Thermally Exfoliated g-C3N4/Ti3C2Tx MXene Schottky Junctions as Photocatalysts for the Removal of Valsartan from Aquatic Environments
Abstract
1. Introduction
2. Results and Discussion
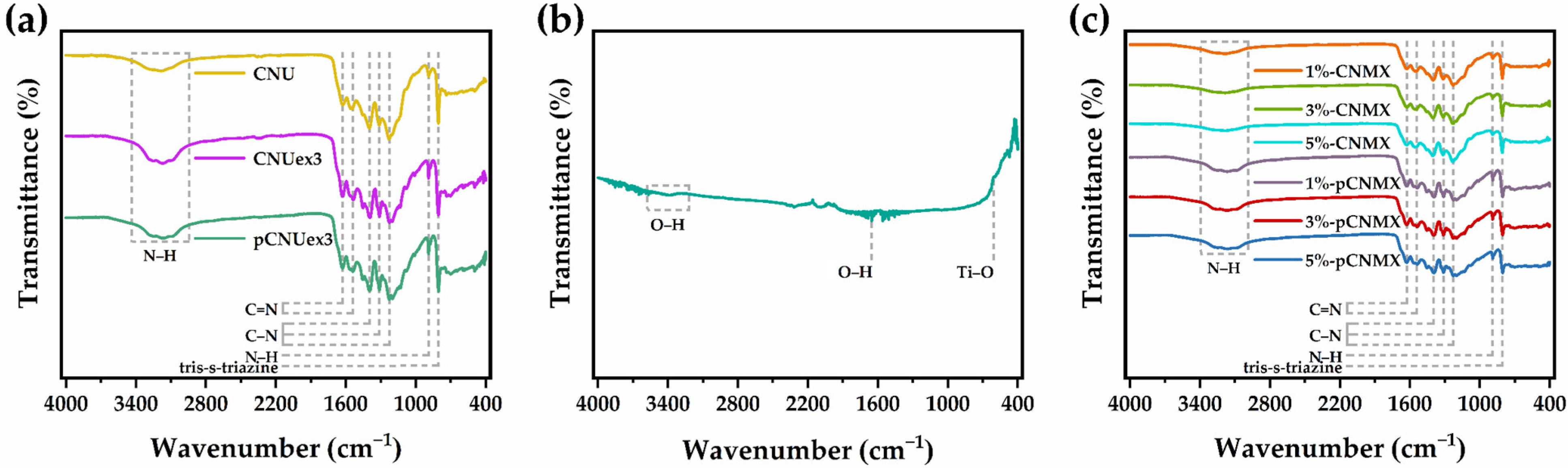
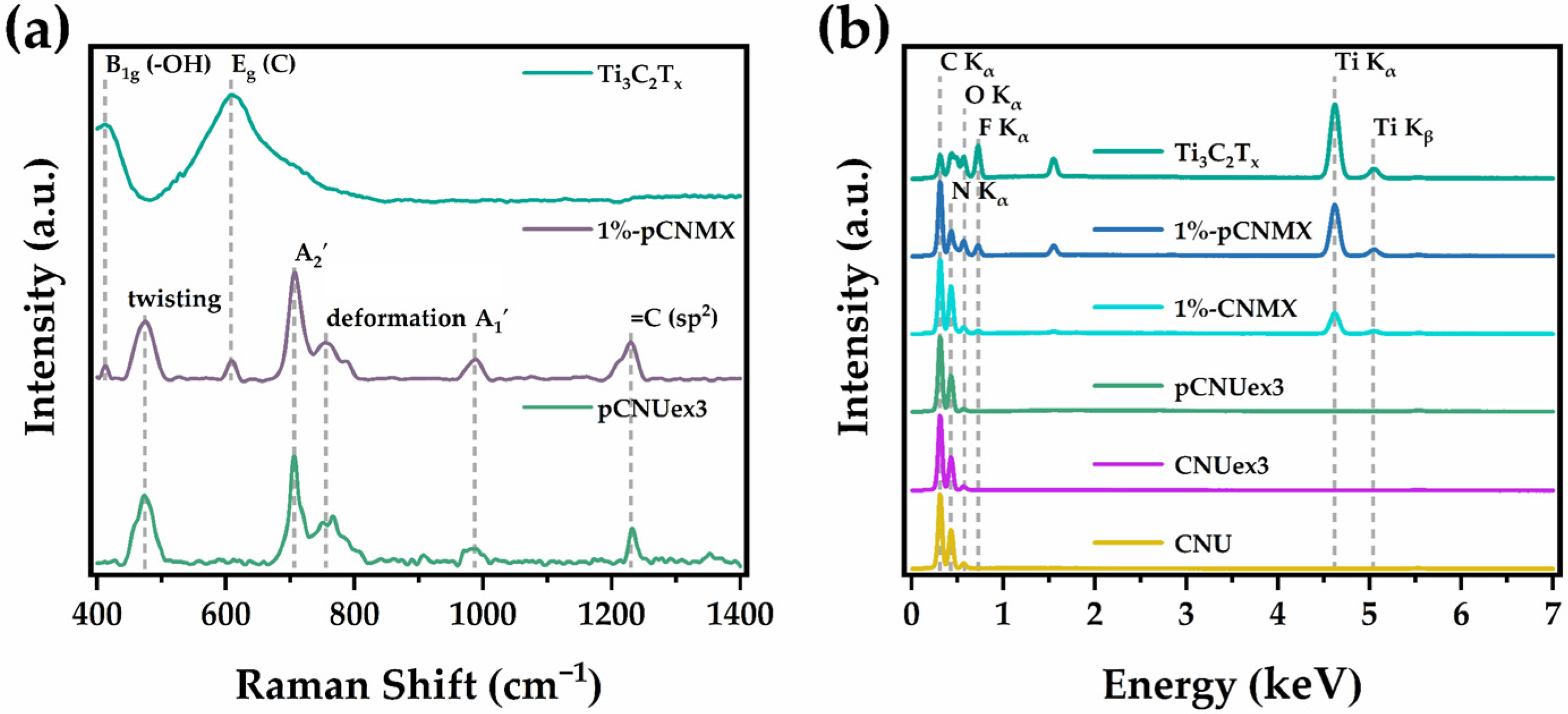
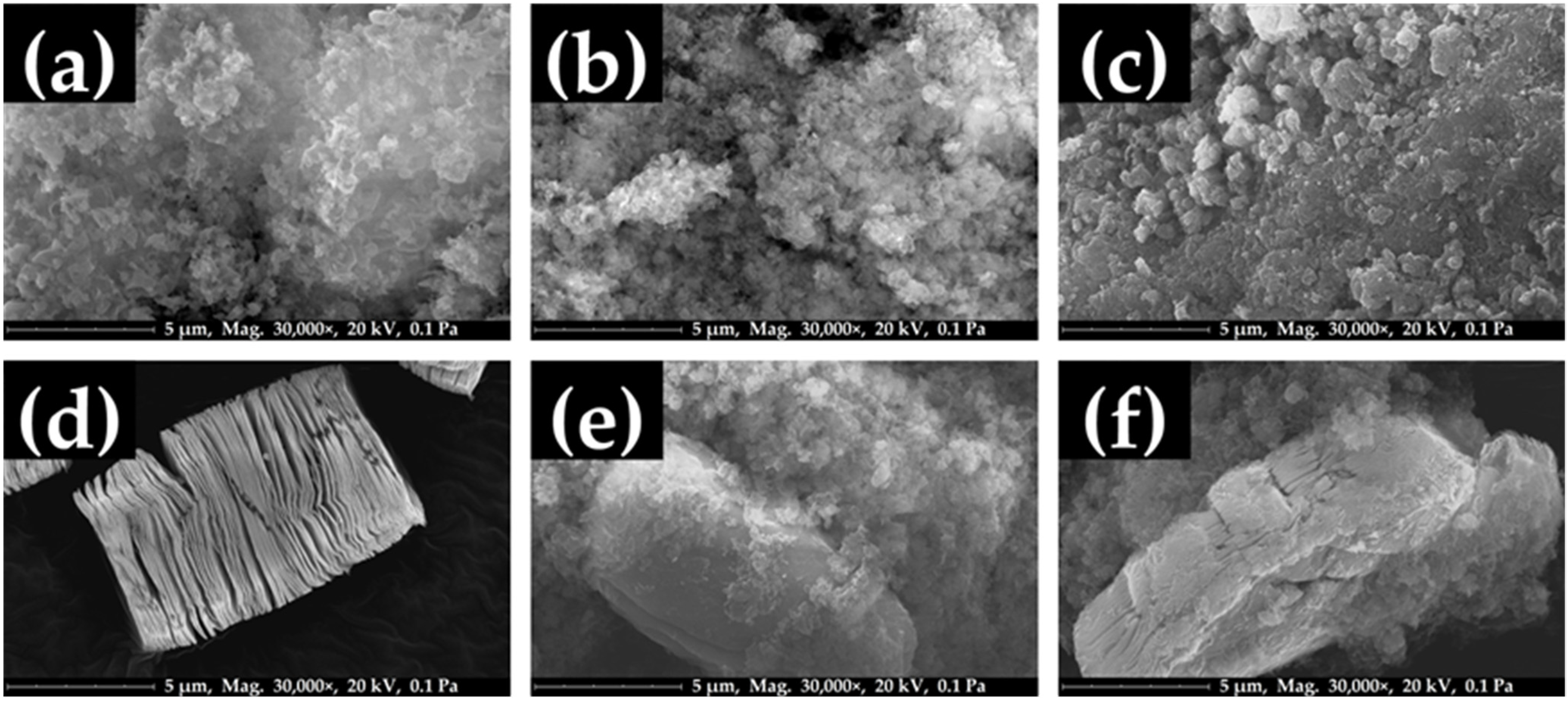
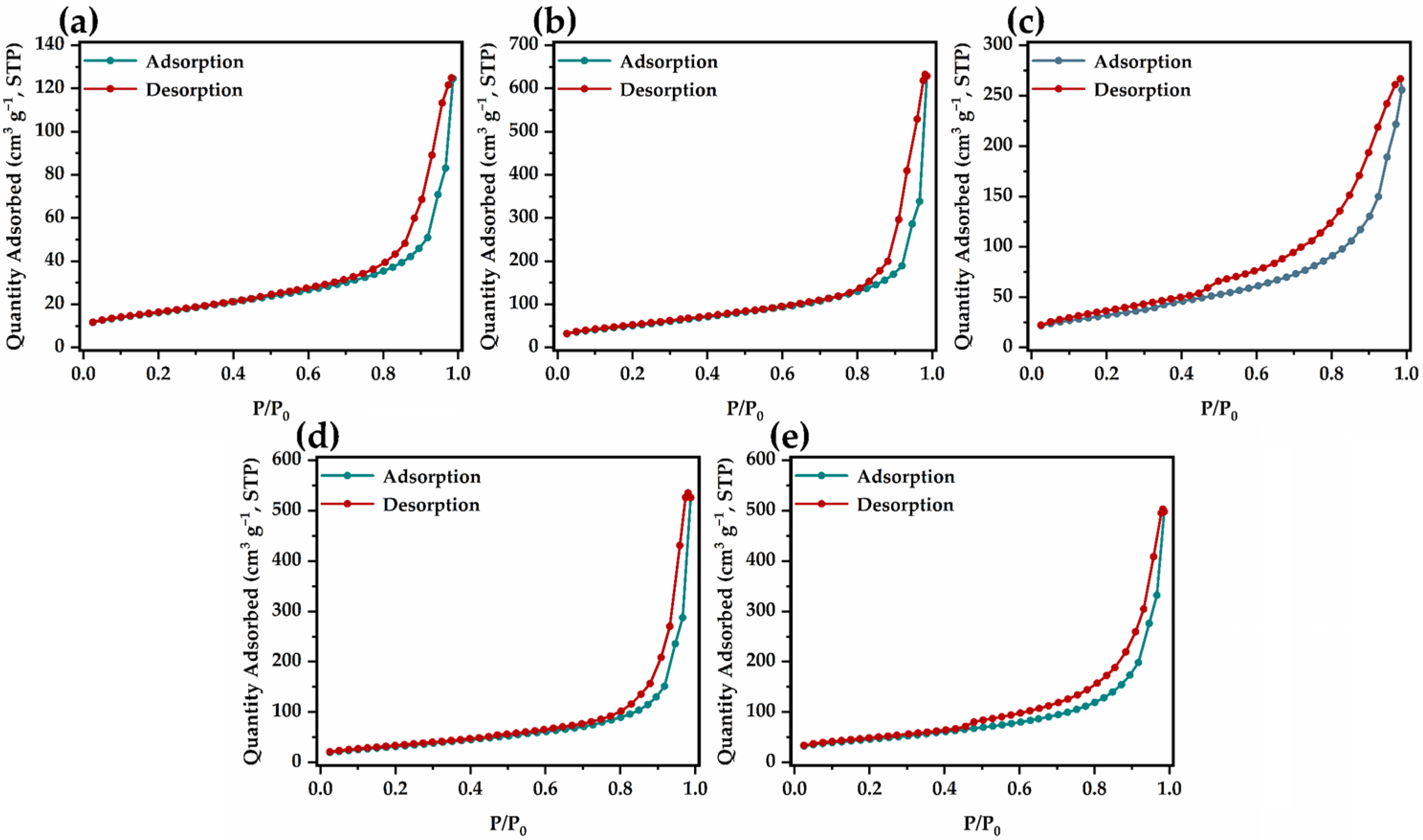
2.1. Material Characterization
2.2. Photocatalytic Degradation of VLS Using the Synthesized Photocatalysts
3. Materials and Methods
3.1. Reagents, Solvents, and Materials
3.2. Synthesis of Bulk g-C3N4
3.3. Synthesis of Thermally Exfoliated CNU
3.4. Synthesis of Protonated CNUex3 (pCNUex3)
3.5. Synthesis of Ti3C2Tx MXene
3.6. Synthesis of x%-CNMX and x%-pCNMX Schottky Junctions
3.7. Material Characterization Techniques
3.7.1. XRD Analysis
3.7.2. ATR-FTIR Spectroscopy
3.7.3. Raman Spectroscopy
3.7.4. FE-SEM and EDS Analysis
3.7.5. Nitrogen Porosimetry Measurements
3.7.6. DRS Measurements
3.7.7. PL Spectroscopy
3.8. Laboratory-Scale Photocatalytic Experiments
3.9. Determination of VLS Concentration in Photocatalysis Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Intisar, A.; Ramzan, A.; Hafeez, S.; Hussain, N.; Irfan, M.; Shakeel, N.; Gill, K.A.; Iqbal, A.; Janczarek, M.; Jesionowski, T. Adsorptive and Photocatalytic Degradation Potential of Porous Polymeric Materials for Removal of Pesticides, Pharmaceuticals, and Dyes-Based Emerging Contaminants from Water. Chemosphere 2023, 336, 139203. [Google Scholar] [CrossRef] [PubMed]
- Simonovic, S.P. World Water Dynamics: Global Modeling of Water Resources. J. Environ. Manag. 2002, 66, 249–267. [Google Scholar] [CrossRef]
- Kumar, R.; Qureshi, M.; Vishwakarma, D.K.; Al-Ansari, N.; Kuriqi, A.; Elbeltagi, A.; Saraswat, A. A Review on Emerging Water Contaminants and the Application of Sustainable Removal Technologies. Case Stud. Chem. Environ. Eng. 2022, 6, 100219. [Google Scholar] [CrossRef]
- Nishmitha, P.S.; Akhilghosh, K.A.; Aiswriya, V.P.; Ramesh, A.; Muthuchamy, M.; Muthukumar, A. Understanding emerging contaminants in water and wastewater: A comprehensive review on detection, impacts, and solutions. J. Hazard. Mater. Adv. 2025, 18, 100755. [Google Scholar] [CrossRef]
- Jelic, A.; Gros, M.; Ginebreda, A.; Cespedes-Sánchez, R.; Ventura, F.; Petrovic, M.; Barcelo, D. Occurrence, Partition and Removal of Pharmaceuticals in Sewage Water and Sludge during Wastewater Treatment. Water Res. 2011, 45, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-S.; You, W.-D.; Chen, C.-E.; Wang, X.-Y.; Yang, B.; Ying, G.-G. Occurrence, Fate and Ecological Risks of 90 Typical Emerging Contaminants in Full-Scale Textile Wastewater Treatment Plants from a Large Industrial Park in Guangxi, Southwest China. J. Hazard. Mater. 2023, 449, 131048. [Google Scholar] [CrossRef]
- Salimi, M.; Esrafili, A.; Gholami, M.; Jafari, A.J.; Kalantary, R.R.; Farzadkia, M.; Kermani, M.; Sobhi, H.R. Contaminants of Emerging Concern: A Review of New Approach in AOP Technologies. Environ. Monit. Assess. 2017, 189, 414. [Google Scholar] [CrossRef]
- Lykos, C.; Kourkouta, T.; Konstantinou, I. Study on the photocatalytic degradation of metronidazole antibiotic in aqueous media with TiO2 under lab and pilot scale. Sci. Total. Environ. 2023, 870, 161877. [Google Scholar] [CrossRef]
- Zawadzki, P. Visible Light–Driven Advanced Oxidation Processes to Remove Emerging Contaminants from Water and Wastewater: A Review. Water Air Soil Pollut. 2022, 233, 374. [Google Scholar] [CrossRef]
- Fast, S.A.; Gude, V.G.; Truax, D.D.; Martin, J.; Magbanua, B.S. A Critical Evaluation of Advanced Oxidation Processes for Emerging Contaminants Removal. Environ. Process. 2017, 4, 283–302. [Google Scholar] [CrossRef]
- Antonopoulou, A.; Konstantinou, I. Photocatalytic degradation and mineralization of tramadol pharmaceutical in aqueous TiO2 suspensions: Evaluation of kinetics, mechanisms and ecotoxicity. Appl. Catal. A Gen. 2016, 515, 136–143. [Google Scholar] [CrossRef]
- Garrido-Cardenas, J.A.; Esteban-García, B.; Agüera, A.; Sánchez-Pérez, J.A.; Manzano-Agugliaro, F. Wastewater Treatment by Advanced Oxidation Process and Their Worldwide Research Trends. Int. J. Environ. Res. Public Health 2019, 17, 170. [Google Scholar] [CrossRef]
