Interfacial Electric Fields and Chemical Bonds in Ti3C2O-Crafted AgI/MoS2 Direct Z-Scheme Heterojunction Synergistically Expedite Photocatalytic Performance
Abstract
1. Introduction
2. Results and Discussion
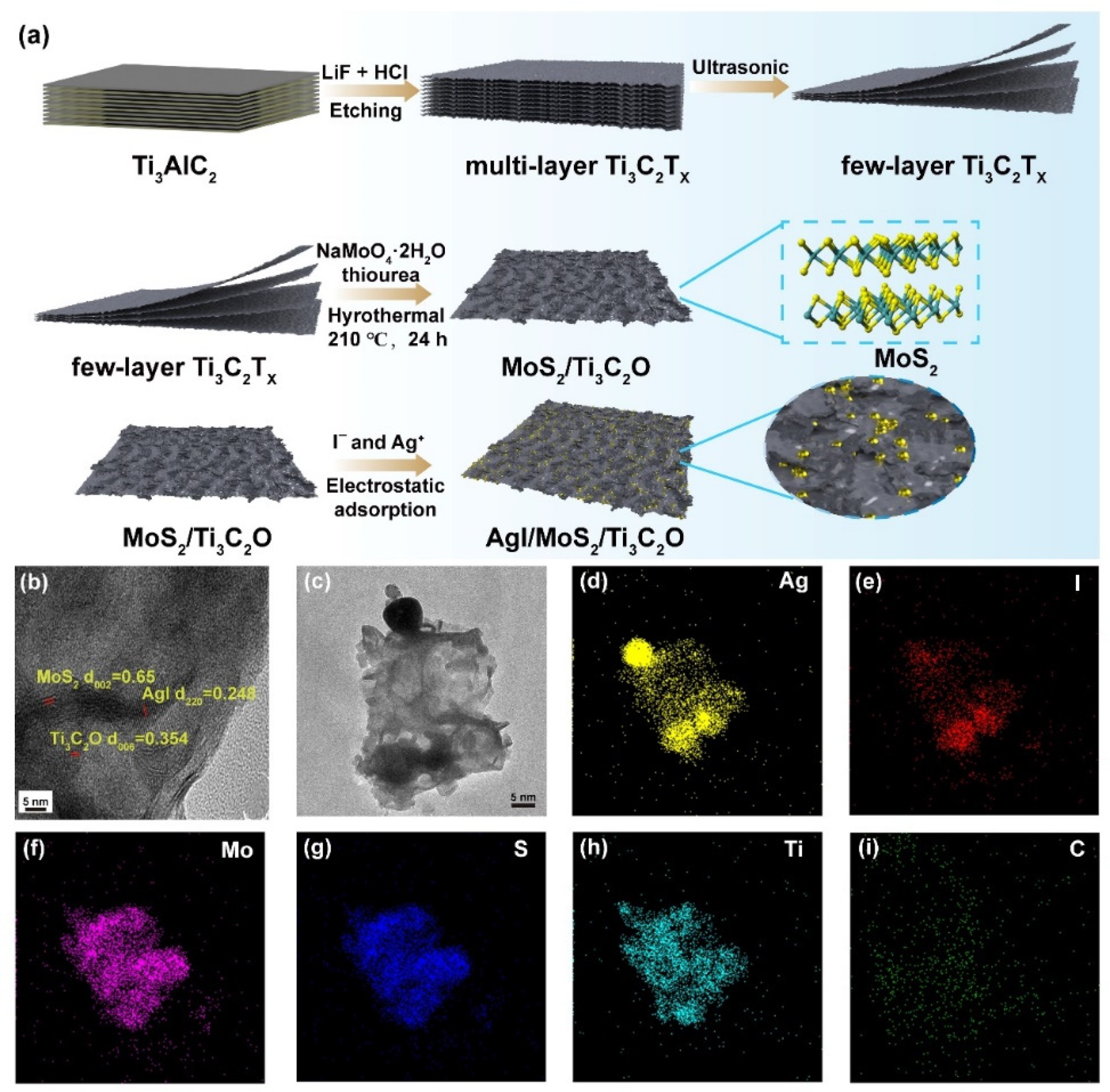
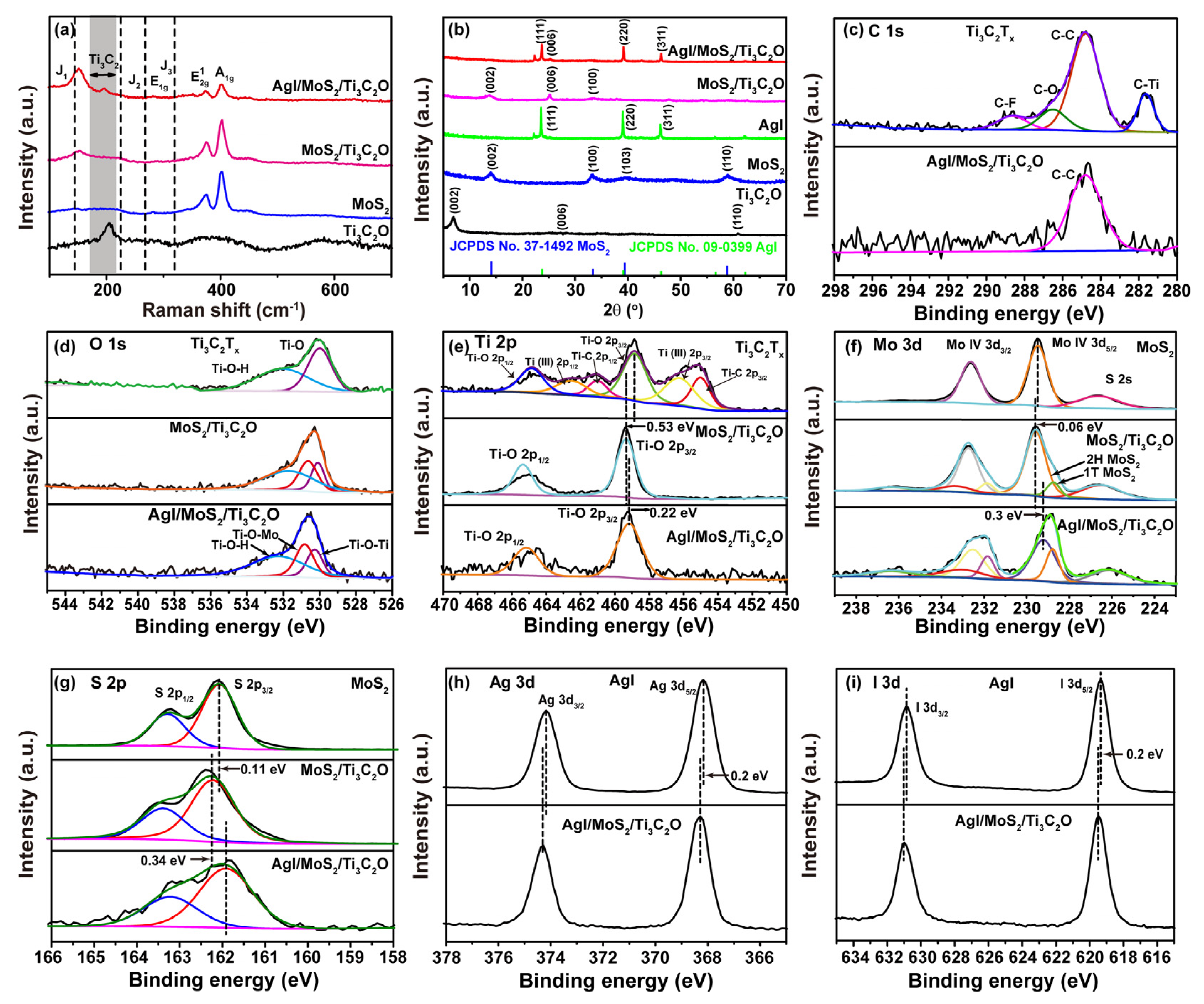
2.1. Characterization of AgI/MoS2/Ti3C2O Z-Scheme Heterojunction
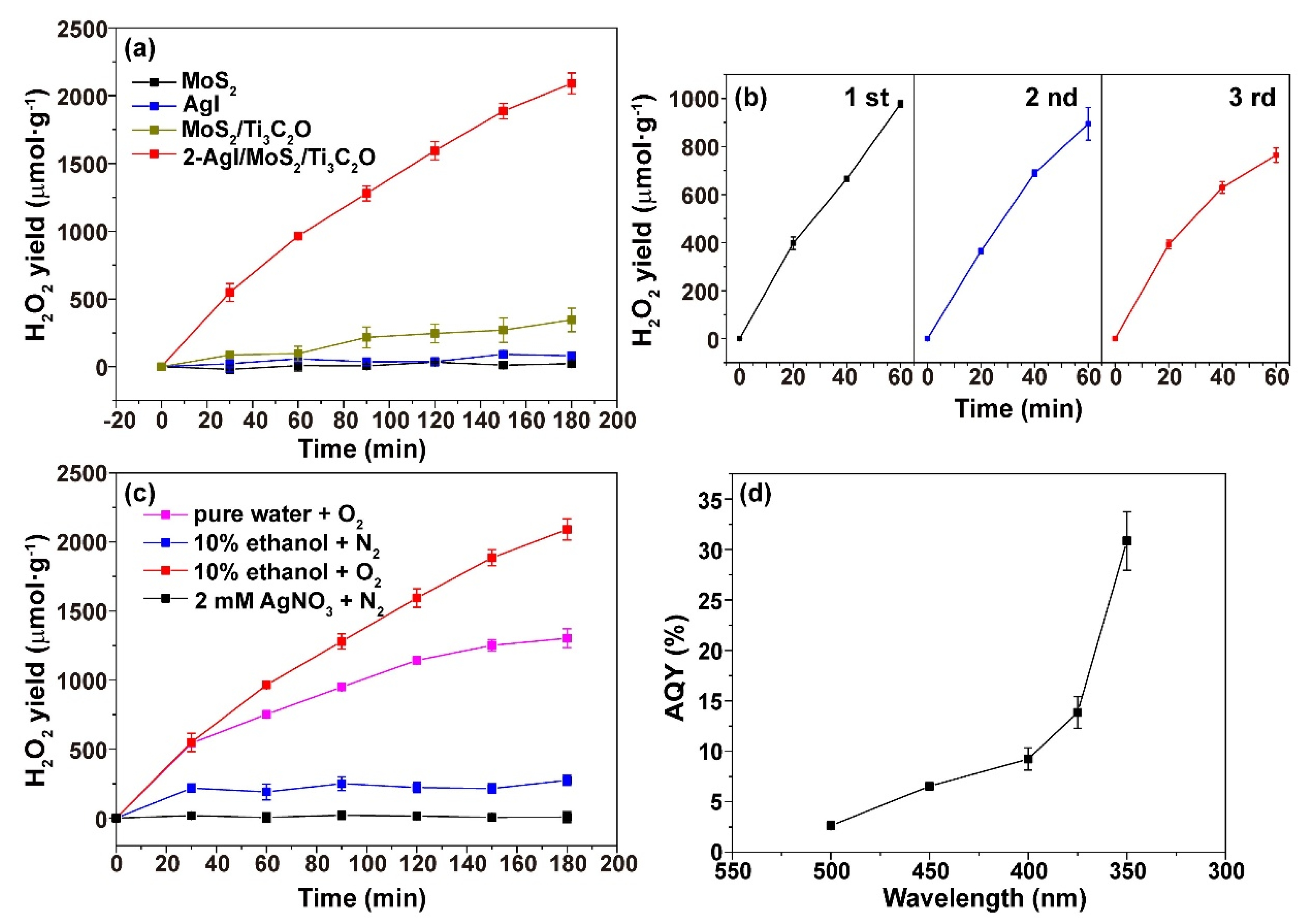
2.2. Photocatalytic H2O2 Production
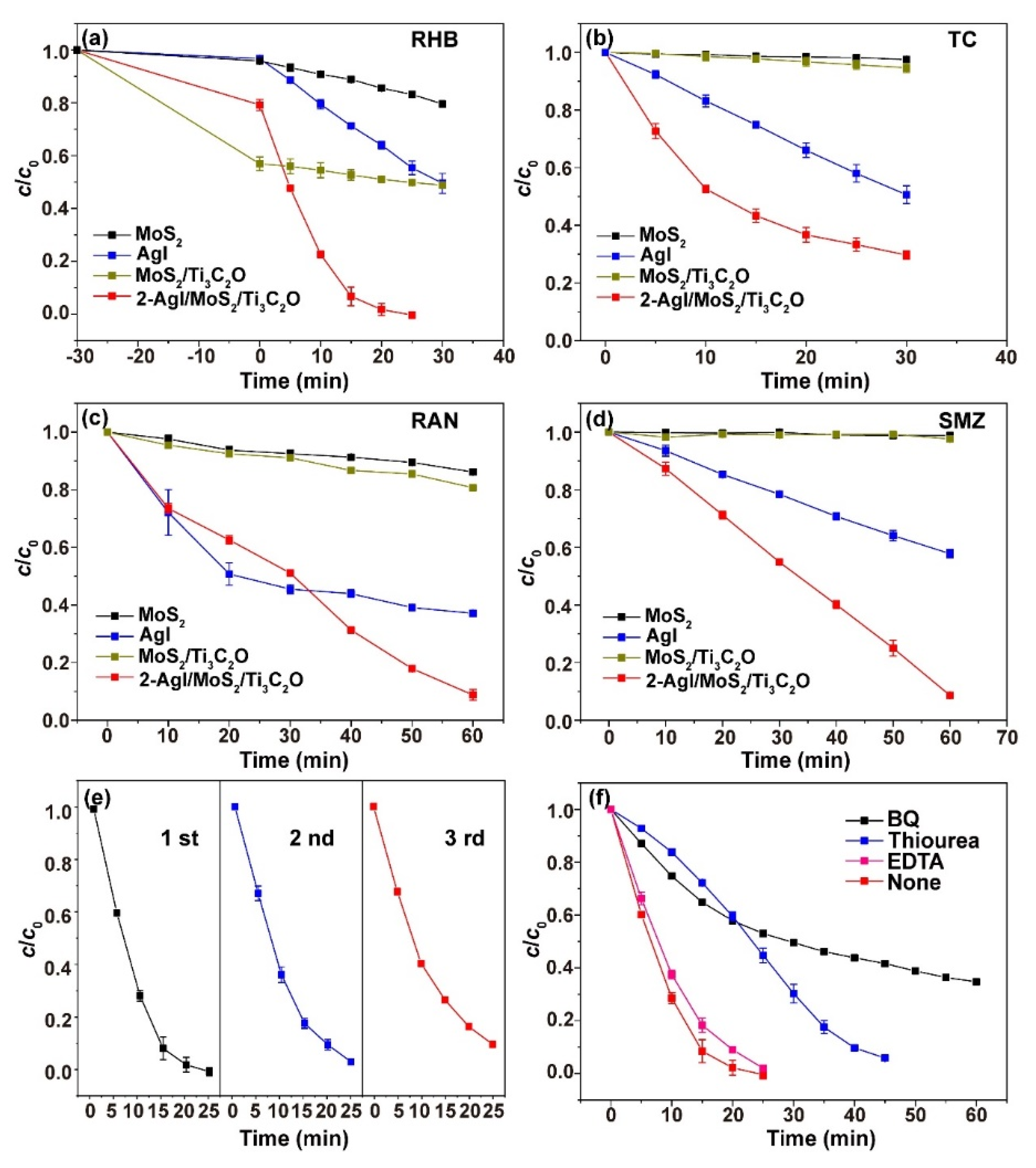
2.3. Photodegradation Activity
2.4. Photocatalytic Mechanism
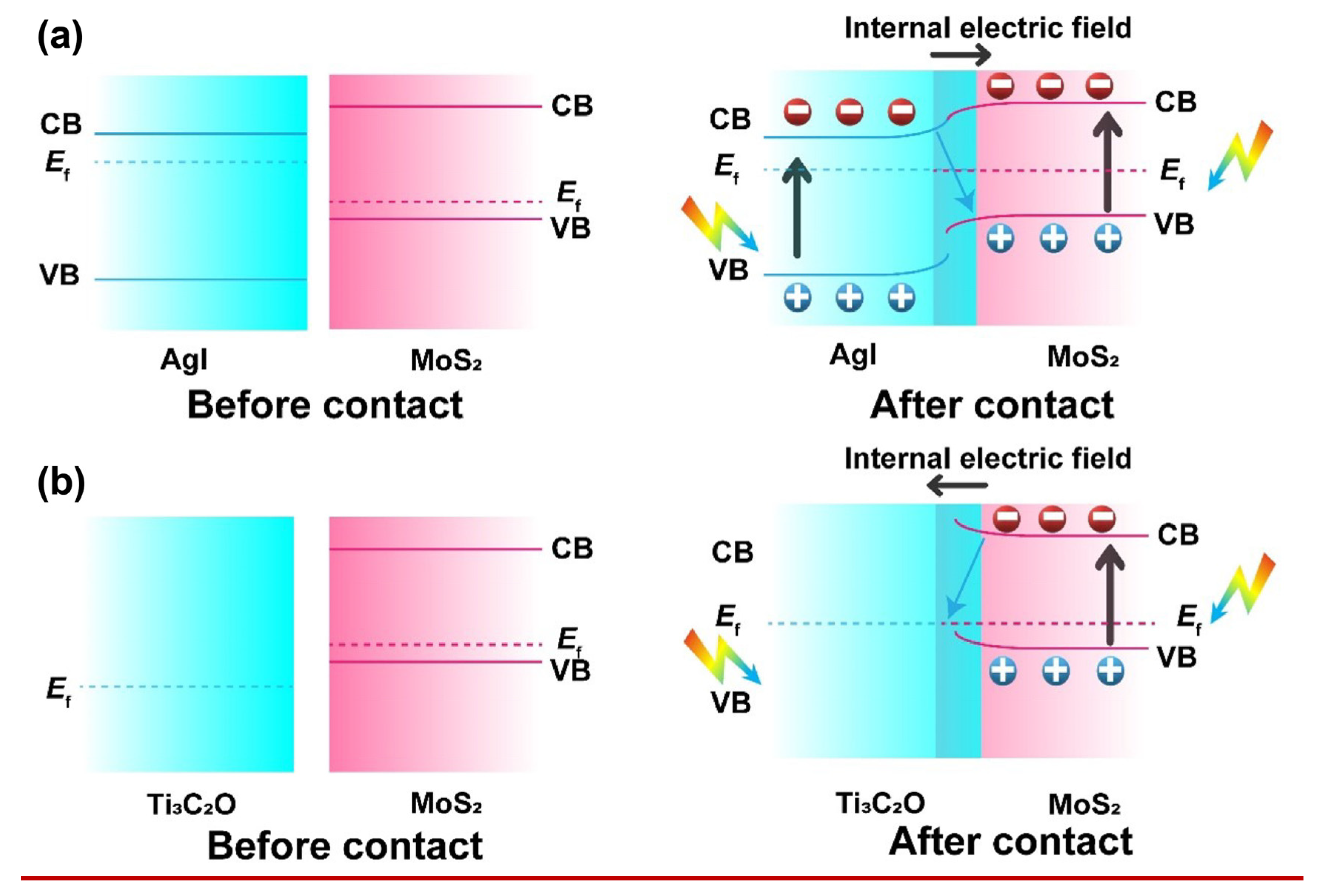
2.4.1. Band Structure
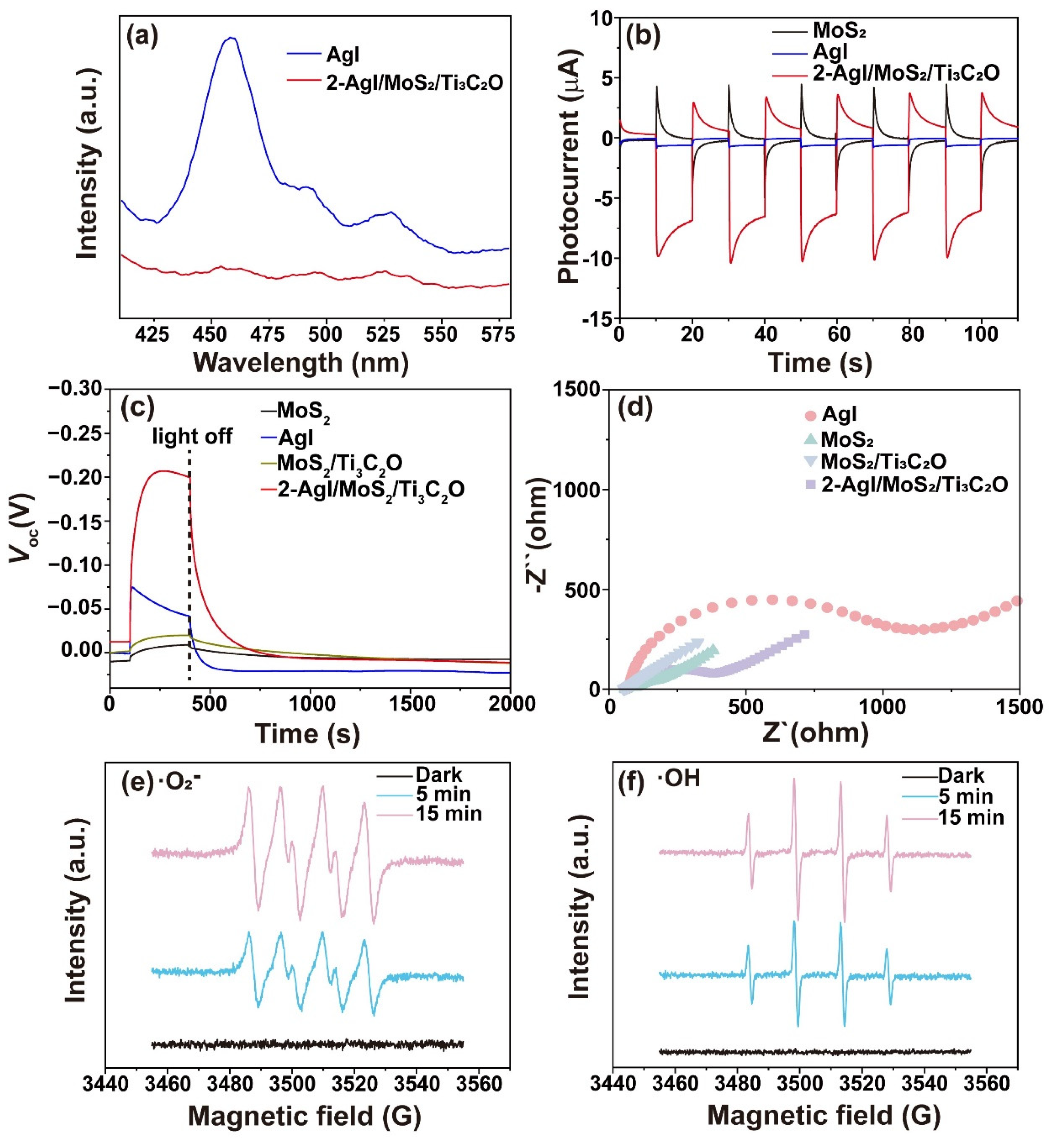
2.4.2. Carrier Transfer Performance
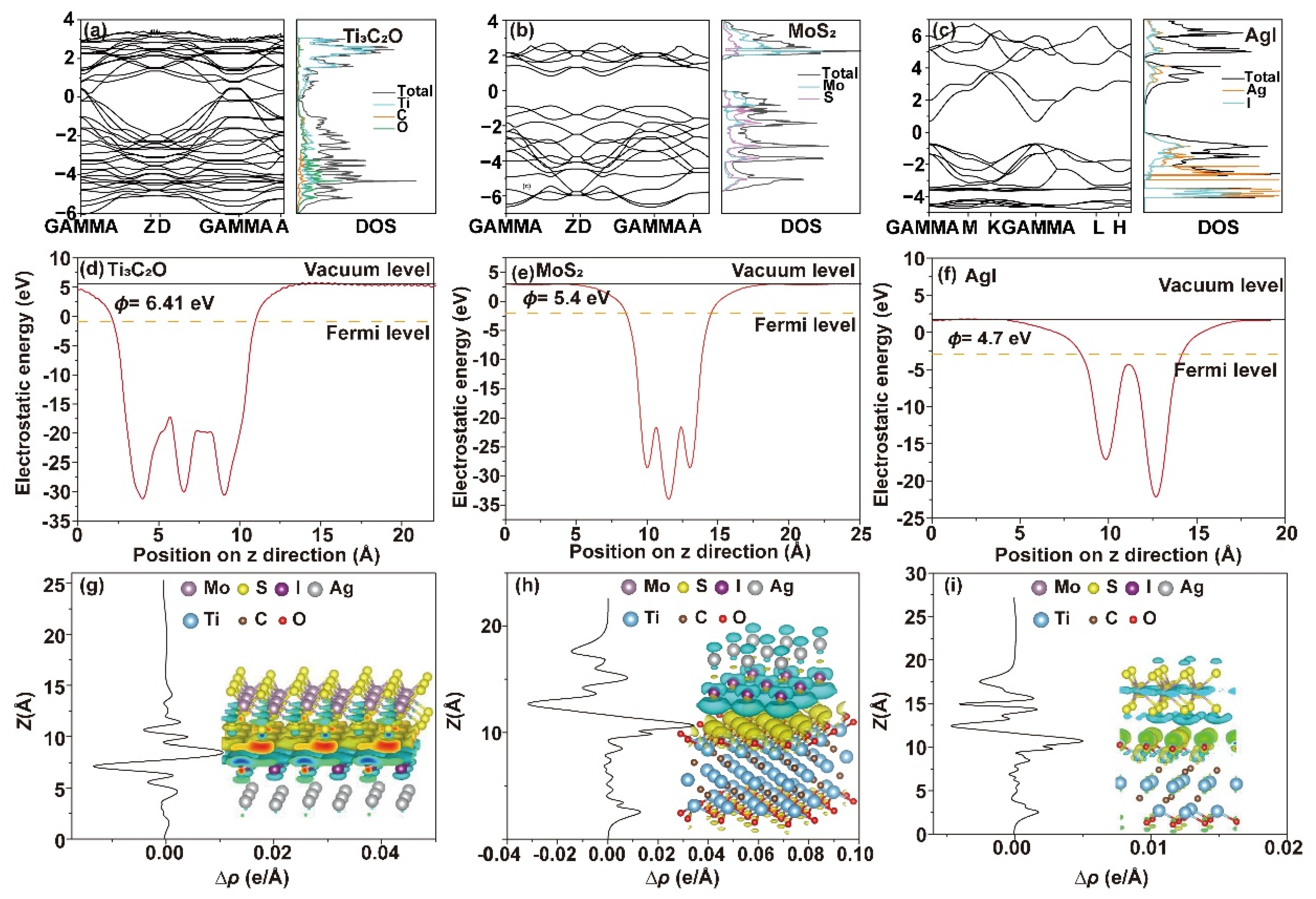
2.4.3. First-Principles Calculations
3. Experimental Section
3.1. Synthesis of AgI/MoS2/Ti3C2O Heterojunction
3.2. Photocatalytic H2O2 Production
3.3. Photocatalytic Degradation of Organic Pollutants
3.4. Photoelectrochemical (PEC) Performance
3.5. Theoretical Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jia, X.; Cao, J.; Sun, H.; Li, X.; Lin, H.; Chen, S. Interfacial engineering of Bi12O17Br2/g-C3N4-x S-scheme junction boosting charge transfer for cooperative tetracycline decomposition and CO2 reduction. Appl. Catal. B Environ. 2024, 343, 123522. [Google Scholar] [CrossRef]
- Yang, Q.; Jiang, Y.; Zhuo, H.; Mitchell, E.M.; Yu, Q. Recent progress of metal single-atom catalysts for energy applications. Nano Energy 2023, 111, 108404. [Google Scholar] [CrossRef]
- Vinoth, S.; Ong, W.-J.; Pandikumar, A. Defect engineering of BiOX (X = Cl, Br, I) based photocatalysts for energy and environmental applications: Current progress and future perspectives. Coord. Chem. Rev. 2022, 464, 214541. [Google Scholar] [CrossRef]
- He, L.; Zeng, X.; Chen, H.; Zhao, L.; Huang, Z.; Wang, D.; He, X.; Fang, W.; Du, X.; Li, W. A hybrid photocatalytic system splits atmospheric water to produce hydrogen. Adv. Funct. Mater. 2024, 34, 2313058. [Google Scholar] [CrossRef]
