Enhanced Peroxydisulfate Activation by Co-Doping of Nitrogen, Chlorine, and Iron: Preparation, Synergistic Effects, and Application
Abstract
1. Introduction
2. Results and Discussion
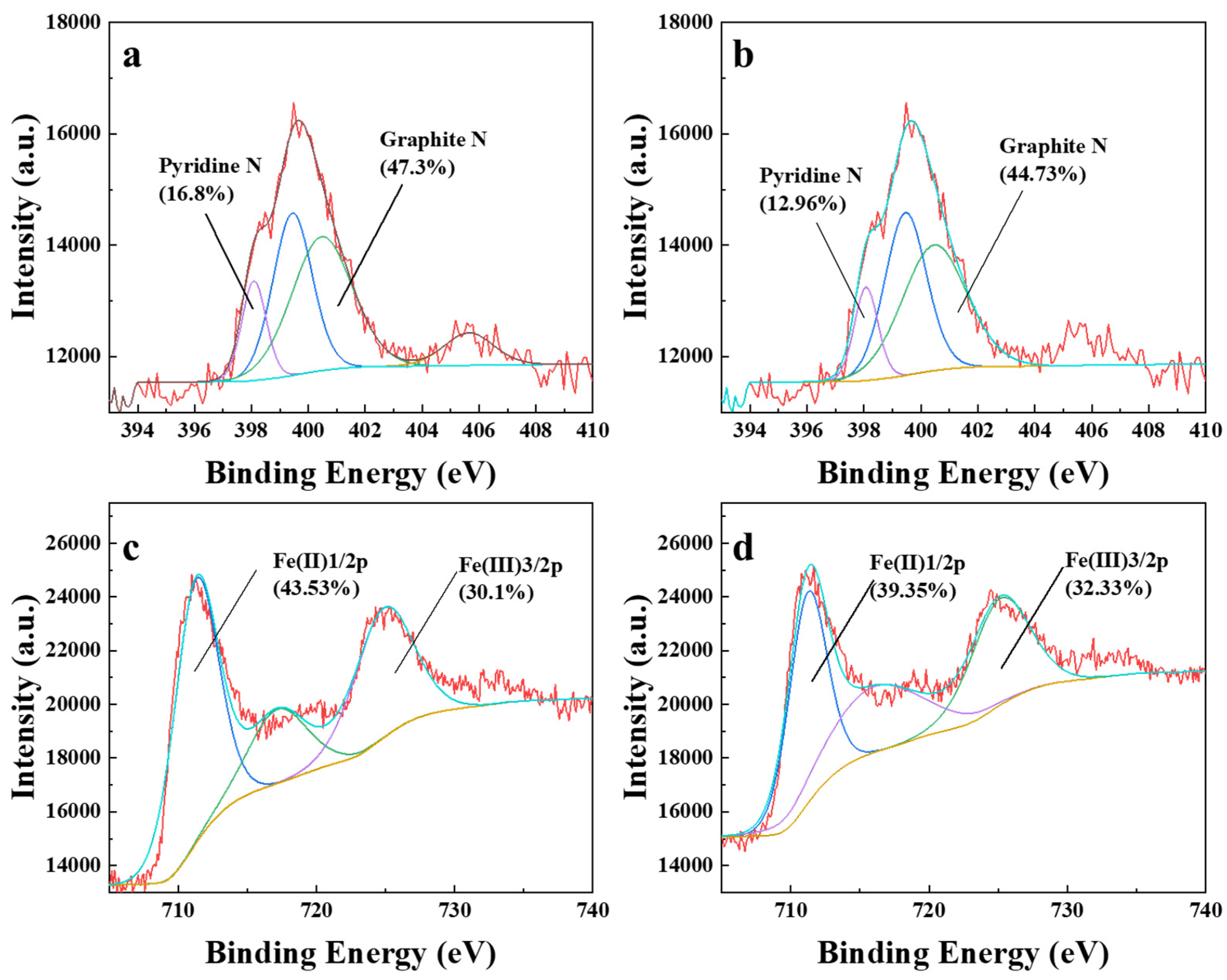
2.1. Characterization of Biochars
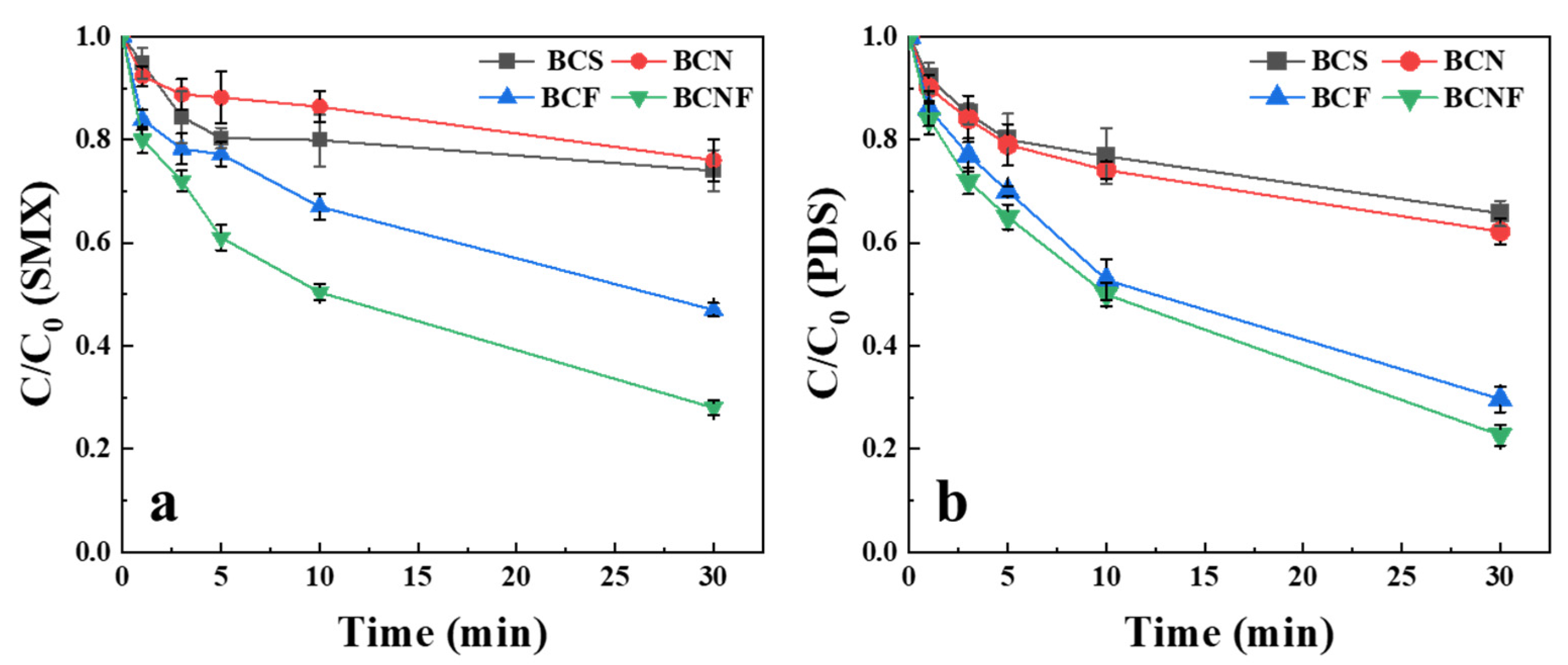
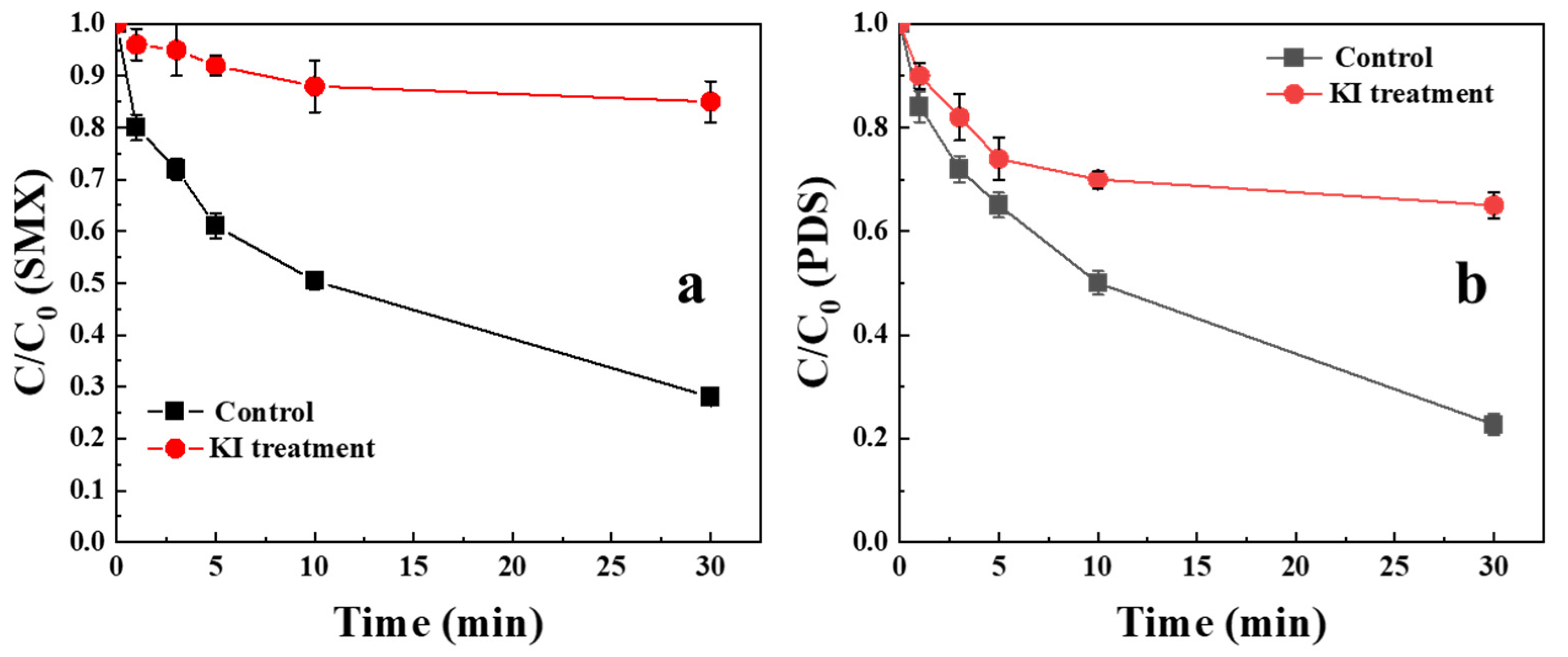
2.2. Degradation of SMX and Decomposition of PDS by Biochar
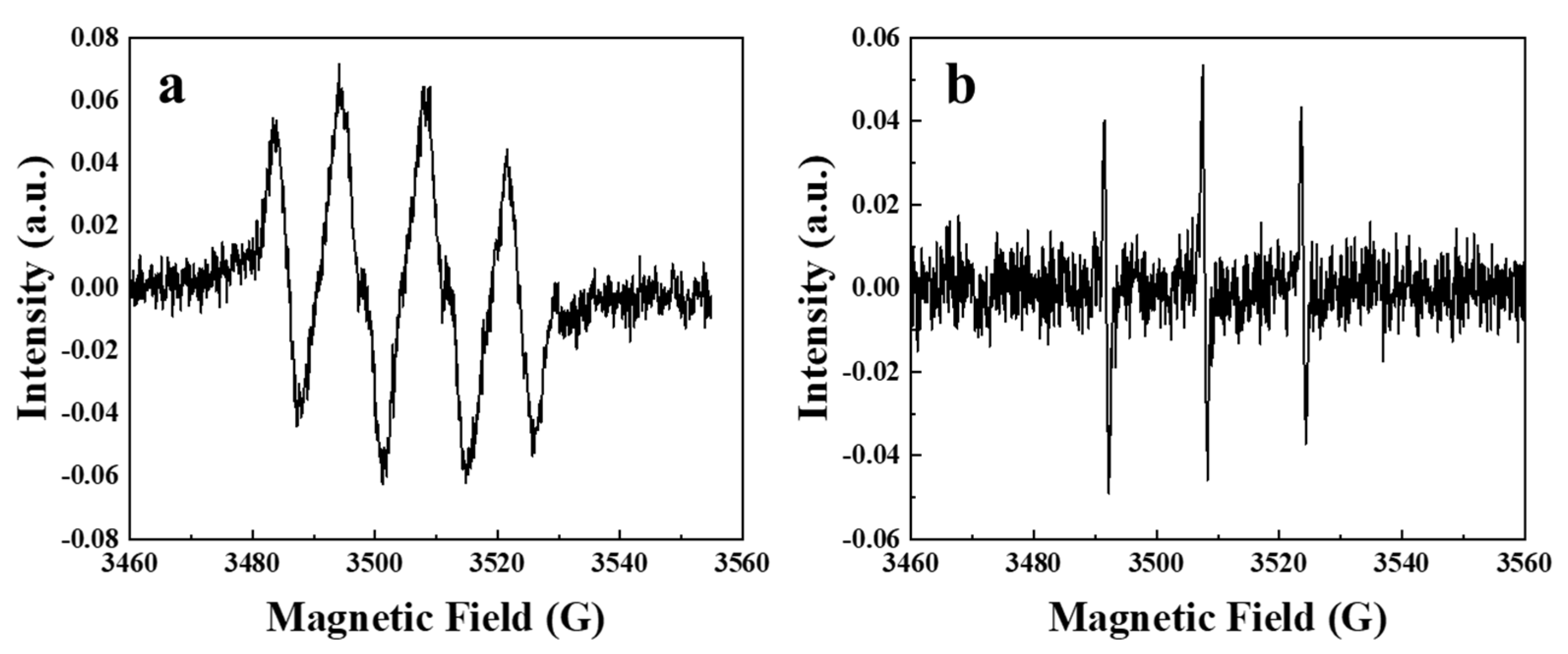
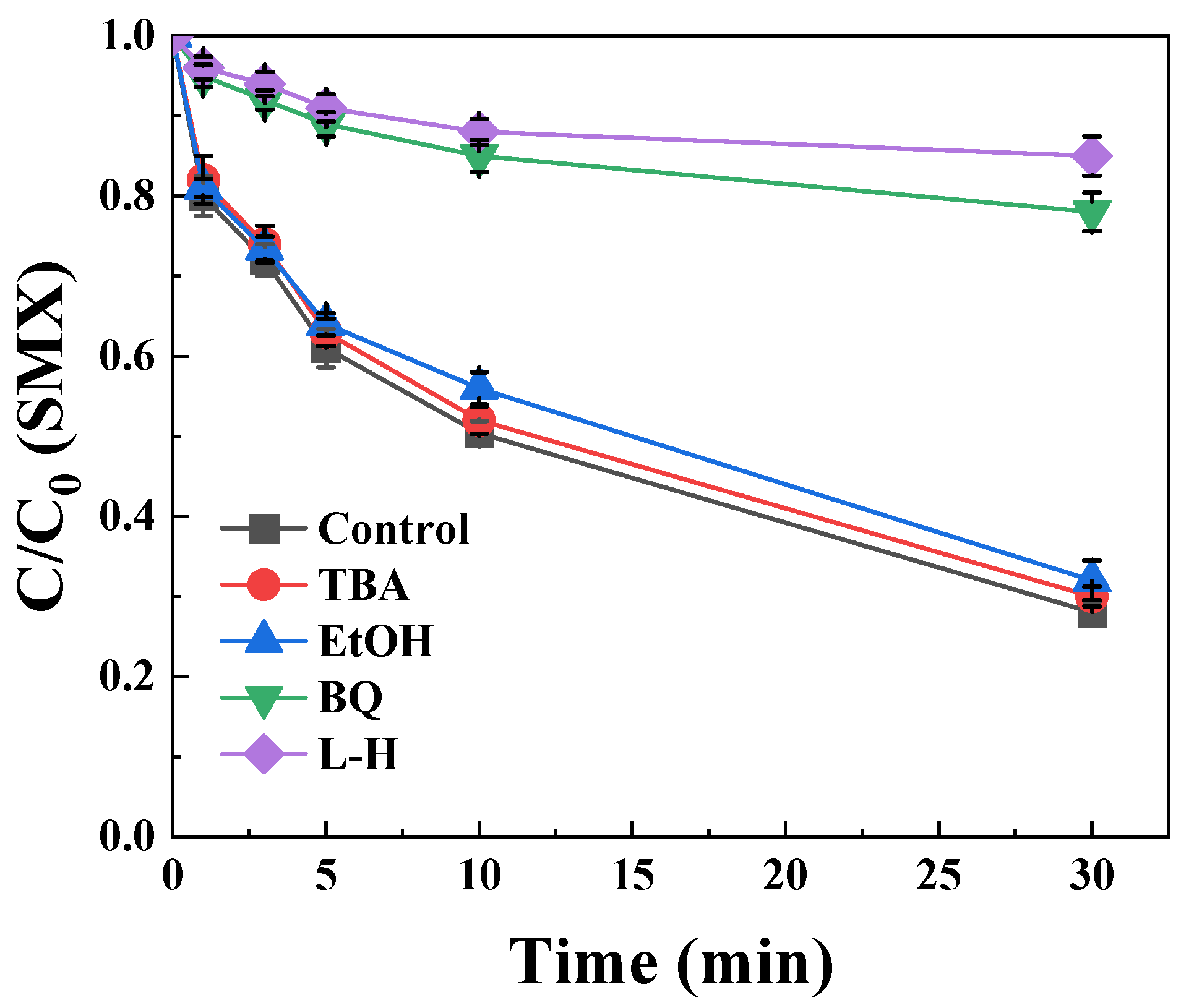
2.3. Elucidation of Reactive Oxygen Species
2.4. Possible Mechanism of PDS Activated by Biochar
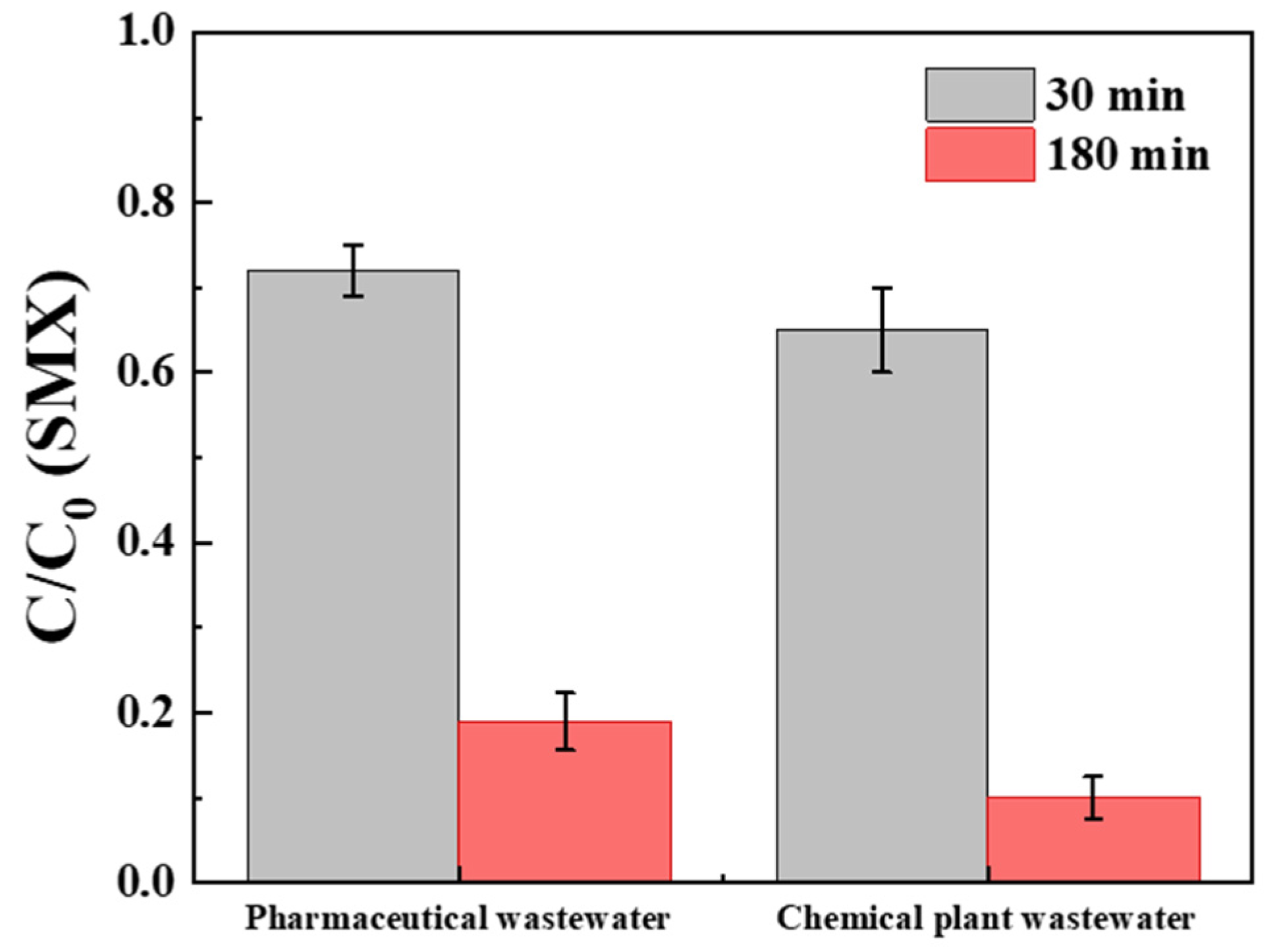
2.5. Application in Wastewater Remediation
2.6. Comparison with Other Materials
2.7. Toxicity of Biochar
3. Experimental
3.1. Materials
3.2. Pyrolysis Experiments
3.3. Characterization of Biochar
3.4. Experimental Procedures
3.5. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Song, Q.; Zhao, H.; Jia, J.; Yang, L.; Lv, W.; Bao, J.; Shu, X.; Gu, Q.; Zhang, P. Pyrolysis of municipal solid waste with iron-based additives: A study on the kinetic, product distribution and catalytic mechanisms. J. Clean. Prod. 2020, 258, 120682. [Google Scholar] [CrossRef]
- Abhishek, K.; Shrivastava, A.; Vimal, V.; Gupta, A.K.; Bhujbal, S.K.; Biswas, J.K.; Singh, L.; Ghosh, P.; Pandey, A.; Sharma, P.; et al. Biochar application for greenhouse gas mitigation, contaminants immobilization and soil fertility enhancement: A state-of-the-art review. Sci. Total Environ. 