- Rapti, I.; Kosma, C.; Albanis, T.; Konstantinou, I. Solar Photocatalytic Degradation of Inherent Pharmaceutical Residues in Real Hospital WWTP Effluents Using Titanium Dioxide on a CPC Pilot Scale Reactor. Catal Today 2022, 423, 113884. [Google Scholar] [CrossRef]
- Rauf, M.A.; Ashraf, S.S. Fundamental principles and application of heterogeneous photocatalytic degradation of dyes in solution. Chem. Eng. J. 2009, 151, 10–18. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, B.; Chen, H.; Yuan, R. Heterogeneous photocatalytic oxidation for the removal of organophosphorus pollutants from aqueous solutions: A review. Sci. Total. Environ. 2022, 856, 159048. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Zhu, Y.; Gao, B.; Li, Q. Progress on Mechanism and Efficacy of Heterogeneous Photocatalysis Coupled Oxidant Activation as an Advanced Oxidation Process for Water Decontamination. Water Res 2024, 251, 121119. [Google Scholar] [CrossRef]
- Morshedy, A.S.; El-Fawal, E.M.; Zaki, T.; El-Zahhar, A.A.; Alghamdi, M.M.; El Naggar, A.M.A. A review on heterogeneous photocatalytic materials: Mechanism, perspectives, and environmental and energy sustainability applications. Inorg. Chem. Commun. 2024, 163, 112307. [Google Scholar] [CrossRef]
- Lykos, C.; Bairamis, F.; Efthymiou, C.; Konstantinou, I. Synthesis and Characterization of Composite WO3 Fibers/g-C3N4 Photocatalysts for the Removal of the Insecticide Clothianidin in Aquatic Media. Nanomaterials 2024, 14, 1045. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, J.; Lodh, B.K.; Sharma, R.; Mahata, N.; Shah, M.P.; Mandal, S.; Ghanta, S.; Bhunia, B. Advanced oxidation process for the treatment of industrial wastewater: A review on strategies, mechanisms, bottlenecks and prospects. Chemosphere 2023, 345, 140473. [Google Scholar] [CrossRef]
- Zhang, Y.; Hawboldt, K.; Zhang, L.; Lu, J.; Chang, L.; Dwyer, A. Carbonaceous Nanomaterial-TiO2 Heterojunctions for Visible-Light-Driven Photocatalytic Degradation of Aqueous Organic Pollutants. Appl. Catal. A Gen. 2022, 630, 118460. [Google Scholar] [CrossRef]
- John, A.; Rajan, M.S.; Thomas, J. Carbon Nitride-Based Photocatalysts for the Mitigation of Water Pollution Engendered by Pharmaceutical Compounds. Environ. Sci. Pollut. Res. 2021, 28, 24992–25013. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Feng, J.; Fan, M.; Pi, Y.; Hu, L.; Han, X.; Liu, M.; Sun, J.; Sun, J. Recent Developments in Heteroge-neous Photocatalytic Water Treatment Using Visible Light-Responsive Photocatalysts: A Review. RSC Adv. 2015, 5, 14610–14630. [Google Scholar] [CrossRef]
- Lee, D.-E.; Kim, M.-K.; Danish, M.; Jo, W.-K. State-of-the-art review on photocatalysis for efficient wastewater treatment: Attractive approach in photocatalyst design and parameters affecting the photocatalytic degradation. Catal. Commun. 2023, 183, 106764. [Google Scholar] [CrossRef]
- Alaghmandfard, A.; Ghandi, K. A Comprehensive Review of Graphitic Carbon Nitride (g-C3N4)–Metal Oxide-Based Nanocomposites: Potential for Photocatalysis and Sensing. Nanomaterials 2022, 12, 294. [Google Scholar] [CrossRef]
- Lykos, C.; Sioulas, S.; Konstantinou, I. g-C3N4 as Photocatalyst for the Removal of Metronidazole Antibiotic from Aqueous Matrices under Lab and Pilot Scale Conditions. Catalysts 2023, 13, 254. [Google Scholar] [CrossRef]
- Inagaki, M.; Tsumura, T.; Kinumoto, T.; Toyoda, M. Graphitic Carbon Nitrides (g-C3N4) with Comparative Discussion to Carbon Materials. Carbon 2019, 141, 580–607. [Google Scholar] [CrossRef]
- Bhanderi, D.; Lakhani, P.; Modi, C.K. Graphitic Carbon Nitride (g-C3N4) as an Emerging Photocatalyst for Sustainable Environmental Applications: A Comprehensive Review. RSC Sustain. 2023, 2, 265–287. [Google Scholar] [CrossRef]
- Bairamis, F.; Rapti, I.; Konstantinou, I. g-C3N4 Based Z-Scheme Photocatalysts for Environmental Pollutants Removal. Curr. Opin. Green Sustain. Chem. 2022, 40, 100749. [Google Scholar] [CrossRef]