- Wang, L.; Chen, T.; Cui, Y.; Wu, J.; Zhou, X.; Xu, M.; Liu, Z.; Mao, W.; Zeng, X.; Shen, W. Rational Design of Environmentally Friendly Carbon Nanotube Embedded Artificial Vesicle-Structured Photocatalysts for Organic Pollutants Degradation. Adv. Funct. Mater. 2024, 34, 2313653. [Google Scholar] [CrossRef]
- Tan, D.; Zhuang, R.; Chen, R.; Ban, M.; Feng, W.; Xu, F.; Chen, X.; Wang, Q. Covalent organic frameworks enable sustainable solar to hydrogen peroxide. Adv. Funct. Mater. 2024, 34, 2311655. [Google Scholar] [CrossRef]
- Gong, Y.-N.; Guan, X.; Jiang, H.-L. Covalent organic frameworks for photocatalysis: Synthesis, structural features, fundamentals and performance. Coord. Chem. Rev. 2023, 475, 214889. [Google Scholar] [CrossRef]
- Zhu, Q.; Xu, Q.; Du, M.; Zeng, X.; Zhong, G.; Qiu, B.; Zhang, J. Recent progress of metal sulfide photocatalysts for solar energy conversion. Adv. Mater. 2022, 34, 2202929. [Google Scholar] [CrossRef]
- Liu, X.; Chen, T.; Xue, Y.; Fan, J.; Shen, S.; Hossain, M.S.A.; Amin, M.A.; Pan, L.; Xu, X.; Yamauchi, Y. Nanoarchitectonics of MXene/semiconductor heterojunctions toward artificial photosynthesis via photocatalytic CO2 reduction. Coord. Chem. Rev. 2022, 459, 214440. [Google Scholar] [CrossRef]
- Song, S.; Xing, Z.; Zhao, H.; Li, Z.; Zhou, W. Recent advances in bismuth-based photocatalysts: Environment and energy applications. Green Energy Environ. 2023, 8, 1232–1264. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Huang, N.-Y.; Xu, Q. Metal halide perovskite materials in photocatalysis: Design strategies and applications. Coord. Chem. Rev. 2023, 481, 215031. [Google Scholar] [CrossRef]
- Yu, Y.; Zeng, Q.; Tao, S.; Xia, C.; Liu, C.; Liu, P.; Yang, B. Carbon dots based photoinduced reactions: Advances and perspective. Adv Sci. 2023, 10, 2207621. [Google Scholar] [CrossRef]
- Tang, J.; Zhao, T.; Solanki, D.; Miao, X.; Zhou, W.; Hu, S. Selective hydrogen peroxide conversion tailored by surface, interface, and device engineering. Joule 2021, 5, 1432–1461. [Google Scholar] [CrossRef]