2022, 853, 158562. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Du, Z.; Zhang, A.; He, C.; Shi, Q.; Tian, L.; Zhang, P.; Li, L.; Wang, J. Long-term biochar addition alters the characteristics but not the chlorine reactivity of soil-derived dissolved organic matter. Water Res. 2020, 185, 116260. [Google Scholar] [CrossRef]
- Ahamed, A.; Veksha, A.; Yin, K.; Weerachanchai, P.; Giannis, A.; Lisak, G. Environmental impact assessment of converting flexible packaging plastic waste to pyrolysis oil and multi-walled carbon nanotubes. J. Hazard. Mater. 2020, 390, 121449. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Meng, Y.; Yao, H.; Cheng, Z.; Chen, G.; Sun, P. Carbon Residue from Copyrolysis of Cartons and Plastics As an Efficient H2O2 Activator. ACS EST Eng. 2023, 3, 1205–1211. [Google Scholar] [CrossRef]
- Fang, Z.; Gao, Y.; Bolan, N.; Shaheen, S.M.; Xu, S.; Wu, X.; Xu, X.; Hu, H.; Lin, J.; Zhang, F.; et al. Conversion of biological solid waste to graphene-containing biochar for water remediation: A critical review. Chem. Eng. J. 2020, 390, 124611. [Google Scholar] [CrossRef]
- Ahmed, M.H.M.; Batalha, N.; Mahmudul, H.M.; Perkins, G.; Konarova, M. A review on advanced catalytic co-pyrolysis of biomass and hydrogen-rich feedstock: Insights into synergistic effect, catalyst development and reaction mechanism. Bio Resour. Technol. 2020, 310, 123457. [Google Scholar] [CrossRef]
