Abstract
The growing accumulation of waste from diverse human activities has intensified the search for sustainable strategies. Mechanochemistry offers a promising pathway, transforming residues into high-value products with reduced energy demand, shorter reaction times, and minimal use of solvents and reagents. Various wastes—including biomass, food residues, fly ash, used batteries, and halogenated polymers—can be converted into environmental adsorbents, industrial biopolymers, biocompatible compounds, electrodes, and catalysts. Unlike previous reviews that addressed specific waste streams, this study provides the first systematic and comparative analysis of mechanochemical valorization across multiple residues, following PRISMA guidelines (2000–2025). A total of 656 studies indexed in Scopus and Web of Science were evaluated. This integrative approach highlights recent advances, current challenges, and future prospects, offering a rigorous and transparent guide for scaling mechanochemistry toward circular and sustainable solutions.
Keywords:
wastes; valorization; mechanochemistry; fly ash; used batteries; biomass; polymer; chitosan 1. Introduction
Mechanochemistry, the science of reactions induced by mechanical force, dates back to ancient times with the grinding of pigments [1]. However, it gained notoriety in the 19th century with M. Carey Lea’s research on photomechanical and mechanochemical reactions, connecting early manual processes with modern mechanochemistry [2]. Recently, it has experienced a resurgence as a modern and sustainable approach to chemical synthesis. Today, mechanochemistry has enabled new chemical processes to create valuable compounds for pharmaceuticals [3,4,5], polymers [6], biopolymers [7,8], and others functional products in many industrial sectors. In this context, mechanochemistry emerges as a solution to the challenge of reducing the dependence on organic solvents in chemical processes, which can be harmful and represent the majority of a process’s composition. Thus, its importance lies in: (i) promoting green chemistry by minimizing or eliminating solvents; (ii) using recyclable raw materials (such as waste), aligning with fundamental sustainability principles [9,10].
Chemical methods used to generate desired products include thermochemical, electrochemical, photochemical, and mechanochemical approaches. All these methods require an energy source to overcome the natural energy barriers involved in breaking chemical bonds, thereby enabling new molecular arrangements and bond formation. Mechanochemical methods, in particular, have attracted increasing interest due to their simplicity, operating under varied conditions such as low temperatures, minimal solvent use, or entirely solid-state systems in inert or reactive environments. The objective of mechanochemical methods is to convert reactants into products through mechanical energy, applied via physical force with tools like crucibles and mortars [9,10]. The friction between components allows the direct absorption of energy, creating favorable conditions to overcome activation energy barriers. This process depends on the contact interfaces of the reactants, as reactions occur at the surface, promoting wear, fracture, and microstructural refinement [11].
Although this technique has employed specific tools since ancient times, its modern evolution is remarkable, as the milling process can now be carried out using ball mills equipped with jars made of resistant materials such as polytetrafluoroethylene (Teflon©), polymethylmethacrylate (PMMA), zirconia, and tungsten carbide. Therefore, the process can be conducted in both open and enclosed systems, using mills that protect the reaction from environmental interference. It can also be monitored in real time when transparent jars are used as reaction vessels. Analytical techniques such as synchrotron XRD (X-ray diffractometers) and Raman spectroscopy facilitate real-time monitoring of reaction kinetics [12,13]. Moreover, mechanochemical methods can incorporate small amounts of liquid additives in the form of “Liquid-Assisted Grinding” (LAG), thereby expanding their applications in pharmaceutical and biological research.
Thanks to its ease, versatility, and solvent-free conditions, mechanochemistry competes effectively with standard synthetic methods, offering efficient, single-step reactions without the need for traditional heating or toxic reagents. Mechanically assisted methods are increasingly recognized for their ability to synthesize a wide array of products. Despite its long history, mechanochemistry’s most significant developments in synthesis have taken place primarily over the past two decades [9,10,14,15]. As shown in Figure 1, studies related to the application of mechanically assisted protocols in organic chemistry and the preparation of new materials have increased considerably, especially over the last 14 years.
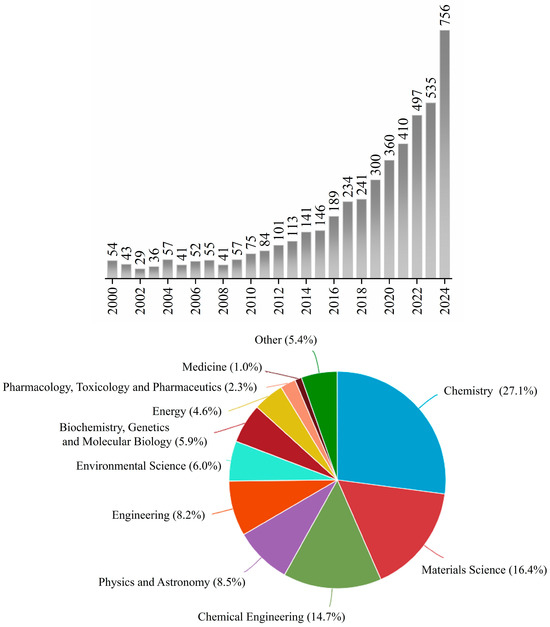
Figure 1.
The graphs show the evolution of research and thematic areas in mechanochemistry: the first presents the number of articles published annually on mechanochemistry, while the second is divided by thematic area of study on mechanochemistry by thematic area, in the period from 2000 to 2025 [16].
The applications of mechanochemical methods encompass catalytic processes such as Suzuki–Miyaura coupling [17,18], olefin metathesis, and C-H activation [12,19]. Other applications of mechanochemical reactions are in the synthesis of metal–organic materials such as MOFs and metal nanoparticles where the extensive use of solvents traditionally required to control particle size is avoided [20,21]. In general, mechanochemistry has found productive applications in the synthesis of porous materials [6]. These protocols have been used to prepare a wide range of products, as represented schematically in Figure 2.
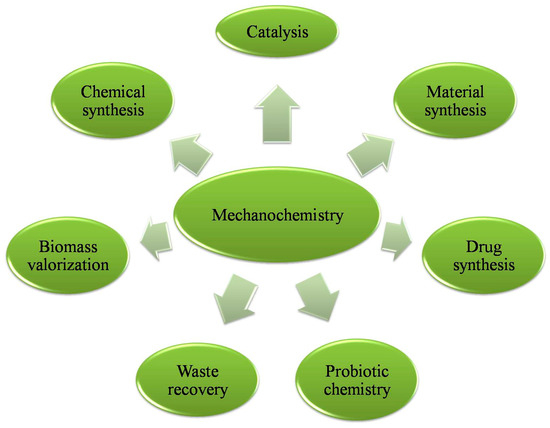
Figure 2.
Schematic representation of the mechanochemical protocols used in the synthesis of highly useful and commercially valuable products.
Significantly reducing the solvent makes mechanochemical methods both cost-effective and broadly applicable. This aligns with the principles of green chemistry and broader environmental protection goals. In this context, waste recovery has emerged as a prominent application. Waste often presents challenges for industrial production, as it can constitute up to 50% of the total output and requires conversion into functional products. At the same time, many synthesis processes require precursors with specific chemical compositions to obtain functional materials, derived from both organic and inorganic sources, such as zeolites, mesoporous silica, biopolymers, nanoflowers, and biochar. These functional products typically undergo physicochemical processes, including hydrothermal reactions, sol–gel synthesis, or thermal treatments. Such precursors can be derived from various waste sources (e.g., minerals, biomass, ashes, and rice husk) [22]. Recent studies have highlighted the central role of mechanochemistry in waste transformation. Indeed, mechanochemistry-assisted techniques can be used for the preparation of new materials or value-added products from various industrial, agro-industrial, and urban wastes [23,24,25,26].
Fly ash is an abundant byproduct generated by various industries, including coal combustion and energy generation from agricultural byproducts such as sugarcane bagasse. In many cases, the incomplete carbonization of these materials results in fly ashes with a high content of unburned carbon [27]. Mechanochemical methods, such as high-energy milling and chemical activation, can be used to upcycle this type of waste. This approach has been applied in several studies, including enhancing adsorption characteristics for the effective removal of pollutants, such as dyes, from aqueous solutions. Furthermore, mechanochemically modified fly ash has shown potential in various applications, ranging from the stabilization of hazardous waste and contaminated soil to the synthesis of sustainable geopolymers and hydraulic cement. These innovations represent significant opportunities for the environmentally friendly utilization of fly ash and the valorization of this commonly overlooked industrial residue through mechanochemical methods [28,29].
The valorization of lignin residues has become an area of great interest, as lignin is one of the primary components of biomass and holds significant potential for the production of high-value chemicals and materials. Various mechanochemical methods have been explored to break lignin bonds and transform it into useful products. Some of these methods include the use of high-energy mills and readily available reagents, avoiding the need for expensive transition metals or solvents. Furthermore, the utilization of mechanochemical approaches has proven effective in producing a variety of products such as monomers, energy storage materials, and bioplastics. Research in this field continues to advance, and it is expected that mechanochemical strategies will play a crucial role in the sustainable valorization of lignin residues, thus contributing to the transition to a greener and more circular economy [30,31,32,33].
Plastic disposal, on both micro- and macro-scales, is a long-term environmental challenge. Sustainable practices are needed to reduce plastic waste, as global plastic production, reaching megatons annually, is heavily used in packaging (40%), construction (20%), and textiles (13%). In 2015, it was estimated that polyolefins, PVC (Polyvinyl Chloride), and PET (Polyethylene Terephthalate) comprised 79% of global plastic production, generating 69% of plastic waste. Besides the use in bottles, PET is included in the production of polyester fibers which has a production of 57 million tons by year that are discarded in landfills or incinerated [34,35]. Mechanochemical depolymerization offers a promising route for reducing plastic waste [36]. Applications include recycling automotive shredder residues through solid-state mechanochemical techniques [37], using mechanoenzymatic methods for polymerization and depolymerization [38], and filling PVC structures with eggshell additives, as described by Skórczewska [39]. These approaches could transform polymer production and waste management, reducing environmental pollution.
Animal-derived feedstocks provide an eco-friendly source for producing functional products using mechanochemistry. Chitin, a polysaccharide of D-glucosamine derived from crustacean shells, is commonly transformed into chitosan, which has wide applications in papermaking, food, bioengineering, and agriculture [40]. Chitosan, synthesized by the deacetylation of chitin, is a biopolymer with high water solubility, biodegradability, and biocompatibility which makes it useful for the production of sustainable derivatives. In textiles, chitosan offers antimicrobial, antiviral, and anti-odor properties [41]. Mechanochemical milling of chitin from European green crabs yields eco-friendly biomaterials, replacing harmful inorganic acids with organic solid acids [33]. Mechanochemical and mechanoenzymatic methods are also applied to produce oligomers of N-acetyl-D-glucosamine and in fine chemistry for the production of 5-hydroxymethylfurfural (5-HMF) [42,43,44]. Thus, it is clear that mechanochemistry has wide applications and has become globally impactful, with research groups advancing its applications in various fields, such as catalyst preparation, depolymerization, geopolymerization, and the synthesis of high-value molecules from agro-industrial and urban biomass, fly ash, and other wastes [4,45,46,47,48,49,50,51,52,53].
Therefore, this systematic review aims to present the current landscape of waste valorization through ecologically sustainable mechanochemical processes, producing high-value functional products with diverse applications. The next section will describe mechanochemically assisted protocols for transforming low-cost, waste-based raw materials into value-added products.
2. Methods
This section describes the research and study selection process. It can be divided into subheadings to detail the search strategy, inclusion and exclusion criteria, and the data extraction and synthesis method.
2.1. Search Strategy
This systematic review was prepared in accordance with the recommendations described in the Preferred Reporting Items for Systematic Review 2020 (PRISMA 2020) methodology [54]. The literature was retrieved from Scopus and Web of Science to search for citations between 15 March 2025 and 30 April 2025 in the Scopus and Web of Science databases, using the keywords “mechanochemistry AND waste OR valorization OR reject OR processing OR waste recovery”, for studies published in the period from 2000 to 2025, limiting only research articles to the areas of chemistry, chemical engineering, materials engineering, materials science, and environmental science. In addition, the inclusion of additional literature was necessary, added from the Capes Journals platform.
2.2. Inclusion and Exclusion Criteria
All mechanochemistry articles that were related to the valorization or beneficiation of waste or rejects were included. The exclusion criteria were articles that did not address mechanochemistry, or mechanochemistry articles unrelated to waste or rejects, duplicate articles, review articles, and studies with insufficient data or low quality.
2.3. Study Selection
The selection of studies was carried out independently by two authors, using the Rayyan selection platform [55]. Initially, the articles were selected after reading the title and abstract, and those that met the eligibility criteria and that had consensus between the two authors were read in full for inclusion or exclusion in the review. Disagreements in the full reading phase were resolved by consensus by a third author [56].
3. Results
Initially, searching with keywords identified 656 potential articles: 336 from the Scopus database and 320 from the Web of Science database (Figure 3). Of the initial 656 articles, 25 were excluded for being duplicates files. The remaining 631 files were evaluated by title, abstract, and keywords, with 203 articles excluded for not being suitable for the scope of the study. There remaining 428 articles were selected for full-text evaluation, 31 of which were not retrieved, 62 were excluded because they were not related to mechanochemistry, 82 articles were not related to mechanochemistry applied to waste or rejects, and another 103 were reviewed articles. Therefore, 150 articles were selected from the Scopus and Web of Science databases and included in the review.
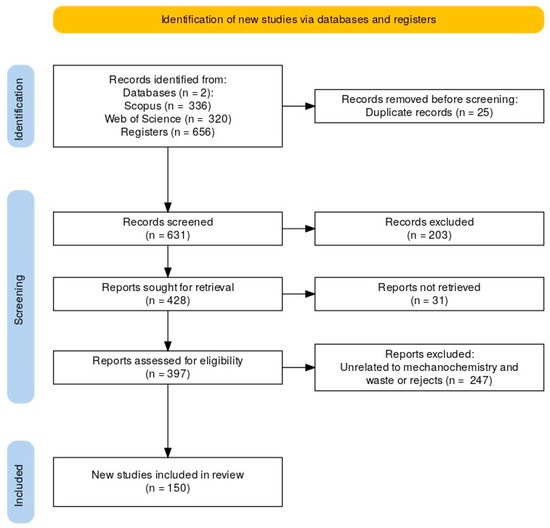
Figure 3.
This PRISMA flowchart illustrates the study selection process, specifying inclusion and exclusion criteria, from initial identification to final inclusion, showing the number of articles at each stagemay improve clarity.
3.1. Mechanochemistry: A Tool to Enhance Waste Valorization into Valuable Products
Mechanochemistry has proven itself as an established method across various research fields and has seen growing interest in recent years. This method contributes significantly to advancements in the utilization of waste in materials science, with a particular focus on catalysis, pretreatment of biomass, and preparation of active materials. Additionally, waste treatment has emerged as a compelling area for the mechanochemical community, with numerous studies employing mechanochemical techniques for waste processing [4,9,10,51,52,57]. In the following subsections, updates will be provided on publications that explore the reuse of various waste materials using a mechanochemical approach for the synthesis of catalytic materials, ceramics, polymers, valuable chemicals, and the degradation of halogenated organic pollutants.
These studies focus on both the synthesis and characterization of materials or products, aiming not only for the economic benefits derived from waste reuse but also for the significant environmental advantages it offers.
3.2. Fly Ashes Waste
Fly ash (FA) is a significant byproduct resulting from the combustion of coal, biomass, or municipal solid waste in power plants and industrial boilers, including those fueled by agricultural residues such as sugarcane bagasse [27]. It is typically defined as a fine, powdery residue composed of inorganic particles expelled from combustion chambers during high-temperature burning (see Figure 4). Based on the source material, fly ash can be classified into three main types: coal fly ash (CFA), biomass fly ash (BFA), and municipal solid waste fly ash (MSWFA) [48,49]. These categories represent the principal residues generated from both fossil fuel and renewable energy combustion processes. From an environmental perspective, fly ash presents considerable concern due to the presence of hazardous contaminants, including polycyclic aromatic hydrocarbons (PAHs), heavy metals, and dioxins [57]. Despite this, its unique physical and chemical properties render it a valuable raw material for environmental applications. Coal fly ash, in particular, has a complex composition, comprising a mixture of aluminosilicates, iron oxides, titanium dioxide, unburned carbon, and various inorganic oxides. Its morphological features, such as particle size distribution, surface area, and pore structure, vary significantly depending on its origin and combustion conditions. Additionally, it contains transition metals (often as oxides) and a range of functional groups that contribute to its reactivity. These properties make fly ash an attractive candidate for the synthesis of adsorbent materials, offering a low-cost and sustainable solution for pollutant removal from aqueous environments. Mechanochemical treatments have been shown to enhance these properties, improving its adsorption capacity and expanding its potential for use in environmental remediation strategies [58].
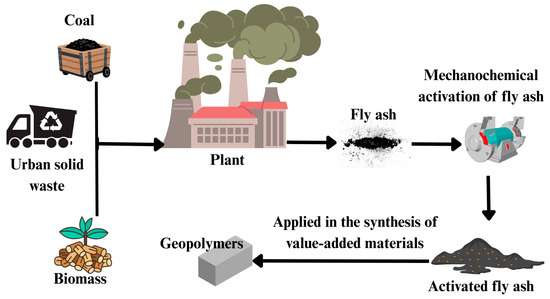
Figure 4.
The graphical diagram shows the process of incinerating coal, municipal solid waste, and biomass in thermoelectric plants. This process produces fly ash as a byproduct. Fly ash can undergo mechanochemical activation to produce active fly ash, which, in turn, is used in the production of highly useful and value-added functional materials.
Mechanochemical techniques have been explored as an effective approach to improve the properties of fly ash for pollutant removal. According to Geng et al. [58], mechanochemical activation—achieved by grinding solid reagents together, with or without solvents—induces surface defects and increases reactivity through frictional and shear forces. In their study, the authors utilized residual coal fly ash from power plants to produce low-cost adsorbents for mercury removal from wastewater. Mechanochemical bromination, performed using a planetary omnidirectional ball mill, was employed to modify the surface of the fly ash. This technique proved to be a cost-effective and efficient alternative to conventional solution-based functionalization, owing to the effective incorporation of bromine onto the adsorbent surface. The improved adsorption capacity was attributed to the formation of carbon–bromine (C–Br) bonds in carbon-rich domains and iron–bromine (Fe–Br) bonds in Fe2O3-containing phases. Based on Fourier-transform infrared spectroscopy (FTIR) and X-ray Photoelectron Spectroscopy (XPS) analyses, the proposed mechanism involved the generation of unsaturated carbon structures within the activated carbon matrix, which subsequently reacted with bromine radicals to form stable C–Br bonds. Overall, the study provides valuable insight into the mechanisms underlying the enhanced mercury adsorption performance of fly ash modified via mechanochemical bromination, reinforcing its potential for cost-effective wastewater treatment applications.
Chumpiboon et al. [27] reported that the use of mechanochemical methods can increase the adsorption capacity of fly ash, such as modifying fly ash through high-energy milling and chemical treatments with acidic and alkaline solutions. In this study, the authors investigated the treatment of sugarcane bagasse fly ash (BFA) with various acids and simultaneous carbonization under a nitrogen atmosphere to remove a cationic dye, methylene blue (MB), from aqueous solutions. This treatment significantly improved both the surface area and adsorption performance of the material. The resulting modified material—referred to as Treated BFA—achieved an MB adsorption capacity of 39.0 mg/g in 90 min, closely approaching that of commercial activated carbon (CAC), which showed a capacity of 42.1 mg/g but with a much larger surface area (1130 m2/g). Kinetic studies revealed that the adsorption process followed pseudo-second-order and intra-particle diffusion models, while equilibrium data were best fitted by the Langmuir isotherm model, with maximum MB adsorption capacities of 27.2 mg/g for untreated BFA, 39.0 mg/g for Treated BFA, and 42.1 mg/g for CAC. A comparative analysis demonstrated the high performance of the modified material relative to the commercial standard. These results highlight that, beyond surface area, surface functional groups and electrostatic interactions also play a critical role in dye adsorption efficiency.
Any material containing silica and alumina phases—whether primary (e.g., kaolinite, illite) [59] or secondary (e.g., fly ash, steel slag, red mud) [60]—is suitable for the production of geopolymers. Among these, fly ash stands out as a promising precursor for geopolymer synthesis through mechanochemical activation. Geopolymers are synthetic aluminosilicate binders formed by the reaction of silicon (Si) and aluminum (Al) oxides under alkaline conditions, resulting in an amorphous framework composed of interconnected SiO4 and AlO4 tetrahedra. Fly ash typically contains these oxides in both crystalline (e.g., mullite, quartz) and reactive amorphous forms. The effectiveness of geopolymerization depends largely on the reactivity of the aluminosilicate phases, which is influenced by factors such as the reactive content of SiO2 and Al2O3, and the particle fineness of the raw material. Insufficient reactivity leads to slower setting and poor mechanical strength in the final geopolymer [28,61]. Mechanochemical activation via high-energy milling plays a key role in enhancing this reactivity by increasing the specific surface area, generating structural defects, and inducing partial amorphization, which facilitates the dissolution of reactive species during alkali activation [29]. These effects have been shown to improve the mechanical performance of the resulting materials. In particular, the incorporation of mechanochemically activated fly ash into cementitious or geopolymeric matrices has led to significant increases in compressive strength, positioning this strategy as a viable route for both waste valorization and the development of sustainable construction materials [28].
Oyun-Erdene and Temuujin [29], investigated the effect of mechanochemical activation of fluidized bed coal fly ash (CFA) on the mechanical performance of geopolymers. In this study, geopolymer samples were prepared from both raw and mechanically activated CFA using alkaline solutions composed of 10 mol/L sodium hydroxide (NaOH) and mixtures of sodium silicate (Na2SiO3) and sodium hydroxide at a 2:1 weight ratio. After 30 min of vibratory milling, the Brunauer–Emmett–Teller (BET) method surface area of CFA increased from 4.8 m2/g to 9.2 m2/g, attributed to particle size reduction and the amorphization of crystalline phases, as confirmed by X-ray Diffraction (XRD), Scanning Electron Microscopy (SEM), and FTIR analyses. The incorporation of sodium silicate into the alkaline solution increased the compressive strength of raw CFA-based geopolymers from 6.03 MPa to 16.23 MPa, while the mechanically activated CFA-based geopolymers exhibited an increase from 27.6 MPa to 32.4 MPa. In addition, geopolymers derived from mechanically treated CFA showed a denser and more homogeneous microstructure. These results demonstrate that mechanochemical activation is an effective strategy to enhance the reactivity of fluidized bed fly ash, thereby improving the geopolymerization process and the resulting mechanical properties of the final material.
In another study, Matsuoka et al. [62] studied the effect of mechanochemical activation on the surface of coal fly ash particles. The article reports that mechanochemical activation modifies the surface morphology and the crystalline phase of the fly ash particles using a friction-type mill (Figure 5A,B). Consequently, an increased dissolution of Si (IV) and Al (III) species in 10 mol/L NaOH for 3 h solution is observed (Figure 5C,D). This increase results in an increased reactivity of the mechanochemical-activated CFA allowing the use of milder geopolymerization conditions (28 days at 70 °C, 7 days at 70 °C, and 28 days at room temperature). Mechanochemical activation of CFA results in improved compressive strength (45.2 MPa for 30 min of treatment after 28 days at 70 °C) of the corresponding geopolymer, along with an increased acid resistance by densification of the hardened body (Figure 5E). The compressive strength of geopolymers after an acid resistance test is shown in Figure 5F. Thus, with these results, it is clearly demonstrated that mechanochemical activation is effective for producing geopolymers with beneficial mechanical properties under milder curing conditions.
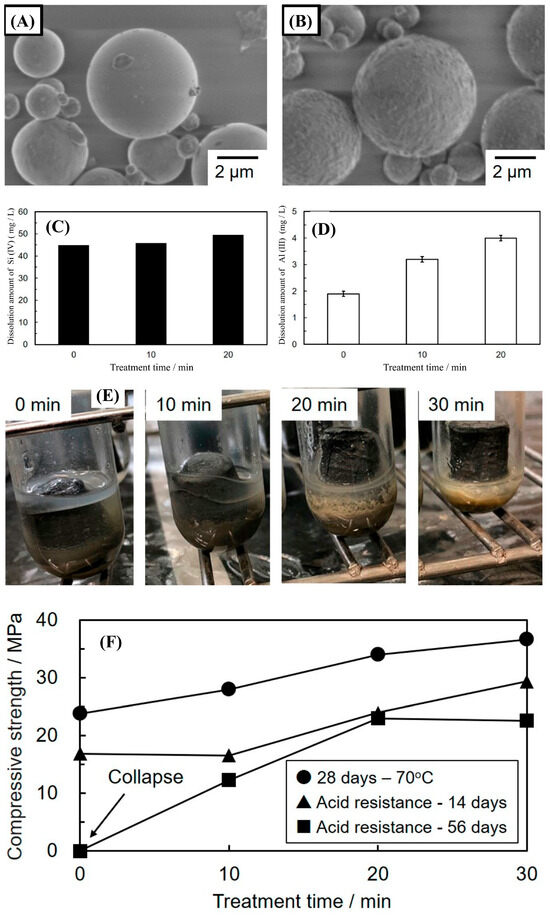
Figure 5.
SEM images of coal fly ash (A) before, (B) after mechanical treatment, Dissolution amount of (C) Si (IV) and (D) Al (III) ions from coal fly ash, (E) appearance of geopolymers after 56 days of the acid resistance test: room temperature curing for 28 days. From left to right, mechanical treatment times are 0, 10, 20, and 30 min, respectively, and (F) Compressive strength of geopolymers (28 days, 70 °C cured) from mechanically treated coal fly ash after acid resistant test (14 and 56 days). Reprinted with permission from Matsuoka et al. [62]. Copyright 2019 MDPI and under the Creative Commons CC BY license.
In another subsequent study, Kalinkin and collaborators [15] examined the synthesis of geopolymers at room temperature using mechanically activated fly ash (FA) milled for 30 s, 180 s, and 400 s, combined with varying contents of natural calcite (0–10 wt%) (Calcium carbonate, CaCO3) and activated with an 8.3 mol/L sodium hydroxide solution. The incorporation of 10 wt% calcite into 90 wt% FA (denoted as GFA10C) resulted in compressive strength values that were 8.0, 3.5, and 2.9 times higher, respectively, than those of the calcite-free geopolymer (GFA0C) for the same milling times, particularly after 7 days of curing (Figure 6A,B). According to specific surface area data (Figure 6C), milling for 180 s led to a substantial strength improvement compared to 30 s, whereas the additional milling from 180 to 400 s yielded only marginal or even negative effects. Furthermore, the main calcite diffraction peak, observed at 2θ ≈ 29.4° in the XRD pattern of GFA10C, showed a slight decrease in intensity relative to the unreacted FA10C blend (Figure 6D), suggesting partial interaction between calcite and the geopolymeric matrix.