- He, K.; Huang, Z.; Chen, C.; Qiu, C.; Zhong, Y.L.; Zhang, Q. Exploring the roles of single atom in hydrogen peroxide photosynthesis. Nano-Micro Lett. 2024, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.; Zhao, J.; Wang, A.; Ji, Q.; Cheng, R.; Liang, H.; Chen, F.; Kannan, P.; Brouzgou, A.; Tsiakaras, P. Efficient photocatalytic production of H2O2 and photodegradation of tetracycline by CdS/square tubular g-C3N4 S-scheme heterojunction photocatalyst. Chem. Eng. J. 2024, 479, 147150. [Google Scholar] [CrossRef]
- Ma, H.; Wang, Y.; Zhang, Z.; Liu, J.; Yu, Y.; Zuo, S.; Li, B. A superior ternary Z-scheme photocatalyst of Bi/Black Phosphorus nanosheets/P-doped BiOCl containing interfacial P–P bond and metallic mediator for H2O2 production and RhB degradation. Chemosphere 2023, 330, 138717. [Google Scholar] [CrossRef]
- Alpysbayeva, G.; Turabekov, M.; Barakhov, B.; Myrzabekov, Z.B.; Ibragimov, P.S.; Tagayev, O.; Alikhanov, K.; Narbayeva, D.; Taipova, A. Development of a disinfectant composition based on hydrogen peroxide. Eng. Sci. 2024, 28, 1080. [Google Scholar] [CrossRef]
- Haddadi, H.; Mokhtari, E.; Farsani, M.R.; Moghadam, A.J. Zinc substituted keggin type polyoxometalate as an effective catalyst for the oxidative desulfurization of dibenzothiophene by hydrogen peroxide as oxidant. ES Mater. Manuf. 2022, 19, 766. [Google Scholar]
- Chen, Z.; Yao, D.; Chu, C.; Mao, S. Photocatalytic H2O2 production systems: Design strategies and environmental applications. Chem. Eng. J. 2023, 451, 138489. [Google Scholar] [CrossRef]
- Hou, H.; Zeng, X.; Zhang, X. Production of hydrogen peroxide by photocatalytic processes. Angew. Chem. Int. Ed. 2020, 59, 17356–17376. [Google Scholar] [CrossRef]
- Chen, H.; Gao, S.; Huang, G.; Chen, Q.; Gao, Y.; Bi, J. Built-in electric field mediated S-scheme charge migration in COF/In2S3 heterojunction for boosting H2O2 photosynthesis and sterilization. Appl. Catal. B Environ. 2024, 343, 123545. [Google Scholar] [CrossRef]
- Chen, H.; Xing, Y.; Liu, S.; Liang, Y.; Fu, J.; Wang, L.; Wang, W. Mechanistic insights into efficient photocatalytic H2O2 production of 2D/2D g-C3N4/In2S3 photocatalyst by tracking charge flow direction. Chem. Eng. J. 2023, 462, 142038. [Google Scholar] [CrossRef]