- Cao, J.; Gao, Y.; Ma, Y. Facile preparation of activated carbon foam via pyrolysis of waste bread under CO2 atmosphere. Biomass Convers. Biorefinery 2019, 9, 521–529. [Google Scholar] [CrossRef]
- Nawaz, A.; Razzak, S.A. Co-pyrolysis of biomass and different plastic waste to reduce hazardous waste and subsequent production of energy products: A review on advancement, synergies, and future prospects. Renew. Energy 2024, 224, 124611. [Google Scholar] [CrossRef]
- Ayiania, M.; Terrell, E.; Dunsmoor, A.; Carbajal-Gamarra, F.M.; Garcia-Perez, M. Characterization of solid and vapor products from thermochemical conversion of municipal solid waste woody fractions. Waste Manag. 2019, 84, 277–285. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, S.; Yang, X.; Li, G.; Zhou, H.; Li, Q.; Zhang, Y. Thermal behaviour and kinetic study of co-pyrolysis of micro-algae with different plastics. Waste Manag. 2021, 126, 331–339. [Google Scholar] [CrossRef]
- Shaaban, M.; Van Zwieten, L.; Bashir, S.; Younas, A.; Núñez-Delgado, A.; Chhajro, M.A.; Kubar, K.A.; Ali, U.; Rana, M.S.; Mehmood, M.A.; et al. A concise review of biochar application to agricultural soils to improve soil conditions and fight pollution. J. Environ. Manag. 2018, 228, 429–440. [Google Scholar] [CrossRef]
- Devi, P.; Das, U.; Dalai, A.K. In-situ chemical oxidation: Principle and applications of peroxide and persulfate treatments in wastewater systems. Sci. Total Environ. 2016, 571, 643–657. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.