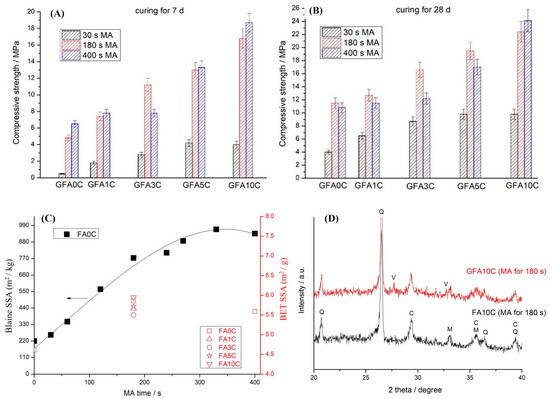
Figure 6.
(A) Effect of calcite content and MA time on the 7-day compressive strength of geopolymers. (B) Effect of calcite content and MA time on the 28-day compressive strength of geopolymers. (C) The X-ray diffraction patterns of the blend containing 10% CaCO3 (FA10C) milled for 180 s and of the geopolymer prepared using this blend after 28 d of curing (GFA10C). Phases marked: Q—quartz; M—mullite; C—calcite; V—vaterite. (D) Blaine-specific surface area (SSA) of the fly ash (black symbols) and Brunauer–Emmett–Teller (BET) SSA of the FA + CaCO3 blends milled for 180 s (red open symbols) vs. mechanochemical activation (MA) time. One Reprinted with permission from Kalinkin et al. [15]. Copyright 2020 MDPI and Adapted from Kalinkin et al. (2020) under the Creative Commons CC BY license.
Research in this field has focused on synthesizing geopolymers from waste and by-products, with fly ash standing out due to its aluminosilicate composition, flow ability, and large waste volume. Geopolymer samples synthesized at a temperature of 27 °C using fly ash mechanically activated for different periods (60–90 min) in an eccentric vibrating mill (EVM) were reported by Kumar et al. [28]. The study also analyzed the effect of MA on the geopolymerization reaction using an isothermal conduction calorimeter. FTIR analysis justified the formation of the new band at 1080–1096 cm−1 in MA samples which is related to the highly polymerized sheet structure corresponding to SiQn (n = 3–4) which is related to the restructuring of quartz, also corroborated by the results from QXRD (Quantitative X-ray Diffraction).
Nikolić et al. [63] investigated the effectiveness of geopolymers derived from FA (initial FA) or mechanically activated FA in lead immobilization. To simulate the ability to use these FAs to trap and immobilize toxic Pb2+ ions during the geopolymerization process, they were mixed, at room temperature, with the geopolymerizing agent (alkali solution) in the presence of Pb(NO3)2. The results of the studies indicated that geopolymers based on mechanically activated FA were more effective in immobilizing lead compared to geopolymers based on initial FA, showing a reduction in the leaching of Pb2+ present in the geopolymers. The greater effectiveness of Pb2+ immobilization was attributed to the decrease in the pore sizes (from 18.8 to 6.9 nm) of the geopolymers based on mechanically activated FA compared to those based on the initial FA. In addition, the mechanically activated FA led to a geopolymer with a significantly increased compressive strength (58.46 N/mm2, with 1% Pb after 28 days of aging). The study reports that mechanochemical activation of FA can replace thermal activation which is of great importance for practical applications of geopolymers.
Regarding mechanical treatment and the presence of heavy metals in FA, studies have focused on the mechanochemical stabilization of Pb. In this sense, Li et al. [64] associated the Pb stabilization mechanism in ground fly ash with the entrapment of Pb in a process of particle re-agglomeration. Interestingly the authors reported a counterintuitive trend of the particle size vs. the mechanochemical process time. Indeed, the size decreases at low treating times while increasing again due to a re-agglomeration process occurring at higher times, to arrive at the size of the starting non-treated particles. In terms of leaching, the results show that 93.11% of Pb was partitioned into the ground ash, and the leaching of Pb was inhibited after short-term grinding (from 5.2 to 1.2 mg/L after 1 h of grinding) and further reduced by around 96% after 96 h of ball milling. This can be attributed to the formation of water-insoluble species, upon interaction with the higher surface energy the freshly ground particles exhibited. Nomura et al. [65] also reported that through mechanochemical treatment of FA, the Pb leaching was reduced by 92.8% due to the formation of water-insoluble species. Among these, is the formation of Pb3O4. Chen et al. [25] also noted that mechanochemical treatment of FA decreases the concentration of leached Pb, Cr, and Cu, while water prewashing effectively increases stabilization efficiency through the removal of soluble salts.
In another study reported by Li et al. [66] red mud (RM) was used as an additive for the stabilization of heavy metals in mechanochemical-treated FA. The results of this study demonstrated that the addition of RM under the optimized conditions of 30% by weight of red mud, 0.1 g/L solid–liquid ratio, and 24 h ball milling time significantly decreased the leaching of Pb, Cr and Zn from FA, in 99%, 81.18% and 100%, respectively. Even in this case, a re-agglomeration phenomenon was reported: FA was broken into small particles and then agglomerated into larger particles after mechanochemical treatment. It was evident that the main reasons responsible for the entrapment of heavy metals were attributed to precipitation and sealing by fine particles, captured by new phases which RM contributed to form.
On the other hand, Li et al. [67] investigate the adsorption properties of methylene blue (MB) on mechanochemically modified FA. The results showed that the modification of the grinding process increased the MB adsorption capacity of FA, with the adsorption capacity value increasing from 5.06 to 7.97 mg/g for raw FA and ground FA, respectively. Kinetic modeling revealed that the adsorption follows a pseudo-second-order reaction rate. Furthermore, the authors mentioned that some assessment of environmental pollution caused by FA should be carried out before application.
Modification of fly ash was carried out by dry grinding in a planetary ball mill was reported by Sundum et al. [68] and studied its efficiency in preparing thermoplastic starch composites (TPS) containing different amounts of unmodified (UFA) or modified (MFA) fly ash powder. It was found that the UFA particle size decreased from 59.60 μm to 13.17 μm while the surface area increased from 3.68 m2/g (UFA) to 4.44 m2/g (MFA), after grinding for 1 h at 400 rpm. Both UFA and MFA powders were incorporated into thermoplastic starch. The high maximum tensile strength (7.78 MPa) was obtained in a composite with 2.50% MFA, which is about 9 times higher than TPS (0.86 MPa) and 2 times higher than the TPS/UFA composite. According to this study, composites with MFA showed better water resistance and delayed degradation compared to TPS and composites with UFA. While the presence of UFA or MFA had a slight effect on the thermal stability of the samples, it promoted the final phase of the thermal decomposition. This property can be exploited for their thermal degradation as waste.
Resuming, the use of mechanochemically treated FA in geopolymers presents several advantages, including transforming waste into a value-added material, improving the mechanical properties of geopolymers, and reducing environmental impact. These and the other results presented in this section are schematically resumed in Table 1.

Table 1.
General summary of studies on mechanochemical activation of fly ash and its applications.
3.3. Biomass
In recent years, concerns about the severity of environmental problems and the growing energy shortage have led to the exploration of renewable resources as a viable alternative. Biomass is the most abundant renewable source globally, with approximately 130 billion tons produced annually. Lignocellulosic biomass, derived from agricultural residues, bioenergy crops, and biomass waste, is composed of three biopolymers: cellulose (38–50%), hemicellulose (20–35%), and lignin (15–25%), varying by species and biomass sources [31,33]. While cellulose and hemicellulose are primarily exploited for conversion into various products such as pentoses, xyloligosaccharides, and liquid fuels, lignin can be employed in the production of aromatic compounds. However, the recalcitrance of biomass and the complexity of its structures pose significant challenges in the efficient separation and utilization of its components [30,31,33,73,74,75,76].
The pre-treatment to isolate lignin from other components is a crucial step in biorefinery operations, presenting a challenge to achieve maximum production efficiency. Various technologies of mechanochemical and biological pre-treatment have been developed for the effective separation of this biomass. Among them, mechanochemical activation is a sustainable approach compared to techniques involving toxic solvents during pre-treatment. This technique disrupts the biomass structure, reducing cellulose crystallinity and enhancing reactive activity [77].
Mechanochemical methods applied in lignin depolymerization, enable the breaking of bonds and the production of economically valuable aromatic compounds. This is because mechanochemistry can fasten reactions and create high-energy microenvironments through localized pressures and friction-induced heating [30,33]. According to Scimmi et al. [31], lignin depolymerization to recover fine chemicals involves hydrogenolysis and oxidation methods, with the latter being more commonly used due to milder conditions and cleaner final product production. Traditional oxidation processes using chlorine and nitrate are considered environmentally unfriendly, posing environmental and health risks. In pursuit of more sustainable alternatives, new oxidative methods have been optimized, replacing oxidants with hydrogen peroxide and molecular oxygen. Additionally, the application of heterogeneous catalysts, following green chemistry principles, aims to improve recoverability, reusability, and waste reduction.
The work of Kleine et al. [74] was a pioneer in reporting the use of mechanochemistry for biomass degradation, demonstrating that with this methodology the use of solvents and metal catalysts can be avoided. This study developed a base-assisted ball milling process, demonstrating its potential as a mechanochemical technique for wood degradation. NMR spectroscopy indicated that cellulose and hemicellulose in beech wood were degraded into low molecular weight compounds, mainly breaking the β-O-4 linkage and hydrolyzing oligosaccharides (Scheme 1). Overall, these observations supported the authors’ initial hypothesis that base-assisted milling can also be applied to the degradation of untreated natural wood.
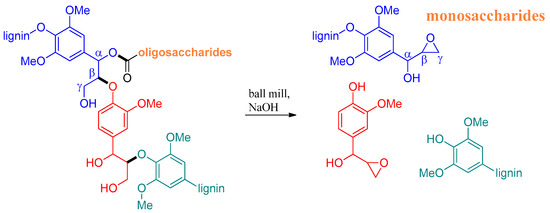
Scheme 1.
Schematic representation of the suggested course of mechanochemical degradation of hemicellulose and lignin in the original wood structure based on spectroscopic results [74].
On the other hand, Dabral et al. [78] reported a mechanochemically activated oxidation of beech lignin using HO–TEMPO, KBr, and Oxone® in the ratio 0.2:0.2:1.5 and using tungsten carbide (WC) as grinding medium. In this study, mechanochemical oxidation of model compounds, methoxy-substituted monolignol derivatives, 1a and 1b proved to be successful, as it provided the corresponding ketones 2a and 2b in 93% and 87% yields, respectively, after purification by column chromatography (Scheme 2).
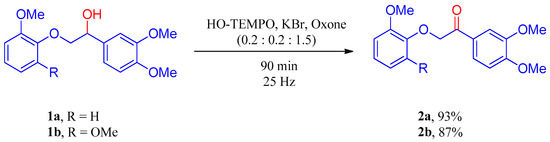
Scheme 2.
Oxidation of Lignin Model Compounds in the Ball Mill using WC Milling Media; Yields after Column Chromatography [78].
The same methodology was applied to a beechwood lignin. The Gas Chromatography coupled to Mass Spectrometry (GC-MS) analysis highlighted the formation of 3,5-dimethoxyquinone as the major monomer (2.5 wt%), along with 2-methoxybenzoquinone (0.5 wt%) (Figure 7). Furthermore, when the reaction was carried out on 10 g of beechwood lignin, gel permeation chromatography showed a strong reduction in molecular weight, demonstrating that the lignin was successfully depolymerized. Overall, this study demonstrates that this oxidative protocol can be applied on a large scale to biomass.
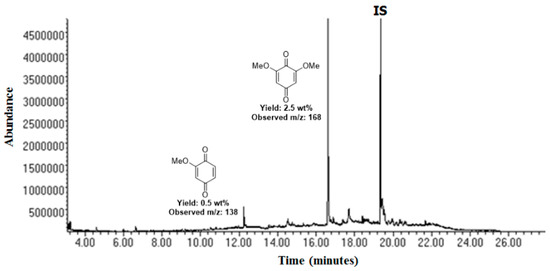
Figure 7.
GC-FID trace of the organic-soluble fraction (diethyl ether filtration) after the mechanochemical oxidation of Organosolv beechwood lignin with HO–TEMPO/KBr/Oxone® in a WC jar at 30 Hz for 90 min in a mixer mill (MM). Quantification performed using n-octadecane as an internal standard (SI). Identification of the major products was achieved by comparing the major products with commercial samples. Reprinted with permission from Dabral et al. [78] Copyright 2018 American Chemical Society.
Another successful solvent-free and environmentally friendly oxidative depolymerization protocol of model lignin (ligninox) was developed by Sun et al. [79], and the results showed successful depolymerization with some selectivity in syringate production (72.7% in weight). Zakaria et al. [80] used lignocellulosic oil palm residues, namely empty fruit bunches (OPEFB) and palm leaf fiber (OPFF), to produce glucose and xylose through enzymatic hydrolysis after ball milling pretreatment. The pretreatment of OPFF for 60 min of ball milling followed by OPEFB for 120 min demonstrated high glucose recovery with yields of 80.3 and 67.5%, respectively. Meanwhile, the highest xylose yield was obtained from OPEFB (80.1%), followed by OPFF (78.6%). The total sugar conversion yield of OPEFB and OPFF was 71.9 and 79.8%, respectively. The BM-treated OPEFB and OPFF produced 4 times more glucose and 4 to 16 times more xylose, respectively, compared to untreated oil palm biomass. The study further showed that higher recoveries of xylose (80.35 and 84.23%), glucose (62.4 and 78.69%) and total conversion (68.1 to 81.5%) were obtained from OPEFB and OPFF samples, respectively, when they were hydrolyzed at different loadings (30 and 40 FPU/g) of acremonium cellulase substrate. This suggested that BM effectively increased the surface area to enhance enzymatic hydrolysis.
Ball milling pretreatment was considered by Pang et al. [81] an efficient method to promote the catalytic conversion of lignocellulosic biomass into ethylene glycol. According to the report, Miscanthus subjected to dry grinding for 6 h at 450 rpm using a planetary ball mill, was converted by a binary catalyst of tungstic acid and Ru/AC into water. The maximum ethylene glycol yield from Miscanthus reached 52.4%, much higher when compared to untreated Miscanthus (22.5%). The study by Schneider et al. [82] describes a procedure for converting lignocellulosic barley straw into total reducing sugars (ATR) by mechanocatalytic pretreatment. Under optimized conditions, it resulted in high ATR release (42%) using oxalic acid dihydrate as catalyst. This study revealed that acid strength plays an important role in the depolymerization of barley straw and furthermore, it showed that the reaction catalyzed by oxalic acid generates low levels of the degradation product 5-hydroxymethylfurfural (HMF).
The ball mill has provided many relevant advantages compared to other lignocellulosic biomass pretreatment techniques, such as easy operation and avoidance of solvents and post-treatments. Zhang et al. [83] compared the feasibility and effectiveness of different biomass pretreatment methods, including microwave-assisted alkali (MAP), ultrasound-assisted alkali (UAP), and ball milling (BMP) to pretreat digested waste (2.5%-DR and 10%-DR) of rice straw, with 2.5% and 10.0% solids, for ethanol production. The result of this study revealed that BMP was found to be the best method to increase ethanol production from 116.65 and 147.42 mg/g to 2.5%-DR and 10%-DR, respectively. Digestion of ruminal fluid from rice straw with 2.5% solids content, combined with ethanol fermentation, produced a total energy production of 7.010 kJ/g, higher than the 5.464 kJ/g with 10% solids content. Overall, the integrated system was an effective biorefinement process with high energy output and reaction time savings.
A combined pretreatment of ball milling and ultrasound (MU) was investigated by Li et al. [77], corn cob with solid acid catalyst (/SiO2−Al2O3/La3+) for the conversion selectively catalytic hydrothermal processing of corncob into furfural. It was evident from this study that combined combination of ball milling and MU pretreatment was an efficient mechanocatalytic approach to destroy the complex structure of corn cob. So much so that the highest furfural yield of 197.76 mg/g corresponding to 82.90% of theoretical productivity was obtained at 190 °C for 30 min.
The work reported by Jiang et al. [76] compared the effects of ionic liquid and ball milling pretreatment on glucose yield from cellulose. According to the report, the glucose yield from untreated cellulose was only 20.9%, while both ball mill and ionic liquid pretreated cellulose exhibited remarkably higher cellulose digestibility for efficient enzymatic hydrolysis. The results proved that the yield of cellulose pretreated by ball milling (84.5%) was higher than that of cellulose pretreated with ionic liquid (78.0%).
Studies prove that ball mill pretreatment is a simple operation with high efficiency and is environmentally friendly. Powders, ball milling is a physical process that eliminates the use of potentially dangerous chemical reagents. The pretreated sample could be used directly without washing and filtration steps. For example, Kim et al. [84] reported that conventional pretreatment processes with sodium hydroxide and aqueous ammonia showed a rice straw loss of 34.2% and 14.8%, respectively. Comparatively, no biomass loss was observed with ball milling. Furthermore, milling produced significantly lower concentrations of soluble phenolics than alkaline treatments.
A solid cellulase acid catalyst (SA-SO3H) prepared without the use of sulfuric acid (H2SO4) was prepared by Shen et al. [85] and applied it to the conversion of microcrystalline cellulose. According to the report, cellulose can be effectively converted to levulinic acid in pure water at 180 °C in 12 h without additives with a maximum yield of 51.5% double SA-SO3H. However, cellulose pretreatment by ball milling improved levulinic acid yields by only a few percent (52.2%), showing that the cellulose binding sites (-Cl) and catalytic sites (-SO3H) of the catalyst are fundamental to the activity of the catalyst. A greater recycling efficiency of 95% is reported in the 5th round, and that the spent catalyst was regenerated with hydrogen peroxide (H2O2) solution. The study investigated the use of glucose as a starting material under the same reaction conditions and with the catalyst it promoted a yield of 61.5% of levulinic acid. The route of converting carbohydrates to levulinic acid in pure water with the biomimetic catalyst prepared with an H2SO4 free method provides an environmentally friendly method for producing bio-based platform chemicals from renewable resources.
Su et al. [86] successfully obtained a solid acid catalyst, notable for its absence of strong sulfonic acid (-SO3H) functional groups. Instead, the material, prepared from cow dung, exhibits the presence of weak acidic groups, namely carboxylic and phenolic. The synthesis process involved the initial carbonization of the dung at 400 °C for 2 h, followed by an activation step at 600 °C under a nitrogen (N2) atmosphere for 2 h, using potassium hydroxide (KOH) in a 1:2 weight ratio (substrate/activator).
A solid carbonaceous acid catalyst functionalized with -SO3H group by co-carbonization of sucralose was synthesized by Qiu et al. [87] and studied its efficiency in converting cellulose to glucose in pure water (Figure 8A). It was established by the study that the mixture of cellulose with the catalyst prepared by ball milling, used in the cellulose hydrolysis process provided a glucose yield of 52.8% in pure water at 200 °C in 1 h, and the glucose yields increased to 71.9% and 88.0% when it was diluted in 0.02% by weight of H2SO4 and hydrochloric acid (HCl) (with the same proton content) aqueous solutions, respectively, (Figure 8B). Furthermore, the prepared solid carbonaceous acid catalyst exhibited relatively stable catalytic activity over five cycles (Figure 8C). According to this study, the excellent catalytic activity for the production of glucose from cellulose was attributed to the reduced degree of crystallinity of cellulose and the improved contact between the active sites and the β-1,4-glycosidic bonds in cellulose during the cellulose and solid acid milling process. The work provided a promising strategy for high-yield glucose production from cellulose hydrolysis in aqueous solutions and offers broad application for transforming lignocellulosic biomass into valuable chemicals.
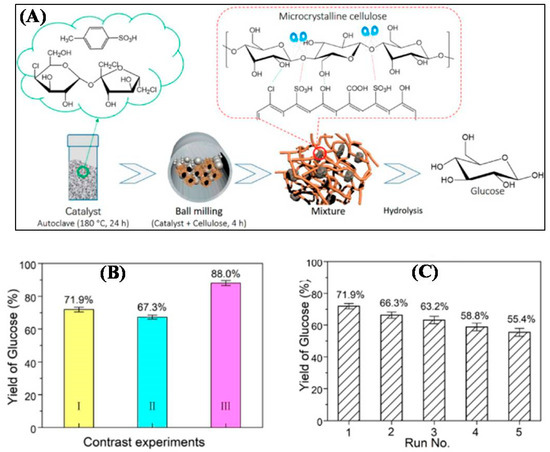
Figure 8.
(A) Hydrolysis of cellulose into glucose by mechanochemical process. (B) Glucose yields obtained under standard conditions for (I) the sucralose-derived catalyst in a 0.02 wt% H2SO4 aqueous solution, (II) the sucrose-derived catalyst in a 0.02 wt% H2SO4 aqueous solution, and (III) the sucralose-derived catalyst in a 0.02 wt% HCl aqueous. solution. (C) Recycling performance of the sucralose-derived catalyst in a 0.02 wt% H2SO4 aqueous solution under standard conditions. Reprinted with permission from Qiu et al. [87]. Copyright 2018 American Chemical Society.
Research conducted by Qi et al. [88], demonstrated a promising method for the synthesis of weakly acidic carbon catalysts from biomass byproducts. The team used black liquor, a substance generated from rice straw pretreated with an aqueous potassium hydroxide (KOH) solution, as the main raw material. These innovative catalysts demonstrated excellent catalytic performance in hydrolysis processes, efficiently converting cellulose and alkali-pretreated rice straw into valuable sugars. The study highlighted remarkable results: cellulose hydrolysis achieved an impressive 76.3% glucose yield. For rice straw, the process resulted in high yields of 52.1% glucose and 66.5% xylose when the reaction was conducted in a 0.015% (by weight) HCl solution at 200 °C for 60 min. In addition to high activity, the carbon catalysts exhibited remarkable stability and recyclability in the aqueous reaction system. One specific catalyst, BC-600, provided a glucose yield of 84.6% and maintained this yield after three recycling cycles, highlighting its robustness and potential for industrial use. In summary, this work not only offers an innovative strategy for the efficient hydrolysis of lignocellulose using catalysts derived from the biomass itself but also represents a significant advance in the comprehensive utilization of lignocellulosic biomass.
The valorization of compounds derived from biomass can benefit from mechanochemical techniques as well. The research group of Oh et al. [89], developed a simple and sustainable mechanochemical technique for the production of polyurethanes (PUs). The method, which does not require the use of solvents and operates at room temperature, uses 2,5-bis(hydroxymethyl)furan (BHMF), a biomass-derived compound, in combination with a vibratory milling process (Figure 9). This approach, in addition to being fast and straightforward, produced PUs with a maximum molecular weight of 163 kJ. The resulting polymers are flexible, with a glass transition temperature (Tg) of 96 °C, and thermally stable, with a decomposition temperature (Td) of 197 °C. The ball milling technique also proved versatile, enabling the synthesis of PU copolymers with different diols and diamines, which allows varying the final properties of the polymer, such as its Tg.
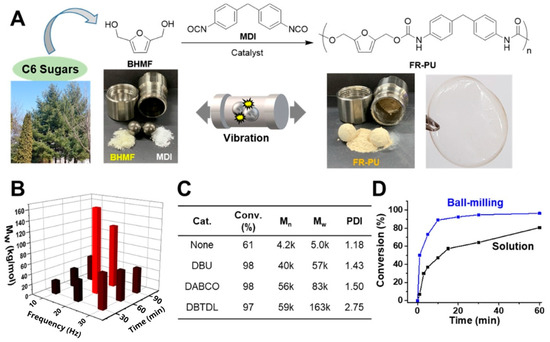
Figure 9.
Schematic illustration of PU synthesis using diols derived from biomass (A). Screening of ball-milling polymerization with BHMF and MDI (B) by controlling frequency and reaction time, and (C) with several catalysts. The reaction with the DBTDL catalyst at 20 Hz for 60 min yielded the highest Mw PU. (D) Comparison of conversion achieved with ball-milling vs. solution synthesis of PU as a function of the reaction time. Reprinted with permission from Oh et al. [89]. Copyright 2020 American Chemical Society.
In summary, mechanochemistry has proven to be a promising tool in transforming biomass, including lignin, into sustainable chemicals and materials. This approach contributes to the search for environmentally friendly solutions for the efficient use of renewable resources and addresses the challenges in biomass separation. As research advances in this field, it is expected that new developments will lead to more effective and sustainable processes for biomass valorization. Table 2 summarizes the effect of ball milling pretreatment on the conversion of lignocellulosic biomass into different chemicals.

Table 2.
Mechanical milling pretreatment on the product yields from different types of biomass.
3.4. Polymer Waste
Polymers are one of the main materials developed in the XX century with the growth of petroleum exploitation, to supply the demand of an extensive range of industrial activities making them ubiquitous. It is estimated that the production of plastics in the world was almost 368 million tons in 2019, with over 80% being waste. In 2020 the production of fibers included polymers such as polyester and polyamide, representing 109 million tons together with natural fibers. In this scenario, polyester is responsible for 52% of fiber production with only polyethylene terephthalate contributing with 57 million tons [34,101].
Plastic waste treatment includes incineration, landfill, mechanical recycling, and chemical upcycling. Among these, chemical upcycling offers a path to obtain highly valuable monomers and oligomers. An example is the reported selective depolymerization of PET and other polymers to originate useful compounds [36,101,102]. The process of chemical upcycling demands the application of heating and the use of chemicals besides catalysts. In addition, classical methods are more time-consuming and less cost-effective than mechanochemical methods which also avoid the use of solvents and additives and offer fewer risks in downgrading the properties of the recycled polymer [102].
The mechanochemical treatment of polymers and their wastes makes the use of mills for the generation of new structures, such as the method called Solid State Shear Milling (SSSM) that allows the formation of ultrafine powder polymer with the application of strong force. Polypropylene (PP) waste and automotive shredder residue (ASR) have been used with this technique to produce a composite with good toughness [37]. The authors fabricated a PP/ASR composite that exhibited robust mechanical performance due to the excellent dispersion of ASR particles and in situ compatibility between the ASR matrix and PP (Figure 10A). The results of this study showed that the 50/50 wt% PP/ASR-10 composite presented excellent mechanical performance combined with good processability and high thermal stability (Figure 10B–E).
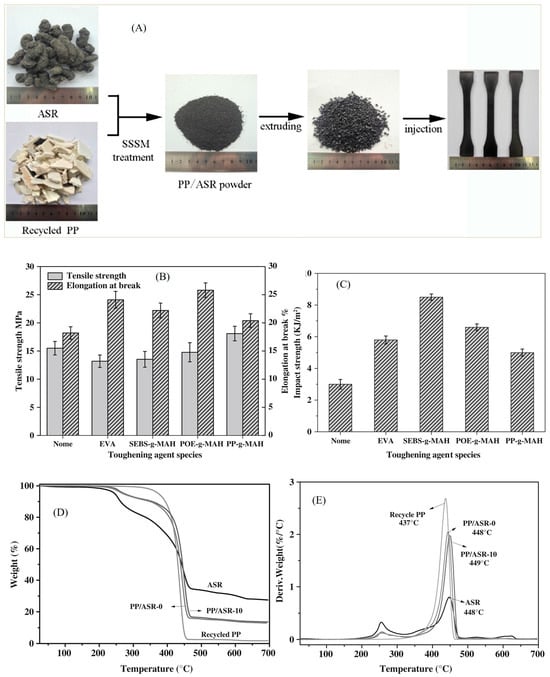
Figure 10.
