Structure of Cu, Ni, and CuNi Bimetallic Small Clusters Incorporated in g-C3N4: A DFT Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Cun-C3N4 (n = 1–4) Structures
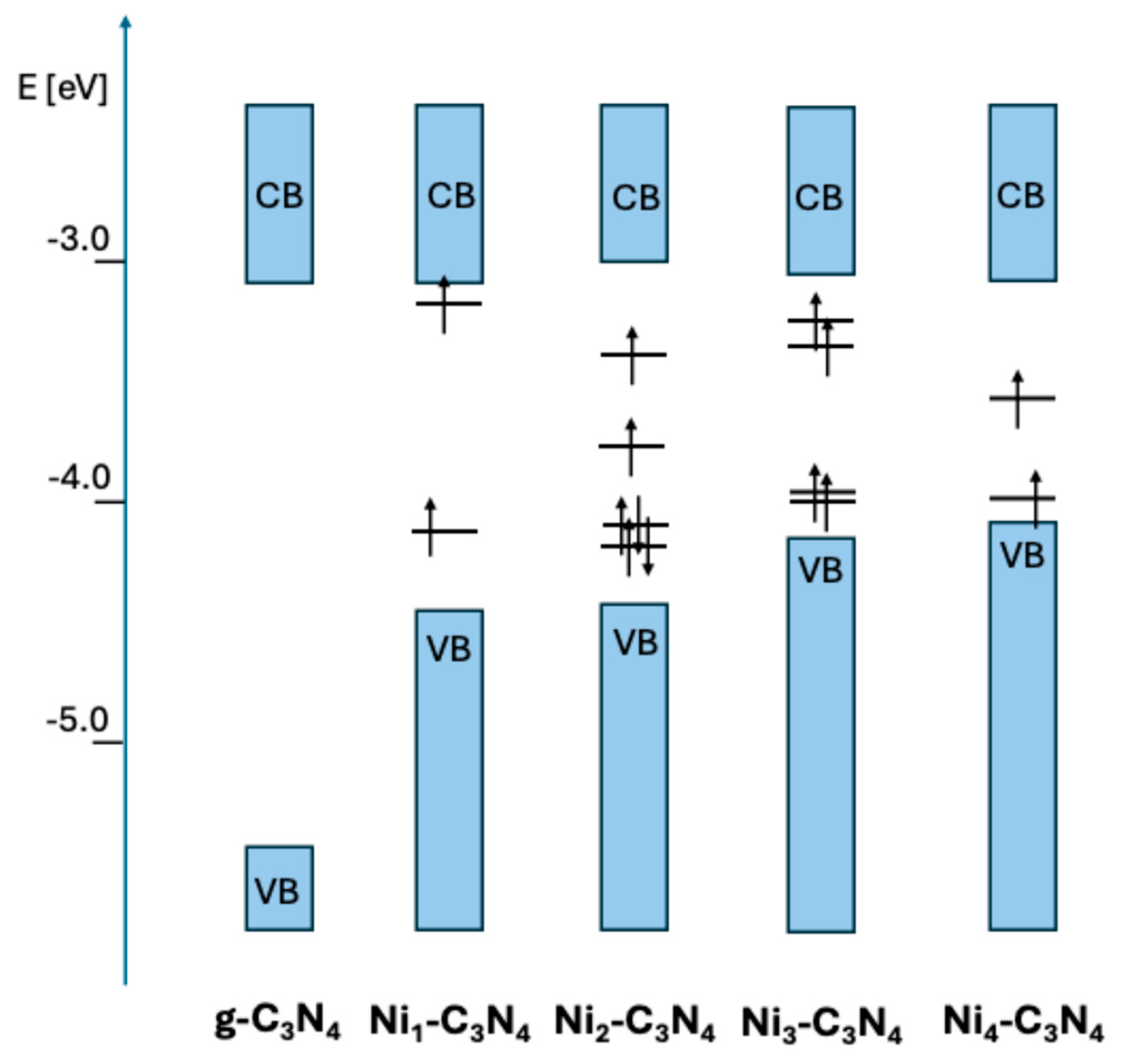
2.2. Structures of Nin-C3N4 (n = 1–4)
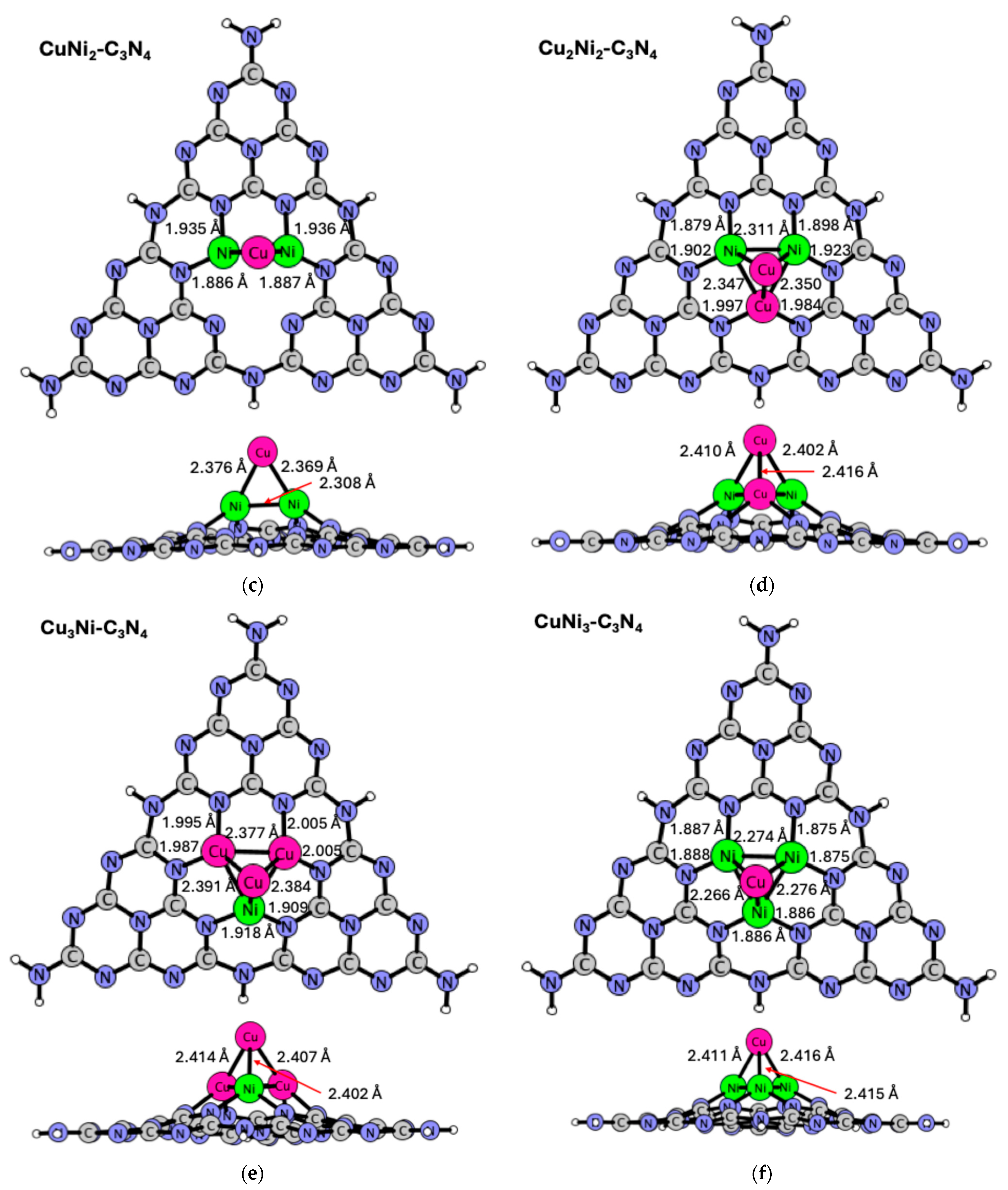
2.3. CunNim-C3N4 (n = 1–3; m = 1–3) Structures
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| g-C3N4 | Graphitic Carbon Nitride |
| DFT | Density Functional Theory |
| NPA | Natural Population Analysis |
| SAC | Single-Atom Catalyst |
References
- Wang, Y.; Mao, J.; Meng, X.; Yu, L.; Deng, D.; Bao, X. Catalysis with Two-Dimensional Materials Confining Single Atoms: Concept, Design, and Applications. Chem. Rev. 2019, 119, 1806–1854. [Google Scholar] [CrossRef] [PubMed]
- Nashim, A.; Parida, K. A Glimpse on the plethora of applications of prodigious material MXene. Sustain. Mater. Technol. 2022, 32, e00439. [Google Scholar] [CrossRef]
- Alam, M.W.; Allag, N.; Naveed-Ur-Rehman, M.; Islam Bhat, S. Graphene-Based Catalysts: Emerging Applications and Potential Impact. Chem. Rec. 2024, 24, e202400096. [Google Scholar] [CrossRef]
- Yruela-Garrido, M.; Campos-Castellanos, E.; Morales, M.V.; Rodríguez-Ramos, I.; Guerrero-Ruiz, A. Boron Nitride-Supported Metal Catalysts for the Synthesis and Decomposition of Ammonia and Formic Acid. Nanomaterials 2025, 15, 212. [Google Scholar] [CrossRef]
- Li, H.; Cheng, B.; Xu, J.; Yu, J.; Cao, S. Crystalline carbon nitrides for photocatalysis. EES Catal. 2024, 2, 411–447. [Google Scholar] [CrossRef]