-D.; Chen, C.-W.; Tsai, M.-L.; Chang, J.-H.; Lyu, S.-Y.; Hung, C.-M. Degradation of 4-nonylphenol in marine sediments by persulfate over magnetically modified biochars. Bioresour. Technol. 2019, 281, 143–148. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xiao, Y.; Tang, J.; Chen, H.; Sun, H. Persulfate activation with sawdust biochar in aqueous solution by enhanced electron donor-transfer effect. Sci. Total Environ. 2019, 690, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.A.; Oh, S. Biochar-mediated oxidation of phenol by persulfate activated with zero-valent iron. J. Chem. Technol. Biotechnol. 2019, 94, 3932–3940. [Google Scholar] [CrossRef]
- Wang, J.; Liao, Z.; Ifthikar, J.; Shi, L.; Du, Y.-A.; Zhu, J.; Xi, S.; Chen, Z.; Chen, Z. Treatment of refractory contaminants by sludge-derived biochar/persulfate system via both adsorption and advanced oxidation process. Chemosphere 2017, 185, 754–763. [Google Scholar] [CrossRef]
- Wang, R.-Z.; Huang, D.-L.; Liu, Y.-G.; Zhang, C.; Lai, C.; Wang, X.; Zeng, G.-M.; Gong, X.-M.; Duan, A.; Zhang, Q.; et al. Recent advances in biochar-based catalysts: Properties, applications and mechanisms for pollution remediation. Chem. Eng. J. 2019, 371, 380–403. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, Z.; Zhang, H.; Chen, T.; Qiu, Y.; Xu, Z.; Yin, D. Efficient removal of several estrogens in water by Fe-hydrochar composite and related interactive effect mechanism of H2O2 and iron with persistent free radicals from hydrochar of pin-ewood. Sci. Total Environ. 