- Zhang, Y.; Di, J.; Zhu, X.; Ji, M.; Chen, C.; Liu, Y.; Li, L.; Wei, T.; Li, H.; Xia, J. Chemical bonding interface in Bi2Sn2O7/BiOBr S-scheme heterojunction triggering efficient N2 photofixation. Appl. Catal. B Environ. 2023, 323, 122148. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Z.; Zhang, J.; Shao, C.; Dai, K.; Fan, K.; Liang, C. Interfacial chemical bond and oxygen vacancy-enhanced In2O3/CdSe-DETA S-scheme heterojunction for photocatalytic CO2 conversion. Adv. Funct. Mater. 2023, 33, 2214470. [Google Scholar] [CrossRef]
- Zhu, Z.; Huang, H.; Liu, L.; Chen, F.; Tian, N.; Zhang, Y.; Yu, H. Chemically bonded α-Fe2O3/Bi4MO8Cl dot-on-plate Z-scheme junction with strong internal electric field for selective photo-oxidation of aromatic alcohols. Angew. Chem. Inter. Ed. 2022, 61, e202203519. [Google Scholar] [CrossRef] [PubMed]
- Deng, A.; Sun, Y.; Gao, Z.; Yang, S.; Liu, Y.; He, H.; Zhang, J.; Liu, S.; Sun, H.; Wang, S. Internal electric field in carbon nitride-based heterojunctions for photocatalysis. Nano Energy 2023, 108, 108228. [Google Scholar] [CrossRef]
- Chen, L.; Ren, J.T.; Yuan, Z.Y. Enabling internal electric fields to enhance energy and environmental catalysis. Adv. Energy Mater. 2023, 13, 2203720. [Google Scholar] [CrossRef]
- Zhu, J.; Bi, Q.; Tao, Y.; Guo, W.; Fan, J.; Min, Y.; Li, G. Mo-modified ZnIn2S4@NiTiO3 S-scheme heterojunction with enhanced interfacial electric field for efficient visible-light-driven hydrogen evolution. Adv. Funct. Mater. 2023, 33, 2213131. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, L.; Yu, X.; Sun, L.; Li, J.; Yang, J.; Liu, Q. Precise regulation of built-in electric field over NH2-MIL-125-Ti/WO3-x S-scheme heterojunction for achieving simultaneous formation of CO and H2O2 from CO2 and H2O. Chem. Eng. J. 2023, 466, 143129. [Google Scholar] [CrossRef]
- Kurra, N.; Ahmed, B.; Gogotsi, Y.; Alshareef, H.N. MXene-on-paper coplanar microsupercapacitors. Adv. Energy Mater. 2016, 6, 1601372. [Google Scholar] [CrossRef]