(A) Schematic of practical processing of PP/ASR composites, Mechanical properties of 50/50 wt% PP/ASR composites with various toughening agents: (B) tensile properties; (C) impact toughness. (D) Thermogravimetric Analysis (TGA) and (E) Derivative Thermogravimetry (DTG) curves of ASR, PP and 50/50 wt% composites with and without SSSM process. Reprinted from Yang et al. [37]. Copyright 2018 with permission from John Wiley and Sons.
Access to new polymer structures based on mechanochemistry methods for recycling polymer waste demands some considerations about experimental parameters such as crushing speed, crushing time, and the knowledge of feed amount. In polyurethane processing for recycling, the action of mechanochemical forces decomposes chemical groups such as methyl and aldehyde groups, generating a material with strong reactivity and plasticity. Improved thermomechanical features are reached in this process [101].
In this scenario, polyurethane thermoset waste recycling is a challenge. A process of milling and grinding in the presence of an organocatalyst, triazabicyclodecene, followed by molding, has been applied to promote a vitrimerization process. This results in a vitrimerized polymer that can relax stress quickly, mainly by a carbamate exchange reaction, allowing the material to be reprocessed by simple techniques such as extrusion and injection molding [103]. Plastic wastes of polyolefins from domestic sources have the potential to be transformed into materials with improved properties by high-energy mechanochemical processes using planetary ball milling [104].
The prospects for eco-friendly methods involving mechanochemical treatments highlight the urgent need to address the disposal of massive amounts of plastic waste. These methods aim to avoid traditional recycling procedures such as incineration and landfill storage, which, while reducing polymer waste, contribute significantly to global pollution. One significant source of waste is car tires, composed of thermosetting polymer materials that can persist in the environment for hundreds of years. The cross-linking in polymer structures has great occurrence [105,106,107,108] and increases their stability. Mechanochemical processing breaks the cross-linking, increasing the surface area and roughness of rubber from tires [109].
The production of polymer blends involves the bonding of a polymer structure to another one. In this process, the mechanochemical technique is applied to split the polymers, with disproportionation or recombination reactions, forming a copolymer block in conditions of high shear. The formation of these structures allows the improvement of mechanical properties in the final material [110]. Mechanochemical ball milling plays a key role in the development of these materials. For example, the formation of polylactic acid (PLA) with improved mechanical properties demonstrates the high efficiency of this method. The high-energy collisions between reactants enable the synthesis of new compounds without the need for solvents. Additionally, mechanochemical processes offer advantages such as shorter polymer degradation times and the ability to avoid C-C bond cleavage. This makes them a versatile route for the synthesis of polymers like polyphenylene/vinylene, polyphenylene, polyazomethine, polylactides, and polyurethane [111].
Polyurethane copolymers can be synthesized via ball milling through the polyaddition of diisocyanates in the presence of a catalyst. To obtain a high molecular weight product, it is crucial to control factors such as reaction time and energy input [89]. In this context, few studies have investigated the kinetics of copolymer formation, such as the melting process of two polymers to produce a copolymer. The mechanical force applied during the process yields a product with an architecture similar to the primary structures involved, often with the addition of a multifunctional reagent that interacts with both polymers. Radical-radical coupling frequently occurs in these reactions. Ball milling, used to pulverize shredded recycled plastic, affects polymer size and has been applied in the treatment of polyolefins, such as in the formation of the polypropylene–low-density polyethylene (PP-LDPE) copolymer. This process acts as a compatibilizer, reducing interfacial tension and improving interfacial bonding [51].
The development of functional materials through the mechanochemical treatment of polymer waste is driving the growth of this research area, offering new concepts for waste management across various industries. In this context, the production of mesoporous carbon materials is particularly attractive for polymer waste-based synthesis, as these materials serve as effective catalyst supports due to their inert nature. They are widely used in reactions such as hydrodeoxygenation, nitrogen reduction, hydrogen evolution, and oxygen evolution. Carbon-based structures can be synthesized from PET waste, as reported by Xu et al. [112]. In this study, functional porous carbons with variable shapes formed in situ and with high surface areas (up to 1001 m2/g) were obtained after carbonization and chemical attack (Figure 11). Furthermore, metallic Mo species were impregnated in a well-dispersed form into the porous carbons through solvent-free extrusion to support solid catalyst design particles (Mo2C@MC). The results indicated that Mo2C@MC not only has good catalytic performance in the reduction reaction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP), but also the linearity of ln(C/C0) over time was consistent with pseudo-first-order kinetics with an apparent rate constant (Kapp) of 0.5812 min−1 (Figure 12).
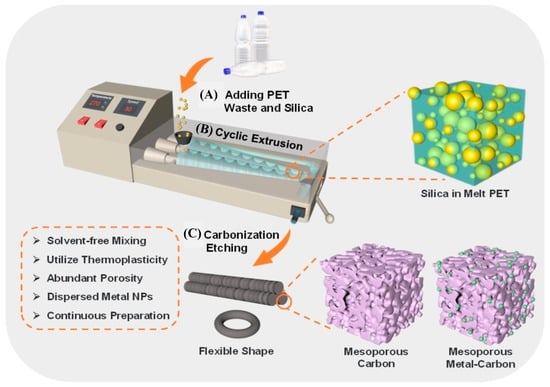
Figure 11.
Schematic illustration of the mechanochemical extrusion processes. (A) Adding PET waste and silica into the extruder. (B) Cyclic extrusion for 60 min. (C) Carbonization at 500–800 °C followed by silica removal. Reprinted from Xu et al. [112]. Copyright 2022 with the permission from John Wiley and Sons.
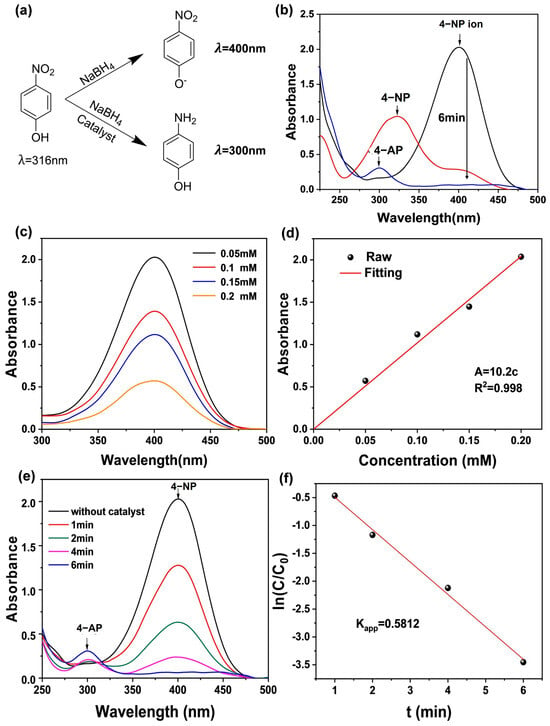
Figure 12.
(a) Maximum UV absorption wavelength of 4-NP, 4-NP ion, and 4-AP. (b) Evolution of UV/Vis absorption spectra for the reduction of 4-NP in 6 min with Mo2C@MC as a catalyst. (c) UV/Vis absorption of 4-NP ion under different concentrations. (d) Variation in absorbance with different solution concentrations of 4-NP ion. (e) UV/Vis spectra of the reaction solution during catalytic reduction by Mo2C@MC. (f) Curves of ln(C/C0) as a function of time. Reaction conditions: C4-NP = 0.2 mm, CNaBH4 = 0.32 m, room temperature, mcat = 10 mg. Reprinted from Xu et al. [112]. Copyright 2022 with the permission from John Wiley and Sons.
Another polymer with potential application in the synthesis of functional materials is polyurethane. Wastes generated from this material are estimated to accumulate at about 19 million tons annually. This is a precursor for N-doped porous carbon materials, used in supercapacitor applications, provided with high surface area and electrical conductivity. The modification with heteroatoms improves features of the carbonous structures in terms of energy storage, being nitrogen incorporated in the carbon skeleton by immersion of it in a liquid medium such as urea or by post-treatment in the gas phase using ammonia (NH3) or nitrogen (N2) at high temperatures [113]. Schneidermann and his collaborators [113] developed a polyurethane upcycling process obtaining porous carbon materials already doped with nitrogen that were applied to supercapacitor electrodes (Figure 13). In this study, polyurethane residues served as a source of carbon (and nitrogen) that were converted via mechanochemistry with K2CO3 and, optionally, urea. Finally, optimized carbon materials with excellent properties were obtained, such as a high specific surface area of 2.150 m2/g and a total pore volume of 0.9 cm3/g (PUUPC-800-1). Furthermore, such N-doped carbon materials performed similarly to commercial carbon material supercapacitors such as YP-50F, showing a specific capacitance of up to 99 F/g in Li2SO4, as well as stable performance in TEA-BF4 with 83 F/g. By mechanochemical upcycling with additional urea, the rate capacity of the supercapacitor was increased and the obtained device exhibits 80% of its capacitance at a high specific current of 10 A/g in aqueous electrolyte. The authors report that such materials have the possibility of being applied to other energy storage systems, such as lithium-ion batteries or wastewater purification, whenever materials with a high surface area and better wettability are required. This study presents a broader perspective, aiming to extend the process described here to other challenging polymeric waste streams, thereby further reducing waste generation.
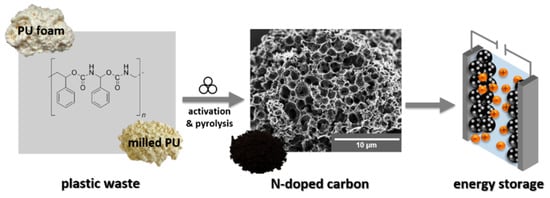
Figure 13.
Upcycling approach consisting of high-energy ball milling and carbonization of a mixture of PU foam as the carbon source and potassium carbonate (K2CO3) as an activation reagent to form nitrogen-doped porous carbon as an electrode material for supercapacitors. Reproduced from Schneidermann et al. [113] under the Creative Commons CC BY license.
The presence of halogenated organics in plastic waste complicates chemical recycling. Therefore, recycling methods for these polymeric wastes focus on developing mechanochemical processes with dehalogenation, such as the removal of bromine, chlorine, and fluorine from polypropylene after ball milling for a certain period [114].
Mechanochemistry has proven to be an effective method for mineralizing halogenated organic pollutants. Balema et al. [115] reported the mechanochemical depolymerization of polystyrene to styrene monomer via ball milling. After the milling process, the molecular weight of polystyrene dropped from 88 kDa to 7–8 kDa, and 7 wt% styrene monomer was detected, as characterized by 1H NMR (hydrogen-1 proton nuclear magnetic resonance) and GC-MS (Gas Chromatography with Mass Spectrometry). Although the depolymerization yield is low, this work showed promise for depolymerizing polystyrene in an economical and scalable manner.
The recycling process for this material involves the use of a solvent (benzyl alcohol) under high-pressure conditions. It was observed that extended soaking periods resulted in less undissolved resin in the final product, improving the quality of the recovered fibers. However, the deposition of the catalyst on the surface may compromise this quality [116]. Some additives such as flame retardants, that use halogens in composition, are present in plastic from vehicle components, that in the end-of-life cycle turn to waste. Hexabromocycledodecane (HBCD) is one of the additives. Plastic components made of ABS (acryl nitrile-butadiene-styrene) and PP/PE (polypropylene/polyethylene) have undergone a mechanochemical dehalogenation process using ball milling with the addition of Al- and Si-based additives. This process is particularly relevant since HBCDs were listed as potential pollutants under the Stockholm Convention on Persistent Organic Pollutants in 2013 [117].
In a previous study, Grause et al. [118] performed the almost complete debromination (98%) of decabromodiphenyl ethane (DBDPE) in high impact polystyrene (HIPS) through 24 h wet grinding with an agitated ball mill. In this study, NaOH in ethylene glycol was used as dehalogenation reagent at a relatively high temperature (90 °C). The diffusion-controlled activation energy was about 205 kJ/mol. The strong reagent, high temperature, and long treatment highlight the weak reactivity of brominated compounds in polymer matrices. However, the ball mill had the advantage of deforming and flattening the plastic particles. Therefore, the thermal stability of treated HIPS was also improved after mechanochemical treatment, according to Thermogravimetric analysis (TGA).
Cagnetta et al. [114] studied the mechanochemical treatment of commercial polypropylene (PP) pellets, which contain 15.4 wt% DBDPE, through high-energy planetary grinding for 8 h. The highest debromination efficiency of 90% was achieved with Fe-SiO2 additives, due to the redox activity of Fe, which reduces DBDPE and facilitates the debromination/degradation. The report estimated the energy consumption, assessing that the polymer matrix absorbed about 85% of the milling energy, while only about 15% was used for effective debromination, approximately proportional to the mass proportion of DBDPE. Furthermore, the proposed mechanochemical process carried out in dry conditions at room temperature with cheap reagents, is significantly faster than previously studied [118].
On the other hand, Wang and collaborators [119] investigated the mechanochemical degradation of polybrominated diphenyl ethers (PBDEs) contained in Waste Printed Circuit Boards (WPCBs). The results showed that a degradation conversion of 65.2% for PBDEs was achieved, with PBDEs with higher bromination being reduced with increasing grinding time, and PBDEs with lower bromination being generated.
Considering the strong need for disposal of waste containing HBCD, mechanochemical pretreatment technology as a dehalogenation method was investigated by Lu et al. [117] to destroy brominated flame retardants (BFRs). In this study, the results showed that HBCD was effectively degraded during mechanochemical treatment with the aid of Si-Al-based additives in the ratio SiO2/Al = 7:2 with a reagent ratio of 15:1. With the optimized additive system, 4 h of ball milling treatment was sufficient to degrade HBCD. According to the experimental and characteristic results, the possible degradation pathway of HBCD was proposed, as shown in Scheme 3. There are two reactions: (a) debromination and (b) fragmentation. Both reactions occur at the same time, but debromination is the dominant reaction. As it is only a qualitative and not a quantitative result, more in-depth research must be carried out on the mechanism.
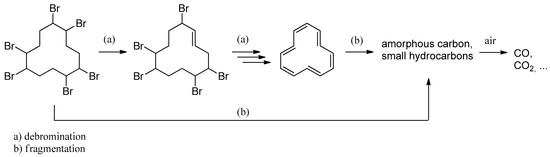
Scheme 3.
The proposed degradation pathways of HBCD in the milling process [117].
The application of recycling techniques enables the exploration of methods beyond ball milling, such as solid-state shear milling, which has been reported as a useful approach for recycling epoxy resin waste with potential applications in coatings and functional materials [120]. To conclude this section, it is important to highlight that mechanochemical methods are as environmentally friendly as other methods of recycling polymeric waste. Giving new life to these materials offers alternatives to obtain composites, blends, catalyst supports, electrodes, and electric components, all products which are essential for many human activities. The mechanochemical processes offer many positive features, e.g., the problem of contaminant presence that can be ignored because contaminants may act as milling assistants. This process can be combined with chemical processing for the recycling of a variety of wastes and for the synthesis of new materials [121], which have already been and will be mentioned throughout this review. It is proven that the mechanochemical method has a certain degradation effect on brominated organic matter. The charge ratio (i.e., ball-to-powder ratio) and the rotation speed are considered fundamental parameters in dehalogenation reactions. These control the specific energy density within the matrix. Essential information on the most effective operating parameters of mechanochemical treatment of the chlorinated, brominated, and fluorinated compounds described in the reviewed papers is reported in Table 3.

Table 3.
Summary of experimental conditions utilized in reviewed papers that achieved best dehalogenation/degradation results.
3.5. Crustaceans
As large amounts of residual shells still remain underexploited, the concept of shell biorefinery is developing rapidly and has attracted increasing attention in recent years. Fractionating crustacean shells into their main components and processing each component into value-added chemicals and materials can bring ecological and economic benefits [164].
Chitosan (CS), a biopolysaccharide composed of glucosamine units linked together by β-1,4 linkages, is generally produced by deacetylation of chitin, which is mainly extracted from crustacean shells, insect cuticles, or other microorganisms such as bacteria, fungi, and others. In particular, low molecular weight chitosan (LMWC) between 2 and 10 kDa exhibits enhanced solubility that can be dissolved in neutral water for direct use under physiological conditions. Furthermore, LMWC is specifically suited for DNA delivery, showing more enhancement in biological, antibacterial, antitumor, and immunostimulatory activities than chitin and high molecular weight chitosan (HMWC), making it attractive in biomedical and pharmaceutical applications. LMWC is also superior compared to HMWC and chitin in materials synthesis due to remarkably improved reactivity in grafting, crosslinking, and other types of modifications [165,166]. Both chitin and chitosan are biocompatible, biodegradable, non-toxic, and quite valuable due to their lower mineral content. However, the deacetylation of chitin into chitosan is still an expensive, difficult process and is usually accompanied by depolymerization, providing low molecular weight chitosan [167].
The first report of a chitinolytic system using combinations of mechanochemical grinding and direct enzymatic degradation of crustacean shells was by Nakagawa and colleagues [168]. This study developed an intensive “convergence” ball mill for rapid mechanochemical conversion of chitin and crustacean shells (crab or shrimp) into amorphous microparticles sensitive to chitinase (Figure 14). The mechanochemical pre-treatment allowed a complete degradation of crab shells into N-acetylglucosamine (GlcNAc).
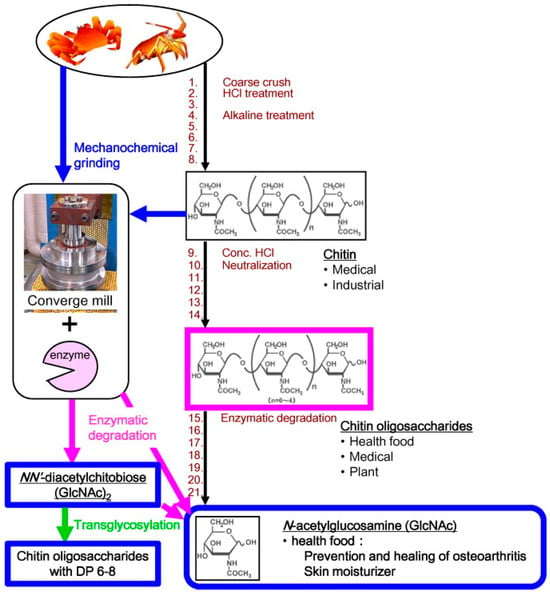
Figure 14.
The traditional method of manufacturing N-acetylglucosamine and decrease the environmental burdens by the innovative technology Left side shows industrially applied process to make chitin, chitin oligomers and GlcNAc from crustacean shells. Using converge mill for pre-treatment, it is possible to decrease 2/3 steps from traditional method. While the traditional method requires many steps (1-21) including acid and alkaline treatments, neutralization to be able to start the enzymatic degradation step to obtain GlcNAc Reprinted from Nakagawa et al. [168]. Copyright 2011, with permission from Elsevier.
Chen et al. [169] transformed shrimp shells into LMWC in the presence of NaOH by single-step mechanochemistry. The results showed that raw shrimp shells were successfully transformed into LMWC (7.9 kDa) with a high purity of 90% under NaOH-catalyzed grinding conditions (8 cycles, 700 rpm). The FTIR spectra of the product demonstrate the formation of chitosan-like compounds, as well as the weak bands at 1536 cm−1 and 1395 cm−1 which suggest the presence of residual CaCO3 and proteins. The content of protein residues in the final product was analyzed using the Bradford method, while analysis by ICP-OES (Inductively Coupled Plasma Optical Emission Spectrometry) revealed a CaCO3 content of 6.7% in weight. This new simple, solvent-free approach avoids the generation of wastewater and the use of oxidants, acids, or other environmentally harmful reagents. Furthermore, the work not only emphasizes that base usage has been drastically reduced to 1/10 compared to traditional methods but also inhibits side reactions under ball milling conditions.
Di Nardo and colleagues [170] reported a new method, to produce high molecular weight chitosan with minimal energy and solvent consumption, based on the combination of mechanochemistry and aging. The results showed that the method was versatile and applicable to a range of chitin sources, including crude crustaceans and insect shells, allowing a deacetylation degree of up to 98% and remarkably high molecular weights. According to reports, this process provides chitosan in a safer way and with lower consumption of materials and energy compared to the classic hydrothermal process. It minimizes material dependency (achieving excellent results with NaOH: chitin ratios of 4 or 5), eliminates the need for solvents during the reaction, and significantly lowers energy consumption, particularly in pure aging experiments. This article describes an innovative methodology for producing chitosan-based materials resistant to dissolution in water. This opens up the possibility of using them in new areas, such as environmental remediation or food packaging production. Because this methodology is remarkably simple to implement, requiring minimal technical equipment, it is highly adaptable to the challenge of bark biorefinery. Overall, this approach offers solutions for the local production of high-value-added products, especially in locations close to fishing areas and insect farms.
Su et al. [171] also developed a contemporary method for reusing shrimp shell waste, transforming it into new value-added materials. This process, called mechanochemistry, is considered gentle and waste-free. One of the main advantages of this method is that it eliminates the use of harsh chemicals such as strong acids and bases, as well as oxidants. It also does not require extreme temperature or pressure conditions and consumes less energy than traditional methods (Table 4). According to the authors, the aim of the study is to contribute to the circular economy by promoting the sustainable use of crustacean waste. With this approach, they aim to achieve United Nations (UN) Sustainable Development Goals (SDG) 3, 6, 12, and 14 [172] proposing a method of economical and sustainable reuse of crustacean waste.

Table 4.
The types, quantities, and energy consumption of chemical reagents required to treat each ton of shrimp shells [171].
Hajiali et al. [33] described a sustainable approach based on mechanochemistry and aging for extracting chitin directly from crab shells using a commercial blender (Figure 15). According to the results, different solid acids (i.e., citric, ascorbic, malic, salicylic, and succinic acids) promoted chitin extraction with yields reaching 27% and low ash contents (0.9%). Using citric acid, chitin from 200 g of crab shells could be extracted using a commercial blender, achieving low values of 190 and 18 kJ/g for process mass intensity (PMI) and energy consumption, respectively. Although 30 min of grinding can provide high-quality materials, yields could be increased further by decreasing the grinding time to 10 min. These results showed viability when compared with other traditional chitin extraction methods and alternative strategies, which is summarized in Figure 16.

Figure 15.
Photos of the whole GC (European Green Crabs) as received by Parks Canada, GC shells after boiling and defleshing, GC shells powder after homogenization using a commercial blender, and chitin extracted after the mechanochemical process developed and proposed. Reprinted from Hajiali et al. [33]. Copyright 2022 with permission from American Chemical Society.
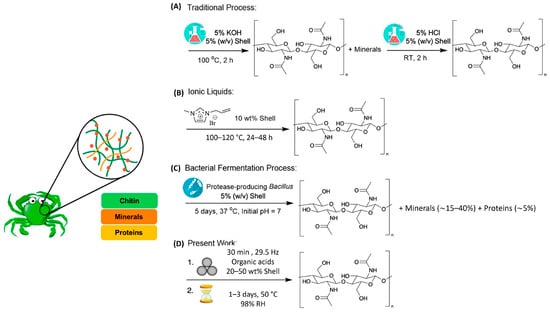
Figure 16.
Comparison between the extraction of chitin from crab shells using (A) the traditional solution-based process, (B) ionic liquids, (C) bacterial fermentation, and (D) the work of Hajiali et al., based on mechanochemistry and aging. Reprinted from Hajiali et al. [33]. Copyright 2022 with permission from American Chemical Society.
Recently, Fatika et al. [173] successfully synthesized chitosan Schiff bases (ChSB) from shrimp shells through a consecutive process involving demineralization, deproteinization, and deacetylation at room temperature, followed by solvent-free mechanochemical grafting using an environmentally friendly method. The resulting ChSB was found to be more amorphous and exhibited increased thermostability compared to chitosan. Notably, solvent-free mechanochemical grafting enabled ChSB synthesis in just 10 min, significantly reducing the processing time compared to conventional methods (>3 h). The authors highlighted the need for further studies to explore this approach for generating ChSB from other raw materials and to investigate its biological activity.
3.6. Synthesis of Materials
Eggs, a widely consumed natural food source, provide essential amino acids, vitamins, and minerals. As a result, chicken eggshells are a common byproduct of food waste. These residues are fully biodegradable, recyclable, and biocompatible [174]. The primary chemical component of eggshells is calcium carbonate (CaCO3) in the form of calcite, comprising approximately 94–97% of their composition. Other constituents include calcium phosphate (Ca3(PO4)2) at 1%, magnesium carbonate (MgCO3) at 1%, and organic matter at 4% [4]. Studies highlight the significance of CaCO3 as the main component of natural waste materials such as oyster and eggshells, both of which have been successfully employed in mechanochemical dechlorination processes [175,176,177]. This review provides an updated overview of recent publications utilizing a mechanochemical approach for eggshell waste treatment, summarizing key applications and findings.
In line with efforts to develop new green chemistry methods, Mosaddegh and Hassankhani [174] explored a simple and rapid ball mill-assisted preparation of nano-CaO (calcium oxide) based on eggshell waste. Additionally, they developed a green, fast, and highly efficient protocol for the one-pot synthesis (Scheme 4) of 2-amino-7-methyl-5-oxo-4-phenyl-4,5-dihydropyran[4,3-b]pyran-3-carbonitrile derivatives (6). The reaction involved a carefully stirred mixture of 4-hydroxy-6-methyl-2H-pyran-2-one (3), substituted benzaldehydes (4), and malononitrile (5) at 120 °C under solvent-free conditions, using a nano-CaO catalyst. This method achieved excellent yields (93–98%) in a remarkably short reaction time. The nano-CaO was still reused several times without loss of catalytic activity. According to the authors, this study represents the first report on the use of eggshell waste as a natural precursor of nanometer-sized CaO catalyst in organic synthesis, offering a sustainable approach to reducing environmental impact.
Scheme 4.
Synthesis of 2-amino-7-methyl-5-oxo-4-phenyl-4,5-dihydropyrano[4,3-b]pyran-3-carbonitrile derivatives.
Focusing on the production of bioceramics, the report Ferro and Guedes [178] is of particular interest, as they managed to prepare hydroxyapatite (HA) using ball milling, without the need for subsequent sintering. Eggshells or cuttlefish bones have been used as sources of calcium (containing Ca in the form of calcite or aragonite, respectively), maintaining a constant Ca/P ratio of 1.67, in the presence of 6.4% by weight of water. It was found that the reaction progress for the synthesis of HA was a function of energy input and was different for each precursor, for the eggshell it was 52.5 kWh/g, while only 6.2 kWh/g was required from the cuttlefish bone. However, the final product was formed more easily when using an aragonite-containing precursor. In all cases, crystalline HA nanoparticles were produced. From a general point of view, the results obtained in this study demonstrate the potential of chicken eggshell and cuttlebone as natural precursors and open a window of opportunity for the development of a reliable, scalable, fast, and economical HA production method. The use of cuttlefish bone as a source of calcium for the mechanochemical production of HA has been previously reported [179], where however, the cuttlefish bone was calcined in CaO before grinding.
