Plasma–Liquid Synthesis of PLA/MXene Composite Films and Their Structural, Optical, and Photocatalytic Properties
Abstract
1. Introduction
2. Results and Discussions
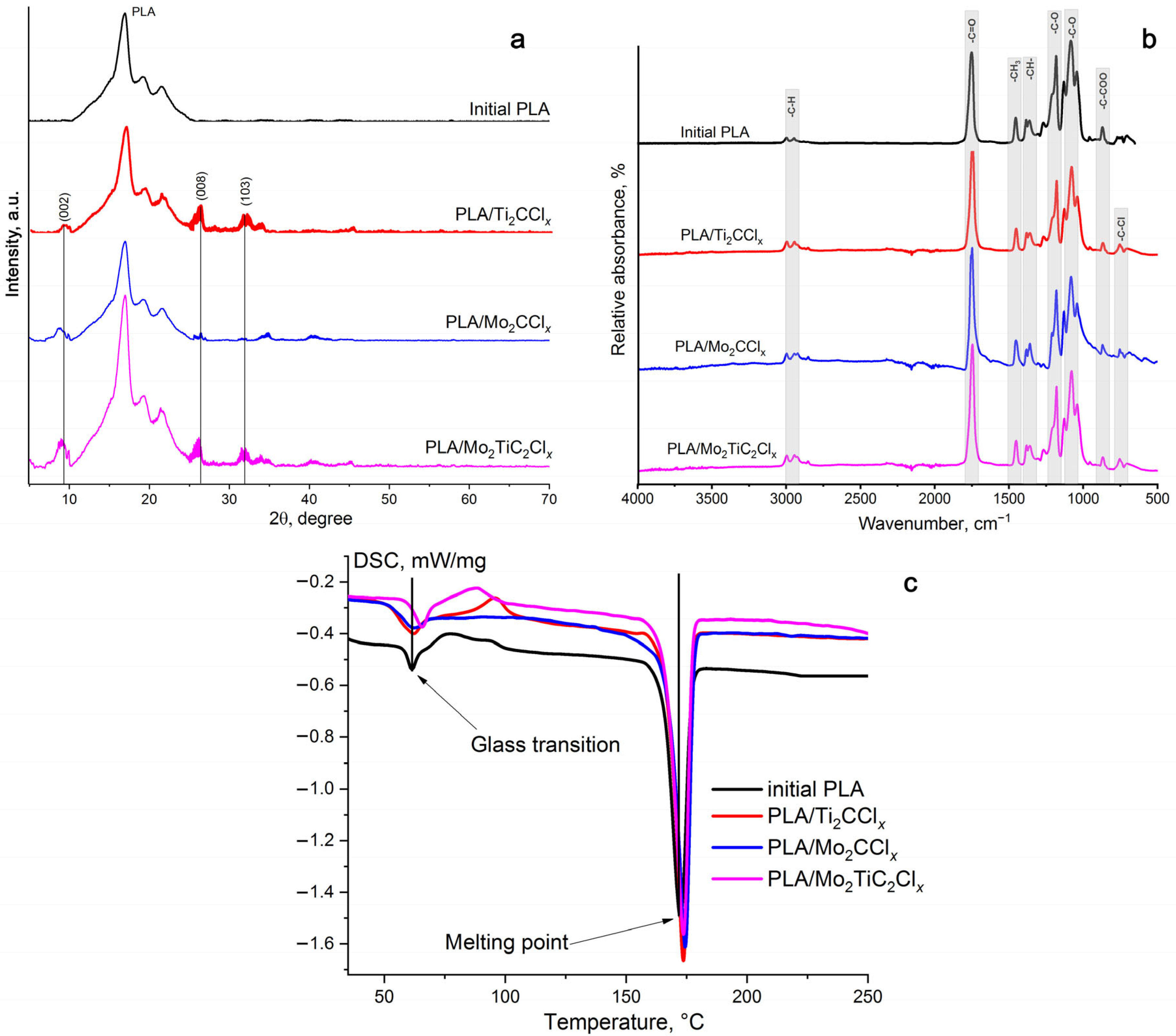
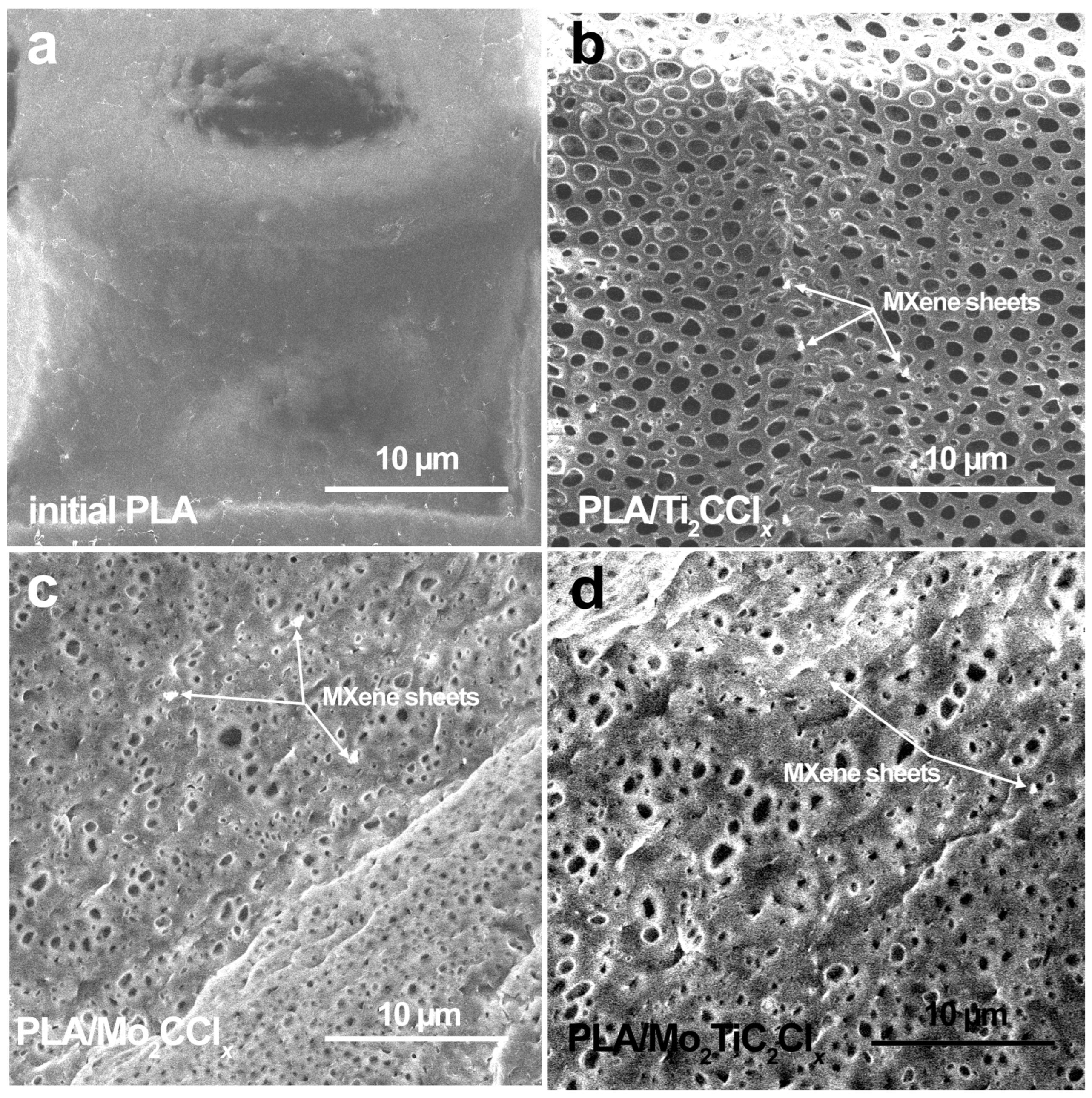
2.1. Structural Properties of Composite Films
2.2. Optical Properties of Composite Films
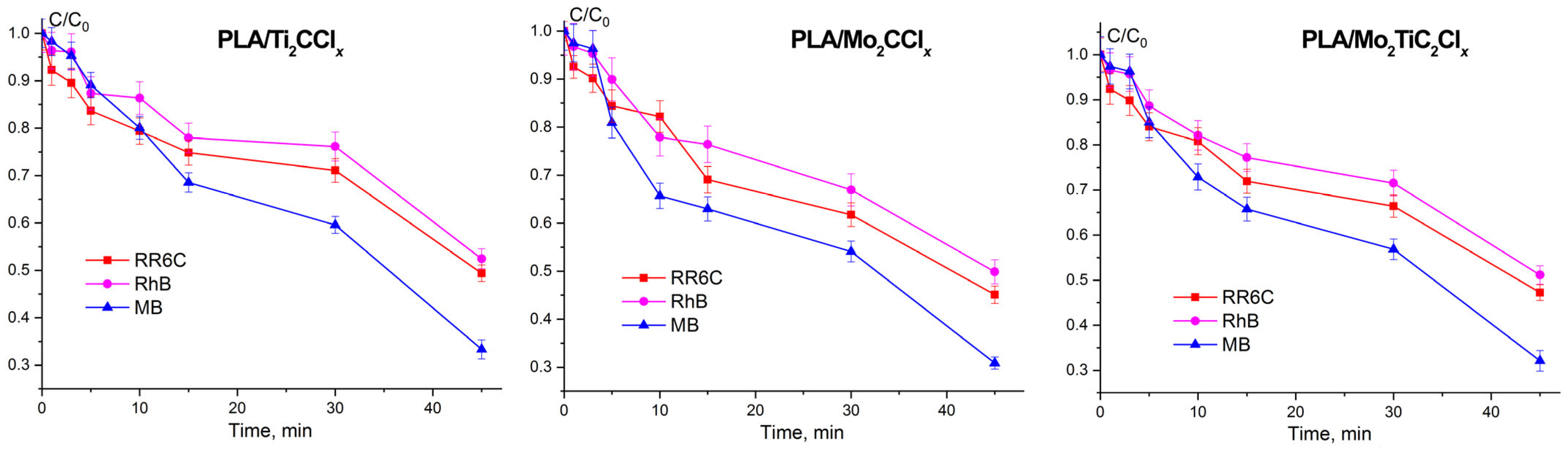
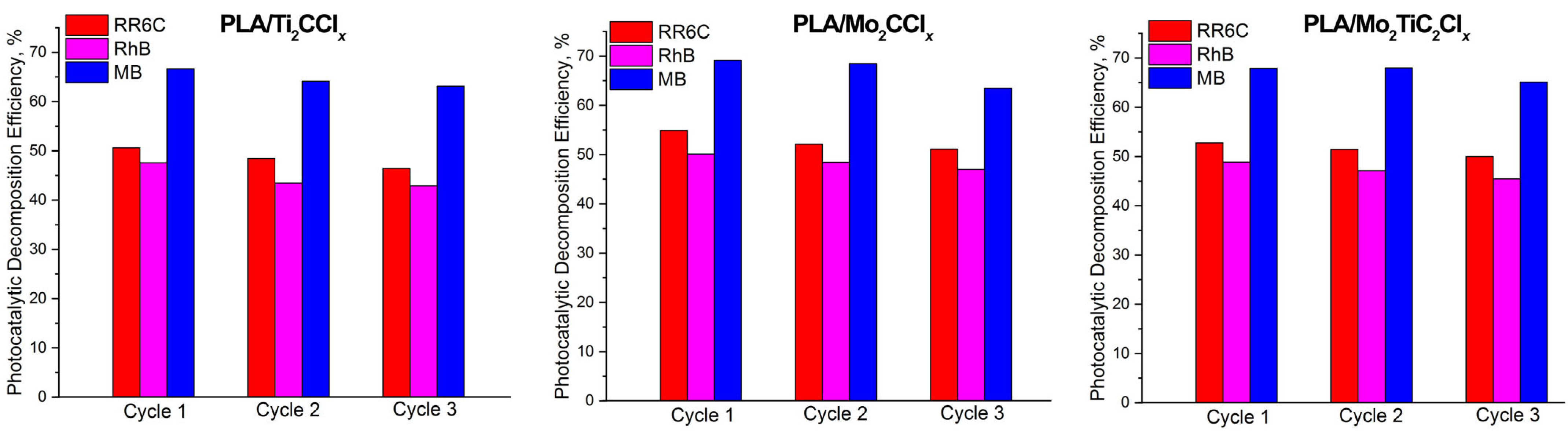
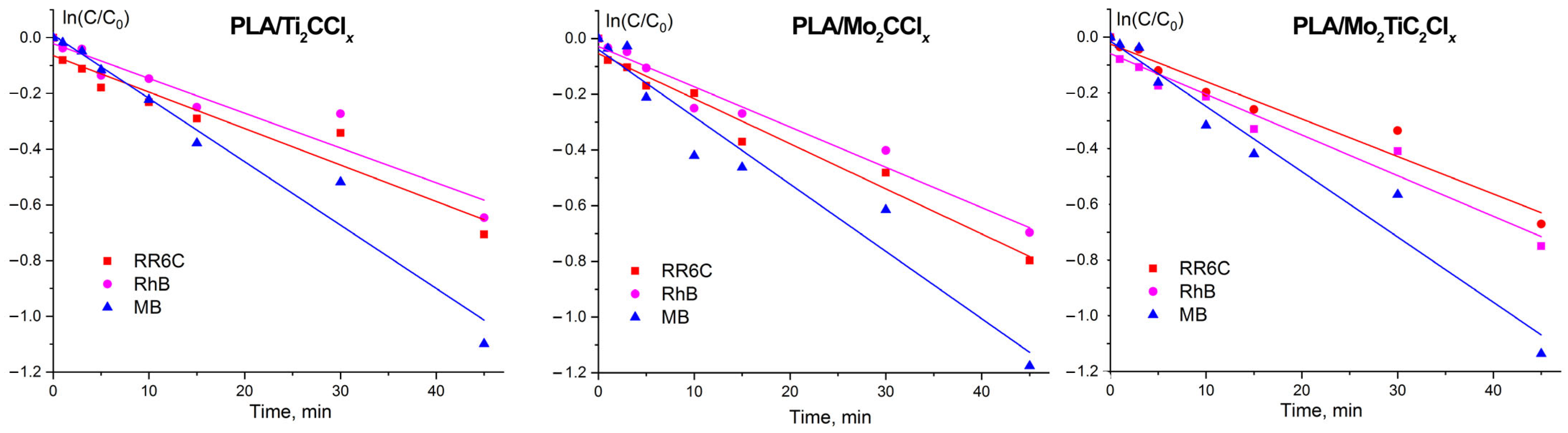
2.3. Photocatalytic Activity of Samples
3. Materials and Methods
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Raquez, J.-M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-Based Nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Kuru, Z.; Kaya, M.A. Poly (Lactic Acid)/Polyester Blends: Review of Current and Future Applications. Eur. J. Res. Dev. 2023, 3, 175–199. [Google Scholar] [CrossRef]
- Khouri, N.G.; Bahú, J.O.; Blanco-Llamero, C.; Severino, P.; Concha, V.O.C.; Souto, E.B. Polylactic Acid (PLA): Properties, Synthesis, and Biomedical Applications—A Review of the Literature. J. Mol. Struct. 2024, 1309, 138243. [Google Scholar] [CrossRef]
- Niu, D.; Yu, W.; Yang, W.; Xu, P.; Liu, T.; Wang, Z.; Yan, X.; Ma, P. Flame-Retardant and UV-Shielding Poly (Lactic Acid) Composites with Preserved Mechanical Properties by Incorporating Cyclophosphazene Derivative and Phosphorus-Modified Lignin. Chem. Eng. J. 2023, 474, 145753. [Google Scholar] [CrossRef]
