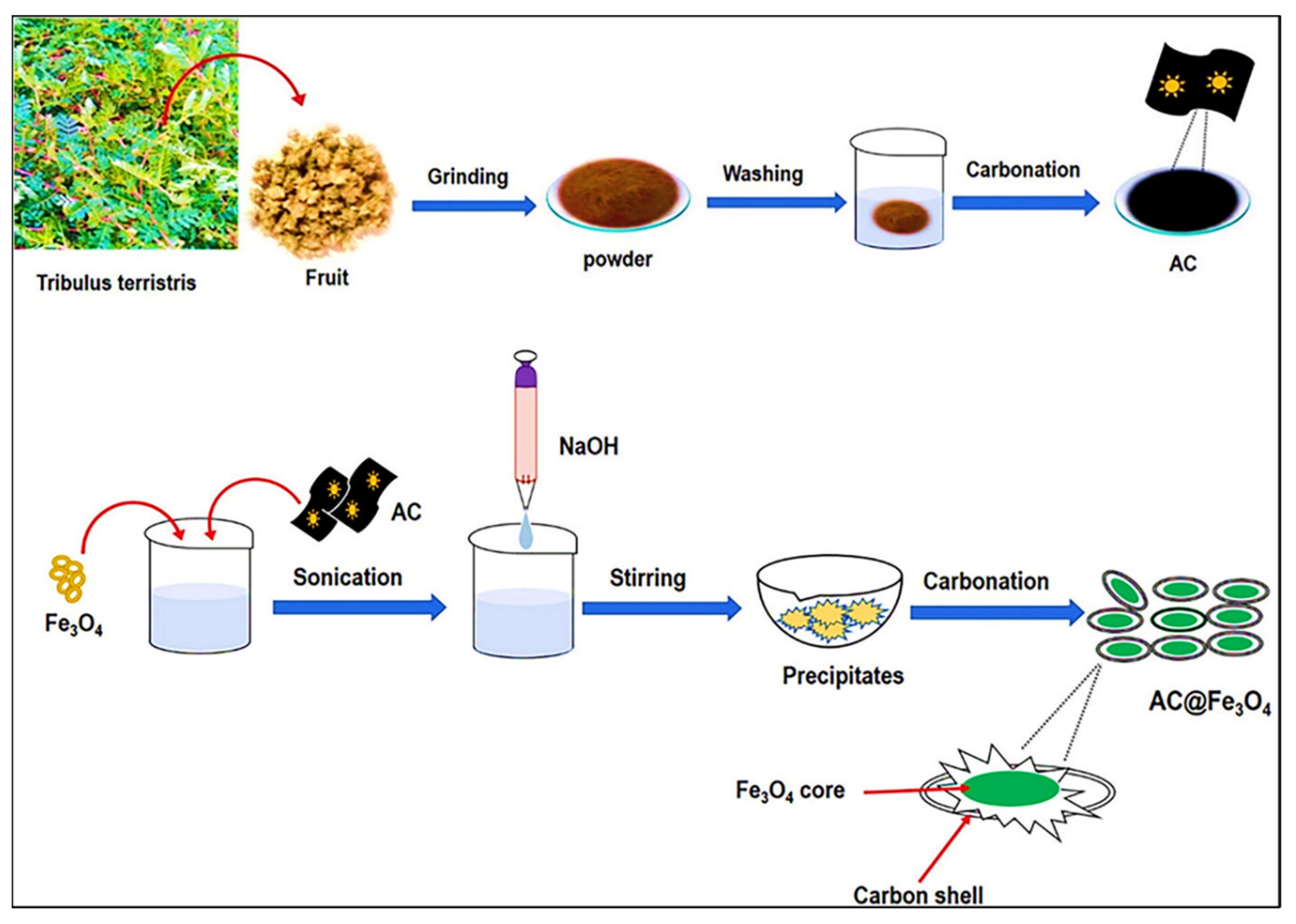
| AC@Fe3O4 | Calcination and coprecipitation | 94.6% | ~1.7 to 2.0 eV | [81] |
| ZnO/g-C3N4 | In situ synthesis | 97% | 3.02 to 2.94 eV | [82] |
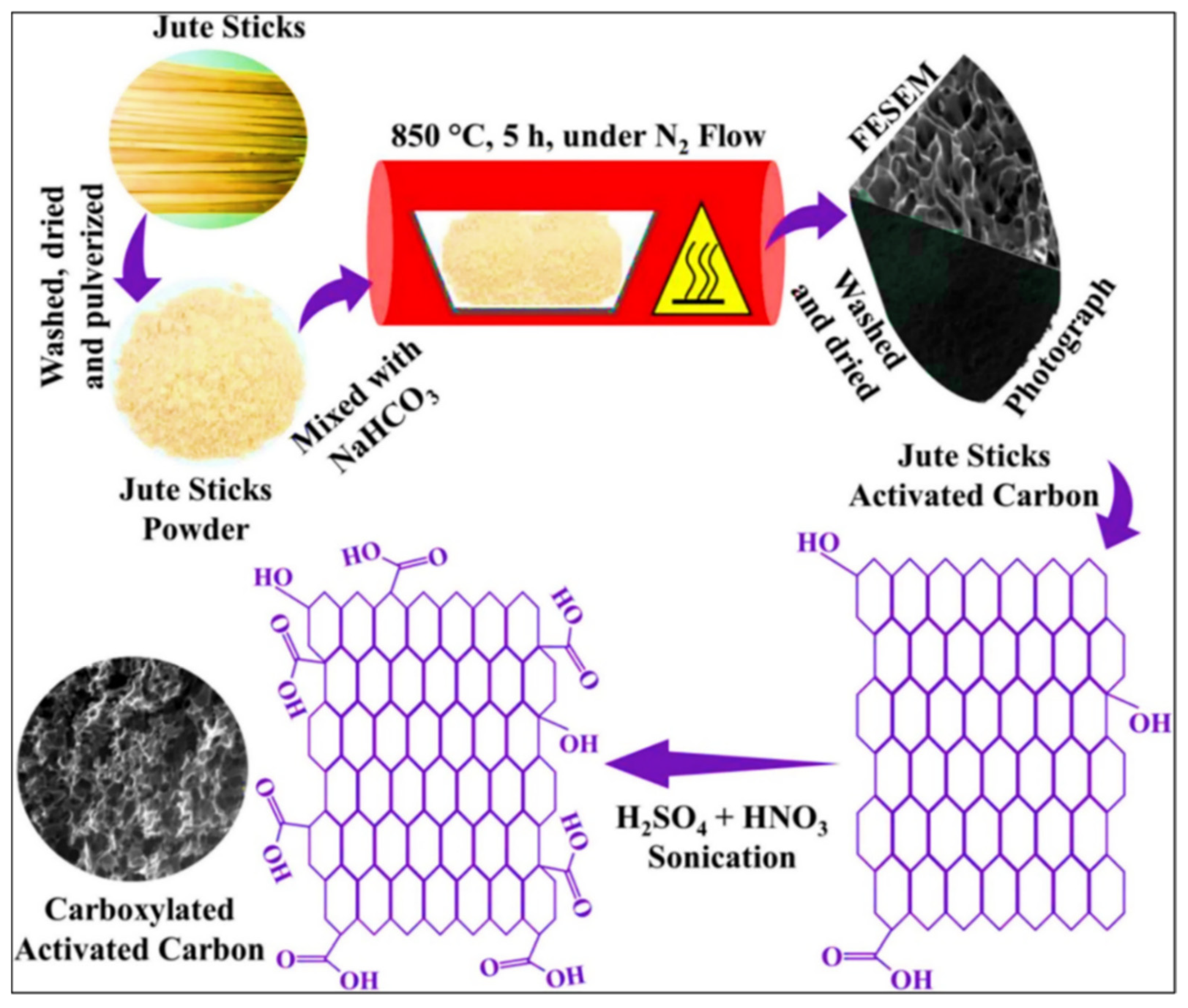
| ZnO/JSAC-COO– | Hydrothermal method | 97.56% | - | [83] |
| MnO2-ZnO-g-C3N4 | Sol-gel method | 94% | 2.0 to 2.5 eV | [84] |
| Ag2S/BSCN | (SILAR) method | 96.48% | ~2.1–2.3 eV | [85] |
| g-C3N4/GO/CuFe2O4 | In situ hydrothermal method | 99% | 2.31 eV | [86] |
| CA/ZnS-Ag | Precipitation method | 68.39% | 2.68 eV | [87] |
| S-scheme/gC3N4/TiO2/SiO2/PAN | Electrospinning, calcination, hydrothermal, and freeze-drying techniques | 99.43% | 2.71 eV | [88] |
| AC-ZrO2/CeO2 NCs | Co-precipitation | 97.91% | 2.2–3.0 eV | [89] |
| Cu-g-C3N4/BC | In situ pyrolysis | 32.7% | 2.06–2.24 eV | [90] |
| WS2/GO/Au | Hydrothermal and laser ablation | 99.00% | - | [91] |
| NiO-doped C3N4 | Ultrasonic method | 92% | 2.95 eV | [92] |
| g-C3N4/WS2 | One-pot hydrothermal method | 95.5% | ∼2.7 eV | [101] |