- Khan, M.A.; Mutahir, S.; Shaheen, I.; Qunhui, Y.; Bououdina, M.; Humayun, M. Recent Advances over the Doped g-C3N4 in Photocatalysis: A Review. Co-ord. Chem. Rev. 2024, 522, 216227. [Google Scholar] [CrossRef]
- Luo, Y.; Wei, X.; Gao, B.; Zou, W.; Zheng, Y.; Yang, Y.; Zhang, Y.; Tong, Q.; Dong, L. Synergistic adsorption-photocatalysis processes of graphitic carbon nitrate (g-C3N4) for contaminant removal: Kinetics, models, and mechanisms. Chem. Eng. J. 2019, 375, 122019. [Google Scholar] [CrossRef]
- Jiang, J.; Cao, S.; Hu, C.; Chen, C. A comparison study of alkali metal-doped g-C3N4 for visible-light photocatalytic hydrogen evolution. Chin. J. Catal. 2017, 38, 1981–1989. [Google Scholar] [CrossRef]
- Yan, W.; Yan, L.; Jing, C. Impact of Doped Metals on Urea-Derived g-C3N4 for Photocatalytic Degradation of Antibiotics: Structure, Photoactivity and Degradation Mechanisms. Appl. Catal. B Environ. 2019, 244, 475–485. [Google Scholar] [CrossRef]
- Ma, T.; Shen, Q.; Zhaoa, J.X.B.; Guan, R.; Liu, X.; Jia, H.; Xu, B. Facile Synthesis of Fe-Doped g-C3N4 for Enhanced Visible-Light Photocatalytic Activity. Inorg. Chem. Commun. 2019, 107, 107451. [Google Scholar] [CrossRef]
- Bao, J.; Bai, W.; Wu, M.; Gong, W.; Yu, Y.; Zheng, K.; Liu, L. Template-Mediated Copper Doped Porous g-C3N4 for Efficient Photodegradation of Antibiotic Contaminants. Chemosphere 2022, 293, 133607. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Wang, M.; Hu, J.; Liu, Q.; Tang, H. The Synergistic Effect of P Doping and Ni(II) Electron Cocatalyst Boosting Photocatalytic H2-Evolution Activity of g-C3N4. Ceram. Int. 2021, 47, 23386–23395. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, H.; Yao, W.; Chen, K.; Chen, D. One Step Synthesis of P-Doped g-C3N4 with the Enhanced Visible Light Photocatalytic Activity. Appl. Surf. Sci. 2018, 430, 309–315. [Google Scholar] [CrossRef]
- Chen, P.; Xing, P.; Chen, Z.; Lin, H.; He, Y. Rapid and Energy-Efficient Preparation of Boron Doped g-C3N4 with Excellent Performance in Photocatalytic H2-Evolution. Int. J. Hydrogen Energy 2018, 43, 19984–19989. [Google Scholar] [CrossRef]
- Ge, L.; Han, C.; Xiao, X.; Guo, L.; Li, Y. Enhanced visible light photocatalytic hydrogen evolution of sulfur-doped polymeric g-C3N4 photocatalysts. Mater. Res. Bull. 2013, 48, 3919–3925. [Google Scholar] [CrossRef]
- Rapti, I.; Bairamis, F.; Konstantinou, I. g-C3N4/MoS2 Heterojunction for Photocatalytic Removal of Phenol and Cr(VI). Photochem 2021, 1, 358–370. [Google Scholar] [CrossRef]
- Obregón, S.; Ruíz-Gómez, M.A.; Rodríguez-González, V.; Vázquez, A.; Hernández-Uresti, D.B. A Novel Type-II Bi2W2O9/g-C3N4 Heterojunction with Enhanced Photocatalytic Performance under Simulated Solar Irradiation. Mater. Sci. Semicond. Process. 2020, 113, 105056. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, P. Advances in Noble Metal-Modified g-C3N4 Heterostructures toward Enhanced Photocatalytic Redox Ability. Int. J. Miner. Metall. Mater. 2024, 31, 2368–2389. [Google Scholar] [CrossRef]
- Nie, L.; Chen, H.; Wang, J.; Yang, Y.; Fang, C. Enhanced Visible-Light H2O2 Production over Pt/g-C3N4 Schottky Junction Photocatalyst. Inorg. Chem. 2024, 63, 4770–4782. [Google Scholar] [CrossRef]
- Saman, F.; Bahruji, H.; Mahadi, A.H.; Ling, C.H.S. Pd/g-C3N4 Photocatalyst for Hydrogen Production: Role of Experimental Condition for Schottky Barrier. Fuel 2023, 349, 128725. [Google Scholar] [CrossRef]
- Wang, H.; Sun, T.; Chang, L.; Nie, P.; Zhang, X.; Zhao, C.; Xue, X. The g-C3N4 Nanosheets Decorated by Plasmonic Au Nanoparticles: A Heterogeneous Electrocatalyst for Oxygen Evolution Reaction Enhanced by Sunlight Illumination. Electrochimica Acta 2019, 303, 110–117. [Google Scholar] [CrossRef]
- Jin, Y.; Geng, J.; Wang, J.; Ren, F.; Chen, Z.; Sun, Z.; Yan, C.; Ren, P.-G. Recent advances of MXenes in the photocatalytic pollutant degradations for environmental applications: A mini review. J. Environ. Chem. Eng. 2023, 11, 110052. [Google Scholar] [CrossRef]
- Pang, X.; Xue, S.; Zhou, T.; Qiao, M.; Li, H.; Liu, X.; Xu, Q.; Liu, G.; Lei, W. Noble Metal-Free Heterojunction of Ultrathin Ti3C2 MXene/WO3 for Boosted Visible-Light-Driven Photoreactivity. Adv. Sustain. Syst. 2022, 7, 2100507. [Google Scholar] [CrossRef]