- Chen, T.; Wang, Y.; Zhang, S.; Gao, Z.; Zhao, D.; Wu, J.; Shen, K.; Sun, B. Cathodic photoelectrochemical sensor developed for glutathione detection based on carrier transport in a Ti3C2Tx/AgI heterojunction. Anal. Chim. Acta 2022, 1233, 340487. [Google Scholar] [CrossRef]
- Fan, X.; Xu, P.; Li, Y.C.; Zhou, D.; Sun, Y.; Nguyen, M.A.T.; Terrones, M.; Mallouk, T.E. Controlled exfoliation of MoS2 crystals into trilayer nanosheets. J. Am. Chem. Soc. 2016, 138, 5143–5149. [Google Scholar] [CrossRef] [PubMed]
- Kwak, I.H.; Kwon, I.S.; Abbas, H.G.; Jung, G.; Lee, Y.; Park, J.; Kang, H.S. Stable methylammonium-intercalated 1T′-MoS2 for efficient electrocatalytic hydrogen evolution. J. Mater. Chem. A 2018, 6, 5613–5617. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Xu, D.; Zhu, Y.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. Hierarchical “nanoroll” like MoS2/Ti3C2Tx hybrid with high electrocatalytic hydrogen evolution activity. Appl. Catal. B Environ. 2019, 241, 89–94. [Google Scholar] [CrossRef]
- Tan, S.M.; Ambrosi, A.; Sofer, Z.; Huber, Š.; Sedmidubský, D.; Pumera, M. Pristine basal-and edge-plane-oriented molybdenite MoS2 exhibiting highly anisotropic properties. Chem.—Eur. J. 2015, 21, 7170–7178. [Google Scholar] [CrossRef]
- Hu, J.; Huang, B.; Zhang, C.; Wang, Z.; An, Y.; Zhou, D.; Lin, H.; Leung, M.K.; Yang, S. Engineering stepped edge surface structures of MoS2 sheet stacks to accelerate the hydrogen evolution reaction. Energy Environ. Sci. 2017, 10, 593–603. [Google Scholar] [CrossRef]
- Wang, J.; Fang, W.; Hu, Y.; Zhang, Y.; Dang, J.; Wu, Y.; Chen, B.; Zhao, H.; Li, Z. Single atom Ru doping 2H-MoS2 as highly efficient hydrogen evolution reaction electrocatalyst in a wide pH range. Appl. Catal. B Environ. 2021, 298, 120490. [Google Scholar] [CrossRef]
- Li, X.; Lv, X.; Sun, X.; Yang, C.; Zheng, Y.-Z.; Yang, L.; Li, S.; Tao, X. Edge-oriented, high-percentage 1T′-phase MoS2 nanosheets stabilize Ti3C2 MXene for efficient electrocatalytic hydrogen evolution. Appl. Catal. B Environ. 2021, 284, 119708. [Google Scholar] [CrossRef]