- Lu, Q.; Eid, K.; Li, W.; Abdullah, A.M.; Xu, G.; Varma, R.S. Engineering graphitic carbon nitride (g-C3N4) for catalytic reduction of CO2 to fuels and chemicals: Strategy and mechanism. Green Chem. 2021, 23, 5394–5428. [Google Scholar] [CrossRef]
- Yang, X.-F.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-Atom Catalysts: A New Frontier in Heterogeneous Catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef]
- Kaiser, S.K.; Chen, Z.; Faust Akl, D.; Mitchell, S.; Pérez-Ramírez, J. Single-Atom Catalysts across the Periodic Table. Chem. Rev. 2020, 120, 11703–11809. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, G.; Li, Y.; Chu, W.; Wang, L.-W. Transition-metal single atoms in nitrogen-doped graphenes as efficient active centers for water splitting: A theoretical study. Phys. Chem. Chem. Phys. 2019, 21, 3024–3032. [Google Scholar] [CrossRef]
- Zhou, P.; Lv, F.; Li, N.; Zhang, Y.; Mu, Z.; Tang, Y.; Lai, J.; Chao, Y.; Luo, M.; Lin, F.; et al. Strengthening reactive metal-support interaction to stabilize high-density Pt single atoms on electron-deficient g-C3N4 for boosting photocatalytic H2 production. Nano Energy 2019, 56, 127–137. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, H.; Tang, R.; Li, H.; Wu, Y. Mechanism of transition-metal-cluster-anchored g-C3N4 for the electrochemical catalytic hydrogenation of carbon dioxide to C1 products. Comput. Theor. Chem. 2025, 1244, 115076. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, H.; Li, C.; Cao, X.; Li, H.; Wu, Y. The reduction mechanism of C1 product from carbon dioxide catalyzed by Ni-doped g-C3N4. Mol. Catal. 2024, 559, 114064. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Sun, C.; Cao, X.; Wang, X.; Yao, J. Defective g-C3N4 supported Ru3 single-cluster catalyst for ammonia synthesis through parallel reaction pathways. Nano Res. 2023, 16, 3580–3587. [Google Scholar] [CrossRef]