| NiO/Cd/g-C3N4 | Microwave-assisted | 81.8% | - | [102] |
| NiO/ZnO/g-C3N4 | Hydrothermal method | ∼95% | ~2.68 eV | [103] |
| GO/Fe3O4 | Chemical precipitation method | 98.68% | 1.96 eV | [93] |
| g-C3N4/ZnO | Hydrothermal method | 97.7% | 2.91 eV | [104] |
| Ni/Mo.S2/MOF-2@g-C3N4 | Solvothermal method | 91% | 1.75 eV | [94] |
| Eggshell-based activated carbon | Chemical activation method | 83% | - | [96] |
| TiO2/C | One-pot liquid phase reaction | 25.1% | - | [97] |
| g-C3N4@wood-derived carbon | Carbonization | 98% | - | [98] |
| Bio-CDs co-doped with S/Cl | Hydrothermal process | 94.2 | - | [105] |
| CDs co-doped with N/S | Hydrothermal process | 2.34 > 100% | - | [106] |
| N-CDs@ZnO | Wet-impregnation method | 99% | 2.97 eV | [99] |
| TiO2@CFs | Thermo-treatment after electrospinning | 94.54% | - | [100] |
| ZCF@MB-MIP | Chemical precipitation | 95.8% | 3.37 eV | [107] |