- Bahar, A.; Hamami, A.E.A.; Benmahiddine, F.; Belhabib, S.; Belarbi, R.; Guessasma, S. The Thermal and Mechanical Behaviour of Wood-PLA Composites Processed by Additive Manufacturing for Building Insulation. Polymers 2023, 15, 3056. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, Y.; Liu, M.; Meng, L.; Li, C. A Review of Research and Application of Polylactic Acid Composites. J. Appl. Polym. Sci. 2023, 140, e53477. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Harussani, M.M.; Hakimi, M.Y.A.Y.; Haziq, M.Z.M.; Atikah, M.S.N.; Asyraf, M.R.M.; Ishak, M.R.; Razman, M.R.; Nurazzi, N.M.; et al. Polylactic Acid (PLA) Biocomposite: Processing, Additive Manufacturing and Advanced Applications. Polymers 2021, 13, 1326. [Google Scholar] [CrossRef]
- Maurya, M.R.; Sha, M.S.; Latrous, L.; Megriche, A.; Sadasivuni, K.K. Engineering PLA-MXene Nanocomposite with Balanced Mechanical Properties for Enhanced Shape Memory Effect. J. Polym. Res. 2024, 31, 345. [Google Scholar] [CrossRef]
- Bigham, A.; Zarepour, A.; Khosravi, A.; Iravani, S.; Zarrabi, A. 3D and 4D Printing of MXene-Based Composites: From Fundamentals to Emerging Applications. Mater. Horiz. 2024, 11, 6257–6288. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D Metal Carbides and Nitrides (MXenes) for Energy Storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Jiang, Y. Applications and Perspectives of Ti3C2Tx MXene in Electrochemical Energy Storage Systems. Int. J. Electrochem. Sci. 2025, 20, 100948. [Google Scholar] [CrossRef]