- An, S.; Zhang, G.; Wang, T.; Zhang, W.; Li, K.; Song, C.; Miller, J.T.; Miao, S.; Wang, J.; Guo, X. High-Density Ultra-small Clusters and Single-Atom Fe Sites Embedded in Graphitic Carbon Nitride (g-C3N4) for Highly Efficient Catalytic Advanced Oxidation Processes. ACS Nano 2018, 12, 9441–9450. [Google Scholar] [CrossRef]
- Dziadyk, E.; Trawczyński, J.; Szyja, B.M. The pathways of the CO2 hydrogenation by NiCu/ZnO from DFT molecular dynamics simulations. J. Mol. Graph. Model. 2020, 100, 107677. [Google Scholar] [CrossRef]
- Wang, C.; Lv, P.; Xue, D.; Cai, Y.; Yan, X.; Xu, L.; Fang, J.; Yang, Y. Zero-Dimensional/Two-Dimensional Au25(Cys)18 Nanoclusters/g-C3N4 Nanosheets Composites for Enhanced Photocatalytic Hydrogen Production under Visible Light. ACS Sustain. Chem. Eng. 2018, 6, 8447–8457. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, J.; Cheng, B.; Yu, J.; Xu, J. Nickel-based cocatalysts for photocatalysis: Hydrogen evolution, overall water splitting and CO2 reduction. Mater. Today Phys. 2020, 15, 100279. [Google Scholar] [CrossRef]
- Wu, Q.; Wei, W.; Lv, X.; Wang, Y.; Huang, B.; Dai, Y. Cu@g-C3N4: An Efficient Single-Atom Electrocatalyst for NO Electrochemical Reduction with Suppressed Hydrogen Evolution. J. Phys. Chem. C 2019, 123, 31043–31049. [Google Scholar] [CrossRef]
- Yang, T.; Mao, X.; Zhang, Y.; Wu, X.; Wang, L.; Chu, M.; Pao, C.-W.; Yang, S.; Xu, Y.; Huang, X. Coordination tailoring of Cu single sites on C3N4 realizes selective CO2 hydrogenation at low temperature. Nat. Commun. 2021, 12, 6022. [Google Scholar] [CrossRef]