| 10%CCO/CeO2-nanocomposite | Co-precipitation method followed by calcination | 85% | 2.75 eV | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majeed, A.; Iqbal, M.A.; Do, T.-O. Advances in Composite Photocatalysts for Efficient Degradation of Organic Pollutants: Strategies, Challenges, and Future Perspectives. Catalysts 2025, 15, 893. https://doi.org/10.3390/catal15090893
Majeed A, Iqbal MA, Do T-O. Advances in Composite Photocatalysts for Efficient Degradation of Organic Pollutants: Strategies, Challenges, and Future Perspectives. Catalysts. 2025; 15(9):893. https://doi.org/10.3390/catal15090893
Chicago/Turabian StyleMajeed, Adnan, Muhammad Adnan Iqbal, and Trong-On Do. 2025. "Advances in Composite Photocatalysts for Efficient Degradation of Organic Pollutants: Strategies, Challenges, and Future Perspectives" Catalysts 15, no. 9: 893. https://doi.org/10.3390/catal15090893
APA StyleMajeed, A., Iqbal, M. A., & Do, T.-O. (2025). Advances in Composite Photocatalysts for Efficient Degradation of Organic Pollutants: Strategies, Challenges, and Future Perspectives. Catalysts, 15(9), 893. https://doi.org/10.3390/catal15090893