- Srinivas, P.; Jacob, L.; Shebeeb, C.M.; Butt, H.; Barsoum, I.; Abu Al-Rub, R.K.; Zaki, W. Mechanical Properties, Energy Absorption, and Shape Memory Behavior of 3D Printed PLA-MXene Nanocomposites and Gyroid Lattices. Adv. Eng. Mater. 2024, 26, 2301698. [Google Scholar] [CrossRef]
- Qiang, T.; Qi, X.; Gao, H.; Qiang, H.; Wang, S.; Hu, L.; Hu, N. UV-Shielding, Flexible and Enhanced Thermal-Conductive Polylactide Composites Modified with Single-Layered, Large-Sized MXene Nano-Sheets. Polym. Bull. 2024, 81, 12243–12265. [Google Scholar] [CrossRef]
- Protyai, M.I.H.; Bin Rashid, A. A Comprehensive Overview of Recent Progress in MXene-Based Polymer Composites: Their Fabrication Processes, Advanced Applications, and Prospects. Heliyon 2024, 10, e37030. [Google Scholar] [CrossRef]
- Sanaka, R.; Sahu, S.K.; Sreekanth, P.S.R.; Giri, J.; Mohammad, F.; Al-Lohedan, H.A.; Saharudin, M.S.; Ma, Q. Heat-Responsive PLA/PU/MXene Shape Memory Polymer Blend Nanocomposite: Mechanical, Thermal, and Shape Memory Properties. Polymers 2025, 17, 338. [Google Scholar] [CrossRef] [PubMed]
- Chanklom, P.; Kreetachat, T.; Chotigawin, R.; Suwannahong, K. Photocatalytic Oxidation of PLA/TiO2 -Composite Films for Indoor Air Purification. ACS Omega 2021, 6, 10629–10636. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.Z.; Fan, X.; Mukhopadhyay, S. Antimicrobial Effects of Pulsed Light Activated TiO2-Polylactic Acid Film. Heliyon 2024, 10, e38891. [Google Scholar] [CrossRef]