- Allasia, N.; Xu, S.; Jafri, S.F.; Borfecchia, E.; Cipriano, L.A.; Terraneo, G.; Tosoni, S.; Mino, L.; Di Liberto, G.; Pacchioni, G.; et al. Resolving the Nanostructure of Carbon Nitride-Supported Single-Atom Catalysts. Small 2025, 21, e2408286. [Google Scholar] [CrossRef]
- Panigrahi, P.; Kumar, A.; Karton, A.; Ahuja, R.; Hussain, T. Remarkable improvement in hydrogen storage capacities of two-dimensional carbon nitride (g-C3N4) nanosheets under selected transition metal doping. Int. J. Hydrogen Energy 2020, 45, 3035–3045. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, S.; Jia, J.; Liu, Y.; Cai, Q.; Zhao, J. Supported Cu3 clusters on graphitic carbon nitride as an efficient catalyst for CO electroreduction to propene. J. Mater. Chem. A 2022, 10, 14460–14469. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, H.; Chen, C. New template for metal decoration and hydrogen adsorption on graphene-like C3N4. Phys. Lett. A 2009, 373, 2778–2781. [Google Scholar] [CrossRef]
- Stottko, R.; Dziadyk-Stopyra, E.; Szyja, B.M. Can Machine Learning Predict the Reaction Paths in Catalytic CO2 Reduction on Small Cu/Ni Clusters? Catalysts 2023, 13, 1470. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, L.; Pei, Y. Single Metal Atom Catalyst Supported on g-C3N4 for Formic Acid Dehydrogenation: A Combining Density Functional Theory and Machine Learning Study. J. Phys. Chem. C 2021, 125, 22513–22521. [Google Scholar] [CrossRef]
- Laboratory, L.B.N. Materials Project Database. Available online: https://next-gen.materialsproject.org/materials/mp-30 (accessed on 29 July 2025).