On the other hand, Cestari et al. [180] studied the use of three different biogenic materials, such as eggshell (calcite), cuttlefish bone (aragonite), and mussel shell (aragonite/calcite) as sources of calcium to produce HA through grinding in an aqueous medium ((NH4)2HPO4 or H3PO4) at relatively low temperature (120–150 °C). Drying temperature was shown to have a fundamental influence on process efficiency, while grinding time appeared to have a negligible impact on HA formation. Furthermore, they confirmed that aragonite from cuttlefish bones was transformed into HA more easily compared to eggshell calcite. They also found that the synthesis was favored by acidic aqueous media compared to basic media.
Another strategy was developed by Shen et al. [181] to prepare hierarchically porous carbons derived from biomass using mechanical grinding with the aid of CaCO3 or eggshell. This work used dairy manure residues and eggshells as carbon precursors (Figure 17). They were pretreated using a ball mill at 350 rpm for 30 min. Subsequently, the mixtures were pyrolyzed at a high temperature (>800 °C) and part of the CaCO3 was decomposed into CO2 (carbon dioxide), which acted as an activating agent to produce micropores in the carbon. The subsequent removal of residual CaCO3 and newly formed CaO by the HCl solution led to the formation of mesopores. The findings were corroborated by nitrogen physisorption analysis. The produced materials applied as supercapacitor electrodes showed a relatively high specific capacitance of 226.6 F/g in KOH aqueous electrolyte (6 mol/L). Furthermore, it exhibited excellent cycling stability with nearly 100% specific capacitance retention after 2500 galvanostatic charge–discharge (GCD) cycles.
Figure 17.
(A) Schematic illustration of synthesis of hierarchical porous carbon from eggshell and dairy manure. (B) Nitrogen adsorption/desorption isotherms and (C) pore-size distributions of the synthesized carbon materials. Reprinted from Shen et al. [181]. Copyright 2018 with permission from John Wiley and Sons.
Seeharaj et al. [182] have manufactured a superhydrophobic coating from eggshell biowaste. The work reports that the coating was prepared by grinding chicken eggshells, followed by surface hydrophobization with stearic acid. The modified eggshell particles were then dispersed in a polystyrene (PS) binder and dip-coated onto a substrate. The resulting material exhibited water contact angle (WCA) values above 150° on cotton fabric substrates when a 4:1 weight ratio of modified eggshell to PS was used, classifying it as superhydrophobic. The material showed good environmental stability, self-cleaning, and oil/water separation properties (Figure 18). These results suggest that biological waste from eggshells can be used for superhydrophobic applications.
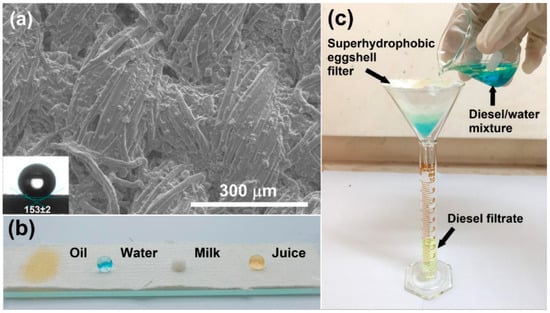
Figure 18.
(a) SEM image of the coating layer, the inset shows WCAs and image of water droplet on it, (b) photograph of soybean oil and various aqueous droplets on the superhydrophobic eggshell filter, and (c) photograph of the experimental setup for oil/water separation test (the water dyed blue with methylene blue). Reprinted from Seeharaj et al. [182]. Copyright 2019 with permission from John Wiley and Sons.
Previously, Tongamp and collaborators [175] had already reported a new process for dechlorinating polyvinyl chloride (PVC) using oyster shell waste. The study reports that with a 1:4 molar ratio of PVC and oyster shell mixture, the complete chlorine extraction rate can be achieved in 2 h of grinding at 700 rpm, while only 65% and 25% of chlorine are extracted to 600 rpm and 500 rpm, respectively. The present process offers a potential route for the handling and disposal of oyster shells and PVC waste.
A semi-industrial approach for simultaneous treatment of eggshell and industrial polyvinyl chloride waste using ball milling tools is reported by Baláž et al. [183]. In this study, the laboratory-scale experiment proved to be more efficient, as complete dechlorination was achieved after 4 h of grinding, while only 56% was achieved after 12 h of treatment in a semi-industrial vibrating ball mill. The Ca:Cl molar ratio was a crucial factor in the reaction’s progression. Increasing it from 2.34 to 5.06 significantly improved the outcome, with the yields rising from 4% to 30% after 8 h of grinding. The rate constant obtained followed the zero-order kinetics with the rate constant 1.23 × 10−5 s−1. The results of XRD and FTIR measurements showed a high probability of calcium chloride formation. This study also highlights the green, environmental and sustainable nature of mechanochemistry and shows the possibility of increasing the process to a semi-industrial scale using the eccentric vibrating mill.
Interesting studies showing the acid resistance properties of a new nanosized eggshell-titanium dioxide material (TiO2-EB), which can potentially be applied in dentistry, were reported by Onwubu et al. [184,185] (see Figure 19). In one study, Onwubu et al. [185] collected bovine enamel and evaluated the resistance properties of TiO2-EB in situ when exposed to HCl in the presence of various toothpaste solutions, as well as pure eggshell and an eggshell-TiO2 composite. The acid-resistant properties could be clearly traced from the SEM images of the enamels obtained after the action of the corresponding solutions (Figure 20). The image observed in Figure 20G visibly confirmed that the titanium dioxide coating on the surface of TiO2-EB effectively improves its resistance in an acidic environment.
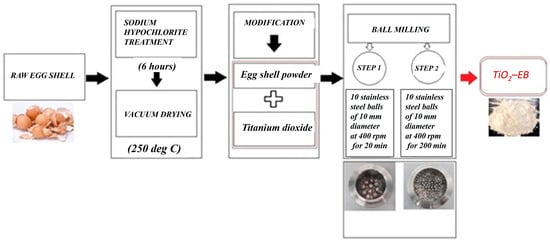
Figure 19.
Schematic illustration of different steps in the preparation of TiO2-EB. Reprinted from Onwubu et al. [185]. Copyright 2019 with permission from Elsevier.
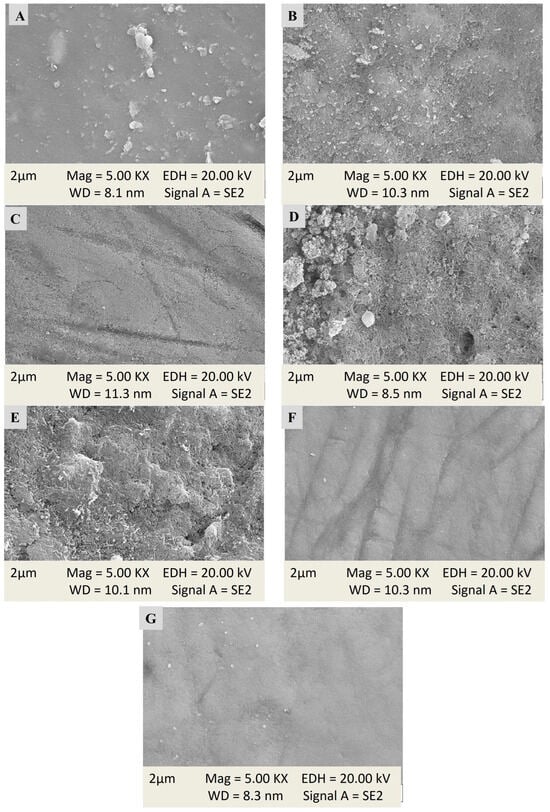
Figure 20.
Enamel surface (A) Unexposed tooth; (B) after exposure to HCI; (C) after exposure to Colgate toothpaste; (D) after exposure to Colgate Sensitive; (E) after exposure to Sensodyne (F) after exposure to eggshell; (G) after exposure to TiO2-EB (All exposed samples were in a solution of 0.2 mL HCl for 30 min). Reprinted from Onwubu et al. [185]. Copyright 2019 with permission from Elsevier.
Calcium titanate (CaTiO3) was successfully synthesized by combustion synthesis using duck eggshell as a source of calcium, mixed with anatase titanium dioxide (A-TiO2) and using magnesium (Mg) as fuel [186]. The eggshell and A-TiO2 were ground for 30 min in a high-energy planetary mill for 30 min at 400 rpm. These powders were then mixed separately with Mg in a ball mill for 120 min. According to XRD patterns, the calcium titanate ceramic sintered at 1350 °C for 180 min presented a single CaTiO3 perovskite phase. Furthermore, the ceramic product exhibited a maximum density of 3.65 g/cm3 and a minimum porosity of 0.54%. The same manufactured product also exhibited the highest dielectric constant (~78) with the lowest dielectric loss (~0.02), which resulted from the simplified charge polarization process.
A biochar composite (CE) was successfully prepared by Liu et al. [187] using eggshell waste as a source of Ca, and rice straw as a source of C through ball milling and pyrolysis steps. Eggshell was co-milled with rice straw and subsequently heated at 800 °C in order to obtain effective adsorbent of phosphate ions. The result of adsorption studies says that CaO-biochar (EC) composite can efficiently remove (231 mg/g) phosphate from aqueous solution (evidenced for eggshell: rice straw 1:1 ratio). The adsorption was very well described by the pseudo-second order (R2 > 0.975) and Langmuir (R2 > 0.979) models. Thermodynamic analysis revealed that the adsorption process was spontaneous (ΔG 0 < 0) and endothermic (ΔH 0 > 0). The FTIR, XRD, SEM and EDS analyzes justified the adsorption of phosphate on the adsorbent (Figure 21A,B and Figure 22A–C). The elevated temperature improved the outcome, which, together with other results in the paper confirmed the chemical character of adsorption. The article also highlights that the prepared CaO-biochar composite is an efficient adsorbent for removing/recovering phosphate from aqueous solution and has potential application as a multifunctional fertilizer after phosphate adsorption.
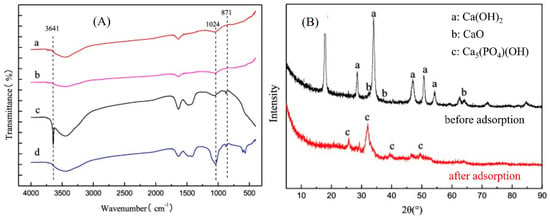
Figure 21.
(A) FTIR spectra of E-C 1:1 and BC materials before and after phosphorus adsorption (a-BC before adsorption, b-BC after adsorption, c-E-C before adsorption, d-E-C after adsorption). (B) XRD spectra of E-C 1:1 before and after phosphorus adsorption. Reprinted from Liu et al. [187]. Copyright 2019 with permission from Elsevier.
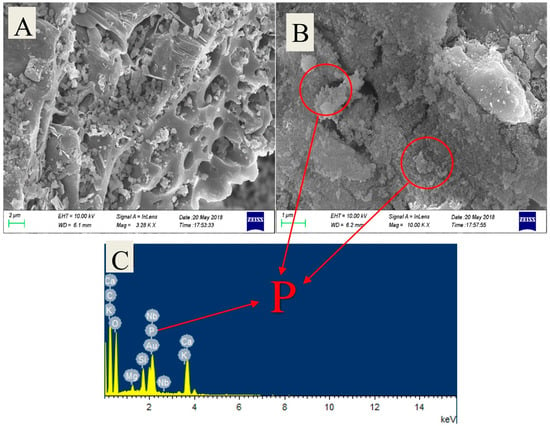
Figure 22.
(A) SEM images of E-C 1:1 before adsorption, (B) SEM images of E-C 1:1 after adsorption and (C) EDS image of E-C 1:1 after adsorption. Reprinted from Liu et al. [187]. Copyright 2019 with permission from Elsevier.
In another study by Sari et al. [188], calcined eggshells were used to produce biogas from palm oil factory effluents and cow dung mixture. The study investigated the effect of particle size reduction achieved by ball milling. According to the results, the increasing amount of nano-Ca led to improved performance; however, the excessive amount of calcium had a detrimental effect on biogas production, as when the Ca concentration is 10 g/L, the result is even worse than in the case of the control experiment. The authors report that further investigation is needed to improve the methane produced during anaerobic digestion.
Egg waste is valuable not only for its calcium-rich inorganic shell but also for its organic inner components, which have been utilized in the mechanochemical preparation of catalytic materials and electrodes. Below are two examples reported in the literature that demonstrate these applications. In a promising contribution on mechanochemistry, Rodríguez-Padrón et al. [189] addressed a facile and environmentally friendly synthesis of a titanium-based nanocomposite (PT-TiO2) using expired egg whites through a model methodology for catalytic and energy storage applications (Figure 23A). The article reports that the catalytic performance of PT-TiO2 sample was evaluated in the microwave-assisted oxidation of diphenyl sulfide (7) to the corresponding sulfoxide (8a) and sulfone (8b) (Scheme 5). The study also showed that the catalytic system remained stable, showing a conversion of 86% after the fifth use (Figure 23D). A comparative study between the prepared material and a commercial one (titania P25), showed that both materials led to comparable results in terms of conversion; however, a critical change in selectivity was observed (Figure 23B,C). On the other hand, the PT-TiO2 nanocomposite was successfully proven as an anode material for lithium-ion batteries, providing a reversible capacity of 107 mA h/g at 0.1C with an excellent Coulombic efficiency of 100% from the second cycle onwards (Figure 24A–D). Furthermore, the synthesized material showed significant capacity retention values of 76% between the 2nd cycle and the 100th cycle. PT-TiO2 turned out to be a versatile material with potential catalytic and energy storage applications.
Figure 23.
(A) Schematic representation of the synthetic protocol of PT-TiO2 material. Catalytic performance of PT-TiO2 and TiO2 P25, in terms of (B) conversion, (C) selectivity and (D) Reusability study of PT-TiO2 nanocomposite. Reprinted from Rodríguez-Padrón et al. [189]. Copyright 2019 with permission from American Chemical Society.
Scheme 5.
Schematic representation of the diphenyl sulfide microwave-assisted oxidation [189].
Figure 24.
Charge–discharge profiles for (A) P25 TiO2 and (B) PT-TiO2 at a current rate of 0.1 A g−1 (approximate to 0.3 C). (C) Specific capacity and coulombic efficiency versus cycle number for PT-TiO2 and P25 TiO2 materials, respectively, at 0.1 A g−1 and (D) Rate capability performances of both anode materials at different current densities from 0.1 C to 2 C, returning to 0.1 C for PT-TiO2 and P25, respectively. (1 C = 335 mA h g−1). Reprinted from Rodríguez-Padrón et al. [189]. Copyright 2019 with permission from American Chemical Society.
Karuppiah et al. [190], prepared a carbon composite lithium iron orthosilicate (LFS@ESM) using a polyol-assisted ball milling method to enhance its poor electronic conductivity, with carbon derived from eggshell membrane. In this study, complete carbonization of the eggshell membrane was achieved through post-grinding calcination in an Ar atmosphere, and the successful iron coating was confirmed by TEM (Transmission Electron Microscopy), which revealed the porous nature of the carbon and the atomic arrangement. The LFS/C composite was characterized as a monolayer, with an average surface area of 24 m2/g, and exhibited excellent electrical properties, including a high Coulombic efficiency of 98.5%, which remained stable for up to 50 cycles. The key findings of the study are summarized in Figure 25.
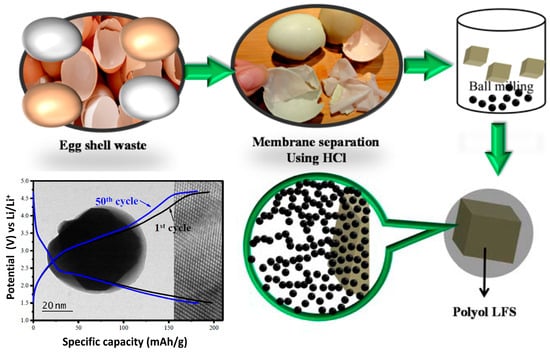
Figure 25.
Schematic illustration of the procedure leading to the production of Li2FeSiO4/C composite. Reprinted from Karuppiah et al. [190]. Copyright 2020 with permission from MDPI under the Creative Commons CC BY license.
Biomass, with an estimated carbon content of 45–50% by weight, is considered a promising and sustainable precursor to prepare advanced carbonaceous materials. Such carbon materials have been widely used in many fields, such as catalysts, adsorbents, and energy storage [181,191]. In general, ordered mesoporous carbons (OMCs) and hierarchically porous carbons (HPCs) are synthesized by hard or soft template methods using toxic regents such as HF (hydrofluoric acid), concentrated NaOH, or formaldehyde among others. These wet processing methods require a large amount of solvent and restrict the widespread application of these materials. The primary self-assembly process for the synthesis of these materials by gentle templating is also induced by the acidic or basic catalyst in the solvent [192]. To overcome these disadvantages, microwave grinding and ball milling are also employed during the synthesis process to enhance the functionality of carbonaceous materials and improve preparation efficiency. Some ball milling methods for preparing biomass-derived carbon materials will be discussed in detail in this section.
In a single-step, solvent-free mechanochemical approach, Zhang and co-workers [193] synthesized ordered mesoporous carbon catalysts from a renewable tannin precursor. The process allowed incorporating cobalt nanoparticles (Co NPs) at high concentrations (up to 21.5 wt%), confining them in an ordered pore structure with tunable diameters (4.3–9.4 nm) and high surface area (up to 700 m2/g) even at high temperatures (Figure 26A). Among the catalysts designated as Co@OMC, the nanocatalyst Co@P1230.8, with 20.9 wt% Co NPs (4.9 nm) uniformly dispersed in 5.1 nm mesoporous channels, stood out with high catalytic efficiency in the deoxygenation of ketones to alkylbenzenes. This catalyst efficiently converted different functional groups (ketones, aldehydes, alcohols) into target molecules in high yields (Scheme 6). The stability of the catalyst was confirmed by the low cobalt leaching (less than 0.1% of total Co, as detected by ICP-AES) in the post-reaction solution. The ultrastability of Co@P1230.8 was evidenced in reuse tests, in which the catalytic performance and textural properties, including the mesoporous structure and dispersion of cobalt nanoparticles, were consistently maintained after multiple reaction cycles (Figure 26B,C).
Figure 26.
(A) Mechanochemical synthesis of Co@OMC catalysts from tannin. (B) Seletive deoxygenation of acetophenone by recycled Co@P1230.8 catalyst. Reaction Conditions: acetophenone 1 mmol, cyclooctane 1 mmol (Internal Standard), hexane 4 mL (Solvent), Co@P1230.8 catalyst 10 mg (Recycled), H2 1 MPa, 120 °C, 23 h. (C) N2 adsorption isotherms of Co@P1230.8 catalyst (Fresh and After use) and the corresponding pore size distributions by using BJH (Barrett-Joyner-Halenda) model. The BET surface area of recycled catalyst was 624 m2/g, a little bit higher than the value (606 m2/g) of fresh one. Reprinted from Zhang et al. [193]. Copyright 2018 with permission from John Wiley and Sons.
Scheme 6.
Selective deoxygenation of ketones, aldehydes, and alcohols by Co@P1230.8 [193].
According to the study by Shan et al. [194] magnetic hybrid materials of biochar/Fe3O4 and AC/Fe3O4 were synthesized and evaluated for the removal of pharmaceuticals, specifically carbamazepine (CBZ) and tetracycline (TC), through adsorption and mechanochemical degradation. Both hybrid materials demonstrated fast adsorption kinetics, with most of the removal occurring within the first hour. Biochar/Fe3O4 exhibited a maximum adsorption capacity of 62.7 mg/g for CBZ and 94.2 mg/g for TC, while AC/Fe3O4 presented values of 135.1 mg/g for CBZ and 45.3 mg/g for TC. Degradation by ball milling was more effective for pharmaceuticals adsorbed on biochar/Fe3O4. After 3 h, TC was 99% degraded on biochar/Fe3O4 and 97% on AC/Fe3O4. For CBZ, degradation was less efficient on both materials. However, the addition of 300 mg SiO2/g of adsorbent significantly improved degradation, reaching 98.4% for CBZ on biochar/Fe3O4 and 88.2% on AC/Fe3O4.
Pure and ball-milled wheat stalk biochars pyrolyzed at 300 °C, 450 °C, 600 °C were studied by Xiang et al. [195] for adsorption of TC hydrochloride from aqueous solution. The result of this study revealed that compared to original biochar, 600 °C pyrolyzed and ball-milled biochar (BM-biochar) adsorbs more TC in the order of 84.54 mg/g. The study also assessed that the adsorption performance of TC was significantly improved largely due to the strong positive correlation between adsorption and specific surface area (257.50 m2/g), total pore volume (0.2678 cm3/g) or mesoporous volume (0.1966 cm3/g). The adsorption results showed a better fit with pseudo second order kinetics. The Langmuir isotherm values show maximum fit for the adsorbent-adsorbate interaction. The paper adequately explains the influence of pH on the maximum adsorption of TC; however, less attention is given to aspects such as the surface charge of the adsorbent, thermodynamics, and the underlying adsorption mechanism, which remain unknown.
Sugarcane bagasse biomass ground into balls and pyrolyzed at 450 °C (BMBG450) was prepared by Lyu et al. [196] to be applied as an adsorbent of methylene blue (MB). Compared to unground bagasse biochar (BG450), BMBG450 showed greater specific surface area (increased from 51 to 331 m2/g), larger pore volume (increased from 0.008 to 0.099 cm3/g), smaller hydrodynamic radius (reduced from 0.5–1 mm to 170 nm), stronger negative zeta potential (about 1.6-fold increase) and more oxygen-containing functional groups (increase 1.05 mmol/g). The adsorption isotherm results indicated that the adsorption fit well with the Langmuir model and the BMBG450 composite showed a maximum removal capacity of 354 mg/g vs. 17.2 mg/g of original BG450. The work pointed out that π-π and electrostatic interactions were dominant mechanisms for MB sorption. The article satisfactorily explained the adsorption mechanism, the influence of pH on adsorption, and the pHpzc of the prepared material and, therefore, justified the adsorption process (Figure 27).
Figure 27.
Illustration of governing mechanisms of methylene blue (MB) adsorption onto unmilled and milled biochars and the adsorption of MB on BMBG450. Reprinted from Lyu et al. [196]. Copyright 2018 with permission from Elsevier.
Dual-functional MgO-biochar nanocomposites were successfully synthesized by Zheng et al. [197], by a novel and facile method that ground walnut biochar with MgO particles under a solvent-free condition. The study reported that MgO-biochar nanocomposites exhibited sorption performance of 62.9% removal of phosphate, and 87.5% removal of methylene blue at low adsorbent dosages of 1.0 g/L and 0.2 g/L, respectively.
Being the byproduct of biodiesel synthesis, glycerol valorization as reagent [198,199,200] and/or solvent [201,202] is a hot topic in scientific community. Searching for an alternative process for the production of calcium diglyceroxide (CaDG) was successfully synthesized by Lukić et al. [26], using mechanochemical treatment between glycerol (18) and CaO in a ratio of 1:5. This was used as a catalyst of the transesterification of sunflower oil to afford biodiesel (F.A.M.E in Scheme 7) under different working conditions: amount of catalyst, stirring speed, temperature and methanol/oil molar ratio. The kinetic model involved the physical effect of CaDG on the reduction in mass transfer resistance, which enhanced the transesterification rate. According to the report, it was found that the maximum (<100%) conversion of triglycerides (15) can be achieved with a molar ratio of methanol (16): sunflower oil of 10:1, 0.5 wt% catalyst, at 60 °C, after 2 h of reaction. However, the reusability of the catalyst and the possible mechanism were not evaluated in this study.
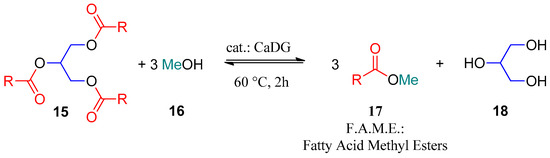
Scheme 7.
Reaction scheme of sunflower oil methanolysis under CaDG [26].
In subsequent studies, the synthesis of CaDG using a continuous mechanochemical reactor (MCR) was reported, using the same equipment used for biodiesel production [203,204]. Specifically, Glycerol and CaO were mixed in two different molar ratios, 3:1 and 5:1, stirred in a mixing tank before being pumped into the MCR (0.5 L volume) at a flow rate of 4 and 150 L/h, respectively. In this study, XRD data was used for both the refinement and quantification of CaDG crystal formation, both of which were carried out using the Rietveld method. According to the results of the Rietveld method, with glycerol: CaO molar ratio of 3:1, the temperature needed to be increased from 25 °C to 50 °C for optimal yield (91 and >99%, in that order). To achieve yields greater than 99% at room temperature (25 °C), it was necessary to increase the molar ratio of glycerol to CaO to 5. The researchers also evaluated the influence of the presence of water due to the hydrophilic nature of glycerol. In the presence of 10 wt% water and glycerol: CaO molar ratio of 5:1, CaDG was obtained in excellent quantitative yield (>99%) with a residence time of 30 min. The same research group investigated the production of biodiesel from both sunflower oil and cooking oil via transesterification using 1.5 wt% CaDG as the catalyst and employing methanol to oil molar ratio close to stoichiometry (4:1). With a continuous inlet flow between 4 and 45 L/h, the same semi-continuous mechanochemical reactor (volume of 0.5 L) containing zirconia spheres doped with yttrium (diameter of 0.3 to 2.0 mm) was used, occupying a volume of 55% to 70% (Figure 28). The subsequent stirring reaction mixture for 4 h at 50 °C or 24 h at room temperature completed the reaction and allowed the production of biodiesel with a yield greater than 90%. According to the authors, this new biodiesel production process proved to be efficient and scalable and can be applied to the conversion of used cooking oils without any significant loss of yield. Furthermore, this new approach proved to be more economical than the conventional batch stirring process. The results showed that the methanol-oil molar ratio can be reduced from 12:1 to 4:1, and the catalyst weight can be reduced from 4% to 1.5% in a standard laboratory-scale reactor, using a more economical process compared to the conventional batch stirring method.
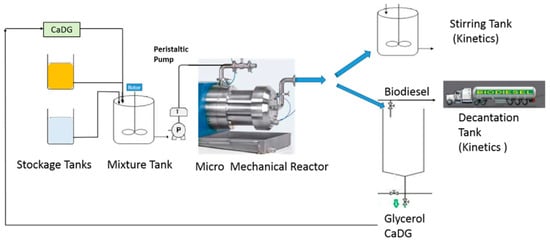
Figure 28.
Flow diagram of CaDG synthesis and biodiesel production, both using the same continuous mechanochemical reactor (CMR). Reprinted from Malpartida et al. [204]. Copyright 2020 with permission from Elsevier.