- Sirotkin, N.A.; Gurina, D.L.; Khlyustova, A.V.; Costerin, D.Y.; Naumova, I.K.; Titov, V.A.; Agafonov, A.V. Experimental and Computational Investigation of Polylactic Acid/Silver-NP Nanocomposite with Antimicrobial Activity Prepared by Plasma in Liquid. Plasma Process. Polym. 2021, 18, 2000169. [Google Scholar] [CrossRef]
- Sirotkin, N.; Khlyustova, A.; Shibaeva, V.; Agafonov, A. Plasma–Liquid Synthesis of Titanium- and Molybdenum-Containing MXenes and Their Photocatalytic Properties. Catalysts 2025, 15, 445. [Google Scholar] [CrossRef]
- Hu, T.; Wang, J.; Zhang, H.; Li, Z.; Hu, M.; Wang, X. Vibrational Properties of Ti3C2 and Ti3C2T2 (T=O, F, OH) Monosheets by First-Principles Calculations: A Comparative Study. Phys. Chem. Chem. Phys. 2015, 17, 9997–10003. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Wan Yunus, W.M.Z.; Hussein, M.Z.; Loo, Y.Y. Effect of Graphene Nanoplatelets as Nanofiller in Plasticized Poly(Lactic Acid) Nanocomposites. J. Therm. Anal. Calorim. 2014, 118, 1551–1559. [Google Scholar] [CrossRef]
- Schippers, C.; Marx, E.; Taubner, R.; Gutmann, J.; Tsarkova, L. Evaluating the Potential of Polylactide Nonwovens as Bio-Based Media for Air Filtration. Textiles 2021, 1, 268–282. [Google Scholar] [CrossRef]
- Toan, L.V.; Thong, N.H.; Quan, D.H.; Huan, P.V.; Trang, T.T.; Thuy, V.T.P.; Giang, N.T.; Tam, P.D.; Hung, N.V.; Pham, V.-H. Synthesis of Polyethylene Glycol–Chitosan–Nano Ag Composites and Their Antibacterial Properties. J. Appl. Spectrosc. 2022, 89, 482–486. [Google Scholar] [CrossRef]