- Sherryna, A.; Tahir, M. Role of Ti3C2MXene as Prominent Schottky Barriers in Driving Hydrogen Production through Photoinduced Water Splitting: A Comprehensive Review. ACS Appl. Energy Mater. 2021, 4, 11982–12006. [Google Scholar] [CrossRef]
- Li, C.; Kan, C.; Meng, X.; Liu, M.; Shang, Q.; Yang, Y.; Wang, Y.; Cui, X. Self-Assembly 2D Ti3C2/g-C3N4 MXene Heterojunction for Highly Efficient Photocatalytic Degradation of Tetracycline in Visible Wavelength Range. Nanomaterials 2022, 12, 4015. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, R.; Huang, J.; Jiang, Y.; Zhao, C.; Yang, X. In Situ Construction of Protonated g-C3N4/Ti3C2 MXene Schottky Heterojunctions for Efficient Photocatalytic Hydrogen Production. Chin. J. Catal. 2021, 42, 107–114. [Google Scholar] [CrossRef]
- Liu, W.; Sun, M.; Ding, Z.; Gao, B.; Ding, W. Ti3C2 MXene Embellished g-C3N4 Nanosheets for Improving Photocatalytic Redox Capacity. J. Alloys Compd. 2021, 877, 160223. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q.; Zou, Y.; Cao, Y.; Cui, W.; Dong, F.; Zhou, Y. Ti3C2 MXene Modified g-C3N4 with Enhanced Visible-Light Photocatalytic Performance for NO Purification. J. Colloid Interface Sci. 2020, 575, 443–451. [Google Scholar] [CrossRef]
- Papailias, I.; Todorova, N.; Giannakopoulou, T.; Ioannidis, N.; Boukos, N.; Athanasekou, C.P.; Dimotikali, D.; Trapalis, C. Chemical vs Thermal Exfoliation of g-C3N4 for NOx Removal under Visible Light Irradiation. Appl. Catal. B Environ. 2018, 239, 16–26. [Google Scholar] [CrossRef]
- Pattnaik, S.P.; Behera, A.; Martha, S.; Acharya, R.; Parida, K. Facile Synthesis of Exfoliated Graphitic Carbon Nitride for Photocatalytic Degradation of Ciprofloxacin under Solar Irradiation. J. Mater. Sci. 2019, 54, 5726–5742. [Google Scholar] [CrossRef]
- Antonopoulou, M.; Bika, P.; Papailias, I.; Zervou, S.-K.; Vrettou, A.; Efthimiou, I.; Mitrikas, G.; Ioannidis, N.; Trapalis, C.; Dallas, P.; et al. Photocatalytic Degradation of Organic Micropollutants under UV-A and Visible Light Irradiation by Exfoliated g-C3N4 Catalysts. Sci. Total. Environ. 2023, 892, 164218. [Google Scholar] [CrossRef]
- Qiu, P.; Chen, H.; Xu, C.; Zhou, N.; Jiang, F.; Wang, X.; Fu, Y. Fabrication of an Exfoliated Graphitic Carbon Nitride as a Highly Active Visible Light Photocatalyst. J. Mater. Chem. A 2015, 3, 24237–24244. [Google Scholar] [CrossRef]
- Lee, D.-E.; Jyothirmai, M.V.; Mameda, N.; Jo, W.-K.; Tonda, S. 2D/2D Schottky-Type Hybrid Heterocatalyst Comprising S-Doped g-C3N4 and Delaminated Ti3C2 MXene: Synergistic Interplay of Dual Strategies for Effective H2 Generation and Pollutant Degradation. Appl. Surf. Sci. 2024, 669, 160516. [Google Scholar] [CrossRef]
- Husain, A.; Lee, D.-E.; Siddiqui, Q.T.; Gunnasegaran, P.; Danish, M.; Jo, W.-K. Scalable Integration of MOFs, COFs, and MXenes with g-C3N4 for Solar H2 Generation: A Review of Energy Conversion Strategies. Int. J. Hydrogen Energy 2025, 150, 150041. [Google Scholar] [CrossRef]
- Huang, K.; Li, C.; Wang, L.; Wang, W.; Meng, X. Layered Ti3C2 MXene and Silver Co-Modified g-C3N4 with Enhanced Visible Light-Driven Photocatalytic Activity. Chem. Eng. J. 2021, 425, 131493. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, X.; Zuo, Y.; Song, N.; Xin, X.; Zheng, B.; Sun, J.; Chen, G.; Li, C. 2D Ti3C2 as Electron Harvester Anchors on 2D g-C3N4 to Create Boundary Edge Active Sites for Boosting Photocatalytic Performance. Appl. Catal. A Gen. 2020, 590, 117367. [Google Scholar] [CrossRef]
- Li, K.; Chen, M.; Chen, L.; Zhao, S.; Xue, W.; Han, Z.; Han, Y. Synthesis of g-C3N4 Derived from Different Precursors for Photodegradation of Sulfamethazine under Visible Light. Processes 2023, 11, 528. [Google Scholar] [CrossRef]
- Xia, P.; Li, G.; Li, X.; Yuan, S.; Wang, K.; Huang, D.; Ji, Y.; Dong, Y.; Wu, X.; Zhu, L.; et al. Synthesis of g-C3N4 from Various Precursors for Photocatalytic H2 Evolution under the Visible Light. Crystals 2022, 12, 1719. [Google Scholar] [CrossRef]