- Yang, L.-M.; Frauenheim, T.; Ganz, E. Properties of the Free-Standing Two-Dimensional Copper Monolayer. J. Nanomater. 2016, 2016, 8429510. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Liu, Y.; Cen, W.; Luo, X. Functional Group Effects on the HOMO–LUMO Gap of g-C3N4. Nanomaterials 2018, 8, 589. [Google Scholar] [CrossRef] [PubMed]
- Li, W. Influence of electronic structures of doped TiO2 on their photocatalysis. Phys. Status Solidi (RRL)-Rapid Res. Lett. 2015, 9, 10–27. [Google Scholar] [CrossRef]
- Li, C.-G.; Shen, Z.-G.; Hu, Y.-F.; Tang, Y.-N.; Chen, W.-G.; Ren, B.-Z. Insights into the structures and electronic properties of Cun+1μ and CunSμ(n = 1–12; μ = 0, ±1) clusters. Sci. Rep. 2017, 7, 1345. [Google Scholar]
- Güven, M.H.; Eryürek, M. Dynamical and Structural Properties of the Ni4 Cluster. Phys. Status Solidi B 1999, 213, 283–295. [Google Scholar] [CrossRef]
- Grigoryan, V.G.; Springborg, M. Structural and energetic properties of nickel clusters: 2 ≤ N ≤ 150. Phys. Rev. B 2004, 70, 205415. [Google Scholar] [CrossRef]
- TURBOMOLE V6.3 2011, a.d.o.U.o.K.a.F.K.G., 1989-2007, and s.a.f.h.w.t.c. TURBOMOLE GmbH, TURBOMOLE V6.3 2011, A Development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, 1989-2007, TURBOMOLE GmbH, Since 2007. Available online: http://www.turbomole.com (accessed on 7 February 2011).
- Gao, W.; Abtew, T.A.; Cai, T.; Sun, Y.-Y.; Zhang, S.; Zhang, P. On the applicability of hybrid functionals for predicting fundamental properties of metals. Solid State Commun. 2016, 234–235, 10–13. [Google Scholar] [CrossRef]
- Haas, P.; Tran, F.; Blaha, P.; Schwarz, K.; Laskowski, R. Insight into the performance of GGA functionals for solid-state calculations. Phys. Rev. B 2009, 80, 195109. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Li, Y. Not One, Not Two, But at Least Three: Activity Origin of Copper Single-Atom Catalysts toward CO2/CO Electroreduction to C2+ Products. J. Am. Chem. Soc. 2024, 146, 14954–14958. [Google Scholar] [CrossRef]
- Fallberg, A.; Ottosson, M.; Carlsson, J.-O. Phase stability and oxygen doping in the Cu–N–O system. J. Cryst. Growth 2010, 312, 1779–1784. [Google Scholar] [CrossRef]
- Mavridou, K.; Zervos, M.; Pinakidou, F.; Brzhezinskaya, M.; Katsikini, M. Oxidation of Cu3N thin films obtained from Cu annealed under NH3:O2 flow: A Raman and N-K-edge NEXAFS study. J. Alloys Compd. 2022, 914, 165293. [Google Scholar] [CrossRef]
- Subhash, K.G.; Benoy, M.D.; Duraimurugan, J.; Siranjeevi, R.; Prabhu, S. Synthesis, and characterization of CuO/g-C3N4 nanocomposites for high performances supercapacitor application. Mater. Lett. 2023, 330, 133288. [Google Scholar] [CrossRef]