- Lee, E.; VahidMohammadi, A.; Prorok, B.C.; Yoon, Y.S.; Beidaghi, M.; Kim, D.-J. Room Temperature Gas Sensing of Two-Dimensional Titanium Carbide (MXene). ACS Appl. Mater. Interfaces 2017, 9, 37184–37190. [Google Scholar] [CrossRef] [PubMed]
- Primc, G.; Mozetič, M. Surface Modification of Polymers by Plasma Treatment for Appropriate Adhesion of Coatings. Materials 2024, 17, 1494. [Google Scholar] [CrossRef]
- Yang, S.; Li, S.-R.; Zhou, S.-Y.; Yang, H.-R.; Xu, L.; Zhong, G.-J.; Xu, J.-Z.; Li, Z.-M.; Tao, X.-M.; Mai, Y.-W. Cold Crystallization Behavior of Poly (Lactic Acid) Induced by Poly (Ethylene Glycol)-Grafted Graphene Oxide: Crystallization Kinetics and Polymorphism. Compos. Sci. Technol. 2024, 258, 110871. [Google Scholar] [CrossRef]
- Zhang, C.; Lan, Q.; Zhai, T.; Nie, S.; Luo, J.; Yan, W. Melt Crystallization Behavior and Crystalline Morphology of Polylactide/Poly (ε-Caprolactone) Blends Compatibilized by Lactide-Caprolactone Copolymer. Polymers 2018, 10, 1181. [Google Scholar] [CrossRef]
- Sirotkin, N.; Khlyustova, A.; Titov, V.; Agafonov, A. Plasma-assisted Synthesis and Deposition of Molybdenum Oxide Nanoparticles on Polyethylene Terephthalate for Photocatalytic Degradation of Rhodamine B. Plasma Process. Polym. 2020, 17, 2000012. [Google Scholar] [CrossRef]