- Reddy, D.A.; Lee, S.; Choi, J.; Park, S.; Ma, R.; Yang, H.; Kim, T.K. Green synthesis of AgI-reduced graphene oxide nanocomposites: Toward enhanced visible-light photocatalytic activity for organic dye removal. Appl. Surf. Sci. 2015, 341, 175–184. [Google Scholar] [CrossRef]
- Chen, D.; Jiang, Z.; Geng, J.; Wang, Q.; Yang, D. Carbon and nitrogen co-doped TiO2 with enhanced visible-light photocatalytic activity. Ind. Eng. Chem. Res. 2007, 46, 2741–2746. [Google Scholar] [CrossRef]
- Liu, L.; Yang, W.; Li, Q.; Gao, S.; Shang, J.K. Synthesis of Cu2O nanospheres decorated with TiO2 nanoislands, their enhanced photoactivity and stability under visible light illumination, and their post-illumination catalytic memory. ACS Appl. Mater. Inter. 2014, 6, 5629–5639. [Google Scholar] [CrossRef]
- Yan, J.; Ren, C.E.; Maleski, K.; Hatter, C.B.; Anasori, B.; Urbankowski, P.; Sarycheva, A.; Gogotsi, Y. Flexible MXene/graphene films for ultrafast supercapacitors with outstanding volumetric capacitance. Adv. Funct. Mater. 2017, 27, 1701264. [Google Scholar] [CrossRef]
- Li, Y.; Yin, Z.; Ji, G.; Liang, Z.; Xue, Y.; Guo, Y.; Tian, J.; Wang, X.; Cui, H. 2D/2D/2D heterojunction of Ti3C2 MXene/MoS2 nanosheets/TiO2 nanosheets with exposed (001) facets toward enhanced photocatalytic hydrogen production activity. Appl. Catal. B Environ. 2019, 246, 12–20. [Google Scholar] [CrossRef]
- Deng, H.; Li, Z.-j.; Wang, L.; Yuan, L.-y.; Lan, J.-h.; Chang, Z.-y.; Chai, Z.-f.; Shi, W.-q. Nanolayered Ti3C2 and SrTiO3 composites for photocatalytic reduction and removal of uranium (VI). ACS Appl. Nano Mater. 2019, 2, 2283–2294. [Google Scholar] [CrossRef]
- Zou, X.; Zhao, X.; Zhang, J.; Lv, W.; Qiu, L.; Zhang, Z. Photocatalytic degradation of ranitidine and reduction of nitrosamine dimethylamine formation potential over MXene–Ti3C2/MoS2 under visible light irradiation. J. Hazard. Mater. 2021, 413, 125424. [Google Scholar] [CrossRef]