- Papamichail, P.; Nannou, C.; Giannakoudakis, D.A.; Bikiaris, N.D.; Papoulia, C.; Pavlidou, E.; Lambropoulou, D.; Samanidou, V.; Deliyanni, E. Maximization of the photocatalytic degradation of diclofenac using polymeric g-C3N4 by tuning the precursor and the synthetic protocol. Catal. Today 2023, 418, 114075. [Google Scholar] [CrossRef]
- Todorova, N.; Papailias, I.; Giannakopoulou, T.; Ioannidis, N.; Boukos, N.; Dallas, P.; Edelmannová, M.; Reli, M.; Kočí, K.; Trapalis, C. Photocatalytic H2 Evolution, CO2 Reduction, and NOx Oxidation by Highly Exfoliated g-C3N4. Catalysts 2020, 10, 1147. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Y.; An, X.; Zhao, J.; Bao, Y.; Hou, L.-A. Exfoliation method matters: The microstructure-dependent photoactivity of g-C3N4 nanosheets for water purification. J. Hazard. Mater. 2022, 424, 127424. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Klahn, M.; Tian, X.; Dai, X.; Rabeah, J.; Aladin, V.; Corzilius, B.; Bartling, S.; Lund, H.; Steinfeldt, N.; et al. Exfoliated Polymeric Carbon Nitride Nanosheets for Photocatalytic Applications. Acs Appl. Nano Mater. 2024, 7, 7442–7452. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, Z.; Zeng, G.; Huang, D.; Xiao, R.; Zhang, C.; Zhou, C.; Xiong, W.; Wang, W.; Cheng, M.; et al. Ti3C2 Mxene/porous g-C3N4 interfacial Schottky junction for boosting spatial charge separation in photocatalytic H2O2 production. Appl. Catal. B Environ. 2019, 258, 117956. [Google Scholar] [CrossRef]
- Hu, J.; Ding, J.; Zhong, Q. Ultrathin 2D Ti3C2 MXene Co-Catalyst Anchored on Porous g-C3N4 for Enhanced Photocatalytic CO2 Reduction under Visible-Light Irradiation. J. Colloid Interface Sci. 2021, 582, 647–657. [Google Scholar] [CrossRef]
- Liu, D.; Li, C.; Ge, J.; Zhao, C.; Zhao, Q.; Zhang, F.; Ni, T.; Wu, W. 3D interconnected g-C3N4 hybridized with 2D Ti3C2 MXene nanosheets for enhancing visible light photocatalytic hydrogen evolution and dye contaminant elimination. Appl. Surf. Sci. 2022, 579, 152180. [Google Scholar] [CrossRef]
- Lou, C.-W.; Xie, M.-M.; Yang, Y.-D.; Wang, H.-Y.; Wang, Z.-K.; Zhang, L.; Hsieh, C.-T.; Liu, L.-Y.; Lin, M.-C.; Li, T.-T. Carbon Nanofiber Membranes Loaded with MXene@g-C3N4: Preparation and Photocatalytic Property. Nanomaterials 2024, 14, 896. [Google Scholar] [CrossRef]
- Anagnostopoulou, K.; Evgenidou, E.; Alampanos, V.; Lambropoulou, D.A. High-resolution mass spectrometry approaches for screening persistent and Mobile organic compounds in wastewaters: Target analysis, suspect analysis and risk assessment. Sci. Total. Environ. 2025, 967, 178777. [Google Scholar] [CrossRef]
- Proctor, K.; Altamirano, J.; Kasprzyk-Hordern, B. Chemicals of Emerging Concern in Wastewater Treatment Plants from Mendoza: Environmental Study in a Semiarid Region of Argentina. J. Hazard. Mater. Adv. 2025, 18, 100662. [Google Scholar] [CrossRef]
- Yao, L.; Hu, Y.; Yang, J.-H.; Wu, R.; Chen, F.-L.; Zhou, X. Wastewater Surveillance for Chronic Disease Drugs in Wastewater Treatment Plants: Mass Load, Removal, and Sewage Epidemiology. J. Hazard. Mater. 2025, 489, 137661. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Herguedas, N.; Irazola, M.; Alvarez-Mora, I.; Orive, G.; Lertxundi, U.; Olivares, M.; Zuloaga, O.; Prieto, A. Comprehensive Micropollutant Characterization of Wastewater during Covid-19 Crisis in 2020: Suspect Screening and Environmental Risk Prioritization Strategy. Sci. Total. Environ. 2023, 873, 162281. [Google Scholar] [CrossRef]
- Ofrydopoulou, A.; Nannou, C.; Evgenidou, E.; Christodoulou, A.; Lambropoulou, D. Assessment of a wide array of organic micropollutants of emerging concern in wastewater treatment plants in Greece: Occurrence, removals, mass loading and potential risks. Sci. Total. Environ. 2022, 802, 149860. [Google Scholar] [CrossRef]
- Mezzelani, M.; Peruzza, L.; D’ERrico, G.; Milan, M.; Gorbi, S.; Regoli, F. Mixtures of environmental pharmaceuticals in marine organisms: Mechanistic evidence of carbamazepine and valsartan effects on Mytilus galloprovincialis. Sci. Total. Environ. 2022, 860, 160465. [Google Scholar] [CrossRef]