- Sung, C.-L.; Wang, R.-H.; Shih, Y.-C.; Wu, Z.-Y.; Alvarado, S.R.; Chang, Y.-H.; Lin, C.-C. g-C3N4 Nanosheet Supported CuO Nanocomposites for the Electrochemical Carbon Dioxide Reduction Reaction. ACS Omega 2023, 8, 7368–7377. [Google Scholar] [CrossRef]
- Suresh, R.; Karthikeyan, N.S.; Gnanasekaran, L.; Rajendran, S.; Soto-Moscoso, M. Facile synthesis of CuO/g-C3N4 nanolayer composites with superior catalytic reductive degradation behavior. Chemosphere 2023, 315, 137711. [Google Scholar] [CrossRef]







| System | Ebin [eV] | Enuc-gas [eV] | Enuc-SAC [eV] | Ereor [eV] |
|---|---|---|---|---|
| Cu1-C3N4 | −2.29 | - | - | - |
| Cu2-C3N4 | −1.35 | −1.14 | 1.15 | 0.02 |
| Cu3-C3N4 | −3.18 | −3.13 | −0.84 | 0.04 |
| Cu4-C3N4 | −3.64 | −3.01 | −0.72 | 1.09 |
| System | Ebin [eV] | Enuc-gas [eV] | Enuc-SAC [eV] | Ereor [eV] |
|---|---|---|---|---|
| Ni1-C3N4 | −4.86 | - | - | - |
| Ni2-C3N4 | −3.71 | −4.43 | 0.43 | 0.00 |
| Ni3-C3N4 | −2.41 | −2.72 | 2.14 | 0.72 |
| Ni4-C3N4 | −5.13 | −7.15 | −2.29 | 0.07 |
| System | Ebin [eV] | Enuc-gas (Cu) [eV] | Enuc-SAC (Cu) [eV] | Enuc-gas (Ni) [eV] | Enuc-SAC (Ni) [eV] | Ereor [eV] |
|---|---|---|---|---|---|---|
| CuNi-C3N4 | −2.72 | −1.38 | 0.91 | −3.95 | 0.91 | 0.02 |
| Cu2Ni-C3N4 | −3.48 | −2.62 | −0.33 | −5.43 | −0.57 | 0.08 |
| CuNi2-C3N4 | −3.83 | −1.99 | 0.30 | −5.03 | −0.18 | 0.09 |
| Cu2Ni2-C3N4 | −5.07 | −3.25 | −0.96 | −5.66 | −0.80 | 0.02 |
| Cu3Ni-C3N4 | −4.27 | −3.10 | −0.81 | −5.40 | −0.54 | 0.52 |
| CuNi3-C3N4 | −5.45 | −5.23 | −2.93 | −5.96 | −1.10 | 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drzewiecka-Matuszek, A.; Sharma, P.; Rutkowska-Zbik, D. Structure of Cu, Ni, and CuNi Bimetallic Small Clusters Incorporated in g-C3N4: A DFT Study. Catalysts 2025, 15, 861. https://doi.org/10.3390/catal15090861
Drzewiecka-Matuszek A, Sharma P, Rutkowska-Zbik D. Structure of Cu, Ni, and CuNi Bimetallic Small Clusters Incorporated in g-C3N4: A DFT Study. Catalysts. 2025; 15(9):861. https://doi.org/10.3390/catal15090861
Chicago/Turabian StyleDrzewiecka-Matuszek, Agnieszka, Priti Sharma, and Dorota Rutkowska-Zbik. 2025. "Structure of Cu, Ni, and CuNi Bimetallic Small Clusters Incorporated in g-C3N4: A DFT Study" Catalysts 15, no. 9: 861. https://doi.org/10.3390/catal15090861
APA StyleDrzewiecka-Matuszek, A., Sharma, P., & Rutkowska-Zbik, D. (2025). Structure of Cu, Ni, and CuNi Bimetallic Small Clusters Incorporated in g-C3N4: A DFT Study. Catalysts, 15(9), 861. https://doi.org/10.3390/catal15090861