- Bhat, M.; Ranjitha, M.T.; Mamatha, S.V.; Nayak, R.; Das, R.; Roymahapatra, G. Removal of Congo red, Patton Reeder’s, and Rhodamine B dyes from aqueous solution using Tabebuiarosea peel as natural adsorbent. ES Food Agrofor. 2024, 16, 1143. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Kubelka, P.; Munk, F. An article on optics of paint layers. Z. Tech. Phys. 1931, 12, 259–274. [Google Scholar]
- Ma, C.; Shi, F.; Liu, J.; Li, T.; Zhu, K.; Liu, J.; Cui, G.; Yang, D.; Xiao, J. Construction of a novel Ag/AgBr/AgI@SiO2 composite aerogel with controlled pore structure: Efficient removal of tetracycline by adsorption/photocatalysis synergism under visible light irradiation. J. Environ. Chem. Eng. 2023, 11, 110157. [Google Scholar] [CrossRef]
- Wang, M.; Han, X.; Zhao, Y.; Li, J.; Ju, P.; Hao, Z. Tuning size of MoS2 in MoS2/graphene oxide heterostructures for enhanced photocatalytic hydrogen evolution. J. Mater. Sci. 2018, 53, 3603–3612. [Google Scholar]
- Saleel Ahammad Saleel, V.P.; Eithiraj, R. Exploring point defects in Rb2O via First-Principles calculations. Eng. Sci. 2023, 24, 925. [Google Scholar]
- Cai, T.; Wang, L.; Liu, Y.; Zhang, S.; Dong, W.; Chen, H.; Yi, X.; Yuan, J.; Xia, X.; Liu, C. Ag3PO4/Ti3C2 MXene interface materials as a Schottky catalyst with enhanced photocatalytic activities and anti-photocorrosion performance. Appl. Catal. B Environ. 2018, 239, 545–554. [Google Scholar] [CrossRef]
- Wu, C.; Teng, Z.; Yang, C.; Chen, F.; Yang, H.B.; Wang, L.; Xu, H.; Liu, B.; Zheng, G.; Han, Q. Polarization engineering of covalent triazine frameworks for highly efficient photosynthesis of hydrogen peroxide from molecular oxygen and water. Adv. Mater. 2022, 34, 2110266. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, Z.; Zeng, G.; Huang, D.; Xiao, R.; Zhang, C.; Zhou, C.; Xiong, W.; Wang, W.; Cheng, M.; et al. Ti3C2 Mxene/porous g-C3N4 interfacial Schottky junction for boosting spatial charge separation in photocatalytic H2O2 production. Appl. Catal. B Environ. 2019, 258, 117956. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, B.; Yu, J.; Wang, L.; Ho, W. TiO2/In2S3 S-scheme photocatalyst with enhanced H2O2-production activity. Nano Res. 2023, 16, 4506–4514. [Google Scholar] [CrossRef]