- Medici, A.; Lavorgna, M.; Isidori, M.; Russo, C.; Orlo, E.; Luongo, G.; Di Fabio, G.; Zarrelli, A. Advanced oxidation process of valsartan by activated peroxymonosulfate: Chemical characterization and ecotoxicological effects of its byproducts. Sci. Total. Environ. 2023, 908, 168337. [Google Scholar] [CrossRef] [PubMed]
- Gallego, S.; Nos, D.; Montemurro, N.; Sanchez-Hernandez, J.C.; Pérez, S.; Solé, M.; Martin-Laurent, F. Ecotoxicological impact of the antihypertensive valsartan on earthworms, extracellular enzymes and soil bacterial communities. Environ. Pollut. 2021, 275, 116647. [Google Scholar] [CrossRef]
- Zheng, C.-J.; Zhang, R.-C.; Xu, K.; Zhang, J.; Wang, K.; Chen, W.; Huang, G.-B.; Yin, H. Protonated g-C3N4/Oxygen-Doped g-C3N4 S-Scheme Homojunction Fabricated by Electrostatic Self-Assembly for Photocatalytic Hydrogen Evolution and Tetracycline Hydrochloride Degradation. Carbon 2024, 226, 119153. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Cai, T.; Wang, L.; Liu, Y.; Zhang, S.; Dong, W.; Chen, H.; Yi, X.; Yuan, J.; Xia, X.; Liu, C.; et al. Ag3PO4/Ti3C2 MXene interface materials as a Schottky catalyst with enhanced photocatalytic activities and anti-photocorrosion performance. Appl. Catal. B Environ. 2018, 239, 545–554. [Google Scholar] [CrossRef]
- Kumar, G.; Ahlawat, A.; Bhardwaj, H.; Sahu, G.K.; Rana, P.S.; Solanki, P.R. Ultrasonication-Assisted Synthesis of Transition Metal Carbide of MXene: An Efficient and Promising Material for Photocatalytic Organic Dyes Degradation of Rhodamine B and Methylene Blue in Wastewater. Environ. Sci. Pollut. Res. 2024, 31, 38232–38250. [Google Scholar] [CrossRef]
- Zhong, R.; Liang, Y.; Huang, F.; Liang, S.; Liu, S. Regulating interfacial coupling of 1D crystalline g-C3N4 nanorods with 2D Ti3C2Tx MXene for boosting photocatalytic CO2 reduction. Chin. J. Catal. 2023, 53, 109–122. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Sun, B.; Qiu, P.; Liang, Z.; Xue, Y.; Zhang, X.; Yang, L.; Cui, H.; Tian, J. The fabrication of 1D/2D CdS nanorod@Ti3C2 MXene composites for good photocatalytic activity of hydrogen generation and ammonia synthesis. Chem. Eng. J. 2021, 406, 127177. [Google Scholar] [CrossRef]
- Wong, K.J.; Foo, J.J.; Siang, T.J.; Khoo, V.; Ong, W. Harnessing the Power of Light: The Synergistic Effects of Crystalline Carbon Nitride and Ti3C2Tx MXene in Photocatalytic Hydrogen Production. Glob. Chall. 2024, 8, 2300235. [Google Scholar] [CrossRef]
- Jin, D.; Lv, Y.; He, D.; Zhang, D.; Liu, Y.; Zhang, T.; Cheng, F.; Zhang, Y.-N.; Sun, J.; Qu, J. Photocatalytic Degradation of COVID-19 Related Drug Arbidol Hydrochloride by Ti3C2 MXene/Supramolecular g-C3N4 Schottky Junction Photocatalyst. Chemosphere 2022, 308, 136461. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.-J.; Tan, L.-L.; Chai, S.-P.; Yong, S.-T.; Mohamed, A.R. Surface charge modification via protonation of graphitic carbon nitride (g-C3N4) for electrostatic self-assembly construction of 2D/2D reduced graphene oxide (rGO)/g-C3N4 nanostructures toward enhanced photocatalytic reduction of carbon dioxide to methane. Nano Energy 2015, 13, 757–770. [Google Scholar] [CrossRef]
- Zhu, B.; Xia, P.; Ho, W.; Yu, J. Isoelectric Point and Adsorption Activity of Porous g-C3N4. Appl. Surf. Sci. 2015, 344, 188–195. [Google Scholar] [CrossRef]
- Karami, P.; Khani, R. Potential of cobalt ferrite-graphitic carbon nitride nanocomposite in trace determination of pyrene as one of the priority pollutants in water and food samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 301, 122969. [Google Scholar] [CrossRef]
- Cao, Y.; Li, Q.; Wang, W. Construction of a Crossed-Layer-Structure MoS2/g-C3N4 Heterojunction with Enhanced Photocatalytic Performance. RSC Adv. 2017, 7, 6131–6139. [Google Scholar] [CrossRef]
- Schwinghammer, K.; Mesch, M.B.; Duppel, V.; Ziegler, C.; Senker, J.; Lotsch, B.V. Crystalline Carbon Nitride Nanosheets for Improved Visible-Light Hydrogen Evolution. J. Am. Chem. Soc. 2014, 136, 1730–1733. [Google Scholar] [CrossRef]
- Narkbuakaew, T.; Sujaridworakun, P. Synthesis of Tri-S-Triazine Based g-C3N4 Photocatalyst for Cationic Rhodamine B Degradation under Visible Light. Top. Catal. 2020, 63, 1086–1096. [Google Scholar] [CrossRef]