To develop a clean production process, Li et al. [205] explored the reaction of CaC2 (calcium carbide) and glycerol (18) under varied conditions. The result of this study revealed that CaC2 can react with glycerol at room temperature in a stirred mill with a rotation speed of 450 rpm, with 96% conversion of glycerol after 1 h of grinding. As a result, the desired acetylene (21) was obtained in good yield, with 96% glycerol conversion (Scheme 8). Importantly, when compared to conventional acetylene production methods, the authors did not detect any PH3, AsH3, and H2S formed as side reactions involving Ca3P2, Ca3As2, and CaS, respectively. It was evident from this study that the mechanochemical reaction performance of CaC2 with glycerol is more efficient than the corresponding thermochemical reaction at high temperatures, and CaC2 shows much greater reactivity than CaO with glycerol.
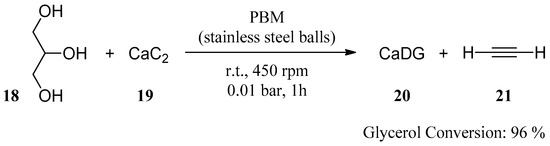
Scheme 8.
Synthesis of acetylene and CaDG starting from calcium carbide and glycerol in a planetary ball mill [205].
In summary, the mechanochemical-assisted method is an efficient way to improve product yield and reduce biomass conversion reaction time compared to the solution-based catalytic method. Literature data on some typical examples of improving the yield of biomass-based products through mechanochemical-assisted methods are listed in Table 5.

Table 5.
Composites synthesized by mechanical grinding from different wastes as precursors.
3.7. Recovery of Used Battery Metals
The lithium-ion battery (LIB) industry has developed rapidly in recent years, accounting for approximately 85% of the rechargeable battery market. And the outlook is that by 2030, the world is expected to have more than 200 million electric vehicles and a battery demand of more than 3 TWh by 2030. The production of LIBs consumes a significant amount of metal resources, especially lithium and cobalt, which will potentially trigger a strategic metals crisis. In addition, compared with other batteries, LIBs do not contain toxic heavy metals such as lead and mercury, but they may cause certain harm to the environment and human health in their production, consumption and disposal. And the development of a recycling process for used lithium-ion batteries is necessary and urgent from the perspective of environmental protection and circular economy of resources [213,214].
Lithium carbonate is an inorganic salt that is used in a variety of applications such as catalysts, ceramics and pharmaceuticals. Recovery of lithium as lithium carbonate salt by precipitation is a common recovery method performed after leaching and LIB recovery processes. The predominant LIB recovery technologies are mainly based on hydrometallurgical approaches and pyrometallurgical methods [215]. Initially, several pretreatment steps are required for both methods, which include classification, discharge, disassembly, crushing, screening and heat treatment. Both techniques require the synergistic use of solvents and heat to drive reactions along the path of selective leaching of high-value metals and enrichment of impurity elements, which can bring environmental problems due to the emission of toxic gases and effluents [216]. As both methods have many disadvantages, an alternative needs to be found to make LIB recycling more environmentally friendly and at the same time profitable for the industry [217]. To promote an even more efficient leaching process, enhanced leaching technologies such as ultrasonication [218], microwave [219] and mechanochemical activation (MCA) were explored.
Saeki et al. [220] and Zhang et al. [221] developed a process for recovering Co and Li metals from waste lithium-ion secondary batteries (LIS) and LiCoO2 powder by co-grinding with polyvinyl chloride (PVC) using the mechanochemical activation method. Meanwhile, more than 90% of Co and nearly 100% of Li were extracted from the used LIS by a mechanochemical method after 30 h of grinding between LiCoO2 and polyvinyl chloride (PVC) to form chlorides that are soluble in water. Consequently, about 90% of the chlorine in the PVC sample was transformed into inorganic chlorides at the time. Wang et al. [222] also achieved conversion rates of 100% to 91.9% to Co, respectively, with 12 h of grinding. During the mechanochemical reaction, PVC played two important roles: one as a chloride source, and the other as a carbon source that acted as a reducing agent of Co(III) in LiCoO2 to Co(II) in cobalt chloride (CoCl2). These studies revealed that the mechanochemical processes not only obviously simplified the leaching of metals but also avoided the generation of liquor residues at the solid–solid reaction point.
On the other hand, Guan and his colleagues [223] examined the feasibility of mechanochemical processes to enhance the acid leaching of Co and Li at room temperature from spent LIBs. In this study, metallic iron powder was used as a reducing reagent and served as grinding aids with good performance for the extraction of valuable metals. A mechanochemical reduction time of 250 min operating at a rotation speed of 650 rpm and a mass ratio of Fe/LiCoO2 of 1:1 enabled a positive leaching of 80% of Co compared to the extraction of Li (63%). The mechanochemical reduction mechanism with Fe powder altered the valence state of Co and played a dominant role in the high-efficiency extraction of Co, supported by the characterization analyses conducted by XRD, SEM, and XPS. In a comparative study with pure materials, the extractions of Li (77%), Co (91.25%), Mn (100%), and Ni (99.9%) were obviously enhanced after 2 h of leaching in HNO3 (nitric acid) solution (1 M) at room temperature. Furthermore, more than 99% of Fe could be leached by this mechanochemical reduction process in acidic solutions.
Meanwhile, Liu et al. [224], in their study, proposed a selective (>90%) and acid-free Li extraction process to successfully achieve isomorphic substitution of LiCl in LiFePO4 crystals with sodium (Na). The method uses LiFePO4 powder co-ground with NaCl in the ratio of 1:2 at a rotation speed of 500 rpm for 6 h. Thus, NaFePO4 and LiCl were obtained due to the substitution of Li and Na. Li was recovered as Li2CO3 and NaCl was regenerated simultaneously using Na2CO3 as the sole reagent, without acid and no detection of leached Fe.
Mechanochemical recovery of Li and Co from pure LiCoO2 and commercial LIB cathodes using Al as a reducing agent was successfully demonstrated by Dolotko et al. [225]. The recovery rates achieved during experiments with pure LiCoO2 are 90% for Co and 76% for Li. In the mechanochemical reduction reaction experiments involving commercial LIB cathodes for 3 h, polyvinylidene fluoride (PVDF) and Al were used as reducing agents. After dispersion in water and magnetic separation, the magnetic material was sonicated in water for 5 min to remove traces of PVDF. Recycling 4 g of the cathode material yields 0.6 g of metallic Co and 0.3 g of Li2CO3 (Figure 29). In a recent publication, Dolotko et al. [226] reported a systematic study of Li recovery up to 70% from various cathodes, such as LiCoO2, LiMn2O4, Li(CoNiMn)O2, LiFePO4 and their mixtures, using Al as a reducing agent in the mechanochemical reaction, without applying corrosive leachates or using high temperatures in the recycling process. Both studies report that the developed processes are simple, fast, energy efficient and do not involve or produce highly corrosive liquid waste or toxic gases, thus offering clear advantages over other known recycling techniques for any type of LIB.
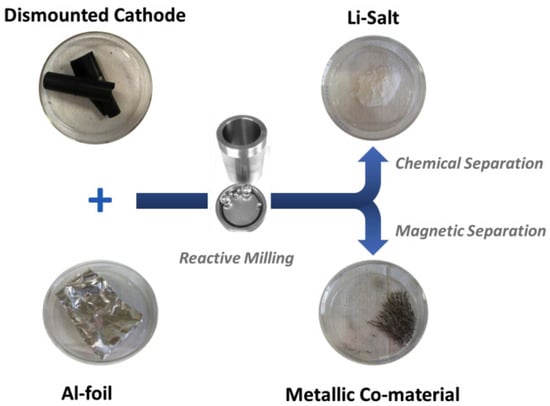
Figure 29.
The process of mechanochemical/chemical recovery of Li and Co from the Li-ion cell cathode, Reprinted from Dolotko et al. [225]. Copyright 2020 with permission from Elsevier.
Liang et al. [213] demonstrated that advanced oxidation processes enhanced by mechanochemical activation can also enhance the effective leaching of valuable metals from spent LIBs. In this study, spent cathode material (LNCM) was subjected to co-milling with oxidant (Ammonium persulfate (NH4)2S2O8 or NHS) and reductant (sucrose, Suc) simultaneously in a ball milling process followed by acid leaching. The study reported that leaching rates of 99.1%, 96.2%, 99.9%, and 99.2% were achieved for Li, Co, Ni, and Mn, respectively, under the following conditions: rotational speed of 800 rpm, grinding time of 3 h, mass ratio of NHS/LNCM and Suc/LNCM of 2:1, leaching temperature of 25 °C, for 15 min at pH 2. The study demonstrated the synergistic effect of ammonium persulfate, iron (0.03 g/g LNCM, activating persulfate to form strong oxidative species), and sucrose in significantly improving the leaching efficiency. The successful leaching of the desired metals within a short period after the mechanochemical activation event could be well tracked by FT-IR, XRD, and XPS analyses that served to support the reaction mechanism.
Zhang et al. [227] reported a one-step mechanochemical leaching process using grape skin powder (GS) as a reducing agent to recover metals from cathode residues of LIBs. It was reported that maximum leaching rates of Li and Co metals reached 99.33% and 98.87%, respectively, using mechanochemical activation at a speed of 400 rpm for 60 min, 0.3 gGS/gLCO of GS added, 0.15 M H3Cit (citric acid), and a solid/liquid ratio of 30 g/L GS. The mechanochemical leaching mechanism was analyzed by BET, PSD, XRD, and XPS. The study proved the superiority of using one-step direct mechanochemical activation as the leaching method for this system over the two-step process because a significantly lower amount of H3Cit can be used in the absence of heating.
Cobalt leaching from spent LIBs using a mechanochemically assisted method in sulfuric acid and hydrogen peroxide system was studied by Qiao et al. [228]. This process was also optimized for better results by response surface methodology (RSM). The optimum conditions determined were H2SO4 concentration of 0.5 M, H2O2 concentration of 2 M, ball-to-powder mass ratio of 35:1, solid-to-liquid ratio of 20 g/L, reaction time of 120 min, revolution speed of 100 rpm, rotation speed of 468 rpm. Under these conditions, the cobalt leaching efficiency reached 98.96%. The work also demonstrated that the Co leaching rate was improved compared to the results in the relevant literature.
Liu and his collaborators [229] developed an advanced and rapid green recovery process for recycling used LiFePO4 (LFP) batteries. In this study, a water leaching process using an ultrafast mechanochemical reaction (UMR) flash metallurgy technology was shown to be highly efficient, acid-free and at room temperature, for selective Li recovery. Recovery rates for Li and Fe of 99.17% and 2.39%, respectively, were achieved under the optimized conditions: a mass ratio of C10H14N2Na2O8 to LFP of 3:1, the addition of 0.5 mL of H2O2, a rotation speed of 30 Hz, 4 min of UMR time, and a filling coefficient of 0.79. After filtration and precipitation, Fe and Li are finally recovered as FePO4 and Li2CO3 precursors, respectively. Furthermore, based on XRD, XPS and TEM characterizations, along with simulations, the study reveals the chemical and mechanical mechanisms of how UMR extracts Li from the orthorhombic structure (Figure 30). Overall, this research highlighted the significant potential that advanced mechanochemical oxidation technology creating an acid-free environment can have to improve industrial efficiency for a greener future that aligns with the era of green chemistry in the entire process that is recycling of used LIBs.
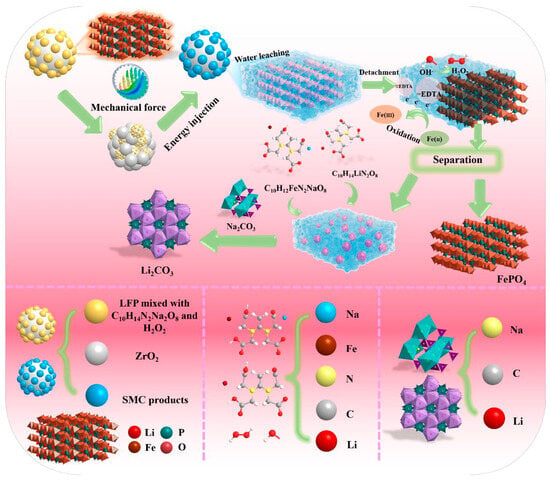
Figure 30.
Possible mechanisms in the UMR process. Reprinted from Liu et al. [229]. Copyright 2024 with permission from Elsevier.
Mechanochemistry has been increasingly applied in recent years to address some of the difficulties in recycling metals from different wastes. As reviewed previously, mechanochemical methods can successfully recover a variety of metals. Table 6 summarizes studies that have applied mechanochemical methods for recycling metals from different wastes, the processing parameters used, and the recycling efficiencies achieved.

Table 6.
Summary of applications of assisted mechanochemical methods in recycling of waste metals.
Table 6.
Summary of applications of assisted mechanochemical methods in recycling of waste metals.
| Materies | Type of Mill | Milling Parameters | Leaching Parameters | Leaching Efficiency (%) | Ref. |
|---|---|---|---|---|---|
| LCO | Planetary | Ratio of the rotational-to-revolution speed is fixed at 1, air atmosphere Time: 36 h, Co-grinding reagent: polyvinyl chloride (PVC)-LiCoO2: = 1:1 | H2O, L/S = 250 mL/g, 1 h, agitation | 90% Co and >90% Li | [221] |
| LCO | Planetary | Ratio of the rotational-to-revolution speed is fixed at 1, Time: 30 h, air atmosphere Co-grinding reagent: polyvinyl chloride (PVC)/LiCoO2 = 1:1 | H2O, L/S = 250 mL/g, 1 h, agitation | 90% Co and 100% Li | [220] |
| CRT | Planetary | Speed: 500 rpm, Time: 240 min | H2O, L/S = 20 mL/g, agitation, 95 °C, 2 h 3 M HNO3, L/S = 150 mL/g, agitation, 95 °C, 2 h | 92.5% Pb | [230] |
| LCO | Planetary | Speed: 600 rpm Time: 240 min Co-grinding reagent: LiCoO2/EDTA = 1:4 | H2O: 100 mL, agitated for 30 min | 98% Co and 99% Li | [231] |
| LCO | Planetary | Speed: 600 rpm Time: 12 h Co-grinding reagent: LCO/PVC/Fe = 1:1:2 | H2O: 100 mL | 81.1% Co, 91.9% Co was rearranged to CoFexOy and 100% Li | [222] |
| LCO | Planetary | Speed: 650 rpm, Time: 250 min Co-grinding reagent: Fe/LiCoO2 = 1:1 | 1 M HNO3; 2 h, 25 °C, magnetic stirring | 99.9% Ni, 91.25% Co, 100% Mn and 77.15% Li | [223] |
| LFP | Planetary | Speed: 550 rpm, Time: 120 min, Co-grinding reagent: LiFePO4/EDTA-2Na = 3:1 | 0.6 M H3PO4, S/L = 50 g/L, 20 min, 25 °C | 97.67% Fe and 94.29% Li | [232] |
| LCO | Planetary | Speed: 500 rpm Time:60 min | 1.0 M L-ascorbic acid; 20 min; 298 K; 10 g/L | 99% Co and 100% Li | [233] |
| LCO | Planetary | Speed: 500 rpm Time:30 min Co-grinding reagent: C | 20 vol% acetic acid; 5 vol% H2O2; 15 min; 298 K | 99.7% Co and 99.8% Li | [234] |
| LFP | Planetary | Speed: 500 rpm Time:120 min Co-grinding reagent: oxalic acid ball milling medium:1 mL H2O | H2O | 94% Fe and 99% Li | [235] |
| LFP | Planetary | Speed: 500 rpm Time: 360 min Co-grinding reagent: NaCl | H2O | >90% Li | [224] |
| WPCB | Planetary | Speed: 400 rpm Time: 240 min Co-grinding reagent: K2S2O8/WPCB = 3:2 | H2O: 50 mL; magnetic stirring; 300 rpm. temperature: 25, 35, 45, and 55 °C | >98% Cu | [236] |
| LCO | SPEX 8000 shaker mil | Speed = 1725 rpm Time = 180 min, Co-grinding reagent: Al/LiCoO2 = 1:1 under air atmosphere and LiCoO2/Li = 1:3 and LiCoO2/Ca = 1:1.5 under argon atmosphere | H2O, stirred for a few minutes in air, 1 M Na2CO3, stirred for 1 h, room temperature | 90% Co and 70% Li | [225] |
| LCO | Planetary | Speed = 700 rpm Time = 90 min, and LiCoO2/Li and LiCoO2/Ca atmospheric pressure Co-grinding reagent: dry ice; mass ratio = 1:20, NaCl, SiO2; LCO/NaCl mass ratio = 1:6, LCO/SiO2 mass ratio = 1:2, room temperature | H2O, magnetic stirring, room temperature, 5 min | 95% Li | [237] |
| LCO | Planetary | Speed: 500 rpm, Time: 720 min, Co-grinding reagent: LiCoO2/NaCl = 1:6 and LiCoO2/SiO2 = 1:2, room temperature and atmospheric pressure | H2O, magnetic stirring, room temperature, 5 min | 92.89% Li | [238] |
| NCM | Planetary | Speed: 550 rpm Time: 120 min Co-grinding reagent: Zn powder | 1.5 M H2SO4; 15 min; 323 K; 20 g/L | 96.2% Ni, 94.3% Co, 91.0% Mn and 99.9% Li | [239] |
| LNCM | Planetary | Speed: 800 rpm Time: 180 min Co-grinding reagent: (NH4)2SO4, sucrose and powder Fe | 1 M H2SO4; 298 K, 15 min; pH 2.0; 25 g/L. | 99.1% Li, 96.2% Co, 99.9% Ni and 99.2% Mn, | [213] |
| LCO | Planetary | Speed: 500 rpm Time: 120 min | 1 M H2SO4; 0.03 M NH4Cl; 60 min; 353 K; 20 g/L | 99.22% Co and 100% Li | [240] |
| LCO | Planetary | Speed: 500 rpm, Time: 240 min, Co-grinding reagent: LCO/alginic acid = 1:10 and 2 mL of H2O2 | H2O, room temperature, and agitated for 5 min | 97.58% Li and 98.59% Co | [241] |
| LCO | Planetary | Speed:400 rpm Time:60 min Co-grinding reagent: Grape skin powder | 0.15 M citric acid; 323 K; 30 min; 30 g/L | 98.87% Co and 99.33% Li | [227] |
| LCO, NMC, LMO and LFP | SPEX 8000 shaker mill | Speed = 1725 rpm Time = 180 min, Co-grinding reagent: Al/LiCoO2 = 1:1, Al/NMC = 1:2.33, LiMn2O4/Al = 1:2.33, LiFePO4/Al = 1:3 and LCO:NMC:LMO:LFP:7.33Al, all experiments were performed in air atmosphere | H2O, stirred for a few minutes in air, room temperature | 29.8–39.6% Li | [226] |
| LCO, NMC, LMO and LFP | SPEX 8000 shaker mill | Speed = 1725 rpm. Time = 180 min, Co-grinding reagent: Al/LiCoO2 = 1:1, Al/NMC = 1:2.33, LiMn2O4/Al = 1:2.33, LiFePO4/Al = 1:3 and LCO:NMC:LMO:LFP:7.33Al, all experiments were performed in air atmosphere | H2O, stirred for a few minutes in air, room temperature, carbonatization and recrystallization at 70 °C | 55.6–75.9% Li | [226] |
| LCO | Planetary | Speed = 650 rpm, time = 90 min, ball-to-powder mass ratio = 60:1 | EDTA 0.052 M, H2O2 0.3 vol.% at room temperature and leaching time = 60 min | 100% Co and 98.2% Li | [242] |
| LCO | Planetary | Speed: 600 rpm, Time: 60 min, Co-grinding reagent: citric acid/LCO = 1 and ascorbic acid/LCO = 0.5 | H2O, S/L = 775 g/L, 15 min, room temperature | 97.2% Co and 99.7% Li | [243] |
| LCO | Planetary | revolution speed of 100 r/min, rotation speed of 468 r/min, Time: 120 min, Co-grinding reagent: H2SO4 0.5 M, H2O2 2 M and LCO | Saturated H2C2O4 solution, S/L = 20 | 98.96% Co | [228] |
| LFP | _ | Speed of 30 Hz Time: 4 min Co-grinding reagent: C10H14N2Na2O8 and H2O2 | H2O; 298 K; 30 min | 99.17% Li and 2.39% Fe | [229] |
3.8. Synthesis of Battery-Based Nanocomposites
An efficient strategy for recycling used LIBs is the direct regeneration of cathode materials from the used cathodes themselves. Successful results have been reported in the literature for the regeneration of cathode materials. Wang et al. [244] synthesized a material in 30 min, Li[Li0.2Mn0.54Ni0.13Co0.13]O2 nanometric, from Li2CO3 cathode materials used by a microwave technique assisted by mechanical and chemical activation. The material showed optimal electrochemical performance with an initial discharge capacity of 239 mA h/g and initial coulombic efficiency of 73% at 25 mA/g and 228, 215, 193 mA h/g at 50, 150 and 300 mA/g, respectively, with almost no capacity loss after 100 cycles at 0.1 C.
Meng et al. [245] regenerated LiMn2O4 used involving a solid-state technique assisted by mechanochemical activation to synthesize a high-performance nano-LiMnPO4/C composite. The obtained material exhibited promising electrochemical performance with discharge capacities of 148.5 mA h/g at a rate of 0.05 C, respectively, in the voltage range of 2.5 to 4.5 V. The result also demonstrated that the LiMnPO4 material has high structural stability and stable cycling performance with a capacity retention of 98% after 100 cycles at a rate of 1 C.
Meng et al. [246] also reported an integrated method of mechanochemical activation and high-temperature solid-state sintering (800 °C for 10 h). In this study, the ball milling process was introduced to facilitate the diffusion of lithium and nickel ions, thereby decreasing the cationic disorder and rebuilding the layered crystal structure. Combined with sintering, the recycled MA-1.20-800 provided a specific discharge capacity of 165 mA h/g at 0.2 C in the first cycle and a capacity retention maintained above 80% after 100 cycles.
The preparation of a highly efficient and easily scalable solid-phase recycling procedure for the cyclic utilization of waste LFP was proposed by Song et al. [247]. In this study, the waste LFP material (W-LFP) was regenerated by mechanical separation (ball milling), removing impurities. Then, 5% carbon nanotubes, 15 wt% glucose, and 5 wt% Li2CO3 were added to the W-LFP (1:1 aqueous ethanol solution as dispersant), followed by ball milling, and the subsequent heat treatment at 650 °C for 12 h. Finally, the obtained regenerated LFP (R-LFP) achieves an excellent specific capacity of 155.47 mA h/g at 0.05 C, which is about 99% of the capacity of a fresh LFP, and long-term cyclic stability (more than 800 cycles at 1 C). The work emphasizes that the main consumption costs in the regeneration process account for only 33.7% of the price of a fresh LFP. Likewise, Liu and his collaborators [248], prepared a new cathode material doped with 3 mol% V, after calcination, to prepare a new material R3, with a discharge capacity of 134.3 mA h/g after 200 cycles at 1 C maintained 99.1% discharge capacity retention. At 2, 5, 10 C, the discharge capacities are 134, 124.1, 111.5 mA h/g, respectively.
Gou et al. [249] described the sample preparation process, first using ethanol or aqueous solution as the dispersant, supplementing the lithium source with waste LiFePO4 cathode powder, and adding melamine as the carbon and nitrogen source. The slurry was mixed by ball milling, dried, and sintered at high temperature under inert gas to obtain a repaired nitrogen-doped carbon-coated LiFePO4 sample (Figure 31). The regenerated R-(C + N)-LiFePO4 exhibits a large discharge capacity of 168 mA h/g. In addition, it exhibited an excellent capacity preservation rate of 99.03% after 200 cycles and an exceptional charge capacity at 5 C, with a discharge capacity of 116 mA h/g.
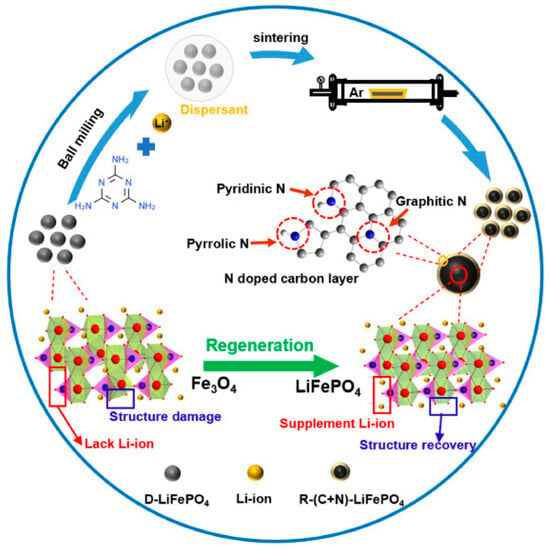
Figure 31.
Diagram of the preparation process of R-(C + N)-LiFePO4. Reprinted from Gou et al. [249]. Copyright 2024 with permission from Elsevier.
Representative publications on direct regeneration of cathode materials from spent cathodes themselves are listed in Table 7. So far, heat treatment is almost an inevitable process for repairing cathodes and effectively improves electrochemical performance.

Table 7.
Electrochemical characteristics of various materials as cathodes.
3.9. Technology Assessment
According to the studies analyzed in this review, there are many advantages of the mechanochemical process over the solution-based wet process. Mechanochemical processing bypasses the requirement for reagent solubility and large solvent consumption, as well as avoiding complex post-treatment steps, such as solvent removal and product purification [48,49,52,191]. Another advantage of mechanochemistry is its operation at room temperature. Therefore, from an environmental and economic point of view, mechanochemistry is an environmentally friendly and labor- and time-saving approach for the synthesis of chemicals and materials from various types of substrates. Throughout this review, several advantages of mechanochemical reactions were highlighted and most of these benefits were related to efficiency and sustainability [252].
Mechanochemistry has become an innovative, advantageous, and very attractive method for producing various materials, polymers, and small chemical products with great added value. Furthermore, most of these advantages are in line with the twelve principles of green chemistry [52]. In general, mechanochemical treatment is well known as a key technology for surface modification and reactivity improvement of various types of substrates (Table 8).

Table 8.
The most important benefits and drawbacks of mechanochemical activation [191].
Another problem is the long grinding time usually required, especially to achieve complete degradation of different residues, such as halogenated pollutants, discarded batteries, residual biomass, among others. This represents an obstacle, since energy consumption is the main weakness of mechanochemical technology. In general, high load rates and rotation speeds reduce grinding times, making the process more efficient, but require more powerful mills that, in most cases, consume more energy. Consequently, energy-intensive grinding for very long periods makes the full-scale mechanochemical process economically impractical [53]. Therefore, the selection of suitable grinding devices and a suitable mixture of reagents to improve the reaction kinetics would allow reducing energy costs, in terms of energy consumption and grinding duration, which continue to be the focus of research.
3.10. Limitations of Technology
As discussed in the previous sections, mechanochemistry emerges as a sustainable alternative to traditional synthesis, reducing the use of solvents and expanding reactive possibilities. However, some obstacles and limitations to the technique can be identified below.
3.10.1. Product Purification
As mentioned in the previous sections, even though mechanochemistry is sometimes a solvent-free technique, it may still require the use of solvents to purify the final product. Removing non-volatile byproducts or traces of reagents may require extraction or recrystallization processes that use solvents. Therefore, mechanochemistry is unlikely to make all chemical syntheses completely solvent-free, but it is important to identify situations in which it offers a clear advantage over conventional methods [9,253].