- Feng, C.; Tang, L.; Deng, Y.; Wang, J.; Liu, Y.; Ouyang, X.; Yang, H.; Yu, J.; Wang, J. A novel sulfur-assisted annealing method of g-C3N4 nanosheet compensates for the loss of light absorption with further promoted charge transfer for photocatalytic production of H2 and H2O2. Appl. Catal. B Environ. 2021, 281, 119539. [Google Scholar] [CrossRef]
- Zhao, X.; You, Y.; Huang, S.; Wu, Y.; Ma, Y.; Zhang, G.; Zhang, Z. Z-scheme photocatalytic production of hydrogen peroxide over Bi4O5Br2/g-C3N4 heterostructure under visible light. Appl. Catal. B Environ. 2020, 278, 119251. [Google Scholar] [CrossRef]
- Zhao, S.; Zhao, X. Polyoxometalates-derived metal oxides incorporated into graphitic carbon nitride framework for photocatalytic hydrogen peroxide production under visible light. J. Catal. 2018, 366, 98–106. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, S.; Chen, T.; Ying, Y.; Liu, Y.; Wu, J. Interfacial Electric Fields and Chemical Bonds in Ti3C2O-Crafted AgI/MoS2 Direct Z-Scheme Heterojunction Synergistically Expedite Photocatalytic Performance. Catalysts 2025, 15, 740. https://doi.org/10.3390/catal15080740
Jiao S, Chen T, Ying Y, Liu Y, Wu J. Interfacial Electric Fields and Chemical Bonds in Ti3C2O-Crafted AgI/MoS2 Direct Z-Scheme Heterojunction Synergistically Expedite Photocatalytic Performance. Catalysts. 2025; 15(8):740. https://doi.org/10.3390/catal15080740
Chicago/Turabian StyleJiao, Suxing, Tianyou Chen, Yiran Ying, Yincheng Liu, and Jing Wu. 2025. "Interfacial Electric Fields and Chemical Bonds in Ti3C2O-Crafted AgI/MoS2 Direct Z-Scheme Heterojunction Synergistically Expedite Photocatalytic Performance" Catalysts 15, no. 8: 740. https://doi.org/10.3390/catal15080740
APA StyleJiao, S., Chen, T., Ying, Y., Liu, Y., & Wu, J. (2025). Interfacial Electric Fields and Chemical Bonds in Ti3C2O-Crafted AgI/MoS2 Direct Z-Scheme Heterojunction Synergistically Expedite Photocatalytic Performance. Catalysts, 15(8), 740. https://doi.org/10.3390/catal15080740