- Liu, H.; Chen, D.; Wang, Z.; Jing, H.; Zhang, R. Microwave-assisted molten-salt rapid synthesis of isotype triazine-/heptazine based g-C3N4 heterojunctions with highly enhanced photocatalytic hydrogen evolution performance. Appl. Catal. B Environ. 2017, 203, 300–313. [Google Scholar] [CrossRef]
- Roshni, C.P.; Jithesh, K.; Manuraj, M.; Raj, K.G.; Rakhi, R.B. β-Ni(OH)2 supported over g-C3N4: A novel catalyst for para-nitrophenol reduction and supercapacitor electrode. Results Chem. 2022, 4, 100498. [Google Scholar] [CrossRef]
- Al Mamari, S.; Thangavel, S.; Kim, Y.; Selvaraj, R. Enhanced photocatalytic activity of-Fe2O3/g-C3N4 composite materials for degradation of toluene in aqueous solution under visible light irradiation. Desalination Water Treat. 2022, 254, 94–103. [Google Scholar] [CrossRef]
- Alkhudaydi, A.M.; Danish, E.Y.; Lima, E.C.; Gabal, M.A.; Abdel Salam, M. Photocatalytic Degradation of Malachite Green Dye Using Ti3C2 MXene Nanosheets Decorated with Fe3O4 NPs under Visible Light Irradiation. Res. Chem. Intermed. 2024, 50, 5117–5135. [Google Scholar] [CrossRef]
- Kiran, N.U.; Deore, A.B.; More, M.A.; Late, D.J.; Rout, C.S.; Mane, P.; Chakraborty, B.; Besra, L.; Chatterjee, S. Comparative Study of Cold Electron Emission from 2D Ti3C2TX MXene Nanosheets with Respect to Its Pre-cursor Ti3SiC2 MAX Phase. Acs Appl. Electron. Mater. 2022, 4, 2656–2666. [Google Scholar] [CrossRef]
- Mahmood, M.; Rasheed, A.; Ayman, I.; Rasheed, T.; Munir, S.; Ajmal, S.; Agboola, P.O.; Warsi, M.F.; Shahid, M. Synthesis of Ultrathin MnO2 nanowire-Intercalated 2D-MXenes for High-Performance Hybrid Supercapacitors. Energy Fuels 2021, 35, 3469–3478. [Google Scholar] [CrossRef]
- Abid, M.Z.; Tanveer, A.; Rafiq, K.; Rauf, A.; Jin, R.; Hussain, E. Proceeding of Catalytic Water Splitting on Cu/Ce@g-C3N4 Photocatalysts: An Exceptional Approach for Sunlight-Driven Hydrogen Generation. Nanoscale 2024, 16, 7154–7166. [Google Scholar] [CrossRef]
- Zhou, J.; Zha, X.; Chen, Z.; Li, K.; Sun, H.; Wang, J.; Lv, K.; Cong, S.; Zhao, Z. Tailoring the coordination microenvironment of single-atom W for efficient photocatalytic CO2 reduction. Appl. Catal. B Environ. 2024, 350, 123911. [Google Scholar] [CrossRef]
- Jiménez-Calvo, P.; Marchal, C.; Cottineau, T.; Caps, V.; Keller, V. Influence of the Gas Atmosphere during the Synthesis of g-C3N4 for Enhanced Photocatalytic H2 Production from Water on Au/g-C3N4 Composites. J. Mater. Chem. A 2019, 7, 14849–14863. [Google Scholar] [CrossRef]
- Li, J.; Chen, S.; Ai, Z.; Zhao, X.; Li, Z.; Tian, L.; Yang, Z.; Liang, H. Solvothermal Synthesis of CdTiO3/Ti3C2 MXene Composite as a New Efficient Visible Light Photocatalyst. Colloids Surf. A Physicochem. Eng. Asp. 2024, 702, 134936. [Google Scholar] [CrossRef]
- Dixit, P.; Maiti, T. A Facile Pot Synthesis of (Ti3AlC2) MAX Phase and Its Derived MXene (Ti3C2Tx). Ceram. Int. 2022, 48, 36156–36165. [Google Scholar] [CrossRef]
- Fan, J.; Qin, H.; Jiang, S. Mn-Doped g-C3N4 Composite to Activate Peroxymonosulfate for Acetaminophen Degradation: The Role of Superoxide Anion and Singlet Oxygen. Chem. Eng. J. 2019, 359, 723–732. [Google Scholar] [CrossRef]
- Shahabi, S.S.; Azizi, N.; Vatanpour, V.; Yousefimehr, N. Novel Functionalized Graphitic Carbon Nitride Incorporated Thin Film Nanocomposite Membranes for High-Performance Reverse Osmosis Desalination. Sep. Purif. Technol. 2020, 235, 116134. [Google Scholar] [CrossRef]
- Zadegan, M.S.J.; Moosaei, R.; Choopani, L.; Salehi, M.M.; Maleki, A.; Zare, E.N. Remediation of Safranin-O and acid fuchsin by using Ti3C2 MXene/rGO-Cu2O nanocomposite: Preparation, characterization, isotherm, kinetic and thermodynamic studies. Environ. Res. 2024, 258, 119469. [Google Scholar] [CrossRef]
- Jiang, X.; Li, X.; Liu, Y.; Zhai, H.; Wang, G. The Oxidative Thermal Exfoliation-Dependent Microstructure and Photocatalytic Performance of g-C3N4 Nanosheets. Diam. Relat. Mater. 2024, 143, 110918. [Google Scholar] [CrossRef]