- Anasori, B.; Xie, Y.; Beidaghi, M.; Lu, J.; Hosler, B.C.; Hultman, L.; Kent, P.R.C.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional, Ordered, Double Transition Metals Carbides (MXenes). ACS Nano 2015, 9, 9507–9516. [Google Scholar] [CrossRef]
- Badr, A. Near Infra Red Spectroscopy. In Wide Spectra of Quality Control; InTech: Houston, TX, USA, 2011. [Google Scholar]
- Weyer, L.G.; Lo, S.-C. Spectra– Structure Correlations in the Near-Infrared. In Handbook of Vibrational Spectroscopy; Griffiths, P.R., Ed.; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar]
- Zhang, L.; Su, W.; Shu, H.; Lü, T.; Fu, L.; Song, K.; Tang, Y. Tuning the photoluminescence of large Ti3C2Tx MXene flakes. Ceram. Int. 2019, 45, 11468–11474. [Google Scholar] [CrossRef]
- Chen, K.; Dong, W.; Huang, Y.; Wang, F.; Zhou, J.L.; Li, W. Photocatalysis for sustainable energy and environmental protection in construction: A review on surface engineering and emerging synthesis. J. Environ. Chem. Eng. 2025, 13, 117529. [Google Scholar] [CrossRef]
- Zeeshan, M.; Javed, T.; Kumari, C.; Thumma, A.; Wasim, M.; Taj, M.B.; Batool, M. Investigating the interactions between dyes and porous/composite materials: A comprehensive study. Sust. Chem. Environ. 2025, 9, 100217. [Google Scholar] [CrossRef]
- Pavel, M.; Anastasescu, C.; State, R.N.; Vasile, A.; Papa, F.; Balint, I. Photocatalytic degradation of organic and inorganic pollutants to harmless end products: Assessment of practical application potential for water and air cleaning. Catalysts 2023, 13, 380. [Google Scholar] [CrossRef]