2019, 658, 1013–1022. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, X.; Geng, M.; Chen, D.; Lin, H.; Zhang, H. Catalytic oxidation of clofibric acid by peroxydisulfate activated with wood-based biochar: Effect of biochar pyrolysis temperature, performance and mechanism. Chem. Eng. J. 2019, 374, 1253–1263. [Google Scholar] [CrossRef]
- Jiang, S.-F.; Ling, L.-L.; Chen, W.-J.; Liu, W.-J.; Li, D.-C.; Jiang, H. High efficient removal of bisphenol A in a peroxymonosul-fate/iron functionalized biochar system: Mechanistic elucidation and quantification of the contributors. Chem. Eng. J. 2019, 359, 572–583. [Google Scholar] [CrossRef]
- Yu, J.; Tang, L.; Pang, Y.; Zeng, G.; Feng, H.; Zou, J.; Wang, J.; Feng, C.; Zhu, X.; Ouyang, X.; et al. Hierarchical porous biochar from shrimp shell for persulfate activation: A two-electron transfer path and key impact factors. Appl. Catal. B Environ. 2020, 260, 118160. [Google Scholar] [CrossRef]
- Zhai, S.; Li, M.; Wang, D.; Zhang, L.; Yang, Y.; Fu, S. In situ loading metal oxide particles on bio-chars: Reusable materials for efficient removal of methylene blue from wastewater. J. Clean. Prod. 2019, 220, 460–474. [Google Scholar] [CrossRef]