- Pérez-Molina, L.; Pastrana-Martínez, L.M.; Morales-Torres, S.; Maldonado-Hódar, F.J. Photodegradation of Cytostatic Drugs by g-C3N4: Synthesis, Properties and Performance Fitted by Selecting the Appropriate Precursor. Catal. Today 2023, 418, 114068. [Google Scholar] [CrossRef]
- Li, J.; Zhao, L.; Wang, S.; Li, J.; Wang, G.; Wang, J. In situ fabrication of 2D/3D g-C3N4/Ti3C2 (MXene) heterojunction for efficient visible-light photocatalytic hydrogen evolution. Appl. Surf. Sci. 2020, 515, 145922. [Google Scholar] [CrossRef]
- Liu, N.; Lu, N.; Su, Y.; Wang, P.; Quan, X. Fabrication of g-C3N4/Ti3C2 composite and its visible-light photocatalytic capability for ciprofloxacin degradation. Sep. Purif. Technol. 2019, 211, 782–789. [Google Scholar] [CrossRef]
- Zheng, Q.; Xu, E.; Park, E.; Chen, H.; Shuai, D. Looking at the Overlooked Hole Oxidation: Photocatalytic Transformation of Organic Contaminants on Graphitic Carbon Nitride under Visible Light Irradiation. Appl. Catal. B Environ. 2019, 240, 262–269. [Google Scholar] [CrossRef]
- Zheng, Q.; Durkin, D.P.; Elenewski, J.E.; Sun, Y.; Banek, N.A.; Hua, L.; Chen, H.; Wagner, M.J.; Zhang, W.; Shuai, D. Visible-Light-Responsive Graphitic Carbon Nitride: Rational Design and Photocatalytic Applications for Water Treatment. Environ. Sci. Technol. 2016, 50, 12938–12948. [Google Scholar] [CrossRef]
- Bairamis, F.; Konstantinou, I. Photocatalytic Degradation Pathways of the Valsartan Drug by TiO2 and g-C3N4 Catalysts. Reactions 2022, 3, 160–171. [Google Scholar] [CrossRef]
- Park, H.; Kim, H.-I.; Moon, G.-H.; Choi, W. Photoinduced Charge Transfer Processes in Solar Photocatalysis Based on Modified TiO2. Energy Environ. Sci. 2015, 9, 411–433. [Google Scholar] [CrossRef]
- Shuck, C.E.; Ventura-Martinez, K.; Goad, A.; Uzun, S.; Shekhirev, M.; Gogotsi, Y. Safe Synthesis of MAX and MXene: Guidelines to Reduce Risk during Synthesis. Acs Chem. Heal. Saf. 2021, 28, 326–338. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Liu, J. Effect of phosphorus doping on electronic structure and photocatalytic performance of g-C3N4: Insights from hybrid density functional calculation. J. Alloys Compd. 2016, 672, 271–276. [Google Scholar] [CrossRef]
- Landi, S.; Segundo, I.R.; Freitas, E.; Vasilevskiy, M.; Carneiro, J.; Tavares, C.J. Use and misuse of the Kubelka-Munk function to obtain the band gap energy from diffuse reflectance measurements. Solid State Commun. 2022, 341, 114573. [Google Scholar] [CrossRef]


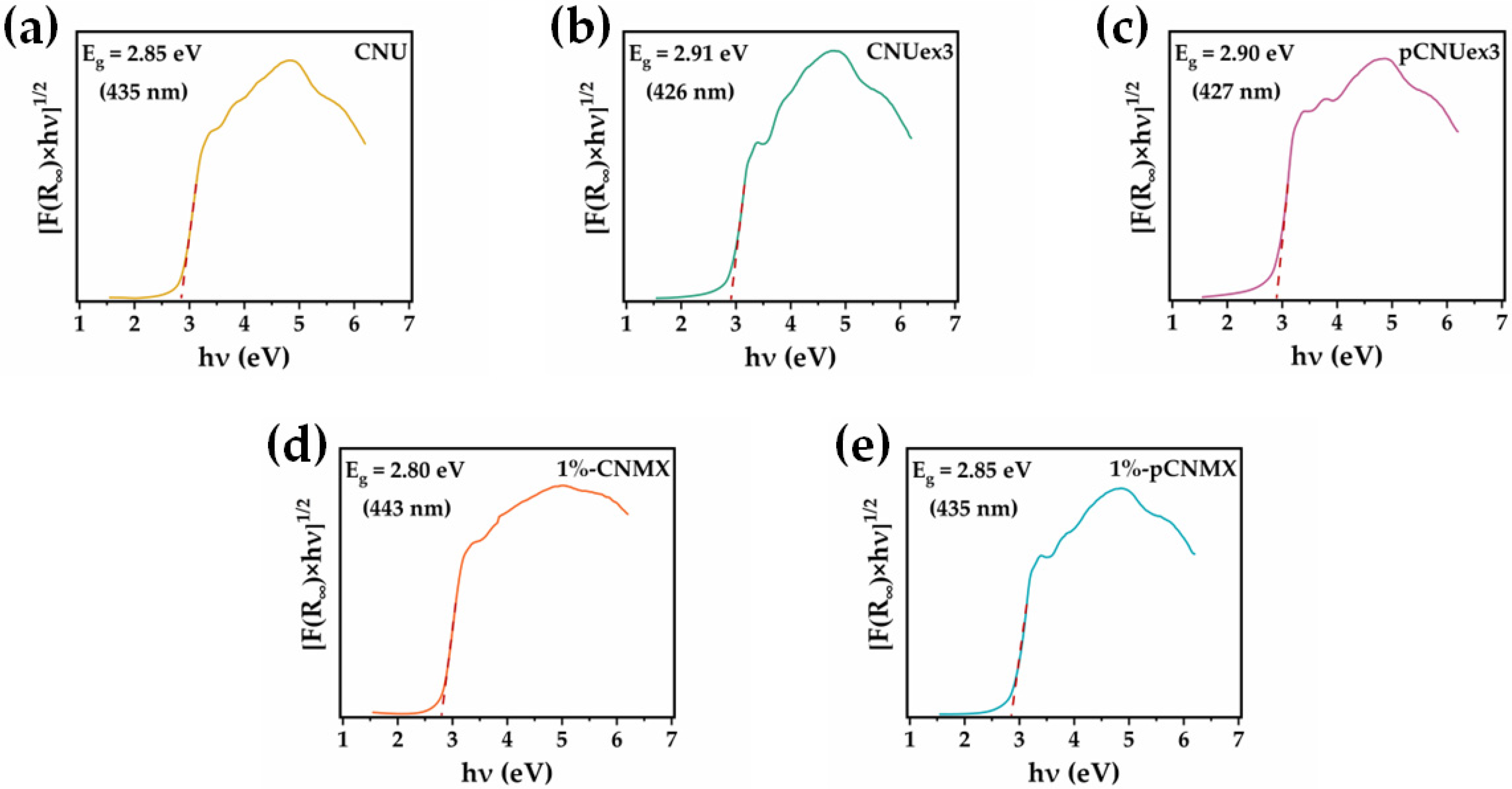

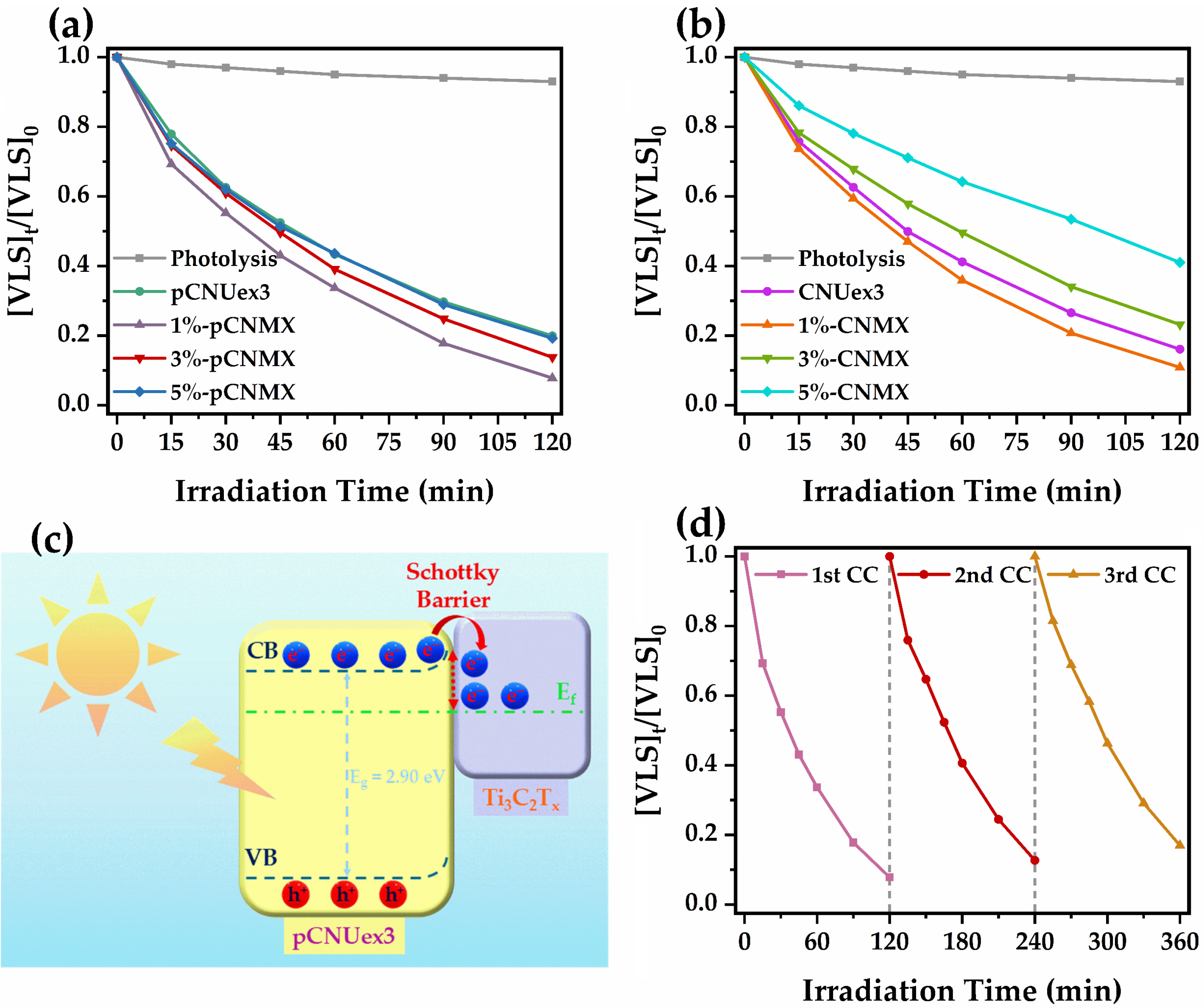
| Photocatalyst | kPC (min−1) | R2 | t1/2 (min) | Removal (%) | ΔkPC (%) |
|---|---|---|---|---|---|
| None (Photolysis) | 0.0007 | 0.940 | 990 | 7.00 | - |
| CNUex3 | 0.0151 | 0.998 | 41.3 | 83.9 | - |
| pCNUex3 | 0.0137 | 0.997 | 50.6 | 80.1 | - |
| 1%-pCNMX | 0.0201 | 0.993 | 34.5 | 92.2 | +46.7 |
| 3%-pCNMX | 0.0161 | 0.997 | 43.1 | 86.2 | +17.5 |
| 5%-pCNMX | 0.0139 | 0.994 | 49.9 | 80.8 | +1.46 |
| 1%-CNMX | 0.0179 | 0.997 | 38.7 | 89.1 | +30.7 |
| 3%-CNMX | 0.0121 | 0.995 | 57.3 | 76.8 | −11.7 |
| 5%-CNMX | 0.0073 | 0.995 | 95.0 | 59.0 | −46.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lykos, C.; Konstantinou, I. Thermally Exfoliated g-C3N4/Ti3C2Tx MXene Schottky Junctions as Photocatalysts for the Removal of Valsartan from Aquatic Environments. Catalysts 2025, 15, 909. https://doi.org/10.3390/catal15090909
Lykos C, Konstantinou I. Thermally Exfoliated g-C3N4/Ti3C2Tx MXene Schottky Junctions as Photocatalysts for the Removal of Valsartan from Aquatic Environments. Catalysts. 2025; 15(9):909. https://doi.org/10.3390/catal15090909
Chicago/Turabian StyleLykos, Christos, and Ioannis Konstantinou. 2025. "Thermally Exfoliated g-C3N4/Ti3C2Tx MXene Schottky Junctions as Photocatalysts for the Removal of Valsartan from Aquatic Environments" Catalysts 15, no. 9: 909. https://doi.org/10.3390/catal15090909
APA StyleLykos, C., & Konstantinou, I. (2025). Thermally Exfoliated g-C3N4/Ti3C2Tx MXene Schottky Junctions as Photocatalysts for the Removal of Valsartan from Aquatic Environments. Catalysts, 15(9), 909. https://doi.org/10.3390/catal15090909