- Yu, C.; Wang, S.; Zhang, K.; Li, M.; Gao, H.; Zhang, J.; Yang, H.; Hu, L.; Jagadeesha, A.V.; Li, D. Visible-Light-Enhanced Photocatalytic Activity of BaTiO3/γ-Al2O3 Composite Photocatalysts for Photodegradation of Tetracycline Hydrochloride. Opt. Mater. 2023, 135, 113364. [Google Scholar] [CrossRef]
- Arbelaiz, A.; Fernández, B.; Valea, A.; Mondragon, I. Mechanical Properties of Short Flax Fibre Bundle/Poly (ε-Caprolactone) Composites: Influence of Matrix Modification and Fibre Content. Carbohydr. Polym. 2006, 64, 224–232. [Google Scholar] [CrossRef]


| Name | Experimental Conditions | Chemical Composition | Content of MXenes in the Composite Film, wt% |
|---|---|---|---|
| Sample 1 | Underwater plasma in CCl4 polylactide solution (2 wt%) at discharge current of 0.25 A; Ti—anode, Ti—cathode | PLA/Ti2CClx | 1.75 ± 0.08 |
| Sample 2 | Underwater plasma in polylactide solution in CCl4 (2 wt%) at discharge current of 0.25 A; Mo—anode, Mo—cathode | PLA/Mo2CClx | 1.54 ± 0.11 |
| Sample 3 | Underwater plasma in polylactide solution in CCl4 (2 wt%) at discharge current of 0.25 A; Ti—anode, Mo—cathode | PLA/Mo2TiC2Clx | 1.69 ± 0.04 |
| Dye | k, min−1 | R2 |
|---|---|---|
| PLA/Ti2CClx | ||
| RR6C | 0.01249 ± 0.00151 | 0.91 |
| RhB | 0.01269 ± 0.00152 | 0.91 |
| MB | 0.02274 ± 0.00117 | 0.96 |
| PLA/Mo2CClx | ||
| RR6C | 0.01419 ± 0.0012 | 0.97 |
| RhB | 0.01662 ± 0.0011 | 0.96 |
| MB | 0.02416± 0.0022 | 0.94 |