- Ababneh, F.A.; Alakhras, A.I.; Heikal, M.; Ibrahim, S.M. Stabilization of lead bearing sludge by utilization in fly ash-slag based geopolymer. Constr. Build. Mater. 2019, 227, 116684. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, R.; Cheng, Z.; Chen, G.; Sun, P. Persulfate Activation by Municipal Solid Waste-Derived Carbon Residue: Mechanism and Application. ACS EST Water 2023, 3, 4004–4010. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Z.; Shen, B.; Liu, L. Insights into biochar and hydrochar production and applications: A review. Energy 2019, 171, 581–598. [Google Scholar] [CrossRef]
- Lai, C.; Huang, F.; Zeng, G.; Huang, D.; Qin, L.; Cheng, M.; Zhang, C.; Li, B.; Yi, H.; Liu, S.; et al. Fabrication of novel magnetic MnFe2O4/biochar composite and heterogeneous photo-Fenton degradation of tetracycline in near neutral pH. Chemosphere 2019, 224, 910–921. [Google Scholar] [CrossRef]
- Lam, S.S.; Yek, P.N.Y.; Ok, Y.S.; Chong, C.C.; Liew, R.K.; Tsang, D.C.W.; Park, Y.-K.; Liu, Z.; Wong, C.S.; Peng, W. Engineering pyrolysis biochar via single-step microwave steam activation for hazardous landfill leachate treatment. J. Hazard. Mater. 2020, 390, 121649. [Google Scholar] [CrossRef]
- Zhang, R.; Li, Y.; Wang, Z.; Tong, Y.; Sun, P. Biochar-activated peroxydisulfate as an effective process to eliminate pharma-ceutical and metabolite in hydrolyzed urine. Water Res. 2020, 177, 115809. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, P.; Wei, K.; Huang, X.; Zhang, X. Enhanced H2O2 activation and sulfamethoxazole degradation by Fe-impregnated biochar. Chem. Eng. J. 2019, 385, 123921. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, R.; Show, P.; Mahlknecht, L.; Wang, C. Degradation of tetracycline by nitrogen-doped biochar as a perox-ydisulfate activator: Nitrogen doping pattern and non-radical mechanism. Sustain. Horizons 2024, 10, 100091. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, Y.; Niu, Q.; Zeng, G.; Lai, C.; Liu, S.; Huang, D.; Qin, L.; Liu, X.; Li, B.; et al. New notion of biochar: A review on the mechanism of biochar applications in advannced oxidation processes. Chem. Eng. J. 2021, 416, 129027. [Google Scholar] [CrossRef]
- Qi, W.; Liu, W.; Zhang, B.; Gu, X.; Guo, X.; Su, D. Oxidative Dehydrogenation on Nanocarbon: Identification and Quantification of Active Sites by Chemical Titration. Angew. Chem. Int. Ed. Engl. 2013, 52, 14224–14228. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, A.; Gao, N.; Li, K.; Ren, J.; Qu, X. Deciphering a Nanocarbon-Based Artificial Peroxidase: Chemical Identification of the Catalytically Active and Substrate-Binding Sites on Graphene Quantum Dots. Angew. Chemie Int. Ed. 2015, 54, 7176–7180. [Google Scholar] [CrossRef]
- Sha, L.; Wu, Z.; Ling, Z.; Liu, X.; Yu, X.; Zhang, S. Investigation on the improvement of activated sludge dewaterability using different iron forms (ZVI vs. Fe (II))/peroxydisulfate combined vertical electro-dewatering processes. Chemosphere 2022, 292, 133416. [Google Scholar] [CrossRef]