3.10.2. Difficulty of Scalability
In the previous sections, it was demonstrated that all mechanochemical processes were performed at laboratory scales, ranging from a few hundred milligrams to a few grams. Although grinding equipment for large-scale material processing is available, the issue of scalability in mechanochemistry has not yet been widely explored. One of the main barriers is the transition from laboratory to industrial scale. While mechanochemistry is ideal for small production runs, its large-scale application is complex. Many mechanochemical processes are performed in batch reactors, which are less efficient than the continuous reactors used in conventional industry. This makes it difficult to produce large volumes of materials economically and reproducibly [9,52,253,254]. The lack of efficient continuous mechanochemical reactors for the chemical industry is a significant challenge [9,253]. Therefore, the perception persists that scaling up this technique presents difficulties.
3.10.3. Parameter Control and Reproducibility
Controlling variables such as temperature, pressure, impact frequency, and degree of grinding is more complex in mechanochemistry than in solution reactions. Small variations in grinding frequency, for example, can significantly influence reaction yield. Mechanical energy and heat dissipation during milling are difficult to control and monitor in real time, which can lead to undesirable variations in the structure and purity of the final product, compromising reproducibility [251,253,254,255]. Knowledge about mechanosynthesis parameters, such as milling conditions and the use of activating agents and precursors, is still developing. Despite notable progress in specific examples presented in this review, the technique has not yet reached the same level of sophistication and versatility as solution-phase synthesis strategies.
3.10.4. Equipment Wear and Contamination
The constant collision of grinding balls and friction can cause wear on the reactors and the balls themselves, leading to product contamination. Although modern mills are designed to minimize this wear, contamination by metal or ceramic particles can be a problem, especially in the production of high-purity materials for the pharmaceutical and electronics industries [52,252,253,254].
In general, mechanochemistry focuses on making known solution-based synthetic procedures more environmentally friendly by avoiding the use of solvents, which is also one of the main disadvantages. Therefore, the development of innovative bond-forming reactions under mechanical milling should be highly valued, as they are inaccessible through solution-phase chemistry.
4. Conclusions
The growing global demand for the sustainable use of natural resources has driven the development of green processes, including the recycling and valorization of waste generated by various anthropogenic activities. These processes enable the production of functional materials with applications across multiple sectors such as medicine, engineering, pharmaceuticals, and chemistry.
Although nature offers a vast diversity of raw materials—many of which are still underexplored—waste materials, often improperly discarded in landfills or natural environments, have demonstrated significant potential for reuse. Over the past decades, a substantial body of literature has emerged highlighting the economic and environmental benefits of converting waste into useful products, reinforcing the growing interest of research groups worldwide in this field.
Among the innovative strategies explored, mechanochemistry has shown particular promise in addressing the challenges associated with waste management, offering a solvent-free, energy-efficient route for the modification and transformation of diverse waste streams. This review presented current advances in the application of mechanochemical techniques to the modification of fly ash, geopolymer synthesis, dehalogenation of electronic waste, metal ion extraction, and the generation of value-added materials.
These studies demonstrate that mechanochemistry is a versatile and environmentally friendly platform with applications in catalysis, electrochemistry, and advanced material production. While this approach offers clear advantages—such as reduced processing time, lower costs, and elimination of harmful reagents—it still remains at a pre-commercial stage, requiring further research, optimization, and scale-up.
The widespread implementation of mechanochemical strategies would represent a significant step forward in waste valorization, contributing to more sustainable production systems, the mitigation of environmental liabilities, and the generation of economically valuable materials from solid waste.
Author Contributions
Conceptualization, R.E., L.A.S.d.N., and A.A.V.; methodology, A.A.V. and. V.A.d.M.; validation, R.E., M.M., R.C.R.N., and L.A.S.d.N.; formal analysis, R.L., R.E., and M.M.; investigation, A.d.N.d.O., R.C.R.N., and A.A.V.; resources, writing—original draft preparation A.A.V., A.d.N.d.O., L.C.P.G., and V.A.d.M.; writing—review and editing R.E., M.M., R.L., R.C.R.N., and L.A.S.d.N.; supervision, R.E., R.C.R.N., and L.A.S.d.N.; project administration, R.L., R.E., and L.A.S.d.N.; funding acquisition, L.A.S.d.N. and R.C.R.N. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank CNPQ under grant number 306665/2025-5, 309711/2023-1, and 444428/2024-0; FAPESPA (073/2023, 167/2024 and 160/2024); Banco da Amazônia 2022/233; and CAPES (LCPG and VAM grants).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors would like to thank PROPESPG/UNIFAP and PROPESP UFPA.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Šepelák, V.; Düvel, A.; Wilkening, M.; Becker, K.-D.; Heitjans, P. Mechanochemical Reactions and Syntheses of Oxides. Chem. Soc. Rev. 2013, 42, 7507. [Google Scholar] [CrossRef] [PubMed]
- Takacs, L.M. Carey Lea, the First Mechanochemist. J. Mater. Sci. 2004, 39, 4987–4993. [Google Scholar] [CrossRef]
- Oliveira, P.F.M.; Baron, M.; Chamayou, A.; André-Barrès, C.; Guidetti, B.; Baltas, M. Solvent-Free Mechanochemical Route for Green Synthesis of Pharmaceutically Attractive Phenolhydrazones. RSC Adv. 2014, 4, 56736–56742. [Google Scholar] [CrossRef]
- Baláž, M.; Boldyreva, E.V.; Rybin, D.; Pavlović, S.; Rodríguez-Padrón, D.; Mudrinić, T.; Luque, R. State-of-the-Art of Eggshell Waste in Materials Science: Recent Advances in Catalysis, Pharmaceutical Applications, and Mechanochemistry. Front. Bioeng. Biotechnol. 2021, 8, 612567. [Google Scholar] [CrossRef] [PubMed]
- Descamps, M.; Willart, J.F. Perspectives on the Amorphisation/Milling Relationship in Pharmaceutical Materials. Adv. Drug Deliv. Rev. 2016, 100, 51–66. [Google Scholar] [CrossRef]
- Krusenbaum, A.; Grätz, S.; Tigineh, G.T.; Borchardt, L.; Kim, J.G. The Mechanochemical Synthesis of Polymers. Chem. Soc. Rev. 2022, 51, 2873–2905. [Google Scholar] [CrossRef]
- Rodríguez-Seoane, P.; Domínguez, H.; Torres, M.D. Mechanical Characterization of Biopolymer-based Hydrogels Enriched with Paulownia Extracts Recovered Using a Green Technique. Appl. Sci. 2020, 10, 8439. [Google Scholar] [CrossRef]
- Nagarajan, S.; Radhakrishnan, S.; Kalkura, S.N.; Balme, S.; Miele, P.; Bechelany, M. Overview of Protein-Based Biopolymers for Biomedical Application. Macromol. Chem. Phys. 2019, 220, 1900126. [Google Scholar] [CrossRef]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for New and Cleaner Synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef] [PubMed]
- Cova, C.M.; Luque, R. Advances in Mechanochemical Processes for Biomass Valorization. BMC Chem. Eng. 2019, 1, 16. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Zeng, L.; Li, X.; Chen, N.; Bai, S.; He, H.; Wang, Q.; Zhang, C. A Review on Mechanochemistry: Approaching Advanced Energy Materials with Greener Force. Adv. Mater. 2022, 34, 2108327. [Google Scholar] [CrossRef] [PubMed]
- Do, J.L.; Mottillo, C.; Tan, D.; Štrukil, V.; Friščić, T. Mechanochemical Ruthenium-Catalyzed Olefin Metathesis. J. Am. Chem. Soc. 2015, 137, 2476–2479. [Google Scholar] [CrossRef] [PubMed]
- Do, J.L.; Friščić, T. Mechanochemistry: A Force of Synthesis. ACS Cent. Sci. J. 2017, 3, 13–19. [Google Scholar] [CrossRef]
- Xu, C.; De, S.; Balu, A.M.; Ojeda, M.; Luque, R. Mechanochemical Synthesis of Advanced Nanomaterials for Catalytic Applications. Chem. Commun. 2015, 51, 6698–6713. [Google Scholar] [CrossRef] [PubMed]
- Kalinkin, A.M.; Gurevich, B.I.; Myshenkov, M.S.; Chislov, M.V.; Kalinkina, E.V.; Zvereva, I.A.; Cherkezova-Zheleva, Z.; Paneva, D.; Petkova, V. Synthesis of Fly Ash-Based Geopolymers: Effect of Calcite Addition and Mechanical Activation. Minerals 2020, 10, 827. [Google Scholar] [CrossRef]
- Web of Science. Available online: https://www.webofscience.com/wos/woscc/summary/af64e11b-2477-4067-bf19-d1e8ecaa29a2-0165a1a177/relevance/1 (accessed on 5 June 2025).
- Zhang, J.; Zhang, P.; Shao, L.; Wang, R.; Ma, Y.; Szostak, M. Mechanochemical Solvent-Free Suzuki–Miyaura Cross-Coupling of Amides via Highly Chemoselective N−C Cleavage. Angew. Chem. Int. Ed. 2022, 61, e202114146. [Google Scholar] [CrossRef]
- Thiery, E.; Delaye, P.O.; Thibonnet, J.; Boudesocque-Delaye, L. Mechanochemical Suzuki-Miyaura Cross-Coupling with Natural Deep Eutectic Solvent as Liquid-Assisted Grinding Additive: Merging Two Fields for a Greener Strategy. Eur. J. Org. Chem. 2023, 26, e202300727. [Google Scholar] [CrossRef]
- Das, D.; Bhosle, A.A.; Panjikar, P.C.; Chatterjee, A.; Banerjee, M. Mn(I)-Catalyzed Mechanochemical C-H Bond Activation: C-2 Selective Alkenylation of Indoles. ACS Sustain. Chem. Eng. 2020, 8, 19105–19116. [Google Scholar] [CrossRef]
- Beamish-Cook, J.; Shankland, K.; Murray, C.A.; Vaqueiro, P. Insights into the Mechanochemical Synthesis of MOF-74. Cryst. Growth Des. 2021, 21, 3047–3055. [Google Scholar] [CrossRef]
- Stolar, T.; Prašnikar, A.; Martinez, V.; Karadeniz, B.; Bjelić, A.; Mali, G.; Friščić, T.; Likozar, B.; Užarević, K. Scalable Mechanochemical Amorphization of Bimetallic Cu-Zn MOF-74 Catalyst for Selective CO2 reduction Reaction to Methanol. ACS Appl. Mater. Interfaces 2021, 13, 3070–3077. [Google Scholar] [CrossRef]
- Vasconcelos, A.A.; Len, T.; Oliveira, A.N.; Costa, A.A.F.; Souza, A.R.S.; Costa, C.E.F.; Luque, R.; Rocha Filho, G.N.; Noronha, R.C.R.; Nascimento, L.A.S. Zeolites: A Theoretical and Practical Approach with Uses in (Bio)Chemical Processes. Appl. Sci. 2023, 13, 1897. [Google Scholar] [CrossRef]
- Rincon, E.; Garcia, A.; Romero, A.A.; Serrano, L.; Luque, R.; Balu, A.M. Mechanochemical Preparation of Novel Polysaccharide-Supported Nb2O5 Catalysts. Catalysts 2019, 9, 38. [Google Scholar] [CrossRef]
- Marjanović, N.; Komljenović, M.; Baščarević, Z.; Nikolić, V. Improving Reactivity of Fly Ash and Properties of Ensuing Geopolymers through Mechanical Activation. Constr. Build. Mater. 2014, 57, 151–162. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, S.; Mao, Q.; Buekens, A.; Chang, W.; Wang, X.; Yan, J. Suppressing Heavy Metal Leaching through Ball Milling of Fly Ash. Energies 2016, 9, 524. [Google Scholar] [CrossRef]
- Lukić, I.; Kesić, Ž.; Zdujić, M.; Skala, D. Calcium Diglyceroxide Synthesized by Mechanochemical Treatment, Its Characterization and Application as Catalyst for Fatty Acid Methyl Esters Production. Fuel 2016, 165, 159–165. [Google Scholar] [CrossRef]
- Chumpiboon, A.; Thongsubsai, K.; Pongsiri, T.; Knijnenburg, J.T.N.; Ngernyen, Y. Removal of Cationic Dye from Textile Wastewater Using Treated Bagasse Fly Ash: An Industrial Waste. Eng. Appl. Sci. Res. 2022, 49, 381–394. [Google Scholar] [CrossRef]
- Kumar, S.; Mucsi, G.; Kristály, F.; Pekker, P. Mechanical Activation of Fly Ash and Its Influence on Micro and Nano-Structural Behaviour of Resulting Geopolymers. Adv. Powder Technol. 2017, 28, 805–813. [Google Scholar] [CrossRef]
- Oyun-Erdene, G.; Temuujin, J. Effect of Mechanical Activation of Fluidized Bed Fly Ash on Geopolymer Properties. Solid State Phenom. 2019, 288, 51–58. [Google Scholar] [CrossRef]
- Hajiali, F.; Jin, T.; Yang, G.; Santos, M.; Lam, E.; Moores, A. Mechanochemical Transformations of Biomass into Functional Materials. ChemSusChem 2022, 15, e202102535. [Google Scholar] [CrossRef]
- Scimmi, C.; Sancineto, L.; Drabowicz, J.; Santi, C. New Insights into Green Protocols for Oxidative Depolymerization of Lignin and Lignin Model Compounds. Int. J. Mol. Sci. 2022, 23, 4378. [Google Scholar] [CrossRef]
- Zheng, L.; Sun, C.; Xu, W.; Dushkin, A.V.; Polyakov, N.; Su, W.; Yu, J. Mechanically Induced Solvent-Free Esterification Method at Room Temperature. RSC Adv. 2021, 11, 5080–5085. [Google Scholar] [CrossRef]
- Hajiali, F.; Vidal, J.; Jin, T.; De La Garza, L.C.; Santos, M.; Yang, G.; Moores, A. Extraction of Chitin from Green Crab Shells by Mechanochemistry and Aging. ACS Sustain. Chem. Eng. 2022, 10, 11348–11357. [Google Scholar] [CrossRef]
- Kaabel, S.; Arciszewski, J.; Borchers, T.H.; Therien, J.P.D.; Friščić, T.; Auclair, K. Solid-State Enzymatic Hydrolysis of Mixed PET/Cotton Textiles**. ChemSusChem 2023, 16, e202201613. [Google Scholar] [CrossRef]
- Zhao, Z.; Abdo, S.M.A.; Wang, X.; Li, H.; Li, X.; Gao, X. Process Intensification on Co-Pyrolysis of Polyethylene Terephthalate Wastes and Biomass via Microwave Energy: Synergetic Effect and Roles of Microwave Susceptor. J. Anal. Appl. Pyrolysis 2021, 158, 105239. [Google Scholar] [CrossRef]
- Al-Ithawi, W.K.A.; Khasanov, A.F.; Kovalev, I.S.; Nikonov, I.L.; Platonov, V.A.; Kopchuk, D.S.; Santra, S.; Zyryanov, G.V.; Ranu, B.C. TM-Free and TM-Catalyzed Mechanosynthesis of Functional Polymers. Polymers 2023, 15, 1853. [Google Scholar] [CrossRef]
- Yang, S.; Bai, S.; Duan, W.; Wang, Q. Preparation of Composites Based on Recycled Polypropylene and Automotive Shredder Residue. Polym. Int. 2018, 67, 936–945. [Google Scholar] [CrossRef]
- Arciszewski, J.; Auclair, K. Mechanoenzymatic Reactions Involving Polymeric Substrates or Products. ChemSusChem 2022, 15, e202102084. [Google Scholar] [CrossRef] [PubMed]
- Skórczewska, K.; Lewandowski, K.; Szewczykowski, P.; Wilczewski, S.; Szulc, J.; Stopa, P.; Nowakowska, P. Waste Eggshells as a Natural Filler for the Poly(Vinyl Chloride) Composites. Polymers 2022, 14, 4372. [Google Scholar] [CrossRef]
- Iber, B.T.; Kasan, N.A.; Torsabo, D.; Omuwa, J.W. A Review of Various Sources of Chitin and Chitosan in Nature. J. Renew. Mater. 2022, 10, 1097–1123. [Google Scholar] [CrossRef]
- Elamri, A.; Zdiri, K.; Hamdaoui, M.; Harzallah, O. Chitosan: A Biopolymer for Textile Processes and Products. Text. Res. J. 2023, 93, 1456–1484. [Google Scholar] [CrossRef]
- Margoutidis, G.; Parsons, V.H.; Bottaro, C.S.; Yan, N.; Kerton, F.M. Mechanochemical Amorphization of α-Chitin and Conversion into Oligomers of N-Acetyl-D-Glucosamine. ACS Sustain. Chem. Eng. 2018, 6, 1662–1669. [Google Scholar] [CrossRef]
- Therien, J.P.D.; Hammerer, F.; Friščić, T.; Auclair, K. Mechanoenzymatic Breakdown of Chitinous Material to N-Acetylglucosamine: The Benefits of a Solventless Environment. ChemSusChem 2019, 12, 3481–3490. [Google Scholar] [CrossRef]
- Zhou, D.; Shen, D.; Lu, W.; Song, T.; Wang, M.; Feng, H.; Shentu, J.; Long, Y. Production of 5-Hydroxymethylfurfural from Chitin Biomass: A Review. Molecules 2020, 25, 541. [Google Scholar] [CrossRef] [PubMed]
- Bychkov, A.; Podgorbunskikh, E.; Bychkova, E.; Lomovsky, O. Current Achievements in the Mechanically Pretreated Conversion of Plant Biomass. Biotechnol. Bioeng. 2019, 116, 1231–1244. [Google Scholar] [CrossRef]
- Baláž, M. Ball Milling of Eggshell Waste as a Green and Sustainable Approach: A Review. Adv. Colloid Interface Sci. 2018, 256, 256–275. [Google Scholar] [CrossRef] [PubMed]
- Piras, C.C.; Fernández-Prieto, S.; De Borggraeve, W.M. Ball Milling: A Green Technology for the Preparation and Functionalisation of Nanocellulose Derivatives. Nanoscale Adv. 2019, 1, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Grabias-Blicharz, E.; Franus, W. A Critical Review on Mechanochemical Processing of Fly Ash and Fly Ash-Derived Materials. Sci. Total Environ. 2023, 860, 160529. [Google Scholar] [CrossRef]
- Guo, X.; Xiang, D.; Duan, G.; Mou, P. A Review of Mechanochemistry Applications in Waste Management. Waste Manag. 2010, 30, 4–10. [Google Scholar] [CrossRef]
- Chu, Y.S.; Davaabal, B.; Kim, D.S.; Seo, S.K.; Kim, Y.; Ruescher, C.; Temuujin, J. Reactivity of Fly Ashes Milled in Different Milling Devices. Rev. Adv. Mater. Sci. 2019, 58, 179–188. [Google Scholar] [CrossRef]
- Yin, S.; Tuladhar, R.; Shi, F.; Shanks, R.A.; Combe, M.; Collister, T. Mechanical Reprocessing of Polyolefin Waste: A Review. Polym. Eng. Sci. 2015, 55, 2899–2909. [Google Scholar] [CrossRef]
- Len, C.; Duhan, V.; Ouyang, W.; Nguyen, R.; Lochab, B. Mechanochemistry and Oleochemistry: A Green Combination for the Production of High-Value Small Chemicals. Front. Chem. 2023, 11, 1306182. [Google Scholar] [CrossRef]
- Cagnetta, G.; Robertson, J.; Huang, J.; Zhang, K.; Yu, G. Mechanochemical Destruction of Halogenated Organic Pollutants: A Critical Review. J. Hazard. Mater. 2016, 313, 85–102. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Pena, S.B.; Guimarães, H.C.Q.C.; Lopes, J.L.; Guandalini, L.S.; Taminato, M.; Barbosa, D.A.; Barros, A.L.B.L. Revisão Sistemática Med o de Cair e o Risco de Queda: Revisão Sistemática e Metanálise. Acta Paul. Enferm. 2019, 32, 456–463. [Google Scholar] [CrossRef]
- Stellacci, P.; Liberti, L.; Notarnicola, M.; Bishop, P.L. Valorization of Coal Fly Ash by Mechano-Chemical Activation. Part I. Enhancing Adsorption Capacity. Chem. Eng. J. 2009, 149, 11–18. [Google Scholar] [CrossRef]
- Geng, X.; Duan, Y.; Zhao, S.; Hu, J.; Zhao, W. Mechanism Study of Mechanochemical Bromination on Fly Ash Mercury Removal Adsorbent. Chemosphere 2021, 274, 129637. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Van Deventer, J.S.J. The Geopolymerisation of Alumino-Silicate Minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef]
- Mucsi, G.; Kumar, S.; Csőke, B.; Kumar, R.; Molnár, Z.; Rácz, Á.; Mádai, F.; Debreczeni, Á. Control of Geopolymer Properties by Grinding of Land Filled Fly Ash. Int. J. Miner. Process. 2015, 143, 50–58. [Google Scholar] [CrossRef]
- Nath, S.K.; Kumar, S. Role of Particle Fineness on Engineering Properties and Microstructure of Fly Ash Derived Geopolymer. Constr. Build. Mater. 2020, 233, 117294. [Google Scholar] [CrossRef]
- Matsuoka, M.; Yokoyama, K.; Okura, K.; Murayama, N.; Ueda, M.; Naito, M. Synthesis of Geopolymers from Mechanically Activated Coal Fly Ash and Improvement of Their Mechanical Properties. Minerals 2019, 9, 791. [Google Scholar] [CrossRef]
- Nikolić, V.; Komljenović, M.; Marjanović, N.; Baščarević, Z.; Petrović, R. Lead Immobilization by Geopolymers Based on Mechanically Activated Fly Ash. Ceram. Int. 2014, 40, 8479–8488. [Google Scholar] [CrossRef]
- Li, M.G.; Sun, C.J.; Gau, S.H.; Chuang, C.J. Effects of Wet Ball Milling on Lead Stabilization and Particle Size Variation in Municipal Solid Waste Incinerator Fly Ash. J. Hazard. Mater. 2010, 174, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Fujiwara, K.; Terada, A.; Nakai, S.; Hosomi, M. Prevention of Lead Leaching from Fly Ashes by Mechanochemical Treatment. Waste Manag. 2010, 30, 1290–1295. [Google Scholar] [CrossRef]
- Li, W.; Wang, W.; Wu, D.; Yang, S.; Fang, H.; Sun, S. Mechanochemical Treatment with Red Mud Added for Heavy Metals Solidification in Municipal Solid Waste Incineration Fly Ash. J. Clean. Prod. 2023, 398, 136642. [Google Scholar] [CrossRef]
- Li, H.; Dai, M.; Dai, S.; Dong, X.; Li, F. Methylene Blue Adsorption Properties of Mechanochemistry Modified Coal Fly Ash. Hum. Ecol. Risk Assess. 2018, 24, 2133–2141. [Google Scholar] [CrossRef]
- Sundum, T.; Szécsényi, K.M.; Kaewtatip, K. Preparation and Characterization of Thermoplastic Starch Composites with Fly Ash Modified by Planetary Ball Milling. Carbohydr. Polym. 2018, 191, 198–204. [Google Scholar] [CrossRef]
- Cristelo, N.; Tavares, P.; Lucas, E.; Miranda, T.; Oliveira, D. Quantitative and Qualitative Assessment of the Amorphous Phase of a Class F Fly Ash Dissolved during Alkali Activation Reactions—Effect of Mechanical Activation, Solution Concentration and Temperature. Compos. Part B 2016, 103, 1–14. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R. Mechanical Activation of Fly Ash: Effect on Reaction, Structure and Properties of Resulting Geopolymer. Ceram. Int. 2011, 37, 533–541. [Google Scholar] [CrossRef]
- Somna, K.; Jaturapitakkul, C.; Kajitvichyanukul, P.; Chindaprasirt, P. NaOH-Activated Ground Fly Ash Geopolymer Cured at Ambient Temperature. Fuel 2011, 90, 2118–2124. [Google Scholar] [CrossRef]
- Temuujin, J.; Williams, R.P.; van Riessen, A. Effect of Mechanical Activation of Fly Ash on the Properties of Geopolymer Cured at Ambient Temperature. J. Mater. Process. Technol. 2009, 209, 5276–5280. [Google Scholar] [CrossRef]
- Rak, M.J.; Friščić, T.; Moores, A. Mechanochemical Synthesis of Au, Pd, Ru and Re Nanoparticles with Lignin as a Bio-Based Reducing Agent and Stabilizing Matrix. Faraday Discuss. 2014, 170, 155–167. [Google Scholar] [CrossRef]
- Kleine, T.; Buendia, J.; Bolm, C. Mechanochemical Degradation of Lignin and Wood by Solvent-Free Grinding in a Reactive Medium. Green Chem. 2013, 15, 160–166. [Google Scholar] [CrossRef]
- Kobayashi, H.; Yabushita, M.; Komanoya, T.; Hara, K.; Fujita, I.; Fukuoka, A. High-Yielding One-Pot Synthesis of Glucose from Cellulose Using Simple Activated Carbons and Trace Hydrochloric Acid. ACS Catal. 2013, 3, 581–587. [Google Scholar] [CrossRef]