| PLA/Mo2TiC2Clx | ||
| RR6C | 0.01345 ± 0.0011 | 0.95 |
| RhB | 0.01442 ± 0.0012 | 0.95 |
| MB | 0.02313 ± 0.0019 | 0.96 |
| Name | Chemical Structure | Class of Dye | λmax, nm |
|---|---|---|---|
| Rhodamine B | 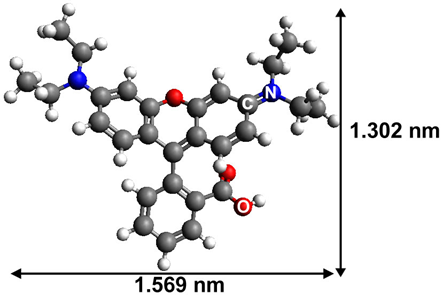 | zwitterionic xanthene | 554 |
| Methylene Blue | 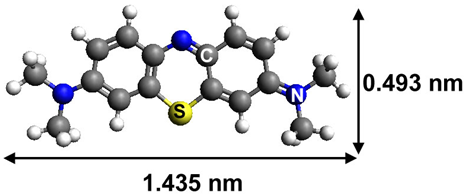 | cationic thiazine | 667 |
| Reactive Red 6C | 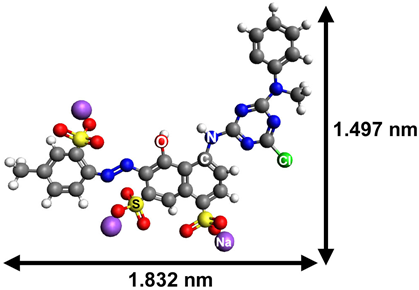 | anionic | 533 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirotkin, N.; Khlyustova, A.; Agafonov, A. Plasma–Liquid Synthesis of PLA/MXene Composite Films and Their Structural, Optical, and Photocatalytic Properties. Catalysts 2025, 15, 890. https://doi.org/10.3390/catal15090890
Sirotkin N, Khlyustova A, Agafonov A. Plasma–Liquid Synthesis of PLA/MXene Composite Films and Their Structural, Optical, and Photocatalytic Properties. Catalysts. 2025; 15(9):890. https://doi.org/10.3390/catal15090890
Chicago/Turabian StyleSirotkin, Nikolay, Anna Khlyustova, and Alexander Agafonov. 2025. "Plasma–Liquid Synthesis of PLA/MXene Composite Films and Their Structural, Optical, and Photocatalytic Properties" Catalysts 15, no. 9: 890. https://doi.org/10.3390/catal15090890
APA StyleSirotkin, N., Khlyustova, A., & Agafonov, A. (2025). Plasma–Liquid Synthesis of PLA/MXene Composite Films and Their Structural, Optical, and Photocatalytic Properties. Catalysts, 15(9), 890. https://doi.org/10.3390/catal15090890