- Xiao, P.-F.; An, L.; Wu, D.-D. The use of carbon materials in persulfate-based advanced oxidation processes: A review. New Carbon Materials 2020, 35, 667–681. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, R.; Huang, J.; Yu, D.; Cheng, Z.; Chen, G.; Sun, P. Carbon residue from co-pyrolysis of cartons and plastics: Characteristics, environmental behaviors and applications. J. Environ. Manag. 2025, 375, 124296. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zhang, H.; Guo, W.; Meng, T.; Cui, H.; Ma, C. Enhanced Peroxydisulfate Activation by Co-Doping of Nitrogen, Chlorine, and Iron: Preparation, Synergistic Effects, and Application. Catalysts 2025, 15, 880. https://doi.org/10.3390/catal15090880
Li Z, Zhang H, Guo W, Meng T, Cui H, Ma C. Enhanced Peroxydisulfate Activation by Co-Doping of Nitrogen, Chlorine, and Iron: Preparation, Synergistic Effects, and Application. Catalysts. 2025; 15(9):880. https://doi.org/10.3390/catal15090880
Chicago/Turabian StyleLi, Zhipeng, Hao Zhang, Wanjiang Guo, Tan Meng, Hongru Cui, and Chao Ma. 2025. "Enhanced Peroxydisulfate Activation by Co-Doping of Nitrogen, Chlorine, and Iron: Preparation, Synergistic Effects, and Application" Catalysts 15, no. 9: 880. https://doi.org/10.3390/catal15090880
APA StyleLi, Z., Zhang, H., Guo, W., Meng, T., Cui, H., & Ma, C. (2025). Enhanced Peroxydisulfate Activation by Co-Doping of Nitrogen, Chlorine, and Iron: Preparation, Synergistic Effects, and Application. Catalysts, 15(9), 880. https://doi.org/10.3390/catal15090880