- Jiang, L.-q.; Zheng, A.-q.; Meng, J.-g.; Wang, X.-b.; Zhao, Z.-l.; Li, H.-b. A Comparative Investigation of Fast Pyrolysis with Enzymatic Hydrolysis for Fermentable Sugars Production from Cellulose. Bioresour. Technol. 2019, 274, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.; Liu, C.; Ren, J.; Zhao, X.; Sun, R.; Wu, A. An Efficient Pretreatment for the Selectively Hydrothermal Conversion of Corncob into Furfural: The Combined Mixed Ball Milling and Ultrasonic Pretreatments. Ind. Crops Prod. 2016, 94, 721–728. [Google Scholar] [CrossRef]
- Dabral, S.; Wotruba, H.; Hernández, J.G.; Bolm, C. Mechanochemical Oxidation and Cleavage of Lignin β-O-4 Model Compounds and Lignin. ACS Sustain. Chem. Eng. 2018, 6, 3242–3254. [Google Scholar] [CrossRef]
- Sun, C.; Zheng, L.; Xu, W.; Dushkin, A.V.; Su, W. Mechanochemical Cleavage of Lignin Models and Ligninviaoxidation and a Subsequent Base-Catalyzed Strategy. Green Chem. 2020, 22, 3489–3494. [Google Scholar] [CrossRef]
- Zakaria, M.R.; Fujimoto, S.; Hirata, S.; Hassan, M.A. Ball Milling Pretreatment of Oil Palm Biomass for Enhancing Enzymatic Hydrolysis. Appl. Biochem. Biotechnol. 2014, 173, 1778–1789. [Google Scholar] [CrossRef]
- Pang, J.; Zheng, M.; Li, X.; Sebastian, J.; Jiang, Y.; Zhao, Y.; Wang, A.; Zhang, T. Unlock the Compact Structure of Lignocellulosic Biomass by Mild Ball Milling for Ethylene Glycol Production. ACS Sustain. Chem. Eng. 2019, 7, 679–687. [Google Scholar] [CrossRef]
- Schneider, L.; Haverinen, J.; Jaakkola, M.; Lassi, U. Solid Acid-Catalyzed Depolymerization of Barley Straw Driven by Ball Milling. Bioresour Technol 2016, 206, 204–210. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, P.; Ye, J.; Wu, Y.; Liu, J.; Fang, W.; Xu, D.; Wang, B.; Yan, L.; Zeng, G. Comparison of Various Pretreatments for Ethanol Production Enhancement from Solid Residue after Rumen Fluid Digestion of Rice Straw. Bioresour. Technol. 2018, 247, 147–156. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, S.; Kim, J.; Mitchell, R.J.; Lee, J.H. Environmentally Friendly Pretreatment of Plant Biomass by Planetary and Attrition Milling. Bioresour. Technol. 2013, 144, 50–56. [Google Scholar] [CrossRef]
- Shen, F.; Smith, R.L.; Li, L.; Yan, L.; Qi, X. Eco-Friendly Method for Efficient Conversion of Cellulose into Levulinic Acid in Pure Water with Cellulase-Mimetic Solid Acid Catalyst. ACS Sustain. Chem. Eng. 2017, 5, 2421–2427. [Google Scholar] [CrossRef]
- Su, J.; Qiu, M.; Shen, F.; Qi, X. Efficient Hydrolysis of Cellulose to Glucose in Water by Agricultural Residue-Derived Solid Acid Catalyst. Cellulose 2018, 25, 17–22. [Google Scholar] [CrossRef]
- Qiu, M.; Bai, C.; Yan, L.; Shen, F.; Qi, X. Efficient Mechanochemical-Assisted Production of Glucose from Cellulose in Aqueous Solutions by Carbonaceous Solid Acid Catalysts. ACS Sustain. Chem. Eng. 2018, 6, 13826–13833. [Google Scholar] [CrossRef]
- Qi, X.; Yan, L.; Shen, F.; Qiu, M. Mechanochemical-Assisted Hydrolysis of Pretreated Rice Straw into Glucose and Xylose in Water by Weakly Acidic Solid Catalyst. Bioresour. Technol. 2019, 273, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.; Choi, E.H.; Choi, E.J.; Premkumar, T.; Song, C. Facile Solid-State Mechanochemical Synthesis of Eco-Friendly Thermoplastic Polyurethanes and Copolymers Using a Biomass-Derived Furan Diol. ACS Sustain. Chem. Eng. 2020, 8, 4400–4406. [Google Scholar] [CrossRef]
- Da Silva, A.S.A.; Inoue, H.; Endo, T.; Yano, S.; Bon, E.P.S. Milling Pretreatment of Sugarcane Bagasse and Straw for Enzymatic Hydrolysis and Ethanol Fermentation. Bioresour. Technol. 2010, 101, 7402–7409. [Google Scholar] [CrossRef] [PubMed]
- Hideno, A.; Inoue, H.; Tsukahara, K.; Fujimoto, S.; Minowa, T.; Inoue, S.; Endo, T.; Sawayama, S. Wet Disk Milling Pretreatment without Sulfuric Acid for Enzymatic Hydrolysis of Rice Straw. Bioresour. Technol. 2009, 100, 2706–2711. [Google Scholar] [CrossRef]
- Hilgert, J.; Meine, N.; Rinaldi, R.; Schüth, F. Mechanocatalytic Depolymerization of Cellulose Combined with Hydrogenolysis as a Highly Efficient Pathway to Sugar Alcohols. Energy Environ. Sci. 2013, 6, 92–96. [Google Scholar] [CrossRef]
- Ribeiro, L.S.; Órfão, J.J.M.; Pereira, M.F.R. Enhanced Direct Production of Sorbitol by Cellulose Ball-Milling. Green Chem. 2015, 17, 2973–2980. [Google Scholar] [CrossRef]
- Su, T.C.; Fang, Z.; Zhang, F.; Luo, J.; Li, X.K. Hydrolysis of Selected Tropical Plant Wastes Catalyzed by a Magnetic Carbonaceous Acid with Microwave. Sci. Rep. 2015, 5, 17538. [Google Scholar] [CrossRef]
- Inoue, H.; Yano, S.; Endo, T.; Sakaki, T.; Sawayama, S. Combining Hot-Compressed Water and Ball Milling Pretreatments to Improve the Efficiency of the Enzymatic Hydrolysis of Eucalyptus. Biotechnol. Biofuels 2008, 1, 2. [Google Scholar] [CrossRef]
- Gu, B.J.; Wang, J.; Wolcott, M.P.; Ganjyal, G.M. Increased Sugar Yield from Pre-Milled Douglas-Fir Forest Residuals with Lower Energy Consumption by Using Planetary Ball Milling. Bioresour. Technol. 2018, 251, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.S.; Órfão, J.J.M.; Pereira, M.F.R. Direct Catalytic Production of Sorbitol from Waste Cellulosic Materials. Bioresour. Technol. 2017, 232, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Huete, F.; Messina, C.; Chen, F.; Cuccia, L.; Ottenwaelder, X.; Forgione, P. Solvent-Free Mechanochemical Oxidation and Reduction of Biomass-Derived 5-Hydroxymethyl Furfural. Green Chem. 2018, 20, 5261–5265. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.X.; Hou, T.; Chen, X.; Gao, C.; Han, L.; Xiao, W. Mechanical Deconstruction of Corn Stover as an Entry Process to Facilitate the Microwave-Assisted Production of Ethyl Levulinate. Fuel Process. Technol. 2018, 174, 53–60. [Google Scholar] [CrossRef]
- Shen, F.; Sun, S.; Zhang, X.; Yang, J.; Qiu, M.; Qi, X. Mechanochemical-Assisted Production of 5-Hydroxymethylfurfural from High Concentration of Cellulose. Cellulose 2020, 27, 3013–3023. [Google Scholar] [CrossRef]
- He, P.; Lu, H.; Ruan, H.; Wang, C.; Zhang, Q.; Huang, Z.; Liu, J. Mechanochemistry: An Efficient Way to Recycle Thermoset Polyurethanes. Polymers 2022, 14, 3277. [Google Scholar] [CrossRef]
- Roy, P.S.; Garnier, G.; Allais, F.; Saito, K. Strategic Approach Towards Plastic Waste Valorization: Challenges and Promising Chemical Upcycling Possibilities. ChemSusChem 2021, 14, 4007–4027. [Google Scholar] [CrossRef]
- Bandegi, A.; Montemayor, M.; Manas-Zloczower, I. Vitrimerization of Rigid Thermoset Polyurethane Foams: A Mechanochemical Method to Recycle and Reprocess Thermosets. Polym. Adv. Technol. 2022, 33, 3750–3758. [Google Scholar] [CrossRef]
- Capuano, R.; Bonadies, I.; Castaldo, R.; Cocca, M.; Gentile, G.; Protopapa, A.; Avolio, R.; Errico, M.E. Valorization and Mechanical Recycling of Heterogeneous Post-Consumer Polymer Waste through a Mechano-Chemical Process. Polymers 2021, 13, 2783. [Google Scholar] [CrossRef]
- Dwivedi, C.; Manjare, S.; Rajan, S.K. Recycling of Waste Tire by Pyrolysis to Recover Carbon Black: Alternative & Environment-Friendly Reinforcing Filler for Natural Rubber Compounds. Compos. B Eng. 2020, 200, 108346. [Google Scholar] [CrossRef]
- Hamed, O.; Lail, B.A.; Deghles, A.; Qasem, B.; Azzaoui, K.; Obied, A.A.; Algarra, M.; Jodeh, S. Synthesis of a Cross-Linked Cellulose-Based Amine Polymer and Its Application in Wastewater Purification. Environ. Sci. Pollut. Res. 2019, 26, 28080–28091. [Google Scholar] [CrossRef]
- Brenner, M.; Weichold, O. Poultry Feather Waste as Bio-Based Cross-Linking Additive for Ethylene Propylene Diene Rubber. Polymers 2021, 13, 3908. [Google Scholar] [CrossRef] [PubMed]
- Hoque, B.; Kolev, S.D.; Cattrall, R.W.; Gopakumar, T.G.; Almeida, M.I.G.S. A Cross-Linked Polymer Inclusion Membrane for Enhanced Gold Recovery from Electronic Waste. Waste Manag. 2021, 124, 54–62. [Google Scholar] [CrossRef]
- Liu, H.L.; Wang, X.P.; Jia, D.M. Recycling of Waste Rubber Powder by Mechano-Chemical Modification. J. Clean. Prod. 2020, 245, 118716. [Google Scholar] [CrossRef]
- Hajian, M.; Sadrmohaghegh, C.; Scott, G. Solid Phase Dispersants Synthesised by a Mechanochemical Procedure. Eur. Polym. J. 1984, 20, 135–138. [Google Scholar] [CrossRef]
- Hong, S.-J.; Jeong, H.; Yuk, J.S.; Park, M.; Kim, G.; Kim, Y.-W.; Shin, J. Semicrystalline-Glassy Multiblock Copolymers via Mechanochemical Synthesis toward Tough Poly(Lactide). ACS Sustain. Chem. Eng. 2022, 10, 14523–14538. [Google Scholar] [CrossRef]
- Xu, J.; Duan, X.; Zhang, P.; Niu, Q.; Dai, S. Processing Poly (Ethylene Terephthalate) Waste into Functional Carbon Materials by Mechanochemical Extrusion. ChemSusChem 2022, 15, e202201576. [Google Scholar] [CrossRef]
- Schneidermann, C.; Otto, P.; Leistenschneider, D.; Grätz, S.; Eßbach, C.; Borchardt, L. Upcycling of Polyurethane Waste by Mechanochemistry: Synthesis of N-Doped Porous Carbon Materials for Supercapacitor Applications. Beilstein J. Nanotechnol. 2019, 10, 1618–1627. [Google Scholar] [CrossRef]
- Cagnetta, G.; Zhang, K.; Zhang, Q.; Huang, J.; Yu, G. Mechanochemical Pre-Treatment for Viable Recycling of Plastic Waste Containing Haloorganics. Waste Manag. 2018, 75, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Balema, V.P.; Hlova, I.Z.; Carnahan, S.L.; Seyedi, M.; Dolotko, O.; Rossini, A.J.; Luzinov, I. Depolymerization of Polystyrene under Ambient Conditions. New J. Chem. 2021, 45, 2935–2938. [Google Scholar] [CrossRef]
- Nzioka, A.M.; Yan, C.Z.; Kim, M.-G.; Sim, Y.-J.; Lee, C.-S.; Kim, Y.-J. Improvement of the Chemical Recycling Process of Waste Carbon Fibre Reinforced Plastics Using a Mechanochemical Process: Influence of Process Parameters. Waste Manag. Res. J. Sustain. Circ. Econ. 2018, 36, 952–964. [Google Scholar] [CrossRef]
- Lu, S.; Guo, X.; He, H.; Peng, Y.; Ding, J.; Ding, J.; Zhu, H.; Muhammad, Q.; Rytöluoto, I.; Tenhunen, A. Mechanochemical Dehalogenation of Brominated Flame Retardants and Preliminary Application for Recycling BFR-Containing Plastic Waste. J. Environ. Chem. Eng. 2023, 11, 109916. [Google Scholar] [CrossRef]
- Grause, G.; Fonseca, J.D.; Tanaka, H.; Bhaskar, T.; Kameda, T.; Yoshioka, T. A Novel Process for the Removal of Bromine from Styrene Polymers Containing Brominated Flame Retardant. Polym. Degrad. Stab. 2015, 112, 86–93. [Google Scholar] [CrossRef]
- Wang, R.; Zhu, Z.; Tan, S.; Guo, J.; Xu, Z. Mechanochemical Degradation of Brominated Flame Retardants in Waste Printed Circuit Boards by Ball Milling. J. Hazard. Mater. 2020, 385, 121509. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.; Yang, S.; Bai, S.; Wang, Q. Novel Application of Mechanochemistry in Waste Epoxy Recycling via Solid-State Shear Milling. ACS Sustain. Chem. Eng. 2021, 9, 11778–11789. [Google Scholar] [CrossRef]
- Zhang, Q.; Kano, J.; Saito, F. Mechanochemical Technology: Application to Material Synthesis and to the Separation and Processing of Recyclable Materials from Wastes. KONA Powder Part. J. 2001, 19, 7–15. [Google Scholar] [CrossRef]
- Song, L.; Lin, L.; Wei, W.; Zhang, S.; Wan, L.; Lou, Z.; Yu, J.; Xu, X. Zero-Valent Iron-Peroxydisulfate as Synergistic Co-Milling Agents for Enhanced Mechanochemical Destruction of 2,4-Dichlorophenol: Coupling Reduction with Oxidation. J. Environ. Manag. 2023, 345, 118571. [Google Scholar] [CrossRef]
- Lou, Z.; Song, L.; Liu, W.; Wu, S.; He, F.; Yu, J. Deciphering CaO-Induced Peroxydisulfate Activation for Destruction of Halogenated Organic Pollutants in a Low Energy Vibrational Mill. Chem. Eng. J. 2022, 431, 134090. [Google Scholar] [CrossRef]
- Hu, J.; Huang, Z.; Yu, J. Highly-Effective Mechanochemical Destruction of Hexachloroethane and Hexachlorobenzene with Fe/Fe3O4 Mixture as a Novel Additive. Sci. Total Environ. 2019, 659, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Sui, H.; Rong, Y.; Song, J.; Zhang, D.; Li, H.; Wu, P.; Shen, Y.; Huang, Y. Mechanochemical Destruction of DDTs with Fe-Zn Bimetal in a High-Energy Planetary Ball Mill. J. Hazard. Mater. 2018, 342, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-h.; Li, X.-d.; Ni, M.-j.; Chen, T.; Yan, J.-h. Remediation of PCB-Contaminated Soil Using a Combination of Mechanochemical Method and Thermal Desorption. Environ. Sci. Pollut. Res. 2017, 24, 11800–11806. [Google Scholar] [CrossRef]
- Cagnetta, G.; Hassan, M.M.; Huang, J.; Yu, G.; Weber, R. Dioxins Reformation and Destruction in Secondary Copper Smelting Fly Ash under Ball Milling. Sci. Rep. 2016, 6, 22925. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, X.; Fei, Q. Dechlorination of Pentachlorophenol by Grinding at Low Rotation Speed in Short Time. Chin. J. Chem. Eng. 2015, 23, 578–582. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Jun, H.; Yu, M.; Wang, F.; Zhou, L.; Yu, G. Acceleration and Mechanistic Studies of the Mechanochemical Dechlorination of HCB with Iron Powder and Quartz Sand. Chem. Eng. J. 2014, 239, 185–191. [Google Scholar] [CrossRef]
- Wang, H.; Huang, J.; Zhang, K.; Yu, Y.; Liu, K.; Yu, G.; Deng, S.; Wang, B. Effects of Zero-Valent Metals Together with Quartz Sand on the Mechanochemical Destruction of Dechlorane plus Coground in a Planetary Ball Mill. J. Hazard. Mater. 2014, 264, 230–235. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, J.; Zhang, W.; Zhang, K.; Deng, S.; Yu, G. Mechanochemical Destruction of Mirex Co-Ground with Iron and Quartz in a Planetary Ball Mill. Chemosphere 2013, 90, 1729–1735. [Google Scholar] [CrossRef]
- Nomura, Y.; Aono, S.; Arino, T.; Yamamoto, T.; Terada, A.; Noma, Y.; Hosomi, M. Degradation of Polychlorinated Naphthalene by Mechanochemical Treatment. Chemosphere 2013, 93, 2657–2661. [Google Scholar] [CrossRef]
- Di Leo, P.; Pizzigallo, M.D.R.; Ancona, V.; Di Benedetto, F.; Mesto, E.; Schingaro, E.; Ventruti, G. Mechanochemical Degradation of Pentachlorophenol onto Birnessite. J. Hazard. Mater. 2013, 244–245, 303–310. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, J.; Zhang, W.; Yu, Y.; Deng, S.; Wang, B.; Yu, G. Coupling the Dechlorination of Aqueous 4-CP with the Mechanochemical Destruction of Solid PCNB Using Fe-Ni-SiO2. J. Hazard. Mater. 2013, 250–251, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Fujiwara, K.; Terada, A.; Nakai, S.; Hosomi, M. Mechanochemical Degradation of γ-Hexachlorocyclohexane by a Planetary Ball Mill in the Presence of CaO. Chemosphere 2012, 86, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Huang, J.; Peng, Z.; Li, X.; Yan, J. Ball Milling 2,4,6-Trichlorophenol with Calcium Oxide: Dechlorination Experiment and Mechanism Considerations. Chem. Eng. J. 2012, 195–196, 62–68. [Google Scholar] [CrossRef]
- Pizzigallo, M.D.R.; Leo, P.D.; Ancona, V.; Spagnuolo, M.; Schingaro, E. Effect of Aging on Catalytic Properties in Mechanochemical Degradation of Pentachlorophenol by Birnessite. Chemosphere 2011, 82, 627–634. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, J.; Xu, F.; Deng, S.; Zhu, W.; Yu, G. Mechanochemical Destruction of Pentachloronitrobenzene with Reactive Iron Powder. J. Hazard. Mater. 2011, 198, 275–281. [Google Scholar] [CrossRef]
- Mitoma, Y.; Miyata, H.; Egashira, N.; Simion, A.M.; Kakeda, M.; Simion, C. Mechanochemical Degradation of Chlorinated Contaminants in Fly Ash with a Calcium-Based Degradation Reagent. Chemosphere 2011, 83, 1326–1330. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, J.; Yu, G.; Deng, S.; Zhu, W. Mechanochemical Destruction of Dechlorane Plus with Calcium Oxide. Chemosphere 2010, 81, 345–350. [Google Scholar] [CrossRef]
- Wei, Y.; Yan, J.; Lu, S.; Li, X. Decomposition of PCDD/Fs by Mechanochemical Means with Calcium-Based Additives. J. Zhejiang Univ. Eng. Sci. 2010, 44, 991–997. [Google Scholar]
- Peng, Z.; Ding, Q.; Sun, Y.; Jiang, C.; Gao, X.; Yan, J. Characterization of Mechanochemical Treated Fly Ash from a Medical Waste Incinerator. J. Environ. Sci. 2010, 22, 1643–1648. [Google Scholar] [CrossRef]
- Wei, Y.; Yan, J.; Lu, S.; Li, X. Mechanochemical Decomposition of Pentachlorophenol by Ball Milling. J. Environ. Sci. 2009, 21, 1761–1768. [Google Scholar] [CrossRef]
- Nah, I.W.; Hwang, K.Y.; Shul, Y.G. Effect of Metal and Glycol on Mechanochemical Dechlorination of Polychlorinated Biphenyls (PCBs). Chemosphere 2008, 73, 138–141. [Google Scholar] [CrossRef]
- Cangialosi, F.; Intini, G.; Liberti, L.; Lupo, D.; Notarnicola, M.; Pastore, T. Mechanochemical Treatment of Contaminated Marine Sediments for PCB Degradation. Chem. Sustain. Dev. 2007, 15, 147–156. [Google Scholar]
- Yan, J.H.; Peng, Z.; Lu, S.Y.; Li, X.D.; Ni, M.J.; Cen, K.F.; Dai, H.F. Degradation of PCDD/Fs by Mechanochemical Treatment of Fly Ash from Medical Waste Incineration. J. Hazard. Mater. 2007, 147, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Zhang, Q.; Saito, F.; Ikoma, T.; Tero-Kubota, S. Dependence of Mechanochemically Induced Decomposition of Mono-Chlorobiphenyl on the Occurrence of Radicals. Chemosphere 2005, 60, 939–943. [Google Scholar] [CrossRef]
- Nomura, Y.; Nakai, S.; Hosomi, M. Elucidation of Degradation Mechanism of Dioxins during Mechanochemical Treatment. Environ. Sci. Technol. 2005, 39, 3799–3804. [Google Scholar] [CrossRef] [PubMed]
- Birke, V.; Mattik, J.; Runne, D. Mechanochemical Reductive Dehalogenation of Hazardous Polyhalogenated Contaminants. J. Mater. Sci. 2004, 39, 5111–5116. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Fragale, C.; Pastore, T. High-Energy Milling to Decontaminate Soils Polluted by Polychlorobiphenyls and Atrazine. Environ. Chem. Lett. 2004, 2, 1–4. [Google Scholar] [CrossRef]
- Aresta, M.; Caramuscio, P.; De Stefano, L.; Pastore, T. Solid State Dehalogenation of PCBs in Contaminated Soil Using NaBH4. Waste Manag. 2003, 23, 315–319. [Google Scholar] [CrossRef]
- Tanaka, Y.; Zhang, Q.; Saito, F. Mechanochemical Dechlorination of Trichlorobenzene on Oxide Surfaces. J. Phys. Chem. B 2003, 107, 11091–11097. [Google Scholar] [CrossRef]
- Ikoma, T.; Zhang, Q.; Saito, F.; Akiyama, K.; Tero-Kubota, S.; Kato, T. Radicals in the Mechanochemical Dechlorination of Hazardous Organochlorine Compounds Using CaO Nanoparticles. Bull. Chem. Soc. Jpn. 2001, 74, 2303–2309. [Google Scholar] [CrossRef]
- Zhang, Q.; Saito, F.; Ikoma, T.; Tero-Kubota, S.; Hatakeda, K. Effects of Quartz Addition on the Mechanochemical Dechlorination of Chlorobiphenyl by Using CaO. Environ. Sci. Technol. 2001, 35, 4933–4935. [Google Scholar] [CrossRef]
- Shintani, M.; Nomura, Y.; Nakashimada, Y.; Hosomi, M. Debromination of Decabromodiphenyl Ether by Mechanochemical Treatment. Organohalogen Compd. 2007, 69, 2677–2680. [Google Scholar]
- Zhang, Q.; Matsumoto, H.; Saito, F.; Baron, M. Debromination of Hexabromobenzene by Its Co-Grinding with CaO. Chemosphere 2002, 48, 787–793. [Google Scholar] [CrossRef]
- Zhang, K.; Huang, J.; Wang, H.; Liu, K.; Yu, G.; Deng, S.; Wang, B. Mechanochemical Degradation of Hexabromocyclododecane and Approaches for the Remediation of Its Contaminated Soil. Chemosphere 2014, 116, 40–45. [Google Scholar] [CrossRef]
- Zhang, K.; Huang, J.; Wang, H.; Yu, G.; Wang, B.; Deng, S.; Kano, J.; Zhang, Q. Mechanochemical Destruction of Decabromodiphenyl Ether into Visible Light Photocatalyst BiOBr. RSC Adv. 2014, 4, 14719–14724. [Google Scholar] [CrossRef]
- Zhang, K.; Huang, J.; Zhang, W.; Yu, Y.; Deng, S.; Yu, G. Mechanochemical Degradation of Tetrabromobisphenol A: Performance, Products and Pathway. J. Hazard. Mater. 2012, 243, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, X.; Zhang, K.; Qi, C. Sodium Persulfate-Assisted Mechanochemical Degradation of Tetrabromobisphenol A: Efficacy, Products and Pathway. Chemosphere 2016, 150, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Shintani, M.; Naito, Y.; Yamada, S.; Nomura, Y.; Zhou, S.; Nakashimada, Y.; Hosomi, M. Degradation of Perfluorooctansulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) by Mechanochemical Treatment. Kagaku Kogaku Ronbun 2008, 34, 539–544. [Google Scholar] [CrossRef]
- Zhang, K.; Huang, J.; Yu, G.; Zhang, Q.; Deng, S.; Wang, B. Destruction of Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) by Ball Milling. Environ. Sci. Technol. 2013, 47, 6471–6477. [Google Scholar] [CrossRef]
- Yan, X.; Liu, X.; Qi, C.; Wang, D.; Lin, C. Mechanochemical Destruction of a Chlorinated Polyfluorinated Ether Sulfonate (F-53B, a PFOS Alternative) Assisted by Sodium Persulfate. RSC Adv. 2015, 5, 85785–85790. [Google Scholar] [CrossRef]
- Chen, X.; Yang, H.; Yan, N. Shell Biorefinery: Dream or Reality? Chem. Eur. J. 2016, 22, 13402–13421. [Google Scholar] [CrossRef]
- Nimesh, S.; Thibault, M.M.; Lavertu, M.; Buschmann, M.D. Enhanced Gene Delivery Mediated by Low Molecular Weight Chitosan/DNA Complexes: Effect of pH and Serum. Mol. Biotechnol. 2010, 46, 182–196. [Google Scholar] [CrossRef]
- Amoozgar, Z.; Park, J.; Lin, Q.; Yeo, Y. Low Molecular-Weight Chitosan as a PH-Sensitive Stealth Coating for Tumor-Specific Drug Delivery. Mol. Pharm. 2012, 9, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Huet, G.; Hadad, C.; González-Domínguez, J.M.; Courty, M.; Jamali, A.; Cailleu, D.; van Nhien, A.N. IL versus DES: Impact on Chitin Pretreatment to Afford High Quality and Highly Functionalizable Chitosan. Carbohydr. Polym. 2021, 269, 118332. [Google Scholar] [CrossRef]
- Nakagawa, Y.S.; Oyama, Y.; Kon, N.; Nikaido, M.; Tanno, K.; Kogawa, J.; Inomata, S.; Masui, A.; Yamamura, A.; Kawaguchi, M.; et al. Development of Innovative Technologies to Decrease the Environmental Burdens Associated with Using Chitin as a Biomass Resource: Mechanochemical Grinding and Enzymatic Degradation. Carbohydr. Polym. 2011, 83, 1843–1849. [Google Scholar] [CrossRef]
- Chen, X.; Yang, H.; Zhong, Z.; Yan, N. Base-Catalysed, One-Step Mechanochemical Conversion of Chitin and Shrimp Shells into Low Molecular Weight Chitosan. Green Chem. 2017, 19, 2783–2792. [Google Scholar] [CrossRef]
- Di Nardo, T.; Hadad, C.; Nguyen Van Nhien, A.; Moores, A. Synthesis of High Molecular Weight Chitosan from Chitin by Mechanochemistry and Aging. Green Chem. 2019, 21, 3276–3285. [Google Scholar] [CrossRef]
- Su, W.; Xu, W.; Polyakov, N.E.; Dushkin, A.V.; Qiao, P.; Su, W. Zero-Waste Utilization and Conversion of Shrimp Shell by Mechanochemical Method. J. Clean. Prod. 2023, 425, 139028. [Google Scholar] [CrossRef]
- Sutherland, D.L.; McCauley, J.; Labeeuw, L.; Ray, P.; Kuzhiumparambil, U.; Hall, C.; Doblin, M.; Nguyen, L.N.; Ralph, P.J. How Microalgal Biotechnology Can Assist with the UN Sustainable Development Goals for Natural Resource Management. Curr. Res. Environ. Sustain. 2021, 3, 100050. [Google Scholar] [CrossRef]
- Fatika, F.A.W.; Anwar, M.; Prasetyo, D.J.; Rizal, W.A.; Suryani, R.; Yuliyanto, P.; Hariyadi, S.; Suwanto, A.; Bahmid, N.A.; Wahono, S.K.; et al. Facile Fabrication of Chitosan Schiff Bases from Giant Tiger Prawn Shells (Penaeus Monodon) via Solvent-Free Mechanochemical Grafting. Int. J. Biol. Macromol. 2023, 247, 125759. [Google Scholar] [CrossRef] [PubMed]
- Mosaddegh, E.; Hassankhani, A. Preparation and Characterization of Nano-CaO Based on Eggshell Waste: Novel and Green Catalytic Approach to Highly Efficient Synthesis of Pyrano [4,3-b]Pyrans. Cuihua Xuebao/Chin. J. Catal. 2014, 35, 351–356. [Google Scholar] [CrossRef]
- Tongamp, W.; Kano, J.; Zhang, Q.; Saito, F. Simultaneous Treatment of PVC and Oyster-Shell Wastes by Mechanochemical Means. Waste Manag. 2008, 28, 484–488. [Google Scholar] [CrossRef]
- Baláž, M.; Baláž, P.; Bujňáková, Z.; Pap, Z.; Kupka, D.; Zorkovská, A. Mechanochemical Dechlorination of PVC by Utilizing Eggshell Waste. In Proceedings of the 8th International Conference on Mechanochemistry and Mechanical Alloying, INCOME 2014, Kraków, Poland, 22–26 June 2014; Volume 126, pp. 884–887. [Google Scholar] [CrossRef]
- Baláž, P.; Calka, A.; Zorkovská, A.; Baláž, M. Processing of Eggshell Biomaterial by Electrical Discharge Assisted Mechanical Milling (EDAMM) and High Energy Milling (HEM) Techniques. Mater. Manuf. Process. 2013, 28, 343–347. [Google Scholar] [CrossRef]
- Ferro, A.C.; Guedes, M. Mechanochemical Synthesis of Hydroxyapatite Using Cuttlefish Bone and Chicken Eggshell as Calcium Precursors. Mater. Sci. Eng. C 2019, 97, 124–140. [Google Scholar] [CrossRef]
- Faksawat, K.; Kaewwiset, W.; Limsuwan, P.; Naemchanthara, K. Comparison of Characteristics of Hydroxyapatite Powders Synthesized from Cuttlefish Bone via Precipitation and Ball Milling Techniques. J. Phys. Conf. Ser. 2017, 901, 012083. [Google Scholar] [CrossRef]
- Cestari, F.; Chemello, G.; Galotta, A.; Sglavo, V.M. Low-Temperature Synthesis of Nanometric Apatite from Biogenic Sources. Ceram. Int. 2020, 46, 23526–23533. [Google Scholar] [CrossRef]
- Shen, F.; Qiu, M.; Hua, Y.; Qi, X. Dual-Functional Templated Methodology for the Synthesis of Hierarchical Porous Carbon for Supercapacitor. ChemistrySelect 2018, 3, 586–591. [Google Scholar] [CrossRef]
- Seeharaj, P.; Sripako, K.; Promta, P.; Detsri, E.; Vittayakorn, N. Facile and Eco-friendly Fabrication of Hierarchical Superhydrophobic Coating from Eggshell Biowaste. Int. J. Appl. Ceram. Technol. 2019, 16, 1895–1903. [Google Scholar] [CrossRef]
- Baláž, M.; Bujňáková, Z.; Achimovičová, M.; Tešinský, M.; Baláž, P. Simultaneous Valorization of Polyvinyl Chloride and Eggshell Wastes by a Semi-Industrial Mechanochemical Approach. Environ. Res. 2019, 170, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Onwubu, S.C.; Mdluli, P.S.; Singh, S. Evaluating the Buffering and Acid-Resistant Properties of Eggshell–Titanium Dioxide Composite against Erosive Acids. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800018809914. [Google Scholar] [CrossRef]
- Onwubu, S.C.; Mdluli, P.S.; Singh, S.; Madikizela, L.; Ngombane, Y. Characterization and in Vitro Evaluation of an Acid Resistant Nanosized Dental Eggshell-Titanium Dioxide Material. Adv. Powder Technol. 2019, 30, 766–773. [Google Scholar] [CrossRef]
- Cherdchom, S.; Rattanaphan, T.; Chanadee, T. Calcium Titanate from Food Waste: Combustion Synthesis, Sintering, Characterization, and Properties. Adv. Mater. Sci. Eng. 2019, 2019, 9639016. [Google Scholar] [CrossRef]
- Liu, X.; Shen, F.; Qi, X. Adsorption Recovery of Phosphate from Aqueous Solution by CaO-Biochar Composites Prepared from Eggshell and Rice Straw. Sci. Total Environ. 2019, 666, 694–702. [Google Scholar] [CrossRef]
- Sari, Y.W.; Listiani, E.; Putri, S.Y.; Abidin, Z. Prospective of Eggshell Nanocalcium in Improving Biogas Production from Palm Oil Mill Effluent. Waste Biomass Valorization 2020, 11, 4631–4638. [Google Scholar] [CrossRef]
- Rodríguez-Padrón, D.; Puente-Santiago, A.R.; Luna-Lama, F.; Caballero, Á.; Muñoz-Batista, M.J.; Luque, R. Versatile Protein-Templated TiO2 Nanocomposite for Energy Storage and Catalytic Applications. ACS Sustain. Chem. Eng. 2019, 7, 5329–5337. [Google Scholar] [CrossRef]
- Karuppiah, D.; Palanisamy, R.; Ponnaiah, A.; Liu, W.-R.; Huang, C.-H.; Rengapillai, S.; Marimuthu, S. Eggshell-Membrane-Derived Carbon Coated on Li2FeSiO4 Cathode Material for Li-Ion Batteries. Energies 2020, 13, 786. [Google Scholar] [CrossRef]
- Shen, F.; Xiong, X.; Fu, J.; Yang, J.; Qiu, M.; Qi, X.; Tsang, D.C.W. Recent Advances in Mechanochemical Production of Chemicals and Carbon Materials from Sustainable Biomass Resources. Renew. Sustain. Energy Rev. 2020, 130, 109944. [Google Scholar] [CrossRef]
- Ma, T.Y.; Liu, L.; Yuan, Z.Y. Direct Synthesis of Ordered Mesoporous Carbons. Chem. Soc. Rev. 2013, 42, 3977–4003. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, N.; Chen, D.; Yang, S.; Liu, X.; Wang, L.; Wu, P.; Phillip, N.; Yang, G.; Dai, S. Ultra-Stable and High-Cobalt-Loaded Cobalt@Ordered Mesoporous Carbon Catalysts: All-in-One Deoxygenation of Ketone into Alkylbenzene. ChemCatChem 2018, 10, 3299–3304. [Google Scholar] [CrossRef]
- Shan, D.; Deng, S.; Zhao, T.; Wang, B.; Wang, Y.; Huang, J.; Yu, G.; Winglee, J.; Wiesner, M.R. Preparation of Ultrafine Magnetic Biochar and Activated Carbon for Pharmaceutical Adsorption and Subsequent Degradation by Ball Milling. J. Hazard. Mater. 2016, 305, 156–163. [Google Scholar] [CrossRef]
- Xiang, W.; Wan, Y.; Zhang, X.; Tan, Z.; Xia, T.; Zheng, Y.; Gao, B. Adsorption of Tetracycline Hydrochloride onto Ball-Milled Biochar: Governing Factors and Mechanisms. Chemosphere 2020, 255, 127057. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Gao, B.; He, F.; Zimmerman, A.R.; Ding, C.; Tang, J.; Crittenden, J.C. Experimental and Modeling Investigations of Ball-Milled Biochar for the Removal of Aqueous Methylene Blue. Chem. Eng. J. 2018, 335, 110–119. [Google Scholar] [CrossRef]
- Zheng, Y.; Wan, Y.; Chen, J.; Chen, H.; Gao, B. MgO Modified Biochar Produced through Ball Milling: A Dual-Functional Adsorbent for Removal of Different Contaminants. Chemosphere 2020, 243, 125344. [Google Scholar] [CrossRef]
- Costa, A.A.F.; Oliveira, A.N.; Esposito, R.; Auvigne, A.; Len, C.; Luque, R.; Noronha, R.C.R.; Nascimento, L.A.S. Glycerol and Microwave-Assisted Catalysis: Recent Progress in Batch and Flow Devices. Sustain. Energy Fuels 2023, 7, 1768–1792. [Google Scholar] [CrossRef]
- Costa, A.A.F.; Oliveira, A.N.; Esposito, R.; Len, C.; Luque, R.; Noronha, R.C.R.; Rocha Filho, G.N.; Nascimento, L.A.S. Glycerol and Catalysis by Waste/Low-Cost Materials—A Review. Catalysts 2022, 12, 570. [Google Scholar] [CrossRef]
- Hambali, E.; Fitria, R.; Sari, V.I. Glycerol and Derivatives. In Biorefinery of Oil Producing Plants for Value-Added Products; Wiley: Weinheim, Germany, 2022; pp. 469–491. [Google Scholar]
- Oliveira, A.N.; Melchiorre, M.; Costa, A.A.F.; Silva, L.S.; Paiva, R.J.; Auvigne, A.; Ouyang, W.; Luque, R.; Rocha Filho, G.N.; Noronha, R.C.R.; et al. Glycerol: A Green Solvent for Synthetic Chemistry. Sustain. Chem. Pharm. 2024, 41, 101656. [Google Scholar] [CrossRef]
- Lenardão, E.J.; Barcellos, A.M.; Penteado, F.; Alves, D.; Perin, G. Glycerol as a Solvent in Organic Synthesis. Rev. Virtual Quim. 2017, 9, 192–237. [Google Scholar] [CrossRef]
- Malpartida, I.; Maireles-Torres, P.; Lair, V.; Halloumi, S.; Thiel, J.; Lacoste, F. New High-Throughput Reactor for Biomass Valorization. Chem. Proc. 2020, 2, 31. [Google Scholar] [CrossRef]
- Malpartida, I.; Maireles-Torres, P.; Vereda, C.; Rodríguez-Maroto, J.M.; Halloumi, S.; Lair, V.; Thiel, J.; Lacoste, F. Semi-Continuous Mechanochemical Process for Biodiesel Production under Heterogeneous Catalysis Using Calcium Diglyceroxide. Renew. Energy 2020, 159, 117–126. [Google Scholar] [CrossRef]
- Li, A.; Song, H.; Xu, X.; Meng, H.; Lu, Y.; Li, C. Greener Production Process of Acetylene and Calcium Diglyceroxide via Mechanochemical Reaction of CaC2 and Glycerol. ACS Sustain. Chem. Eng. 2018, 6, 9560–9565. [Google Scholar] [CrossRef]
- Filiciotto, L.; Balu, A.M.; Romero, A.A.; Rodríguez-Castellón, E.; Van Der Waal, J.C.; Luque, R. Benign-by-Design Preparation of Humin-Based Iron Oxide Catalytic Nanocomposites. Green Chem. 2017, 19, 4423–4434. [Google Scholar] [CrossRef]
- Senthil, C.; Vediappan, K.; Nanthagopal, M.; Seop Kang, H.; Santhoshkumar, P.; Gnanamuthu, R.; Lee, C.W. Thermochemical Conversion of Eggshell as Biological Waste and Its Application as a Functional Material for Lithium-Ion Batteries. Chem. Eng. J. 2019, 372, 765–773. [Google Scholar] [CrossRef]
- Naghdi, M.; Taheran, M.; Brar, S.K.; Rouissi, T.; Verma, M.; Surampalli, R.Y.; Valero, J.R. A Green Method for Production of Nanobiochar by Ball Milling—Optimization and Characterization. J. Clean. Prod. 2017, 164, 1394–1405. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, Y.; Gao, B.; Cao, X. N-Doped Biochar Synthesized by a Facile Ball-Milling Method for Enhanced Sorption of CO2 and Reactive Red. Chem. Eng. J. 2019, 368, 564–572. [Google Scholar] [CrossRef]
- Rak, M.J.; Friščić, T.; Moores, A. One-Step, Solvent-Free Mechanosynthesis of Silver Nanoparticle-Infused Lignin Composites for Use as Highly Active Multidrug Resistant Antibacterial Filters. RSC Adv. 2016, 6, 58365–58370. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, S.S.; Tsang, D.C.W.; Clark, J.H.; Budarin, V.L.; Hu, C.; Wu, K.C.W.; Zhang, S. Microwave-Assisted Depolymerization of Various Types of Waste Lignins over Two-Dimensional CuO/BCN Catalysts. Green Chem. 2020, 22, 725–736. [Google Scholar] [CrossRef]
- Torres-Pastor, M.Á.; Espro, C.; Selva, M.; Perosa, A.; Reyes, A.A.R.; Osman, S.M.; Luque, R.; Rodríguez-Padrón, D. Glycerol Valorization towards a Benzoxazine Derivative through a Milling and Microwave Sequential Strategy. Molecules 2022, 27, 632. [Google Scholar] [CrossRef]
- Liang, Z.; Peng, G.; Hu, J.; Hou, H.; Cai, C.; Yang, X.; Chen, S.; Liu, L.; Liang, S.; Xiao, K.; et al. Mechanochemically Assisted Persulfate Activation for the Facile Recovery of Metals from Spent Lithium Ion Batteries. Waste Manag. 2022, 150, 290–300. [Google Scholar] [CrossRef]
- Liu, W.; Liu, W.; Li, X.; Liu, Y.; Ogunmoroti, A.E.; Li, M.; Bi, M.; Cui, Z. Dynamic Material Flow Analysis of Critical Metals for Lithium-Ion Battery System in China from 2000–2018. Resour. Conserv. Recycl. 2021, 164, 105122. [Google Scholar] [CrossRef]
- Winslow, K.M.; Laux, S.J.; Townsend, T.G. A Review on the Growing Concern and Potential Management Strategies of Waste Lithium-Ion Batteries. Resour. Conserv. Recycl. 2018, 129, 263–277. [Google Scholar] [CrossRef]
- Fan, M.-C.; Zhao, Y.; Kang, Y.-Q.; Wozny, J.; Liang, Z.; Wang, J.-X.; Zhou, G.-M.; Li, B.-H.; Tavajohi, N.; Kang, F.-Y. Room-Temperature Extraction of Individual Elements from Charged Spent LiFePO4 Batteries. Rare Met. 2022, 41, 1595–1604. [Google Scholar] [CrossRef]
- Geiß, D.; Dolotko, O.; Indris, S.; Neemann, C.; Bologa, A.; Bergfeldt, T.; Knapp, M.; Ehrenberg, H. Revealing the Mechanism of Reductive, Mechanochemical Li Recycling from LiFePO4. RSC Mechanochem. 2024, 1, 349–360. [Google Scholar] [CrossRef]
- Jiang, F.; Chen, Y.; Ju, S.; Zhu, Q.; Zhang, L.; Peng, J.; Wang, X.; Miller, J.D. Ultrasound-Assisted Leaching of Cobalt and Lithium from Spent Lithium-Ion Batteries. Ultrason. Sonochem. 2018, 48, 88–95. [Google Scholar] [CrossRef]
- Diaz, F.; Wang, Y.; Moorthy, T.; Friedrich, B. Degradation Mechanism of Nickel-Cobalt-Aluminum (NCA) Cathode Material from Spent Lithium-Ion Batteries in Microwave-Assisted Pyrolysis. Metals 2018, 8, 565. [Google Scholar] [CrossRef]
- Saeki, S.; Lee, J.; Zhang, Q.; Saito, F. Co-Grinding LiCoO2 with PVC and Water Leaching of Metal Chlorides Formed in Ground Product. Int. J. Miner. Process. 2004, 74, S373–S378. [Google Scholar] [CrossRef]
- Zhang, Q.; Saeki, S.; Tanaka, Y.; Kano, J.; Saito, F. A Soft-Solution Process for Recovering Rare Metals from Metal/Alloy-Wastes by Grinding and Washing with Water. J. Hazard. Mater. 2007, 139, 438–442. [Google Scholar] [CrossRef]
- Wang, M.-M.; Zhang, C.-C.; Zhang, F.-S. Recycling of Spent Lithium-Ion Battery with Polyvinyl Chloride by Mechanochemical Process. Waste Manag. 2017, 67, 232–239. [Google Scholar] [CrossRef]
- Guan, J.; Li, Y.; Guo, Y.; Su, R.; Gao, G.; Song, H.; Yuan, H.; Liang, B.; Guo, Z. Mechanochemical Process Enhanced Cobalt and Lithium Recycling from Wasted Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2017, 5, 1026–1032. [Google Scholar] [CrossRef]
- Liu, K.; Tan, Q.; Liu, L.; Li, J. Acid-Free and Selective Extraction of Lithium from Spent Lithium Iron Phosphate Batteries via a Mechanochemically Induced Isomorphic Substitution. Environ. Sci. Technol. 2019, 53, 9781–9788. [Google Scholar] [CrossRef]
- Dolotko, O.; Hlova, I.Z.; Mudryk, Y.; Gupta, S.; Balema, V.P. Mechanochemical Recovery of Co and Li from LCO Cathode of Lithium-Ion Battery. J. Alloys Compd. 2020, 824, 153876. [Google Scholar] [CrossRef]
- Dolotko, O.; Gehrke, N.; Malliaridou, T.; Sieweck, R.; Herrmann, L.; Hunzinger, B.; Knapp, M.; Ehrenberg, H. Universal and Efficient Extraction of Lithium for Lithium-Ion Battery Recycling Using Mechanochemistry. Commun. Chem. 2023, 6, 49. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, C.; Zhang, X.; Ma, E. A Mechanochemical Method for One-Step Leaching of Metals from Spent LIBs. Waste Manag. 2023, 161, 245–253. [Google Scholar] [CrossRef]
- Qiao, Q.; Li, X.; Li, Y.; Wang, K.; Yu, H.; Xing, W.; Li, N.; Sun, Y.; Wang, B. Mechanochemically-Aided Leaching of Cobalt from the Cathode of Spent Lithium-Ion Batteries. Braz. J. Chem. Eng. 2024, 41, 461–473. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, G.; Cheng, L.; Gu, J.; Yang, J.; Yuan, H.; Chen, Y.; Wu, Y. Ultra-Fast Mechanochemistry Reaction Process: An Environmentally Friendly Instant Recycling Method for Spent LiFePO4 Batteries. Sep. Purif. Technol. 2024, 335, 126174. [Google Scholar] [CrossRef]
- Yuan, W.; Li, J.; Zhang, Q.; Saito, F. Innovated Application of Mechanical Activation To Separate Lead from Scrap Cathode Ray Tube Funnel Glass. Environ. Sci. Technol. 2012, 46, 4109–4114. [Google Scholar] [CrossRef]
- Wang, M.-M.; Zhang, C.-C.; Zhang, F.-S. An Environmental Benign Process for Cobalt and Lithium Recovery from Spent Lithium-Ion Batteries by Mechanochemical Approach. Waste Manag. 2016, 51, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zheng, X.; Cao, H.; Zhao, C.; Lin, X.; Ning, P.; Zhang, Y.; Jin, W.; Sun, Z. A Closed-Loop Process for Selective Metal Recovery from Spent Lithium Iron Phosphate Batteries through Mechanochemical Activation. ACS Sustain. Chem. Eng. 2017, 5, 9972–9980. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Y.; Lou, X.; Guan, J.; Li, Y.; Mai, X.; Liu, H.; Zhao, C.X.; Wang, N.; Yan, C.; et al. Improved Extraction of Cobalt and Lithium by Reductive Acid from Spent Lithium-Ion Batteries via Mechanical Activation Process. J. Mater. Sci. 2018, 53, 13790–13800. [Google Scholar] [CrossRef]
- Wang, M.; Tan, Q.; Li, J. Unveiling the Role and Mechanism of Mechanochemical Activation on Lithium Cobalt Oxide Powders from Spent Lithium-Ion Batteries. Environ. Sci. Technol. 2018, 52, 13136–13143. [Google Scholar] [CrossRef]
- Fan, E.; Li, L.; Zhang, X.; Bian, Y.; Xue, Q.; Wu, J.; Wu, F.; Chen, R. Selective Recovery of Li and Fe from Spent Lithium-Ion Batteries by an Environmentally Friendly Mechanochemical Approach. ACS Sustain. Chem. Eng. 2018, 6, 11029–11035. [Google Scholar] [CrossRef]
- Liu, K.; Yang, J.; Hou, H.; Liang, S.; Chen, Y.; Wang, J.; Liu, B.; Xiao, K.; Hu, J.; Deng, H. Facile and Cost-Effective Approach for Copper Recovery from Waste Printed Circuit Boards via a Sequential Mechanochemical/Leaching/Recrystallization Process. Environ. Sci. Technol. 2019, 53, 2748–2757. [Google Scholar] [CrossRef]
- Wang, M.; Tan, Q.; Huang, Q.; Liu, L.; Chiang, J.F.; Li, J. Converting Spent Lithium Cobalt Oxide Battery Cathode Materials into High-Value Products via a Mechanochemical Extraction and Thermal Reduction Route. J. Hazard. Mater. 2021, 413, 125222. [Google Scholar] [CrossRef]
- Wang, M.; Tan, Q.; Liu, L.; Li, J. Selective Regeneration of Lithium from Spent Lithium-Ion Batteries Using Ionic Substitution Stimulated by Mechanochemistry. J. Clean. Prod. 2021, 279, 123612. [Google Scholar] [CrossRef]
- Xie, J.; Huang, K.; Nie, Z.; Yuan, W.; Wang, X.; Song, Q.; Zhang, X.; Zhang, C.; Wang, J.; Crittenden, J.C. An Effective Process for the Recovery of Valuable Metals from Cathode Material of Lithium-Ion Batteries by Mechanochemical Reduction. Resour. Conserv. Recycl. 2021, 168, 105261. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, C.; Zhang, X.; Ma, E. Recovery of Li and Co from Spent Li-Ion Batteries by Mechanochemical Integration with NH4Cl. ACS Sustain. Chem. Eng. 2022, 10, 5611–5620. [Google Scholar] [CrossRef]
- Cai, L.; Lin, J.; Fan, E.; Wu, F.; Chen, R.; Li, L. Eco-Friendly Organic Acid-Assisted Mechanochemical Process for Metal Extraction from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2022, 10, 10649–10657. [Google Scholar] [CrossRef]
- Gao, G.; Luo, X.; Liu, N.; Yang, T.; Zhang, X.; Guan, J.; Chen, S.; Zhang, R.-Q.; Guo, Y. Exploration of Sequential Mechanochemical Activation and Complexation Leaching for Enhanced Recovery of Valuable Metals from Spent Lithium-Ion Batteries. Ionics 2023, 29, 3585–3596. [Google Scholar] [CrossRef]
- Vauloup, J.; Bouilhac, C.; Coppey, N.; Lacroix-Desmazes, P.; Stievano, L.; Monconduit, L.; Sougrati, M.T. Towards a More Sustainable Leaching Process for Li-Ion Battery Cathode Material Recycling: Mechanochemical Leaching of LiCoO2 Using Citric Acid. ACS Sustain. Resour. Manag. 2024, 1, 2032–2040. [Google Scholar] [CrossRef]
- Wang, W.; Hu, G.; Peng, Z.; Du, K.; Cao, Y.; Duan, J. Nano-Sized over-Lithiated Oxide by a Mechano-Chemical Activation-Assisted Microwave Technique as Cathode Material for Lithium Ion Batteries and Its Electrochemical Performance. Ceram. Int. 2018, 44, 1425–1431. [Google Scholar] [CrossRef]
- Meng, Q.; Duan, J.; Zhang, Y.; Dong, P. Novel Efficient and Environmentally Friendly Recovering of High Performance Nano-LiMnPO4/C Cathode Powders from Spent LiMn2O4 Batteries. J. Ind. Eng. Chem. 2019, 80, 633–639. [Google Scholar] [CrossRef]
- Meng, X.; Hao, J.; Cao, H.; Lin, X.; Ning, P.; Zheng, X.; Chang, J.; Zhang, X.; Wang, B.; Sun, Z. Recycling of LiNi1/3Co1/3Mn1/3O2 Cathode Materials from Spent Lithium-Ion Batteries Using Mechanochemical Activation and Solid-State Sintering. Waste Manag. 2019, 84, 54–63. [Google Scholar] [CrossRef]
- Song, L.; Qi, C.; Wang, S.; Zhu, X.; Zhang, T.; Jin, Y.; Zhang, M. Direct Regeneration of Waste LiFePO4 Cathode Materials with a Solid-Phase Method Promoted by Activated CNTs. Waste Manag. 2023, 157, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, Y.; Dong, P.; Zhang, Y.; Meng, Q.; Zhou, S.; Yang, X.; Zhang, M.; Yang, X. Direct Regeneration of Spent LiFePO4 Cathode Materials with Pre-Oxidation and V-Doping. J. Alloys Compd. 2021, 860, 157909. [Google Scholar] [CrossRef]
- Gou, Y.; Qi, C.; Li, R.; Liu, X.; Zhou, Z.; Zhang, M.; Sun, Q.; Song, L.; Jin, Y. Direct Regeneration of High-Value LiFePO4 Cathode Materials with Nitrogen Doped Carbon Coating. Electrochim. Acta 2024, 488, 144180. [Google Scholar] [CrossRef]
- Han, Y.; Fang, Y.; Yan, M.; Qiu, H.; Han, Y.; Chen, Y.; Lin, L.; Qian, J.; Mei, T.; Wang, X. Direct Regeneration of Fluorine-Doped Carbon-Coated LiFePO4 Cathode Materials from Spent Lithium-Ion Batteries. Green Chem. 2024, 26, 9791–9801. [Google Scholar] [CrossRef]
- Falah, E.N.; Widyastuti; Noerochim, L.; Asih, R.; Ananda, M.B.; Wibisono, A.T.; Pradesar, Y.; Zulfa, L.L.; Yulamda, I. A Study of the Addition of G-C3N4 in Direct Regeneration of Spent LiFePO4 Battery Cathodes on the Electrochemical Performance of Lithium-Ion Batteries (LIB). Mater. Res. Bull. 2025, 187, 113378. [Google Scholar] [CrossRef]
- Ardila-Fierro, K.J.; Hernández, J.G. Sustainability Assessment of Mechanochemistry by Using the Twelve Principles of Green Chemistry. ChemSusChem 2021, 14, 2145–2162. [Google Scholar] [CrossRef]
- Pagola, S. Outstanding Advantages, Current Drawbacks, and Significant Recent Developments in Mechanochemistry: A Perspective View. Crystals 2023, 13, 124. [Google Scholar] [CrossRef]
- Cindro, N.; Tireli, M.; Karadeniz, B.; Mrla, T.; Užarević, K. Investigations of Thermally Controlled Mechanochemical Milling Reactions. ACS Sustain. Chem. Eng. 2019, 7, 16301–16309. [Google Scholar] [CrossRef]
- Achar, T.K.; Bose, A.; Mal, P. Mechanochemical Synthesis of Small Organic Molecules. Beilstein J. Org. Chem. 2017, 13, 1907–1931. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).